Enhancing Patient Safety in Refractory Ventricular Fibrillation: A Systematic Review of Double Sequential and Vector Change Defibrillation Barriers
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. PICOT Framework
- P (Population): Adult patients (≥18 years) who experienced refractory ventricular fibrillation (RVF) during cardiac arrest.
- I (Intervention): Double sequential external defibrillation (DSED) or vector change (VC) defibrillation.
- C (Comparison): Conventional single-shock defibrillation or standard resuscitation protocol.
- O (Outcomes): Return of spontaneous circulation (ROSC), survival to hospital discharge, neurological outcomes, and identification of practical barriers, especially those affecting patient safety.
- T (Time): Studies published between January 2015 and August 2025.
2.3. Eligibility Criteria
2.4. Study Selection
2.5. Data Extraction and Management
2.6. Use of Generative AI
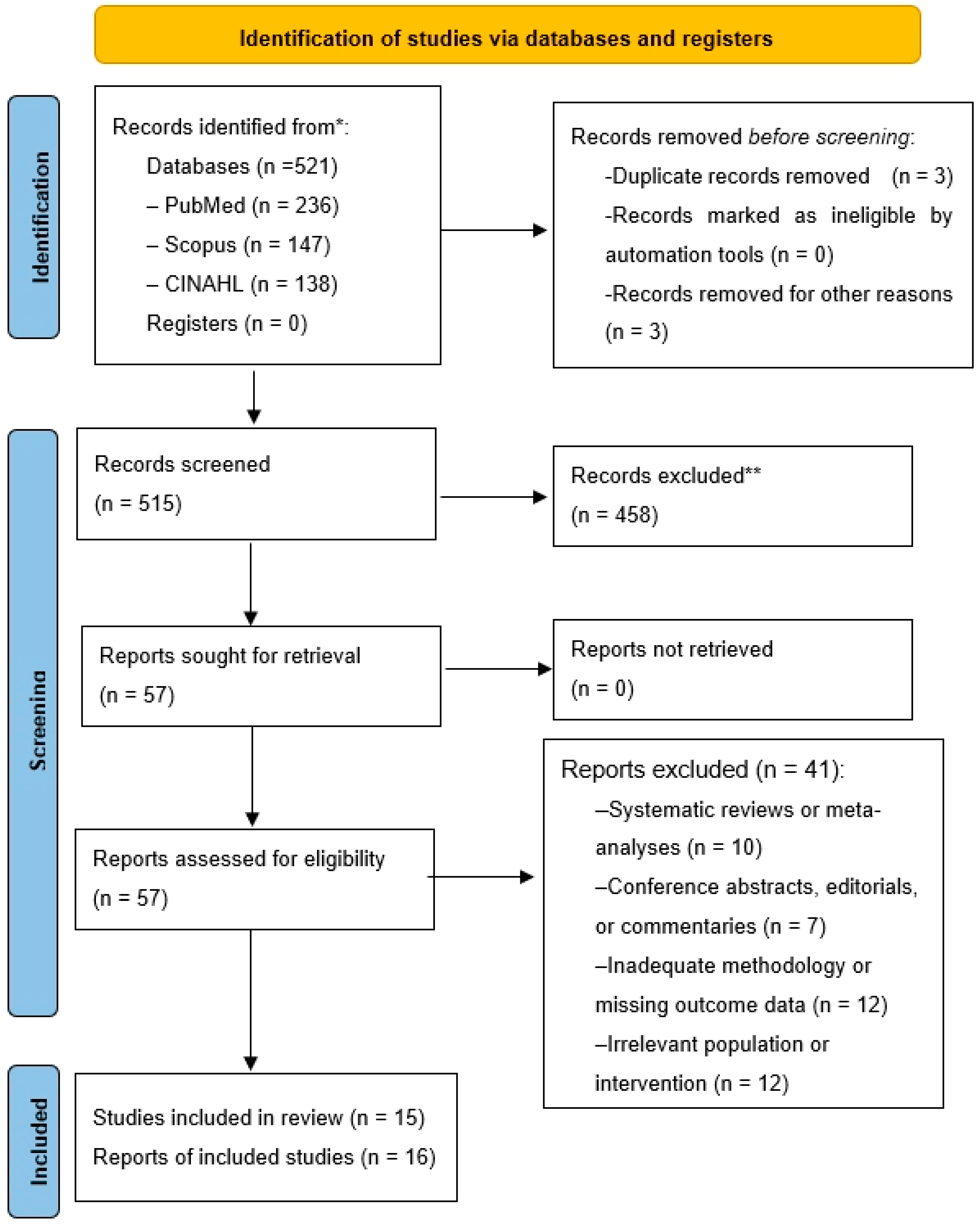
3. Results
3.1. Study Characteristics
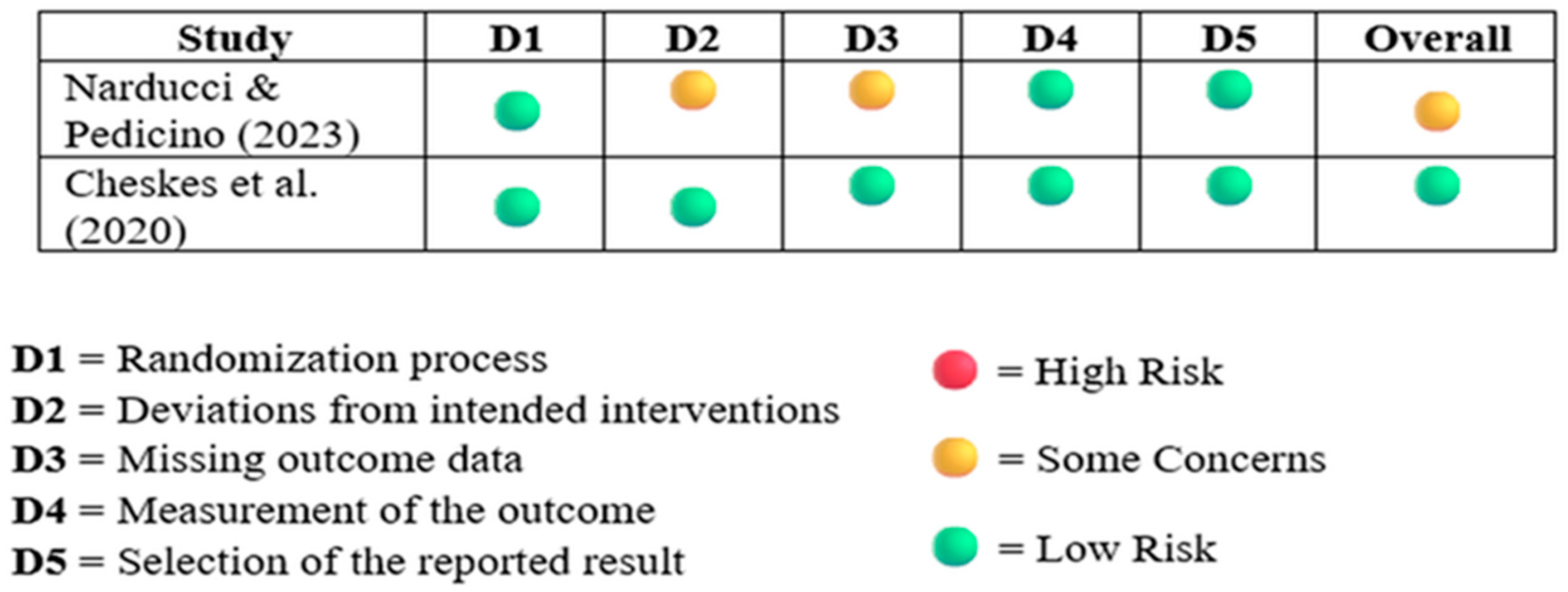
3.2. Quality Assessment
3.2.1. Newcastle–Ottawa Scale
3.2.2. Revised Cochrane Risk of Bias Tool for RCT 2
3.2.3. The Joanna Briggs Institute Critical Appraisal Checklist for Case Series
3.3. Practical Barriers and Patient Safety Implications in the Implementation of DSED and VC Techniques
3.3.1. Equipment and Resource Limitations
3.3.2. Coordination and Timing Complexity
3.4. Training and Personnel Constraints
3.5. Protocol Inconsistency and Limited Integration
3.6. Limitations of the Evidence
3.6.1. Small Sample Sizes and Underpowered Designs
3.6.2. Retrospective and Observational Methodologies
3.6.3. Lack of Standardized Outcomes and Comparator Arms
3.6.4. Simulation-Based and Non-Generalizable Settings
3.7. Comparative Effectiveness of DSED, VC, and Conventional Defibrillation
4. Discussion
4.1. Limitations of This Review
4.2. Future Research Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| VF | Ventricular Fibrillation |
| RVF | Refractory Ventricular Fibrillation |
| ERC | European Resuscitation Council |
| AHA | American Heart Association |
| RCT | Randomized Controlled Trial |
| DSED | Double Sequential External Defibrillation |
| VC | Vector Change |
| OHCA | Out-of-Hospital Cardiac Arrest |
| IHCA | In-Hospital Cardiac Arrest |
| CPR | Cardiopulmonary Resuscitation |
| ROSC | Return of Spontaneous Circulation |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| NOS | Newcastle–Ottawa Scale |
| RoB 2 | Cochrane Risk of Bias 2 Tool |
| EMS | Emergency Medical Services |
References
- Cabañas, J.G.; Myers, J.B.; Williams, J.G.; De Maio, V.J.; Bachman, M.W. Double sequential external defibrillation in out-of-hospital refractory ventricular fibrillation: A report of ten cases. Prehosp. Emerg. Care 2015, 19, 126–130. [Google Scholar] [CrossRef]
- Deakin, C.D.; Morley, P.; Soar, J.; Drennan, I.R. Double (dual) sequential defibrillation for refractory ventricular fibrillation cardiac arrest: A systematic review. Resuscitation 2020, 155, 24–31. [Google Scholar] [CrossRef]
- Delorenzo, A.; Nehme, Z.; Yates, J.; Bernard, S.; Smith, K. Double sequential external defibrillation for refractory ventricular fibrillation out-of-hospital cardiac arrest: A systematic review and meta-analysis. Resuscitation 2019, 135, 124–129. [Google Scholar] [CrossRef]
- Kane, A.D.; Nolan, J.P. Changes to the European Resuscitation Council Guidelines for Adult Resuscitation. BJA Educ. 2022, 22, 265–272. [Google Scholar] [CrossRef]
- Perman, S.M.; Elmer, J.; Maciel, C.B.; Uzendu, A.; May, T.; Mumma, B.E.; Bartos, J.A.; Rodriguez, A.J.; Kurz, M.C.; Panchal, A.R.; et al. 2023 American Heart Association Focused Update on Adult Advanced Cardiovascular Life Support: An Update to the American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2024, 149, e254–e273. [Google Scholar] [CrossRef]
- Stankovic, N.; Høybye, M.; Holmberg, M.J.; Lauridsen, K.G.; Andersen, L.W.; Granfeldt, A. Factors Associated with Shockable versus Non-Shockable Rhythms in Patients with In-Hospital Cardiac Arrest. Resuscitation 2021, 158, 166–174. [Google Scholar] [CrossRef]
- Perna, B.; Guarino, M.; De Fazio, R.; Esposito, L.; Portoraro, A.; Rossin, F.; Spampinato, M.D.; De Giorgio, R. Beyond standard shocks: A critical review of alternative defibrillation strategies in refractory ventricular fibrillation. J. Clin. Med. 2025, 14, 5016. [Google Scholar] [CrossRef]
- Lybeck, A.M.; Moy, H.P.; Tan, D.K. Double sequential defibrillation for refractory ventricular fibrillation: A case report. Prehosp. Emerg. Care 2015, 19, 554–557. [Google Scholar] [CrossRef]
- Cortez, E.; Krebs, W.; Davis, J.; Keseg, D.P.; Panchal, A.R. Use of double sequential external defibrillation for refractory ventricular fibrillation during out-of-hospital cardiac arrest. Resuscitation 2016, 108, 82–86. [Google Scholar] [CrossRef]
- Nordviste, V.; Rehn, M.; Jørstad Krüger, A.; Rødseth Brede, J. Time difference between pad placement in single versus double external defibrillation: A live patient simulation model. Resusc. Plus 2024, 19, 100741. [Google Scholar] [CrossRef]
- Cheskes, S.; Dorian, P.; Feldman, M.; McLeod, S.; Scales, D.C.; Pinto, R.; Turner, L.; Morrison, L.J.; Drennan, I.R.; Verbeek, P.R. DOuble Sequential External Defibrillation for Refractory Ventricular Fibrillation: The DOSE VF Pilot Randomized Controlled Trial. Resuscitation 2020, 150, 178–184. [Google Scholar] [CrossRef]
- Drennan, I.R.; Seidler, D.; Cheskes, S. A survey of the incidence of defibrillator damage during double sequential external defibrillation for refractory ventricular fibrillation. Resusc. Plus 2022, 11, 100287. [Google Scholar] [CrossRef]
- Eraniyan, K.; Banerjee, A.; Persaud, N.A.; Banerjee, P. Double Sequential Defibrillation for Refractory Ventricular Fibrillation. Acad. Med. Surg. 2025. [Google Scholar] [CrossRef]
- Rahimi, M.; Drennan, I.R.; Turner, L.; Dorian, P.; Cheskes, S. The impact of double sequential shock timing on outcomes during refractory out-of-hospital cardiac arrest. Resuscitation 2024, 194, 110082. [Google Scholar] [CrossRef] [PubMed]
- Verkaik, B.J.; Walker, R.G.; Taylor, T.G.; Ekkel, M.M.; Marx, R.; Stieglis, R.; van Eeden, V.G.M.; Doeleman, L.C.; Hulleman, M.; Chapman, F.W.; et al. Defibrillation and refractory ventricular fibrillation. Eur. Heart J. 2024, 46, 582–584. [Google Scholar] [CrossRef]
- Kim, H.E.; Lee, K.J.; Jo, Y.H.; Lee, J.H.; Kim, Y.J.; Kim, J.H.; Kim, J.H.; Lee, D.K.; Kim, D.W.; Park, S.M.; et al. Refractory Ventricular Fibrillation Treated with Double Simultaneous Defibrillation: Pilot Study. Emerg. Med. Int. 2020, 2020, 5470912. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Narducci, M.L.; Pedicino, D. A New Defibrillation Strategy for Refractory Ventricular Fibrillation during Out-of-Hospital Cardiac Arrest: Are Two Better than One? Eur. Heart J. 2023, 44, 919–920. [Google Scholar] [CrossRef]
- Mapp, J.G.; Hans, A.J.; Darrington, A.M.; Ross, E.M.; Ho, C.C.; Miramontes, D.A.; Harper, S.A.; Wampler, D.A. Prehospital Double Sequential Defibrillation: A Matched Case–Control Study. Acad. Emerg. Med. 2019, 26, 994–1001. [Google Scholar] [CrossRef]
- Beck, L.R.; Ostermayer, D.G.; Ponce, J.N.; Srinivasan, S.; Wang, H.E. Effectiveness of Prehospital Dual Sequential Defibrillation for Refractory Ventricular Fibrillation and Ventricular Tachycardia Cardiac Arrest. Prehosp. Emerg. Care 2019, 23, 597–602. [Google Scholar] [CrossRef]
- Cheskes, S.; Wudwud, A.; Turner, L.; McLeod, S.; Summers, J.; Morrison, L.J.; Verbeek, P.R. The Impact of Double Sequential External Defibrillation on Termination of Refractory Ventricular Fibrillation during Out-of-Hospital Cardiac Arrest. Resuscitation 2019, 144, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Emmerson, A.C.; Whitbread, M.; Fothergill, R.T. Double Sequential Defibrillation Therapy for Out-of-Hospital Cardiac Arrests: The London Experience. Resuscitation 2017, 118, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Ross, E.M.; Redman, T.T.; Harper, S.A.; Mapp, J.G.; Wampler, D.A.; Miramontes, D.A. Dual Defibrillation in Out-of-Hospital Cardiac Arrest: A Retrospective Cohort Analysis. Resuscitation 2016, 106, 14–17. [Google Scholar] [CrossRef]
- Merlin, M.A.; Tagore, A.; Bauter, R.; Arshad, F.H. A Case Series of Double Sequence Defibrillation. Prehosp. Emerg. Care 2016, 20, 550–553. [Google Scholar] [CrossRef]
- Cheskes, S.; Drennan, I.R.; Turner, L.; Pandit, S.V.; Dorian, P. The Impact of Alternate Defibrillation Strategies on Shock-Refractory and Recurrent Ventricular Fibrillation: A Secondary Analysis of the DOSE VF Cluster Randomized Controlled Trial. Resuscitation 2024, 198, 110186. [Google Scholar] [CrossRef]
- Li, Y.; He, X.; Li, Z.; Li, D.; Yuan, X.; Yang, J. Double Sequential External Defibrillation versus Standard Defibrillation in Refractory Ventricular Fibrillation: A Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 2022, 9, 1017935. [Google Scholar] [CrossRef] [PubMed]
| Author (Year) | Country | Study Design | Sample/Intervention | Outcomes | Clinical Barriers/Limitations |
|---|---|---|---|---|---|
| Eraniyan et al. (2025) [13] | USA | Retrospective case series | 29 OHCA patients with RVF, DSED used | ROSC 24%, Survival 21%, Neurological outcome NR | Delays due to equipment/setup; small sample; registry design |
| Rahimi et al. (2024) [14] | Canada | Retrospective cohort | 106 OHCA, DSED intervals analyzed | ROSC 24% (<75 ms); no survival/neurological difference | Timing precision critical; incomplete data; low power |
| Nordviste et al. (2024) [10] | Norway | Observational simulation | 108 procedures by EMS teams | DSED delay ~13.7s vs. standard; feasible in simulation | Simulation only; no CPR/shocks; generalizability limited |
| Cheskes et al. (2024) [25] | Canada | Secondary analysis of RCT | 345 OHCA, DSED/VC vs. standard | DSED: ROSC/survival benefit; VC improved VF termination | Small subgroup sizes; no post-ROSC care data |
| Verkaik et al. (2024) [15] | Netherlands | Observational registry | 436 OHCA with ≥3 shocks | True RVF 5%; VF terminated in 95% | Hard to differentiate VF types in real-time; outcome scope limited |
| Narducci & Pedicino (2023) [18] | Italy | Cluster-RCT | 405 OHCA with RVF | DSED: survival 30%, ROSC 46%, neuro 27% | COVID-related early stop; no long-term outcomes |
| Kim et al. (2020) [16] | South Korea | Retrospective pilot | 38 IHCA with RVF/VT | DSiD better early outcomes; neuro not significant | Small sample; ED setting; coordination issues |
| Cheskes et al. (2020) [11] | Canada | Pilot cluster-RCT | 152 OHCA, DSED/VC vs. standard | Feasibility 89.5%, ROSC improved | Pilot size; generalizability concerns; no power for outcomes |
| Mapp et al. (2019) [19] | USA | Matched case–control | 205 OHCA (64 survivors matched) | No survival/neuro difference between DSD and standard | Late DSD use; small subgroups; variable CPR quality |
| Beck et al. (2019) [20] | USA | Retrospective cohort | 310 OHCA with RVF | Lower ROSC/survival in DSD vs. standard | Selection bias; non-standardized DSD application |
| Cheskes et al. (2019) [21] | Canada | Retrospective cohort | 252 OHCA, DSED vs. standard | Better early ROSC with DSED; NS overall | Online approval delays; observational design |
| Emmerson et al. (2017) [22] | UK | Retrospective observational | 220 OHCA (45 DSED) | ROSC 38%, survival 7% with DSED | Late DSED, AP-only staff; small group |
| Ross et al. (2016) [23] | USA | Retrospective cohort | 279 OHCA with RVF | NS differences in ROSC/survival/neuro | Inconsistent timing; missing data; selection bias |
| Cortez et al. (2016) [9] | USA | Retrospective case series | 12 OHCA with RVF ≥5 shocks | ROSC 25%, neuro intact 17% | Delays in DSED; small, non-comparative study |
| Merlin et al. (2016) [24] | USA | Retrospective case series | 7 OHCA with ≥3 shocks | Survival 43%, neuro intact 43% | Tiny sample; protocol adherence concerns |
| Cabañas et al. (2015) [1] | USA | Retrospective case series | 10 OHCA with ≥5 shocks | ROSC 30%, no discharge survival | Very small group; no neuro/post-arrest data |
| Study | Selection | Comparability | Outcomes | Total | |||||
|---|---|---|---|---|---|---|---|---|---|
| Study | Representativeness | Selection (Non-Exposed) | Ascertainment | Outcome Not Present | Comparability | Assessment | Follow-Up Length | Follow-Up Adequacy | Total |
| Cheskes et al. (2024) [25] | * | * | * | * | ** | * | * | 8 | |
| Rahimi et al. (2024) [14] | * | * | * | * | * | * | * | 7 | |
| Verkaik et al. (2024) [15] | * | * | * | * | * | * | 6 | ||
| Nordviste et al. (2024) [10] | * | * | * | * | * | * | * | 7 | |
| Kim et al. (2020) [16] | * | * | * | * | * | * | 6 | ||
| Mapp et al. (2019) [19] | * | * | * | * | ** | * | * | 8 | |
| Beck et al. (2019) [20] | * | * | * | * | * | * | * | 7 | |
| Cheskes et al. (2019) [21] | * | * | * | * | * | * | * | 7 | |
| Emmerson et al. (2017) [22] | * | * | * | * | * | * | * | 7 | |
| Ross et al. (2016) [23] | * | * | * | * | * | * | * | 7 | |
| Study | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Eraniyan et al. 2025 [13] | Yes | Yes | Yes | Yes | Yes | No | Unclear | Yes | Yes | No |
| Merlin et al. 2016 [24] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Cortez et al. 2016 [9] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No |
| Cabañas et al. 2015 [1] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alexandrou, K.; Khattab, E.; Asimakopoulou, E. Enhancing Patient Safety in Refractory Ventricular Fibrillation: A Systematic Review of Double Sequential and Vector Change Defibrillation Barriers. Healthcare 2025, 13, 2645. https://doi.org/10.3390/healthcare13202645
Alexandrou K, Khattab E, Asimakopoulou E. Enhancing Patient Safety in Refractory Ventricular Fibrillation: A Systematic Review of Double Sequential and Vector Change Defibrillation Barriers. Healthcare. 2025; 13(20):2645. https://doi.org/10.3390/healthcare13202645
Chicago/Turabian StyleAlexandrou, Kyriakos, Elina Khattab, and Evanthia Asimakopoulou. 2025. "Enhancing Patient Safety in Refractory Ventricular Fibrillation: A Systematic Review of Double Sequential and Vector Change Defibrillation Barriers" Healthcare 13, no. 20: 2645. https://doi.org/10.3390/healthcare13202645
APA StyleAlexandrou, K., Khattab, E., & Asimakopoulou, E. (2025). Enhancing Patient Safety in Refractory Ventricular Fibrillation: A Systematic Review of Double Sequential and Vector Change Defibrillation Barriers. Healthcare, 13(20), 2645. https://doi.org/10.3390/healthcare13202645








