Abstract
The seeds of 111 Malus sp. different fruit use (dessert and cider apples) cultivars/genotypes developed in 18 countries were analysed to evaluate composition of tocopherol homologues and identify crop-specific profile, including diploid, triploid, and tetraploid apple cultivars with and without scab-resistance to ensure high genetic diversity. The percentage of individual tocopherols was as follows: alpha-tocopherol (alpha-T) (38.36%), beta-tocopherol (beta-T) (40.74%), gamma-tocopherol (gamma-T) (10.93%), and delta-tocopherol (delta-T) (9.97%), represented by average measurements of 17.48, 18.56, 4.98, and 4.54 mg/100 g dry weight, respectively. The values of the variation coefficient showed high variability for delta (0.695) and gamma (0.662) homologue content, whereas measurements of alpha-T and beta-T were less variable (coefficient of variation 0.203 and 0.256, respectively). The unweighted pair group method with arithmetic mean (UPGMA) revealed three main cultivar groups characterised by almost equal content of all four tocopherol homologues (Group I), high concentrations of alpha-T and beta-T, but very low content of gamma-T and delta-T (Group II), and relatively high average content of alpha-T and beta-T, but higher gamma-T and delta-T content (Group III). Specific tocopherol homologues showed association with certain valuable traits, such as harvesting time (total content of tocopherols) and resistance to apple scab (alpha-T and total content of tocopherols). This study represents the first large-scale tocopherol homologue (alpha, beta, gamma, and delta) screening in apple seeds. The dominant tocopherol homologues in cultivated apple cultivars are alpha-T and beta-T, with the prevalence of alpha-T or beta-T depending on genotype. It is a unique finding due to the rare occurrence of beta-T in the plant world and is considered a unique feature of the species.
1. Introduction
Apples are among the most popular temperate fruit crops—the third most widely grown fruit, with global harvest reaching over 86 million tons in 2020 [1]. More than 30,000 apple cultivars are registered [2], and every year breeding programs release new cultivars to develop high-quality and disease-resistant apples. Cultivars present a broad range of apple quality attributes [3,4]. To streamline the breeding programs, it is necessary to introduce new, modern tools in apple breeding, as it is limited by the current long and complex multi-step process [4]. Phytochemicals in apples may assist the selection of Malus genotypes with specific nutraceutical traits suitable for establishing innovative breeding strategies [5]. One such group of compounds is tocochromanols, which have not yet been investigated or applied as potential biomarkers in apple seeds.
Tocochromanols are lipophilic antioxidants composed of a chromanol ring with varying structure and saturated or unsaturated side chains of different length. Tocochromanols include such bioactive compounds as tocopherols, tocotrienols, plastochromanol-8, and other prenyllipids [6]. Tocopherols are involved in several physiological processes of plant seeds: germination, growth, leaf senescence, responses to abiotic stresses, antioxidant function, and export of photoassimilates [7]. Although there are still many questions in plant biology related to plant resistance to biotic factors, tocopherols can be particularly important in plants as alternative defence mechanism activators under specific growing conditions [7]. Considering this, a number of studies have demonstrated the value of these lipophilic molecules in chemotaxonomy and therefore can play a significant role for taxonomic studies in some plant families [8,9,10]. For instance, tocopherol homologues can be useful biomarkers to distinguish between seeds of different oak species—Quercus rubra L. vs. Quercus robur L. [11]. Other studies confirm that tocopherols are suitable as plant functional trait biomarkers and can play an essential role in monitoring the physiological response of plants, for instance, to stress [12]. Despite significant knowledge about tocopherols, tocotrienols, and other tocochromanol functions and roles in plants, many issues associated with those unique lipophilic molecules are still unclear [7,12].
Despite many chemical composition studies on apples, few are concerned with seed tocopherols, and a limited number of genotypes has been included—only one to thirty apple genotypes were analysed [13,14,15,16,17,18]. In apple seeds, four tocopherol homologues (alpha, beta, gamma, and delta) have been found, and their proportions vary among cultivars [13]. The aim of the present study was to investigate the tocopherol homologue composition in seeds of a comprehensive number of the Malus sp. (dessert and cider apples) genotypes to understand the variability of these secondary metabolites and to find regularities in crop-specific profiles.
2. Results and Discussion
This study is the first large-scale screening of all four tocopherol homologues (alpha, beta, gamma and delta) in apple seeds. Tocopherol homologues were determined according to the previously validated RP-HPLC-FLD method [19]. All four tocopherol homologues were identified and quantified in one hundred eleven apple genotypes (Table 1). Each genotype was represented by three biological samples, ANOVA did not show statistically significant differences between biological samples or repetitions. A wide range of each tocopherol homologue concentration and high variability among tested apple samples was observed (Figure 1). The content of delta-T (0.695) and gamma-T (0.662) showed high diversity, whereas alpha-T and beta-T were less variable and more consistent among genotypes (coefficient of variation 0.203 and 0.256, respectively). The total tocopherol content ranged from 31.82 to 56.75 mg/100 g dw for cvs. ‘Mc Shay’ and ‘Kurzemes Svītrainais’, respectively. A similar range of total tocopherol concentration was reported in twelve cultivars of dessert and crab apples [13]. The mean percentage of individual tocochromanols was as follows: alpha-T (38.36%), beta-T (40.74%), gamma-T (10.93%), delta-T (9.97%), represented by mean content 17.48 (alpha-T), 18.56 (beta-T), 4.98 (gamma-T), and 4.54 (delta-T) mg/100 g, respectively.

Table 1.
Tocopherol homologue (mg/100 g dw) contents in seeds of different various origin and harvest time cider and dessert apple genotypes.
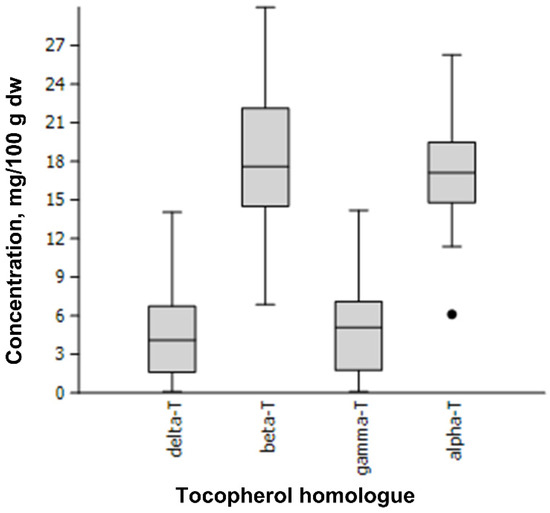
Figure 1.
Content of tocopherol homologues (alpha, beta, gamma and delta) in 111 apple genotypes, mg/100 g dw.
Figure 1 presents the median value, upper and lower quartile and upper and lower extreme measurements. The bullet represents the extreme values determined for the apple cultivar ‘Uspeh’.
The dominance of alpha-T and beta-T in seeds of cultivated apples is unique in the plant world [13,14,15,16,17], and the present study. In contrast, the seeds of other pome fruits, such as pears, quince, and Japanese quince [14,20,21], or fruit crops of the Prunus genus [22,23,24,25] are dominated mainly by gamma-T, and quinces only contain alpha-T. The varied prevalence between alpha-T and beta-T has been observed in other studies: predominance of alpha-T over beta-T [13,15] or beta-T over alpha-T [14,16,17], which can be explained by the specificity of tested cultivars. In the current study, moderate prevalence of beta-T over alpha-T was observed in 54.1% of apple samples. The highest content of alpha-T was noted for cv. ‘Alro’ (26.29 mg/100 g dw), whereas the lowest for cv. ‘Uspeh’ (6.12 mg/100 g dw), an outlying measurement (Figure 1). The highest content of beta-T was found in cv. ‘Anīss Svītrainais’ (29.99 mg/100 g dw), the lowest—in cv. ‘Uspeh’ (6.88 mg/100 g dw) seeds. Tocopherol homologues gamma-T and delta-T had significantly lower concentrations: the highest content of gamma-T was observed in red leaf cv. ‘Carnikava’ (14.20 mg/100 g dw), whereas cv. ‘Ligol’ had the highest content of delta-T (14.05 mg/100 g dw). Cultivars ‘Katja’ and ‘Ruhm von Kirchwerder’ contained the least gamma-T (0.13 mg/100 g dw) and delta-T (0.12 mg/100 g dw), respectively. ANOVA consistently showed statistically significant differences between tested cultivars for all tocopherol homologues as well as their total content (p < 0.001), which allowed complete discrimination of all tested apple genotypes (Figure 2).
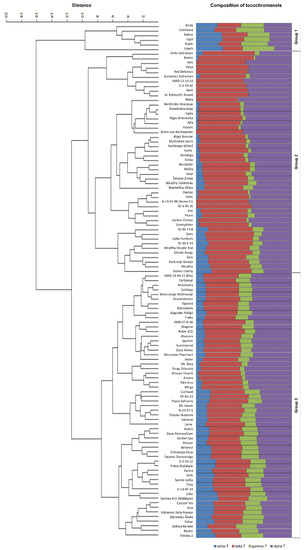
Figure 2.
Apple genotypes grouped according to of individual tocopherol homologue (alpha, beta, gamma, and delta) content (mg/100 g dw) and their proportion (percentage, %) (Euclidean distance, UPGMA clustering).
The analysed set of apples includes genotypes with different harvesting times: early, medium, and late (13, 67, and 31 genotypes, respectively). Using this parameter as a factor, ANOVA showed statistically significant differences between the total content of tocopherols (early apple genotypes were significantly different, average values for early—47.36 mg/100 g dw, medium—45.35 mg/100 g dw, late—45.32 mg/100 g dw), but not significant for individual tocopherol homologues. The content of tocopherols increases in seeds during fruit development in three cultivars of Japanese quince, which, same as apples, are pome fruits [26]. Based on this finding, the slightly higher total content of tocopherols in seeds of early cultivars (summer/autumn) could be explained by the higher maturity of early apple genotypes compared to medium and late varieties. Similarly, the relationship of tocopherol content and apple scab resistance was evaluated. The dataset contained 12 genotypes that were apple scab-resistant (with Rvi5 and Rvi6 resistance genes) and 99 susceptible genotypes. While delta-T, beta-T, and gamma-T did not show a statistically significant difference, alpha-T and total tocopherol content did. The average values for apple scab susceptible genotypes were 17.67 mg/100 g dw alpha-T and 45.85 mg/100 g dw total tocopherols content. Lower content was observed in resistant genotypes—15.75 mg/100 g dw alpha-T and 43.21 mg/100 g dw total tocopherol content. To explain the inconsistency, additional studies are needed, using material from several harvest years.
The unweighted pair group method with arithmetic mean (UPGMA), an agglomerative hierarchical clustering method based on Euclidean distances among tested apple genotypes, revealed three main cultivar groups (Figure 2), characterised by different proportions of tocopherol homologues (alpha, beta, gamma, and delta), supported by Principal Component Analysis (PCA) (Figure 3).
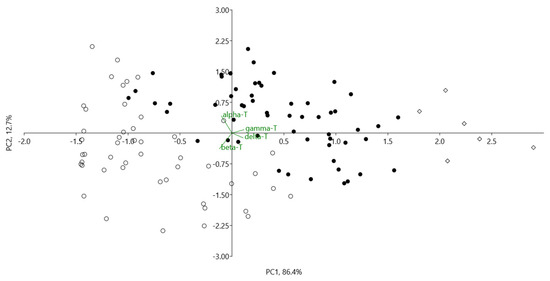
Figure 3.
PC1 verses PC2 of 111 apples genotypes based on composition of tocopherol homologues content (alpha, beta, gamma and delta) (mg/100 g dw). Apple cultivar groups: ●—I, ○—II and ◊—III (groups identified by UPGMA cluster analysis, corresponds with Figure 2).
ANOVA of cultivar groups was performed to evaluate their reliability. Statistically significant differences were found between all analysed tocopherol homologues (p < 0.001) (Figure 4). Group I included six cultivars with very similar content of all four tocopherol homologues: 12.51/11.64/13.18/11.79 mg/100 g dw for alpha/beta/gamma/delta, respectively. All cultivars of this group descend from East and Central Europe (Belarus, Estonia, Latvia, Poland, and Russia) or Central Asia (Kazakhstan). In the case of Kazakhstan, the breeding parents of the analysed cultivar also had East-European origin. Like in the previous study, some cultivars (in this case, Group I) showed almost equal proportions of tocopherol homologues, for example, cv. ‘Antej’ has also been analysed previously, and an equal proportion of tocopherol homologues was observed [13]. Group II (forty-eight apple cultivars) conversely includes cultivars with very different proportions of tocopherol homologues—high concentrations of alpha-T and beta-T, very low content of gamma-T and delta-T (19.82, 22.87, 2.45, and 2.57 mg/100 g dw, respectively). The fifty-seven apple cultivars in Group III also had a comparatively high mean content of alpha-T (16.14 mg/100 g dw) and beta-T (15.69 mg/100 g dw), but higher content of gamma-T (6.45 mg/100 g dw) and delta-T (5.56 mg/100 g dw) than Group II (Figure 2). Groups II and III include cultivars of very distant origin both geographically and by parentage. Group II and III can be separated based on the proportion of alpha-T and beta-T. Although the general moderate prevalence of beta-T over alpha-T was observed and discussed above, significant differences in this parameter were observed for UPGMA-identified cultivar groups. In Group II, 22.9% of cultivars had a prevalence of alpha-T, whereas in Group III—the predominance of alpha-T was stated for 68.4% of cultivars. These observations explain contradictions in different publications about the tocopherol homologue profile in apple seed oils, discussed above. Since previous studies have analysed a relatively small number (one up to twelve) of Malus sp. varieties [13,14,15,16,17], they fit well into one or the other group dominated by alpha-T or beta-T. Further analysis of tocopherol content showed a close correlation between particular homologues, e.g., positive correlations: gamma-T and delta-T (r = 0.935, p < 0.01), alpha-T and beta-T (r = 0.676, p < 0.01), negative correlations: alpha-T and delta-T (r = −0.682, p < 0.01), alpha-T and gamma-T (r = −0.650, p < 0.01), gamma-T and beta-T (r = −0.654, p < 0.01). A similar observation was reported in acorns of Q. robur and Q. rubra [11]: positive correlations between gamma-T and delta-T as well as alpha-T and beta-T, and negative correlations between alpha-T and delta-T, alpha-T and gamma-T, beta-T and gamma-T, and, lastly, beta-T and delta-T. In the biosynthetic pathway of tocopherols, delta-T and gamma-T have two precursors: 2,3-dimethyl-6-phytyl-1,4-benzoquinol (DMPBQ) and 2-methyl-6-phytyl-1,4-benzoquinol (MPBQ), a cyclised form of MPBQ produced by methyltransferase (MT), a tocopherol cyclase. These primary products (delta-T and gamma-T) are precursors of beta-T and alpha-T, respectively, catalysed by gamma-T methyltransferase (gamma-TMT) [27,28]. Thus the obtained positive and negative correlations are related to the varying activity of enzymes involved in each of the steps of compound biosynthesis, favoring either primary or secondary products.
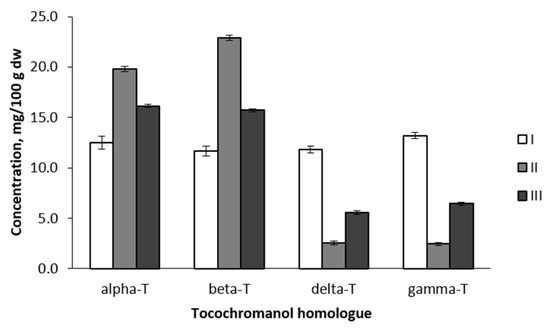
Figure 4.
Comparison of tocopherol homologue content between apple groups identified in 111 tested genotypes. I, II and III—apple cultivar groups, identified by UPGMA cluster analysis (corresponds with Figure 2).
No relation between the calculated cultivar groups and either the harvesting time or the pedigree of the cultivars has been found.
3. Materials and Methods
3.1. Reagents
Sigma–Aldrich (Taufkirchen, Germany) provided all required solvents and reagents of analytical to HPLC grade. Standards of alpha-, beta-, gamma-, and delta-T, with purity over 95%, were obtained from Merck (Darmstadt, Germany).
3.2. Plant Material
The tocopherol homologues composition was evaluated in the seeds of 111 apple genotypes of selected in different geographical regions with different harvesting time, including advanced modern cultivars, selected hybrids and landraces, diverse pedigrees as well as biological and growing properties (Table 1). Genotypes included in this study were developed in 18 countries (Belarus, Canada, Czech Republic, Estonia, Finland, France, Germany, Japan, Kazakhstan, Latvia, Lithuania, New Zealand, Poland, Russia, Sweden, Ukraine, United Kingdom, and the United States), ensuring sufficient genetic diversity. A broad range of apple cultivars was analysed to evaluate the tocopherol homologue content and to find crop-specific profiles: diploid, triploid, and tetraploid cultivars, as well as cultivars with different tree structures (columnar and standard tree), leaf colour (red leaf cv. ‘Carnikava’), fruit use (dessert and cider apples), scab resistant cultivars (with Rvi5 or Rvi6 apple scab resistance genes). The seeds were collected in 1 September–30 November 2013 from fruits at the experimental garden/orchard (GPS location: N: 56°36′39″ E: 23°17′50″) at Institute of Horticulture, Dobele, Latvia. Three biological replicates were obtained from one to five apple trees for each genotype. If only one tree was available, apples were split into three replications. Ten to twenty apples were collected randomly from different sides of each tree. All recovered seeds from apple flesh and cores, except for those not fully developed, were used for phytochemical analysis. The seeds were immediately frozen for 24 h at −18 °C, and freeze-dried for 24 h using the Labconco FreeZone system (Kansas City, MO, USA). Lyophilised seeds were stored at −18 ± 1 °C for no longer than six months before analysis. The homogenized amount of 2 to 5 g of dry apple seeds was milled using an A 11 basic mill (IKA, Staufen, Germany). The apple seed powder (≤0.5 mm, mesh size) was immediately used for extraction of tocopherols. The solids content (dry weight; dw) of the apple seeds was measured gravimetrically. All analyses were performed at the end of 2013 and beginning of 2014.
3.3. Tocopherol Homologues Analysis
Samples were prepared using the same micro-saponification and extraction protocols as in previous reports [13]. Briefly, 0.1 g of powdered seeds, 0.05 g of pyrogallol, 2.5 mL of absolute ethanol, and 0.25 mL of 60% (w/v) aqueous KOH were placed in a 15 mL glass tube, and saponified (25 min, at 80 °C). Then, the sample was cooled in an ice-water bath (5 min) and 2.5 mL of 1% (w/v) NaCl solution was added and mixed (5 s). Tocopherols were extracted using 2.5 mL n-hexane:ethyl acetate (9:1; v/v) and mixing (15 s). The layers were separated by centrifugation (1000× g at 4 °C for 5 min). The organic layer was transferred to a round-bottom flask, while residues were re-extracted twice as described above. The combined extracts were evaporated in a vacuum rotary evaporator until dry, dissolved in ethanol (1 mL), filtered through a syringe filter (0.22 μm) into a vial, and injected into the RP-HPLC/FLD system (Shimadzu Corporation, Kyoto, Japan). Tocochromanols were separated in a Luna PFP column (150 × 4.6 mm, 3 μm) with a connected guard column (4 × 3 mm, 3 μm) obtained from Phenomenex (Torrance, CA, USA). The method was validated previously [19].
3.4. Statistical Analysis
The results were presented as means (n = 3). Each apple genotype is represented by three independent measurements of different biological replication of plant material. Data was processed using ANOVA. Significant differences (alpha = 0.05) were determined using the Duncan Post-hoc test for different independent samples and selected genotypes, using IBM SPSS Statistics 25 software (IBM Corp., Armonk, NY, USA, 2017). An unweighted pair group method with arithmetic mean (UPGMA) and Euclidean distance was applied to explore genotype groups using the PAST 4 (Hammer et al., 2001) software. The same software was used for principal components analysis (PCA) to identify the type of variable interaction.
4. Conclusions
The content and proportion of tocopherol homologues alpha-T, beta-T, gamma-T, and delta-T are highly varied, especially gamma-T and delta-T, in cultivated apple genotypes. Specific tocopherol homologues showed association with certain valuable traits, such as harvesting time (total content of tocopherols) and resistance to apple scab (alpha-T and total tocopherol content). Significant relation between the geographical origin of apple genotypes and the specific tocochromanol profile in seeds was not found. The dominant tocopherol homologues in cultivated apple cultivars are alpha-T and beta-T, with the prevalence of alpha-T or beta-T depending on genotype.
Although the alpha-T and total tocopherol content showed significant statistical differences related to scab resistance, their use for genotype evaluation can be limited by the sensitivity of the tocopherol biosynthetic pathway to various environmental factors, which can cause a false positive or false negative expectation of a certain trait.
Author Contributions
P.G.: Methodology, Investigation, Formal analysis, Data Curation, Writing—Original Draft, Writing—Review & Editing, Funding acquisition; G.L.: Conceptualization, Data Curation, Software, Visualization, Writing—Original Draft, Writing—Review & Editing; I.M.: Formal analysis; L.I.: Writing—Original Draft, Writing—Review & Editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Latvian Council of Science, project “Dicotyledonous plant families and green tools as a promising alternative approach to increase the accessibility of tocotrienols from unconventional sources”, project No. lzp-2020/1-0422”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to the fact that all data are based on the presented data in Table 1.
Conflicts of Interest
The authors declare no conflict of interest.
References
- FAOSTAT. FAO Statistical Database. Available online: http://www.fao.org (accessed on 4 December 2022).
- Strohm, K. Of the 30,000 Apple Varieties Found All over the World Only 30 Are Used and Traded Commercially. Available online: www.agribenchmark.org (accessed on 4 December 2022).
- Musacchi, S.; Serra, S. Apple fruit quality: Overview on pre-harvest factors. Sci. Hortic. 2018, 234, 409–430. [Google Scholar] [CrossRef]
- Sheick, R.; Serra, S.; Tillman, J.; Luby, J.; Evans, K.; Musacchi, S. Characterization of a novel S-RNase allele and genotyping of new apple cultivars. Sci. Hortic. 2020, 273, 109630. [Google Scholar] [CrossRef]
- Farneti, B.; Masuero, D.; Costa, F.; Magnago, P.; Malnoy, M.; Costa, G.; Vrhovsek, U.; Mattivi, F. Is there room for improving the nutraceutical composition of apple? J. Agric. Food Chem. 2015, 63, 2750–2759. [Google Scholar] [CrossRef] [PubMed]
- Górnaś, P.; Baškirovs, G.; Siger, A. Free and esterified tocopherols, tocotrienols and other extractable and non-extractable tocochromanol-related molecules: Compendium of knowledge, future perspectives and recommendations for chromatographic techniques, tools, and approaches used for tocochromanol determination. Molecules 2022, 27, 6560. [Google Scholar]
- Falk, J.; Munne-Bosch, S. Tocochromanol functions in plants: Antioxidation and beyond. J. Exp. Bot. 2010, 61, 1549–1566. [Google Scholar] [CrossRef]
- Horvath, G.; Wessjohann, L.; Bigirimana, J.; Jansen, M.; Guisez, Y.; Caubergs, R.; Horemans, N. Differential distribution of tocopherols and tocotrienols in photosynthetic and non-photosynthetic tissues. Phytochemistry 2006, 67, 1185–1195. [Google Scholar] [CrossRef]
- Goffman, F.D.; Thies, W.; Velasco, L. Chemotaxonomic value of tocopherols in Brassicaceae. Phytochemistry 1999, 50, 793–798. [Google Scholar] [CrossRef]
- Siger, A.; Górnaś, P. Free tocopherols and tocotrienols in 82 plant species’ oil: Chemotaxonomic relation as demonstrated by PCA and HCA. Food Res. Int. 2023, 164, 112386. [Google Scholar] [CrossRef]
- Górnaś, P. Oak Quercus rubra L. and Quercus robur L. acorns as an unconventional source of gamma-and beta-tocopherol. Eur. Food Res. Technol. 2019, 245, 257–261. [Google Scholar] [CrossRef]
- De Agostini, A.; Cogoni, A.; Cortis, P.; Vacca, A.; Becerril, J.M.; Hernández, A.; Esteban, R. Heavy metal tolerance strategies in metallicolous and non-metallicolous populations of mosses: Insights of γ+ β-tocopherol regulatory role. Environ. Exp. Bot. 2022, 194, 104738. [Google Scholar] [CrossRef]
- Górnaś, P.; Segliņa, D.; Lācis, G.; Pugajeva, I. Dessert and crab apple seeds as a promising and rich source of all four homologues of tocopherol (a, b, g and d). LWT Food Sci. Technol. 2014, 59, 211–214. [Google Scholar] [CrossRef]
- Fromm, M.; Bayha, S.; Kammerer, D.R.; Carle, R. Identification and quantitation of carotenoids and tocopherols in seed oils recovered from different rosaceae species. J. Agric. Food Chem. 2012, 60, 10733–10742. [Google Scholar] [CrossRef]
- Arain, S.; Sherazi, S.T.H.; Bhanger, M.I.; Memon, N.; Mahesar, S.A.; Rajput, M.T. Prospects of fatty acid profile and bioactive composition from lipid seeds for the discrimination of apple varieties with the application of chemometrics. Grasas Aceites 2012, 63, 175–183. [Google Scholar]
- Pieszka, M.; Migdał, W.; Gąsior, R.; Rudzińska, M.; Bederska-Łojewska, D.; Pieszka, M.; Szczurek, P. Native oils from apple, blackcurrant, raspberry, and strawberry seeds as a source of polyenoic fatty acids, tocochromanols, and phytosterols: A health implication. J. Chem. 2015, 2015, 659541. [Google Scholar] [CrossRef]
- Rodríguez Madrera, R.; Suárez Valles, B. Characterization of apple seeds and their oils from the cider-making industry. Eur. Food Res. Technol. 2018, 244, 1821–1827. [Google Scholar] [CrossRef]
- Górnaś, P.; Mišina, I.; Ikase, L. Crab apple (Malus spp.) seed tocopherol profile: Impact of genotype, species, purpose and rootstock. Agronomy 2022, 12, 2736. [Google Scholar] [CrossRef]
- Górnaś, P.; Siger, A.; Czubinski, J.; Dwiecki, K.; Segliņa, D.; Nogala-Kalucka, M. An alternative RP-HPLC method for the separation and determination of tocopherol and tocotrienol homologues as butter authenticity markers: A comparative study between two European countries. Eur. J. Lipid Sci. Technol. 2014, 116, 895–903. [Google Scholar] [CrossRef]
- Górnaś, P.; Mišina, I.; Lāce, B.; Lācis, G.; Segliņa, D. Tocochromanols composition in seeds recovered from different pear cultivars: RP-HPLC/FLD and RP-UPLC-ESI/MSn study. LWT Food Sci. Technol. 2015, 62, 104–107. [Google Scholar] [CrossRef]
- Sipeniece, E.; Mišina, I.; Grygier, A.; Qian, Y.; Rudzińska, M.; Kaufmane, E.; Segliņa, D.; Siger, A.; Górnaś, P. Impact of the harvest year of three cultivars of Japanese quince (Chaenomeles japonica) on the oil content and its composition. Sci. Hortic. 2021, 275, 109683. [Google Scholar] [CrossRef]
- Górnaś, P.; Mišina, I.; Grāvīte, I.; Soliven, A.; Kaufmane, E.; Segliņa, D. Tocochromanols composition in kernels recovered from different apricot varieties: RP-HPLC/FLD and RP-UPLC-ESI/MSn study. Nat. Prod. Res. 2015, 29, 1222–1227. [Google Scholar] [CrossRef]
- Górnaś, P.; Mišina, I.; Ruisa, S.; Rubauskis, E.; Lācis, G.; Segliņa, D. Composition of tocochromanols in kernels recovered from different sweet cherry (Prunus avium L.) cultivars: RP-HPLC/FLD and RP-UPLC-ESI/MSn study. Eur. Food Res. Technol. 2015, 240, 663–667. [Google Scholar] [CrossRef]
- Górnaś, P.; Rudzinska, M.; Raczyk, M.; Mišina, I.; Soliven, A.; Lācis, G.; Seglina, D. Impact of species and variety on concentrations of minor lipophilic bioactive compounds in oils recovered from plum kernels. J. Agric. Food Chem. 2016, 64, 898–905. [Google Scholar] [CrossRef] [PubMed]
- Górnaś, P.; Rudzińska, M.; Raczyk, M.; Mišina, I.; Soliven, A.; Segliņa, D. Composition of bioactive compounds in kernel oils recovered from sour cherry (Prunus cerasus L.) by-products: Impact of the cultivar on potential applications. Ind. Crops Prod. 2016, 82, 44–50. [Google Scholar] [CrossRef]
- Mišina, I.; Sipeniece, E.; Rudzińska, M.; Grygier, A.; Radzimirska-Graczyk, M.; Kaufmane, E.; Segliņa, D.; Lācis, G.; Górnaś, P. Associations between oil yield and profile of fatty acids, sterols, squalene, carotenoids, and tocopherols in seed oil of selected Japanese quince genotypes during fruit development. Eur. J. Lipid Sci. Technol. 2020, 122, 1900386. [Google Scholar] [CrossRef]
- Mène-Saffrané, L. Vitamin E biosynthesis and its regulation in plants. Antioxidants 2017, 7, 2. [Google Scholar] [CrossRef]
- Muñoz, P.; Munné-Bosch, S. Vitamin E in plants: Biosynthesis, transport, and function. Trends Plant Sci. 2019, 24, 1040–1051. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).