Long-Term Outcomes and Predictors of Artificial Urinary Sphincter Survival After Prostate Cancer Treatment: A Multicenter Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. AUS (AMS 800) Implantation Procedure and Surgical Follow-Up
2.3. Assessment of Continence Status
2.4. Outcomes to Be Studied
2.5. Statistical Analysis
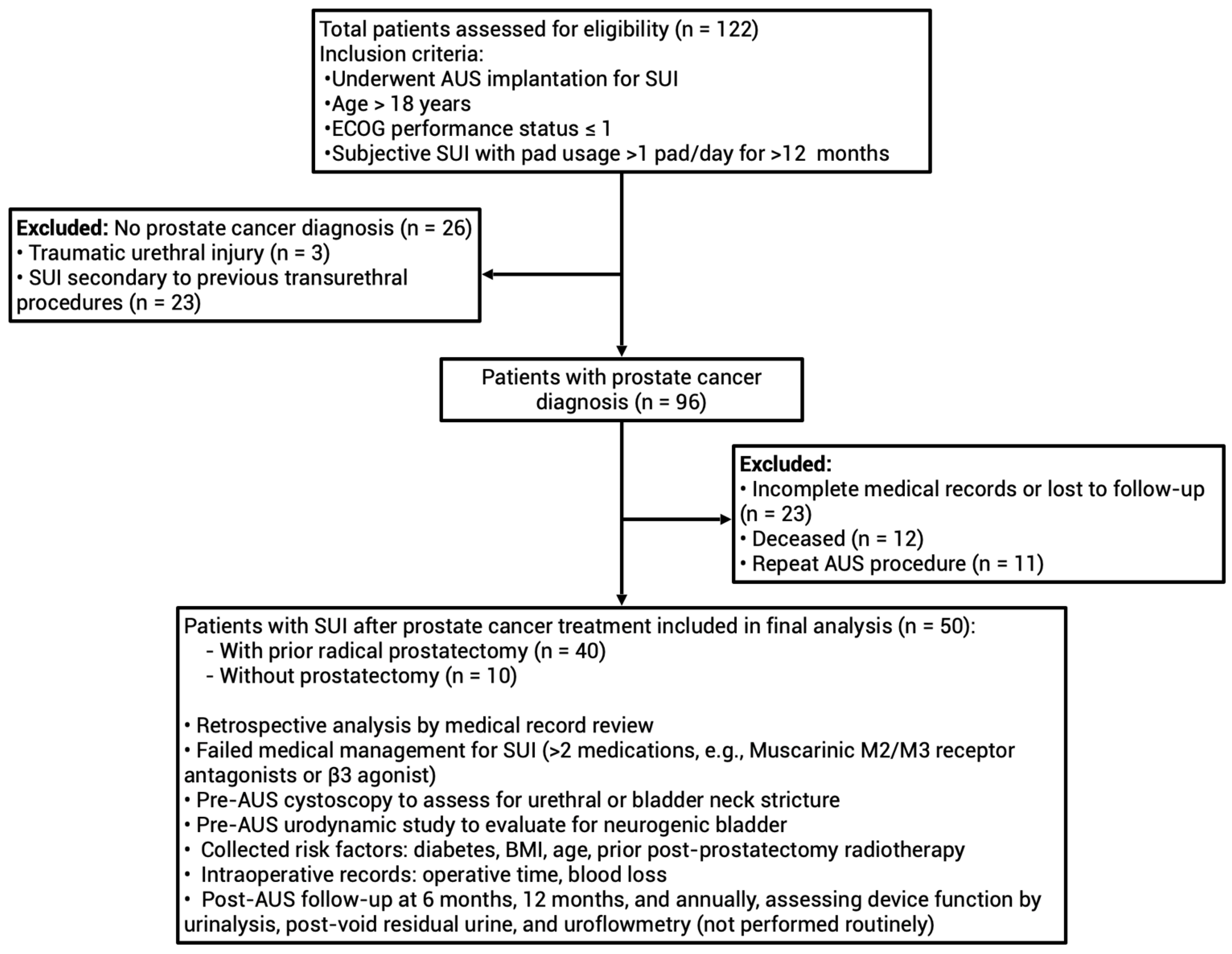
3. Results
3.1. Study Design and Subject Characteristics
3.2. Perioperative and Postoperative Outcomes of AUS (AMS 800) Implantation
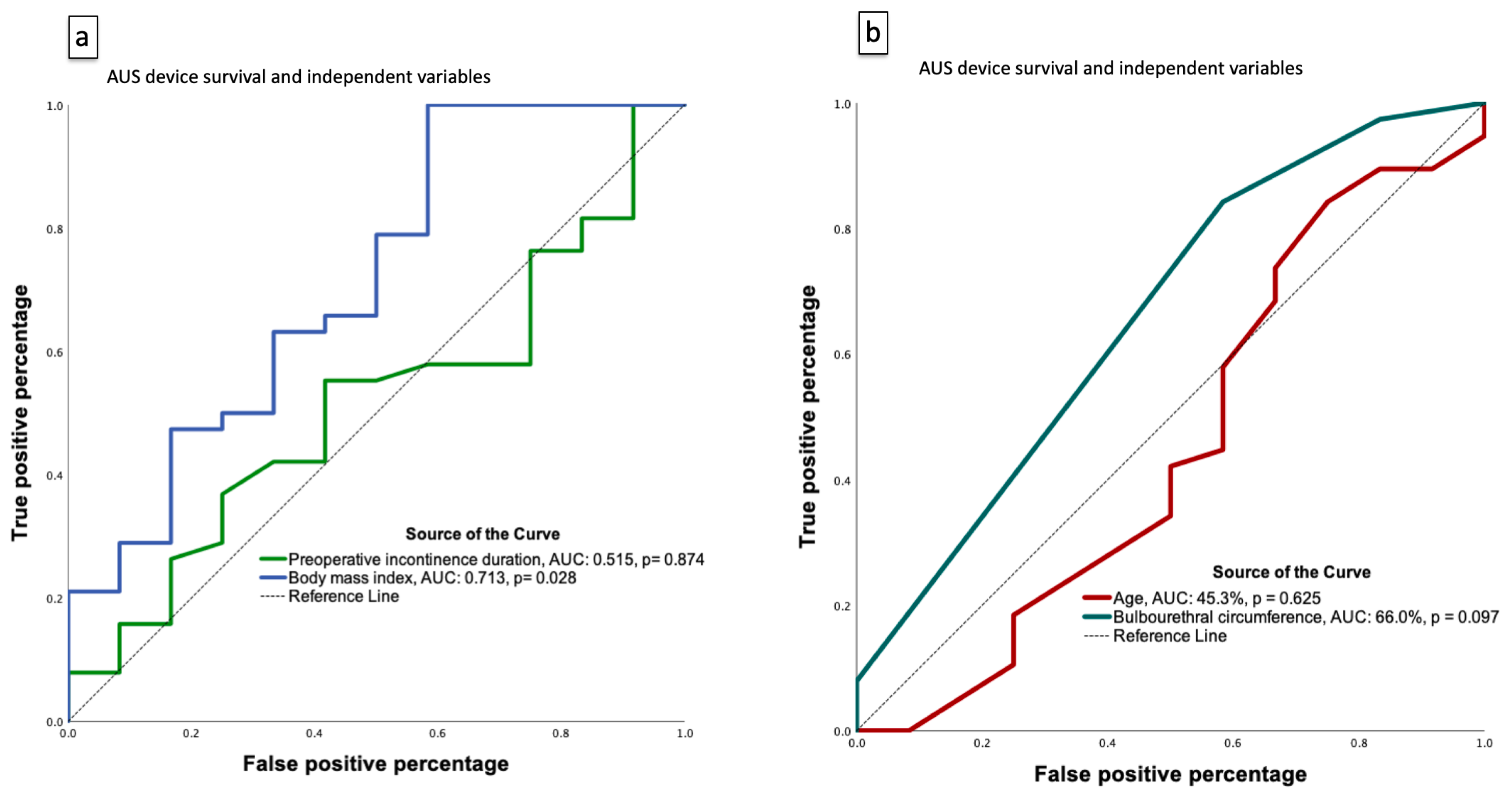
3.3. Predictors of Device Failure Following AUS Implantation
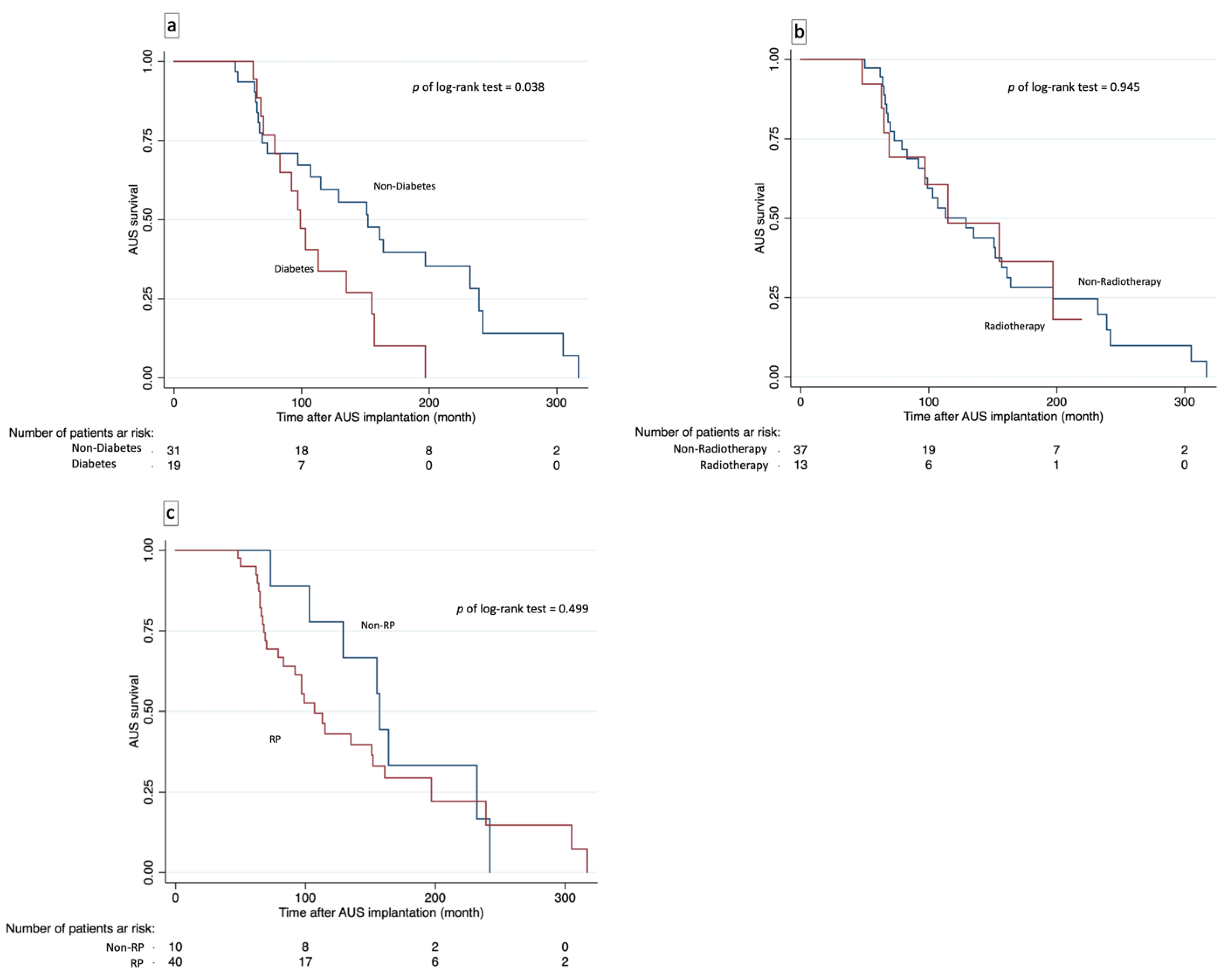
3.4. Device Survival Analysis and Risk Factors for Device Failure
3.5. Model Calibration and Individualized Risk Prediction
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Global Burden of Disease Cancer Collaboration; Fitzmaurice, C.; Allen, C.; Barber, R.M.; Barregard, L.; Bhutta, Z.A.; Brenner, H.; Dicker, D.J.; Chimed-Orchir, O.; Dandona, R.; et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived with Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2017, 3, 524–548. [Google Scholar] [CrossRef] [PubMed]
- Ziglioli, F.; Baciarello, M.; Maspero, G.; Bellini, V.; Bocchialini, T.; Cavalieri, D.; Bignami, E.G.; Maestroni, U. Oncologic outcome, side effects and comorbidity of high-intensity focused ultrasound (HIFU) for localized prostate cancer. A review. Ann. Med. Surg. 2020, 56, 110–115. [Google Scholar] [CrossRef]
- Mercader, C.; Musquera, M.; Franco, A.; Alcaraz, A.; Ribal, M.J. Primary cryotherapy for localized prostate cancer treatment. Aging Male 2020, 23, 1460–1466. [Google Scholar] [CrossRef]
- Bekelman, J.E.; Rumble, R.B.; Chen, R.C.; Pisansky, T.M.; Finelli, A.; Feifer, A.; Nguyen, P.L.; Loblaw, D.A.; Tagawa, S.T.; Gillessen, S.; et al. Clinically Localized Prostate Cancer: ASCO Clinical Practice Guideline Endorsement of an American Urological Association/American Society for Radiation Oncology/Society of Urologic Oncology Guideline. J. Clin. Oncol. 2018, 36, 3251–3258. [Google Scholar] [CrossRef]
- Wilson, L.C.; Gilling, P.J. Post-prostatectomy urinary incontinence: A review of surgical treatment options. BJU Int. 2011, 107 (Suppl. S3), 7–10. [Google Scholar] [CrossRef]
- Goluboff, E.T.; Saidi, J.A.; Mazer, S.; Bagiella, E.; Heitjan, D.F.; Benson, M.C.; Olsson, C.A. Urinary continence after radical prostatectomy: The Columbia experience. J. Urol. 1998, 159, 1276–1280. [Google Scholar] [CrossRef] [PubMed]
- Roberts, C.B.; Jang, T.L.; Shao, Y.H.; Kabadi, S.; Moore, D.F.; Lu-Yao, G.L. Treatment profile and complications associated with cryotherapy for localized prostate cancer: A population-based study. Prostate Cancer Prostatic Dis. 2011, 14, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Resnick, M.J.; Koyama, T.; Fan, K.H.; Albertsen, P.C.; Goodman, M.; Hamilton, A.S.; Hoffman, R.M.; Potosky, A.L.; Stanford, J.L.; Stroup, A.M.; et al. Long-term functional outcomes after treatment for localized prostate cancer. N. Engl. J. Med. 2013, 368, 436–445. [Google Scholar] [CrossRef]
- Peretsman, S.J.; Emberton, M.; Fleshner, N.; Shoji, S.; Bahler, C.D.; Miller, L.E. High-intensity focused ultrasound with visually directed power adjustment for focal treatment of localized prostate cancer: Systematic review and meta-analysis. World J. Urol. 2024, 42, 175. [Google Scholar] [CrossRef]
- Anderson, C.A.; Omar, M.I.; Campbell, S.E.; Hunter, K.F.; Cody, J.D.; Glazener, C.M. Conservative management for postprostatectomy urinary incontinence. Cochrane Database Syst. Rev. 2015, 1, CD001843. [Google Scholar] [CrossRef]
- Gacci, M.; Sakalis, V.I.; Karavitakis, M.; Cornu, J.N.; Gratzke, C.; Herrmann, T.R.W.; Kyriazis, I.; Malde, S.; Mamoulakis, C.; Rieken, M.; et al. European Association of Urology Guidelines on Male Urinary Incontinence. Eur. Urol. 2022, 82, 387–398. [Google Scholar] [CrossRef]
- Choiniere, R.; Violette, P.D.; Morin, M.; Tu, L.M.; Guyatt, G.H.; Reed, C.; Philie, C.A.; Legault, B.; Beaudry, M.M.; Ahmed, M.M.; et al. Evaluation of Benefits and Harms of Surgical Treatments for Post-radical Prostatectomy Urinary Incontinence: A Systematic Review and Meta-analysis. Eur. Urol. Focus 2021, 8, 1042–1052. [Google Scholar] [CrossRef]
- Scott, F.B.; Bradley, W.E.; Timm, G.W. Treatment of urinary incontinence by implantable prosthetic sphincter. Urology 1973, 1, 252–259. [Google Scholar] [CrossRef]
- Elliott, D.S.; Barrett, D.M. Mayo Clinic long-term analysis of the functional durability of the AMS 800 artificial urinary sphincter: A review of 323 cases. J. Urol. 1998, 159, 1206–1208. [Google Scholar] [CrossRef] [PubMed]
- Kaiho, Y.; Masuda, H.; Takei, M.; Hirayama, T.; Mitsui, T.; Yokoyama, M.; Kitta, T.; Kawamorita, N.; Nakagawa, H.; Iwamura, M.; et al. Surgical and Patient Reported Outcomes of Artificial Urinary Sphincter Implantation: A Multicenter, Prospective, Observational Study. J. Urol. 2018, 199, 245–250. [Google Scholar] [CrossRef]
- Radomski, S.B.; Ruzhynsky, V.; Wallis, C.J.D.; Herschorn, S. Complications and Interventions in Patients with an Artificial Urinary Sphincter: Long-Term Results. J. Urol. 2018, 200, 1093–1098. [Google Scholar] [CrossRef]
- Lambert, E.; Chartier-Kastler, E.; Vaessen, C.; Beaugerie, A.; Cotte, J.; Roupret, M.; Mozer, P.; Parra, J.; Seisen, T.; Lenfant, L.; et al. Robot-assisted Periprostatic Artificial Urinary Sphincter Implantation in Men with Neurogenic Stress Urinary Incontinence: Description of the Surgical Technique and Comparison of Long-term Functional Outcomes with the Open Approach. Eur. Urol. 2024, 85, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Linder, B.J.; Rivera, M.E.; Ziegelmann, M.J.; Elliott, D.S. Long-term Outcomes Following Artificial Urinary Sphincter Placement: An Analysis of 1082 Cases at Mayo Clinic. Urology 2015, 86, 602–607. [Google Scholar] [CrossRef]
- Leon, P.; Chartier-Kastler, E.; Roupret, M.; Ambrogi, V.; Mozer, P.; Phe, V. Long-term functional outcomes after artificial urinary sphincter implantation in men with stress urinary incontinence. BJU Int. 2015, 115, 951–957. [Google Scholar] [CrossRef]
- Ko, K.J.; Suh, Y.S.; Chung, J.Y.; Jeong, J.Y.; Lee, K. Long-term functional outcomes and durability of artificial urinary sphincter (AMS 800™) implantation in men with stress urinary incontinence. In Proceedings of the 33rd Annual EAU Congress (EAU 2018), Copenhagen, Denmark, 16–20 March 2018; p. Poster 072. [Google Scholar]
- Viers, B.R.; Linder, B.J.; Rivera, M.E.; Andrews, J.R.; Rangel, L.J.; Ziegelmann, M.J.; Elliott, D.S. The Impact of Diabetes Mellitus and Obesity on Artificial Urinary Sphincter Outcomes in Men. Urology 2016, 98, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Moses, R.A.; Broghammer, J.A.; Breyer, B.N.; Voelzke, B.B.; Buckley, J.C.; Erickson, B.A.; Elliott, S.; Vanni, A.J.; Ramkumar, N.; Myers, J.B. Patient Risk Factors Associated with Reported Urinary Quality of Life Following Artificial Urinary Sphincter Placement: A Paired Pre and Postoperative Analysis. Urology 2022, 169, 226–232. [Google Scholar] [CrossRef]
- Bates, A.S.; Martin, R.M.; Terry, T.R. Complications following artificial urinary sphincter placement after radical prostatectomy and radiotherapy: A meta-analysis. BJU Int. 2015, 116, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Mamane, J.; Sanchez, S.; Lellouch, A.G.; Gaillard, V.; Poussot, B.; Tricard, T.; Saussine, C.; Brierre, T.; Game, X.; Beraud, F.; et al. Impact of radiation therapy on artificial urinary sphincter implantation in male patients: A multicenter study. Neurourol. Urodyn. 2022, 41, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Sacomani, C.A.R.; Zequi, S.C.; Costa, W.H.D.; Benigno, B.S.; Campos, R.S.M.; Bachega, W., Jr.; Guimaraes, G.C. Long-term results of the implantation of the AMS 800 artificial sphincter for post-prostatectomy incontinence: A single-center experience. Int. Braz. J. Urol. 2018, 44, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Pitout, A.; Lecoanet, P.; Mazeaud, C.; Gaillard, V.; Poussot, B.; Tricard, T.; Saussine, C.; Brierre, T.; Game, X.; Beraud, F.; et al. Risk Factors for Artificial Urinary Sphincter Explantation and Erosion in Male Nonneurological Patients. Int. Neurourol. J. 2024, 28, 147–155. [Google Scholar] [CrossRef]
- Slevin, F.; Zattoni, F.; Checcucci, E.; Cumberbatch, M.G.K.; Nacchia, A.; Cornford, P.; Briers, E.; De Meerleer, G.; De Santis, M.; Eberli, D.; et al. A Systematic Review of the Efficacy and Toxicity of Brachytherapy Boost Combined with External Beam Radiotherapy for Nonmetastatic Prostate Cancer. Eur. Urol. Oncol. 2024, 7, 677–696. [Google Scholar] [CrossRef]
- Solomon, E.; Veeratterapillay, R.; Malde, S.; Harding, C.; Greenwell, T.J. Can filling phase urodynamic parameters predict the success of the bulbar artificial urinary sphincter in treating post-prostatectomy incontinence? Neurourol. Urodyn. 2017, 36, 1557–1563. [Google Scholar] [CrossRef]
- Pan, W.H.; Flegal, K.M.; Chang, H.Y.; Yeh, W.T.; Yeh, C.J.; Lee, W.C. Body mass index and obesity-related metabolic disorders in Taiwanese and US whites and blacks: Implications for definitions of overweight and obesity for Asians. Am. J. Clin. Nutr. 2004, 79, 31–39. [Google Scholar] [CrossRef]
- International Diabetes Federation. Diabetes Prevalence in Taiwan. Available online: https://idf.org/our-network/regions-and-members/western-pacific/members/taiwan/ (accessed on 20 July 2025).
- National Diabetes Statistics Report. Prevalence of Both Diagnosed and Undiagnosed Diabetes. Available online: https://www.cdc.gov/diabetes/php/data-research/index.html (accessed on 20 July 2025).
- Guercio, A.; Lombardo, R.; Turchi, B.; Romagnoli, M.; Franco, A.; D’Annunzio, S.; Fusco, F.; Pastore, A.L.; Al Salhi, Y.; Fuschi, A.; et al. Patient satisfaction and decision regret in patients undergoing radical prostatectomy: A multicenter analysis. Int. Urol. Nephrol. 2025, 57, 3207–3213. [Google Scholar] [CrossRef]

| Overall (n = 50) | RT Group (n = 13) | Non-RT Group (n = 37) | p Value | |
|---|---|---|---|---|
| Age, years | 68.72 ± 6.38 | 69.31 ± 6.61 | 68.51 ± 6.38 | 0.704 |
| Diabetes, n (%) | 19 (38) | 4 (30.8) | 15 (40.5) | 0.742 |
| eGFR, mL/min/1.73 m2 | 71.87 ± 22.56 | 70.96 ± 22.06 | 72.18 ± 23.03 | 0.868 |
| Body-mass index, kg/m2 | 25.73 ± 3.07 | 27.45 ± 2.89 | 25.12 ± 2.93 | 0.017 * |
| 5-year AUS device survival, n (%) | 47 (94) | 12 (92.3) | 35 (94.6) | 0.765 |
| 10-year AUS device survival, n (%) | 20 (40) | 4 (30.8) | 16 (43.2) | 0.430 |
| Prostate cancer stage, n (%) | 0.057 | |||
| I | 3 (60) | 0 (0) | 3 (8.1) | |
| II | 33 (66) | 6 (46.2) | 27 (73.0) | |
| III | 13 (26) | 7 (53.8) | 6 (16.2) | |
| IV | 1 (2) | 0 (0) | 1 (2.7) | |
| Preoperative incontinence duration, months (median [IQR]) | 26.5 (13.75–47) | 32 (14–52) | 26 (13–45) | 0.921 |
| Postoperative continence duration, months (mean ± SD) | 126.50 ± 68.11 | 113.62 ± 53.50 | 131.03 ± 72.65 | 0.433 |
| Continence at last follow-up since AUS, n (%) | 12 (24) | 5 (38.5) | 7 (18.9) | 0.256 |
| Number of preoperative anti-incontinence medications, n (%) | 0.303 | |||
| 1 | 10 (20) | 4 (30.8) | 6 (16.2) | |
| >1 | 29 (58) | 8 (61.5) | 21 (56.8) | |
| Preoperative botulinum toxin treatment, n (%) | 1 (2) | 0 (0) | 1 (2.7) | 0.740 |
| Preoperative MUCP, cmH2O (median [IQR]) | 33 (24.25–42.75) | 20 (10–42) | 36 (26.5–58) | 0.062 |
| Preoperative PFM training (Kegel exercises or biofeedback), n (%) | 18 (36) | 6 (46.2) | 12 (32.4) | 0.375 |
| Prostate cancer treatment history, n (%) | ||||
| Radical prostatectomy # | 40 (80) | 12 (92.3) | 28 (75.7) | 0.258 |
| HIFU or Cryotherapy | 5 (10) | 1 (7.7) | 4 (10.8) | 1.000 |
| TURP & | 12 (24) | 4 (30.8) | 8 (21.6) | 0.707 |
| OIU after prostate treatment | 6 (12) | 2 (15.4) | 4 (10.8) | 0.643 |
| Overall (n = 50) | RT Group (n = 13) | Non-RT Group (n = 37) | p Value | |
|---|---|---|---|---|
| AUS operation time, min (median [IQR]) | 142 (107.00–180.25) | 148.0 (113.0–197.5) | 138.0 (107.0–179.5) | 0.611 |
| Estimated blood loss, mL | 5.0 (5.0–42.5) | 5.0 (5.0–7.5) | 5 (5–50) | 0.460 |
| Bulbourethral circumference, mm (after prostate cancer treatment) | 4 (4–4) | 4.0 (4.0–4.5) | 4 (4–4) | 0.441 |
| Postoperative pain score (NRS), median (IQR) | 2 (1–2) | 2 (1–2) | 2 (1–2) | 0.953 |
| Days to Foley catheter removal after surgery | 14 (1–28) | 14 (14–35) | 14 (1–28) | 0.858 |
| RU, mL (after prostate cancer treatment) | 1 (0–15) | 3.0 (0–23.5) | 1.0 (0–13.5) | 0.474 |
| Postoperative stress urinary incontinence, n (%) | 0.474 | |||
| Mild (1 pad/day) | 10 (20) | 3 (23.1) | 7 (18.9) | |
| Moderate to severe (≥2 pads/day) | 5 (10) | 2 (15.4) | 3 (8.1) | |
| Postoperative urinary retention (RU > 50 mL), n (%) | 3 (6) | 3 (23.1) | 0 (0) | 0.015 * |
| Failure Type | n (%) | Median Time to Failure, Months (IQR) |
|---|---|---|
| Revision | 1 (2.6) | 151 (151–151) |
| Explant * | 28 (73.7) | 94.5 (67.8–158.0) |
| Persistent incontinence | 9 (23.7) | 107 (97–155) |
| Total | 38 (100) | 101 (68.3–156.5) |
| Univariate, Crude | Multivariable, Model 1 | Multivariable, Model 2 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p Value | OR | 95% CI | p Value | OR | 95% CI | p Value | |
| Age | 1.049 | 0.947–1.161 | 0.361 | 1.019 | 0.901–1.153 | 0.761 | — | — | — |
| Radiotherapy | 0.373 | 0.093–1.495 | 0.164 | 0.741 | 0.149–3.688 | 0.714 | — | — | — |
| eGFR | 0.995 | 0.966–1.025 | 0.753 | — | — | — | — | — | — |
| Prostate cancer stage | 0.962 | 0.318–2.909 | 0.946 | — | — | — | — | — | — |
| Diabetes | 1.304 | 0.333–5.108 | 0.703 | 2.480 | 0.443–13.886 | 0.301 | 2.712 | 0.487–15.115 | 0.255 |
| BMI | 0.704 | 0.544–0.911 | 0.008 * | 0.684 | 0.506–0.925 | 0.014 * | 0.685 | 0.508–0.923 | 0.013 * |
| TURP | 4.481 | 0.515–39.009 | 0.174 | — | — | — | — | — | — |
| RP | 0.750 | 0.136–4.133 | 0.741 | — | — | — | — | — | — |
| Bulbourethral circumference | 0.112 | 0.014–0.874 | 0.037 * | — | — | — | 0.169 | 0.017–1.668 | 0.128 |
| Postoperative pain score | 0.954 | 0.452–2.016 | 0.903 | — | — | — | — | — | — |
| Preoperative incontinence duration | 0.999 | 0.983–1.015 | 0.905 | — | — | — | — | — | — |
| Univariate, Crude | Multivariable, Model 1 | Multivariable, Model 2 | Multivariable, Model 3 | |||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Age | 1.002 (0.953–1.055) | 0.930 | — | — | — | — | 1.004 (0.940–1.073) | 0.898 |
| Radiotherapy | 1.028 (0.463–2.285) | 0.946 | 1.168 (0.478–2.854) | 0.733 | 1.141 (0.465–2.802) | 0.773 | 0.760 (0.264–2.190) | 0.611 |
| eGFR | 1.008 (0.994–1.023) | 0.267 | 1.010 (0.994–1.0260 | 0.236 | 1.004 (0.986–1.022) | 0.678 | 1.000 (0.980–1.021) | 0.965 |
| Prostate cancer stage | 1.311 (0.699–2.461) | 0.399 | — | — | 1.363 (0.702–2.646) | 0.360 | 1.473 (0.684–3.169) | 0.322 |
| Diabetes | 2.082 (1.024–4.233) | 0.043 * | 2.281 (1.029–4.910) | 0.035 * | — | — | 2.966 (1.114–7.900) | 0.030 * |
| BMI | 0.919 (0.812–1.040) | 0.181 | — | — | — | — | 0.941 (0.798–1.108) | 0.463 |
| TURP | 1.731 (0.840–3.563) | 0.137 | 1.628 (0.733–3.617) | 0.232 | 1.507 (0.697–3.258) | 0.297 | 1.601 (0.673–3.812) | 0.287 |
| RP | 1.311 (0.594–2.895) | 0.502 | 1.221 (0.521–2.862) | 0.647 | 1.103 (0.480–2.538) | 0.817 | 1.311 (0.505–3.404) | 0.578 |
| Bulbourethral circumference | 0.315 (0.095–1.044) | 0.059 | 0.402 (0.110–1.476) | 0.170 | 0.324 (0.077–1.367) | 0.125 | 0.305 (0.070–1.323) | 0.113 |
| Postoperative pain score | 0.879 (0.589–1.311) | 0.527 | 0.819 (0.543–1.236) | 0.342 | 0.707 (0.460–1.088) | 0.115 | 0.636 (0.401–1.011) | 0.056 |
| Preoperative incontinence duration | 1.003 (0.995–1.010) | 0.482 | 1.004 (0.995–1.013) | 0.382 | 1.004 (0.995–1.013) | 0.372 | 1.004 (0.994–1.013) | 0.458 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, C.-F.; Chen, H.-Y.; Wu, C.-T.; Liu, K.-L.; Lin, C.-C.; Hsu, H.-J.; Lee, C.-C.; Chen, C.-Y. Long-Term Outcomes and Predictors of Artificial Urinary Sphincter Survival After Prostate Cancer Treatment: A Multicenter Cohort Study. Healthcare 2025, 13, 2812. https://doi.org/10.3390/healthcare13212812
Lin C-F, Chen H-Y, Wu C-T, Liu K-L, Lin C-C, Hsu H-J, Lee C-C, Chen C-Y. Long-Term Outcomes and Predictors of Artificial Urinary Sphincter Survival After Prostate Cancer Treatment: A Multicenter Cohort Study. Healthcare. 2025; 13(21):2812. https://doi.org/10.3390/healthcare13212812
Chicago/Turabian StyleLin, Cheng-Feng, Hung-Yi Chen, Chun-Te Wu, Kuan-Lin Liu, Cheng-Chia Lin, Heng-Jung Hsu, Chin-Chan Lee, and Chun-Yu Chen. 2025. "Long-Term Outcomes and Predictors of Artificial Urinary Sphincter Survival After Prostate Cancer Treatment: A Multicenter Cohort Study" Healthcare 13, no. 21: 2812. https://doi.org/10.3390/healthcare13212812
APA StyleLin, C.-F., Chen, H.-Y., Wu, C.-T., Liu, K.-L., Lin, C.-C., Hsu, H.-J., Lee, C.-C., & Chen, C.-Y. (2025). Long-Term Outcomes and Predictors of Artificial Urinary Sphincter Survival After Prostate Cancer Treatment: A Multicenter Cohort Study. Healthcare, 13(21), 2812. https://doi.org/10.3390/healthcare13212812







