Potential Impacts of Certain N2-Fixing Bacterial Strains and Mineral N Doses for Enhancing the Growth and Productivity of Maize Plants
Abstract
1. Introduction
2. Results
2.1. Optimization of the N2-Fixing Isolates
2.2. Germination (%)
2.3. Identification of the N2-Fixing Isolate
2.3.1. Cultural and Biochemical Tests
2.3.2. Molecular Test
2.4. Pots Trial
2.5. Field Trials
2.5.1. Growth Parameters
2.5.2. Biochemical Measurements
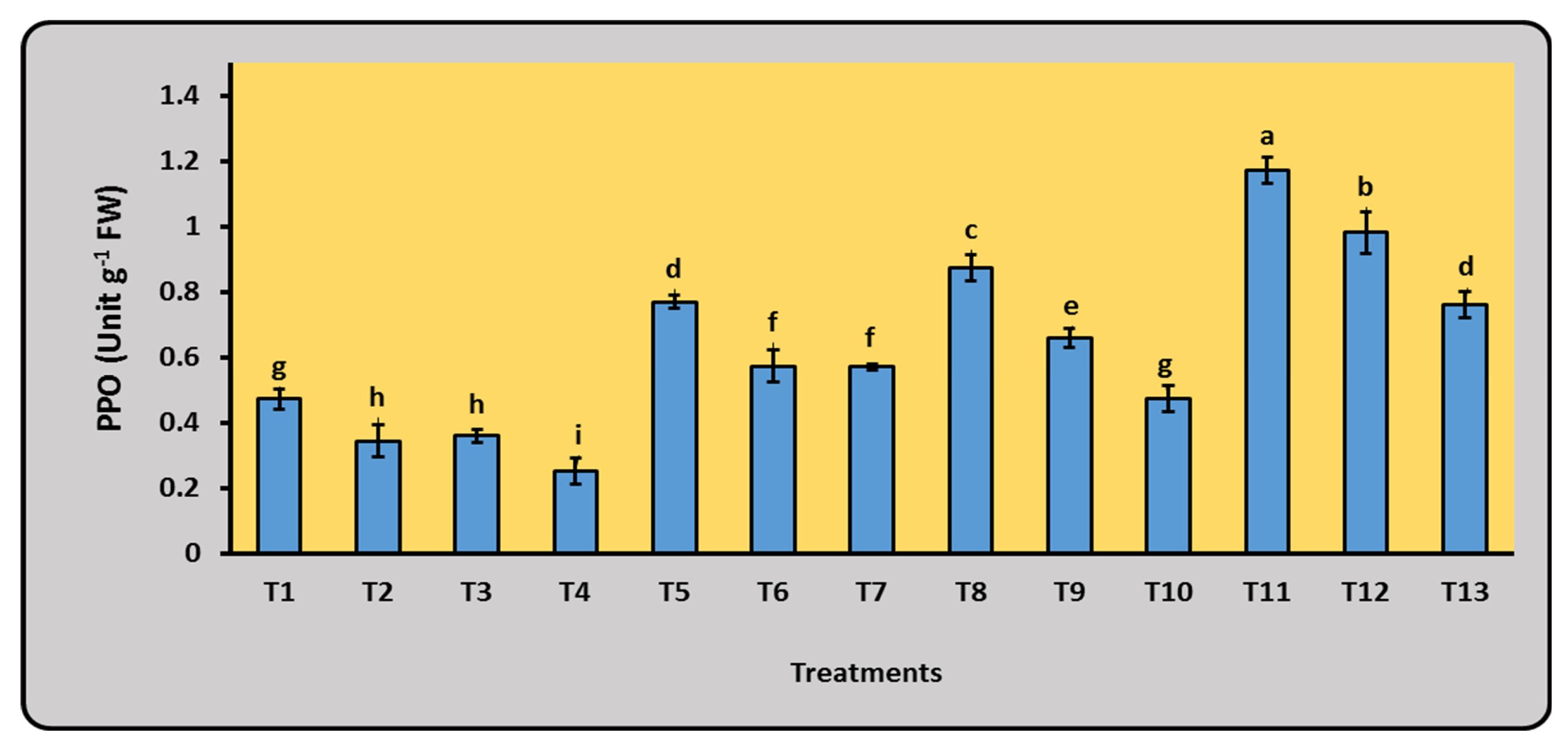
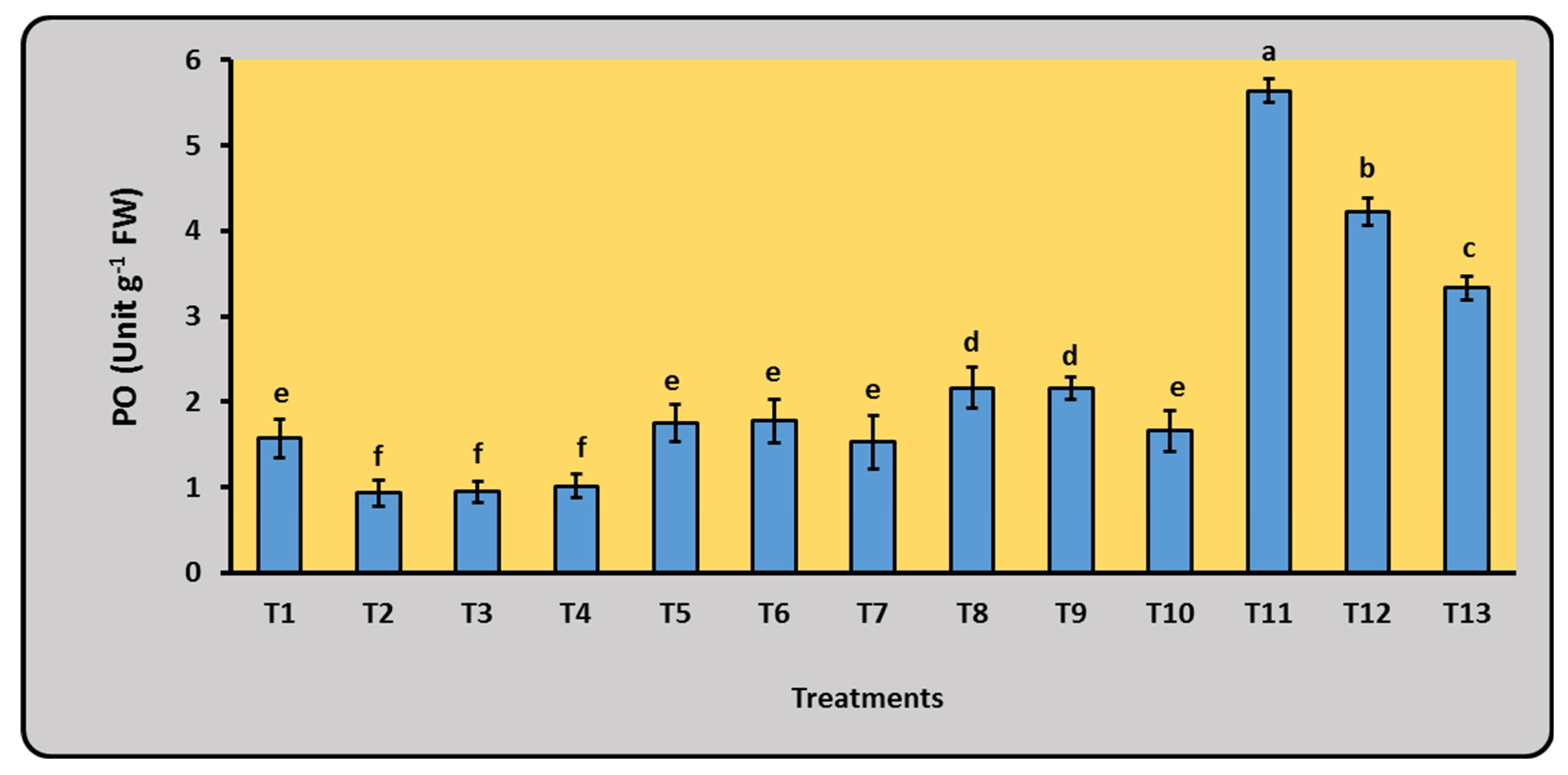
2.5.3. Enzymes Activities
2.5.4. Yield Parameters
3. Discussion
4. Materials and Methods
4.1. Soil Sampling
4.2. Isolation of N2-Fixing Bacteria
4.3. Optimization of the N2-Fixing Isolates
4.3.1. Total Nitrogen Content
4.3.2. Nitrogenase Activity
4.3.3. Production of IAA
4.3.4. Detection of NH3
4.4. Germination Test
4.5. Identification of the N2-Fixing Isolates
4.5.1. Cultural and Biochemical Tests
4.5.2. Molecular Test
4.6. In Vivo Trials
4.6.1. Pot Experiment
4.6.2. Field Experiment
Plant Growth and Yield Parameters
Biochemical Measurements
- Chlorophyll
- Proline
- Total nitrogen and protein
- Enzymes activities
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Revilla, P.; Alves, M.L.; Andelković, V.; Balconi, C.; Dinis, I.; Mendes-Moreira, P.; Redaelli, R.; de Galarreta, J.I.R.; Patto, M.C.V.; Žilić, S.; et al. Traditional Foods from Maize (Zea mays L.) in Europe. Front. Nutr. 2022, 8, 683399. [Google Scholar] [CrossRef] [PubMed]
- FAOStat. Food and Agriculture Organization of the United Nations, Food and Agriculture Statistics; FAOStat: Rome, Italy, 2021.
- Wen, A.; Havens, K.L.; Bloch, S.E.; Shah, N.; Higgins, D.A.; Davis-Richardson, A.G.; Sharon, J.; Rezaei, F.; Mohiti-Asli, M.; Johnson, A.; et al. Enabling Biological Nitrogen Fixation for Cereal Crops in Fertilized Fields. ACS Synth. Biol. 2021, 10, 3264–3277. [Google Scholar] [CrossRef] [PubMed]
- Arora, N.K. Agricultural sustainability and food security. Environ. Sustain. 2018, 1, 217–219. [Google Scholar] [CrossRef]
- Meena, S.S.; Mankoti, M.; Rout, P.R.; Mohanty, A. Plant-microbe interactions and its effect on crop productivity. In Advances in Agricultural and Industrial Microbiology: Applications of Microbes for Sustainable Agriculture and In-Silico Strategies; Springer Nature Singapore: Singapore, 2022; Volume 2, pp. 29–60. [Google Scholar]
- Tabacchioni, S.; Passato, S.; Ambrosino, P.; Huang, L.; Caldara, M.; Cantale, C.; Hett, J.; Del Fiore, A.; Fiore, A.; Schlüter, A.; et al. Identification of Beneficial Microbial Consortia and Bioactive Compounds with Potential as Plant Biostimulants for a Sustainable Agriculture. Microorganisms 2021, 9, 426. [Google Scholar] [CrossRef] [PubMed]
- de Andrade, L.A.; Santos, C.H.B.; Frezarin, E.T.; Sales, L.R.; Rigobelo, E.C. Plant Growth-Promoting Rhizobacteria for Sustainable Agricultural Production. Microorganisms 2023, 11, 1088. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Diksha; Sindhu, S.S.; Kumar, R. Biofertilizers: An ecofriendly technology for nutrient recycling and environmental sustainability. Curr. Res. Microb. Sci. 2022, 3, 100094. [Google Scholar] [CrossRef] [PubMed]
- Xavier, G.R.; da Conceição Jesus, E.; Dias, A.; Coelho, M.R.R.; Molina, Y.C.; Rumjanek, N.G. Contribution of Biofertilizers to Pulse Crops: From Single-Strain Inoculants to New Technologies Based on Microbiomes Strategies. Plants 2023, 12, 954. [Google Scholar] [CrossRef] [PubMed]
- Jacoby, R.; Peukert, M.; Succurro, A.; Koprivova, A.; Kopriva, S. The Role of Soil Microorganisms in Plant Mineral Nutrition—Current Knowledge and Future Directions. Front. Plant Sci. 2017, 8, 1617. [Google Scholar] [CrossRef]
- Asoegwu, C.R.; Awuchi, C.G.; Nelson, K.C.T.; Orji, C.G.; Nwosu, O.U.; Egbufor, U.C.; Awuchi, C.G. A review on the role of biofertilizers in reducing soil pollution and increasing soil nutrients. Himal. J. Agric. 2020, 1, 34–38. [Google Scholar]
- Etesami, H.; Adl, S.M. Plant Growth-Promoting Rhizobacteria (PGPR) and Their Action Mechanisms in Availability of Nutrients to Plants. In Phyto-Microbiome in Stress Regulation; Springer: Singapore, 2020; pp. 147–203. [Google Scholar]
- Guo, K.; Yang, J.; Yu, N.; Luo, L.; Wang, E. Biological nitrogen fixation in cereal crops: Progress, strategies, and perspectives. Plant Commun. 2023, 4, 100499. [Google Scholar] [CrossRef]
- Yassen, A.A.; Abou Seeda, M.A.; Abou El-Nour, E.A.A.; Sahar, M.; Zaghloul, A.S.S.; Abo Sedera, S.A. Response of maize Plant to bio, chemical fertilizers and their combination on growth, yield and some nutrient contents. Middle East J. Agric. Res. 2019, 8, 561–568. [Google Scholar]
- El-Nahrawy, S.; Omara, A.E.-D. Effectiveness of Co-inoculation with Pseudomonas koreensis and Rhizobia on Growth, Nodulation and Yield of Common Bean (Phaseolus vulgaris L.). Microbiol. Res. J. Int. 2017, 21, 1–16. [Google Scholar] [CrossRef]
- Hafez, E.; Omara, A.E.D.; Ahmed, A. The Coupling Effects of Plant Growth Promoting Rhizobacteria and Salicylic Acid on Physiological Modifications, Yield Traits, and Productivity of Wheat under Water Deficient Conditions. Agronomy 2019, 9, 524. [Google Scholar] [CrossRef]
- Amer, M.M.; Elbagory, M.; El-Nahrawy, S.; Omara, A.E.D. Impact of Gypsum and Bio-Priming of Maize Grains on Soil Properties, Physiological Attributes and Yield under Saline–Sodic Soil Conditions. Agronomy 2022, 12, 2550. [Google Scholar] [CrossRef]
- Rashid, M.I.; Mujawar, L.H.; Shahzad, T.; Almeelbi, T.; Ismail, I.M.; Oves, M. Bacteria and fungi can contribute to nutrients bioavailability and aggregate formation in degraded soils. Microbiol. Res. 2016, 183, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Sant’Anna, F.H.; Almeida, L.G.; Cecagno, R.; Reolon, L.A.; Siqueira, F.M.; Machado, M.R.; Vasconcelos, A.T.; Schrank, I.S. Genomic insights into the versatility of the plant growth-promoting bacterium Azospirillum amazonense. BMC Genom. 2011, 12, 409. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, K.H.; Al-Barakah, F.N.; Assafed, A.M.; Dar, B.A.M. Isolation and identification of Azospirillum and Azotobacter species from Acacia spp. at Riyadh, Saudi Arabia. Bangladesh J. Bot. 2019, 48, 239–251. [Google Scholar] [CrossRef]
- Yogaraj, M.R.; Thamizh, R.V.; Kumutha, K.; Veeramani, A.; Ramalingam, J. Studies on Developing Salt Tolerant Azospirillum Strains from the Coastal Saline Soils of Tamil Nadu. Int. J. Curr. Microbiol. Appl. Sci. 2021, 10, 1778–1785. [Google Scholar]
- Nawadkar, R.B.; Jadhav, D.B.; Shaikh, N.R. Isolation of Azotobacter spp. from saline soil and its applications on wheat (Tritium aestivum) plant for future use in reclamation of saline soil with wheat plant. RJLBPCS 2015, 1, 1–62. [Google Scholar]
- Gouda, S.; Kerry, R.G.; Das, G.; Paramithiotis, S.; Shin, H.-S.; Patra, J.K. Revitalization of plant growth promoting rhizobacteria for sustainable development in agriculture. Microbiol. Res. 2018, 206, 131–140. [Google Scholar] [CrossRef]
- Numan, M.; Bashir, S.; Khan, Y.; Mumtaz, R.; Shinwari, Z.K.; Khan, A.L.; Khan, A.; Al-Harrasi, A. Plant growth promoting bacteria as an alternative strategy for salt tolerance in plants: A review. Microbiol. Res. 2018, 209, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Rad, H.E.; Aref, F.; Rezaei, M. Response of rice to different salinity levels during different growth stages. Res. J. Appl. Sci. Eng. Technol. 2012, 4, 3040–3047. [Google Scholar]
- Omer, A.M.; Emara, H.M.; Zaghloul, R.A.; Abdel, M.; Dawwam, G. Potential of Azotobacter salinestris as plant growth promoting rhizobacteria under saline stress conditions. Res. J. Pharm. Biol. Chem. Sci. 2015, 7, 2572–2583. [Google Scholar]
- Xie, C.-H.; Yokota, A. Azospirillum oryzae sp. nov., a nitrogen-fixing bacterium isolated from the roots of the rice plant Oryza sativa. Int. J. Syst. Evol. Microbiol. 2005, 55, 1435–1438. [Google Scholar] [CrossRef] [PubMed]
- Abdelsattar, M.; El-Sawah, A.M.; El-Kady, S.M.; Hauka, F.I. Evaluation of Plant Growth Promoting of Salt-tolerant Rhizobacteria Isolated from Egyptian Saline Soils. J. Agric. Chem. Biotechnol. 2022, 13, 95–100. [Google Scholar] [CrossRef]
- Gao, C.; El-Sawah, A.M.; Ali, D.F.I.; Hamoud, Y.A.; Shaghaleh, H.; Sheteiwy, M.S. The Integration of Bio and Organic Fertilizers Improve Plant Growth, Grain Yield, Quality and Metabolism of Hybrid Maize (Zea mays L.). Agronomy 2020, 10, 319. [Google Scholar] [CrossRef]
- Taha, R.S.; Mahdi, A.H.; El-Rahman, H.A.A. Effect of Biofertilizers as a Partial Substitute for Mineral Fertilizers on Growth, Anatomical Structure, Mineral Elements and Yield of Wheat under Newly Reclaimed Soil Conditions. Int. J. Curr. Microbiol. Appl. Sci. 2016, 5, 458–469. [Google Scholar] [CrossRef][Green Version]
- Abdelaal, K.; AlKahtani, M.; Attia, K.; Hafez, Y.; Király, L.; Künstler, A. The Role of Plant Growth-Promoting Bacteria in Alleviating the Adverse Effects of Drought on Plants. Biology 2021, 10, 520. [Google Scholar] [CrossRef]
- Bagheri, N.; Ahmadzadeh, M.; Mariotte, P.; Jouzani, G.S. Behavior and interactions of the plant growth-promoting bacteria Azospirillum oryzae NBT506 and Bacillus velezensis UTB96 in a co-culture system. World J. Microbiol. Biotechnol. 2022, 38, 101. [Google Scholar] [CrossRef]
- Kordrostami, M.; Rabiei, B.; Kumleh, H.H. Biochemical, physiological and molecular evaluation of rice cultivars differing in salt tolerance at the seedling stage. Physiol. Mol. Biol. Plants 2017, 23, 529–544. [Google Scholar] [CrossRef]
- Fasciglione, G.; Casanovas, E.M.; Quillehauquy, V.; Yommi, A.K.; Goñi, M.G.; Roura, S.I.; Barassi, C.A. Azospirillum inoculation effects on growth, product quality and storage life of lettuce plants grown under salt stress. Sci. Hortic. 2015, 195, 154–162. [Google Scholar] [CrossRef]
- Shalaby, M.E.; Ghoniem, K.E.; Morsy, S.M.; El-Diehi, M.A. Molecular aspects, biochemical and pathogenic induction of defense responses in onion plants infected with Sclerotium cepivorum. Fresenius Environ. Bull. 2020, 29, 11197–11208. [Google Scholar]
- Sreedevi, B.M.; Charitha, D.; Saigopal, D.V. Induction of defense enzymes in Trichoderma harzianum treated groundnut plants against Macrophomina phaseolina. J. Biol. Control 2011, 25, 33–39. [Google Scholar]
- Seleim, M.A.; Abo-Elyousr, K.A.M.; Mohamed, A.A.A.; Al-Marzoky, H.A. Peroxidase and polyphenol oxidase activities as biochemical markers for biocontrol efficacy in the control of tomato bacterial wilt. J. Plant Physiol. Pathol. 2014, 2, 1–4. [Google Scholar] [CrossRef]
- Latef, A.A.H.A.; Abu Alhmad, M.F.; Kordrostami, M.; Abo–Baker, A.-B.A.-E.; Zakir, A. Inoculation with Azospirillum lipoferum or Azotobacter chroococcum Reinforces Maize Growth by Improving Physiological Activities Under Saline Conditions. J. Plant Growth Regul. 2020, 39, 1293–1306. [Google Scholar] [CrossRef]
- Enebe, M.C.; Babalola, O.O. The influence of plant growth-promoting rhizobacteria in plant tolerance to abiotic stress: A survival strategy. Appl. Microbiol. Biotechnol. 2018, 102, 7821–7835. [Google Scholar] [CrossRef] [PubMed]
- Tarang, E.; Ramroudi, M.; Galavi, M.; Dahmardeh, M.; Mohajeri, F. Evaluation grain yield and quality of corn (Maxima Cv) in responses to Nitroxin bioferilizer and chemical fertilizers. Int. J. Agric. Crop Sci. 2013, 5, 683. [Google Scholar]
- Yazdani, M.; Bahmanyar, M.A.; Pirdashti, H.; Esmaili, M.A. Effect of phosphate solubilization microorganisms (PSM) and plant growth promoting rhizobacteria (PGPR) on yield and yield components of corn (Zea mays L.). World Acad. Sci. Eng. Technol. 2009, 49, 90–92. [Google Scholar]
- Jackson, M.L. Soil Chemical Analysis: Advanced Course; UW-Madison Libraries Parallel Press: Madison, WI, USA, 2005; Volume 13, pp. 595–649. [Google Scholar]
- El-Badry, M.A.; Elbarbary, T.A.; Ibrahim, I.A.; Abdel-Fatah, Y.M. Azotobacter vinelandii evaluation and optimization of Abu Tartur Egyptian phosphate ore dissolution. Saudi J. Pathol. Microbiol. 2016, 1, 80–93. [Google Scholar]
- Atlas, R.M. Handbook of Microbiological Media, 4th ed.; CRC Press: Washington, DC, USA, 2010. [Google Scholar]
- Nandish, M.S.; Shilpa, H.C.; Suchitha, Y. Development and Evaluation of Nitrogen Fixing and Phosphate Solubilizing Microbial Consortia on Spinach (Spinacia oleracea). Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 239–248. [Google Scholar]
- Hardy, R.; Burns, R.; Holsten, R. Applications of the acetylene-ethylene assay for measurement of nitrogen fixation. Soil Biol. Biochem. 1973, 5, 47–81. [Google Scholar] [CrossRef]
- Zaki, R.M.; Mehesen, A.A.M.; Ashour, E.H.; Afify, A.H. Characterization of Soil-Indigenous Cyanobacterial Strains and Bioactivity Assessment. J. Agric. Chem. Biotechnol. 2021, 12, 195–199. [Google Scholar] [CrossRef]
- Ahmad, F.; Ahmad, I.; Khan, M.S. Indole acetic acid production by the indigenous isolates of Azotobacter and fluorescent Pseudomonas in the presence and absence of tryptophan. Turk. J. Biol. 2005, 29, 29–34. [Google Scholar]
- Gang, S.; Sharma, S.; Saraf, M.; Buck, M.; Schumacher, J. Analysis of indole-3-acetic acid (IAA) production in Klebsiella by LC-MS/MS and the Salkowski method. Bio-protocol 2019, 9, e3230. [Google Scholar] [CrossRef] [PubMed]
- Avan Ginkel, J.H. Determination of total nitrogen in plant material with Nessler’s reagent by continuous-flow analysis. Analyst 1980, 105, 1199–1203. [Google Scholar] [CrossRef]
- Hylemon, P.B.; Wells, J.S., Jr.; Krieg, N.R.; Jannasch, H.W. The genus Spirillum: A taxonomic study. Int. J. Syst. Bacteriol. 1973, 23, 340–380. [Google Scholar] [CrossRef]
- Weisburg, W.G.; Barns, S.M.; Pelletier, D.A.; Lane, D.J. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 1991, 173, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- Shalaby, M.E.; El-Moghazy, S.M.; Abdelrasoul, E.A.; Mehesen, A.A. Effect of some plant-growth promoters in controlling late wilt disease and enhancing nutritive value of maize plants. Egypt. J. Appl. Sci. 2011, 26, 369–385. [Google Scholar]
- El-Khateeb, N.M.M.; Metwaly, M.S.M. Influence of some bio-fertilizers on wheat plants grown under graded levels of nitrogen fertilization. Int. J. Environ. 2019, 8, 43–56. [Google Scholar]
- Moran, R. Formulae for determination of chlorophyllous pigments extracted with N,N-dimethylformamide. Plant Physiol. 1982, 69, 1376–1381. [Google Scholar] [CrossRef] [PubMed]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Azam, A.K.M.S.; Mansur, M.A.; Asadujjaman, M.; Rahman, M.; Sarwer, M.G. Quality and safety aspects of fresh and frozen prawn (Macrobrachium rosenbergii), Bangladesh. Am. J. Food Sci. Technol. 2013, 1, 77–81. [Google Scholar]
- Lagrimini, L.M.; Gingas, V.; Finger, F.; Rothstein, S.; Liu, T. Characterization of Antisense Transformed Plants Deficient in the Tobacco Anionic Peroxidase. Plant Physiol. 1997, 114, 1187–1196. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, D.P.; Bateman, D.F. Changes in the activities of some oxidases in extracts of Rhizoctonia-infected bean hypocotyls in relation to lesion maturation. Phytopathology 1967, 57, 132–136. [Google Scholar]
- Pütter, J. Peroxidases. In Methods of Enzymatic Analysis; Academic Press: New York, NY, USA, 1974; pp. 685–690. [Google Scholar]
- Duncan, D.B. Multiple Range and Multiple F Tests. Biometrics 1955, 11, 1–42. [Google Scholar] [CrossRef]





| Isolate Code | Total Nitrogen Content (%) | Nitrogenase Activity (Nano-Mole mL−1 h−1) | IAA Production (µg mL−1) | NH3 Detection (+/-) |
|---|---|---|---|---|
| OT-H1 | 14.01 ± 1.23 a | 517 ± 22.19 a | 539 ± 32.89 a | + |
| OT-S1 | 7.70 ± 1.09 d | 320 ± 17.93 e | 361 ± 26.19 e | + |
| OT-D2 | 6.30 ± 0.97 f | 401 ± 24.28 c | 394 ± 25.10 c | + |
| OT-M2 | 7.05 ± 1.11 e | 305 ± 19.20 f | 357 ± 27.02 e | - |
| OT-B1 | 10.50 ±1.09 c | 378 ± 20.11 d | 303 ± 22.76 f | - |
| OS-H2 | 4.90 ± 0.92 g | 242 ± 15.17 g | 263 ± 23.35 h | - |
| OS-Q3 | 3.50 ± 0.69 i | 173 ± 11.30 i | 304 ± 23.11 f | + |
| OS-D1 | 4.20 ± 0.93 h | 200 ± 17.90 h | 285 ± 20.87 g | - |
| OS-M1 | 11.20 ± 1.12 b | 412 ± 22.19 b | 450 ± 28.29 b | + |
| OS-S6 | 3.09 ±0.91 j | 201 ± 19.97 h | 379 ± 26.95 d | - |
| Characteristics | OT-H1 Isolate | |
|---|---|---|
| Colony | Watery and mucilaginous | |
| Pigmentation transparent | Brown | |
| Cell shape | Short rods to oval | |
| Motility | + | |
| Endospore formation | - | |
| Gram reaction | - | |
| Catalase induction | + | |
| Phosphate solubilization | + | |
| Utilization of carbon sources | Glucose | + |
| Fructose | + | |
| Mannitol | + | |
| pH | 6.5 | + |
| 7.5 | + | |
| 8.5 | + | |
| 9.5 | + | |
| Temperature °C | 25 | + |
| 30 | + | |
| 35 | + | |
| 40 | + | |
| NaCl % | 2.0 | + |
| 3.0 | + | |
| 4.0 | + | |
| Treatment | Dry Matter of Shoot (%) | Dry Matter of Leaf (%) | Flag Leaf Area (cm2) | Chlorophyll (µg/cm2) | Total –N of Leaf (%) | |
|---|---|---|---|---|---|---|
| a | b | |||||
| T1 | 93.89 ± 6.12 ab | 55.12 ± 4.08 e | 121.86 ± 3.65 c | 20.21 ± 1.07 f | 5.57 ± 1.11 i | 0.84 ± 0.22 cd |
| T2 | 81.44 ± 5.22 def | 49.16 ± 3.62 f | 68.31 ± 3.41 g | 14.95 ± 1.09 h | 8.5 ± 1.23 f | 0.63 ± 0.19 e |
| T3 | 84.48 ± 4.92 cde | 43.20 ± 3.11 g | 43.39 ± 3.87 i | 12.84 ± 0.95 i | 6.39 ± 0.93 h | 0.42 ± 0.09 f |
| T4 | 76.18 ± 3.88 f | 40.94 ± 3.01 g | 15.61 ± 1.97 k | 10.77 ± 0.89 j | 3.53 ± 1.01 k | 0.28 ± 0.11 g |
| T5 | 91.06 ± 5.72 abc | 83.63 ± 4.12 b | 85.23 ± 4.12 f | 33.62 ± 1.02 c | 6.77 ± 1.02 h | 0.89 ± 0.19 bc |
| T6 | 86.48 ± 4.19 b–e | 75.28 ± 4.85 c | 70.75 ± 3.82 h | 21.13 ± 1.01 e | 10.52 ± 1.47 d | 0.63 ± 0.18 e |
| T7 | 80.62 ± 4.28 ef | 63.01 ± 3.86 d | 18.97 ± 1.11 jk | 17.93 ± 1.01 g | 8.03 ± 1.22 g | 0.42 ± 0.22 f |
| T8 | 93.29 ± 5.38 ab | 84.70 ± 4.29 b | 84.01 ± 3.90 e | 35.19 ± 1.15 b | 5.84 ± 1.09 i | 0.98 ± 0.31 ab |
| T9 | 92.70 ± 4.92 ab | 83.32 ± 4.91 b | 56.70 ± 3.86 h | 21.78 ± 0.85 de | 11.74 ± 0.87 c | 0.77 ± 0.21 d |
| T10 | 88.77 ± 5.35 a–d | 86.26 ± 4.87 b | 21.90 ± 1.17 j | 18.01 ± 0.92 g | 9.47 ± 1.01 e | 0.56 ± 0.12 e |
| T11 | 95.61 ± 6.09 a | 92.27 ± 4.95 a | 179.70 ± 6.97 a | 38.12 ± 1.09 a | 20.62 ± 1.65 a | 1.07 ± 0.31 a |
| T12 | 95.04 ± 6.18 a | 83.62 ± 4.54 b | 147.42 ± 7.22 b | 33.13 ± 1.15 c | 13.42 ± 0.59 b | 0.84 ± 0.13 cd |
| T13 | 92.25 ± 5.86 ab | 82.36 ± 4.81 b | 107.63 ± 5.96 d | 22.60 ± 0.98 d | 7.74 ± 0.55 g | 0.63 ± 0.19 e |
| Treatment | Plant Height (cm) | Dry Matter of Leaf (%) | Flag Leaf Area (cm2) | Total –N of Leaf (%) |
|---|---|---|---|---|
| T1 | 120.70 ± 5.66 d | 53.47 ± 2.34 f | 462.21 ± 23.11 g | 1.12 ± 0.11 cd |
| T2 | 107.70 ± 5.18 f | 49.44 ± 2.87 g | 351.39 ± 26.19 j | 0.91 ± 0.19 e |
| T3 | 97.70 ± 5.09 h | 45.64 ± 2.51 h | 301.86 ± 31.09 k | 0.70 ± 0.15 f |
| T4 | 83.70 ± 4.87 k | 44.39 ± 1.97 h | 264.00 ± 34.29 l | 0.56 ± 0.13 g |
| T5 | 123.70 ± 4.98 c | 75.10 ± 2.39 bc | 480.96 ± 35.98 f | 1.16 ± 0.23 bc |
| T6 | 100.70 ± 4.93 g | 68.23 ± 2.65 d | 406.80 ± 39.10 i | 0.91 ± 0.27 e |
| T7 | 86.70 ± 4.57 j | 58.97 ± 2.34 e | 353.73 ± 41.90 j | 0.72 ± 0.12 f |
| T8 | 126.70 ± 6.09 b | 76.25 ± 2.86 bc | 573.87 ± 48.12 d | 1.26 ± 0.31 ab |
| T9 | 106.70 ± 5.61 f | 74.42 ± 2.89 c | 495.60 ± 43.10 e | 1.05 ± 0.33 d |
| T10 | 89.70 ± 5.18 i | 75.58 ± 2.61 bc | 436.77 ± 38.98 h | 0.84 ± 0.26 e |
| T11 | 146.70 ± 6.09 a | 85.69 ± 2.75 a | 909.72 ± 66.76 a | 1.35 ± 0.34 a |
| T12 | 126.70 ± 5.72 b | 77.78 ± 2.78 b | 746.40 ± 45.19 b | 1.12 ± 0.38 cd |
| T13 | 115.70 ± 5.90 e | 76.20 ± 2.81 bc | 677.82 ± 47.71 c | 0.91 ± 0.27 e |
| Treatment | Yield Parameters | |||
|---|---|---|---|---|
| Grain Count (cob−1) | 1000 Kernel (g plot−1) | N Content | Crude Protein | |
| (% g−1 Dry Weight) | ||||
| T1 | 563.66 ± 41.19 b | 604.33 ± 44.10 c | 5.34 ± 1.02 i | 5.61 ± 1.01 d |
| T2 | 402.00 ± 38.90 g | 473.00 ± 41.98 j | 3.29 ± 1.17 k | 2.70 ± 0.59 g |
| T3 | 337. 30 ± 36.11 j | 450.00 ± 40.12 k | 2.82 ± 1.29 l | 2.20 ± 0.29 h |
| T4 | 283.00 ± 28.98 m | 294.03 ± 29.90 m | 1.61 ± 1.02 m | 0.82 ± 0.19 i |
| T5 | 475.33 ± 39.10 e | 594.00 ± 43.19 e | 6.80 ± 1.04 d | 7.03 ± 1.09 c |
| T6 | 357.00 ± 35.39 h | 532.00 ± 44.67 g | 6.06 ± 1.28 g | 5.61 ± 0.99 d |
| T7 | 312.00 ± 33.34 k | 486.00 ± 41.69 h | 4.03 ± 0.95 j | 3.40 ± 0.81 f |
| T8 | 466.00 ± 40.19 f | 582.00 ± 47.89 f | 6.96 ± 1.02 c | 7.05 ± 1.11 c |
| T9 | 346.66 ± 32.45 i | 422.00 ± 39.20 l | 6.52 ± 1.10 f | 4.79 ± 1.01 e |
| T10 | 296.66 ± 27.45 l | 480.00 ± 38.98 i | 5.73 ± 0.89 h | 4.78 ± 0.92 e |
| T11 | 694.00 ± 45.23 a | 754.33 ± 49.87 a | 7.73 ± 1.09 a | 10.14 ± 1.87 a |
| T12 | 522.00 ± 41.98 c | 694.00 ± 50.10 b | 7.15 ± 1.18 b | 8.63 ± 1.29 b |
| T13 | 485.00 ± 41.45 d | 601.00 ± 49.23 d | 6.68 ± 1.21 e | 6.98 ± 1.05 c |
| No | Isolates Code | Location | pH | EC (dS m−1) |
|---|---|---|---|---|
| 1 | OT-H1 | Elhamoul (31°20′58″ N latitude and 31°09′01″ E longitude) | 9.00 ± 1.22 | 2.70 ± 0.25 |
| 2 | OT-S1 | Sakha (31°04′26″ N latitude and 30°54′52″ E longitude) | 8.67 ± 1.02 | 0.26 ± 0.12 |
| 3 | OT-D2 | Disouq (31°09′12″ N latitude and 30°39′45″ E longitude) | 8.75 ± 1.23 | 0.83 ± 0.19 |
| 4 | OT-M2 | Motobas (31°16′44″ N latitude and 30°30′58″ E longitude) | 8.10 ± 1.17 | 0.46 ± 0.11 |
| 5 | OT-B1 | Baltim (31°30′59″ N latitude and 31°06′07″ E longitude) | 9.11 ± 1.31 | 1.03 ± 0.29 |
| 6 | OS-H2 | Elhamoul (31°20′58″ N latitude and 31°09′01″ E longitude) | 9.00 ± 1.29 | 2.70 ± 0.25 |
| 7 | OS-Q3 | Qulin (31°04′34″ N latitude and 30°51′47″ E longitude) | 8.16 ± 1.11 | 1.74 ± 0.31 |
| 8 | OS-D1 | Disouq (31°09′12″ N latitude and 30°39′45″ E longitude) | 8.75 ± 1.09 | 0.83 ± 0.18 |
| 9 | OS-M1 | Motobas (31°16′44″ N latitude and 30°30′58″ E longitude) | 8.10 ± 1.02 | 0.46 ± 0.14 |
| 10 | OS-S6 | Sidi Salim (31°07′52″ N latitude and 30°57′29″ E longitude) | 8.59 ± 1.12 | 0.70 ± 0.19 |
| Mechanical Analysis (%) | Texture | pH (1:2.5) | EC (dSm−1) | OM (g Kg−1) | Available Elements (mg Kg−1) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sand | Silt | Clay | N | P | K | |||||
| Pots | 7.4 | 7.5 | 85.1 | Clayey | 8.76 | 3.80 | 15.18 | 8.44 | 8.08 | 316.55 |
| Field | 3.1 | 7.7 | 89.2 | Clayey | 9.10 | 4.18 | 17.87 | 8.98 | 9.86 | 401.83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shalaby, M.; Elbagory, M.; EL-Khateeb, N.; Mehesen, A.; EL-Sheshtawy, O.; Elsakhawy, T.; Omara, A.E.-D. Potential Impacts of Certain N2-Fixing Bacterial Strains and Mineral N Doses for Enhancing the Growth and Productivity of Maize Plants. Plants 2023, 12, 3830. https://doi.org/10.3390/plants12223830
Shalaby M, Elbagory M, EL-Khateeb N, Mehesen A, EL-Sheshtawy O, Elsakhawy T, Omara AE-D. Potential Impacts of Certain N2-Fixing Bacterial Strains and Mineral N Doses for Enhancing the Growth and Productivity of Maize Plants. Plants. 2023; 12(22):3830. https://doi.org/10.3390/plants12223830
Chicago/Turabian StyleShalaby, Moustafa, Mohssen Elbagory, Nagwa EL-Khateeb, Ahlam Mehesen, Omaima EL-Sheshtawy, Tamer Elsakhawy, and Alaa El-Dein Omara. 2023. "Potential Impacts of Certain N2-Fixing Bacterial Strains and Mineral N Doses for Enhancing the Growth and Productivity of Maize Plants" Plants 12, no. 22: 3830. https://doi.org/10.3390/plants12223830
APA StyleShalaby, M., Elbagory, M., EL-Khateeb, N., Mehesen, A., EL-Sheshtawy, O., Elsakhawy, T., & Omara, A. E.-D. (2023). Potential Impacts of Certain N2-Fixing Bacterial Strains and Mineral N Doses for Enhancing the Growth and Productivity of Maize Plants. Plants, 12(22), 3830. https://doi.org/10.3390/plants12223830









