Efficacy and Safety of Accelerated Transepithelial Corneal Crosslinking in Non-Pediatric Patients with Progressive Keratoconus: Insights from a Retrospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Design
2.2. Inclusion and Exclusion Criteria
2.3. Surgical Technique
2.4. Measurements
2.5. Statistical Analysis
3. Results
4. Discussion
4.1. Visual Acuity and Refractive Improvements
4.2. Corneal Remodeling and Stability
4.3. Efficacy in Advanced and Pediatric Keratoconus
4.4. Long-Term Considerations and Progression Risks
4.5. Limitations
4.6. Future Lines of Research
4.7. Clinical Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BAC | Benzalkonium Chloride |
| BCV | Baiocchi–Calossi–Versaci Index |
| BSS | Balanced Salt Solution |
| CCT | Central Corneal Thickness |
| CDVA | Corrected Distance Visual Acuity |
| CXL | Corneal Collagen Crosslinking |
| EDTA | Ethylenediaminetetraacetic Acid |
| HOA RMS | Higher-Order Aberrations Root Mean Square |
| KV | Keratoconus Vertex |
| Kmax | Maximum Keratometry |
| LogMAR | Logarithm of the Minimum Angle of Resolution |
| PRC | Posterior Radius of Curvature |
| Si | Symmetry Index |
| TCT | Thinnest Corneal Thickness |
| TE-ACXL | Transepithelial Accelerated Corneal Crosslinking |
| TE-CXL | Transepithelial Corneal Crosslinking |
| Tris | Trometamol |
| UDVA | Uncorrected Distance Visual Acuity |
| UV-A | Ultraviolet-A |
| VE-TPGS | Vitamin E TPGS (D-α-Tocopheryl Polyethylene Glycol-1000 Succinate) |
| aCFXL | Accelerated Custom-Fast Corneal Crosslinking |
| epi-off CXL | Epithelium-Off Corneal Crosslinking |
| epi-on CXL | Epithelium-On Corneal Crosslinking |
| sCXL | Standard Dresden Protocol Corneal Crosslinking |
References
- Cronin, B.; Gunn, D.; Chang, C.Y. Oxygen-Supplemented and Topography-Guided Epithelium-on Corneal Crosslinking with Pulsed Irradiation for Progressive Keratoconus. J. Cataract Refract. Surg. 2024, 50, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Vilares-Morgado, R.; Ferreira, A.M.; Cunha, A.M.; Moreira, R.; Torrão, L.; Neves-Cardoso, P.; Pinheiro-Costa, J. Transepithelial Accelerated Crosslinking for Progressive Keratoconus: A Critical Analysis of Medium-Term Treatment Outcomes. Clin. Ophthalmol. 2024, 18, 393–407. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, B. Efficacy and Safety of Transepithelial Corneal Collagen Crosslinking Surgery versus Standard Corneal Collagen Crosslinking Surgery for Keratoconus: A Meta-Analysis of Randomized Controlled Trials. BMC Ophthalmol. 2017, 17, 262. [Google Scholar] [CrossRef]
- Ozer, M.D.; Batur, M.; Mesen, S.; Tekin, S.; Seven, E. Long-Term Results of Accelerated Corneal Cross-Linking in Adolescent Patients with Keratoconus. Cornea 2019, 38, 992–997. [Google Scholar] [CrossRef]
- Oliverio, G.W.; Vagge, A.; Gargano, R.; Aragona, P.; Roszkowska, A.M. Clinical Results of Accelerated Iontophoresis-Assisted Epithelium-on Corneal Cross-Linking for Progressive Keratoconus in Children. J. Pediatr. Ophthalmol. Strabismus 2023, 61, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Shajari, M.; Kolb, C.M.; Agha, B.; Steinwender, G.; Müller, M.; Herrmann, E.; Schmack, I.; Mayer, W.J.; Kohnen, T. Comparison of Standard and Accelerated Corneal Cross-Linking for the Treatment of Keratoconus: A Meta-Analysis. Acta Ophthalmol. 2019, 97, e22–e35. [Google Scholar] [CrossRef]
- Mazzotta, C.; Raiskup, F.; Hafezi, F.; Torres-Netto, E.A.; Armia Balamoun, A.; Giannaccare, G.; Bagaglia, S.A. Long Term Results of Accelerated 9 MW Corneal Crosslinking for Early Progressive Keratoconus: The Siena Eye-Cross Study 2. Eye Vis. 2021, 8, 16. [Google Scholar] [CrossRef]
- Henriquez, M.A.; Rodríguez, A.M.; Izquierdo, L. Accelerated Epi-on versus Standard Epi-off Corneal Collagen Cross-Linking for Progressive Keratoconus in Pediatric Patients. Cornea 2017, 36, 1503–1508. [Google Scholar] [CrossRef]
- Mazzotta, C.; Pandolfi, A.; Ferrise, M. Progressive High-Fluence Epithelium-on Accelerated Corneal Crosslinking: A Novel Corneal Photodynamic Therapy for Early Progressive Keratoconus. Front. Med. 2023, 10, 1198246. [Google Scholar] [CrossRef]
- Yuksel, E.; Cubuk, M.; Yalcin, N. Accelerated Epithelium-on or Accelerated Epithelium-off Corneal Collagen Cross-Linking: Contralateral Comparison Study. Taiwan J. Ophthalmol. 2020, 10, 37–44. [Google Scholar] [CrossRef]
- Henriquez, M.A.; Hernandez-Sahagun, G.; Camargo, J.; Izquierdo, L. Accelerated Epi-On Versus Standard Epi-Off Corneal Collagen Cross-Linking for Progressive Keratoconus in Pediatric Patients: Five Years of Follow-Up. Cornea 2020, 39, 1493–1498. [Google Scholar] [CrossRef] [PubMed]
- Boschetti, F.; Conti, D.; Soriano, E.M.; Mazzotta, C.; Pandolfi, A. Experimental In-Vitro Investigation on Epi-Off-Crosslinking on Porcine Corneas. PLoS ONE 2021, 16, e0249949. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, K.; Kanayama, S.; Takahashi, M.; Shoji, N. Visual and Topographic Improvement with Epithelium-on, Oxygen-Supplemented, Customized Corneal Cross-Linking for Progressive Keratoconus. J. Clin. Med. 2020, 9, 3222. [Google Scholar] [CrossRef]
- Sun, L.; Zhao, J.; Zhang, X.; Shen, Y.; Tian, M.; Zhou, X. Transepithelial Accelerated Corneal Crosslinking for Keratoconus Eyes with Maximum Keratometry Values Larger than 58 Diopters. J. Cataract Refract. Surg. 2022, 48, 208–214. [Google Scholar] [CrossRef]
- Vidas Pauk, S.; Jandroković, S.; Lešin Gaćina, D.; Tomić, M.; Bulum, T.; Pupić Bakrač, A.; Kuzman, T.; Knežević, J.; Kalauz, M. Short-Term Effect of Conventional Versus Accelerated Corneal Cross-Linking Protocol on Corneal Geography and Stability. Medicina 2023, 59, 1043. [Google Scholar] [CrossRef] [PubMed]
- Cronin, B.; Ghosh, A.; Chang, C.Y. Oxygen-Supplemented Transepithelial-Accelerated Corneal Crosslinking with Pulsed Irradiation for Progressive Keratoconus: 1 Year Outcomes. J. Cataract Refract. Surg. 2022, 48, 1175–1182. [Google Scholar] [CrossRef]
- Hamida Abdelkader, S.M.; Fernández, J.; Sebastián, J.; Piñero, D.P. Preliminary Characterization of Predictive Factors of the Visual Change after Epi-On and Epi-Off Corneal Collagen Crosslinking Techniques. J. Ophthalmol. 2021, 2021, 9680253. [Google Scholar] [CrossRef]
- Mazzotta, C.; Baiocchi, S.; Bagaglia, S.A.; Fruschelli, M.; Meduri, A.; Rechichi, M. Accelerated 15 MW Pulsed-Light Crosslinking to Treat Progressive Keratoconus: Two-Year Clinical Results. J. Cataract Refract. Surg. 2017, 43, 1081–1088. [Google Scholar] [CrossRef]
- Mazzotta, C.; Balamoun, A.A.; Chabib, A.; Rechichi, M.; D’Oria, F.; Hafezi, F.; Bagaglia, S.A.; Ferrise, M. Transepithelial Enhanced Fluence Pulsed Light M Accelerated Crosslinking for Early Progressive Keratoconus with Chemically Enhanced Riboflavin Solutions and Air Room Oxygen. J. Clin. Med. 2022, 11, 5039. [Google Scholar] [CrossRef]
- Hill, J.; Liu, C.; Deardorff, P.; Tavakol, B.; Eddington, W.; Thompson, V.; Gore, D.; Raizman, M.; Adler, D.C. Optimization of Oxygen Dynamics, UV-A Delivery, and Drug Formulation for Accelerated Epi-On Corneal Crosslinking. Curr. Eye Res. 2020, 45, 450–458. [Google Scholar] [CrossRef]
- Caruso, C.; Epstein, R.L.; Troiano, P.; Napolitano, F.; Scarinci, F.; Costagliola, C. Topo-Pachimetric Accelerated Epi-on Cross-Linking Compared to the Dresden Protocol Using Riboflavin with Vitamin E TPGS: Results of a 2-Year Randomized Study. J. Clin. Med. 2021, 10, 3799. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Radi, M.; Anwar, M.; Aly, M.A.M.; Eata, W.E.S.; Goda, I. Management of Tomographically Suspicious Fellow Eyes of Young Patients with Unilateral Clinically Evident Keratoconus: Accelerated Epithelium-On Corneal Crosslinking Versus Observation. Cornea 2024. [Google Scholar] [CrossRef] [PubMed]
- Nicula, C.A.; Nicula, D.; Rednik, A.M.; Bulboacǎ, A.E. Comparative Results of ‘Epi-Off’ Conventional versus ‘Epi-Off’ Accelerated Cross-Linking Procedure at 5-Year Follow-Up. J. Ophthalmol. 2020, 2020, 4745101. [Google Scholar] [CrossRef] [PubMed]

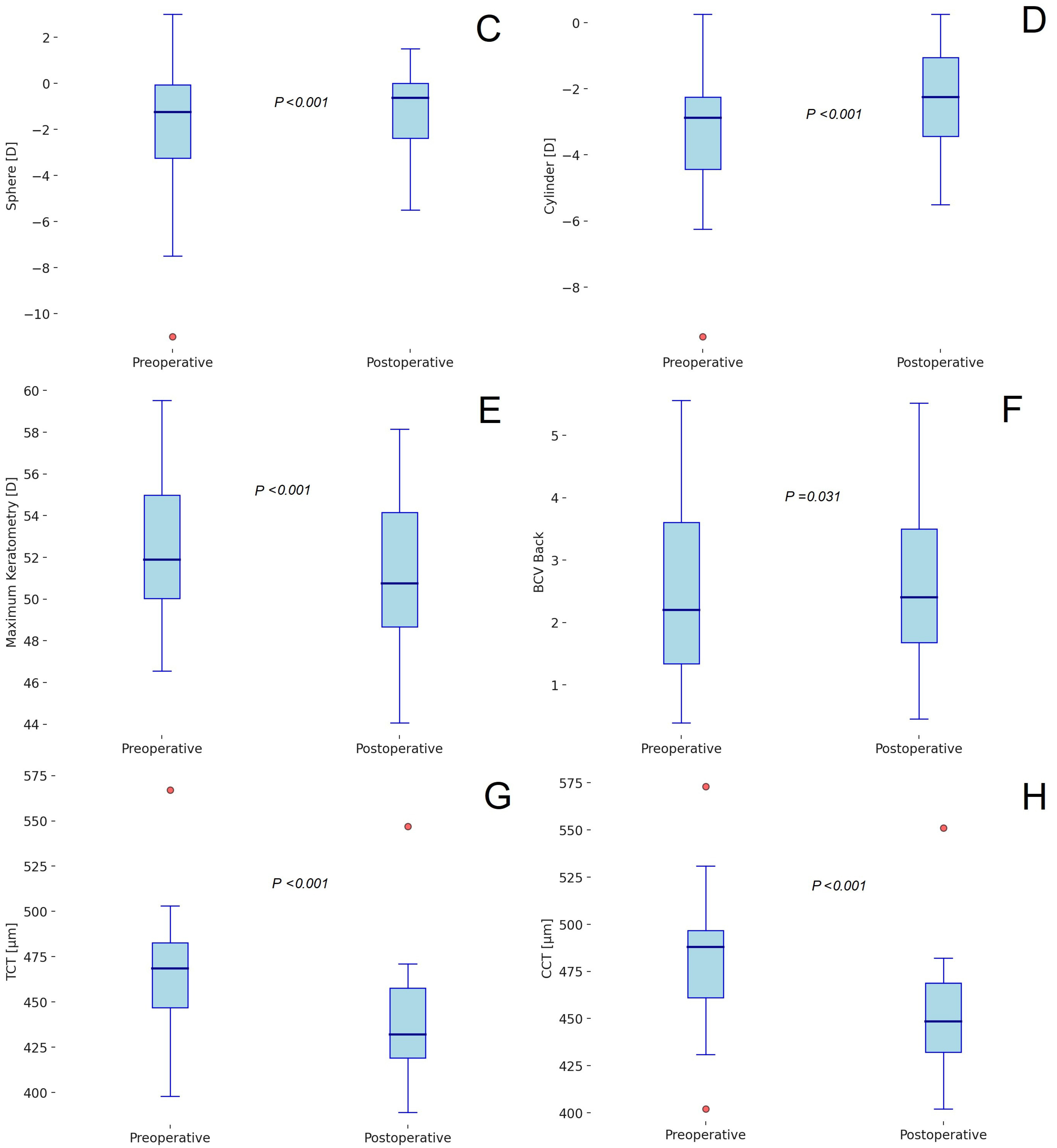
| Variables [Units] | Preoperative Data | Postoperative Data | p-Value |
|---|---|---|---|
| Sphere [D] | −2.18 ± 3.05 (−11.00 to 3.00) | −1.31 ± 1.84 (−5.50 to 1.50) | <0.001 |
| Cylinder [D] | −3.33 ± 1.98 (−9.50 to 0.25) | −2.33 ± 1.52 (−5.50 to 0.25) | <0.001 |
| UDVA [decimal] | 0.44 ± 0.30 (0.05 to 1.00) | 0.61 ± 0.29 (0.05 to 1.00) | <0.001 |
| UDVA [LogMAR] | 0.47 ± 0.35 (0.00 to 1.00) | 0.29 ± 0.30 (0.00 to 1.00) | <0.001 |
| CDVA [decimal] | 0.71 ± 0.25 (0.10 to 1.00) | 0.90 ± 0.17 (0.40 to 1.00) | 0.022 |
| CDVA [LogMAR] | 0.20 ± 0.22 (0.00 to 1.00) | 0.06 ± 0.11 (0.00 to 0.40) | 0.004 |
| Variables [Units] | Preoperative Data | Postoperative Data | p-Value |
|---|---|---|---|
| Kmax [D] | 52.33 ± 3.51 (46.55 to 59.52) | 51.19 ± 3.63 (44.06 to 58.14) | <0.001 |
| Si front | 5.38 ± 3.01 (0.37 to 12.44) | 5.37 ± 2.75 (0.28 to 12.01) | 0.479 |
| Si back | 1.43 ± 0.74 (0.29 to 2.78) | 1.46 ± 0.69 (0.33 to 2.67) | 0.153 |
| KV front | 24.43 ± 13.41 (4 to 55) | 24.17 ± 11.53 (4 to 48) | 0.373 |
| KV back | 53.53 ± 30.10 (8 to 128) | 55.53 ± 30.62 (9 to 136) | 0.070 |
| BCV front | 2.55 ± 1.40 (0.36 to 5.67) | 2.56 ± 1.26 (0.37 to 5.03) | 0.420 |
| BCV back | 2.52 ± 1.43 (0.39 to 5.56) | 2.64 ± 1.36 (0.46 to 5.52) | 0.031 |
| TCT [µm] | 466.43 ± 31.24 (398 to 567) | 438.63 ± 30.54 (389 to 547) | <0.001 |
| CCT [µm] | 480.80 ± 33.24 (402 to 573) | 451.23 ± 29.26 (402 to 551) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiraples, A.-C.; Munteanu, M.; Stanca, H.T.; Darabus, D.-M.; Barakat, D.; Negru, A.-G. Efficacy and Safety of Accelerated Transepithelial Corneal Crosslinking in Non-Pediatric Patients with Progressive Keratoconus: Insights from a Retrospective Cohort Study. Healthcare 2025, 13, 567. https://doi.org/10.3390/healthcare13050567
Chiraples A-C, Munteanu M, Stanca HT, Darabus D-M, Barakat D, Negru A-G. Efficacy and Safety of Accelerated Transepithelial Corneal Crosslinking in Non-Pediatric Patients with Progressive Keratoconus: Insights from a Retrospective Cohort Study. Healthcare. 2025; 13(5):567. https://doi.org/10.3390/healthcare13050567
Chicago/Turabian StyleChiraples, Alina-Cristina, Mihnea Munteanu, Horia T. Stanca, Diana-Maria Darabus, Diana Barakat, and Alina-Gabriela Negru. 2025. "Efficacy and Safety of Accelerated Transepithelial Corneal Crosslinking in Non-Pediatric Patients with Progressive Keratoconus: Insights from a Retrospective Cohort Study" Healthcare 13, no. 5: 567. https://doi.org/10.3390/healthcare13050567
APA StyleChiraples, A.-C., Munteanu, M., Stanca, H. T., Darabus, D.-M., Barakat, D., & Negru, A.-G. (2025). Efficacy and Safety of Accelerated Transepithelial Corneal Crosslinking in Non-Pediatric Patients with Progressive Keratoconus: Insights from a Retrospective Cohort Study. Healthcare, 13(5), 567. https://doi.org/10.3390/healthcare13050567









