Micronutrient Supplementation in Frailty: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Highlights
- The quality of published evidence for vitamin D and multicomponent supplementation is low or very low for most outcomes, and clinicians should be aware of this fact before prescribing them.
- Frailty is inconsistently measured in published clinical trials.
- Future research should focus on patient-oriented outcomes, such as changes in frailty levels, functional status, and cognitive function.
- Providers should be aware of the low certainty of evidence when prescribing micronutrient supplements.
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.1.1. Types of Studies
2.1.2. Types of Participants
2.1.3. Types of Interventions (And Co-Interventions)
2.2. Search Strategy
2.3. Study Selection and Data Extraction
2.4. Risk of Bias Evaluation and GRADE Assessment
2.5. Statistical Synthesis
3. Results
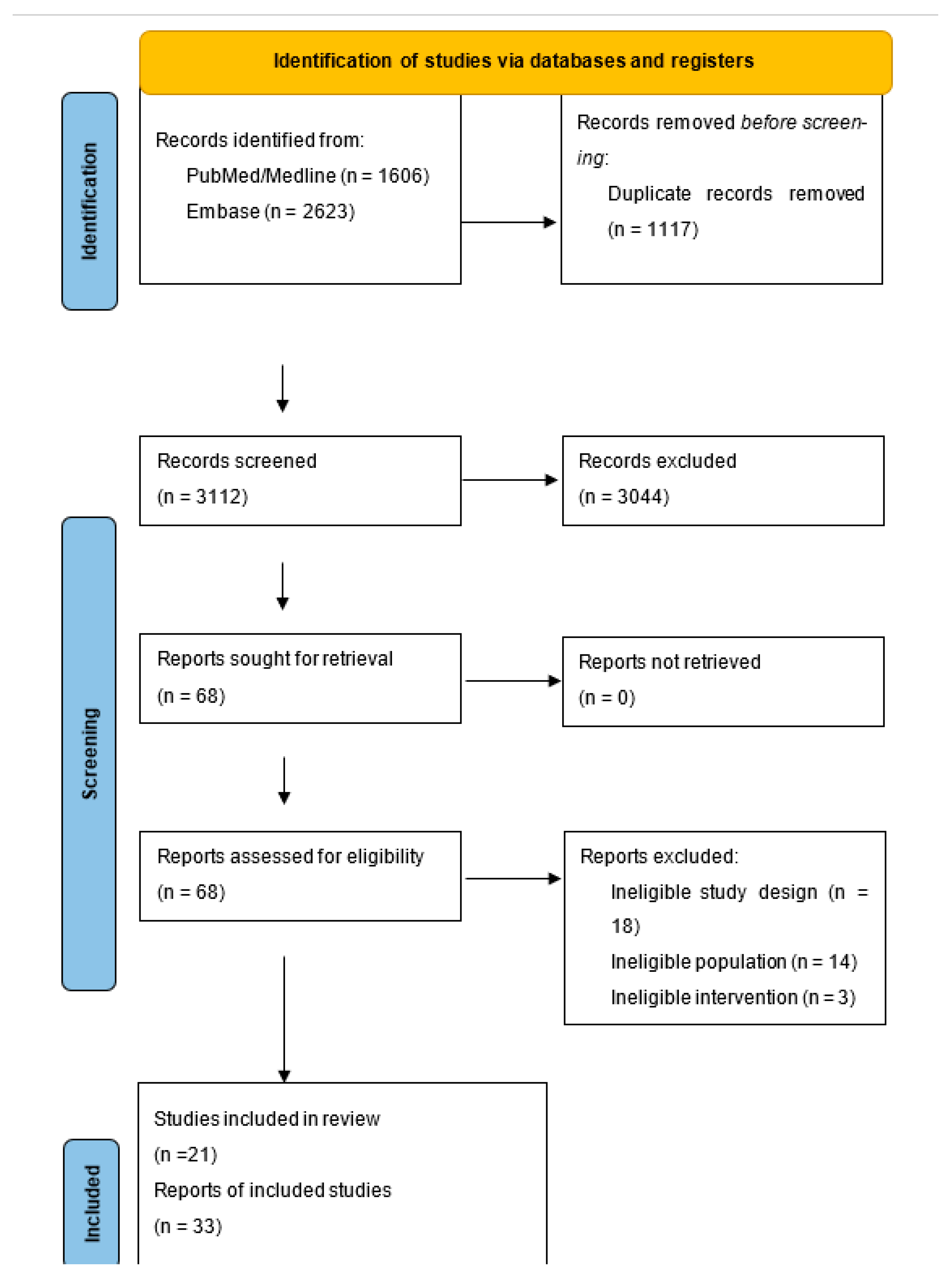
3.1. Results of the Search
3.2. Included Studies
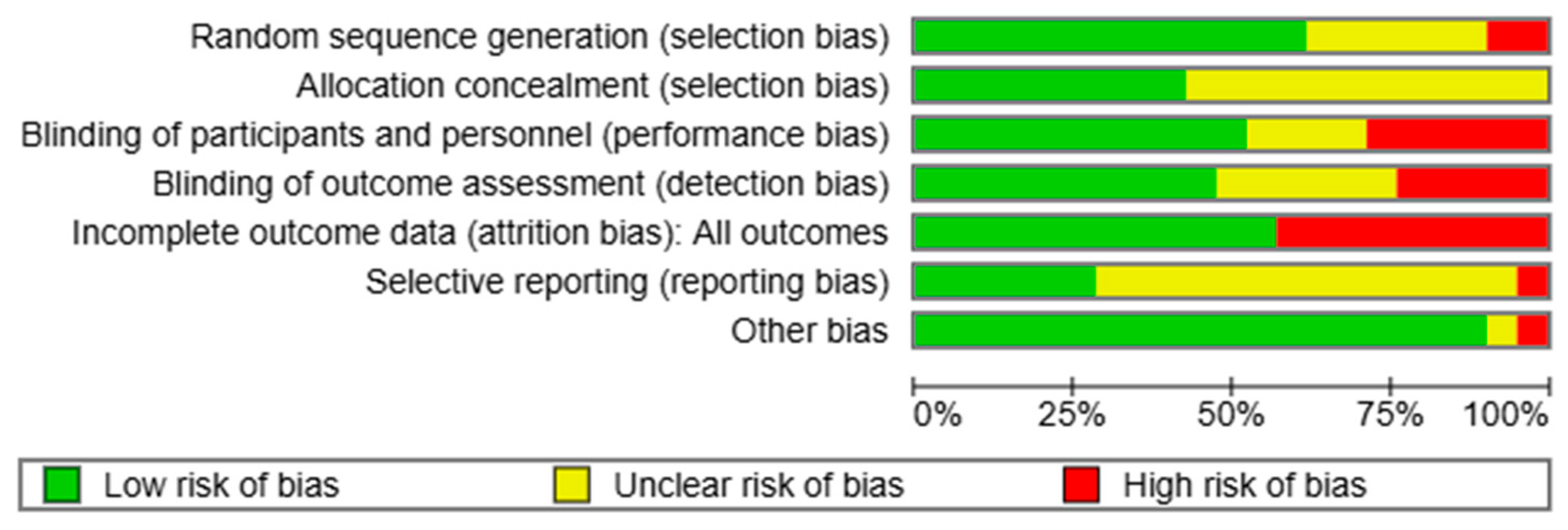
3.3. Risk of Bias in Included Studies
3.4. Vitamin D Supplementation
Outcomes
- 1.
- Vitamin D Supplementation: All-cause mortality
- 2.
- Vitamin D Supplementation: Frailty levels, as measured by validated frailty scales, such as the Frailty Index and Fried’s Frailty Phenotype
- 3.
- Vitamin D Supplementation: Falls
- 4.
- Vitamin D Supplementation: Fractures, including hip fractures and vertebral fractures, among others
- 5.
- Vitamin D Supplementation: Muscle strength as measured by handgrip strength
- 6.
- Vitamin D Supplementation: Gait speed
- 7.
- Vitamin D Supplementation: Body mass measures, including lean mass, fat-free mass, total mass, and body mass index.
- 8.
- Vitamin D Supplementation: Cognitive function as measured by validated scales such as the Mini-Mental State Exam
- 9.
- Vitamin D Supplementation: Inflammatory markers, including but not limited to cytokines and CRP
- 10.
- Vitamin D Supplementation: Functionality
3.5. Multicomponent Supplementation
- 1.
- Multicomponent Supplementation: All-cause mortality
- 2.
- Multicomponent Supplementation: Frailty levels, as measured by validated frailty scales, such as the Frailty Index and Fried’s Frailty Phenotype
- 3.
- Multicomponent Supplementation: Falls
- 4.
- Multicomponent Supplementation: Fractures, including hip fractures and vertebral fractures, among others
- 5.
- Multicomponent Supplementation: Muscle strength as measured by handgrip strength
- 6.
- Multicomponent Supplementation: Gait speed
- 7.
- Multicomponent Supplementation: Body mass measures, including lean mass, fat-free mass, total mass, and body mass index
- 8.
- Multicomponent Supplementation: Cognitive function as measured by validated scales such as the Mini-Mental State Examination
- 9.
- Multicomponent Supplementation: Inflammatory markers, including but not limited to cytokines and CRP
- 10.
- Multicomponent Supplementation: Functionality
3.6. Nicotinamide (Vitamin B3) Supplementation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2001, 56, M146–M156. [Google Scholar] [CrossRef]
- Ofori-Asenso, R.; Chin, K.L.; Mazidi, M.; Zomer, E.; Ilomaki, J.; Zullo, A.R.; Gasevic, D.; Ademi, Z.; Korhonen, M.J.; LoGiudice, D.; et al. Global Incidence of Frailty and Prefrailty Among Community-Dwelling Older Adults: A Systematic Review and Meta-analysis. JAMA Netw. Open 2019, 2, e198398. [Google Scholar] [CrossRef]
- Hajek, A.; Kretzler, B.; König, H.H. Prevalence of Prefrailty and Frailty Among Older Adults in Germany: A Systematic Review, Meta-Analysis and Meta-Regression. Front. Med. 2022, 9, 870714. [Google Scholar] [CrossRef]
- Bolland, M.J.; Avenell, A.; Smith, K.; Witham, M.D.; Grey, A. Vitamin D supplementation and testing in the UK: Costly but ineffective? BMJ (Clin. Res. Ed.) 2021, 372, n484. [Google Scholar] [CrossRef]
- National Collaborating Centre for Acute Care (UK). Nutrition Support for Adults: Oral Nutrition Support, Enteral Tube Feeding and Parenteral Nutrition; National Collaborating Centre for Acute Care (UK): London, UK, 2006.
- Bruyère, O.; Cavalier, E.; Buckinx, F.; Reginster, J.Y. Relevance of vitamin D in the pathogenesis and therapy of frailty. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 26–29. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ (Clin. Res. Ed.) 2011, 343, d5928. [Google Scholar] [CrossRef]
- Schünemann, H.; Brożek, J.; Guyatt, G.; Oxman, A. (Eds.) GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations; GRADEpro: Kraków, Poland; Updated October 2013. Available online: https://gdt.gradepro.org/app/handbook/handbook.html (accessed on 1 September 2025).
- Vaes, A.M.M.; Tieland, M.; Toussaint, N.; Nilwik, R.; Verdijk, L.B.; van Loon, L.J.C.; de Groot, L.C.P.G.M. Cholecalciferol or 25-Hydroxycholecalciferol Supplementation Does Not Affect Muscle Strength and Physical Performance in Prefrail and Frail Older Adults. J. Nutr. 2018, 148, 712–720. [Google Scholar] [CrossRef] [PubMed]
- Neelemaat, F.; Bosmans, J.E.; Thijs, A.; Seidell, J.C.; van Bokhorst-de van der Schueren, M.A. Post-discharge nutritional support in malnourished elderly individuals improves functional limitations. J. Am. Med. Dir. Assoc. 2011, 12, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Flicker, L.; MacInnis, R.J.; Stein, M.S.; Scherer, S.C.; Mead, K.E.; Nowson, C.A.; Thomas, J.; Lowndes, C.; Hopper, J.L.; Wark, J.D. Should older people in residential care receive vitamin D to prevent falls? Results of a randomized trial. J. Am. Geriatr. Soc. 2005, 53, 1881–1888. [Google Scholar] [CrossRef]
- Appel, L.J.; Michos, E.D.; Mitchell, C.M.; Blackford, A.L.; Sternberg, A.L.; Miller, E.R., 3rd; Juraschek, S.P.; Schrack, J.A.; Szanton, S.L.; Charleston, J.; et al. The Effects of Four Doses of Vitamin D Supplements on Falls in Older Adults: A Response-Adaptive, Randomized Clinical Trial. Ann. Intern. Med. 2021, 174, 145–156. [Google Scholar] [CrossRef]
- Latham, N.K.; Anderson, C.S.; Lee, A.; Bennett, D.A.; Moseley, A.; Cameron, I.D.; Fitness Collaborative Group. A randomized, controlled trial of quadriceps resistance exercise and vitamin D in frail older people: The Frailty Interventions Trial in Elderly Subjects (FITNESS). J. Am. Geriatr. Soc. 2003, 51, 291–299. [Google Scholar] [CrossRef]
- Gloth, F.M., 3rd; Smith, C.E.; Hollis, B.W.; Tobin, J.D. Functional improvement with vitamin D replenishment in a cohort of frail, vitamin D-deficient older people. J. Am. Geriatr. Soc. 1995, 43, 1269–1271. [Google Scholar] [CrossRef]
- Rizka, A.; Setiati, S.; Harimurti, K.; Sadikin, M.; Mansur, I.G. Effect of Alfacalcidol on Inflammatory markers and T Cell Subsets in Elderly with Frailty Syndrome: A Double Blind Randomized Controlled Trial. Acta Medica Indones 2018, 50, 215–221. [Google Scholar]
- Bjorkman, M.P.; Sorva, A.J.; Tilvis, R.S. C-reactive protein and fibrinogen of bedridden older patients in a six-month vitamin D supplementation trial. J. Nutr. Health Aging 2009, 13, 435–439. [Google Scholar] [CrossRef]
- Cai, Y.; Wanigatunga, A.A.; Mitchell, C.M.; Urbanek, J.K.; Miller, E.R., 3rd; Juraschek, S.P.; Michos, E.D.; Kalyani, R.R.; Roth, D.L.; Appel, L.J.; et al. The effects of vitamin D supplementation on frailty in older adults at risk for falls. BMC Geriatr. 2022, 22, 312. [Google Scholar] [CrossRef]
- Meyer, H.E.; Smedshaug, G.B.; Kvaavik, E.; Falch, J.A.; Tverdal, A.; Pedersen, J.I. Can vitamin D supplementation reduce the risk of fracture in the elderly? A randomized controlled trial. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2002, 17, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Bischoff-Ferrari, H.A.; Kistler-Fischbacher, M.; Gaengler, S.; Münzer, T.; Dawson-Hughes, B.; Lang, W.; Theiler, R.; Egli, A.; Orav, E.J.; Freystaetter, G. Effects of testosterone and vitamin D on fall risk in pre-frail hypogonadal men: A factorial design RCT. J. Nutr. Health Aging 2024, 28, 100217. [Google Scholar] [CrossRef] [PubMed]
- Dwimartutie, N.; Setiati, S.; Tamin, T.Z.; Prijanti, A.R.; Harahap, A.R.; Purnamasari, D.; Harimurti, K.; Pramantara, I.D.P.; Suwarto, S.; Kojima, T. Effect of cholecalciferol supplementation on hand grip strength, walking speed, and expression of vitamin D receptor, interleukin-6, and insulin-like growth factor-1 in monocyte in pre-frail older adults: A randomized double-blind placebo-controlled trial. Geriatr. Gerontol. Int. 2024, 24, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Imaoka, M.; Higuchi, Y.; Emiko, T.; Kitagwa, T.; Ueda, T. Low-frequency Exercise and Vitamin D Supplementation Reduce Falls Among Institutionalized Frail Elderly. Int. J. Gerontol. 2016, 10, 202–206. [Google Scholar] [CrossRef]
- Bonnefoy, M.; Cornu, C.; Normand, S.; Boutitie, F.; Bugnard, F.; Rahmani, A.; Lacour, J.R.; Laville, M. The effects of exercise and protein-energy supplements on body composition and muscle function in frail elderly individuals: A long-term controlled randomised study. Br. J. Nutr. 2003, 89, 731–739. [Google Scholar] [CrossRef]
- Biesek, S.; Vojciechowski, A.S.; Filho, J.M.; Menezes Ferreira, A.C.R.; Borba, V.Z.C.; Rabito, E.I.; Gomes, A.R.S. Effects of Exergames and Protein Supplementation on Body Composition and Musculoskeletal Function of Prefrail Community-Dwelling Older Women: A Randomized, Controlled Clinical Trial. Int. J. Environ. Res. Public Health 2021, 18, 9324. [Google Scholar] [CrossRef] [PubMed]
- Na, W.; Kim, J.; Kim, H.; Lee, Y.; Jeong, B.; Lee, S.P.; Sohn, C. Evaluation of Oral Nutritional Supplementation in the Management of Frailty among the Elderly at Facilities of Community Care for the Elderly. Clin. Nutr. Res. 2021, 10, 24–35. [Google Scholar] [CrossRef]
- Gosney, M.A.; Hammond, M.F.; Shenkin, A.; Allsup, S. Effect of micronutrient supplementation on mood in nursing home residents. Gerontology 2008, 54, 292–299. [Google Scholar] [CrossRef]
- de Jong, N.; Paw, M.J.; de Groot, L.C.; de Graaf, C.; Kok, F.J.; van Staveren, W.A. Functional biochemical and nutrient indices in frail elderly people are partly affected by dietary supplements but not by exercise. J. Nutr. 1999, 129, 2028–2036. [Google Scholar] [CrossRef]
- Abe, S.; Ezaki, O.; Suzuki, M. Medium-Chain Triglycerides in Combination with Leucine and Vitamin D Increase Muscle Strength and Function in Frail Elderly Adults in a Randomized Controlled Trial. J. Nutr. 2016, 146, 1017–1026. [Google Scholar] [CrossRef] [PubMed]
- Wouters-Wesseling, W.; Wagenaar, L.W.; de Groot, L.C.; Bindels, J.G.; van Staveren, W.A. Biochemical antioxidant levels respond to supplementation with an enriched drink in frail elderly people. J. Am. Coll. Nutr. 2003, 22, 232–238. [Google Scholar] [CrossRef]
- Wouters-Wesseling, W.; Wagenaar, L.W.; Rozendaal, M.; Deijen, J.B.; de Groot, L.C.; Bindels, J.G.; van Staveren, W.A. Effect of an enriched drink on cognitive function in frail elderly persons. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2005, 60, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Akasaka, H.; Nakagami, H.; Sugimoto, K.; Yasunobe, Y.; Minami, T.; Fujimoto, T.; Yamamoto, K.; Hara, C.; Shiraki, A.; Nishida, K.; et al. Effects of nicotinamide mononucleotide on older patients with diabetes and impaired physical performance: A prospective, placebo-controlled, double-blind study. Geriatr. Gerontol. Int. 2023, 23, 38–43. [Google Scholar] [CrossRef]
- Prokopidis, K.; Giannos, P.; Katsikas Triantafyllidis, K.; Kechagias, K.S.; Mesinovic, J.; Witard, O.C.; Scott, D. Effect of vitamin D monotherapy on indices of sarcopenia in community-dwelling older adults: A systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 2022, 13, 1642–1652. [Google Scholar] [CrossRef]
- Reid, I.R.; Bolland, M.J. Controversies in medicine: The role of calcium and vitamin D supplements in adults. Med. J. Aust. 2019, 211, 468–473. [Google Scholar] [CrossRef]
- Murphy, J. Do oral nutritional supplements effectively reduce malnutrition or its adverse outcomes in older people with frailty? lancet. Healthy Longev. 2022, 3, e637–e638. [Google Scholar] [CrossRef]
- Bisset, E.S.; Howlett, S.E. The Use of Dietary Supplements and Amino Acid Restriction Interventions to Reduce Frailty in Pre-Clinical Models. Nutrients 2022, 14, 2806. [Google Scholar] [CrossRef] [PubMed]
- Seldeen, K.L.; Berman, R.N.; Pang, M.; Lasky, G.; Weiss, C.; MacDonald, B.A.; Thiyagarajan, R.; Redae, Y.; Troen, B.R. Vitamin D Insufficiency Reduces Grip Strength, Grip Endurance and Increases Frailty in Aged C57Bl/6J Mice. Nutrients 2020, 12, 3005. [Google Scholar] [CrossRef] [PubMed]
- Kotlarczyk, M.P.; Perera, S.; Ferchak, M.A.; Nace, D.A.; Resnick, N.M.; Greenspan, S.L. Vitamin D deficiency is associated with functional decline and falls in frail elderly women despite supplementation. Osteoporos. Int. J. Establ. Result Coop. Between Eur. Found. Osteoporos. Natl. Osteoporos. Found. USA 2017, 28, 1347–1353. [Google Scholar] [CrossRef] [PubMed]
- Balboa-Castillo, T.; Struijk, E.A.; Lopez-Garcia, E.; Banegas, J.R.; Rodríguez-Artalejo, F.; Guallar-Castillon, P. Low vitamin intake is associated with risk of frailty in older adults. Age Ageing 2018, 47, 872–879. [Google Scholar] [CrossRef]
- Sharma, Y.; Popescu, A.; Horwood, C.; Hakendorf, P.; Thompson, C. Prevalence of Hypovitaminosis C and its Relationship with Frailty in Older Hospitalised Patients: A Cross-Sectional Study. Nutrients 2021, 13, 2117. [Google Scholar] [CrossRef]
- Marcos-Pérez, D.; Sánchez-Flores, M.; Proietti, S.; Bonassi, S.; Costa, S.; Teixeira, J.P.; Fernández-Tajes, J.; Pásaro, E.; Valdiglesias, V.; Laffon, B. Low Vitamin D Levels and Frailty Status in Older Adults: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 2286. [Google Scholar] [CrossRef]
- Zhou, J.; Huang, P.; Liu, P.; Hao, Q.; Chen, S.; Dong, B.; Wang, J. Association of vitamin D deficiency and frailty: A systematic review and meta-analysis. Maturitas 2016, 94, 70–76. [Google Scholar] [CrossRef]
- Semba, R.D.; Bartali, B.; Zhou, J.; Blaum, C.; Ko, C.W.; Fried, L.P. Low serum micronutrient concentrations predict frailty among older women living in the community. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2006, 61, 594–599. [Google Scholar] [CrossRef]
- Zupo, R.; Castellana, F.; De Nucci, S.; Sila, A.; Aresta, S.; Buscemi, C.; Randazzo, C.; Buscemi, S.; Triggiani, V.; De Pergola, G.; et al. Role of Dietary Carotenoids in Frailty Syndrome: A Systematic Review. Biomedicines 2022, 10, 632. [Google Scholar] [CrossRef]
- Giovannini, S.; Onder, G.; Lattanzio, F.; Bustacchini, S.; Di Stefano, G.; Moresi, R.; Russo, A.; Bernabei, R.; Landi, F. Selenium Concentrations and Mortality among Community-Dwelling Older Adults: Results from IlSIRENTE Study. J. Nutr. Health Aging 2018, 22, 608–612. [Google Scholar] [CrossRef]
- Kaimoto, K.; Yamashita, M.; Suzuki, T.; Makizako, H.; Koriyama, C.; Kubozono, T.; Takenaka, T.; Ohishi, M.; Kanouchi, H.; The Tarumizu Study Diet Group. Association of Protein and Magnesium Intake with Prevalence of Prefrailty and Frailty in Community-Dwelling Older Japanese Women. J. Nutr. Sci. Vitaminol. 2021, 67, 39–47. [Google Scholar] [CrossRef]
- Lin, Y.; Meng, L.; Guo, F.; Zhang, S.; Jiang, H.; Jin, M.; Wang, J.; Tang, M.; Chen, K. Association of dietary intake and whole blood essential trace elements with frailty in older adults: A cross-sectional study. Aging Clin. Exp. Res. 2025, 37, 213. [Google Scholar] [CrossRef]
- Crichton, M.; Craven, D.; Mackay, H.; Marx, W.; de van der Schueren, M.; Marshall, S. A systematic review, meta-analysis and meta-regression of the prevalence of protein-energy malnutrition: Associations with geographical region and sex. Age Ageing 2019, 48, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Antoniak, A.E.; Greig, C.A. The effect of combined resistance exercise training and vitamin D3 supplementation on musculoskeletal health and function in older adults: A systematic review and meta-analysis. BMJ Open 2017, 7, e014619. [Google Scholar] [CrossRef] [PubMed]
- Gandham, A.; Mesinovic, J.; Jansons, P.; Zengin, A.; Bonham, M.P.; Ebeling, P.R.; Scott, D. Falls, fractures, and areal bone mineral density in older adults with sarcopenic obesity: A systematic review and meta-analysis. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2021, 22, e13187. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, F.; Hashimoto, Y.; Kaji, A.; Sakai, R.; Kawate, Y.; Okamura, T.; Kitagawa, N.; Okada, H.; Nakanishi, N.; Majima, S.; et al. Association between Geriatric Nutrition Risk Index and The Presence of Sarcopenia in People with Type 2 Diabetes Mellitus: A Cross-Sectional Study. Nutrients 2021, 13, 3729. [Google Scholar] [CrossRef]
- Khor, P.Y.; Vearing, R.M.; Charlton, K.E. The effectiveness of nutrition interventions in improving frailty and its associated constructs related to malnutrition and functional decline among community-dwelling older adults: A systematic review. J. Hum. Nutr. Diet. Off. J. Br. Diet. Assoc. 2022, 35, 566–582. [Google Scholar] [CrossRef]
- Liu, C.; Xu, H.; Chen, L.; Zhu, M. Exercise and Nutritional Intervention for Physical Function of the Prefrail: A Systematic Review and Meta-Analysis. J. Am. Med. Dir. Assoc. 2022, 23, 1431.e1–1431.e19. [Google Scholar] [CrossRef]
- Thomson, K.; Rice, S.; Arisa, O.; Johnson, E.; Tanner, L.; Marshall, C.; Sotire, T.; Richmond, C.; O’Keefe, H.; Mohammed, W.; et al. Oral nutritional interventions in frail older people who are malnourished or at risk of malnutrition: A systematic review. Health Technol. Assess. 2022, 26, 1–112. [Google Scholar] [CrossRef]
- Lee, S.H.; Yu, S. Effectiveness of multifactorial interventions in preventing falls among older adults in the community: A systematic review and meta-analysis. Int. J. Nurs. Stud. 2020, 106, 103564. [Google Scholar] [CrossRef]
- Huang, C.Y.; Mayer, P.K.; Wu, M.Y.; Liu, D.H.; Wu, P.C.; Yen, H.R. The effect of Tai Chi in elderly individuals with sarcopenia and frailty: A systematic review and meta-analysis of randomized controlled trials. Ageing Res. Rev. 2022, 82, 101747. [Google Scholar] [CrossRef]
- Kirwan, R.P.; Mazidi, M.; Rodríguez García, C.; Lane, K.E.; Jafari, A.; Butler, T.; Perez de Heredia, F.; Davies, I.G. Protein interventions augment the effect of resistance exercise on appendicular lean mass and handgrip strength in older adults: A systematic review and meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2022, 115, 897–913. [Google Scholar] [CrossRef] [PubMed]
- Weng, W.H.; Cheng, Y.H.; Yang, T.H.; Lee, S.J.; Yang, Y.R.; Wang, R.Y. Effects of strength exercises combined with other training on physical performance in frail older adults: A systematic review and meta-analysis. Arch. Gerontol. Geriatr. 2022, 102, 104757. [Google Scholar] [CrossRef] [PubMed]
- Gagesch, M.; Wieczorek, M.; Vellas, B.; Kressig, R.W.; Rizzoli, R.; Kanis, J.; Willett, W.C.; Egli, A.; Lang, W.; Orav, E.J.; et al. Effects of Vitamin D, Omega-3 Fatty Acids and a Home Exercise Program on Prevention of Pre-Frailty in Older Adults: The DO-HEALTH Randomized Clinical Trial. J. Frailty Aging 2023, 12, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Orkaby, A.R.; Dushkes, R.; Ward, R.; Djousse, L.; Buring, J.E.; Lee, I.M.; Cook, N.R.; LeBoff, M.S.; Okereke, O.I.; Copeland, T.; et al. Effect of Vitamin D3 and Omega-3 Fatty Acid Supplementation on Risk of Frailty: An Ancillary Study of a Randomized Clinical Trial. JAMA Netw. Open 2022, 5, e2231206. [Google Scholar] [CrossRef]
- Cavalcante, B.R.; Falck, R.S.; Silva, D.T.; Campos, I.R.A.; Silva, M.S.; Eckardt, N.; Batista, G.A.; Ferreira, F.M.; Pirauá, A.L.T.; Souza, M.F.; et al. Effects of Instability Resistance Training on Physical and Cognitive Function in Adults. Int. J. Sports Med. 2025. advance online publication. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Παρασκευάς Θεμιστοκλής, Συμπληρώματα μικροθρεπτικών συστατικών στο σύνδρομο ευθραυστότητας: μια συστηματική ανασκόπηση και μέτα-ανάλυση τυχαιοποιημένων κλινικών δοκιμών. 2023. Available online: https://pergamos.lib.uoa.gr/uoa/dl/object/3294695 (accessed on 1 September 2025).

| Intervention | Outcome | Studies/Participants | Summary Effect Measure | Heterogeneity | GRADE | |
|---|---|---|---|---|---|---|
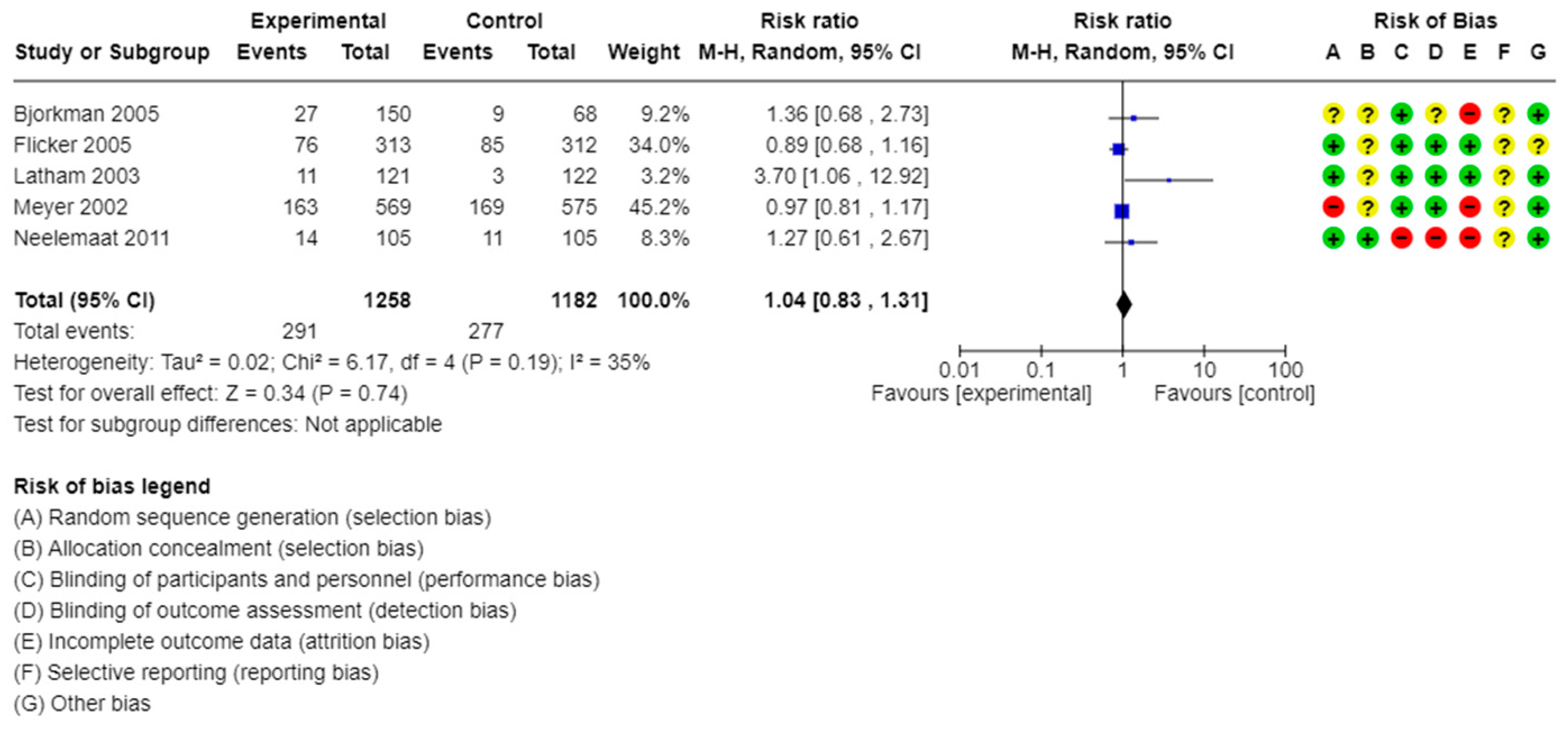
| Vitamin D supplementation vs. placebo or control | All-cause mortality | 7 studies/2600 participants | RR: 1.04, 95% CI: 0.83 to 1.31 | I2 = 35% | Moderate | |
| Frailty levels | - | |||||
| At least one fall | 2 studies/847 participants | RR: 0.99, 95% CI: 0.82 to 1.21 | I2 = 55% | Moderate | ||
| Fracture | 2 studies/1769 participants | RR: 0.77, 95% CI: 0.59 to 1.01 | I2 = 0% | Low | ||
| Muscle strength (kg) | 3 studies/27 participants | MD: −0.62, 95% CI: −1.74 to 0.50 | I2 = 0% | Very low | ||
| Gait speed | No quantitative synthesis | |||||
| Weight Indices | No quantitative synthesis | |||||
| Cognitive function | No data | |||||
| Inflammatory markers | No quantitative synthesis | |||||
| Functional measures | No quantitative synthesis | |||||
| Multicomponent supplementation vs. placebo or control | All-cause mortality | 4 studies/180 participants (only two deaths were reported in total) | Very low | |||
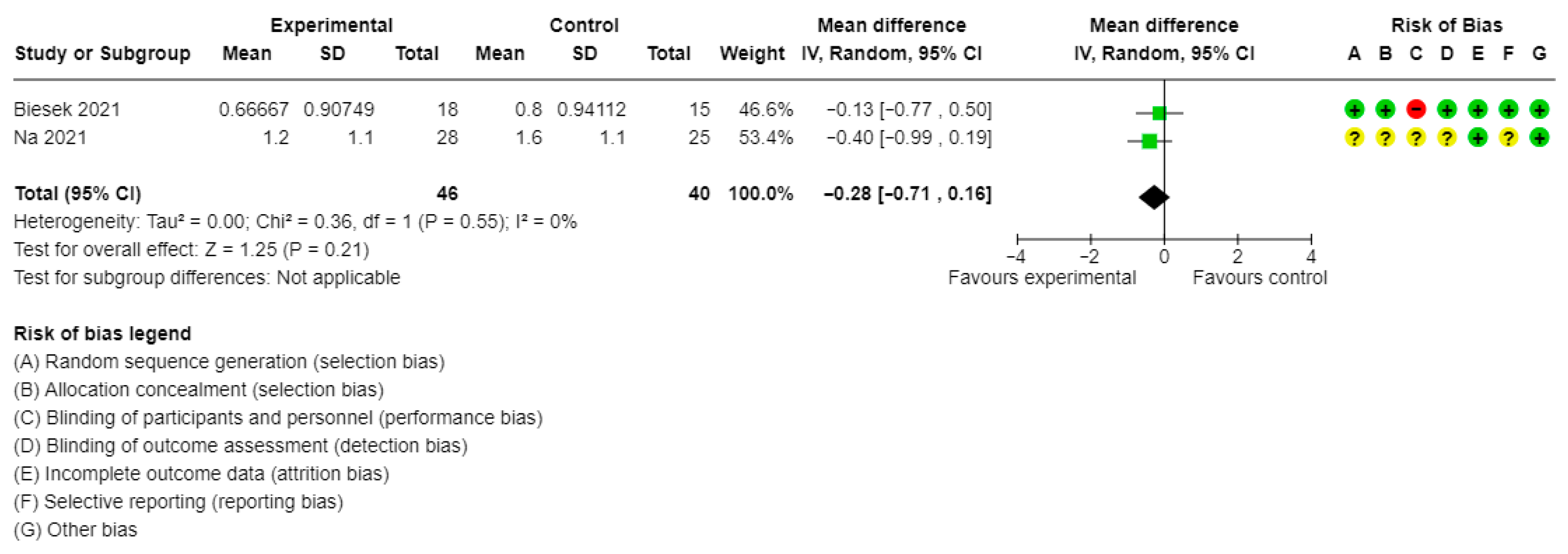
| Frailty levels (mean Fried’s Frailty Phenotype score) | 2 studies/86 participants | MD: −0.28, 95% CI: −0.71 to 0.16 | I2 = 0% | Very low | ||
| At least one fall | No quantitative synthesis | |||||
| Fracture | - | |||||
| Muscle strength (kg) | 4 studies/153 participants | MD: 0.76, 95% CI: −1.35, 2.8 | I2 = 31% | Very low | ||
| Gait speed | No quantitative synthesis | |||||
| Weight Indices | BMI | 2 studies/157 participants | MD: 0.69, 95% CI: −0.78 to 2.16 | I2 = 21% | Very low | |
| Body weight (kg) | 4 studies/188 participants | MD: 1.23, 95% CI: −0.91 to 3.37 | I2 = 30% | Very low | ||
| Cognitive function (MMSE mean score) | 2 studies/89 participants | MD: 1.34, 95% CI: −1.45 to 4.14 | I2 = 0 | Very low | ||
| Inflammatory markers | No quantitative synthesis | |||||
| Functional measures | No quantitative synthesis | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paraskevas, T.; Kotrokois, K.; Vassilakou, T.; Halvatsiotis, P.; Psaltopoulou, T.; Sarafis, P.; Sergentanis, T.N. Micronutrient Supplementation in Frailty: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Healthcare 2025, 13, 2828. https://doi.org/10.3390/healthcare13222828
Paraskevas T, Kotrokois K, Vassilakou T, Halvatsiotis P, Psaltopoulou T, Sarafis P, Sergentanis TN. Micronutrient Supplementation in Frailty: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Healthcare. 2025; 13(22):2828. https://doi.org/10.3390/healthcare13222828
Chicago/Turabian StyleParaskevas, Themistoklis, Konstantinos Kotrokois, Tonia Vassilakou, Panagiotis Halvatsiotis, Theodora Psaltopoulou, Pavlos Sarafis, and Theodoros N. Sergentanis. 2025. "Micronutrient Supplementation in Frailty: A Systematic Review and Meta-Analysis of Randomized Controlled Trials" Healthcare 13, no. 22: 2828. https://doi.org/10.3390/healthcare13222828
APA StyleParaskevas, T., Kotrokois, K., Vassilakou, T., Halvatsiotis, P., Psaltopoulou, T., Sarafis, P., & Sergentanis, T. N. (2025). Micronutrient Supplementation in Frailty: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Healthcare, 13(22), 2828. https://doi.org/10.3390/healthcare13222828









