PEDOT:PSS/PEDOT Film Chemiresistive Sensors for Hydrogen Peroxide Vapor Detection under Ambient Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
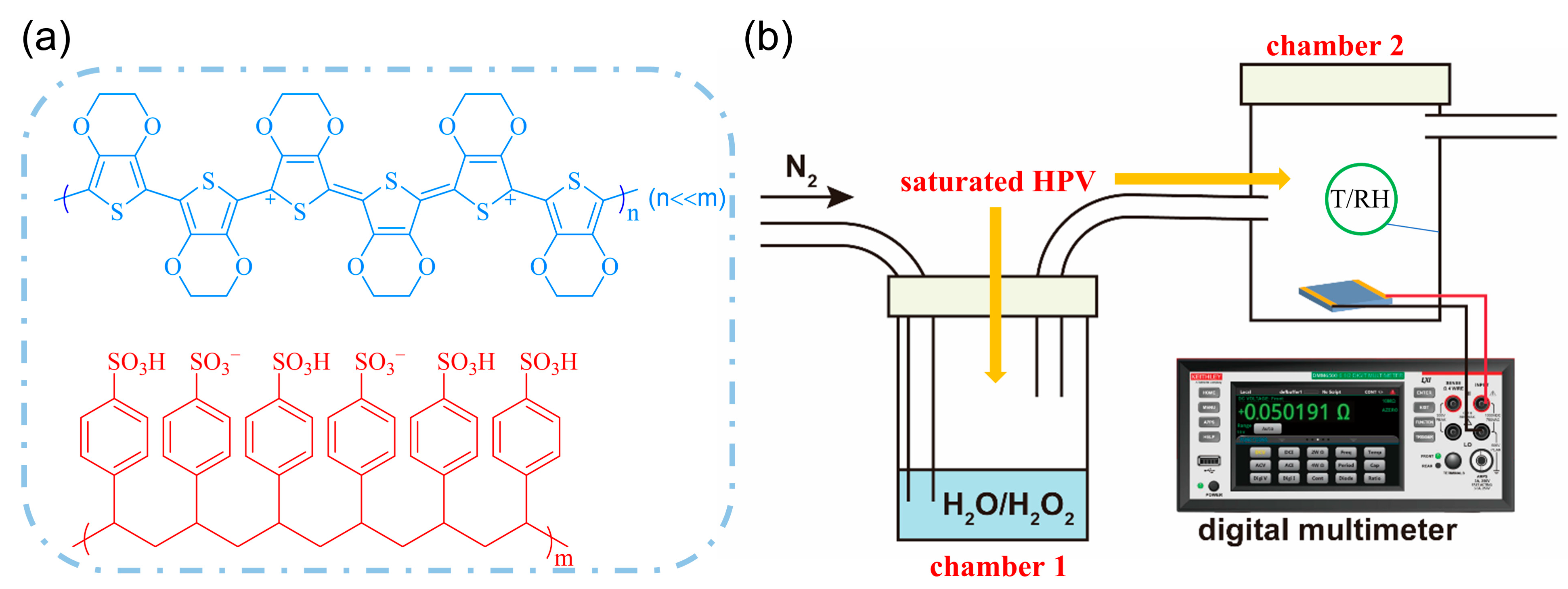
2.2. Preparation of PEDOT-Based Composite Films
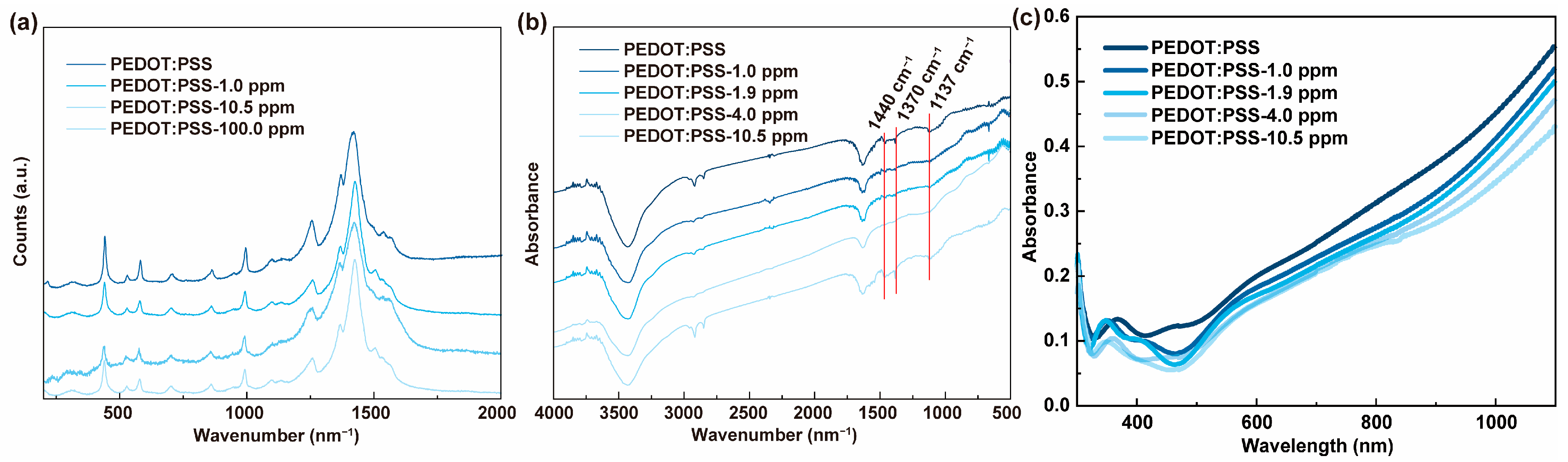
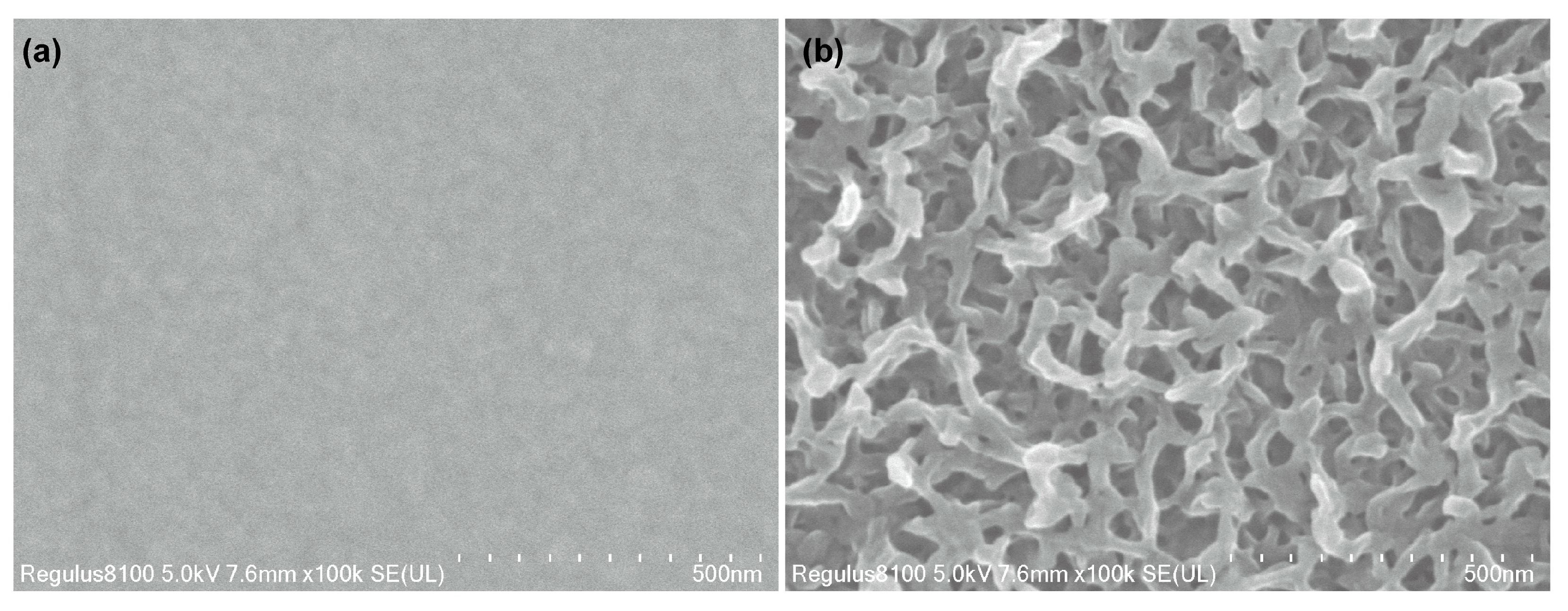
2.3. Characterization
2.4. Construction of HPV Detection System
3. Results and Discussion
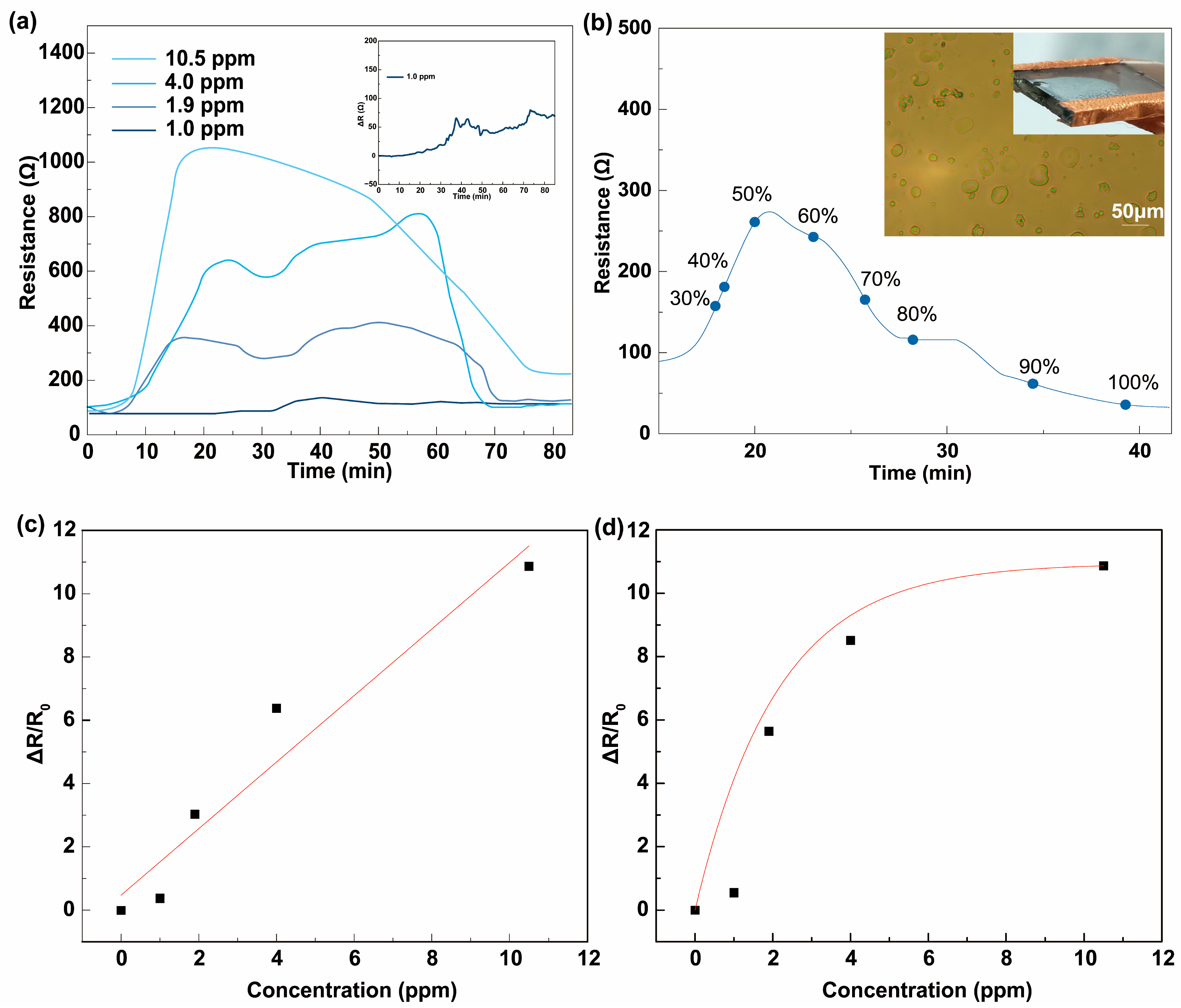
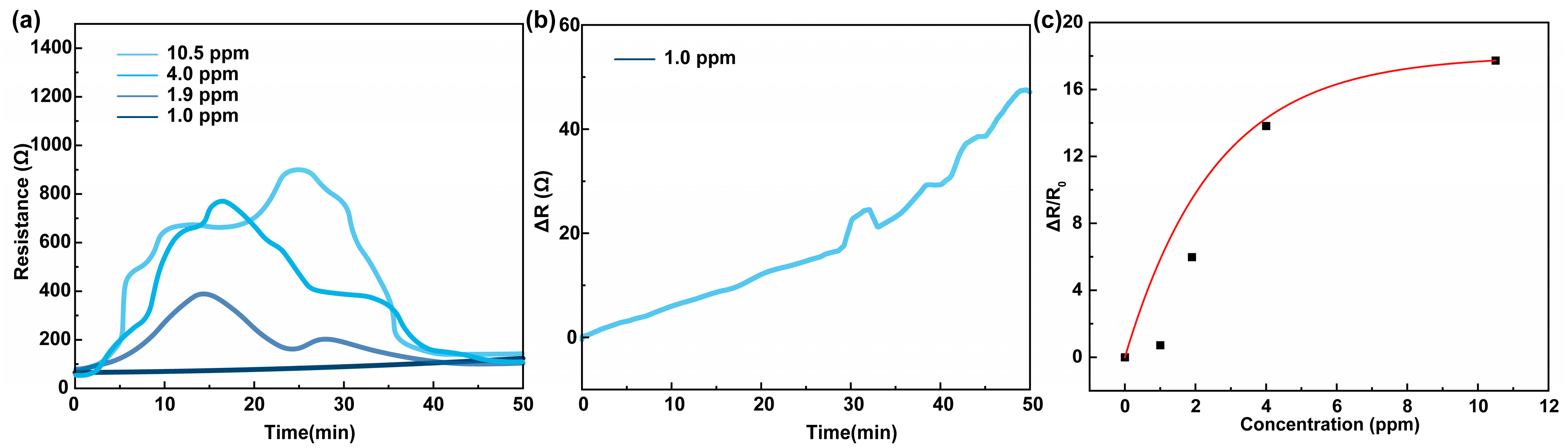
3.1. Chemiresistive HPV Sensing Based on PEDOT:PSS Film
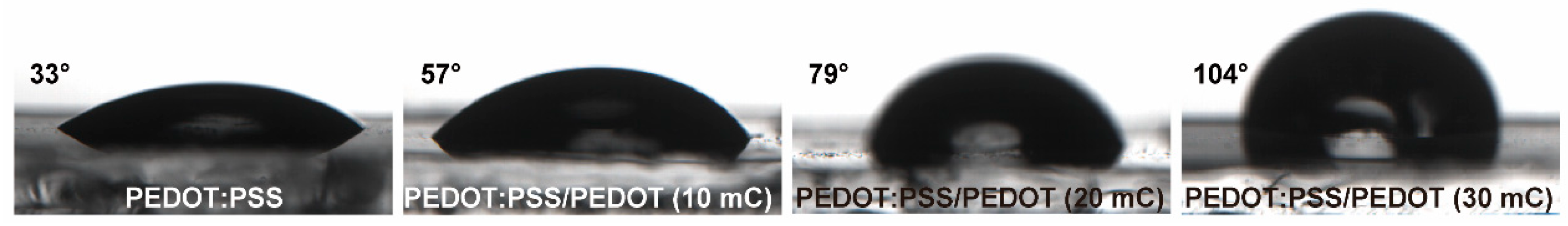
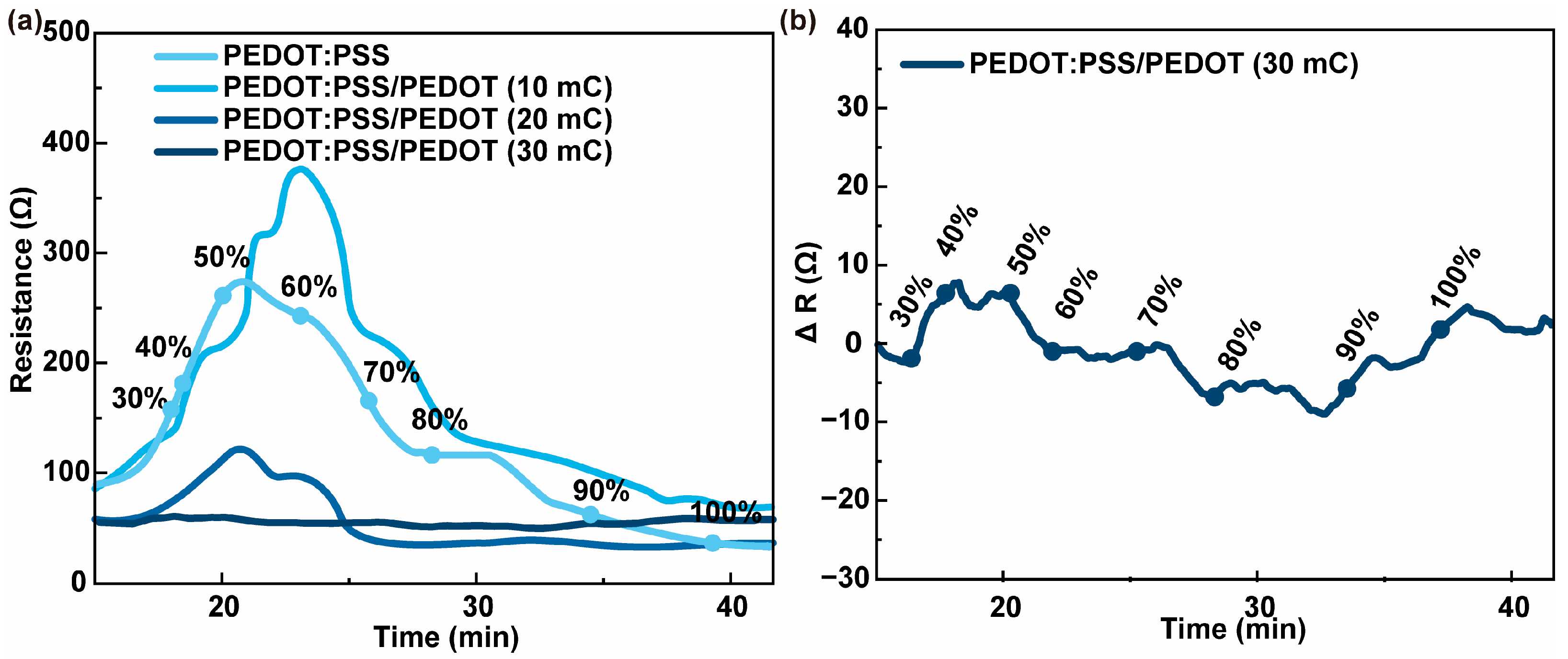
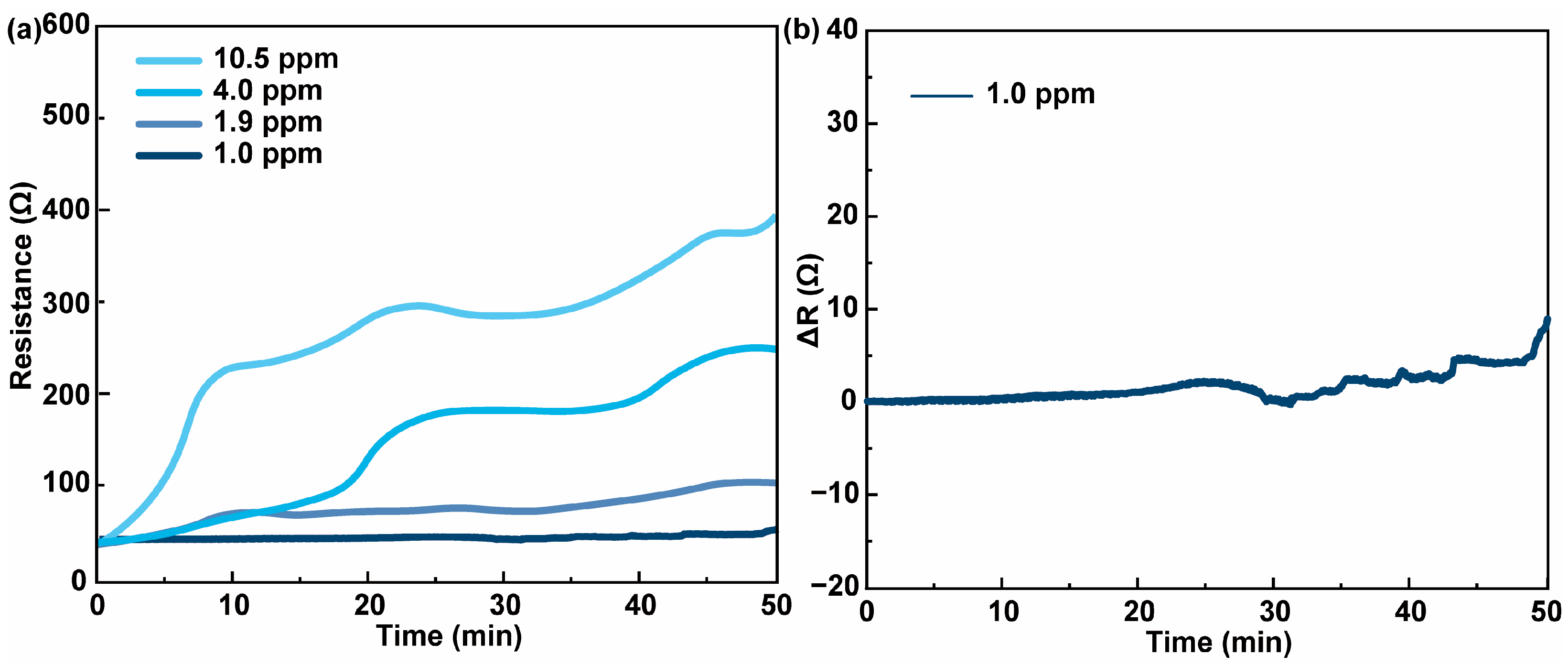
3.2. Chemiresistive HPV Sensing Based on PEDOT:PSS/PEDOT Film
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Berger, D.; Gundermann, G.; Sinha, A.; Moroi, M.; Goyal, N.; Tsai, A. Review of aerosolized hydrogen peroxide, vaporized hydrogen peroxide, and hydrogen peroxide gas plasma in the decontamination of filtering facepiece respirators. Am. J. Infect. Control 2022, 50, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Dewey, H.M.; Jones, J.M.; Keating, M.R.; Budhathoki-Uprety, J. Increased use of disinfectants during the COVID-19 pandemic and its potential impacts on health and safety. ACS Chem. Health Saf. 2022, 29, 27–38. [Google Scholar] [CrossRef]
- Holm, S.M.; Leonard, V.; Durrani, T.; Miller, M.D. Do we know how best to disinfect child care sites in the United States? A review of available disinfectant efficacy data and health risks of the major disinfectant classes. Am. J. Infect. Control 2019, 47, 82–91. [Google Scholar] [CrossRef]
- Mucci, N.; Dugheri, S.; Bonari, A.; Farioli, A.; Rapisarda, V.; Garzaro, G.; Cappelli, G.; Arcangeli, G. Health risk assessment related to hydrogen peroxide presence in the workplace atmosphere-analytical methods evaluation for an innovative monitoring protocol. Int. J. Occup. Med. Environ. Health 2020, 33, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Reisert, S.; Schneider, B.; Geissler, H.; Van Gompel, M.; Wagner, P.; Schöning, M.J. Multi-sensor chip for the investigation of different types of metal oxides for the detection of H2O2 in the ppm range. Phys. Status Solidi A Appl. Mater. Sci. 2013, 210, 898–904. [Google Scholar] [CrossRef]
- Nguyen, N.D.; Van Nguyen, T.; Chu, A.D.; Tran, H.V.; Tran, L.T.; Huynh, C.D. A label-free colorimetric sensor based on silver nanoparticles directed to hydrogen peroxide and glucose. Arab. J. Chem. 2017, 11, 1134–1143. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, P.; Xu, Z.; Chen, M.; Ding, Y.; Yue, K.; Xu, J. A facile strategy to prepare porphyrin functionalized ZnS nanoparticles and their peroxidase-like catalytic activity for colorimetric sensor of hydrogen peroxide and glucose. Sens. Actuators B 2017, 251, 339–348. [Google Scholar] [CrossRef]
- Siao, H.W.; Chen, S.M.; Lin, K.C. Electrochemical study of PEDOT-PSS-MDB-modified electrode and its electrocatalytic sensing of hydrogen peroxide. J. Solid State Electrochem. 2010, 15, 1121–1128. [Google Scholar] [CrossRef]
- Xu, J.; Peng, R.; Ran, Q.; Xian, Y.; Tian, Y.; Jin, L. A highly soluble poly(3,4-ethylenedioxythiophene)-poly(styrene sulfonic acid)/Au nanocomposite for horseradish peroxidase immobilization and biosensing. Talanta 2010, 82, 1511–1515. [Google Scholar] [CrossRef]
- Moßhammer, M.; Kühl, M.; Koren, K. Possibilities and challenges for quantitative optical sensing of hydrogen peroxide. Chemosensors 2017, 5, 28. [Google Scholar] [CrossRef]
- Xu, M.; Bunes, B.R.; Zang, L. Paper-based vapor detection of hydrogen peroxide: Colorimetric sensing with tunable interface. ACS Appl. Mater. Interfaces 2011, 3, 642–647. [Google Scholar] [CrossRef]
- Xu, M.; Han, J.M.; Zhang, Y.Q.; Yang, X.M.; Zang, L. A selective fluorescence turn-on sensor for trace vapor detection of hydrogen peroxide. Chem. Commun. 2013, 49, 11779–11781. [Google Scholar] [CrossRef]
- Xu, M.; Han, J.M.; Wang, C.; Yang, X.M.; Pei, J.; Zang, L. Fluorescence ratiometric sensor for trace vapor detection of hydrogen peroxide. ACS Appl. Mater. Interfaces 2014, 6, 8708–8714. [Google Scholar] [CrossRef]
- Chen, Q.Q.; Yang, L.; Guo, K.K.; Yang, J.L.; Han, J.M. Expedite fluorescent sensor prototype for hydrogen peroxide detection with long-life test substrates. ACS Omega 2021, 6, 11447–11457. [Google Scholar] [CrossRef]
- Gao, N.; Yu, J.Y.; Tian, Q.Y.; Shi, J.; Zhang, M.; Chen, S.; Zang, L. Application of PEDOT:PSS and its composites in electrochemical and electronic chemosensors. Chemosensors 2021, 9, 79. [Google Scholar] [CrossRef]
- Aroutiounian, V.; Arakelyan, V.; Aleksanyan, M.; Shahnazaryan, G.; Kacer, P.; Picha, P.; Kovarik, J.; Pekarek, J.; Joost, B. Thin-film SnO2 and ZnO detectors of hydrogen peroxide vapors. J. Sens. Sens. Syst. 2018, 7, 281–288. [Google Scholar] [CrossRef]
- Shahkhatuni, G.H.; Aroutiounian, V.M.; Arakelyan, V.M.; Aleksanyan, M.S.; Shahnazaryan, G.E. Investigation of sensor made of ZnO:La for detection of hydrogen peroxide vapours by impedance spectroscopy method. J. Contemp. Phys. Armen. Acad. Sci. 2019, 54, 188–195. [Google Scholar] [CrossRef]
- Adamyan, Z.N.; Sayunts, A.G.; Khachaturyan, E.A.; Araqelyan, V.M.; Aroutiounian, V.M.; Joost, B. Study of hydrogen peroxide vapors sensors based on carbon nanotubes coated with tin oxide nanoparticles. J. Contemp. Phys. Armen. Acad. Sci. 2019, 54, 57–64. [Google Scholar] [CrossRef]
- Verma, A.L.; Saxena, S.; Saini, G.S.S.; Gaur, V.; Jain, V.K. Hydrogen peroxide vapor sensor using metal-phthalocyanine functionalized carbon nanotubes. Thin Solid Film. 2011, 519, 8144–8148. [Google Scholar] [CrossRef]
- Lee, D.J.; Choi, S.W.; Byun, Y.T. Room temperature monitoring of hydrogen peroxide vapor using platinum nanoparticles-decorated single-walled carbon nanotube networks. Sens. Actuators B Chem. 2018, 256, 744–750. [Google Scholar] [CrossRef]
- Hasani, A.; Dehsari, H.S.; Gavgani, J.N.; Shalamzari, E.K.; Salehi, A.; Afshar Taromi, F.; Mahyari, M. Sensor for volatile organic compounds using an interdigitated gold electrode modified with a nanocomposite made from poly(3,4-ethylenedioxythiophene)-poly(styrenesulfonate) and ultra-large graphene oxide. Microchim. Acta 2015, 182, 1551–1559. [Google Scholar] [CrossRef]
- Shinde, S.; Jiang, C.Y.; Zheng, C.X.; Wang, Y.Z.; Lin, K.M.; Koinkar, P.M. Room-temperature and flexible PEDOT:PSS-WO3 gas sensor for nitrogen dioxide detection. Mod. Phys. Lett. B 2019, 33, 1940013. [Google Scholar] [CrossRef]
- Tang, L.; Wu, S.; Qu, J.; Gong, L.; Tang, J. A review of conductive hydrogel used in flexible strain Ssensor. Materials 2020, 13, 3947. [Google Scholar] [CrossRef] [PubMed]
- Rahimzadeh, Z.; Naghib, S.M.; Zare, Y.; Rhee, K.Y. An overview on the synthesis and recent applications of conducting poly(3,4-ethylenedioxythiophene) (PEDOT) in industry and biomedicine. J. Mater. Sci. 2020, 55, 7575–7611. [Google Scholar] [CrossRef]
- Giaretta, J.E.; Oveissi, F.; Dehghani, F.; Naficy, S. Paper-based, chemiresistive sensor for hydrogen peroxide detection. Adv. Mater. Technol. 2021, 6, 2001148. [Google Scholar] [CrossRef]
- Manatt, S.L.; Manatt, M.R.R. On the analyses of mixture vapor pressure data: The hydrogen peroxide/water system and its excess thermodynamic functions. Chem. A Eur. J. 2004, 10, 6540–6557. [Google Scholar] [CrossRef]
- Taccola, S.; Greco, F.; Zucca, A.; Innocenti, C.; Fernández, C.D.J.; Campo, G.; Sangregorio, C.; Mazzolai, B.; Mattoli, V. Characterization of free-standing PEDOT:PSS/iron oxide nanoparticle composite thin films and application as conformable humidity sensors. ACS Appl. Mater. Interfaces 2013, 5, 6324–6332. [Google Scholar] [CrossRef]
- Aziz, S.; Chang, D.E.; Doh, Y.H.; Kang, C.U.; Choi, K.H. Humidity sensor based on PEDOT:PSS and zinc stannate nano-composite. J. Electron. Mater. 2015, 44, 3992–3999. [Google Scholar] [CrossRef]
- Romero, F.J.; Rivadeneyra, A.; Becherer, M.; Morales, D.P.; Rodríguez, N. Fabrication and characterization of humidity sensors based on graphene oxide-PEDOT:PSS composites on a flexible substrate. Micromachines 2020, 11, 148. [Google Scholar] [CrossRef]
- Popov, V.I.; Kotin, I.A.; Nebogatikova, N.A.; Smagulova, S.A.; Antonova, I.V. Graphene-PEDOT:PSS humidity sensors for high sensitive, low-cost, highly-reliable, flexible, and printed electronics. Materials 2019, 12, 3477. [Google Scholar] [CrossRef]
- Sakamoto, S.; Okumura, M.; Zhao, Z.; Furukawa, Y. Raman spectral changes of PEDOT-PSS in polymer light-emitting diodes upon operation. Chem. Phys. Lett. 2005, 412, 395–398. [Google Scholar] [CrossRef]
- Bhowal, A.C.; Talukdar, H.; Kundu, S. Preparation, characterization and electrical behaviors of PEDOT:PSS-Au/Ag nanocomposite thin films: An ecofriendly approach. Polym. Bull. 2019, 76, 5233–5251. [Google Scholar] [CrossRef]
- Yan, Y.; Yang, G.; Xu, J.L.; Zhang, M.; Kuo, C.C.; Wang, S.D. Conducting polymer-inorganic nanocomposite-based gas sensors: A review. Sci. Technol. Adv. Mater. 2021, 21, 768–786. [Google Scholar] [CrossRef]
- Andò, B.; Baglio, S.; Di Pasquale, G.; Pollicino, A.; Graziani, S.; Gugliuzzo, C.; Lombardo, C.; Marletta, V. Direct printing of a multi-layer sensor on pet substrate for CO2 detection. Energies 2019, 12, 557. [Google Scholar] [CrossRef]
- Dunst, K.; Karczewski, J.; Jasiński, P. Nitrogen dioxide sensing properties of PEDOT polymer films. Sens. Actuators B Chem. 2017, 247, 108–113. [Google Scholar] [CrossRef]
- Yang, B.G.; Yao, F.L.; Ye, L.; Hao, T.; Zhang, Y.B.; Zhang, L.; Dong, D.Y.; Fang, W.C.; Wang, Y.; Zhang, X.Y.; et al. A conductive PEDOT/alginate porous scaffold as a platform to modulate the biological behaviors of brown adipose-derived stem cells. Biomater. Sci. 2020, 8, 3173–3185. [Google Scholar] [CrossRef]
- Pendyala, P.; Kim, H.N.; Grewal, H.S.; Chae, U.; Yang, S.; Cho, I.J.; Song, S.; Yoon, E.S. Internal-flow-mediated, tunable one-dimensional Cassie-to-Wenzel wetting transition on superhydrophobic microcavity surfaces during evaporation. Nanoscale Microscale Thermophys. Eng. 2019, 23, 275–288. [Google Scholar] [CrossRef]
- Azimi, A.; He, P. Effect of gravity in the Cassie-to-Wenzel transition on a micropatterned surface. MRS Commun. 2020, 10, 129–134. [Google Scholar] [CrossRef]
| HPV (ppm) | PEDOT:PSS Film | PEDOT:PSS/PEDOT Film |
|---|---|---|
| 1.0 | 0.54 | 0.72 |
| 1.9 | 5.64 | 5.98 |
| 4.0 | 8.51 | 13.82 |
| 10.5 | 10.87 | 17.73 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, X.; Gao, N.; Zhu, L.; Hunter, M.; Chen, S.; Zang, L. PEDOT:PSS/PEDOT Film Chemiresistive Sensors for Hydrogen Peroxide Vapor Detection under Ambient Conditions. Chemosensors 2023, 11, 124. https://doi.org/10.3390/chemosensors11020124
Xie X, Gao N, Zhu L, Hunter M, Chen S, Zang L. PEDOT:PSS/PEDOT Film Chemiresistive Sensors for Hydrogen Peroxide Vapor Detection under Ambient Conditions. Chemosensors. 2023; 11(2):124. https://doi.org/10.3390/chemosensors11020124
Chicago/Turabian StyleXie, Xiaowen, Nan Gao, Ling Zhu, Matthew Hunter, Shuai Chen, and Ling Zang. 2023. "PEDOT:PSS/PEDOT Film Chemiresistive Sensors for Hydrogen Peroxide Vapor Detection under Ambient Conditions" Chemosensors 11, no. 2: 124. https://doi.org/10.3390/chemosensors11020124
APA StyleXie, X., Gao, N., Zhu, L., Hunter, M., Chen, S., & Zang, L. (2023). PEDOT:PSS/PEDOT Film Chemiresistive Sensors for Hydrogen Peroxide Vapor Detection under Ambient Conditions. Chemosensors, 11(2), 124. https://doi.org/10.3390/chemosensors11020124








