The Effect of Cognitive Training with Neurofeedback on Cognitive Function in Healthy Adults: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Eligibility Criteria
2.3. Search Strategy
2.4. Study Outcome
2.5. Data Extraction
2.6. Risk of Bias Assessment
2.7. Data Synthesis and Statistical Analysis
3. Results
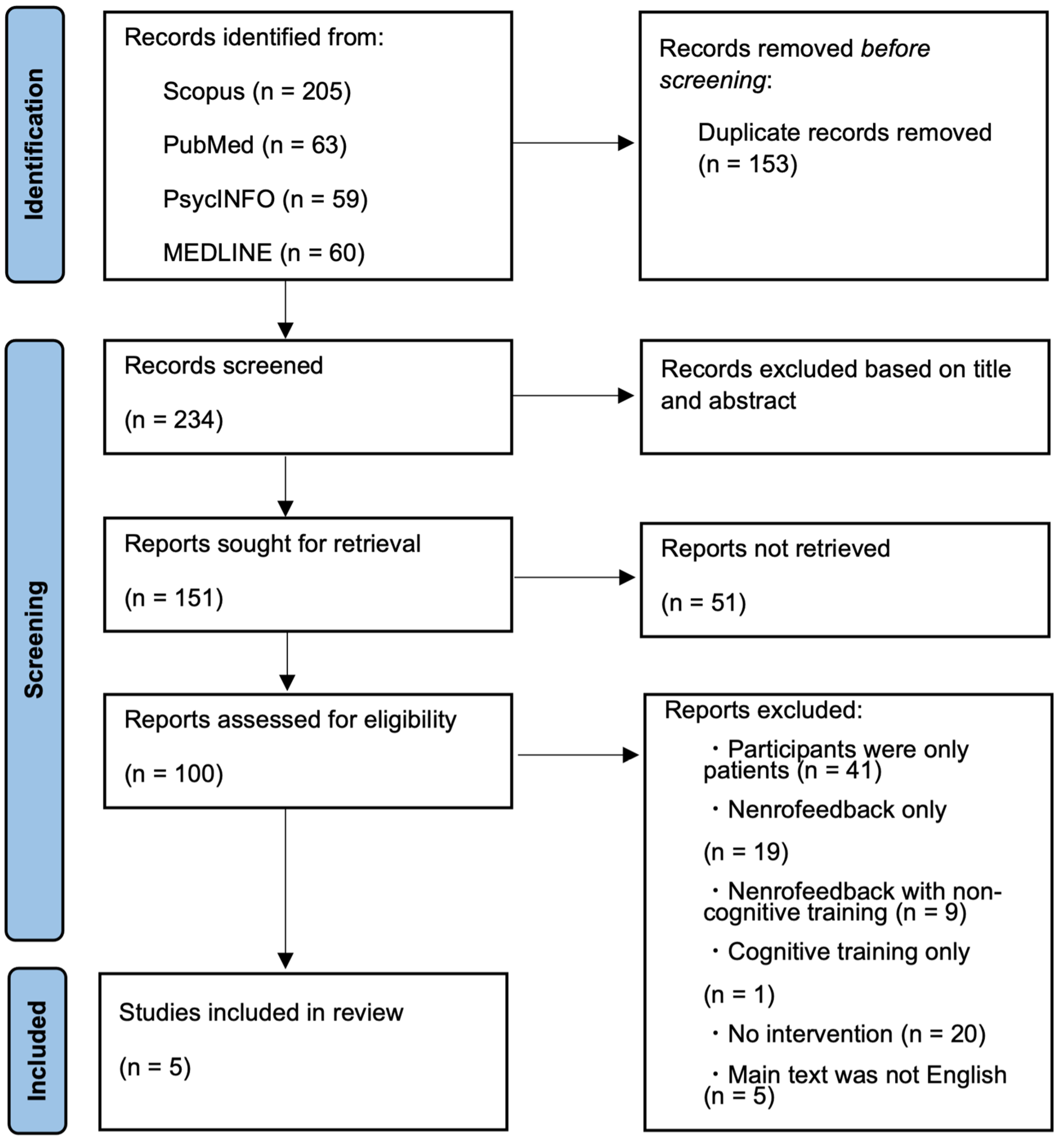
3.1. Literature Search and Study Selection
3.2. Characteristics of Included Studies
3.3. Episodic Memory/Long-Term Memory
3.4. Executive Function
3.5. Working Memory
3.6. Short-Term Memory
3.7. Processing Speed
3.8. Attention
3.9. Visuo-Spatial Performance
3.10. Quality Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Toh, W.X.; Yang, H.; Hartanto, A. Executive Function and Subjective Well-being in Middle and Late Adulthood. J. Gerontol. Ser. B 2020, 75, e69–e77. [Google Scholar] [CrossRef] [PubMed]
- Merten, N.; Pinto, A.A.; Paulsen, A.J.; Chen, Y.; Dillard, L.K.; Fischer, M.E.; Ryff, C.D.; Schubert, C.R.; Cruickshanks, K.J. The Association of Psychological Well-Being with Sensory and Cognitive Function and Neuronal Health in Aging Adults. J. Aging Health 2022, 34, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Ali, T.; Nilsson, C.J.; Weuve, J.; Rajan, K.B.; de Leon, C.F.M. Effects of social network diversity on mortality, cognition and physical function in the elderly: A longitudinal analysis of the Chicago Health and Aging Project (CHAP). J. Epidemiol. Community Health 2018, 72, 990–996. [Google Scholar] [CrossRef] [PubMed]
- Khodabakhsh, S. Factors Affecting Life Satisfaction of Older Adults in Asia: A Systematic Review. J. Happiness Stud. 2022, 23, 1289–1304. [Google Scholar] [CrossRef]
- Kane, M.J.; Brown, L.H.; McVay, J.C.; Silvia, P.J.; Myin-Germeys, I.; Kwapil, T.R. For Whom the Mind Wanders, and When: An Experience-Sampling Study of Working Memory and Executive Control in Daily Life. Psychol. Sci. 2007, 18, 614–621. [Google Scholar] [CrossRef]
- Pe, M.L.; Koval, P.; Kuppens, P. Executive well-being: Updating of positive stimuli in working memory is associated with subjective well-being. Cognition 2013, 126, 335–340. [Google Scholar] [CrossRef]
- Salthouse, T.A. Selective review of cognitive aging. J. Int. Neuropsychol. Soc. 2010, 16, 754–760. [Google Scholar] [CrossRef]
- Sakaki, K.; Nouchi, R.; Matsuzaki, Y.; Saito, T.; Dinet, J.; Kawashima, R. Benefits of VR Physical Exercise on Cognition in Older Adults with and without Mild Cognitive Decline: A Systematic Review of Randomized Controlled Trials. Healthcare 2021, 9, 883. [Google Scholar] [CrossRef]
- Nouchi, R.; Suiko, T.; Kimura, E.; Takenaka, H.; Murakoshi, M.; Uchiyama, A.; Aono, M.; Kawashima, R. Effects of Lutein and Astaxanthin Intake on the Improvement of Cognitive Functions among Healthy Adults: A Systematic Review of Randomized Controlled Trials. Nutrients 2020, 12, 617. [Google Scholar] [CrossRef]
- Nouchi, R.; Kawashima, R. Improving Cognitive Function from Children to Old Age: A Systematic Review of Recent Smart Ageing Intervention Studies. Adv. Neurosci. 2014, 2014, 235479. [Google Scholar] [CrossRef]
- Nouchi, R.; Kobayashi, A.; Nouchi, H.; Kawashima, R. Newly Developed TV-Based Cognitive Training Games Improve Car Driving Skills, Cognitive Functions, and Mood in Healthy Older Adults: Evidence from a Randomized Controlled Trial. Front. Aging Neurosci. 2019, 11, 99. [Google Scholar] [CrossRef]
- Nouchi, R.; Hu, Q.; Saito, T.; dos Santos Kawata, N.Y.; Nouchi, H.; Kawashima, R. Brain Training and Sulforaphane Intake Interventions Separately Improve Cognitive Performance in Healthy Older Adults, Whereas a Combination of These Interventions Does Not Have More Beneficial Effects: Evidence from a Randomized Controlled Trial. Nutrients 2021, 13, 352. [Google Scholar] [CrossRef]
- Brehmer, Y.; Westerberg, H.; Bäckman, L. Working-memory training in younger and older adults: Training gains, transfer, and maintenance. Front. Hum. Neurosci. 2012, 6, 63. [Google Scholar] [CrossRef]
- Nouchi, R.; Saito, T.; Nouchi, H.; Kawashima, R. Small Acute Benefits of 4 Weeks Processing Speed Training Games on Processing Speed and Inhibition Performance and Depressive Mood in the Healthy Elderly People: Evidence from a Randomized Control Trial. Front. Aging Neurosci. 2016, 8, 302. [Google Scholar] [CrossRef]
- Soveri, A.; Antfolk, J.; Karlsson, L.; Salo, B.; Laine, M. Working memory training revisited: A multi-level meta-analysis of n-back training studies. Psychon. Bull. Rev. 2017, 24, 1077–1096. [Google Scholar] [CrossRef]
- Traut, H.J.; Guild, R.M.; Munakata, Y. Why Does Cognitive Training Yield Inconsistent Benefits? A Meta-Analysis of Individual Differences in Baseline Cognitive Abilities and Training Outcomes. Front. Psychol. 2021, 12, 1–20. [Google Scholar] [CrossRef]
- Baykara, E.; Könen, T.; Unger, K.; Karbach, J. MRI Predictors of Cognitive Training Outcomes. J. Cogn. Enhanc. 2021, 5, 245–258. [Google Scholar] [CrossRef]
- Nouchi, R.; Kawata, N.Y.D.S.; Saito, T.; Himmelmeier, R.M.; Nakamura, R.; Nouchi, H.; Kawashima, R. Dorsolateral Prefrontal Cortex Activity during a Brain Training Game Predicts Cognitive Improvements after Four Weeks’ Brain Training Game Intervention: Evidence from a Randomized Controlled Trial. Brain Sci. 2020, 10, 560. [Google Scholar] [CrossRef]
- Vermeij, A.; Kessels, R.P.C.; Heskamp, L.; Simons, E.M.F.; Dautzenberg, P.L.J.; Claassen, J.A.H.R. Prefrontal activation may predict working-memory training gain in normal aging and mild cognitive impairment. Brain Imaging Behav. 2017, 11, 141–154. [Google Scholar] [CrossRef]
- Rogala, J.; Jurewicz, K.; Paluch, K.; Kublik, E.; Cetnarski, R.; Wróbel, A. The Do’s and Don’ts of Neurofeedback Training: A Review of the Controlled Studies Using Healthy Adults. Front. Hum. Neurosci. 2016, 10, 301. [Google Scholar] [CrossRef]
- Crosson, B.; Ford, A.; McGregor, K.M.; Meinzer, M.; Cheshkov, S.; Li, X.; Walker-Batson, D.; Briggs, R.W. Functional imaging and related techniques: An introduction for rehabilitation researchers. J. Rehabil. Res. Dev. 2010, 47, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Loriette, C.; Ziane, C.; Ben Hamed, S. Neurofeedback for cognitive enhancement and intervention and brain plasticity. Rev. Neurol. 2021, 177, 1133–1144. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, J.C.; De Souza, M.L. Neurofeedback training for cognitive performance improvement in healthy subjects: A systematic review. Psychol. Neurosci. 2021, 14, 262–279. [Google Scholar] [CrossRef]
- Kawata, N.Y.S.; Nouchi, R.; Oba, K.; Matsuzaki, Y.; Kawashima, R. Auditory Cognitive Training Improves Brain Plasticity in Healthy Older Adults: Evidence from a Randomized Controlled Trial. Front. Aging Neurosci. 2022, 14, 140. [Google Scholar] [CrossRef]
- Takeuchi, H.; Magistro, D.; Kotozaki, Y.; Motoki, K.; Nejad, K.K.; Nouchi, R.; Jeong, H.; Sato, C.; Sessa, S.; Nagatomi, R.; et al. Effects of Simultaneously Performed Dual-Task Training with Aerobic Exercise and Working Memory Training on Cognitive Functions and Neural Systems in the Elderly. Neural Plast. 2020, 2020, 3859824. [Google Scholar] [CrossRef]
- Gordon, S.; Todder, D.; Deutsch, I.; Garbi, D.; Alkobi, O.; Shriki, O.; Shkedy-Rabani, A.; Shahar, N.; Meiran, N. Effects of neurofeedback and working memory-combined training on executive functions in healthy young adults. Psychol. Res. 2020, 84, 1586–1609. [Google Scholar] [CrossRef]
- Nouchi, R.; Nouchi, H.; Dinet, J.; Kawashima, R. Cognitive Training with Neurofeedback Using NIRS Improved Cognitive Functions in Young Adults: Evidence from a Randomized Controlled Trial. Brain Sci. 2022, 12, 5. [Google Scholar] [CrossRef]
- Hosseini, S.H.; Pritchard-Berman, M.; Sosa, N.; Ceja, A.; Kesler, S.R. Task-based neurofeedback training: A novel approach toward training executive functions. Neuroimage 2016, 134, 153–159. [Google Scholar] [CrossRef]
- Quaresima, V.; Ferrari, M. A Mini-Review on Functional Near-Infrared Spectroscopy (fNIRS): Where Do We Stand, and Where Should We Go? Photonics 2019, 6, 87. [Google Scholar] [CrossRef]
- Kohl, S.H.; Mehler, D.M.A.; Lührs, M.; Thibault, R.T.; Konrad, K.; Sorger, B. The Potential of Functional Near-Infrared Spectroscopy-Based Neurofeedback—A Systematic Review and Recommendations for Best Practice. Front. Neurosci. 2020, 14, 594. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Akobeng, A.K. Principles of evidence based medicine. Arch. Dis. Child. 2005, 90, 837–840. [Google Scholar] [CrossRef]
- Schardt, C.; Adams, M.B.; Owens, T.; Keitz, S.; Fontelo, P. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med. Inform. Decis. Mak. 2007, 7, 16. [Google Scholar] [CrossRef]
- Ito, E.; Nouchi, R.; Dinet, J.; Cheng, C.-H.; Husebø, B.S. The Effect of Music-Based Intervention on General Cognitive and Executive Functions, and Episodic Memory in People with Mild Cognitive Impairment and Dementia: A Systematic Review and Meta-Analysis of Recent Randomized Controlled Trials. Healthcare 2022, 10, 1462. [Google Scholar] [CrossRef]
- Schneider, J.; McGrew, K. The Cattell-Horn-Carroll (CHC) Model of Intelligence. In Contemporary Intellectual Assessment: Theories, Tests, and Issues; Flanagan, D., Harrison, P., Eds.; Guilford: New York, NY, USA, 2012. [Google Scholar]
- Gualtieri, C.T.; Johnson, L.G. Reliability and validity of a computerized neurocognitive test battery, CNS Vital Signs. Arch. Clin. Neuropsychol. 2006, 21, 623–643. [Google Scholar] [CrossRef]
- Jonides, J.; Schumacher, E.H.; Smith, E.E.; Lauber, E.J.; Awh, E.; Minoshima, S.; Koeppe, R.A. Verbal Working Memory Load Affects Regional Brain Activation as Measured by PET. J. Cogn. Neurosci. 1997, 9, 462–475. [Google Scholar] [CrossRef]
- Sternberg, S. The discovery of processing stages: Extensions of Donders’ method. Acta Psychol. 1969, 30, 276–315. [Google Scholar] [CrossRef]
- Fan, J.; McCandliss, B.D.; Sommer, T.; Raz, A.; Posner, M.I. Testing the Efficiency and Independence of Attentional Networks. J. Cogn. Neurosci. 2002, 14, 340–347. [Google Scholar] [CrossRef]
- Nouchi, R.; Taki, Y.; Takeuchi, H.; Hashizume, H.; Nozawa, T.; Kambara, T.; Sekiguchi, A.; Miyauchi, C.M.; Kotozaki, Y.; Nouchi, H.; et al. Brain Training Game Boosts Executive Functions, Working Memory and Processing Speed in the Young Adults: A Randomized Controlled Trial. PLoS ONE 2013, 8, e55518. [Google Scholar] [CrossRef]
- Anderson, P. Assessment and Development of Executive Function (EF) During Childhood. Child Neuropsychol. 2002, 8, 71–82. [Google Scholar] [CrossRef]
- Hakoda, Y.; Watanabe, M. Manual for New Stroop Test Ⅱ; Toyo Psysical: Fukuoka, Japan, 2004. [Google Scholar]
- Lezak, M.D.; Howieson, D.B.; Bigler, E.D.; Tranel, D. Neuropsychological Assessment, 5th ed.; Oxford University Press: New York, NY, USA, 2012. [Google Scholar]
- Kesler, S.; Blayney, D.W. Mobile cognitive assessment battery (MCAB) for assessment of cancer-related cognitive changes. J. Clin. Oncol. 2014, 32, 9571. [Google Scholar] [CrossRef]
- de Morton, N.A. The PEDro scale is a valid measure of the methodological quality of clinical trials: A demographic study. Aust. J. Physiother. 2009, 55, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Maher, C.G.; Sherrington, C.; Herbert, R.D.; Moseley, A.M.; Elkins, M. Reliability of the PEDro Scale for Rating Quality of Randomized Controlled Trials. Phys. Ther. 2003, 83, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Balduzzi, S.; Rücker, G.; Schwarzer, G. How to perform a meta-analysis with R: A practical tutorial. Evid. Based Ment. Health 2019, 22, 153–160. [Google Scholar] [CrossRef]
- Viechtbauer, W. Conducting Meta-Analyses in R with the Metafor. J. Stat. Softw. 2010, 36, 1–48. [Google Scholar] [CrossRef]
- Acevedo, B.P.; Dattatri, N.; Le, J.; Lappinga, C.; Collins, N.L. Cognitive Training with Neurofeedback Using fNIRS Improves Cognitive Function in Older Adults. Int. J. Environ. Res. Public Health 2022, 19, 5531. [Google Scholar] [CrossRef]
- Hou, J.; Jiang, T.; Fu, J.; Su, B.; Wu, H.; Sun, R.; Zhang, T. The Long-Term Efficacy of Working Memory Training in Healthy Older Adults: A Systematic Review and Meta-Analysis of 22 Randomized Controlled Trials. J. Gerontol. Ser. B 2020, 75, E174–E188. [Google Scholar] [CrossRef]
- Yeh, W.-H.; Ju, Y.-J.; Liu, Y.-T.; Wang, T.-Y. Systematic Review and Meta-Analysis on the Effects of Neurofeedback Training of Theta Activity on Working Memory and Episodic Memory in Healthy Population. Int. J. Environ. Res. Public Health 2022, 19, 11037. [Google Scholar] [CrossRef]
- Kelly, M.E.; Loughrey, D.; Lawlor, B.A.; Robertson, I.H.; Walsh, C.; Brennan, S. The impact of cognitive training and mental stimulation on cognitive and everyday functioning of healthy older adults: A systematic review and meta-analysis. Ageing Res. Rev. 2014, 15, 28–43. [Google Scholar] [CrossRef]
- Yeh, W.-H.; Hsueh, J.-J.; Shaw, F.-Z. Neurofeedback of Alpha Activity on Memory in Healthy Participants: A Systematic Review and Meta-Analysis. Front. Hum. Neurosci. 2021, 14, 562360. [Google Scholar] [CrossRef]
- Blumenfeld, R.S.; Ranganath, C. Prefrontal Cortex and Long-Term Memory Encoding: An Integrative Review of Findings from Neuropsychology and Neuroimaging. Neuroscientist 2007, 13, 280–291. [Google Scholar] [CrossRef]
- Chai, W.J.; Hamid, A.I.A.; Abdullah, J.M. Working Memory from the Psychological and Neurosciences Perspectives: A Review. Front. Psychol. 2018, 9, 401. [Google Scholar] [CrossRef]
- Rajabi, S.; Pakize, A.; Moradi, N. Effect of combined neurofeedback and game-based cognitive training on the treatment of ADHD: A randomized controlled study. Appl. Neuropsychol. Child 2020, 9, 193–205. [Google Scholar] [CrossRef]
- Luo, X.; Guo, X.; Zhao, Q.; Zhu, Y.; Chen, Y.; Zhang, D.; Jiang, H.; Wang, Y.; Johnstone, S.; Sun, L. A randomized controlled study of remote computerized cognitive, neurofeedback, and combined training in the treatment of children with attention-deficit/hyperactivity disorder. Eur. Child Adolesc. Psychiatry 2022, 1–12. [Google Scholar] [CrossRef]


| Study and Year | Country | No. of Participants | Mean Age | Female (%) | Domain of Cognitive Training | Type of Neurofeedback | Brain Regions Used for Neurofeedback | Comparison Groups | Intervention Period | Intervention Frequency (Time per Session) | Study Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Nouchi et al., 2022 [27] | Japan | 60 | 21.43 | 50 | Processing speed, Memory span, Attention | NIRS (2ch) | Left and right DLPFC | CT, ACT (Tetris) | Four weeks | Every day (20 min) | Cd, ST, D-CAT, DS-F, DS-B, LM-I, LM-D, MR |
| Acevedo et al., 2022 [49] | USA | 86 | 65.96 | 75.58 | Multiple domain | NIRS (1ch) | Right prefrontal cortex | ACT (Tetris) | Four weeks | 5–7 days/week (10–15 min) | VS-VBM, VS-VSM, VS-M, VS-PS, VS-EF, VS-WM, VS-SA |
| Gordon et al., 2020 [26] | Israel | 140 | 22.08 | 39.29 | Working memory | EEG (3ch) | Parietal midline (Pz) | NFT + ACT (VS), NFT, WMT, ACT (VS), Silent Control | Five weeks | 2 times/week (30–60 min) | Executive function and mental rotaion test |
| Hosseini et al., 2016 [28] | USA | 20 | 24.6 | 50 | Working memory | NIRS (52ch) | Left and right DLPFC | CT + SHAM feedback | Two weeks | 4 sessions (25 min) | nB, TMT, CW, MCAB-Sw, STB |
| No. | Items | Nouchi et al. (2022) [27] | Acevedo et al. (2022) [49] | Hosseini et al. (2016) [28] |
|---|---|---|---|---|
| 1 | Eligibility criteria were specified (additional) | Yes | No | Yes |
| 2 | Subjects were randomly allocated to groups (in a crossover study, subjects were randomly allocated an order in which treatments were received | Yes | Yes | No |
| 3 | Allocation was concealed | Yes | No | No |
| 4 | The groups were similar at baseline regarding the most important prognostic indicators | Yes | No | Yes |
| 5 | There was blinding of all subjects | Yes | No | No |
| 6 | There was blinding of all therapists who administered the therapy | Yes | No | No |
| 7 | There was blinding of all assessors who measured at least one key outcome | Yes | No | No |
| 8 | Measures of at least one key outcome were obtained from more than 85% of the subjects initially allocated to groups | Yes | No | Yes |
| 9 | All subjects for whom outcome measures were available received the treatment or control condition as allocated, or, where this was not the case, data for at least one outcome was analyzsed by “intention to treat” | Yes | Yes | Yes |
| 10 | The results of between-group statistical comparisons are reported for at least one key outcome | Yes | Yes | Yes |
| 11 | The study provides both point measures and measures of variability for at least one key outcome | No | No | No |
| Total (Max 10) | 9 | 3 | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsuzaki, Y.; Nouchi, R.; Sakaki, K.; Dinet, J.; Kawashima, R. The Effect of Cognitive Training with Neurofeedback on Cognitive Function in Healthy Adults: A Systematic Review and Meta-Analysis. Healthcare 2023, 11, 843. https://doi.org/10.3390/healthcare11060843
Matsuzaki Y, Nouchi R, Sakaki K, Dinet J, Kawashima R. The Effect of Cognitive Training with Neurofeedback on Cognitive Function in Healthy Adults: A Systematic Review and Meta-Analysis. Healthcare. 2023; 11(6):843. https://doi.org/10.3390/healthcare11060843
Chicago/Turabian StyleMatsuzaki, Yutaka, Rui Nouchi, Kohei Sakaki, Jérôme Dinet, and Ryuta Kawashima. 2023. "The Effect of Cognitive Training with Neurofeedback on Cognitive Function in Healthy Adults: A Systematic Review and Meta-Analysis" Healthcare 11, no. 6: 843. https://doi.org/10.3390/healthcare11060843
APA StyleMatsuzaki, Y., Nouchi, R., Sakaki, K., Dinet, J., & Kawashima, R. (2023). The Effect of Cognitive Training with Neurofeedback on Cognitive Function in Healthy Adults: A Systematic Review and Meta-Analysis. Healthcare, 11(6), 843. https://doi.org/10.3390/healthcare11060843







