Adaptive Distribution and Priority Protection of Endangered Species Cycas balansae
Abstract
1. Introduction
2. Results
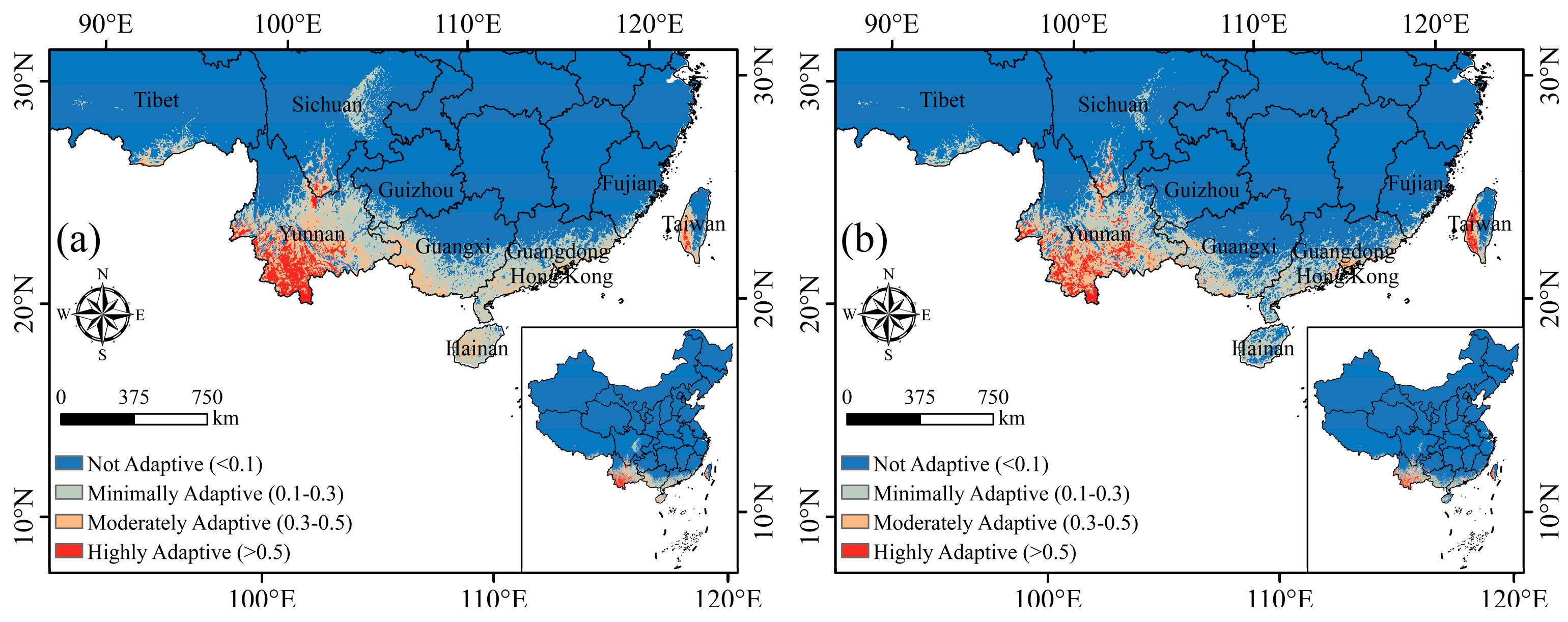
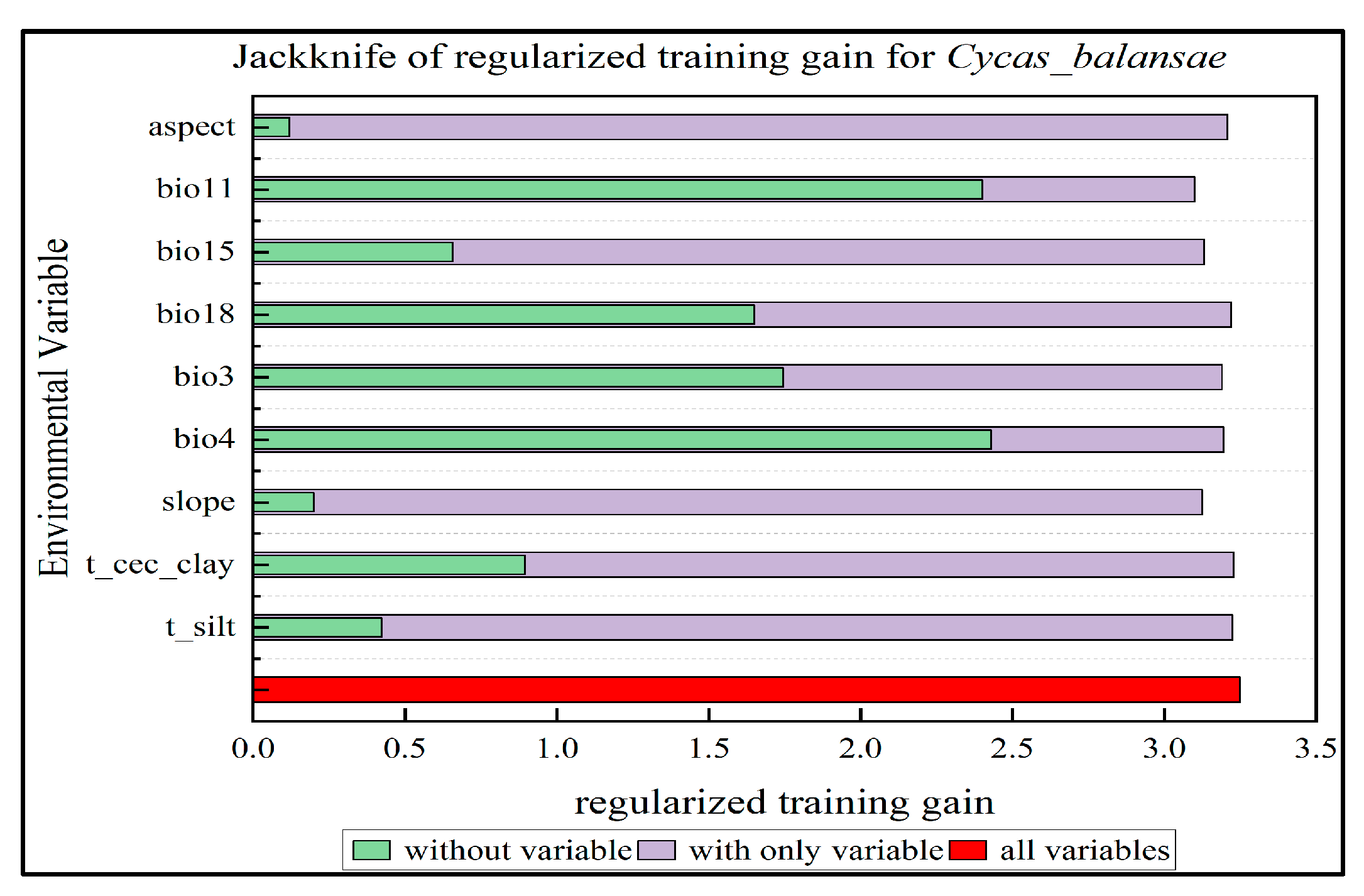
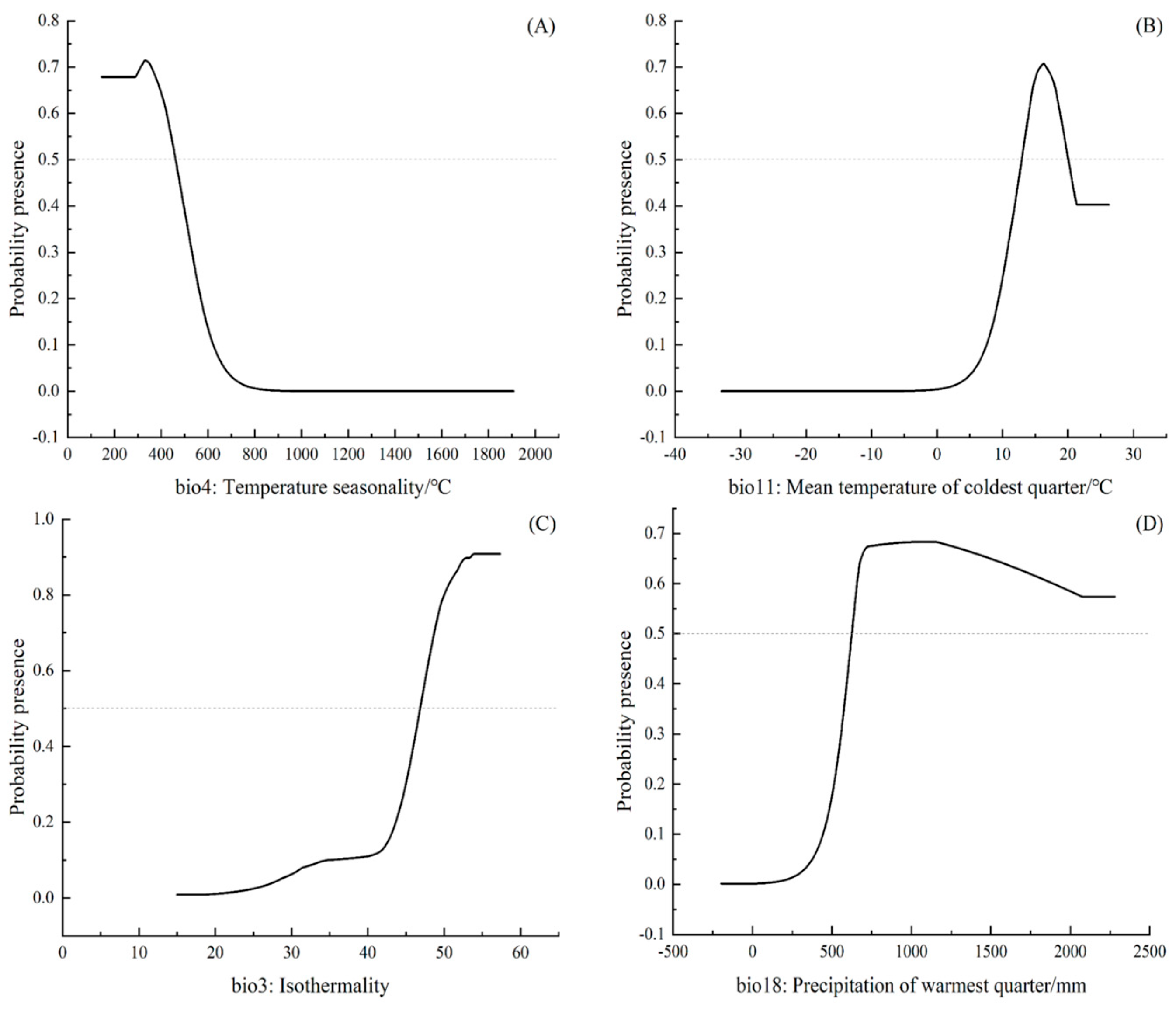
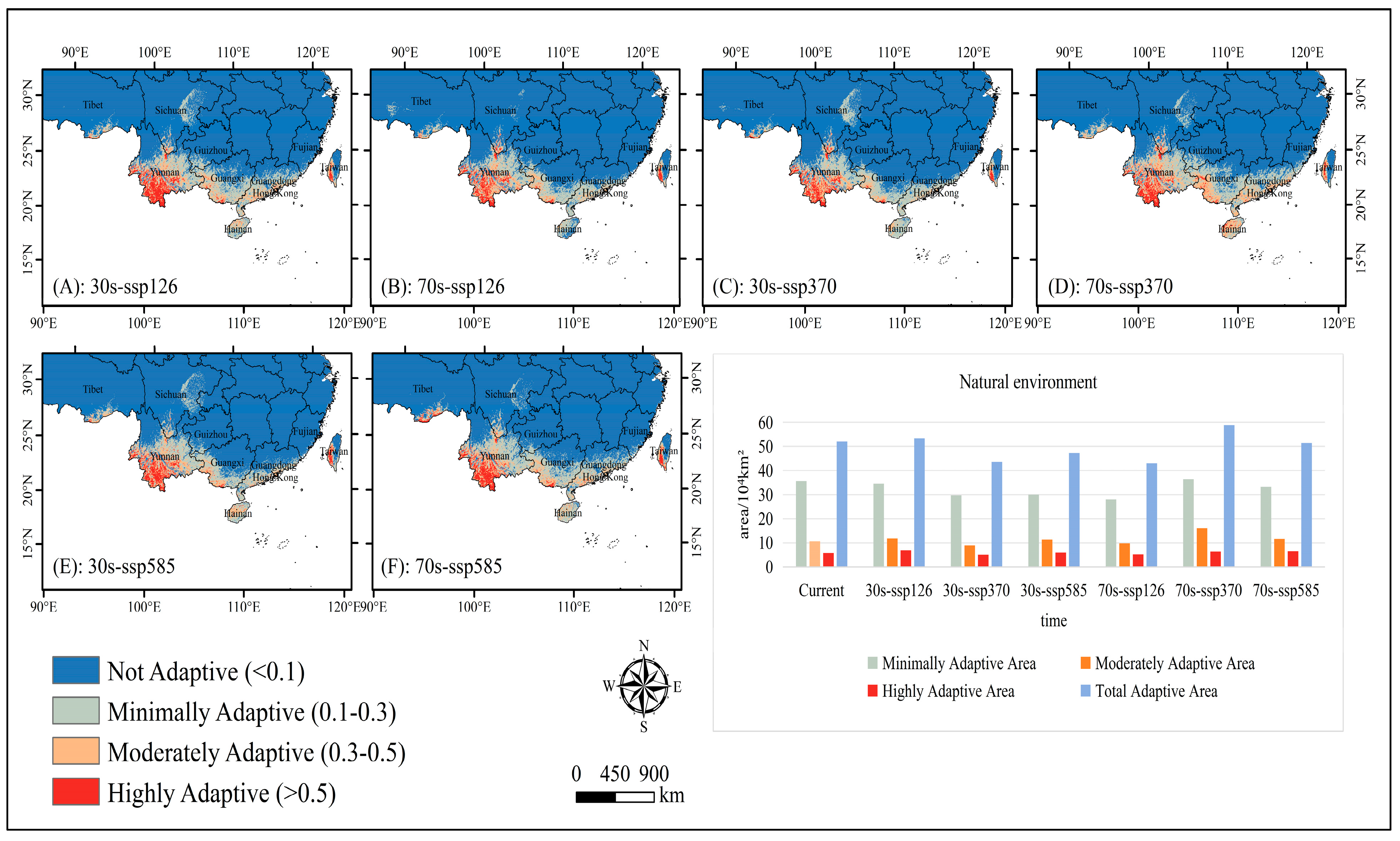
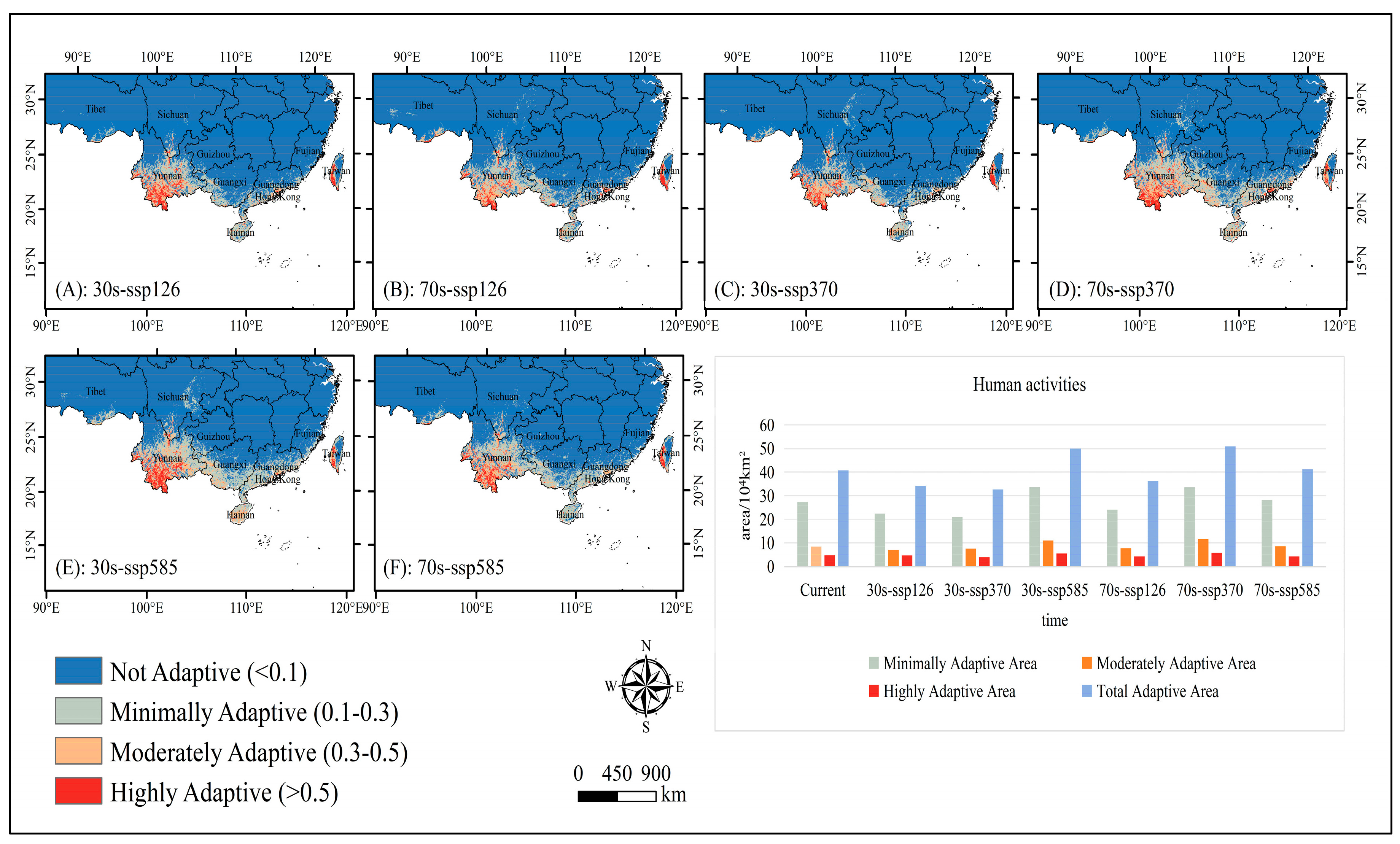
2.1. Adaptive Distribution and Environmental Drivers
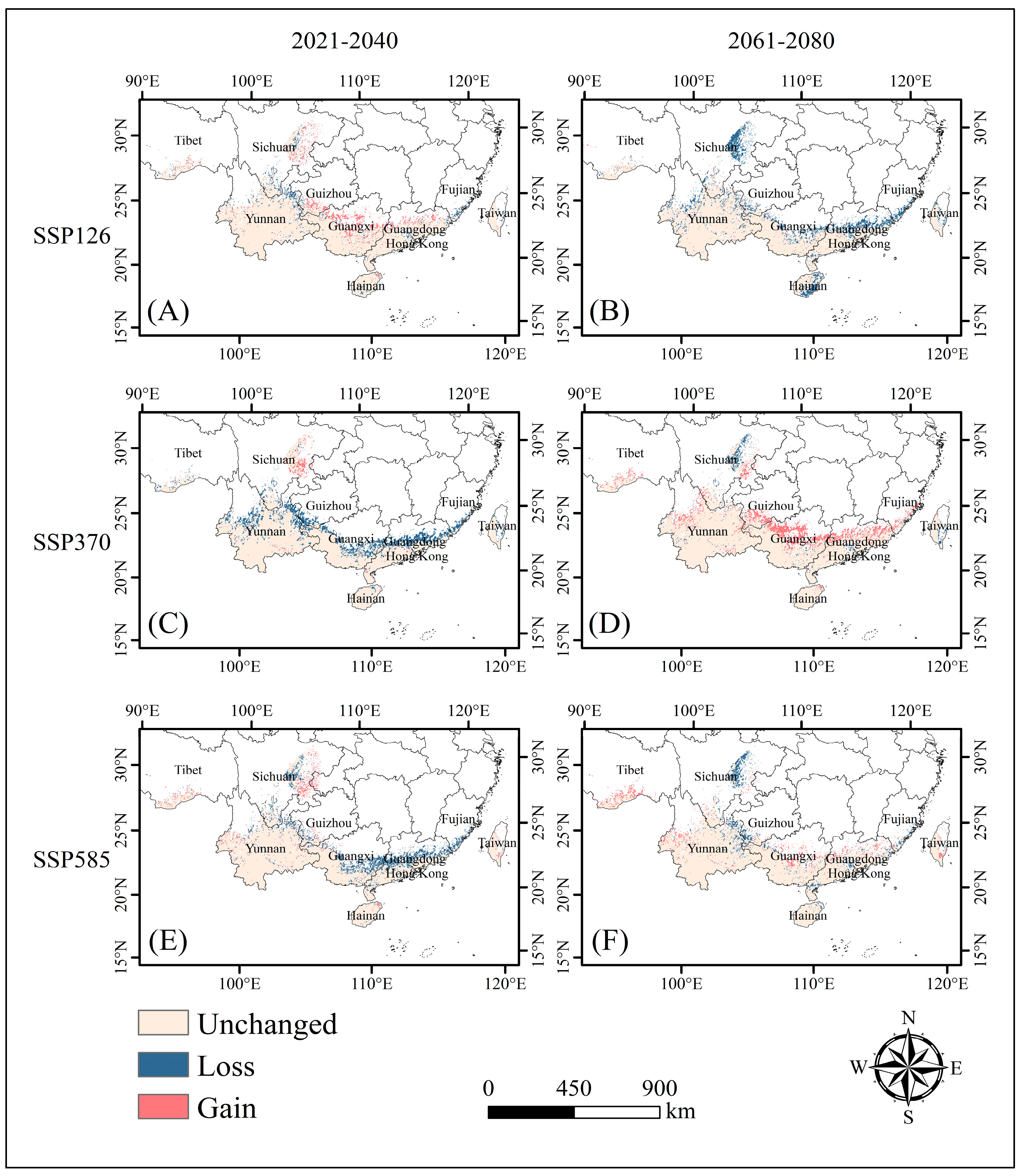
2.2. Adaptive Distribution Driven by Climate
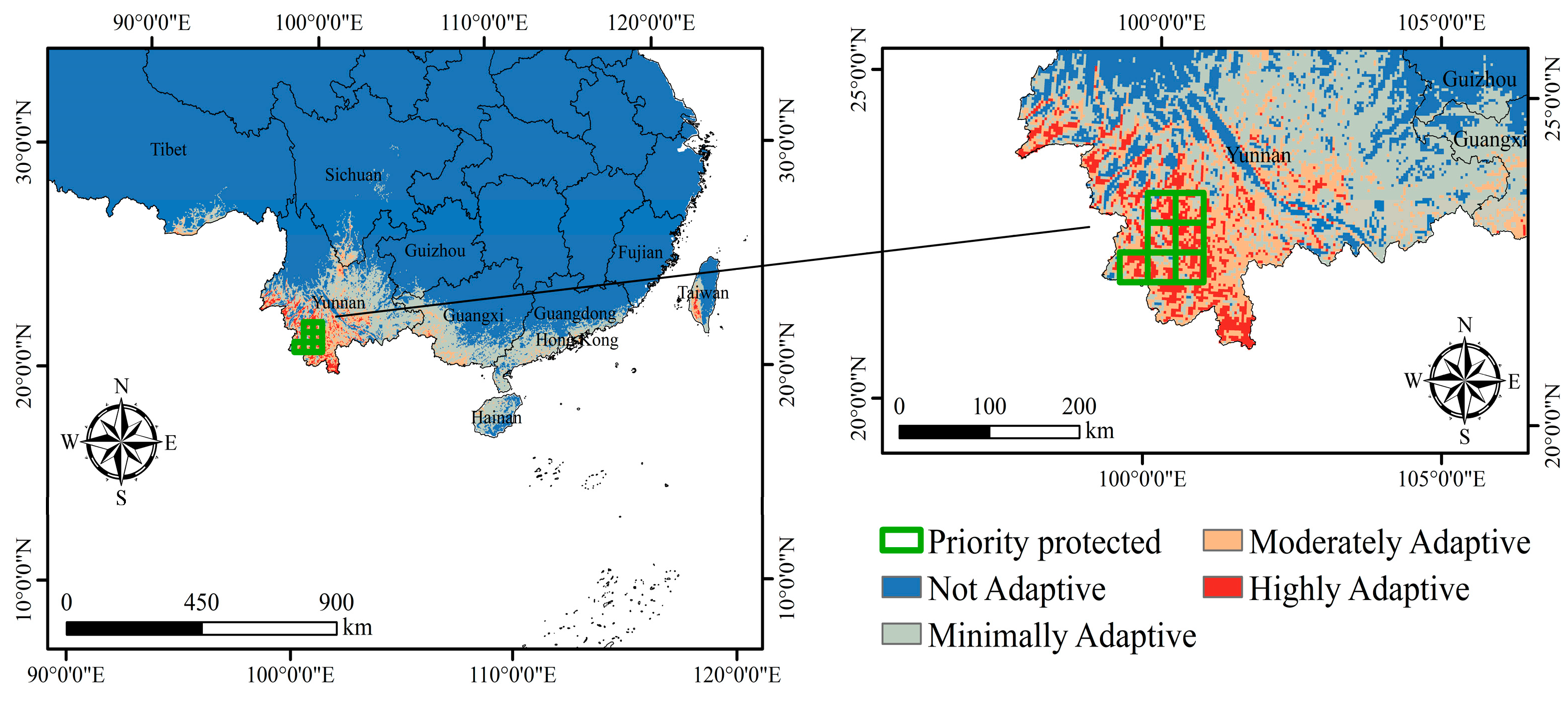
2.3. Protection Priority Area
3. Discussion
4. Materials and Methods
4.1. Data Sources and Data Processing
4.2. Model Prediction and Accuracy Assessment
4.3. Calculation of Adaptive Distribution and Identification of Environmental Drivers
4.4. Determination of Priority Protected Areas
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Parmesan, C.; Yohe, G. A globally coherent fingerprint of climate change impacts across natural systems. Nature 2003, 421, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Pereira, H.M.; Leadley, P.W.; Proenca, V.; Alkemade, R.; Scharlemann, J.P.W.; Fernandez-Manjarres, J.F.; Araujo, M.B.; Balvanera, P.; Biggs, R.; Cheung, W.W.L.; et al. Scenarios for Global Biodiversity in the 21st Century. Science 2010, 330, 1496–1501. [Google Scholar] [CrossRef] [PubMed]
- Newbold, T. Future effects of climate and land-use change on terrestrial vertebrate community diversity under different scenarios. Proc. R. Soc. B-Biol. Sci. 2018, 285, 20180792. [Google Scholar] [CrossRef]
- Veloz, S.D.; Williams, J.W.; Blois, J.L.; He, F.; Otto-Bliesner, B.; Liu, Z. No-analog climates and shifting realized niches during the late quaternary: Implications for 21st-century predictions by species distribution models. Glob. Change Biol. 2012, 18, 1698–1713. [Google Scholar] [CrossRef]
- Bellard, C.; Bertelsmeier, C.; Leadley, P.; Thuiller, W.; Courchamp, F. Impacts of climate change on the future of biodiversity. Ecol. Lett. 2012, 15, 365–377. [Google Scholar] [CrossRef]
- Yu, F.; Wang, T.; Groen, T.A.; Skidmore, A.K.; Yang, X.; Ma, K.; Wu, Z. Climate and land use changes will degrade the distribution of Rhododendrons in China. Sci. Total Environ. 2019, 659, 515–528. [Google Scholar] [CrossRef]
- Sun, J.; Feng, L.; Wang, T.; Tian, X.; He, X.; Xia, H.; Wang, W. Predicting the Potential Habitat of Three Endangered Species of Carpinus Genus under Climate Change and Human Activity. Forests 2021, 12, 1216. [Google Scholar] [CrossRef]
- Yi, Z.; Cannon, C.H.; Chen, J.; Ye, C.; Swetnam, R.D. Developing indicators of economic value and biodiversity loss for rubber plantations in Xishuangbanna, southwest China: A case study from Menglun township. Ecol. Indic. 2014, 36, 788–797. [Google Scholar] [CrossRef]
- Zheng, G.; Li, S.; Yang, X. Spider diversity in canopies of Xishuangbanna rainforest (China) indicates an alarming juggernaut effect of rubber plantations. For. Ecol. Manag. 2015, 338, 200–207. [Google Scholar] [CrossRef]
- Schemske, D.W.; Husband, B.C.; Ruckelshaus, M.H.; Goodwillie, C.; Parker, I.M.; Bishop, J.G. Evaluating Approaches to the Conservation of Rare and Endangered Plants. Ecology 1994, 75, 584–606. [Google Scholar] [CrossRef]
- Liu, M.; Li, L.; Wang, S.; Xiao, S.; Mi, J. Forecasting the future suitable growth areas and constructing ecological corridors for the vulnerable species Ephedra sinica in China. J. Nat. Conserv. 2023, 73, 126401. [Google Scholar] [CrossRef]
- Zheng, Y.; Liu, J.; Feng, X.; Gong, X. The distribution, diversity, and conservation status of Cycas in China. Ecol. Evol. 2017, 7, 3212–3224. [Google Scholar] [CrossRef] [PubMed]
- Elgorriaga, A.; Atkinson, B.A. Cretaceous pollen cone with three-dimensional preservation sheds light on the morphological evolution of cycads in deep time. New Phytol. 2023, 238, 1695–1710. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Thomas, B.A. A review of fossil cycad megasporophylls, with new evidence of Crossozamia pomel and its associated leaves from the lower permian of Taiyuan, China. Rev. Palaeobot. Palynol. 1989, 60, 205–223. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, S.; Li, L.; Yang, T.; Dong, S.; Wei, T.; Wu, S.; Liu, Y.; Gong, Y.; Feng, X.; et al. The Cycas genome and the early evolution of seed plants. Nat. Plants 2022, 8, 389–401. [Google Scholar] [CrossRef]
- Tang, J.; Zou, R.; Chen, T.; Pan, L.; Zhu, S.; Ding, T.; Chai, S.; Wei, X. Comparative Analysis of the Complete Chloroplast Genomes of Six Endangered Cycas Species: Genomic Features, Comparative Analysis, and Phylogenetic Implications. Forests 2023, 14, 2069. [Google Scholar] [CrossRef]
- Wang, Y.D.; Zhang, W.; Zheng, S.L.; Saiki, K.; Nan, L. New discovery of fossil cycad-like plants from the middle Jurassic of West Liaoning, China. Chin. Sci. Bull. 2005, 50, 1804–1807. [Google Scholar] [CrossRef]
- Bamigboye, S.; Tshisikhawe, M.P. The impacts of bark harvesting on a population of Encephalartos transvenosus (Limpopo cycad), in Limpopo Province, South Africa. Biodiversitas J. Biol. Divers. 2020, 21, 8–13. [Google Scholar] [CrossRef]
- Tao, Y.; Chen, B.; Kang, M.; Liu, Y.; Wang, J. Genome-Wide Evidence for Complex Hybridization and Demographic History in a Group of Cycas From China. Front. Genet. 2021, 12, 717200. [Google Scholar] [CrossRef]
- Osborne, R. The world cycad census and a proposed revision of the threatened species status for cycad taxa. Biol. Conserv. 1995, 71, 1–12. [Google Scholar] [CrossRef]
- Ling, Z.; Lu, E.; Peng, X.; Yang, Y.; Zheng, Y. The physiochemical characteristics and glycerolipid profile of Cycas panzhihuaensis in response to individual and combined drought and freezing temperature stress. Plant Physiol. Biochem. 2024, 214, 108907. [Google Scholar] [CrossRef] [PubMed]
- Hill, K.E.; Hill, R.S.; Watling, J.R. Pinnule and Stomatal Size and Stomatal Density of Living and Fossil Bowenia and Eobowenia Specimens Give Insight into Physiology during Cretaceous and Eocene Paleoclimates. Int. J. Plant Sci. 2019, 180, 323–336. [Google Scholar] [CrossRef]
- Suárez-Moo, P.d.J.; Vovides, A.P.; Griffith, M.P.; Barona-Gómez, F.; Cibrián-Jaramillo, A. Unlocking a high bacterial diversity in the coralloid root microbiome from the cycad genus Dioon. PLoS ONE 2019, 14, e0211271. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, Y.; Li, Y.; Zhu, Y. Re-investigation of the Mesozoic cycad genus Chilinia: Fossil record, diversity, spatio-temporal distribution, and palaeoclimate implications. Cretac. Res. 2024, 162, 105920. [Google Scholar] [CrossRef]
- Xi, H.; Wang, Y.; Pan, Y.; Xu, T.; Zhan, Q.; Liu, J.; Feng, X.; Gong, X. Resources and protection of Cycas plants in China. Biodivers. Sci. 2020, 30, 21495. [Google Scholar] [CrossRef]
- Tam, N.M.; Trang, N.T.P.; Giang, V.T.H.; Triest, L.T. Effects of Habitat Fragmentation on Genetic Diversity in Cycas Balansae (Cycadaceae). ASEAN J. Sci. Technol. Dev. 2017, 23, 193–205. [Google Scholar] [CrossRef]
- Wu, P.; Zhang, K. Status of Cycas spp. Plants in Yunnan and Measures for Their Protection. For. Inventory Plan. 2008, 33, 116–119. [Google Scholar] [CrossRef]
- Liu, S.; Huang, X.; Bin, Z.; Yu, B.; Lu, Z.; Hu, R.; Long, C. Wild edible plants and their cultural significance among the Zhuang ethnic group in Fangchenggang, Guangxi, China. J. Ethnobiol. Ethnomedicine 2023, 19, 52. [Google Scholar] [CrossRef]
- Tan, H.; Yang, Y.; Dong, S.; He, Q.; Jia, Y.; Zhao, L.; Yu, S.; Liu, H.; Liu, B.; Yan, Y.; et al. Threatened Species List of China’s Higher Plants. Biodivers. Sci. 2017, 25, 696–744. [Google Scholar] [CrossRef]
- Wei, Y.; Chen, J.; Zheng, Y.; Zhu, Y.; Zhou, A.; Ma, H.; Wu, J. Diversity and adaptability of endophytic microorganisms in coralloid roots of different species of Cycas. Acta Microbiol. Sin. 2022, 62, 2835–2849. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudík, M. Modeling of species distributions with Maxent: New extensions and a comprehensive evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudík, M.; Chee, Y.E.; Yates, C.J. A statistical explanation of MaxEnt for ecologists. Divers. Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- Cai, C.; Zhang, X.; Zha, J.; Li, J.; Li, J. Predicting Climate Change Impacts on the Rare and Endangered Horsfieldia tetratepala in China. Forests 2022, 13, 1051. [Google Scholar] [CrossRef]
- Wang, Z.; Jia, Y.; Li, P.; Tang, Z.; Guo, Y.; Wen, L.; Yu, H.; Cui, F.; Hu, F. Study on environmental factors affecting the quality of codonopsis radix based on MaxEnt model and all-in-one functional factor. Sci. Rep. 2023, 13, 20726. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, S.; Ren, L.; Tian, X.; Tang, S.; Xian, Y.; Wu, X.; Zhang, Z. Prediction of Chinese suitable habitats of Panax notoginseng under climate change based on MaxEnt and chemometric methods. Sci. Rep. 2024, 14, 16434. [Google Scholar] [CrossRef]
- Wang, Z.; Luo, M.; Ye, L.; Peng, J.; Luo, X.; Gao, L.; Huang, Q.; Chen, Q.; Zhang, L. Prediction of the Potentially Suitable Areas of Actinidia latifolia in China Based on Climate Change Using the Optimized MaxEnt Model. Sustainability 2024, 16, 5975. [Google Scholar] [CrossRef]
- Shi, X.; Wang, J.; Zhang, L.; Chen, S.; Zhao, A.; Ning, X.; Fan, G.; Wu, N.; Zhang, L.; Wang, Z. Prediction of the potentially suitable areas of Litsea cubeba in China based on future climate change using the optimized MaxEnt model. Ecol. Indic. 2023, 148, 110093. [Google Scholar] [CrossRef]
- Xiao, F.; Liu, Q.; Qin, Y. Predicting the Potential Distribution of Haloxylon ammodendron under Climate Change Scenarios Using Machine Learning of a Maximum Entropy Model. Biology 2024, 13, 3. [Google Scholar] [CrossRef]
- Hernández, P.; Graham, C.H.; Master, L.L.; Albert, D.L. The effect of sample size and species characteristics on performance of different species distribution modeling methods. Ecography 2006, 29, 773–785. [Google Scholar] [CrossRef]
- Huang, P.; Xiao, Y.; Sun, Y.; Huang, H.; Gong, Z.; Zhu, Y. Distribution changes of Ormosia microphylla under different climatic scenarios. Sci. Rep. 2025, 15, 2607. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Song, Y.; Zeng, B.; Dong, M. Potential distribution of the extremely endangered species Ostrya rehderiana (Betulaceae) in China under future climate change. Environ. Sci. Pollut. Res. 2022, 29, 7782–7792. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.D.; Cameron, A.; Green, R.E.; Bakkenes, M.; Beaumont, L.J.; Collingham, Y.C.; Erasmus, B.F.N.; de Siqueira, M.F.; Grainger, A.; Hannah, L.; et al. Extinction risk from climate change. Nature 2004, 427, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Mori, A.S.; Furukawa, T.; Sasaki, T. Response diversity determines the resilience of ecosystems to environmental change. Biol. Rev. 2013, 88, 349–364. [Google Scholar] [CrossRef]
- Weiskopf, S.R.; Rubenstein, M.A.; Crozier, L.G.; Gaichas, S.; Griffis, R.; Halofsky, J.E.; Hyde, K.J.W.; Morelli, T.L.; Morisette, J.T.; Munoz, R.C.; et al. Climate change effects on biodiversity, ecosystems, ecosystem services, and natural resource management in the United States. Sci. Total Environ. 2020, 733, 137782. [Google Scholar] [CrossRef]
- Wei, J.; Zhao, Q.; Zhao, W.; Zhang, H. Predicting the potential distributions of the invasive cycad scale Aulacaspis yasumatsui (Hemiptera: Diaspididae) under different climate change scenarios and the implications for management. PeerJ 2018, 6, e4832. [Google Scholar] [CrossRef]
- He, Z.; Yao, Z.; Wang, K.; Li, Y.; Liu, Y. Genetic Structure and Differentiation of Endangered Cycas Species Indicate a Southward Migration Associated with Historical Cooling Events. Diversity 2023, 15, 643. [Google Scholar] [CrossRef]
- Hao, Y.; Dong, P.; Wang, L.; Ke, X.; Hao, X.; He, G.; Chen, Y.; Guo, F. Predicting the Potential Distribution of Hypericum perforatum under Climate Change Scenarios Using a Maximum Entropy Model. Biology 2024, 13, 452. [Google Scholar] [CrossRef]
- Luo, J.; Ma, Y.; Liu, Y.; Zhu, D.; Guo, X. Predicting Polygonum capitatum distribution in China across climate scenarios using MaxEnt modeling. Sci. Rep. 2024, 14, 20020. [Google Scholar] [CrossRef]
- Yang, Z.; Bai, Y.; Alatalo, J.M.; Huang, Z.; Yang, F.; Pu, X.; Wang, R.; Yang, W.; Guo, X. Spatio-temporal variation in potential habitats for rare and endangered plants and habitat conservation based on the maximum entropy model. Sci. Total Environ. 2021, 784, 147080. [Google Scholar] [CrossRef]
- Xu, L.; Fan, Y.; Zheng, J.; Guan, J.; Lin, J.; Wu, J.; Liu, L.; Wu, R.; Liu, Y. Impacts of climate change and human activity on the potential distribution of Aconitum leucostomum in China. Sci. Total Environ. 2024, 912, 168829. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, L.; Wang, C. Predicting possible distribution of rice leaf roller (Cnaphalocrocis medinalis) under climate change scenarios using MaxEnt model in China. Sci. Rep. 2024, 14, 21245. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, R.; Lenoir, J.; Piedallu, C.; Riofrio-Dillon, G.; de Ruffray, P.; Vidal, C.; Pierrat, J.-C.; Gegout, J.-C. Changes in plant community composition lag behind climate warming in lowland forests. Nature 2011, 479, 517–520. [Google Scholar] [CrossRef]
- Alexander, J.M.; Diez, J.M.; Levine, J.M. Novel competitors shape species’ responses to climate change. Nature 2015, 525, 515–518. [Google Scholar] [CrossRef] [PubMed]
- Rumpf, S.B.; Huelber, K.; Wessely, J.; Willner, W.; Moser, D.; Gattringer, A.; Klonner, G.; Zimmermann, N.E.; Dullinger, S. Extinction debts and colonization credits of non-forest plants in the European Alps. Nat. Commun. 2019, 10, 4293. [Google Scholar] [CrossRef]
- Balint, M.; Domisch, S.; Engelhardt, C.H.M.; Haase, P.; Lehrian, S.; Sauer, J.; Theissinger, K.; Pauls, S.U.; Nowak, C. Cryptic biodiversity loss linked to global climate change. Nat. Clim. Change 2011, 1, 313–318. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, L.; Li, Y.; Zhu, W.; Chen, Y. Maxent Modelling Predicts a Shift in Suitable Habitats of a Subtropical Evergreen Tree (Cyclobalanopsis glauca (Thunberg) Oersted) under Climate Change Scenarios in China. Forests 2022, 13, 126. [Google Scholar] [CrossRef]
- Zhang, Y.-F.; Chen, S.-T.; Gao, Y.; Yang, L.; Yu, H. Prediction of global potential suitable habitats of Nicotiana alata Link et Otto based on MaxEnt model. Sci. Rep. 2023, 13, 4851. [Google Scholar] [CrossRef]
- Hu, W.; Wang, Y.; Dong, P.; Zhang, D.; Yu, W.; Ma, Z.; Chen, G.; Liu, Z.; Du, J.; Chen, B.; et al. Predicting potential mangrove distributions at the global northern distribution margin using an ecological niche model: Determining conservation and reforestation involvement. For. Ecol. Manag. 2020, 478, 118517. [Google Scholar] [CrossRef]
- Reddy, V. Micropropagation of rare and threatened medicinal plant species of South Africa—For propagation and preservation: An overview. Acta Hortic. 2017, 1155, 619–624. [Google Scholar] [CrossRef]
- Berihun, M.L.; Tsunekawa, A.; Haregeweyn, N.; Meshesha, D.T.; Adgo, E.; Tsubo, M.; Masunaga, T.; Fenta, A.A.; Sultan, D.; Yibeltal, M. Exploring land use/land cover changes, drivers and their implications in contrasting agro-ecological environments of Ethiopia. Land Use Policy 2019, 87, 104052. [Google Scholar] [CrossRef]
- Puttker, T.; Crouzeilles, R.; Almeida-Gomes, M.; Schmoeller, M.; Maurenza, D.; Alves-Pinto, H.; Pardini, R.; Vieira, M.V.; Banks-Leite, C.; Fonseca, C.R.; et al. Indirect effects of habitat loss via habitat fragmentation: A cross-taxa analysis of forest-dependent species. Biol. Conserv. 2020, 241, 108368. [Google Scholar] [CrossRef]
- Lian, Y.; Bai, Y.; Huang, Z.; Ali, M.; Wang, J.; Chen, H. Spatio-Temporal Changes and Habitats of Rare and Endangered Species in Yunnan Province Based on MaxEnt Model. Land 2024, 13, 240. [Google Scholar] [CrossRef]
- Chape, S.; Harrison, J.; Spalding, M.; Lysenko, I. Measuring the extent and effectiveness of protected areas as an indicator for meeting global biodiversity targets. Philos. Trans. R. Soc. B-Biol. Sci. 2005, 360, 443–455. [Google Scholar] [CrossRef]
- Huang, Z.; Lu, L.; Dai, N.; Jiao, G. Vacancy analysis on the development of nature reserves in Jiangxi Province. Acta Ecol. Sin. 2014, 34, 3099–3106. [Google Scholar] [CrossRef]
- Swart, C.; Donaldson, J.; Barker, N. Predicting the distribution of Encephalartos latifrons, a critically endangered cycad in South Africa. Biodivers. Conserv. 2018, 27, 1961–1980. [Google Scholar] [CrossRef]
- Chen, J.; Shi, H.; Wang, X.; Zhang, Y.; Zhang, Z. Effectiveness of China’s Protected Areas in Mitigating Human Activity Pressure. Int. J. Environ. Res. Public Health 2022, 19, 9335. [Google Scholar] [CrossRef]
- GBIF Occurrence. Available online: https://www.gbif.org/occurrence/download/0064101-231002084531237 (accessed on 18 November 2023).
- Sanderson, E.W.; Jaiteh, M.; Levy, M.A.; Redford, K.H.; Wannebo, A.V.; Woolmer, G. The Human Footprint and the Last of the Wild. BioScience 2002, 52, 891–904. [Google Scholar] [CrossRef]
- Harvey, B.J.; Cook, P.; Shaffrey, L.C.; Schiemann, R. The Response of the Northern Hemisphere Storm Tracks and Jet Streams to Climate Change in the CMIP3, CMIP5, and CMIP6 Climate Models. J. Geophys. Res.-Atmos. 2020, 125, e2020JD032701. [Google Scholar] [CrossRef]
- Tokarska, K.B.; Stolpe, M.B.; Sippel, S.; Fischer, E.M.; Smith, C.J.; Lehner, F.; Knutti, R. Past warming trend constrains future warming in CMIP6 models. Sci. Adv. 2020, 6, eaaz9549. [Google Scholar] [CrossRef]
- Yang, J.; Jiang, P.; Huang, Y.; Yang, Y.; Wang, R.; Yang, Y. Potential geographic distribution of relict plant Pteroceltis tatarinowii in China under climate change scenarios. PLoS ONE 2022, 17, e0266133. [Google Scholar] [CrossRef] [PubMed]
- Birhane, E.; Gidey, T.; Abrha, H.; Brhan, A.; Zenebe, A.; Gebresamuel, G.; Noulekoun, F. Impact of land-use and climate change on the population structure and distribution range of the rare and endangered Dracaena ombet and Dobera glabra in northern Ethiopia. J. Nat. Conserv. 2023, 76, 126506. [Google Scholar] [CrossRef]
- Zhou, Y.; Lu, X.; Zhang, G. Potentially differential impacts on niche overlap between Chinese endangered Zelkova schneideriana and its associated tree species under climate change. Front. Ecol. Evol. 2023, 11, 1218149. [Google Scholar] [CrossRef]
- Wei, L.; Wang, G.; Xie, C.; Gao, Z.; Huang, Q.; Jim, C.Y. Predicting suitable habitat for the endangered tree Ormosia microphylla in China. Sci. Rep. 2024, 14, 10330. [Google Scholar] [CrossRef]
- Saupe, E.E.; Barve, V.; Myers, C.E.; Soberon, J.; Barve, N.; Hensz, C.M.; Peterson, A.T.; Owens, H.L.; Lira-Noriega, A. Variation in niche and distribution model performance: The need for a priori assessment of key causal factors. Ecol. Model. 2012, 237, 11–22. [Google Scholar] [CrossRef]
- Raes, N.; Cannon, C.H.; Hijmans, R.J.; Piessens, T.; Saw, L.G.; van Welzen, P.C.; Slik, J.W.F. Historical distribution of Sundaland’s Dipterocarp rainforests at Quaternary glacial maxima. Proc. Natl. Acad. Sci. USA 2014, 111, 16790–16795. [Google Scholar] [CrossRef]
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carre, G.; Garcia Marquez, J.R.; Gruber, B.; Lafourcade, B.; Leitao, P.J.; et al. Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography 2013, 36, 27–46. [Google Scholar] [CrossRef]
- Li, D.; Li, Z.; Liu, Z.; Yang, Y.; Khoso, A.G.; Wang, L.; Liu, D. Climate change simulations revealed potentially drastic shifts in insect community structure and crop yields in China’s farmland. J. Pest Sci. 2023, 96, 55–69. [Google Scholar] [CrossRef]
- Warren, D.L.; Seifert, S.N. Ecological niche modeling in Maxent: The importance of model complexity and the performance of model selection criteria. Ecol. Appl. 2011, 21, 335–342. [Google Scholar] [CrossRef]
- Moreno, R.; Zamora, R.; Ramon Molina, J.; Vasquez, A.; Angel Herrera, M. Predictive modeling of microhabitats for endemic birds in South Chilean temperate forests using Maximum entropy (Maxent). Ecol. Inform. 2011, 6, 364–370. [Google Scholar] [CrossRef]
- Ahmad, R.; Khuroo, A.A.; Hamid, M.; Charles, B.; Rashid, I. Predicting invasion potential and niche dynamics of Parthenium hysterophorus (Congress grass) in India under projected climate change. Biodivers. Conserv. 2019, 28, 2319–2344. [Google Scholar] [CrossRef]
- Jessica, B.-S.; Christine, R.W.; Karen, V.R. Developing macrohabitat models for bats in parks using maxent and testing them with data collected by citizen scientists. Int. J. Biodivers. Conserv. 2014, 6, 171–183. [Google Scholar] [CrossRef]
- Walden-Schreiner, C.; Leung, Y.-F.; Kuhn, T.; Newburger, T.; Tsai, W.-L. Environmental and managerial factors associated with pack stock distribution in high elevation meadows: Case study from Yosemite National Park. J. Environ. Manag. 2017, 193, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Jiang, F.; Li, G.; Qin, W.; Wu, T.; Xu, F.; Hou, Y.; Song, P.; Cai, Z.; Zhang, T. The four antelope species on the Qinghai-Tibet plateau face habitat loss and redistribution to higher latitudes under climate change. Ecol. Indic. 2021, 123, 107337. [Google Scholar] [CrossRef]
- Merow, C.; Smith, M.J.; Silander, J.A., Jr. A practical guide to MaxEnt for modeling species’ distributions: What it does, and why inputs and settings matter. Ecography 2013, 36, 1058–1069. [Google Scholar] [CrossRef]
- Liu, C.R.; Berry, P.M.; Dawson, T.P.; Pearson, R.G. Selecting thresholds of occurrence in the prediction of species distributions. Ecography 2005, 28, 385–393. [Google Scholar] [CrossRef]
- Jimenez-Valverde, A.; Lobo, J.M. Threshold criteria for conversion of probability of species presence to either-or presence-absence. Acta Oecologica-Int. J. Ecol. 2007, 31, 361–369. [Google Scholar] [CrossRef]
- Wang, Q.; Fan, B.; Zhang, G. Prediction of potential distribution area of Corylus mandshurica in China under climate change. Chin. J. Ecol. 2020, 39, 3774–3884. [Google Scholar] [CrossRef]
- Margules, C.R.; Pressey, R.L. Systematic conservation planning. Nature 2000, 405, 243–253. [Google Scholar] [CrossRef]
- Wintle, B.A.; Kujala, H.; Whitehead, A.; Cameron, A.; Veloz, S.; Kukkala, A.; Moilanen, A.; Gordon, A.; Lentini, P.E.; Cadenhead, N.C.R.; et al. Global synthesis of conservation studies reveals the importance of small habitat patches for biodiversity. Proc. Natl. Acad. Sci. USA 2019, 116, 909–914. [Google Scholar] [CrossRef]
- Zhang, L.; Ouyang, Z.; Xu, W. Theory, work frame and hot issues of systematic conservation planning. Acta Ecol. Sin. 2015, 35, 1284–1295. [Google Scholar] [CrossRef]
- Mirea, M.D.; Miu, I.V.; Popescu, V.D.; Brodie, B.S.; Chiriac, S.; Rozylowicz, L. Priority conservation areas for protected saproxylic beetles in Romania under current and future climate scenarios. Biodivers. Conserv. 2024, 33, 2949–2973. [Google Scholar] [CrossRef]
- Goettsch, B.; Paz Duran, A.; Gaston, K.J. Global gap analysis of cactus species and priority sites for their conservation. Conserv. Biol. 2019, 33, 369–376. [Google Scholar] [CrossRef] [PubMed]
- De Alban, J.D.T.; Leong, B.P.I.; Venegas-Li, R.; Connette, G.M.; Jamaludin, J.; Latt, K.T.; Oswald, P.; Reeder, C.; Webb, E.L. Conservation beyond the existing protected area network is required to improve species and habitat representation in a global biodiversity hotspot. Biol. Conserv. 2021, 257, 109105. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, K.; Zhao, R.; Xie, L.; Li, Y.; Zhao, G.; Zhang, F.-G. Prediction of potential suitable habitats in the 21st century and GAP analysis of priority conservation areas of Chionanthus retusus based on the MaxEnt and Marxan models. Front. Plant Sci. 2024, 15, 1304121. [Google Scholar] [CrossRef]
- He, P.; Li, J.; Li, Y.; Xu, N.; Gao, Y.; Guo, L.; Huo, T.; Peng, C.; Meng, F. Habitat protection and planning for three Ephedra using the MaxEnt and Marxan models. Ecol. Indic. 2021, 133, 108399. [Google Scholar] [CrossRef]
- Tang, J.; Lu, H.; Xue, Y.; Li, J.; Li, G.; Mao, Y.; Deng, C.; Li, D. Data-driven planning adjustments of the functional zoning of Houhe National Nature Reserve. Glob. Ecol. Conserv. 2021, 29, e01708. [Google Scholar] [CrossRef]
- Zhang, H.; Wei, Y.; Yue, J.; Wang, Z.; Zou, H.; Ji, X.; Zhang, S.; Liu, Z. Prediction of Potential Suitable Areas and Priority Protection for Cupressus gigantea on the Tibetan Plateau. Plants 2024, 13, 896. [Google Scholar] [CrossRef]
- Luck, G.W.; Ricketts, T.H.; Daily, G.C.; Imhoff, M. Alleviating spatial conflict between people and biodiversity. Proc. Natl. Acad. Sci. USA 2004, 101, 182–186. [Google Scholar] [CrossRef]
- Venter, O.; Sanderson, E.W.; Magrach, A.; Allan, J.R.; Beher, J.; Jones, K.R.; Possingham, H.P.; Laurance, W.F.; Wood, P.; Fekete, B.M.; et al. Sixteen years of change in the global terrestrial human footprint and implications for biodiversity conservation. Nat. Commun. 2016, 7, 12558. [Google Scholar] [CrossRef]
- Carrasco, J.; Price, V.; Tulloch, V.; Mills, M. Selecting priority areas for the conservation of endemic trees species and their ecosystems in Madagascar considering both conservation value and vulnerability to human pressure. Biodivers. Conserv. 2020, 29, 1841–1854. [Google Scholar] [CrossRef]
| Code | Variables Name | Code | Variables Name |
|---|---|---|---|
| Bio1 | Annual mean temperature | Bio15 | Precipitation seasonality |
| Bio2 | Mean diurnal range | Bio16 | Precipitation of wettest quarter |
| Bio3 | Isothermality | Bio17 | Precipitation of driest quarter |
| Bio4 | Temperature seasonality | Bio18 | Precipitation of warmest quarter |
| Bio5 | Max temperature of warmest month | Bio19 | Precipitation of coldest quarter |
| Bio6 | Min temperature of coldest month | ele | Elevation |
| Bio7 | Temperature annual range | slo | Slope |
| Bio8 | Mean temperature of wettest quarter | asp | Aspect |
| Bio9 | Mean temperature of driest quarter | T_SILT | Topsoil silt fraction |
| Bio10 | Mean temperature of warmest quarter | T_SAND | Topsoil sand fraction |
| Bio11 | Mean temperature of coldest quarter | T_CLAY | Topsoil clay fraction |
| Bio12 | Annual precipitation | T_pH_H2O | Topsoil pH (H2O) |
| Bio13 | Precipitation of wettest month | T_ESP | Topsoil sodicity (ESP) |
| Bio14 | Precipitation of driest month | T_CEC_CLAY | Topsoil CEC (clay) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Zhou, Y.; Zhang, S.; Wang, Z.; Liu, Z. Adaptive Distribution and Priority Protection of Endangered Species Cycas balansae. Plants 2025, 14, 815. https://doi.org/10.3390/plants14050815
Zhang H, Zhou Y, Zhang S, Wang Z, Liu Z. Adaptive Distribution and Priority Protection of Endangered Species Cycas balansae. Plants. 2025; 14(5):815. https://doi.org/10.3390/plants14050815
Chicago/Turabian StyleZhang, Huayong, Yanxia Zhou, Shijia Zhang, Zhongyu Wang, and Zhao Liu. 2025. "Adaptive Distribution and Priority Protection of Endangered Species Cycas balansae" Plants 14, no. 5: 815. https://doi.org/10.3390/plants14050815
APA StyleZhang, H., Zhou, Y., Zhang, S., Wang, Z., & Liu, Z. (2025). Adaptive Distribution and Priority Protection of Endangered Species Cycas balansae. Plants, 14(5), 815. https://doi.org/10.3390/plants14050815







