Abstract
Urinary tract infections (UTIs) represent one of the most frequent low genital tract diseases in the female population. When UTIs occur with a frequency of at least three times per year or two times in the last six month, we speak of recurrent UTI (rUTI) and up to 70% of women will have rUTI within 1 year. It was previously thought that antibiotic resistance was principally responsible for the recurrence of UTIs, but nowadays new diagnostic technologies have shown the role of microbiota in the pathophysiology of these diseases. Much research has been conducted on the role of gut microbiome in the development of rUTI, while little is known yet about vaginal and urinary microbiome and the possible immunological and microscopical mechanisms through which they trigger symptoms. New discoveries and clinical perspectives are arising, and they all agree that a personalized, multi-modal approach, treating vaginal and urinary dysbiosis, may reduce rUTIs more successfully.
1. Introduction
A total of 1.5 million people suffers from urinary tract infections (UTIs) every year, making it one of the most prevalent health problems []. Women experience UTIs eight times more often than men [], and 50–60% of adult women will have at least one UTI in their lifetime, affecting their quality of life and psychological wellbeing [,].
Anatomical characteristics, sexual behaviour, urogenital aging, pelvic organ prolapse, urethral diverticula, vescico-vaginal fistula, urinary incontinence, menopause, and pregnancy all represent possible risk factors for women [].
In clinical practice it may be useful to distinguish between uncomplicated and complicated UTIs. Complicated UTIs are caused by urological anomalies, including indwelling catheters, renal insufficiency, neurogenic bladder, pregnancy, previous urological surgery, and conditions causing an immunocompromised state [], and these may also progress to sepsis and other systemic illnesses, which mostly impact the kidneys [].
Recurrent UTIs (rUTIs) are characterised as complicated and/or uncomplicated UTIs that happen at least three times yearly or twice over six months [,], differently from persistent infections in which the pathogen is not eradicated but instead persists in some of the infected people’s cells []. Recurrent UTIs are common; after getting one, 24% of women will get another within 6 months, and up to 70% will get another within a year [,]. Six or more episodes of rUTIs occur in at least 35 million women worldwide each year (1% of all women) [,,].
The pathophysiology of rUTIs is not well understood. However, in the 80s, it was already clear that recurrence of UTIs was closely linked to antibiotic resistance [,,]. The increased use of antibiotics globally, along with prophylactic therapy, contributed to the development of multiresistant bacteria, such as extended-spectrum beta-lactamase-producing bacteria, carbapenemase-resistant organisms, and pan-resistant bacteria [,].
Furthermore, the myth that urine is sterile has been dispelled only in recent years by developments in technology and molecular biology, concomitantly with the discovery of the role of microbiota of the bladder, vagina, and gut in the pathophysiology of rUTIs [,,]. While little is known about the vaginal and urinary microbiomes, a great deal of study has been done on the function of the gut microbiome in the development of rUTIs [].
The purpose of this review is to highlight potential mechanisms by which the vaginal and urinary microbiomes, as well as the potential role of the urothelial immunological microenvironment, contribute to rUTIs onset in women of different ages (Figure 1).
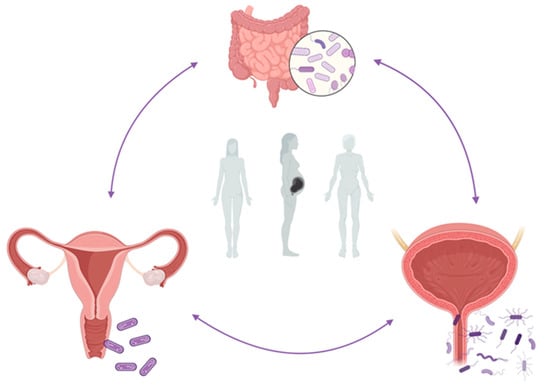
Figure 1.
Abstract figure. The interplay between the gut, vaginal, and urinary microbiome in the onset of rUTIs during woman different ages. Figure created with BioRender.com (accessed on 29 January 2023).
2. Materials and Methods
The most significant medical databases, including PubMed, Cochrane Database of Systematic Reviews, EMBASE, and Web of Science, were consulted, according to a combination of the following keywords: “recurrent urinary tract infection, recurrent cystitis, vaginal microbiome, vaginal microbiota, urinary microbiota, urinary microbiome, urobiome, dysbiosis, urinary bladder disease”, including pluralization and English spelling variations and suffixes/prefixes. From 2000 until 11 November 2022, we collected all publications, including case studies, literature reviews, and prospective or retrospective trials. Two authors (MD and ALS) independently evaluated the references to incorporate the literature data into the review. Preferred reporting items for systematic reviews and meta-analyses (PRISMA) method was applied to conduct a systematic search (Figure 2).
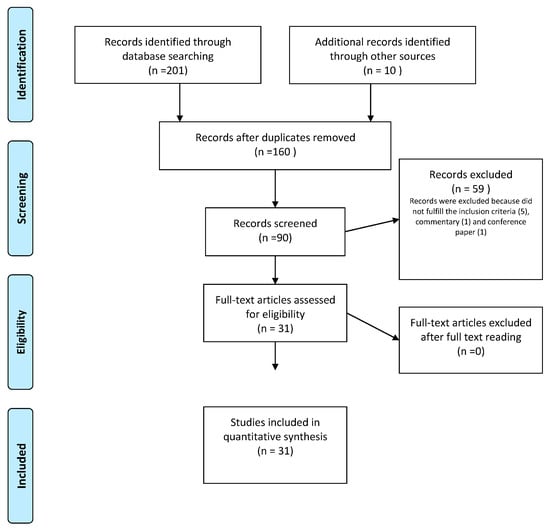
Figure 2.
PRISMA flow diagram of study selection process.
In the first step, the authors considered the title of the paper, then the abstract, and finally the manuscript. Consequently, the data obtained was collected. Studies were considered qualified if they met the following criteria: (I) the involvement of the vaginal microbiota and microbiome in the onset of rUTIs in female population, (II) the role of the urinary microbiota and microbiome in rUTIs in women, (III) dysbiosis as a cause of recurrent cystitis, and (IV) novel therapeutics approaches in the field of study. Instead, the following were considered exclusion criteria: (I) case reports; (II) conference abstracts, editorials, and pre-prints manuscripts; (III) multimedia; and (IV) papers written in languages other than English.
To ensure validity and prevent any selection, performance, detection, attrition, and reporting bias, two researchers (MD and ALS) independently assessed the risk of bias for each selected study, in accordance with the Cochrane Handbook for Systematic Reviews of Interventions [,]. Conflicts were resolved through discussion between researchers. Finally, the two researchers examined and extracted data separately.
3. Results
The search method provided 201 papers in total and another 10 studies were included through the references. In total, 90 publications were screened by title and/or abstract following the elimination of articles not published in English or published before 2000. Duplicate papers, and irrelevant works were excused from the analysis. In the end, 31 research articles were included and analysed (Figure 1). Table 1 reports the main findings derived from the literature data about the internship between microbiota, immunology, and rUTIs.
3.1. Vaginal Microbiome and rUTIs
The pathogenesis of rUTIs is significantly influenced by the vaginal microenvironment, in contrast to widespread assumption, which attested that bacteria causing UTIs typically originate from the gut altered microbiota, as the only way of infection [].
Community state types (CSTs) represents a classification used to describe at least five major subcategories of cervico-vaginal bacterial species involved in the maintenance of vaginal balancing between physiological and pathological flora. Each group has a unique mix of bacteria, each with a different relative characteristic. Four of these are dominated by one of the following four species: L. crispatus (CST I), L. gasseri (CST II), L. iners (CST III), or L. jensenii (CST V) [,].
Instead, CST IV predominantly contains anaerobic bacteria, such G. vaginalis, Atopobium vaginae, and Megasphaera spp., similar to the vaginal microbiota in bacterial vaginosis [,]. Interestingly, a recent meta-analysis reported that Prevotella bivia, G. vaginalis, Chlamydia trachomatis, and Human Papillomavirus infections are more common in women with lower levels of Lactobacillus in their CST IV cervico-vaginal microbiota than in women with higher levels of Lactobacillus [].
Several Lactobacillus species, such as L. crispatus, L. jensenii, L. gasseri, and L. iners, constitute most of the vaginal microbiota in women of reproductive age [,,]. These bacteria produce lactic acid, helping the maintenance of the vaginal acidic pH [,].
By creating bacteriocins and hydrogen peroxide, vaginal lactobacilli perform a protective function, inhibiting the colonisation of other potential pathogens, particularly E. coli [,,,,]. For this reason, lower lactobacilli levels promote the insurgence of bacterial vaginosis or vaginal E. coli colonisation, which increase the risk of UTI insurgence [,,,].
The vagina also represents a reservoir for pathogens: The literature data underlines that women with a history of UTI exhibit more E. coli colonisation in the vaginal introitus (>105 CFU/mL), highlighting the importance of vaginal microenvironment in the pathogenesis of rUTIs [].
S. saprophyticus is the most frequent gram-positive bacterial source of community-acquired UTI. S. saprophyticus virulence in in vitro and rat UTI model is based on the following components: Secreted surface-associated proteins Aas (hemagglutinin) and Ssp (lipase), the proteins UafA of cell wall, SdrI, SssF, and UafB, which mediate adherence, and the ureases [].
By contrast, S. aureus and S. epidermidis can also cause UTI, especially during catheterization or pregnancy: Experimental models highlighted that the nickel ABC-transporters Opp2 and Opp5a are involved in the pathogenesis of S. aureus urinary infection [,,,,].
Finally, vaginal bacteria, such as Actinobacteria, other Firmicutes, and gram-negative anaerobic organisms, which are not common uropathogens, may colonise the urinary system, alter the physiological microbiota, and change the immunological assessment of vaginal and bladder mucosa. In other words, even if specific vaginal bacteria do not colonise the bladder or are eliminated by the host prior to the diagnosis of a UTI, brief contact with these bacteria in the urinary tract can still have a significant impact on UTI pathogenesis []. This phenomenon is called “covert pathogenesis” [,]. For instance, group B streptococcus and G. vaginalis promote the survival of E. coli in the bladder, permitting the development of UTIs [,,]. Numerous studies have connected Streptococcus agalactiae (GBS) colonisation to vulvo-vaginitis [] and urinary tract infections [], but none have looked at the connection between vaginal GBS and GBS UTI []. GBS colonisation is typically asymptomatic. By contrast, Gardnerella vaginalis can also cause UTI and is connected to sepsis, renal disease, and urgency incontinence [,], as we better described later (Section 4.2).
3.2. Urinary Microbiome and rUTIs
Modern urine culture techniques have shown that several bacteria allow the maintenance of urothelium homeostasis [,,]. The host characteristics, which change across people, life, and geographical areas, as well as environmental exposure and behavioural factors, are the most important factors for maintaining the balance of this microbial ecosystem [].
The most often identified colonising microorganisms in the urine microbiome are Lactobacillus and Streptococcus, which constitute a barrier against infections, producing factors, which inhibit the adhesion of pathogens to epithelium, such as lactic acid. Alloscardovia, Burkholderia, Jonquetella, Klebsiella, Saccharofermentans, Rhodanobacter, and Veillonella are less often identified bacteria []. Proteobacteria (35.6%), Firmicutes (31.3%), Actinobacteria (22.4%), Bacteroidetes (6.4%), and others (4.3%) are the key phyla of the human urinary tract, according to Morand et al. []. The authors attested that the urine microbiota ordinarily contains several uropathogens, but pathogenicity results from an imbalance in their relative percentages and from the host immunological response [].
From 435 urine samples, Dubourg et al. identified 450 different bacterial species, of which 256 had never been discovered before in urine, while 18 were entirely new [].
Other recent research suggests that many urinary system diseases, including UTIs and rUTIs, may be influenced by the urine microbiome (or urobiome), which plays a key role in the maintenance of the homeostasis of urothelial microenvironment []. Urinary microbiota abnormalities precede UTI onset, and the urobiome normalizes following therapy, as Bossa et al. demonstrated [].
The knowledge of the urobiome is fundamental in clinical practice because different clinical manifestations are probably connected to a specific urine microbiota modification, as attested by Burnett et al. []. Additionally, non-infectious urologic conditions, such as neurogenic bladder dysfunction, interstitial cystitis, and urgency urine incontinence have been associated to changes in the urinary microbiota spectrum [].
It is interesting to consider in the knowledge of a UTI’s pathology and in the importance of health urobiome is the evidence that uropathogenic Escherichia coli (UPEC) has a reservoir also in the bladder epithelium; many investigations in both adults and children, as well as in bladder biopsies, demonstrated intracellular UPEC in bladder epithelial cells that release in urine [,,,,]. In addition, 82% of rUTIs are brought on by the same UPEC strain as in the prior infection, even when the proper antibiotic therapy is administered [,,,,,]. The pathogenesis of UPEC is described in Section 4.2.
4. Discussion and Conclusions
4.1. Risk Factors for rUTIs in Women
Hormonal fluctuations have a significant role in the changes in vaginal and urine microbiomes composition; oestrogen promotes Lactobacillus development in the bladder and vagina, increasing their defensive role against pathogens and infections. Consequently, loss of oestrogen in postmenopausal women causes a reduction in vaginal lactobacilli and an increase in rUTIs [,]. The genitourinary syndrome of menopause is constituted by vaginal epithelium thinning, a decrease in extracellular matrix, proteoglycans and collagens synthesis, and vulvovaginal atrophy [], which facilitate the penetration of bacteria in urothelium and vaginal epithelium.
For this reason, post-menopausal women are more prone to develop rUTIs, with a rate of 8–11% [,,,]. Regarding vaginal and urinary microbiome composition after menopause, some authors demonstrated that post-menopausal women present fewer distinct bacterial species [,,], while others supported the development of an increased diversity of species [].
UTIs are also a common problem among pregnant women, representing the most common infection during this period of life, particularly asymptomatic bacteriuria, affecting 2 to 7% of pregnant women []. As we all know, pregnancy is characterized by physiological changes in immune response, the vaginal microbiome also undergoes major changes. Indeed, pregnancy reduces the differences in microbiome diversity across women; particularly, pregnancy-related vaginal alterations result in an increased Lactobacillus dominance and a reduced species diversity []. These changes are protective regarding a preterm birth rate because they boost infection resistance and support the production of anti-inflammatory cytokines []. Furthermore, they regard also racioethnic differences in the vaginal microbiome, so particularly in women of African ancestry, the configuration of the vaginal microbiome during pregnancy may have predictive value for premature birth [].
Other factors that influence vaginal microbiome composition are:
- Contraceptive methods: Spermicidal products containing nonoxynol-9 deplete lactobacilli and favor E. coli colonization [,,,,,]. Instead, oral contraceptives seem to decrease the rate of bacterial vaginosis [], but they do not influence the risk of rUTIs;
- Sexual activity: Vaginal activity either promotes the entry of possible germs into the urethral meatus from the vagina or facilitates the transfer of potential uropathogens to the vagina [,,,,,,];
- New antimicrobial treatments (oral or topical) for the risk of the development of antibiotic resistance [,].
4.2. NGS as a Better Diagnostic Tool
Nowadays, several studies and research articles have re-written the idea that most UTI bacteria originate in the gut [], and recent research has clarified the role that urine and vaginal bacteria play in the onset and recurrence of these diseases [,]. The microbiomes of the vagina and urinary tract are inextricably related and together participate in the maintenance of a healthy balance in the genital and urinary tracts []. In addition, from a microbiological perspective, around one-third of the bladder microbiota only resides in the vagina []. Mechanical transfer is one of the main risk factors for UTIs and rUTIs [,,,,,,,] because it enables vaginal bacteria to enter the urinary system, such as during sexual activity [,].
In this new perspective, the myth that urine is sterile was disproved also thanks to the development of new analytical techniques, such as NGS and metagenomic approaches [], which allowed for the detection of a microbiome in the healthy urogenital tract [,,]. Microbial ecologists created the culture-independent DNA-based identification of microorganisms with the aim of identifying bacterial species without the need for culture. Particularly, NGS employs PCR amplification and high-throughput sequencing of essential 16S rRNA genes, using polymorphisms of the 16S rRNA gene amplicon to distinguish bacterial species, even those that are closely related [,]. A urine sample is sequenced using a multi-step process that starts with the isolation and purification of microbial DNA, follows with 16S rRNA amplification and sequencing, and ends with bioinformatic analysis through a variety of software database platforms. As a result, there are still a lot of restrictions with this technology, particularly in terms of its clinical uses [].
Yoo et al. demonstrated that the clinical application of urine NGS in cases of acute uncomplicated cystitis and rUTIs reported a better sensitivity than the application of conventional urine culture [], which is consistent with prior research [,,,]. Indeed, it appears that a typical urine culture misses roughly 90% of non-UPEC pathogens [], and anaerobic bacteria or a multi-microbial illness may be to blame for negative findings in routine urine cultures [,,].
Most importantly, NGS is not greatly impacted by antibiotic usage, because bacteria do not need to be alive as for a traditional culture method []. Furthermore, NGS is highly sensitive to atypical bacteria, anaerobes, or multimicrobial urinary tract infections []. Another crucial element that facilitates prompt clinical decision-making and medication is represented by the faster NGS technique for the detection of pathogens with respect to culture; this reduces testing times from several days to just 24 h [].
4.3. Pathophysiology and Immunology in rUTIs
While analysing the immunological assessment of urinary infections, data in the literature reported that intracellular bacterial communities (IBCs) and quiescent intracellular reservoirs (QIRs) are two methods that allow pathogens to survive antibiotic treatment and to host an immune response in the bladder, developing a chronic colonization [,].
Regarding UPEC, adhesive organelles, such as type 1, P, S, and F1C pili, are used to first infiltrate the host cells in the urothelium. Then, UPEC creates IBCs, which consist of the development of a biofilm formed of a polysaccharide matrix wrapped in a uroplakin coating, enabling UPEC to proliferate and thrive in a secure manner [,,,,,]. As opposed to this, QIRs are made up of a subgroup of bacteria that have remained undetected by the host immune system for a considerable amount of time in cells after receiving antibiotics [,]. Dormant bacteria may begin to reproduce and lead to reinfection because of the urothelium’s turnover. IBCs are transitory, developing within a few hours in the cytosol as opposed to QIRs, which might spend months quiescent within the endosomes [].
Lipopolysaccharide (LPS), a key component of UPEC pathogenicity, affects UPEC life cycles and promotes reservoir development [] by activating intracellular signalling pathways and innate and adaptive immune responses []. By raising cytosolic calcium through a Toll-like receptor (TRL 4-mediated increase), LPS suppresses the synthesis of cytokines []. Additionally, NLRP3 inflammasome activation by pathogen-associated molecules, such as flagellin and hemolysin as well as LPS can cause urothelial cells to exfoliate and let UPEC to enter deeply [,].
Regarding the relationship between the innate and adaptive immune systems and rUTIs, this is not completely understood yet [].
The functions of pentraxin 3 (PTX3) and uroplakin IIIa (UPIIIa) signalling have received little attention in the literature. A crucial function of PTX3 is that polymorphisms or a deficit in it may impair the body’s capacity to control infection, which may promote infection spread []. The endocytic process is instead induced by UPIIIa signalling, which enters the intracellular space [].
Instead, particular attention has been paid to the relation between vaginal microenvironment and urinary tract inflammatory diseases. The assumption that the vagina is the main source of bladder colonising pathogens was made since women have UTIs at a greater rate than males [,]. The vaginal canal can operate as a reservoir for E. coli and other bacteria, becoming a significant player in the pathogenesis of UTI.
E. coli can penetrate vaginal cells and remain in the vagina during UTI, according to preliminary research in murine UTI models []. Regarding this, more research has been conducted on the relationship between Gardenerella vaginalis and E. coli.
Animal experiments that exposed the urinary system to different common vaginal bacteria (especially Gardnerella) in the setting of E. coli UTI corroborate the previous reported theory of “covert pathogenesis” [,].
Gardnerella can frequently be found in urine samples from healthy, asymptomatic women. Three patterns of patients who tested positive for Gardnerella were proposed by Yoo et al. []: (I) the Escherichia-dominant group; (II) the Gardnerella-dominant group; and (III) the Lactobacillus-dominant group. They emphasised that all Escherichia dominant groups were linked to rUTI, but Gardnerella- and Lactobacillus-dominant groups might be linked to rUTI but not necessarily be symptomatic. This supported the idea that bladder dysbiosis can cause various symptoms by altering the immune system’s reaction to bacterial colonisation []. Furthermore, it was shown that Gardnerella may be a “covert” pathogen that causes E. coli activation [], and UTI can also happen in the Lactobacillus-dominant group even if a minor amount of Gardnerella is present, if Lactobacillus has a poor protective effect [].
Other research confirmed that the development of UPEC from bladder reservoirs is significantly influenced by Gardnerella. Indeed, it influences urothelial apoptosis and exfoliation and other mucosal immune system-related activities as demonstrated in a mouse model [,]. Among these, immediate-early (IE) genes, including the orphan nuclear receptor Nur77 (also known as Nr4a1), are increased in mice exposed to Gardnerella [,]; at the same time, animals lacking Nur77 are not at risk from recurrent UPEC UTI after Gardnerella exposure.
Numerous cellular functions are controlled by Nur77, including apoptosis in various tissues [,]. Additionally, Nur77 regulates inflammation [] and has a specific impact on T-cell responses [] and Ly6C-monocytes []. As a result, the IE response could play a role in the Gardnerella-related recurrent UPEC UTI [].
Instead, KEGG pathways and GO keywords are significantly changed after several Gardnerella exposures []. IL-12, IFN-g, and RANTES levels rise in bladder homogenates after exposure to Gardnerella [], and pathways associated to T and B cells are also activated.
Finally, Kirjavainen et al. investigated immune defence anomalies in women with rUTIs and discovered that peripheral monocytes and myeloid dendritic cells (DCs) produced elevated level of interleukin-12 and did not induce the T cell activation. In the case of rUTIs, the T cell polarisation is avoided. In addition, there was a decrease in levels of vascular endothelial growth factor (VEGF) related with tissue healing and a reduction in concentrations of monocyte chemotactic protein 1, the main chemoattractant for DC and monocytes [].
All these factors may promote the insurgence of urinary infection and chronic colonisation due to the deficiency of immune response and the imbalance of host response to bacterial injuries. It is likely that the host immune response depends on the phenotypic and behaviour characteristics (such as smoking, sexual activity, alcohol abuse, and menopausal status) of a host as previous described.
4.4. New Perspectives of Therapy and Prevention
Recurrent UTIs are strictly associated with urinary tract dysbiosis [,]. The importance of lactobacilli and oestrogens in the prevention of rUTIs was confirmed by Neugent et al., who described the correlations between Bifidobacterium, Lactobacillus, and urinary oestrogens in women with no history of UTIs []. According to this theory, some researchers suggested that administering probiotics may be more beneficial in treating rUTIs [,] as opposed to administering antibiotics or taking antibiotics prophylactically at low doses to prevent recurrent infections, both of which promote the evolution of pathogenic resistance by causing bacterial persister cells [].
A considerable decrease in rUTIs is linked to the use of Lactobacillus vaginal suppositories [,,]. Sadahira et al. showed that the administration GAI 98,322 strain of L. crispatus had a significant effect in reducing the recurrent cystitis in 86% of patients. However, more importantly, the suppressive effect persisted in 77% of patients for at least a year after the end of the therapy, with a significant decrease in the mean number of cystitis episodes both during and after administration []. The oral treatment with Lactobacillus reuteri RC-14 and Lactobacillus rhamnosus GR-1 also improved the population profiles of vaginal lactobacilli and reduced the colonisation of potentially dangerous bacteria [].
In order to support the host’s immunological assessment against bacterial invasion and prevent recurrent infection, functional restoration should be the main focus of therapy, according to the recent literature data on the urinary tract urobiome and the importance the local and systemic immune system response in the prevention of UTIs recurrence [].
As was already mentioned, oestrogen regulates the balance of the urogenital microbiome; it promotes lactobacilli growth, whereas oestrogen insufficiency results in a decrease in vaginal lactobacilli, which raises the risk of rUTIs [,]. Therefore, rUTIs may be decreased by oestrogen replacement treatment [,,,,,], and intravaginal oestrogen may provide great benefit with less risk when compared to oral oestrogen [].
Recently, research has been conducted on the use of natural sources for therapy and prevention of rUTIs. For example, Mehta et al. studied the potential antibacterial role of the oroxindin from Bacopa monnieri against UTIs caused by Klebsiella pneumoniae and Proteuns mirabilis. B. monnieri is a medicinal plant growing in the world’s wetlands and warmer regions; the authors demonstrated that K. pneumoniae and P. mirabilis can be effectively eliminated by B. monnieri, also establishing its safety [].
5. Conclusions
In conclusion the complex correlation among microbiota, low genital tract, and urinary system is based on the balance between host characteristics, immunological microenvironment and pathogens. Further investigation may provide an accurate analysis of the urogenital microbiome, especially to promote a tailoring therapy in order to reduce antibiotic resistance and increase the physiological mechanism of urothelium response.

Table 1.
Main findings from the studies included in the review.
Table 1.
Main findings from the studies included in the review.
| Variables | Main Findings |
|---|---|
| Role of vaginal microbiome [,,,,,,,,,] | The vaginal microbiome is involved in rUTIs pathogenesis: if its balance is maintained, it constitutes a barrier against pathogens. However, every change, which we know as bacterial vaginosis, is an important risk factor for the development of urinary tract infections. |
| This may be a consequence of the decrease in vaginal lactobacilli, which seems to allow the growth of gram-positive bacteria, (especially Staphylococcus saprophyticus, Escherichia coli, Enterococcus faecalis, and Streptococcus agalactiae) or Gardnerella vaginalis. | |
| These vaginal bacteria may be present in the vaginal canal and colonize the urinary system, avoiding the immune response and allowing the formation of E.coli reservoirs. | |
| Role of urinary microbiome [,,] | Urinary system microbiota has a key role in preserving urinary health. So, the pathophysiology of rUTI is influenced by urobiome. |
| Indeed, urinary microbiome composition differs between healthy and rUTIs subjects. Specific urine microorganisms are linked to distinct clinical features in women with rUTI. | |
| Risk factors of rUTIs and dysbiosis [,,,,] | Risk factors for the development of symptoms include host variables, host behaviours, and bacterial features. Among these, menopause influences the urine microbiota composition following aging and the decrease in oestreogens protection. First of all, it brings altered Lactobacillus composition, increasing the risk of rUTIs. |
| Possible immunological pathways [,,,,] | Several microscopic pathways have been identified, including the intracellular bacterial community, QIR, LPS, multimicrobial infection, and urothelial mucosal remodelling. These mechanisms allow uropathogens to persist in the bladder and survive antibiotic therapy and host immune response. Furthermore, immunological defences show some abnormalities in UTI-prone women, such as increased levels of IL-12, absence of T-cell response, less VEGF, lower level of monocyte chemotactic protein 1, the upregulation of immediate-early (IE) genes, such Nur77. |
| New perspectiver of diagnosis [] | NGS is more sensitive than a conventional urine culture in the detection of uropathogens, highlighting an increased microbiome diversity in the recurrent cystitis group. Additional NGS tests can facilitate rapid decision-making and therapeutic advancement. |
| New perspectives of theraphy [,,,,,,,] | Following the understanding of the importance of lactobacilli and oestrogen in the pathophysiology of rUTIs, several studies demonstrated their benefits as therapies. |
| The intravaginal administration of lactobacillus and/or oestrogens is associated with a significant reduction in rUTIs, especially if they are integrated with nonantibiotic therapeutical options as well as modification of behaviour, specific diet, integration with probiotics, and d-mannos, use of local oestrogens therapy, and systemic or local immunostimulants. The administration of one or more of these approaches provides the beneficial treatment to reduce rUTI risk. |
Author Contributions
B.G. and M.D. designed the structure of the manuscript. M.L.V., C.M., A.G., M.F.P., S.B. and M.T. contributed to the literature search. A.L.S. and M.D. wrote the manuscript. M.D., B.G. and A.L.S. reviewed and revised the initial manuscript and approved the final manuscript as submitted. All authors approved the submitted version. All authors have read and agreed to the published version of the manuscript.
Funding
The study is supported by grants of the Italian ministry of health, “ricerca corrente” to the IRCCS Fondazione Policlinico San Matteo, Pavia, Italy.
Institutional Review Board Statement
Ethical review and approval were waived for this study because it is a systematic review of literature.
Informed Consent Statement
Patient consent was waived for this study because it is a systematic review of literature.
Data Availability Statement
The literature data analyzed during the current review are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Stamm, W.E.; Norrby, S.R. Urinary Tract Infections: Disease Panorama and Challenges. J. Infect. Dis. 2001, 183 (Suppl. 1), S1–S4. [Google Scholar] [CrossRef] [PubMed]
- Cox, C.E.; Lacy, S.S.; Hinman, F., Jr. The Urethra and its Relationship to Urinary Tract Infection. II. The Urethral Flora of the Female with Recurrent Urinary Infection. J. Urol. 1968, 99, 632–638. [Google Scholar] [CrossRef]
- Al-Badr, A.; Al-Shaikh, G. Recurrent Urinary Tract Infections Management in Women: A review. Sultan Qaboos Univ. Med. J. 2013, 13, 359–367. [Google Scholar] [CrossRef]
- Renard, J.; Ballarini, S.; Mascarenhas, T.; Zahran, M.; Quimper, E.; Choucair, J.; Iselin, C.E. Recurrent Lower Urinary Tract Infections Have a Detrimental Effect on Patient Quality of Life: A Prospective, Observational Study. Infect. Dis. Ther. 2014, 4, 125–135. [Google Scholar] [CrossRef]
- Dason, S.; Dason, J.T.; Kapoor, A. Guidelines for the diagnosis and management of recurrent urinary tract infection in women. Can. Urol. Assoc. J. 2013, 5, 316–322. [Google Scholar] [CrossRef]
- Sabih, A.; Leslie, S.W. Complicated Urinary Tract Infections. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www-ncbi-nlm-nih-gov.offcampus.lib.washington.edu/books/NBK436013/ (accessed on 12 August 2021).
- Bonkat, G.; Pickard, R.; Bartoletti, R.; Bruyère, F.; Geerlings, S.; Wagenlehner, F.; Wullt, B.; Pradere, B.; Veeratterapillay, R. EAU Guidelines on Urological Infections; European Association of Urology: Arnhem, The Netherlands, 2018. [Google Scholar]
- Sihra, N.; Goodman, A.; Zakri, R.; Sahai, A.; Malde, S. Nonantibiotic prevention and management of recurrent urinary tract infection. Nat. Rev. Urol. 2018, 15, 750–776. [Google Scholar] [CrossRef] [PubMed]
- Boldogh, I.; Albrecht, T.; Porter, D.D. Persistent Viral Infections. In Medical Microbiology, 4th ed.; Baron, S., Ed.; University of Texas Medical Branch at Galveston: Galveston, TX, USA, 1996; Chapter 46. Available online: https://www.ncbi.nlm.nih.gov/books/NBK8538/ (accessed on 30 January 2023).
- Foxman, B. Urinary Tract Infection Syndromes: Occurrence, recurrence, bacteriology, risk factors, and disease burden. Infect. Dis. Clin. N. Am. 2014, 28, 1–13. [Google Scholar] [CrossRef]
- Foxman, B.; Gillespie, B.; Koopman, J.; Zhang, L.; Palin, K.; Tallman, P.; Marsh, J.V.; Spear, S.; Sobel, J.D.; Marty, M.J.; et al. Risk Factors for Second Urinary Tract Infection among College Women. Am. J. Epidemiol. 2000, 151, 1194–1205. [Google Scholar] [CrossRef] [PubMed]
- Foxman, B.; Manning, S.; Tallman, P.; Bauer, R.; Zhang, L.; Koopman, J.S.; Gillespie, B.; Sobel, J.D.; Marrs, C.F. Uropathogenic Escherichia coli Are More Likely than Commensal E. coli to Be Shared between Heterosexual Sex Partners. Am. J. Epidemiol. 2002, 156, 1133–1140. [Google Scholar] [CrossRef]
- Murray, B.E.; Rensimer, E.R.; DuPont, H.L. Emergence of high-level trimethoprim resistance in fecal Escherichia coli during oral ad-ministration of trimethoprim or trimethoprim—Sulfamethoxazole. N. Engl. J. Med. 1982, 306, 130–135. [Google Scholar] [CrossRef]
- Wright, S.W.; Wrenn, K.D.; Haynes, M.L. Trimethoprim-sulfamethoxazole resistance among urinary coliform isolates. J. Gen. Intern. Med. 1999, 14, 606–609. [Google Scholar] [CrossRef] [PubMed]
- Kristiansen, J.E. The antimicrobial activity of non-antibiotics. Report from a congress on the antimicrobial effect of drugs other than antibiotics on bacteria, viruses, protozoa, and other organisms. APMIS. Suppl. 1992, 30, 7–14. [Google Scholar] [PubMed]
- Ulett, G.C.; Schembri, M.A. Bacterial pathogenesis: Remodelling recurrent infection. Nat. Microbiol. 2016, 2, 16256. [Google Scholar] [CrossRef]
- Langford, B.J.; Brown, K.A.; Diong, C.; Marchand-Austin, A.; Adomako, K.; Saedi, A.; Schwartz, K.L.; Johnstone, J.; MacFadden, D.R.; Matukas, L.M.; et al. The Benefits and Harms of Antibiotic Prophylaxis for Urinary Tract Infection in Older Adults. Clin. Infect. Dis. 2021, 73, e782–e791. [Google Scholar] [CrossRef] [PubMed]
- Gasiorek, M.; Hsieh, M.H.; Forster, C.S. Utility of DNA Next-Generation Sequencing and Expanded Quantitative Urine Culture in Diagnosis and Management of Chronic or Persistent Lower Urinary Tract Symptoms. J. Clin. Microbiol. 2019, 58, e00204-19. [Google Scholar] [CrossRef] [PubMed]
- Thomas-White, K.; Brady, M.; Wolfe, A.J.; Mueller, E.R. The Bladder Is Not Sterile: History and Current Discoveries on the Urinary Microbiome. Curr. Bladder Dysfunct. Rep. 2016, 11, 18–24. [Google Scholar] [CrossRef]
- Pohl, H.G.; Groah, S.L.; Pérez-Losada, M.; Ljungberg, I.; Sprague, B.M.; Chandal, N.; Caldovic, L.; Hsieh, M. The Urine Microbiome of Healthy Men and Women Differs by Urine Collection Method. Int. Neurourol. J. 2020, 24, 41–51. [Google Scholar] [CrossRef]
- Ley, R.E.; Peterson, D.A.; Gordon, J.I. Ecological and Evolutionary Forces Shaping Microbial Diversity in the Human Intestine. Cell 2006, 124, 837–848. [Google Scholar] [CrossRef]
- Higgins, J.; Altman, D.; Sterne, J. Chapter 8: Assessing risk of bias in included studies. In Cochrane Handbook for Systematic Reviews of Interventions, Version 5.2.0; Higgins, J.P.T., Churchill, R., Chandler, J., Cumpston, M.S., Eds.; John Wiley and Sons: Chichester, UK, 2017. [Google Scholar]
- Schünemann, H.J.; Higgins, J.P.; Vist, G.E.; Glasziou, P.; Akl, E.A.; Skoetz, N.; Guyatt, G.H.; Cochrane GRADEing Methods Group; Cochrane Statistical Methods Group. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In Cochrane Handbook for Systematic Reviews of Interventions, Version 6.2; Higgins, J.P., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; John Wiley and Sons: Chichester, UK, 2021. [Google Scholar]
- Czaja, C.A.; Stamm, W.E.; Stapleton, A.E.; Roberts, P.L.; Hawn, T.R.; Scholes, D.; Samadpour, M.; Hultgren, S.J.; Hooton, T.M. Prospective Cohort Study of Microbial and Inflammatory Events Immediately Preceding Escherichia coli Recurrent Urinary Tract Infection in Women. J. Infect. Dis. 2009, 200, 528–536. [Google Scholar] [CrossRef]
- Ravel, J.; Gajer, P.; Abdo, Z.; Schneider, G.M.; Koenig, S.S.K.; McCulle, S.L.; Karlebach, S.; Gorle, R.; Russell, J.; Tacket, C.O.; et al. Vaginal microbiome of reproductive-age women. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. S1), 4680–4687. [Google Scholar] [CrossRef]
- Romero, R.; Hassan, S.S.; Gajer, P.; Tarca, A.L.; Fadrosh, D.W.; Nikita, L.; Galuppi, M.; Lamont, R.F.; Chaemsaithong, P.; Miranda, J.; et al. The composition and stability of the vaginal microbiota of normal pregnant women is different from that of non-pregnant women. Microbiome 2014, 2, 4. [Google Scholar] [CrossRef]
- Tamarelle, J.; Thiébaut, A.; de Barbeyrac, B.; Bébéar, C.; Ravel, J.; Delarocque-Astagneau, E. The vaginal microbiota and its association with human papillomavirus, Chlamydia trachomatis, Neisseria gonorrhoeae and Mycoplasma genitalium infections: A systematic review and meta-analysis. Clin. Microbiol. Infect. 2019, 25, 35–47. [Google Scholar] [CrossRef]
- Borges, S.; Silva, J.; Teixeira, P. The role of lactobacilli and probiotics in maintaining vaginal health. Arch. Gynecol. Obstet. 2013, 289, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Tachedjian, G.; Aldunate, M.; Bradshaw, C.S.; Cone, R.A. The role of lactic acid production by probiotic Lactobacillus species in vaginal health. Res. Microbiol. 2017, 168, 782–792. [Google Scholar] [CrossRef] [PubMed]
- Lewis, A.L.; Gilbert, N.M. Roles of the vagina and the vaginal microbiota in urinary tract infection: Evidence from clinical correlations and experimental models. GMS Infect. Dis. 2020, 8, DOC02. [Google Scholar] [CrossRef] [PubMed]
- Hudson, P.L.; Hung, K.J.; Bergerat, A.; Mitchell, C. Effect of Vaginal Lactobacillus Species on Escherichia coli Growth. Urogynecology 2020, 26, 146–151. [Google Scholar] [CrossRef]
- Vagios, S.; Hesham, H.; Mitchell, C. Understanding the potential of lactobacilli in recurrent UTI prevention. Microb. Pathog. 2020, 148, 104544. [Google Scholar] [CrossRef]
- Pfau, A.; Sacks, T. The Bacterial Flora of the Vaginal Vestibule, Urethra and Vagina in Premenopausal Women with Recurrent Urinary Tract Infections. J. Urol. 1981, 126, 630–634. [Google Scholar] [CrossRef]
- Gupta, K.; Stapleton, A.; Hooton, T.M.; Roberts, P.L.; Fennell, C.L.; Stamm, W.E. Inverse Association of H2O2-Producing Lactobacilli and Vaginal Escherichia coli Colonization in Women with Recurrent Urinary Tract Infections. J. Infect. Dis. 1998, 178, 446–450. [Google Scholar] [CrossRef]
- Hooton, T.M.; Fihn, S.D.; Johnson, C.; Roberts, P.L.; Stamm, W.E. Association between bacterial vaginosis and acute cystitis in women using diaphragms. Arch. Intern. Med. 1989, 149, 1932–1936. [Google Scholar] [CrossRef]
- Hooton, T.M.; Roberts, P.L.; Stamm, W.E. Effects of Recent Sexual Activity and Use of a Diaphragm on the Vaginal Microflora. Clin. Infect. Dis. 1994, 19, 274–278. [Google Scholar] [CrossRef]
- Harmanli, O.H.; Cheng, G.Y.; Nyirjesy, P.; Chatwani, A.; Gaughan, J.P. Urinary Tract Infections in Women with Bacterial Vaginosis. Obstet. Gynecol. 2000, 95, 710–712. [Google Scholar] [CrossRef]
- Stapleton, A.E.; Au-Yeung, M.; Hooton, T.M.; Fredricks, D.N.; Roberts, P.L.; Czaja, C.A.; Yarova-Yarovaya, Y.; Fiedler, T.; Cox, M.; Stamm, W.E. Randomized, Placebo-Controlled Phase 2 Trial of a Lactobacillus crispatus Probiotic Given Intravaginally for Prevention of Recurrent Urinary Tract Infection. Clin. Infect. Dis. 2011, 52, 1212–1217. [Google Scholar] [CrossRef] [PubMed]
- Amatya, R.; Bhattarai, S.; Mandal, P.K.; Tuladhar, H.; Karki, B.M.S. Urinary tract infection in vaginitis: A condition often overlooked. Nepal. Med. Coll. J. 2013, 15, 65–67. [Google Scholar]
- Navas-Nacher, E.L.; Dardick, F.; Venegas, M.F.; Anderson, B.E.; Schaeffer, A.J.; Duncan, J.L. Relatedness of Escherichia coli Colonizing Women Longitudinally. Mol. Urol. 2001, 5, 31–36. [Google Scholar] [CrossRef]
- Muder, R.R.; Brennen, C.; Rihs, J.D.; Wagener, M.M.; Obman, A.; Stout, J.E.; Yu, V.L. Isolation of Staphylococcus aureus from the Urinary Tract: Association of Isolation with Symptomatic Urinary Tract Infection and Subsequent Staphylococcal Bacteremia. Clin. Infect. Dis. 2006, 42, 46–50. [Google Scholar] [CrossRef]
- Baraboutis, I.G.; Tsagalou, E.P.; Lepinski, J.L.; Papakonstantinou, I.; Papastamopoulos, V.; Skoutelis, A.T.; Johnson, S. Primary Staphylococcus aureus urinary tract infection: The role of undetected hematogenous seeding of the urinary tract. Eur. J. Clin. Microbiol. Infect. Dis. 2010, 29, 1095–1101. [Google Scholar] [CrossRef]
- Gilbert, N.M.; O’Brien, V.P.; Hultgren, S.; Macones, G.; Lewis, W.G.; Lewis, A.L. Urinary Tract Infection as a Preventable Cause of Pregnancy Complications: Opportunities, Challenges, and a Global Call to Action. Glob. Adv. Health Med. 2013, 2, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Kline, K.A.; Lewis, A.L. Gram-Positive Uropathogens, Polymicrobial Urinary Tract Infection, and the Emerging Microbiota of the Urinary Tract. Microbiol. Spectr. 2016, 4, UTI-0012-2012. [Google Scholar] [CrossRef] [PubMed]
- Allsworth, J.E.; Lewis, V.A.; Peipert, J.F. Viral Sexually Transmitted Infections and Bacterial Vaginosis: 2001–2004 National Health and Nutrition Examination Survey Data. Sex. Transm. Dis. 2008, 35, 791–796. [Google Scholar] [CrossRef]
- Klein, S.; Nurjadi, D.; Horner, S.; Heeg, K.; Zimmermann, S.; Burckhardt, I. Significant increase in cultivation of Gardnerella vaginalis, Alloscardovia omnicolens, Actinotignum schaalii, and Actinomyces spp. in urine samples with total laboratory automation. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 1305–1311. [Google Scholar] [CrossRef]
- Sumati, A.; Saritha, N. Association of urinary tract infection in women with bacterial vaginosis. J. Glob. Infect. Dis. 2009, 1, 151–152. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, N.M.; O’Brien, V.P.; Lewis, A.L. Transient microbiota exposures activate dormant Escherichia coli infection in the bladder and drive severe outcomes of recurrent disease. PLoS Pathog. 2017, 13, e1006238. [Google Scholar] [CrossRef] [PubMed]
- Donders, G.G.; Vereecken, A.; Bosmans, E.; Dekeersmaecker, A.; Salembier, G.; Spitz, B. Definition of a type of abnormal vaginal flora that is distinct from bacterial vaginosis: Aerobic vaginitis. BJOG Int. J. Obstet. Gynaecol. 2002, 109, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.K.; Ulett, K.B.; Steele, M.; Benjamin, W.H., Jr.; Ulett, G.C. Prognostic value of semi-quantitative bacteruria counts in the diagnosis of group B streptococcus urinary tract infection: A 4-year retrospective study in adult patients. BMC Infect. Dis. 2012, 12, 273. [Google Scholar] [CrossRef] [PubMed]
- Gottschick, C.; Deng, Z.-L.; Vital, M.; Masur, C.; Abels, C.; Pieper, D.H.; Wagner-Döbler, I. The urinary microbiota of men and women and its changes in women during bacterial vaginosis and antibiotic treatment. Microbiome 2017, 5, 99. [Google Scholar] [CrossRef]
- Groah, S.L.; Pérez-Losada, M.; Caldovic, L.; Ljungberg, I.H.; Sprague, B.M.; Castro-Nallar, E.; Chandel, N.J.; Hsieh, M.H.; Pohl, H. Redefining Healthy Urine: A Cross-Sectional Exploratory Metagenomic Study of People with and Without Bladder Dysfunction. J. Urol. 2016, 196, 579–587. [Google Scholar] [CrossRef]
- Forster, C.S.; Pohl, H. Diagnosis of Urinary Tract Infection in the Neuropathic Bladder: Changing the Paradigm to Include the Microbiome. Top. Spinal Cord Inj. Rehabil. 2019, 25, 222–227. [Google Scholar] [CrossRef]
- Whiteside, S.A.; Razvi, H.; Dave, S.; Reid, G.; Burton, J.P. The microbiome of the urinary tract—A role beyond infection. Nat. Rev. Urol. 2015, 12, 81–90. [Google Scholar] [CrossRef]
- Atassi, F.; Ahn, D.L.P.V.; Moal, V.L.-L. Diverse Expression of Antimicrobial Activities Against Bacterial Vaginosis and Urinary Tract Infection Pathogens by Cervicovaginal Microbiota Strains of Lactobacillus gasseri and Lactobacillus crispatus. Front. Microbiol. 2019, 10, 2900. [Google Scholar] [CrossRef]
- De Seta, F.; Lonnee-Hoffmann, R.M.; Campisciano, G.; Comar, M.; Verstraelen, H.M.; Vieira-Baptista, P.; Ventolini, G.M.; Lev-Sagie, A. The Vaginal Microbiome: III. The Vaginal Microbiome in Various Urogenital Disorders. J. Low. Genit. Tract Dis. 2022, 26, 85–92. [Google Scholar] [CrossRef]
- Morand, A.; Cornu, F.; Dufour, J.-C.; Tsimaratos, M.; Lagier, J.-C.; Raoult, D. Human Bacterial Repertoire of the Urinary Tract: A Potential Paradigm Shift. J. Clin. Microbiol. 2019, 57, e00675-18. [Google Scholar] [CrossRef]
- Dubourg, G.; Morand, A.; Mekhalif, F.; Godefroy, R.; Corthier, A.; Yacouba, A.; Diakite, A.; Cornu, F.; Cresci, M.; Brahimi, S.; et al. Deciphering the Urinary Microbiota Repertoire by Culturomics Reveals Mostly Anaerobic Bacteria from the Gut. Front. Microbiol. 2020, 11, 513305. [Google Scholar] [CrossRef]
- Neugent, M.L.; Hulyalkar, N.V.; Nguyen, V.H.; Zimmern, P.E.; De Nisco, N.J. Advances in Understanding the Human Urinary Microbiome and Its Potential Role in Urinary Tract Infection. Mbio 2020, 11, e00218-20. [Google Scholar] [CrossRef]
- Bossa, L.; Kline, K.; McDougald, D.; Lee, B.B.; Rice, S.A. Urinary catheter-associated microbiota change in accordance with treatment and infection status. PLoS ONE 2017, 12, e0177633. [Google Scholar] [CrossRef] [PubMed]
- Burnett, L.A.; Hochstedler, B.R.; Weldon, K.; Wolfe, A.J.; Brubaker, L. Recurrent urinary tract infection: Association of clinical profiles with urobiome composition in women. Neurourol. Urodyn. 2021, 40, 1479–1489. [Google Scholar] [CrossRef] [PubMed]
- Elliott, T.; Reed, L.; Slack, R.; Bishop, M. Bacteriology and ultrastructure of the bladder in patients with urinary tract infections. J. Infect. 1985, 11, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Robino, L.; Scavone, P.; Araujo, L.; Algorta, G.; Zunino, P.; Pírez, M.C.; Vignoli, R. Intracellular Bacteria in the Pathogenesis of Escherichia coli Urinary Tract Infection in Children. Clin. Infect. Dis. 2014, 59, e158–e164. [Google Scholar] [CrossRef]
- Rosen, D.A.; Hooton, T.M.; Stamm, W.E.; Humphrey, P.A.; Hultgren, S.J. Detection of Intracellular Bacterial Communities in Human Urinary Tract Infection. PLoS Med. 2007, 4, e329. [Google Scholar] [CrossRef]
- Liu, S.-C.; Han, X.-M.; Shi, M.; Pang, Z.-L. Persistence of uropathogenic Escherichia Coli in the bladders of female patients with sterile urine after antibiotic therapies. J. Huazhong Univ. Sci. Technol. 2016, 36, 710–715. [Google Scholar] [CrossRef]
- De Nisco, N.J.; Neugent, M.; Mull, J.; Chen, L.; Kuprasertkul, A.; de Souza Santos, M.; Palmer, K.L.; Zimmern, P.; Orth, K. Direct detection of tissue-resident bacteria and chronic inflammation in the bladder wall of postmenopausal women with recurrent urinary tract infection. J. Mol. Biol. 2019, 431, 4368–4379. [Google Scholar] [CrossRef] [PubMed]
- Ejrnaes, K.; Sandvang, D.; Lundgren, B.; Ferry, S.; Holm, S.; Monsen, T.; Lundholm, R.; Frimodt-Moller, N. Pulsed-Field Gel Electrophoresis Typing of Escherichia coli Strains from Samples Collected before and after Pivmecillinam or Placebo Treatment of Uncomplicated Community-Acquired Urinary Tract Infection in Women. J. Clin. Microbiol. 2006, 44, 1776–1781. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Ma, Y.; Zhao, Q.; Wang, L.; Guo, L.; Ye, L.; Zhang, Y.; Yang, J. Similarity and Divergence of Phylogenies, Antimicrobial Susceptibilities, and Virulence Factor Profiles of Escherichia coli Isolates Causing Recurrent Urinary Tract Infections That Persist or Result from Reinfection. J. Clin. Microbiol. 2012, 50, 4002–4007. [Google Scholar] [CrossRef] [PubMed]
- Skjøt-Rasmussen, L.; Olsen, S.; Jakobsen, L.; Ejrnæs, K.; Scheutz, F.; Lundgren, B.; Frimodt-Møller, N.; Hammerum, A. Escherichia coli clonal group A causing bacteraemia of urinary tract origin. Clin. Microbiol. Infect. 2013, 19, 656–661. [Google Scholar] [CrossRef] [PubMed]
- Kõljalg, S.; Truusalu, K.; Stsepetova, J.; Pai, K.; Vainumäe, I.; Sepp, E.; Mikelsaar, M. The Escherichia coli phylogenetic group B2 with integrons prevails in childhood recurrent urinary tract infections. Apmis 2014, 122, 452–458. [Google Scholar] [CrossRef]
- Yoo, J.-J.; Song, J.S.; Bin Kim, W.; Yun, J.; Shin, H.B.; Jang, M.-A.; Ryu, C.B.; Kim, S.S.; Chung, J.C.; Kuk, J.C.; et al. Gardnerella vaginalis in Recurrent Urinary Tract Infection Is Associated with Dysbiosis of the Bladder Microbiome. J. Clin. Med. 2022, 11, 2295. [Google Scholar] [CrossRef]
- Stapleton, A.E. The Vaginal Microbiota and Urinary Tract Infection. Microbiol. Spectr. 2016, 4, 79–86. [Google Scholar] [CrossRef]
- Raz, R. Urinary Tract Infection in Postmenopausal Women. Korean J. Urol. 2011, 52, 801–808. [Google Scholar] [CrossRef]
- Muhleisen, A.L.; Herbst-Kralovetz, M.M. Menopause and the vaginal microbiome. Maturitas 2016, 91, 42–50. [Google Scholar] [CrossRef]
- Mitchell, C.M.; Waetjen, L.E. Genitourinary changes with aging. Obstet. Gynecol. Clin. N. Am. 2018, 45, 737–750. [Google Scholar] [CrossRef]
- Bhide, A.; Tailor, V.; Khullar, V. Interstitial cystitis/bladder pain syndrome and recurrent urinary tract infection and the potential role of the urinary microbiome. Post Reprod. Health 2020, 26, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.; Brubaker, L. The etiology and management of recurrent urinary tract infections in postmenopausal women. Climacteric 2019, 22, 242–249. [Google Scholar] [CrossRef]
- Hugenholtz, F.; van der Veer, C.; Terpstra, M.L.; Borgdorff, H.; van Houdt, R.; Bruisten, S.; Geerlings, S.E.; van de Wijgert, J.H.H.M. Urine and vaginal microbiota compositions of postmenopausal and premenopausal women differ regardless of recurrent urinary tract infection and renal transplant status. Sci. Rep. 2022, 12, 2698. [Google Scholar] [CrossRef]
- Vaughan, M.H.; Mao, J.; Karstens, L.A.; Ma, L.; Amundsen, C.L.; Schmader, K.E.; Siddiqui, N.Y. The Urinary Microbiome in Postmenopausal Women with Recurrent Urinary Tract Infections. J. Urol. 2021, 206, 1222–1231. [Google Scholar] [CrossRef] [PubMed]
- Curtiss, N.; Balachandran, A.; Krska, L.; Peppiatt-Wildman, C.; Wildman, S.; Duckett, J. Age, menopausal status and the bladder microbiome. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 228, 126–129. [Google Scholar] [CrossRef]
- Biagi, E.; Candela, M.; Fairweather-Tait, S.; Franceschi, C.; Brigidi, P. Ageing of the human metaorganism: The microbial counterpart. Age 2011, 34, 247–267. [Google Scholar] [CrossRef]
- Yoo, J.-J.; Shin, H.B.; Song, J.S.; Kim, M.; Yun, J.; Kim, Z.; Lee, Y.M.; Lee, S.W.; Lee, K.W.; Kim, W.b.; et al. Urinary Microbiome Characteristics in Female Patients with Acute Uncomplicated Cystitis and Recurrent Cystitis. J. Clin. Med. 2021, 10, 1097. [Google Scholar] [CrossRef]
- Ansaldi, Y.; Weber, B.M.d.T. Urinary tract infections in pregnancy. Clin. Microbiol. Infect. 2022. [Google Scholar] [CrossRef] [PubMed]
- DiGiulio, D.B.; Callahan, B.J.; McMurdie, P.J.; Costello, E.K.; Lyell, D.J.; Robaczewska, A.; Sun, C.L.; Goltsman, D.S.A.; Wong, R.J.; Shaw, G.; et al. Temporal and spatial variation of the human microbiota during pregnancy. Proc. Natl. Acad. Sci. USA 2015, 112, 11060–11065. [Google Scholar] [CrossRef]
- Fettweis, J.M.; Serrano, M.G.; Brooks, J.P.; Edwards, D.J.; Girerd, P.H.; Parikh, H.I.; Huang, B.; Arodz, T.J.; Edupuganti, L.; Glascock, A.L.; et al. The vaginal microbiome and preterm birth. Nat. Med. 2019, 25, 1012–1021. [Google Scholar] [CrossRef]
- Dominguez-Bello, M.G. Gestational shaping of the maternal vaginal microbiome. Nat. Med. 2019, 25, 882–883. [Google Scholar] [CrossRef] [PubMed]
- Hooton, T.M.; Gupta, K. Recurrent Urinary Tract Infection in Women; UpToDate: Waltham, MA, USA, 2016. [Google Scholar]
- Eschenbach, D.A.; Patton, D.L.; Meier, A.; Thwin, S.S.; Aura, J.; Stapleton, A.; Hooton, T.M. Effects of oral contraceptive pill use on vaginal flora and vaginal epithelium. Contraception 2000, 62, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Hillier, S.L.; Hooton, T.M.; Roberts, P.L.; Stamm, W.E. Effects of Contraceptive Method on the Vaginal Microbial Flora: A Prospective Evaluation. J. Infect. Dis. 2000, 181, 595–601. [Google Scholar] [CrossRef]
- Hooton, T.M.; Hillier, S.; Johnson, C.; Roberts, P.L.; Stamm, W.E. Escherichia coli Bacteriuria and Contraceptive Method. JAMA 1991, 265, 64–69. [Google Scholar] [CrossRef]
- Hooton, T.M.; Scholes, D.; Roberts, P.L.; Stapleton, A.; Stergachis, A.; Stamm, W.E. A prospective cohort study of the association between UTI and contraceptive method. Abstr. Intersci. Conf. Antimicrob. Agents Chemother. 1994, 34, 134. [Google Scholar]
- Hooton, T.M.; Fennell, C.L.; Clark, A.M.; Stamm, W.E. Nonoxynol-9: Differential Antibacterial Activity and Enhancement of Bacterial Adherence to Vaginal Epithelial Cells. J. Infect. Dis. 1991, 164, 1216–1219. [Google Scholar] [CrossRef] [PubMed]
- Achilles, S.L.; Hillier, S.L. The complexity of contraceptives: Understanding their impact on genital immune cells and vaginal microbiota. AIDS 2013, 27 (Suppl 1), S5–S15. [Google Scholar] [CrossRef]
- Raz, R.; Stamm, W.E. A Controlled Trial of Intravaginal Estriol in Postmenopausal Women with Recurrent Urinary Tract Infections. N. Engl. J. Med. 1993, 329, 753–756. [Google Scholar] [CrossRef]
- Stapleton, A.; Latham, R.H.; Johnson, C.; Stamm, W.E. Postcoital antimicrobial prophylaxis for recurrent urinary tract infection. A randomized, double-blind, placebo-controlled trial. JAMA 1990, 264, 703–706. [Google Scholar] [CrossRef]
- Stamatiou, C.; Bovis, C.; Panagopoulos, P.; Petrakos, G.; Economou, A.; Lycoudt, A. Sex-induced cystitis--patient burden and other epidemiological features. Clin. Exp. Obstet. Gynecol. 2005, 32, 180–182. [Google Scholar]
- Hooton, T.M.; Scholes, D.; Hughes, J.P.; Winter, C.; Roberts, P.L.; Stapleton, A.E.; Stergachis, A.; Stamm, W.E. A Prospective Study of Risk Factors for Symptomatic Urinary Tract Infection in Young Women. N. Engl. J. Med. 1996, 335, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Scholes, D.; Hooton, T.M.; Roberts, P.L.; Stapleton, A.; Gupta, K.; Stamm, W.E. Risk Factors for Recurrent Urinary Tract Infection in Young Women. J. Infect. Dis. 2000, 182, 1177–1182. [Google Scholar] [CrossRef]
- Shreiner, A.B.; Kao, J.Y.; Young, V.B. The gut microbiome in health and in disease. Curr. Opin. Gastroenterol. 2015, 31, 69–75. [Google Scholar] [CrossRef]
- Cumpanas, A.A.; Bratu, O.G.; Bardan, R.; Ferician, O.C.; Cumpanas, A.D.; Horhat, F.G.; Licker, M.; Pricop, C.; Cretu, O.M. Urinary Microbiota—Are We Ready for Prime Time? A Literature Review of Study Methods’ Critical Steps in Avoiding Contamination and Minimizing Biased Results. Diagnostics 2020, 10, 343. [Google Scholar] [CrossRef] [PubMed]
- Meštrović, T.; Matijašić, M.; Perić, M.; Čipčić Paljetak, H.; Barešić, A.; Verbanac, D. The Role of Gut, Vaginal, and Urinary Microbiome in Urinary Tract Infections: From Bench to Bedside. Diagnostics 2021, 11, 7. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, A.J.; Brubaker, L. Urobiome updates: Advances in urinary microbiome research. Nat. Rev. Urol. 2019, 16, 73–74. [Google Scholar] [CrossRef]
- Lewis, D.A.; Brown, R.; Williams, J.; White, P.; Jacobson, S.K.; Marchesi, J.R.; Drake, M.J. The human urinary microbiome; bacterial DNA in voided urine of asymptomatic adults. Front. Cell. Infect. Microbiol. 2013, 3, 41. [Google Scholar] [CrossRef]
- Finucane, T.E. ‘Urinary tract infection’ and the microbiome. Am. J. Med. 2017, 130, e97–e98. [Google Scholar] [CrossRef]
- Ackerman, A.L.; Chai, T.C. The Bladder is Not Sterile: An Update on the Urinary Microbiome. Curr. Bladder Dysfunct. Rep. 2019, 14, 331–341. [Google Scholar] [CrossRef]
- Amabebe, E.; Anumba, D.O.C. Female Gut and Genital Tract Microbiota-Induced Crosstalk and Differential Effects of Short-Chain Fatty Acids on Immune Sequelae. Front. Immunol. 2020, 11, 2184. [Google Scholar] [CrossRef]
- Brubaker, L.; Wolfe, A.J. The new world of the urinary microbiota in women. Am. J. Obstet. Gynecol. 2015, 213, 644–649. [Google Scholar] [CrossRef]
- Wolfe, A.J.; Toh, E.; Shibata, N.; Rong, R.; Kenton, K.; FitzGerald, M.; Mueller, E.R.; Schreckenberger, P.; Dong, Q.; Nelson, D.E.; et al. Evidence of Uncultivated Bacteria in the Adult Female Bladder. J. Clin. Microbiol. 2012, 50, 1376–1383. [Google Scholar] [CrossRef]
- Hilt, E.E.; McKinley, K.; Pearce, M.M.; Rosenfeld, A.B.; Zilliox, M.J.; Mueller, E.R.; Brubaker, L.; Gai, X.; Wolfe, A.J.; Schreckenberger, P.C. Urine Is Not Sterile: Use of Enhanced Urine Culture Techniques to Detect Resident Bacterial Flora in the Adult Female Bladder. J. Clin. Microbiol. 2014, 52, 871–876. [Google Scholar] [CrossRef]
- Price, T.K.; Dune, T.; Hilt, E.E.; Thomas-White, K.J.; Kliethermes, S.; Brincat, C.; Brubaker, L.; Wolfe, A.J.; Mueller, E.R.; Schreckenberger, P.C. The Clinical Urine Culture: Enhanced Techniques Improve Detection of Clinically Relevant Microorganisms. J. Clin. Microbiol. 2016, 54, 1216–1222. [Google Scholar] [CrossRef]
- Pearce, M.M.; Hilt, E.E.; Rosenfeld, A.B.; Zilliox, M.J.; Thomas-White, K.; Fok, C.; Kliethermes, S.; Schreckenberger, P.C.; Brubaker, L.; Gai, X.; et al. The Female Urinary Microbiome: A Comparison of Women with and without Urgency Urinary Incontinence. Mbio 2014, 5, e01283-14. [Google Scholar] [CrossRef] [PubMed]
- Khasriya, R.; Sathiananthamoorthy, S.; Ismail, S.; Kelsey, M.; Wilson, M.; Rohn, J.L.; Malone-Lee, J. Spectrum of Bacterial Colonization Associated with Urothelial Cells from Patients with Chronic Lower Urinary Tract Symptoms. J. Clin. Microbiol. 2013, 51, 2054–2062. [Google Scholar] [CrossRef] [PubMed]
- McDonald, M.; Kameh, D.; Johnson, M.E.; Johansen, T.E.B.; Albala, D.; Mouraviev, V. A Head-to-Head Comparative Phase II Study of Standard Urine Culture and Sensitivity Versus DNA Next-generation Sequencing Testing for Urinary Tract Infections. Rev. Urol. 2017, 19, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, T.; Watanabe, N.; Inoue, S.; Aoki, H.; Tsuji, T.; Yamamoto, B.; Yanagi, H.; Oki, M.; Kryukov, K.; Nakagawa, S.; et al. Usefulness of next-generation DNA sequencing for the diagnosis of urinary tract infection. Drug Discov. Ther. 2020, 14, 42–49. [Google Scholar] [CrossRef]
- Bennett, J.E.; Dolin, R.; Blaser, M.J. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases; Elsevier Health Sciences: Philadelphia, PA, USA, 2014; Volume 2. [Google Scholar]
- Hooton, T.M. Recurrent urinary tract infection in women. Int. J. Antimicrob. Agents 2001, 17, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Brannon, J.R.; Dunigan, T.L.; Beebout, C.J.; Ross, T.; Wiebe, M.A.; Reynolds, W.S.; Hadjifrangiskou, M. Invasion of vaginal epithelial cells by uropathogenic Escherichia coli. Nat. Commun. 2020, 11, 2803. [Google Scholar] [CrossRef]
- Rosen, D.A.; Pinkner, J.S.; Jones, J.M.; Walker, J.N.; Clegg, S.; Hultgren, S.J. Utilization of an Intracellular Bacterial Community Pathway in Klebsiella pneumoniae Urinary Tract Infection and the Effects of FimK on Type 1 Pilus Expression. Infect. Immun. 2008, 76, 3337–3345. [Google Scholar] [CrossRef] [PubMed]
- Robino, L.; Scavone, P.; Araujo, L.; Algorta, G.; Zunino, P.; Vignoli, R. Detection of intracellular bacterial communities in a child with Escherichia coli recurrent urinary tract infections. Pathog. Dis. 2013, 68, 78–81. [Google Scholar] [CrossRef]
- Schilling, J.D.; Lorenz, R.G.; Hultgren, S.J. Effect of Trimethoprim-Sulfamethoxazole on Recurrent Bacteriuria and Bacterial Persistence in Mice Infected with Uropathogenic Escherichia coli. Infect. Immun. 2002, 70, 7042–7049. [Google Scholar] [CrossRef]
- Mysorekar, I.U.; Hultgren, S.J. Mechanisms of uropathogenic Escherichia coli persistence and eradication from the urinary tract. Proc. Natl. Acad. Sci. USA 2006, 103, 14170–14175. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; Ahn, J.H.; Choi, W.S.; Park, H.K.; Kim, S.; Paick, S.H.; Kim, H.G. What is the Cause of Recurrent Urinary Tract Infection? Contemporary Microscopic Concepts of Pathophysiology. Int. Neurourol. J. 2021, 25, 192–201. [Google Scholar] [CrossRef]
- Simpson, B.W.; May, J.M.; Sherman, D.J.; Kahne, D.; Ruiz, N. Lipopolysaccharide transport to the cell surface: Biosynthesis and extraction from the inner membrane. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015, 370, 20150029. [Google Scholar] [CrossRef]
- Zhang, G.; Meredith, T.C.; Kahne, D. On the essentiality of lipopolysaccharide to Gram-negative bacteria. Curr. Opin. Microbiol. 2013, 16, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Hunstad, D.A.; Justice, S.S.; Hung, C.S.; Lauer, S.R.; Hultgren, S.J. Suppression of Bladder Epithelial Cytokine Responses by Uropathogenic Escherichia coli. Infect. Immun. 2005, 73, 3999–4006. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, H.; Kouadir, M.; Song, H.; Shi, F. Recent advances in the mechanisms of NLRP3 inflammasome activation and its inhibitors. Cell Death Dis. 2019, 10, 128. [Google Scholar] [CrossRef]
- Hughes, F.M., Jr.; Vivar, N.P.; Kennis, J.G.; Pratt-Thomas, J.D.; Lowe, D.W.; Shaner, B.E.; Nietert, P.J.; Spruill, L.S.; Purves, J.T. Inflammasomes are important mediators of cyclo¬phosphamide-induced bladder inflammation. Am. J. Physiol. Ren. Physiol. 2014, 306, F299–F308. [Google Scholar] [CrossRef]
- Jaillon, S.; Moalli, F.; Ragnarsdottir, B.; Bonavita, E.; Puthia, M.; Riva, F.; Barbati, E.; Nebuloni, M.; Krajinovic, L.C.; Markotic, A.; et al. The Humoral Pattern Recognition Molecule PTX3 Is a Key Component of Innate Immunity against Urinary Tract Infection. Immunity 2014, 40, 621–632. [Google Scholar] [CrossRef]
- Bishop, B.L.; Duncan, M.J.; Song, J.; Li, G.; Zaas, D.; Abraham, S.N. Cyclic AMP–regulated exocytosis of Escherichia coli from infected bladder epithelial cells. Nat. Med. 2007, 13, 625–630. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, V.P.; Lewis, A.L.; Gilbert, N.M. Bladder Exposure to Gardnerella Activates Host Pathways Necessary for Escherichia coli Recurrent UTI. Front. Cell. Infect. Microbiol. 2021, 11, 788229. [Google Scholar] [CrossRef]
- Yoon, K.; Lee, S.-O.; Cho, S.-D.; Kim, K.; Khan, S.; Safe, S. Activation of nuclear TR3 (NR4A1) by a diindolylmethane analog induces apoptosis and proapoptotic genes in pancreatic cancer cells and tumors. Carcinog. 2011, 32, 836–842. [Google Scholar] [CrossRef]
- Gao, H.; Chen, Z.; Fu, Y.; Yang, X.; Weng, R.; Wang, R.; Lu, J.; Pan, M.; Jin, K.; McElroy, C.; et al. Nur77 exacerbates PC12 cellular injury in vitro by aggravating mitochondrial impairment and endoplasmic reticulum stress. Sci. Rep. 2016, 6, 34403. [Google Scholar] [CrossRef] [PubMed]
- Rajpal, A.; Cho, Y.A.; Yelent, B.; Koza-Taylor, P.H.; Li, D.; Chen, E.; Whang, M.; Kang, C.; Turi, T.G.; Winoto, A. Transcriptional activation of known and novel apoptotic pathways by Nur77 orphan steroid receptor. EMBO J. 2003, 22, 6526–6536. [Google Scholar] [CrossRef] [PubMed]
- Herring, J.A.; Elison, W.S.; Tessem, J.S. Function of Nr4a Orphan Nuclear Receptors in Proliferation, Apoptosis and Fuel Utilization Across Tissues. Cells 2019, 8, 1373. [Google Scholar] [CrossRef]
- Rodríguez-Calvo, R.; Tajes, M.; Vázquez-Carrera, M. The NR4A subfamily of nuclear receptors: Potential new therapeutic targets for the treatment of inflammatory diseases. Expert Opin. Ther. Targets 2017, 21, 291–304. [Google Scholar] [CrossRef]
- Liebmann, M.; Hucke, S.; Koch, K.; Eschborn, M.; Ghelman, J.; Chasan, A.I.; Glander, S.; Schädlich, M.; Kuhlencord, M.; Daber, N.M.; et al. Nur77 serves as a molecular brake of the metabolic switch during T cell activation to restrict autoimmunity. Proc. Natl. Acad. Sci. USA 2018, 115, E8017–E8026. [Google Scholar] [CrossRef]
- Hanna, R.N.; Carlin, L.M.; Hubbeling, H.G.; Nackiewicz, D.; Green, A.M.; Punt, J.A.; Geissmann, F.; Hedrick, C.C. The transcription factor NR4A1 (Nur77) controls bone marrow differentiation and the survival of Ly6C− monocytes. Nat. Immunol. 2011, 12, 778–785. [Google Scholar] [CrossRef]
- O’Brien, V.P.; Joens, M.S.; Lewis, A.L.; Gilbert, N.M. Recurrent Escherichia coli Urinary Tract Infection Triggered by Gardnerella vaginalis Bladder Exposure in Mice. J. Vis. Exp. 2020, 166, e61967. [Google Scholar] [CrossRef]
- Kirjavainen, P.V.; Pautler, S.; Baroja, M.L.; Anukam, K.; Crowley, K.; Carter, K.; Reid, G. Abnormal Immunological Profile and Vaginal Microbiota in Women Prone to Urinary Tract Infections. Clin. Vaccine Immunol. 2009, 16, 29–36. [Google Scholar] [CrossRef]
- Neugent, M.L.; Kumar, A.; Hulyalkar, N.V.; Lutz, K.C.; Nguyen, V.H.; Fuentes, J.L.; Zhang, C.; Nguyen, A.; Sharon, B.M.; Kuprasertkul, A.; et al. Recurrent urinary tract infection and estrogen shape the taxonomic ecology and function of the postmenopausal urogenital microbiome. Cell Rep. Med. 2022, 3, 100753. [Google Scholar] [CrossRef]
- Akgul, T.; Karakan, T. The role of probiotics in women with recurrent urinary tract infections. Turk. J. Urol. 2018, 44, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Boswell-Ruys, C.L.; Toh, S.-L.; Lee, B.S.B.; Simpson, J.M.; Clezy, K.R. Probiotics for preventing urinary tract infection in people with neuropathic bladder. Cochrane Database Syst. Rev. 2017, 9, 010723. [Google Scholar] [CrossRef]
- Goneau, L.W.; Yeoh, N.S.; MacDonald, K.W.; Cadieux, P.A.; Burton, J.P.; Razvi, H.; Reid, G. Selective Target Inactivation Rather than Global Metabolic Dormancy Causes Antibiotic Tolerance in Uropathogens. Antimicrob. Agents Chemother. 2014, 58, 2089–2097. [Google Scholar] [CrossRef]
- Uehara, S.; Monden, K.; Nomoto, K.; Seno, Y.; Kariyama, R.; Kumon, H. A pilot study evaluating the safety and effectiveness of Lactobacillus vaginal suppositories in patients with recurrent urinary tract infection. Int. J. Antimicrob. Agents 2006, 28 (Suppl. 1), 30–34. [Google Scholar] [CrossRef]
- Sadahira, T.; Wada, K.; Araki, M.; Mitsuhata, R.; Yamamoto, M.; Maruyama, Y.; Iwata, T.; Watanabe, M.; Watanabe, T.; Kariyama, R.; et al. Efficacy of Lactobacillus vaginal suppositories for the prevention of recurrent cystitis: A phase II clinical trial. Int. J. Urol. 2021, 28, 1026–1031. [Google Scholar] [CrossRef]
- Reid, G.; Charbonneau, D.; Erb, J.; Kochanowski, B.; Beuerman, D.; Poehner, R.; Bruce, A.W. Oral use of Lactobacillus rhamnosus GR-1 and L. fermentum RC-14 significantly alters vaginal flora: Randomized, placebo-controlled trial in 64 healthy women. FEMS Immunol. Med. Microbiol. 2003, 35, 131–134. [Google Scholar] [CrossRef]
- Garofalo, L.; Nakama, C.; Hanes, D.; Zwickey, H. Whole-Person, Urobiome-Centric Therapy for Uncomplicated Urinary Tract Infection. Antibiotics 2022, 11, 218. [Google Scholar] [CrossRef]
- Heinemann, C.; Reid, G. Vaginal microbial diversity among postmenopausal women with and without hormone replacement therapy. Can. J. Microbiol. 2005, 51, 777–781. [Google Scholar] [CrossRef]
- Raz, R. Hormone Replacement Therapy or Prophylaxis in Postmenopausal Women with Recurrent Urinary Tract Infection. J. Infect. Dis. 2001, 183, S74–S76. [Google Scholar] [CrossRef] [PubMed]
- Cauci, S.; Driussi, S.; De Santo, D.; Penacchioni, P.; Iannicelli, T.; Lanzafame, P.; De Seta, F.; Quadrifoglio, F.; de Aloysio, D.; Guaschino, S. Prevalence of Bacterial Vaginosis and Vaginal Flora Changes in Peri- and Postmenopausal Women. J. Clin. Microbiol. 2002, 40, 2147–2152. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, B.C. A randomized, open, parallel-group study on the preventive effect of an estradiol-releasing vaginal ring (Estring) on recurrent urinary tract infections in postmenopausal women. Am. J. Obstet. Gynecol. 1999, 180, 1072–1079. [Google Scholar] [CrossRef] [PubMed]
- Krause, M.; Wheeler, T.L., 2nd; Snyder, T.E.; Richter, H.E. Local Effects of Vaginally Administered Estrogen Therapy: A Review. J. Pelvic Med. Surg. 2009, 15, 105–114. [Google Scholar] [CrossRef]
- Mehta, J.; Utkarsh, K.; Fuloria, S.; Singh, T.; Sekar, M.; Salaria, D.; Rolta, R.; Begum, M.Y.; Gan, S.H.; Rani, N.N.I.M.; et al. Antibacterial Potential of Bacopa monnieri (L.) Wettst. and Its Bioactive Molecules against Uropathogens—An In Silico Study to Identify Potential Lead Molecule(s) for the Development of New Drugs to Treat Urinary Tract Infections. Molecules 2022, 27, 4971. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).