Abstract
Background: With increasing work pace and stress, sedentary office habits and insufficient physical activity (PA) pose significant threats to employee health and organizational productivity. Physical activity-led workplace health interventions (PAWHIs) have gained attention due to their multifaceted benefits for employees’ physical and mental health. This systematic review aims to evaluate the effectiveness of PAWHIs and examine the success rates of PA combined with various supplementary intervention approaches in improving employee health. Methods: This study was conducted according to the PRISMA 2020 statement guidelines. A systematic search was performed across four databases (PubMed, Web of Science, EBSCO, and Scopus) for randomized controlled trials (RCTs) published between 2013 and 2023. The Cochrane risk-of-bias tool was used to assess the risk of bias in the included studies. Results: After screening, 40 studies meeting the criteria were finally identified and subjected to quality assessment. The primary intervention measures of PAWHIs focused on education, physical activity, and dietary interventions. Fifty percent of the studies adopted multimodal combined intervention schemes involving two or more types of interventions. The most common intervention durations were 12 weeks (9 studies) and 24 weeks (24 studies). An analysis of the various intervention effects of PAWHIs revealed that the most successfully improved outcomes were increased physical activity levels (26/32), reduced psychological stress (4/5), and improved dietary habits (12/19), with over 60% of the related studies reporting positive effects. Additionally, improvements were also commonly observed in body composition (16/29) and clinical health outcomes (15/27). Conclusions: PAWHIs have positive effects on improving employee health status and promoting healthy behaviors, particularly in increasing PA levels and reducing psychological stress. However, interventions need to be contextualized and further optimized to achieve more comprehensive and sustainable health outcomes.
1. Introduction
With the increasing health awareness among enterprises and employees, the workplace has gradually become a key environment influencing employee health and well-being [1,2]. Accelerated work pace, poor work habits, sedentary behavior, overtime work, and other unhealthy lifestyles pose serious threats to employees’ physical and mental health [3,4,5]. These issues not only affect employees’ personal quality of life but also negatively impact corporate productivity and economic performance [6,7]. For instance, a 2022 employee health report from a Fortune 500 company in the IT industry revealed that the prevalence of metabolic syndrome among long-term sedentary programmers was as high as 32%, significantly exceeding the 15% rate in the general population. These health issues result in an average increase of 3.5 days of absence per employee per year, causing a direct loss of production efficiency of $2800 [6].
Current research indicates that the workplace accounts for up to 60% of employees’ waking hours [8], making it an internationally recognized setting for health promotion and disease prevention [9]. Workplace health intervention programs have garnered widespread attention due to their potential to enhance employees’ physical and mental well-being [10,11,12]. Among these, physical activity-led workplace health interventions (PAWHIs) have emerged as a popular approach. PAWHI refers to comprehensive health promotion programs implemented in workplace settings, with the core objectives of increasing physical activity (PA), fostering regular exercise habits, and ultimately improving employees’ physical and mental health and work performance. Numerous studies have demonstrated the effectiveness of PA in reducing chronic disease risks, improving body composition and physiological function, alleviating psychological stress and symptoms of depression and anxiety, and enhancing job satisfaction and work performance [13,14,15]. A meta-analysis specifically showed that increasing PA levels led to a 23% reduction in hypertension incidence, a 31% decrease in depression symptom scores, and a 14% improvement in work performance [12]. Additionally, a Fortune 500 company reported that through multifaceted interventions such as workplace exercises, sports clubs, and step counting via smart wristbands, employees’ average daily steps increased by 42%, waist circumference significantly decreased, and annual medical expenditures were reduced by 18% [16]. These findings corroborate the health-improving effects of PAWHIs, leading more enterprises to favor PAWHIs over traditional health intervention measures.
However, critical gaps remain in current PAWHI research, necessitating systematic reviews to address them. First, while the overall effectiveness of PA-led health interventions has been established, significant variations exist across studies in terms of intervention strategies (e.g., exercise type, intensity, duration), supplementary approaches (e.g., health education, psychological support, environmental modifications), and target populations. Further synthesis and summarization of the literature are required to guide the selection of appropriate intervention types and strategies that align with corporate objectives. Second, there is a lack of systematic analysis on the success rates of different PAWHI strategies. Research has indicated that standalone exercise interventions yield limited improvements in physical and mental health, whereas integrated approaches combining exercise with cognitive and technical enhancements demonstrate superior outcomes [17].
Therefore, this study aims to systematically review the relevant workplace health intervention literature, focusing on empirical PAWHI research. By analyzing intervention characteristics—including methods, evaluation metrics, and outcomes—we seek to elucidate the features of PAWHI and assess the success rates of various supplementary intervention approaches and combinations. The findings will provide a scientific basis for designing and implementing workplace health intervention programs while offering practical guidance and recommendations for enterprises and health managers.
2. Materials and Methods
This systematic review adheres to the PRISMA 2020 guidelines (Preferred Reporting Items for Systematic Reviews and Meta-Analyses), a standardized protocol comprising 27 items grouped into 7 domains. This system evaluation has submitted a registration application on the PROSPERO platform and is currently under review. The registration submission number is PROSPERO 2025 CRD420251047695. The final registration number will be updated through the Supplementary Materials or the officially published version. The primary research objectives and specific study questions are detailed in Supplementary Material S1.
2.1. Literature Search
To comprehensively capture diverse studies in this field, a systematic literature search was conducted across four major academic databases: PubMed, Web of Science, EBSCO, and Scopus. The search covered publications from January 2013 to December 2023, ensuring broad temporal coverage of relevant research. The search terms included the following: “Physical Activity”, “Occupational Group”, and ”Workplace”. The search strategy combined subject headings (MeSH terms) and free-text keywords, with the specific details presented in Table 1. Boolean operators (“AND” and “OR”) were applied to construct precise search queries. Search strategy: #1 AND #2 AND #3.

Table 1.
The terms used in the search process.
2.2. Eligibility Criteria
This systematic review examines PAWHI. The analysis evaluates intervention effectiveness across multiple health domains, including the following: physical activity levels, dietary and nutritional status, psychological stress, body composition, and disease-related health outcomes. A narrative summary analysis of intervention success rates was performed [18]. This study established literature selection criteria following the PICOS framework (Participants, Interventions, Comparisons, Outcomes, Study Design), with specific inclusion/exclusion criteria detailed in Table 2.

Table 2.
Inclusion and exclusion criteria based on the PICOS framework.
2.3. Data Extraction
Two authors (S.C. and M.N.) independently screened the literature following a standardized procedure. First, potential studies were evaluated based on their titles and abstracts to identify those relevant to the research question and meeting the predefined inclusion criteria. Duplicate records and studies deemed irrelevant after abstract or full-text review were excluded. Finally, the selected studies underwent full-text analysis to determine their eligibility for final inclusion. Any discrepancies between the researchers were resolved through discussion until consensus was reached.
The entire screening process was documented in a flowchart following the PRISMA guidelines. For data extraction, team members worked independently, compiling the key characteristics of the selected articles into a structured table containing the following information: author/year, country, occupation/company type, study objective, intervention duration, intervention type, and outcome type. This tabulated data facilitated the systematic understanding and interpretation of the included studies. The specific content can be found in Table 3.
The intervention types are classified into Educational Interventions; Physical Activity Interventions; Dietary Interventions; Other Intervention Types. The outcome types are classified as Clinical Health Outcomes, Body Composition, Diet and Nutrition, Physical Activity and Function, Sleep Habits and Quality, Psychological Health Outcomes (e.g., Depression, Anxiety, Self-Efficacy, Stress), and Work-Related or Organizational Outcomes.
2.4. Success Rate Definition
Based on the significant improvements observed across studies, this research defined the “success rate” as follows: the proportion of studies that demonstrated statistically significant improvements (p < 0.05) or clinically relevant improvements in at least one primary outcome measure when comparing the intervention group with the control group, either post-intervention or during follow-up [19]. The specific criteria were as follows:
- (1)
- Statistical significance: determined based on the p-values reported in each study.
- (2)
- Clinical relevance: for clinical health indicators (e.g., blood pressure and blood glucose), the degree of improvement had to meet the minimal clinically important difference (MCID) recommended by clinical guidelines.
- (3)
- Handling of mixed results: if some indicators within a given health domain showed improvement while others remained unchanged in the same study, it was still considered a partial success for that domain.
2.5. Risk-of-Bias Assessment
The Cochrane risk-of-bias tool is widely recognized as the gold standard methodology for evaluating the quality of RCTs. In this study, all included RCTs were systematically assessed using the Cochrane risk-of-bias criteria, which examines seven key domains: random sequence generation, allocation concealment, blinding of participants and researchers, blinding of outcome assessment, completeness of outcome data, selective reporting, and other potential sources of bias. In accordance with the Cochrane risk-of-bias tool, two authors classified each study as “high risk”, “unclear risk”, or “low risk”. Specific evaluation criteria are provided in Supplementary Material S2. Based on these criteria, study quality was categorized into three levels: studies meeting five or more criteria were classified as low risk of bias, those meeting three to four criteria were classified as moderate risk of bias, and those meeting fewer than three criteria were classified as high risk of bias [20]. The results of the risk-of-bias assessments were graphically represented using the Review Manager 5.4 software.

Table 3.
Characteristics of the selected studies.
Table 3.
Characteristics of the selected studies.
| Author/Year | N | Occupation or Company Type | Objective | Intervention Duration | Intervention Type | Outcome Type | Risk of Bias |
|---|---|---|---|---|---|---|---|
| Gerstel et al. 2013 [21] | 129 | Employees of a nursing agency | Metabolic syndrome | 48 weeks | PA Intervention and Health Education Intervention (obesity, PA, diet) | Clinical Health Outcomes, Body Composition, PA, and Diet and Nutrition | M |
| Andersen et al. 2013 [22] | 160 | Programmers | Insufficient PA | 9 weeks | PA Intervention and Health Education Intervention | Clinical Health Outcomes, Body Composition, and PA | M |
| Christensen et al. 2013 [23] | 144 | Employees of a nursing agency | Improve work efficiency | 12 weeks | PA Intervention, Dietary Intervention (restricted diet), and Health Education Intervention (diet) | Attendance Rate, Absenteeism Rate, and Work-Related or Organizational Outcomes (Productivity) | M |
| Gussenhoven et al. 2013 [24] | 1288 | Programmer, hospital, and insurance company | Obesity | 24 weeks | Health Education Intervention (PA and diet) | Work-Related (Absenteeism Rate, GLPD, and NLPD) | H |
| Weinhold et al. 2015 [25] | 69 | University lecturer | Diabetes | 16 weeks | PA Intervention, Dietary Intervention, and Health Education Intervention | Clinical Health Outcomes, Body Composition, PA, and Diet and Nutrition | L |
| Mitchell et al.2015 [26] | 300 | Farm grower | Obesity | 12 weeks | Health Education Intervention (PA) | Clinical Health Outcomes, Body Composition, PA, and Diet and Nutrition | M |
| Miller et al. 2015 [27] | 70 | University worksite among employees | Diabetes | 16 weeks | PA Intervention and Health Education Intervention | Clinical Health Outcomes, Diet and Nutrition, PA, and Work-Related Indicators | M |
| Audrey et al. 2015 [28] | 84 | Office workers | Insufficient PA | 8 weeks | PA Intervention and Health Education Intervention (PA) | PA and Work-Related Indicators | M |
| Solenhill et al. 2016 [29] | 3876 | Employees in the transport services | Improve lifestyle habits | 36 weeks | Health Education Intervention (PA and diet) | Body Composition, PA, Diet and Nutrition, and Sleep Quality | M |
| Gregoski et al. 2016 [30] | 54 | Bank employees | Obesity | 10 weeks | PA Intervention and Health Education Intervention (PA and diet) | Body Composition, PA, Diet and Nutrition, Sleep Habits, and Work-Related Indicators | H |
| Chandrasiri et al. 2016 [31] | 81 | Administrative office staff | Non-communicable diseases | 12 weeks | Health Education Intervention (PA, diet, smoking cessation) | Clinical Health Outcomes and Body Composition | H |
| Hendriksen et al. 2016 [32] | 502 | Insurance company | Improve lifestyle habits | 40 weeks | Health Education Intervention (PA mainly) | Clinical Health Outcomes, Body Composition, and Work-Related Indicators | H |
| Jamal et al. 2016 [33] | 194 | University lecturer | Obesity | 12 weeks | Health Education Intervention (PA and diet) | Clinical Health Outcomes, Body Composition, PA, Diet and Nutrition, Depression and Anxiety, and Self-Efficacy | M |
| Balk-Moller et al. 2017 [34] | 566 | The Social Welfare and Health Care Sector | Obesity | 38 weeks | Health Education Intervention (PA and diet) | Clinical Health Outcomes and Body Composition | H |
| Maylor et al. 2018 [35] | 89 | Office workers | Insufficient PA | 8 weeks | PA Intervention and Health Education Intervention (PA, chronic disease) | Clinical Health Outcomes, Body Composition and PA | H |
| Viester et al. 2018 [36] | 314 | Construction workers | Overweight | 24 weeks | Health Education Intervention (PA and diet) | Clinical Health Outcomes, Body Composition, PA, and Diet and Nutrition | M |
| Ing et al. 2018 [37] | 217 | The Social Welfare and Health Care Sector and academic institutions | Obesity | 36 weeks | Health Education Intervention | Clinical Health Outcomes, Body Composition, PA and Physical Fitness, Diet and Nutrition, and Self-Efficacy | M |
| Kouwenhoven-Pasmooij et al. 2018 [38] | 491 | Police and hospital | Cardiovascular disease | 24 weeks | Health Education Intervention (lifestyle education) | Clinical Health Outcomes, Body Composition, PA, Diet and Nutrition, and Work-Related Indicators | M |
| Oftedal et al. 2019 [39] | 40 | Shift workers | Improve lifestyle habits | 4 weeks | Health Education Intervention (PA, diet, sleep) | Clinical Health Outcomes, Body Composition, PA, Diet and Nutrition, Sleep Quality, and Work-Related Indicators | M |
| Fang et al. 2019 [40] | 98 | High-tech industries | Obesity | 12 weeks | PA Intervention | Clinical Health Outcomes, Body Composition, PA and Physical Fitness, Stress, and Work-Related Indicators | H |
| Edman et al. 2019 [41] | 54 | Healthcare system employees | Cardiovascular disease | 12 weeks | Health Education Intervention (PA, diet, stress management) | Body Composition, PA, Stress, Depression, and Sleep Habits | H |
| Haufe et al. 2019 [42] | 314 | Automobile factory workers | Metabolic syndrome | 24 weeks | PA Intervention | Clinical Health Outcomes, PA and Physical Fitness, Diet and Nutrition, Depression and Anxiety, and Work-Related Indicators | L |
| Stein et al. 2019 [43] | 1000 | Healthcare system employees and university worksite among employees | Obesity | 96 weeks | Health Education Intervention | PA | M |
| Bonn et al. 2019 [44] | 209 | White-collar office employees | Improve lifestyle habits | 12 weeks | PA Intervention and Health Education Intervention | Clinical Health Outcomes, Body Composition, PA, Diet and Nutrition, Sleep Quality, and Psychological Health Outcomes | M |
| Sareban et al. 2020 [45] | 73 | Hospital staff | Cardiovascular disease | 48 weeks | PA Intervention | Clinical Health Outcomes, Body Composition, and PA | M |
| Linnan et al. 2020 [46] | 553 | Child care center | Improve lifestyle habits | 24 weeks | Health Education Intervention | PA | M |
| Piao et al. 2020 [47] | 121 | Office workers | Insufficient PA | 12 weeks | PA Intervention and Health Education Intervention (PA) | Sleep Quality and Habits | M |
| Reich et al. 2020 [48] | 66 | Doctor | Cardiovascular disease | 48 weeks | PA Intervention | Clinical Health Outcomes, Body composition, PA and Physical Fitness | M |
| Haufe et al. 2021 [49] | 314 | Automobile factory workers | Metabolic syndrome | 24 weeks | PA Intervention, Dietary Intervention, and Health Education Intervention (PA) | Clinical Health Outcomes, Body Composition, PA and Physical Fitness, Depression and Anxiety, and Work-Related Indicators | M |
| Neshteruk et al. 2021 [50] | 250 | Child care center | Insufficient PA | 24 weeks | PA Intervention and Health Education Intervention (PA) | PA | M |
| Mamede et al. 2021 [51] | 298 | Administrative office staff | Insufficient PA | 10 weeks | PA Intervention and Health Education Intervention | Clinical Health Outcomes, Body Composition, PA, and Work-Related Indicators | H |
| Noori et al. 2021 [52] | 80 | Fruit factory workers | Improve lifestyle habits | 8 weeks | Health Education Intervention (PA, diet, mental health) | Clinical Health Outcomes, Body Composition, PA, Diet and Nutrition, Sleep Habits, Stress, and Work-Related Indicators | H |
| Stephenson et al. 2021 [53] | 56 | Office workers | Insufficient PA | 8 weeks | PA Intervention and Health Education Intervention | Clinical Health Outcomes, Body Composition, PA, Diet and Nutrition, Psychological Health Outcomes, and Work-Related Indicators | L |
| Garcia Perez de Sevilla et al. 2021 [54] | 24 | University employees | Improve lifestyle habits | 18 weeks | PA Intervention, Dietary Intervention, and Health Education Intervention (PA, diet) | Clinical Health Outcomes, PA, Diet and Nutrition, and Psychological Health Outcomes | M |
| Kong et al. 2022 [55] | 955 | Office workers | Obesity | 48 weeks | PA Intervention, Dietary Intervention, and Health Education Intervention (PA, diet) | Body Composition, PA, Diet and Nutrition, and Self-Efficacy | M |
| Shiri et al. 2022 [56] | 159 | Healthcare system employees | Improve lifestyle habits | 8 weeks | PA Intervention, Dietary Intervention, and Health Education Intervention (PA, diet) | Depression and Anxiety, Sleep Habits, and Work-Related Indicators | M |
| Bayerle et al. 2022 [57] | 129 | Automobile factory workers | Metabolic syndrome | 24 weeks | PA Intervention and Health Education Intervention (PA) | Clinical Health Outcomes, Body Composition, PA, Depression, and Work-Related Indicators | M |
| Edwardson et al. 2022 [58] | 756 | Office workers | Insufficient PA | 12 weeks | PA Intervention and Health Education Intervention (PA) | Clinical Health Outcomes, Body Composition, PA, Diet and Nutrition, Sleep Quality, Stress, Anxiety, and Work-Related Indicators | H |
| Silva et al. 2022 [59] | 31 | Office workers | Insufficient PA | 16 weeks | PA Intervention | Clinical Health Outcomes, Body Composition, PA and Physical Fitness, Diet and Nutrition, and Stress | L |
| Brinkmann et al. 2023 [60] | 206 | Automobile factory workers | Diabetes | 15 weeks | PA Intervention, Dietary Intervention, Health Education Intervention, and Smoking Cessation | Clinical Health Outcomes and Body Composition | H |
PA interventions, which directly engaged employees in exercise or enhanced PA levels. “L” refers to low risk of bias; “M” refers to moderate risk of bias; “H” refers to high risk of bias.
3. Results
3.1. Study Selection
This study ultimately included 40 RCTs investigating workplace interventions primarily focused on PA. The systematic literature selection process is illustrated in Figure 1. Of the 40 finally included studies, 22 were from PubMed, 10 from WOS, 6 from Scopus, and 2 from EBSCO/SPORTDiscus. This distribution shows the centralization trend of RCT-related PAWHI studies in biomedical and multidisciplinary databases.
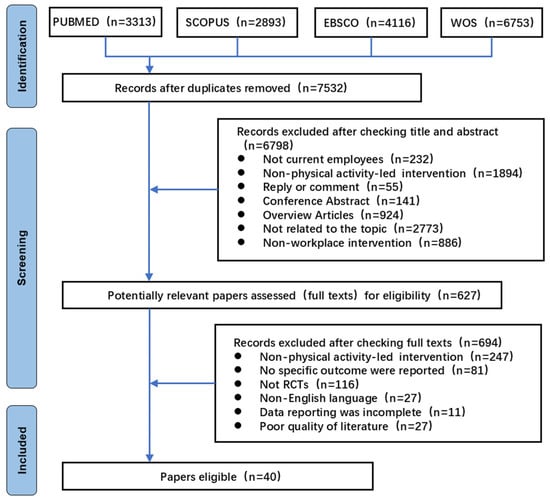
Figure 1.
PRISMA flow diagram of the selection of studies.
3.2. Characteristics of the Samples
The analyzed studies encompassed a broad sample size distribution, totaling 14,414 participants across all included trials. The average intervention sample size was relatively large (mean = 360.35; median = 195.5). Participants were recruited from 11 countries: Sweden, the United States, Australia, China, Germany, Finland, the United Kingdom, Iran, Sri Lanka, Denmark, and the Netherlands. Intervention durations varied significantly, ranging from 4 weeks (shortest) to 96 weeks (longest). The most common intervention periods were 12 weeks (9/40 studies) and 24 weeks (24/40 studies). In terms of intervention design, 20 studies adopted multi-component intervention programs among single-component interventions: health education was the predominant approach (14/40 studies); physical activity interventions accounted for 5/40 studies.
3.3. Characteristics of the Interventions
3.3.1. Educational Interventions
Health education represents the most prevalent strategy in workplace health interventions and serves as a critical supportive measure for PA interventions. In this review, 35 studies implemented health education as a primary supplementary approach in workplace health intervention programs.
The educational content encompassed diverse themes, primarily including the following: increasing PA and reducing sedentary behavior [36,58], improving dietary patterns and nutrition (including low-fat diets, Mediterranean dietary patterns, etc.) [21,24,27,32,34,41,43,46,54,55,56], weight management (including weight loss goal setting and energy intake and expenditure calculations) [21,23,27,33,34,37,43,45,55,58], knowledge regarding the benefits of PA for chronic disease management [31,35,38,40], mechanisms and methods of exercise for mental health promotion [21,27,28,32,38,41,50,51], and guidance on healthy lifestyle practices such as smoking cessation and alcohol management.
The health education interventions employed diverse delivery formats, including the following: educational manuals and video materials [24,27,37,39,50,54], online or in-person health assessments and personalized guidance by professionals (such as customized PA and dietary plans) [24,31,36,38,43], daily health behavior monitoring tools (including Polar heart rate monitors, pedometers, health diaries) [23,28,33,34,39,43,58], educational lectures or group meetings [21,27,31,32,35,37,45,50], multi-channel health information dissemination (including bulletin boards and posters) [35,46,50,55], internet and mobile technology-based health information delivery (including emails, text messages, WeChat groups) [29,34,43,50,55], and specialized health management applications or systems [28,39,47].
3.3.2. Physical Activity Interventions
This systematic review analyzed 25 studies implementing workplace PA interventions, which directly engaged employees in exercise or enhanced PA levels. The findings indicate that these interventions primarily aimed to promote employee health through improved physical functioning. The interventions employed diverse approaches, including the following: (1) promoting active commuting, such as encouraging cycling or walking to and from work [21,28,35,42,45,48,50,51,55], (2) structured “work break exercises” during work breaks, such as workplace exercises [30], and (3) redesigning office environments by strategically placing waste bins and printers to increase routine PA [35,58]. Additionally, several studies implemented team-based collective activities, such as stair-climbing competitions [22,47] and step-counting challenges [30,35], while establishing incentive mechanisms and role models. As organizations increasingly prioritize employee health, various monitoring and exercise-facilitating devices were utilized to enhance motivation and accessibility, including pedometers [55], video monitoring systems [30], sit–stand workstations [58], and mobile applications or digital platforms [53,57] for exercise guidance and monitoring, effectively increasing PA levels.
3.3.3. Dietary Interventions
This review examined 18 studies involving dietary and nutritional supportive interventions in workplace settings. Through systematic analysis, these interventions primarily comprised the following: (1) dietary and nutritional health education, such as food nutrient composition analysis and balanced diet guidance [21,23,24,25,27,49,60], (2) practical tool applications, including food measuring instruments [29], (3) metabolic monitoring, encompassing daily recording of energy intake and expenditure, and (4) dietary journals for the systematic documentation and analysis of individual eating behaviors [49], aimed at promoting rational, healthy eating and controlling low-fat dietary intake. (5) Additionally, dietary intervention studies employed various group participation strategies and workplace environment modification approaches. These included establishing nutrition and health-focused groups to foster peer motivation among members, organizing healthy eating competitions or challenges [55], implementing environmental interventions such as improving healthy food options in cafeterias and workplace supermarkets [55].
3.3.4. Other Intervention Types
This review analyzed 4 studies involving smoking cessation [31,39,45,60] and alcohol reduction [39,44] interventions. Smoking cessation interventions primarily employed diverse health education approaches, including group promotional activities and individual face-to-face counseling, with some studies also utilizing nicotine replacement therapy, such as nicotine patches, to facilitate cessation. Regarding alcohol consumption issues, studies mainly implemented dietary education and self-monitoring strategies through food diaries to reduce excessive drinking behaviors. Additionally, 7 studies [32,41,43,45,52,56] explored stress reduction interventions for employees, predominantly employing mindfulness or meditation techniques, combined with breathing exercises and muscle relaxation training, enhanced by musical or verbal guidance. Notably, other non-medical intervention types emerged in the research, such as measures targeting improved sleep quality among employees [39,41].
3.4. Measures Used
3.4.1. Clinical Health Outcomes
Clinical health outcomes refer to changes or results in patients’ health status following medical interventions or health management measures, typically used to evaluate treatment efficacy, disease progression, or the effectiveness of health intervention strategies. In this review, 27 studies assessed clinical health outcomes, primarily through patient-reported outcomes (PROs) and biological outcomes.
Patient-reported outcomes are data directly provided by patients regarding their health status, including symptoms, functional status, and quality of life. Twelve studies employed standardized self-administered questionnaires, primarily including the following: (1) the Symptom Checklist-90 (SCL-90) [21] for assessing mental disorders and psychological conditions; (2) quality of life-related scales, such as the 36-Item Short-Form Health Survey (SF-36) [38,42,45,48,54,57], the World Health Organization Quality of Life-BREF (WHOQOL-BREF) [27,33,40,59], and the EuroQol 5-Dimension 5-Level (EQ-5D-5L) [58], which comprehensively evaluate the health status and quality of life from different dimensions; and (3) the Symptom and Neurotoxicity Questionnaire (SNQ) [27], specifically designed for diagnosing neurological and musculoskeletal symptoms.
Biological outcomes are objective indicators obtained through laboratory tests or imaging examinations, which served as key assessment metrics in 18 studies. These biological outcomes primarily included the following: (1) cardiovascular-related indicators, such as blood pressure [22,25,27,32,34,35,36,37,40,45,57,58,60], electrocardiograms [48,57], and cardiovascular risk scores [45,48,60]; (2) metabolism-related indicators, including blood glucose [21,25,26,40,45,48,57] and blood lipids (e.g., total cholesterol, low-density lipoprotein, high-density lipoprotein, and triglycerides) [21,25,32,34,35,36,40,45,48,57,58,59,60]; and (3) inflammation and disease-specific markers, such as high-sensitivity C-reactive protein (hs-CRP) [60] as a predictor of inflammation and cardiovascular events, glycated hemoglobin (HbA1c) [58,60] for long-term glycemic control assessment, and liver fibrosis-related indices (FIB-4, APRI, and AST) [49] for evaluating the degree of hepatic fibrosis.
3.4.2. Body Composition
Body Composition refers to the proportional distribution of various tissues, organs, and substances within the human body, serving as a critical indicator for assessing individual health status, nutritional condition, and disease risk. In this systematic review, 29 studies evaluated body composition-related outcomes. Among the reported body composition outcome parameters, the most frequently included metrics were as follows: (1) body mass index (BMI) [22,26,29,30,31,33,36,37,38,39,40,41,45,48,51,52,53,55,57,59,60], serving as a fundamental indicator for assessing overall obesity; (2) visceral fat area (VFA) or body fat percentage [21,32,34,40,42,45,48,55,58,59], used to evaluate fat distribution and accumulation patterns; and (3) waist-to-hip ratio [21,25,26,33,34,35,36,40,45,48,55,58,59,60], functioning as a predictor of central obesity and metabolic risk. Regarding body composition assessment methodologies, the most commonly employed techniques in these studies included Bioelectrical Impedance Analysis (BIA) [21,40,44,45,55,59], anthropometric methods (such as circumference measurements), and skinfold thickness measurements [48]. Notably, although dual-energy X-ray absorptiometry (DEXA) is considered the gold standard for body composition assessment, it was not utilized in any of the studies included in this review.
3.4.3. Diet and Nutrition
This review analyzed dietary and nutritional outcome indicators from 19 studies, primarily designed to assess individual nutritional status, dietary behaviors, and their health impacts. The findings indicate that the core assessment framework for diet and nutrition encompasses dietary reference intakes and nutritional status classification, with specific indicators covering nutrient intake, nutritional status, dietary behaviors, and health outcomes, measuring changes in food variety, dietary intake volume, water consumption, health status, eating habits, and dietary perceptions.
Regarding assessment methodologies, studies predominantly employed standardized questionnaires and dietary recording techniques. Standardized questionnaire instruments included the following: (1) Food Frequency Questionnaire (FFQ) [21,25,27,39,44,45,59], assessing specific food intake frequency; (2) Weight Efficacy Lifestyle Questionnaire (WEL) [33], measuring individual self-efficacy in controlling diet across different situations; (3) Mediterranean Diet Adherence Screener (MEDAS) [53], evaluating adherence to the Mediterranean dietary pattern; (4) Australian Recommended Food Score (ARFS) [39], gauging dietary quality; (5) Health-Promoting Lifestyle Profile (HPLP) [52,54], assessing health behaviors and lifestyle; (6) Three-Factor Eating Questionnaire (TFEQ-R21) [44], measuring relationships between individual eating behaviors and emotional and cognitive factors; and (7) Alcohol Use Disorders Identification Test—Consumption (AUDIT-C) [29], screening for drinking behaviors and alcoholism risk.
Beyond questionnaire assessments, multiple studies implemented a 24 h dietary recall or dietary record methods (3-day or 7-day records) [26,30,36,37,38,55,58], documenting daily dietary intake in detail and integrating food energy tools or reference manuals to estimate caloric content, thereby promoting dietary self-monitoring and management [33,42].
3.4.4. Physical Activity and Function
This systematic review identified 32 studies that evaluated outcome indicators related to PA levels and physical function changes, with assessment methods categorized into objective measurements and subjective assessments.
Objective measurement methods directly record PA data through specialized equipment or technologies, providing high-precision results suitable for individualized assessment and precise scientific research. These methods primarily include the following motion sensors: (1) accelerometers (e.g., ActiGraph and GENEActiv), typically worn on the waist, wrist, or ankle, capable of recording body acceleration and distinguishing low, moderate, and high-intensity PA [25,27,28,35,43,44,50,51,53,55,58,59]; (2) pedometers, used for recording daily step counts, simple to operate but unable to differentiate activity intensity [43,55]; (3) smart bands/watches, such as Fitbit, Garmin, and Apple Watch, capable of simultaneously monitoring the heart rate, step count, calorie expenditure, and activity duration [43,51]; and (4) heart rate monitors, which estimate energy expenditure and activity intensity through heart rate data, particularly suitable for assessing moderate-to-high-intensity activities [45,48].
Subjective assessment methods rely on individuals’ self-reporting or recall of PA patterns, applicable to large-scale epidemiological surveys and population studies. Standardized questionnaires employed in these studies include the following: Physical Activity Frequency Questionnaire (PAFQ) [21,38], International Physical Activity Questionnaire (IPAQ) [22,26,33,36,48], Global Physical Activity Questionnaire (GPAQ) [54], Freiburg Physical Activity Questionnaire (FPAQ) [49,57], Occupational Sitting and Physical Activity Questionnaire (OSPAQ) [58], Godin–Shephard Leisure-Time Physical Activity Questionnaire (GSLTPAQ) [55], and the Active Australia Questionnaire (AAQ) [39], used to evaluate activity frequency, intensity, and duration. Additionally, some studies utilized comprehensive scales such as the Health-Promoting Lifestyle Profile-II (HPLP-II) [52], assessing PA patterns during specific time periods through its PA dimension. Multiple studies also implemented PA logs to document participants’ exercise habits and training elements in detail, ensuring the rational implementation of exercise interventions [29,30,43,45,46,51].
Beyond directly increasing employee PA levels, studies also measured intervention effectiveness by assessing employees’ physical fitness and functional capacity, including specialized tests for muscle strength, cardiorespiratory endurance, and flexibility [37,40,41,42,59].
3.4.5. Sleep Habits and Quality
In this review, eight studies evaluated sleep status as an outcome indicator. These studies primarily employed subjective assessment methods, including standardized scales such as the Pittsburgh Sleep Quality Index (PSQI) [39,58], the Karolinska Sleep Scale (KSS) [29], and Numerical Rating Scales (NRSs) [41]. Additionally, several studies utilized sleep status self-recording forms or online sleep monitoring software systems to conduct multidimensional assessments of employees’ sleep habits and sleep quality [30,44,56]. These assessment tools collectively formed a comprehensive indicator system for evaluating the impact of workplace interventions on employee sleep health.
3.4.6. Psychological Health Outcomes
Based on a comprehensive integration of research analyses, workplace health interventions predominantly focused on PA demonstrate a concentrated emphasis on three key dimensions of psychological health outcomes: psychological stress management, depression and anxiety symptomatology, and exercise-related psychological factors (such as self-efficacy and motivational adherence). These interventions operate through diverse mechanisms to positively influence the psychological well-being of working populations.
(1) Psychological stress assessment: Five studies focused on the evaluation of psychological stress and stress management. The most commonly utilized instrument for assessing individual psychological stress was the Perceived Stress Scale (PSS) [41,45,58,59], while work environment-related stress was evaluated using the Occupational Stress Scale [40]. Additionally, the Health-Promoting Lifestyle Profile-II (HPLP-II) was employed to assess participants’ stress management levels [52].
(2) Emotional state assessment: Seven studies addressed the evaluation of depression, anxiety, and burnout. These studies primarily employed the Hospital Anxiety and Depression Scale (HADS) [42,49,57,58], the Generalized Anxiety Disorder-7 (GAD-7), and the Patient Health Questionnaire-9 (PHQ-9) [56] for relevant assessments. Furthermore, the Automatic Thoughts Questionnaire (ATQ) was used to evaluate negative cognitive patterns [33], while Numerical Rating Scales (NRSs) for fatigue and pain were utilized to assess individual levels of fatigue and pain during specific timeframes [41].
(3) Exercise-Related Psychological Factor Assessment: Three studies examined exercise self-efficacy and exercise motivation, primarily through employee self-report questionnaires. Specific assessment tools included the Weight Efficacy Lifestyle Questionnaire (WEL) [33] and the Self-Efficacy for Exercise Scale (SEE) [37,55], which were used to evaluate participants’ exercise motivation and self-efficacy levels.
3.4.7. Work-Related or Organizational Outcomes
This review included 16 studies that evaluated work-related outcome indicators using multiple standardized assessment tools. These instruments can be categorized as follows: (1) work engagement assessment, such as the Utrecht Work Engagement Scale (UWES) [58], measuring employees’ energy, dedication, and absorption in their work; (2) work limitation assessment, including the Work Limitations Questionnaire (WLQ-8) [38,58] and the Work Productivity and Activity Impairment Questionnaire (WPAI-GH 2.0) [28], evaluating barriers and limiting factors encountered during work processes; (3) work ability assessment, primarily conducted through the World Health Organization Health and Work Performance Questionnaire (WHO-HPQ) [58] and the Work Ability Index (WAI) [38,42,49,56,57]; (4) employee vitality and performance assessment, such as the Energy and Performance Scan (EPS) [32], comprehensively evaluating employees’ vitality, work performance, and attendance; and (5) job satisfaction assessment, employing the Minnesota Satisfaction Questionnaire (MSQ) [40] to measure employees’ satisfaction with their work. Additionally, multiple studies assessed work efficiency through objective indicators, including weekly workday counts and working hours statistics [51,52], Lost Productivity Days (LPDs) [24], as well as records of sick leave days, attendance rates, and absenteeism rates [23,27,30,32,53].
3.5. Overall Effects of the Interventions
3.5.1. Clinical Health Outcomes
Compared to baseline levels, intervention results from studies included in this review demonstrated significant improvements in employees’ overall health status and quality of life, as assessed through standardized self-report questionnaires such as the WHOQOL-BREF [33,40,59] and SF-36 [21,38,42,45,48,54]. Further analysis indicated that PAWHIs also yielded positive changes in clinical indicators, including significant reductions in metabolic syndrome scores [42] and cardiovascular risk scores [60], as well as improvements in FIB-4, APRI, and AST [49]. Total cholesterol, low-density lipoprotein, high-density lipoprotein, triglycerides, and blood pressure monitoring, which served as the most common health assessment measures, showed positive changes across multiple studies [21,22,25,37,40,45,48,49,59,60]. Notably, among the 27 studies involving clinical health outcomes, 15 studies reported statistically significant health improvements [21,22,25,33,37,38,40,42,45,48,49,54,58,59,60], indicating that workplace lifestyle interventions have a significant positive impact on employees’ clinical health indicators.
3.5.2. Body Composition
The systematic review identified 16 studies demonstrating significant improvements in at least one body composition parameter following workplace health management interventions. These improvements encompassed various anthropometric measures, including body weight, BMI, waist circumference, hip circumference, waist-to-height ratio, body fat percentage, and visceral adipose tissue [21,22,25,26,30,33,34,36,37,38,40,41,49,55,59,60].
3.5.3. Diet and Nutrition
Among the 19 studies reporting nutrition or healthy eating outcomes, 12 studies demonstrated significant improvements in dietary patterns or nutritional status following interventions [21,25,26,27,29,33,36,39,42,52,54,55], primarily manifested as transformations in dietary concepts, the optimization of the dietary structure, and the enhancement of dietary scores. Workplace dietary interventions facilitated the development of scientifically sound eating habits among employees through comprehensive strategies, including nutrition education, healthy menu design, and environmental optimization. Specific improvement indicators encompassed increased dietary quality scores [42,52,54], reduced daily caloric intake [21,25,55], optimized dietary structure [25,26,27,29,33,42], and improved hydration habits coupled with decreased sugar-sweetened beverage consumption [36]. Notably, in five of these studies, researchers integrated PA interventions with dietary interventions, incorporating individualized exercise goals [25] and increasing the frequency of aerobic activities such as walking or cycling [21,27,42,55], thereby significantly enhancing the effectiveness of comprehensive health interventions. However, some studies also indicated that the efficacy of dietary and nutritional interventions was constrained by multiple factors during implementation, including insufficient employee compliance, varying levels of cafeteria management quality, and cost control pressures (for instance, some employees opted for takeout or fast food due to busy work schedules), which to some extent diminished the effectiveness of the interventions.
3.5.4. Physical Activity and Function
Twenty-six studies demonstrated significant improvements in PA-related outcomes following interventions [21,22,25,27,29,33,35,36,37,38,39,40,41,43,45,46,48,49,50,51,52,53,54,55,57,58]. Among these studies, improvements in PA were manifested across multiple dimensions: increased activity frequency [37,41], extended duration (reflected by increased step counts, extended cycling distances, and increased daily intervention time) [21,27,45,51,55], and enhanced exercise intensity (transitioning from light-to-moderate or vigorous activity) [21,51]. Additionally, the interventions significantly improved employees’ cognition regarding PA [33,52,54], enhanced motivation for exercise participation [29,37,55], and effectively reduced sedentary behavior [35,54,58]. The majority of studies employed PA assessment questionnaires or objective tools such as accelerometers to evaluate intervention effects, confirming significant improvements in PA levels [22,25,27,29,33,35,36,38,43,46,49,50,53,54,55,57,58]. Notably, following workplace healthy lifestyle interventions, employees’ physical fitness parameters also exhibited marked improvements, including increased vital capacity, enhanced aerobic endurance, improved strength, and better flexibility [37,40,41,48,49,57].
3.5.5. Sleep Habits and Quality
Among all studies included in the analysis, only two studies reported positive effects of PAWHI on employees’ sleep quality [46,56]. This notably low proportion may reveal the multifactorial etiology of sleep disorders. Employees’ sleep quality is subject to synergistic regulation by multidimensional factors, including occupation-related factors (such as workload, occupational stress, and work environment), psychosocial factors (such as family relationships and life events), and individual health status (such as anxiety, depression, and other psychological issues). PA-only interventions may not be sufficient to address the multifactorial causes of sleep disturbances. Therefore, their effects on sleep quality may be limited. This finding suggests that when designing workplace health promotion programs, it may be necessary to integrate diversified strategies, including psychological interventions, environmental optimization, and lifestyle adjustments, to more effectively address employee sleep problems.
3.5.6. Psychological Health Outcomes
Among the included studies, 14 recorded and evaluated the impact of interventions on psychological status. Of these, five studies focused on psychological stress and coping abilities, with four demonstrating significant improvements in stress-related indicators following intervention, primarily manifested as enhanced stress source identification, reduced stress levels, improved stress management skills, and optimized coping strategies [40,41,52,58]. Additionally, seven studies addressed negative emotional states such as depression and anxiety, with three confirming that workplace health interventions effectively alleviated employees’ depressive or anxiety symptoms [42,56,57]. Furthermore, three studies investigated the impact of interventions on self-efficacy and exercise motivation, with one observing significantly improved exercise self-efficacy among employees post-intervention [33]. These results suggest that workplace health interventions have potential for improving employees’ psychological health, though effects vary across different psychological domains, indicating that future intervention designs need to be customized according to specific psychological health dimensions.
3.5.7. Work-Related or Organizational Outcomes
In this review, 16 studies included assessments of at least one work-related or organizational outcome. Among these, seven studies demonstrated statistically significant positive impacts of PAWHIs on work-related outcomes, primarily reflected in enhanced productivity and work ability [23,32,42,56], increased attendance rates [27,30,32], and improved job satisfaction [40]. However, several studies also revealed the limitations of such interventions, with some reporting that interventions failed to deliver expected work-related benefits to organizations while incurring relatively high implementation costs [24], and isolated studies even observed slight decreases in productivity following intervention [38]. Notably, none of the studies included in this analysis reported intervention effects on long-term organizational indicators, such as early retirement intentions, employee retention rates, or staff turnover rates, suggesting that future research needs to expand the range of outcome measures to comprehensively evaluate the organizational benefits of workplace health promotion programs.
3.6. Success Rate of the Interventions
The significantly improved outcome measures from the included studies were systematically compiled, with the specific improvement status for each outcome type detailed in Table 4.

Table 4.
Success rates of interventions based on statistical significance.
This study systematically evaluated the impact of PAWHIs on multidimensional health outcomes, with results summarized in Table 5. Analysis revealed significant variations in intervention effectiveness across different health domains. Health domains with relatively high intervention success rates included PA and physical function improvement (81.3%), stress management (80.0%), and dietary and nutritional status (63.2%). Domains with moderate intervention effectiveness encompassed clinical health indicators (55.6%), body composition (55.2%), work-related outcomes (43.8%), and depressive and anxiety symptoms (42.9%). Domains showing comparatively lower intervention effects included self-efficacy enhancement (33.3%) and sleep quality improvement (28.6%). Notably, the number of intervention studies addressing psychological health dimensions such as stress management, depressive and anxiety symptoms, and self-efficacy was relatively limited, potentially affecting the robustness of related conclusions. This suggests that future research should further expand sample sizes in these domains to obtain more reliable evidence.

Table 5.
The success rate of the interventions in improving outcomes.
3.7. Risk-of-Bias Results
The risk of bias for all included studies was independently assessed by two reviewers using the Cochrane risk-of-bias assessment tool, with discrepancies resolved through reassessment by a third reviewer and consensus reached through discussion.
Comprehensive bias risk analysis revealed that among all included RCTs (n = 40), the inadequate blinding of participants and/or personnel represented the most prevalent source of high-risk bias (26/40, 65.0%). Other high-risk biases, in descending order of frequency, included insufficient or absent allocation concealment (17/40, 42.5%), incomplete outcome data (15/40, 37.5%), inadequate random sequence generation (9/40, 22.5%), other sources of bias (9/40, 22.5%), and a lack of outcome assessment blinding (7/40, 17.5%), while selective reporting demonstrated the lowest proportion of high-risk bias (4/40, 10.0%). Notably, a substantial number of studies (19/40, 47.5%) inadequately described the blinding status of outcome assessment, resulting in an “unclear” risk rating for this dimension. Beyond issues with participant and personnel blinding, an insufficient description of allocation concealment and randomization methods constituted common methodological limitations. Regarding outcome assessment blinding, as most studies employed objective measurement indicators (such as blood biochemical parameters, electrocardiographic examinations, and standardized exercise tests), the practical impact of detection bias may be relatively limited. The final assessment indicated that only seventeen studies (42.5%) achieved four or more “low-risk” ratings across the seven dimensions, and only four studies (10.0%) achieved five or more ‘low-risk’ ratings, suggesting that the overall methodological quality of included studies requires improvement. The risk-of-bias rating results of each study are shown in Table 3. The specific results are shown in Figure 2 and Figure 3.
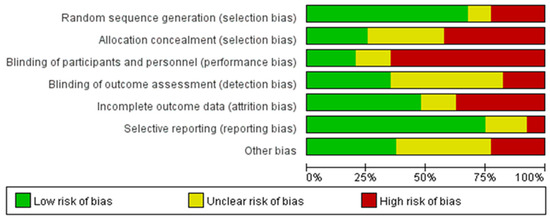
Figure 2.
Estimated risk of bias across all studies (1).
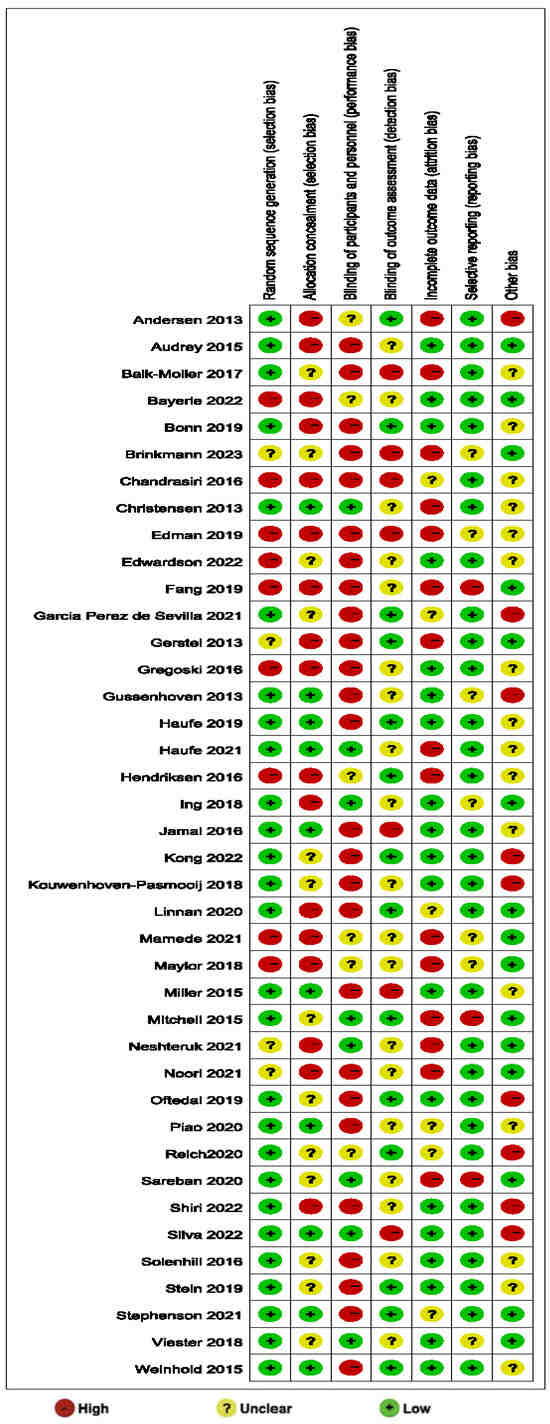
Figure 3.
Estimated risk of bias across all studies (2) [21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60].
4. Discussion
4.1. Summary of the Characteristics of the Studies
The literature included in this study shared the following key characteristics: the study population comprised employed adults aged 18 to 65 years, with particular emphasis on those in unhealthy working conditions; all studies employed RCT methodology; interventions featured PAWHI; and intervention settings were restricted to occupational environments. The intervention measures primarily included the following: (1) diversified health education (encompassing PA guidance, dietary and nutritional knowledge, exercise-based weight reduction principles, mechanisms of exercise for chronic disease improvement, strategies for exercise-promoted mental health, and smoking/alcohol cessation guidance); (2) structured exercise organization and PA promotion; (3) dietary pattern adjustment and nutritional intervention; (4) meditation and mindfulness training; and (5) tobacco and alcohol dependency behavioral interventions. The main outcome measures evaluated in these studies addressed seven health-related domains: (1) clinical health indicators (e.g., blood pressure, blood lipids, blood glucose); (2) body composition parameters (e.g., weight, BMI, body fat percentage); (3) dietary behavior and nutritional status; (4) sleep quality and patterns; (5) psychological stress levels; (6) depressive and anxiety symptoms; and (7) work-related performance metrics.
4.2. Successfulness of Interventions
This study systematically evaluated PAWHI, revealing significant health benefits, relatively low implementation costs, and sustainable long-term health promotion effects. In addition, due to high heterogeneity in intervention design, duration, and outcome measurement, meta-analysis was not feasible.
4.2.1. Clinical Health Outcomes
Among 27 RCTs assessing clinical health outcomes, 15 (55.6%) reported significant improvements. Recent research confirms that integrated interventions combining dietary adjustments, increased PA, and psychological support effectively improve metabolic health indicators such as blood glucose and lipid profiles, thereby reducing the risk of cardiovascular disease (CVD) and diabetes [39,61]. Systematic reviews further support workplaces as ideal controlled environments for increasing moderate-to-vigorous physical activity (MVPA) levels and enhancing cardiometabolic health [62].
However, some studies indicate that because these interventions are typically implemented uniformly at the workplace level rather than tailored to individual differences, their impact on improving employee health status and reducing healthcare costs may be limited. An 18-month intervention study involving 32,974 employees showed limited improvements in self-reported health status, cholesterol, and blood pressure [63]. This suggests that despite positive progress in improving clinical health indicators through workplace health interventions, attention must be paid to the long-term effectiveness and sustainability of intervention measures. Clinical practice indicates that single health outcome indicators have limited predictive ability, necessitating the integration of multiple indicators to optimize risk assessment [64]. Additionally, successful implementation requires the thorough consideration of employee engagement and personalized needs [65].
4.2.2. Body Composition
Among the 29 studies that looked at body composition-related outcome indicators, 16 of them, which accounted for 55.2%, showed significant improvements. Intervention duration emerged as a critical factor influencing effectiveness, with short-term interventions (<3 months) potentially yielding only temporary improvements [66]. Research indicates that the most effective workplace exercise interventions combine at least 4 months of supervised guidance, moderate-intensity aerobic exercise, and strength training [67].
The combination of aerobic exercise and strength training demonstrates synergistic effects in improving body composition: aerobic exercise (such as moderate-intensity continuous training) effectively reduces total fat mass (TFM) and visceral adipose tissue (VAT) [68], while strength training helps maintain or increase fat-free mass (FFM), thereby optimizing the muscle-to-fat ratio. Although high-intensity interval training (HIIT) offers advantages in time efficiency, its fat reduction effects show no significant difference compared to moderate-intensity aerobic exercise [69].
Notably, the diversity of body composition assessment tools (such as DXA, BIA, and skinfold thickness) may introduce measurement bias. DXA precisely differentiates fat and muscle distribution, while BIA is significantly affected by fluid changes and may overestimate fat-free mass [70]. Similarly, waist circumference as a proxy indicator for visceral fat, though operationally simple, correlates with VAT in ways that are influenced by gender and ethnicity, suggesting the need to combine multimodal measurements to enhance result reliability [71].
4.2.3. Diet and Nutrition
Workplace dietary interventions are influenced by multiple factors, including work schedules, environment, and culture [72]. This study shows a 63.2% success rate for dietary interventions. As an auxiliary intervention measure in workplaces, dietary interventions significantly improve employees’ dietary quality and body mass index [73]. Workplace healthy eating policies and management measures, such as cafeteria renovations, healthy food options, nutritional labeling, and environmental cues, effectively support employees in forming healthy lifestyles [74].
Single dietary interventions often have a limited impact, whereas combining them with exercise and behavioral strategies tends to yield stronger results. A workplace dietary intervention study involving 602 employees found that two years of intervention influenced employees’ food choices to some extent, but the single dietary intervention model did not significantly improve employee weight status [75]. In contrast, RCTs confirmed that intensive lifestyle interventions combining diet and exercise significantly improved blood glucose control, weight management, and cardiopulmonary function in type 2 diabetes patients when implemented in the workplace [76]. Gepner et al. integrated dietary interventions with workplace exercise interventions, achieving clinically meaningful improvements in body composition [77]. Therefore, workplace dietary interventions should use combined strategies, particularly those integrating physical activity.
4.2.4. Physical Activity
Comprehensive assessment indicates that increased PA levels and healthy exercise habits demonstrate significant intervention effects in improving employee body composition, functional status, disease management, and work-related indicators. The central role of PA interventions in workplace health promotion cannot be overlooked [78,79]. Numerous studies confirm that PA interventions not only bring significant health benefits to employees but also improve work performance and reduce medical costs [21,25,80].
PA interventions provide both immediate short-term benefits, such as significantly increased PA levels [81] and preliminary improvements in health awareness and behavior [82,83], as well as long-term health benefits through sustained exercise habit formation, including overall physical health improvements [84,85], body composition optimization [86], enhanced mental health and cognitive function [87], and increased job satisfaction and engagement [88]. PA interventions address not just single health issues but serve as comprehensive strategies for addressing multidimensional health challenges [89].
The team incentives and social attributes of workplace PA interventions show significant advantages in promoting employee participation [90,91,92]. Research indicates that team-based economic incentives (such as team rankings and financial rewards) significantly increase employees’ daily step counts, while “supervisory” information in team communications (such as encouraging more active member participation) correlates significantly with individual step count increases [93]. Additionally, mobile health technologies in workplace interventions provide convenient means for self-monitoring and PA level assessment [94].
Based on the literature review, successful PAWHIs should include the following core elements: (1) comprehensive interventions at the workplace level (including text messages, online platforms, etc.), providing personalized health guidance for employees; (2) establishing health lifestyle management systems such as platforms for data collection and management; (3) conducting health education and promotion through multiple channels such as training meetings, website resources, and educational materials; (4) focusing on behavioral habit improvement as the core objective, optimizing work routines (such as increasing standing, stretching, and scheduled hydration); (5) promoting self-management, reducing management costs, enhancing employee autonomy, and stimulating intrinsic motivation and self-efficacy (such as setting personalized PA goals); (6) organizing sports events and challenges with incentive mechanisms; and (7) implementing comprehensive monitoring and feedback systems to ensure intervention quality.
4.2.5. Mental Health and Self-Efficacy
Research indicates that PAWHIs (including regular exercise, balanced diet, and sleep management) help reduce stress and improve emotional stability [95]. A systematic review assessed the effectiveness of organizational-level workplace mental health interventions on stress, burnout, non-clinical depression and anxiety symptoms, and well-being among construction workers [96]. Most studies show that workplace interventions demonstrate mild-to-moderate effects in improving mental health conditions (such as burnout, insomnia, and stress) and enhancing positive mental health (such as well-being) [97,98].
Specific studies emphasize that short-duration activities such as lunchtime walks or yoga help employees temporarily escape high-pressure work environments and restore psychological resilience [99,100,101]. However, these benefits depend on sustained participation, and their effects are significantly influenced by individual compliance. One study applying Bandura’s self-efficacy model to explore employee participation in lifestyle intervention occupational rehabilitation programs found that enhanced self-efficacy helps participants more effectively cope with work stress, improve health behaviors, and enhance work performance [102]. Another study indicated that workplace health education and mental health promotion significantly increase employee self-efficacy, effectively promoting weight management and health behavior change [103].
It should be noted that this study included a limited number of studies on self-efficacy and depression/anxiety-related outcomes. Constrained by the overall research scale, the strength of the related evidence is relatively insufficient, suggesting future research should further expand sample sizes to obtain more reliable evidence. Future studies should use longitudinal designs with control groups to clarify causal relationships and mechanisms of change, particularly for self-efficacy and psychological outcomes.
4.2.6. Work-Related Outcomes
Work-related outcomes in this study showed moderate improvement. Among eighteen studies evaluating work-related outcomes, eight demonstrated improvement in one or more indicators: four studies observed enhanced productivity and work ability, three found increased attendance rates, and one showed increased employee job satisfaction.
Currently, no clear consensus exists regarding the effectiveness of workplace interventions in reducing absenteeism. A systematic review of positive workplace interventions for reducing sick leave concluded that existing evidence is insufficient to support the effectiveness of such interventions for reducing sick leave [104]. However, another systematic review on the effectiveness of PAWHIs for work-related and health-related outcomes (musculoskeletal diseases, mental health issues, or other health conditions) found that for the musculoskeletal disease subgroup, such interventions effectively reduce sick leave [105]. Similarly, a systematic review indicated that comprehensive low-back pain interventions positively impacted absenteeism, cost control, and the prevention of new-onset low-back pain [106].
Research shows that work ability typically declines by 0.5–0.7 percentage points annually with age, and improvements in work ability can be viewed as an important indicator of corporate economic benefits [107]. A meta-analysis confirmed that workplace interventions improve employee work ability and increase productivity, although the magnitude of effects is relatively small [108].
4.2.7. Sleep Quality
The sleep improvement results in the included studies demonstrated relatively limited efficacy, with only two out of seven studies (28.6%) reporting statistically significant improvements. This finding contrasts with general research on physical activity (PA) and sleep, where appropriate PA typically shows positive effects on sleep quality—particularly in enhancing deep sleep, reducing sleep latency, and improving sleep efficiency [109,110]. However, individual variability and the complex regulatory mechanisms of sleep, combined with external influencing factors, may explain the inconsistent intervention effects. In the two studies that reported significant improvements, participants were office workers with fixed schedules and predominantly sedentary behaviors. In the two studies with significant improvements, the inclusion of educational and structured PA components may have contributed to positive outcomes, though broader conclusions are limited by the small sample size. Existing research on sleep and PA suggests that achieving a certain exercise intensity is crucial: moderate-intensity PA has been shown to significantly enhance sleep quality, whereas low-intensity PA yields minimal effects [111,112]. Additionally, age is a key moderating factor, with more pronounced sleep benefits observed in older adults and children [113]. Additionally, balanced nutrition positively affects sleep quality [109,114,115]. Research confirms that diets containing the appropriate amounts of protein, carbohydrates, and healthy fats are crucial for maintaining sleep quality [116]. To improve sleep quality, it is recommended to prioritize foods containing complex carbohydrates, a low glycemic index, low glycemic load, and high fiber content, while avoiding processed foods rich in saturated fatty acids [117].
Due to the limited number of included studies, further investigation is needed to clarify the effectiveness of physical activity-led workplace health interventions (PAWHIs) on employee sleep outcomes. Future research should explore differential effects by age, occupational demands, and baseline activity levels to clarify which subgroups benefit most from PAWHI in sleep-related outcomes.
4.3. Risk-of-Bias Results
The risk-of-bias assessment revealed methodological limitations in the included studies. A high prevalence of performance bias (65.0%, 26/40 studies) due to the inadequate blinding of participants and personnel was observed. The insufficiency of blinding is largely limited by the characteristics of the intervention measures. If the intervention involves visible health management activities (such as fitness classes and dietary guidance), it will be difficult to set blinding methods for both the intervener and the participants.
Allocation concealment and random sequence generation were insufficiently described in 42.5% and 22.5% of studies, respectively, compromising baseline comparability. Among the included PAWHI studies, some did not strictly implement the randomization method. They merely grouped companies or departments as a whole instead of randomly assigning individuals.
It is worth noting that in selective reporting, the proportion of high-risk situations is the lowest, which to some extent reduces the misleading conclusions caused by “selective emphasis”. Meanwhile, most of the primary outcome measures included in the studies were objective measures. Compared with subjective measures (such as self-reported stress or dietary habits), objective outcome evaluations (such as blood biomarkers and body composition) had the potential to offset the risk of bias because these measurements were less likely to produce explanatory variability.
Only 10.0% of studies met the ≥5 low-risk criteria, underscoring the need for improved methodological rigor in future workplace intervention trials. Emphasis should be placed on the transparent reporting of randomization, allocation concealment, and blinding protocols, particularly for studies relying on subjective outcomes. The integration of objective biomarkers and standardized tools could further enhance reliability.
This aligns with the review’s broader finding that PAWHI efficacy is robust for objective outcomes but more variable for subjective domains, where bias risks are higher. Especially in small-sample studies, attention should be paid. Future research should address these limitations to strengthen evidence quality.
4.4. Study Limitations and Prospects
Several notable limitations exist in the current study: First, PAWHIs are typically implemented uniformly at the organizational level. While this approach facilitates workplace culture transformation and provides extensive social support networks for health behavior change, the implementation across diverse demographic profiles, geographical regions, and employment contexts makes it challenging to precisely identify the potential determinants of outcome heterogeneity.
Second, the systematic reporting of organizational support levels and employee engagement metrics is conspicuously insufficient. Existing evidence suggests that active employee participation and strong management support are key predictors of intervention success; therefore, exploring strategies to optimize employee engagement and secure sustained management commitment to strengthen intervention effectiveness should be prioritized in future research.
Furthermore, current research indicates that there is limited attention paid to the cost-benefit analysis of the intervention. Although most research confirms the positive impacts of interventions on health indicators, their economic cost-effectiveness remains incomprehensively evaluated, particularly regarding long-term return on investment. Future research should incorporate economic evaluation methodologies to provide decision-makers with more comprehensive investment value assessment criteria.
Tension exists between intervention universality and personalized customization. Different employee subgroups (based on age cohorts, health status, job nature, and occupational strata) likely require differentiated intervention strategies. Consequently, determining how to maintain core intervention elements while making appropriate adjustments according to target population characteristics to enhance intervention precision and effectiveness represents a critical area requiring in-depth exploration. Concurrently, to achieve broader and more sustainable health intervention outcomes, future program designs should incorporate greater personalization, enhance long-term sustainability, and secure more comprehensive organizational support.
Finally, in the analysis of the intervention effect, future studies should prioritize standardized, objective measurement tools (e.g., DEXA for body composition and accelerometers for PA) to minimize methodological variability and enhance cross-study comparability.
5. Conclusions
This systematic review provides a comprehensive evaluation of PAWHIs in terms of specific intervention strategies and their multidimensional effects. The findings demonstrate that such workplace interventions exhibit varying degrees of effectiveness in improving employee health status, optimizing body composition, enhancing physical function, promoting mental well-being, and improving work-related outcomes The results of the intervention success rate indicate that, particularly for physical activity (81.3%), stress management (80.0%), and diet/nutrition status (63.2%), this confirms PAWHI have significant efficacy in these specific assessment indicators.
In conclusion, PAWHI represents an effective intervention strategy for enhancing employee health, fostering healthy lifestyle habits, and improving work performance. It offers corporate leaders innovative approaches to workforce management and employee health improvement.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/healthcare13111292/s1.
Author Contributions
Conceptualization, S.Z. and H.R.; methodology, S.Z.; software, S.Z. and M.N.; validation, S.Z.; formal analysis, S.Z.; investigation, S.Z. and J.P.; resources, S.Z.; data curation, S.Z.; writing—original draft preparation, S.Z.; writing—review and editing, S.Z.; visualization, S.Z. and J.P.; supervision, H.R. and M.N.; project administration, S.Z. and J.P.; funding acquisition, H.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| PAWHI | Physical activity-led workplace health interventions |
| PA | Physical activity |
References
- Terada, T.M.; Mistura, H.; Tulloch, A.P.; Reed, J. Dietary Behaviour Is Associated with Cardiometabolic and Psychological Risk Indicators in Female Hospital Nurses-a Post-Hoc, Cross-Sectional Study. Nutrients 2019, 11, 2054. [Google Scholar] [CrossRef] [PubMed]
- Ryu, H.; Jung, J.; Moon, J. Health Promotion Program for Office Workers with Sem Based on the Who’s Healthy Workplace Framework. Health Promot. Int. 2020, 35, 1369–1382. [Google Scholar] [CrossRef] [PubMed]
- Schluter, P.J.; Turner, C.; Benefer, C. Long working hours and alcohol risk among Australian and New Zealand nurses and midwives: A cross-sectional study. Int. J. Nurs. Stud. 2012, 49, 701–709. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, W.; Kim, H.R. Long working hours and the risk of hypothyroidism in healthy Korean workers: A cohort study. Epidemiol. Health 2022, 44, e2022104. [Google Scholar] [CrossRef]
- Nagaya, T.; Hibino, M.; Kondo, Y. Long working hours directly and indirectly (via short sleep duration) induce headache even in healthy white-collar men: Cross-sectional and 1-year follow-up analyses. Int. Arch. Occup. Environ. Health 2018, 91, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Baicker, K.; Cutler, D.; Song, Z. Workplace wellness programs can generate savings. Health Aff. 2010, 29, 304–311. [Google Scholar] [CrossRef]
- Goetzel, R.Z.; Ozminkowski, R.J. The health and cost benefits of work site health-promotion programs. Annu. Rev. Public Health 2008, 29, 303–323. [Google Scholar] [CrossRef]
- Priano, S.M.; Hong, O.S.; Chen, J.L. Lifestyles and Health-Related Outcomes of U.S. Hospital Nurses: A Systematic Review. Nurs. Outlook 2018, 66, 66–76. [Google Scholar] [CrossRef]
- Nilan, K.; McKeever, T.M.; McNeill, A.; Raw, M.; Murray, R.L. Prevalence of tobacco use in healthcare workers: A systematic review and meta-analysis. PLoS ONE 2019, 14, e0220168. [Google Scholar] [CrossRef]
- Prieske, O.; Dalager, T.; Herz, M.; Hortobagyi, T.; Sjogaard, G.; Sogaard, K.; Granacher, U. Effects of Physical Exercise Training in the Workplace on Physical Fitness: A Systematic Review and Meta-analysis. Sports Med. 2019, 49, 1903–1921. [Google Scholar] [CrossRef]
- Vilela, B.L.; Benedito Silva, A.A.; de Lira, C.A.; Andrade Mdos, S. Workplace exercise and educational program for improving fitness outcomes related to health in workers: A randomized controlled trial. J. Occup. Environ. Med. 2015, 57, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Proper, K.I.; van Oostrom, S.H. The effectiveness of workplace health promotion interventions on physical and mental health outcomes—A systematic review of reviews. Scand. J. Work. Environ. Health 2019, 45, 546–559. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.; Gordt-Oesterwind, K.; Bongartz, M.; Zimmermann, S.; Seide, S.; Braun, V.; Schwenk, M. Effects of Physical Activity Interventions on Strength, Balance and Falls in Middle-Aged Adults: A Systematic Review and Meta-Analysis. Sports Med. Open 2023, 9, 61. [Google Scholar] [CrossRef]
- Larinier, N.; Vuillerme, N.; Balaguier, R. Effectiveness of warm-up interventions on work-related musculoskeletal disorders, physical and psychosocial functions among workers: A systematic review. BMJ Open 2023, 13, e056560. [Google Scholar] [CrossRef]
- Armaou, M.; Konstantinidis, S.; Blake, H. The Effectiveness of Digital Interventions for Psychological Well-Being in the Workplace: A Systematic Review Protocol. Int. J. Environ. Res. Public Health 2019, 17, 255. [Google Scholar] [CrossRef]
- Zhou, L.; Deng, X.; Guo, K.; Hou, L.; Hui, X.; Wu, Y.; Xu, M.; Wang, Y.; Liang, S.; Yang, K. Effectiveness of multicomponent interventions in office-based workers to mitigate occupational sedentary behavior: Systematic review and meta-analysis. JMIR Public Health Surveill. 2023, 9, e44745. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, H.; Xu, R. The impact of technology on promoting physical activities and mental health: A gender-based study. BMC Psychol. 2023, 11, 298. [Google Scholar] [CrossRef]
- Hersch, R.K.; Cook, R.F.; Deitz, D.K.; Kaplan, S.; Hughes, D.; Friesen, M.A.; Vezina, M. Reducing nurses’ stress: A randomized controlled trial of a web-based stress management program for nurses. Appl. Nurs. Res. 2016, 32, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Stanulewicz, N.; Knox, E.; Narayanasamy, M.; Shivji, N.; Khunti, K.; Blake, H. Effectiveness of Lifestyle Health Promotion Interventions for Nurses: A Systematic Review. Int. J. Environ. Res. Public Health 2019, 17, 17. [Google Scholar] [CrossRef]
- O’Donoghue, G.; Blake, C.; Cunningham, C.; Lennon, O.; Perrotta, C. What exercise prescription is optimal to improve body composition and cardiorespiratory fitness in adults living with obesity? A network meta-analysis. Obes. Rev. 2021, 22, e13137. [Google Scholar] [CrossRef]
- Gerstel, E.; Pataky, Z.; Busnel, C.; Rutschmann, O.; Guessous, I.; Zumwald, C.; Golay, A. Impact of lifestyle intervention on body weight and the metabolic syndrome in home-care providers. Diabetes Metab. 2013, 39, 78–84. [Google Scholar] [CrossRef]
- Andersen, L.L.; Sundstrup, E.; Boysen, M.; Jakobsen, M.D.; Mortensen, O.S.; Persson, R. Cardiovascular health effects of internet-based encouragements to do daily workplace stair-walks: Randomized controlled trial. J. Med. Internet Res. 2013, 15, e127. [Google Scholar] [CrossRef]
- Christensen, J.R.; Overgaard, K.; Hansen, K.; Sogaard, K.; Holtermann, A. Effects on presenteeism and absenteeism from a 1-year workplace randomized controlled trial among health care workers. J. Occup. Environ. Med. 2013, 55, 1186–1190. [Google Scholar] [CrossRef] [PubMed]
- Gussenhoven, A.H.; van Wier, M.F.; Bosmans, J.E.; Dekkers, J.C.; van Mechelen, W. Cost-effectiveness of a distance lifestyle counselling programme among overweight employees from a company perspective, ALIFE@Work: A randomized controlled trial. Work 2013, 46, 337–346. [Google Scholar] [CrossRef]
- Weinhold, K.R.; Miller, C.K.; Marrero, D.G.; Nagaraja, H.N.; Focht, B.C.; Gascon, G.M. A Randomized Controlled Trial Translating the Diabetes Prevention Program to a University Worksite, Ohio, 2012–2014. Prev. Chronic Dis. 2015, 12, E210. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, D.C.; Andrews, T.; Schenker, M.B. Pasos Saludables: A Pilot Randomized Intervention Study to Reduce Obesity in an Immigrant Farmworker Population. J. Occup. Environ. Med. 2015, 57, 1039–1046. [Google Scholar] [CrossRef]
- Miller, C.K.; Weinhold, K.; Marrero, D.G.; Nagaraja, H.N.; Focht, B.C. A Translational Worksite Diabetes Prevention Trial Improves Psychosocial Status, Dietary Intake, and Step Counts among Employees with Prediabetes: A Randomized Controlled Trial. Prev. Med. Rep. 2015, 2, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Audrey, S.; Cooper, A.R.; Hollingworth, W.; Metcalfe, C.; Procter, S.; Davis, A.; Campbell, R.; Gillison, F.; Rodgers, S.E. Study protocol: The effectiveness and cost effectiveness of an employer-led intervention to increase walking during the daily commute: The Travel to Work randomised controlled trial. BMC Public Health 2015, 15, 154. [Google Scholar] [CrossRef]
- Solenhill, M.; Grotta, A.; Pasquali, E.; Bakkman, L.; Bellocco, R.; Trolle Lagerros, Y. The Effect of Tailored Web-Based Feedback and Optional Telephone Coaching on Health Improvements: A Randomized Intervention Among Employees in the Transport Service Industry. J. Med. Internet Res. 2016, 18, e158. [Google Scholar] [CrossRef]
- Gregoski, M.J.; Newton, J.; Ling, C.G.; Blaylock, K.; Smith, S.A.; Paguntalan, J.; Treiber, F.A. Effective weight-loss using an e-health delivered physical activity and dietary intervention: A federal credit union pilot study. Work 2016, 54, 127–134. [Google Scholar] [CrossRef]
- Chandrasiri, A.; Dissanayake, A.; de Silva, V. Health promotion in workplaces as a strategy for modification of risk factors for Non Communicable Diseases (NCDs): A practical example from Sri Lanka. Work 2016, 55, 281–284. [Google Scholar] [CrossRef]
- Hendriksen, I.J.; Snoijer, M.; de Kok, B.P.; van Vilsteren, J.; Hofstetter, H. Effectiveness of a Multilevel Workplace Health Promotion Program on Vitality, Health, and Work-Related Outcomes. J. Occup. Environ. Med. 2016, 58, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Jamal, S.N.; Moy, F.M.; Azmi Mohamed, M.N.; Mukhtar, F. Effectiveness of a Group Support Lifestyle Modification (GSLiM) Programme among Obese Adults in Workplace: A Randomised Controlled Trial. PLoS ONE 2016, 11, e0160343. [Google Scholar] [CrossRef] [PubMed]
- Balk-Moller, N.C.; Poulsen, S.K.; Larsen, T.M. Effect of a Nine-Month Web- and App-Based Workplace Intervention to Promote Healthy Lifestyle and Weight Loss for Employees in the Social Welfare and Health Care Sector: A Randomized Controlled Trial. J. Med. Internet Res. 2017, 19, e108. [Google Scholar] [CrossRef]
- Maylor, B.D.; Edwardson, C.L.; Zakrzewski-Fruer, J.K.; Champion, R.B.; Bailey, D.P. Efficacy of a Multicomponent Intervention to Reduce Workplace Sitting Time in Office Workers: A Cluster Randomized Controlled Trial. J. Occup. Environ. Med. 2018, 60, 787–795. [Google Scholar] [CrossRef]
- Viester, L.; Verhagen, E.; Bongers, P.M.; van der Beek, A.J. Effectiveness of a Worksite Intervention for Male Construction Workers on Dietary and Physical Activity Behaviors, Body Mass Index, and Health Outcomes: Results of a Randomized Controlled Trial. Am. J. Health Promot. 2018, 32, 795–805. [Google Scholar] [CrossRef]
- Ing, C.T.; Miyamoto, R.E.S.; Fang, R.; Antonio, M.; Paloma, D.; Braun, K.L.; Kaholokula, J.K. Comparing Weight Loss-Maintenance Outcomes of a Worksite-Based Lifestyle Program Delivered via DVD and Face-to-Face: A Randomized Trial. Health Educ. Behav. 2018, 45, 569–580. [Google Scholar] [CrossRef] [PubMed]
- Kouwenhoven-Pasmooij, T.A.; Robroek, S.J.W.; Kraaijenhagen, R.A.; Helmhout, P.H.; Nieboer, D.; Burdorf, A.; Myriam Hunink, M.G. Effectiveness of the blended-care lifestyle intervention ‘PerfectFit’: A cluster randomised trial in employees at risk for cardiovascular diseases. BMC Public Health 2018, 18, 766. [Google Scholar] [CrossRef]
- Oftedal, S.; Burrows, T.; Fenton, S.; Murawski, B.; Rayward, A.B.; Duncan, M.J. Feasibility and Preliminary Efficacy of an m-Health Intervention Targeting Physical Activity, Diet, and Sleep Quality in Shift-Workers. Int. J. Environ. Res. Public Health 2019, 16, 34. [Google Scholar] [CrossRef]
- Fang, Y.Y.; Huang, C.Y.; Hsu, M.C. Effectiveness of a physical activity program on weight, physical fitness, occupational stress, job satisfaction and quality of life of overweight employees in high-tech industries: A randomized controlled study. Int. J. Occup. Saf. Ergon. 2019, 25, 621–629. [Google Scholar] [CrossRef]
- Edman, J.S.; Galantino, M.L.; Hutchinson, J.; Greeson, J.M. Health coaching for healthcare employees with chronic disease: A pilot study. Work 2019, 63, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Haufe, S.; Kerling, A.; Protte, G.; Bayerle, P.; Stenner, H.T.; Rolff, S.; Sundermeier, T.; Kuck, M.; Ensslen, R.; Nachbar, L.; et al. Telemonitoring-supported exercise training, metabolic syndrome severity, and work ability in company employees: A randomised controlled trial. Lancet Public Health 2019, 4, e343–e352. [Google Scholar] [CrossRef]
- Stein, R.I.; Strickland, J.R.; Tabak, R.G.; Dale, A.M.; Colditz, G.A.; Evanoff, B.A. Design of a randomized trial testing a multi-level weight-control intervention to reduce obesity and related health conditions in low-income workers. Contemp. Clin. Trials 2019, 79, 89–97. [Google Scholar] [CrossRef]
- Bonn, S.E.; Lof, M.; Ostenson, C.G.; Trolle Lagerros, Y. App-technology to improve lifestyle behaviors among working adults—The Health Integrator study, a randomized controlled trial. BMC Public Health 2019, 19, 273. [Google Scholar] [CrossRef]
- Sareban, M.; Fernandez La Puente de Battre, M.D.; Reich, B.; Schmied, C.; Loidl, M.; Niederseer, D.; Niebauer, J. Effects of active commuting to work for 12 months on cardiovascular risk factors and body composition. Scand. J. Med. Sci. Sports 2020, 30 (Suppl. S1), 24–30. [Google Scholar] [CrossRef] [PubMed]
- Linnan, L.A.; Vaughn, A.E.; Smith, F.T.; Westgate, P.; Hales, D.; Arandia, G.; Neshteruk, C.; Willis, E.; Ward, D.S. Results of caring and reaching for health (CARE): A cluster-randomized controlled trial assessing a worksite wellness intervention for child care staff. Int. J. Behav. Nutr. Phys. Act. 2020, 17, 64. [Google Scholar] [CrossRef]
- Piao, M.; Ryu, H.; Lee, H.; Kim, J. Use of the Healthy Lifestyle Coaching Chatbot App to Promote Stair-Climbing Habits Among Office Workers: Exploratory Randomized Controlled Trial. JMIR mHealth uHealth 2020, 8, e15085. [Google Scholar] [CrossRef]
- Reich, B.; Niederseer, D.; Loidl, M.; Fernandez La Puente de Battre, M.D.; Rossi, V.A.; Zagel, B.; Caselli, S.; Schmied, C.; Niebauer, J. Effects of active commuting on cardiovascular risk factors: GISMO-a randomized controlled feasibility study. Scand. J. Med. Sci. Sports 2020, 30 (Suppl. S1), 15–23. [Google Scholar] [CrossRef] [PubMed]
- Haufe, S.; Hupa-Breier, K.L.; Bayerle, P.; Boeck, H.T.; Rolff, S.; Sundermeier, T.; Kerling, A.; Eigendorf, J.; Kuck, M.; Hanke, A.A.; et al. Telemonitoring-Supported Exercise Training in Employees With Metabolic Syndrome Improves Liver Inflammation and Fibrosis. Clin. Transl. Gastroenterol. 2021, 12, e00371. [Google Scholar] [CrossRef]
- Neshteruk, C.D.; Willis, E.; Smith, F.; Vaughn, A.E.; Grummon, A.H.; Vu, M.B.; Ward, D.S.; Linnan, L. Implementation of a workplace physical activity intervention in child care: Process evaluation results from the Care2BWell trial. Transl. Behav. Med. 2021, 11, 1430–1440. [Google Scholar] [CrossRef]
- Mamede, A.; Noordzij, G.; Jongerling, J.; Snijders, M.; Schop-Etman, A.; Denktas, S. Combining Web-Based Gamification and Physical Nudges With an App (MoveMore) to Promote Walking Breaks and Reduce Sedentary Behavior of Office Workers: Field Study. J. Med. Internet Res. 2021, 23, e19875. [Google Scholar] [CrossRef] [PubMed]
- Noori, F.; Behboodimoghadam, Z.; Haghani, S.; Pashaeypoor, S. The Effect of an Empowerment Program on the Health-promoting Behaviors of Iranian Women Workers: A Randomized Controlled Trial. J. Prev. Med. Public Health 2021, 54, 275–283. [Google Scholar] [CrossRef]
- Stephenson, A.; Garcia-Constantino, M.; Murphy, M.H.; McDonough, S.M.; Nugent, C.D.; Mair, J.L. The “Worktivity” mHealth intervention to reduce sedentary behaviour in the workplace: A feasibility cluster randomised controlled pilot study. BMC Public Health 2021, 21, 1416. [Google Scholar] [CrossRef]
- Garcia Perez de Sevilla, G.; Barcelo Guido, O.; De la Cruz, M.P.; Blanco Fernandez, A.; Alejo, L.B.; Montero Martinez, M.; Perez-Ruiz, M. Adherence to a Lifestyle Exercise and Nutrition Intervention in University Employees during the COVID-19 Pandemic: A Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2021, 18, 7510. [Google Scholar] [CrossRef]
- Kong, J.; Chen, Y.; Zheng, Y.; Zhu, L.; Chen, B.; Cheng, X.; Song, M.; Patrick, D.L.; Beresford, S.A.A.; Wang, H. Effectiveness of a Worksite-Based Lifestyle Intervention on Employees’ Obesity Control and Prevention in China: A Group Randomized Experimental Study. Int. J. Environ. Res. Public Health 2022, 19, 6738. [Google Scholar] [CrossRef]
- Shiri, R.; Vaananen, A.; Mattila-Holappa, P.; Kauppi, K.; Borg, P. The Effect of Healthy Lifestyle Changes on Work Ability and Mental Health Symptoms: A Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2022, 19, 13206. [Google Scholar] [CrossRef] [PubMed]
- Bayerle, P.; Kerling, A.; Kuck, M.; Rolff, S.; Boeck, H.T.; Sundermeier, T.; Ensslen, R.; Tegtbur, U.; Lauenstein, D.; Bothig, D.; et al. Effectiveness of wearable devices as a support strategy for maintaining physical activity after a structured exercise intervention for employees with metabolic syndrome: A randomized controlled trial. BMC Sports Sci. Med. Rehabil. 2022, 14, 24. [Google Scholar] [CrossRef] [PubMed]
- Edwardson, C.L.; Biddle, S.J.H.; Clemes, S.A.; Davies, M.J.; Dunstan, D.W.; Eborall, H.; Granat, M.H.; Gray, L.J.; Healy, G.N.; Jaicim, N.B.; et al. Effectiveness of an intervention for reducing sitting time and improving health in office workers: Three arm cluster randomised controlled trial. BMJ 2022, 378, e069288. [Google Scholar] [CrossRef]
- Silva, F.M.; Duarte-Mendes, P.; Carvalho, E.; Soares, C.M.; Farinha, C.; Serrano, J.; Paulo, R.; Massart, A.; Rodrigues, R.N.; Teixeira, A.M.; et al. Effects of combined training during the COVID-19 pandemic on metabolic health and quality of life in sedentary workers: A randomized controlled study. Front. Public Health 2022, 10, 1040714. [Google Scholar] [CrossRef]
- Brinkmann, C.; Hof, H.; Gysan, D.B.; Albus, C.; Millentrup, S.; Bjarnason-Wehrens, B.; Latsch, J.; Herold, G.; Wegscheider, K.; Heming, C.; et al. Lifestyle intervention reduces risk score for cardiovascular mortality in company employees with pre-diabetes or diabetes mellitus—A secondary analysis of the PreFord randomized controlled trial with 3 years of follow-up. Front. Endocrinol. 2023, 14, 1106334. [Google Scholar] [CrossRef]
- Xiao, E.; Yu, R.; Cai, X.; Jiang, L.; Li, J.; Ma, C.; Liu, Y.; Liu, L.; Su, G.; Wang, X. Development and validation of a novel metabolic health-related nomogram to improve predictive performance of cardiovascular disease risk in patients with prediabetes. Lipids Health Dis. 2025, 24, 45. [Google Scholar] [CrossRef] [PubMed]
- Ramezani, M.; Tayefi, B.; Zandian, E.; SoleimanvandiAzar, N.; Khalili, N.; Hoveidamanesh, S.; Massahikhaleghi, P.; Rampisheh, Z. Workplace interventions for increasing physical activity in employees: A systematic review. J. Occup. Health 2022, 64, e12358. [Google Scholar] [CrossRef] [PubMed]
- Reed, J.L.; Prince, S.A.; Elliott, C.G.; Mullen, K.A.; Tulloch, H.E.; Hiremath, S.; Cotie, L.M.; Pipe, A.L.; Reid, R.D. Impact of Workplace Physical Activity Interventions on Physical Activity and Cardiometabolic Health Among Working-Age Women: A Systematic Review and Meta-Analysis. Circ. Cardiovasc. Qual. Outcomes 2017, 10, e003516. [Google Scholar] [CrossRef]
- Song, Z.; Baicker, K. Effect of a Workplace Wellness Program on Employee Health and Economic Outcomes: A Randomized Clinical Trial. JAMA 2019, 321, 1491–1501. [Google Scholar] [CrossRef] [PubMed]
- Sawchuk, C.N.; Russo, J.; Ferguson, G.; Williamson, J.; Sabin, J.A.; Goldberg, J.; Madesclaire, O.; Bogucki, O.E.; Buchwald, D. Health Promotion Programs and Policies in the Workplace: An Exploratory Study With Alaska Businesses. Prev. Chronic Dis. 2020, 17, E125. [Google Scholar] [CrossRef]
- Zhang, Z.; Pang, Y.; Shen, J.; Chen, W.; Hao, C.; Lei, Z. The new definition of metabolic syndrome including hyperuricemia improves its prognostic value: Results from NHANES database. BMC Cardiovasc. Disord. 2025, 25, 93. [Google Scholar] [CrossRef]
- Iturriaga, T.; Barcelo, O.; Diez-Vega, I.; Cordero, J.; Pulgar, S.; Fernandez-Luna, A.; Perez-Ruiz, M. Effects of a short workplace exercise program on body composition in women: A randomized controlled trial. Health Care Women Int. 2020, 41, 133–146. [Google Scholar] [CrossRef]
- de Sevilla, G.G.P.; Vicente-Arche, F.C.; Thuissard, I.J.; Barcelo, O.; Perez-Ruiz, M. Effectiveness of Workplace Exercise Interventions on Body Composition: A Systematic Review and Meta-Analysis. Am. J. Health Promot. 2021, 35, 1150–1161. [Google Scholar] [CrossRef]
- Chiu, C.H.; Ko, M.C.; Wu, L.S.; Yeh, D.P.; Kan, N.W.; Lee, P.F.; Hsieh, J.W.; Tseng, C.Y.; Ho, C.C. Benefits of different intensity of aerobic exercise in modulating body composition among obese young adults: A pilot randomized controlled trial. Health Qual. Life Outcomes 2017, 15, 168. [Google Scholar] [CrossRef]
- Sultana, R.N.; Sabag, A.; Keating, S.E.; Johnson, N.A. The Effect of Low-Volume High-Intensity Interval Training on Body Composition and Cardiorespiratory Fitness: A Systematic Review and Meta-Analysis. Sports Med. 2019, 49, 1687–1721. [Google Scholar] [CrossRef]
- Cristi-Montero, C.; Martinez-Flores, R.; Espinoza-Puelles, J.P.; Favero-Ramirez, L.; Zurita-Corvalan, N.; Canete, I.C.; Leppe, J.; Ferrari, G.; Sadarangani, K.P.; Cancino-Lopez, J.; et al. Study protocol and rationale of “the UP project”: Evaluating the effectiveness of active breaks on health indicators in desk-based workers. Front. Public Health 2024, 12, 1363015. [Google Scholar] [CrossRef]
- Jablonowska-Lietz, B.; Wrzosek, M.; Wlodarczyk, M.; Nowicka, G. New indexes of body fat distribution, visceral adiposity index, body adiposity index, waist-to-height ratio, and metabolic disturbances in the obese. Kardiol. Pol. 2017, 75, 1185–1191. [Google Scholar] [CrossRef] [PubMed]
- Gibson, R.; Rogerson, G.A.F.; Theobald, H.E.; Alawfi, J.S.; Philippou, E.; David, D.; Sum, K.; Durrant, L.R.; Nutrition Society Workplace, D.; Health Special Interest, G. The Nutrition Society Satellite Symposium, Nutrition Congress: Workplace diet and health. Proc. Nutr. Soc. 2024, 6, 1–4. [Google Scholar] [CrossRef]
- Salinger, M.R.; Levy, D.E.; McCurley, J.L.; Gelsomin, E.D.; Rimm, E.B.; Thorndike, A.N. Employees’ Baseline Food Choices and the Effect of a Workplace Intervention to Promote Healthy Eating: Secondary Analysis of the ChooseWell 365 Randomized Controlled Trial. J. Acad. Nutr. Diet. 2023, 123, 1586–1595.e1584. [Google Scholar] [CrossRef]
- Wolfenden, L.; Goldman, S.; Stacey, F.G.; Grady, A.; Kingsland, M.; Williams, C.M.; Wiggers, J.; Milat, A.; Rissel, C.; Bauman, A.; et al. Strategies to improve the implementation of workplace-based policies or practices targeting tobacco, alcohol, diet, physical activity and obesity. Cochrane Database Syst. Rev. 2018, 11, CD012439. [Google Scholar] [CrossRef]
- Thorndike, A.N.; McCurley, J.L.; Gelsomin, E.D.; Anderson, E.; Chang, Y.; Porneala, B.; Johnson, C.; Rimm, E.B.; Levy, D.E. Automated Behavioral Workplace Intervention to Prevent Weight Gain and Improve Diet: The ChooseWell 365 Randomized Clinical Trial. JAMA Netw. Open 2021, 4, e2112528. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, M.; Yoshino, J.; Smith, G.I.; Stein, R.I.; Bittel, A.J.; Bittel, D.C.; Reeds, D.N.; Sinacore, D.R.; Cade, W.T.; Patterson, B.W.; et al. Worksite-based intensive lifestyle therapy has profound cardiometabolic benefits in people with obesity and type 2 diabetes. Cell Metab. 2022, 34, 1431–1441.e1435. [Google Scholar] [CrossRef]
- Gepner, Y.; Shelef, I.; Schwarzfuchs, D.; Zelicha, H.; Tene, L.; Yaskolka Meir, A.; Tsaban, G.; Cohen, N.; Bril, N.; Rein, M.; et al. Effect of Distinct Lifestyle Interventions on Mobilization of Fat Storage Pools: CENTRAL Magnetic Resonance Imaging Randomized Controlled Trial. Circulation 2018, 137, 1143–1157. [Google Scholar] [CrossRef] [PubMed]
- Keadle, S.K.; Conroy, D.E.; Buman, M.P.; Dunstan, D.W.; Matthews, C.E. Targeting Reductions in Sitting Time to Increase Physical Activity and Improve Health. Med. Sci. Sports Exerc. 2017, 49, 1572–1582. [Google Scholar] [CrossRef]
- Yaghoubitajani, Z.; Gheitasi, M.; Bayattork, M.; Andersen, L.L. Corrective exercises administered online vs at the workplace for pain and function in the office workers with upper crossed syndrome: Randomized controlled trial. Int. Arch. Occup. Environ. Health 2022, 95, 1703–1718. [Google Scholar] [CrossRef]
- Freak-Poli, R.; Cumpston, M.; Albarqouni, L.; Clemes, S.A.; Peeters, A. Workplace pedometer interventions for increasing physical activity. Cochrane Database Syst. Rev. 2020, 7, CD009209. [Google Scholar] [CrossRef]
- Gray, C.M. Reducing sedentary behaviour in the workplace. BMJ 2018, 363, k4061. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.N.; Gao, J.L.; Dai, J.M.; Zheng, P.P.; Li, Z.Y.; Li, G.Y.; Fu, H. A comprehensive evaluation of intervention effects on workplace health promotion in 10 government agencies. Chin. J. Ind. Hyg. Occup. Dis. 2018, 36, 251–253. [Google Scholar] [CrossRef]
- Amatori, S.; Ferri Marini, C.; Gobbi, E.; Sisti, D.; Giombini, G.; Rombaldoni, R.; Rocchi, M.B.L.; Lucertini, F.; Federici, A.; Perroni, F.; et al. Short High-Intensity Interval Exercise for Workplace-Based Physical Activity Interventions: A Systematic Review on Feasibility and Effectiveness. Sports Med. 2023, 53, 887–901. [Google Scholar] [CrossRef]
- Reif, J.; Chan, D.; Jones, D.; Payne, L.; Molitor, D. Effects of a Workplace Wellness Program on Employee Health, Health Beliefs, and Medical Use: A Randomized Clinical Trial. JAMA Intern. Med. 2020, 180, 952–960. [Google Scholar] [CrossRef]
- Mulchandani, R.; Chandrasekaran, A.M.; Shivashankar, R.; Kondal, D.; Agrawal, A.; Panniyammakal, J.; Tandon, N.; Prabhakaran, D.; Sharma, M.; Goenka, S. Effect of workplace physical activity interventions on the cardio-metabolic health of working adults: Systematic review and meta-analysis. Int. J. Behav. Nutr. Phys. Act. 2019, 16, 134. [Google Scholar] [CrossRef]
- Sandercock, V.; Andrade, J. Evaluation of Worksite Wellness Nutrition and Physical Activity Programs and Their Subsequent Impact on Participants’ Body Composition. J. Obes. 2018, 2018, 1035871. [Google Scholar] [CrossRef] [PubMed]
- Nooijen, C.F.J.; Blom, V.; Ekblom, O.; Ekblom, M.M.; Kallings, L.V. Improving office workers’ mental health and cognition: A 3-arm cluster randomized controlled trial targeting physical activity and sedentary behavior in multi-component interventions. BMC Public Health 2019, 19, 266. [Google Scholar] [CrossRef] [PubMed]
- Arrogi, A.; Schotte, A.; Bogaerts, A.; Boen, F.; Seghers, J. Short- and long-term effectiveness of a three-month individualized need-supportive physical activity counseling intervention at the workplace. BMC Public Health 2017, 17, 52. [Google Scholar] [CrossRef]
- Demou, E.; MacLean, A.; Cheripelli, L.J.; Hunt, K.; Gray, C.M. Group-based healthy lifestyle workplace interventions for shift workers: A systematic review. Scand. J. Work Environ. Health 2018, 44, 568–584. [Google Scholar] [CrossRef]
- Hutchinson, A.D.; Wilson, C. Improving nutrition and physical activity in the workplace: A meta-analysis of intervention studies. Health Promot. Int. 2012, 27, 238–249. [Google Scholar] [CrossRef] [PubMed]
- Seppala, T.; Hankonen, N.; Korkiakangas, E.; Ruusuvuori, J.; Laitinen, J. National policies for the promotion of physical activity and healthy nutrition in the workplace context: A behaviour change wheel guided content analysis of policy papers in Finland. BMC Public Health 2017, 18, 87. [Google Scholar] [CrossRef]
- Shiri, R.; Nikunlaakso, R.; Laitinen, J. Effectiveness of Workplace Interventions to Improve Health and Well-Being of Health and Social Service Workers: A Narrative Review of Randomised Controlled Trials. Healthcare 2023, 11, 1792. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Xiao, Y.; Chen, H.; Gebel, K.; Li, C.; Sun, S.; Yang, Q.; Wang, S.; Zhang, L.; Wang, J.; et al. Effects of group communication norms on daily steps in a team-based financial incentive mobile phone intervention in Shanghai, China. Int. J. Behav. Nutr. Phys. Act. 2025, 22, 9. [Google Scholar] [CrossRef]
- Abadiyan, F.; Hadadnezhad, M.; Khosrokiani, Z.; Letafatkar, A.; Akhshik, H. Adding a smartphone app to global postural re-education to improve neck pain, posture, quality of life, and endurance in people with nonspecific neck pain: A randomized controlled trial. Trials 2021, 22, 274. [Google Scholar] [CrossRef] [PubMed]
- Hogg, B.; Medina, J.C.; Gardoki-Souto, I.; Serbanescu, I.; Moreno-Alcazar, A.; Cerga-Pashoja, A.; Coppens, E.; Toth, M.D.; Fanaj, N.; Greiner, B.A.; et al. Workplace interventions to reduce depression and anxiety in small and medium-sized enterprises: A systematic review. J. Affect. Disord. 2021, 290, 378–386. [Google Scholar] [CrossRef]
- Greiner, B.A.; Leduc, C.; O’Brien, C.; Cresswell-Smith, J.; Rugulies, R.; Wahlbeck, K.; Abdulla, K.; Amann, B.L.; Pashoja, A.C.; Coppens, E.; et al. The effectiveness of organisational-level workplace mental health interventions on mental health and wellbeing in construction workers: A systematic review and recommended research agenda. PLoS ONE 2022, 17, e0277114. [Google Scholar] [CrossRef]
- Miguel, C.; Amarnath, A.; Akhtar, A.; Malik, A.; Baranyi, G.; Barbui, C.; Karyotaki, E.; Cuijpers, P. Universal, selective and indicated interventions for supporting mental health at the workplace: An umbrella review of meta-analyses. Occup. Environ. Med. 2023, 80, 225–236. [Google Scholar] [CrossRef]
- Gray, P.; Senabe, S.; Naicker, N.; Kgalamono, S.; Yassi, A.; Spiegel, J.M. Workplace-Based Organizational Interventions Promoting Mental Health and Happiness among Healthcare Workers: A Realist Review. Int. J. Environ. Res. Public Health 2019, 16, 4396. [Google Scholar] [CrossRef]
- Cohen, C.; Pignata, S.; Bezak, E.; Tie, M.; Childs, J. Workplace interventions to improve well-being and reduce burnout for nurses, physicians and allied healthcare professionals: A systematic review. BMJ Open 2023, 13, e071203. [Google Scholar] [CrossRef]
- Axen, I.; Follin, G. Medical yoga in the workplace setting-perceived stress and work ability-a feasibility study. Complement. Ther. Med. 2017, 30, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Della Valle, E.; Palermi, S.; Aloe, I.; Marcantonio, R.; Spera, R.; Montagnani, S.; Sirico, F. Effectiveness of Workplace Yoga Interventions to Reduce Perceived Stress in Employees: A Systematic Review and Meta-Analysis. J. Funct. Morphol. Kinesiol. 2020, 5, 33. [Google Scholar] [CrossRef]
- Linge, A.D.; Bjorkly, S.K.; Jensen, C.; Hasle, B. Bandura’s Self-Efficacy Model Used to Explore Participants’ Experiences of Health, Lifestyle, and Work After Attending a Vocational Rehabilitation Program with Lifestyle Intervention—A Focus Group Study. J. Multidiscip. Healthc. 2021, 14, 3533–3548. [Google Scholar] [CrossRef] [PubMed]
- Faghri, P.D.; Simon, J.; Huedo-Medina, T.; Gorin, A. Perceived Self-Efficacy and Financial Incentives: Factors Affecting Health Behaviors and Weight Loss in a Workplace Weight Loss Intervention. J. Occup. Environ. Med. 2017, 59, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Odeen, M.; Magnussen, L.H.; Maeland, S.; Larun, L.; Eriksen, H.R.; Tveito, T.H. Systematic review of active workplace interventions to reduce sickness absence. Occup. Med. 2013, 63, 7–16. [Google Scholar] [CrossRef]
- van Oostrom, S.H.; Driessen, M.T.; de Vet, H.C.; Franche, R.L.; Schonstein, E.; Loisel, P.; van Mechelen, W.; Anema, J.R. Workplace interventions for preventing work disability. Cochrane Database Syst. Rev. 2009, 15, CD006955. [Google Scholar] [CrossRef]
- Tveito, T.H.; Hysing, M.; Eriksen, H.R. Low back pain interventions at the workplace: A systematic literature review. Occup. Med. 2004, 54, 3–13. [Google Scholar] [CrossRef]
- Pak, K.; Kooij, T.A.M.; De Lange, A.H.; Van den Heuvel, S.; Van Veldhoven, M. Successful ageing at work: The role of job characteristics in growth trajectories of work ability and motivation to work amongst older workers. Acta Psychol. 2023, 239, 104012. [Google Scholar] [CrossRef]
- Murray, K.; Godbole, S.; Natarajan, L.; Full, K.; Hipp, J.A.; Glanz, K.; Mitchell, J.; Laden, F.; James, P.; Quante, M.; et al. The relations between sleep, time of physical activity, and time outdoors among adult women. PLoS ONE 2017, 12, e0182013. [Google Scholar] [CrossRef]
- Wilckens, K.A.; Erickson, K.I.; Wheeler, M.E. Physical Activity and Cognition: A Mediating Role of Efficient Sleep. Behav. Sleep Med. 2018, 16, 569–586. [Google Scholar] [CrossRef]
- Christie, A.D.; Seery, E.; Kent, J.A. Physical activity, sleep quality, and self-reported fatigue across the adult lifespan. Exp. Gerontol. 2016, 77, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Hartescu, I.; Morgan, K.; Stevinson, C.D. Increased physical activity improves sleep and mood outcomes in inactive people with insomnia: A randomized controlled trial. J. Sleep Res. 2015, 24, 526–534. [Google Scholar] [CrossRef]
- Zhao, H.; Lu, C.; Yi, C. Physical Activity and Sleep Quality Association in Different Populations: A Meta-Analysis. Int. J. Environ. Res. Public Health 2023, 20, 1864. [Google Scholar] [CrossRef] [PubMed]
- Sutanto, C.N.; Loh, W.W.; Toh, D.W.K.; Lee, D.P.S.; Kim, J.E. Association Between Dietary Protein Intake and Sleep Quality in Middle-Aged and Older Adults in Singapore. Front. Nutr. 2022, 9, 832341. [Google Scholar] [CrossRef] [PubMed]
- Trommelen, J.; van Loon, L.J. Pre-Sleep Protein Ingestion to Improve the Skeletal Muscle Adaptive Response to Exercise Training. Nutrients 2016, 8, 763. [Google Scholar] [CrossRef]
- Haghighatdoost, F.; Karimi, G.; Esmaillzadeh, A.; Azadbakht, L. Sleep deprivation is associated with lower diet quality indices and higher rate of general and central obesity among young female students in Iran. Nutrition 2012, 28, 1146–1150. [Google Scholar] [CrossRef]
- Nathan, N.; Murawski, B.; Hope, K.; Young, S.; Sutherland, R.; Hodder, R.; Booth, D.; Toomey, E.; Yoong, S.L.; Reilly, K.; et al. The Efficacy of Workplace Interventions on Improving the Dietary, Physical Activity and Sleep Behaviours of School and Childcare Staff: A Systematic Review. Int. J. Environ. Res. Public Health 2020, 17, 4998. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).