Abstract
Background: Our research hypothesis was that most French indicators of quality of care have been validated by experts who are not clinicians and might not always be meaningful for clinicians. Our objective was to define a core set of measurable indicators of care quality during delivery and the immediate postpartum period relevant to clinical practice. Methods: A steering committee comprising nine specialists in obstetrics and/or public health conducted a literature review to develop potential indicators. A panel of obstetrician-gynecologists and midwives working in a delivery unit rated each indicator for appropriateness in a two-round Rand-modified Delphi procedure and a physical meeting. The consensus among the panelists was assessed. Results: In the first round, 145 panelists (110 obstetrician-gynecologists and 35 midwives) assessed 77 indicators and 3 definitions: 6 related to labor onset, 20 to delivery, 3 to pain management, 23 to neonatal morbidity/mortality, and 28 to maternal morbidity. In the second round, 132 panelists (98 obstetrician-gynecologists and 34 midwives) assessed 42 indicators and 1 definition. The final set comprised 50 indicators and 2 definitions. Conclusions: This Delphi procedure selected 50 indicators that reflect the quality of perinatal care. These indicators should be recorded in each French maternity ward’s birth register for each delivery.
1. Introduction
Reducing maternal and perinatal severe morbidity and mortality around the world remains a major public health concern [1]. Accordingly, different indicators assessing the quality of perinatal care have been proposed [2,3,4,5,6,7,8,9,10,11,12], although their promulgation and distribution have not resulted in a notable reduction in perinatal and neonatal outcomes around the world [13,14,15,16], or even only in France [17,18,19,20]. These findings, especially given that many are regularly reported to perinatal professionals [19,20,21,22], suggest that these indicators remain less than optimal in France. There are always, of course, variations between countries, between maternity departments within a country, and even between professionals within the same maternity unit [22,23]. One explanation may be that most current maternity indicators derived from routinely collected hospital data are not always easy to understand. This is due mainly to the lack of clinical information contained in these medical-administrative databases and the absence of evidence for the best practice in specific obstetric situations [18,24]. Moreover, indicators derived from routine hospital data can lack validity. For example, “good” maternity wards are considered to be those with a global cesarean rate near the mean for the country. However, what is a good cesarean rate? We do not know; maternity units with low cesarean rates may have high neonatal morbidity and/or mortality, perhaps because their cesarean rates are too low [25]. Several French obstetric guidelines do not provide indicators to assess their impact on the practice or appropriate outcome indicators [26]. Worse, some French national guidelines do not clearly identify the indicators they are intended to affect. For example, the French guidelines about planned cesarean deliveries at term do not define a planned cesarean [27].
Our research hypothesis was that as most of the indicators currently used were developed by working groups of experts in quality, public health, or epidemiology, they might not always make sense to clinicians working in delivery rooms. However, it is important to have quality indicators that are well accepted by professionals working in maternity units and are easy to collect so that they can be recorded continuously and enable valid comparisons over time. The National College of French Gynecologists and Obstetricians (CNGOF) and the National College of Midwives (CNSF) have therefore decided to study the opinions of French clinicians practicing in the birth sector about the relevance of quality indicators in maternity units.
Our objective was to use a modified Delphi study to define a core set of quality indicators, measurable and relevant to clinical practice, during delivery and the immediate postpartum period. This technique is widely used to select quality indicators in health care [28].
2. Materials and Methods
2.1. Design and Setting
A modified RAND/University of California at Los Angeles (UCLA) appropriateness method Delphi study took place in four stages (Figure 1) to develop a set of indicators of the quality of care during delivery and the immediate postpartum period for deliveries or births ≥22 weeks (or birth weight ≥ 500 g when the date of conception was unknown) [29]. The first stage was the constitution of a French multidisciplinary steering committee, and the second was the extensive review of the literature it was assigned to perform to identify quality indicators in obstetrics. The third phase consisted of two Delphi consensus rounds in which potential indicators were rated by a panel of informed persons. Delphi is a formalized technique for determining consensus from the collective wisdom of the panelists about, in this context, appropriate indicators. The benefits of this approach are anonymity, iteration (allowing participants to change their opinions), not requiring the physical presence of the panelist, controlled feedback in which panelists can have the panel’s previous responses, and the derivation of summary measures of agreement [30]. In the fourth and last phase, the steering committee and an external board approved the final set of quality indicators.
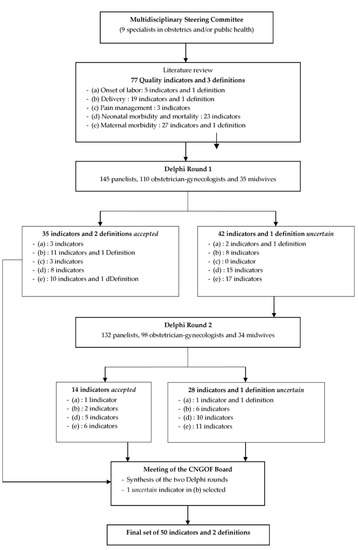
Figure 1.
The modified Delphi process for the selection of indicators of quality of care during delivery and the immediate postpartum period.
We modified the RAND/UCLA appropriateness method by beginning the process with a set of selected quality indicators. The French panelists did not meet physically, and indicators could be discarded between the two rounds.
2.2. Literature Review
The French steering committee established comprised nine specialists in obstetrics and/or public health, recognized for their expertise in quality indicators and/or the Delphi method. This committee reviewed the literature from January 2003 through December 2013. A PubMed search was conducted for the keywords “quality indicators” and “obstetrics”. All reports on perinatal quality indicators issued by learned societies in France or abroad were also collected. A database of 318 references was identified.
During face-to-face and telephone meetings, completed by email exchanges, the steering committee used the literature search results to establish an exhaustive list of potential quality indicators. The indicators to be chosen were to be collected and calculated from data in patient files, collected immediately intrapartum, during delivery, and early postpartum (in the delivery or operating unit, according to the type of delivery).
The Delphi questionnaire was then drafted, with special attention paid to the clarity of each indicator. The web-based questionnaire and instructions were tested before each round for face validity.
2.3. Modified Delphi Process
2.3.1. Participants
The list of quality of care indicators was presented for rating in a two-round Delphi study. Obstetrician-gynecologists who were members of CNGOF and midwives belonging to the CNSF—all practicing in French delivery rooms—were invited via each organization’s email list to compose the panel of the Delphi study. They received a cover letter by email explaining the study and inviting them to participate by completing the electronic questionnaire accessible with the URL in the email. No financial incentive was proposed. The panelists gave their consent to participate in the Delphi survey when they replied to the first round. Under French law, this study was exempt from approval by an ethics committee.
2.3.2. Data Collection
Round 1 data were collected from 11 December 2014 to 28 January 2015, and round 2 data from 25 March to 17 May 2015. The participants were asked to answer within four weeks for each round. No reminder was sent out during the first round, whereas two reminder emails were sent during the second. Only participants who completed the first-round electronic questionnaire received the URL to participate in the second round.
During both rounds, the panelists were invited to rate individually and independently each quality indicator for its clinical relevance on a 9-point Likert scale from 1 (totally inappropriate/irrelevant) to 9 (totally appropriate/relevant), with 5 for no preference, indecision, etc. They were also invited to comment on each indicator.
For the second round, the panelists received descriptive statistics of the distribution of the panel’s scores for each indicator rated in the first round. Each panelist was invited to re-score on the same 9-point Likert scale the indicators that had not been accepted in the first round, taking into account the previous answers of the panel.
2.3.3. Data Analysis
After each of the two rounds, the distribution of scores was described by the number and frequencies of each score of the rating scale, the number and frequencies in the lowest tertile (between 1 and 3), the number and frequencies in the upper tertile (between 7 and 9), and the median score.
Judgment of the indicators and consensus followed the RAND/UCLA method [29]. A median score was used to judge an indicator by measuring the central tendency of the distribution for each indicator’s rating. An indicator was judged appropriate if the panelists’ median score ranged from 7 through 9. An indicator with a median score of 1–3 was judged inappropriate. To evaluate the consensus between the panelists (that is, the panelists’ agreement with one another), a continuous statistical measure of dispersion among the individual scores was used: the Disagreement Index. We adapted the Rand Working Group definition and defined the Disagreement Index as the 10–90% interpercentile range (IPR) divided by the interpercentile range adjusted for symmetry (IPRAS) [29], which applies to any size panel. In the RAND method, a Disagreement Index lower than 1 indicates consensus or agreement between panelists (low dispersion of scores, with the IPRAS larger than the IPR), and a Disagreement Index higher than 1 indicates a lack of consensus or disagreement (high score dispersion with the IPRAS smaller than the IPR).
If an indicator was judged appropriate with agreement among the panelists, then it was considered accepted. Indicators consensually judged inappropriate were rejected. An indicator with a median score ranging between 3.5 and 6.5 or scored with disagreement between panelists was considered uncertain. Based on the findings of round 1, uncertain indicators were resubmitted for further evaluation and discussion in the second rating round.
Statistical analyses were performed with SAS software (version 9.3, SAS Institute, Cary, NC, 2002–2012).
2.4. External Validity
The steering committee received the detailed results after each round and discussed them. In November 2015, after the second round, the Delphi results were sent for validation to the CNGOF and CNSF board members. The final set of quality indicators was established.
3. Results
3.1. Modified Delphi Survey Questionnaire
The steering committee proposed a total of 77 indicators and 3 definitions for evaluation in the two Delphi rounds, categorized as follows: “onset of labor” (5 indicators and 1 definition), “delivery” (19 indicators and 1 definition), “pain management” (3 indicators), “neonatal morbidity and mortality” (23 indicators), and “maternal morbidity” (27 indicators and 1 definition) (Figure 1). Indicator results were expressed as percentages. Numerators (number of the exposed population by the relevant event) and denominators (target population) were detailed for each quality indicator.
3.2. Description of the Modified Delphi Survey Panel
The first round was completed by 145 panelists, 110 obstetrician-gynecologists (75.9%) practicing for 20.5 ± 10.1 years, and 35 midwives (24.1%), practicing for 21.7 ± 11.6 years (Table 1). Men accounted for 54.5%, and the mean age of the entire panel was 49.7 ± 10.6 years. Overall, 34.5% practiced in academic hospitals, 41.4% in general public hospitals, and 24.1% in private hospitals; 20.7% worked in level I maternity units, 40.7% in level II facilities, and 38.6% in level III hospitals, with 50.3% practicing in a maternity unit with an average of more than 2500 deliveries annually.

Table 1.
Panelists’ characteristics.
Participating in the second round were 132 panelists who had responded to the first round—98 obstetrician-gynecologists and 34 midwives (89.1% and 97.1%, respectively) (Table 1). They had been in practice for a mean of 21.5 ± 10.6 years, and 53.0% practiced in a maternity unit with a mean of more than 2500 annual deliveries.
3.3. Modified Delphi Survey Analysis
Figure 1 presents the modified Delphi process used to select the quality indicators.
After the first round, 35 indicators and 2 definitions were accepted: 3 indicators in the domain “onset of labor”, 11 indicators and 1 definition in “delivery”, all the indicators in “pain management” (i.e., 3), 8 indicators in “neonatal morbidity and mortality”, and 10 indicators and 1 definition in “maternal morbidity”. No indicator was rejected. Forty-two indicators and 1 definition were considered uncertain and proposed for further evaluation in the second round (Table 2).

Table 2.
Rating scores of the proposed quality indicators and definitions during the two-round Delphi survey.
After the second round, 14 indicators were accepted: 1 indicator in the “onset of labor” domain, 2 in “delivery”, 5 in “neonatal morbidity and mortality”, and 6 in “maternal morbidity”. No indicator was rejected, while 28 indicators and 1 definition remained uncertain (Table 2). The indicators (including their definitions) that remained uncertain after the two rounds are listed in Supplementary Table S1.
3.4. External Validity
A synthesis of the two rounds of the Delphi process was presented and discussed in a face-to-face meeting of the CNGOF board members. Among the indicators that had not been accepted, one was judged highly important based on the international literature review and was included in the final set of quality indicators: the Robson classification [31], advocated by the International Federation of Gynecology and Obstetrics (FIGO) [32].
Finally, a set of 50 quality indicators and 2 definitions was established: 4 indicators in the domain of “onset of labor”, 15 indicators and 1 definition in “delivery”, 3 indicators in “pain management”, 13 indicators in “neonatal morbidity and mortality”, and 16 indicators and 1 definition in “maternal morbidity”. Table 3 presents the detailed definition (numerator and denominator) for each final quality indicator.

Table 3.
Final set of quality indicators and definitions.
4. Discussion
Using a modified RAND-UCLA Delphi method, we developed a clinically relevant set of 50 quality indicators and 2 definitions in obstetrics that covered care during delivery and in the immediate postpartum period. They were based simultaneously on medical literature and on the judgments of a large panel. One indicator—the Robson classification for cesareans—was selected after the Delphi process by the CNGOF in view of international guidelines and to promote international comparability by the FIGO [32].
The number of panelists in the two-round Delphi survey is one of the strengths of our study. To the best of our knowledge, there are currently no clear guidelines for the sample size of a Delphi panel [33]. It has been suggested that a minimum number of panelists should range from 7 to 15 [29,34]. The large panel of obstetrician-gynecologists and midwives who participated in this Delphi survey should certainly have increased the stability of our results and the reliability of the final set of quality indicators [35,36]. Second, only a few panelists did not respond to the second round of Delphi, and the follow-up response rate exceeded the 70% suggested by Sumsion [37]. Another strength of our study was the assessment of external validity. Indeed, the final set of quality indicators was submitted for an external independent review for approval by two French professional societies specialized in childbirth: CNGOF and CNSF—whose members are the obstetricians and midwives who complete the delivery register in the maternity units for each delivery. Similarly, the only eligible panelists were obstetricians and midwives working in a delivery unit. Our final objective was to select indicators that can be routinely filled in and monitored via the computerization of the delivery registers available in all French maternity units to guide the development of quality improvement programs at the local and national levels.
This study has some limitations. The indicators were developed in a French setting, so the results may not be generalizable to other countries as practices vary internationally. Nonetheless, the recommendations were based on references extracted from an international literature review, which should provide good external validity. The self-selection of the panelists may have influenced the results. Those who participated in the Delphi rounds may not have had the same characteristics and/or may not have rated the indicators the same as those who did not participate. To facilitate a high level of continued participation, the number of rounds for the Delphi procedure was limited to 2. This left some indicators or definitions neither accepted nor rejected. This is the case for the definition of a planned cesarean, which remains a problem in France. We have guidelines about planned cesareans and a national indicator (rate of cesareans [%]) but still no clear definition of a planned cesarean [27,38].
Globally, the panelists did not select any of the risk-adjusted indicators or adverse outcome index models suggested by various authors [12,39,40,41]. However, they did select the main’s cesarean indicator “nulliparous term singleton vertex cesarean birth” (NTSV CB) [42]. This underlines the incomprehensibility of risk-adjusted indicators for clinicians without substantial training and experience in statistics.
During the first Delphi round, the panelists accepted the international definitions about perineal lacerations, which will enable comparisons of French studies with non-French studies and quality indicators on this topic. To our surprise, the definition of low risk at the end of pregnancy was accepted during the first Delphi round, although there was no formal consensus in France on this subject at that time.
We expected a lower number of quality indicators to be selected after the second round because mandatory care quality and safety indicators in French hospitals are poorly accepted by caregivers [43]. The results of these indicators contribute to the pay-for-performance mechanism of French health establishments [44]. However, the bulk of hospital revenues is still allocated according to medical activities. This medical resistance to guideline implementation, assessment by indicators, and audits point to the physicians’ feeling of loss of autonomy through the demands of standardization of medical practices [45,46,47]. We were therefore surprised by the high number of perinatal care providers participating in the Delphi procedure.
For many indicators, we do not know what the right rate is [48]. For example, the planned cesarean rate is a good indicator of the quality of care in a maternity ward. Nonetheless, it is difficult to tell physicians what the correct rate of this intervention is. The mean rate is usually recommended, on the assumption that the highest and lowest rates are inappropriate, but this is not necessarily true. In a study, we assessed the incidence of postpartum hemorrhage (PPH) and second-line procedures in maternity units according to the quality of their PPH protocol [49]. We find that maternity units with higher scores identified PPH better and used fewer curative second-line procedures. So the ideal rate is that associated with the lowest level of maternal and/or neonatal morbidity or mortality, but it is difficult to ascertain in practice.
The number of indicators selected at the end of our study is reduced compared to some reviews of the literature [50,51]. This can be explained by the fact that we have focused on indicators that can be recorded in the daily routine in the birth unit and so we do not have structure or health-determinant quality indicators [50].
5. Conclusions
In conclusion, our study has identified a list of 50 quality indicators and 2 definitions suitable for routine monitoring in maternity units. However, the feasibility of these quality indicators will need to be assessed by research studies conducted under the conditions of everyday practices. To improve the calculation and monitoring of these indicators, birth registers, still widely kept on paper in French maternity wards, should be required to be computerized. Further research should study the impact of monitoring these indicators on improving maternal and neonatal morbidity and mortality at a national level.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/healthcare11060848/s1, Supplementary Table S1: Quality indicators (numerator and denominator) and definitions that were not accepted after both rounds of the Delphi survey.
Author Contributions
Conceptualization, F.V.; methodology, C.G.-A. and L.G.; validation, C.G.-A., L.G. and F.V.; formal analysis, C.G.-A.; investigation, F.V.; data curation, C.G.-A. and O.R.; writing—original draft preparation, C.G.-A. and F.V.; writing—review and editing, C.G.-A., L.G. and F.V.; visualization, C.G.-A. and F.V.; supervision, O.R. and F.V.; project administration, F.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study did not require ethics review and approval given that no personal medical data regarding panelists were collected. Under French legislation, ethics approval is not required for this type of study. All information and data collected were treated anonymously.
Informed Consent Statement
All panelists gave their informed consent to participate in the study.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
The authors would like to thank all obstetrician-gynecologists and midwives who participated in the Delphi process for their contribution to the study and their time in responding to the questionnaires. We acknowledge the CNSF and the CNGOF for their support and the Audipog association for its free logistic support. We acknowledge Catherine Crenn-Hébert (Audipog, APHP, Hôpital Louis-Mourier), Jeanne Fresson (CHU de Nancy), Pascal Thibon (CHU de Caen), Sandra David-Tchouda (CHU de Grenoble), Marie-José D’Alché-Gautier (CHU de Caen), and Rym Boulkedid (APHP, Hôpital Robert Debré) for their participation in the steering committee of the study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- World Health Organization—Millennium Development Goals (MDGs). Available online: https://www.who.int/news-room/fact-sheets/detail/millennium-development-goals-(mdgs) (accessed on 9 February 2023).
- Talungchit, P.; Liabsuetrakul, T.; Lindmarck, G. Development and assessment of indicators for quality of care in severe preeclampsia/eclampsia and postpartum hemorrhage. J. Healthc. Qual. 2013, 35, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, S.; Mainz, J.; Bartels, P. Selection of indicators for continuous monitoring of patient safety: Recommendations of the project ’safety improvement for patients in Europe’. Int. J. Qual. Health Care 2009, 21, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Bonfill, X.; Roqué, M.; Aller, M.B.; Osorio, D.; Foradada, C.; Vives, A.; Rigau, D. Development of quality of care indicators from systematic reviews: The case of hospital delivery. Implement. Sci. 2013, 8, 42. [Google Scholar] [CrossRef] [PubMed]
- Kesmodel, U.S.; Jolving, L.R. Measuring and improving quality in obstetrics—The implementation of national indicators in Denmark. Acta Obstet. Gynecol. Scand. 2011, 90, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Korst, L.M.; Gregory, K.D.; Lu, M.C.; Reyes, C.; Hobel, C.J.; Chavez, G.F. A framework for the development of maternal quality of care indicators. Matern. Child Health J. 2005, 9, 317–341. [Google Scholar] [CrossRef]
- Sibanda, T.; Fox, R.; Draycott, T.J.; Mahmood, T.; Richmond, D.; Simms, R.A. Intrapartum care quality indicators: A systematic approach for achieving consensus. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 166, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, A.; Gissler, M.; Bolumar, F.; Rasmussen, S. The availability of perinatal health indicators in Europe. Eur. J. Obstet. Gynecol. Reprod. Biol. 2003, 111, S15–S32. [Google Scholar] [CrossRef]
- Wildman, K.; Blondel, B.; Nijhuis, J.; Defoort, P.; Bakoula, C. European indicators of health care during pregnancy, delivery and the postpartum period. Eur. J. Obstet. Gynecol. Reprod. Biol. 2003, 111, S53–S65. [Google Scholar] [CrossRef]
- Melman, S.; Schoorel, E.C.N.; de Boer, K.; Burggraaf, H.; Derks, J.B.; van Dijk, D.; van Dillen, J.; Dirksen, C.D.; Duvekot, J.J.; Franx, A.; et al. Development and measurement of guidelines-based quality indicators of caesarean section care in the Netherlands: A Rand-modified Delphi procedure and retrospective chart review. PLoS ONE 2016, 11, e0145771. [Google Scholar] [CrossRef]
- Zeitlin, J.; Wildman, K.; Bréart, G.; Alexander, S.; Barros, H.; Blondel, B.; Buitendijk, S.; Gissler, M.; Macfarlane, A. Selecting an indicator set for monitoring and evaluating perinatal health in Europe: Criteria, methods and results from the PERISTAT project. Eur. J. Obstet. Gynecol. Reprod. Biol. 2003, 111, S5–S14. [Google Scholar] [CrossRef]
- Bailit, J.L. Measuring the quality of inpatient care. Obstet. Gynecol. Surv. 2007, 62, 207–213. [Google Scholar] [CrossRef]
- Alkema, L.; Chou, D.; Hogan, D.; Zhang, S.; Moller, A.B.; Gemmill, A.; Fat, D.M.; Boerma, T.; Temmerman, M.; Mathers, C.; et al. Global, regional, and national levels and trends in maternal mortality between 1990 and 2015, with scenario-based projections to 2030: A systematic analysis by the UN Maternal Mortality Estimation Inter-Agency Group. Lancet 2016, 38, 462–474. [Google Scholar] [CrossRef] [PubMed]
- Say, L.; Chou, D.; Gemmill, A.; Tunçalp, Ö.; Moller, A.B.; Daniels, J.; Gülmezoglu, A.M.; Temmerman, M.; Alkema, L. Global causes of maternal death: A WHO systematic analysis. Lancet Glob. Health 2014, 2, e323–e333. [Google Scholar] [CrossRef] [PubMed]
- Haller, G.; Camparini-Righini, N.; Kern, C.; Pfister, R.E.; Morales, M.; Berner, M.; Clergue, F.; Irion, O. Patient safety indicators for obstetrics: A Delphi based study. J. Gynecol. Obstet. Biol. Reprod. 2010, 39, 371–378. [Google Scholar] [CrossRef]
- Bunch, K.J.; Allin, B.; Jolly, M.; Hardie, T.; Knight, M. Developing a set of consensus indicators to support maternity service quality improvement: Using Core Outcome Set methodology including a Delphi process. BJOG 2018, 125, 1612–1618. [Google Scholar] [CrossRef] [PubMed]
- Boulkedid, R.; Sibony, O.; Goffinet, F.; Fauconnier, A.; Branger, B.; Alberti, C. Quality indicators for continuous monitoring to improve maternal and infant health in maternity departments: A modified Delphi survey of an international multidisciplinary panel. PLoS ONE 2013, 8, e60663. [Google Scholar] [CrossRef] [PubMed]
- Sauvegrain, P.; Chantry, A.A.; Chiesa-Dubruille, C.; Keita, H.; Goffinet, F.; Deneux-Tharaux, C. Monitoring quality of obstetric care from hospital discharge databases: A Delphi survey to propose a new set of indicators based on maternal health outcomes. PLoS ONE 2019, 14, e0211955. [Google Scholar] [CrossRef]
- Deneux-Tharaux, C.; Morau, E.; Dreyfus, M. Maternal mortality in France 2013–2015: An evolving profile. Gynecol. Obstet. Fertil. Senol. 2021, 49, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Zeitlin, J.; Alexander, S.; Barros, H.; Blondel, B.; Delnord, M.; Durox, M.; Gissler, M.; Hindori-Mohangoo, A.D.; Hocquette, A.; Szamotulska, K.; et al. Perinatal health monitoring through a European lens: Eight lessons from the Euro-Peristat report on 2015 births. BJOG 2019, 126, 1518–1522. [Google Scholar] [CrossRef] [PubMed]
- Blondel, B.; Durox, M.; Zeitlin, J. How perinatal health in France compared with other European countries in 2015: Some progress but also some concerns about newborn health. Arch. Pediatr. 2019, 26, 249–251. [Google Scholar] [CrossRef]
- European Perinatal Health Report—Core Indicators of the Health and Care of Pregnant Women and Babies in Europe from 2015 to 2019. Available online: https://www.europeristat.com/images/Euro-Peristat_Fact_sheets_2022_for_upload.pdf (accessed on 9 February 2023).
- Corallo, A.N.; Croxford, R.; Goodman, D.C.; Bryan, E.L.; Srivastava, D.; Stukel, T.A. A systematic review of medical practice variation in OECD countries. Health Policy 2014, 114, 5–14. [Google Scholar] [CrossRef]
- The Royal College of Obstetricians and Gynaecologists. Patterns of Maternity Care in English NHS Hospitals, 2011–2012; RCOG Press: London, UK, 2013. [Google Scholar]
- Vendittelli, F.; Tassié, M.C.; Gerbaud, L.; Lémery, D. Appropriateness of elective casarean deliveries in a perinatal network: A cross-sectional study. BMC Pregnancy Childbirth 2014, 14, 135. [Google Scholar] [CrossRef] [PubMed]
- Vendittelli, F.; Rivière, O.; Crenn-Hébert, C.; Giraud-Roufast, A.; Audipog Sentinel Network. Do perinatal guidelines have an impact on obstetric practices? Rev. Epidemiol. Sante Publique 2012, 60, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Haute Autorité de Santé—Indications de la Césarienne Programmée à Terme. Méthode Recommandations pour la Pratique Clinique. Available online: https://www.has-sante.fr/upload/docs/application/pdf/2012-03/indications_cesarienne_programme_-_argumentaire.pdf (accessed on 9 February 2023).
- Boulkedid, R.; Abdoul, H.; Loustau, M.; Sibony, O.; Alberti, C. Using and Reporting the Delphi Method for Selecting Healthcare Quality Indicators: A Systematic Review. PLoS ONE 2011, 6, e20476. [Google Scholar] [CrossRef] [PubMed]
- Fitch, K.; Bernstein, S.J.; Aguilar, M.D.; Burnand, B.; LaCalle, J.R.; Lazaro, P.; van het Loo, M.; McDonnell, J.; Vader, J.; Kahan, J.P. The RAND/UCLA Appropriateness Method User’s Manual; RAND Corporation: Santa Monica, CA, USA, 2001. [Google Scholar]
- Jones, J.; Hunter, D. Consensus methods for medical and health services research. BMJ 1995, 311, 376–380. [Google Scholar] [CrossRef] [PubMed]
- Robson, M.S. Can we reduce the cesarean section rate? Best Pract. Res. Clin. Obstet. Gynaecol. 2001, 15, 179–194. [Google Scholar] [CrossRef] [PubMed]
- FIGO Working Group on Challenges in Care of Mothers and Infants during Labour and Delivery. Best practice advice on the 10-Group Classification System for cesarean deliveries. Int. J. Gynecol. Obstet. 2016, 135, 232–233. [Google Scholar] [CrossRef]
- Jorm, A.F. Using the Delphi expert consensus method in mental health research. Aust. N.Z.J. Psychiatry 2015, 49, 887–897. [Google Scholar] [CrossRef] [PubMed]
- Okoli, C.; Pawlowski, S.D. The Delphi method as a research tool: An example, design considerations and applications. Inf. Manag. 2004, 42, 15–29. [Google Scholar] [CrossRef]
- Dalkey, N.C. The Delphi Method: An Experimental Study of Group Opinion; RAND Corporation: Santa Monica, CA, USA, 1969. [Google Scholar]
- Murphy, M.K.; Black, N.A.; Lamping, D.L.; McKee, C.M.; Sanderson, C.F.; Askham, J.; Marteau, T. Consensus development methods, and their use in clinical guideline development. Health Technol. Assess. 1998, 2, 1–88. [Google Scholar] [CrossRef] [PubMed]
- Sumsion, T. The Delphi technique: An adaptive research tool. Br. J. Occup. Ther. 1998, 61, 153–156. [Google Scholar] [CrossRef]
- République Française, Santé Publique France, Inserm—Enquête Nationale Périnatale. Rapport 2021. Les naissances, le suivi à deux mois et les établissements. Situation et évolution depuis 2016. Available online: https://www.santepubliquefrance.fr/maladies-et-traumatismes/maladies-de-la-mere-et-de-l-enfant/surdite-permanente-neonatale/documents/enquetes-etudes/enquete-nationale-perinatale.-rapport-2021.-les-naissances-le-suivi-a-deux-mois-et-les-etablissements (accessed on 9 February 2023).
- David, S.; Mamelle, N.; Riviere, O. Estimation of an expected caesarean section rate, taking into account the case mix of a maternity hospital. Analysis from the AUDIPOG Sentinelle Network (France). Obstetricians of AUDIPOG. Association of Users of Computerised Files in Perinatalogy, Obstetrics and Gynaecology. BJOG 2001, 108, 919–926. [Google Scholar] [CrossRef] [PubMed]
- Mann, S.; Pratt, S.; Gluck, P.; Nielsen, P.; Risser, D.; Greenberg, P.; Marcus, R.; Goldman, M.; Shapiro, D.; Pearlman, M.; et al. Assessing quality obstetrical care: Development of standardized measures. Jt. Comm. J. Qual. Patient Saf. 2006, 32, 497–505. [Google Scholar] [CrossRef]
- Foglia, L.M.; Nielsen, P.E.; Hemann, E.A.; Walker, S.; Pates, J.A.; Napolitano, P.G.; Deering, S. Accuracy of the Adverse Outcome Index: An Obstetrical Quality Measure. Jt. Comm. J. Qual. Patient Saf. 2015, 41, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Main, E.K.; Moore, D.; Farrell, B.; Schimmel, L.D.; Altman, R.J.; Abrahams, C.; Campbell Bliss, M.; Polivy, L.; Sterling, J. Is there a useful cesarean birth measure? Assessment of the nulliparous term singleton vertex cesarean birth rate as a tool for obstetric quality improvement. Am. J. Obstet. Gynecol. 2006, 194, 1644–1651. [Google Scholar] [CrossRef] [PubMed]
- Bertillot, H. How quality indicators take hold in French hospital: Mechanisms of a soft institutionalization. Sociologies 2020, 1–15. (In French) [Google Scholar] [CrossRef]
- Ministère des Solidarités et de la Santé—Décret n° 2021-1613 du 9 décembre 2021 portant modification De dispositions réglementaires du code de la sécurité sociale relatives à l’amélioration de la qualité et de la sécurité des soins. Available online: https://www.legifrance.gouv.fr/download/file/k9J4QampA_S_KWuxcrXD3mPztg_RyZO4BL7nbsvBufc=/JOE_TEXTE (accessed on 9 February 2023).
- Abelhauser, A.; Gori, R.; Sauret, M.J. La Folie Évaluation. Les Nouvelles Fabriques De La Servitude; Edition Fayard/Mille et Une Nuits: Paris, France, 2011. [Google Scholar]
- Todd, R. The End of Average: How We Succeed in A World That Values Sameness; HarperCollins: Toronto, ON, Canada, 2016. [Google Scholar]
- Dumesnil, J. Art Médical Et Normalisation Du Soin; Presse Universitaire de France: Paris, France, 2011. [Google Scholar]
- Wennberg, J. Which rate is right? N. Engl. J. Med. 1986, 314, 310–311. [Google Scholar] [CrossRef]
- Vendittelli, F.; Barasinski, C.; Rivière, O.; Da Costa Correia, C.; Crenn-Hébert, C.; Dreyfus, M.; Legrand, A.; Gerbaud, L. Does the Quality of Postpartum Hemorrhage Local Protocols Improve the Identification and Management of Blood Loss after Vaginal Deliveries? A Multicenter Cohort Study. Healthcare 2022, 10, 992. [Google Scholar] [CrossRef] [PubMed]
- Saturno-Hernández, P.J.; Martínez-Nicolás, I.; Moreno-Zegbe, E.; Fernández-Elorriaga, M.; Poblano-Verástegui, O. Indicators for monitoring maternal and neonatal quality care: A systematic review. BMC Pregnancy Childbirth 2019, 19, 25. [Google Scholar] [CrossRef]
- Lazzaretto, E.; Nespoli, A.; Fumagalli, S.; Colciago, E.; Perego, S.; Locatelli, A. Intrapartum care quality indicators: A literature review. Minerva Ginecol. 2018, 70, 346–356. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).