Abstract
Background: Filgotinib is an emerging Janus kinase 1 (JAK1) inhibitor being investigated for inflammatory bowel disease. This systematic review and network meta-analysis (NMA) evaluated the efficacy and safety of filgotinib in adult patients with moderate-to-severe crohn’s disease. Methods: We systematically searched PubMed, EMBASE, and Scopus through April 2025. Randomized controlled trials evaluating filgotinib versus placebo in adults with moderate-to-severe Crohn’s disease were included. Primary outcomes were clinical remission and endoscopic response. Study quality was assessed using the Cochrane Risk of Bias 2.0 tool. A network meta-analysis was performed to integrate direct and indirect evidence, reporting risk ratios (RRs) with 95% confidence intervals (CIs). Results: Five randomized controlled trials (from 4 publications) met the inclusion criteria. Filgotinib 200 mg significantly improved clinical remission compared with placebo (RR: 1.75 [1.40–2.19]) and 100 mg (RR: 1.38 [1.11–1.71]), while 100 mg showed no significant difference versus placebo (RR: 1.27 [0.99–1.63]). For endoscopic response, both 200 mg (RR: 1.72 [1.09–2.69]) and 100 mg (RR: 1.65 [1.02–2.69]) demonstrated significant benefit over placebo, though no difference was observed between active doses (RR: 1.04 [0.64–1.68]; I2 = 57%). In the two-item patient-reported outcome, 200 mg showed significant improvement versus placebo (RR: 1.47 [1.20–1.80]) and 100 mg (RR: 1.26 [1.02–1.55]), while 100 mg remained insignificant versus placebo (RR: 1.17 [0.93–1.46]). Neither dose increased the risk of treatment-emergent adverse events, serious adverse events, or infections compared with placebo, with consistent homogeneity across analyses. Conclusions: Filgotinib 200 mg demonstrated superior efficacy across clinical, endoscopic, and patient-reported outcomes compared with 100 mg and placebo, with a favorable safety profile. The 100 mg dose showed limited efficacy and no advantage over placebo. Filgotinib represents a promising oral therapeutic option, particularly for biologic-naïve patients and in maintenance therapy, while also showing potential benefit in perianal fistulising crohn’s disease. Future trials should explore long-term safety and head-to-head comparisons with established biologics.
1. Introduction
Crohn’s disease (CD) represents a chronic inflammatory disorder of the gastrointestinal tract characterized by transmural inflammation, often presenting with abdominal pain, diarrhea, weight loss, and extraintestinal manifestations [1]. With a global prevalence of 3–20 cases per 100,000 individuals and rising incidence rates particularly in Western populations, CD imposes substantial disease burden, diminished quality of life, and increased healthcare expenditures [2]. Despite significant advancements in therapeutic approaches over the past two decades, approximately 30–40% of patients demonstrate primary non-response to current biological therapies, with an additional 30–50% experiencing secondary loss of response over time, underscoring the critical need for novel therapeutic agents with distinct mechanisms of action [3,4].
Janus kinase (JAK) inhibitors have emerged as a promising therapeutic class in immune-mediated inflammatory disorders through their capacity to modulate multiple cytokine signaling pathways simultaneously [5]. Filgotinib, a selective JAK1 inhibitor, has demonstrated particular interest due to its preferential inhibition profile that potentially enhanced efficacy while minimizing adverse events commonly associated with pan-JAK inhibition [6]. The oral administration route of filgotinib presents a noteworthy advantage over currently available parenteral biological therapies, potentially improving treatment adherence and patient satisfaction [7].
Recent clinical trials investigating filgotinib in moderate-to-severe CD have reported encouraging outcomes regarding clinical remission, endoscopic response, and mucosal healing parameters. The FITZROY phase II trial provided initial evidence of efficacy with a favorable short-term safety profile [8]. Subsequent phase III investigations within the DIVERSITY clinical program have expanded the evidence base regarding filgotinib’s therapeutic potential in both induction and maintenance settings across various CD patient populations, including those with prior anti-TNF exposure or failure [9].
Previous conventional meta-analysis suggested that filgotinib 200 mg provides superior short-term clinical remission and mucosal healing compared with placebo, while the 100 mg dose shows limited efficacy, with both doses maintaining acceptable safety profiles [10]. However, those analyses were restricted to pairwise comparisons and did not formally assess the relative efficacy between doses.
This network meta-analysis (NMA) aims to evaluate the comparative efficacy and safety of filgotinib 100 mg and 200 mg versus placebo in moderate-to-severe CD. By integrating both direct and indirect evidence, we assessed clinical remission rates, patient-reported outcomes, endoscopic response, and adverse events, thereby providing a comprehensive and dose-specific evaluation of filgotinib’s therapeutic profile.
2. Materials and Methods
This Frequentist NMA was conducted in accordance with the guidelines of the Cochrane Handbook for Systematic Reviews of Interventions and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [11,12]. The protocol was registered on OSF (https://doi.org/10.17605/OSF.IO/WT7DK).
2.1. Eligibility Criteria
We included randomized controlled trials (RCTs) that evaluated at least one of the predefined outcomes related to clinical remission, endoscopic response, patient-reported outcomes, or adverse events of filgotinib in adult patients (≥18 years) with moderate-to-severe CD. We excluded non-randomized trials, observational studies, reviews, letters, conference abstracts, and ongoing or unpublished trials without sufficient data. Participants were required to have a clinical diagnosis of CD, with no restrictions placed on prior treatment exposure, disease duration, or sex.
Eligible interventions included filgotinib administered orally at a dose of either 100 mg or 200 mg once daily, compared to placebo. The primary efficacy outcomes were clinical remission, defined as a CD Activity Index (CDAI) score of less than 150, and endoscopic response, defined as a reduction of at least 50% in the Simple Endoscopic Score for CD (SES-CD). Secondary efficacy outcomes included improvement in the two-item patient-reported outcome (PRO2), a composite score based on daily stool frequency and self-reported abdominal pain, with remission defined as “7 × (mean daily number of liquid or very soft stools) + 7 × (mean daily self-reported abdominal pain)”. Safety outcomes included the incidence of treatment-emergent adverse events (TEAEs), serious TEAEs, and any infection.
2.2. Information Sources, Search Strategy, and Study Selection
We searched PubMed, EMBASE, and Scopus from database inception until April 2025, without language or publication date restrictions. We also manually screened the reference lists of included studies and relevant review articles to identify additional eligible trials. The search strategy combined MeSH terms and keywords related to filgotinib and CD. Full search strategies for each database are provided in Table S1. All identified records were imported into EndNote software 21.3, and duplicates were removed. Four reviewers independently screened titles and abstracts to assess eligibility. Full-text articles of potentially relevant studies were retrieved and assessed for inclusion. Discrepancies were resolved by consensus or consultation with a fifth reviewer.
2.3. Data Collection Process
A standardized data extraction form was used to collect relevant information from each included study. Three reviewers independently extracted data on study characteristics, participant demographics, intervention and comparator details, outcome definitions and results, follow-up duration, baseline data, and outcomes. Discrepancies between reviewers were resolved through discussion.
2.4. Risk of Bias Assessment
The methodological quality of each included study was assessed using the Cochrane Risk of Bias 2.0 (RoB 2) tool [13]. Three reviewers independently evaluated the risk of bias across five domains: the randomization process, deviations from intended interventions, missing outcome data, measurement of the outcome, and selection of the reported result. Each domain was rated as low risk of bias, some concerns, or high risk of bias. Any disagreements were resolved through consensus.
2.5. Statistical Analysis
A network meta-analysis was performed to compare filgotinib 100 mg, 200 mg, and placebo by synthesizing both direct and indirect evidence using R programming. The analysis was conducted within a frequentist statistical framework. For dichotomous outcomes, we calculated pooled risk ratios (RRs) with 95% confidence intervals (CIs). A random-effects model and leave-one-out test were used to account for expected clinical and methodological heterogeneity across studies. Heterogeneity was assessed using the Chi-squared test and quantified using the I2 statistic, with a p value less than 0.10 considered indicative of substantial heterogeneity. Publication bias and small-study effects were assessed using comparison-adjusted funnel plots and Egger’s regression test for each outcome.
The assumption of transitivity was evaluated by ensuring that the included trials were sufficiently similar in terms of patient characteristics, interventions, and outcome definitions to justify indirect comparisons within the network framework.
2.6. Certainty of Evidence
The certainty of evidence for each outcome was assessed using the GRADE (Grading of Recommendations, Assessment, Development and Evaluation) approach [14]. The assessment considered study design, risk of bias, inconsistency, indirectness, imprecision, and potential publication bias. Randomized controlled trials (RCTs) were initially rated as high-quality evidence and subsequently downgraded based on the applicable domains. The overall quality of evidence for each outcome was categorized as high, moderate, low, or very low.
3. Results
Out of 679 records identified from PubMed, EMBASE, and Scopus, 306 duplicates were removed. After screening 373 records, 363 were excluded. Of the 10 reports assessed for eligibility, 6 were excluded because they were not RCTs or conference abstracts. Finally, 5 studies (in 4 manuscripts) were included in both the systematic review and meta-analysis [8,9,15,16], Figure 1.
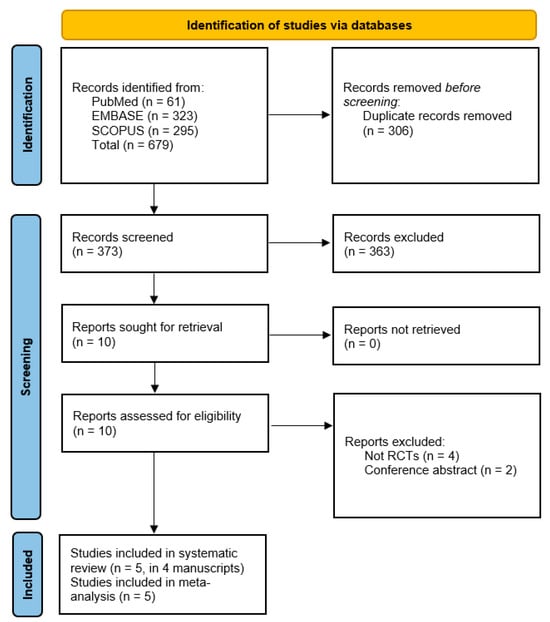
Figure 1.
PRISMA flow diagram of study selection.
3.1. Baseline Characteristics and Summary of the Included Studies
The average age ranged from 35.1 to 46 years, with female representation varying from 43% to 71.9%. Disease duration averaged between 6.8 and 14.6 years, indicating a chronic condition. Baseline CDAI scores ranged from 190 to 309, while SES-CD scores were reported in some studies, indicating endoscopic disease activity. Most participants had a history of previous interventions, including anti-TNF agents and immunomodulators, and were on concomitant medications such as oral steroids and antibiotics. Further details are shown in Table 1 and Table 2.

Table 1.
Baseline characteristics of the included studies.

Table 2.
Summary of included studies.
3.2. Quality Assessment
Using the ROB 2 tool, the quality assessment of the included studies revealed low risk of bias in most domains. Vermeire et al. (2017), Vermeire et al. (2025) (study A and Study B), and D’Haens et al. (2023) were rated as low risk across all five domains, indicating high methodological quality [8,9,16]. Reinisch et al. (2024) showed “some concerns” in missing outcomes data and the overall risk of bias, despite being rated low in the other domains [15], Figure 2.
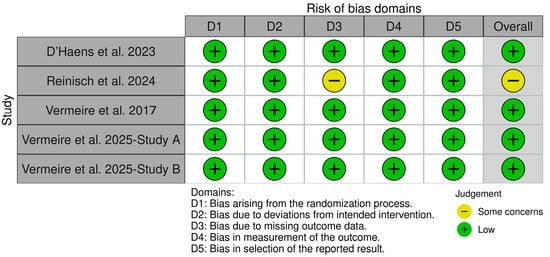
Figure 2.
Risk of bias assessment of included studies using the ROB 2 tool [8,9,15,16].
3.3. Outcomes
3.3.1. Efficacy Outcomes
Clinical Remission CDAI < 150 Points
Filgotinib 200 mg demonstrated a significant improvement in the clinical remission rate compared with both 100 mg (1.38 [1.11–1.71]) and placebo (1.75 [1.40–2.19]). In contrast, filgotinib 100 mg did not differ significantly from placebo (1.27 [0.99–1.63]). The analysis indicated homogeneity across studies (p = 0.88, I2 = 0), Figure 3A, Table S2 and Figure S1.
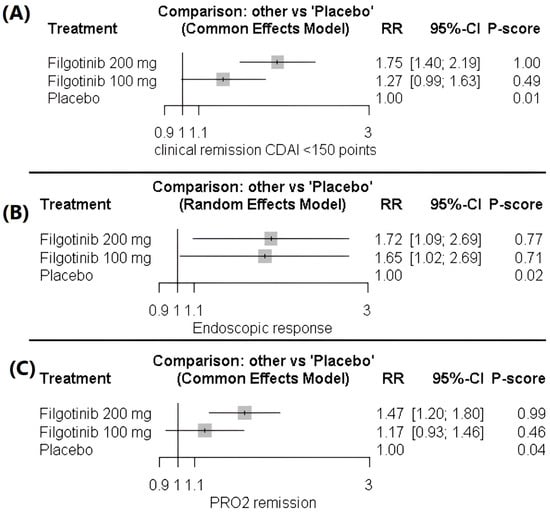
Figure 3.
(A) Forest plot of clinical remission (CDAI < 150). (B) Forest plot of endoscopic response (≥50% reduction in SES-CD). (C) Forest plot of improvement in two-item patient-reported outcome (PRO2).
Endoscopic Response (Reduction of at Least 50% in Centrally Read SES-CD)
For the endoscopic response outcome, both filgotinib 200 mg (1.72 [1.09–2.69]) and 100 mg (1.65 [1.02–2.69]) were associated with significant improvements compared with placebo. In contrast, the two active doses did not differ significantly (1.04 [0.64–1.68]), and the data were heterogeneous (p = 0.072, I2 = 57%), Figure 3B, Table S3 and Figure S2.
Heterogeneity was resolved after excluding Vermeire et al. (2025) [9]—Study B, reducing inconsistency to non-significant levels (p = 0.53, I2 = 0). Following this adjustment, Filgotinib 200 mg demonstrated an endoscopic response of RR 2.32 (95% CI 1.66–3.24), and Filgotinib 100 mg showed RR 2.06 (95% CI 1.44–2.97), both compared with placebo. There remained no significant difference between the 200 mg and 100 mg doses after adjustment, with an RR of 0.89 (95% CI 0.60–1.31), Table S4, and Figures S3 and S4.
Two-Item Patient-Reported Outcome (PRO2)
Filgotinib 200 mg demonstrated a significant improvement in the two-item patient-reported outcome (PRO2) compared with both filgotinib 100 mg (1.26 [1.02–1.55]) and placebo (1.47 [1.20–1.80]). In contrast, filgotinib 100 mg did not differ significantly from placebo (1.17 [0.93–1.46]). The data showed homogeneity (p = 0.3, I2 = 16.5%), Figure 3C, Table S5 and Figure S5.
3.3.2. Safety Outcomes
Treatment-Emergent Adverse Events (TEAEs)
No significant differences were observed in the risk of any TEAE with filgotinib 200 mg (1.02 [0.93; 1.11]) or 100 mg (0.98 [0.90; 1.08]) compared with placebo. Likewise, the two active doses did not differ significantly (1.03 [0.95; 1.12]). The findings were consistent across studies, indicating homogeneity. The data were homogeneous (p = 0.54, I2 = 0), Figure 4A, Table S6 and Figure S6.
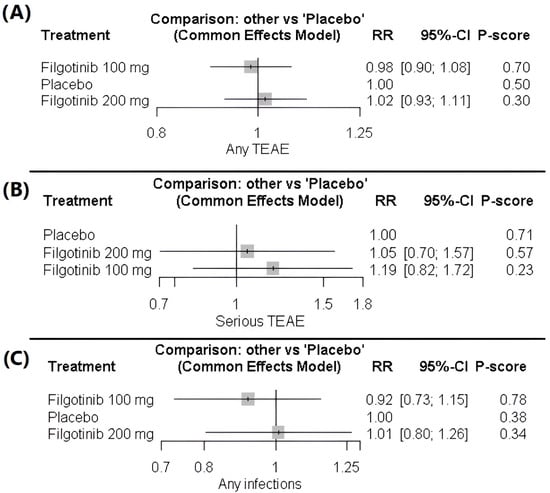
Figure 4.
(A) Forest plot of treatment-emergent adverse events (TEAEs). (B) Forest plot of serious treatment-emergent adverse events (TEAEs). (C) Forest plot of infection risk.
Serious Treatment-Emergent Adverse Events
The analysis of serious TEAEs did not differ significantly across treatment groups. Filgotinib 200 mg (1.05 [0.70–1.57]) and filgotinib 100 mg (1.19 [0.82–1.72]) were insignificant compared with placebo, and the difference between the two active doses was also insignificant (0.89 [0.60–1.31]). The data showed homogeneity (p = 0.14, I2 = 37.6%), Figure 4B, Table S7 and Figure S7.
Occurrence of Any Infection
No significant differences were observed in the risk of any infection with filgotinib 200 mg (1.01 [0.80–1.26]) or 100 mg (0.92 [0.73–1.15]) compared with placebo. Similarly, there was no significant difference between the two doses (1.10 [0.87–1.39]). The data indicated homogeneity across studies (p = 0.717, I2 = 0), Figure 4C, Table S8 and Figure S8.
3.3.3. Qualitative Synthesis
Role of Filgotinib in Perianal Fistulizing CD (PFCD)
Filgotinib has shown promise in treating PFCD as demonstrated in the DIVERGENCE 2 trial [15]. This phase 2 study revealed that filgotinib 200 mg led to significant reductions in the number of draining perianal fistulas compared to placebo, with a combined fistula response rate of 47.1%. The treatment was generally well tolerated, highlighting its potential as a therapeutic option for patients with PFCD who have experienced prior treatment failures.
Difference Between Prior Treatments and Naive Patients
In the DIVERSITY trial, biologic-naive patients exhibited different responses to filgotinib compared to biologic-experienced patients [9]. Biologic-naive patients showed a nominally significant improvement in clinical remission rates with filgotinib 200 mg, while biologic-experienced patients had higher rates of prior treatment failures. This suggests that treatment history may influence the efficacy of filgotinib, indicating a need for tailored therapeutic strategies based on patient history.
Maintenance Role of Filgotinib
The maintenance phase of the DIVERSITY study demonstrated that patients who achieved clinical remission or an endoscopic response at week 10 with filgotinib 200 mg continued to benefit from treatment [9]. At week 58, significantly more patients maintained PRO2 clinical remission and endoscopic response compared to placebo. This underscores the importance of ongoing therapy in sustaining remission and highlights filgotinib’s potential as a long-term treatment option for CD.
3.4. GRADE Assessment
Efficacy outcomes showed moderate-quality evidence, with the 200 mg dose significantly improving clinical remission and PRO2 scores, while the 100 mg dose did not. The endoscopic response also had moderate-quality evidence, but moderate heterogeneity (I2 = 57%). Safety outcomes were supported by high-quality evidence, showing no significant increase in adverse events or infection risk compared to placebo, Table 3.

Table 3.
GRADE assessment for study outcomes.
3.5. Publication Bias
Across all outcomes, the comparison-adjusted funnel plots are generally symmetric, and Egger’s tests are non-significant. This indicates no evidence of small-study effects or publication bias, with any observed scatter reflecting imprecision rather than true asymmetry, Figures S9–S14.
4. Discussion
Our network meta-analysis demonstrated a clear dose-dependent efficacy profile for filgotinib in moderate-to-severe CD. The 200 mg dose significantly improved clinical remission based on CDAI compared with both placebo and 100 mg, while the 100 mg dose did not differ from placebo. This superiority of 200 mg extended to patient-reported outcomes (PRO2), whereas the 100 mg dose again showed limited efficacy, consistent with the DIVERGENCE 2 trial [9]. The superior efficacy of filgotinib 200 mg likely reflects dose-dependent JAK1 inhibition, with higher doses more effectively suppressing key pro-inflammatory cytokines (e.g., IL-6, IL-10, interferons) and achieving greater modulation of immune activation and mucosal inflammation than the subtherapeutic 100 mg dose [10].
For endoscopic response outcomes, both filgotinib doses (200 mg and 100 mg) were associated with significant improvements compared with placebo, while no significant difference was observed between the two doses. However, this comparison showed moderate heterogeneity. Importantly, filgotinib maintained a favorable safety profile across both dosages. The NMA confirmed no significant differences between either dose and placebo for treatment-emergent adverse events, serious adverse events, or infections, with consistent homogeneity across studies.
The differential efficacy between biologic-naïve and biologic-experienced populations represents another crucial finding. Based on qualitative synthesis, biologic-naïve patients demonstrate a better response to filgotinib, particularly at the 200 mg dose [9]. This pattern aligns with established observations in inflammatory bowel disease therapeutics, where treatment-naïve patients typically exhibit higher response rates than those with previous treatment failures [17]. The underlying mechanisms may involve less immunological complexity, lower disease burden, or absence of anti-drug antibodies from previous biological therapies [18]. The maintenance efficacy demonstrated in the DIVERSITY trial further supports filgotinib’s potential role in long-term disease management [9]. Sustained PRO2 clinical remission and endoscopic response at week 58 indicate durable efficacy, addressing a critical unmet need given the significant proportion of patients who experience secondary loss of response to current biological therapies.
The previous systematic review and meta-analysis by Elgendy et al. highlighted the dose-dependent efficacy of filgotinib, with 200 mg achieving significant improvements in clinical remission and mucosal healing compared with placebo, while 100 mg showed limited benefit, and both doses demonstrated acceptable safety [10]. Building on this foundation, our NMA provides an important extension by synthesizing both direct and indirect evidence, enabling head-to-head comparisons between 100 mg and 200 mg. This allowed us to demonstrate the superior efficacy of 200 mg across clinical, patient-reported, and endoscopic outcomes compared with 100 mg, while confirming comparable safety profiles across doses.
The differential efficacy between 100 mg and 200 mg doses provides valuable clinical guidance for optimal dosing strategies. Based on our results, the 200 mg dose should be considered the preferred option for most patients, particularly those with more severe disease or previous treatment failures. However, individual benefit-risk assessments remain essential, especially in populations with elevated baseline risk factors for JAK inhibitor-associated adverse events.
The maintenance efficacy demonstrated in the DIVERSITY trial suggests filgotinib could address the significant problem of secondary loss of response to biological therapies. Approximately 30–50% of patients experience diminished efficacy over time with current biologics, necessitating dose escalation, switching within class, or exploring alternative mechanisms of action [19]. Filgotinib’s distinct mechanism potentially offers a valuable option for these patients, potentially delaying or preventing surgical interventions. The encouraging results in PFCD from the DIVERGENCE 2 trial merit particular attention [15]. PFCD represents one of the most challenging manifestations of Crohn’s disease, with limited effective medical therapies available [20]. From a mechanistic perspective, these findings reinforce the central role of JAK-STAT signaling in Crohn’s disease pathophysiology. The efficacy of selective JAK1 inhibition highlights the importance of cytokines that signal through this pathway, including IL-6, IL-10, type I and II interferons, and several other inflammatory mediators implicated in disease pathogenesis [5].
The positioning of filgotinib within the evolving Crohn’s disease treatment algorithm warrants careful consideration. Traditional treatment paradigms have followed a stepwise approach from conventional immunomodulators to biological therapies, typically anti-TNF agents as first-line biologics [21]. However, the emergence of oral targeted synthetic small molecules with favorable efficacy-safety profiles challenges this conventional approach. Given its oral administration route, lack of immunogenicity, rapid onset of action, and efficacy across various disease manifestations, including fistulizing disease, filgotinib may offer advantages as an earlier treatment option rather than being reserved for anti-TNF failures. This represents a potential paradigm shift in treatment algorithms, particularly for patients with contraindications or preferences against injectable therapies.
The beneficial effects observed in both clinical and endoscopic outcomes suggest that filgotinib addresses both symptomatic improvement and underlying mucosal inflammation, addressing both patient-reported symptoms and objective disease activity markers. This comprehensive approach to disease control aligns with emerging treat-to-target strategies in inflammatory bowel disease management that emphasize the importance of achieving deep remission to modify the long-term disease course [22].
A key strength of our analysis lies in its inclusion of high-quality randomized controlled trials with consistent outcome definitions, enabling clinically meaningful conclusions. By assessing both induction and maintenance phases, we offer insights into filgotinib’s potential role throughout the disease course. The inclusion of both symptomatic and objective endpoints—clinical response, remission, and endoscopic improvement—enhances the overall relevance of our findings. Furthermore, our systematic evaluation of safety outcomes across several domains, including treatment-emergent adverse events, serious adverse events, and infections, provides detailed safety data to support clinical decision-making. The low heterogeneity observed in most outcomes adds to the strength and reliability of the evidence. Importantly, by employing a network meta-analysis, we were able to integrate both direct and indirect comparisons, allowing a more robust evaluation of dose-dependent effects between 100 mg and 200 mg. However, several limitations warrant acknowledgment. First, the relatively small number of included studies limits the strength of our conclusions, particularly for subgroup analyses. Second, the variable follow-up periods across studies (ranging from 10 to 58 weeks) challenge the assessment of long-term efficacy and safety. Additional long-term extension data will be crucial to evaluate filgotinib’s sustained benefit-risk profile (e.g., malignancy and thromboembolism). The studies included predominantly white populations from Western countries, which may limit their generalizability to other ethnicities and regions with different environmental exposures and genetic backgrounds. Additionally, the trials excluded patients with certain comorbidities, which limits their applicability to more complex real-world populations.
5. Conclusions
Our NMA demonstrates that filgotinib 200 mg offers superior efficacy over both placebo and filgotinib 100 mg in achieving clinical remission, patient-reported outcomes, and endoscopic response in moderate-to-severe CD, while maintaining a favorable safety profile. The 100 mg dose provided limited or insignificant benefits compared with placebo, confirming a clear dose–response relationship. Importantly, both doses were well tolerated, with no significant increase in adverse events, serious adverse events, or infections.
Based on these findings, filgotinib 200 mg should be considered the preferred dose for most patients, particularly those who are biologic-naïve or have more severe disease activity. Clinicians should individualize treatment decisions, weighing potential risks in patients with elevated susceptibility to JAK inhibitor–related adverse events. Important research gaps remain regarding filgotinib’s long-term safety, real-world effectiveness, and comparative efficacy against established biologics such as anti-TNF and anti-IL-23 agents. Future large-scale, head-to-head, and longitudinal studies should clarify its therapeutic role, including in perianal fistulizing Crohn’s disease and potential combination strategies.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/healthcare14010005/s1, Table S1: search strategy for each database (Date 28 April 2025); Table S2: Netleague of clinical remission CDAI < 150 points; Table S3: Netleague of Endoscopic response before resolving heterogeneity; Table S4: Netleague of Endoscopic response after resolving heterogeneity; Table S5: Netleague of PRO2 remission; Table S6: Netleague of any TEAEs; Table S7: Netleague of serious TEAEs; Table S8: Netleague of any infection; Figure S1: Net Graph of clinical remission CDAI < 150 points; Figure S2: Net Graph of Endoscopic Response before resolving heterogeneity; Figure S3: Net Graph of Endoscopic Response after resolving heterogeneity; Figure S4: Comparison Graph of Endoscopic Response after resolving heterogeneity; Figure S5: Net Graph of PRO2 remission; Figure S6: Net Graph of any TEAE; Figure S7: Net Graph of serious TEAE; Figure S8: Net Graph of the occurrence of any infection; Figure S9: funnel plot of clinical remission CDAI < 150 points; Figure S10: funnel plot of Endoscopic response; Figure S11: funnel plot of PRO2 remission; Figure S12: funnel plot of any TEAEs; Figure S13: funnel plot of serious TEAEs; Figure S14: funnel plot of any infection.
Author Contributions
Y.A.K., Y.Z.H. and M.S.Z.: Conceptualization, methodology. Y.A.K., Y.Z.H., A.G.D., R.K.A., Y.S.A., A.S., S.A.A., D.T.A., H.S.B., A.J.S. and O.A.A.: resources, data curation, investigation, validation, writing—original draft preparation. M.S.Z. and Y.A.K.: formal analysis. M.S.Z. supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. The data presented in this study will be made available by the authors upon request.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| CD | Crohn’s Disease |
| CDAI | Crohn’s Disease Activity Index |
| SES-CD | Simple Endoscopic Score for Crohn’s Disease |
| JAK1 | Janus Kinase 1 |
| RR | Risk Ratios |
| CI | Confidence Intervals |
| SD | Standard Deviation |
| NMA | Network Meta-Analysis |
| PRISMA | The Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| RCT | Randomized Controlled Trials |
| PRO2 | Two-Item Patient-Reported Outcome |
| TEAEs | Treatment-Emergent Adverse Events |
| PFCD | Perianal Fistulizing Crohn’s Disease |
References
- Feuerstein, J.D.; Cheifetz, A.S. Crohn Disease: Epidemiology, Diagnosis, and Management. Mayo Clin. Proc. 2017, 92, 1088–1103. [Google Scholar] [CrossRef]
- Molodecky, N.A.; Soon, I.S.; Rabi, D.M.; Ghali, W.A.; Ferris, M.; Chernoff, G.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Barkema, H.W.; et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 2012, 142, 46–54.e42, quiz-e30. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; George, J.; Boland, B.S.; Vande Casteele, N.; Sandborn, W.J. Primary Non-Response to Tumor Necrosis Factor Antagonists is Associated with Inferior Response to Second-line Biologics in Patients with Inflammatory Bowel Diseases: A Systematic Review and Meta-analysis. J. Crohns Colitis 2018, 12, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Papamichael, K.; Gils, A.; Rutgeerts, P.; Levesque, B.G.; Vermeire, S.; Sandborn, W.J.; Vande Casteele, N. Role for therapeutic drug monitoring during induction therapy with TNF antagonists in IBD: Evolution in the definition and management of primary nonresponse. Inflamm. Bowel Dis. 2015, 21, 182–197. [Google Scholar] [CrossRef]
- Schwartz, D.M.; Kanno, Y.; Villarino, A.; Ward, M.; Gadina, M.; O’Shea, J.J. JAK inhibition as a therapeutic strategy for immune and inflammatory diseases. Nat. Rev. Drug Discov. 2017, 17, 78. [Google Scholar] [CrossRef] [PubMed]
- Benucci, M.; Bardelli, M.; Cazzato, M.; Laurino, E.; Bartoli, F.; Damiani, A.; Li Gobbi, F.; Panaccione, A.; Di Cato, L.; Niccoli, L.; et al. ReLiFiRa (Real Life Filgotinib in Rheumatoid Arthritis): Retrospective Study of Efficacy and Safety in Common Clinical Practice. J. Pers. Med. 2023, 13, 1303. [Google Scholar] [CrossRef]
- Dudek, P.; Fabisiak, A.; Zatorski, H.; Malecka-Wojciesko, E.; Talar-Wojnarowska, R. Efficacy, Safety and Future Perspectives of JAK Inhibitors in the IBD Treatment. J. Clin. Med. 2021, 10, 5660. [Google Scholar] [CrossRef]
- Vermeire, S.; Schreiber, S.; Petryka, R.; Kuehbacher, T.; Hebuterne, X.; Roblin, X.; Klopocka, M.; Goldis, A.; Wisniewska-Jarosinska, M.; Baranovsky, A.; et al. Clinical remission in patients with moderate-to-severe Crohn’s disease treated with filgotinib (the FITZROY study): Results from a phase 2, double-blind, randomised, placebo-controlled trial. Lancet 2017, 389, 266–275. [Google Scholar] [CrossRef]
- Vermeire, S.; Schreiber, S.; Rubin, D.T.; D’Haens, G.; Reinisch, W.; Watanabe, M.; Mehta, R.; Roblin, X.; Beales, I.; Gietka, P.; et al. Efficacy and safety of filgotinib as induction and maintenance therapy for Crohn’s disease (DIVERSITY): A phase 3, double-blind, randomised, placebo-controlled trial. Lancet Gastroenterol. Hepatol. 2025, 10, 138–153. [Google Scholar] [CrossRef]
- Elgendy, M.S.; Raza, A.; Rifai, M.; Rehman, W.U.; Emara, A.; Khan, M.H.; Jan, A.; Younas, M.; Nawaz, A.; Khan, U. Dose-dependent efficacy and safety of Filgotinib in moderate to severe Crohn’s disease: A grade-assessed systematic review and meta-analysis of randomized controlled trials. Eur. J. Clin. Pharmacol. 2025, 81, 1517–1531. [Google Scholar] [CrossRef]
- Cumpston, M.; Li, T.; Page, M.J.; Chandler, J.; Welch, V.A.; Higgins, J.P.T.; Thomas, J. Updated guidance for trusted systematic reviews: A new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst. Rev. 2019, 10, Ed000142. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J. Clin. Epidemiol. 2009, 62, e1–e34. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H.J. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef]
- Reinisch, W.; Colombel, J.F.; D’Haens, G.R.; Rimola, J.; Masior, T.; McKevitt, M.; Ren, X.; Serone, A.; Schwartz, D.A.; Gecse, K.B. Efficacy and Safety of Filgotinib for the Treatment of Perianal Fistulising Crohn’s Disease [DIVERGENCE 2]: A Phase 2, Randomised, Placebo-controlled Trial. J. Crohns Colitis 2024, 18, 864–874. [Google Scholar] [CrossRef]
- D’Haens, G.R.; Lee, S.; Taylor, S.A.; Serone, A.; Rimola, J.; Colombel, J.F. Filgotinib for the Treatment of Small Bowel Crohn’s Disease: The DIVERGENCE 1 Trial. Gastroenterology 2023, 165, 289–292.e283. [Google Scholar] [CrossRef]
- Ma, C.; Solitano, V.; Danese, S.; Jairath, V. The Future of Clinical Trials in Inflammatory Bowel Disease. Clin. Gastroenterol. Hepatol. 2025, 23, 480–489. [Google Scholar] [CrossRef]
- Moss, A.C. Approach to Treatment Failure in Inflammatory Bowel Disease. Gastroenterol. Hepatol. 2022, 18, 360–363. [Google Scholar]
- Marsal, J.; Barreiro-de Acosta, M.; Blumenstein, I.; Cappello, M.; Bazin, T.; Sebastian, S. Management of Non-response and Loss of Response to Anti-tumor Necrosis Factor Therapy in Inflammatory Bowel Disease. Front. Med. 2022, 9, 897936. [Google Scholar] [CrossRef]
- Lopez, N.; Ramamoorthy, S.; Sandborn, W.J. Recent advances in the management of perianal fistulizing Crohn’s disease: Lessons for the clinic. Expert. Rev. Gastroenterol. Hepatol. 2019, 13, 563–577. [Google Scholar] [CrossRef]
- Berg, D.R.; Colombel, J.F.; Ungaro, R. The Role of Early Biologic Therapy in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2019, 25, 1896–1905. [Google Scholar] [CrossRef]
- Srinivasan, A.R. Treat to target in Crohn’s disease: A practical guide for clinicians. World J. Gastroenterol. 2024, 30, 50–69. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.