Abstract
A new diatom genus Cymbosellaphora Kulikovskiy, Glushchenko, Genkal and Kociolek gen. nov., was described with species Cymbosellaphora vietnamensis Glushchenko, Kulikovskiy and Kociolek sp. nov. C. vietnamensis sp. nov. was described from Vietnam and characterized by the presence of morphological features such as valves with naviculoid symmetry, slight dorsiventrality, the presence of tectula as pore occlusions, uniseriate striae, and a very broad mantle. Four species were transferred to the new genus. These are C. absoluta comb. nov., C. circumborealis comb. nov., C. geisslerae comb. nov., and C. laterostrata comb. nov. Previously, these species were members of genera Navicula Bory, Sellaphora Mereschkowsky, and Naviculadicta Lange-Bertalot. The taxonomic history of these species and genera are discussed. The tectulum is known only from the cymbelloid diatoms, and our new genus is placed within the Cymbellaceae. The presence of a tectulum demonstrates that these species cannot be placed in Sellaphora, as indicated in the literature. The recent proposal to transfer a large number of species with different morphologies to the genus Sellaphora is also discussed. Additionally, we compare pore occlusions with tectula between different genera of the Cymbellaceae with naviculoid symmetry.
1. Introduction
Wetzel et al. [1] recently proposed more than 60 new taxonomical combinations in the genus Sellaphora Mereschkowsky. They discussed the morphology and taxonomy of many small-celled naviculoid diatoms that play important roles in ecological analyses; many of these taxa are widespread. New combinations were proposed, transferring many species previously known to be members of Navicula Bory, Naviculadicta Lange-Bertalot, and Eolimna Lange-Bertalot and Schiller to Sellaphora. Wetzel et al. [1] comprehensively investigated the type material of some species with light and scanning electron microscopy. However, some new combinations were proposed without investigating morphology with the benefit of SEM, and without referring to published observations where SEM investigations were conducted. Examples include Sellaphora absoluta (Hustedt) C.E. Wetzel, Ector, Van de Vijver, Compere and D.G. Mann; and Sellaphora circumborealis (Lange-Bertalot) C.E. Wetzel, Ector, Van de Vijver, Compere and D.G. Mann. As a result, genera such as Naviculadicta and Eolimna were left without taxa, and the genus Sellaphora grew dramatically, going on to be one of the largest freshwater naviculoid genera.
The genus Sellaphora includes species that differ in size and also such morphological features as presence or absence of conopeum, longitudinal grooves, apical pits, and uniseriate or biseriate striae, among others [2,3]. Sellaphora was established by Mereschkowsky [4] based on the presence of an H-shaped plastid containing an invaginated pyrenoid. D.G. Mann and his colleagues have provided comprehensive investigations of the silica valve structure, as well as the chloroplast structure, sexual reproduction, and mating system [2,5,6,7,8,9,10]. According to above-mentioned investigations and morphological description given by Round et al. [11] and Wetzel et al. [1], Sellaphora, as a genus, is characterized by naviculoid and solitary cells with a number of morphological features, including the following:
- (1)
- Striae that are uniseriate or biseriate, radiate, or parallel;
- (2)
- A nonporous conopeum that can be present adjacent to the axial area;
- (3)
- A flat valve face that curves fairly gently into shallow or moderately deep mantles;
- (4)
- Striae that contain small round poroids, which are occluded near their internal apertures by hymenes;
- (5)
- A central and straight raphe system, with distal raphe ends deflected or hooked;
- (6)
- A central external raphe endings that are expanded and slightly deflected towards the primary side;
- (7)
- A central internal raphe endings turned or deflected towards the primary side;
- (8)
- The presence of helictoglossae.
This concept of Sellaphora describes the genus as a typical naviculoid taxon with a centrally positioned filiform raphe, having uniseriate or biseriate striae with pores that are covered internally. This description differentiates Sellaphora from the genus Eolimna, which has large areolae (relative to cell size) occluded by hymenes positioned medially in the areolae. Comparison with the genus Naviculadicta is not productive because this genus was created during a period of taxonomical instability related to naviculoid diatom genera, and it was suggested that it was only a matter of time until species would be transferred from this artificial group into a natural group [12]. Molecular studies of species from the genus [13,14,15,16,17] have suggested that Sellaphora possibly represents a monophyletic group that also includes Eolimna Lange-Bertalot and Schiller in Schiller et Lange-Bertalot [18] and Diprora Main [19]. However, the number of species used in these phylogenetic reconstructions is not large, with only 17 having been identified to the species level, 4 taxa having been identified presumptively, and 18 not having been identified [20].
Morphological investigations of diatoms continue to be important to understanding taxonomic identifications and for assessing phylogenetic relationships. Taxonomical investigations based on morphology are important because diatoms play important roles in the ecological monitoring of aquatic ecosystems, stratigraphy, and many other applied disciplines [1]. Different morphological features play different roles in diatom taxonomy [21] since they can diagnose groups at different levels in the hierarchy of classification according to formal analyses of morphological data.
Pore occlusions have been used to delineate taxonomic groups at the Family and Order levels [12]. Mann [22], who discussed a phenetic approach to diatom systematics, discussed the important role of pore occlusion in understanding of diatom phylogeny. Similar pore occlusions should characterize “natural” monophyletic groups, and these groups should share the same pore occlusion types—an idea that was later supported by Cox [23]. Widely, three velum types (cribrum, rota, and vola) have been used for describing pore occlusions in pennate diatoms [11,24,25]. Later, hymenate occlusions were described by D.G. Mann [22]. He described hymenes as tiny and slim perforations that are dappled by very small (in many cases) circular holes with a diameter less than 5 nm or 5–10 nm (see Round et al.) [11]. Hymenes are mainly known in members of the Order Naviculales Bessey 1907 sensu emend. Round, Crawford and D.G. Mann 1900 [12].
Cox [26] discussed and presented a reassessment of the structure and terminology of pore occlusions in the members of the Cymbellales with the description of two new types of pore occlusions, namely, tectula and foricula. Cox [23] described foricula as a “thinner flange-like outgrowth with a wide point of attachment, which partially occludes an areola, usually on the outer surface” and tectulum as “a round or squarish flap-like covering, attached to the edges of an areola by several, regularly arranged small struts”. According to Cox [23], foricula are known from members of Cymbella Agardh sensu stricto; Gomphonema Ehrenberg; Gomphoneis P.T. Cleve; and Reimeria Kociolek and Stoermer. Tectula, according to Cox [23], are known in the genus Placoneis Mereschkowsky. Based on these observations, we can distinguish naviculoid diatom genera from cymbelloid on the basis of pore occlusion.
The aim of this publication is to provide additional morphological evidence of some naviculoid species, previously belonging to the genera Sellaphora, Navicula, and Naviculadicta, and, based on the results of this study, to describe the genus Cymbosellaphora gen. nov.
2. Results and Discussion
Cymbosellaphora Kulikovskiy, Glushchenko, Genkal and Kociolek gen. nov.
Type species (designated here): Cymbosellaphora vietnamensis Glushchenko, Kulikovskiy and Kociolek sp. nov.
Description
LM. Valves nearly linear–elliptic to almost rhombic–lanceolate. Valves naviculoid or slightly dorsiventral. Ends broadly protracted and broadly rounded, obtusely rounded, sometimes rostrate or sub-capitate. Striae uniseriate, parallel or slightly radiate, radiate in the center, convergent near the ends, puncta usually resolvable. Axial area narrow, straight. Central area variable, small-to-medium size, transversely widened and irregularly delimited. Raphe straight and filiform. Central raphe ends straight and puncta-like, curved to opposite from distal raphe end side. Distal raphe ends hooked, extending onto the mantle.
SEM, external view. Valve face flat without visible sternum. Raphe lying in the middle of the valve. Central raphe ends straight, point-like and slightly curved to the side opposite from the distal raphe ends. Central nodule broad. Distal raphe ends hook-like and curved to mantle. Striae uniseriate with distantly spaced circle or linear, zig-zag linear areolae. Striae at both ends are regularly spaced or slightly approximating. Pore fields not detected. Mantle very wide with striae and areolae continuing from valve face. Girdle bands with two rows of perforations.
SEM, internal view. Axial area straight and with narrow sternum evident or slightly evident. Raphe with curved central raphe ends, distal raphe ends straight and finished by evident helictoglossae. Central nodule broad. Interstriae not elevated, wide and broader than striae. Striae uniseriate and includes areolae. Areolae covered with a thin imperforate flat silica layer. This silica layer covers the areolae at one level with the inner surface of the valve. When this layer is destroyed, the internal structure of the areola becomes visible. Every one hole has two levels. At one level, the opening is broader and circular or rectangular, and on this level, a silica layer that covers areola internally is present. The second level has a smaller hole. These smaller holes are circular or linear and extend through the valve. Every one circle or circular areola is divided by rectangular strut. During the cleaning of the valves, these rectangular struts are destroyed and are represented by two outgrowths situated parallel to the valve level and opposite one another. These struts or tectula are present between areolar openings within each stria. Voigt discontinuity is present on the secondary side of the valves.
Etymology: This genus name is a combination of the genus names Sellaphora and Cymbella, indicating that they share some similar morphological features of both genera.
Cymbosellaphora vietnamensis Glushchenko, Kulikovskiy and Kociolek sp. nov. (Figure 1, Figure 2 and Figure 3)
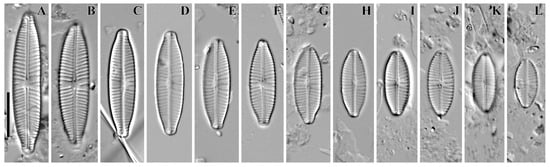
Figure 1.
(A–L) Cymbosellaphora vietnamensis Glushchenko, Kulikovskiy and Kociolek sp. nov. Light microscopy, differential interference contrast, size diminution series. Vietnam. Slide no. 02151. (A) Holotype. Scale bar = 10 μm.
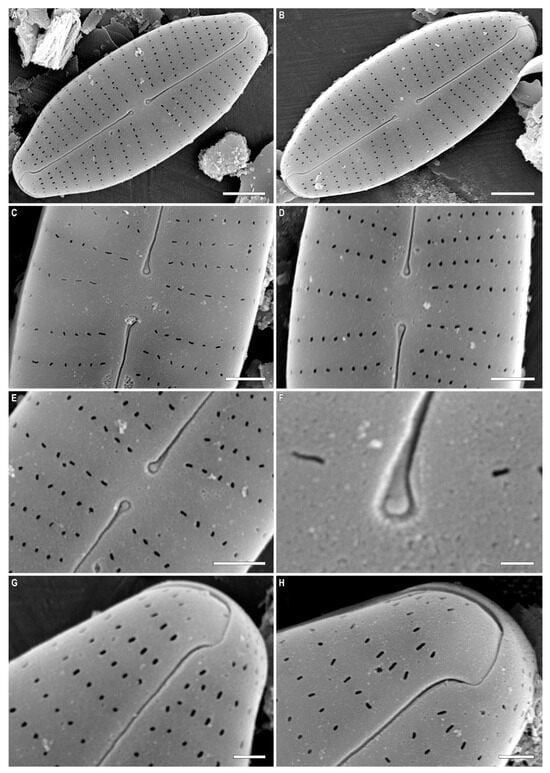
Figure 2.
(A–H) Cymbosellaphora vietnamensis Glushchenko, Kulikovskiy and Kociolek sp. nov. Scanning electron microscopy, external views. (A,B) The whole valve. (C–E) Central area. (F) Central raphe end. (G,H) Valve ends. Scale bar (A,B) = 2 μm; (C–E) = 1 μm; (G,H) = 0.5 μm (F) = 0.2 μm.
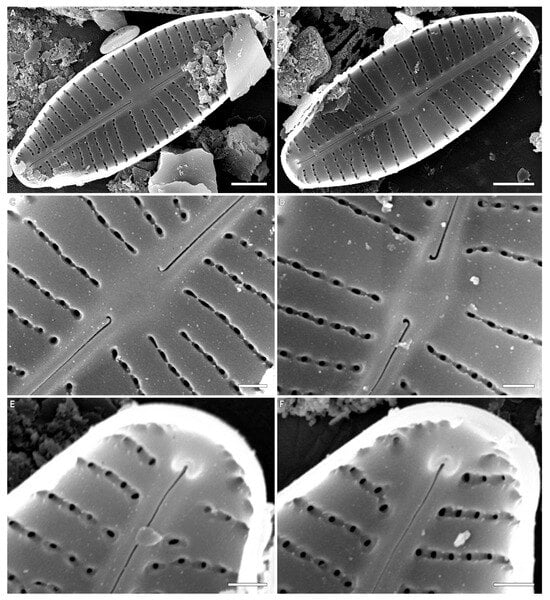
Figure 3.
(A–F) Cymbosellaphora vietnamensis Glushchenko, Kulikovskiy and Kociolek sp. nov. Scanning electron microscopy, internal views. (A,B) The whole valve. (C,D) Central area. (E,F) Valve ends. Tectula, as small struts, are present between areolar openings within each stria. Voigt discontinuity is clearly visible. Scale bar (A,B) = 2 μm; (C–F) = 0.5 μm.
Holotype. Deposited in the herbarium of MHA, Main Botanical Garden Russian Academy of Science, Moscow, Russia, the holotype here designated, slide no. 12151 (Figure 1A).
Isotype. Collection of Maxim Kulikovskiy at the Herbarium of the Institute of Plant Physiology Russian Academy of Science, Moscow, Russia, slide no. 12151a.
Etymology: The specific epithet “vietnamensis” refers to the name of the country where this species was discovered.
Description. LM (Figure 1A–L). Valves almost linear to linear–elliptic. Valves naviculoid, slightly dorsiventral in longer specimens. Length 10.7–31.4 µm; breadth 5.0–6.7 µm. Ends broadly protracted. Axial area narrow, straight. Central area variable, small-to-medium size, transversely widened and irregularly delimited. Raphe straight and filiform. Central raphe ends straight and puncta-like, curved to opposite from distal raphe end side. Distal raphe ends hooked, going to the mantle. Striae uniseriate, slightly radiate, convergent near the ends, 14–16 in 10 µm. Areolae noticeable in some specimens.
SEM, external view (Figure 2A–F). Valve face flat without visible sternum. Raphe positioned in the middle of the valve. Central raphe ends straight, point-like, and slightly curved to the side opposite the distal raphe ends. Central nodule broad. Distal raphe ends hook-like and curved onto the mantle. Striae uniseriate with distantly spaced circular or linear areolae. Striae at both ends are regularly spaced or slightly approximating. Pore fields not detected. Mantle very wide with striae and areolae continuing from valve face. Areolae 35–40 in 10 µm.
SEM, internal view (Figure 3A–F). Axial area straight and present by evident or slightly evident narrow sternum. Raphe with curved central raphe ends, distal raphe ends straight, and they terminate as evident helictoglossae. Central nodule broad. Interstriae not elevated, wide and broader than striae. Striae uniseriate. Areolae covered by a thin imperforate flat silica layer. This silica layer covers the areolae at one level with the inner surface of the valve. When this layer is destroyed, the internal structure of the areola becomes visible. Every opening has two levels. At one level, the opening is broader and circular or rectangular. On this level, a silica layer is present that covers areolae internally. Voigt discontinuity is present on the secondary side of the valves.
The openings at the second level have a smaller aperture, are circular or linear in shape, and extend through the valves. Every areola is divided by a rectangular strut. During the digestion of valve, these rectangular struts can be destroyed, and what is revealed are two outgrowths situated parallel to valve level and opposite one another.
New combinations:
Cymbosellaphora absoluta (Hustedt) Kulikovskiy, Glushchenko, Genkal and Kociolek comb. nov. (Figure 4, Figure 5, Figure 6, Figure 7 and Figure 8)
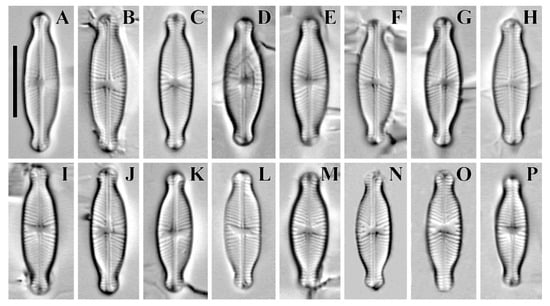
Figure 4.
(A–P) Cymbosellaphora absoluta Kulikovskiy, Glushchenko, Genkal and Kociolek comb. nov. Light microscopy, differential interference contrast, size diminution series. Mongolia. Slide no. 02431 (L,P); 02440 (A,J); 02441 (C,M); 02442 (E); 02445 (H); 02446 (K); 02447 (B,F,N,O); 02451 (I); 02457 (D,G). Scale bar = 10 μm.

Figure 5.
(A–F) Cymbosellaphora absoluta Kulikovskiy, Glushchenko, Genkal and Kociolek comb. nov. Scanning electron microscopy, external views. (A–E) The whole valve. (F) Fragment of valve. Scale bar (B,D) = 5 μm; (A,C,E,F) = 2 μm.
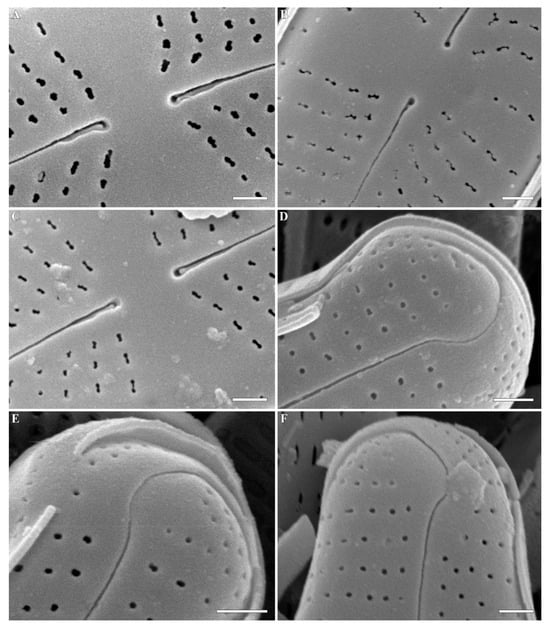
Figure 6.
(A–F) Cymbosellaphora absoluta Kulikovskiy, Glushchenko, Genkal and Kociolek comb. nov. Scanning electron microscopy, external views. (A–C) Central area. (D–F) Valve ends. Scale bar (A–F) = 0.5 μm.
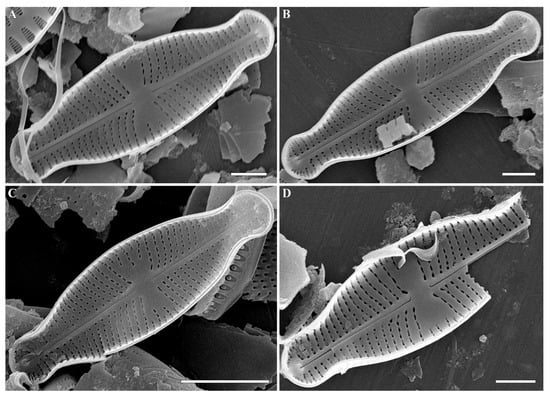
Figure 7.
(A–D) Cymbosellaphora absoluta Kulikovskiy, Glushchenko, Genkal and Kociolek comb. nov. Scanning electron microscopy, internal views. (A–C) The whole valve. Voigt discontinuity is clearly visible. (D) Fragment of valve. Scale bar (C) = 5 μm; (A,B,D) = 2 μm.
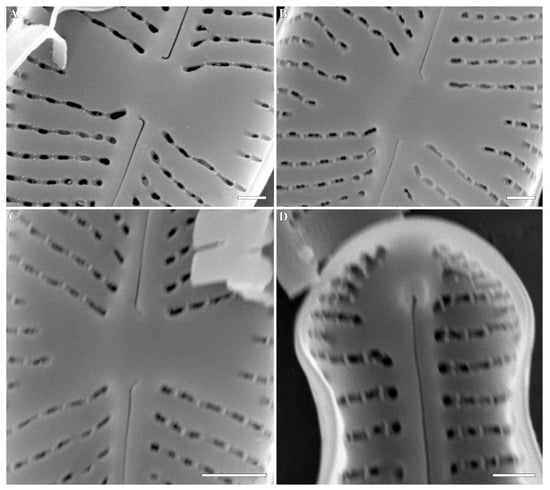
Figure 8.
(A–D) Cymbosellaphora absoluta Kulikovskiy, Glushchenko, Genkal and Kociolek comb. nov. Scanning electron microscopy, internal views. (A–C) Central area. (D) Valve end. Tectula, as struts, are present between areolar openings within each stria. Scale bar (C) = 1 μm; (A,B,D) = 0.5 μm.
Basionym: Navicula absoluta Hustedt 1950. Archiv für Hydrobiologie. Band 43, P. 435, Pl. 38, Fig. 80–85.
Synonyms: Naviculadicta absoluta (Hustedt) Lange-Bertalot in Moser and Lange-Bertalot 1994; Sellaphora absoluta (Hustedt) Wetzel, Ector, Van de Vijver, Compère and D.G. Mann 2015.
Comments: The specimens in our sample were 15.4–19.2 μm long, 4.5–5.5 μm wide, and had 25–26 striae in 10 μm and 25–30 areolae in 10 μm.
Cymbosellaphora circumborealis (Lange-Bertalot) Kulikovskiy, Glushchenko, Genkal and Kociolek comb. nov. (Figure 9)
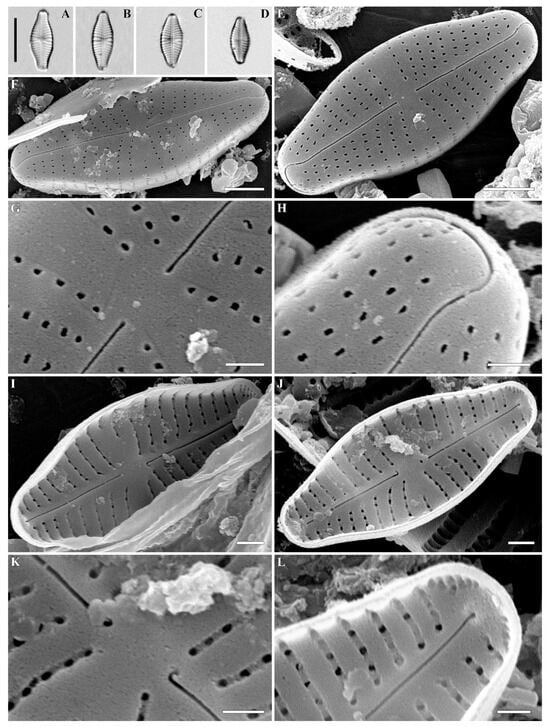
Figure 9.
(A–L) Cymbosellaphora circumborealis Kulikovskiy, Glushchenko, Genkal and Kociolek comb. nov. (A–D) Light microscopy, differential interference contrast, size diminution series. Mongolia. Slide no. 02663 (B); 02684 (A); 02695 (C); 03009 (D). (E–H) SEM, external view. (I–L) SEM, internal view. (E,F,I,J) The whole valve. (G,K) Central area. (H,L) Valve ends. Scale bar = (A–D) = 10 μm; (E,F) = 2 μm; (I,J) = 1 μm; (G,H,K,L) = 0.5 μm.
Basionym: Naviculadicta circumborealis Lange-Bertalot in Lange-Bertalot and Moser 1994. Bibliotheca Diatomologica. Vol. 29. P. 84–85. Pl. 52. Figs 29–36.
Comments: The specimens in our sample were 10.4–13.6 μm long, 4.2–5.1 μm wide, and had 20 striae in 10 μm and 35–40 areolae in 10 μm.
Cymbosellaphora geisslerae (Jahn) Kulikovskiy, Glushchenko, Genkal and Kociolek comb. nov. (Figure 10 and Figure 11)
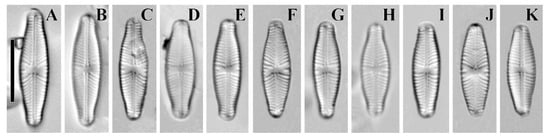
Figure 10.
(A–K) Cymbosellaphora geisslerae (Jahn) Kulikovskiy, Glushchenko, Genkal, and Kociolek comb. nov. Light microscopy, differential interference contrast, size diminution series. Mongolia. Slide no. 02642 (A–C,E–G,I–K); 02684 (D,H). Scale bar = 10 μm.
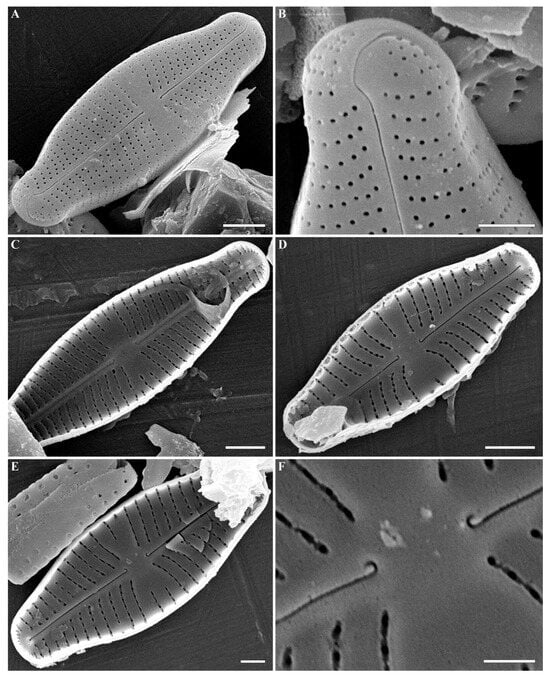
Figure 11.
(A–F) Cymbosellaphora geisslerae Kulikovskiy, Glushchenko, Genkal and Kociolek comb. nov. Scanning electron microscopy. (A,B) External views. (C–F) Internal views. (A–E) The whole valve. (B) Valve end. (F) Central area. Tectula, as small struts, are present between areolar openings within each stria. Scale bar (A,C,D) = 2 μm; (B,E) = 1 μm; (F) = 0.5 μm.
Basionym: Navicula geisslerae Jahn 1992. Diatom Research. Vol. 7. Issue 1. P. 69–75. Figs 1–15.
Synonym: Naviculadicta geisslerae (Jahn) Jahn in Lange-Bertalot and Moser 1994.
Comments: The specimens in our sample were 14.3–18.8 μm long, 4.3–4.8 μm wide, and had 19–21 striae in 10 μm and 38–40 areolae in 10 μm.
Cymbosellaphora laterostrata (Hustedt) Kulikovskiy, Glushchenko, Genkal and Kociolek comb. nov.
Basionym: Navicula laterostrata Hustedt 1925. Bacillariales aus Schlesien. II. Nachtrag. Internationale Revue der gesamten Hydrobiologie und Hydrographie. Band 13. S. 345–357.
Synonym: Navicula mournei R.M. Patrick 1959.
All of the species discussed herein, transferred to the genus Cymbosellaphora gen. nov., i.e., C. vietnamensis sp. nov., Cymbosellaphora absoluta comb. nov., Cymbosellaphora circumborealis comb. nov., Cymbosellaphora geisslerae comb. nov., Cymbosellaphora laterostrata comb. nov., share the same morphological features. The feature that they all share, and which distinguishes them from Sellaphora, is the internal pore occlusions. In these taxa, areolae are covered internally by a nonperforated silica layer with presence of rectangular struts that divide every areola. During the cleaning of the valves, these rectangular struts can be destroyed, revealing two outgrowths situated parallel to the valve level and opposite one another. The presence of nonperforated silica covers, as well as struts and outgrowths, are similar to the pore occlusions of Placoneis described by Cox [23]. Differences between Cymbosellaphora gen. nov. and Placoneis include the number of vertical points around the areola internally that are covered by a flap-like covering (tectulum, “little silica roof”) in the sense of Cox [23]. However, these two genera are similar, based on the presence of two outgrowths situated parallel to valve level and opposite one another (see Kulikovskiy et al.) [27]. This morphological feature is present in every species assigned to our new genus, suggesting that Cymbosellaphora gen. nov. is characterized by pore occlusions that are like the tectulum of cymbelloid diatoms, not the hymenate occlusions characteristic of Navicula or Sellaphora. On this basis, we assign our new genus to the family Cymbellaceae Greville. This family, according to Kulikovskiy et al. [12], includes genera with almost naviculoid symmetry, such as Cymbellafalsa Lange-Bertalot and Metzeltin; Geissleria Lange-Bertalot and Metzeltin; Khursevichia Kulikovskiy, Lange-Bertalot and Metzeltin; Ochigma Kulikovskiy, Lange-Bertalot and Metzeltin; Paraplaconeis Kulikovskiy, Lange-Bertalot and Metzeltin; Placoneis and Rexlowea Kociolek and Thomas.
The new genus, Cymbosellaphora gen. nov., shares morphological features such as the tectulum, naviculoid symmetry with slight dorsiventrality, and perforated girdle bands with cymbelloid diatoms [25,26]. However, genera in the cymbelloid lineage are distinguished from each other by slightly different pore structures.
All taxa have pore occlusions covered internally by flap-like coverings called tectula, but the presence or absence of different struts, the shape of the tectulum, and the number and aperture morphology are different. For example, Geissleria is a very similar genus to Cymbosellaphora gen. nov., but it differs from it by the presence of two broad outgrowths situated parallel to valve level and opposite one another ([12], Figure 2.14.6; 41). Additionally, Geissleria is characterized by the presence of special areolae at each end, called an annulus [28]. Paraplaconeis is characterized by having a much more complicated areolar structure than Cymbosellaphora gen. nov. and Placoneis. Paraplaconeis has uniform slits that have a stalked flap and long struts separating the areolae internally, forming a polygonal pattern over the alternating biseriate areolae of the valve face [27]. Areolae are also covered by tectula internally (see discussion in Kulikovskiy et al.) ([12], Figure 2.6.2). Rexlowea is characterized also by having naviculoid symmetry, but this genus possesses a prominent undulate raphe and striae with internal occlusions with narrow bases and flared tips protruding into the areolae [12,29,30]. Khursevichia differs by having areolae with noticeable oval flat hollows—circuses, on which the small outgrowths are present [31]. These outgrowths link up with their tops, closing the opening of the areolae. Pore occlusions of Ochigma are much more complicated and characterized by large areolae with circular hollows. Each areola has a flat circus, going deep into the valve with a tapered round or oblong hole in the form of a funnel. This circus-like plane has a large number of rather long, irregularly shaped nodules, which have a silica layer on the top [31].
Cymbellafalsa is a genus very similar to Cymbosellaphora gen. nov. Cymbellafalsa was described by Lange-Bertalot and Metzeltin in 2009 from Mongolian material [32]. Species assigned to this genus were previously members of the genera Cymbella and Navicula. The referral to Cymbella was based on the visible dorsiventral shape and the presence of pore fields at both valve ends. The presence of pore fields is an important morphological feature that divides Cymbellafalsa from Cymbosellaphora gen. nov. Pore fields in Cymbellafalsa are characterized by the presence of an undulate flap–like silica strip above the internal apertures of each pervalvar row of foramina, not completely occluding internal apertures ([32], Figure 266: 4). Members of Cymbosellaphora gen. nov. have slightly compressed striae near the valve ends but never with the special flap-like silica strips seen in Cymbellafalsa. The latter genus is similar to Cymbosellaphora gen. nov., based on striae structure and pore occlusion type. However, valve shape and striae structure are not helpful features for combining genera relative to morphological variation associated with pore occlusions. This situation was discussed and is shown with respect to the genus Karthickia [33,34]. Our new genus is easily distinguished from Navicula and Sellaphora by the internal absence of hymenes.
The taxonomic position of species now assigned to the genus Cymbosellaphora gen. nov. was discussed previously. Jahn [25], who described C. geisslerae comb. nov. as a member of Navicula, discussed the similarity of this species with the genus Placoneis. Jahn [25] undertook a comprehensive morphological investigation of this small-celled, widespread species and showed it to have pore occlusions that are typical for Cymbellaceae. However, the description of the tectulum and its differences from other pore occlusions of naviculoid genera was not given until later [23]. Our investigations of different cymbelloid genera, using molecular data as well as morphological data, showed that pore occlusions are an important morphological feature for differentiating between cymbelloid and naviculoid genera [20,35]. The description of new genera with scanning electron microscopy reveals that pore occlusions are different between cymbelloid genera. The structure of the tectulum across cymbelloid genera differs mainly by presence/absence, as well as the number and shape of struts into the areolae. This morphological feature will receive further investigation.
C. vietnamensis sp. nov., C. absoluta comb. nov., C. circumborealis comb. nov., C. geisslerae comb. nov. and C. laterostrata comb. nov. can easily be distinguished from each other. The morphologies of these species are summarized in Table 1.

Table 1.
Comparison of Cymbosellaphora vietnamensis sp. nov. and four species transferred to Cymbosellaphora gen. nov.
Morphological investigation of the populations of the species studied herein are similar to previous descriptions [25,36,39,40,41,42]. Chudaev and Gololobova [41] prepared a comprehensive light and scanning electron investigation of C. geisslerae comb. nov. ([41]: Figs 229: 1–35 and 230: 1–5) and C. laterostrata comb. nov. ([41]: Figs 231: 1–6 and 232: 1–14]). The populations of the species studied herein share morphological features documented in the literature cited above.
The species Cymbosellaphora vietnamensis sp. nov. is known only from the type locality (Lake Ba Bể, Vietnam). This is the first documented finding of this genus in Southeast Asia. Reports of Navicula absoluta and Navicula laterostrata in Indonesia are not supported by illustrations [43,44].
All other documented species of the genus are known from Holarctic habitats. Cymbosellaphora absoluta comb. nov. was described as Navicula absoluta from Northern Germany (from Lake Garrensee) [36]. The species was also previously found in Mongolia, in the sediments of the Great Lakes Depression, sine loco [38]. We recorded this species in the benthos, periphyton, and sediments of Lake Davaa, as well as in the periphyton of the unnamed river flowing out of Lake Davaa.
Cymbosellaphora circumborealis comb. nov. was described as Navicula circumborealis from Lake James, Canada [40]. The species is known from Sweden, Finland, and the Aleutian Islands [40]. In Mongolia, this species was found by us in the periphyton and benthos of Boreug Bay and Heeguer Bay of Lake Khövsgöl.
Cymbosellaphora geisslerae comb. nov. was described as Navicula geisslerae from Spree River, Berlin [39]. This species was also illustrated from Lake James, Canada, Central Europe [39,40], and from Lake Glubokoe, Russia [41]. This species was recorded by us from Mongolia, from the benthos of Khövsgöl Lake (Heeguer Bay), as well as from Unnamed Lake near Khövsgöl Lake, separated by a sandbar.
Cymbosellaphora laterostrata comb. nov. was invalidly described as Navicula laterostrata from Germany and reported from lakes Schlawasee, Ogglisch-Mühlensee, Kleiner Tarnauersee [42]. Later, Reimer Simonsen validated this taxon [37]. It has also been illustrated from Großes Heiliges Meer lake [40]. The species was found in Russia, in Lake Glubokoe [41]. This species was also previously found in Mongolia, in the sediments of Har Us Lake, Dood Lake, Davaa Lake, and Terhiyn Tsagaan Lake [38]. This species was not noted in our material for the present report.
3. Conclusions
Review of taxa previously transferred from Navicula, Naviculadicta, and Eolimna to Sellaphora show they have features that are found in the genera and species of the Cymbellales rather than the Naviculales. The tectula morphology in these taxa are different from other genera in Cymbellales, warranting the creation of a new genus for them; thus, the genus Cymbosellaphora is proposed to accommodate these species with unique tectular morphology. The genus is found in the Holarctic but also from Vietnam. A further review of the structure of tectula is warranted in order to document further variability in its structure and its potential to diagnose natural groups. Further research on small species recently allocated to the genus Sellaphora may uncover additional cryptic diversity in this catch-all genus.
4. Materials and Methods
Sampling. A list of all samples examined in this study, along with their geographic position, is presented in Table 2.

Table 2.
List of the collected samples and their characteristics.
Preparation of slides and microscope investigation. Samples were processed by means of a standard procedure involving treatment with 10% HCl and concentrated hydrogen peroxide. After treatment with HCl, the samples were washed with deionized water. Permanent diatom preparations were mounted in Naphrax® (Brunel Microscopes, Chippenham, UK). Light microscopic (LM) observations were performed with a Zeiss Axio Scope A1 (Zeiss, Oberkochen, Germany) microscope equipped with an oil immersion objective (×100/n.a.1.4, DIC). For scanning electron microscopy (SEM), parts of the suspensions were fixed on aluminum stubs after air drying. The stubs were sputter-coated with 50 nm of gold in an Eiko IB 3 (Eiko Engineering, Yamazaki, Hitachinaka Shi, Ibaraki Ken, Japan). Valve ultrastructure was examined by means of a JSM-6510LV scanning electron microscope (JEOL Ltd., Akishima, Tokyo, Japan), operated at 10 kV and 11 mm distance.
Samples and slides are deposited in the collection of Maxim Kulikovskiy at the Herbarium of the Institute of Plant Physiology Russian Academy of Sciences, Moscow, Russia.
Author Contributions
Conceptualization, M.S.K.; methodology, M.S.K. and A.M.G.; validation, A.M.G., M.S.K. and J.P.K.; investigation, A.M.G. and M.S.K.; resources, M.S.K. and A.M.G.; writing—original draft preparation, A.M.G. and Y.I.M.; writing—review and editing, J.P.K.; visualization, A.M.G., I.V.K., S.I.G. and Y.I.M.; supervision, M.S.K.; funding acquisition, M.S.K. All authors have read and agreed to the published version of the manuscript.
Funding
Publication is based on research carried out with financial support by the Russian Science Foundation no. 19-14-00320Π (90% financing) for LM and SEM and by the framework of state assignment of the Ministry of Science and Higher Education of the Russian Federation, theme no. 122042700045-3 (10% financing) for finishing manuscript.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Wetzel, C.E.; Ector, L.; Van de Vijver, B.; Compère, P.; Mann, D.G. Morphology, typification and critical analysis of some ecologically important small naviculoid species (Bacillariophyta). Fottea 2015, 15, 203–234. [Google Scholar] [CrossRef]
- Mann, D.G.; Thomas, S.J.; Evans, K.M. Revision of the diatom genus Sellaphora: A first account of the larger species in the British Isles. Fottea 2008, 8, 15–78. [Google Scholar] [CrossRef]
- Liu, Y.; Kociolek, J.P.; Lu, X.; Fan, Y. A new Sellaphora Mereschkowsky species (Bacillariophyceae) from Hainan Island, China, with comments on the current state of the taxonomy and morphology of the genus. Diatom Res. 2020, 35, 85–98. [Google Scholar] [CrossRef]
- Mereschkowsky, C. On Sellaphora, a new genus of diatoms. Ann. Mag. Nat. Hist. 1902, 9, 185–195. [Google Scholar] [CrossRef]
- Mann, D.G.; Chepurnov, V.A.; Droop, S.J.M. Sexuality, incompatibility, size variation, and preferential polyandry in natural populations and clones of Sellaphora pupula (Bacillariophyceae). J. Phycol. 1999, 35, 152–170. [Google Scholar] [CrossRef]
- Mann, D.G. The systematics of the Sellaphora pupula complex: Typification of S. pupula. In Studies on Diatoms. Lange-Bertalot Festschrift; Jahn, R., Kociolek, J.P., Witkowski, A., Compere, P., Eds.; Koeltz: Koenigstein, Germany, 2001. [Google Scholar]
- Mann, D.G.; McDonald, S.M.; Bayer, M.M.; Droop, S.J.M.; Chepurnov, V.A.; Loke, R.E.; Ciobanu, A.; du Buf, J.M.H. The Sellaphora pupula species complex (Bacillariophyceae): Morphometric analysis, ultrastructure and mating data provide evidence for five new species. Phycologia 2004, 43, 459–482. [Google Scholar] [CrossRef]
- Mann, D.G.; Evans, K.M.; Chepurnov, V.A.; Nagai, S. Morphology and formal description of Sellaphora bisexualis sp. nov. (Bacillariophyta). Fottea 2009, 9, 199–209. [Google Scholar] [CrossRef]
- Mann, D.G.; Stickle, A.S. Cytological characteristics of the Sellaphoraceae. Acta Bot. Croat. 2009, 68, 239–250. [Google Scholar]
- Vanormelingen, P.; Evans, K.M.; Chepurnov, V.A.; Vyverman, W.; Mann, D.G. Molecular species discovery in the diatom Sellaphora and its congruence with mating trials. Fottea 2013, 13, 133–148. [Google Scholar] [CrossRef]
- Round, F.E.; Crawford, R.M.; Mann, D.G. The Diatoms. Biology and Morphology of the Genera; Cambridge University Press: Cambridge, UK, 1990; 747p. [Google Scholar]
- Kulikovskiy, M.S.; Glushchenko, A.M.; Genkal, S.I.; Kuznetsova, I.V. Identification Book of Diatoms from Russia; Filigran: Yaroslavl, Russia, 2016; 804p. [Google Scholar]
- Evans, K.M.; Wortley, A.H.; Mann, D.G. An assessment of potential diatom “barcode” genes (cox1, rbcL, 18S and ITS rDNA) and their effectiveness in determining relationships in Sellaphora (Bacillariophyta). Protist 2007, 158, 349–364. [Google Scholar] [CrossRef]
- Evans, K.M.; Wortley, A.H.; Simpson, G.E.; Chepurnov, V.A.; Mann, D.G. A molecular systematic approach to explore diversity within the Sellaphora pupula species complex (Bacillariophyta). J. Phycol. 2008, 44, 215–231. [Google Scholar] [CrossRef] [PubMed]
- Evans, K.M.; Chepurnov, V.A.; Sluiman, H.J.; Thomas, S.J.; Spears, B.M.; Mann, D.G. Highly differentiated populations of the freshwater diatom Sellaphora capitata suggest limited dispersal and opportunities for allopatric speciation. Protist 2009, 160, 386–396. [Google Scholar] [CrossRef]
- Behnke, A.; Friedl, T.; Chepurnov, V.A.; Mann, D.G. Reproductive compatibility and rDNA sequence analyses in the Sellaphora pupula species complex (Bacillariophyta). J. Phycol. 2004, 40, 193–208. [Google Scholar] [CrossRef]
- Mann, D.G.; Poulíčková, A. Homothallism, morphology and phylogenetic position of a new species of Sellaphora (Bacillariophyta), S. pausariae. Plant Ecol. Evol. 2019, 152, 203–218. [Google Scholar] [CrossRef]
- Schiller, W.; Lange-Bertalot, H. Eolimna martinii n. gen., n. sp. (Bacillariophyceae) aus dem Unter-Oligozän von Sieblos/Rhön im Vergleich mit ähnlichen rezenten Taxa. Paläontol. Z. 1997, 71, 163–172. [Google Scholar] [CrossRef]
- Kociolek, J.P.; Stepanek, J.G.; Lowe, R.L.; Johansen, J.R.; Sherwood, A.R. Molecular data show the enigmatic cave-dwelling diatom Diprora (Bacillariophyceae) to be a raphid diatom. Eur. J. Phycol. 2013, 48, 474–484. [Google Scholar] [CrossRef]
- Glushchenko, A.M.; Maltsev, Y.I.; Kociolek, J.P.; Kuznetsova, I.V.; Kulikovskiy, M.S. Molecular and Morphological Investigations of Two Giant Diatom Cymbella Species from the Transbaikal Area (Russia, Siberia) with Comments on Their Distributions. Plants 2022, 11, 2445. [Google Scholar] [CrossRef]
- Kulikovskiy, M.S.; Andreeva, S.A.; Gusev, E.S.; Kuznetsova, I.V.; Annenkova, N.V. Molecular phylogeny of monoraphid diatoms and raphe significance in evolution and taxonomy. Biol. Bull. 2016, 43, 398–407. [Google Scholar] [CrossRef]
- Mann, D.G. Sieves and flaps: Siliceous minutae in the pores of raphid diatoms. In Proceedings of the 6th Symposium on Reecent and Fossil Diatoms, Budapest, Hungary, 1–5 September 1980; Ross, R., Ed.; O. Koeltz, Koenigstein: Koenigstein-Taunus, Germany, 1981; pp. 279–300. [Google Scholar]
- Cox, E.J. Pore occlusions in raphid diatoms—A reassessment of their structure and terminology, with particular reference to members of the Cymbellales. Diatom 2004, 20, 33–46. [Google Scholar]
- Ross, R.; Sims, P. The fine structure of the frustule in centric diatoms: A suggested terminology. Br. Phycol. J. 1972, 7, 139–163. [Google Scholar] [CrossRef]
- Jahn, R. Navicula geisslerae sp. nov.—A small species from the River Spree (Berlin, Germany). Diatom Res. 1992, 7, 69–75. [Google Scholar] [CrossRef]
- Cox, E.J. Placoneis Mereschkowsky: The re-evaluation of a diatom genus originally characterized by its chloroplast type. Diatom Res. 1987, 2, 145–157. [Google Scholar] [CrossRef]
- Kulikovskiy, M.S.; Lange-Bertalot, H.; Metzeltin, D.; Witkowski, A. Lake Baikal: Hotspot of endemic diatoms I. Iconogr. Diatomol. 2012, 23, 7–608. [Google Scholar]
- Lange-Bertalot, H. Navicula sensu stricto, 10 genera separated from Navicula sensu lato, Frustulia. Diatoms Eur. 2001, 2, 1–526. [Google Scholar]
- Kociolek, J.P.; Thomas, E.W. Taxonomy and ultrastructure of five naviculoid diatoms (class Bacillariophyceae) from the Rocky Mountains of Colorado (USA), with the description of a new genus and four new species. Nova Hedwig. 2010, 90, 195–214. [Google Scholar] [CrossRef]
- Kociolek, J.P.; Kulikovskiy, M.; Genkal, S.; Kuznetsova, I. Morphological investigation and transfer of Naviculadicta parasemen Lange-Bertalot to the genus Rexlowea Kociolek & Thomas. Diatom Res. 2017, 32, 477–481. [Google Scholar] [CrossRef]
- Kulikovskiy, M.S.; Kuznetsova, I.V. Morphology, taxonomical position, and distribution of the genera of diatoms Ochigma and Khursevichia from Lake Baikal. Inland Water Biol. 2016, 9, 226–233. [Google Scholar] [CrossRef]
- Metzeltin, D.; Lange-Bertalot, H.; Soninkhishig, N. Diatoms in Mongolia. Iconogr. Diatomol. 2009, 20, 1–691. [Google Scholar]
- Glushchenko, A.; Kuznetsova, I.; Kociolek, J.P.; Kulikoviskiy, M. Karthickia verestigmata gen. et sp. nov.—An interesting diatom with frustular morphology similar to several different cymbelloid diatom genera. Phycologia 2019, 58, 605–613. [Google Scholar] [CrossRef]
- Yana, E.; Nakkaew, S.; Pekkoh, S.; Peerapornpisal, Y.; Tuji, A.; Davis, M.P.; Julius, M.L.; Mayama, S. Valve and ‘Stigma’ Structure and Phylogeny of an Enigmatic Cymbelloid Diatom Karthickia verestigmata Glushchenko, Kulikovskiy & Kociolek. Diatom Res. 2022, 37, 293–305. [Google Scholar] [CrossRef]
- Glushchenko, A.; Gusev, E.; Maltsev, Y.; Kociolek, J.P.; Kuznetsova, I.; Kulikovskiy, M. Cymbopleura natellia—A new species from Transbaikal area (Russia, Siberia) described on the basis of molecular and morphological investigation. PhytoKeys 2021, 183, 95–105. [Google Scholar] [CrossRef]
- Hustedt, F. Die Diatomeenflora norddeutscher Seen mit besonderer Berücksichtigung des holsteinischen Seengebiets. V.-VII. Seen in Mecklenburg, Lauenburg und Nordostdeutschland. Arch. fur Hydrobiol. 1950, 43, 329–458. [Google Scholar]
- Simonsen, R. Atlas and Catalogue of the Diatom Types of Friedrich Hustedt; J. Cramer in der Gebrüder Borntraeger Velagsbuchhandlung: Berlin/Stuttgart, Germany, 1987; Vol. 1. Catalogue. Vol. 2. pls 1–395. Vol. 3. pls. 396–772. pp. 1–525, 772 pls. [Google Scholar]
- Dorofeyuk, N.I.; Kulikovskiy, M.S. Diatoms of Mongolia. Proc. Jt. Russ. Mong. Complex Biol. Exped. 2012, 59, 1–367. [Google Scholar]
- Lange-Bertalot, H.; Hofmann, G.; Werum, M.; Cantonati, M. Freshwater Benthic Diatoms of Central Europe: Over 800 Common Species Used in Ecological Assessment. English Edition with Updated Taxonomy and Added Species; Koeltz Botanical Books: Schmitten-Oberreifenberg, Germany, 2017; 942p. [Google Scholar]
- Lange-Bertalot, H.; Moser, G. Brachysira. Monographie der Gattung und Naviculadicta nov. gen. Bibl. Diatomol. 1994, 29, 1–212. [Google Scholar]
- Chudaev, D.A.; Gololobova, M.A. Diatoms of the Glubokoe Lake (Moscow Region); KMK: Moscow, Russia, 2016; pp. 1–446, 266 pls. (In Russian) [Google Scholar]
- Hustedt, F. Bacillariales aus Schlesien. II. Nachtrag. Int. Rev. Gesamten Hydrobiol. Hydrogr. 1925, 13, 345–357. [Google Scholar] [CrossRef]
- Sumita, M.; Watanabe, T. Epilithic freshwater diatoms in Jakarta, Surabaya and Singapore. Jpn. J. Phycol. 1979, 27, 1–6. [Google Scholar]
- Watanabe, T.; Usman, R. Epilithic freshwater diatoms in Central Sumatra. Diatom 1987, 3, 33–87. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).