Abstract
Iron (Fe) deficiency is among the most important agronomical concerns under alkaline conditions. Bicarbonate is considered an important factor causing Fe deficiency in dicot plants, mainly on calcareous soils. Current production systems are based on the use of high-yielding varieties and the application of large quantities of agrochemicals, which can cause major environmental problems. The use of beneficial rhizosphere microorganisms is considered a relevant sustainable alternative to synthetic fertilizers. The main purpose of this work has been to analyze the impact of the inoculation of tomato (Solanum lycopersicum L.) seedlings with the WCS417 strain of Pseudomonas simiae, in the presence or absence of bicarbonate, on plant growth and other physiological parameters. To conduct this research, three different inoculation methods were implemented: root immersion, foliar application, and substrate inoculation by irrigation. The results obtained show the ability of the P. simiae WCS417 strain to induce medium acidification in the presence of bicarbonate to increase the SPAD index and to improve the growth and development of the tomato plants in calcareous conditions provoked by the presence of bicarbonate, which indicates that this bacteria strain could have a great potential as an Fe biofertilizer.
1. Introduction
From a microbiological perspective, soil is probably the most complex of all natural environments due to the size of its microbial community and its diversity. It is estimated that a gram of cultivated or pasture soil contains about 2 × 109 prokaryotic cells [1].
The portion of the soil in contact with plant roots is called the rhizosphere, ranging from 2 to 80 mm from the root [2]. The rhizosphere harbors a rich microbial community of up to 1010 bacteria per gram of soil [3,4] and encompasses a great diversity of taxa [5,6]. The majority of available information regarding the plant–microbe relationship is based on the pathogenic effect of microorganisms on plants [7]. However, despite numerous studies focused on phytopathology, most microorganisms do not cause harm to plants; on the contrary, many exert beneficial effects [8,9] by promoting plant growth and improving health through direct and indirect mechanisms [10,11].
Beneficial interactions between plants and microorganisms are symbiotic interactions where costs and benefits are shared by both [12,13]. Mutualistic symbioses correspond to the intimate, and mostly obligatory, interactions between microorganisms and a strict range of compatible host plants. They generally require the formation of structures specifically dedicated to this interaction (e.g., nodules during symbiosis between rhizobia and legumes, arbuscular mycorrhizas) [14,15,16]. These interactions also involve soil bacteria capable of colonizing the root system’s surface (sometimes even internal root tissues) and stimulate plant growth and health, known as plant growth-promoting rhizobacteria (PGPR) [17]. The colonization of roots by PGPR is heterogeneous along the root system, and their competitiveness during this process is an essential condition for the promotion of plant growth [18,19,20]. In contrast to mutualistic interactions, PGPR interact with a wide range of plant species and encompass a broad taxonomic diversity, especially within the Firmicutes and Proteobacteria phyla [20,21].
The mechanisms by which development and growth promotion occur can be diverse. For example, they can occur through the solubilization of specific nutrients, the production of certain compounds that directly promote growth (exudates), or the stimulation of the production of phytohormones [22]. Regarding the increase in nutrient supply through solubilization, the following two main types of bacterial activities need to be considered: (i) the production of organic acids, and (ii) the release of H+ to the outer surface inducing the H+-ATPase action. It is well known that many PGPR, such as species from the genera Pseudomonas, Bacillus, or Rhizobium, can solubilize insoluble forms of phosphate [11] by acidifying the external medium through the secretion of low molecular weight organic molecules that chelate cations bound to phosphate [23], or by producing phosphatases and phytases that hydrolyze phosphate-rich organic molecules. Another example is the activation of the plants machinery necessary for Fe acquisition, induced by the production of volatile organic compounds emitted by Bacillus subtilis GB03. These compounds act at two levels; firstly, they acidify the medium and subsequently induce the expression of genes encoding various molecules involved in Fe assimilation, including a Fe3+ chelate reductase enzyme, a Fe2+ transporter, and a transcription factor that induces the expression of these two genes [24,25]. Another important aspect of soil microbiology is the action of well-known Fe-bacteria, particularly, schizomycetes capable of promoting oxidative processes at the expense of Fe compounds. In calcareous soils where the bicarbonate fraction is in equilibrium with the carbonate fraction, ferrous carbonate is oxidized to insoluble ferric hydroxide with the release of CO2 by the chemoautotrophic Fe-bacteria in the soil [26].
On the other hand, many bacteria fix the atmospheric nitrogen that becomes available to the plant. There is evidence supporting the involvement of PGPR in the N2 supply to various plants, especially sugarcane [27,28,29]. However, the impact of N2 fixation by PGPR is still debated, and it is not entirely clear whether the nitrogen fixed by these bacteria substantially contributes to plant growth [30,31,32]. Finally, some non-nitrogen-fixing rhizobacteria promote plant growth, demonstrating that there must be other mechanisms, independent of nitrogen supply, by which these bacteria promote plant growth. For example, Phyllobacterium brassicacearum STM196 is unlikely to fix N2, yet it has been shown to promote the growth of rapeseed and Arabidopsis thaliana plants [33,34,35].
Regarding exudate production, microorganisms can act on the plant by modulating the composition of its exudates, contributing to the recruitment of beneficial ones and the suppression/inhibition of non-beneficial and pathogenic ones to avoid infections and the proliferation of non-beneficial microbial communities [24]. These exudates also interact with organic and inorganic molecules in the soil, regulating their availability in the soil and their absorption by the plant [36]. The growth and composition of the root microbial environment are largely determined by the composition of radical exudates, as many of these compounds attract bacteria, especially those that can metabolize some of their components and proliferate in that environment [20,37,38,39]. Also, concerning the stimulation of phytohormone production, certain plant responses are modulated by ethylene. Ethylene produced due to stress factors (extreme temperature increase, presence of heavy metals, lack of water, nutritional deficiencies, and the presence of microorganisms) can lead to responses that may enhance plant survival in adverse conditions. Various microbial strains have been shown to induce ACC synthase, which catalyzes the production of ACC, a precursor of ethylene, from S-adenosylmethionine [40]. It is interesting to note the effect that some microorganisms have on certain plant species. For example, the inoculation of Pachycereus pringlei seedlings with the bacterium Azospirillum brasiliense can not only promote plant growth but also reduce the pH of the rhizosphere [41].
Although the experiments have been conducted in hydroponic solution, we cannot overlook the presence of humic and fulvic acids in the soil. Considering that the study focuses on calcareous substrates, it is appropriate to highlight the following: (a) Humic acids have chelating properties, binding to nutrients in the soil and facilitating their uptake. (b) There exists high cation exchange capacity (CEC). (c) Humic acids are water-soluble at alkaline pH levels. Their highest concentration is found in calcareous or neutral soils [42,43].
Bicarbonate is a key factor contributing to iron (Fe) chlorosis in dicot plants, particularly in calcareous soils [44,45,46]. However, its exact mechanism of action remains unclear. Traditionally, the primary effect of bicarbonate on Fe nutrition has been attributed to its pH-buffering capacity. By maintaining a high pH level (7.5–8.0) in the surrounding medium, bicarbonate can reduce Fe solubility and inhibit ferric reductase activity, which operates optimally at around pH 5.0 [47,48]. While this pH-buffering effect is significant, other studies indicate that bicarbonate may impair Fe nutrition by inhibiting ferric reductase activity and limiting other Fe-acquisition mechanisms [49,50,51,52,53].
Bicarbonate’s inhibition of Fe responses may also be linked to its suppressive effect on transcription factors such as SlFER and AtFIT, and subsequently, on Fe-acquisition genes like FRO, IRT, and CsHA1 [51]. Conversely, other studies have observed that bicarbonate can stimulate ferric reductase activity and the expression of Fe-acquisition genes [49,51,54]. These seemingly contradictory findings could be attributed to variations in experimental conditions, such as differences in bicarbonate and Fe concentrations, the treatment duration, and plant species. For instance, Lucena et al. [51] applied bicarbonate and 0 μM Fe to Arabidopsis plants for two days, whereas Msilini et al. [55] used bicarbonate and 5 μM Fe over a 30-day period. Generally, bicarbonate appears to inhibit ferric reductase activity and other Fe responses more significantly at higher concentrations and in the absence of Fe [51]. This suggests that bicarbonate’s influence on Fe nutrition is more complex than its pH-buffering capacity alone would indicate.
In addition to bicarbonate, several environmental factors can trigger or worsen Fe chlorosis in soils, including high moisture, poor drainage, inadequate aeration, and soil compaction—each associated with hypoxic stress, which can exacerbate Fe chlorosis [44,45,46,56,57,58]. Most studies link hypoxia’s negative impact on Fe nutrition to its role in increasing soil bicarbonate levels. García et al. [52] provided initial evidence that hypoxia may directly inhibit Fe-acquisition gene expression. Under these conditions, elevated bicarbonate levels may further compromise Fe nutrition [45,46,56,58,59]. In waterlogged or compacted soils, water saturation fills soil pores, raising the partial pressure of CO2 in the soil air and, consequently, the bicarbonate concentration [44,45,46,57].
The WCS417 strain is known to be a plant growth promoter. It has been determined that in Arabidopsis, it can favor both the increase in the size of the aboveground part in in vitro and substrate cultures, as well as in chlorophyll levels. Similarly, it also affects root structure, promoting greater development, resulting in the formation of more lateral roots [60]. Other strains belonging to the Pseudomonas genus have been shown to have a growth-promoting effect on A. thaliana seedlings due to the production of pyoverdine, a siderophore synthesized by the bacterium under iron-deficient conditions [61]. Under general nutrient deficiency in the soil and high pressure or a CO2 environment, the WCS417 strain is capable of both promoting plant growth expressed as fresh weight and increasing leaf surface [62]. It is known that auxins produced by certain microorganisms are molecules attributed to this growth-promoting effect [63]. In recent years, it has been discovered that this growth-promoting effect is dependent on auxin synthesis by the WCS417 strain [63,64]. Inoculation with the WCS417 strain or exposure to volatiles produced by it can induce the transcription of other genes involved in iron deficiency in Arabidopsis seedlings, such as the IRT1 and FRO2 genes [65,66].
The objective of this article has been to determine the effect of inoculation with the WCS417 strain of P. simiae on the physiological response mechanisms to iron deficiency in tomato seedlings and to study the possible effect of this bacterial strain on promoting the growth of tomato plants in alkaline pH.
2. Materials and Methods
2.1. Bacteria Strain, Plant Variety, Growth Conditions
Pseudomonas simiae WCS417 was cultured at 27 °C in King’s B medium [67] (20 g/L, 1.5 g/L K2HPO4, 1.5 mM MgSO4, 15 mL/L glycerol, pH 7.2 ± 0.2), supplemented with Rifampicin (50 μg/mL). The cells were preserved in glycerol at −80 °C. Regarding the tomato seeds used, this study employed tomato seeds of the ‘Tres Cantos’ variety.
2.2. Seed Germination
Seeds were subjected to surface sterilization using 20% sodium hypochlorite solution for 1 min, followed by rinsing with distilled water. Subsequently, the seeds were placed on a moist perlite substrate in a tray. A 5 mM CaCl2 solution (20 mL) was used to facilitate germination. The seeds remained under growth chamber conditions. Around 20–25 days after planting in a tray with perlite, the seedlings had already developed cotyledons, and the first two true leaves were beginning to emerge. Carefully, they were individually removed from the perlite, their roots were washed with ample distilled water, and they transferred to the hydroponic cultivation system [68]. Each individual plant was inserted in plastic lids and held in the holes of a thin polyurethane raft floating on an aerated nutrient solution containing 2 mM Ca(NO3), 0.75 mM K2SO4, 0.65 mM MgSO4, 0.5 mM KH2PO4, 50 µM KCl, 10 µM H3BO3, 1 µM MnSO4, 0.5 µM CuSO4, 0.5 µM ZnSO4, 0.05 µM (NH4)6Mo7O24, and 20 µM Fe-EDDHA.
This hydroponic system was maintained in the growth chamber at 22 °C day/20 °C night temperatures with a relative humidity of 70% and a 14 h photoperiod at an irradiance of 300 µmol m−2 s−1.
2.3. Cultivation of Bacteria and Inoculum Preparation
The P. simiae WCS417 inoculum was obtained from a stock preserved in glycerol at −80 °C. They were cultured on KB agar plates (King’s medium B) [67] supplemented with 50 μg mL−1 of rifampicin at 27 °C for 24 h. Subsequently, the cells were harvested using 10 mM MgSO4 after being washed twice by centrifugation for 5 min at 4500× g. Finally, the cells were resuspended in 10 mM MgSO4, and the optical density (OD) at 600 nm was determined before inoculation.
2.4. Experimental Conditions
Plants aged 20–25 days were used for the experiments. The plants were selected to ensure similar size and growth, aiming to minimize potential variability among seedlings.
Treatments were designed with a concentration of 40 μM Fe-EDDHA (until that moment, the plants had been growing with a lower Fe concentration of 20 μM Fe-EDDHA), and each plant was housed in an individual 0.5 L container, as detailed earlier, connected to an aeration system to prevent anoxic conditions. To simulate the alkaline conditions of calcareous soils, 40 mM NaHCO3 was added to the nutrition solution, since bicarbonate is one of the most important factors inducing Fe chlorosis [51]. Three different types of inoculation were carried out as follows: (1) inoculation in nutrient solution; (2) inoculation through foliar spraying; and (3) inoculation through substrate irrigation. Based on the various variables considered in this experimental design, the following treatments were applied: Control, Inoculation in solution, Foliar inoculation, Control + NaHCO3, Inoculation in solution + NaHCO3, and Foliar inoculation + NaHCO3. For the substrate experiments conducted in 0.7 L capacity pots, the following two types of different solid substrates were used: (1) perlite and (2) black peat. For each substrate type, a control treatment and an inoculated treatment were set up. Twelve tomato plants were used for each treatment.
Regarding the inoculation, in all cases, the plants were inoculated with a concentration of 107 CFU mL−1 of bacteria. For foliar inoculation, the aerial part of the seedlings was sprayed with a bacterial suspension containing 107 CFU mL−1 using a sprayer. In substrate-based trials, the seedlings were irrigated with 100 mL of a bacterial suspension containing 107 CFU mL−1.
2.5. Physiological Determinations
2.5.1. Growth Promotion
Periodic measurements of the height of all treatment plants were taken. At the time of plant harvest, the fresh and dry weight of both the aboveground and root parts were determined.
2.5.2. SPAD
The level of chlorosis in the plants was determined through SPAD readings. A portable chlorophyll meter, Minolta SPAD-502 (Konica Minolta, Tokyo [Japan]), was employed. Three readings per leaf were taken, and the arithmetic mean of these readings was calculated.
2.5.3. pH Determination
In the experiments conducted in hydroponic cultivation, the pH of the nutrient solution was monitored using a portable pH meter. Different pH meters were used to prevent potential contamination. After the determinations, the pH meter electrode was washed with a bactericide.
2.5.4. Bacterial Colonization
To measure the colonization and survival of bacteria under the study conditions, samples of both root and nutrient solutions were taken at two intervals, one at 26 days and the other at 37 days after inoculation. These samples were inoculated onto rifampicin-supplemented KB plates at 27 °C for one day. Subsequently, the number of colony-forming units (CFU g−1 for root samples and CFU mL−1 for nutrient solution samples) was calculated.
2.6. Statistical Analysis
The statistical analysis and graphs were performed using GraphPad Prism 8. To compare data from the treatments, Student’s t-test was used for parametric data and the Mann–Whitney test for non-parametric data. For multiple comparisons, the ANOVA test was used for parametric data and the Kruskal–Wallis test for non-parametric data. The significance level was determined by asterisks, with * p < 0.05, ** p < 0.01, or *** p < 0.001 indicating the presence of significant differences between the treatments.
3. Results
3.1. Monitoring the Growth of Tomato Plants and the External pH After Inoculation in the Nutrient Solution
Inoculation with the WCS417 strain in tomato plants grown in a hydroponic system supplemented with 40 μM Fe-EDDHA showed no visible effects on growth, as determined by plant length (Figure 1B). Monitoring the nutrient solution pH revealed significant differences only at 21 days, although the trend suggested that plants inoculated with the bacteria tended to acidify the medium (Figure 1D). On the other hand, in the presence of 40 mM NaHCO3 (sodium bicarbonate) with a strong alkalizing effect on the medium, the inoculated plants exhibited greater growth than the control plants from day 15 onwards. The control plants ceased to grow (Figure 1A). After 37 days, at the final harvest, 4 out of 6 of the remaining control plants had dried up, while the inoculated treatment plants remained alive (Figure 1E). Regarding the pH, the WCS417 strain triggered the acidification response in plants starting from the fifth day after inoculation (Figure 1C).
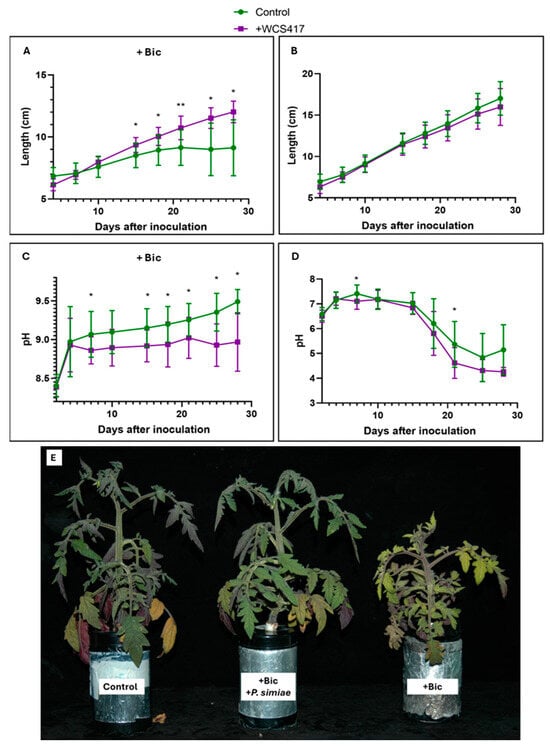
Figure 1.
Effect of inoculation with the WCS417 strain on the growth and acidification of tomato plants grown in a hydroponic system. Once the nutrient solution was inoculated with 107 CFU mL−1 bacterial suspension of the WCS417 strain, seedlings were transferred to individual containers. Shoot growth and the pH of the nutrient solution were monitored over time. (A) Growth evolution of tomato plants over 28 days cultured in the presence of 40 mM NaHCO3. (B) Growth evolution of tomato plants over 28 days cultured in the absence of NaHCO3. (C) pH evolution in the nutrient solution over 28 days of tomato plants grown in the presence of 40 mM NaHCO3. (D) pH evolution in the nutrient solution over 28 days of tomato plants grown in the absence of NaHCO3. (E) Comparison between control, inoculated treatments with P. simiae in the presence of NaHCO3, and non-inoculated treatments of tomato plants grown in the presence of NaHCO3 and after 37 days. * p < 0.05 and ** p < 0.01 indicate significant differences between inoculated and control treatments. Values are the means ± S.E. of 12 replicates.
Additionally, two harvests were conducted, each consisting of six randomly selected plants from each treatment, one at 26 days and another at 37 days. Both the aboveground and root parts of the plants were weighed. In the first harvest, there were no significant differences in growth between plants from the inoculated and control treatments. In the second harvest, the hydroponically grown inoculated plants in the absence of NaHCO3 exhibited a significantly greater root weight than the control treatment plants. There were no significant differences for the aboveground part (Figure 2A). On the other hand, in the presence of 40 mM NaHCO3, the inoculated plants showed a significantly higher weight for both the root and aboveground parts compared to the control treatment plants (Figure 2B).
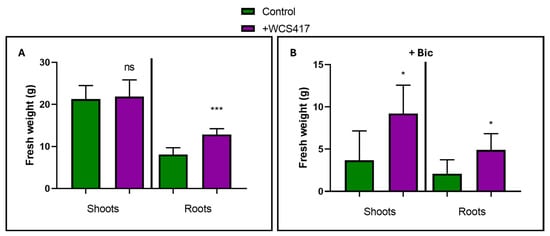
Figure 2.
Growth promotion of shoots and roots of tomato plants inoculated with the WCS417 strain after 37 days of cultivation in a hydroponic system. Shoots were excised from roots and weighed separately. (A) Tomato plants grown in the absence of NaHCO3. (B) Tomato plants grown in the presence of 40 mM NaHCO3. * p <0.05 and *** p < 0.001 indicate significant differences between inoculated and control treatments, ns (no significant differences). Values are the means ± S.E. of 12 replicates.
When counting bacteria during the first harvest, it was determined that both in the nutrient solution and in the roots, the bacterial count was much higher in the absence of NaHCO3 (Figure 3A,C). On the other hand, in the second harvest, the number of CFUs per gram or milliliter clearly decreased compared to the first harvest. In the presence of NaHCO3, the bacteria had completely disappeared in the nutrient solution. In the absence of NaHCO3, there were some samples where bacterial presence still persisted (Figure 3D). In the roots, although the quantity of bacteria decreased significantly compared to the first harvest, the survival was higher compared to the nutrient solution. Survival was also higher in the absence of NaHCO3 (Figure 3B).
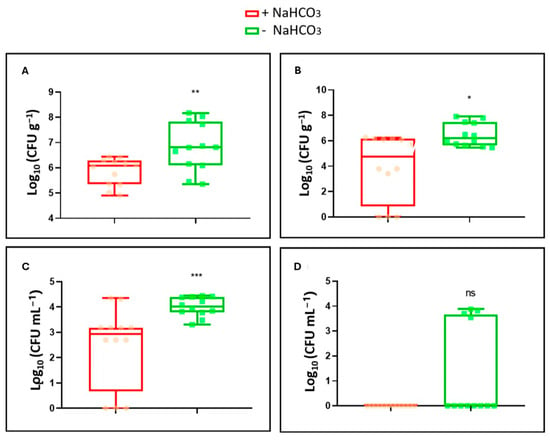
Figure 3.
Bacterial colonization of roots and the nutrient solution of tomato plants grown in a hydroponic system. Samples of root and nutrient solution were taken in two periods, one at 26 days and the other at 37 days after inoculation. CFU g−1 and CFU mL−1 numbers were estimated. (A) Root colonization of the WCS417 strain after 26 days in the presence and absence of NaHCO3. (B) Root colonization of the WCS417 strain after 37 days in the presence and absence of NaHCO3. (C) Growth of the WCS417 strain after 26 days in nutrient solution in the presence and absence of NaHCO3. (D) Growth of the WCS417 strain after 37 days in nutrient solution in the presence and absence of NaHCO3. * p < 0.05, ** p < 0.01 and *** p < 0.001 indicate significant differences between control and inoculated treatments, ns (no significant differences). Values are the means ± S.E. of 12 replicates.
3.2. Monitoring the Growth of Tomato Plants and the External pH After Foliar Inoculation
Tomato plants cultivated in the presence of NaHCO3 experienced a growth-promoting effect through foliar inoculation with the WCS417 strain. In contrast, plants in the control treatment did not exhibit any growth (Figure 4A). Foliar inoculation in this same treatment favored the root’s rhizosphere acidification response, as shown in Figure 4C. Chlorophyll levels in the control treatment gradually declined, becoming significant only at the end of the trial on day 15 (Figure 4E). There is nothing noteworthy regarding plants grown in the absence of NaHCO3, as there were no significant differences between the control and the inoculated treatment plants (Figure 4B,D,F).
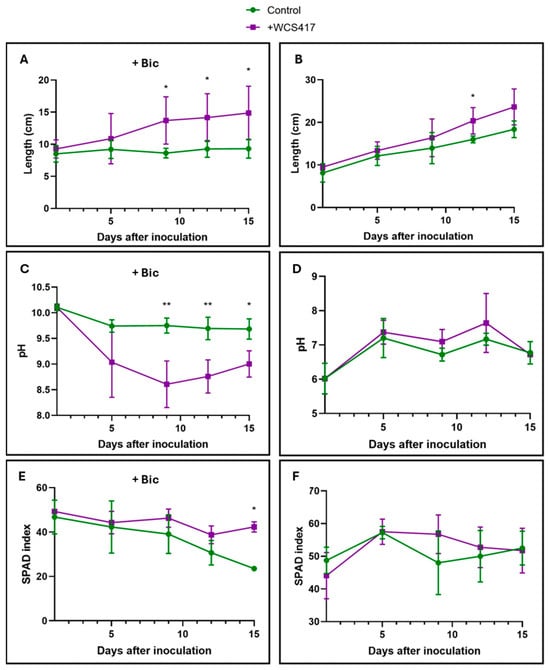
Figure 4.
Effect of foliar inoculation with the WCS417 strain on shoot growth, acidification, and SPAD of tomato plants grown in a hydroponic system. After spraying the aerial part of tomato plants with 107 CFU mL−1 of strain WCS417, plants were transferred to individual containers. Subsequently, growth and pH evolution over time as well as chlorophyll levels were monitored. (A) Growth of tomato plants over 15 days in the presence of 40 mM NaHCO3. (B) Growth of tomato plants over 15 days in the absence of NaHCO3. (C) pH evolution in nutrient solution over 15 days of tomato plants grown in the presence of 40 mM NaHCO3. (D) Evolution of pH in nutrient solution of tomato plants grown in the absence of NaHCO3 over 15 days. (E) Evolution of chlorophyll levels of tomato plants grown in the presence of 40 mM NaHCO3 over 15 days; (F) Evolution of chlorophyll levels of tomato plants grown in the absence of NaHCO3 over 15 days. * p < 0.05, and ** p < 0.01 indicate significant differences between control and inoculated treatments. Values are the means ± S.E. of 12 replicates.
3.3. Effects of Inoculation with the WCS417 Strain on Tomato Plants Cultivated in Pots with Solid Substrate (Peat or Perlite)
Inoculation with the WCS417 strain only had significant effects on the growth of tomato plants cultivated in black peat (Figure 5A). Regarding chlorophyll levels, no significant differences were detected between the control and inoculated treatments, either in black peat or perlite (Figure 5C,D). In perlite, there were also no significant differences between the control and inoculated treatments in terms of growth over time (Figure 5B).
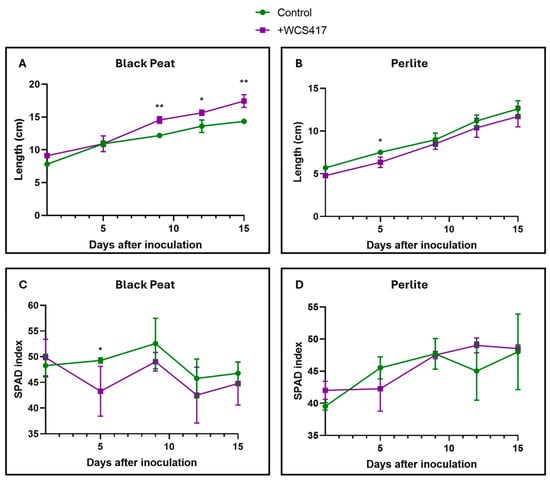
Figure 5.
Effect of the inoculation of the WCS417 strain by the irrigation of tomato plants grown in the substrate. After the inoculation of tomato plants with 100 mL of a 107 CFU mL−1 bacterial suspension, SPAD and height data were collected for a period of 15 days. (A) Height of tomato plants grown in black peat. (B) Height of tomato plants grown in perlite. (C) Chlorophyll levels of tomato plants grown in black peat. (D) Chlorophyll levels of tomato plants grown in perlite. * p < 0.05, and ** p < 0.01 indicate significant differences between control and inoculated treatments. Values are the means ± S.E. of 12 replicates.
4. Discussion
The WCS417 strain of P. simiae is one of the best-characterized PGPR. It can colonize the external surface of the roots of plants such as Arabidopsis thaliana, and it also has the ability to colonize endophytically tomato plants [63]. There is evidence suggesting that the growth-promoting effect and changes in root architecture are due to the production of auxins by this strain [69]. Similarly, it is credited for the promotion of growth along with other mechanisms, such as inhibiting the growth of other microorganisms [70]. The inoculation of dicotyledonous plant roots with the WCS417 strain has been shown to trigger the induction of ISR in distal tissues, providing protection against a wide range of pathogens and herbivores [63]. It is known that the WCS417 strain can induce the MYB72 gene, encoding the homonymous transcription factor involved in both ISR and the response to Fe deficiency [70]. In the same line, it has been demonstrated that volatiles produced by the WCS417 strain can induce the expression of genes related to Fe deficiency in A. thaliana seedlings [66].
The results obtained in this study demonstrate the growth-promoting effect of the WCS417 strain on tomato plants grown in a hydroponic system subjected to nutritional stress due to the presence of bicarbonate (Figure 1A, Figure 2B and Figure 4A). Other authors have already highlighted this growth-promoting effect [71]. Similarly, inoculating Vigna radiata plants with the GRP3A strain of Pseudomonas clearly produced a growth-promoting effect under both Fe sufficiency and deficiency conditions. Additionally, this strain is capable of producing siderophores under Fe deficiency conditions, aiding the plant in overcoming conditions of Fe deficiency [72]. Similarly, Ali et al. [73] have demonstrated that inoculating wheat plants with the AKMP7 strain of Pseudomonas putida promoted both root and shoot growth (measured by length and dry weight). Similarly to what is shown in this chapter with the inoculation of tomato plants in the presence of bicarbonate, Sandhya et al. [74] demonstrated that five different Pseudomonas strains were able to induce growth promotion in maize plants cultivated under water stress conditions, demonstrating the agronomic potential of members of this bacterial genus under different environmental stress conditions.
In relation to the effect of the WCS417 strain of P. simiae on foliar application, it is worth highlighting that our results are still preliminary. Our research group’s subsequent investigations will focus on elucidating the mode of action of the strain of P. simiae. We hypothesize that foliar application triggers a series of signaling mechanisms that travel from the leaf to the root, activating the mechanisms responsible for responding to nutrient deficiencies [9,25,75]. The activation of that signaling route could be the response leading to growth promotion, medium acidification, or the SPAD value incrementation in inoculated plants. Previous studies by our group have shown that hormones such as ethylene or nitric oxide are involved in these signaling systems [9,25,75].
Regarding alkaline pH levels, it is known that growth-promoting microorganisms, especially alkali-tolerant ones, can promote plant resistance to these conditions by producing IAA (auxins) and ACC, and by allowing an increase in internal potassium levels. In this way, the plant’s relative humidity and ionic homeostasis are maintained [76]. Ipek et al. [77] used five different strains with a growth-promoting effect on strawberry plants cultivated in calcareous soil, resulting in a highly significant growth-promoting effect across all strains. However, inoculation with the WCS417 strain only had relevant effects on the growth of tomato plants grown in peat (Figure 5A). Regarding chlorophyll levels, no significant differences were detected between the control and inoculated treatment in either peat or perlite (Figure 5C,D). In perlite, no significant differences were detected between the control and inoculated treatment in terms of growth over time (Figure 5B). Black peat and perlite are different solid substrates with distinct textures and compositions. In the preliminary trials that shaped this paper, a viability study of the bacterium in both substrates was not conducted. From our perspective, inoculation through irrigation was more successful in peat than in perlite because it was more capable of remaining in the medium. The inoculum may have been washed away with subsequent irrigations in perlite but not in peat. For future experiments, we plan to conduct viability analyses in these and other substrates (cultivated soils) to confirm whether or not the bacterium is suitable for use.
It is interesting to note that, at 26 and 37 days after the application of the treatments, tomato roots still showed the presence of CFU g−1 both in the presence and absence of bicarbonate (Figure 3A,B). However, bicarbonate reduced the survival of the bacterium in both the nutrient solution and the roots. In both cases, there was a decrease in the number of CFU g−1 compared to the roots and solution of tomato plants cultivated in the absence of NaCO3 (Figure 3). More specifically, NaCO3 caused the disappearance of the bacterium toward the end of the experiment in the nutrient solution. However, the WCS417 strain still existed in the plant roots (Figure 3B). Similarly, Carrillo et al. [41] also detected root colonization by the GB03 strain of B. subtilis at the level of 106 CFU mg−1. The number of CFU mg−1 in this case was influenced by the nitrogen source used in the nutrient solution. Ahmad et al. [78] used several strains to test the root colonization of cotton plants after one week. They determined that two strains, one of B. subtilis and another of Paenibacillus sp., showed high efficiency in colonization, almost reaching the order of 106. These also had a strong growth-promoting effect on the plant. Some years ago, Lucena et al. [51] demonstrated that bicarbonate could cause Fe deficiency by inhibiting the expression of several Fe acquisition genes in dicot plants. Authors underlined the difference between this bicarbonate effect (inhibition of expression of Fe acquisition genes) and other effects due to its pH buffer capacity. In calcareous soils, the ability of plants to induce the ferric reductase, the iron transporter, and the H+-ATPase genes allows them to locally acidify (by the subapical regions of the roots) the rhizosphere and to mobilize Fe from soil particles. However, if the expression of the genes is blocked by a high concentration of bicarbonate in the soil (as found by Lucena et al. [51]), this capacity does not exist, and plants are not able to remobilize Fe from the soil. In conclusion, the P. simiae WCS417 strain induces an acidification response in tomato plants cultured in the presence of 40 mM NaCO3. This acidification was observed both when the bacteria were inoculated by foliar spraying and by root immersion. In addition, our results demonstrate the growth-promoting effect of the WCS417 strain in tomato plants subjected to nutritional stress due to the presence of bicarbonate.
5. Conclusions
The results presented in this work show the ability of the P. simiae WCS417 strain to induce medium acidification in the presence of bicarbonate, increasing the SPAD index and improving the growth and development of tomato plants in calcareous conditions provoked by bicarbonate presence. This indicates that this bacteria strain could have a great potential as an Fe biofertilizer. However, further research is necessary to fully understand its mechanisms of action and to optimize its application for agricultural purposes. Continued investigation and experimentation is crucial for harnessing the full potential of the P. simiae WCS417 strain as a biofertilizer that enhances Fe uptake and improves crop productivity in tomato, and potentially other agricultural systems and species. The potential of the P. simiae WCS417 strain to be used as an Fe biofertilizer opens up new possibilities for its application in more sustainable and environmentally friendly agriculture.
Author Contributions
M.A.A.: methodology, investigation, software, formal analysis, data curation, writing—original draft; F.J.R.-C.: methodology, investigation, software, formal analysis, data curation, writing—original draft and editing; J.R.: conceptualization, supervision, review and editing, funding acquisition, project administration; F.J.R.: conceptualization, supervision, review and editing, funding acquisition, project administration; C.L.: methodology, investigation, software, formal analysis, data curation, writing—original draft and editing, supervision, project administration. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in the study are included in the article.: All the data included in this article are publicly available. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Paul, E.A.; Clark, F.E. Soil Microbiology and Biochemistry; Academic Press: San Diego, CA, USA, 1989; 272p. [Google Scholar]
- Koo, B.-J.; Adriano, D.C.; Bolan, N.S.; Barton, C.D. Root exudates and microorganisms. In Encyclopedia of Soils in the Environment; Elsevier: Amsterdam, The Netherlands, 2005; pp. 421–428. ISBN 978-0-12-348530-4. [Google Scholar]
- Gans, J.; Wolinsky, M.; Dunbar, J. Computational Improvements Reveal Great Bacterial Diversity and High Metal Toxicity in Soil. Science 2005, 309, 1387–1390. [Google Scholar] [CrossRef] [PubMed]
- Roesch, L.F.W.; Camargo, F.A.O.; Bento, F.M.; Triplett, E.W. Biodiversity of Diazotrophic Bacteria within the Soil, Root and Stem of Field-Grown Maize. Plant Soil 2008, 302, 91–104. [Google Scholar] [CrossRef]
- Kyselková, M.; Kopecký, J.; Frapolli, M.; Défago, G.; Ságová-Marečková, M.; Grundmann, G.L.; Moënne-Loccoz, Y. Comparison of Rhizobacterial Community Composition in Soil Suppressive or Conducive to Tobacco Black Root Rot Disease. ISME J. 2009, 3, 1127–1138. [Google Scholar] [CrossRef]
- Gomes, N.C.M.; Cleary, D.F.R.; Pinto, F.N.; Egas, C.; Almeida, A.; Cunha, A.; Mendonça-Hagler, L.C.S.; Smalla, K. Taking Root: Enduring Effect of Rhizosphere Bacterial Colonization in Mangroves. PLoS ONE 2010, 5, e14065. [Google Scholar] [CrossRef] [PubMed]
- Zilber-Rosenberg, I.; Rosenberg, E. Role of microorganisms in the evolution of animals and plants: The hologenome theory of evolution. FEMS Microbiol. Rev. 2008, 32, 723–735. [Google Scholar] [CrossRef] [PubMed]
- Andreote, F.D.; Pereira E Silva, M.D.C. Microbial Communities Associated with Plants: Learning from Nature to Apply It in Agriculture. Curr. Opin. Microbiol. 2017, 37, 29–34. [Google Scholar] [CrossRef]
- Aparicio, M.A.; Lucena, C.; García, M.J.; Ruiz-Castilla, F.J.; Jiménez-Adrián, P.; López-Berges, M.S.; Prieto, P.; Alcántara, E.; Pérez-Vicente, R.; Ramos, J.; et al. The Nonpathogenic Strain of Fusarium Oxysporum FO12 Induces Fe Deficiency Responses in Cucumber (Cucumis Sativus L.) Plants. Planta 2023, 257, 50. [Google Scholar] [CrossRef] [PubMed]
- Couillerot, O.; Prigent-Combaret, C.; Caballero-Mellado, J.; Moënne-Loccoz, Y. Pseudomonas Fluorescens and Closely-Related Fluorescent Pseudomonads as Biocontrol Agents of Soil-Borne Phytopathogens. Lett. Appl. Microbiol. 2009, 48, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Richardson, A.E.; Barea, J.-M.; McNeill, A.M.; Prigent-Combaret, C. Acquisition of Phosphorus and Nitrogen in the Rhizosphere and Plant Growth Promotion by Microorganisms. Plant Soil 2009, 321, 305–339. [Google Scholar] [CrossRef]
- Odum, E.P.; Barrett, G.W. Fundamentals of Ecology, 5th ed.; Thomson Brooks/Cole: Belmont, CA, USA, 2005; ISBN 978-0-534-42066-6. [Google Scholar]
- Bulgarelli, D.; Schlaeppi, K.; Spaepen, S.; Van Themaat, E.V.L.; Schulze-Lefert, P. Structure and Functions of the Bacterial Microbiota of Plants. Annu. Rev. Plant Biol. 2013, 64, 807–838. [Google Scholar] [CrossRef]
- Parniske, M. Arbuscular Mycorrhiza: The Mother of Plant Root Endosymbioses. Nat. Rev. Microbiol. 2008, 6, 763–775. [Google Scholar] [CrossRef] [PubMed]
- Masson-Boivin, C.; Giraud, E.; Perret, X.; Batut, J. Establishing Nitrogen-Fixing Symbiosis with Legumes: How Many Rhizobium Recipes? Trends Microbiol. 2009, 17, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Di Martino, C.; Fioretto, A.; Palmieri, D.; Torino, V.; Palumbo, G. Influence of tomato plant mycorrhization on nitrogen metabolism, growth and fructification on P-limited soil. J. Plant Growth Regul. 2019, 38, 1183–1195. [Google Scholar] [CrossRef]
- Barea, J.-M.; Pozo, M.J.; Azcón, R.; Azcón-Aguilar, C. Microbial Co-Operation in the Rhizosphere. J. Exp. Bot. 2005, 56, 1761–1778. [Google Scholar] [CrossRef] [PubMed]
- Benizri, E.; Baudoin, E.; Guckert, A. Root Colonization by Inoculated Plant Growth-Promoting Rhizobacteria. Biocontrol Sci. Technol. 2001, 11, 557–574. [Google Scholar] [CrossRef]
- Dutta, S.; Podile, A.R. Plant Growth Promoting Rhizobacteria (PGPR): The Bugs to Debug the Root Zone. Crit. Rev. Microbiol. 2010, 36, 232–244. [Google Scholar] [CrossRef] [PubMed]
- Drogue, B.; Doré, H.; Borland, S.; Wisniewski-Dyé, F.; Prigent-Combaret, C. Which Specificity in Cooperation between Phytostimulating Rhizobacteria and Plants? Res. Microbiol. 2012, 163, 500–510. [Google Scholar] [CrossRef]
- Lugtenberg, B.; Kamilova, F. Plant-Growth-Promoting Rhizobacteria. Annu. Rev. Microbiol. 2009, 63, 541–556. [Google Scholar] [CrossRef] [PubMed]
- Dobbelaere, S.; Croonenborghs, A.; Thys, A.; Vande Broek, A.; Vanderleyden, J. Phytostimulatory Effect of Azospirillum Brasilense Wild Type and Mutant Strains Altered in IAA Production on Wheat. Plant Soil 1999, 212, 153–162. [Google Scholar] [CrossRef]
- Miller, S.H.; Browne, P.; Prigent-Combaret, C.; Combes-Meynet, E.; Morrissey, J.P.; O’Gara, F. Biochemical and Genomic Comparison of Inorganic Phosphate Solubilization in Pseudomonas Species. Environ. Microbiol. Rep. 2010, 2, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.-S.; Enns, C.A. Iron Homeostasis: Recently Identified Proteins Provide Insight into Novel Control Mechanisms. J. Biol. Chem. 2009, 284, 711–715. [Google Scholar] [CrossRef]
- Romera, F.J.; García, M.J.; Lucena, C.; Martínez-Medina, A.; Aparicio, M.A.; Ramos, J.; Alcántara, E.; Angulo, M.; Pérez-Vicente, R. Induced Systemic Resistance (ISR) and Fe Deficiency Responses in Dicot Plants. Front. Plant Sci. 2019, 10, 287. [Google Scholar] [CrossRef]
- Daliran, T.; Halajnia, A.; Lakzian, A. Thiobacillus bacteria-enhanced iron biofortification of soybean in a calcareous soil enriched with ferrous sulfate, mill scale, and pyrite. J. Soil Sci. Plant Nutr. 2022, 22, 2221–2234. [Google Scholar] [CrossRef]
- Boddey, R.M.; Urquiaga, S.; Alves, B.J.R.; Reis, V. Endophytic Nitrogen Fixation in Sugarcane: Present Knowledge and Future Applications. Plant Soil 2003, 252, 139–149. [Google Scholar] [CrossRef]
- Singh, J.S.; Singh, D.P. Plant Growth Promoting Rhizobacteria (PGPR): Microbes in Sustainable Agriculture. In Management of Microbial Resources in the Environment; Malik, A., Grohmann, E., Alves, M., Eds.; Springer: Dordrecht, The Netherlands, 2013; pp. 361–385. ISBN 978-94-007-5930-5. [Google Scholar]
- Chauhan, H.; Bagyaraj, D.J.; Sharma, A. Plant growth-promoting bacterial endophytes from sugarcane and their potential in promoting growth of the host under field conditions. Ex. Agric. 2013, 49, 43–52. [Google Scholar] [CrossRef]
- Dobbelaere, S.; Vanderleyden, J.; Okon, Y. Plant Growth-Promoting Effects of Diazotrophs in the Rhizosphere. Crit. Rev. Plant Sci. 2003, 22, 107–149. [Google Scholar] [CrossRef]
- Muthukumarasamy, R.; Cleenwerck, I.; Revathi, G.; Vadivelu, M.; Janssens, D.; Hoste, B.; Ui Gum, K.; Park, K.-D.; Young Son, C.; Sa, T.; et al. Natural Association of Gluconacetobacter Diazotrophicus and Diazotrophic Acetobacter Peroxydans with Wetland Rice. Syst. Appl. Microbiol. 2005, 28, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Luna, M.F.; Aprea, J.; Crespo, J.M.; Boiardi, J.L. Colonization and Yield Promotion of Tomato by Gluconacetobacter Diazotrophicus. Appl. Soil Ecol. 2012, 61, 225–229. [Google Scholar] [CrossRef]
- Bertrand, H.; Plassard, C.; Pinochet, X.; Touraine, B.; Normand, P.; Cleyet-Marel, J.C. Stimulation of the Ionic Transport System in Brassica napus by a Plant Growth-Promoting Rhizobacterium (Achromobacter sp.). Can. J. Microbiol. 2000, 46, 229–236. [Google Scholar] [CrossRef]
- Bertrand, H.; Nalin, R.; Bally, R.; Cleyet-Marel, J.-C. Isolation and Identification of the Most Efficient Plant Growth-Promoting Bacteria Associated with Canola (Brassica napus). Biol. Fertil. Soils 2001, 33, 152–156. [Google Scholar] [CrossRef]
- Mantelin, S.; Desbrosses, G.; Larcher, M.; Tranbarger, T.J.; Cleyet-Marel, J.-C.; Touraine, B. Nitrate-Dependent Control of Root Architecture and N Nutrition Are Altered by a Plant Growth-Promoting Phyllobacterium sp. Planta 2006, 223, 591–603. [Google Scholar] [CrossRef]
- Bertin, C.; Yang, X.; Weston, L.A. The Role of Root Exudates and Allelochemicals in the Rhizosphere. Plant Soil 2003, 256, 67–83. [Google Scholar] [CrossRef]
- Bais, H.P.; Weir, T.L.; Perry, L.G.; Gilroy, S.; Vivanco, J.M. The role of root exudates in rhizosphere interactions with plants and other organisms. Annu. Rev. Plant Biol. 2006, 57, 233–266. [Google Scholar] [CrossRef] [PubMed]
- Pothier, J.F.; Wisniewski-Dyé, F.; Weiss-Gayet, M.; Moënne-Loccoz, Y.; Prigent-Combaret, C. Promoter-Trap Identification of Wheat Seed Extract-Induced Genes in the Plant-Growth-Promoting Rhizobacterium Azospirillum brasilense Sp245. Microbiology 2007, 153, 3608–3622. [Google Scholar] [CrossRef][Green Version]
- Badri, D.V.; Weir, T.L.; Van Der Lelie, D.; Vivanco, J.M. Rhizosphere Chemical Dialogues: Plant–Microbe Interactions. Curr. Opin. Biotechnol. 2009, 20, 642–650. [Google Scholar] [CrossRef] [PubMed]
- Glick, B.R. Plant Growth-Promoting Bacteria: Mechanisms and Applications. Scientifica 2012, 2012, 1–15. [Google Scholar] [CrossRef]
- Carrillo, A.; Li, C.; Bashan, Y. Increased Acidification in the Rhizosphere of Cactus Seedlings Induced by Azospirillum brasilense. Naturwissenschaften 2002, 89, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Delgado, A.; Madrid, A.; Kassem, S.; Andreu, L.; del Carmen del Campillo, M. Phosphorus fertilizer recovery from calcareous soils amended with humic and fulvic acids. Plant Soil 2002, 245, 277–286. [Google Scholar] [CrossRef]
- Farid, I.M.; El-Ghozoli, M.A.; Abbas, M.H.H.; El-Atrony, D.S.; Abbas, H.H.; Elsadek, M.; Saad, H.A.; El Nahhas, N.; Mohamed, I. Organic materials and their chemically extracted humic and fulvic acids as potential soil amendments for Faba Bean cultivation in soils with varying CaCO3 contents. Horticulturae 2021, 7, 205. [Google Scholar] [CrossRef]
- Lindsay, W.L.; Thorne, D.W. Bicarbonate ion and oxygen level as related to chlorosis. Soil Sci. 1954, 77, 271–280. [Google Scholar] [CrossRef]
- Chaney, R.L. Diagnostic Practices to Identify Iron Deficiency in Higher Plants. J. Plant Nutr. 1984, 7, 47–67. [Google Scholar] [CrossRef]
- Loeppert, R.H. Reactions of Iron and Carbonates in Calcareous Soils. J. Plant Nutr. 1986, 9, 195–214. [Google Scholar] [CrossRef]
- Römheld, V.; Marschner, H. Mechanism of Iron Uptake by Peanut Plants: I. FeIII Reduction, Chelate Splitting, and Release of Phenolics. Plant Physiol. 1983, 71, 949–954. [Google Scholar] [CrossRef]
- Moog, P.R.; Brüggemann, W. Iron Reductase Systems on the Plant Plasma Membrane—A Review. Plant Soil 1994, 165, 241–260. [Google Scholar] [CrossRef]
- Romera, F.J.; Alcántara, E.; De La Guardia, M.D. Effects of Bicarbonate, Phosphate and High pH on the Reducing Capacity of Fe-deficient Sunflower and Cucumber Plants. J. Plant Nutr. 1992, 15, 1519–1530. [Google Scholar] [CrossRef]
- Molassiotis, A.N.; Diamantidis, G.C.; Therios, I.N.; Tsirakoglou, V.; Dimassi, K.N. Oxidative Stress, Antioxidant Activity and Fe(III)-Chelate Reductase Activity of Five Prunus Rootstocks Explants in Response to Fe Deficiency. Plant Growth Regul. 2005, 46, 69–78. [Google Scholar] [CrossRef]
- Lucena, C.; Romera, F.J.; Rojas, C.L.; García, M.J.; Alcántara, E.; Pérez-Vicente, R. Bicarbonate Blocks the Expression of Several Genes Involved in the Physiological Responses to Fe Deficiency of Strategy I Plants. Functional. Plant Biol. 2007, 34, 1002. [Google Scholar] [CrossRef] [PubMed]
- García, M.J.; García-Mateo, M.J.; Lucena, C.; Romera, F.J.; Rojas, C.L.; Alcántara, E.; Pérez-Vicente, R. Hypoxia and Bicarbonate Could Limit the Expression of Iron Acquisition Genes in Strategy I Plants by Affecting Ethylene Synthesis and Signaling in Different Ways. Physiol. Plant. 2014, 150, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Dong, X.; Chen, Y.; Ma, B.; Yao, C.; Ma, F.; Liu, Z. Direct and Bicarbonate-Induced Iron Deficiency Differently Affect Iron Translocation in Kiwifruit Roots. Plants 2020, 9, 1578. [Google Scholar] [CrossRef] [PubMed]
- Waters, B.M.; Troupe, G.C. Natural Variation in Iron Use Efficiency and Mineral Remobilization in Cucumber (Cucumis sativus). Plant Soil 2012, 352, 185–197. [Google Scholar] [CrossRef]
- Msilini, N.; Attia, H.; Bouraoui, N.; M’rah, S.; Ksouri, R.; Lachaâl, M.; Ouerghi, Z. Responses of Arabidopsis thaliana to Bicarbonate-Induced Iron Deficiency. Acta Physiol. Plant 2009, 31, 849–853. [Google Scholar] [CrossRef]
- Boxma, R. Bicarbonate as the Most Important Soil Factor in Lime-Induced Chlorosis in the Netherlands. Plant Soil 1972, 37, 233–243. [Google Scholar] [CrossRef]
- Bloom, P.; Inskeep, W. Factors Affecting Bicarbonate Chemistry and Iron Chlorosis in Soils. J. Plant Nutr. 1986, 9, 215–228. [Google Scholar] [CrossRef]
- Zuo, Y.; Ren, L.; Zhang, F.; Jiang, R.-F. Bicarbonate Concentration as Affected by Soil Water Content Controls Iron Nutrition of Peanut Plants in a Calcareous Soil. Plant Physiol. Biochem. 2007, 45, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Brand, J.D.; Tang, C.; Graham, R.D. The Effect of Soil Moisture on the Tolerance of Lupinus pilosus Genotypes to a Calcareous Soil. Plant Soil 2000, 219, 263–271. [Google Scholar] [CrossRef]
- Van Loon, L.C. Plant Responses to Plant Growth-Promoting Rhizobacteria. Eur. J. Plant Pathol. 2007, 119, 243–254. [Google Scholar] [CrossRef]
- Trapet, P.; Avoscan, L.; Klinguer, A.; Pateyron, S.; Citerne, S.; Chervin, C.; Mazurier, S.; Lemanceau, P.; Wendehenne, D.; Besson-Bard, A. The Pseudomonas Fluorescens Siderophore Pyoverdine Weakens Arabidopsis Thaliana Defense in Favor of Growth in Iron-Deficient Conditions. Plant Physiol. 2016, 171, 675–693. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.; Pétriacq, P.; Beerling, D.J.; Cotton, T.E.A.; Ton, J. Impacts of Atmospheric CO2 and Soil Nutritional Value on Plant Responses to Rhizosphere Colonization by Soil Bacteria. Front. Plant Sci. 2018, 9, 1493. [Google Scholar] [CrossRef] [PubMed]
- Pieterse, C.M.J.; Berendsen, R.L.; De Jonge, R.; Stringlis, I.A.; Van Dijken, A.J.H.; Van Pelt, J.A.; Van Wees, S.C.M.; Yu, K.; Zamioudis, C.; Bakker, P.A.H.M. Pseudomonas simiae WCS417: Star Track of a Model Beneficial Rhizobacterium. Plant Soil 2021, 461, 245–263. [Google Scholar] [CrossRef]
- Zamioudis, C.; Mastranesti, P.; Dhonukshe, P.; Blilou, I.; Pieterse, C.M.J. Unraveling Root Developmental Programs Initiated by Beneficial Pseudomonas spp. Bacteria. Plant Physiol. 2013, 162, 304–318. [Google Scholar] [CrossRef]
- Stringlis, I.A.; Yu, K.; Feussner, K.; De Jonge, R.; Van Bentum, S.; Van Verk, M.C.; Berendsen, R.L.; Bakker, P.A.H.M.; Feussner, I.; Pieterse, C.M.J. MYB72-Dependent Coumarin Exudation Shapes Root Microbiome Assembly to Promote Plant Health. Proc. Natl. Acad. Sci. USA 2018, 115, E5213–E5222. [Google Scholar] [CrossRef] [PubMed]
- Zamioudis, C.; Korteland, J.; Van Pelt, J.A.; Van Hamersveld, M.; Dombrowski, N.; Bai, Y.; Hanson, J.; Van Verk, M.C.; Ling, H.; Schulze-Lefert, P.; et al. Rhizobacterial Volatiles and Photosynthesis-related Signals Coordinate MYB 72 Expression in Arabidopsis Roots During Onset of Induced Systemic Resistance and Iron-deficiency Responses. Plant J. 2015, 84, 309–322. [Google Scholar] [CrossRef] [PubMed]
- King, E.D.; Ward, M.K.; Raney, D.E. Two simple media for the demonstration of pyocyanin and fluorescin. J. Lab. Clin. Med. 1954, 44, 301–307. [Google Scholar]
- Lucena, C.; Waters, B.M.; Romera, F.J.; Garcia, M.J.; Morales, M.; Alcantara, E.; Perez-Vicente, R. Ethylene Could Influence Ferric Reductase, Iron Transporter, and H+-ATPase Gene Expression by Affecting FER (or FER-like) Gene Activity. J. Exp. Bot. 2006, 57, 4145–4154. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Asif, M.; Zaheer, A.; Malik, A.; Ali, Q.; Rasool, M. Plant growth promoting rhizobacteria and sustainable agriculture: A review. Afr. J. Microbiol. Res. 2013, 7, 704–709. [Google Scholar]
- Zamioudis, C.; Hanson, J.; Pieterse, C.M.J. β-Glucosidase BGLU 42 Is a MYB 72-dependent Key Regulator of Rhizobacteria-induced Systemic Resistance and Modulates Iron Deficiency Responses in A. rabidopsis Roots. New Phytol. 2014, 204, 368–379. [Google Scholar] [CrossRef] [PubMed]
- Verbon, E.H.; Trapet, P.L.; Kruijs, S.; Temple-Boyer-Dury, C.; Rouwenhorst, T.G.; Pieterse, C.M.J. Rhizobacteria-Mediated Activation of the Fe Deficiency Response in Arabidopsis Roots: Impact on Fe Status and Signaling. Front. Plant Sci. 2019, 10, 909. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Johri, B.N. Combat of Iron-Deprivation through a Plant Growth Promoting Fluorescent Pseudomonas Strain GRP3A in Mung Bean (Vigna radiata L. Wilzeck). Microbiol. Res. 2003, 158, 77–81. [Google Scholar] [CrossRef]
- Ali, S.Z.; Sandhya, V.; Grover, M.; Linga, V.R.; Bandi, V. Effect of Inoculation with a Thermotolerant Plant Growth Promoting Pseudomonas putida Strain AKMP7 on Growth of Wheat (Triticum spp.) under Heat Stress. J. Plant Interact. 2011, 6, 239–246. [Google Scholar] [CrossRef]
- Sandhya, V.; Ali, S.Z.; Grover, M.; Reddy, G.; Venkateswarlu, B. Effect of Plant Growth Promoting Pseudomonas spp. on Compatible Solutes, Antioxidant Status and Plant Growth of Maize Under Drought Stress. Plant Growth Regul. 2010, 62, 21–30. [Google Scholar] [CrossRef]
- Sevillano-Caño, J.; García, M.J.; Córdoba-Galván, C.; Luque-Cruz, C.; Agustí-Brisach, C.; Lucena, C.; Ramos, J.; Pérez-Vicente, R.; Romera, F.J. Exploring the Role of Debaryomyces hansenii as Biofertilizer in Iron-Deficient Environments to Enhance Plant Nutrition and Crop Production Sustainability. Int. J. Mol. Sci. 2024, 25, 5729. [Google Scholar] [CrossRef] [PubMed]
- Msimbira, L.A.; Smith, D.L. The Roles of Plant Growth Promoting Microbes in Enhancing Plant Tolerance to Acidity and Alkalinity Stresses. Front. Sustain. Food Syst. 2020, 4, 106. [Google Scholar] [CrossRef]
- Ipek, M.; Pirlak, L.; Esitken, A.; Figen Dönmez, M.; Turan, M.; Sahin, F. Plant Growth-Promoting Rhizobacteria (Pgpr) Increase Yield, Growth and Nutrition of Strawberry Under High-Calcareous Soil Conditions. J. Plant Nutr. 2014, 37, 990–1001. [Google Scholar] [CrossRef]
- Ahmad, M.; Ahmad, I.; Hilger, T.H.; Nadeem, S.M.; Akhtar, M.F.; Jamil, M.; Hussain, A.; Zahir, Z.A. Preliminary Study on Phosphate Solubilizing Bacillus Subtilis Strain Q3 and Paenibacillus sp. Strain Q6 for Improving Cotton Growth under Alkaline Conditions. PeerJ 2018, 6, e5122. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).