Abstract
Acute heart failure (AHF) is a major public health concern, affecting 26 million worldwide. Sodium-glucose cotransporter 2 (SGLT2) inhibitors are a class of glucose-lowering drugs, comprising canagliflozin, dapagliflozin, and empagliflozin that are being explored for AHF. We aim to meta-analyze the effectiveness of SGLT2 inhibitors compared to placebo for primary outcomes including all-cause and cardiovascular mortality, heart failure events, symptomatic improvement, and readmissions. Our secondary outcome is the risk of serious adverse events. This meta-analysis has been designed in accordance with the PRISMA Statement 2020. A systematic search across PubMed, Scopus, and Cochrane Library was conducted through August 13, 2022. The following keywords were utilized: sglt2, sodium-glucose transporter 2 inhibitors, sglt2 inhibitors, decompensated heart failure, de-novo heart failure, and/or acute heart failure. Only randomized controlled trials (RCTs) with adult patients (>18 years), hospitalized with de-novo AHF, acutely decompensated chronic heart failure with reduced, borderline, or preserved ejection, and receiving SGLT2 inhibitors were included. A quantitative analytical methodology was applied where the standardized mean difference (SMD) applying 95% confidence intervals (CI) for continuous outcomes and risk ratio (RR) with 95% CI was yielded. All tests were carried out on Review Manager 5.4 (Cochrane). In total, three RCTs were included pooling in a total of 1831 patients where 49.9% received SGLT2 inhibitors. The mean age was 72.9 years in the interventional group compared to 70.6 years in the placebo. Only 33.7% of the sample was female. The follow-up spanned 2–9 months. Heart failure events were reduced by 62% in the interventional group (RR = 0.66, p < 0.0001). readmissions had a reduced risk of 24% with SGLT2 inhibitors (RR = 0.76, p = 0.03). We assessed the efficacy and safety of SGLT2 inhibitors in preventing complications post-AHF. The odds of all-cause mortality, cardiovascular mortality, heart failure events, and re-admissions rates were substantially reduced within the first 1–9 months of hospitalization.
1. Introduction
Acute heart failure (AHF) is a major public health problem, affecting nearly 6 million people in the United States and 26 million people globally [1]. AHF is defined as a rapid onset or worsening of signs and symptoms of heart failure (HF) that require urgent medical care [2]. With over 1 million annual hospitalizations in the US, AHF is the leading cause of admissions in the elderly [3]. Approximately 1 in 5 patients with AHF are readmitted within 30 days of being discharged and over 3 in 5 patients are readmitted during the first year [4]. The 1-year mortality rate is 10–30% with the highest risk of mortality within 30 days of the index hospitalization [5,6,7]. Despite therapeutic advances in chronic heart failure (CHF) [8], AHF has a dismal prognosis since no therapy is proven to have long-term mortality benefits [9]. Loop diuretics are the mainstay of therapy for AHF but have a negative impact on renal function as well as potential neurohormonal imbalance [10,11,12].
Sodium-glucose cotransporter 2 (SGLT2) inhibitors are a new class of glucose-lowering drugs, including canagliflozin, dapagliflozin, and empagliflozin, that block the SGLT2 protein located in the proximal convoluted tubule of the nephron for adults with type 2 diabetes [13]. The SGLT2 protein is responsible for the resorption of nearly 90% of filtered glucose [14,15,16,17]. In 2015, among patients with cardiovascular disease (CVD), the Empagliflozin Outcome Event Trial in Type 2 Diabetes Mellitus Patients–Removing Excess Glucose (EMPA-REG OUTCOME) trial indicated a significant reduction in composite risk of cardiovascular death, myocardial infarction, or stroke by 14%; the risk of all-cause mortality was reduced by 32% over a mean duration of 3.1 years [18,19]. While such promising evidence was encouraging, there were concerns about several adverse events that include acute kidney injury (AKI), diabetic ketoacidoses (DKA), and urinary tract infections (UTIs) based on initial reports [18,19]. Since then, many randomized controlled trials (RCTs) evaluated SGLT2 as a potential adjunctive pharmacotherapy for HF patients, which has shown significant benefits. SGLT2 inhibitors, empagliflozin, and dapagliflozin demonstrated efficacy for patients with CHF who had a reduced left ventricular ejection fraction (LVEF) [20,21]. More recently, the U.S. Food and Drug Administration (FDA) approved empagliflozin in February 2022, for patients with HF regardless of LVEF to reduce the risk of cardiovascular death and hospitalization [22].
Whether SGLT2 inhibitors provide clinical benefits in patients with AHF is being explored. Despite emerging trials, there are limitations in systematically assessing the efficacy and safety of SGLT2 inhibitors among patients hospitalized with AHF, therefore, our objective is to address the current knowledge gap surrounding the effectiveness of SGLT2 inhibitors compared with placebo in patients with or without type 2 diabetes. Applying different endpoint measures, our primary outcome comprises estimating risks of all-cause and cardiovascular mortality, heart failure events, readmissions, and symptomatic improvement. Our secondary outcome is the risk evaluation of serious adverse events.
2. Methods
2.1. Study Design and Strategy
This study has been designed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement 2020 guidelines [23]. This meta-analysis was registered with PROSPERO 2022 CRD42022365431. A comprehensive literature search of digital databases, including PubMed, Scopus, and Cochrane Library was performed from inception to August 13, 2022. Keywords and medical subject headings (MESH) were combined and run on the PubMed database with the following terms: “sglt2,” “sodium-glucose transporter 2 inhibitors,” “sglt2 inhibitors,” “decompensated heart failure,” “de-novo heart failure,” “acute heart failure”. The Boolean (and/or) logic was applied. Search filters were not applied to ensure maximal, relevant publications were obtained. This strategy was tailored to other databases including Scopus, and Cochrane Library. The reference lists were also reviewed for review articles to identify all relevant studies (umbrella methodology). Potential unpublished studies on ClinicalTrials.gov were also reviewed to locate any ongoing trials and include any relevant data.
2.2. Eligibility Criteria
All RCTs, including patients aged > 18 years, hospitalized with de-novo acute heart failure (AHF) or acutely decompensated chronic heart failure with reduced, borderline, or preserved ejection fraction, and intervention group receiving SGLT2 inhibitors that have been approved or are currently investigational were included. All observational studies, letters to the editor, single-arm studies, and two-arm studies that did not report any outcomes of interest were excluded. There was no restriction placed with respect to the diagnosis of type 2 diabetes or lack thereof. Outcome measures were decided a priori based on already-published data on heart failure (HF) patients who received SGLT2 inhibitors for non-AHF conditions. Efficacy outcomes included all-cause mortality, patients with heart failure events, total heart failure events, cardiovascular mortality, readmissions, and change in Kansas City Cardiomyopathy Questionnaire (KCCQ) Total Symptom Score. Safety outcomes included serious adverse events, acute kidney injury, hepatic injury, hypotension, hypoglycemia, urinary tract infections (UTIs), and diabetic ketoacidosis (DKA).
2.3. Study Selection and Data Extraction
Two investigators (A.S. and Z.S.) independently conducted the screening and finalization of the articles. The titles and abstracts were screened in the first stage by each investigator independently. Any discrepancies were resolved by a third investigator (I.C.O.) in case of any disagreements. Shortlisted articles were screened for full-text eligibility by the two investigators independently. In the final stage, the discrepancies were solved by the third investigator, and the studies were included in the review. Data were systematically collected for efficacy and safety outcomes. For all identified studies, the findings were tabulated as (i) clinical trial identifier, (ii) author-year, (iii) country, (iv) dose of SGLT2, (v) frequency of intervention, (vi) total sample size, (vii) intervention arm sample size, (viii) control arm sample size, (ix) follow-up duration, (x) primary outcome, and (xi) secondary outcome.
2.4. Statistical Analysis
All studies identified from the databases were stored in Endnote X9 (Clarivate Analytics, London, UK). The duplicates were removed using the Endnote X9 deduplication tool. No duplicates were found using the online resources (i.e., ClinicalTrials.Gov). The methodology was both quantitative and analytical to ascertain the benefit of SGLT2 in reducing the risk of adverse outcomes following hospitalization for AHF. The KCCQ scale scores were continuous, and the difference in means along with standard deviation was computed. On noting these values, the standardized mean difference (SMD) was computed, reported as Cohen’s d, applying 95% confidence intervals (CI). A random-effects model was applied as it was assumed that the observed estimates of treatment effect can vary across studies because of real differences in the treatment effect in each study as well as sampling variability (chance). Forest plots were generated for every outcome documenting Risk Ratio (RR), SMD, 95% CI, heterogeneity, and overall results. The formula for RR and SMD is as follows:
The minimum requirement to meta-analyze the findings was two or more studies reporting the same outcome measure. While a funnel plot was not generated to test for publication bias due to the limited number of studies (<10), the heterogeneity between the included studies was tested using the χ2-based Q test and the I2 index. The statistical analysis was conducted using Review Manager 5.4 (RevMan, Cochrane).
2.5. Risk of Bias Assessment
The risk of bias was assessed for the included studies individually by two investigators (A.S. and Z.S.). The risk-of-bias tool for randomized trials (RoB 2) version 2 available in the Cochrane Handbook for Systematic Reviews of Interventions was utilized [24]. Five fixed sets of domains of bias were graded as “low,” “high,” or “some concerns.” The five domains comprised: (i) bias from the randomization process, (ii) bias due to deviations from intended interventions, (iii) bias due to missing outcome data, (iv) bias in the measurement of the outcome, and (v) bias in the selection of reported results. In case of discrepancies between the two investigators, the third investigator (I.C.O.) resolved it through consensus.
3. Results
3.1. PRISMA
We identified 2627 articles, and 1985 titles and abstracts were screened after duplicates were removed. There were 529 articles that met the criteria for full-text review and 3 articles were included. The trial selection process is presented in a PRISMA flow diagram (Figure 1). There was a strong agreement among the reviewers for the inclusion of the articles; the title and abstract screening stage yielded a score of: κ = 0.86; and for full-text screening (κ = 0.88).
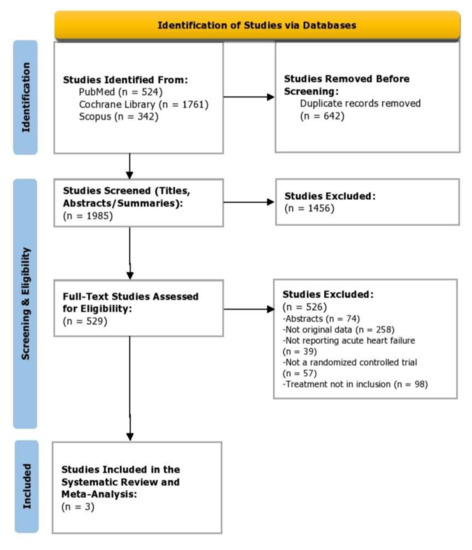
Figure 1.
PRISMA flow diagram depicting study selection.
3.2. Risk of Bias
Each included study was critically appraised using the revised tool for Risk of Bias (ROB 2) for randomized trials at the level of five outcomes: all-cause mortality, heart-failure events, re-admissions, symptom score, and adverse events (Figure 2). All studies were considered to have a “low” risk of bias based on the five pre-specified domains in the ROB-2 tool. One trial was identified as having “some concerns” due to deviation in reporting pre-specified primary outcomes that may introduce bias in the selection of the reported result (Domain 5).
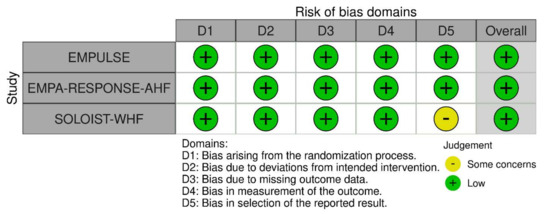
Figure 2.
Risk of bias assessment using the Risk of Bias 2.0 tool [25,26,27].
3.3. Baseline Characteristics
A total of 1831 patients were included of whom 913 received intervention (49.9%). The drug of choice was Empagliflozin in EMPULSE [25] and EMPA-RESPONSE-WHF [26] and Sotagiflozin in SOLOIST-WHF [27]. Baseline characteristics were mostly similar between interventional and control groups. The overall mean age in the interventional arm was 72.9 (SD: 5.3) years. In the control arm, the overall mean age was 70.6 (SD: 2.1) years. Only 33.7% (n = 617) of the total sample was female. Follow-up duration ranged from 60 days to 9 months. A total of 770 (84.3%) of the patients in the intervention arm and 758 (82.6%) of the patients in the control arm had diabetes as a comorbid. Key characteristics are summarized in Table 1.

Table 1.
Trial characteristics of the included studies. Only patients who had acute heart failure with or without diabetes were included in the trials.
3.4. All-Cause Mortality
All three trials reported the data on all-cause mortality at different time points. Compared with the placebo group, the risk of mortality was reduced by 27% in the intervention group (RR: 0.73, 95% CI: 0.49–1.09, p = 0.12, I2 = 18%) (Figure 3). For the sensitivity test, SOLOIST-WHF [27] had the highest weight and was thus removed to determine whether there will be changes in the risk. The post-sensitivity analysis was conducted to further explore the risk after the SOLOIST-WHF trial was removed which had the highest weight [27]. It demonstrated an even larger reduction in risk of death such that the group receiving SGLT2 inhibitors still had a lowered risk of all-cause mortality at 51% compared to the placebo group, which was significant (RR: 0.49, 95% CI: 0.25–0.95, p = 0.04, I2 = 0%). Both EMPA-RESPONSE-AHF [26] and SOLOIST-WHF [27] were analyzed separately as patients with acute decompensated chronic HF (ADCHF) were included in both these trials; the mortality risk reduction was 15% in patients with ADCHF who took SGLT2 inhibitors compared to placebo (RR:0.85, 95% CI: 0.62–1.15, p = 0.39, I2 = 0%).
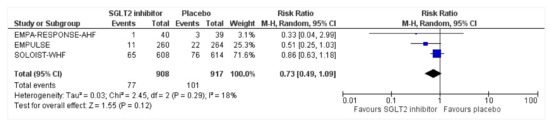
Figure 3.
Forest plot for all-cause mortality of patients with acute heart failure receiving sodium-glucose cotransporter 2 inhibitor or placebo. The total number of events is presented in the given study duration [25,26,27]. CI: confidence interval; M-H: Mantel–Haenszel; SGLT2: sodium-glucose cotransporter 2.
3.5. Heart Failure Events
Heart failure events (HFEs) were defined as a hospitalization/ER visit, an urgent care visit, or an outpatient visit requiring intensification of management (intravenous therapy, mechanical support, renal, or circulatory support) for worsening signs and/or symptoms of heart failure. All three trials reported heart failure events (HFEs) among the participants. Compared to the placebo group, the intervention group had a significant risk reduction of 62% in HFEs (RR: 0.66, 95% CI: 0.58–0.75, p < 0.0001, I2 = 0%) (Figure 4A). After removing the SOLOIST-WHF trial [27], the sensitivity analysis still indicated a 45% reduction in HFEs in favor of the intervention group which did not reach statistical significance (RR: 0.55, 95% CI: 0.24–1.26, p = 0.16, I2 = 48%). The total number of HFEs presented was identified during the entire duration of the study. Two of three trials reported the total number of HFEs across participants. There was a significant risk reduction of 35% in the number of HFEs across the trials (RR: 0.65, 95% CI: 0.46–0.91, p = 0.01, I2 = 0) (Figure 4B). The total number of patients who had worsening heart failure or had died is attributable to cardiovascular causes till the end-of-trial visit was included. Two trials reported a combined endpoint attributable to the total number of patients who died of cardiovascular causes or had HFEs. There was a significant reduction of 28% in the intervention group for risk of either cardiovascular death or HFEs (RR: 0.82, 95% CI: 0.73–0.93, p = 0.002, I2 = 0%) (Figure 4C).
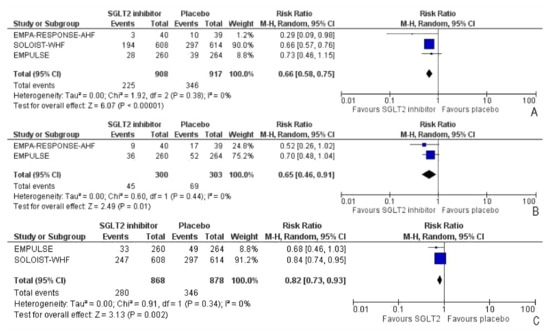
Figure 4.
Forest plot for (A) the number of participants with heart failure events (the total number of participants presented who had one or more HFEs in the duration of the study), (B) the number of heart failure events, and (C) the number of patients with either HFEs or death attributable to cardiovascular causes [25,26,27].
3.6. Readmissions
All three trials reported readmissions due to HFEs. The endpoints for readmissions in EMPULSE [25] and EMPA-RESPONSE-AHF [26] were 30 and 60 days, respectively. The SOLOIST-WHF [27] reported readmission rates during the entire duration of the study. There was a 24% risk reduction of first-time readmission among the intervention group who had been discharged following acute heart failure, compared to the placebo group, which was significant (RR: 0.76, 95% CI: 0.60–0.98, p = 0.03, I2 = 4%) (Figure 5). A sensitivity analysis was conducted and SOLOIST-WHF [27] was removed to identify the readmission risk within 60 days as reported in EMPULSE [25] and EMPA-RESPONSE-AHF [26]. The readmission risk was reduced by 15% which did not receive statistical significance due to the smaller sample size of the remaining two trials (RR: 0.85, 95% CI: 0.31–2.31, p = 0.75, I2 = 36%).
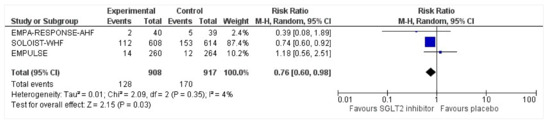
Figure 5.
Forest plot for the number of patients requiring readmission following discharge for acute heart failure in either group [25,26,27].
3.7. Kansas City Cardiomyopathy Questionnaire Total Symptom Score (KCCQ TSS)
Two of three trials noted the adjusted mean reduction in the Kansas City Cardiomyopathy Questionnaire Total Symptom Score (KCCQ TSS). An analysis was conducted to note the effect size of intervention vs. placebo for KCCQ TSS. While a small effect size in favor of intervention was found, the KCCQ-TSS reduction was significant which indicates improvement in the group receiving SGLT2 inhibitors (Cohen’s d = −0.27, 95% CI = −0.40, −0.14, p < 0.0001, I2 = 41%) (Figure 6).
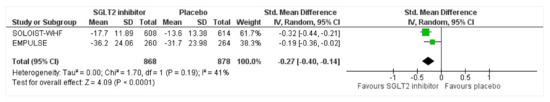
Figure 6.
Forest plot for the adjusted mean change in KCCQ-TSS (mean values [SD]) at 90 days from baseline in either group [25,27].
3.8. Serious Adverse Events
All three trials reported safety outcomes of participants receiving either intervention or placebo. The risk for serious adverse events was slightly lowered in the intervention group by 15% which was insignificant, demonstrating no safety concerns in the three trials concerning SGLT2 inhibitor use in acute heart failure (RR: 0.85, 95% CI: 0.70–1.03, p = 0.1, I2 = 44%) (Figure 7). Compared to the placebo, there was a 22% reduced risk of developing acute kidney injury with SGLT2 inhibitors yet did not reach statistical significance (RR: 0.78, 95% CI: 0.54–1.14, p = 0.2 I2 = 0%) (Figure 8). There was a comparative risk reduction of 23% of developing hepatic injury with SGLT2 inhibitors compared to placebo yet did not reach statistical significance (RR: 0.77, 95% CI: 0.38–1.55, p = 0.46, I2 = 0%) (Figure 9). There was a comparative increase in risk by 18% for the development of hypotension with SGLT2 inhibitors compared to placebo yet did not reach statistical significance (RR: 1.18, 95% CI: 0.76–1.84, p = 0.45, I2 = 0%) (Figure 10). There was a relatively increased risk of hypoglycemia by 49% with SGLT2 inhibitors compared to placebo yet did not reach statistical significance (RR: 1.49, 95% CI: 0.86–2.58, p = 0.79, I2 = 0%) (Figure 11). Compared to the placebo, there was a 16% reduced risk of developing urinary tract infections with SGLT2 inhibitors yet did not reach statistical significance (RR: 0.84, 95% CI: 0.56–1.27, p = 0.41, I2 = 0%) (Figure 12). There was a notable risk reduction of 54% in the group receiving SGLT2 inhibitors when compared to placebo but did not reach statistical significance (RR: 0.46, 95% CI: 0.10–2.04, p = 0.31, I2 = 0%) (Figure 13).
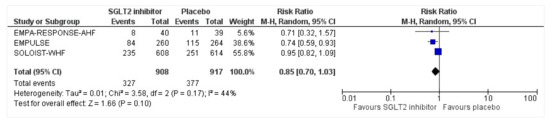
Figure 7.
Forest plot of the total number of patients who had at least one serious adverse event in either group [25,26,27].
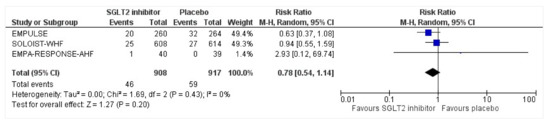
Figure 8.
Forest plot for the total number of patients who had acute renal failure as a serious adverse event during the duration of treatment in either group [25,26,27].
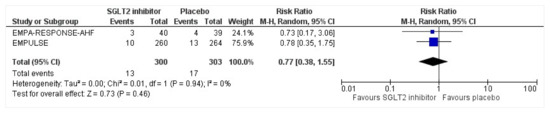
Figure 9.
Forest plot for the total number of patients who had a hepatic injury as a serious adverse event during the duration of treatment in either group [25,26].
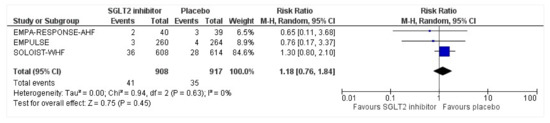
Figure 10.
Forest plot for the total number of patients who had hypotension as a serious adverse event in either group [25,26,27].
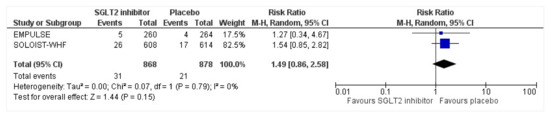
Figure 11.
Forest plot for the total number of patients who had hypoglycemia as a serious adverse event in either group [25,27].
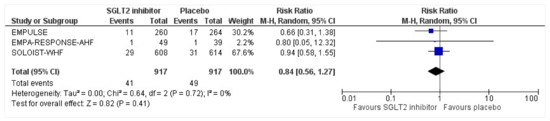
Figure 12.
Forest plot for the total number of patients who had a urinary tract infection as a serious adverse event in either group [25,26,27].
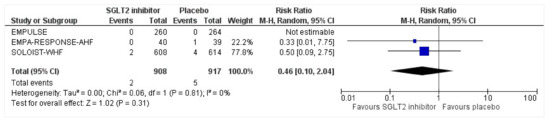
Figure 13.
Forest plot for the total number of patients who had DKA as a serious adverse event in either group [25,26,27].
3.9. Ongoing Clinical Trials of SGLT2 inhibitors and Heart Failure
As of 17th November 2022, 15 ongoing clinical trials are being conducted testing the efficacy and safety of SGLT2 inhibitors on heart failure, diabetes mellites type 2, acute myocardial infarction, and chronic kidney disease (Table 2). The interventions consist of Empagliflozin (10 mg, 25 mg), Dapagliflozin (10 mg), and Canagliflozin. Control and comparator group standard care approaches consist of either placebo or loop diuretics, vasodilators, inotropic agents, digoxin, and/or vasopressors.

Table 2.
Ongoing clinical trial characteristics of SGLT2 inhibitors and varying heart failure conditions.
With a total enrollment of 17,503 participants, the ongoing trials are being conducted worldwide in 27 countries: Argentina, Australia, Brazil, Bulgaria, Canada, China, Denmark, Egypt, France, Germany, Hong Kong, Hungary, India, Israel, Japan, Japan, Korea, Mexico, Netherlands, Poland, Romania, Russian Federation, Serbia, Sweden, United Kingdom, Switzerland, Ukraine, and the United States. The outcome measures, phase of study, enrollment, and completion date for ongoing trials are enlisted in Table 3.

Table 3.
Ongoing clinical trial characteristics of outcome measures, phase of study, enrollment, completion date, and locations.
4. Discussion
The primary objective of our systematic review and meta-analysis was to examine the efficacy of SGLT2 inhibitors compared to placebo for reduction of all-cause and cardiovascular mortality, heart failure events (HFEs), readmissions, and symptomatic improvement in patients admitted for acute heart failure. Our secondary objective was to assess the risk of serious adverse events in patients admitted for acute heart failure, receiving either SGLT2 inhibitors or a placebo. Our meta-analysis compiled the findings of three studies that utilized SGLT2 inhibitors in either acutely decompensated chronic HF (ADCHF) or de novo acute HF (AHF). We found that SGLT2 inhibitors reduced the risk of all-cause mortality by 27% and were significant at 51% when a post-sensitivity analysis was conducted. Furthermore, we also found a significant reduction in cardiovascular mortality and HFEs by 28% among patients receiving SGLT2 inhibitors. Specifically, the absolute number of HFEs and total patients with HFEs were significantly reduced by 62% and 35% among those who used SGLT2 inhibitors, respectively. Moreover, we found a significant reduction in first-time re-admission rates by 24% among patients with SGLT2 inhibitors. These findings were supported by significant symptomatic improvement and no additional safety concerns [28].
The initial evidence of the efficacy of SGLT2 inhibitors in the clinical trial setting was noted in EMPA-REG-OUTCOME [19]—where it was determined that individuals hospitalized for heart failure, and who were randomized into treatment groups had a two-fold reduced risk of being re-hospitalized or dying in the first 1–3 months of the first heart failure event [19]. The EMPA-RESPONSE-AHF trial was the first prospective clinical trial to have evaluated the clinical benefits of SGLT2 inhibitors among acute heart failure patients [26]. This trial was a double-blind, randomized, placebo-based, parallel-group study based in multiple centers; 80 individuals with acute heart failure with or without type 2 diabetes mellitus were randomized to either 10 mg/day of empagliflozin or to the control group; all randomized and medicinal administration was pursued within 24 h of admission [26]. The EMPULSE (EMPagliflozin in patients hospitalized with acUte heart faiLure who have been StabilizEd) trial was based in multiple countries/centers, randomized with a double-blind setting where the effects of the SGLT2 inhibitor (empagliflozin) was assessed for safety, clinical benefit and tolerability for acute heart failure [25,29]. In this trial, patients underwent an initial stabilization period which had a median time span of 3 days. The patients also received 10 mg per day of the SGLT2 inhibitor (empagliflozin) or no intervention with standard care for 90 days. The EMPULSE trial met its endpoint, where patients showed more clinical benefits as compared to placebo; the stratified win ratio was 1.36 (95% CI = 1.09 to 1.68, p = 0.0054) [25]. Notably, the benefits were unanimous throughout the different subgroups including those that had decompensated chronic heart failure and ventricular ejection fractions upwards or downwards of 40%. The intervention was considered to be both well-tolerated and safe for the patients.
Other representatives of the class including dapagliflozin are currently being investigated. The DISTATE-AHF, which is a multicenter, prospective, open-label, randomized trial is enrolling 240 patients in the US [30]. The patient population consists of patients with type 2 diabetes mellitus hospitalized with hypervolemic AHF and glomerular filtration rate above 30 mL/min/1.73m2 [30]. With endpoints consisting of diuretic response, inpatient AHF, 30-day readmission rate, and safety metrics, it is yet to be quantified whether dapagliflozin will be a candidate therapeutic for AHF among patients with diabetes [30]. In the CHIEF-HF trial, 467 participants with heart failure regardless of diabetes or ejection fraction status were randomized to canagliflozin (100 mg) or placebo intervention [31]. While the enrollment was stopped early because of sponsor priorities, Spertus and colleagues (2022) report that the primary outcome of KCCQ TSS was changed at 12 weeks by 4.3 points (p = 0.016)—favoring canagliflozin [31]. The CHIEF-HF met its primary endpoint; with a shift in the paradigm of care for heart failure imminent [31], it is important to further test and review the findings of SGLT2 inhibitors in randomized, double-blind settings.
In our meta-analysis, we ascertain the pooled findings of all three RCTs published in this arena so far. The EMPULSE trial works as an add-on/complement to the results of the previous two trials that administered SGLT2 inhibitors; these trials depict the patient journey from undergoing acute heart failure to in-hospital admission and use of the intervention. Our findings are noteworthy as we collate proof to showcase that SGLT2 inhibitors can be considered an element of usual care, translating to clinical benefit [32]. Our study findings support that SGLT2 inhibitors help manage AHF patients following hospitalization.
4.1. Clinical Practice Recommendations
With abundant data on the use of SGLT2 inhibitors for heart failure, it is essential to review the feasibility in clinical practice. So far, literature reports that SGLT2 inhibition interacts with key pathways at the cellular level, thereby providing cardioprotective effects to patient populations. The mechanisms include cardiac remodeling, myocardial calcium handling, lipolysis, and modification of the epicardial thickness [33].
SGLT2 inhibitors reduce the risk of composite and specific heart failure outcomes. The benefits of SGLT2 inhibitor use are large among those with a history of heart failure and with diabetes. Moreover, benefits are also noted in weight control, blood pressure regulation, and hemoglobin levels. It may be worthwhile to consider SGLT2 inhibitor treatment initiation if heart failure predominates since the intervention is an excellent target for blood glucose and control. No notable adverse events are reported among patients with heart failure being treated with SGLT2 inhibitors as compared to the standard of care. The benefits of treatment on heart failure outcomes are insofar high based on current evidence when compared to other therapies used for heart failure management including ACE inhibitors.
4.2. Limitations
Our meta-analysis has certain limitations that must be reported. First, the duration of the intervention was variable across all trials. Second, there was limited uniformity in how the trials defined HFEs. Third, the follow-up duration was inconsistent. Certain aspects of care were administered for longer periods across the trials. Fourth, the outcome measures were conducted at different time points e.g., readmission rates. Fifth, the sample size was fairly small, with SGLT2 inhibitor group enrolled participants not reaching generalizability. Lastly, a majority of the sample was diabetic, which may lead to biases when making broadly characterizable decisions for patient care.
5. Conclusions
The current study looked into the efficacy and safety of SGLT2 inhibitors in preventing complications following AHF. By compiling the findings of three trials, we analyzed a total sample of 1831 patients irrespective of their diabetes status. Moreover, we also qualitatively critiqued 15 ongoing clinical trials administering SGLT2 inhibitors across 27 countries for a variety of cardiovascular conditions including acute heart failure. We found that SGLT2 inhibitors decrease the odds of all-cause mortality, cardiovascular mortality, heart failure events, and re-admission rates within the first 1–9 months of hospitalization. Additional studies are necessary to fully understand the beneficial impact of SGLT2 inhibitors in managing AHF patients without diabetes.
Author Contributions
Conceptualization, N.U.A., F.S. and T.A.; methodology, N.U.A., F.S., T.A. and A.S.; software, Z.S. and I.C.-O.; investigation, N.U.A., F.S., T.A. and A.S.; resources, K.R.-V.; writing—original draft preparation, N.U.A., F.S., T.A., Z.S., A.S., K.R.-V. and I.C.-O.; writing—review and editing, N.U.A., F.S., T.A., Z.S., A.S., K.R.-V. and I.C.-O.; visualization, Z.S. and A.S.; supervision, I.C.-O.; project administration, A.S. and K.R.-V. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data utilized for the purpose of this study are available publicly and online.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fountoulaki, K.; Ventoulis, I.; Drokou, A.; Georgarakou, K.; Parissis, J.; Polyzogopoulou, E. Emergency Department Risk Assessment and Disposition of Acute Heart Failure Patients: Existing Evidence and Ongoing Challenges. Heart Fail. Rev. 2022; online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Arrigo, M.; Jessup, M.; Mullens, W.; Reza, N.; Shah, A.M.; Sliwa, K.; Mebazaa, A. Acute Heart Failure. Nat. Rev. Dis. Prim. 2020, 6, 16. [Google Scholar] [CrossRef]
- Farmakis, D.; Papingiotis, G.; Parissis, J. Acute Heart Failure: Epidemiology and Socioeconomic Burden. Contin. Cardiol. Educ. 2017, 3, 88–92. [Google Scholar] [CrossRef]
- Khan, M.S.; Sreenivasan, J.; Lateef, N.; Abougergi, M.S.; Greene, S.J.; Ahmad, T.; Anker, S.D.; Fonarow, G.C.; Butler, J. Trends in 30-and 90-Day Readmission Rates for Heart Failure. Circ. Heart Fail. 2021, 14, e008335. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, Y.; Kohsaka, S.; Sato, N.; Takano, T.; Kitai, T.; Yoshikawa, T.; Matsue, Y. 9-year Trend in the Management of Acute Heart Failure in Japan: A Report from the National Consortium of Acute Heart Failure Registries. J. Am. Heart Assoc. 2018, 7, e008687. [Google Scholar] [CrossRef]
- Bytyçi, I.; Bajraktari, G. Mortality in Heart Failure Patients. Anatol. J. Cardiol. 2015, 15, 63. [Google Scholar] [CrossRef] [PubMed]
- Buddeke, J.; Valstar, G.B.; Van Dis, I.; Visseren, F.L.J.; Rutten, F.H.; Den Ruijter, H.M.; Vaartjes, I.; Bots, M.L. Mortality after Hospital Admission for Heart Failure: Improvement over Time, Equally Strong in Women as in Men. BMC Public Health 2020, 20, 36. [Google Scholar] [CrossRef]
- Tsutsui, H. Recent Advances in the Pharmacological Therapy of Chronic Heart Failure: Evidence and Guidelines. Pharmacol. Ther. 2022, 238, 108185. [Google Scholar] [CrossRef]
- Mazza, A.; Townsend, D.M.; Torin, G.; Schiavon, L.; Camerotto, A.; Rigatelli, G.; Cuppini, S.; Minuz, P.; Rubello, D. The Role of Sacubitril/Valsartan in the Treatment of Chronic Heart Failure with Reduced Ejection Fraction in Hypertensive Patients with Comorbidities: From Clinical Trials to Real-World Settings. Biomed. Pharmacother. 2020, 130, 110596. [Google Scholar] [CrossRef]
- Mullens, W.; Damman, K.; Harjola, V.; Mebazaa, A.; Brunner-La Rocca, H.; Martens, P.; Testani, J.M.; Tang, W.H.W.; Orso, F.; Rossignol, P. The Use of Diuretics in Heart Failure with Congestion—A Position Statement from the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2019, 21, 137–155. [Google Scholar] [CrossRef]
- Felker, G.M.; O’Connor, C.M.; Braunwald, E. Loop Diuretics in Acute Decompensated Heart Failure: Necessary? Evil? A Necessary Evil? Circ. Heart Fail. 2009, 2, 56–62. [Google Scholar] [CrossRef]
- Chiong, J.R.; Cheung, R.J. Loop Diuretic Therapy in Heart Failure: The Need for Solid Evidence on a Fluid Issue. Clin. Cardiol. 2010, 33, 345–352. [Google Scholar] [CrossRef]
- Boorsma, E.M.; Beusekamp, J.C.; Ter Maaten, J.M.; Figarska, S.M.; Danser, A.H.J.; van Veldhuisen, D.J.; van Der Meer, P.; Heerspink, H.J.L.; Damman, K.; Voors, A.A. Effects of Empagliflozin on Renal Sodium and Glucose Handling in Patients with Acute Heart Failure. Eur. J. Heart Fail. 2021, 23, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Ghani, M.A.; Norton, L.; DeFronzo, R.A. Renal Sodium-Glucose Cotransporter Inhibition in the Management of Type 2 Diabetes Mellitus. Am. J. Physiol. Physiol. 2015, 309, F889–F900. [Google Scholar] [CrossRef]
- Liu, J.; Lee, T.; DeFronzo, R.A. Why Do SGLT2 Inhibitors Inhibit Only 30–50% of Renal Glucose Reabsorption in Humans? Diabetes 2012, 61, 2199–2204. [Google Scholar] [CrossRef]
- Gallo, L.A.; Wright, E.M.; Vallon, V. Probing SGLT2 as a Therapeutic Target for Diabetes: Basic Physiology and Consequences. Diabetes Vasc. Dis. Res. 2015, 12, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Perez Lopez, G.; Gonzalez Albarran, O.; Cano Megias, M. Type 2 Sodium-Glucose Cotransporter (SGLT2) Inhibitors: From Familial Renal Glucosuria to the Treatment of Type 2 Diabetes Mellitus. Nefrologia 2010, 30, 618–625. [Google Scholar] [PubMed]
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Woerle, H.J. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N. Engl. J. Med. 2015, 373, 2117–2128. [Google Scholar] [CrossRef] [PubMed]
- Savarese, G.; Sattar, N.; Januzzi, J.; Verma, S.; Lund, L.H.; Fitchett, D.; Zeller, C.; George, J.T.; Brueckmann, M.; Ofstad, A.P. Empagliflozin Is Associated with a Lower Risk of Post-Acute Heart Failure Rehospitalization and Mortality: Insights from the EMPA-REG OUTCOME Trial. Circulation 2019, 139, 1458–1460. [Google Scholar] [CrossRef]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Pocock, S.J.; Carson, P.; Januzzi, J.; Verma, S.; Tsutsui, H.; Brueckmann, M. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N. Engl. J. Med. 2020, 383, 1413–1424. [Google Scholar] [CrossRef]
- FDA. FDA Approves Treatment for Wider Range of Patients with Heart Failure. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-treatment-wider-range-patients-heart-failure (accessed on 7 October 2022).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. PLoS Med. 2021, 18, e1003583. [Google Scholar] [CrossRef]
- Cochrane RoB 2: A Revised Cochrane Risk-of-Bias Tool for Randomized Trials. Available online: https://methods.cochrane.org/bias/resources/rob-2-revised-cochrane-risk-bias-tool-randomized-trials (accessed on 7 August 2022).
- Voors, A.A.; Angermann, C.E.; Teerlink, J.R.; Collins, S.P.; Kosiborod, M.; Biegus, J.; Ferreira, J.P.; Nassif, M.E.; Psotka, M.A.; Tromp, J. The SGLT2 Inhibitor Empagliflozin in Patients Hospitalized for Acute Heart Failure: A Multinational Randomized Trial. Nat. Med. 2022, 28, 568–574. [Google Scholar] [CrossRef]
- Damman, K.; Beusekamp, J.C.; Boorsma, E.M.; Swart, H.P.; Smilde, T.D.J.; Elvan, A.; van Eck, J.W.M.; Heerspink, H.J.L.; Voors, A.A. Randomized, Double-blind, Placebo-controlled, Multicentre Pilot Study on the Effects of Empagliflozin on Clinical Outcomes in Patients with Acute Decompensated Heart Failure (EMPA-RESPONSE-AHF). Eur. J. Heart Fail. 2020, 22, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, D.L.; Szarek, M.; Steg, P.G.; Cannon, C.P.; Leiter, L.A.; McGuire, D.K.; Lewis, J.B.; Riddle, M.C.; Voors, A.A.; Metra, M. Sotagliflozin in Patients with Diabetes and Recent Worsening Heart Failure. N. Engl. J. Med. 2021, 384, 117–128. [Google Scholar] [CrossRef]
- Kosiborod, M.N.; Angermann, C.E.; Collins, S.P.; Teerlink, J.R.; Ponikowski, P.; Biegus, J.; Comin-Colet, J.; Ferreira, J.P.; Mentz, R.J.; Nassif, M.E. Effects of Empagliflozin on Symptoms, Physical Limitations and Quality of Life in Patients Hospitalized for Acute Heart Failure-Results from the EMPULSE Trial. Circulation 2022, 146, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Tromp, J.; Ponikowski, P.; Salsali, A.; Angermann, C.E.; Biegus, J.; Blatchford, J.; Collins, S.P.; Ferreira, J.P.; Grauer, C.; Kosiborod, M. Sodium–Glucose Co-transporter 2 Inhibition in Patients Hospitalized for Acute Decompensated Heart Failure: Rationale for and Design of the EMPULSE Trial. Eur. J. Heart Fail. 2021, 23, 826–834. [Google Scholar] [CrossRef]
- Cox, Z.L.; Collins, S.P.; Aaron, M.; Hernandez, G.A.; McRae, A.T., III; Davidson, B.T.; Fowler, M.; Lindsell, C.J.; Harrell, F.E., Jr.; Jenkins, C.A. Efficacy and Safety of Dapagliflozin in Acute Heart Failure: Rationale and Design of the DICTATE-AHF Trial. Am. Heart J. 2021, 232, 116–124. [Google Scholar] [CrossRef]
- Spertus, J.A.; Birmingham, M.C.; Nassif, M.; Damaraju, C.V.; Abbate, A.; Butler, J.; Lanfear, D.E.; Lingvay, I.; Kosiborod, M.N.; Januzzi, J.L. The SGLT2 Inhibitor Canagliflozin in Heart Failure: The CHIEF-HF Remote, Patient-Centered Randomized Trial. Nat. Med. 2022, 28, 809–813. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, S.; Yamada, T.; Watanabe, T.; Morita, T.; Furukawa, Y.; Kawasaki, M.; Kikuchi, A.; Kawai, T.; Seo, M.; Abe, M. Effect of Empagliflozin as an Add-on Therapy on Decongestion and Renal Function in Patients with Diabetes Hospitalized for Acute Decompensated Heart Failure: A Prospective Randomized Controlled Study. Circ. Heart Fail. 2021, 14, e007048. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.S.; Singh, T.; Newby, D.E.; Singh, J. Sodium-Glucose Co-Transporter 2 Inhibitor Therapy: Mechanisms of Action in Heart Failure. Heart 2021, 107, 1032–1038. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).