Abstract
Phenylphenalenone-type compounds accumulated in the tissues of two banana cultivars—Musa acuminata cv. “Grande Naine” (AAA) and Musa acuminata × balbisiana Colla cv. “Bluggoe” (ABB)—when these were fed on by the banana weevil (Cosmopolites sordidus (Germ.) (Coleoptera: Curculionidae)) and the banana stem weevil (Odoiporus longicollis (Oliver) (Coleoptera: Curculionidae)). The chemical constituents of the banana material were separated by means of chromatographic techniques and identified by NMR spectroscopy. One new compound, 2-methoxy-4-phenylphenalen-1-one, was found exclusively in the corm material of “Bluggoe” that had been fed on by the weevils.
1. Introduction
Bananas (Musa spp.) are a major staple food for more than 400 million people in developing countries in the tropics and subtropics, and an important export product for the Philippines and many Latin American countries [1,2]. Production is threatened by a variety of pests and pathogens, such as plant parasitic nematodes, ascomycetous fungi, viruses, bacteria, and insects [3,4,5,6,7,8]. The most important banana production and productivity limiting insect-pests are the banana weevil, Cosmopolites sordidus (CS), and the banana (pseudo)stem weevil, Odoiporus longicollis (OL) [9]. Adult weevils can be distinguished from one another by differences in body length, body width, and rostrum length: CS has a shorter and less slender rostrum, and OL a broader pronotum tapered anterior and a broader third tarsal segment (Figure 1) [10]. CS is native to Southeast Asia, but has been transported throughout the banana growing regions of the world, and is now established in southern Asia, Africa, many Pacific islands, Australia, northern South America, most of Central America, Cuba, the West Indies, and in Mexico, Florida, and Hawaii [11]. OL is also native to Southeast Asia, but its distribution is restricted to the banana growing belts of India, Southeast Asia, southern China, and the southern islands of Japan.

Figure 1.
Adult banana (pseudo)stem weevil, Odoiporus longicollis (OL)—larger weevil facing top right corner of photo and adult banana weevil, Cosmopolites sordidus (CS)—smaller weevil at bottom of photo facing ruler.
CS is regarded as the main insect pest in bananas [11], though OL is a major limiting factor of banana production in India, which is the country that produces the most bananas worldwide. OL causes 10%–90% yield losses, depending upon the growth stage of the crop and management efficiency [12,13]. CS larvae create complex tunnel systems in the corm and rhizome of banana plants, leading to their toppling, often shortly before fruit harvest. In contrast, the larvae of OL produce long tunnels in the banana pseudostem, weakening the plant, reducing flowering rates, and finally producing undersized or no fruit [12]. Recently, phenylphenalenones have been identified as effective plant secondary metabolites involved in the defense response of particular banana cultivars to both the burrowing nematode (Radopholus similis) [14] and the fungal disease Black Sigatoka, caused by Mycosphaerella fijiensis [15]. The biosynthesis of the phenylphenalenones is closely related to that of flavonoids. Both classes of natural products are derived from the phenylpropanoid/plant polyketide pathway [16,17]. The present study focuses on phenylphenalenone-type compounds produced by two different banana varieties, Musa acuminata cv. “Grande Naine” (GN) and M. acuminata × balbisiana Colla cv. “Bluggoe” (BG) in reaction to banana weevils (CS) and banana pseudostem weevils (OL) feeding on the plants.
2. Results
Six weeks after starting the feeding experiments, the banana pseudostem and corm material of BG and GN were inspected for damage caused by the beetles. A change of the coloration from green to orange-red in the case of the pseudostem-material and from pale-brown to dark red-brown for the corm material was detected. Control material of BG and GN showed no such discoloration. Secondary metabolites were extracted from the plant material using ethanol. Liquid–liquid separation of all the samples and controls resulted in CHCl3, ethyl acetate, 1-butanol, and aqueous subfractions. Similar to the procedure of nematode-infected banana material [14], only the CHCl3 subfractions of the wounded plant material contained phenylphenalenones. These CHCl3 subfractions were separated by thin-layer chromatography. The purified compounds were subjected to 1H NMR spectroscopy. The structures of all the compounds (Figure 2) were identified as phenylphenalenones, which are typical metabolites and major phytoalexins of Musa species. Compounds (1–4) (Figure 2) have been previously reported [18], and 2-methoxy-4-phenylphenalen-1-one (5) is a new natural product.
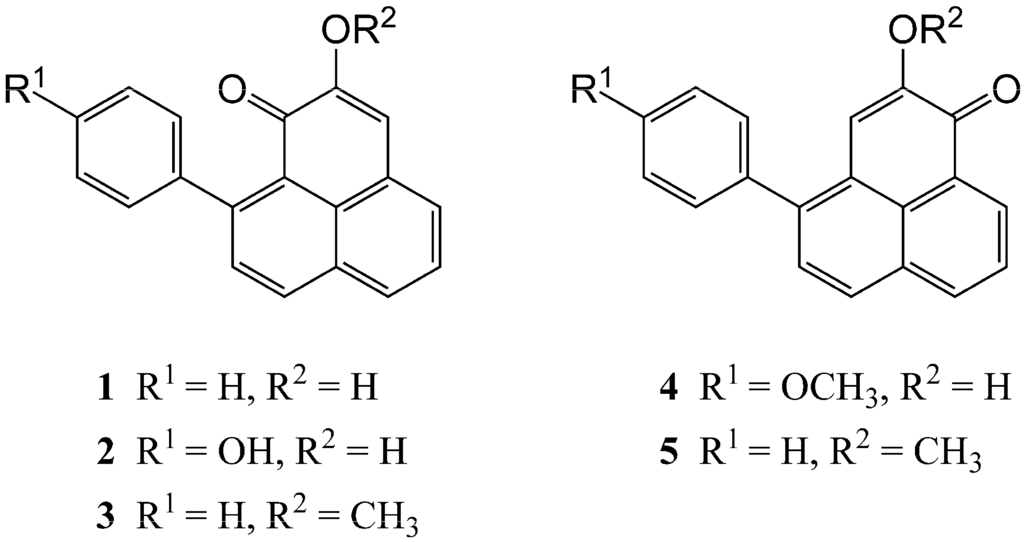
Figure 2.
Structures of phenylphenalenone-type phytoalexins (1–5). (1) Anigorufone; (2) hydroxyanigorufone; (3) methoxyanigorufone; (4) 4-O-methylirenolone; (5) 2-methoxy-4-phenylphenalen-1-one isolated from pseudostem or corm material from Musa AAA cv. “Grande Naine” and Musa ABB cv. “Bluggoe” that had been fed on by Odoiporus longicollis or Cosmopolites sordidus.
Generally, the corm material contained greater quantities of secondary metabolites than pseudostem material (Figure 3).
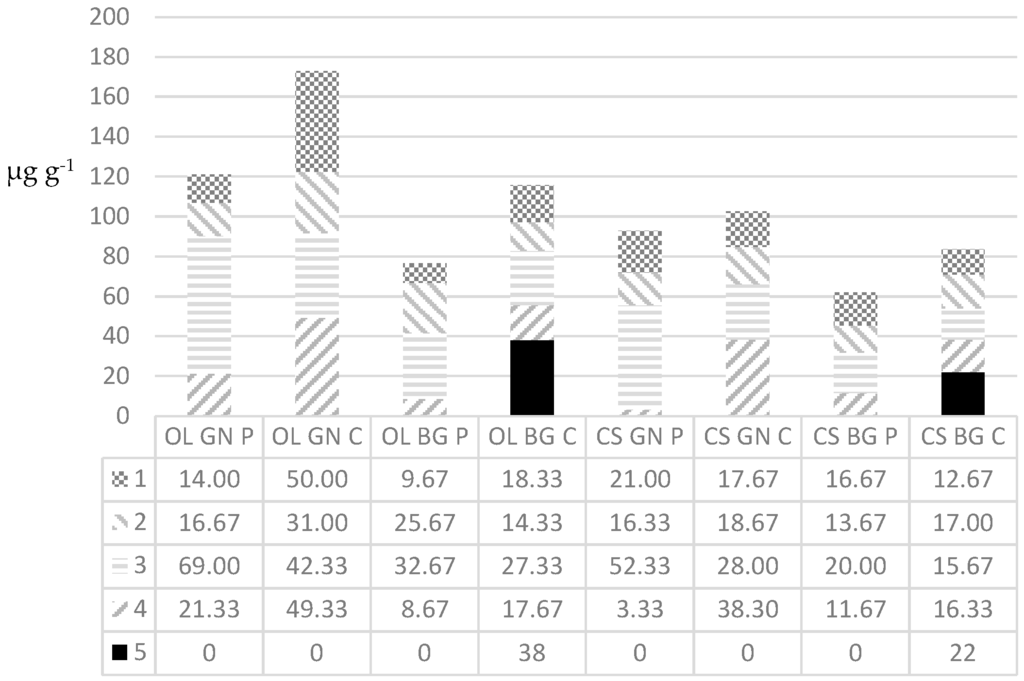
Figure 3.
Average masses (µg) of the five phenylphenalenones (1–5) detected per g pseudostem (P) or corm material (C) from Musa AAA cv. “Grande Naine” (GN) or Musa ABB cv. “Bluggoe” (BG) after being fed on by either Cosmopolites sordidus (CS) or Odoiporus longicollis (OL) for 6 weeks. The values are the averages of two very similar measured masses.
Generally, for each weevil and cultivar combination, greater quantities of phenylphenalenones were produced in corm material than in pseudostem material, with GN corm material fed on by OL producing the greatest cumulative mass and with BG material fed on by CS producing the smallest cumulative mass. The corm material of GN always produced greater cumulative quantities than the BG corm pieces for both the weevil species. Compounds 1, 2, 3, and 4 occur in greater quantities in GN- than BG corm material fed on by OL or CS. The same results were observed with pseudostem material, with the exception of 2 and 4 for BG pseudostem material. The new compound 5 was only detectable in relatively high quantities in the BG-corm material, fed on by CS or OL. The structural elucidation of 5 followed that for 2-hydroxy-4-phenylphenalen-1-one reported by [19]. The position of the additional 2-O-methyl group of 5 was confirmed by the Heteronuclear Multiple-Bond Correlation (HMBC) cross signal between the methoxy protons (δ 3.72) and C-2 (δ 153.8).
3. Discussion
Phenylphenalenones have been identified as typical phytoalexins of Musa species [18]. The objective of this study was to investigate which defense metabolites are produced by two Musa cultivars (GN and BG) in response to herbivory by two important banana insect pests (OL and CS). The phytochemical profiles of the different combinations of plant material, cultivar, and weevil revealed that each treatment produced a unique mix of 1–5, with none of these metabolites dominating across treatments. The concentration of a specific compound can be crucial in the effectiveness of the defense response of plant materials attacked by pests and pathogens, as was shown for 1 in the interaction of the burrowing nematode R. similis with two different Musa cultivars with different levels of susceptibility [14]. Recently, the role of methoxyanigorufone (3) and the non-methylated analogue of 5, isoanigorufone, were reported to be the most relevant metabolites for the defense of Musa plants against M. fijiensis [15]. The new phenylphenalenone, 2-methoxy-4-phenylphenalen-1-one (5) was only produced in BG-corm material, and may also play a role in the defense of Musa spp. against weevil attack. However, further studies are needed to confirm the importance of this compound in the plant–pest interaction.
4. Materials and Methods
4.1. Plant Material
“Grande Naine” (ITC1256, GN) in vitro plantlets were provided by the International Transit Centre (ITC), Bioversity International, Katholieke Universiteit Leuven, Belgium. Plants with roots were first transplanted into small pots containing a sterile quartz–peat mixture (2:1). At weekly intervals, each plant received a dose of commercial liquid fertilizer mixture of 2% Ferty® 3 (Planta Düngemittel GmbH, Regenstauf, Germany; www.plantafert.de) and 0.2% Folicin-Bor (Jost GmbH, Iserlohn, Germany; www.jost-group.com). Additionally, an aqueous solution of 0.05% Biplantol® agrar (Bioplant Naturverfahren GmbH, Konstanz, Germany; www.biplantol.com) was applied. Later, the plants were transplanted into larger 8 L pots containing the same potting mix and were maintained under greenhouse conditions at an ambient temperature of 27/20 °C (day/night), 80% relative humidity, 12-h photoperiod, and irrigated as needed. Suckers of “Bluggoe” (BG) plants were originally collected in Oman [20] and cultivated under the same greenhouse conditions as described for GN.
4.2. Banana Weevils
Adult CS were originally provided from the International Institute of Tropical Agriculture (IITA), Kampala, Uganda. Adult OL were provided by the Department of Agronomy, Yezin Agriculture University, Yezin, Nay Pyi Taw, Myanmar. The weevils were reared and propagated in small plastic boxes (inside diameter 11 cm) on wet tissue paper and fed with small pieces of pseudostem material from different Musa cultivars. These plastic boxes were kept in a growth chamber at an ambient temperature of 26 °C, 55% relative humidity, and a 16-h photoperiod.
4.3. Weevil Feeding Experiments
A total of 16 feeding containers were used, each containing 30 g of either pseudostem or corm material from either GN or BG and four adult CS or OL. Additionally, the same number of containers (16) without weevils served as non-feeding control. Each week, the containers were carefully sprayed with water. Six weeks after set-up, the banana material pieces left in the 32 containers were snap frozen in liquid nitrogen and stored at −80 °C in preparation for phytochemical analysis.
4.4. Isolation and Structure Elucidation of Phenylphenalenones
The frozen banana material was ground and exhaustively extracted with ethanol at room temperature. The crude extract was evaporated (<40 °C) and partitioned between CHCl3-H2O and ethyl-acetate-H2O, followed by 1-butanol-H2O. Purifications were achieved by means of thin layer chromatography (TLC: silica gel 60 F254, toluene–Me2CO (2:1)).
4.5. NMR Spectroscopy
The individually collected compounds were subjected to 1H NMR spectroscopy for identification. All compounds were identified as phenylphenalenones (1–5, Figure 2). Metabolites 1–4 are known typical metabolites and major phytoalexins of Musa species, while 2-methoxy-4-phenylphenalen-1-one (5) is reported here for the first time. 1H NMR and 2D NMR spectra (1H-1H COSY, HSQC, HMBC) were measured on an AV 500 NMR spectrometer (Bruker, www.bruker.com) at 500.13 MHz. The spectrometer was equipped with a 5-mm Bruker TCI Cryoprobe. Standard Bruker pulse sequences were used to record spectra in acetone-d6 at 300 K. Spectra were referenced to tetramethylsilane, which was used as an internal standard.
4.6. Analytical Data of 2-Methoxy-4-phenyl-1H-phenalen-1-one (5)
1H NMR (500 MHz, acetone-d6): δ 8.65 (1H, dd, J = 7.1, 1.2 Hz, H-9), 8.42 (1H, dd, J = 7.9, 1.2 Hz, H-7), 8.12 (1H, d, J = 8.5 Hz, H-6), 7.89 (1H, dd, J = 7.9, 7.1 Hz, H-8), 7.64 (1H, d, J = 8.5 Hz, H-5), 7.61–7.59 (5H, m, H-2’–H-6’), 7.10 (1H, s, H-3), 3.72 (3H, s, OMe). 13C NMR (125 MHz, acetone-d6, chemical shifts extracted from HMQC and HMBC): 179.0 (C-1), 153.8 (C-2), 142.7 (C-4), 140.4 (C-1’), 135.9 (C-7), 132.2 (C-6a), 130.8 (C-9), 129.1 (C-3’/C-5’), 130.2 (C-5), 130.2 (C-9a), 129.8 (C-6), 128.6 (C-4’), 130.7 (C-2’/C-6’), 127.3 (C-8), 125.5 (C-9b), 125.1 (C-3a), 111.1 (C-3), 55.2 (OMe). HRESIMS: m/z 287.1068 [M + 1]+ (calc. for C20H15O2 287.1072).
Acknowledgments
We wish to thank Tamara Krügel and the greenhouse team of the Max Planck Institute for Chemical Ecology (Jena, Germany) and Rainer Braukmann and the team of the Greenhouse for Tropical Crops (Universität Kassel, Witzenhausen, Germany) for raising the Musa acuminata cv. “Grande Naine” (AAA) and Musa acuminata x balbisiana Colla cv. “Bluggoe” (ABB) plants. The authors gratefully acknowledge Alexandra zum Felde for editorial help and Sybille Lorenz for providing the high resolution mass spectrum of 2-methoxy-4-phenylphenalenone. The authors are especially grateful to Thin Nwe Htwe (Department of Agronomy, Yezin Agriculture University, Yezin, Nay Pyi Taw, Myanmar) for providing the adult beetles of Odoiporus longicollis. This study was supported by the Deutsche Forschungsgemeinschaft (HO 4380/1 and SCHN 450/10 to D.H. and B.S.), respectively.
Author Contributions
Dirk Hölscher conceived, designed and performed the experiments; Dirk Hölscher analyzed the data; Dirk Hölscher, Andreas Bürkert and Bernd Schneider wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Food and Agriculture Organization of United Nations (FAOSTAT). Production (Crops) Quantities of Banana and Plantains from 2013 to 2014. Available online: http://faostat3.fao.org/browse/Q/QC/E (accessed on 19 August 2016).
- Food and Agriculture Organization of United Nations (FAOSTAT). Trade (Crops and livestock products) Quantities of Banana and Plantains for 2012. Available online: http://faostat3.fao.org/browse/P/*/E (accessed on 19 August 2016).
- Dale, J.L. Banana bunchy top virus: An economically important tropical plant virus disease. Adv. Virus Res. 1987, 33, 301–325. [Google Scholar] [PubMed]
- Chabrier, C.; Queneherve, P. Control of the burrowing nematode (Radopholus similis Cobb) on banana: Impact of the banana field destruction method on the efficiency of the following fallow. Crop Prot. 2003, 22, 121–127. [Google Scholar] [CrossRef]
- Gold, C.S.; Kagezi, G.H.; Night, G.; Ragama, P.E. The effects of banana weevil, Cosmopolites sordidus, damage on highland banana growth, yield and stand duration in Uganda. Ann. Appl. Biol. 2004, 145, 263–269. [Google Scholar] [CrossRef]
- Ploetz, R.C. Fusarium-induced diseases of tropical, perennial crops. Phytopathology 2006, 96, 648–652. [Google Scholar] [CrossRef] [PubMed]
- Biruma, M.; Pillay, M.; Tripathi, L.; Blomme, G.; Abele, S.; Mwangi, M.; Bandyopadhyay, R.; Muchunguzi, P.; Kassim, S.; Nyine, M.; et al. Banana Xanthomonas wilt: A review of the disease, management strategies and future research directions. Afr. J. Biotechnol. 2007, 6, 953–962. [Google Scholar]
- Etebu, E.; Young-Harry, W. Control of black Sigatoka disease: Challenges and prospects. Afr. J. Agric. Res. 2011, 6, 508–514. [Google Scholar]
- Ostmark, H.E. Economic insect pests of bananas. Annu. Rev. Entomol. 1974, 19, 161–176. [Google Scholar] [CrossRef]
- Thu, M. Biology, Population Dynamics and Management of Banana Pseudostem Borer Odoiporus longicollis Olivier (Coleoptera: Curculionidae) on ‘LAKATAN’ AA, Musa acuminata Colla. Ph.D. Thesis, University of the Philippines, Los Baños, Philippines, 2010. [Google Scholar]
- Gold, C.S.; Pena, J.E.; Karamura, E.B. Biology and integrated pest management for the banana weevil Cosmopolites sordidus (Germar) (Coleoptera: Curculionidae). Integr. Pest Manag. Rev. 2001, 6, 79–155. [Google Scholar] [CrossRef]
- Prasuna, A.L.; Jyothi, K.N.; Prasad, A.R.; Yadav, J.S.; Padmanaban, B. Olfactory responses of banana pseudostem weevil, Odoiporus longicollis Olivier (Coleoptera: Curculionidae) to semiochemicals from conspecifics and host plant. Curr. Sci. 2008, 94, 896–900. [Google Scholar]
- Sahayaraj, K.; Kombiah, P.; Dikshit, A.K.; Rathi, J.M. Chemical constituents of the essential oils of Tephrosia purpurea and Ipomoea carnea and their repellent activity against Odoiporus longicollis. J. Serb. Chem. Soc. 2015, 80, 465–473. [Google Scholar] [CrossRef]
- Hölscher, D.; Dhakshinamoorthy, S.; Alexandrov, T.; Becker, M.; Bretschneider, T.; Buerkert, A.; Crecelius, A.C.; de Waele, D.; Elsen, A.; Heckel, D.G.; et al. Phenalenone-type phytoalexins mediate resistance of banana plants (Musa spp.) to the burrowing nematode Radopholus similis. Proc. Natl. Acad. Sci. USA 2014, 111, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, W.; Chandran, J.N.; Menezes, R.C.; Otálvaro, F.; Schneider, B. Phenylphenalenones protect banana plants from infection by Mycosphaerella fijiensis and are deactivated by metabolic conversion. Plant Cell Environ. 2016, 39, 492–513. [Google Scholar] [CrossRef] [PubMed]
- Vogt, T. Phenylpropanoid Biosynthesis. Mol. Plant 2010, 3, 2–20. [Google Scholar] [CrossRef] [PubMed]
- Brand, S.; Hölscher, D.; Schierhorn, A.; Svatoš, A.; Schröder, J.; Schneider, B. A type III polyketide synthase from Wachendorfia thyrsiflora and its role in diarylheptanoid and phenylphenalenone biosynthesis. Planta 2006, 224, 413–428. [Google Scholar]
- Otálvaro, F.; Nanclares, J.; Vásquez, L.E.; Quiñones, W.; Echeverri, F.; Arango, R.; Schneider, B. Phenalenone-Type compounds from Musa acuminata var. “Yangambi km 5” (AAA) and their activity against Mycosphaerella fijiensis. J. Nat. Prod. 2007, 70, 887–890. [Google Scholar] [CrossRef] [PubMed]
- Luis, J.G.; Lahlou, E.H.; Andrés, L.S. 4′-Dehydroxy-irenolone. A new phytoanticipin from the resistant Musa selected hybrid Sh-3481. Nat. Prod. Res. 1999, 13, 299–304. [Google Scholar]
- Behrendt, S.; zum Felde, A.; De Langhe, E.; Al Khanjari, S.; Brinkmann, K.; Buerkert, A. Distribution and diversity of banana (Musa spp.) in Wadi Tiwi, northern Oman. Genet. Resour. Crop Evol. 2015, 62, 1135–1145. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).