Abstract
Plant species usually have either annual or perennial life cycles, but facultative annual species have annual or perennial populations depending on their environment. In terrestrial angiosperms, facultative annual species are rare, with wild rice being one of the few examples. Our review shows that in marine angiosperms (seagrasses) facultative annual species are more common: six (of 63) seagrass species are facultative annual. It concerns Zostera marina, Z. japonica, Halophila decipiens, H. beccarii, Ruppia maritima, and R. spiralis. The annual populations generally produce five times more seeds than their conspecific perennial populations. Facultative annual seagrass species occur worldwide. Populations of seagrasses are commonly perennial, but the facultative annual species had annual populations when exposed to desiccation, anoxia-related factors, shading, or heat stress. A system-wide ‘experiment’ (closure of two out of three connected estuaries for large-scale coastal protection works) showed that the initial annual Z. marina population could shift to a perennial life cycle within 5 years, depending on environmental circumstances. We discuss potential mechanisms and implications for plant culture. Further exploration of flexible life histories in plant species, and seagrasses in particular, may aid in answering questions about trade-offs between vegetative and sexual reproduction, and preprogrammed senescence.
1. Introduction
Facultative annual species are perennial species that have some populations displaying annual life histories under certain conditions, completing their life cycles from germination to seed production followed by death within one year. Although it is known that the lengths of the life cycles of plants can vary depending on the environment, especially in biennials [1], facultative annual life histories are uncommon for angiosperms. To our knowledge, it is described in only two, unrelated, terrestrial species: wild rice Oryza perennis [2] and the herb Erythrante guttata (syn. Mimulus guttatus [3]). Facultative perennials, i.e., annual plants that may have perennial populations, are also uncommon [4,5]. The marine environment is colonized by a few angiosperm species that are all clonal and perennial [6] (Supplementary Information S1), but annual populations of the well-investigated seagrass species Zostera marina were already described in the 1970s [7]. From a biological and ecological perspective, such a flexible life-cycle strategy is an interesting phenomenon worth exploring.
The marine environment has posed special challenges to angiosperms which required physiological and reproductive adaptations [8,9]. Possibly, flexible life cycles are another, until now underexplored, response to the marine environment, which we wish to address here. We study whether other (perennial) seagrass species, in addition to the well-studied Z. marina, present annual populations. Secondly, we review the varying environmental settings of facultative annual seagrasses. In this review, we specifically question the following. (1) How common is facultative annual life history among seagrass species? (2) Is the occurrence of facultative annual life history widespread geographically? (3) Does the annual seed production differ with life cycle lengths? (4) Is there a relationship between life history and environmental settings? (5) Is there evidence for shifts between life histories?
2. A Facultative Annual Life History Is Widespread among Seagrass Species and Occurs Worldwide
Literature review shows that there are no true annual seagrass species. An annual life cycle was suggested by Kuo et al. [10] for the understudied deep water dioecious Halophila tricostata, but recent work by Chartrand [11] showed that this species overwintered with quiescent rhizomes, although yearly recurring seedling recruitment was important for persistence. Similar life history strategies with vegetative quiescent phases have been revealed for other seagrass species (Supplementary Information S1).
Based on available evidence, at least 6 out of 63 seagrass species display a facultative annual life history, with true annual populations, namely Zostera marina, Z. japonica, Halophila decipiens, H. beccarii, Ruppia maritima, and R. spiralis (Supplementary Information S2). The trait is polyphyletic, as these species belong to different families (Hydrocharitaceae, Ruppiaceae, and Zosteraceae) [12]. Z. marina is the best-known facultative annual seagrass species. This species occurs in the temperate and tropical northern hemispheres, with annual populations recorded at several locations (Figure 1).
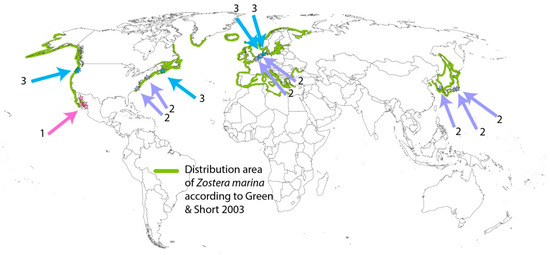
Figure 1.
Map of locations of annual Zostera marina populations (arrows) and the total distribution of Z. marina (green coastlines). Numbers indicate three types of environments. 1: Environments with yearly recurrent heat stress. 2: Subtidal or permanently submersed environments experiencing anoxia-related stress or shading stress. 3: Mid-intertidal environments with twice-daily exposure to air. More explanation in Section 4 and Supplementary Information S3.
3. Seed Production Is Higher in Annual Than Perennial Populations
The seed production of annual populations tends to be higher than that of perennial populations in the six facultative annual seagrass species identified in our study (Figure 2). Overall, seed production is five times higher in annual populations compared to conspecific perennial populations. However, populations vary greatly in seed production, and some perennial populations also present high seed outputs; for example, a perennial population of Z. marina in Chesapeake Bay had a potential maximum seed production of 40,000 seeds/m2 [13] vs. 100,000 seeds/m2 of an annual population in the subtropical Gulf of California [14]. Annual Z. marina plants typically have limited rhizome development and allocate most of the aboveground biomass to reproductive shoots [7]. Such differential allocation to vegetative and reproductive structures has been found for terrestrial angiosperms when comparing annual and perennial congeneric species [4,15,16].
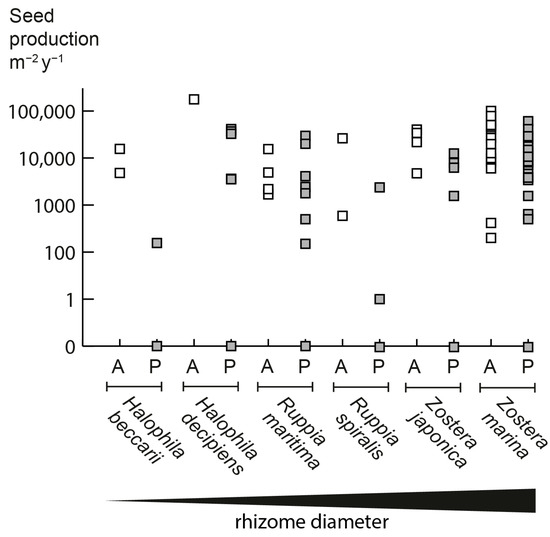
Figure 2.
Annual seed production in the six facultative annual seagrass species. A = Annual (open symbols), P = Perennial (grey symbols). If a range was presented, both minimal and maximal values are indicated. Please note that the perennial populations of all species can also have zero seed production, which is quite common for seagrasses. Literature sources and data are in Supplementary Information S2.
4. Annual Populations Live in More Stressful Environments
Assuming that there is a trade-off between vegetative (clonal) growth and sexual reproduction [16,17,18] and that sexual reproduction competes with the vegetative functions for necessary resources for plant growth and maintenance, an annual life cycle should only be favored over a perennial cycle when the survivorship of the established plant is lower than that of the seed or seedling. Such unfavorable conditions for vegetative development may recur periodically (often seasonally) or at stochastic intervals in highly unpredictable environments [19].
In seagrasses, such periodically unfavorable conditions may be low temperatures combined with high turbidity, as was found in Zostera japonica (British Columbia [20]) and Ruppia maritima (Baltic Sea [21]). Ruppia spp. may colonize shallow coastal lagoons that are only flooded during part of the year, and annual growth forms are reported to be a response to desiccation (Supplementary Information S2). Halophila beccarii forms annual populations as a response to decreased salinities on tidal flats in Malaysia [22]. Additionally, the subtidal delicate and shallow-rooted Halophila decipiens does not have a broad tolerance to salinity or temperature changes and may therefore be susceptible to removal or die-off during winter (Supplementary Information S2).
Annual populations of the relatively well-studied Z. marina are encountered in a myriad of situations (Figure 3 and Box 1). Comparing habitats of annual populations with the nearest perennial ones, the first seems to be more stressful than the latter. They experience either desiccation, heat stress, anoxia-related stress, shading stress, or a combination of all these well-known stressors of Z. marina and other seagrass species [23]. Populations are usually annual in the intertidal, where they experience periodic desiccation, but in water-retaining depressions and in moist air intertidal, plants have a perennial live history (Box 1). Subtidal or submersed annual populations seem to be exposed to higher levels of anoxia compared to those in neighboring populations (Box 1). Anoxia-related stress includes excessive eutrophication and/or organic matter loading, at times accompanied by lower salinity (as a covariate of enhanced nutrient input from freshwater sources), increased shading, warmer circumstances (decreasing dissolved oxygen and likely enhancing microbial processes leading to anoxia), or muddier sediments (mud is often correlated with organic matter and occurs in areas with less flushing). Anoxia results in the microbial production of sulfide and ammonia, which are toxic to Zostera spp. [24,25]. In addition, tidal or submersed annual populations occur in heat-stressed environments and in light-limited (deep) habitats (Box 1).
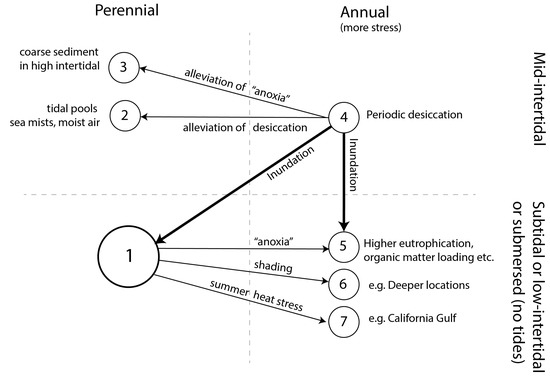
Figure 3.
Overview of reported habitat characteristics of annual and perennial Zostera marina populations and alleged drivers for transitions between life histories (arrows). The numbers refer to types of reported habitats listed in Box 1. Number 1 is the most typical growth strategy and environment for Z. marina. Thin arrows depict relative differences between environments of annual populations versus nearest perennial populations (correlative). Thick arrows refer to a system-scale inundation “experiment”; see Section 5. The term “anoxia” refers to a situation of more nutrient loading and/or muddier or more organic sediments and/or more anoxic sediment, often accompanied by higher turbidity and lower salinity due to freshwater origin of the nutrient or organic matter loads. These factors usually covariate in eutrophic situations [26].
Box 1. In what environments can we find annual and perennial populations of Zostera marina?
Annual populations of Zostera marina may recur at the same sites for decades, without perennial neighbors [27] (Supplementary Information S3), and thus are likely self-sustaining.
Perennial populations can be encountered as follows:
- In subtidal or submersed environments with low or moderate eutrophication (this is the typical environment and life cycle for Z. marina);
- Exceptionally, in mid-intertidal environments that probably remain sufficiently moist during low tide, namely (a) in tidal pools where the plants remain submersed (US [28,29,30]; probably NW Europe [31]) and (b) where high air moisture during the growing season (humid climate and sea mists) may protect the plants from desiccation in the mid-intertidal zone: along the eastern shores of the UK and Ireland [32,33]; Z. marina is here referred to as Z. angustifolia), and probably also along the Southwest coast of US, as suggested by the low flowering frequency (33% in Carlsbad [28]), and the robust perennial growth form encountered in San Diego, pers. obs. first author);
- Even more exceptionally, in coarse sanded mid-intertidal areas, at a slightly higher tidal level than the nearby annual population, where they experience even more desiccation. They lose aboveground biomass during summer as a consequence, but rhizomes survive both during summer and winter, the latter likely due to the coarse sediments that allow for flushing (observed in the southern and northern Wadden Sea [34]).
Annual populations can be encountered as follows:
- 4.
- In mid-intertidal environments that are twice-daily exposed to air on the east and west coast of North America and in NW Europe. All seedlings may develop into reproductive shoots [7], or, alternatively, a consistent part of the population may consist of vegetative shoots during the growing season, but they disappear (including belowground parts) during winter (e.g., in Zandkreek, Europe [35,36]). In North America (both east and west coast), transitions from annual to perennial populations coincide with the tidal depth gradient; from the mid-intertidal towards the low tide level, an increasing number of plants becomes perennial [7,29,30];
- 5.
- Permanently submersed environments on the east coast of the USA, in NW Europe, Japan, and Korea, with muddier, more turbid, warmer, more eutrophicated, and/or less saline conditions as compared to those of nearby perennial populations [37,38,39]. Generally, not all shoots are reproductive; some shoots are vegetative and may last longer than the reproductive shoots until they finally disappear (including belowground parts) during winter [13,36]. These populations may represent a transition between perennial and annual life histories;
- 6.
- Deep submersed environments where light is limiting. Nearby perennial populations are located shallower, described for Korea [40] and NW Europe [41];
- 7.
- Permanently submersed environments with yearly recurrent heat stress. There are no perennial populations nearby, described for several populations in the Gulf of California, at the southern distribution limit of this species. All shoots of these plants become reproductive [42].
Note: Some populations are called ‘annual’ or a separate
ecotype but seem to occupy marginal habitats incidentally colonized by
incoming seed from nearby populations; thus, they are not self-sustaining
populations [43,44].
5. Shifts between Annual and Perennial Life Histories in Zostera marina
System scale ‘experiments’ in the Southwest Netherlands have shown that annual populations can become perennial within 5 years after a change in environment. Three estuary branches were modified for coastal protection during 1961–1986: one branch was modified into an oligotrophic saline lake [45], one branch was modified into a brackish and eutrophic lake [46], whereas one branch remained intertidal with a modified hydrodynamic regime [47]. Prior to the modifications, the branches were connected, and they all hosted intertidal, annual populations of Z. marina [48]. In the newly formed oligotrophic saline lake, the population became perennial upon submergence within 5 years [41]. However, in the newly formed brackish and eutrophic lake as well as the intertidal branch, the populations continued to be annual ([36,39]; Figure 4). This shift in life history, or absence thereof, after modification of the environment, is evidence that population life history traits can be induced by the environment. When the plants became perennial, they presented lower seed production and a number of flowering shoots, higher belowground biomass, and the vegetative shoots showed vigorous growth earlier in the season than before, when the population was still annual and seasonal timing is earlier, suggesting that rhizomes give the shoots a head start as compared to the seed [36].
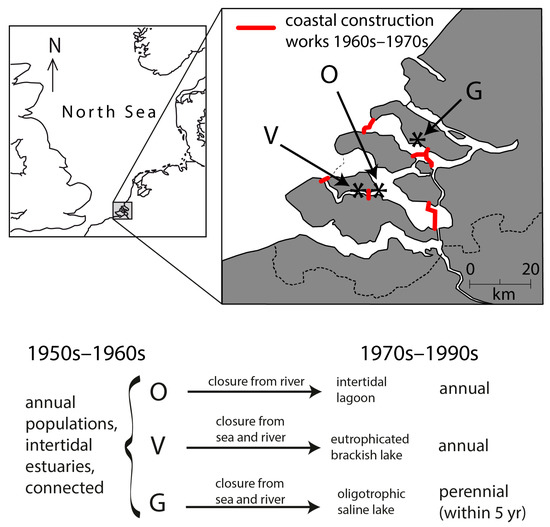
Figure 4.
Dutch waterworks resulting in an unintended system-scale “experiment” in the southwest Netherlands. Prior to coastal defense works, three arms of the Meuse-Rhine estuary harbored annual, mid-intertidal Zostera marina [48]. During the 1960s and 1970s, two of the branches were closed, forming a brackish lake (V = Veere) and a saline lake (G = Grevelingen), whereas the third arm (O = Oosterschelde) remained tidal, though cut off from river water. The freshwater input varied between the lakes; as a result, the brackish Lake Veere had a low salinity, high nutrient loading, macroalgal blooms, high turbidity, and periods of anoxia, whereas the saline Lake Grevelingen had a higher salinity, lower nutrient loading, higher water clarity, and lower algal growth [39]. In the Oosterschelde and the eutrophic brackish lake Veere, Z. marina plants remained annual, but in the oligotrophic saline lake Grevelingen, the plants became perennial within 5 years (comparing [48] with [36,41]). All populations went (near) extinct during the last 3 decades.
Transplantation experiments in NW Europe and in North America confirm that seedlings from annual populations can become perennial plants during the first winter (NW Europe [34], Izembek Lagoon, Alaska [49], although their reproductive effort remains high (NW Europe [34], Willapa bay, Washington [50]). Keddy and Patriquin [7] cultivated seedlings in the laboratory from seeds originating from annual and perennial populations in Nova Scotia and found that 28 out of 29 of the seedlings from the ‘annual’ seeds developed into annual plants and 1 developed into a perennial plant. Vice versa, 26 of 28 seedlings from ‘perennial’ seeds developed into perennial plants, whereas 2 of 28 developed into an annual plant. Thus, the findings of Keddy and Patriquin [7] suggest that annual populations have the potential to produce perennial offspring and vice versa.
It is intriguing that the seedlings of the reviewed annual populations produce reproductive shoots very early in development; in other words, they are “programmed for scenescence” several months later. Secondly, it is intriguing that they, nevertheless, may shift to a perennial life history when the environment becomes more favorable for vegetative survival in critical periods.
6. What Mechanisms May Induce an Annual or Perennial Life Cycle? Future Avenues of Research
During early growth, the seedlings of annual Zostera marina plants may not receive any indications from their environment that they will encounter adverse conditions for perennial growth later in the season, and the rapid development of generative shoots and early scenescence are perhaps “programmed”. Chartrand [11] found indications for such programming in deep water annual populations of Halophila decipiens in tropical Australia. However, it is also possible that a more stressful environment may already manifest early in the season and induce lower productivity/respiration ratios in the seedlings (see Figure 3 and Box 1). This lower P/R ratio may induce the plant to invest more resources into sexual reproduction, which is also suggested by a review of the effect of disturbance on sexual reproduction in seagrasses by Cabaço and Santos [51], and supported by later studies, for example, showing relations between sexual reproductive effort and temperature [52,53,54], but see [55], desiccation [43,56], nutrients [57,58], mechanical disturbance [59], and high salinity [60].
Population genetic studies in NW Europe [61] and in San Francisco Bay US [62] suggest a lack of genetic differentiation between annual and perennial populations, as well as high rates of gene flow between them, although genetic diversity is generally larger in the annual than in perennial populations [63]. Muñoz-Salazar and coworkers [64] found significant genetic differentiation between perennial Z. marina populations from the Pacific coast and annual ones in the Gulf of California (the summer annuals, type number 1 in Figure 1). This genetic divergence may be explained by the different life histories (annual vs. perennial), but it could also have been generated by limited gene flow between the two regions, as the tropical waters and current patterns of the southern Gulf of California have presented a barrier to gene flow and migration since the end of the Pleistocene. Oetjen and coworkers [65], using a genome scanning approach (using SNP and microsatellite markers), found some indications of selection between the subtidal perennial and intertidal annual populations in NW Europe. Divergent selections between the types of populations were detected at six loci, of which three were linked to genes involved in osmoregulation, water balance, and sexual reproduction (seed maturation). Selection could be enhanced by the different timing of the flowering initiation, even if annual populations are located in the immediate proximity of perennial populations via reproductive isolation [18,66].
Our review suggests that the annual vs. perennial life cycles in facultative annual Z. marina (and possibly the other facultative annual seagrass species) may be reversible, involving tradeoffs between vegetative and generative functions. Genetic evidence of such inflection of tradeoff was, for example, found in the terrestrial annual Arabidopsis thaliana. Modulation of the activities of only three genes influenced the indeterminacy of meristems and longevity of the plants, resulting in a growth form with the increasing development of vegetative buds, higher longevity, and extensive woodiness, indicative of perennial plants [67]. In the two terrestrial facultative annuals described in the literature, Erythrante guttata and Oryza sativa, possible genetic mechanisms for such reversibility between life histories have been investigated. Friedman and coworkers [3], when identifying phenotypic and genetic trade-offs between flowering and vegetative growth in E. guttata, found that differential responses to photoperiod and vernalization (the induction of a plant’s flowering process by exposure to the prolonged cold of winter) of plants from annual or perennial populations involved quantitative trait loci (QTL) and differential gene expression. QTL was also found to influence resource allocation in annual and perennial populations of rice Oryza [68,69].
In general, gene expression may be involved in frequent and precocious flowering. Perennial plants require reprogramming of some meristems to start the production of reproductive organs. Overexpression of the Flowering Locus (FT) gene from A. thaliana resulted in precocious flower development independent of photoperiod [70]. In the same plant, it was found that micro RNAs are involved in gene expression; miRNA 172 (miR172) caused early flowering through disruption of the downregulation of floral repression genes [71]. Interspecies gene transfer between perennial Arabis alpina and A. thaliana, showed a perennial and an annual signaling pathway to flowering, involving Squamosa promotor binding protein-like 15 (SPL15) and FL pathways, respectively [72]. The functional overlap between the pathways may enable flexible responses to shifting environments, as well as life history variation [72]. In general, from an evolutionary perspective, life history traits are among the most labile trait syndromes in flowering plants and annuality has evolved convergently in different lineages of flowering plants, though mechanisms underlying transitions are still unclear [16].
Chartrand [11] found that the general condition of the seagrass plants of the deep-water annual population of the seagrass H. decipiens declined before the light levels fell below the critical threshold for growth, from which she suggests that senescence and sexual reproduction were programmed. She observed shifts in hormones involved in these processes similar to shifts previously reported in terrestrial plants [4]. Up- and downregulation of corresponding areas could be confirmed with metabolomic profile analysis. Such changes in metabolomic expression may be heritable (epigenetic); epigenetic changes may last through cell divisions for the duration of the plant’s life and may also last for multiple generations, even though they do not involve changes in the underlying DNA sequence of the organism [73]. In short, annual life cycles in facultative annual species seem to be induced by the environment (for example, by low P/R ratios) or (epi-) genetic programming. Further research into mechanisms that induce the annual life cycle is needed, and the six seagrass species detected in this review may be good candidates for such studies.
7. Perspectives for Plant Culture
The tradeoff between investment in generative or vegetative plant parts becomes visible and tangible in facultative annual species, making this type of species of interest for plant biological and ecological studies. Moreover, when seagrass plants are to be cultured at a large scale, for example, to rewild the sea with domesticated seagrass when donor populations are scarce [74], knowing the factors that determine the life history may help to maximize seed production. Though seagrass domestication is still in its infancy, the facultative annual live strategy may allow a culture aiming at a balance between seed production and whole-year maintenance of ecosystem services provided by vegetative plant parts typically provided by perennial populations (such as carbon sequestration and erosion control). Such an ‘ideal’ tradeoff is presently targeted in terrestrial crops such as wheat and rice for a more sustainable agricultural practice, where perennial strains or species are domesticated to not only maintain food security but also ecosystem services such as erosion control and improved nitrogen use efficiency [4,75,76,77,78,79,80]. It is probably not a coincidence that perennial rice development, being one of the rare terrestrial facultative annual species, is more advanced than the development of perennial wheat, which requires de novo domestication of a congeneric species [78]. Our review shows that facultative annual species could potentially be brought to an optimum of seed production and vegetative development. Further research should elucidate whether this could be accomplished via manipulation of stresses or through other means such as (epi)genetic selection. Considering this, the study of facultative annual seagrasses may reveal a “rice from the sea” in the future.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants12102002/s1, Supplementary Information S1: Seagrasses with vegetative quiescent phases [11,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113], Supplementary Information S2: Facultative annual species [7,11,13,14,20,21,22,28,29,30,35,36,37,38,39,40,42,43,44,52,54,59,92,99,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176,177,178,179,180,181,182,183,184,185,186,187,188], Supplementary Information S3: Table with reproductive traits of Zostera marina populations, comparing annual populations with the nearest perennial population [7,13,14,28,29,30,31,34,35,36,37,38,39,40,42,43,64,92,166,173,175,176,177,178,189,190].
Author Contributions
Conceptualization, M.M.v.K. and B.I.v.T.; investigation, B.I.v.T. and M.M.v.K. writing—original draft preparation, M.M.v.K. and B.I.v.T.; writing—review and editing, M.M.v.K. and B.I.v.T. All authors have read and agreed to the published version of the manuscript.
Funding
B.I.v.T. received a grant for a sabbatical period from the Dirección General Asuntos del Personal Académico, Universidad Nacional Autónoma de México.
Data Availability Statement
The data presented in this study are available in the Supplementary Material.
Acknowledgments
The authors thank Lucien Hanssen for the provided feedback and discussions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kelly, D. On strict and facultative biennials. Oecologia 1985, 67, 292–294. [Google Scholar] [CrossRef]
- Sano, Y. Adaptive strategy in wild rice. Jpn. J. Genet. 1980, 55, 507–508. [Google Scholar]
- Friedman, J.; Twyford, A.D.; Willis, J.H.; Blackman, B.K. The extent and genetic basis of phenotypic divergence in life history traits in Mimulus guttatus. Mol. Ecol. 2015, 24, 111–122. [Google Scholar] [CrossRef]
- Friedman, J. The evolution of annual and perennial plant life histories: Ecological correlates and genetic mechanisms. Annu. Rev. Ecol. Evol. Syst. 2020, 51, 461–481. [Google Scholar] [CrossRef]
- Verma, R.; Kaul, V. Annual to perennial reproductive shift in Anisomeles indica (L.) Kuntze: A strategic seasonal switch. Nord. J. Bot. 2022, 2022, e03203. [Google Scholar] [CrossRef]
- den Hartog, C. The Sea-Grasses of the World; North-Holland: Amsterdam, The Netherlands, 1970; pp. 1–275. [Google Scholar]
- Keddy, C.J.; Patriquin, D.G. An annual form of eelgrass in Nova Scotia. Aquat. Bot. 1978, 5, 163–170. [Google Scholar] [CrossRef]
- Duarte, C.M.; Planas, D.; Penuelas, J. Macrophytes, taking control of an ancestor home. In Limnology Now: A Paradigm of Planetary Problems; Margalet, R., Ed.; Elsevier: Amsterdam, The Netherlands, 1994; pp. 59–79. [Google Scholar]
- van Tussenbroek, B.I.; Marquéz Guzmán, J.; Wong, R. Phenology of marine angiosperms [seagrasses]: Reporductive synchrony in the sea. In Functional Approach to Sexual Plant Reproduction; Gamboa-deBuen, A., Orozco-Segovia, A., Cruz-Garcia, F., Eds.; Research Signpost: Kerala, India, 2009; pp. 17–46. [Google Scholar]
- Kuo, J.; Long, W.L.; Coles, R.G. Occurrence and fruit and seed biology of Halophila tricostata Greenway (Hydrocharitaceae). Aust. J. Mar. Freshw. Res. 1993, 44, 43–57. [Google Scholar] [CrossRef]
- Chartrand, K.M. Growth Dynamics and Drivers of Deep-Water Seagrasses from the Great Barrier Reef Lagoon. Doctoral Dissertation, University of Technology, Sydney, Australia, 2021. [Google Scholar]
- Les, D.H.; Cleland, M.A.; Waycott, M. Phylogenetic studies in Alismatidae, II: Evolution of marine angiosperms (seagrasses) and hydrophily. Syst. Bot. 1997, 22, 443–463. [Google Scholar] [CrossRef]
- Jarvis, J.C.; Moore, K.A.; Kenworthy, W.J. Characterization and ecological implication of eelgrass life history strategies near the species’ southern limit in the western North Atlantic. Mar. Ecol. Prog. Ser. 2012, 444, 43–56. [Google Scholar] [CrossRef]
- Meling-Lopez, A.E.; Ibarra-Obando, S.E. Annual life cycles of two Zostera marina L. populations in the Gulf of California: Contrasts in seasonality and reproductive effort. Aquat. Bot. 1999, 65, 59–69. [Google Scholar] [CrossRef]
- Vico, G.; Manzoni, S.; Nkurunziza, L.; Murphy, K.; Weih, M. Trade-offs between seed output and life span-a quantitative comparison of traits between annual and perennial congeneric species. N. Phytol. 2016, 209, 104–114. [Google Scholar] [CrossRef]
- Hjertaas, A.C.; Preston, J.C.; Kainulainen, K.; Humphreys, A.M.; Fjellheim, S. Convergent evolution of the annual life history syndrome from perennial ancestors. Front. Plant Sci. 2023, 13, 1048656. [Google Scholar] [CrossRef]
- Cook, R.E. Growth and development in clonal plant populations. In Population Biology and Evolution of Clonal Organisms; Jackson, J.B.C.B.L.W., Cook, R.E., Eds.; Yale University Press: New Haven, CT, USA, 1985; pp. 259–296. [Google Scholar]
- Ruesink, J.L.; Ortiz, B.A.B.; Mawson, C.H.; Boardman, F.C. Tradeoffs in life history investment of eelgrass Zostera marina across estuarine intertidal conditions. Mar. Ecol. Prog. Ser. 2022, 686, 61–70. [Google Scholar] [CrossRef]
- Mayfield, M.M.; Dwyer, J.M.; Main, A.; Levine, J.M. The germination strategies of widespread annual plants are unrelated to regional climate. Glob. Ecol. Biogeogr. 2014, 23, 1430–1439. [Google Scholar] [CrossRef]
- Harrison, P.G.; Bigley, R.E. The Recent Introduction of the Seagrass Zostera japonica Aschers and Graebn to the Pacific Coast of North-America. Can. J. Fish. Aquat. Sci. 1982, 39, 1642–1648. [Google Scholar] [CrossRef]
- Kautsky, L. Seed and tuber banks o3f aquatic macrophytes in the Askoe area, northern Baltic proper. Holarct. Ecol. 1990, 13, 143–148. [Google Scholar]
- Zakaria, M.H.; Bujang, J.S.; Arshad, A. Flowering, fruiting and seedling of annual Halophila beccarii Aschers in peninsular Malaysia. Bull. Mar. Sci. 2002, 71, 1199–1205. [Google Scholar]
- Roca, G.; Alcoverro, T.; Krause-Jensen, D.; Balsby, T.J.S.; van Katwijk, M.M.; Marba, N.; Santos, R.; Arthur, R.; Mascaro, O.; Fernandez-Torquemada, Y.; et al. Response of seagrass indicators to shifts in environmental stressors: A global review and management synthesis. Ecol. Indic. 2016, 63, 310–323. [Google Scholar] [CrossRef]
- Lamers, L.P.M.; Govers, L.L.; Janssen, I.C.J.M.; Geurts, J.J.M.; van der Welle, M.E.W.; van Katwijk, M.M.; van der Heide, T.; Roelofs, J.G.M.; Smolders, A.J.P. Sulfide as a soil phytotoxin-a review. Front. Plant Sci. 2013, 4, 268. [Google Scholar] [CrossRef]
- Govers, L.L.; de Brouwer, J.H.F.; Suykerbuyk, W.; Bouma, T.J.; Lamers, L.P.M.; Smolders, A.J.P.; van Katwijk, M.M. Toxic effects of increased sediment nutrient and organic matter loading on the seagrass Zostera noltii. Aquat. Toxicol. 2014, 155, 253–260. [Google Scholar] [CrossRef]
- van der Heide, T.; Peeters, E.T.H.M.; Hermus, D.C.R.; van Katwijk, M.M.; Roelofs, J.G.M.; Smolders, A.J.P. Predicting habitat suitability in temperate seagrass ecosystems. Limnol. Oceanogr. 2009, 54, 2018–2024. [Google Scholar] [CrossRef]
- Dolch, T.; Buschbaum, C.; Reise, K. Persisting intertidal seagrass beds in the northern Wadden Sea since the 1930s. J. Sea Res. 2013, 82, 134–141. [Google Scholar] [CrossRef]
- Phillips, R.C.; Grant, W.S.; McRoy, C.P. Reproductive strategies of eelgrass (Zostera marina L.). Aquat. Bot. 1983, 16, 1–20. [Google Scholar] [CrossRef]
- Bayer, R.D. Intertidal zonation of Zostera marina in the Yaquina Estuary, Oregon. Syesis 1979, 12, 147–154. [Google Scholar]
- Keddy, C.J. Reproduction of annual eelgrass: Variation among habitats and comparison with perennial eelgrass (Zostera marina L.). Aquat. Bot. 1987, 27, 243–256. [Google Scholar] [CrossRef]
- Reigersman, C.J.A.; Houben, G.F.H.; Havinga, B. Rapport Omtrent Den Invloed Van De Wierziekte Op Den Achteruitgang Van De Wierbedrijven, Met Bijlagen; Provinciale Waterstaat in Noord-Holland: Haarlem, Holland, 1939; pp. 1–67. [Google Scholar]
- Tutin, T.G. Zostera marina L. J. Ecol. 1942, 30, 217–226. [Google Scholar] [CrossRef]
- Davison, D.M.H.D.J. Zostera biotopes (Volume I). An Overview of Dynamics and Sensitivity Characteristics for Conservation Management of Marine SACs; Scottish Association for Marine Science (UK Marine SACs Project): Oban, UK, 1998; p. 95. [Google Scholar]
- van Katwijk, M.M.; Schmitz, G.H.W.; Hanssen, L.S.A.M.; den Hartog, C. Suitability of Zostera marina populations for transplantation to the Wadden Sea as determined by a mesocosm shading experiment. Aquat. Bot. 1998, 60, 283–305. [Google Scholar] [CrossRef]
- Harrison, P.G. Variations in demography of Zostera marina and Zostera noltii on an intertidal gradient. Aquat. Bot. 1993, 45, 63–77. [Google Scholar] [CrossRef]
- van Lent, F.; Verschuure, J.M. Intraspecific variability of Zostera marina L. (eelgrass) in the estuaries and lagoons of the southwestern Netherlands: I. Population dynamics. Aquat. Bot. 1994, 48, 31–58. [Google Scholar] [CrossRef]
- Harlin, M.M.; Thorne-Miller, B.; Boothroyd, J. Seagrass-sediment dynamics of a flood-tidal delta in Rhode Island (U.S.A.). Aquat. Bot. 1982, 14, 127–138. [Google Scholar] [CrossRef]
- Verhoeven, J.T.A.; Van Vierssen, W. Distribution and structure of communities dominated by Ruppia, Zostera and Potamogeton species in the inland waters of ‘De Bol’, Texel, The Netherlands. Estuar. Coast. Mar. Sci. 1978, 6, 417–428. [Google Scholar] [CrossRef]
- van Lent, F.; Verschuure, J.M. Intraspecific variability of Zostera marina L. (eelgrass) in the estuaries and lagoons of the southwestern Netherlands: II. Relation with environmental factors. Aquat. Bot. 1994, 48, 59–75. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, J.H.; Park, S.R.; Lee, K.S. Annual and perennial life history strategies of Zostera marina populations under different light regimes. Mar. Ecol. Prog. Ser. 2014, 509, 1–13. [Google Scholar] [CrossRef]
- Nienhuis, P.H.; de Bree, B.H.H. Production and growth dynamics of eelgrass (Zostera marina) in Brackish Lake Grevelingen (The Netherlands). Neth. J. Sea Res. 1980, 14, 102–118. [Google Scholar] [CrossRef]
- Santamaria-Gallegos, N.A.; Sanchez-Lizaso, J.L.; Felix-Pico, E.F. Phenology and growth cycle of annual subtidal eelgrass in a subtropical locality. Aquat. Bot. 2000, 66, 329–339. [Google Scholar] [CrossRef]
- Qin, L.Z.; Li, W.T.; Zhang, X.M.; Nie, M.; Li, Y. Sexual reproduction and seed dispersal pattern of annual and perennial Zostera marina in a heterogeneous habitat. Wetl. Ecol. Manag. 2014, 22, 671–682. [Google Scholar] [CrossRef]
- Becheler, R.; Diekmann, O.; Hily, C.; Moalic, Y.; Arnaud-Haond, S. The concept of population in clonal organisms: Mosaics of temporally colonized patches are forming highly diverse meadows of Zostera marina in Brittany. Mol. Ecol. 2010, 19, 2394–2407. [Google Scholar] [CrossRef]
- van Katwijk, M.M.; Cronau, R.J.T.; Lamers, L.P.M.; Kamermans, P.; van Tussenbroek, B.I.; de Jong, D.J. Salinity-Induced extinction of Zostera marina in Lake Grevelingen? How strong habitat modification may require introduction of a suitable ecotype. Sustainability 2023, 15, 3472. [Google Scholar] [CrossRef]
- Wijnhoven, S.; Escaravage, V.; Daemen, E.; Hummel, H. The Decline and Restoration of a Coastal Lagoon (Lake Veere) in the Dutch Delta. Estuaries Coasts 2010, 33, 1261–1278. [Google Scholar] [CrossRef][Green Version]
- Louters, T.; van den Berg, J.H.; Mulder, J.P.M. Geomorphological changes of the Oosterschelde tidal system during and after the implementation of the delta project. J. Coast. Res. 1998, 14, 1134–1151. [Google Scholar]
- Beeftink, W.G. De zoutvegetatie van ZW-Nederland beschouwd in Europees verband. Meded. Landbouwhogesch. Wagening. 1965, 65–1, 82–85. [Google Scholar]
- Phillips, R.C.; Lewis, R.L., III. Influence of environmental gradients on variations in leaf width and transplant success in North American seagrasses. Mar. Technol. Soc. J. 1983, 17, 59–68. [Google Scholar]
- Ruesink, J.L. Size and fitness responses of eelgrass (Zostera marina L.) following reciprocal transplant along an estuarine gradient. Aquat. Bot. 2018, 146, 31–38. [Google Scholar] [CrossRef]
- Cabaço, S.; Santos, R. Seagrass reproductive effort as an ecological indicator of disturbance. Ecol. Indic. 2012, 23, 116–122. [Google Scholar] [CrossRef]
- Xu, S.C.; Xu, S.; Zhou, Y.; Yue, S.D.; Qiao, Y.L.; Liu, M.J.; Gu, R.T.; Song, X.Y.; Zhang, Y.; Zhang, X.M. Sonar and in situ surveys of eelgrass distribution, reproductive effort, and sexual recruitment contribution in a eutrophic bay with intensive human activities: Implication for seagrass conservation. Mar. Pollut. Bull. 2020, 161, 111706. [Google Scholar] [CrossRef]
- Ito, M.A.; Lin, H.J.; O’Connor, M.I.; Nakaoka, M. Large-scale comparison of biomass and reproductive phenology among native and non-native populations of the seagrass Zostera japonica. Mar. Ecol. Prog. Ser. 2021, 675, 1–21. [Google Scholar] [CrossRef]
- Yue, S.D.; Zhang, X.M.; Xu, S.C.; Zhang, Y.; Zhao, P.; Wang, X.D.; Zhou, Y. Reproductive strategies of the seagrass Zostera japonica under different geographic conditions in northern China. Front. Mar. Sci. 2020, 7, 574790. [Google Scholar] [CrossRef]
- Vercaemer, B.M.; Scarrow, M.A.; Roethlisberger, B.; Krumhansl, K.A.; Wong, M.C. Reproductive ecology of Zostera marina L. (eelgrass) across varying environmental conditions. Aquat. Bot. 2021, 175, 103444. [Google Scholar] [CrossRef]
- van Tussenbroek, B.I.; Soissons, L.M.; Bouma, T.J.; Asmus, R.; Auby, I.; Brun, F.G.; Cardoso, P.G.; Desroy, N.; Fournier, J.; Ganthy, F.; et al. Pollen limitation may be a common Allee effect in marine hydrophilous plants: Implications for decline and recovery in seagrasses. Oecologia 2016, 182, 595–609. [Google Scholar] [CrossRef]
- Johnson, A.J.; Moore, K.A.; Orth, R.J. The influence of resource availability on flowering intensity in Zostera marina (L.). J. Exp. Mar. Biol. Ecol. 2017, 490, 13–22. [Google Scholar] [CrossRef]
- Suonan, Z.; Kim, S.H.; Qin, L.; Kim, H.; Zhang, F.; Lee, K.S. Increased Coastal Nutrient Loading Enhances Reproductive Intensity of Zostera marina: Implications for Seagrass Meadow Resilience. Front. Mar. Sci. 2022, 9, 832035. [Google Scholar] [CrossRef]
- Suonan, Z.; Kim, S.H.; Qin, L.Z.; Lee, K.S. Reproductive strategy of the intertidal seagrass Zostera japonica under different levels of disturbance and tidal inundation. Estuar. Coast. Shelf Sci. 2017, 197, 185–193. [Google Scholar] [CrossRef]
- Strazisar, T.; Koch, M.S.; Santangelo, C.W.; Madden, C.J. Abiotic and biotic interactions control Ruppia maritima life history development within a heterogeneous coastal landscape. Estuaries Coasts 2021, 44, 1975–1993. [Google Scholar] [CrossRef]
- Reusch, T.B.H. Microsatellites reveal high population connectivity in eelgrass (Zostera marina) in two contrasting coastal areas. Limnol. Oceanogr. 2002, 47, 78–85. [Google Scholar] [CrossRef]
- Ort, B.S.; Cohen, C.S.; Boyer, K.E.; Wyllie-Echeverria, S. Population structure and genetic diversity among eelgrass (Zostera marina) beds and depths in San Francisco Bay. J. Hered. 2012, 103, 533–546. [Google Scholar] [CrossRef]
- Reynolds, L.K.; Stachowicz, J.J.; Hughes, A.R.; Kamel, S.J.; Ort, B.S.; Grosberg, R.K. Temporal stability in patterns of genetic diversity and structure of a marine foundation species (Zostera marina). Heredity 2017, 118, 404–412. [Google Scholar] [CrossRef]
- Muñiz-Salazar, R.; Talbot, S.L.; Sage, G.K.; Ward, D.H.; Cabello-Pasini, A. Population genetic structure of annual and perennial populations of Zostera marina L. along the Pacific coast of Baja California and the Gulf of California. Mol. Ecol. 2005, 14, 711–722. [Google Scholar] [CrossRef]
- Oetjen, K.; Ferber, S.; Dankert, I.; Reusch, T.B.H. New evidence for habitat-specific selection in Wadden Sea Zostera marina populations revealed by genome scanning using SNP and microsatellite markers. Mar. Biol. 2010, 157, 81–89. [Google Scholar] [CrossRef]
- von Staats, D.A.; Hanley, T.C.; Hays, C.G.; Madden, S.R.; Sotka, E.E.; Hughes, A.R. Intra-Meadow Variation in Seagrass Flowering Phenology Across Depths. Estuaries Coasts 2021, 44, 325–338. [Google Scholar] [CrossRef]
- Melzer, S.; Lens, F.; Gennen, J.; Vanneste, S.; Rohde, A.; Beeckman, T. Flowering-time genes modulate meristem determinacy and growth form in Arabidopsis thaliana. Nat. Genet. 2008, 40, 1489–1492. [Google Scholar] [CrossRef]
- Cai, H.W.; Morishima, H. QTL clusters reflect character associations in wild and cultivated rice. Theor. Appl. Genet. 2002, 104, 1217–1228. [Google Scholar] [CrossRef]
- Onishi, K.; Horiuchi, Y.; Ishigoh-Oka, N.; Takagi, K.; Ichikawa, N.; Maruoka, M.; Sano, Y. A QTL cluster for plant architecture and its ecological significance in Asian wild rice. Breed. Sci. 2007, 57, 7–16. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Kaya, H.; Goto, K.; Iwabuchi, M.; Araki, T. A pair of related genes with antagonistic roles in mediating flowering signals. Science 1999, 286, 1960–1962. [Google Scholar] [CrossRef] [PubMed]
- Aukerman, M.J.; Sakai, H. Regulation of flowering time and floral organ identity by a microRNA and its APETALA2-like target genes. Plant Cell 2003, 15, 2730–2741. [Google Scholar] [CrossRef] [PubMed]
- Hyun, Y.; Vincent, C.; Tilmes, V.; Bergonzi, S.; Kiefer, C.; Richter, R.; Martinez-Gallegos, R.; Severing, E.; Coupland, G. A regulatory circuit conferring varied flowering response to cold in annual and perennial plants. Science 2019, 363, 409–412. [Google Scholar] [CrossRef] [PubMed]
- Berger, S.L.; Kouzarides, T.; Shiekhattar, R.; Shilatifard, A. An operational definition of epigenetics. Genes Dev. 2009, 23, 781–783. [Google Scholar] [CrossRef]
- van Katwijk, M.M.; van Tussenbroek, B.I.; Hanssen, S.V.; Hendriks, A.J.; Hanssen, L. Rewilding the Sea with Domesticated Seagrass. Bioscience 2021, 71, 1171–1178. [Google Scholar] [CrossRef]
- Glover, J.D.; Reganold, J.P.; Bell, L.W.; Borevitz, J.; Brummer, E.C.; Buckler, E.S.; Cox, C.M.; Cox, T.S.; Crews, T.E.; Culman, S.W.; et al. Increased Food and Ecosystem Security via Perennial Grains. Science 2010, 328, 1638–1639. [Google Scholar] [CrossRef]
- Asbjornsen, H.; Hernandez-Santana, V.; Liebman, M.; Bayala, J.; Chen, J.; Helmers, M.; Ong, C.K.; Schulte, L.A. Targeting perennial vegetation in agricultural landscapes for enhancing ecosystem services. Renew. Agric. Food Syst. 2014, 29, 101–125. [Google Scholar] [CrossRef]
- Toensmeier, E. The Carbon Farming Solution: A Global Toolkit of Perennial Crops and Regenerative Agriculture Practices for Climate Change Mitigation and Food Security; Chelsea Green Publishing: London, UK, 2016. [Google Scholar]
- DeHaan, L.R.; Van Tassel, D.L. Gourmet grasslands: Harvesting a perennial future. One Earth 2022, 5, 14–17. [Google Scholar] [CrossRef]
- Windes, S.; Carrijo, D.; Curwen-McAdams, C.; Hayes, P. Improving the sustainability of malting barley production: Prospects for perennial and annual growth habit varieties. Crop Sci. 2019, 59, 2289–2296. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Huang, G.F.; Zhang, S.L.; Zhang, J.; Gan, S.X.; Cheng, M.; Hu, J.; Huang, L.Y.; Hu, F.Y. An innovated crop management scheme for perennial rice cropping system and its impacts on sustainable rice production. Eur. J. Agron. 2021, 122, 11. [Google Scholar] [CrossRef]
- Les, D.H. Breeding systems, population-structure, and evolution in hydrohilous angiosperms. Ann. Mo. Bot. Gard. 1988, 75, 819–835. [Google Scholar] [CrossRef]
- Barrett, S.C.H.; Eckert, C.G.; HUSBAND, B.C. Evolutionary processes in aquatic plant populations. Aquat. Bot. 1993, 44, 105–145. [Google Scholar] [CrossRef]
- Grace, J.B. The adaptive significance of clonal reproduction in angiosperms: An aquatic perspective. Aquat. Bot. 1993, 44, 159–180. [Google Scholar] [CrossRef]
- van Groenendael, J.M.; Klimes, L.; Klimesova, J.; Hendriks, R.J.J. Comparative ecology of clonal plants. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 1996, 351, 1331–1339. [Google Scholar] [CrossRef]
- Edgeloe, J.M.; Severn-Ellis, A.A.; Bayer, P.E.; Mehravi, S.; Breed, M.F.; Krauss, S.L.; Batley, J.; Kendrick, G.A.; Sinclair, E.A. Extensive polyploid clonality was a successful strategy for seagrass to expand into a newly submerged environment. Proc. R. Soc. B Biol. Sci. 2022, 289, 20220538. [Google Scholar] [CrossRef]
- Procaccini, G.; Mazzella, L. Population genetic structure and gene flow in the seagrass Posidonia oceanica assessed using microsatellite analysis. Mar. Ecol. Prog. Ser. 1998, 169, 133–141. [Google Scholar] [CrossRef]
- Migliaccio, M.; De Martino, F.; Silvestre, F.; Procaccini, G. Meadow-scale genetic structure in Posidonia oceanica. Mar. Ecol. Prog. Ser. 2005, 304, 55–65. [Google Scholar] [CrossRef]
- Arnaud-Haond, S.; Duarte, C.M.; Diaz-Almela, E.; Marba, N.; Sintes, T.; Serrao, E.A. Implications of extreme life span in clonal organisms: Millenary clones in meadows of the threatened seagrass Posidonia oceanica. PLoS ONE 2012, 7, e30454. [Google Scholar] [CrossRef]
- Reusch, T.B.H.; Böstrom, C.; Stam, W.T.; Olsen, J.L. An ancient eelgrass clone in the Baltic. Mar. Ecol. Prog. Ser. 1999, 183, 301–304. [Google Scholar] [CrossRef]
- van Dijk, J.K.; Van Tussenbroek, B.I. Clonal diversity and structure related to habitat of the marine angiosperm Thalassia testudinum along the Atlantic coast of Mexico. Aquat. Bot. 2010, 92, 63–69. [Google Scholar] [CrossRef]
- Bricker, E.; Calladine, A.; Virnstein, R.; Waycott, M. Mega clonality in an aquatic plant-A potential survival strategy in a changing environment. Front. Plant Sci. 2018, 9, 435. [Google Scholar] [CrossRef] [PubMed]
- Hootsmans, M.J.M.; Vermaat, J.E.; Van Vierssen, W. Seed-bank development, germination and early seedling survival of two seagrass species from the Netherlands: Zostera marina L and Z. noltii Hornemann. Aquat. Bot. 1987, 28, 275–285. [Google Scholar] [CrossRef]
- Vermaat, J.E.; Verhagen, F.C.A. Seasonal variation in the intertidal seagrass Zostera noltii Hornem.: Coupling demographic and physiological patterns. Aquat. Bot. 1996, 52, 259–281. [Google Scholar] [CrossRef]
- Zipperle, A.M.; Coyer, J.A.; Reise, K.; Gitz, E.; Stam, W.T.; Olsen, J.L. Clonal architecture in an intertidal bed of the dwarf eelgrass Zostera noltii in the Northern Wadden Sea: Persistence through extreme physical perturbation and the importance of a seed bank. Mar. Biol. 2009, 156, 2139–2148. [Google Scholar] [CrossRef] [PubMed]
- Zipperle, A.M.; Coyer, J.A.; Reise, K.; Stam, W.T.; Olsen, J.L. Evidence for persistent seed banks in dwarf eelgrass Zostera noltii in the German Wadden Sea. Mar. Ecol. Prog. Ser. 2009, 380, 73–80. [Google Scholar] [CrossRef]
- Wood, E.J.F. Some east Australian sea-grass communities. Proc. Linn. Soc. New South Wales 1959, 84, 218–226. [Google Scholar]
- Hamdorf, I.; Kirkman, H. Status of Australian Seagrass: Issues Paper; Fisheries Pollution and Marine Environment Committee: Canberra, Australia, 1995; pp. 1–32. [Google Scholar]
- Maher, D.; Squire, P.; Eyre, B. Benthic Habitat Mapping, Primary Productivity Measurements and Macrofauna Surveys in the Camden Haven and Hastings River Estuaries; Centre for coastal Biogeochemistry, Southern Cross University: Lismore, Australia, 2007. [Google Scholar]
- Angsupanich, S. Seagrasses and epiphytes in Thale Sap Songkhla, Soujythern Thailand. Mer 1996, 34, 67–73. [Google Scholar]
- Rasheed, M.A.; McKenna, S.A.; Carter, A.B.; Coles, R.G. Contrasting recovery of shallow and deep water seagrass communities following climate associated losses in tropical north Queensland, Australia. Mar. Pollut. Bull. 2014, 83, 491–499. [Google Scholar] [CrossRef]
- Brock, M.A. Biology of the salinity tolerant genus Ruppia L. in saline lakes in South-Australia. 1. Morphological variation wsithin and between species and ecophysiology. Aquat. Bot. 1982, 13, 219–248. [Google Scholar] [CrossRef]
- Brock, M.A. Biology of the salinity tolerant genus Ruppia L. in saline lakes in South-Australia 2. Population ecology and reproductive biology. Aquat. Bot. 1982, 13, 249–268. [Google Scholar] [CrossRef]
- Brock, M.A. Reproductive allocation in annual and perennial species of the submerged aquatic halophyte Ruppia. J. Ecol. 1983, 71, 811–818. [Google Scholar] [CrossRef]
- Porter, J.L. Constrasting emergence patterns of Lamprothamnium macropogon (Characeae, Charophyceae) and Ruppia tuberosa (Potamogetonaceae) from arid-zone saline wetlands in Australia. Charophytes 2007, 1, 19–27. [Google Scholar]
- Jacobs, S.W.L.; Brock, M.A. A revision of the genus Ruppia (Potamogetonaceae) in Australia. Aquat. Bot. 1982, 14, 325–337. [Google Scholar] [CrossRef]
- Burnell, O.W.; Connell, S.D.; Irving, A.D.; Russell, B.D. Asymmetric patterns of recovery in two habitat forming seagrass species following simulated overgrazing by urchins. J. Exp. Mar. Biol. Ecol. 2013, 448, 114–120. [Google Scholar] [CrossRef]
- Christianen, M.J.A.; van Katwijk, M.M.; van Tussenbroek, B.I.; Pages, J.F.; Ballorain, K.; Kelkar, N.; Arthur, R.; Alcoverro, T. A dynamic view of seagrass meadows in the wake of successful green turtle conservation. Nat. Ecol. Evol. 2021, 5, 553–555. [Google Scholar] [CrossRef]
- Pérez, D.; Galindo, L. Efectos de la hiposalinidad en Thalassia testudinum (Hydrocharitaceae) del Parque Nacional Morrocoy, Venezuela. Rev. Biol. Trop. 2000, 48, 251–260. [Google Scholar]
- Chollett, I.; Bone, D.; Perez, D. Effects of heavy rainfall on Thalassia testudinum beds. Aquat. Bot. 2007, 87, 189–195. [Google Scholar] [CrossRef]
- Van Tussenbroek, B.I.; Galindo, C.A.; Marquez, J. Dormancy and foliar density regulation in Thalassia testudinum. Aquat. Bot. 2000, 68, 281–295. [Google Scholar] [CrossRef]
- Lipkin, Y. Seagrass Vegetation of Sinai and Israel; McRoy, C.P., Helfferich, C., Eds.; M. Dekker Inc.: New York, NY, USA, 1977; pp. 263–293. [Google Scholar]
- Lipkin, Y.; Beer, S.; Zakai, D. The Seagrasses of the Eastern Mediterranean and the Red Sea; Green, E.P., Short, F.T., Eds.; UNEP, University of California Press: Berkeley, CA, USA, 2003; pp. 65–73. [Google Scholar]
- Fraser, M.W.; Kendrick, G.A.; Statton, J.; Hovey, R.K.; Zavala-Perez, A.; Walker, D.I. Extreme climate events lower resilience of foundation seagrass at edge of biogeographical range. J. Ecol. 2014, 102, 1528–1536. [Google Scholar] [CrossRef]
- Den Hartog, C.; Triest, L. A profound view and discourse on the typification and status of three confused taxa: Ruppia maritima, R. spiralis and R. cirrhosa. Bot. Mar. 2020, 63, 229–239. [Google Scholar] [CrossRef]
- Abu Hena, M.K.; Short, F.T.; Sharifuzzaman, S.M.; Hasan, M.; Rezowan, M.; Ali, M. Salt marsh and seagrass communities of Bakkhali Estuary, Cox’s Bazar, Bangladesh. Estuar. Coast. Shelf Sci. 2007, 75, 72–78. [Google Scholar] [CrossRef]
- Phan, T.T.H.; De Raeymaeker, M.; Luong, Q.D.; Triest, L. Clonal and genetic diversity of the threatened seagrass Halophila beccarii in a tropical lagoon: Resilience through short distance dispersal. Aquat. Bot. 2017, 142, 96–104. [Google Scholar] [CrossRef]
- Aye, A.A.; Hsan, A.M.; Soe-Htun, U. The morphotaxonomy and phytosociology of Halophila beccarii (Family: Hydrocharitaceae) inKalegauk Island, Mon State. Mawlamyine Univ. Res. J. 2014, 5, 1–5. [Google Scholar]
- Jagtap, T.G.; Untawale, A.G. Ecology of seagrass sed of Halophila beccarii (Aschers) in Mandovi Estuary, Goa. Indian J. Mar. Sci. 1981, 10, 402–404. [Google Scholar]
- Zakaria, M.H.; Sidik, B.J.; Hishamuddin, O. Flowering, fruiting and seedling of Halophila beccarii Aschers. (Hydrocharitaceae) from Malaysia. Aquat. Bot. 1999, 65, 199–207. [Google Scholar] [CrossRef]
- Kuo, J.; Kirkman, H. Halophila decipiens Ostenfeld in estuaries of southwestern Australia. Aquat. Bot. 1995, 51, 335–340. [Google Scholar] [CrossRef]
- Kenworthy, W.J. The role of sexual reproduction in maintaining populations of Halophila decipiens: Implications for the biodiversity and conservation of tropical seagrass ecosystems. Pac. Conserv. Biol. 2000, 5, 260–268. [Google Scholar] [CrossRef]
- Kuo, J.; Kanamoto, Z.; Toma, T.; Nishihira, M. Occurrence of Halophila decipiens Ostenfeld (hydrocharitaceae) in Okinawa island, Japan. Aquat. Bot. 1995, 51, 329–334. [Google Scholar] [CrossRef]
- Bell, S.S.; Fonseca, M.S.; Kenworthy, W.J. Dynamics of a subtropical seagrass landscape: Links between disturbance and mobile seed banks. Landsc. Ecol. 2008, 23, 67–74. [Google Scholar] [CrossRef]
- Kuo, J.; Kanamoto, Z.; Iizumi, H.; Mukai, H. Seagrasses of the genus Halophila Thouars (Hydrocharitaceae) from Japan. Acta Phytotaxon. Geobot. 2006, 57, 129–154. [Google Scholar]
- York, P.H.; Carter, A.B.; Chartrand, K.; Sankey, T.; Wells, L.; Rasheed, M.A. Dynamics of a deep-water seagrass population on the Great Barrier Reef: Annual occurrence and response to a major dredging program. Sci. Rep. 2015, 5, 13167. [Google Scholar] [CrossRef] [PubMed]
- Lewmanomont, K.; Supanwanid, C. Species composition of seagrasses at Haad Chao Mai National Part, Trang province, Thailand. Kasetsart Univ. Fish. Res. Bull. 2000, 22, 1–9. [Google Scholar]
- Hammerstrom, K.K.; Kenworthy, W.J.; Fonseca, M.S.; Whitfield, P.E. Seed bank, biomass, and productivity of Halophila decipiens, a deep water seagrass on the west Florida continental shelf. Aquat. Bot. 2006, 84, 110–120. [Google Scholar] [CrossRef]
- Santamaria-Gallegos, N.A.; Riosmena-Rodriguez, R.; Sanchez-Lizaso, J.L. Occurrence and seasonality of Halophila decipiens Ostenfeld in the Gulf of California. Aquat. Bot. 2006, 84, 363–366. [Google Scholar] [CrossRef]
- McDermid, K.J.; Gregoritza, M.C.; Freshwater, D.W. A new record of a second seagrass species from the Hawaiian archipelago: Halophila decipiens Ostenfeld. Aquat. Bot. 2002, 74, 257–262. [Google Scholar] [CrossRef]
- Josselyn, M.; Fonseca, M.; Niesen, T.; Larson, R. Biomass, production and decomposition of a deep-water seagrass, Halophila decipiens Ostenf. Aquat. Bot. 1986, 25, 47–61. [Google Scholar] [CrossRef]
- Williams, S.L. Disturbance and recovery of a deep-water Caribbean seagrass bed. Mar. Ecol. Prog. Ser. 1988, 42, 63–71. [Google Scholar] [CrossRef]
- McMillan, C.; Soong, K. An annual cycle of flowering, fruiting and seed reserve for Halophila decipiens Ostenfeld (Hydrocharitaceae) in Panama. Aquat. Bot. 1989, 34, 375–379. [Google Scholar] [CrossRef]
- Verhoeven, J.T.A. Ecology of Ruppia-dominated communities in western Europe. 1. Distribution of Ruppia representatives in relation to their autecology. Aquat. Bot. 1979, 6, 197–268. [Google Scholar] [CrossRef]
- Gesti, J.; Badosa, A.; Quintana, X.D. Reproductive potential in Ruppia cirrhosa (Petagna) Grande in response to water permanence. Aquat. Bot. 2005, 81, 191–198. [Google Scholar] [CrossRef]
- Riddin, T.; Adams, J.B. Influence of mouth status and water level on the macrophytes in a small temporarily open/closed estuary. Estuar. Coast. Shelf Sci. 2008, 79, 86–92. [Google Scholar] [CrossRef]
- Riddin, T.; Adams, J.B. The seed banks of two temporarily open/closed estuaries in South Africa. Aquat. Bot. 2009, 90, 328–332. [Google Scholar] [CrossRef]
- Vromans, D.C.; Adams, J.B.; Riddin, T. The phenology of Ruppia cirrhosa (Petagna) Grande and Chara sp. in a small temporarily open/closed estuary, South Africa. Aquat. Bot. 2013, 110, 1–5. [Google Scholar] [CrossRef]
- Kiorboe, T. Production of Ruppia cirrhosa (Petagna) Grande in mixed beds in Ringkobing Fjord (Denmark). Aquat. Bot. 1980, 9, 135–143. [Google Scholar] [CrossRef]
- Ménendez, M.; Comín, F.A. Seasonal patterns of biomass variation of Ruppia cirrhosa (Petagna) Grande and Potamogeton pectinatus L. in a coastal lagoon. Scient. Mar. 1989, 53, 633–638. [Google Scholar]
- Ménendez, M. Net production of Ruppia cirrhosa in the Ebro Delta. Aquat. Bot. 2002, 73, 107–113. [Google Scholar] [CrossRef]
- Ballester, R. Biomasa, estacionalidad y distribución de tres macrófitos: Ruppia cirrhosa, Cymodocea nodosa 7 Caulerpa prolifera en al mar Menor (Murcia, SE de España). An. De Biol. 1985, 4, 31–36. [Google Scholar]
- Calado, G.; Duarte, P. Modelling growth of Ruppia cirrhosa. Aquat. Bot. 2000, 68, 29–44. [Google Scholar] [CrossRef]
- Casagranda, C.; Boudouresque, C.F. Biomass of Ruppia cirrhosa and Potamogeton pectinatus in a Mediterranean brackish lagoon, Lake Ichkeul, Tunisia. Fundam. Appl. Limnol. 2007, 168, 243–255. [Google Scholar] [CrossRef]
- Bonis, A.; Lepart, J.; Grillas, P. Seed bank dynamics and coexistence of annual macrophytes in a temporary and variable habitat. Oikos 1995, 74, 81–92. [Google Scholar] [CrossRef]
- Malea, P.; Kevrekidis, T.; Mogias, A. Annual versus perennial growth cycle in Ruppia maritima L.: Temporal variation in population characteristics in Mediterranean lagoons (Monolimni and Drana Lagoons, Northern Aegean Sea). Bot. Mar. 2004, 47, 357–366. [Google Scholar] [CrossRef]
- Bigley, R.E. The Population Biology of Two Intertidal Seagrasses, Zostera japonica and Ruppia maritima, at Roberts Bank, British Columbia. Master’s Thesis, University of British Columbia, Vancouver, BC, Canada, 1981. [Google Scholar]
- Richardson, F.D. Ecology of Ruppia maritima L. in New Hampshire (USA) tidal marshes. Rhodora 1980, 82, 403–439. [Google Scholar]
- Acosta, L.W.; Sabbatini, M.R.; Fernandez, O.A.; Burgos, M.A. Propagule bank and plant emergence of macrophytes in artificial channels of a temperate irrigation area in Argentina. Hydrobiologia 1999, 415, 1–5. [Google Scholar] [CrossRef]
- Silberhorn, G.M.; Dewing, S.; Mason, P.A. Production of reproductive shoots, vegetative shoots, and seeds in populations of Ruppia maritima L from the Chesapeake Bay, Virginia. Wetlands 1996, 16, 232–239. [Google Scholar] [CrossRef]
- Koch, E.W.; Seeliger, U. Germination ecology of 2 Ruppia maritima L. populations in southern Brazil. Aquat. Bot. 1988, 31, 321–327. [Google Scholar] [CrossRef]
- McGovern, T.M. Growth and reproduction of Ruppia maritima in the northern Gulf of Mexico. Gulf Mex. Sci. 2009, 2, 91–101. [Google Scholar] [CrossRef]
- Dunton, K.H. Production ecology of Ruppia maritima l. S1 and Halodule wrightii Aschers in 2 subtropical estuaries. J. Exp. Mar. Biol. Ecol. 1990, 143, 147–164. [Google Scholar] [CrossRef]
- Flores-Verdugo, F.J.; Day, J.W.; Mee, L.; Briseño-Dueñas, R. Phytoplankton production and seasonal biomass variation of seagrass, Ruppia maritima L., in a tropical Mexican lagoon with an ephemeral inlet. Estuaries 1988, 11, 51–56. [Google Scholar] [CrossRef]
- Rosenzweig, M.S.; Parker, B.C. Turion production by Ruppia maritima in Chesapeake Bay. Rhodora 2002, 104, 309–311. [Google Scholar]
- Taxeira da Silva, E.; Asmus, M.L. A dynamic simulation model of the widgeon grass Ruppia maritima and its epiphytes in the estuary of the Patos Lagoon, RS, Brazil. Ecol. Model. 2001, 137, 161–179. [Google Scholar] [CrossRef]
- Cho, H.J.; Poirrier, M.A. Seasonal growth and reproduction of Ruppia maritima L. s.l. in Lake Pontchartrain, Louisiana, USA. Aquat. Bot. 2005, 81, 37–49. [Google Scholar] [CrossRef]
- Pulich, W.M. Seasonal growth dynamics of Ruppia maritima L SL and Halodule wrightii Aschers in southern Texas and evaluation of sediment fertility status. Aquat. Bot. 1985, 23, 53–66. [Google Scholar] [CrossRef]
- McMillan, C. The seed reserve for Halodule wrightii, Syringodium filiforme and Ruppia maritima in Laguna Madre, Texas. Contrib. Mar. Sci. 1985, 28, 141–149. [Google Scholar]
- Lazar, A.C.; Dawes, C.J. A seasonal study of the seagrass Ruppia maritima L. in Tampa Bay, Florida (USA): Organic constituents and tolerances to salinity and temperature. Bot. Mar. 1991, 34, 265–269. [Google Scholar] [CrossRef]
- Strazisar, T.; Koch, M.S.; Dutra, E.; Madclen, C.J. Ruppia maritima L. seed bank viability at the Everglades-Florida Bay ecotone. Aquat. Bot. 2013, 111, 26–34. [Google Scholar] [CrossRef]
- Lopez-Calderon, J.; Riosmena-Rodriguez, R.; Rodriguez-Baron, J.M.; Carrion-Cortez, J.; Torre, J.; Meling-Lopez, A.; Hinojosa-Arango, G.; Hernandez-Carmona, G.; Garcia-Hernandez, J. Outstanding appearance of Ruppia maritima along Baja California Sur, Mexico and its influence in trophic networks. Mar. Biodivers. 2010, 40, 293–300. [Google Scholar] [CrossRef]
- Congdon, R.A.; McComb, A.J. Productivity of Ruppia—Seasonal—Changes and dependence on light in an Australian estuary. Aquat. Bot. 1979, 6, 121–132. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, Y.; Xu, S.; Wang, P.; Zhao, P.; Yue, S.; Gu, R.; Song, X.; Xu, S.; Liu, J.X.; et al. Differences in reproductive effort and sexual recruitment of the seagrass Zostera japonica between two geographic populations in northern China. Mar. Ecol. Prog. Ser. 2020, 638, 65–81. [Google Scholar] [CrossRef]
- Lee, S.Y.; Oh, J.H.; Choi, C.I.; Suh, Y.; Mukai, H. Leaf growth and population dynamics of intertidal Zostera japonica on the western coast of Korea. Aquat. Bot. 2005, 83, 263–280. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kim, J.B.; Lee, S.M. Temporal dynamics of subtidal Zostera marina and intertidal Zostera japonica on the southern coast of Korea. Mar. Ecol. Evol. Perspect. 2006, 27, 133–144. [Google Scholar] [CrossRef]
- Nakaoka, M.; Aioi, K. Ecology of seagrasses Zostera spp. (Zosteraceae) in Japanese waters: A review. Otsuchi Mar. Sci. 2001, 26, 7–22. [Google Scholar]
- Park, S.R.; Kim, Y.K.; Kim, J.H.; Kang, C.K.; Lee, K.S. Rapid recovery of the intertidal seagrass Zostera japonica following intense Manila clam (Ruditapes philippinarum) harvesting activity in Korea. J. Exp. Mar. Biol. Ecol. 2011, 407, 275–283. [Google Scholar] [CrossRef]
- Lee, S.Y. Annual cycle of biomass of a threatened population of the intertidal seagrass Zostera japonica in Hong Kong. Mar. Biol. 1997, 129, 183–193. [Google Scholar] [CrossRef]
- Huong, T.T.L.; Vermaat, J.E.; Terrados, J.; Van Tien, N.; Duarte, C.M.; Borum, J.; Tri, N.H. Seasonality and depth zonation of intertidal Halophila ovalis and Zostera japonica in Ha Long Bay (northern Vietnam). Aquat. Bot. 2003, 75, 147–157. [Google Scholar] [CrossRef]
- Ruesink, J.L.; Hong, J.S.; Wisehart, L.; Hacker, S.D.; Dumbauld, B.R.; Hessing-Lewis, M.; Trimble, A.C. Congener comparison of native (Zostera marina) and introduced (Z. japonica) eelgrass at multiple scales within a Pacific Northwest estuary. Biol. Invasions 2010, 12, 1773–1789. [Google Scholar] [CrossRef]
- Kaldy, J.E. Production ecology of the non-indigenous seagrass, dwarf eelgrass (Zostera japonica Ascher. & Graeb.), in a Pacific Northwest estuary, USA. Hydrobiologia 2006, 553, 201–217. [Google Scholar]
- Lopez-Calderon, J.M.; Riosmena-Rodriguez, R.; Torre, J.; Meling, A.; Basurto, X. Zostera marina meadows from the Gulf of California: Conservation status. Biodivers. Conserv. 2016, 25, 261–273. [Google Scholar] [CrossRef]
- Morita, T.; Okumura, H.; Abe, M.; Kurashima, A.; Maegawa, M. Density and distribution of seeds in bottom sediments in Zostera marina beds in Ago Bay, central Japan. Aquat. Bot. 2007, 87, 38–42. [Google Scholar] [CrossRef]
- Imao, K.; Fushimi, H. Ecology of the eelgrass (Zostera marina), especially environmental factors determining the occurrence of annual eelgrass in Lake Hamana-ko (Japan). Jpn. J. Phycol. 1985, 33, 320–327. [Google Scholar]
- Boese, B.L.; Robbins, B.D.; Thursby, G. Desiccation is a limiting factor for eelgrass (Zostera marina L.) distribution in the intertidal zone of a northeastern Pacific (USA) estuary. Bot. Mar. 2005, 48, 274–283. [Google Scholar] [CrossRef]
- Robertson, A.I.; Mann, K.H. Disturbance by ice and life-history adaptations of the seagrass Zostera marina. Mar. Biol. 1984, 80, 131–141. [Google Scholar] [CrossRef]
- van Katwijk, M.M.; Bos, A.R.; Kennis, P.; de Vries, R. Vulnerability to eutrophication of a semi-annual life history: A lesson learnt from an extinct eelgrass (Zostera marina) population. Biol. Conserv. 2010, 143, 248–254. [Google Scholar] [CrossRef]
- Erftemeijer, P.L.A.; van Beek, J.K.L.; Ochieng, C.A.; Jager, Z.; Los, H.J. Eelgrass seed dispersal via floating generative shoots in the Dutch Wadden Sea: A model approach. Mar. Ecol. Prog. Ser. 2008, 358, 115–124. [Google Scholar] [CrossRef]
- Cabello-Pasini, A.; Muniz-Salazar, R.; Ward, D.H. Annual variations of biomass and photosynthesis in Zostera marina at its southern end of distribution in the North Pacific. Aquat. Bot. 2003, 76, 31–47. [Google Scholar] [CrossRef]
- Poumian-Tapia, M.; Ibarra-Obando, S.E. Demography and biomass of the seagrass Zostera marina in a Mexican coastal lagoon. Estuaries 1999, 22, 837–847. [Google Scholar] [CrossRef]
- Lee, K.S.; Park, J.I.; Kim, Y.K.; Park, S.R.; Kim, J.H. Recolonization of Zostera marina following destruction caused by a red tide algal bloom: The role of new shoot recruitment from seed banks. Mar. Ecol. Prog. Ser. 2007, 342, 105–115. [Google Scholar] [CrossRef]
- Xu, S.C.; Wang, P.M.; Zhou, Y.; Zhang, X.M.; Gu, R.T.; Liu, X.J.; Liu, B.J.; Song, X.Y.; Xu, S.; Yue, S.D. New Insights into Different Reproductive Effort and Sexual Recruitment Contribution between Two Geographic Zostera marina L. Populations in Temperate China. Front. Plant Sci. 2018, 9, 15. [Google Scholar] [CrossRef]
- Jarvis, J.C.; Moore, K.A. The role of seedlings and seed bank viability in the recovery of Chesapeake Bay, USA, Zostera marina populations following a large-scale decline. Hydrobiologia 2010, 649, 55–68. [Google Scholar] [CrossRef]
- Silberhorn, G.M.; Orth, R.J.; Moore, K.A. Anthesis and seed production in Zostera marina L. (Eelgrass) from the Chesapeake Bay. Aquat. Bot. 1983, 15, 133–144. [Google Scholar] [CrossRef]
- Harwell, M.C.; Orth, R.J. Seed bank patterns in Chesapeake Bay eelgrass (Zostera marina L.): A bay-wide perspective. Estuaries 2002, 25, 1196–1204. [Google Scholar] [CrossRef]
- Greve, T.M.; Krause-Jensen, D.; Rasmussen, M.B.; Christensen, P.B. Means of rapid eelgrass (Zostera marina L.) recolonisation in former dieback areas. Aquat. Bot. 2005, 82, 143–156. [Google Scholar] [CrossRef]
- Olesen, B.; Krause-Jensen, D.; Christensen, P.B. Depth-related changes in reproductive strategy of a cold-temperate Zostera marina meadow. Estuaries Coasts 2017, 40, 553–563. [Google Scholar] [CrossRef]
- Olesen, B. Reproduction in Danish eelgrass (Zostera marina L.) stands: Size-dependence and biomass partitioning. Aquat. Bot. 1999, 65, 209–219. [Google Scholar] [CrossRef]
- Thorne-Miller, B.; Harlin, M.M. The production of Zostera marina L. and other submerged macrophytes in a coastal lagoon in Rhode Island, U.S.A. Bot. Mar. 1984, 27, 539–546. [Google Scholar] [CrossRef]
- Harmsen, G.W. Systematische Beobachtungen der Nordwest-Europäischen Seegrasformen. Ned. Kruidkd. Arch. 1936, 46, 852–877. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).