Abstract
The amount of atmospheric nitrogen-containing aerosols has increased dramatically due to the globally rising levels of nitrogen from fertilization and atmospheric deposition. Although the balance of carbon and nitrogen in plants is a crucial component of physiological and biochemical indexes and plays a key role in adaptive regulation, our understanding of how nitrogen-containing aerosols affect this remains limited; in particular, regarding the associated mechanisms. Using a fumigation particle generator, we generated ammonium nitrate solution (in four concentrations of 0, 15, 30, 60 kg N hm−2 year−1) into droplets, in 90% of which the diameters were less than 2.5 μm, in the range of 0.35–4 μm, and fumigated Iris germanica L. and Portulaca grandiflora Hook. for 30 days in April and August. We found that the weight percentage of nitrogen in the upper epidermis, mesophyll tissue, and bulk of leaves decreased significantly with the N addition rate, which caused a decrease of carbon:nitrogen ratio, due to the enhanced net photosynthetic rate. Compared with Portulaca grandiflora Hook., Iris germanica L. responded more significantly to the disturbance of N addition, resulting in a decrease in the weight percentage of nitrogen in the roots, due to a lower nitrogen use efficiency. In addition, the superoxide dismutase activity of the two plants was inhibited with a higher concentration of nitrogen sol; a reduction of superoxide dismutase activity in plants means that the resistance of plants to various environmental stresses is reduced, and this decrease in superoxide dismutase activity may be related to ROS signaling. The results suggest that inorganic nitrogen-containing aerosols caused excessive stress to plants, especially for Iris germanica L.
1. Introduction
The extensive use of nitrogen-containing fertilizers, industrial emission, automobile exhaust, and fossil fuels has dramatically increased the amount of atmospheric nitrogen-containing aerosols [1]. Excessive deposition of nitrogen leads to soil acidification and water eutrophication, which threatens the stability of nitrogen-related ecological processes [2,3,4], and has a substantial negative impact on the structure and function of ecosystems globally [5,6,7,8]. According to their aerodynamic diameter, the aerosols in particulate matters (PM) can be classified as PM10 (Ø ≤ 10 μm), PM2.5 (Ø ≤ 2.5 μm), and PM0.1 (Ø ≤ 0.1 μm) [9]. Among them, PM2.5 is known for its difficult settlement, a wide range of influences, great harm to the human body, control difficulties due to a small particle size, and easy enrichment [10]. Furthermore, water soluble inorganic salt is the main component of PM2.5, and its contribution rate to the mass concentration of PM2.5 is more than 40% [11]. NH4+ and NO3− are the main components of water-soluble inorganic salts [12]. Therefore, study of the environmental effects of inorganic-nitrogen-formed PM2.5 is important to understand the effect of aerosols on the physiological and ecological processes of animals and plants.
As a natural purifier to improve the environment, plants can effectively block and absorb aerosols and other particles in the air, and play a leading role in improving air quality [13,14]. The dust retention ability of plants is closely related to their leaf morphology and leaf surface characteristics. Leaves with a rough surface, or that are fluffy or mucus secreting, are more likely to absorb aerosols and other particles in the atmosphere [15]. Meanwhile, increased emissions of inorganic-nitrogen-formed aerosols have influences on plants, either via affecting the soil chemistry and other abiotic and biotic interactions, which have been well studied in the form of traditional nitrogen addition or fertilization [16,17,18], or via surface penetration on above ground organs directly [19], about which knowledge remains limited. It used to be believed that PM0.1, which represents only a small proportion of aerosols, was the main component that can pass through the plant stomata. However, Lehndorff et al. [20] showed evidence that PM2.5 can also penetrate into the leaves through the stomata, and it became the dominate component affecting physiological and biochemical process in leaves, due to its high proportion in aerosols comparing to PM0.1.
It has been reported that, due to the proportional dependence on carbon and nitrogen caused by the long-term evolution of plants, proper application of nitrogen to the leaves will lead to an increase of photosynthetic rate, to maintain the carbon and nitrogen balance [21,22], and some plants growing in adversity can also actively increase their photosynthetic rate, to improve nitrogen metabolism [23,24]. The high concentration of nitrogen in air can have a negative effect on the physiology and growth of individual plants [25,26], caused by the cellular acidosis and the destruction of electron transport in chloroplasts, which usually results in yellowing, slowed growth, and necrosis of leaves [27]. When a nitrogen-containing droplet enters the plant through the stomata on the leaves, it dissolves rapidly in the continuous area of the surrounding cell wall [28]. An overdose of nitrogen in the cell wall will inhibit the activity and content of photosynthetic enzymes in leaves, and eventually lead to a decrease of photosynthetic rate and carbon nitrogen ratios. At the same time, it can depress the production of NADP+ in chloroplasts, increase the content of active oxygen, and cause oxidative damage [29,30], which can be reflected by the change of superoxide dismutase (SOD) activity. SOD is the first antioxidant enzyme shown to play a role in the process of scavenging reactive oxygen species [31]. In addition, nitrous oxide dissolved in cells will be reduced to ammonium by nitrous reductase and then combined with other substances, to form macromolecular amino acids or proteins [32].
However, these results usually came from experiments spraying or smearing the nitrogenous solution on the leaves of fruit trees or other crops, while the spraying of nitrogen fertilizer on leaves, known as foliar fertilization, is an important management method in agriculture [33], in which the effect of nitrogen application is more through infiltration than through penetration into the sub-pores [34]. The nitrogen concentration in PM2.5 is usually represented by the unit μg/m3 [35,36] (such as 13.9–14.7 μg m−3 in 2004–2005 from Hangzhou City, China) [37], which is much less than the application concentration in foliar fertilization that usually uses the unit g N [38,39] (such as leaves spraying 0.5–1 g N plant−1 week−1 in a foliar fertilization experiment) [40]. In addition, the amount of nitrogen penetrating through stomata and participating in the process of metabolism and circulation in plants is also much less than the amount of nitrogen applied for fertilization. Therefore, whether the effects of inorganic nitrogen PM2.5 applications (with lower nitrogen concentrations and lesser penetration processes) on the photosynthesis and other physiological processes of plant leaves are all negative is worth exploring.
Iris germanica L. and Portulaca grandiflora Hook. are widely used garden plants and are representative C3 and C4 plants. The hypothesis that a C4 plant has much higher nitrogen use efficiency (NUE) than a C3 plant was put forward by Brown [5]. It can be summarized as follows: (1) C4 plants can assimilate NH4+ in both mesophyll cells and vascular bundle sheath cells, to synthesize amino acids and proteins, while C3 plants reduce nitrogen only in mesophyll cells, resulting in low NUE. (2) Compared with the CO2 fixed by C3, the nitrogen demand from the C4 photosynthetic pathway will increase correspondingly, to maintain the carbon–nitrogen balance [41]. Therefore, in the long-term evolution process, C4 evolved a complex biochemical adaptation mechanism to improve NUE, such as higher cytoplasmic nitratase [42,43], and lower Ribulose bisphosphate carboxylase/oxygenase (Rubisco) [44,45] and Calvin-Benson cycle enzyme contents [46]. Therefore, it has been reported that C4 plants suffer a competitive disadvantage during nitrogen addition treatments [47,48]. However, whether the amount of nitrogen penetrating across the stomata from PM2.5 is sufficient for the relief of nitrogen limitation remains unclear, although it had been reported that the photosynthetic rate of C3 plants increased slightly in the low concentration of gaseous nitrogen dioxide, without thorough discussion, because of the total decreased pattern, along with the increase of nitrogen addition concentration [49]. Iris germanica L. and Portulaca grandiflora Hook. have similar differences in light and coping, so experimental verification of their differences is required.
Therefore, inorganic nitrogen containing aerosol fumigation needs to be conducted to improve our understanding of the response of carbon and nitrogen balance in plants. The objectives of this study were (1) to examine the effects of inorganic nitrogen PM2.5 on photosynthetic rate and carbon/nitrogen assignment, and (2) to evaluate the different strategies of Iris germanica L. and Portulaca grandiflora Hook. under its influence, due to their different NUE.
2. Materials and Methods
2.1. Materials Preparation
Iris germanica L. and Portulaca grandiflora Hook. were chosen as the experiment materials, due to their common use as urban greening herb species. The two species were sown in a greenhouse in February with uniform nursery soil (Model 422, Klasmann-Deilmann GMBH Incorporated, Geeste, German), located in Jiangsu Academy of Forestry, Nanjing, China. Thirty two plants growing in unison for both species were chosen in April and August, which was the vegetative stage for both.
2.2. Experimental Design
A six-jet Atomizer (Model: 9306A, TSI Incorporated, Shoreview, MN 55126, USA) with compressed air pump (Model 36-7, Jiebao Incorporated, Shanghai, China) was used as the fumigation particle generator and generated ammonium nitrate solution into droplets, in 90% of which the diameters were less than 2.5 μm, in the range of 0.35–4 μm. This was calibrated by adjusting nozzle the valve and monitored using a Dusttrak II particulate monitor (Model: 8530, TSI Incorporated, Shoreview, MN 55126, USA). The plants were placed in a series of chambers, alternately connected with rubber hose and PVC pipe (Figure 1). The size of each chamber was 30 × 30 × 65 cm, with a side sliding door. In order to prevent nitrogen-contained aerosols from being absorbed by the soil, a plastic film was used to wrap the containers for planting plants, to eliminate the influence of fumigation on the soil. For watering, a PVC pipe with a lid on the top was inserted into the soil.
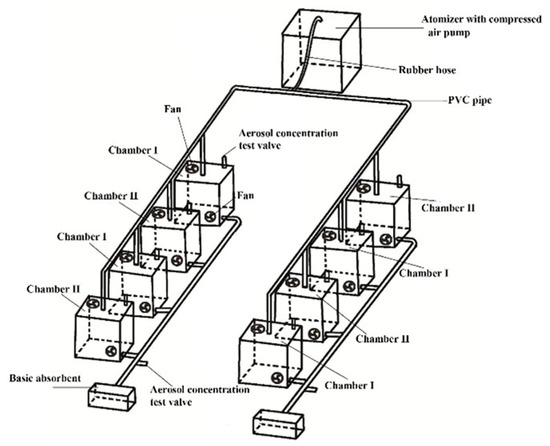
Figure 1.
Experimental setup diagram of one application concentration of inorganic nitrogen fumigation. Chambers I and II were for the two types of tested plants (Iris germanica L. and Portulaca grandiflora Hook.). The aerosol concentration test valve was for the monitoring of aerosol concentration, which was only opened for concentration checking during fumigating. The basic absorbent was sodium bicarbonate solution. The fans were for the uniform distribution of aerosols, temperature, and CO2, vertically and horizontally.
The concentration of aerosols was set at 50 μg/m3 through the adjustment of a flux valve, while the value of Nanjing city was 43–74 μg/m3 in 2014–2016 [50]. The nitrogen levels were 0, 15 (half dose), 30 (background dose), and 60 (double dose) kg N hm−2 year−1 (32 plants were needed for each species), while bulk deposition fluxes of inorganic nitrogen averaged 35.8 kg N hm−2 year−1, and wet deposition fluxes of inorganic nitrogen were 28.7 kg N hm−2 year−1 [51]. In order to maintain the uniformity of carbon dioxide and temperature, the control was fumigated using pure water vapor, to ensure the uniformity of environmental factors (such as temperature, CO2 concentration, etc.)
2.3. Sampling and Measurements
From 1 April to 30 April (non-growing season) and from 15 July to 15 August (growing season) 2018, under natural light conditions, leaves with the same status and complete shape were selected to measure the physiological indexes of net photosynthetic rate, transpiration rate, and stomatal conductance, using a portable photosynthetic instrument (Model: LI-6400, LI-COR Incorporated, Lincoln, Nebraska 68504, USA), which was completed before 12:00 a.m. in sunny weather (once each week during fumigations and lasting for one month (5 measurements)). On 1 May (non-growing season) and 16 August (growing season) of 2018, leaves with the same growth, complete shape, and healthy maturity from each plant were taken, and the roots were collected, from 10:00 a.m. to 12:00 a.m., and then stored with dry ice and brought back to the laboratory for testing.
The preparation process was as follows: Weigh about 0.2 g of fresh leaves, cut them into pieces, add 10 mL of precooled PBS 7.8 solution (added twice), grind and extract, then centrifuge at 4 °C in 10,500 rpm for 15 min, take the supernatant, and store at 4 °C. SOD activity was tested with the nitro blue tetrazolium (NBT) photoreduction method [52]. An enzyme activity unit determined 50% inhibition of NBT photoreduction. It was calculated as follows:
where ACK is the light absorption value of illumination to the care; AE is the light absorption value of the sample tube; V is the total volume of sample solution; Vt is the dosage of sample solution in determination; and W is the weight of samples.
SOD activity = [(ACK − AE) × V]/(ACK × 0.5 × W × Vt)
Total C and N were measured using an element analyzer (Elementar Vario EL, Hanau, Germany) from part of the leaves and roots, which had already been oven baked for 30 min at 105 °C, dried to constant weight at 55–65 °C, and ground.
The other parts of leaves were prepared as portrait section slices with a square blade, with side length of 5 mm, after freeze-drying, and the chemical composition of their upper epidermis and mesophyll tissue (Example diagram in Figure 2) was studied using a field emission scanning electron microscope (Model: JSM-7600F, JEOL Incorporated, Akishima, Japan).
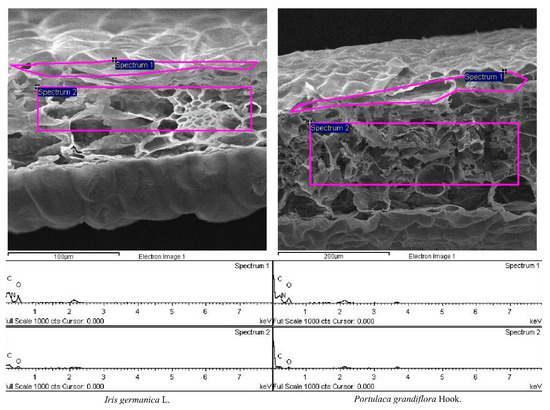
Figure 2.
Example of field emission scanning electron microscope output for the leaves of two species. Spectrum 1 and 2 are the sample area for the weight percentage (%) of carbon (C), nitrogen (N), and oxygen (O) in the upper epidermis and mesophyll tissue of leaves, respectively.
2.4. Statistical Analysis
To test the effects of species, nitrogen addition, weeks in fumigation, and season on the net photosynthetic rate, stomatal conductance, and the weight percentage of carbon and nitrogen in the upper epidermis, mesophyll tissue, and bulk of leaves, we used the following linear mixed model:
where Yijklmn is the net photosynthetic rate, stomatal conductance, and the weight percentage of carbon and nitrogen in the upper epidermis, mesophyll tissue, and bulk of leaves; Sn (n = 1, 2); Ni (i = 0, 1, 2, 3) is the level of nitrogen application (0, 15, 30, 60 kg N hm−2 year−1); Mj (j = 1, 2) is the month (April and August); Dk (k = 1, 2, 3, 4, 5) is sample date (3, 8, 15, 22, 30 days in fumigations), which was excluded in the analysis of the weight percentage of carbon and nitrogen, because they were measured only once at the end of each experimental period; πl represents the random plot effect (l = 1, 2, …16) nested in the two random blocks; and ɛm(ijkl) (m = 1, 2) is the sampling error. We conducted a linear mixed effect analysis, using the restricted maximum likelihood estimation with the “lme4” package [53]. We then calculated the absolute values of spring and summer plant measurements: photosynthetic rate (Ph), transpiration rate (Tr), stomatal conductance (gs) and the corresponding standard errors (Table 1).

Table 1.
The absolute values of plant measurements in spring and summer: photosynthetic rate (Ph), transpiration rate (Tr), stomatal conductance (gs), and corresponding standard errors.
To better understand the mechanisms associated with changes in these physiological and biochemical indexes, we used Pearson correlation analysis, which was performed using the “PerformanceAnalysis” package [54], to examine the extent of associations. All analyses were performed using R Statistical Software (Version: 4.1.3, The R Foundation for Statistical Computing c/o Institute for Statistics and Mathematics, Vienna, Australia) [55].
3. Results
3.1. The Effect on Carbon and Nitrogen Distribution in Leaves
Our result showed that the percentage weight of nitrogen was enhanced and carbon was depressed with the increase of nitrogen concentration in the form of aerosols, which caused an increase of carbon and nitrogen weight ratios. The concentration of inorganic nitrogen in the form of aerosols affected the nitrogen in the upper epidermis and the bulk of Iris germanica L. leaves, and only the percentage weight of carbon in the mesophyll tissue of its leaves varied significantly, while several interaction effects were statistically significant (Table 2). The weight ratios of carbon and nitrogen in the upper epidermis and mesophyll tissue of Iris germanica L. leaves increased significantly as the application was increased to 60 kg N hm−2 year−1, due to the dramatic increase (rising by 19.4% comparing to 0 kg N hm−2 year−1) of carbon and decrease of nitrogen (declined 26.4% comparing to 0 kg N hm−2 year−1), respectively, which was not found in Portulaca grandiflora Hook. (Figure 3A–F). Furthermore, the nitrogen containing PM2.5 induced a decrease of nitrogen percentage weight and caused a decrease of the nitrogen percentage weight in the bulk leaves, although it did not show significant differences for Portulaca grandiflora Hook., due to the considerable standard errors (Figure 3H).

Table 2.
The effects of nitrogen-containing PM2.5 addition concentration (N), sampling date (M), and their interaction (N × M) on the weight percentage of carbon (C), nitrogen (N), and C/N in the upper epidermis (UE), mesophyll tissue (MT), bulk of leaves (BL), and roots (R) in Iris germanica L. and Portulaca grandiflora Hook. The linear mixed-effects model used the Kenward–Roger method as a denominator of degrees of freedom.
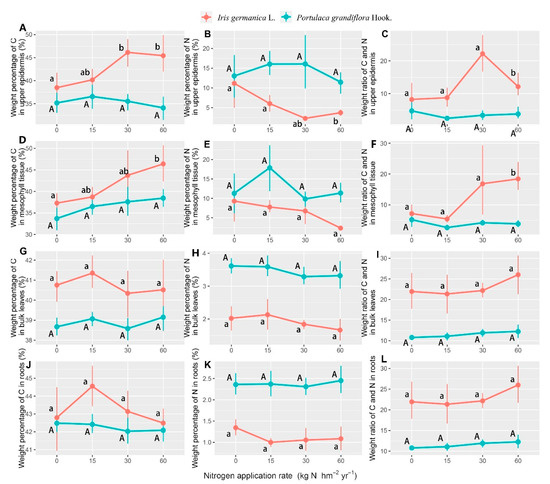
Figure 3.
The response of the percentage weight of carbon (C), nitrogen (N), and C/N in the roots (R) (A–C), the upper epidermis (UE) (D–F), mesophyll tissue (MT) (G–I), and bulk of leaves (BL) (J–L) to the nitrogen application rate in the form of aerosols in Iris germanica L. and Portulaca grandiflora Hook. (averages of two sampling seasons). Values are means with 95% bootstrapped confidence intervals (CI). Differences are significant at α = 0.05, when the CI does not overlap the subsequent mean. Upper and lower case letters indicate the significantly different results of Portulaca grandiflora Hook. and Iris Germanica L. on applied nitrogen concentration, respectively.
3.2. Effect on the Net Photosynthetic Rate, Transpiration Rate, and Stomatal Conductance
A significantly increased net photosynthetic rate and stomatal conductance were also found in response to the nitrogen application rate, while the transpiration rate and SOD decreased with the nitrogen application rate up to 30 and 60 kg N hm−2 year−1 (Table 3, Figure 4). The net photosynthetic rate showed no significant differences at the nitrogen application rates of 0 and 15 kg N hm−2 year−1, and Iris germanica L. showed a more significant increase up to the highest nitrogen application (Figure 4A), which was totally opposite for the SOD activities. Portulaca grandiflora Hook. had higher SOD activities at the nitrogen application rates of 0 and 15 kg N hm−2 year−1, but this effect disappeared under the trend of a rapid decline at higher nitrogen application rates (Figure 4D). The transpiration rate showed significant variation between the two species, but no significant variation existed for the stomatal conductance (Figure 4B,C).

Table 3.
The effects of inorganic nitrogen PM2.5 (N), species(S), days in fumigation (D), experimental month (M), and their interactions on the net photosynthetic rate, transpiration rate, and stomatal conductance of Iris germanica L. and Portulaca grandiflora Hook. The linear mixed-effects model used the Kenward–Roger method as a denominator for degrees of freedom.
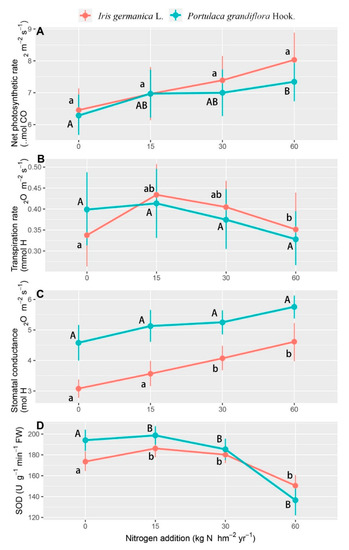
Figure 4.
The response of the net photosynthetic rate (Npr) (A), transpiration rate (Tr) (B), stomatal conductance (gs) (C), and SOD activity (D) to nitrogen application rate in the form of aerosols in Iris germanica L. and Portulaca grandiflora Hook. over sampling dates. Values are means with 95% bootstrapped confidence intervals (CI). Differences are significant at α = 0.05, when the CI does not overlap the subsequent mean. Upper and lower case letters indicate significantly different results for Portulaca grandiflora Hook. and Iris Germanica L. on applied nitrogen concentration, respectively.
3.3. Correlation Analysis between Possible Drivers and Nitrogen Distribution in Plants
According to the correlation analysis, the net photosynthetic rate played an important role in the decreasing weight percentage of nitrogen in the leaves of Iris germanica L. but did not show the same pattern for Portulaca grandiflora Hook. (Figure 5), which could explain the differences in Figure 3H between these two species. Although the correlation between the net photosynthetic rate and nitrogen weight percentage in the upper epidermis of leaves was still uniformly positive in the two species, the nitrogen densities in the leaves and roots showed a contrary pattern (negative), in which the main effects of nitrogen application concentration were also only significant in Iris germanica L. (Table 2). In addition, the Pearson correlation analysis also showed the positive relationship between (1) the net photosynthetic rate and the stomatal conductance, and (2) the nitrogen density of leaves and roots in both Iris germanica L. and Portulaca grandiflora Hook. (Figure 5).
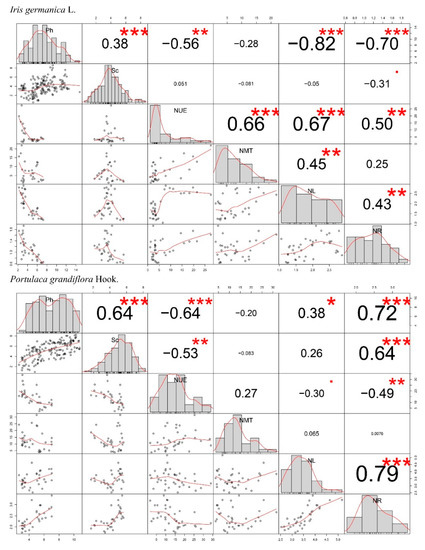
Figure 5.
Pearson correlations between the net photosynthetic rate (Ph), stomatal conductance (gs), the weight percentage of nitrogen (N) in the upper epidermis (UE), mesophyll tissue (MT), bulk of leaves (BL), and in the roots (R). At the bottom of the plot, bivariate scatter plots with a smooth line are displayed. At the top of the plot, the value of the correlation plus the significance level are displayed as asterisks (*** p < 0.001, ** p < 0.01, * p < 0.05). Units associated with the variables are shown in Figure 3 and Figure 4.
4. Discussion
4.1. Nitrogen-Containing Aerosol Affected the Balance of Carbon and Nitrogen
It is common for plants to maintain their internal carbon and nitrogen balance by regulating net the photosynthetic rate, to a ensure normal physiological process, which was widely recognized as the theory of carbon nitrogen ratio and often used as the theoretical basis for the management of nitrogen fertilizer addition to promote photosynthesis [56,57]. However, our results show that a nitrogen-containing aerosol induced an excessive increase of net photosynthetic rate and led to an imbalance of carbon and nitrogen, showing that the percentage of nitrogen in plant leaves decreased. To our knowledge, this is the first report to prove that nitrogen enters leaves directly through a stomata-induced increase of carbon and nitrogen ratios, which could be explained by the formation of excess photosynthetic products.
The carbon and nitrogen assimilation process is closely related and interacts through competition for photosynthesis. The photoreaction of photosynthesis produces ATP and reduces ferredoxin as intermediates through photophosphorylation. The reduced ferritin temporarily fixes electrons by reducing NADP+ to NADPH (for CO2 assimilation), and nitrate and nitrite ions to ammonium ion (for the synthesis of nitrogen-containing substances), which both involve the coupling of carbon and nitrogen [58,59]. Therefore, exogenous nitrogen can significantly affect the photosynthetic rate of plants [60], even the formation of chloroplasts [61], because plants tend to maintain a stable carbon nitrogen ratio [62]. Our results also showed a trend of net photosynthetic rate increasing with the increase of nitrogen concentration (Figure 4A), but not an inhibition due to the overdose of nitrogen, as some research reported [63,64,65], which showed that the nitrogen applied in this study was still at a low concentration level for plants.
However, a difference between this study and previous studies is the method of nitrogen application. Increases of photosynthetic rate caused by nitrogen application are mainly due to the active increase of stomatal conductance [66,67]. Nevertheless, in our case, the increase of stomatal conductance induced a further increase in the amount of nitrogen entering the pores and the sub-pores [68], which led to a temporary but large increase of nitrogen concentration in the gas exchange space in direct contact with the chloroplast. This is the mechanism by which “excessive photosynthesis” could happen. The chloroplast was misled by the increase of nitrogen concentration in the local tissues and formed excessive photosynthetic products, in order to balance the extra exogenous nitrogen. This can be supported by the aerosols dramatically inducing enhanced carbon nitrogen ratios in the upper epidermis and mesophyll tissue (Table 2). In addition, nitrogen can further closely regulate the activity of cell protective enzymes related to plant senescence, by regulating SOD activity, so as to regulate leaf senescence [69]. Our results showed that the decrease of relative nitrogen content (Figure 3) caused by excessive photosynthesis led to a decrease of SOD activity in leaves (Figure 4D), which may increase the malondialdehyde content in plants and induce the premature senescence of leaves [70].
4.2. The Different Response of Iris germanica L. and Portulaca grandiflora Hook. to Nitrogen Containing Aerosols
Our results showed that although the nitrogen application (p = 0.032) and species (p < 0.001) affected the percentage weight of nitrogen in leaves significantly, the response of Iris germanica L. (p = 0.043) and Portulaca grandiflora Hook. (p = 0.558) plants were different, which indicated that the depression of nitrogen percentage weight in leaves (Figure 3), due to “excessive photosynthesis”, had been alleviated in Portulaca grandiflora Hook. The results that the net photosynthetic rate of Portulaca grandiflora Hook. increased with the increase of nitrogen concentration, and that the change of leaf nitrogen mass percentage was different from that of Iris germanica L. (Figure 3 and Figure 4), may be explained by the following: The typical structural difference between C4 and C3 plants is the vascular bundle sheath cells with a Kranz ring structure [71]. C4 plants have a higher NUE, due to different characteristics of carbon metabolism (i.e., different positions of nitrogen reducing and assimilating enzymes in the cells) and different mechanisms of nitrogen absorption, reduction, and assimilation [5,72]. C4 plants can assimilate ammonium ions in both mesophyll cells and vascular bundle sheath cells, to synthesize amino acids and proteins, while C3 plants only reduce nitrogen in mesophyll cells, resulting in a low NUE and photosynthetic efficiency [73,74]. At the same time, in terms of cell anatomical structure, the distance between the veins of C4 plants is generally smaller than that of C3 plants, the density of veins is higher, and the number of mesophyll cell layers between veins of C4 plants is also less [75,76]. The higher NUE and photosynthetic efficiency of C4 plants caused a higher tolerance to the low nitrogen concentration, in the form of an aerosol-induced imbalance of carbon and nitrogen. This was also supported by the different correlation patterns between the net photosynthetic rate and weight percentage of nitrogen in leaves (Figure 5).
Furthermore, our results showed that the nitrogen in plant roots was transported upward, in order to alleviate the phenomenon of the decrease of nitrogen content in leaves, while the carbon and nitrogen balance in the leaves of Iris germanica L. was disturbed as discussed, which led to the significant decrease of nitrogen weight percentage in the roots (Figure 3K, Table 2). The organic nitrogen in the root system is transported to the leaves through the xylem, and glutamine and glutamate are formed through the assimilation reaction, to participate in nitrogen metabolism [77]. At the same time, the amino acids produced by nitrogen metabolism in the leaves can also be transported back to the root system through the phloem [78]. Therefore, there must be a linear positive correlation between the nitrogen content in leaves and roots [79,80], as shown in Figure 5. This determines that when the relative content of nitrogen in leaves decreases, in order to maintain the balance of carbon and nitrogen, the root system will increase the transport of nitrogen [81]. However, for Iris germanica L., with a low NUE, the additional nitrogen transport from roots could not offset the decline of nitrogen relative content caused by “excessive photosynthesis”, which eventually led to the decrease of nitrogen percentage weight in roots.
5. Conclusions
In summary, we found that the inorganic nitrogen-containing aerosols caused a decrease of nitrogen percentage weight in leaves, due to an excessively enhanced net photosynthetic rate, which led to an imbalance of carbon and nitrogen in the plants. Portulaca grandiflora Hook. had a higher tolerance due to its higher NUE, while the nitrogen weight percentage in the roots of Iris germanica L. was also affected. In addition, the SOD activities were depressed with higher nitrogen concentrations of aerosols for both species, which might have caused the premature senescence of leaves; hence, the effect of inorganic nitrogen containing aerosols on plants was generally negative.
Author Contributions
Conceptualization, Z.G. and W.X.; methodology, Z.G. and Y.M.; software, Y.M., Z.G. and W.X.; validation, L.M.; formal analysis, Y.W. and S.P.; investigation, Z.G., Y.M., Y.W., S.P., L.M. and Z.M.; resources, L.M. and S.P.; data curation, L.M. and Z.G.; writing—original draft, Z.G. and L.M.; writing—review and editing, Z.G. and Y.M.; visualization, L.M. and W.X.; supervision, Z.M. and Y.W.; project administration, Z.G. and L.M.; funding acquisition, Z.G., L.M. and Z.M. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Strategic Priority Research Program of Chinese Academy of Sciences (grant number XDB31000000), the National Natural Science Foundation of China (grant numbers 31870506, 41601254), the Natural Science Foundation of Jiangsu Province (grant number BK20181398, BK20190109), Jiangsu Forestry Science and Technology Innovation and Promotion Program (LYKJ [2021] 25) and Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We would like to thank the members of the Biodiversity and Conservation Research Group at Nanjing Forestry University for their valuable insights during the writing of this paper. Thanks to Han Y.H. Chen from Lakehead University, Canada, for his valuable comments in the process of improving the first draft of the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wu, L.; Yue, S.; Shi, Z.; Hu, W.; Chen, J.; Ren, H.; Deng, J.; Ren, L.; Fang, Y.; Yan, H.; et al. Source forensics of inorganic and organic nitrogen using δ15N for tropospheric aerosols over Mt. Tai. npj Clim. Atmos. Sci. 2021, 4, 1–8. [Google Scholar] [CrossRef]
- Chen, D.; Xing, W.; Lan, Z.; Saleem, M.; Wu, Y.; Hu, S.; Bai, Y. Direct and indirect effects of nitrogen enrichment on soil organisms and carbon and nitrogen mineralization in a semi-arid grassland. Funct. Ecol. 2019, 33, 175–187. [Google Scholar] [CrossRef]
- Lu, X.T.; Lu, Y.L.; Chen, D.L.; Su, C.; Song, S.; Wang, T.Y.; Tian, H.Q.; Liang, R.Y.; Zhang, M.; Khan, K. Climate change induced eutrophication of cold-water lake in an ecologically fragile nature reserve. J. Environ. Sci. China 2019, 75, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Wani, S.H.; Vijayan, R.; Choudhary, M.; Kumar, A.; Zaid, A.; Singh, V.; Kumar, P.; Yasin, J.K. Nitrogen use efficiency (NUE): Elucidated mechanisms, mapped genes and gene networks in maize (Zea mays L.). Physiol. Mol. Plant Pathol. 2021, 27, 2875–2891. [Google Scholar] [CrossRef]
- Brown, R.H. A Difference in N Use Efficiency in C3 and C4 Plants and its Implications in Adaptation and Evolution1. Crop Sci. 1978, 18, 93–98. [Google Scholar] [CrossRef]
- Kuang, Y.; Sun, F.; Wen, D.; Xu, Z.; Huang, L.; Li, J. Nitrogen deposition influences nitrogen isotope composition in soil and needles of Pinus massoniana forests along an urban-rural gradient in the Pearl River Delta of south China. J. Soils Sediments 2011, 11, 589–595. [Google Scholar] [CrossRef]
- Lu, X.K.; Vitousek, P.M.; Mao, Q.G.; Gilliam, F.S.; Luo, Y.Q.; Zhou, G.Y.; Zou, X.M.; Bai, E.; Scanlon, T.M.; Hou, E.Q.; et al. Plant acclimation to long-term high nitrogen deposition in an N-rich tropical forest. Proc. Natl. Acad. Sci. USA 2018, 115, 5187–5192. [Google Scholar] [CrossRef]
- Zhang, T.; Chen, H.Y.H.; Ruan, H. Global negative effects of nitrogen deposition on soil microbes. ISME J. 2018, 12, 1817–1825. [Google Scholar] [CrossRef]
- Gugamsetty, B.; Wei, H.; Liu, C.N.; Awasthi, A.; Hsu, S.C.; Tsai, C.J.; Roam, G.D.; Wu, Y.C.; Chen, C.F. Source Characterization and Apportionment of PM10, PM2.5 and PM0.1 by Using Positive Matrix Factorization. Aerosol Air Qual Res 2012, 12, 476–491. [Google Scholar] [CrossRef]
- Pachauri, T.; Satsangi, A.; Singla, V.; Lakhani, A.; Kumari, K.M. Characteristics and Sources of Carbonaceous Aerosols in PM2.5 during Wintertime in Agra, India. Aerosol Air Qual. Res. 2013, 13, 977–991. [Google Scholar] [CrossRef] [Green Version]
- Lin, G.Y.; Chen, H.W.; Chen, B.J.; Yang, Y.C. Characterization of temporal PM2.5, nitrate, and sulfate using deep learning techniques. Atmos. Pollut. Res. 2022, 13, 101260. [Google Scholar] [CrossRef]
- Xie, M.; Feng, W.; He, S.; Wang, Q. Seasonal variations, temperature dependence, and sources of size-resolved PM components in Nanjing, east China. J. Environ. Sci. China 2022, 121, 175–186. [Google Scholar] [CrossRef]
- Wang, X.; Wu, J.; Chen, M.; Xu, X.T.; Wang, Z.H.; Wang, B.; Wang, C.Z.; Piao, S.L.; Lin, W.L.; Miao, G.F.; et al. Field evidences for the positive effects of aerosols on tree growth. Glob. Change Biol. 2018, 24, 4983–4992. [Google Scholar] [CrossRef] [PubMed]
- McDonald, A.G.; Bealey, W.J.; Fowler, D.; Dragosits, U.; Skiba, U.; Smith, R.I.; Donovan, R.G.; Brett, H.E.; Hewitt, C.N.; Nemitz, E. Quantifying the effect of urban tree planting on concentrations and depositions of PM10 in two UK conurbations. Atmos. Environ. 2007, 41, 8455–8467. [Google Scholar] [CrossRef]
- McMurry, P.H.; Rader, D.J. Studies of aerosol formation in power plant plumes—I. Growth laws for secondary aerosols in power plant plumes: Implications for chemical conversion mechanisms. Atmos. Environ. 1967, 15, 2315–2327. [Google Scholar] [CrossRef]
- Ge, Z.W.; Fang, S.Y.; Chen, H.; Zhu, R.W.; Peng, S.L.; Ruan, H.H. Soil Aggregation and Organic Carbon Dynamics in Poplar Plantations. Forests 2018, 9, 508. [Google Scholar] [CrossRef]
- Matsumoto, K.; Ogawa, T.; Ishikawa, M.; Hirai, A.; Watanabe, Y.; Nakano, T. Organic and inorganic nitrogen deposition on the red pine forests at the northern foot of Mt. Fuji, Japan. Atmos. Environ. 2020, 237, 117676. [Google Scholar] [CrossRef]
- Zhong, M.X.; Miao, Y.; Han, S.J.; Wang, D. Nitrogen addition decreases seed germination in a temperate steppe. Ecol. Evol. 2019, 9, 8441–8449. [Google Scholar] [CrossRef]
- Adamson, J.; Jaunky, T.; Thorne, D.; Gaca, M.D. Characterisation of the borgwaldt LM4E system for in vitro exposures to undiluted aerosols from next generation tobacco and nicotine products (NGPs). Food Chem. Toxicol. 2018, 113, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Lehndorff, E.; Urbat, M.; Schwark, L. Accumulation histories of magnetic particles on pine needles as function of air quality. Atmos. Environ. 2006, 40, 7082–7096. [Google Scholar] [CrossRef]
- Magnani, F.; Mencuccini, M.; Borghetti, M.; Berbigier, P.; Berninger, F.; Delzon, S.; Grelle, A.; Hari, P.; Jarvis, P.G.; Kolari, P.; et al. The human footprint in the carbon cycle of temperate and boreal forests. Nature 2007, 447, 849–851. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Keenan, T.F.; Chen, J.M.; Croft, H.; Colin Prentice, I.; Smith, N.G.; Walker, A.P.; Wang, H.; Wang, R.; Xu, C.; et al. Global variation in the fraction of leaf nitrogen allocated to photosynthesis. Nat. Commun. 2021, 12, 4886. [Google Scholar] [CrossRef]
- Xu, Z.Z.; Zhou, G.S. Nitrogen metabolism and photosynthesis in Leymus chinensis in response to long-term soil drought. J. Plant Growth Regul. 2006, 25, 252–266. [Google Scholar] [CrossRef]
- Kelly, S. The Amount of Nitrogen Used for Photosynthesis Modulates Molecular Evolution in Plants. Mol. Biol. Evol. 2018, 35, 1616–1625. [Google Scholar] [CrossRef]
- Bobbink, R.; Hicks, K.; Galloway, J.; Spranger, T.; Alkemade, R.; Ashmore, M.; Bustamante, M.; Cinderby, S.; Davidson, E.; Dentener, F.; et al. Global assessment of nitrogen deposition effects on terrestrial plant diversity: A synthesis. Ecol. Appl. 2010, 20, 30–59. [Google Scholar] [CrossRef]
- Sheng, Q.; Zhu, Z. Physiological Response of European Hornbeam Leaves to Nitrogen Dioxide Stress and Self-recovery. J. Am. Soc. Hortic. Sci. 2019, 144, 23–30. [Google Scholar] [CrossRef]
- Stevens, C.J.; David, T.I.; Storkey, J. Atmospheric nitrogen deposition in terrestrial ecosystems: Its impact on plant communities and consequences across trophic levels. Funct. Ecol. 2018, 32, 1757–1769. [Google Scholar] [CrossRef]
- Hu, Y.B.; Bellaloui, N.; Tigabu, M.; Wang, J.H.; Diao, J.; Wang, K.; Yang, R.; Sun, G.Y. Gaseous NO2 effects on stomatal behavior, photosynthesis and respiration of hybrid poplar leaves. Acta Physiol. Plant. 2015, 37, 39. [Google Scholar] [CrossRef]
- Vranová, E.; Inzé, D.; Van, B.F. Signal transduction during oxidative stress. J. Exp. Bot. 2002, 53, 1227–1236. [Google Scholar] [CrossRef]
- Wei, T.B.; Wei, Y.J.; Yang, Z.H.; Zang, G.P.; Wu, Z.T.; Bing, S.X. Effects of Planting Density and Nitrogen Fertilizer on Active Components and Yield of Astragalus mongolica in Oasis Region. J. Agr. Nucl. Sin. 2022, 36, 1664–1675. [Google Scholar]
- Zhu, J.; Li, G.; Zhou, J.; Xu, Z.; Xu, J. Cytoprotective effects and antioxidant activities of acteoside and various extracts of Clerodendrum cyrtophyllum Turcz leaves against t-BHP induced oxidative damage. Sci. Rep. 2022, 12, 12630. [Google Scholar] [CrossRef] [PubMed]
- Dawar, K.; Sardar, K.; Zaman, M.; Müller, C.; Sanz-Cobena, A.; Khan, A.; Borzouei, A.; Perez -Castillo, A.G. Effects of the nitrification inhibitor nitrapyrin and the plant growth regulator gibberellic acid on yield-scale nitrous oxide emission in maize fields under hot climatic conditions. Pedosphere 2021, 31, 323–331. [Google Scholar] [CrossRef]
- Guak, S.; Neilsen, D.; Millard, P.; Looney, N.E. Leaf absorption, withdrawal and remobilization of autumn-applied urea-N in apple. Can. J. Plant Sci. 2004, 84, 259–264. [Google Scholar] [CrossRef]
- Liu, Q.Q.; Shi, J.; Cao, X.H.; Liu, C.; Xia, X.L.; Guo, H.H. Absorption and Distribution of PM2.5 NH4+ and NO3− in Populus euramericana Neva. Acta Ecol. Sin. 2015, 35, 6541–6548. [Google Scholar]
- Gu, X.K.; Yin, S.S.; Lu, X.; Zhang, H.; Wang, L.L.; Bai, L.; Wang, C.; Zhang, R.Q.; Yuan, M.H. Recent development of a refined multiple air pollutant emission inventory of vehicles in the Central Plains of China. J. Environ. Sci. China 2019, 84, 80–96. [Google Scholar] [CrossRef]
- Pilz, V.; Wolf, K.; Breitner, S.; Ruckerl, R.; Koenig, W.; Rathmann, W.; Cyrys, J.; Peters, A.; Schneider, A. C-reactive protein (CRP) and long-term air pollution with a focus on ultrafine particles. Int. J. Hyg. Environ. Health 2018, 221, 510–518. [Google Scholar] [CrossRef]
- Liu, G.; Teng, W.L.; Yang, Z.Q. Carbon, Nitrogen, Hydrogen and Heavy metals in Airborne PM2.5 in Hangzhou. J. Environ. Health 2007, 11, 890–892. [Google Scholar]
- Klanian, M.G.; Diaz, M.D.; Aranda, J.; Juarez, C.R. Integrated effect of nutrients from a recirculation aquaponic system and foliar nutrition on the yield of tomatoes Solanum lycopersicum L. and Solanum pimpinellifolium. Environ. Sci. Pollut. Res. 2018, 25, 17807–17819. [Google Scholar] [CrossRef]
- Liu, M.Y.; Tang, D.D.; Shi, Y.Z.; Ma, L.F.; Zhang, Q.F.; Ruan, J.Y. Foliar N Application on Tea Plant at Its Dormancy Stage Increases the N Concentration of Mature Leaves and Improves the Quality and Yield of Spring Tea. Front. Plant Sci. 2021, 12, 753086. [Google Scholar] [CrossRef]
- Nguyen, T.N.; Tang, L.H.; Peng, Y.K.; Ni, J.Y.; Chang, Y.N. Effects of Composite Inorganic, Organic Fertilizer and Foliar Spray of Multi-nutrients on Growth, Yield and Quality of Cherry Tomato. J. Agric. Sci. Technol. 2015, 17, 1781–1788. [Google Scholar]
- LeCain, D.R.; Morgan, J.A. Growth, gas exchange, leaf nitrogen and carbohydrate concentrations in NAD-ME and NADP-ME C4 grasses grown in elevated CO2. Physiol. Plant. 1998, 102, 297–306. [Google Scholar] [CrossRef]
- Sehtiya, H.L.; Goyal, S.S. Comparative uptake of nitrate by intact seedlings of C3 (barley) and C4 (corn) plants: Effect of light and exogenously supplied sucrose. Plant Soil 2000, 227, 185–190. [Google Scholar] [CrossRef]
- Sekhar, K.M.; Kota, V.R.; Reddy, T.P.; Rao, K.V.; Reddy, A.R. Amelioration of plant responses to drought under elevated CO2 by rejuvenating photosynthesis and nitrogen use efficiency: Implications for future climate-resilient crops. Photosynth. Res. 2021, 150, 21–40. [Google Scholar] [CrossRef]
- Wessinger, M.E.; Edwards, G.E.; Ku, M.S. Quantity and Kinetic Properties of Ribulose 1,5-Bisphosphate Carboxylase in C3, C4, and C3-C4 Intermediate Species of Flaveria (Asteraceae). Plant Cell Physiol. 1989, 30, 665–671. [Google Scholar]
- Zhang, Y.; Zhou, Y.; Sun, Q.; Deng, D.X.; Liu, H.H.; Chen, S.H.; Yin, Z.T. Genetic determinants controlling maize rubisco activase gene expression and a comparison with rice counterparts. BMC Plant Biol. 2019, 19, 351. [Google Scholar] [CrossRef] [Green Version]
- Bräutigam, A.; Gowik, U. Photorespiration connects C3 and C4 photosynthesis. J. Exp. Bot. 2016, 67, 2953–2962. [Google Scholar] [CrossRef]
- Boyer, K.E.; Zedler, J.B. Nitrogen addition could shift plant community composition in a restored California salt marsh. Restor. Ecol. 1999, 7, 74–85. [Google Scholar] [CrossRef]
- Haddad, N.M.; Haarstad, J.; Tilman, D. The effects of long-term nitrogen loading on grassland insect communities. Oecologia 2000, 124, 73–84. [Google Scholar] [CrossRef]
- Ma, C.Y.; Feng, S.D.; Huang, L.L.; Wang, Y.; Li, N.; Xu, X.; Zhou, B.; Jia, K.; Xu, Q.A.; Li, R.G.; et al. Exogenous salicylic acid prevents nitrogen dioxide-induced oxidative injury and nitrate accumulation in Brassica campestris L. ssp chinensis seedlings. J. Hortic. Sci. Biotechnol. 2010, 85, 241–247. [Google Scholar] [CrossRef]
- Ji, Y.; Zhang, J.; Song, J. Analysis of PM2.5 Pollution in Jiangsu Province and Determination of Building Outdoor PM2.5 Design Concentration. J. Chang. Univ. Nat. Sci. Ed. 2018, 30, 53–58. [Google Scholar]
- Sun, L.Y.; Li, B.; Ma, Y.C.; Wang, J.Y.; Xiong, Z.Q. Year-Round Atmospheric Wet and Dry Deposition of Nitrogen and Phosphorus on Water and Land Surfaces in Nanjing, China. Water Environ. Res. 2013, 85, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Takakubo, M.; Faure, J. Riboflavin sensitized photoreduction of nitro blue tetrazolium ion (NBT2+) in degassed aqueous solution. Photochem. Photobiol. 1983, 38, 137–140. [Google Scholar] [CrossRef]
- Bates, D.M.M.B. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Peterson, B.G.; Carl, P.; Boudt, K.; Bennett, R.; Ulrich, J.; Zivot, E.; Lestel, M.; Balkissoon, K.; Wuertz, D. Package ‘PerformanceAnalytics’, 2018. CRAN-Package Performance Analytics (microsoft.com). Available online: https://cran.r-project.org/web/packages/PerformanceAnalytics/index.html (accessed on 1 August 2022).
- Team, R. R: A Language and Environment for for Statistical Computing. Version 3.6.3. MSOR Connections. 2014. Available online: https://www.semanticscholar.org/paper/R%3A-A-language-and-environment-for-statistical-Team/659408b243cec55de8d0a3bc51b81173007aa89b (accessed on 1 August 2022).
- Avnimelech, Y. Carbon/nitrogen ratio as a control element in aquaculture systems. Aquaculture 1999, 176, 227–235. [Google Scholar] [CrossRef]
- Sinha, A.; Haider, T.; Narula, K.; Ghosh, S.; Chakraborty, N.; Chakraborty, S. Integrated Seed Proteome and Phosphoproteome Analyses Reveal Interplay of Nutrient Dynamics, Carbon-Nitrogen Partitioning, and Oxidative Signaling in Chickpea. Proteomics 2020, 20, 1900267. [Google Scholar] [CrossRef]
- Sui, Y.H.; Tian, R.; Lu, N.H. NADPH oxidase is a primary target for antioxidant effects by inorganic nitrite in lipopolysaccharide-induced oxidative stress in mice and in macrophage cells. Chem. Biol. Nitric Oxide 2019, 89, 46–53. [Google Scholar] [CrossRef]
- Tan, Y.; Zhang, Q.S.; Zhao, W.; Liu, Z.; Ma, M.Y.; Zhong, M.Y.; Wang, M.X. The highly efficient NDH-dependent photosystem I cyclic electron flow pathway in the marine angiosperm Zostera marina. Photosynth. Res. 2020, 144, 49–62. [Google Scholar] [CrossRef]
- Ye, M.; Peng, S.B.; Li, Y. Intraspecific variation in photosynthetic nitrogen-use efficiency is positively related to photosynthetic rate in rice (Oryza sativa L.) plants. Photosynthetica 2019, 57, 311–319. [Google Scholar] [CrossRef]
- Kramer, B.J.; Davis, T.W.; Meyer, K.A.; Rosen, B.H.; Goleski, J.A.; Dick, G.J.; Oh, G.; Gobler, C.J. Nitrogen limitation, toxin synthesis potential, and toxicity of cyanobacterial populations in Lake Okeechobee and the St. Lucie River Estuary, Florida, during the 2016 state of emergency event. PLoS ONE 2018, 13, e0196278. [Google Scholar] [CrossRef]
- Cullen, J.J.; Horrigan, S.G. Effects of nitrate on the diurnal vertical migration, carbon to nitrogen ratio, and the photosynthetic capacity of the dinoflagellate Gymnodinium splendens. Mar. Biol. 1981, 62, 81–89. [Google Scholar] [CrossRef]
- Zeng, W.Z.; Xu, C.; Wu, J.W.; Huang, J.S.; Zhao, Q.; Wu, M.S. Impacts of Salinity and Nitrogen on the Photosynthetic Rate and Growth of Sunflowers (Helianthus annuus L.). Pedosphere 2014, 24, 635–644. [Google Scholar] [CrossRef]
- Zhang, X.N.A.U.; Huang, G.N.A.U.; Bian, X.N.A.U.; Zhao, Q.C.A.O. Effects of root interaction and nitrogen fertilization on the chlorophyll content, root activity, photosynthetic characteristics of intercropped soybean and microbial quantity in the rhizosphere. Plant Soil Environ. 2013, 59, 80–88. [Google Scholar] [CrossRef]
- Shangguan, Z.; Shao, M.; Dyckmans, J. Effects of nitrogen nutrition and water deficit on net photosynthetic rate and chlorophyll fluorescence in winter wheat. J. Plant Physiol. 2000, 156, 46–51. [Google Scholar] [CrossRef]
- Green, T.H.; Mitchell, R.J. Effects of nitrogen on the response of loblolly pine to water stress. New Phytol. 1992, 122, 627–633. [Google Scholar] [CrossRef]
- Shen, H.; Dong, S.; DiTommaso, A.; Li, S.; Xiao, J.; Yang, M.; Zhang, J.; Gao, X.; Xu, Y.; Zhi, Y.; et al. Eco-physiological processes are more sensitive to simulated N deposition in leguminous forbs than non-leguminous forbs in an alpine meadow of the Qinghai-Tibetan Plateau. Sci. Total Environ. 2020, 744, 140612. [Google Scholar] [CrossRef]
- Ion, A.C.; Vermeylen, R.; Kourtchev, I.; Cafmeyer, J.; Chi, X.; Gelencser, A.; Maenhaut, W.; Claeys, M. Polar organic compounds in rural PM2.5 aerosols from K-puszta, Hungary, during a 2003 summer field campaign: Sources and diel variations. Atmos. Chem. Phys. 2005, 5, 1805–1814. [Google Scholar] [CrossRef] [Green Version]
- Dong, R.L.; Xu, X.Z.; Li, G.; Feng, W.J.; Zhao, G.; Zhao, J.J.; Wang, D.W.; Tu, L. Bradykinin inhibits oxidative stress-Induced cardiomyocytes senescence via regulating redox state. PLoS ONE 2013, 8, e770334. [Google Scholar]
- Perl, A. Oxidative stress in the pathology and treatment of systemic lupus erythematosus. Nat. Rev. Rheumatol. 2013, 9, 674–686. [Google Scholar] [CrossRef]
- Kinsman, E.A.; Pyke, K.A. Bundle sheath cells and cell-specific plastid development in Arabidopsis leaves. Development 1998, 125, 1815–1822. [Google Scholar] [CrossRef]
- Yadav, S.; Mishra, A. Ectopic expression of C4 photosynthetic pathway genes improves carbon assimilation and alleviate stress tolerance for future climate change. Physiol. Mol. Plant Pathol. 2020, 26, 195–209. [Google Scholar] [CrossRef] [PubMed]
- Attia, Z.; Dalal, A.; Moshelion, M. Vascular bundle sheath and mesophyll cells modulate leaf water balance in response to chitin. Plant J. 2020, 101, 1368–1377. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Prieto, M.A.; Foster, C.; Watson-Lazowski, A.; Ghannoum, O.; Chen, M. Comparative analysis of thylakoid protein complexes in the mesophyll and bundle sheath cells from C3, C4 and C3 -C4 Paniceae grasses. Physiol. Plant 2019, 166, 134–147. [Google Scholar] [CrossRef]
- Langdale, J.A. C4 Cycles: Past, Present, and Future Research on C4 Photosynthesis. Plant Cell 2011, 23, 3879–3892. [Google Scholar] [CrossRef]
- Sedelnikova, O.V.; Hughes, T.E.; Langdale, J.A. Understanding the Genetic Basis of C4 Kranz Anatomy with a View to Engineering C3 Crops. Annu. Rev. Genet. 2018, 52, 249–270. [Google Scholar] [CrossRef]
- Shahid, M.A.; Balal, R.M.; Khan, N.; Zotarelli, L.; Liu, G.D.; Ghazanfar, M.U.; Rathinasabapathi, B.; Mattson, N.S.; Martinez-Nicolas, J.J.; Garcia-Sanchez, F. Ploidy level of citrus rootstocks affects the carbon and nitrogen metabolism in the leaves of Chromium-stressed Kinnow mandarin plants. Environ. Exp. Bot. 2018, 149, 70–80. [Google Scholar] [CrossRef]
- Lu, M.Z.; Snyder, R.; Grant, J.; Tegeder, M. Manipulation of sucrose phloem and embryo loading affects pea leaf metabolism, carbon and nitrogen partitioning to sinks as well as seed storage pools. Plant J. 2020, 101, 217–236. [Google Scholar] [CrossRef]
- Schoning, C.; Wurst, S. Positive effects of root-knot nematodes (Meloidogyne incognita) on nitrogen availability do not outweigh their negative effects on fitness in Nicotiana attenuata. Plant Soil 2016, 400, 381–390. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Wang, P.; Zhang, Q.A.; Yan, C.; Yu, F.; Yi, J.; Fang, L. Effect of different levels of nitrogen, phosphorus, and potassium on root activity and chlorophyll content in leaves of Brassica oleracea seedlings grown in vegetable nursery substrate. Hortic. Environ. Biotechnol. 2017, 58, 5–11. [Google Scholar] [CrossRef]
- Kafkafi, U. Root Zone Temperature and the Preferred Form of Nitrogen to Crops. Acta Hortic. 2009, 807, 321–326. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).