Abstract
Trigger finger is a common yet vastly understudied fibroproliferative hand pathology, severely affecting patients’ quality of life. Consistent trauma due to inadequate positioning within the afflicted finger’s tendon/pulley system leads to cellular dysregulation and eventual fibrosis. While the genetic characteristics of the fibrotic tissue in the trigger finger have been studied, the pathways that govern the initiation and propagation of fibrosis are still unknown. The complete gene expression profile of the trigger finger has never been explored. Our study has used the Nanostring nCounter gene expression assay to investigate the molecular signaling involved in trigger finger pathogenesis. We collected samples from patients undergoing trigger finger (n = 4) release surgery and compared the gene expression to carpal tunnel tissue (n = 4). Nanostring nCounter analysis identified 165 genes that were differentially regulated; 145 of these genes were upregulated, whereas 20 genes were downregulated. We found that several collagen genes were significantly upregulated, and a regulatory matrix metalloproteinase (MMP), MMP-3, was downregulated. Bioinformatic analysis revealed that several known signaling pathways were dysregulated, such as the TGF-β1 and Wnt signaling pathways. We also found several novel signaling pathways (e.g., PI3K, MAPK, JAK-STAT, and Notch) differentially regulated in trigger finger. The outcome of our study helps in understanding the molecular signaling pathway involved in the pathogenesis of the trigger finger.
1. Introduction
Trigger finger, also known as stenosing tenosynovitis, is a musculoskeletal disorder in which a finger gets “locked” in either a flexed or extended position due to the disproportion between the diameter of that finger’s flexor tendon and pulley system. Friction is generated as the flexor tendon glides through the pulley and creates an intratendinous lump, leading to common manifestations of the trigger finger [1,2]. Histologically, the normal musculoskeletal connective tissue found in the pulley system shows abnormal characteristics with small collagen fibers and abundant extracellular matrix (ECM) proteins, along with fibrocartilage metaplasia [3,4,5]. The most common symptom of this disorder is the “catching” of the finger in question in a flexed position, in addition to pain, clicking, and loss of motion in the finger. These symptoms characterize trigger finger as one of the most common causes of hand pain in adults. Although not defined as a life-threatening condition, the pain and discomfort due to untreated trigger fingers are reported to cause significant debilitation for patients [6,7]. Treatment options of those suffering from trigger finger vary between noninvasive and invasive options, depending upon the severity of the condition. Patients can opt for treatment that ranges from noninvasive splinting, corticosteroid injections, shockwave therapy, or invasive surgical release [8,9]. Studies have suggested that the best and most cost-effective treatment of trigger finger is an immediate surgical release or corticosteroid injections followed by an eventual surgical release [9].
The gene expression profiling of the trigger finger pathogenesis has not been fully explored and only investigated a few selected extracellular matrix (ECM)-related genes. Previously, our group [10] and others [11] have reported elevated levels of ECM (collagen type 1a1, collagen type 3a1, aggrecan, and biglycan) and downregulation of MMP-3 and TIMP-3 [12]. The changes in expression levels of these genes can result in ECM imbalance and possibly eventual molecular pathogenesis of the trigger finger. The studies mentioned above [10,11,12] focused on ECM and growth factors genes, which did not provide a complete gene expression profile of trigger finger pathogenesis. Our study attempted to investigate the comprehensive gene expression profile of ECM and inflammatory signaling pathways using Nanostring technology to uncover possible trigger finger molecular etiologies.
The Nanostring nCounter Gene Expression Assay is a high-fidelity, simple protocol that allows the detection of up to 800 genes in a single reaction. The assay digitally detects mRNA molecules of interest using specific probes. The first probe anneals to the 5′-end of the target gene, which enables molecular barcoding downstream. The second probe carries a biotin marker which allows the anchoring of the gene for downstream detection. The genes are then immobilized and analyzed using their corresponding color codes to identify the expression levels of each of the molecules of interest [13]. The Nanostring nCounter Gene Expression Assay removed the need for any tedious enzymatic reactions and has also been proven simpler and more effective compared to other alternatives such as SYBR Green real-time PCR [14,15,16,17,18,19,20]. The Nanostring nCounter Gene Expression Assay tool has also been previously used to profile pathogenic gene expression profiles during infection [21,22]. We aimed to understand the molecular pathways that lead to fibrotic tissue generation in trigger finger. To the best of our knowledge, no studies have investigated the full breadth of differential gene expression in the trigger finger condition.
In this study, we collected tissue samples from the patients visited for trigger finger and carpal tunnel release surgery. We considered the carpal tunnel tissue samples as a control. Total RNA was isolated, and the Nanostring nCounter Gene Expression Assay was performed. We identified several differentially regulated genes in the trigger finger. Our goal for this study was to identify possible molecular pathways that lead to the pathogenesis of the trigger finger. Identifying potential genes or biomarkers would serve as valuable information for the future treatment of patients suffering from trigger finger.
2. Materials and Methods
2.1. Ethical Approval and Informed Consent
All relevant national policies and institutional regulations were followed according to the Helsinki Declaration to conduct our research on human tissue samples. All steps of this protocol were reviewed, audited, and accepted by the Augusta University Institutional Review Board (IRBNet ID: 611626-4) or the equivalent governing body. Informed consent was obtained from all patients undergoing the indicated procedures.
2.2. Obtaining Patient Samples
Experimental tissue specimens were collected from the patients undergoing A1 pulley trigger finger release surgery for symptomatic trigger finger (TF) at the Augusta University Medical Center. Control tissue specimens were collected from the patients undergoing carpal tunnel release surgery at the Augusta University Medical Center. We confirmed that patients with carpal tunnel syndrome had no clinical evidence or history of previous trigger finger before collecting tissue samples. Patient characteristics are described in Table 1. All surgeries were performed by a practicing, board-certified hand and upper-extremity surgeon employed by the Department of Orthopedic Surgery. Patient samples were then classified into two groups: trigger finger (n = 4) and carpal tunnel syndrome (n = 4) as the control samples. Specimens were then directly transported from the operating room to the laboratory. They were all snap-frozen and kept at −80 °C [10].

Table 1.
Characteristics of Patients used for tissue samples.
2.3. RNA Isolation and NanoString’s nCounter XT Gene Expression Assay
Total RNA was isolated from tissues as per the published method [10]. In brief, the frozen tissue samples were ground with liquid N2 using a mortar and pestle. The RNA was isolated using Trizol as per the manufacturer’s instructions. The quality of the RNA was measured by absorbance at 260 nm and 280 nm (Helios-Gamma, Thermo Spectronic, Rochester, NY, USA). We used NanoString’s nCounter (NanoString Technologies, Inc. 530 Fairview Ave N, Seattle, WA, USA) technology for gene expression comparison between different groups at GEM labs, LLC, (Department of Pathology, Augusta University). NanoString’s nCounter technology is based on digital detection and direct molecular barcoding of individual target molecules through the use of a unique probe pair for each target of interest. The probe pair consists of a color-coded Reporter probe, which carries the visible signal on its 5′ end, and a Capture probe, which carries a biotin moiety on the 3′ end. One hundred nanograms of total RNA (OD260/280 ratio 1.7–2.2) is hybridized overnight (>12 h) with reporter and capture code set at 65 °C, and excess probes are washed away using a two-step magnetic bead-based purification on an nCounter instrument. Finally, the purified target-probe complexes are eluted off the beads, immobilized on the cartridge, and aligned for data collection. Data collection was performed using epifluorescence microscopy and CCD capture technology on an nCounter instrument to yield hundreds of thousands of target molecule counts. Digital images are processed within the nCounter instrument, and the Reporter Probe counts are tabulated in a comma separated value (CSV) format for convenient data analysis with NanoString’s free nSolver™ Analysis Software V.3 (NanoString Technologies, Inc. 530 Fairview Ave N, Seattle, WA, USA).
2.4. Statistical Method
In this study, the nCounter PanCancer Pathways panel that included 770 genes from 13 canonical pathways (see Supplementary Table S1 for gene and probe information). These gene sets covered diverse biological pathways such as Notch, Wnt, Hedgehog, chromatin modification, transcriptional misregulation, DNA damage repair, TGFβ, MAPK, JAK-STAT, PI3K, Ras, cell cycle, and apoptosis. The samples were read at 555 FOV (Field of view) and resulting RCC data files were analyzed for QC in nSolver 3.0. Subsequent analyses were performed using the nCounter Advanced Analysis 2.0 plug-in (NanoString Technologies, Inc. 530 Fairview Ave N, Seattle, Washington, USA). The gene expression normalization was performed using the geNorm algorithm that selected the best housekeeping genes from the initial list of 40 genes (attached). To visualize the results, unsupervised clustering was used to generate heatmap based on the QC passed, normalized data counts of individual genes. Differential expression was graphed as a volcano plot with individual genes −log10 (p-value) and log2 fold change compared to the control group. Pathview module was used to display overexpressed genes (gold color) or downregulated genes (blue color) overlaid on KEGG pathways.
3. Results
3.1. Global Gene Expression Profile of Trigger Finger Samples Compared to Control
We used the Nanostring nSolver software to elucidate the differentially regulated genes in trigger finger samples compared to carpal tunnel control samples. The heatmap generated after raw data analysis (Figure 1) indicates distinct expression profiles for both up- and downregulated genes. The volcano plot (Figure 2) shows all samples plotted as a function of fold change vs. p-value.
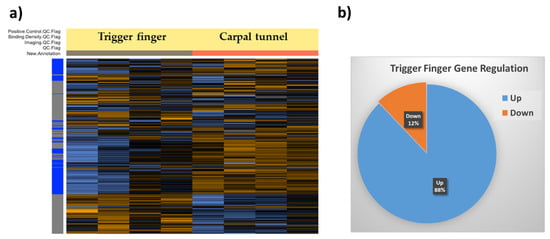
Figure 1.
(a) Heatmap of normalized data generated via unsupervised clustering by the Nanostring nSolver software. Heatmap is scaled to give all genes equal variance. Control samples (n = 4) are organized under the orange column, and trigger finger samples (n = 4) are organized under the gray column. Within the gene clusters, orange indicates high expression, and blue indicates low expression. (b) Pie chart showing the percentage of genes up- and downregulated in trigger finger compared to carpal tunnel samples.
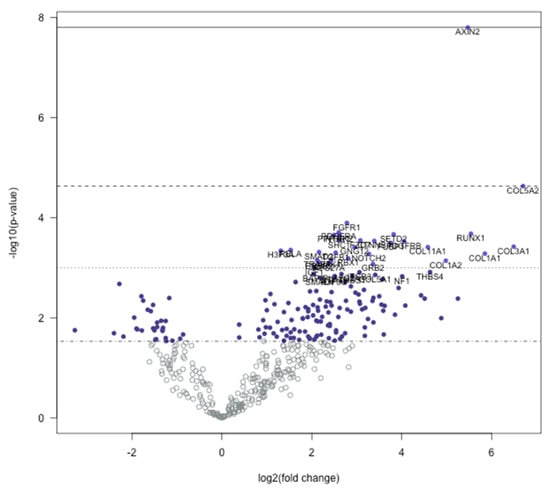
Figure 2.
Volcano plot displaying each gene tested plotted comparing −log10 (p-value) and log2 fold change. Horizontal lines on the plot describe statistical significance; thus, highly significant values are at the top of the plot. Highly differentially expressed genes are at the horizontal extremes of the plot. The 40 most statistically significant values are highlighted in the plot.
Genes that exhibited a significant (p < 0.05) and 1.4-fold change in expression compared to the control group were selected. Overall, 165 genes were differentially regulated; 145 genes were upregulated, whereas 20 were downregulated. The overall fold changes of each of these genes and the pathways they impact are shown in Table 2. It is encouraging that our findings coincide with those previously reported by us [10] and others [12].

Table 2.
Table of log2 fold change, p-value, and genetic pathway impact for 165 genes with fold change values ±1.40. Twenty genes were downregulated, whereas 145 genes were upregulated for trigger finger samples, as compared to controls.
The genes with the highest positive fold-change differences were three collagen genes, COL5A2 (6.7), COL3A1 (6.49), and COL1A1 (5.85). In addition to these three upregulated collagen genes, four other collagen transcribing genes were upregulated within the 145 isolated upregulated genes, COL1A2 (4.98), COL11A1 (4.58), COL5A1 (3.41), and COL2A1 (2.67). All upregulated collagen-transcribing genes impacted the PI3K genetic signaling pathway. In addition to these collagen transcribing genes, RUNX1 and IGF1 genes were also upregulated, impacting the common transcriptional misregulation pathway. Other notable upregulated genes included AXIN2 (5.47), PPP3CB (2.76), PPP3R1 (2.49), CCND1 (2.33), SMAD4 (2.18), SMAD2 (2.16), and RAC1 (2.03). These genes all impacted the Wnt signaling pathway.
The gene with the most negative fold-change difference was MMP-3 (−3.27) with a primary impact on the transcriptional misregulation pathway. There were no collagen-transcribing genes with negative fold-change values <−1.40. Other notable genes that were downregulated included NODAL (−2.4) and LEFTY1 (0.00994), both with a primary impact on the TGF-beta signaling pathway.
3.2. Signaling Pathway Predictions
The Nanostring nSolver software allowed for signaling pathway prediction through its directed global significance score ratings (Table 3). This statistic measures the extent of up- and downregulation compared to the control of a distinct signaling pathway. In addition to the global significance score ratings, a comprehensive roadmap generated by the Nanostring nSolver software of the genetic pathway known as PathView with both positive and negative regulatory effects is shown in Supplementary Figure S1.

Table 3.
Global significance ratings comparing overall differential expression of selected pathways relative to control.
The pathway with the highest global significance rating in trigger finger samples compared to controls was the Wnt signaling pathway with a score of 6.268 (Figure 3). Other significant upregulated pathways included the PI3K signaling pathway (3.283), the TGF-beta signaling pathway (2.951), and the transcriptional misregulation pathway (2.648). Two pathways with global significance score ratings less than 1 were the chromatin modification pathway (0.579) and the Hedgehog pathway (0.273).
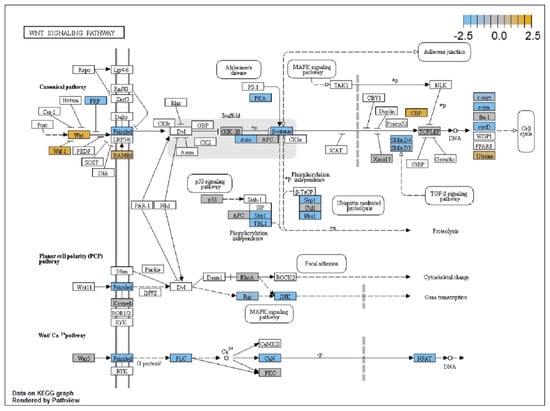
Figure 3.
Pathview analysis done by NanoString nSolver software showing a comprehensive pathway roadmap for differentially expressed genes within WNT signaling pathways. Elements overexpressed are shown in gold, elements underexpressed are shown in blue, and elements with unchanged expression are shown in gray.
4. Discussion
Trigger finger is widely understood as a “mild” hand pathology but is a condition that renders significant pain in patients, which greatly impacts quality of life [23]. The molecular mechanism of the trigger finger and the potential pathways that lead to trigger finger pathogenesis are still unknown. Previously, our group [10] and others [12] demonstrated alteration in extracellular matrix (ECM) (collagen 1a1, collagen 3a1, matrix metallopeptidase (MMP)-2, MMP-3, ADAMTS-5, TIMP-3, aggrecan, biglycan, decorin, and versican) and growth factor (TGF-b and IGF) genes.
Our study utilized the Nanostring nCounter Gene Expression Assay, which simultaneously detects up to 800 genes in a single reaction. We identified 165 statistically significant genes that were differentially regulated in trigger finger, compared to carpal tunnel. To our knowledge, our study is the first study to conduct a comprehensive gene expression analysis on trigger finger to understand its pathogenesis. ECM genes (seven collagens) were significantly upregulated, which is no surprise. Collagens have long been known to be the most abundant fibrous protein in the ECM that provides structural support and cellular strength, along with tissue repair and remodeling capabilities [24,25,26,27]. In the context of tendinopathies, it has been previously reported that collagen types I, III, and V are increased in proportion to other collagens and contribute to the mechanical weakness of the diseased tendon [28,29,30]. Basal production and degradation of collagen is a balanced equilibrium that ensures proper systemic functioning of the ECM and body. This equilibrium is further maintained through the function of MMP enzymes that work to degrade various ECM proteins such as collagens, proteoglycans, and many other ECM components [31,32,33]. In our study, MMP-3 was significantly (−3.27) downregulated in trigger finger samples. MMP-3 is an enzyme that degrades fibronectin, gelatin, and type 1 collagen, among many other ECM components, and it directly activates pro-collagenases such as MMP-1, MMP-7, MMP-8, MMP-9, and MMP-13 [34,35,36]. Thus, the downregulation of MMP-3 has wide-ranging effects that could potentially explain the vast build-up of collagen proteins in trigger finger [37]. Previously, Riley et al. [38] reported that the activity of MMP-3 (compared to MMP-1 and MMP-2 in tendon pathologies) was significantly reduced, which leads to increased turnover and deterioration in the quality of the collagen network [38]. The change in ECM remodeling activity has been known to be associated with an onset of tendinopathy, and this phenomenon could be due to the imbalance between collagen production and MMP-mediated collagen degradation [39]. Thus, the overabundance of collagen can be attributed to decreased MMPs expression, potentially leading to the fibroproliferation of formerly healthy finger tendons and, ultimately, trigger finger.
Fibrosis is defined as the overgrowth, hardening, and/or scarring of tissues due to the abnormal deposition of ECM components, such as collagen [40]. Fibrotic tissue generation is dependent on the production of collagen from myofibroblast cells that are dependent on various signaling pathways triggered by a multitude of genetic factors [5,40,41,42]. In the trigger finger, persistent tissue injury on the pathological flexor tendon eventually triggers fibrosis, but the exact signaling and/or molecular pathway is still a mystery [43,44]. One factor that was considerably upregulated in our study is TGF-β1 (2.53). TGF-β1 is a known stimulator in the molecular pathogenesis of fibrosis in another notable musculoskeletal fibroproliferative hand pathology, Dupuytren’s contracture [42,45,46,47]. In Dupuytren’s contracture, TGF-β1 acts as a growth factor that induces fibroblast contraction within pathological tissues, leading to deformation at the cellular level [48]. Overstimulation of TGF-β1 stimulates the Wnt/β-catenin pathway by decreasing the expression of the Wnt pathway antagonist, Dickkopf-1 [49,50]. Multiple Wnt signaling genes such as RAC1 (2.03), SMAD2 (2.16), SMAD4 (2.18), CCND1 (2.33), PPP3R1 (2.49), PPP3CB (2.76), and AXIN2 (5.47) were significantly upregulated in the trigger finger. Bioinformatics analysis showed that Wnt signaling was the most upregulated cellular pathway, with a directed differential expression rating of 6.268 compared to control. TGF-β1-mediated Wnt signaling has been proven in other studies to regulate fibroproliferation in lung fibrosis, renal fibrosis, skin fibrosis, musculoskeletal fibrosis, and liver fibrosis which could potentially mediate fibrosis in trigger finger [51,52]. Lederhose disease [53,54], adhesive capsulitis [55,56,57], and Peyronie’s disease [45,58,59] are prominent fibroproliferative disorders that share molecular characteristics with Dupuytren’s contracture. We believe that the trigger finger also shares many of the same molecular characteristics as these fibrotic disorders.
We also noted that the “transcriptional misregulation” pathway was upregulated. One of the genes of this pathway, RUNX1 (RUNX family transcription factor 1), was upregulated with a foldchange of 5.54. RUNX1 interacts with other proteins to play important and dynamic roles in ribosome biogenesis, cell-cycle regulation, and TGF-β1 signaling regulation [60,61]. Upregulation of RUNX1 is known to play a role in the increased cellular commitment of mesenchymal stem cells to myofibroblasts [62]. Elevated levels of RUNX1 could lead to many manifestations of the trigger finger: increased myofibroblast activity, increased collagen production, and fibrosis of the finger tendon. Another gene that was upregulated was IGF-1 (insulin-like growth factor 1) (2.06), a known hormone that has diverse roles in regulating growth on almost every cell in the body [63]. In the context of tissue repair, IGF-1 can modulate the conversion of fibroblasts to myofibroblasts and, thus, stimulate the production of collagen [64,65,66,67]. The upregulation of IGF-1 and its downstream effects on collagen production could also contribute to collagen’s overabundance leading to fibrotic tissue generation. Both of these genetic factors, IGF-1 and RUNX1, being a regulatory hormone and a transcription factor, respectively, have a multitude of effects outside of tissue repair and collagen production. Our study identified several genes (Table 1) and signaling pathways (Table 2) dysregulated in the trigger finger and might be involved in the pathogenesis.
Our study had certain limitations. We used a limited number of samples but enough for a proof-of-concept study. Our control group was also not an “actual” control as carpal tunnel tissue is not healthy but diseased tissue. It was complicated to obtain healthy controls due to age-matching restrictions and the ethical limitations of conducting surgery on healthy individuals. Overall, our pilot study found several novel genes and signaling pathways involved in the pathophysiology of trigger finger. The outcome of our study will further help us in understanding the molecular signaling pathways involved in the pathogenesis and designing therapeutic strategies for the treatment of the trigger finger.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/healthcare9111592/s1, Table S1: Gene and Probe Information. Figure S1: Pathview analysis done by NanoString nSolver software showing a comprehensive pathway roadmap for differentially expressed genes within various KEGG pathways of our samples. Elements over-expressed are shown in gold, elements under-expressed are shown in blue, and elements with unchanged expression are shown in gray.
Author Contributions
S.F. and M.F. conceived the idea for the manuscript, and drafted the initial manuscript with U.G., and R.K.; methodology, A.K.M., C.P., U.G.; software, P.A., N.S.S.; validation, A.K.M., C.P., N.S.S. and C.M.I.; formal analysis, J.C.; resources, S.F., R.K., C.M.I.; data curation, J.C., A.K.M., C.P.; writing—original draft preparation, S.F., U.G., R.K.; writing—review and editing, C.M.I., M.F., R.K., J.C.; supervision, S.F.; project administration, S.F.; funding acquisition, S.F., R.K. All authors have read and agreed to the published version of the manuscript.
Funding
Funding was provided by Department of Orthopedic Surgery, Department of Pathology, and Division of Endocrine, Augusta University, GA.
Institutional Review Board Statement
All relevant national policies and institutional regulations were followed according to the Helsinki Declaration to conduct our research on human tissue samples. All steps of this protocol were reviewed, audited, and accepted by the Augusta University Institutional Review Board (IRBNet ID: 611626-4) or the equivalent governing body.
Informed Consent Statement
Informed consent was obtained from all patients.
Data Availability Statement
The data that support the findings of this study will be available from the corresponding author, upon reasonable request.
Acknowledgments
We would like to thank the Department of Orthopedic Surgery, Department of Pathology, and Division of Endocrine for their support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fiorini, H.J.; Tamaoki, M.J.; Lenza, M.; Gomes Dos Santos, J.B.; Faloppa, F.; Belloti, J.C. Surgery for trigger finger. Cochrane Database Syst. Rev. 2018, 2, CD009860. [Google Scholar] [CrossRef]
- Jeanmonod, R.; Waseem, M. Trigger Finger. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2019. [Google Scholar]
- Sbernardori, M.C.; Bandiera, P. Histopathology of the A1 pulley in adult trigger fingers. J. Hand Surg. Eur. Vol. 2007, 32, 556–559. [Google Scholar] [CrossRef]
- Sampson, S.P.; Badalamente, M.A.; Hurst, L.C.; Seidman, J. Pathobiology of the human A1 pulley in trigger finger. J. Hand Surg. Am. 1991, 16, 714–721. [Google Scholar] [CrossRef]
- Niumsawatt, V.; Mao, D.; Salerno, S.; Rozen, W.M. Trigger finger release with stepwise preservation of the A1 pulley: A functional pulley-preserving technique. Int. Surg. 2013, 98, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Langer, D.; Luria, S.; Michailevich, M.; Maeir, A. Long-term functional outcome of trigger finger. Disabil. Rehabil. 2018, 40, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Langer, D.; Maeir, A.; Michailevich, M.; Luria, S. Evaluating Hand Function in Clients with Trigger Finger. Occup. Ther. Int. 2017, 2017, 9539206. [Google Scholar] [CrossRef] [PubMed]
- Makkouk, A.H.; Oetgen, M.E.; Swigart, C.R.; Dodds, S.D. Trigger finger: Etiology, evaluation, and treatment. Curr. Rev. Musculoskelet. Med. 2008, 1, 92–96. [Google Scholar] [CrossRef]
- Matthews, A.; Smith, K.; Read, L.; Nicholas, J.; Schmidt, E. Trigger finger: An overview of the treatment options. JAAPA 2019, 32, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Cain, M.; Awad, M.E.; Kolhe, R.; Mondal, A.K.; Ghilzai, U.; Isales, C.; Fulcher, M.; Fulzele, S. Dysregulation of epigenetic related genes in Diabetic Trigger finger Patients; preliminary analysis of Patient-Derived Samples. Biomol. Concepts 2020, 11, 221–229. [Google Scholar] [CrossRef]
- Abate, M.; Schiavone, C.; Salini, V.; Andia, I. Occurrence of tendon pathologies in metabolic disorders. Rheumatology 2013, 52, 599–608. [Google Scholar] [CrossRef]
- Lundin, A.C.; Aspenberg, P.; Eliasson, P. Trigger finger, tendinosis, and intratendinous gene expression. Scand. J. Med. Sci. Sports 2014, 24, 363–368. [Google Scholar] [CrossRef]
- Kulkarni, M.M. Digital multiplexed gene expression analysis using the NanoString nCounter system. Curr. Protoc. Mol. Biol. 2011, 94, 25B.10.1–25B.10.17. [Google Scholar] [CrossRef]
- Geiss, G.K.; Bumgarner, R.E.; Birditt, B.; Dahl, T.; Dowidar, N.; Dunaway, D.L.; Fell, H.P.; Ferree, S.; George, R.D.; Grogan, T.; et al. Direct multiplexed measurement of gene expression with color-coded probe pairs. Nat. Biotechnol. 2008, 26, 317–325. [Google Scholar] [CrossRef]
- Veldman-Jones, M.H.; Brant, R.; Rooney, C.; Geh, C.; Emery, H.; Harbron, C.G.; Wappett, M.; Sharpe, A.; Dymond, M.; Barrett, J.C.; et al. Evaluating Robustness and Sensitivity of the NanoString Technologies nCounter Platform to Enable Multiplexed Gene Expression Analysis of Clinical Samples. Cancer Res. 2015, 75, 2587–2593. [Google Scholar] [CrossRef]
- Saba, N.F.; Wilson, M.; Doho, G.; DaSilva, J.; Benjamin Isett, R.; Newman, S.; Chen, Z.G.; Magliocca, K.; Rossi, M.R. Mutation and Transcriptional Profiling of Formalin-Fixed Paraffin Embedded Specimens as Companion Methods to Immunohistochemistry for Determining Therapeutic Targets in Oropharyngeal Squamous Cell Carcinoma (OPSCC): A Pilot of Proof of Principle. Head Neck Pathol. 2015, 9, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Scott, D.W.; Chan, F.C.; Hong, F.; Rogic, S.; Tan, K.L.; Meissner, B.; Ben-Neriah, S.; Boyle, M.; Kridel, R.; Telenius, A.; et al. Gene expression-based model using formalin-fixed paraffin-embedded biopsies predicts overall survival in advanced-stage classical Hodgkin lymphoma. J. Clin. Oncol. 2013, 31, 692–700. [Google Scholar] [CrossRef] [PubMed]
- Norton, N.; Sun, Z.; Asmann, Y.W.; Serie, D.J.; Necela, B.M.; Bhagwate, A.; Jen, J.; Eckloff, B.W.; Kalari, K.R.; Thompson, K.J.; et al. Gene expression, single nucleotide variant and fusion transcript discovery in archival material from breast tumors. PLoS ONE 2013, 8, e81925. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, T.; Wallden, B.; Schaper, C.; Ferree, S.; Liu, S.; Gao, D.; Barry, G.; Dowidar, N.; Maysuria, M.; Storhoff, J. Analytical validation of the PAM50-based Prosigna Breast Cancer Prognostic Gene Signature Assay and nCounter Analysis System using formalin-fixed paraffin-embedded breast tumor specimens. BMC Cancer 2014, 14, 177. [Google Scholar] [CrossRef]
- Dowsett, M.; Sestak, I.; Lopez-Knowles, E.; Sidhu, K.; Dunbier, A.K.; Cowens, J.W.; Ferree, S.; Storhoff, J.; Schaper, C.; Cuzick, J. Comparison of PAM50 risk of recurrence score with oncotype DX and IHC4 for predicting risk of distant recurrence after endocrine therapy. J. Clin. Oncol. 2013, 31, 2783–2790. [Google Scholar] [CrossRef]
- Xu, W.; Solis, N.V.; Filler, S.G.; Mitchell, A.P. Pathogen Gene Expression Profiling During Infection Using a Nanostring nCounter Platform. Methods Mol. Biol. 2016, 1361, 57–65. [Google Scholar] [CrossRef]
- Tsang, H.F.; Xue, V.W.; Koh, S.P.; Chiu, Y.M.; Ng, L.P.; Wong, S.C. NanoString, a novel digital color-coded barcode technology: Current and future applications in molecular diagnostics. Expert Rev. Mol. Diagn. 2017, 17, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Zychowicz, M.E. A closer look at hand and wrist complaints. Nurse Pract. 2013, 38, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Frantz, C.; Stewart, K.M.; Weaver, V.M. The extracellular matrix at a glance. J. Cell Sci. 2010, 123, 4195–4200. [Google Scholar] [CrossRef]
- Gordon, M.K.; Hahn, R.A. Collagens. Cell Tissue Res. 2010, 339, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Kadler, K.E.; Baldock, C.; Bella, J.; Boot-Handford, R.P. Collagens at a glance. J. Cell Sci. 2007, 120, 1955–1958. [Google Scholar] [CrossRef]
- Rozario, T.; DeSimone, D.W. The extracellular matrix in development and morphogenesis: A dynamic view. Dev. Biol. 2010, 341, 126–140. [Google Scholar] [CrossRef]
- Birch, H.L.; Bailey, A.J.; Goodship, A.E. Macroscopic ’degeneration’ of equine superficial digital flexor tendon is accompanied by a change in extracellular matrix composition. Equine Vet. J. 1998, 30, 534–539. [Google Scholar] [CrossRef]
- Mead, M.P.; Gumucio, J.P.; Awan, T.M.; Mendias, C.L.; Sugg, K.B. Pathogenesis and Management of Tendinopathies in Sports Medicine. Transl. Sports Med. 2018, 1, 5–13. [Google Scholar] [CrossRef]
- Riley, G.P.; Harrall, R.L.; Constant, C.R.; Chard, M.D.; Cawston, T.E.; Hazleman, B.L. Tendon degeneration and chronic shoulder pain: Changes in the collagen composition of the human rotator cuff tendons in rotator cuff tendinitis. Ann. Rheum. Dis. 1994, 53, 359–366. [Google Scholar] [CrossRef]
- Nagase, H.; Visse, R.; Murphy, G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc. Res. 2006, 69, 562–573. [Google Scholar] [CrossRef]
- Newby, A.C. Dual role of matrix metalloproteinases (matrixins) in intimal thickening and atherosclerotic plaque rupture. Physiol. Rev. 2005, 85, 1–31. [Google Scholar] [CrossRef]
- Visse, R.; Nagase, H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: Structure, function, and biochemistry. Circ. Res. 2003, 92, 827–839. [Google Scholar] [CrossRef]
- Ye, S.; Eriksson, P.; Hamsten, A.; Kurkinen, M.; Humphries, S.E.; Henney, A.M. Progression of coronary atherosclerosis is associated with a common genetic variant of the human stromelysin-1 promoter which results in reduced gene expression. J. Biol. Chem. 1996, 271, 13055–13060. [Google Scholar] [CrossRef]
- Flores-Pliego, A.; Espejel-Nunez, A.; Castillo-Castrejon, M.; Meraz-Cruz, N.; Beltran-Montoya, J.; Zaga-Clavellina, V.; Nava-Salazar, S.; Sanchez-Martinez, M.; Vadillo-Ortega, F.; Estrada-Gutierrez, G. Matrix Metalloproteinase-3 (MMP-3) Is an Endogenous Activator of the MMP-9 Secreted by Placental Leukocytes: Implication in Human Labor. PLoS ONE 2015, 10, e0145366. [Google Scholar] [CrossRef]
- Sternlicht, M.D.; Bissell, M.J.; Werb, Z. The matrix metalloproteinase stromelysin-1 acts as a natural mammary tumor promoter. Oncogene 2000, 19, 1102–1113. [Google Scholar] [CrossRef]
- Milner, J.M.; Elliott, S.F.; Cawston, T.E. Activation of procollagenases is a key control point in cartilage collagen degradation: Interaction of serine and metalloproteinase pathways. Arthritis Rheum. 2001, 44, 2084–2096. [Google Scholar] [CrossRef]
- Riley, G.P.; Curry, V.; DeGroot, J.; van El, B.; Verzijl, N.; Hazleman, B.L.; Bank, R.A. Matrix metalloproteinase activities and their relationship with collagen remodelling in tendon pathology. Matrix Biol. 2002, 21, 185–195. [Google Scholar] [CrossRef]
- Riley, G. Tendinopathy--from basic science to treatment. Nat. Clin. Pract. Rheumatol. 2008, 4, 82–89. [Google Scholar] [CrossRef]
- Wynn, T.A. Cellular and molecular mechanisms of fibrosis. J. Pathol. 2008, 214, 199–210. [Google Scholar] [CrossRef]
- Graham, J.G.; Wang, M.L.; Rivlin, M.; Beredjiklian, P.K. Biologic and mechanical aspects of tendon fibrosis after injury and repair. Connect. Tissue Res. 2019, 60, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Hinz, B. Formation and function of the myofibroblast during tissue repair. J. Investig. Dermatol. 2007, 127, 526–537. [Google Scholar] [CrossRef]
- Ceran, F.; Basat, S.O.; Basaran, K.; Saydam, F.A. Five-Years Trigger Finger Due to Partial Flexor Tendon Laceration in a Child. J. Hand Microsurg. 2015, 7, 228–229. [Google Scholar] [CrossRef] [PubMed]
- Nichols, A.E.C.; Best, K.T.; Loiselle, A.E. The cellular basis of fibrotic tendon healing: Challenges and opportunities. Transl. Res. 2019, 209, 156–168. [Google Scholar] [CrossRef] [PubMed]
- O’Gorman, D.B.; Vi, L.; Gan, B.S. Molecular mechanisms and treatment strategies for Dupuytren’s disease. Ther. Clin. Risk Manag. 2010, 6, 383–390. [Google Scholar] [CrossRef]
- Badalamente, M.A.; Sampson, S.P.; Hurst, L.C.; Dowd, A.; Miyasaka, K. The role of transforming growth factor beta in Dupuytren’s disease. J. Hand Surg. Am. 1996, 21, 210–215. [Google Scholar] [CrossRef]
- Kloen, P.; Jennings, C.L.; Gebhardt, M.C.; Springfield, D.S.; Mankin, H.J. Transforming growth factor-beta: Possible roles in Dupuytren’s contracture. J. Hand Surg. Am. 1995, 20, 101–108. [Google Scholar] [CrossRef]
- Zhou, C.; Liu, F.; Gallo, P.H.; Baratz, M.E.; Kathju, S.; Satish, L. Anti-fibrotic action of pirfenidone in Dupuytren’s disease-derived fibroblasts. BMC Musculoskelet. Disord. 2016, 17, 469. [Google Scholar] [CrossRef][Green Version]
- Akhmetshina, A.; Palumbo, K.; Dees, C.; Bergmann, C.; Venalis, P.; Zerr, P.; Horn, A.; Kireva, T.; Beyer, C.; Zwerina, J.; et al. Activation of canonical Wnt signalling is required for TGF-beta-mediated fibrosis. Nat. Commun. 2012, 3, 735. [Google Scholar] [CrossRef] [PubMed]
- Cheon, S.S.; Nadesan, P.; Poon, R.; Alman, B.A. Growth factors regulate beta-catenin-mediated TCF-dependent transcriptional activation in fibroblasts during the proliferative phase of wound healing. Exp. Cell Res. 2004, 293, 267–274. [Google Scholar] [CrossRef]
- Piersma, B.; Bank, R.A.; Boersema, M. Signaling in Fibrosis: TGF-beta, WNT, and YAP/TAZ Converge. Front. Med. (Lausanne) 2015, 2, 59. [Google Scholar] [CrossRef]
- Guo, Y.; Xiao, L.; Sun, L.; Liu, F. Wnt/beta-catenin signaling: A promising new target for fibrosis diseases. Physiol. Res. 2012, 61, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Classen, D.A.; Hurst, L.N. Plantar fibromatosis and bilateral flexion contractures: A review of the literature. Ann. Plast. Surg. 1992, 28, 475–478. [Google Scholar] [CrossRef]
- Donato, R.R.; Morrison, W.A. Dupuytren’s disease in the feet causing flexion contractures in the toes. J. Hand Surg. Br. 1996, 21, 364–366. [Google Scholar] [CrossRef]
- Bunker, T.D.; Reilly, J.; Baird, K.S.; Hamblen, D.L. Expression of growth factors, cytokines and matrix metalloproteinases in frozen shoulder. J. Bone Jt. Surg. Br. 2000, 82, 768–773. [Google Scholar] [CrossRef]
- Bunker, T.D.; Anthony, P.P. The pathology of frozen shoulder. A Dupuytren-like disease. J. Bone Joint Surg. Br. 1995, 77, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.P.; Devaraj, V.S.; Bunker, T.D. The association between frozen shoulder and Dupuytren’s disease. J. Shoulder Elbow Surg. 2001, 10, 149–151. [Google Scholar] [CrossRef]
- Qian, A.; Meals, R.A.; Rajfer, J.; Gonzalez-Cadavid, N.F. Comparison of gene expression profiles between Peyronie’s disease and Dupuytren’s contracture. Urology 2004, 64, 399–404. [Google Scholar] [CrossRef]
- Carrieri, M.P.; Serraino, D.; Palmiotto, F.; Nucci, G.; Sasso, F. A case-control study on risk factors for Peyronie’s disease. J. Clin. Epidemiol. 1998, 51, 511–515. [Google Scholar] [CrossRef]
- Chuang, L.S.; Ito, K.; Ito, Y. RUNX family: Regulation and diversification of roles through interacting proteins. Int. J. Cancer 2013, 132, 1260–1271. [Google Scholar] [CrossRef]
- Cai, X.; Gao, L.; Teng, L.; Ge, J.; Oo, Z.M.; Kumar, A.R.; Gilliland, D.G.; Mason, P.J.; Tan, K.; Speck, N.A. Runx1 Deficiency Decreases Ribosome Biogenesis and Confers Stress Resistance to Hematopoietic Stem and Progenitor Cells. Cell Stem Cell 2015, 17, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Barron, D.A.; San Martin, R.; Chan, K.S.; Tran, L.L.; Yang, F.; Ressler, S.J.; Rowley, D.R. RUNX1 is essential for mesenchymal stem cell proliferation and myofibroblast differentiation. Proc. Natl. Acad. Sci. USA 2014, 111, 16389–16394. [Google Scholar] [CrossRef]
- Yakar, S.; Rosen, C.J.; Beamer, W.G.; Ackert-Bicknell, C.L.; Wu, Y.; Liu, J.L.; Ooi, G.T.; Setser, J.; Frystyk, J.; Boisclair, Y.R.; et al. Circulating levels of IGF-1 directly regulate bone growth and density. J. Clin. Investig. 2002, 110, 771–781. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Lee, D.Y. Insulin-like growth factor (IGF)-I and IGF binding proteins axis in diabetes mellitus. Ann. Pediatr. Endocrinol. Metab. 2015, 20, 69–73. [Google Scholar] [CrossRef]
- Dahlgren, L.A.; van der Meulen, M.C.; Bertram, J.E.; Starrak, G.S.; Nixon, A.J. Insulin-like growth factor-I improves cellular and molecular aspects of healing in a collagenase-induced model of flexor tendinitis. J. Orthop. Res. 2002, 20, 910–919. [Google Scholar] [CrossRef]
- Kurtz, C.A.; Loebig, T.G.; Anderson, D.D.; DeMeo, P.J.; Campbell, P.G. Insulin-like growth factor I accelerates functional recovery from Achilles tendon injury in a rat model. Am. J. Sports Med. 1999, 27, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Achar, R.A.; Silva, T.C.; Achar, E.; Martines, R.B.; Machado, J.L. Use of insulin-like growth factor in the healing of open wounds in diabetic and non-diabetic rats. Acta Cirurgica Bras. 2014, 29, 125–131. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).