Abstract
Cardiovascular diseases (CVDs) are the leading cause of mortality worldwide and, together with associated risk factors such as diabetes, hypertension, and dyslipidaemia, greatly impact patients’ quality of life and health care systems. This burden can be alleviated by fomenting lifestyle modifications and/or resorting to pharmacological approaches. However, due to several side effects, current therapies show low patient compliance, thus compromising their efficacy and enforcing the need to develop more amenable preventive/therapeutic strategies. In this scenario, medicinal and aromatic plants are a potential source of new effective agents. Specifically, plants from the Allioideae subfamily (formerly Alliaceae family), particularly those from the genus Allium and Tulbaghia, have been extensively used in traditional medicine for the management of several CVDs and associated risk factors, mainly due to the presence of sulphur-containing compounds. Bearing in mind this potential, the present review aims to gather information on traditional uses ascribed to these genera and provide an updated compilation of in vitro and in vivo studies validating these claims as well as clinical trials carried out in the context of CVDs. Furthermore, the effect of isolated sulphur-containing compounds is presented, and whenever possible, the relation between composition and activity and the mechanisms underlying the beneficial effects are pointed out.
1. Introduction
Cardiovascular diseases (CVDs) continue to lead mortality rates worldwide [1], accounting for nearly 18 million annual deaths, primarily due to coronary heart disease and stroke [2]. Unfortunately, these numbers tend to increase as several non-modifiable and modifiable risk factors associated with the onset and development of these disorders are also escalating. While non-modifiable risk factors such as aging, gender, genetic predisposition, family history of heart-related problems and ethnicity cannot be altered [3,4,5], modifiable risk factors are changeable. These include hypertension, dyslipidaemia, diabetes, obesity, smoking, alcohol misuse, unhealthy diet, sedentary lifestyle, and psychosocial factors [6] and are recognised as relevant targets to manage CVDs. For example, the INTERHEART case–control study pointed out that 90% of acute myocardial infarction cases are due to these risk factors and that controlling or eliminating them per se could lead to a drastic decrease in CVD mortality [7,8]. Indeed, due to their huge impact on CVDs, these risk factors are included in the World Health Organisation (WHO) target list that aims to reduce their prevalence by 2025 [9]. The negative impact of CVDs is further fuelled by the fact that 60% of patients fail to correctly adhere to the therapeutic regimen [10], mainly due to the cost of CVD therapies [11]. Therefore, new therapeutic interventions and/or preventive strategies with fewer side effects are mandatory, with aromatic and medicinal plants emerging as promising agents to manage both CVDs and associated risk factors. In fact, herbal medicines are relevant sources of bioactive molecules, used by ca. 80% of the world’s population in basic health care [12]. Moreover, many of these medicinal plants have already been used in the treatment of chronic and acute conditions including CVDs [13,14,15,16] and are part of the Mediterranean-style diet with proven beneficial effects on cardiovascular risk factors [17], as pointed out in several meta-analysis and critical reviews [18,19,20,21,22,23]. Interestingly, these effects are associated with the increased consumption of fruit, vegetables, spices, garlic, and onions [24]. Overall, the preventive/therapeutic potential of aromatic and medicinal plants is mainly attributed to the presence of secondary metabolites [25] including phenolic compounds, terpenes, alkaloids, and organosulfur compounds [26]. Organosulfur compounds are widely found in plants from the Allioideae subfamily (ex-Alliaceae family) and, together with extracts or raw bulbs from these plants, are widely reported for their medicinal properties [27]. Therefore, bearing in mind the bioactive potential of these plants, a systematised review gathering information on the effects of sulphur-containing extracts/compounds on major CVD risk factors, namely hypertension and dyslipidaemia/diabetes, is presented. Additionally, whenever reported, the mechanisms underlying the observed effects are referred to and the relation between composition and activity pointed out. To achieve this, a bibliographic search was conducted using Pubmed, Scopus and Google scholar databases, combining the keywords “Allium”, “Tulbaghia”, “Alliaceae” or “Allioideae” with “cardiovascular”, “diabetes”, “obesity”, “dyslipidaemia”, “hypertension” or “vasorelaxation”. Studies published over the last 20 years that had an available DOI were considered.
2. Importance of Allioideae Species in Cardiovascular Diseases
In the following sections, both the relevance and potential of plants from the Allioideae subfamily are described. First, the traditional uses ascribed to these plants in several ethnobotanical surveys is shown in order to highlight their importance in local health care systems. Then, studies validating some of these effects are systematised, considering pre-clinical approaches and clinical trials. The effect of isolated sulphur-containing compounds is also presented, and whenever possible, the relation between composition and activity is discussed, thus opening new avenues for further investigations in the field.
2.1. Traditional Uses of Allioideae
A plethora of traditional uses are ascribed to Allioideae plants or plant-based preparations, as summarised in Table 1. Plants’ scientific and common names are included as well as the region of use. In addition, the plant part or preparations used (with reference to the preparation method and posology, when known) and beneficial effects on the cardiovascular system are pointed out. Overall, the majority of the studies focus on the genus Allium, with only a few studies reporting the effects of two species from the Tulbaghia genus. In traditional preparations, the plant bulb is commonly used (7/11 total studies), with leaves (1/11), aerial parts (1/11) or whole plants (1/11) referred to in much less often. In addition, the use of a combination of plants is frequent and, therefore, this information is also provided. A list of abbreviations, used throughout the table, is provided at the end of the table.

Table 1.
Traditional uses ascribed to plants from the Allioideae subfamily.
2.2. Pre-Clinical Studies Validating the Cardioprotective Effects of Allioideae
Given the importance of Allioideae plants in the management of CVDs and associated risk factors in ethnopharmacological studies, we next compile several pre-clinical studies validating these effects. First, the effect of plants or their extracts is pointed out (Table 2) followed by the effect of isolated sulphur-containing compounds (Table 3) and then clinical trials.

Table 2.
Effects of plant parts/extracts from the Allioideae on the cardiovascular system.
2.2.1. The Effect of Plant Parts or Extracts
In Table 2, studies reporting the beneficial effects of plant parts or extracts is presented with reference to the species name, the plant part/extract used (with reference to the preparation method and concentration), the study model and the main findings regarding the effect observed in the cardiovascular system. Unless stated, a daily administration was used. Studies are grouped considering the cardiovascular disease and/or risk factor assessed with plants organised in alphabetical order of their scientific name. A list of abbreviations, used throughout the table, is provided at the end of the table.
Plants from this subfamily are rich in cysteine sulfoxide derivatives, such as alliin [132], which, by the action of alliinase, are converted into thiosulfinates, e.g., allicin, which in turn are instable and change into organosulfur compounds like ajoene [133]. Thiosulfinates are considered to be the main class of compounds responsible for the biological activities reported for plants from Allioideae subfamily [134]. Accordingly, several studies have assessed the role of alliinase activity on the effect of the extracts. Indeed, the antihypertensive effect of onions (Allium cepa) is lost or is much weaker upon boiling [81]. Similarly, the antiplatelet aggregation potential of these bulbs is also compromised, since longer heating times in either a conventional oven or microwave led to a pro-aggregatory effect rather than the expected anti-aggregatory potential [109]. Additionally, with the loss of alliinase activity, the vasorelaxant properties of Allium sativum, were abolished in aortic rings pre-contracted with phenylephrine [95]. Similar dependency on alliinase activity was reported for the hypolipidemic activity of A. sativum where long heating times or microwave heating compromised this effect [135].
On the other hand, the antidyslipidaemic and antidiabetic effects of A. sativum seem to depend on the PI3K/Akt/Nrf2 [87] or IGFIR/PI3K/Akt [67,68] pathways since, upon treatment, activation of these pathways is observed.
In order to better disclose the putative factors underlying the hypolipidemic effect of A. hookeri, a metabolomic analysis on the serum of hamsters consuming a high-fat diet and administered A. hookeri powder orally was carried out. The authors found 25 putative markers which could explain the lipid-lowering effect of this species, with phosphatidylcholines, lysophosphatidylcholines and lysophosphatidylethanolamines the most common targets. Furthermore, the metabolism for glycerophospholipids was increased in the treated group [57].
2.2.2. The Effect of Isolated Sulphur-Containing Compounds
In this section, the effect of isolated sulphur-containing compounds found in the Allioideae subfamily is presented. Then, a composition–activity relation is discussed in order to bring attention to potential active extracts. Table 3 systematises the main studies performed in these compounds, with the compound name, chemical structure, study model used, and the main findings of the study pointed out. Additionally, whenever reported, the route of administration and concentration used is highlighted. A list of abbreviations, used throughout the table, is provided at the end of the table.

Table 3.
Effects of sulphur-containing compounds on the cardiovascular system.
Table 3.
Effects of sulphur-containing compounds on the cardiovascular system.
| Compound | Study Model: Insult or Injury (Route of Administration; Concentration) | Main Findings | Ref. |
|---|---|---|---|
Ajoene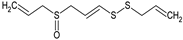 | Smooth muscle cells (1–50 μM) | ↓ Proliferation, cholesterol biosynthesis | [136] |
Allicin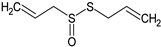 | Mice: ApoE-deficient and LDLR-deficient (p.o.; 9 mg/kg) | ↓ Atherosclerotic plaque, uptake and degradation of oxLDL by macrophages | [137] |
| HUVEC: oxLDL-induced damage (10, 30, 100 μM) | ↓ Apoptosis | [138] | |
| In chemico: Cu2+-induced oxidation of LDL from treated ApoE/LDLR-deficient mice (p.o.; 9 mg/kg) | ↓ LDL oxidation | [137] | |
| In chemico: Cu2+-induced LDL oxidation (0.1, 1 and 10 mM) | ↑ LDL oxidation (at higher doses) | [60] | |
| Phe-contracted PA rings (0.1, 0.3 and 1.0 µg/mL) | Induced relaxation | [95] | |
| Rat: SHR (p.o. for 6 weeks; 80 mg/kg on chow) | ↓ SBP and TG | [139] | |
Alliin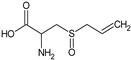 | Rat: High fructose (p.o. for 3 weeks; 0.111 and 0.222 mg/kg) | ↑ Heart function; ↓ SBP | [88] |
| Rat: ISO-induced myocardial infarction (gastric intubation for 35 days; 40 and 80 mg/kg) | ↓ CK, CK-MB, LDH, ALT, AST, TC, LDL, VLDL, TG, FFA, PL, MDA levels, HMGR activity; ↑ HDL levels, LCAT activity | [140] | |
Diallyl disulphide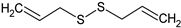 | HEPC: In vitro neovasculogenesis (0.1, 1, and 10 μM) | ↑ Tube formation, c-kit/PI3K/Akt pathway | [141] |
| Rat: Diabetic cardiomyopathy (gavage every other day for 16 days; 40 mg/kg) | ↓ Cardiac apoptosis and apoptotic markers dependent of death receptor and mitochondria; ↑ PI3K/Akt pathway | [68] | |
| HUVEC: Ox-LDL-induced damage (100 and 200 µM) | ↑ eNOS phosphorylation at Ser1177, NO and cGMP levels; stabilised eNOS/Cav-1 interaction; ↓ eNOS degradation, proteosome activity | [142] | |
| HUVEC: Non-stimulated and stimulated (0.2 to 500 µM) | Non-stimulated: ↓ MMP-2 secretion and activity and TIMP-1 secretion Stimulated: ↓ MMP-9 and TIMP-1 secretion | [143] | |
| In chemico: Isolated xanthine-oxidase activity (5 and 10 µM) | Restored activity in the presence of Cu2+ | [144] | |
| In chemico: Cu2+ and amphotericin-induced LDL oxidation (5 and 10 µM) | ↓ MDA | ||
| Rat: ISO-induced myocardial necrosis (p.o. for 14 days; 8.94 mg/kg) | ↓ HW, LDH, CK-MB, cTnC and systemic inflammation; ↑ SOD and cat | [48] | |
Diallyl trisulphide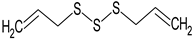 | Rat: Diabetic cardiomyopathy (gavage every other day for 16 days; 40 mg/kg) | ↓ Cardiac apoptosis | [68] |
| HUVEC: Ox-LDL-induced damage (20 and 50 µM) | ↑ eNOS phosphorylation at Ser1177, NO and cGMP levels; stabilised eNOS/Cav-1 interaction; ↓ eNOS degradation, proteosome activity | [142] | |
| Rat: metabolic syndrome (gavage every second day for 3 weeks; 40 mg/kg) | ↓ TG, LDL, homocysteine, BG, insulin, MDA, O22+, NF-κB, IL-17A, Bax, caspase-3 and -9 mRNA; ↑ HDL, H2S, NO2−, cat, GSH, SOD, cardiac function, eNOS, SOD1/2 and Bcl-2 mRNA | [145] | |
| HEK293 cells: Whole cell patch clamp (n/a) | ↓ IKr and hERG channel trafficking | [146] | |
| Cardiomyocytes: HG-induced apoptosis (10 μM) | ↓ Apoptosis | [147,148] | |
| Rat: STZ-induced diabetic (i.p. for 14 days; 500 μg/kg) | ↑ NO, eNOS proteins and phosphorylation levels, blood perfusion and capillary density | [149] | |
| HUVEC (1.3, 2.5, 5, and 10 µM) | ↓ Tube formation, VEGF2 release and VEGF2R expression | [150] | |
| HEPC: In vitro neovasculogenesis (0.1, 1, and 10 μM) | In vitro: ↑ tube formation | [141] | |
| Rat: In vivo neovasculogenesis (gavage for 2 weeks; 10 mg/kg) | In vivo: ↑ new vessels in a xenograft model of neovasculogenesis | ||
Dimethyl disulphide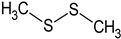 | PA: Phe-induced contractions (cumulative doses from 100 nM to 3 μM) | Induced relaxation; ↑ NOS phosphorylation and Ca2+ influx to ECs | [84] |
S-allylcysteine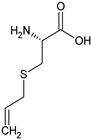 | Rat: Acute myocardial infarction (i.p. for 7 days pre-surgery + 2 days post-surgery; 50 mg/kg) | ↓ Mortality, infarct size; ↑ CTH activity | [151] |
| Cu2+-induced LDL oxidation (0.1, 1 and 10 mM) | ↓ Oxidation | [60,152] | |
| Macrophages and HUVEC: oxLDL stimulated (2.5, 5, 10 and 20 mM) | ↓ H2O2 production | [152] | |
| HUVEC: TNF-α and H2O2 stimulated (2.5, 5, 10 and 20 mM) | ↓ NF-κB activation | ||
| HUVEC and macrophages: LPS- and IFNγ stimulated (20, 40 and 80 µM) | HUVEC: ↑ eNOS activity, cGMP levels Macrophages: ↓ iNOS activity | [127] | |
| Rat: ISO-induced myocardial infarction (p.o. every other day for 3 weeks; 13.1 mg/kg and 32.76 mg/kg) | ↓ LDH, CK-MB; ↑ heart function; SOD and Cat | [42,43,153] |
↑—Increase; ↓—Decrease; Akt—v-Akt Murine thymoma viral oncogene/Protein kinase-B; ALT—Alanine aminotransferase; ApoE—Apoliprotein E; AST—Aspartate aminotransferase; Bax—Bcl-2-associated X protein; Bcl-2—B-cell lymphoma 2; BG—Blood glucose; Cat—Catalase; Cav-1—Caveolin-1; cGMP—Cyclic guanosine monophosphate; CK—Creatine kinase; CK-MB—Creatine kinase muscle/brain isoform; CTH—Cystathionine-γ-lyase; cTnC—Cardiac troponin C; Cu2+ —Copper (II); EC—Endothelial cell; eNOS—Endothelial nitric oxide synthase; FFA—Free fatty acid; GSH—Glutathione; H2O2—Hydrogen peroxide; H2S—Hydrogen sulphide; HDL—High-density lipoprotein; HDL—High-density lipoprotein; HEK293—Human embryonic kidney 293 cell; HEPC—Human endothelial progenitor cell; hERG—Human ether-a-go-go-related gene; HG—High glucose; HMGR—3-Hydroxy-3-methylglutaryl-Coenzyme A reductase; HUVEC—Human umbilical cord vein endothelial cell; HW—Heart weight; IFNγ—Interferon gamma; IKr—Delayed rectifier potassium current; IL—Interleukin; i.p.—Intraperitoneal injection; ISO—Isoproterenol; LCAT—Lecithin-cholesterol acyltransferase; LDH—Lactate dehydrogenase; LDL—Low-density lipoprotein; LDLR—Low-density lipoprotein receptor; LPS—Lipopolysaccharide; MMP—Matrix metalloproteinase; mRNA—Messenger RNA; n/a- Not available; NF-κB—Nuclear factor kappa-light-chain-enhancer of activated B cells; NO—Nitric oxide; NO2−—Nitrite; O22+ —Superoxide; oxLDL—Oxidised low-density lipoprotein; PA—Pulmonary artery; Phe- Phenylephrine; PI3K—Phosphoinositide 3-kinase; PL—Phospholipid; p.o.—Per os (orally); SBP—Systolic blood pressure; SHR—Spontaneously hypertensive rat; SOD—Superoxide dismutase; STZ—Streptozotocin; TC—Total cholesterol; TG—Triglycerides; TIMP-1—Tissue inhibitor of metalloproteinase-1; TNF-α—Tumour necrosis factor alpha; VEGF2—Vascular endothelial growth factor 2; VEGF2R—Vascular endothelial growth factor 2 receptor; VLDL—Very low-density lipoprotein.
Despite the interest in these compounds and their potential, only one study from those listed in Table 3 focused on the mechanisms of action underlying the observed effects. Indeed, it was shown that allicin reduced oxidised low-density lipoprotein-induced damage by inhibiting apoptosis and decreasing oxidative stress [138].
Although the compounds presented in Table 3 are commonly found in plants from the Allioideae subfamily, there are others that, despite being found in lower amounts, have been assessed for their cardioprotective effect. For example, the antidyslipidaemic effects reported for garlic (A. sativum) seem to be due to the capacity of S-allyl cysteine, N-acetyl-S-allyl cysteine, alliin, allixin, and allylmercaptocysteine to suppress low-density lipoprotein oxidation since all these compounds were able to reduce LDL oxidation induced by copper (II) [60]. Additionally, S-methylcysteine sulfoxide in high cholesterol-fed rats, was able to reduce the levels of total cholesterol, triglycerides and phospholipids. Furthermore, this compound reduced the activity of lipoprotein lipase without affecting the activity of other lipogenic proteins, while decreasing the levels of free fatty acids. In addition, the excretion of bile acids and sterols was enhanced in the treated group [154].
Furthermore, the antiplatelet activity of aged garlic extract was related to the presence of S-ethylcysteine, S-methyl-L-cysteine, S-1-proponyl-L-cysteine, since the remaining constituents of the extract (alliin, cycloalliin, S-allyl-L-cysteine, S-allylmercapto-L-cysteine, and fructosyl-arginine) failed to significantly inhibit platelet aggregation [116]. Moreover, two compounds, sodium n-propyl thiosulfate and sodium 2-propenyl thiosulfate decreased adenosine diphosphate-induced platelet aggregation in both dogs and human blood [155].
Regarding the vascular protective effect of garlic, it seems that allithiamine (vitamin B analogue found in garlic) might play a relevant role. Indeed, the presence of this compound in HUVEC growing in high glucose conditions showed a lower level of advanced glycation end products as well as a lower inflammatory profile when compared to high glucose-only treated cells. In addition, this compound also showed a very potent antioxidant potential [156]. Moreover, 2-vinyl-4H-1,3-dithiin, an organosulfur compound found in macerated garlic oil or in stir-fried garlic, decreased spontaneously hypertensive rat’s vascular smooth muscle cells proliferation and cell migration and arrested cell cycle at G2 phase. Furthermore, it decreased reactive oxygen species production induced by angiotensin II [157]. Also, diallyl disulphide and diallyl trisulphide have been reported for their capacity to induce neovasculogenesis via PI3K/Akt pathway activation [68,141]. In addition, reduction of cell death dependent on death receptor and mitochondria is also reported for both compounds [68]. Furthermore, for diallyl trisulphide, the promotion of neovasculogenesis is also attributed to a decrease in the microRNA 221 [68,141]. This compound also activated Nrf2 via the PI3K/Akt pathway [147] and induced the release of hydrogen sulphide by cystathionine-γ-lyase [148] using in vitro conditions mimicking diabetes. The reported effects for ajoene might be due to its capacity to inhibit protein prenylation, particularly that dependent on protein farnesyltransferase and protein geranylgeranyltransferase type I [136].
Some studies also assessed the activity of synthetic derivatives of naturally occurring sulphur-containing compounds. A study compared the antihypercholesterolaemic properties of diallyl disulphide analogues and showed that all the tested analogues lowered serum and hepatic levels of several lipids, including low-density lipoprotein while increasing those of high-density lipoprotein. The authors suggested that this lipid-lowering effect is due to the modulation of the 3-hydroxy-3-methylglutaryl-CoA reductase activity since a decrease in mRNA levels with a concomitant inactivation of sterol regulatory element-binding protein-2 and cyclic adenosine monophosphate response element-binding protein is observed [158]. Another study assessed the antihypertensive and vasorelaxant properties of five synthetic derivatives of diallyl disulphide. The results showed that all analogues were able to decrease systolic blood pressure in the Nω-nitro-L-arginine methyl ester-induced hypertensive animal model. Similarly, all compounds restored the antioxidant defences as observed by an increase in the activity of glutathione peroxidase, glutathione and superoxide dismutase with concomitant decrease in malondialdehyde and protein carbonyl levels. Furthermore, nitric oxide metabolites and cyclic guanosine monophosphate levels were restored by all the analogues, while the activity of angiotensin-converting enzyme was decreased [159].
The effect of sulphur-containing compounds on the pharmacodynamic and pharmacokinetics of other drugs was also assessed. Indeed, it was reported that the oral co-consumption of diallyl trisulphide and nifedipine led to a higher maximum concentration and area under the curve, thus suggesting that the compound might affect the gastrointestinal metabolism of nifedipine, since no effect on the pharmacokinetics was observed when nifedipine was given intravenously [160].
2.2.3. Clinical Trials
The importance of plants from the Allioideae subfamily is also validated by a small number of clinical trials. However, some contradictory results have been reported that may be related to the different doses used, duration of the treatment and/or association with other compounds. For example, in a small placebo-controlled and double-blind trial, firefighters were given four tablets containing 300 mg/table of aged garlic extract and 30 mg/table of coenzyme Q10 for up to 1 year. The results showed that the consumption improved their vascular elasticity and endothelial function [161]. In another study, the consumption of 1200 mg of this extract daily for 4 weeks followed by 4 weeks of washout had no effect on several parameters assessed such as glycated haemoglobin A1c, blood pressure, total cholesterol, triglycerides and high-density lipoprotein, and did not prevent endothelial dysfunction, oxidative stress or inflammation in patients with type 2 diabetes with high cardiovascular risk [162]. Furthermore, the administration of aged garlic extract (250 mg) supplemented with vitamins B12 and B6, folic acid and L-arginine daily for a 12-month period increased the ratio between brown and white epicardial adipose tissues with concomitant increase in the temperature-rebound index, while decreasing homocysteine levels and preventing the progression of coronary artery calcification [163]. In patients with coronary artery calcification and increased cardiovascular disease risk, the consumption of 2400 mg of aged garlic extract daily for 1 year inhibited the progression of the calcification. Regarding secondary outcomes, the extract decreased interleukin-6 levels as well as the glucose levels and blood pressure [164]. The same concentration increased cutaneous microcirculation in diabetic patients, thus suggesting that aged garlic extract might promote wound healing in these patients [165]. Overall, it seems that longer treatment durations (up to 1 year) have better outcomes.
Regarding other extracts, the consumption of 125 mL of red wine extract of onion twice daily for 10 weeks by healthy individuals showed hypocholesterolaemic, antioxidant and anti-inflammatory effects [166]. Additionally, the consumption of 300 mg of A. sativum standardised powder for 8 weeks by patients undergoing haemodialysis decreased the absolute values for oxidised low-density lipoprotein and homocysteine. In addition, the powder significantly ameliorated the values of calcium, triglycerides, oxidised low-density lipoprotein and homocysteine [167]. The consumption of quercetin-rich A. cepa extract daily for 6 weeks decreased systolic blood pressure in hypertensive individuals when compared to the placebo group [168]. The consumption of A. cepa peel extract twice daily for 12 weeks improved the flow-mediated dilation as well as the number of circulating endothelial progenitor cells in healthy overweight and obese patients. Indeed, the rate of patients with endothelial dysfunction decreased from 26% to 9% after extract administration [169].
Concerning CVD risk factors, some studies have been performed, such as the Tehran Lipid and Glucose study that assessed the effect of dietary consumption of A. sativum and A. cepa in cardiometabolic risk factors (body mass index, waist circumference, systolic blood pressure, diastolic blood pressure, fasting plasma glucose, triglycerides-to-high-density lipoprotein ratio, insulin, creatinine, estimated glomerular filtration rate and creatinine clearance) for 6 years. The results showed that high consumption of these vegetables led to a 64% reduction in CVD outcomes, as well as a lower incidence of chronic kidney disease and hypertension while no association was made with type 2 diabetes. Furthermore, it improved TG levels and creatine clearance [170].
Although the majority of the reported clinical trials are conducted using a small cohort of patients and are usually single-centre studies, they highlight the potential of plants from the Allioideae subfamily in the management of CVDs and associated risk factors. Nevertheless, these effects should be validated in more complete clinical trials with access to bigger multicentre cohorts to account for the genetic polymorphisms which impact the activity of drug metabolising enzymes leading to altered pharmacokinetics [171].
Drug interactions between conventional drugs or between these and herbal medicines are common, with both beneficial and detrimental effects reported. For example, the consumption of capsules containing 0.5 g of A. macrostemon bulb extract powder (three times a day) for eight weeks by patients undergoing baseline therapy for unstable angina, led to lower oxidised low-density lipoprotein and plasminogen activator inhibitor-1 level, while increasing plasminogen activity [172]. Moreover, in patients undergoing simvastatin therapy, supplementation with fenugreek and garlic for 8 weeks significantly reduced total cholesterol, triglycerides, non-high-density lipoprotein and low-density lipoprotein levels and increased those of high-density lipoprotein [173]. On the other hand, care must be taken with antiplatelet drugs, particularly warfarin and aspirin, as a simultaneous consumption of garlic or onion with these drugs can increase the risk of bleeding [174,175]. This interaction is attributed to their capacity to decrease platelet adhesion and aggregation, by inhibiting plasminogen activating factor and fibrinogen receptors and by decreasing thromboxane X2 synthesis [176]. In addition, garlic consumption is known to inhibit CYP3A4, the enzyme responsible for warfarin metabolism [175].
3. Final Remarks
The present review sheds light on the potential of plants from the Allioideae subfamily in the management of CVDs and associated risk factors. Traditional uses of some of these species are widely recognised, with garlic (Allium sativum) and onions (Allium cepa) being the most common. Additionally, pre-clinical studies and clinical trials validating their beneficial potential are frequent, thus confirming their importance. Nevertheless, other species such as A. jacquermontii, A. rotundum and Tulbaghia alliacea, despite being used in traditional remedies in some regions, lack scientific validation while other plants have undergone clinical trials but with no beneficial effects on the cardiovascular system.
Regarding CVD risk factors, plants from the Allioideae subfamily showed promising antiplatelet aggregation, antidiabetic, and dyslipidaemic effects, and were able to exert protection against atherosclerotic events.
Overall, we gathered information on both the tapped and untapped potential of plants belonging to the Allioideae subfamily, by highlighting scientific gaps as well as well-validated effects that pave the way for the development of new preventive/therapeutic approaches for CVDs.
Author Contributions
Conceptualization, J.M.A.-S., M.Z., H.G. and L.S.; validation, J.M.A.-S. and M.Z.; formal analysis, J.M.A.-S. and M.Z., investigation, J.M.A.-S.; resources, H.G. and L.S.; writing—original draft preparation, J.M.A.-S.; writing—review and editing, M.Z., H.G. and L.S.; visualization, J.M.A.-S. and M.Z.; supervision, L.S.; project administration, L.S.; funding acquisition, L.S. and H.G. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the European Regional Development Fund (ERDF) through the Operational Program for Competitiveness Factors (COMPETE) under the projects: HealthyAging2020 CENTRO-01-0145-FEDER-000012-N2323, CENTRO-01-0145-FEDER-032179, CENTRO-01-0145-FEDER-032414, POCI-01-0145-FEDER-022122, UIDB/04539/2020 and UIDP/04539/2020; by the COST Action EU-CARDIOPROTECTION CA16225 supported by the European Cooperation in Science and Technology (COST); by the Portuguese Foundation for Science and Technology (FCT) under the project POCI-01-0145-FEDER-032414 and through a PhD grant attributed to Jorge M. Alves-Silva (SFRH/BD/120692/2016); and by FCT and the “La Caixa” Foundation under the project PLANTS4AGEING.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Townsend, N.; Wilson, L.; Bhatnagar, P.; Wickramasinghe, K.; Rayner, M.; Nichols, M. Cardiovascular Disease in Europe: Epidemiological Update 2016. Eur. Heart J. 2016, 37, 3232–3245. [Google Scholar] [CrossRef] [PubMed]
- WHO Cardiovascular Diseases (CVDs). Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 15 March 2022).
- North, B.J.; Sinclair, D.A. The Intersection between Aging and Cardiovascular Disease. Circ. Res. 2012, 110, 1097–1108. [Google Scholar] [CrossRef] [PubMed]
- Buttar, H.S.; Li, T.; Ravi, N. Prevention of Cardiovascular Diseases: Role of Exercise, Dietary Interventions, Obesity and Smoking Cessation. Exp. Clin. Cardiol. 2005, 10, 229–249. [Google Scholar]
- WHO Cardiovascular Diseases. Available online: https://www.who.int/health-topics/cardiovascular-diseases/ (accessed on 20 March 2022).
- Mackay, J.; Mensah, G.A. Risk Factors in the Atlas of Heart Disease and Stroke; World Health Organization: Geneva, Switzerland, 2002; pp. 24–25. [Google Scholar]
- Timmis, A.; Townsend, N.; Gale, C.P.; Torbica, A.; Lettino, M.; Petersen, S.E.; Mossialos, E.A.; Maggioni, A.P.; Kazakiewicz, D.; May, H.T.; et al. European Society of Cardiology: Cardiovascular Disease Statistics 2019. Eur. Heart J. 2020, 41, 12–85. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, S.; Hawken, S.; Ôunpuu, S.; Dans, T.; Avezum, A.; Lanas, F.; McQueen, M.; Budaj, A.; Pais, P.; Varigos, J.; et al. Effect of Potentially Modifiable Risk Factors Associated with Myocardial Infarction in 52 Countries (the INTERHEART Study): Case-Control Study. Lancet 2004, 364, 937–952. [Google Scholar] [CrossRef]
- World Health Organization. Noncommunicable Diseases: Campaign for Action—Meeting the NCD Targets; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Baroletti, S.; Dell’Orfano, H. Medication Adherence in Cardiovascular Disease. Circulation 2010, 121, 1455–1458. [Google Scholar] [CrossRef]
- Jahromi, B.; Pirvulescu, I.; Candido, K.D.; Knezevic, N.N. Herbal Medicine for Pain Management: Efficacy and Drug Interactions. Pharmaceutics 2021, 13, 251. [Google Scholar] [CrossRef]
- Dhara, A.K.; Nayak, A.K. Introduction to Herbal Biomolecules in Herbal Biomolecules in Healthcare Applications; Elsevier: Amsterdam, The Netherlands, 2022; pp. 1–19. [Google Scholar]
- Alves-Silva, J.M.; Zuzarte, M.; Marques, C.; Viana, S.; Preguiça, I.; Baptista, R.; Ferreira, C.; Cavaleiro, C.; Domingues, N.; Sardão, V.A.; et al. 1, 8-Cineole Ameliorates Right Ventricle Dysfunction Associated with Pulmonary Arterial Hypertension by Restoring Connexin 43 and Mitochondrial Homeostasis. Pharmacol. Res. 2022, 180, 106151. [Google Scholar] [CrossRef]
- Alves-Silva, J.M.; Zuzarte, M.; Girão, H.; Salgueiro, L. The Role of Essential Oils and Their Main Compounds in the Management of Cardiovascular Disease Risk Factors. Molecules 2021, 26, 3506. [Google Scholar] [CrossRef]
- Alves-Silva, J.M.; Monica, Z.; Carla, M.; Lígia, S.; Henrique, G. Protective Effects of Terpenes on the Cardiovascular System: Current Advances and Future Perspectives. Curr. Med. Chem. 2016, 23, 4559–4600. [Google Scholar] [CrossRef]
- Wachtel-Galor, S.; Benzie, I.F.F. (Eds.) Herbal Medicine: An Introduction to Its History, Usage, Regulation, Current Trends, and Research Needs. In Herbal Medicine: Biomolecular and Clinical Aspects; CRC Press: Boca Raton, FL, USA, 2011; pp. 1–10. ISBN 9781439807132. [Google Scholar]
- Tuso, P.; Stoll, S.R.; Li, W.W. A Plant-Based Diet, Atherogenesis, and Coronary Artery Disease Prevention. Perm. J. 2015, 19, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Martínez-González, M.A.; Gea, A.; Ruiz-Canela, M. The Mediterranean Diet and Cardiovascular Health. Circ. Res. 2019, 124, 779–798. [Google Scholar] [CrossRef] [PubMed]
- Dontas, A.S.; Zerefos, N.S.; Panagiotakos, D.B.; Vlachou, C.; Valis, D.A. Mediterranean Diet and Prevention of Coronary Heart Disease in the Elderly. Clin. Interv. Aging 2007, 2, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Widmer, R.J.; Flammer, A.J.; Lerman, L.O.; Lerman, A. The Mediterranean Diet, Its Components, and Cardiovascular Disease. Am. J. Med. 2015, 128, 229–238. [Google Scholar] [CrossRef]
- Temple, N.J.; Guercio, V.; Tavani, A. The Mediterranean Diet and Cardiovascular Disease: Gaps in the Evidence and Research Challenges. Cardiol. Rev. 2019, 27, 127–130. [Google Scholar] [CrossRef] [PubMed]
- Karageorgou, D.; Micha, R.; Zampelas, A. Chapter 9—Mediterranean Diet and Cardiovascular Disease: An Overview of Recent Evidence. In The Mediterranean Diet; Preedy, V.R., Watson, R.R., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 91–104. ISBN 978-0-12-407849-9. [Google Scholar]
- Becerra-Tomás, N.; Mejía, S.B.; Viguiliouk, E.; Khan, T.; Kendall, C.W.C.; Kahleova, H.; Rahelić, D.; Sievenpiper, J.L.; Salas-Salvadó, J. Mediterranean Diet, Cardiovascular Disease and Mortality in Diabetes: A Systematic Review and Meta-Analysis of Prospective Cohort Studies and Randomized Clinical Trials. Crit. Rev. Food Sci. Nutr. 2020, 60, 1207–1227. [Google Scholar] [CrossRef] [PubMed]
- D’Innocenzo, S.; Biagi, C.; Lanari, M. Obesity and the Mediterranean Diet: A Review of Evidence of the Role and Sustainability of the Mediterranean Diet. Nutrients 2019, 11, 1306. [Google Scholar] [CrossRef]
- Jenke-Kodama, H.; Müller, R.; Dittmann, E. Evolutionary Mechanisms Underlying Secondary Metabolite Diversity. Prog. Drug Res. 2008, 65, 121–140. [Google Scholar] [CrossRef]
- Hartmann, T. From Waste Products to Ecochemicals: Fifty Years Research of Plant Secondary Metabolism. Phytochemistry 2007, 68, 2831–2846. [Google Scholar] [CrossRef]
- Bastaki, S.M.A.; Ojha, S.; Kalasz, H.; Adeghate, E. Chemical Constituents and Medicinal Properties of Allium Species. Mol. Cell. Biochem. 2021, 476, 4301–4321. [Google Scholar] [CrossRef]
- Jarić, S.; Mačukanović-Jocić, M.; Djurdjević, L.; Mitrović, M.; Kostić, O.; Karadžić, B.; Pavlović, P. An Ethnobotanical Survey of Traditionally Used Plants on Suva Planina Mountain (South-Eastern Serbia). J. Ethnopharmacol. 2015, 175, 93–108. [Google Scholar] [CrossRef] [PubMed]
- Belda, A.; Zaragozí, B.; Martínez, I.; Seva, E. Traditional Knowledge of Medicinal Plants in the Serra de Mariola Natural Park, South-Eastern Spain. Afr. J. Tradit. Complement. Altern. Med. 2013, 10, 299–309. [Google Scholar] [CrossRef]
- Mrabti, H.N.; Jaradat, N.; Kachmar, M.R.; Ed-Dra, A.; Ouahbi, A.; Cherrah, Y.; Faouzi, M.E.A. Integrative Herbal Treatments of Diabetes in Beni Mellal Region of Morocco. J. Integr. Med. 2019, 17, 93–99. [Google Scholar] [CrossRef]
- Bading Taika, B.; Bouckandou, M.; Souza, A.; Bourobou Bourobou, H.P.; MacKenzie, L.S.; Lione, L. An Overview of Anti-Diabetic Plants Used in Gabon: Pharmacology and Toxicology. J. Ethnopharmacol. 2018, 216, 203–228. [Google Scholar] [CrossRef]
- Gbolade, A. Ethnobotanical Study of Plants Used in Treating Hypertension in Edo State of Nigeria. J. Ethnopharmacol. 2012, 144, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Esakkimuthu, S.; Mutheeswaran, S.; Arvinth, S.; Paulraj, M.G.; Pandikumar, P.; Ignacimuthu, S. Quantitative Ethnomedicinal Survey of Medicinal Plants given for Cardiometabolic Diseases by the Non-Institutionally Trained Siddha Practitioners of Tiruvallur District, Tamil Nadu, India. J. Ethnopharmacol. 2016, 186, 329–342. [Google Scholar] [CrossRef]
- Ahmad, L.; Semotiuk, A.; Zafar, M.; Ahmad, M.; Sultana, S.; Liu, Q.-R.; Zada, M.P.; Abidin, S.Z.U.; Yaseen, G. Ethnopharmacological Documentation of Medicinal Plants Used for Hypertension among the Local Communities of DIR Lower, Pakistan. J. Ethnopharmacol. 2015, 175, 138–146. [Google Scholar] [CrossRef]
- Özdemir, E.; Alpınar, K. An Ethnobotanical Survey of Medicinal Plants in Western Part of Central Taurus Mountains: Aladaglar (Nigde-Turkey). J. Ethnopharmacol. 2015, 166, 53–65. [Google Scholar] [CrossRef]
- Odeyemi, S.; Bradley, G. Medicinal Plants Used for the Traditional Management of Diabetes in the Eastern Cape, South Africa: Pharmacology and Toxicology. Molecules 2018, 23, 2759. [Google Scholar] [CrossRef]
- Karou, S.D.; Tchacondo, T.; Djikpo Tchibozo, M.A.; Abdoul-Rahaman, S.; Anani, K.; Koudouvo, K.; Batawila, K.; Agbonon, A.; Simpore, J.; Souza, C.d. Ethnobotanical Study of Medicinal Plants Used in the Management of Diabetes Mellitus and Hypertension in the Central Region of Togo. Pharm. Biol. 2011, 49, 1286–1297. [Google Scholar] [CrossRef]
- Barkaoui, M.; Katiri, A.; Boubaker, H.; Msanda, F. Ethnobotanical Survey of Medicinal Plants Used in the Traditional Treatment of Diabetes in Chtouka Ait Baha and Tiznit (Western Anti-Atlas), Morocco. J. Ethnopharmacol. 2017, 198, 338–350. [Google Scholar] [CrossRef]
- Hyun, S.-W.; Jang, M.; Park, S.W.; Kim, E.J.; Jung, Y.-S. Onion (Allium cepa) Extract Attenuates Brain Edema. Nutrition 2013, 29, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kim, M.-Y.; Lee, D.H.; Lee, S.H.; Baik, E.J.; Moon, C.-H.; Park, S.W.; Ko, E.Y.; Oh, S.-R.; Jung, Y.-S. Methanolic Extract of Onion (Allium cepa) Attenuates Ischemia/Hypoxia-Induced Apoptosis in Cardiomyocytes via Antioxidant Effect. Eur. J. Nutr. 2009, 48, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Peng, Y.; Li, J.; Shen, H.; Shen, M.; Fang, T. Gualou Xiebai Decoction Prevents Myocardial Fibrosis by Blocking TGF-Beta/Smad Signalling. J. Pharm. Pharmacol. 2013, 65, 1373–1381. [Google Scholar] [CrossRef] [PubMed]
- Asad, M.; Avula, P.; Asdaq, S. Effect of Aged Garlic Extract and S-Allyl Cysteine and Their Interaction with Atenolol during Isoproterenol Induced Myocardial Toxicity in Rats. Indian J. Pharmacol. 2014, 46, 94. [Google Scholar] [CrossRef] [PubMed]
- Asdaq, S.M.B.; Challa, O.; Alamri, A.S.; Alsanie, W.F.; Alhomrani, M.; Almutiri, A.H.; Alshammari, M.S. Cytoprotective Potential of Aged Garlic Extract (AGE) and Its Active Constituent, S-Allyl-l-Cysteine, in Presence of Carvedilol during Isoproterenol-Induced Myocardial Disturbance and Metabolic Derangements in Rats. Molecules 2021, 26, 3203. [Google Scholar] [CrossRef]
- Asdaq, S.M.B.; Inamdar, M.N. Pharmacodynamic Interaction of Captopril with Garlic in Isoproterenol-Induced Myocardial Damage in Rat. Phytother. Res. 2010, 24, 720–725. [Google Scholar] [CrossRef]
- Banerjee, S.K.; Sood, S.; Dinda, A.K.; Das, T.K.; Maulik, S.K. Chronic Oral Administration of Raw Garlic Protects against Isoproterenol-Induced Myocardial Necrosis in Rat. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2003, 136, 377–386. [Google Scholar] [CrossRef]
- Banerjee, S.K.; Dinda, A.K.; Manchanda, S.C.; Maulik, S.K. Chronic Garlic Administration Protects Rat Heart against Oxidative Stress Induced by Ischemic Reperfusion Injury. BMC Pharm. 2002, 2, 16. [Google Scholar] [CrossRef]
- Czompa, A.; Szoke, K.; Prokisch, J.; Gyongyosi, A.; Bak, I.; Balla, G.; Tosaki, A.; Lekli, I. Aged (Black) versus Raw Garlic against Ischemia/Reperfusion-Induced Cardiac Complications. Int. J. Mol. Sci. 2018, 19, 1017. [Google Scholar] [CrossRef]
- Asdaq, S.M.B.; Alamri, A.S.; Alsanie, W.F.; Alhomrani, M. Cardioprotective Potential of Garlic Oil and Its Active Constituent, Diallyl Disulphide, in Presence of Carvedilol during Chronic Isoprenaline Injection-Mediated Myocardial Necrosis in Rats. Molecules 2021, 26, 5137. [Google Scholar] [CrossRef] [PubMed]
- Rankovic, M.; Krivokapic, M.; Bradic, J.; Petkovic, A.; Zivkovic, V.; Sretenovic, J.; Jeremic, N.; Bolevich, S.; Kartashova, M.; Jeremic, J.; et al. New Insight Into the Cardioprotective Effects of Allium Ursinum L. Extract Against Myocardial Ischemia-Reperfusion Injury. Front. Physiol. 2021, 12, 690696. [Google Scholar] [CrossRef] [PubMed]
- Murugesan, S.; Pandiyan, A.; Saravanakumar, L.; Moodley, K.; Mackraj, I. Protective Role of Wild Garlic on Isoproterenol-Induced Myocardial Necrosis in Wistar Rats. J. Ethnopharmacol. 2019, 237, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yang, C.; Mei, X.; Huang, R.; Zhang, S.; Tang, Y.; Dong, Q.; Zhou, C. Effect of the Polyphenol-rich Extract from Allium Cepa on Hyperlipidemic Sprague-dawley Rats. J. Food Biochem. 2021, 45, e13565. [Google Scholar] [CrossRef]
- Ostrowska, E.; Gabler, N.K.; Sterling, S.J.; Tatham, B.G.; Jones, R.B.; Eagling, D.R.; Jois, M.; Dunshea, F.R. Consumption of Brown Onions (Allium Cepa Var. Cavalier and Var. Destiny) Moderately Modulates Blood Lipids, Haematological and Haemostatic Variables in Healthy Pigs. Br. J. Nutr. 2004, 91, 211–218. [Google Scholar] [CrossRef]
- Safaeian, L.; Zolfaghari, B.; Karimi, S.; Talebi, A.; Ghazvini, M. The Effects of Hydroalcoholic Extract of Allium Elburzense Wendelbo Bulb on Dexamethasone-Induced Dyslipidemia, Hyperglycemia, and Oxidative Stress in Rats. Res. Pharm. Sci. 2018, 13, 22. [Google Scholar] [CrossRef]
- Janahmadi, Z.; Nekooeian, A.A.; Mozafari, M. Hydroalcoholic Extract of Allium Eriophyllum Leaves Attenuates Cardiac Impairment in Rats with Simultaneous Type 2 Diabetes and Renal Hypertension. Res. Pharm. Sci. 2015, 10, 125–133. [Google Scholar]
- Khaleghi, S.K. Ethyl Acetate Fraction of Allium Hirtifolium Improves Functional Parameters of Isolated Hearts of Diabetic Rats. Anatol. J. Cardiol. 2017, 17, 452. [Google Scholar] [CrossRef]
- Lee, J. The Hypolipidemic Effect of Allium Hookeri in Rats Fed with a High Fat Diet. Korean J. Community Living Sci. 2016, 27, 137–145. [Google Scholar] [CrossRef]
- Jang, G.-J.; Sung, M.J.; Hur, H.J.; Yoo, M.; Choi, J.H.; Hwang, I.K.; Lee, S. Metabolomics Analysis of the Lipid-Regulating Effect of Allium Hookeri in a Hamster Model of High-Fat Diet-Induced Hyperlipidemia by UPLC/ESI-Q-TOF Mass Spectrometry. Evid.-Based Complement. Altern. Med. 2018, 2018, 1–8. [Google Scholar] [CrossRef]
- Park, S.; No, K.; Lee, J. Anti-Obesity Effect of Allium hookeri Leaf Extract in High-Fat Diet-Fed Mice. J. Med. Food 2018, 21, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Torres, I.; Torres-Narváez, J.; Pedraza-Chaverri, J.; Rubio-Ruiz, M.; Díaz-Díaz, E.; Valle-Mondragón, L.d.; Martínez-Memije, R.; López, E.V.; Guarner-Lans, V. Effect of the Aged Garlic Extract on Cardiovascular Function in Metabolic Syndrome Rats. Molecules 2016, 21, 1425. [Google Scholar] [CrossRef]
- Lau, B.H.S. Suppression of LDL Oxidation by Garlic Compounds Is a Possible Mechanism of Cardiovascular Health Benefit. J. Nutr. 2006, 136, 765S–768S. [Google Scholar] [CrossRef]
- Morihara, N.; Hino, A.; Yamaguchi, T.; Suzuki, J. Aged Garlic Extract Suppresses the Development of Atherosclerosis in Apolipoprotein E–Knockout Mice. J. Nutr. 2016, 146, 460S–463S. [Google Scholar] [CrossRef] [PubMed]
- El-Sayyad, H.I.; Abou-El-Naga, A.M.; Gadallah, A.A.; Bakr, I.H. Protective Effects of Allium Sativum against Defects of Hypercholesterolemia on Pregnant Rats and Their Offspring. Int. J. Clin. Exp. Med. 2010, 3, 152–163. [Google Scholar] [PubMed]
- Sohn, C.W.; Kim, H.; You, B.R.; Kim, M.J.; Kim, H.J.; Lee, J.Y.; Sok, D.-E.; Kim, J.H.; Lee, K.J.; Kim, M.R. High Temperature- and High Pressure-Processed Garlic Improves Lipid Profiles in Rats Fed High Cholesterol Diets. J. Med. Food 2012, 15, 435–440. [Google Scholar] [CrossRef]
- Abdel-Mageid, A.D.; Abou-Salem, M.E.S.; Salaam, N.M.H.A.; El-Garhy, H.A.S. The Potential Effect of Garlic Extract and Curcumin Nanoparticles against Complication Accompanied with Experimentally Induced Diabetes in Rats. Phytomedicine 2018, 43, 126–134. [Google Scholar] [CrossRef]
- Sultana, M.R.; Bagul, P.K.; Katare, P.B.; Anwar Mohammed, S.; Padiya, R.; Banerjee, S.K. Garlic Activates SIRT-3 to Prevent Cardiac Oxidative Stress and Mitochondrial Dysfunction in Diabetes. Life Sci. 2016, 164, 42–51. [Google Scholar] [CrossRef]
- Amor, S.; González-Hedström, D.; Martín-Carro, B.; Inarejos-García, A.; Almodóvar, P.; Prodanov, M.; García-Villalón, A.; Granado, M. Beneficial Effects of an Aged Black Garlic Extract in the Metabolic and Vascular Alterations Induced by a High Fat/Sucrose Diet in Male Rats. Nutrients 2019, 11, 153. [Google Scholar] [CrossRef]
- Cheng, Y.-C.; Chang, M.-H.; Tsai, C.-C.; Chen, T.-S.; Fan, C.-C.; Lin, C.-C.; Lai, C.-H.; Tsai, F.-J.; Lin, J.A.; Huang, C.-Y. Garlic Oil Attenuates the Cardiac Apoptosis in Hamster-Fed with Hypercholesterol Diet. Food Chem. 2013, 136, 1296–1302. [Google Scholar] [CrossRef]
- Huang, Y.-T.; Yao, C.-H.; Way, C.-L.; Lee, K.-W.; Tsai, C.-Y.; Ou, H.-C.; Kuo, W.-W. Diallyl Trisulfide and Diallyl Disulfide Ameliorate Cardiac Dysfunction by Suppressing Apoptotic and Enhancing Survival Pathways in Experimental Diabetic Rats. J. Appl. Physiol. 2013, 114, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Aziz, H.A.; Tan, Y.T.F.; Peh, K.K.; Yam, M.F. Direct Effect of Khat and Garlic Extracts on Blood Lipids Contents: Preliminary in Vitro Study. Obes. Res. Clin. Pract. 2010, 4, e247–e252. [Google Scholar] [CrossRef]
- Baluchnejadmojarad, T.; Roghani, M. Endothelium-Dependent and -Independent Effect of Aqueous Extract of Garlic on Vascular Reactivity on Diabetic Rats. Fitoterapia 2003, 74, 630–637. [Google Scholar] [CrossRef]
- Baluchnejadmojarad, T.; Roghani, M.; Homayounfar, H.; Hosseini, M. Beneficial Effect of Aqueous Garlic Extract on the Vascular Reactivity of Streptozotocin-Diabetic Rats. J. Ethnopharmacol. 2003, 85, 139–144. [Google Scholar] [CrossRef]
- Baluchnejadmojarad, T.; Roghani, M. Garlic Extract Attenuates Time-Dependent Changes in the Reactivity of Isolated Aorta in Streptozotocin-Diabetic Rats. Life Sci. 2003, 73, 2281–2289. [Google Scholar] [CrossRef]
- Patumraj, S.; Tewit, S.; Amatyakul, S.; Jariyapongskul, A.; Maneesri, S.; Kasantikul, V.; Shepro, D. Comparative Effects of Garlic and Aspirin on Diabetic Cardiovascular Complications. Drug Deliv. 2000, 7, 91–96. [Google Scholar] [CrossRef]
- Supakul, L.; Pintana, H.; Apaijai, N.; Chattipakorn, S.; Shinlapawittayatorn, K.; Chattipakorn, N. Protective Effects of Garlic Extract on Cardiac Function, Heart Rate Variability, and Cardiac Mitochondria in Obese Insulin-Resistant Rats. Eur. J. Nutr. 2014, 53, 919–928. [Google Scholar] [CrossRef]
- Lee, S.; Joo, H.; Kim, C.-T.; Kim, I.-H.; Kim, Y. High Hydrostatic Pressure Extract of Garlic Increases the HDL Cholesterol Level via Up-Regulation of Apolipoprotein A-I Gene Expression in Rats Fed a High-Fat Diet. Lipids Health Dis. 2012, 11, 1–7. [Google Scholar] [CrossRef]
- Madkor, H.R.; Mansour, S.W.; Ramadan, G. Modulatory Effects of Garlic, Ginger, Turmeric and Their Mixture on Hyperglycaemia, Dyslipidaemia and Oxidative Stress in Streptozotocin–Nicotinamide Diabetic Rats. Br. J. Nutr. 2011, 105, 1210–1217. [Google Scholar] [CrossRef]
- Sobenin, I.A.; Andrianova, I.V.; Lakunin, K.Y.; Karagodin, V.P.; Bobryshev, Y.V.; Orekhov, A.N. Anti-Atherosclerotic Effects of Garlic Preparation in Freeze Injury Model of Atherosclerosis in Cholesterol-Fed Rabbits. Phytomedicine 2016, 23, 1235–1239. [Google Scholar] [CrossRef]
- Mukthamba, P.; Srinivasan, K. Protective Effect of Dietary Fenugreek (Trigonella foenum-graecum) Seeds and Garlic (Allium sativum) on Induced Oxidation of Low-Density Lipoprotein in Rats. J. Basic Clin. Physiol. Pharmacol. 2016, 27, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Bombicz, M.; Priksz, D.; Varga, B.; Gesztelyi, R.; Kertesz, A.; Lengyel, P.; Balogh, P.; Csupor, D.; Hohmann, J.; Bhattoa, H.; et al. Anti-Atherogenic Properties of Allium Ursinum Liophylisate: Impact on Lipoprotein Homeostasis and Cardiac Biomarkers in Hypercholesterolemic Rabbits. Int. J. Mol. Sci. 2016, 17, 1284. [Google Scholar] [CrossRef] [PubMed]
- Olorunnisola, O.S.; Bradley, G.; Afolayan, A.J. Protective Effect of Tulbaghia Violacea Harv. on Aortic Pathology, Tissue Antioxidant Enzymes and Liver Damage in Diet-Induced Atherosclerotic Rats. Int. J. Mol. Sci. 2012, 13, 12747–12760. [Google Scholar] [CrossRef]
- Kawamoto, E.; Sakai, Y.; Okamura, Y.; Yamamoto, Y. Effects of Boiling on the Antihypertensive and Antioxidant Activities of Onion. J. Nutr. Sci. Vitaminol. 2004, 50, 171–176. [Google Scholar] [CrossRef][Green Version]
- Chen, J.-H.; Tsai, S.-J.; Chen, H. Welsh Onion (Allium fistulosum L.) Extracts Alter Vascular Responses in Rat Aortae. J. Cardiovasc. Pharmacol. 1999, 33, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Aoyama, S.; Hamaguchi, N.; Rhi, G.-S. Antioxidative and Antihypertensive Effects of Welsh Onion on Rats Fed with a High-Fat High-Sucrose Diet. Biosci. Biotechnol. Biochem. 2005, 69, 1311–1317. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Qi, J.; Gao, S.; Li, C.; Ma, Y.; Wang, J.; Bai, Y.; Zheng, X. Vasodilation Effect of Volatile Oil from Allium Macrostemon Bunge Are Mediated by PKA/NO Pathway and Its Constituent Dimethyl Disulfide in Isolated Rat Pulmonary Arterials. Fitoterapia 2017, 120, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Hara, Y.; Noda, A.; Miyata, S.; Minoshima, M.; Sugiura, M.; Kojima, J.; Otake, M.; Furukawa, M.; Cheng, X.W.; Nagata, K.; et al. Effects of Aged Garlic Extract on Left Ventricular Diastolic Function and Fibrosis in a Rat Hypertension Model. Exp. Anim. 2013, 62, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Takashima, M.; Kanamori, Y.; Kodera, Y.; Morihara, N.; Tamura, K. Aged Garlic Extract Exerts Endothelium-Dependent Vasorelaxant Effect on Rat Aorta by Increasing Nitric Oxide Production. Phytomedicine 2017, 24, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Padiya, R.; Chowdhury, D.; Borkar, R.; Srinivas, R.; Pal Bhadra, M.; Banerjee, S.K. Garlic Attenuates Cardiac Oxidative Stress via Activation of PI3K/AKT/Nrf2-Keap1 Pathway in Fructose-Fed Diabetic Rat. PLoS ONE 2014, 9, e94228. [Google Scholar] [CrossRef]
- Asdaq, S.M.; Inamdar, M.N. Potential of Garlic and Its Active Constituent, S-Allyl Cysteine, as Antihypertensive and Cardioprotective in Presence of Captopril. Phytomedicine 2010, 17, 1016–1026. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Ku, D.D. Allicin in Garlic Protects against Coronary Endothelial Dysfunction and Right Heart Hypertrophy in Pulmonary Hypertensive Rats. Am. J. Physiol.-Heart Circ. Physiol. 2006, 291, H2431–H2438. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.Z.; Hussain, M.E.; Fahim, M. Endothelium Mediated Vasorelaxant Response of Garlic in Isolated Rat Aorta: Role of Nitric Oxide. J. Ethnopharmacol. 2004, 90, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, A.M.; Darabi, R.; Akbarloo, N. Investigation of Antihypertensive Mechanism of Garlic in 2K1C Hypertensive Rat. J. Ethnopharmacol. 2003, 86, 219–224. [Google Scholar] [CrossRef]
- Ganado, P.; Sanz, M.; Padilla, E.; Tejerina, T. An In Vitro Study of Different Extracts and Fractions of Allium Sativum (Garlic): Vascular Reactivity. J. Pharmacol. Sci. 2004, 94, 434–442. [Google Scholar] [CrossRef]
- Grman, M.; Misak, A.; Cacanyiova, S.; Kristek, F.; Tomaskova, Z.; Bertova, A.; Ondrias, K. The Aqueous Garlic, Onion and Leek Extracts Release Nitric Oxide from S-Nitrosoglutathione and Prolong Relaxation of Aortic Rings. Gen. Physiol. Biophys. 2012, 30, 396–402. [Google Scholar] [CrossRef]
- Kim-Park, S.; Ku, D.D. Garlic Elicits A Nitric Oxide-Dependent Relaxation And Inhibits Hypoxic Pulmonary Vasoconstriction In Rats. Clin. Exp. Pharmacol. Physiol. 2000, 27, 780–786. [Google Scholar] [CrossRef]
- Ku, D.D.; Abdel-Razek, T.T.; Dai, J.; Kim-Park, S.; Fallon, M.B.; Abrams, G.A. Garlic And Its Active Metabolite Allicin Produce Endothelium- And Nitric Oxide-Dependent Relaxation In Rat Pulmonary Arteries. Clin. Exp. Pharmacol. Physiol. 2002, 29, 84–91. [Google Scholar] [CrossRef]
- Radenkovic, M.; Brankovic, S.; Kitic, D.; Veljkovic, S.; Ivetic, V.; Nesić, M.; Miladinovic, B. Inhibitory Effect of Aqueous and Ethanolic Garlic (Allium sativum L., Lilliaceae) Extracts on the Rat Atria. Clin. Exp. Hypertens. 2010, 32, 251–255. [Google Scholar] [CrossRef]
- Bombicz, M.; Priksz, D.; Varga, B.; Kurucz, A.; Kertész, A.; Takacs, A.; Posa, A.; Kiss, R.; Szilvassy, Z.; Juhasz, B. A Novel Therapeutic Approach in the Treatment of Pulmonary Arterial Hypertension: Allium Ursinum Liophylisate Alleviates Symptoms Comparably to Sildenafil. Int. J. Mol. Sci. 2017, 18, 1436. [Google Scholar] [CrossRef]
- Moodley, K.; Mackraj, I.; Naidoo, Y. Cardiovascular Effects of Tulbaghia Violacea Harv. (Alliaceae) Root Methanolic Extract in Dahl Salt-Sensitive (DSS) Rats. J. Ethnopharmacol. 2013, 146, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Alpsoy, S.; Aktas, C.; Uygur, R.; Topcu, B.; Kanter, M.; Erboga, M.; Karakaya, O.; Gedikbasi, A. Antioxidant and Anti-Apoptotic Effects of Onion (Allium cepa) Extract on Doxorubicin-Induced Cardiotoxicity in Rats. J. Appl. Toxicol. 2013, 33, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Alpsoy, S.; Uygur, R.; Aktas, C.; Topcu, B.; Kanter, M.; Erboga, M.; Karakaya, O.; Gedikbasi, A. The Effects of Onion (Allium cepa) Extract on Doxorubicin-Induced Apoptosis in Aortic Endothelial Cells. J. Appl. Toxicol. 2013, 33, 364–369. [Google Scholar] [CrossRef]
- Alpsoy, S.; Kanter, M.; Aktas, C.; Erboga, M.; Akyuz, A.; Akkoyun, D.C.; Oran, M. Protective Effects of Onion Extract on Cadmium-Induced Oxidative Stress, Histological Damage, and Apoptosis in Rat Heart. Biol. Trace Elem. Res. 2014, 159, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Ige, S.F.; Akhigbe, R.E. Common Onion (Allium cepa) Extract Reverses Cadmium-Induced Organ Toxicity and Dyslipidaemia via Redox Alteration in Rats. Pathophysiology 2013, 20, 269–274. [Google Scholar] [CrossRef]
- Alkreathy, H.M.; Damanhouri, Z.A.; Ahmed, N.; Slevin, M.; Osman, A.-M.M. Mechanisms of Cardioprotective Effect of Aged Garlic Extract Against Doxorubicin-Induced Cardiotoxicity. Integr. Cancer Ther. 2012, 11, 364–370. [Google Scholar] [CrossRef]
- Alkreathy, H.; Damanhouri, Z.A.; Ahmed, N.; Slevin, M.; Ali, S.S.; Osman, A.-M.M. Aged Garlic Extract Protects against Doxorubicin-Induced Cardiotoxicity in Rats. Food Chem. Toxicol. 2010, 48, 951–956. [Google Scholar] [CrossRef]
- Mukherjee, S.; Banerjee, S.K.; Maulik, M.; Dinda, A.K.; Talwar, K.K.; Maulik, S.K. Protection against Acute Adriamycin-Induced Cardiotoxicity by Garlic: Role of Endogenous Antioxidants and Inhibition of TNF-Alpha Expression. BMC Pharm. 2003, 3, 16. [Google Scholar] [CrossRef][Green Version]
- Gomaa, A.M.S.; Abdelhafez, A.T.; Aamer, H.A. Garlic (Allium sativum) Exhibits a Cardioprotective Effect in Experimental Chronic Renal Failure Rat Model by Reducing Oxidative Stress and Controlling Cardiac Na+/K+-ATPase Activity and Ca2+ Levels. Cell Stress Chaperones 2018, 23, 913–920. [Google Scholar] [CrossRef]
- Pop, R.M.; Bocsan, I.C.; Buzoianu, A.D.; Chedea, V.S.; Socaci, S.A.; Pecoraro, M.; Popolo, A. Evaluation of the Antioxidant Activity of Nigella Sativa L. and Allium Ursinum Extracts in a Cellular Model of Doxorubicin-Induced Cardiotoxicity. Molecules 2020, 25, 5259. [Google Scholar] [CrossRef]
- Beretta, H.V.; Bannoud, F.; Insani, M.; Berli, F.; Hirschegger, P.; Galmarini, C.R.; Cavagnaro, P.F. Relationships Between Bioactive Compound Content and the Antiplatelet and Antioxidant Activities of Six Allium Vegetable Species. Food Technol. Biotechnol. 2017, 55, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Cavagnaro, P.F.; Galmarini, C.R. Effect of Processing and Cooking Conditions on Onion (Allium cepa L.) Induced Antiplatelet Activity and Thiosulfinate Content. J. Agric. Food Chem. 2012, 60, 8731–8737. [Google Scholar] [CrossRef] [PubMed]
- Ro, J.-Y.; Ryu, J.-H.; Park, H.-J.; Cho, H.-J. Onion (Allium cepa L.) Peel Extract Has Anti-Platelet Effects in Rat Platelets. Springerplus 2015, 4, 1–8. [Google Scholar] [CrossRef]
- Ko, E.Y.; Nile, S.H.; Jung, Y.-S.; Keum, Y.S. Antioxidant and Antiplatelet Potential of Different Methanol Fractions and Flavonols Extracted from Onion (Allium cepa L.). 3 Biotech 2018, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Briggs, W.H.; Folts, J.D.; Osman, H.E.; Goldman, I.L. Administration of Raw Onion Inhibits Platelet-Mediated Thrombosis in Dogs. J. Nutr. 2001, 131, 2619–2622. [Google Scholar] [CrossRef]
- Chen, J.-H.; Chen, H.; Tsai, S.-J.; Jen, C.J. Chronic Consumption of Raw But Not Boiled Welsh Onion Juice Inhibits Rat Platelet Function. J. Nutr. 2000, 130, 34–37. [Google Scholar] [CrossRef][Green Version]
- Chen, J.-H.; Chen, H.; Wang, J.-S.; Tsai, S.-J.; Jen, C.J. Effects of Welsh Onion Extracts on Human Platelet Function in Vitro. Life Sci. 2000, 66, 1571–1579. [Google Scholar] [CrossRef]
- Allison, G.L.; Lowe, G.M.; Rahman, K. Aged Garlic Extract Inhibits Platelet Activation by Increasing Intracellular CAMP and Reducing the Interaction of GPIIb/IIIa Receptor with Fibrinogen. Life Sci. 2012, 91, 1275–1280. [Google Scholar] [CrossRef]
- Allison, G.L.; Lowe, G.M.; Rahman, K. Aged Garlic Extract and Its Constituents Inhibit Platelet Aggregation through Multiple Mechanisms. J. Nutr. 2006, 136, 782S–788S. [Google Scholar] [CrossRef]
- Allison, G.L.; Lowe, G.M.; Rahman, K. Aged Garlic Extract May Inhibit Aggregation in Human Platelets by Suppressing Calcium Mobilization. J. Nutr. 2006, 136, 789S–792S. [Google Scholar] [CrossRef]
- Morihara, N.; Hino, A. Aged Garlic Extract Suppresses Platelet Aggregation by Changing the Functional Property of Platelets. J. Nat. Med. 2017, 71, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Rahman, K.; Billington, D. Dietary Supplementation with Aged Garlic Extract Inhibits ADP-Induced Platelet Aggregation in Humans. J. Nutr. 2000, 130, 2662–2665. [Google Scholar] [CrossRef] [PubMed]
- Rahman, K.; Lowe, G.M.; Smith, S. Aged Garlic Extract Inhibits Human Platelet Aggregation by Altering Intracellular Signaling and Platelet Shape Change. J. Nutr. 2016, 146, 410S–415S. [Google Scholar] [CrossRef] [PubMed]
- Hiyasat, B.; Sabha, D.; Grötzinger, K.; Kempfert, J.; Rauwald, J.-W.; Mohr, F.-W.; Dhein, S. Antiplatelet Activity of Allium Ursinum and Allium Sativum. Pharmacology 2009, 83, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Fukao, H.; Yoshida, H.; Tazawa, Y.; Hada, T. Antithrombotic Effects of Odorless Garlic Powder Both in Vitro and in Vivo. Biosci. Biotechnol. Biochem. 2007, 71, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Seyfi, P.; Mostafaie, A.; Mansouri, K.; Arshadi, D.; Mohammadi-Motlagh, H.-R.; Kiani, A. In Vitro and in Vivo Anti-Angiogenesis Effect of Shallot (Allium ascalonicum): A Heat-Stable and Flavonoid-Rich Fraction of Shallot Extract Potently Inhibits Angiogenesis. Toxicol. In Vitro 2010, 24, 1655–1661. [Google Scholar] [CrossRef]
- Hiramatsu, K.; Tsuneyoshi, T.; Ogawa, T.; Morihara, N. Aged Garlic Extract Enhances Heme Oxygenase-1 and Glutamate-Cysteine Ligase Modifier Subunit Expression via the Nuclear Factor Erythroid 2–Related Factor 2–Antioxidant Response Element Signaling Pathway in Human Endothelial Cells. Nutr. Res. 2016, 36, 143–149. [Google Scholar] [CrossRef]
- Yeh, Y.-Y.; Yeh, S. Homocysteine-Lowering Action Is Another Potential Cardiovascular Protective Factor of Aged Garlic Extract. J. Nutr. 2006, 136, 745S–749S. [Google Scholar] [CrossRef]
- Lee, E.N.; Choi, Y.W.; Kim, H.K.; Park, J.K.; Kim, H.J.; Kim, M.J.; Lee, H.W.; Kim, K.-H.; Bae, S.S.; Kim, B.S.; et al. Chloroform Extract of Aged Black Garlic Attenuates TNF-α-Induced ROS Generation, VCAM-1 Expression, NF-ΚB Activation and Adhesiveness for Monocytes in Human Umbilical Vein Endothelial Cells. Phytother. Res. 2011, 25, 92–100. [Google Scholar] [CrossRef]
- Kim, K.-M.; Chun, S.-B.; Koo, M.-S.; Choi, W.-J.; Kim, T.-W.; Kwon, Y.-G.; Chung, H.-T.; Billiar, T.R.; Kim, Y.-M. Differential Regulation of NO Availability from Macrophages and Endothelial Cells by the Garlic Component S-Allyl Cysteine. Free Radic. Biol. Med. 2001, 30, 747–756. [Google Scholar] [CrossRef]
- Louis, X.L.; Murphy, R.; Thandapilly, S.J.; Yu, L.; Netticadan, T. Garlic Extracts Prevent Oxidative Stress, Hypertrophy and Apoptosis in Cardiomyocytes: A Role for Nitric Oxide and Hydrogen Sulfide. BMC Complement. Altern. Med. 2012, 12, 140. [Google Scholar] [CrossRef] [PubMed]
- Lima, P.R.d.S.; Bandeira, F.C.V.; Rolim, J.C.; Nogueira, M.R.S.; Pordeus, M.A.A.; Oliveira, A.F.B.d.; Pitta, G.B.B. Allium Sativum Compared to Cilostazol as an Inhibitor of Myointimal Hyperplasia. Braz. J. Cardiovasc. Surg. 2016, 31, 291–299. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Recinella, L.; Chiavaroli, A.; Masciulli, F.; Fraschetti, C.; Filippi, A.; Cesa, S.; Cairone, F.; Gorica, E.; Leo, M.D.; Braca, A.; et al. Protective Effects Induced by a Hydroalcoholic Allium Sativum Extract in Isolated Mouse Heart. Nutrients 2021, 13, 2332. [Google Scholar] [CrossRef] [PubMed]
- Rassoul, F.; Salvetter, J.; Reissig, D.; Schneider, W.; Thiery, J.; Richter, V. The Influence of Garlic (Allium sativum) Extract on Interleukin 1α-Induced Expression of Endothelial Intercellular Adhesion Molecule-1 and Vascular Cell Adhesion Molecule-1. Phytomedicine 2006, 13, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Rose, P.; Whiteman, M.; Moore, P.K.; Zhu, Y.Z. Bioactive S-Alk(En)Yl Cysteine Sulfoxide Metabolites in the Genus Allium: The Chemistry of Potential Therapeutic Agents. Nat. Prod. Rep. 2005, 22, 351–368. [Google Scholar] [CrossRef]
- Fukaya, M.; Nakamura, S.; Hayashida, H.; Noguchi, D.; Nakashima, S.; Yoneda, T.; Matsuda, H. Structures of Cyclic Organosulfur Compounds From Garlic (Allium sativum L.) Leaves. Front. Chem. 2020, 8, 282. [Google Scholar] [CrossRef]
- Golubkina, N.; Caruso, G. Chapter 5—Onion. In Nutritional Composition and Antioxidant Properties of Fruits and Vegetables; Jaiswal, A.K., Ed.; Academic Press: San Diego, CA, USA, 2020; pp. 73–87. ISBN 978-0-12-812780-3. [Google Scholar]
- Najman, K.; Sadowska, A.; Buczak, K.; Leontowicz, H.; Leontowicz, M. Effect of Heat-Treated Garlic (Allium Sativum L.) on Growth Parameters, Plasma Lipid Profile and Histological Changes in the Ileum of Atherogenic Rats. Nutrients 2022, 14, 336. [Google Scholar] [CrossRef]
- Ferri, N.; Yokoyama, K.; Sadilek, M.; Paoletti, R.; Apitz-Castro, R.; Gelb, M.H.; Corsini, A. Ajoene, a Garlic Compound, Inhibits Protein Prenylation and Arterial Smooth Muscle Cell Proliferation. Br. J. Pharmacol. 2003, 138, 811–818. [Google Scholar] [CrossRef]
- Gonen, A.; Harats, D.; Rabinkov, A.; Miron, T.; Mirelman, D.; Wilchek, M.; Weiner, L.; Ulman, E.; Levkovitz, H.; Ben-Shushan, D.; et al. The Antiatherogenic Effect of Allicin: Possible Mode of Action. Pathobiology 2005, 72, 325–334. [Google Scholar] [CrossRef]
- Chen, X.; Pang, S.; Lin, J.; Xia, J.; Wang, Y. Allicin Prevents Oxidized Low-Density Lipoprotein-Induced Endothelial Cell Injury by Inhibiting Apoptosis and Oxidative Stress Pathway. BMC Complement. Altern. Med. 2016, 16, 133. [Google Scholar] [CrossRef]
- Elkayam, A.; Peleg, E.; Grossman, E.; Shabtay, Z.; Sharabi, Y. Effects of Allicin on Cardiovascular Risk Factors in Spontaneously Hypertensive Rats. Isr. Med. Assoc. J. 2013, 15, 170–173. [Google Scholar] [PubMed]
- Sangeetha, T.; Quine, S.D. Preventive Effect of S-Allyl Cysteine Sulfoxide (Alliin) on Cardiac Marker Enzymes and Lipids in Isoproterenol-Induced Myocardial Injury. J. Pharm. Pharmacol. 2010, 58, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Chiang, E.-P.I.; Chiu, S.-C.; Pai, M.-H.; Wang, Y.-C.; Wang, F.-Y.; Kuo, Y.-H.; Tang, F.-Y. Organosulfur Garlic Compounds Induce Neovasculogenesis in Human Endothelial Progenitor Cells through a Modulation of MicroRNA 221 and the PI3-K/Akt Signaling Pathways. J. Agric. Food Chem. 2013, 61, 4839–4849. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.-P.; Liu, C.-T.; Sheen, L.-Y.; Chen, H.-W.; Lii, C.-K. Diallyl Disulfide and Diallyl Trisulfide Protect Endothelial Nitric Oxide Synthase against Damage by Oxidized Low-Density Lipoprotein. Mol. Nutr. Food Res. 2010, 54, S42–S52. [Google Scholar] [CrossRef] [PubMed]
- Meyer, K.; Ueberham, E.; Gebhardt, R. Influence of Organosulphur Compounds from Garlic on the Secretion of Matrix Metalloproteinases and Their Inhibitor TIMP-1 by Cultured HUVEC Cells. Cell Biol. Toxicol. 2004, 20, 253–260. [Google Scholar] [CrossRef]
- Ou, C.; Tsao, S.; Lin, M.; Yin, M. Protective Action on Human LDL against Oxidation and Glycation by Four Organosulfur Compounds Derived from Garlic. Lipids 2003, 38, 219–224. [Google Scholar] [CrossRef]
- Jeremic, J.N.; Jakovljevic, V.L.; Zivkovic, V.I.; Srejovic, I.M.; Bradic, J.V.; Milosavljevic, I.M.; Mitrovic, S.L.; Jovicic, N.U.; Bolevich, S.B.; Svistunov, A.A.; et al. Garlic Derived Diallyl Trisulfide in Experimental Metabolic Syndrome: Metabolic Effects and Cardioprotective Role. Int. J. Mol. Sci. 2020, 21, 9100. [Google Scholar] [CrossRef]
- Li, G.; Cheng, G.; Wu, J.; Ma, S.; Zhang, A.; Han, W.; Sun, C. Allitridin Reduces IKr Current by Disrupting the Trafficking of Human Ether-à-Go-Go-Related Gene Channels. Cardiology 2014, 128, 1–8. [Google Scholar] [CrossRef]
- Tsai, C.-Y.; Wang, C.-C.; Lai, T.-Y.; Tsu, H.-N.; Wang, C.-H.; Liang, H.-Y.; Kuo, W.-W. Antioxidant Effects of Diallyl Trisulfide on High Glucose-Induced Apoptosis Are Mediated by the PI3K/Akt-Dependent Activation of Nrf2 in Cardiomyocytes. Int. J. Cardiol. 2013, 168, 1286–1297. [Google Scholar] [CrossRef]
- Tsai, C.-Y.; Wen, S.-Y.; Shibu, M.A.; Yang, Y.-C.; Peng, H.; Wang, B.; Wei, Y.-M.; Chang, H.-Y.; Lee, C.-Y.; Huang, C.-Y.; et al. Diallyl Trisulfide Protects against High Glucose-Induced Cardiac Apoptosis by Stimulating the Production of Cystathionine Gamma-Lyase-Derived Hydrogen Sulfide. Int. J. Cardiol. 2015, 195, 300–310. [Google Scholar] [CrossRef]
- Yang, H.-B.; Liu, H.-M.; Yan, J.-C.; Lu, Z.-Y. Effect of Diallyl Trisulfide on Ischemic Tissue Injury and Revascularization in a Diabetic Mouse Model. J. Cardiovasc. Pharmacol. 2018, 71, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; Li, M.; Herman-Antosiewicz, A.; Antosiewicz, J.; Xiao, H.; Lew, K.L.; Zeng, Y.; Marynowski, S.W.; Singh, S.V. Diallyl Trisulfide Inhibits Angiogenic Features of Human Umbilical Vein Endothelial Cells by Causing Akt Inactivation and Down-Regulation of VEGF and VEGF-R2. Nutr. Cancer 2006, 55, 94–107. [Google Scholar] [CrossRef]
- Chuah, S.C.; Moore, P.K.; Zhu, Y.Z. S-Allylcysteine Mediates Cardioprotection in an Acute Myocardial Infarction Rat Model via a Hydrogen Sulfide-Mediated Pathway. Am. J. Physiol.-Heart Circ. Physiol. 2007, 293, H2693–H2701. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.E.; Ide, N.; Lau, B.H.S. S-Allyl Cysteine Reduces Oxidant Load in Cells Involved in the Atherogenic Process. Phytomedicine 2001, 8, 39–46. [Google Scholar] [CrossRef]
- Padmanabhan, M.; Prince, P.S.M. Preventive Effect of S-Allylcysteine on Lipid Peroxides and Antioxidants in Normal and Isoproterenol-Induced Cardiotoxicity in Rats: A Histopathological Study. Toxicology 2006, 224, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Kumari, K.; Augusti, K.T. Lipid Lowering Effect of S-Methyl Cysteine Sulfoxide from Allium Cepa Linn in High Cholesterol Diet Fed Rats. J. Ethnopharmacol. 2007, 109, 367–371. [Google Scholar] [CrossRef]
- Chang, H.S.; Yamato, O.; Sakai, Y.; Yamasaki, M.; Maede, Y. Acceleration of Superoxide Generation in Polymorphonuclear Leukocytes and Inhibition of Platelet Aggregation by Alk(En)Yl Thiosulfates Derived from Onion and Garlic in Dogs and Humans. Prostaglandins Leukot. Essent. Fat. Acids 2004, 70, 77–83. [Google Scholar] [CrossRef]
- Biró, A.; Markovics, A.; Fazekas, M.É.; Fidler, G.; Szalóki, G.; Paholcsek, M.; Lukács, J.; Stündl, L.; Remenyik, J. Allithiamine Alleviates Hyperglycaemia-Induced Endothelial Dysfunction. Nutrients 2020, 12, 1690. [Google Scholar] [CrossRef]
- Torres-Palazzolo, C.; de Paola, M.; Quesada, I.; Camargo, A.; Castro, C. 2-Vinyl-4H-1,3-Dithiin, a Bioavailable Compound from Garlic, Inhibits Vascular Smooth Muscle Cells Proliferation and Migration by Reducing Oxidative Stress. Plant Foods Hum. Nutr. 2020, 75, 355–361. [Google Scholar] [CrossRef]
- Rai, S.K.; Sharma, M.; Tiwari, M. Inhibitory Effect of Novel Diallyldisulfide Analogs on HMG-CoA Reductase Expression in Hypercholesterolemic Rats: CREB as a Potential Upstream Target. Life Sci. 2009, 85, 211–219. [Google Scholar] [CrossRef]
- Kumar Sharma, D.; Manral, A.; Saini, V.; Singh, A.; Srinivasan, B.P.; Tiwari, M. Novel Diallyldisulfide Analogs Ameliorate Cardiovascular Remodeling in Rats with L-NAME-Induced Hypertension. Eur. J. Pharmacol. 2012, 691, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zou, M.-J.; Zhao, N.; Ren, J.-G.; Zhou, H.; Cheng, G. Effect of Diallyl Trisulfide on the Pharmacokinetics of Nifedipine in Rats. J. Food Sci. 2011, 76, T30–T34. [Google Scholar] [CrossRef] [PubMed]
- Larijani, V.N.; Ahmadi, N.; Zeb, I.; Khan, F.; Flores, F.; Budoff, M. Beneficial Effects of Aged Garlic Extract and Coenzyme Q10 on Vascular Elasticity and Endothelial Function: The FAITH Randomized Clinical Trial. Nutrition 2013, 29, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Atkin, M.; Laight, D.; Cummings, M.H. The Effects of Garlic Extract upon Endothelial Function, Vascular Inflammation, Oxidative Stress and Insulin Resistance in Adults with Type 2 Diabetes at High Cardiovascular Risk. A Pilot Double Blind Randomized Placebo Controlled Trial. J. Diabetes Its Complicat. 2016, 30, 723–727. [Google Scholar] [CrossRef]
- Ahmadi, N.; Nabavi, V.; Hajsadeghi, F.; Zeb, I.; Flores, F.; Ebrahimi, R.; Budoff, M. Aged Garlic Extract with Supplement Is Associated with Increase in Brown Adipose, Decrease in White Adipose Tissue and Predict Lack of Progression in Coronary Atherosclerosis. Int. J. Cardiol. 2013, 168, 2310–2314. [Google Scholar] [CrossRef]
- Wlosinska, M.; Nilsson, A.-C.; Hlebowicz, J.; Hauggaard, A.; Kjellin, M.; Fakhro, M.; Lindstedt, S. The Effect of Aged Garlic Extract on the Atherosclerotic Process—A Randomized Double-Blind Placebo-Controlled Trial. BMC Complement. Med. Ther. 2020, 20, 132. [Google Scholar] [CrossRef]
- Wlosinska, M.; Nilsson, A.; Hlebowicz, J.; Malmsjö, M.; Fakhro, M.; Lindstedt, S. Aged Garlic Extract Preserves Cutaneous Microcirculation in Patients with Increased Risk for Cardiovascular Diseases: A Double-blinded Placebo-controlled Study. Int. Wound J. 2019, 16, 1487–1493. [Google Scholar] [CrossRef]
- Chiu, H.-F.; Shen, Y.-C.; Huang, T.-Y.; Venkatakrishnan, K.; Wang, C.-K. Cardioprotective Efficacy of Red Wine Extract of Onion in Healthy Hypercholesterolemic Subjects. Phytother. Res. 2016, 30, 380–385. [Google Scholar] [CrossRef]
- Asgharpour, M.; Khavandegar, A.; Balaei, P.; Enayati, N.; Mardi, P.; Alirezaei, A.; Bakhtiyari, M. Efficacy of Oral Administration of Allium Sativum Powder “Garlic Extract” on Lipid Profile, Inflammation, and Cardiovascular Indices among Hemodialysis Patients. Evid.-Based Complement. Altern. Med. 2021, 2021, 1–7. [Google Scholar] [CrossRef]
- Brüll, V.; Burak, C.; Stoffel-Wagner, B.; Wolffram, S.; Nickenig, G.; Müller, C.; Langguth, P.; Alteheld, B.; Fimmers, R.; Naaf, S.; et al. Effects of a Quercetin-Rich Onion Skin Extract on 24 h Ambulatory Blood Pressure and Endothelial Function in Overweight-to-Obese Patients with (Pre-)Hypertension: A Randomised Double-Blinded Placebo-Controlled Cross-over Trial. Br. J. Nutr. 2015, 114, 1263–1277. [Google Scholar] [CrossRef]
- Choi, E.-Y.; Lee, H.; Woo, J.S.; Jang, H.H.; Hwang, S.J.; Kim, H.S.; Kim, W.-S.; Kim, Y.-S.; Choue, R.; Cha, Y.-J.; et al. Effect of Onion Peel Extract on Endothelial Function and Endothelial Progenitor Cells in Overweight and Obese Individuals. Nutrition 2015, 31, 1131–1135. [Google Scholar] [CrossRef] [PubMed]
- Bahadoran, Z.; Mirmiran, P.; Momenan, A.A.; Azizi, F. Allium Vegetable Intakes and the Incidence of Cardiovascular Disease, Hypertension, Chronic Kidney Disease, and Type 2 Diabetes in Adults. J. Hypertens. 2017, 35, 1909–1916. [Google Scholar] [CrossRef] [PubMed]
- Boobis, A.R.; Shiga, T.; Edwards, R.J. Genetic Polymorphisms and Cardiovascular Drug Metabolism. In Cardiovascular Pharmacogenetics. Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2004; Volume 160, pp. 39–77. [Google Scholar]
- Liu, Y.; Zhang, L.; Liu, Y.-F.; Yan, F.-F.; Zhao, Y.-X. Effects of Bulbus Allii Macrostemi on Clinical Outcomes and Oxidized Low-Density Lipoprotein and Plasminogen in Unstable Angina/Non-ST-Segment Elevation Myocardial Infarction Patients. Phytother. Res. 2008, 22, 1539–1543. [Google Scholar] [CrossRef]
- Alobaidi, A. Effect of Nigella Sativa and Allium Sativum Coadminstered with Simvastatin in Dyslipidemia Patients: A Prospective, Randomized, Double-Blind Trial. Anti-Inflamm. Anti-Allergy Agents Med. Chem. 2014, 13, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Capasso, F.; Gaginella, T.S.; Grandolini, G.; Izzo, A.A. Phytotherapy: A Quick Reference to Herbal Medicine; Springer: Berlin/Heidelberg, Germany, 2003; ISBN 3540000526. [Google Scholar]
- Leite, P.M.; Martins, M.A.P.; Castilho, R.O. Review on Mechanisms and Interactions in Concomitant Use of Herbs and Warfarin Therapy. Biomed. Pharmacother. 2016, 83, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Leite, P.M.; Martins, M.A.P.; Carvalho, M.D.G.; Castilho, R.O. Mechanisms and Interactions in Concomitant Use of Herbs and Warfarin Therapy: An Updated Review. Biomed. Pharmacother. 2021, 143, 112103. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).