Abstract
The search for sustainable agriculture has increased interest in using endophytic bacteria to reduce fertilizer use and increase stress resilience. Stress-adapted plants are a potential source of these bacteria. Some species of these plants have not yet been evaluated for this, such as pangolão grass, from which we considered endophytic bacteria as potential plant growth promoters. Bacteria from the root, colm, leaves, and rhizospheric soil were isolated, and 132 strains were evaluated for their in vitro biological nitrogen fixation, IAA and siderophores production, and phosphate solubilization. Each mechanism was also assessed under low N availability, water stress, and low-solubility Fe and P sources in maize greenhouse experiments. All strains synthesized IAA; 63 grew on N-free media, 114 synthesized siderophores, and 46 solubilized P, while 19 presented all four mechanisms. Overall, these strains had better performance than commercial inoculant in all experiments. Still, in vitro responses were not good predictors of in vivo effects, which indicates that the former should not be used for strain selection, since this could lead to not testing strains with good plant growth promotion potential. Their heterologous growth promotion in maize reinforces the potential of stress-adapted plant species as potential sources of strains for inoculants.
1. Introduction
Population growth demands increased food production, while current and forecasted environmental conditions require a significant decrease in agriculture’s ecological footprint. This has led to the increasing use of plant-growth-promoting bacteria (PGPB), since these can reduce fertilizer use and increase crops’ resilience to environmental stresses [1]. These are usually endophytic or rhizospheric bacteria that do not provoke any harm to the plant and may, under some conditions, promote their growth or reduce the effects of environmental stresses [2].
Endophytic bacteria may increase plant growth and adaptation to environmental stress [3]. This plant growth promotion is variously credited to several mechanisms, such as biological nitrogen fixation, synthesis and release of phytohormones such as IAA, phytopathogen control through synthesis and release of siderophores, or phosphate solubilization, several of which are frequently evaluated under in vitro conditions [4,5,6].
These mechanisms are widely distributed in several bacterial genera, such as Acetobacter, Aerobacter, Aeromonas, Agrobacterium, Azospirillum, Bacillus, Burkholderia, Chryseomonas, Curtobacterium, Enterobacter, Erwinia, Flavimonas, Gluconacetobacter, Herbaspirillum, Klebsiella, Pseudomonas, Rhizobium, and Sphingomonas, which have been isolated as endophytic from a broad spectrum of plant species, such as maize, wheat, rice, sugarcane, sorghum, and signal grass [7,8,9,10,11,12].
On the other hand, several plant species well adapted to stressful environments, such as pangolão grass (Digitaria eriantha cv. Survenola), have not been well studied until now, even though we have found it to harbor a very diverse bacterial endophyte population [13], since the literature contains several examples of plants from these environments being effective sources of plant-growth-promoting bacteria [14,15,16]. At the same time, it is also known that bacteria isolated from one species may be highly effective on another; for example, as happens with the commercial inoculants based on Azospirillum brasilense in Brazil [17]. Other example is the use of bacteria isolated from Stipa tenacissima L. and used to reduce salt stress on tomato [16], from Opuntia ficus-indica increasing wheat growth under drought [18], from garlic on common beans growth [19], or from Suaeda nudiflora on Amaranthus viridis under salt stress [3].
We aim, thus, to evaluate this diversity as a source of plant-growth-promoter bacteria both in vitro and for maize plants.
2. Results
2.1. Plant-Growth-Promoting Characteristics of Endophytic Isolates
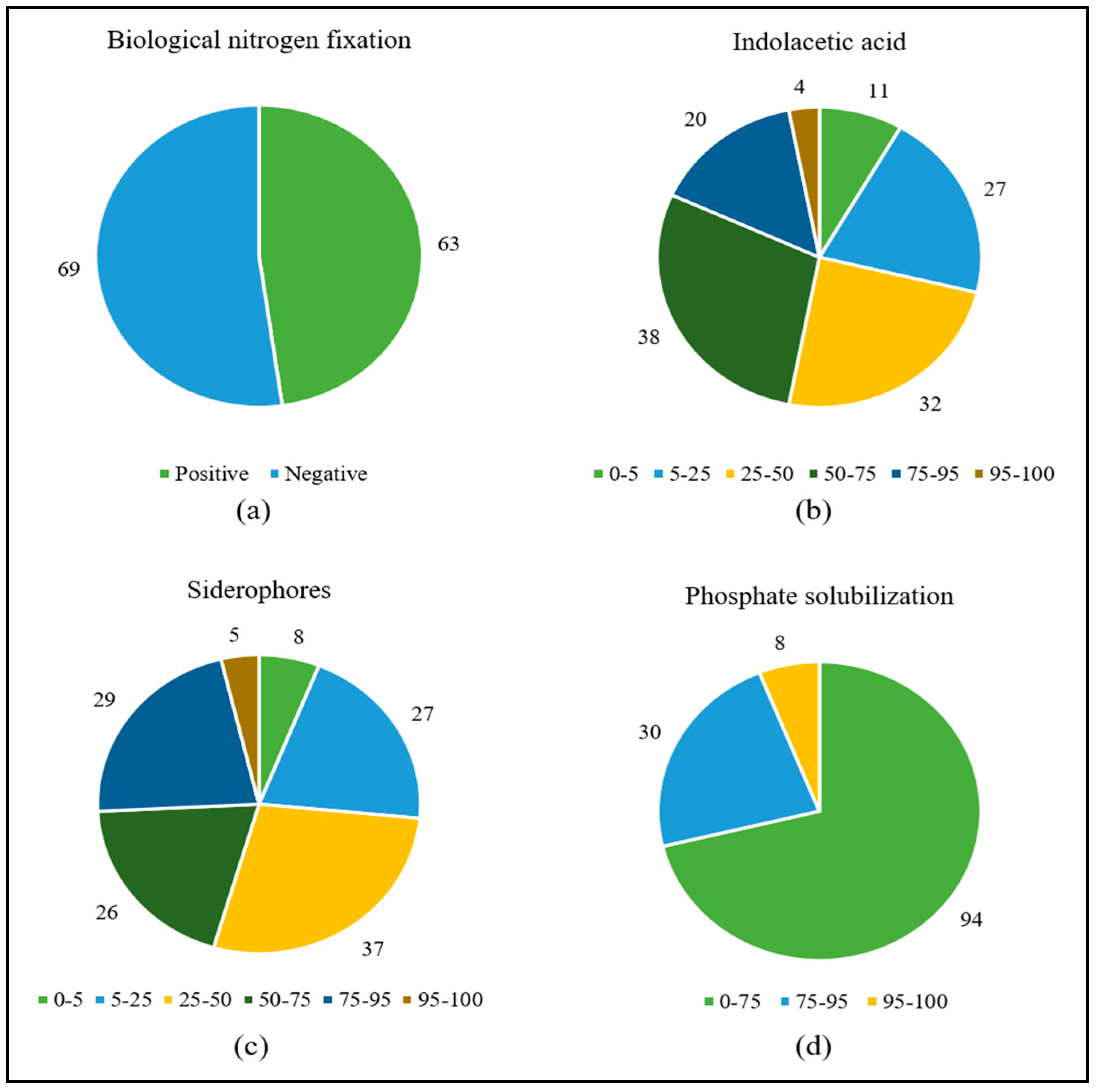
The most frequent in vitro growth-promoting mechanism was IAA production, which occurred in all strains ranging from ca. 4 to ca. 212 μg mL−1, followed by siderophores (86%, ranging from 1 to 83%), BFN (48%), and phosphate solubilization (35%, with SI from 1 to 3) (Figure 1) (Table S1).
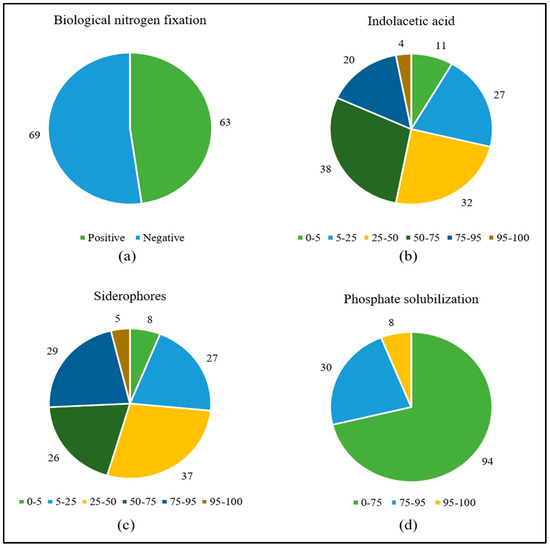
Figure 1.
Number of pangolão grass endophytic bacteria positive for growth in N-free media (a) or presenting given percentiles of IAA synthesis (b), siderophore production (c), and calcium phosphate solubilization (d).
2.2. Binding of In Vitro Growth-Promoting Mechanisms
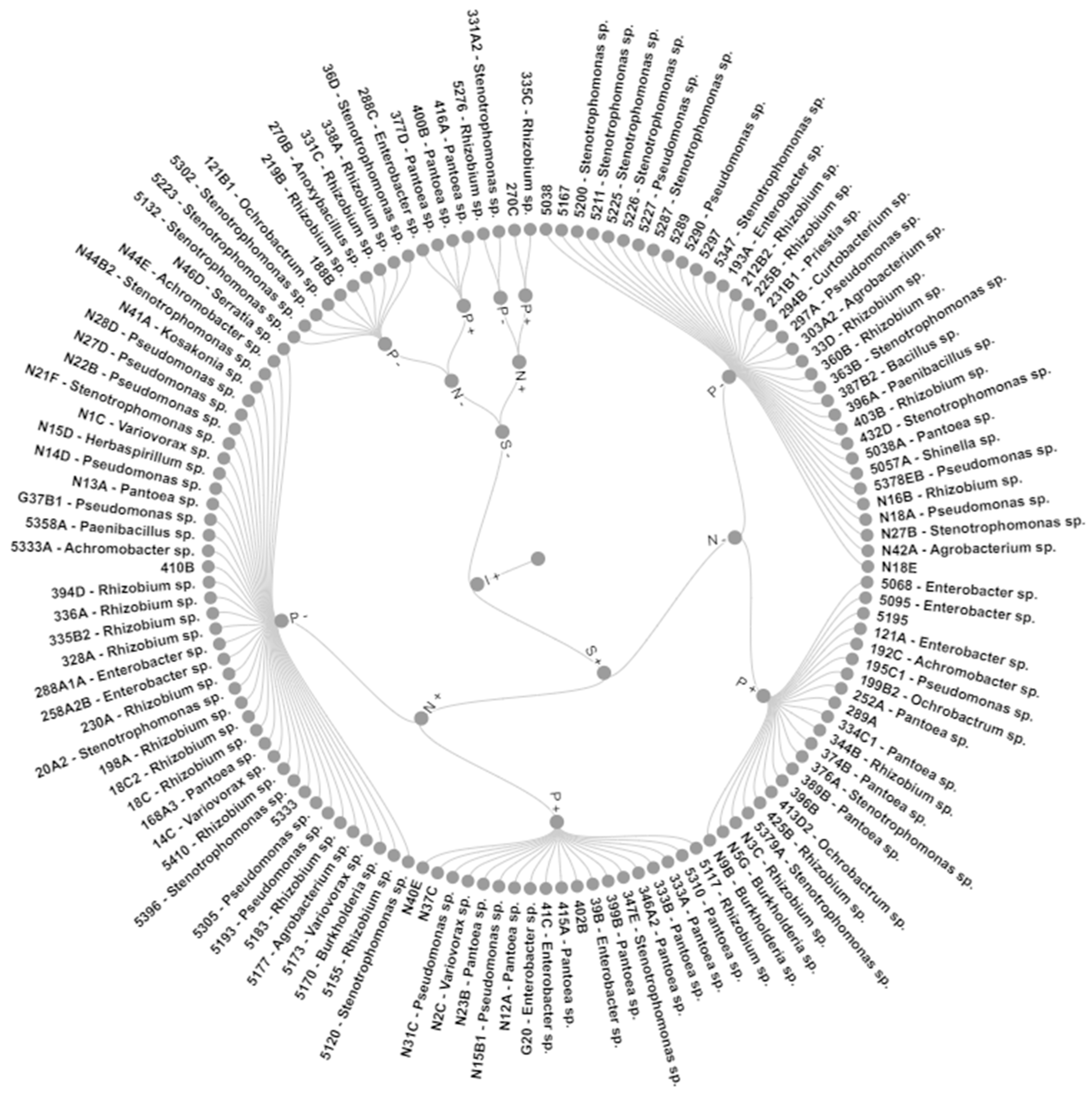
Grouping these characteristics led to eight groups with 100% similarity (Figure 2). Group I (GI) included two strains that grew without N and produced IAA, while GII also had two strains that, besides the above, also solubilized phosphate. GIII with four strains was like GII but did not grow in N-free media. In comparison, the 10 strains of GIV could only produce IAA, the 19 strains of GV presented all mechanisms, and the 40 strains of GVI had all but P solubilization. GVII is formed by 34 IAA- and siderophore-producing strains, and GVIII by 21 strains with all mechanisms bar growth in N-free media.
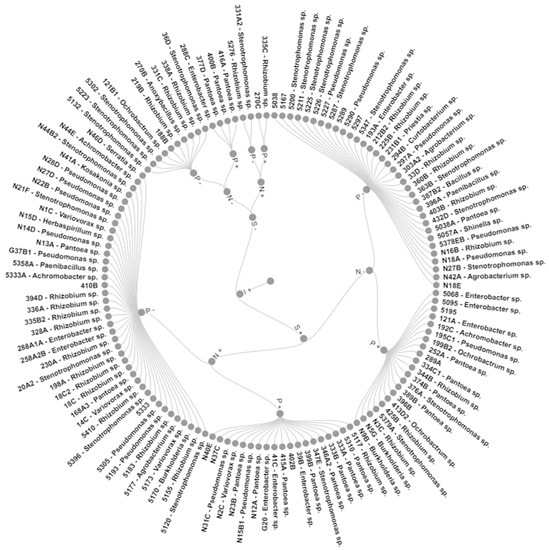
Figure 2.
Pangolão grass endophytic bacteria grouped by in vitro growth promotion mechanisms. N—growth in N-free media; I—IAA production; S—siderophore production; P—calcium phosphate solubilization; +—bacteria presents the trait; −—bacteria does not present the trait.
2.3. Efficiency of Growth-Promoting Bacteria in the Early Stage of Maize
Generally, the pangolão isolated strains yielded higher results than CI for at least two variables in the N-restricted experiment (Table 1). Although no strain led to significantly higher SDM than CI, several were significantly higher in RECI, indicating an overall higher growth promotion activity. However, all were significantly lower in RE + C than the +C treatment, indicating that the growth promotion could not fully overcome N deficiency effects.

Table 1.
Growth performance of maize inoculated with pangolão endophytic bacteria under restricted N availability.
Although no strain from Pangolão led to higher LA than CI, most other variables had at least two strains with higher results (Table 2). Most importantly, two strains had higher SDM and RECI, while several increased RDM, and four achieved higher RE + C than fully irrigated plants.

Table 2.
Growth performance of maize inoculated with pangolão endophytic bacteria under restricted water availability.
Although no strain significantly differed from CI for any of the variables on the low-Fe solubility experiment (Table S2), plants with strain 252 were significantly higher, and strain 5227 had a higher SDM. While no strain achieved higher SDM, SAN, or relative efficiencies than CI, several led to significantly higher PH, CD, LA, RDM, and SAP (Table 3). Some had non-significant higher RE + C than +C plants.

Table 3.
Growth performance of maize inoculated with pangolão endophytic bacteria with low solubility P.
2.4. Binding of In Vitro and In Vivo Growth-Promoting Mechanisms
Most in vitro plant-growth-promoting characteristics were not significantly correlated to plant growth effect in maize (Table 4), and even those which were significant, such as SI and PH for the siderophore experiment (r = 0.25, p < 0.01), or between SI and SDM in the P solubilization experiment (r = 0.30, p < 0.01), had relatively low predictive values.

Table 4.
Pearson’s correlation between in vitro growth promoting characteristics of the strains and results obtained in the BNF, IAA, siderophore, and phosphate solubilization experiments.
As a group, strains that did not grow in N-free media had lower leaf area in the N-deficient experiment and increased RDM in the low-solubility Fe one (Table 5). At the same time, those positive for this mechanism reduced SAN and SAP in the water-deficit experiment. While low or average IAA synthesis led to generally higher results in the water-deficit experiment, those with high IAA synthesis increased RDM in the low-solubility P experiment. Medium SI strains had generally higher RDM in the low-solubility P experiment, while this was found for the high SI strains in the low Fe solubility experiment. These results, coupled with the low and mostly non-significant correlations between in vitro and maize results, indicate that these in vitro methods are not good predictors of plant effects.

Table 5.
Pangolão endophytic bacteria, grouped according to their in vitro growth promotion mechanisms performance, and their effects on maize growth under different environmental stresses, reduced N availability, water deficit stress, low P solubility, and low Fe solubility.
3. Discussion
This is the first evaluation of the plant-growth-promoting potential of Pangolão grass endophytic bacteria, which is particularly interesting since endophytic bacteria from plants from stressful environments such as this might help reduce environmental stress effects on crops [5,18]. Strains previously isolated from this plant species [13] included several genera known to harbor plant-growth-promoting strains. Indeed, all 132 strains tested under in vitro conditions here had at least one commonly held plant-growth-promoting trait.
These traits occurred in different proportions when other plant species were evaluated. For example, while 48% of the strains grew in N-free media, strains from other grasses grown in the Brazilian tropical semiarid included 66% diazotrophs [20], and signal grass root and rhizosphere bacteria had 58% diazotrophs [21].
IAA production, on the other hand, seems to be much more widely spread, since 100% of the strains were positive for this trait, as also happened with strains from cold-tolerant rice [22,23], and strains from plants under saline conditions also had 86% occurrence for this trait [24]. A similarly frequent feature seems to be siderophore production since we found 86% of siderophore-producing strains and signal grass had very similar results at 84% occurrence [25].
Most Pangolão-isolated strains had at least one of the commonly evaluated in vitro growth-promoting characteristics [26], while strains with more than one of these tended to have stronger plant-growth-promoting effects under environmental stress [8]. This may indicate that these bacteria are an important part of Pangolão adaptation mechanisms to the environmental stress typical of where it was collected. This is furthered by the presence of Pantoea, Enterobacter, Rhizobium, Pseudomonas, and Stenotrophomonas strains, which are frequently described as promoting plant growth for several species [14,27,28,29,30,31,32].
The high proportion of in vitro plant growth characteristics found here agree with the frequent consideration that plants adapted to stressful environments tend to have plant-growth-promoting endophytic bacteria [8,26,33]. Although we evaluated only the initial maize growth, several strains increased some of the plant variables found in plants receiving a CI with known effective strains for this crop under low N availability [17]. This is likely due to IAA effects on root growth, and thus on water and nitrogen absorption [2].
Interestingly, the strains with higher N accumulation in the low-water-availability experiment were all identified as Rhizobium, Paenibacillus, and Agrobacterium species, all of which are known as potentially diazotrophic [34,35], which may indicate some level of unmeasured biological nitrogen fixation.
On the other hand, the small effects of both low P and Fe solubility experiments might be due to the early stage on which we harvested the maize, as also observed for wheat [32]. Still, even under those limiting conditions, some strains induced higher growth than the CI.
A common occurrence in our experiments was bacteria with low values for a given in vitro mechanism achieving higher than CI values for a plant-growth promotion in the experiment designed to evaluate that trait. This is coupled with a generally low and non-significant correlation between in vitro and maize effects and might be due to most of the strains presenting more than one of the mechanisms (Figure 2), as also proposed in the literature and in the typical indication of using a mix of strains for inoculant production [36,37,38,39,40].
4. Materials and Methods
Plant sampling, bacterial sampling, isolation, DNA extraction, BOX-PCR grouping, and sampling were all described in Alves et al. [13]. In short, endophytic and rhizospheric bacteria from Pangolão grass from three Pernambuco state municipalities were isolated, through serial dilutions in sterile saline solution, in semi-solid, N-free, NFB media [41], while in one of the locations, JNFB and JMV media were also used [42,43]. Where growth was observed in the N-free media, bacteria were transferred to YMA media for phenotypical characterization [44], followed by DNA extraction and BOX-PCR fingerprinting. Representative strains of the BOX-PCR 90% similarity groups had their 16S rRNA sequenced. They were chosen for the in vitro evaluation of biological nitrogen fixation [41], IAA production [45], calcium phosphate solubilization [46], and siderophore production [47].
Each of these mechanisms was later evaluated in a separate greenhouse experiment with maize to evaluate specific environmental stresses, such as low available nitrogen, water deficit, and low-solubility P and Fe sources, respectively.
All experiments were conducted in 5 L plastic vessels filled with sterile sand: vermiculite 1:1 mixture, using maize cultivar BR-5026, also known as São José. This cultivar is recommended for low-resource agriculture in tropical semiarid regions and was developed as a multipurpose cultivar for forage production, immature corn, or for grain production. It has dented yellow cobs, 300 cm tall plants, a 120-day cycle, and a stable yield.
Seeds were surface-disinfested with 70% ethanol for 30 s, immersed in 2.5% sodium hypochlorite for 2 min, washed eight times with sterile distilled water, and inoculated with 1 mL per seed of 109 cells.mL−1 bacterial broth of the strain under evaluation, according to standard Brazilian recommendations for the commercial inoculation of corn [17]. All experiments were conducted on a randomized block design with three replicates.
As biological nitrogen fixation is not considered to support all plant growth for non-legumes [48], all plants in this experiment were supplied with 30% of the recommended N dose at seeding. Twenty strains were evaluated; seven were positive for N-free media growth, and the remainder did not grow in this media (Table 6). The control treatments for this experiment were non-inoculated plants inoculated with the commercial Azospirillum brasilense inoculant AzzoFix® (CI). This is a liquid inoculant for corn with strains Ab-V5 and Ab-V6 and 2.0 × 108 UFC·mL−1, as defined and authorized according to Brazilian legislation [17], and 100% N dose supply (equivalent to 20 kg N·ha−1) (+C) under a low N input recommendation for the tropical semi-arid condition in Pernambuco state [49].

Table 6.
Strains selected for the maize growth-promotion experiments. BNF—reduced N availability, IAA—water deficit stress, P—low P solubility, and Siderophore—low Fe solubility. Grey cells indicate those in common to all experiments. + and - indicate presence or absence of the trait, respectively.
IAA was evaluated under water deficit stress since this mechanism is supposed to increase root growth, maintained by keeping the plants at ca. 30% pot-water-holding capacity after complete seed germination. Twenty-four strains were selected representing the 0–5, 5–25, 25–50, 50–75, 75–95, and 95–100 percentiles for in vitro IAA production (Table 6), with the same control treatments as above, except for the positive control substituted for the use of 100% pot-water holding capacity (+C).
For the P solubilization capacity, tri-calcium phosphate was used as the sole P source, and 21 bacterial strains were selected to represent the 0–75, 75–95, and 95–100 percentiles for in vitro P solubilization, respecting the proportion of non-solubilizing bacteria effectively found similarly to the BNF experiment (Table 6). Control treatments were similar to the previous experiments, with the positive control being triple superphosphate as the phosphorus source (+C).
Siderophore was evaluated by replacing the Fe source in the nutrient solution with a low-solubility one. In this case, 24 strains were selected to represent the 0–5, 5–25, 25–50, 50–75, 75–95, and 95–100 percentiles for in vitro siderophore production (Table 6), and the control treatments were similar to the previous experiments, with the positive control being a high-solubility Fe source (+C).
For all experiments, harvest was 20 days after emergence, plant height (PH), colm diameter (CD), and leaf area (LA) were measured, and root (RDM) and shoot dry matter (SDM) was determined. Traditional wet methods determined N and P contents, and shoot accumulated N (SAN) and P (SAP) were calculated by the product of shoot dry matter and the appropriate content.
We also calculated the relative efficiencies of each treatment in relation to the non-inoculated control (REN), commercial inoculant (RECI), and each positive control (RE + C). In all cases, these were calculated according to this equation.
The in vitro data were grouped at 100% similarity using the Jaccard dissimilarity index and UPGMA. Maize data were evaluated for homoscedastic and outliers. When needed, they were transformed by log10 and elimination of outliers before ANOVA and Dunnett’s test at 5% significance using the commercial inoculant as the control treatment. Later, only the inoculant treatments were evaluated, considering their grouping from the in vitro evaluations, as described for each experiment, and considering the variation within each group as a random effect. The Pearson linear correlation was evaluated for each experiment, considering the in vitro values and the plant variables.
5. Conclusions
Endophytic bacteria strains from pangolão grass increased initial plant growth for at least some variables of those provided by known effective Azospirillum strains under four different environmental stress conditions, likely due to this plant being adapted to stressful environments. Thus, its microbiome is an essential component of this adaptation.
The lack of substantial predictive value for the in vitro plant-growth-promotion characteristics, as seen by the low and generally non-significant correlations and the apparent disconnect between these and plant effects, indicates that the common practice of choosing strains for plant evaluation based on the in vitro evaluation might be flawed and needlessly reduce the genetic diversity in the plant evaluation.
Although the maize experiments were designed to evaluate specific environmental stresses purportedly linked to each in vitro plant-growth-promoting characteristic, the plant promotion is not directly related to individual mechanisms.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants12142589/s1, Table S1: In vitro growth promotion evaluation. BNF—growth in N-free media. IAA—IAA synthesis. SI—calcium phosphate solubilization index. Siderophore—siderophore synthesis; Table S2: Evaluation of strains associated with pangolan grass in terms of in vivo siderophore production and corn growth 20 days after germination.
Author Contributions
Conceptualization, M.J.G.A. and M.A.L.J.; Methodology, M.J.G.A., J.J.M., G.M.V., E.X.C. and M.A.L.J.; Investigation, M.J.G.A., J.J.M. and G.M.V.; Formal Analysis, M.J.G.A., E.X.C. and M.A.L.J.; Funding Acquisition, E.X.C., J.P.O. and M.A.L.J.; Supervision, E.X.C., J.P.O., F.J.C.F., G.G.M.F. and M.A.L.J.; Writing—Original Draft, M.J.G.A.; Writing—Review and Editing, M.J.G.A. and M.A.L.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Conselho Nacional de Pesquisa e Desenvolvimento Científico e Tecnológico, Brazil, Grant Numbers 304107/2020-4, 306252/2021-0, 401896/2013-7 and 483287/2013-0; Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Brazil, Finance Code 001 and Fundação de Amparo à Ciência e Tecnologia do Estado de Pernambuco, Brazil, Grant Numbers BCT-0406-5.03/21, APQ-0453-5.01/15 and BPV-0008-5.01/19.
Data Availability Statement
Data are available upon request to contact author.
Acknowledgments
We acknowledge the Instituto Agronômico de Pernambuco for overall support through several staff members.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zuluaga, M.Y.A.; Milani, K.M.L.; Miras-Moreno, B.; Lucini, L.; Valentinuzzi, F.; Mimmo, T.; Pii, Y.; Cesco, S.; Rodrigues, E.P.; Oliveira, A.L.M.D. Inoculation with plant growth-promoting bacteria alters the rhizosphere functioning of tomato plants. Appl. Soil Ecol. 2021, 158, 103784. [Google Scholar] [CrossRef]
- Faria, P.S.A.; Marques, V.d.O.; Selari, P.J.R.G.; Martins, P.F.; Silva, F.G.; Sales, J.d.F. Multifunctional potential of endophytic bacteria from Anacardium othonianum Rizzini in promoting in vitro and ex vitro plant growth. Microbiol. Res. 2021, 242, 126600. [Google Scholar] [CrossRef]
- Patel, M.; Vurukonda, S.S.K.P.; Patel, A. Multi-trait Halotolerant Plant Growth-promoting Bacteria Mitigate Induced Salt Stress and Enhance Growth of Amaranthus Viridis. J. Soil Sci. Plant Nutr. 2023, 23, 1860–1883. [Google Scholar] [CrossRef]
- Batista, B.D.; Bonatelli, M.L.; Quecine, M.C. Fast screening of bacteria for plant growth promoting traits. Methods Mol. Biol. 2021, 2232, 61–75. [Google Scholar] [CrossRef]
- Jain, R.; Bhardwaj, P.; Pandey, S.S.; Kumar, S. Arnebia euchroma, a Plant Species of Cold Desert in the Himalayas, Harbors Beneficial Cultivable Endophytes in Roots and Leaves. Front. Microbiol. 2021, 12, 696667. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Xu, J.; Liu, J.; Guo, W.H.; Li, X.B.; Xia, J.B.; Xie, W.J.; Yao, Z.G.; Zhang, Y.M.; Wang, R.Q. Characterization and Initial Application of Endophytic Bacillus safensis Strain ZY16 for Improving Phytoremediation of Oil-Contaminated Saline Soils. Front. Microbiol. 2019, 10, 991. [Google Scholar] [CrossRef] [PubMed]
- Govindasamy, V.; Raina, S.K.; George, P.; Kumar, M.; Rane, J.; Minhas, P.S.; Vittal, K.P.R. Functional and phylogenetic diversity of cultivable rhizobacterial endophytes of sorghum [Sorghum bicolor (L.) Moench]. Antonie Van Leeuwenhoek 2017, 110, 925–943. [Google Scholar] [CrossRef]
- Mutai, C.; Njuguna, J.; Ghimire, S. Brachiaria Grasses (Brachiaria spp.) harbor a diverse bacterial community with multiple attributes beneficial to plant growth and development. MicrobiologyOpen 2017, 6, e00497. [Google Scholar] [CrossRef]
- Carpentieri-Pipolo, V.; De Almeida Lopes, K.B.; Degrassi, G. Phenotypic and genotypic characterization of endophytic bacteria associated with transgenic and non-transgenic soybean plants. Arch. Microbiol. 2019, 201, 1029–1045. [Google Scholar] [CrossRef]
- Tapia-García, E.Y.; Hernández-Trejo, V.; Guevara-Luna, J.; Rojas-Rojas, F.U.; Arroyo-Herrera, I.; Meza-Radilla, G.; Vásquez-Murrieta, M.S.; Estrada-de los Santos, P. Plant growth-promoting bacteria isolated from wild legume nodules and nodules of Phaseolus vulgaris L. trap plants in central and southern Mexico. Microbiol. Res. 2020, 239, 126522. [Google Scholar] [CrossRef]
- Daraz, U.; Li, Y.; Sun, Q.; Zhang, M.; Ahmad, I. Inoculation of Bacillus spp. Modulate the soil bacterial communities and available nutrients in the rhizosphere of vetiver plant irrigated with acid mine drainage. Chemosphere 2021, 263, 128345. [Google Scholar] [CrossRef] [PubMed]
- Leandro, M.R.; Vespoli, L.d.S.; Andrade, L.F.; Soares, F.S.; Boechat, A.L.; Pimentel, V.R.; Moreira, J.R.; Passamani, L.Z.; Silveira, V.; de Souza Filho, G.A. DegP protease is essential for tolerance to salt stress in the plant growth-promoting bacterium Gluconacetobacter diazotrophicus PAL5. Microbiol. Res. 2021, 243, 126654. [Google Scholar] [CrossRef]
- Alves, M.J.G.; Oliveira, C.S.; Vitalino, G.M.; Carvalho, E.X.D.; Oliveira, J.D.P.; Fracetto, G.G.M.; Fracetto, F.J.C.; Lira Junior, M.A. Associative bacterial diversity of pangolão, a stress-resilient tropical grass. Bragantia 2022, 81, 20220071. [Google Scholar]
- Cherif-Silini, H.; Thissera, B.; Bouket, A.C.; Saadaoui, N.; Silini, A.; Eshelli, M.; Alenezi, F.N.; Vallat, A.; Luptakova, L.; Yahiaoui, B.; et al. Durum Wheat Stress Tolerance Induced by Endophyte Pantoea agglomerans with Genes Contributing to Plant Functions and Secondary Metabolite Arsenal. Int. J. Mol. Sci. 2019, 20, 3989. [Google Scholar] [CrossRef] [PubMed]
- Jochum, M.D.; McWilliams, K.L.; Borrego, E.J.; Kolomiets, M.V.; Niu, G.; Pierson, E.A.; Jo, Y.K. Bioprospecting Plant Growth-Promoting Rhizobacteria That Mitigate Drought Stress in Grasses. Front. Microbiol. 2019, 10, 2106. [Google Scholar] [CrossRef]
- Dif, G.; Belaouni, H.A.; Goudjal, Y.; Yekkour, A.; Djemouai, N.; Zitouni, A. Potential for plant growth promotion of Kocuria arsenatis Strain ST19 on tomato under salt stress conditions. S. Afr. J. Bot. 2021, 138, 94–104. [Google Scholar] [CrossRef]
- Brasil, S.d.D.A.-M.d.A.P.e.A. Instrução Normativa Nº13, de 24 de março de 2011. In Diário Oficial da União—Seção 1; 2011; pp. 3–7. [Google Scholar]
- Govindasamy, V.; George, P.; Ramesh, S.V.; Sureshkumar, P.; Rane, J.; Minhas, P.S. Characterization of root-endophytic actinobacteria from cactus (Opuntia ficus-indica) for plant growth promoting traits. Arch. Microbiol. 2022, 204, 150. [Google Scholar] [CrossRef]
- Gupta, S.; Pandey, S. ACC deaminase producing bacteria with multifarious plant growth promoting traits alleviates salinity stress in French Bean (Phaseolus vulgaris) plants. Front. Microbiol. 2019, 10, 1506. [Google Scholar] [CrossRef]
- Antunes, G.d.R.; Santana, S.R.A.; Escobar, I.E.C.; Brasil, M.d.S.; Araújo, G.G.L.d.; Voltolini, T.V.; Fernandes-Júnior, P.I. Associative diazotrophic bacteria from forage grasses in the Brazilian semi-arid region are effective plant growth promoters. Crop Pasture Sci. 2019, 70, 899–907. [Google Scholar] [CrossRef]
- Oliveira, J.T.C.; Pereira, A.P.A.; Souza, A.J.; Silva, G.T.; Diniz, W.P.S.; Figueredo, E.F.; Kuklinsky-Sobral, J.; Freire, F.J. Plant growth-promoting mechanisms and genetic diversity of bacteria strains isolated from Brachiaria humidicola and Brachiaria decumbens. An. Acad. Bras. Ciências 2021, 93, e20191123. [Google Scholar] [CrossRef]
- Habibi, S.; Djedidi, S.; Ohkama-Ohtsu, N.; Sarhadi, W.A.; Kojima, K.; Rallos, R.V.; Ramirez, M.D.A.; Yamaya, H.; Sekimoto, H.; Yokoyama, T. Isolation and screening of indigenous plant growth-promoting rhizobacteria from different rice cultivars in afghanistan soils. Microbes Environ. 2019, 34, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Valle Expósito, C.D.; López, J.Á.; Liu, J.; Bao, N.; Liang, J.; Zhang, J. Development of a cold-active microbial compound biofertilizer on the improvement for rice (oryza sativa L.) tolerance at low-temperature. Rhizosphere 2022, 24, 100586. [Google Scholar] [CrossRef]
- Enquahone, S.; van Marle, G.; Simachew, A. Plant growth-promoting characteristics of halotolerant endophytic bacteria isolated from Sporobolus specatus (Vahr) Kunth and Cyperus laevigatus L. of Ethiopian rift valley lakes. Arch. Microbiol. 2022, 204, 403. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, N.V.S.; Vidal, M.S.; Barrios, S.C.L.; Baldani, V.L.D.; Baldani, J.I. Genetic diversity and growth promoting characteristics of diazotrophic bacteria isolated from 20 genotypes of Brachiaria spp. Plant Soil 2020, 451, 187–205. [Google Scholar] [CrossRef]
- Panichikkal, J.; Krishnankutty, R.E. Root exudate components induced production of plant beneficial metabolites in rhizospheric Pseudomonas spp. Rhizosphere 2021, 19, 100366. [Google Scholar] [CrossRef]
- Afzal, I.; Shinwari, Z.K.; Sikandar, S.; Shahzad, S. Plant beneficial endophytic bacteria: Mechanisms, diversity, host range and genetic determinants. Microbiol. Res. 2019, 221, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Costa-Gutierrez, S.B.; Lami, M.J.; Santo, M.C.C.; Zenoff, A.M.; Vincent, P.A.; Molina-Henares, M.A.; Espinosa-Urgel, M.; de Cristobal, R.E. Plant growth promotion by Pseudomonas putida KT2440 under saline stress: Role of eptA. Appl. Microbiol. Biotechnol. 2020, 104, 4577–4592. [Google Scholar] [CrossRef]
- Kaleh, A.M.; Singh, P.; Mazumdar, P.; Chua, K.O.; Harikrishna, J.A. Halotolerant rhizobacteria isolated from a mangrove forest alleviate saline stress in Musa acuminata cv. Berangan. Microbiol. Res. 2022, 265, 127176. [Google Scholar] [CrossRef]
- Singh, S.; Choure, K.; Rai, P.K.; Gour, S.S.; Agnihotri, V.K. Evaluation of plant growth-promoting activities of endophytic bacteria of Musa acuminata and their characterization. J. Appl. Biol. Biotechnol. 2022, 10, 94–101. [Google Scholar] [CrossRef]
- Rana, K.L.; Kour, D.; Kaur, T.; Negi, R.; Devi, R.; Yadav, N.; Rai, P.K.; Singh, S.; Rai, A.K.; Yadav, A.; et al. Endophytic nitrogen-fixing bacteria: Untapped treasurer for agricultural sustainability. J. Appl. Biol. Biotechnol. 2022, 11, 75–93. [Google Scholar] [CrossRef]
- Rasul, M.; Yasmin, S.; Yahya, M.; Breitkreuz, C.; Tarkka, M.; Reitz, T. The wheat growth-promoting traits of Ochrobactrum and Pantoea species, responsible for solubilization of different P sources, are ensured by genes encoding enzymes of multiple P-releasing pathways. Microbiol. Res. 2021, 246, 126703. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Jiang, F.; Han, B.; Zhang, H.; Huang, D.; Shao, X. New insight into the divergent responses of plants to warming in the context of root endophytic bacterial and fungal communities. PeerJ 2021, 9, e11340. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Cordero, A.; Chamorro-Anaya, L.; Zootec, A.D.M. Endophytes bacterial growth promoters isolated to colosoana grass, Department of Sucre, Colombia. Rev. MVZ Cordoba 2018, 23, 6696–6709. [Google Scholar] [CrossRef]
- Li, Y.; Wang, M.; Chen, S. Application of N2-fixing Paenibacillus triticisoli BJ-18 changes the compositions and functions of the bacterial, diazotrophic, and fungal microbiomes in the rhizosphere and root/shoot endosphere of wheat under field conditions. Biol. Fertil. Soils 2021, 57, 347–362. [Google Scholar] [CrossRef]
- Alkahtani, M.D.F.; Fouda, A.; Attia, K.A.; Al-Otaibi, F.; Eid, A.M.; Ewais, E.E.-D.; Hijri, M.; St-Arnaud, M.; Hassan, S.E.-D.; Khan, N.; et al. Isolation and Characterization of Plant Growth Promoting Endophytic Bacteria from Desert Plants and Their Application as Bioinoculants for Sustainable Agriculture. Agronomy 2020, 10, 1325. [Google Scholar] [CrossRef]
- Garcia, M.V.C.; Nogueira, M.A.; Hungria, M. Combining microorganisms in inoculants is agronomically important but industrially challenging: Case study of a composite inoculant containing Bradyrhizobium and Azospirillum for the soybean crop. AMB Express 2021, 11, 71. [Google Scholar] [CrossRef]
- Khan, S.T. Consortia-based microbial inoculants for sustaining agricultural activities. App.. Soil Ecol. 2022, 176, 104503. [Google Scholar] [CrossRef]
- Saad, M.M.; Eida, A.A.; Hirt, H.; Doerner, P. Tailoring plant-associated microbial inoculants in agriculture: A roadmap for successful application. J. Exp. Bot. 2020, 71, 3878–3901. [Google Scholar] [CrossRef]
- Li, X.; Geng, X.; Xie, R.; Fu, L.; Jiang, J.; Gao, L.; Sun, J. The endophytic bacteria isolated from elephant grass (Pennisetum purpureum Schumach) promote plant growth and enhance salt tolerance of Hybrid Pennisetum. Biotechnol. Biofuels 2016, 9, 190. [Google Scholar] [CrossRef]
- Döbereiner, J.; Baldani, V.L.D.; Baldani, J.I. Como Isolar e Identificar Bactérias Diazotróficas de Plantas Não-Leguminosas; Embrapa SPI: Brasilia, Brazil, 1995. [Google Scholar]
- Baldani, V.L.D.; Baldani, J.I.; Dobereiner, J. Meios de cultura específicos para o isolamento de bactérias endofíticas que fixam N2 atmosférico. In Comunicado Técnico; EMBRAPA-CNPAB: Rio de Janeiro, Brazil, 1996; Volume 12, pp. 1–4. [Google Scholar]
- Baldani, J.I.; Baldani, V.L.D.; Seldin, L.; Dobereiner, J. Characterization of Herbaspirillum seropedicae gen. nov., sp. nov., a Root-Associated Nitrogen-Fixing Bacterium. Int. J. Syst. Bacteriol. 1986, 36, 86–93. [Google Scholar] [CrossRef]
- Silva, M.d.O.; Freire, F.J.; Lira Junior, M.A.; Kuklinsky-Sobral, J.; Costa, D.P.d.; Lira-Cadete, L. Isolamento e prospecção de bactérias endofíticas e epifíticas na cana-de-açúcar em áreas com e sem cupinicida. Rev. Bras. Ciência Do Solo 2012, 36, 1113–1122. [Google Scholar]
- Kuss, A.V.; Kuss, V.V.; Lovato, T.; Flôres, M.L. Nitrogen fixation and in vitro production of indolacetic acid by endophytic diazotrophic bacteria. Pesqui. Agropecu. Bras. 2007, 42, 1459–1465. [Google Scholar]
- Nautiyal, C.S. An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiol. Lett. 1999, 170, 265–270. [Google Scholar] [PubMed]
- Schwyn, B.; Neilands, J.B. Universal chemical assay for the detection and determination of siderophores. Anal. Biochem. 1987, 160, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Fukami, J.; Nogueira, M.A.; Araujo, R.S.; Hungria, M. Accessing inoculation methods of maize and wheat with Azospirillum brasilense. AMB Express 2016, 6, 3. [Google Scholar] [CrossRef]
- Pernambuco, C.F.E. Recomendações de Adubação Para o Estado de Pernambuco. 2a. Aproximação; Empresa Pernambucana de Pesquisa Agropecuria: Recife, Brazil, 2008; p. 198. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).