Abstract
Tissue engineering endeavors to regenerate tissues and organs through appropriate cellular and molecular interactions at biological interfaces. To this aim, bio-mimicking scaffolds have been designed and practiced to regenerate and repair dysfunctional tissues by modifying cellular activity. Cellular activity and intracellular signaling are performances given to a tissue as a result of the function of elaborated electrically conductive materials. In some cases, conductive materials have exhibited antibacterial properties; moreover, such materials can be utilized for on-demand drug release. Various types of materials ranging from polymers to ceramics and metals have been utilized as parts of conductive tissue engineering scaffolds, having conductivity assortments from a range of semi-conductive to conductive. The cellular and molecular activity can also be affected by the microstructure; therefore, the fabrication methods should be evaluated along with an appropriate selection of conductive materials. This review aims to address the research progress toward the use of electrically conductive materials for the modulation of cellular response at the material-tissue interface for tissue engineering applications.
1. Introduction
According to statistics, only in the US, one person is listed as waiting for an organ transplant every fifteen minutes [1,2,3]. Unfortunately, less than half of the waiting patients are lucky enough to receive an appropriate organ from a pardoner due to exponential growth in the list of expectants. This raising dearth, however, is unable to be met by an accumulation of transplantable organs that has stagnated over the previous decade. One of the most undertaken strategies is tissue engineering, which reduces the organ shortage catastrophe thanks to artificial tissue design by the use of a combination of cells, engineering principles, and materials [4,5,6]. Tissue engineering techniques have been frequently applied to many types of tissues and organs such as skin, heart, muscle, nerve, bone, cartilage, and cornea [7,8,9,10]. In the body, tissue cells are besieged by a sophisticated mechanical, chemical, and electrical milieu. Commonly-used in vitro culture techniques have limited choices for mimicking all micro-environmental factors to direct stem cell differentiation in a developing organ [11,12,13]. Tissue properties such as stiffness and biosignals determine the cellular activity, including adhesion, proliferation, differentiation, and growth, that the architected scaffold should display to mimic the native tissue properties for damaged tissue to guarantee required regeneration. For instance, the stiffness of a scaffold is responsible for the formation of a brindled structure for skeletal myoblasts, stimulation of capillary tubes for endothelial cells, and neurite outgrowth for neuron cells [14]. Since cellular fate is modulated by cell-scaffold interactions, efforts have been done to regulate cellular responses by controlling the topography, 3D geometry, or chemical composition of cell substrates [15,16]. Additionally, some external factors can potentially affect cell–material interactions and biocompatibility including: Physical stimulation using surface topology; biochemical stimulations using release of growth factors; and mechanical and electrical stimulation (ES) [17,18,19]. The impact of electrical inducement on tissues has been defined since the 1960s when Bassett et al. proved that the electrical stimulation affects the bone formation [20]. It has been proved and explained how tissue microenvironment experiences a field of 1 V/cm during wound healing [21]. It has also been demonstrated that in vitro application of electrophysiologically DC fields (1–10 V/cm) and AC currents (10 to 100 mA) governs cellular behavior via interference in migration, cytoskeleton organization, alignment of neural cells, vascular endothelial, cardiofibroblasts, and myoblast cells, and enhances neurite outgrowth in nerve cells, differentiation, collagen production, and enhances calcification of osteoblasts [22,23]. Altogether, these preliminary studies have confirmed that the electrical and mechanical properties of scaffolds should be properly controlled for the development of physiologically healthy artificial tissues [24,25]. While there are several studies on conducting polymers for microelectronic and optoelectronic applications, researchers are exerting their focus toward biomedical applications; especially, biosensing, drug delivery, bioactuators, bioimaging and tissue engineering that benefit from developments in electroactive biomaterials [26,27,28]. The common attributes of conducting polymers (CPs) such as polyaniline (PANi), poly(3, 4-ethylenedioxythiophene) (PEDOT), and polypyrrole (PPy) are demanded for tissue engineering and regenerative medicine applications such as electroactivity, reversible oxidation, hydrophobicity, biocompatibility and surface topography. Nevertheless, elongated in vivo degradation time of conducting polymers may result in inflammation and requirement of surgical removal. To overcome such problems, researchers are now working on the development of biodegradable CPs [29,30].
2. Conductive Materials in Tissue Engineering
It is known that biomaterials surface properties have an important impact on cellular activities and cell–substrate interactions. The ability to keeping cells on the surface rather than within the hydrophobic scaffolds is one of the challenging issues in scaffold design. In this sense, surface treatment strategies have been developed to prepare substrates with high cell attachment potential. Along with the surface treatment, tissue engineering strategies can lead cells fate into particular, favorable lineages. Although electrical stimulation currents can be propagated via ionically conductive culture media, a more intended successful delivery requires electrical conductivity within three dimensional scaffolds for better tissue repair [31]. Electrical stimulation was revealed to have affirmative influences on the function and behavior of electroactive tissues [32,33]. Electroactive materials along with preparation of the proper substrate for cell adhesion and growth can make possible stimulation of cellular activity using electrical transfer [34,35]. In order to prepare proper environmental stimulus to develop healthy cell function and tissue regeneration, there is a need to develop scaffolds with all requirements, i.e., electrical, mechanical and chemical properties. The conductivity of tissues (ventricular muscle, nerve, lung, cardiac, and skeletal muscle) lies in an ordered manner in between 0.03 and 0.6 S/m [14,36].
Conductive biomaterials are a member of a novel generation of “smart” biomaterials that let direct transference of electrical, electrochemical and electromechanical stimuli to cells. Electrically conductive organic polymers are a new type of ‘synthetic metals’ that merge the chemical and mechanical properties of polymers with the electronic confidants of metals and semiconductors, together [37,38]. These π-conjugated polymers have unconstrained electrons in their segments. Within the unsaturated segments, by free motion of the loosely held π-electrons an electrical path can be opened for itinerant charge carriers [32,39]. The CPs possessing useful electrical and optical properties effectively control the electrical motive, as well as have a high conductivity/weight ratio and can be manufactured with some key characteristics such as being biocompatible, biodegradable and porous. Moreover, the changes of surface zeta potential and polymer surface properties like wettability and spatial conformation can influence the cell’s behavior during ES [40]. Alternatively, cellular functions, such as cell growth, migration, adhesion, proliferation, and differentiation, can be corrected by conductive polymers with/without ES [41,42]. A great advantage of CPs is the ability to adapting their properties to the specific requirements of their usage by accommodating antibodies, enzymes and other biological segments [43]. As illustrated in Figure 1, conductive materials, due to versatility, can be designed for targeted tissue to enhance the regeneration. Substrate conductivity, which can be adjusted using synthesis method, can affect drug release pattern, physical properties, cell behavior and regeneration rate. Tissue properties can be encoded on the conductive substrate on which the designed platform recapitulates the tissue properties to achieve maximum regeneration.
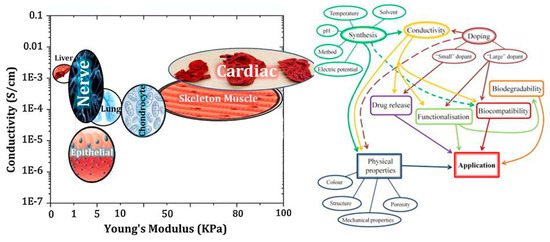
Figure 1.
Conducive platform’s properties are adjustable with various tissues [14,43]. The plot in the left-hand side gives advice on selection of biomaterials for a target tissue considering their conductivity and mechanical properties, while the right-hand one provides the investigator with a brief view over microstructure–property–performance relationship when one takes first step in selection of conductive biomaterials for tissue engineering and regenerative medicine uses.
Langer and Ingber are the pioneers who verified the cell function interaction with CP by seeding mammalian cells on the conductive films based on PPy, affected by its redox state [39]. Later, Williams and Doherty exerted the PPy as a scaffold in nerve tissue engineering. Their results offered that this biocompatible conductive substrate could be used as a nerve conduit and as a substrate for electrical currents delivery simultaneously [44]. Furthermore, Thrikvikraman et al. showed the external electric stimulation of the stem cell can determine its fate to specific lineage. As illustrated in Figure 2, electrical stimulation and its response pattern have significant effect on cell morphology, proliferation and behavior. In this review article, conductive materials used to conduct the scaffolds for various tissue engineering applications have also been considered. Table 1 presents a brief view over conductive materials used in tissue scaffolds.
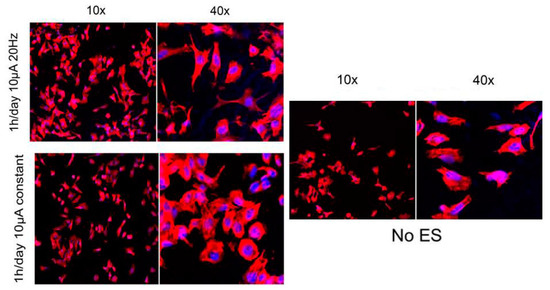
Figure 2.
Effect of the electrical stimulation on the cell morphology and proliferation. Fluorescence microscopy of PC12 cells without stimulation, with constant 10 µA of stimulation and 10 µA, 20 Hz of stimulation. Electrical stimulation enhances cell proliferation. Amplitude stimulation affects the cell morphology [45], copyright Elsevier, 2011.

Table 1.
Conductive materials used in tissue engineering.
2.1. Polypyrrole
Polypyrrole (PPy) is the most common choice among other conductive polymers due to high electrical conductivity, pliable procedure of preparation, various surface modification, great environmental resistance, proper biocompatibility, ion exchange capacity, and support cellular activities [48,49]. PPy should be doped with various anions such as Cl−, Br−, or NO−3 [48]. When opting to dopant it should be performed meticulously, for dopant affects the cell growth, proliferation and behavior. Runge et al. [50] As an example, the synthesized polycaprolactone (PCL)/PPY platform with various types of dopants and their effects are illustrated in Figure 3.
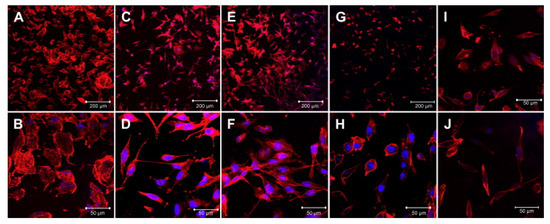
Figure 3.
PC12 behavior on polypyrrole (PPy)/polycaprolactone (PCL) platform with various dopants. Cell proliferation on conductive polymer has been affected by dopant type which dramatically affect the cell proliferation, morphology and behavior. The different scaffolds are (A,B) PCL, (C,D) PCL/PPY-NSA, (E,F) PCL/PPY-DBSA, (G,H) PCL/PPY-DOSS, (I) PCL/PPY-PI, (J) PCL/PPY-lysine. Dodecylbenzene sulfonic acid (DBSA) and naphthalene sulfonic acid (NSA) as a dopant enhanced cell proliferation than others [50], copyright Elsevier, 2010.
PPy is an attracting CP that has been frequently investigated for its efficacy towards the cell functions [46]. Since the nineties, PPy has been studied as a cell culture substrate within in vitro culture methods. In addition, animal models have been used to study the effects of implantation in vivo [33,49]. PPy have been utilized in artificial muscles, biosensors, drug delivery system, carrier of immobilized enzymes and tissue engineering [51,52]. For example, Bueno et al. electropolymerized PPy in xanthan hydrogels (XCA). Under stress XCA-PPy showed larger strain than the XCA, probably due to the slipping of planar PPy chains. Fibroblast proliferation was more enunciated onto XCA-PPy than onto XCA, due to its higher hydrophobicity and surface roughness [53]. Moreover, Haixia Liu and Ran Wan designed a biodegradable and electroactive scaffold consisting of magnesium (Mg), PPy-block-ploycaprolactone (PPy-PCL), and poly (lactic-co-glycolic acid) (PLGA) as a core-shell-frame mode for tissue engineering usage. Conductive PPy-PCL layer coated the Mg nanoparticles due to corrosion stability, through the UV-induced photo-polymerization procedure, and then PLGA is added to control the biodegradation of the illative composite. In vitro experiments using 293FT-GFP cells demonstrated that the scaffold was biocompatible [54]. Additionally, Guixin Shi and Mahmoud Rouabhia synthesized a new electrically conductive biodegradable material based on PPy nanoparticles and PLA using emulsion polymerization. Such substrate maintained a biologically significant DC current in a physiological milieu for 1000 h. Fibroblasts growth on such composite membranes was enhanced by the direct electron current applied through the membranes [52]. Deng et al. have synthesized cryogel based on PPY in which the platform exhibited the thermal sensitivity, shape memory and photothermal properties [55].
2.2. Polyaniline
Polyaniline (PANi) is another best-determined CPs, which has variety of structural appearances, proper environmental durability and facility of charge transfer by the ‘doping/dedoping’ procedure [56,57,58,59]. In 1985, MacDiarmid et al. investigated PANi as an electrically reliable material [60,61,62,63]. Among CPs, PANi and its derivatives have achieved a growing portion of electroactive filed in tissue engineering. This was due to inimitable properties of this polymer including facile synthesis, variety of structural forms, superior thermal stability, high environmental constancy, in vitro compatibility, comfort accumulation of raw materials, and low cost. PANi has also been exhibited to have the potency to clean insidious free radicals from the environment, being a well troth to be used where tissues tolerate high oxidative stress especially post infarction [64,65,66,67]. Moreover, according to some reports PANi has proper antibacterial function, especially for Gram-positive bacterium [67,68,69]. Mattioli-Belmonte et al. illustrated and explained the biocompatibility of PANi in vitro and in vivo. A conductive form of PANi appeared when the nonconductive emeraldine base is doped with an acid [70]. PANi is the only CP whose electrical properties can be adjusted properly via charge-transfer doping and/or protonation, Furthermore, the amount of electrons associated with the polymer backbone does not alter during the procedure, being capable to be introduced as the only non-redox doping CP [71,72,73,74]. PANi has been determined to adjust cellular activities [75,76]. However, degradability is commonly a favorable characteristic in tissue engineering scaffolds, the key restriction factor is non-biodegradability of CPs, causing the inflammation and contributing to the further surgery for obviation [77]. To gadget this issue, supplying of low molecular weight oligoanilines (aniline trimer, tetramer and pentamer) has been examined by different researchers to display progressed processability and biodegradability [78]. Oligoanilines were observed to undertake conductivity versus high molecular weight analogous, while their low molecular weight nature assisted direct digestion by macrophages following kidney clearance. This, in turn, decreases the chance of adverse foreign body reactions. Therefore, representing these moieties into the backbone of intrinsically biodegradable material may represent a promising method for the manufacturing of conductive biodegradable scaffolds. It was speculated that the molecular weight, functional end group, and dopant exhibit strong effect on oligoaniline biocompatibility [79,80]. Self-dopant oligoaniline-based biomaterials can reduce the cell toxicity [81]. Numerous studies have considered this issue through blending oligoanilines with materials having biodegradable segments like ester linkages, fast degradable polymers like PLA, or combining with natural polymers. In fact, it has been proved that the electrical conductivity of the hyper branched biodegradable CPs is much higher than that of their linear counterpoint with similar amounts of aniline pentamer [82]. Aniline oligomer-based biomaterials have been used as an on-demand electrical drug release. In aqueous media, aniline oligomers tend to self-assemble and form vesicle and particle, which can encapsulate loaded drugs in scaffold. Applying electrical current can rupture the vesicle and release the drug (Figure 4) [83].
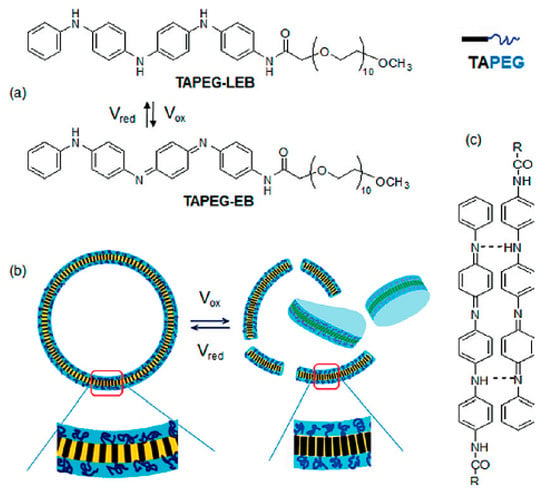
Figure 4.
Electro-responsive tetraaaniline-PEG rod-coils in aquatic media, (a) chemical structures of TetraAaniline-PEG in the various oxidation states. (b) Redox switching results in vesicles rupture. (c) Hydrogen bonding of TAPEG [83], copyright American Chemical Society, 2011.
2.3. Poly (3, 4-ethylenedioxythiophene)
Poly (3, 4-ethylenedioxythiophene) (PEDOT) has lately been prospected as an option to PPy since it has higher resistance to oxidation and more conductivity. Contrary to PPy, PEDOT can preserve 89% of its conductivity under same conditions [84]. Its high surface area and unique structures resulted in lower impedance which enhance its usage in bioelectrode coatings. In vitro toxicity and biocompatibility tests have shown that PEDOT, like PPy, has no cytotoxic effects on cells [85]. Xufeng Niu and Mahmoud Rouabhia showed that a sorely thin coating of PEDOT on micro fibrous PLA network is of adequate conductivity and durability in hydrous milieu to maintain ES to cultured fibroblasts. PEDOT-coated fibers exhibited higher hydrophilicity, thermal stability, and lower glass transition temperature contrasted with the pristine PLA fiber. In a cytotoxicity test result, PLLA/PEDOT scaffold showed no cytotoxicity and supported human dermal fibroblast migration, adhesion, and proliferation [86]. PEDOT was utilized for neural recording applications which in comparison with gold exhibit low impedance and high charge density; moreover nanofiber structure augmented its performance [87,88].
2.4. Polythiophene
The polythiophenes (PTh) are a group of CPs, which chemical modifications manifold their properties to suit various applications. The advantages of PThs over other conductive monomers (such as PPy) are that they are bowed to functionalization using a wide range of reaction conditions [89], which can be used in doped and neutral states with various properties [90]. Thiophene, terthiophene, and bithiophene molecules as parent monomers are used to synthesize the polythiphene derivatives with different functionality and tailorable properties for various applications [91]. Equated to investigations on PPy and PANi, studies on the suitability of PTh and its formatives in tissue engineering are fewer, and proportionately recent. The PThs were showed to have properties similar to, and in some cases more convenient, than other CPs [92].
2.5. Carbon
Naturall-y-occurring carbon allotropes come from variations in covalent binds of carbon atoms. Each of the carbon allotropes has specific properties based on the unparalleled spatial regulation of carbon atoms. Carbon allotropes comprise fullerene, diamond, DLC, graphite, and carbon nanotubes (CNT) [47,93,94]. “Graphene”, referring to the isolated two-dimensional crystal structures made of single atomic layers of graphite, presents great thermal and electrical conductivity due to its inimitable structure and strong carbon–carbon bonding. Furthermore, low defect density in the crystal lattice provides superior thermal and electrical conductivity in single layer graphene. Excellent electrical conductivity and thermal properties of graphene can be beneficial not only in electrical instruments but also in biomedical devices in order to measure cell potential and as a conductive platform in tissue engineering and biosensors [95,96,97,98]. It has been shown that accompanying graphene to polymers can elevate the electrical, mechanical, and thermal properties of the originating nanocomposites [99]. Recently, researches have shown that graphene membranes and hydrogels with high in-plane stiffness, can potentially be used as a biocompatible and transferable substrate for stem cell culture [100,101]. CNTs can be assumed to form when a graphene sheet is twisted into a cylinder. Single walled carbon nanotubes (SWNT) form when one graphene sheet is rolled up, while extra concentric graphene sheets create a multiple wall carbon nanotube (MWNT). In addition, carbon nanofibers (CNFs) with poor arrangement of atoms form when graphene sheets are bent at some angle to form embankment of nanocones [102,103,104,105]. CNTs are hollow nanostructures containing carbon atoms bound to each other using sp2 bonds, of which the key roles are bringing CNTs’ high electrical/thermal conductivity and mechanical properties. Excellent electrical conductivity of this aromatic structure is due to the fourth valence electron which is shared and mobile [106]. In 1991, Lijima discovered CNTs for the first time [107]. Since then, CNTs and CNFs have possessed increasing consideration due to their thermal, mechanical, optical, electrical, and structural features [102,108,109]. Carbon meshes used in tissue engineering have frequently been provided from CNFs and CNTs, either single wall or multiwall and graphene [110,111,112]. Their inimitable electrical and mechanical properties can be handled to form biomimetic tailored scaffolds [113]. Some arguments exist in concerns to the biocompatibility of SWNT and MWNT, with some in vitro studies demonstrating that CNT has a cytotoxic effect while other studies reporting CNT to be an optimum platform for cellular growth [110,111,112]. Functionalization of CNTs with active molecules makes them biocompatible which can be utilized in biomedical applications [114]. Rare attempts have been carried out to expand pristine CNT-based substrate with lack of mechanical properties and elevated CNT toxicity, where the concatenation of CNTs within polymer composites has appeared as an arresting mostly invested [115]. For example, in 2008 Sanjib Bhattacharyya and Samuel Guillot designed a nanocomposite consist of CNTs, by dispersing functionalized SWNTs in hyaluronic acid (HA) solutions in order to form hybrid HA hydrogels with SWNTs cross-linking using divinyl sulfone. This resulted in a significant variation in the topology of the hydrogels. The authors observed that the incorporation of 2% wt SWNT (vs HA) does not change water uptake capacity, while dramatically altering the dynamic mechanical properties of the hybrid hydrogels versus pristine ones [96]. Furthermore, Hermant et al. reported the development of two species of SWNTs, namely, HiPCO SWNTs and Carbolex SWNTs in a SWNTPS/PEDOT: PSS system. The authors determined that in the composite with PEDOT: PSS the SWNTs do not mostly chip in the conductivity and reasonably act as a scaffold that assists the formation of an electrically infiltrating network of the PEDOT:PSS [116].
2.6. Silicon
The amorphous SiOx cover on single crystalline silicon core enables several modifications on Silicon nanowires (SiNWs) which are pertaining for biosensor and tissue engineering applications [117]. SiNWs have tunable electrical conductivity, harmonic dimensions, and comfortable surface adjustability [118,119]. Additionally, many researches proved the SiNWs’ biodegradablity, and that Si(OH)4, their main degradation residues, are metabolically enduring in vivo. This makes them profitable compared to other non-biodegradable, electrically conductive nanomaterials, particularly for in vivo applications’ capability [119]. Nanostructured silicon as a biomaterial application has been largely reinforced by reports of easily calcium phosphate growth on the Si surface as an important bioactive material [120].
2.7. Gold
Gold nanoparticles (AuNPs) are achieving considerable attention due to their biocompatibility and relative ease of functionalization with various organic and biological moieties [121,122]. AuNPs have been successfully applied in many biological implementations to make a inimitable cellular environment that merges controllable conductivity and elasticity, which are favorable for tissue engineering [123]. McKeon-Fischer and Freeman demonstrated that low cell proliferation is not due to Au toxicity but may be a sign for myotube differentiation. An electroactive, biocompatible, and biodegradable scaffold containing electrospun PLLA nanofibers with high amounts of AuNps, was prepared for skeletal muscle tissue engineering that could perhaps need lower voltages to develop myotube formation [124]. Despite the fact that AuNps are settled of an inactive material, biocompatibility distributions have to be taken into account. Modification with AuNP alters material nanotopography which could affect a vast range of cell activities. Cells in contact to AuNps go under a phagocytosis process and therefore the particles are accumulated inside the cells in perinuclear sections (structures adjacent to the cell nucleus). Regarding cytotoxic effects, one has to recognize the effects related to the nature of the material as well as the effects prevalent to nanoparticles. At the same time, inert particles such as gold expressed tissue inflammations due to the presence of gold nanoparticles within the cell [125]. However, in cell culture experiments AuNps are considered as biocompatible, and serious cytotoxicity has not been observed yet [125,126], as well as in in vivo and pre-clinical scenarios [127,128,129,130].
2.8. Melanin
Melanin is a light absorber polymer achieved from the oxidation of tyrosine. Melanin is widely interspersed in animals and plants. It is the main pigment existing in the vertebrate’s surface [131]. Eumelanins is the main component of melanin, in which it could be the extended heteropolymers of 5, 6-dihydroxyindole and 5, 6dihydroxyindole-2-carboxylic acid. These heteropolymers can gather to form accumulates with strong π-π interactions, which supply the chemical foundation for the inimitable features of melanins.
Although the exact conduction mechanisms are not clear, physical form, temperature, and the hydration state are three factors that affect the electrical conductance of melanins, which largely ranges from 10−8 S/m to 10−3 S/m. The inimitable electrical properties of melanins propose their potential application as conductive biomaterial scaffolds in cardiac tissue engineering [132,133]. Melanin is a natural photoprotectant of skin and hair with antioxidant features, which can be used in Parkinson’s disease [134]. For instant, Dan Kai et al. suggested conductive nanofibers including 10% melanin that ameliorates cell interaction. Furthermore, improved electrical stimuli transfer (rectangular, 150 ms, 1 V/cm, 1 Hz) through the scaffolds exhibited improved cell proliferation, alignment, coupling, and the expression of connexin-43 [132].
2.9. Calcium Titanate
Calcium titanate (CaTiO3) possesses an orthorhombic architecture at ambient temperature, and the structure turns into tetragonal at 600 °C and cubic at 1000 °C. From the optical density studies on single-crystal CaTiO3, Linz and Herrington determined the band-gap energy of 3.4 eV (at 300 °K) [135,136]. Thrivikraman and Mallik used spark plasma sintered HA-CaTiO3 as a pattern system to determine the influence of altering conductivity on cell behaviors. In addition, mouse myoblast cells (C2C12) were seeded on scaffold which observed that the cell proliferation was enhanced. Generally, this work convincingly appoints the favorable effect of the platform conductivity to cell proliferation and differentiation besides corroborates the efficiency of HA-CaTiO3 biocomposites as conductive substrate to comfort the growth and proliferation of myoblasts, even when seeded without exterior electric field [137].
3. Application of Conductive Materials in Tissue Engineering
3.1. Nerve Tissue Engineering
In advanced community, recuperation from spinal cord injuries (SCI) and neurodegenerative diseases (NDD) enumerates one of the biggest universal general health challenges [138]. For in vitro/vivo studies, the principal demands for the substrate are non-cytotoxicity and mechanical properties of scaffolds necessitate to be appropriate for neural tissue expansion. Moreover, surface topography and intrinsic porosity of the scaffold affect cell proliferation and differentiation. Human body reacts to electrical stimulations and the key incorporator of neural transmission in the body is the action potential created at the synapse. This infers that a perfect neural scaffold should also own electrical conductivity to assist neurite outgrowth and thereby elevate nerve regeneration in culture [139].
Neurons have a potential of using comparatively weak electrochemical currents in mV range for controlling cellular activities. Electrically-conductive substrates can help to transfer these necessary signals among neurons, which have a positive effect on the expansion of neural tissue. Conductive scaffolds were used in nerve tissue engineering. Pires et al. cross-linked PEDOT: PSS, then neural stem cells were cultured in laminin coated substrate, and differentiated over eight days in the lack of those factors under 100 Hz pulsed DC electrical stimulation, 1 V with 10 ms pulses. The total number of neurons was 1.6 times higher with longer neurite for cells cultured under electrical motivation. Such stimulations were also directed to longer neurons. It was the first time that PEDOT:PSS combination was used to extend human neural stem cells through the implementation of pulsed signals, influencing on their differentiation directed to neurons and promoting to longer neurite [140]. Huang et al. [141] fabricated a biodegradable conductive composite containing PPy and chitosan for electrically stimulation of Schwann cells. Their results revealed that low potential (100 mV/mm) stimulations can have useful effects on cellular activities but superior potentials (300–1000 mV/mm) have damaging influences. Neurite outgrowth was highly elevated by electrical stimulation when electrical stimulation was applied through the conductive scaffold in vivo. Altogether, Schwann cell production of nerve growth factor (NGF) and Brain-derived neurotrophic factor (BDNF) was considerably elevated by electrical stimulation, which might further contribute neurite outgrowth and nerve regeneracy [142].
Thitima et al. constructed a new biomaterial for neural tissue engineering applications by coating electrospun PLA nanofibers with an electroactive polymer, PPy, via admicellar polymerization. Cell culture experiments demonstrated that PPy-coated electrospun PLA scaffold has no toxicity in vitro and could promote adhesion and immigration of neural progenitor cells. It should be noted that the PPy-coated random fibers were accidently oriented and had innumerable connections between coated fibers, while the organized fibers gave more electron current path along the PPy surface [143,144]. One of the main hindrances involving the field of electrically CPs such as PANi is difficult processing and therefore electrospinning of PANi still continues a great problem. To solve this challenge, most of the researchers have electrospun PANi by blending it with other spinnable polymers, though it reduced the conductivity of the composite fibers. For example, Prabhakaran et al. in their study designed electrospun conductive nanofibers of PANi/PLLA. Electrical stimulation along this conductive nanofibrous scaffolds showed elevated cell proliferation and neurite outgrowth versus the PANi/PLLA scaffolds that were not subjected to electrical stimulation [21]. As it was discussed recently, PANi is prevalently being used for the preparation of scaffolds which can electrically motivate cells so that regulate specific cellular functions and, eventually, the procedure of tissue regeneration.
Engineered scaffold can affect the cell alignment and elongation. Micropatterned conductive substrate based on poly (glycerol sebacate) and aniline pentamer was synthesized and Schwann cell and PC12 were seeded on. It was observed that, such platform along with enhancement of the cells alignment and neurite elongation increases the nerve growth factor (NGF) gene expression of Schwann cells. Comparison between Figure 5a–c and Figure 5d–f, it divulged that seeded cells on conductive substrate exhibit the multiple neurite from cell bodies, more neurite terminus and longer neurite length [145].
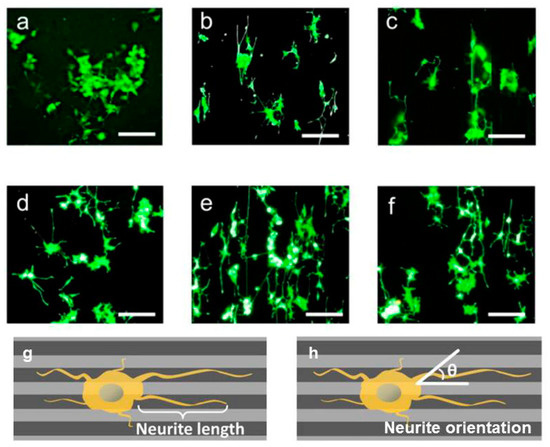
Figure 5.
Culturing Schwann cell on conductive substrate affected the cell morphology and orientation. Moreover, micropatterning along with conductivity affect the cell morphology and alignment. (a) flat substrate, (b) grooved substrate 50 μm, (c) grooved substrate 100 μm, (d) flat conductive substrate, (e) grooved conductive substrate 50 μm, (f) grooved conductive substrate 100 μm, (g) schematic of neurite length, (h) neurite orientation [145], copyright Elsevier, 2018.
Guarino et al. synthesized PANi and hydrogel cross-linked incorporated to allow the preparation of materials with good conductive manner. The attendance of PANi obviously picked up the conductivity of the material to (1.1 ± 0.5) × 10−3 mS/cm with a PANi content of 3% wt. In vitro studies corroborated that 3% wt PANi also promotes the biological reaction of PC12 and hMSC cells. PANi/Polyethyleneglycol diacrylate macroporous hydrogel add new usefulness regarding morphological and conductive attributes, both of which are necessary requirement to guide neural cells in regenerative pathways [146]. Wu et al. synthesized the conductive polyurethane based on aniline oligomer which ameliorated the Schwann cells myelin gene expression. It was speculated that the CaSR and PLCβ pathway was blocked by conductive substrate and then Ca2+ level of intracellular decreased. Intracellular Ca2+ level and DAG decrement resulted in PKC inactivation; hence, protein kinase enzyme activated other signal pathways like mitogen-activated protein kinase (MAPK) which can affect the cell behavior like differentiation, migration and secretion (Figure 6) [25].
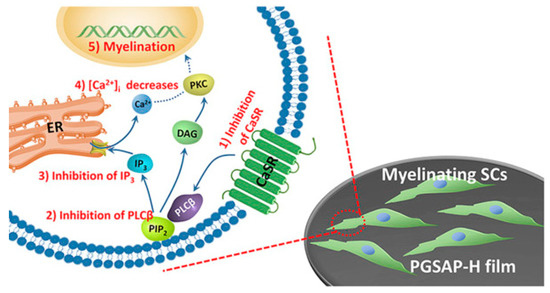
Figure 6.
Mechanism of Schwann cells’ (SCs’) myelination on conductive platform. Conductive films inhibit CaSR and PLCβ pathway, and then decline the intracellular Ca2 þ level [25], copyright Elsevier, 2016.
Zarrintaj et al. synthesized the polysaccharide based conductive substrate with various length of aniline oligomer [147,148,149,150]. It was reported that the conductive polysaccharide can be good candidate for neural regeneration because of resembling ECM [151,152,153].
Conductive platform along with directs electrical signal transfer, assists neural growth. Functionalization and bioactive coating are suggested to ameliorate the biocompatibility of CNT-based scaffolds. According to Mattson et al. aldehyde 4-hydroxynonenal is desired because of its influences in raising intracellular Ca2+ concentration, improving cytoskeletal proteins and signaling pathways that direct neurite expansion [154]. Using this plan, MWNTs were coated with 4-HNE to control neural cell activities. Results demonstrated that SWNTs can be functionalized to promote neuron growth and expanding that is decisive in neural regeneration [102]. Moreover, Kabiri et al. compared the neural differentiation and proliferation of stem cells on three distinctive aligned electrospun scaffolds composed of PLLA complemented with either SWNT or MWNT. Adding nanotube impregnated conductivity to the scaffolds and directed mouse embryonic stem cells for neural differentiation as proved by a development of mature neuronal markers expression [155].
The neural stem cells as a multipotent cell type in the CNS, which display undertaking outlooks in expansion cell therapies for neural regeneration. A scaffold that adjusts neural stem cell action and tissue improvement by constructing milieu has been a perfume in clinical usages. At the present time, the combining carbon nanomaterials propose numerous occasions to prepare novel scaffolds as neural tissue engineering. Li et al. [156] revealed that the application of graphene foam as a novel platform for neural stem cells in vitro. A good electrical connecting of 3D-GFs with differentiated NSCs for impressive electrical stimulation was seen. Their research signified 3D-GFs could tender a formidable intent for neural stem cell research, nerve tissue engineering, and neural interface. The cyclic voltammetry results implied that it involves low risk for three-dimensional graphene foams (3D-GF) to electrically stimulate cells via capacitive charge injection like 2D graphene electrode. However, due to its high surface area, it could supply much stronger charge injection capability than 2D graphene films with the same geometrical area. As a result, the 3D architecture of GFs can impressively enhance the electrical stimulation actions of conductive scaffold. Furthermore, it was found that in contrast to two-dimensional graphene films, 3D-GFs can promote neural stem cells growth and Ki67 expression [30]. Table 2 summarizes the conductive scaffold for neural regeneration.

Table 2.
Conductive scaffolds used in neural tissue engineering.
3.2. Cardiovascular Tissue Engineering
Cardiovascular disease is the most prevalent reasons of death in developed countries and it is becoming an epizootic menace of the 21st century [188,189]. Among different strategies, tissue engineering and cell biology have recently found some innovative ways to shed light over finding new treatment approaches. It is believed that the exact function of cardiomyocytes (CM: contractile muscle cells that are specialized for myocardium tissue) and neurons is supported by on persevering conductivity. It is also known that heart disease or improper functioning may interrupt such conductivity [190,191]. Myocardial infarction (MI) is the most usual reason of heart failure and distraction [123,192,193]. Behind an immense CM loss owing to MI, the myocardial tissue misses considerable intrinsic regenerative ability to swap the lost cells, thus the disorder of the heart wall muscle is everlasting [194,195].
Numerous methods have been used to enhance CM and neuron development near defunct tissue after a MI. Such projects possess ex vivo culture of CM on cardiac patches for direct cell injection, probable implantation, scaffolds based on collagen, PLA, PCL, 3D printing, and injectable scaffolds using materials adorning from fibrin to CNFs. Each method has its own superiorities however usually all of the above can be detached into two categories: (a) Conductive patches and (b) non-conductive patches [196]. One of the most trustworthy tactics among the above is cardiac tissue engineering [197]. The major difficulty in tissue engineering is to imitate the structural and functional properties of ECM, thus constructing a bioactive platform possess suitable chemical, biological, and conductive properties [198]. Versus the natural heart tissue milieus, the porous substrate have a few artificial characteristics. First, the scaffolds are non-conductive at biological frequencies, but heart tissues have a DC conductivity of about 0.1 S/m and are emphasizing with electrically conductive Purkinje fibers. Secondly, many scaffolds do not have nanofibrous structures at nano scale round 10–100 nm diameters, which are plentiful in natural ECMs and possess a crucial role in controlling cellular functions. Thirdly, the scaffolds have typically more weak mechanical properties than the native heart muscles [199]. Wu et el. synthesized the interwoven directed conductive nanofiber to recapitulate the cardiac tissue. Figure 7 illustrates the cardiac tissue structure which was the inspiration of 3D scaffold fabrication. 3D yarn/hydrogel scaffold mimicked the cardiac structure and conducting 3D cellular arrangement. Such scaffold enhanced the cellular orientation and proliferation which can be a harbinger for ultimate cardiac regeneration [200].
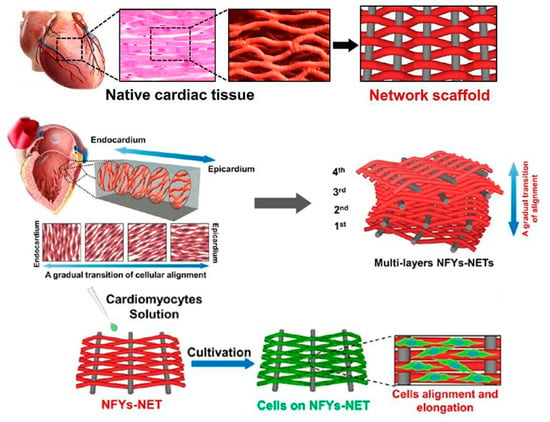
Figure 7.
An interwoven structure of cardiac with aligned cell layers and biomimic scaffold with similar structure. Multiple layers of Yarn nanofiber can recapitulate the cardiac muscles which cause cell alignment and elongation [200], copyright American Chemical Society 2017.
Electroconductive materials have been acknowledged to be useful for myocardial tissue engineering due to the capability to be used as a substrate that controls electrical stimulation [132]. Li and colleagues showed that nanofibrous scaffolds made of gelatin and PANi as a conductive platform supported H9C2 annex and proliferation. This study demonstrates the first phase in their long-term tactics of seeding cells on nano-fibrous scaffolds made of CP. Conditional upon the concentrations of PANi, the cells primarily showed different topologies on the fibrous scaffold [38]. Later Dan Kai and coworkers reported an electrospun PCL-gelatin-PPy scaffold that advanced CM annex, proliferation and presentation of cardiac-specific proteins. Nanofibers made of 15% PPy represented the most equivalent properties of conductivity, mechanical properties, and biodegradation, corresponding to the provisions for repairing of cardiac tissue [192]. After that Benjamin Spearman et al. demonstrated that after culturing CM on the conductive PPy-PCL substrates, more cells were distinguished to possess peripheral localization of the gap junction protein connexin-43 [201]. This composite had a resistance of 1.0 ± 0.4 kΩ cm, which is equivalent to natural cardiac muscle. Consequentially, faster calcium wave spreading rate and shorter calcium temporary period for CM monolayers on PPy-PCL associated to cells on PCL [201]. Liang et al. synthesized the cardiac patch based on PPy-dopamin which was applied using injection (Figure 8). Such method attracted a significant attention because of its non-invasive nature and elimination of surgical operation. After injection, hydrogel formed and due to its conductivity, which is equal to normal myocardium is powerfully bonded to the beating heart. It is a promising method to eliminate the surgical operation [202].
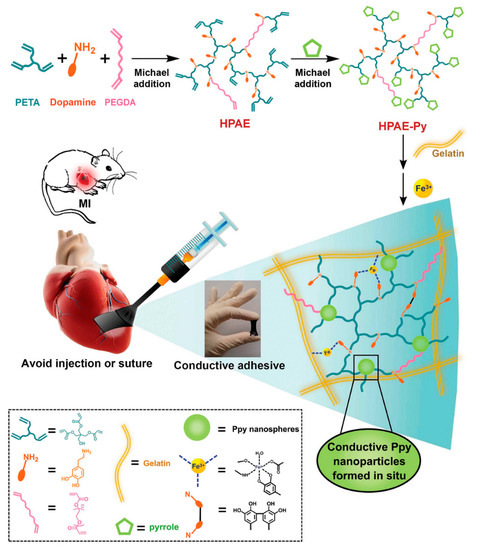
Figure 8.
Conductive adhesive hydrogel patches synthesis attached on myocardial infarction (MI) site. Pyrrole was capped dopamine, simultaneously polymerized using Fe3+ oxidation which acted as an adhesive and conductive substrate [202], copyright John Wiley and Sons, 2018.
The cooperation between conductive scaffold and stem cells offers a chance to prevail MI. Borriello et al. determined that the conducting PCL/PANi platform could be done as a suitable substrate to promote MSC differentiation to CM lineage. Their results proposed that PANi short fibers conduct electrical stimulations impressively. The authors revealed that the viability of CM-like cells onto PCL/PANi substance was considerably superior to that on the PCL. Furthermore, the presentation of sarcomeric α-actinin was also approved on a conductive platform [203]. Moreover, Spencer W Crowder and colleagues introduces an electrospun PCL including CNT to specify MSCs fate [204]. This scaffold surrounded inherent potency enhancing rod-like and prolonged morphology in 3D culture. Outcomes showed that differentiation of hMSC can be improved by culturing on conductive scaffolds [204].
Additionally, the exhorting actions gained by using CPs, for instance, CNTs with nanostructure can emulate the ECM. Valentina Martinelli and colleagues showed that CNT platforms assist CM growth and puberty by changing the gene expression program, doing the cell electrophysiological attributes and enhancing networking and puberty of syncytia. They showed that ventricular myocytes cultured on scaffolds of MWNT communicate with CNTs by forming firm osculations and show enhanced survival rate and proliferation [205,206]. Later Xia Li et al. indicated that the poly (N-isopropylacrylamide) (PNIPAAm)/SWNTs hydrogel showed considerably higher bioactivities to encapsulated stem cells compared to one-fold PNIPAAm hydrogel, containing improving cell attachment and proliferation, in vitro. Furthermore, when acting as a vehicle for intramyocardial delivery of stem cells after MI, the PNIPAAm/SWNTs gel considerably assisted the hybridization of culturing cells in infarct myocardium and increased their therapeutic efficacies [207]. Lately, Stout et al. [190] presented a PLGA-CNF composite for cardiac tissue engineering. Aortic endothelial, fibroblast, and CM cells were seeded onto a PLGA: CNF scaffold to distinguish if CNF concentration has an influence on cellular activity. Throughout consecutive ES, CM cell density increased compared to its static counterpoint. A lesser raise in Troponin I excretion in ES in comparison to the static state demonstrated nominal CM cell activity within cell cultures. Fibroblast and endothelial cell growth studies demonstrated the material prevented or stopped proliferation during both static and ES, thus promoting the growth of CM onto the damaged tissue area. Moreover, the findings showed that CNF concentration exhibited an influence on PLGA:CNF scaffold in vitro compatibility features with the proper results achieving from the 50:50 (PLGA:CNF) [208]. Furthermore Kharaziha et al. fabricated hard and flexible hybrid scaffolds with improved electrical attributes containing CNTs detruded aligned poly (glycerol sebacate): Gelatin nanofibers [196]. The resulting CNT-PG scaffolds showed more powerful instinctive and simultaneous beating action evaluated to those seeded on PG scaffold. Totally, their results showed that aligned CNT-PG scaffold have excellent mechanical attributes with elevated CM beating attributes [196].
Dissident to the usefulness of cell-based therapies in regenerating infarcted myocardial tissues, lack of electrical communications between donor cells and the host myocardium is caused by the absence of functional merging of them. To reconstruct the contractile heart muscle, electroactive scaffolds are employed in order to graft implanted cells with the host tissue in a simultaneous strategy. The electroactive portion of scaffolds signifies identical stimulatory of implanted and host cells, and also promote distribution of the electrical wave front [119,209]. Chun-Wen Hsiao et al. suggested a nanofiber mesh of PANi and PLGA, as a conductive layer for adapting the beatings of the seeded CMs simultaneously [209]. Accordingly, after electrical stimulation contractions of the single clusters grown on the conductive platform were contemporized, and the cell beating frequency was similar to the electrical potential [209]. Su Ryon Shin and coworkers specified that the presence of CNT as a conductive factor in gelatin-methacrylate hydrogel resulted in promoting myocardial cell attachment, organization, and cell–cell communication [199]. Moreover, 2D engineered cardiac tissue on CNT-gelatin-methacrylate showed suitable mechanical unity tolerating tissue constriction. Combination CNT to the structure decreased stimulation limen about 85% lower. Actually, CNT networks connected the insulating pore walls of the hydrogel, supplied additional paths for straight electrical charge flow, and decreased the impedance between cells for charge redistribution and action potential dissemination [199]. Furthermore, Hongyu Sun et al. introduced SWNTs blended into collagen scaffolds were used as growth carriers for CMs, which promoted CM adhesion and proliferation [210]. Moreover, they found that the presence of CNTs significantly incremented intercalated disc (ID: a well-organized structure that attaches myocytes into a syncitium electromechanically and supply propagation of electrical diagonals in every part of the heart) protein expression and improved ID congregation and activity. On that foundation, they further probed the fundamental mechanism for how CNTs promoted ID assembly. They discovered that the signaling pathway of β1-integrin-mediated intervened CNT-induced regulation of mechanical and electrical junction proteins. Particularly, CNTs significantly precipitated gap junction formation using β1- integrin-mediated FAK/ERK/GATA4 pathway activation. These results supply a noteworthy understanding into the mechanistic influences that CNTs cause on neonatal CM actions [210].
Electrical signals’ dissemination through natural myocardium causes ordered constriction of the heart, then blood pumping throughout the body. An unfit mechanical junction between CMs terminates to an annihilated cardiac pumping; presented improper electrical junction may end to incomplete conduction of the electrical impulse and pursuant pickup of cardiac arrhythmias. Tal Dvir et al. declared that flexible gold nanostructure in alginate hydrogel can merge the insulated pore walls of alginate and promote electrical connection between cardiac cells and their neighbors. In this way, thicker- and further-aligned tissue grown on this platform and cells in these tissues contracted simultaneously [211]. Elsewhere, Tan et al. specified that adhesion of a trace amount of conductive silicon wires in differently scaffold-free cardiac spheroids can create an electroactive mesh, outstanding to simultaneous and considerably improved constriction, terminating in more developed contractile maturation and cellular structural [119].
The study mentioned earlier is the first explanation of using nanostructure semiconductors to advance cardiac tissue formation and CM maturation without importing contractual scaffolding materials [119]. Notwithstanding all these attempts, it is regarded that the usage of electroactive biomaterials is just restricted to in vitro maturing engineered cardiac tissues, and it subsists to be elucidated whether electroactive biomaterials can advocate functional engineered cardiac tissue-formation in vivo, perform beneficial traces on the heart function, or claim the structural and functional accretion between engineered cardiac tissues and infarcted myocardium based on their nanoscale attributes [204,212]. One challenge is weak cellular totality in engineered cardiac tissues constructed using current procedures. This is particularly reflected in the insufficient regenerating of IDs [210,211,213].
Arterial blood vessels have a multi-layer construction including collagen and elastin fibers, smooth muscle, and a complex construction of endothelium. Blood vessels have a different round structure due to the orientation of their fibrous parts [214]. A.S. Rowlands for the first time showed that vascular smooth muscle cells (VSMCs) seeded on a CP layer and subject to ES not only present improved proliferation but can be contemporaneously patronized to excess contractile protein expression [168]. In this research VSMCs were seeded on PPy layer and were exposed to a 50-mA sinusoidal ES at 0.05, 5, and 500 Hz. Such layers were coated with collagen IV followed by Matrigel and doped with HA in order to imitate the tissue and promote cell adhesion. Enhanced proliferation and expression of smooth muscle phenotype markers (smooth muscle α-actin and smooth muscle myosin heavy chain) were monitored in cultures stimulated at 5 and 500 Hz [215]. Moreover, Mihardja et al. prepared an injectable hydrogel of alginate merged to PPy as a conductive platform. Local injection of polymer mixture in to the infarct area produced considerably better levels of arteriogenesis. Moreover, this scaffold considerably improved migration of myofibroblasts into the infarct zone [216]. The conductive scaffold for cardiovascular regeneration is summarized in Table 3.

Table 3.
Conductive scaffolds used in cardiovascular tissue engineering.
3.3. Bone Tissue Engineering
In 1950, bone was found to present inherent electrical attributes such as piezoelectricity [229]. These attributes create an internal electrical field in response to tensions that change cell proliferation, which can describe why exterior electric and electromagnetic excitation have an advanced effect in bone healing therapy [230,231,232,233]. The electrical potentials happened in bone due to the mechanical loading, which is described in terms of both the piezoelectric features of the collagen in bone and by the motion of ionic fluids through the structure. These potentials have been connected to the mechanical conformity of bone in response to loading, outstanding to the recommendation that using an electrically active part in an implant material may enhance healing and conformity of the circumambient tissue [234]. It was shown that such excitation improve osteoblasts activity. Figure 9 exhibits various electrical stimulation methods which have been utilized for bone regeneration. DC decreases the level of oxygen and enhances the pH; hence, osteoblast cell proliferation increases. In second method, capacitive coupling results in an increment in cystolic calcium through voltage gated calcium channels and finally inductive coupling result in a direct increment in intracellular calcium, which increases activated calmodulin stores [235].
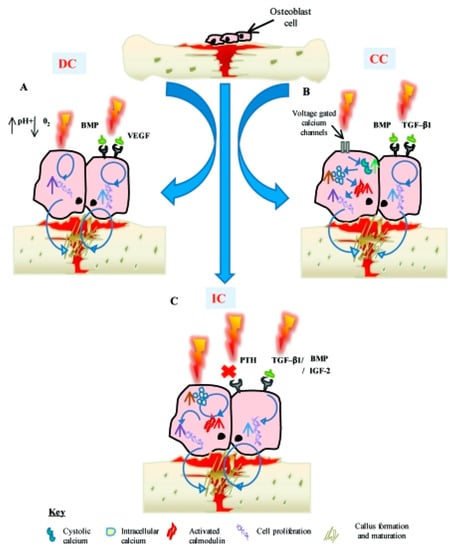
Figure 9.
(A) Direct current decreases the oxygen level and enhances the pH, which causes to increase of osteoblast proliferation (B) capacitive coupling results in increment of cystolic calcium through voltage gated calcium channels. (C) Inductive coupling stimulation results in a direct enhancement in intracellular calcium [235].
Progress studies in 3D scaffolds prepared for bone tissue engineering are frequently afforded to enhance the attributes of the scaffolds in regard to their chemical and mechanical attributes. To incorporate the tissue engineering methods with the idea of improving the bone healing by electrical stimulation, the electrical attributes of the scaffolds needs to be corrected [230]. The CP such as PPy and PANi have been vastly used and researched due to their comfortable and helpful synthesis, manageable electrochemical activity, and great compatibility in vitro. Haitao Cui et al. in their research prepared a novel electroactive polyelectrolyte based on tetreaniline and poly (L-glutamic acid), that manufactured through layer-by-layer (LbL) assembly strategy. In comparison to the nongrafted polyelectrolyte multilayer films, the tetreaniline-grafted samples demonstrated superior stiffness and roughness in micro/nano structures. The surface specifications and the typical electroconductive attributes were more useful for cellular activity of preosteoblasts MC3T3-E1 cells. Furthermore, the improved influences were seen on the incorporation of MC3T3-E1 cells, when the electroactive polyelectrolyte multilayer films were coupled with ES, particularly in the initial phase of the osteoblasts differentiation [236].
Since the 1980s, CPs with admissible biocompatibility have been applied in several biomedical usages. CPs intervene ES and have the capability to be the motivating factor that increases bone regeneration. PEDOT is a biocompatible CP which is lately being operated in biomedical usages particularly in bone tissue engineering. Shahini et al. in their research fabricated 3D conductive scaffolds by hiring a biocompatible CP, i.e., PEDOT:PSS in the optimized nanocomposite of gelatin and bioactive glass. Adult human MSCs were cultured on the substrates, for in vitro examination. Their outcomes demonstrated that such conductive scaffolds are not only structurally desirable for bone tissue engineering, but also can be an approach in incorporating the tissue engineering methods with the method of improving the bone healing by electrical stimulation [237]. In 2014, Liu and Cui showed the cytocompatibility of the aniline pentamer-graft-gelatin/PLLA nanofibers in vitro by the attachment and proliferation of MC3T3-E1 cells. The cellular expansion was considerably larger on electroactive aniline pentamer-graft-gelatin/PLLA nanofibers than on PLLA nanofibers. Moreover, the aniline pentamer–graft–gelatin/PLLA nanofibers motivated by an electrical pulsed indication could enhance the differentiation of MC3T3-E1 cells evaluated with pristine PLLA nanofibers. Their outcomes showed that the conductive and biodegradable PLLA/aniline pentamer/gelatin nanofibers had capability of being as bone scaffold materials in tissue engineering in vivo [238].
Graphene has obtained exceptional attention in various applications according to its inimitable physical attributes. Lately, several research groups have demonstrated that graphene boosts cellular activities. In 2015, Lyu et al. tested the influence of applying a graphene hydrogel to compel the osteogenic differentiation of hASCs. Compared on arbitrary graphene and carbon fiber films, the hydrogel had enhanced mechanical stability and malleability. Moreover, they discovered that the hydrogel has no cytotoxicity and is biocompatible. One advantage is that film could motive the osteogenic differentiation of hASCs by oneself free of extra chemical signals. Such influences are more powerful for such hydrogel than others; the induction valiancy of the hydrogel is not as high as that of the osteogenic induced medium. The superior osteoinductivity of the hydrogel is nearly linked to its considerable physical attributes that contain special nanoscale structures, surface topology, cell adhesion, surface hydrophilicity, and protein absorption [239]. In 2011, Meng et al. seeded osteoblasts-like Saos-2 cells on an electroactive layer made of PLA and bioactivated PPy using heparin (PPy/HE). The influence of multiple electrical stimulations on osteoblast mineralization was considered at many culture periods using electrical cell culture plates. As confirmed by surface analysis, the electrical stimulation was capable of elevating osteoblast growth and adhesion, causing considerably higher calcium and phosphate concentration in the mineral precipitation of the electrically motivated meshes with similar characteristic features to hydroxyapatite. Electrical stimulation also considerably up ordered the expression of the osteoblasts-specific markers Runt Related Transcription Factor 2 (RRTF-2), Alkaline phosphatase (AP), Bone morphogenetic protein 2 (BMP2), and Osteocalcin. Hence electrical stimulation through a synthetic CPs platform shows a vital factor to enhance bone regeneration [240]. In 2013, Shiyun Meng et al. seeded Saos-2 cells on conductive substrates containing biodegradable PLA and the heparin-PPy to study their response to ES intervened through such scaffolds. Interval and strength of electrical stimulation improved cell proliferation, generating an inimitable electrical intensity and provisional “window” within which osteoblasts proliferation was up-modulated in comparison to the down modulation or ineffectiveness in other electrical stimulation zones. The desirable electrical stimulation intensity around 200 mV/mm was more considered gene activation and protein production of two significant osteoblasts markers described by ECM maturation and mineralization that is AP and osteocalcin [241]. In 2007, Melanie A. Whitehead and Dongmei Fan discussed about of preparation and analysis of an electrically conductive composite material containing PCL, PANi, and silicon. The efficacy of PANi/silicon on calcium phosphate infusion was determined through ex vitro experiment using ES. Formation of calcium phosphate is one conceivable eligible specifications of “intelligent” synthetic scaffolds for orthopedic-pertinent usages. Moreover, electrical consistency evaluations were done in DMEM to assess the constancy of such structures to bias in a reliable electrolyte via a classic cell research. The composites cytocompatibility was measured in vitro via HEK293 cell proliferation, together with more orthopedically pertaining MSCs from mouse stroma. Significantly, these composites show precipitated calcification in simulated body fluid when electrical bias is used catholically to the scaffold. Moreover, these substrates display non-cytotoxicity in the vicinity of fibroblasts during culture period, and annex of stromal cells to the semiconducting scaffold was directly assessed via scanning electron microscopy. Generally, these outcomes propose that such materials are capable to be as a biomaterial [242]. Table 4 abridges the conductive scaffold in bone tissue engineering

Table 4.
Conductive scaffolds used in bone tissue engineering.
3.4. Muscular Tissue Engineering
Skeletal muscle tissue includes brindled nanoscale fibrous morphologies convened into fiber fardel which contract upon motivation by an attached nerve [124,252,253,254]. Electrical stimulation has been applied in some clinical trials to considerably help spinal fusion and in the functional recovery and regenerating of muscle in patients who have tolerated denervation. It has been presented that lasting low-frequency electrical stimulation affects myoblast growth and differentiation via duplicating some bioelectric signals [255]. The progression of new tissue engineering experiments for skeletal muscle is serious for the renovation of lost or defective muscle that can happen as a result of traumatic damage or neuromuscular perturbations, such as the Muscular dystrophies [34,252,256]. From a biomimetic outlook, functional engineered skeletal muscle tissues must display indigenous-like structural attributes and, particularly, include compactly packed and uniformly aligned myofibers all over a relevantly large tissue volume [253,254,257].
Another option contains the implantation of prefabricated muscle tissue formed by the in vitro differentiation and puberty of muscle pioneer cells on a matrix or layer. New muscle tissue is expanded in vitro by managing the environmental situations and containing differentiation, with the procedure being seriously related to the material acting as the scaffold for the cells [34,253,256]. Recently, engineering of compact, directed, feigned muscle tissues with a comparatively large area was assayed by consecutively layering collagen matrix and culturing myoblasts in a culture dish [254,257]. One group of materials with potential as a breathtaking candidate for skeletal muscle tissue engineering is CPs. CPs are surmised wonderful as they not only prepare a scaffold for mechanical support, but through their natural conductive attributes can also transfer different stimulation to the cells [34,252,256]. Sung In Jeong and coworkers demonstrated that blending PANi to poly (L-lactide-co-e-caprolactone) scaffold makes ameliorated myoblast cell annex and metabolic activity. The growth of NIH-3T3 fibroblasts is improved under the incitement of several direct current flows between 0-200 mA [75]. In the same study Indong Jun et al. demonstrated that blending of PANi to PLA-PCL scaffold, the number of myocyte cells positive for sarcomeric myosin was 3.6-times higher on the electrically conductive fibers after 4 days of culture. Moreover, the level of myogenin expression deciphered on day 8 of culture on PLA-PCL/PANi-15 (containing 15% PANi) was nearly 1.6-fold greater than the PLA-PCL/PANi-0 fibers. Equivalent outcomes were seen for the expression of other genes containing troponin T (2-fold greater) and the myosin heavy chain gene (3-fold greater) [258]. Wang et al. synthesized the nanofiber yarn/hydrogel core–shell scaffolds for mimicking skeletal muscle (Figure 10). The synthesized scaffold recapitulated the native skeletal muscle tissue which resulted in 3D cellular alignment inducement and elongated myotube formation. The aligned core-shell nanofiber was fabricated by electrospinning based on PCL/PANi/Slik which 3D structure enhanced the nutrient exchange and provided the proper milieu for better alignment and differentiation [259].
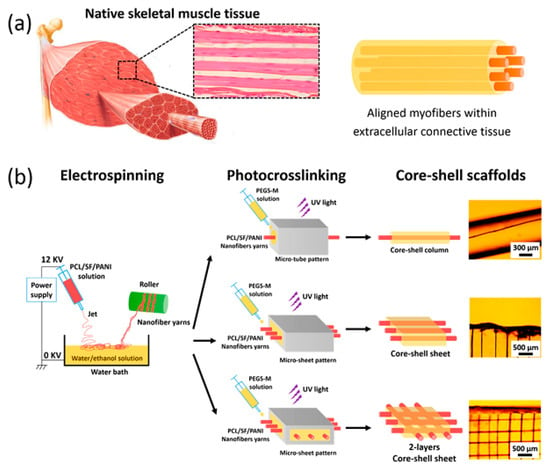
Figure 10.
(a) Composite similar to the skeletal muscle structure, contain aligned myofibers formed through myoblast fusion together into multinucleated myotubes surrounded within the extracellular connective tissue. (b) Scheme of scaffolds fabrication [259], copyright American Chemical Society, 2015.
A great attention for the engineering of muscle is the capability to restitute tissue in suitable orientation reflecting. Particularly, myotubes should be designed in a linear arrangement to express native muscle structure, which is organized as extremely linear, non-branched bundles in vivo. This organization is in part interposed through the physical and biological attributes of the ECM. The ECM of skeletal muscle includes a nanofibrous network of proteins. Such structure has been repeated ex vivo resulting in the linear orientation of differentiated initial skeletal muscle cells grown on microstructured CP platforms. This efficacy has also been attained at the nanoscale using biodegradable nanofibers, showing that nanoengineered scaffolds prepare the capability to control muscle fiber orientation. The capability to restrain the expansion of myoblasts into orientated myotubes is important for efficient muscle engineering [260]. As an example, Quigley et al. conceived a novel nanostructured conductive scaffold made of aligned MWNT with and without para-toluene sulphonic acid doped PPy. Electrochemical analysis of these substrates demonstrated better electrochemical activity in MWNT after coating. Myoblasts attached, proliferated and differentiated on all collected surfaces sans the use of interpolation molecules. Myotubes grown on nanostructured surfaces showed alignment.
A considerable increment in myotube alignment and length was also discovered on linear functionalized MWNT arrays. The gamut of myotube alignment was discovered to reduce with increasing film thickness. A considerable increment in cell density and myotube formation was distinguished in the electrically motivated group [260]. In two outstanding projects, Ku et al. and Chen et al. divulged that myosin heavy chain expression, multinucleate myotube formation, the emanation of differentiation special genes, the differentiation of myoblasts on PCL/PANi electrospun nanofibers was strongly dependent on both nanofiber alignment and PANi concentration. These outcomes displayed that a composed effect of both guidance cues were more efficacious than a single cue [261,262]. Conductive scaffold which are utilized for muscular tissue engineering is listed in Table 5.

Table 5.
Conductive scaffolds used in muscular tissue engineering.
4. Conclusions and Future Perspectives
CPs were demonstrated to be able to tune cellular actions via ES such as cell growth and cell migration, leading to a significant interest in CPs and their derivatives for tissue engineering usages. Several research studies are linked to various tissues, which are susceptible to electrical stimulation. This exhibits the significance of CPs in tissue engineering, because the regulation of cellular demeanor is conclusive for the regeneration of blemished tissues. However, there are applied obstacles when the CPs are employed in tissue engineering. The original impediments with the available systems are poor polymer–cell interactions, the absenteeism of cell interaction sites, hydrophobicity, poor solubility and processability, as well as uncontrollable mechanical attributes. Their incapacitation to degrade is one of the greatest constraints for tissue engineering usages. Keeping CPs in vivo for a long time may clasp an inflammatory reaction and the requirement for a second surgical process. The synthesis of materials with both electroactive and degradable attributes is extremely favorable and is still a challenge. There are different fabrication and synthesis paths of biodegradable and electrically CPs using both CPs to form mixes and composites as well as conducting oligomers to form biodegradable and electroactive copolymers [30,265].
Provision of clinically appropriate CPs-based tissue scaffolds with biomimetic chemical, mechanical, and topological attributes is the other challenge in this field. These materials can be constructed in a diversity of ways. Biomimetic chemical methods are innovative approaches recently attained that non-covalently combine both high and low molecular weight components/derivatives of the ECM as dopants during electropolymerization reactions. An ECM-mimicking structure can also be exposed to materials by covalently modifying their surfaces with ECM derivatives, generally employing carbodiimide chemistry. Materials composed of CPs only tend to be relevantly inelastic because the polymers have confined conformational freedom in 3D, result in film preparation via electropolymerization rip. This demonstrates a significant problem, as the handling of biomedical products are of key significance to their successful repented from the laboratory to the clinic. Flexible conductive biomaterials can be constructed by interspersing a large-enough quantity of conductive filler within an elastomeric matrix, such as PCL or polyurethane or gathered from multiblock copolymers composed of intermittent blocks of conducting and elastomeric blocks, such as PPy and PCL. The development of tissue engineering scaffolds that mimic wrapped structured natural tissues will be the focus of significant interest in the next few years. Among different strategies, electrospinning is a general method of preparing nanofibrous tissue engineering scaffolds with a tunable degree of fiber alignment nearly similar to the native tissue structure [266].
One of the most challenging issues in tissue engineering is achieving tissue-specific functions. As an example, since hepatocyte cells are anchorage-dependent cells and highly susceptible to the ECM milieu for the keeping of their viability and differentiated functions, liver tissue engineering requires a suitable ECM for hepatocyte cell culture. On the other hand, since initial hepatocytes lose their phenotype quickly after isolation, impounding liver-specific functions has been the main goal of these studies. By implementing innovative physical and chemical strategies, favorable aspects of tissue engineering can be mimic for specific interactions between scaffolds and cell surface receptors. Hence, the design and selection of biomaterials for tissue engineering scaffolds are of great importance. To gain higher levels of tissue-specific function and mechanical consistency, culturing cells have been tested on different conductive biomaterials.
Surface charge and conductivity of biomaterials and different kinds of cells ascertain a communication between the surface voltage, the rest potential, and a control over differentiation and proliferation. It has been determined that the rest potential of cancer and stem cells, which have excellent proliferation rates, are much lower than any kind of adult differentiated cells. After cancer and stem cells, among all the differentiated cells, liver cells are the most depolarized cells within the human body [267]. This phenomenon can be affirmatively influenced in the attendance of conducting biomaterials. In fact, the CPs will conduct the specific charge of the cells that will be resisted in other scaffolds due to resistant materials (e.g., natural polymers) used in the scaffold compositions. The conducted charge can make local electrical fields inside the seeded scaffolds, which will cause regulation of the ion transfer and movement across the membrane and further influence the cellular behaviors, such as cell attachment, cell proliferation, and proteins expression. Some recent studies on hepatocyte cells in tissue engineering scaffolds with different compositions showed that the scaffolds including (PEDOT) could provide the best situation for annex and proliferation of the cells. More particularly, the mixture of hyaluronan, PEDOT, and collagen (I) as dopants in gelatin–chitosan-based scaffolds could introduce the best cell/scaffold actions for regeneration of cells [268,269].
The development of innovative biomaterials as structural and bioactive scaffolds is not only essential for tissue engineering but also important for cellular biophysics. Bozhi Tian et al. [270] have recently designed a new type of macroporous nanowire nanoelectronic scaffolds. This class of nanoelectronic scaffolds could mimic the structure of normal tissue scaffolds, organized by self-organization of coplanar reticular networks with built-in strain and by manipulation of 2D mesh matrices. This is one of the important studies showing robust electronic characteristics that have been used lonely or combined with other biomaterials as biocompatible extracellular scaffolds for 3D culture of neurons, CMs and smooth muscle cells [270].
The current researches can potentially solve problems associated with the conventional scaffolds that could not electrically probe the physicochemical and biological microenvironments throughout their 3D interior, which can have a noticeable impact in both electronics and biomaterials. Furthermore, the integrated sensory capability of conductive scaffolds by real-time monitoring of the local electrical activity within the constructs could revolutionize the response of neural and cardiac tissue models to drugs, and vital characteristics inside and outside vascular smooth muscle constructs.
Author Contributions
A.S. and F.J. wrote the first draft. P.Z. and M.R.S. wrote some parts and edited the text. M.M. initiated the idea, guided the direction of the article and edited the text.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| ECM | extracellular matrix |
| SCI | spinal cord injuries |
| NDD | neurodegenerative diseases |
| CM | cardiomyocyte |
| MI | Myocardial infarction |
| hASCs | human adipose stem cells |
| MSC | mesenchymal stem cell |
| NGF | Nerve growth factor |
| BDNF | Brain-derived neurotrophic factor |
| CP | conductive polymer |
| PPy | polypyrrole |
| PANi | polyaniline |
| XCA | xanthan hydrogels |
| PLA | poly(lactic acid) |
| PEDOT | poly (3, 4-ethylenedioxythiophene) |
| PLLA | poly(L-lactic acid) |
| PDLLA | poly (d,l-lactide) |
| CNT | carbon nanotube |
| SWNT | single wall carbon nanotube |
| MWNT | multiple wall carbon nanotube |
| CNFs | carbon nanofibers |
| 3D-GFs | three-dimensional graphene foams |
| PCL | polycaprolactone |
| AuNPs | Gold nanoparticles |
| SiNWs | Silicon nanowires |
| HA | hyaluronic acid |
| PNIPAAm | poly (N-isopropylacrylamide) |
| SGH | self-supporting graphene hydrogel |
| PABS | poly (aminobenzene sulfonic acid) |
References
- Liu, Y.; Lim, J.; Teoh, S.-H. Review: Development of clinically relevant scaffolds for vascularised bone tissue engineering. Biotechnol. Adv. 2013, 31, 688–705. [Google Scholar] [CrossRef] [PubMed]
- Farokhi, M.; Mottaghitalab, F.; Samani, S.; Shokrgozar, M.A.; Kundu, S.C.; Reis, R.L.; Fattahi, Y.; Kaplan, D.L. Silk fibroin/hydroxyapatite composites for bone tissue engineering. Biotechnol. Adv. 2018, 36, 68–91. [Google Scholar] [CrossRef] [PubMed]
- Nilforoushzadeh, M.A.; Zare, M.; Zarrintaj, P.; Alizadeh, E.; Taghiabadi, E.; Heidari-Kharaji, M.; Amirkhani, M.A.; Saeb, M.R.; Mozafari, M. Engineering the niche for hair regeneration–a critical review. Nanomed. Nanotechnol. Biol. Med. 2018, 15, 70–85. [Google Scholar] [CrossRef] [PubMed]
- Kargozar, S.; Mozafari, M. Nanotechnology and Nanomedicine: Start small, think big. Mater. Today Proc. 2018, 5, 15492–15500. [Google Scholar] [CrossRef]
- Zarrintaj, P.; Moghaddam, A.S.; Manouchehri, S.; Atoufi, Z.; Amiri, A.; Amirkhani, M.A.; Nilforoushzadeh, M.A.; Saeb, M.R.; Hamblin, M.R.; Mozafari, M. Can regenerative medicine and nanotechnology combine to heal wounds? The search for the ideal wound dressing. Nanomedicine 2017, 12, 2403–2422. [Google Scholar] [CrossRef] [PubMed]
- Zarrintaj, P.; Jouyandeh, M.; Ganjali, M.R.; Hadavand, B.S.; Mozafari, M.; Sheiko, S.S.; Russell, A.J.; Vatankhah-Varnoosfaderani, M.; Gutiérrez, T.J.; Saeb, M.R. Thermo-sensitive polymers in medicine: A review. Eur. Polym. J. 2019, 117, 402–403. [Google Scholar] [CrossRef]
- Sefat, F.; Youseffi, M.; Khaghani, S.A.; Soon, C.F.; Javid, F. Effect of transforming growth factor-β3 on mono and multilayer chondrocytes. Cytokine 2016, 83, 118–126. [Google Scholar] [CrossRef]
- Zarrintaj, P.; Bakhshandeh, B.; Saeb, M.R.; Sefat, F.; Rezaeian, I.; Ganjali, M.R.; Ramakrishna, S.; Mozafari, M. Oligoaniline-based Conductive Biomaterials for Tissue Engineering. Acta Biomater. 2018, 72, 16–34. [Google Scholar] [CrossRef]
- Zarrintaj, P.; Ahmadi, Z.; Saeb, M.R.; Mozafari, M. Poloxamer-based stimuli-responsive biomaterials. Mater. Today Proc. 2018, 5, 15516–15523. [Google Scholar] [CrossRef]
- Farokhi, M.; Mottaghitalab, F.; Fatahi, Y.; Saeb, M.R.; Zarrintaj, P.; Kundu, S.C.; Khademhosseini, A. Silk fibroin scaffolds for common cartilage injuries: Possibilities for future clinical applications. Eur. Polym. J. 2019, 115, 251–267. [Google Scholar] [CrossRef]
- Fouladiha, H.; Marashi, S.-A.; Shokrgozar, M.A.; Farokhi, M.; Atashi, A. Applications of a metabolic network model of mesenchymal stem cells for controlling cell proliferation and differentiation. Cytotechnology 2018, 70, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Rahmati, M.; Pennisi, C.P.; Mobasheri, A.; Mozafari, M. Bioengineered Scaffolds for Stem Cell Applications in Tissue Engineering and Regenerative Medicine. Adv. Exp. Med. Biol. 2018, 3, 73–89. [Google Scholar]
- Touri, M.; Moztarzadeh, F.; Osman, N.A.A.; Dehghan, M.M.; Mozafari, M. 3D-printed biphasic calcium phosphate scaffolds coated with an oxygen generating system for enhancing engineered tissue survival. Mater. Sci. Eng. C 2018, 84, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Zarrintaj, P.; Manouchehri, S.; Ahmadi, Z.; Saeb, M.R.; Urbanska, A.M.; Kaplan, D.L.; Mozafari, M. Agarose-based biomaterials for tissue engineering. Carbohydr. Polym. 2018, 187, 66–84. [Google Scholar] [CrossRef] [PubMed]
- Kalantari, E.; Naghib, S.M.; Naimi-Jamal, M.R.; Aliahmadi, A.; Iravani, N.J.; Mozafari, M. Nanostructured monticellite for tissue engineering applications-Part I: Microstructural and physicochemical characteristics. Ceram. Int. 2018, 44, 12731–12738. [Google Scholar] [CrossRef]
- Naserzadeh, P.; Mortazavi, S.A.; Ashtari, K.; Salimi, A.; Farokhi, M.; Pourahmad, J. Evaluation of the toxicity effects of silk fibroin on human lymphocytes and monocytes. J. Biochem. Mol. Toxicol. 2018, 32, e22056. [Google Scholar] [CrossRef] [PubMed]
- Jazayeri, M.; Shokrgozar, M.A.; Haghighipour, N.; Bolouri, B.; Mirahmadi, F.; Farokhi, M. Effects of electromagnetic stimulation on gene expression of mesenchymal stem cells and repair of bone lesions. Cell J. 2017, 19, 34–44. [Google Scholar]
- Jazayeri, M.; Shokrgozar, M.A.; Haghighipour, N.; Mahdian, R.; Farrokhi, M.; Bonakdar, S.; Mirahmadi, F.; Abbariki, T.N. Evaluation of mechanical and chemical stimulations on osteocalcin and Runx2 expression in mesenchymal stem cells. Mol. Cell Biomech. 2015, 12, 197–213. [Google Scholar]
- Derakhshandeh, M.R.; Eshraghi, M.J.; Javaheri, M.; Khamseh, S.; Sari, M.G.; Zarrintaj, P.; Saeb, M.R.; Mozafari, M. Diamond-like carbon-deposited films: A new class of biocorrosion protective coatings. Surf. Innov. 2018, 6, 266–276. [Google Scholar] [CrossRef]
- Bassett, C.A.L.; Becker, R.O. Generation of electric potentials by bone in response to mechanical stress. Science 1962, 137, 1063–1064. [Google Scholar] [CrossRef]
- Prabhakaran, M.P.; Ghasemi-Mobarakeh, L.; Jin, G.; Ramakrishna, S. Electrospun conducting polymer nanofibers and electrical stimulation of nerve stem cells. J. Biosci. Bioeng. 2011, 112, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Ning, C.; Zhou, Z.; Tan, G.; Zhu, Y.; Mao, C. Electroactive polymers for tissue regeneration: Developments and perspectives. Prog. Polym. Sci. 2018, 81, 144–162. [Google Scholar] [CrossRef] [PubMed]
- Leppik, L.; Zhihua, H.; Mobini, S.; Parameswaran, V.T.; Eischen-Loges, M.; Slavici, A.; Helbing, J.; Pindur, L.; Oliveira, K.M.; Bhavsar, M.B. Combining electrical stimulation and tissue engineering to treat large bone defects in a rat model. Sci. Rep. 2018, 8, 6307. [Google Scholar] [CrossRef] [PubMed]
- Memic, A.; Aldhahri, M.; Tamayol, A.; Mostafalu, P.; Abdel-Wahab, M.S.; Samandari, M.; Moghaddam, K.M.; Annabi, N.; Bencherif, S.A.; Khademhosseini, A. Nanofibrous silver-coated polymeric scaffolds with tunable electrical properties. Nanomaterials 2017, 7, 63. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, L.; Guo, B.; Shao, Y.; Ma, P.X. Electroactive biodegradable polyurethane significantly enhanced Schwann cells myelin gene expression and neurotrophin secretion for peripheral nerve tissue engineering. Biomaterials 2016, 87, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Zarrintaj, P.; Saeb, M.R.; Ramakrishna, S.; Mozafari, M. Biomaterials Selection for Neuroprosthetics. Curr. Opin. Biomed. Eng. 2018, 6, 99–109. [Google Scholar] [CrossRef]
- Zarrintaj, P.; Ahmadi, Z.; Vahabi, H.; Ducos, F.; Saeb, M.R.; Mozafari, M. Polyaniline in retrospect and prospect. Mater. Today Proc. 2018, 5, 15852–15860. [Google Scholar] [CrossRef]
- Babanejad, N.; Nabid, M.R.; Farhadian, A.; Dorkoosh, F.; Zarrintaj, P.; Saeb, M.; Mozafari, M. Sustained delivery of olanzapine from sunflower oil-based polyol-urethane nanoparticles synthesized through a cyclic carbonate ring-opening reaction. IET Nanobiotechnol. 2019. [Google Scholar] [CrossRef]
- Guo, B.; Ma, P.X. Conducting polymers for tissue engineering. Biomacromolecules 2018, 19, 1764–1782. [Google Scholar] [CrossRef]
- Guo, B.; Glavas, L.; Albertsson, A.-C. Biodegradable and electrically conducting polymers for biomedical applications. Prog. Polym. Sci. 2013, 38, 1263–1286. [Google Scholar] [CrossRef]
- Hiltunen, M.; Pelto, J.; Ellä, V.; Kellomäki, M. Uniform and electrically conductive biopolymer-doped polypyrrole coating for fibrous PLA. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 104, 1721–1729. [Google Scholar] [CrossRef] [PubMed]
- Jin, G.; Li, K. The electrically conductive scaffold as the skeleton of stem cell niche in regenerative medicine. Mater. Sci. Eng. C 2014, 45, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Collier, J.H.; Camp, J.P.; Hudson, T.W.; Schmidt, C.E. Synthesis and characterization of polypyrrole–hyaluronic acid composite biomaterials for tissue engineering applications. J. Biomed. Mater. Res. 2000, 50, 574–584. [Google Scholar] [CrossRef]
- Breukers, R.; Gilmore, K.J.; Kita, M.; Wagner, K.; Higgins, M.; Moulton, S.; Clark, G.M.; Officer, D.; Kapsa, R.; Wallace, G. Creating conductive structures for cell growth: Growth and alignment of myogenic cell types on polythiophenes. J. Biomed. Mater. Res. Part A 2010, 95, 256–268. [Google Scholar] [CrossRef] [PubMed]
- Ferris, C.J. Conducting bio-materials based on gellan gum hydrogels. Soft Matter 2009, 5, 3430–3437. [Google Scholar] [CrossRef]
- You, J.-O.; Rafat, M.; Ye, G.J.; Auguste, D.T. Nanoengineering the heart: Conductive scaffolds enhance connexin 43 expression. Nano Lett. 2011, 11, 3643–3648. [Google Scholar] [CrossRef] [PubMed]
- Anand, J.; Palaniappan, S.; Sathyanarayana, D. Conducting polyaniline blends and composites. Prog. Polym. Sci. 1998, 23, 993–1018. [Google Scholar] [CrossRef]
- Li, M.; Guo, Y.; Wei, Y.; MacDiarmid, A.G.; Lelkes, P.I. Electrospinning polyaniline-contained gelatin nanofibers for tissue engineering applications. Biomaterials 2006, 27, 2705–2715. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.Y.; Langer, R.; Ingber, D.E. Electrically conducting polymers can noninvasively control the shape and growth of mammalian cells. Proc. Natl. Acad. Sci. USA 1994, 91, 3201–3204. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Shao, J.; Wang, Y.; Zhang, P.; Chen, X.; Wei, Y. PLA-PEG-PLA and Its Electroactive Tetraaniline Copolymer as Multi-interactive Injectable Hydrogels for Tissue Engineering. Biomacromolecules 2013, 14, 1904–1912. [Google Scholar] [CrossRef]
- Huang, K.; Niu, Y.; Wang, L.J.; Liu, Y.; Chen, J.S.; Wang, R.Z. pH-Induced Cross-Linking of Dopamine-Containing Block Copolymers with Fe3+ to Form Self-Healing Hydrogels. Adv. Mater. Res. 2012, 569, 11–14. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, W.; Wang, C.; Wen, T.-C.; Wei, Y. One-dimensional conducting polymer nanocomposites: Synthesis, properties and applications. Prog. Polym. Sci. 2011, 36, 671–712. [Google Scholar] [CrossRef]
- Balint, R.; Cassidy, N.J.; Cartmell, S.H. Conductive polymers: Towards a smart biomaterial for tissue engineering. Acta Biomater. 2014, 10, 2341–2353. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.; Doherty, P. A preliminary assessment of poly (pyrrole) in nerve guide studies. J. Mater. Sci. Mater. Med. 1994, 5, 429–433. [Google Scholar] [CrossRef]
- Moroder, P.; Runge, M.B.; Wang, H.; Ruesink, T.; Lu, L.; Spinner, R.J.; Windebank, A.J.; Yaszemski, M.J. Material properties and electrical stimulation regimens of polycaprolactone fumarate–polypyrrole scaffolds as potential conductive nerve conduits. Acta Biomater. 2011, 7, 944–953. [Google Scholar] [CrossRef] [PubMed]
- Mozafari, M.; Vashaee, D.; Tayebi, L.; Mehraien, M. Electroconductive nanocomposite scaffolds: A new strategy into tissue engineering and regenerative medicine. In Nanocomposites—New Trends and Developments; Ebrahimi, F., Ed.; INTECH Open Access Publisher: London, UK, 2012. [Google Scholar]
- Derakhshandeh, M.R.; Eshraghi, M.J.; Hadavi, M.M.; Javaheri, M.; Khamseh, S.; Sari, M.G.; Zarrintaj, P.; Saeb, M.R.; Mozafari, M. Diamond-like carbon thin films prepared by pulsed-DC PE-CVD for biomedical applications. Surf. Innov. 2018, 6, 167–175. [Google Scholar] [CrossRef]
- Bendrea, A.-D.; Cianga, L.; Cianga, I. Review paper: Progress in the field of conducting polymers for tissue engineering applications. J. Biomater. Appl. 2011, 26, 3–84. [Google Scholar] [CrossRef]
- Ateh, D.; Navsaria, H.; Vadgama, P. Polypyrrole-based conducting polymers and interactions with biological tissues. J. R. Soc. Interface 2006, 3, 741–752. [Google Scholar] [CrossRef]
- Runge, M.B.; Dadsetan, M.; Baltrusaitis, J.; Knight, A.M.; Ruesink, T.; Lazcano, E.A.; Lu, L.; Windebank, A.J.; Yaszemski, M.J. The development of electrically conductive polycaprolactone fumarate–polypyrrole composite materials for nerve regeneration. Biomaterials 2010, 31, 5916–5926. [Google Scholar] [CrossRef]
- Moreno, J.S.; Panero, S.; Artico, M.; Filippini, P. Synthesis and characterization of new electroactive polypyrrole–chondroitin sulphate A substrates. Bioelectrochemistry 2008, 72, 3–9. [Google Scholar] [CrossRef]
- Shi, G.; Rouabhia, M.; Wang, Z.; Dao, L.H.; Zhang, Z. A novel electrically conductive and biodegradable composite made of polypyrrole nanoparticles and polylactide. Biomaterials 2004, 25, 2477–2488. [Google Scholar] [CrossRef] [PubMed]
- Bueno, V.B.; Takahashi, S.H.; Catalani, L.H.; de Torresi, S.I.C.; Petri, D.F.S. Biocompatible xanthan/polypyrrole scaffolds for tissue engineering. Mater. Sci. Eng. C 2015, 52, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, R.; Chu, H.K.; Sun, D. Design and characterization of a conductive nanostructured polypyrrole-polycaprolactone coated magnesium/PLGA composite for tissue engineering scaffolds. J. Biomed. Mater. Res. Part A 2015, 103, 2966–2973. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Guo, Y.; Ma, P.X.; Guo, B. Rapid thermal responsive conductive hybrid cryogels with shape memory properties, photothermal properties and pressure dependent conductivity. J. Colloid Interface Sci. 2018, 526, 281–294. [Google Scholar] [CrossRef] [PubMed]
- Saeb, M.R.; Zarrintaj, P. Chapter 10—Polyaniline/graphene-based nanocomposites. In Fundamentals and Emerging Applications of Polyaniline; Mozafari, M., Chauhan, N.P.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 165–175. [Google Scholar]
- Saeb, M.R.; Zarrintaj, P.; Khandelwal, P.; Chauhan, N.P.S. Chapter 2—Synthetic route of polyaniline (I): Conventional oxidative polymerization. In Fundamentals and Emerging Applications of Polyaniline; Mozafari, M., Chauhan, N.P.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 17–41. [Google Scholar]
- Zarrintaj, P.; Yazdi, M.K.; Jouyandeh, M.; Saeb, M.R. Chapter 7—PANI-based nanostructures. In Fundamentals and Emerging Applications of Polyaniline; Mozafari, M., Chauhan, N.P.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 121–130. [Google Scholar]
- Zarrintaj, P.; Khalili, R.; Vahabi, H.; Saeb, M.R.; Ganjali, M.R.; Mozafari, M. Chapter 8—Polyaniline/metal oxides nanocomposites. In Fundamentals and Emerging Applications of Polyaniline; Mozafari, M., Chauhan, N.P.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 131–141. [Google Scholar]
- Zarrintaj, P.; Yazdi, M.K.; Vahabi, H.; Moghadam, P.N.; Saeb, M.R. Towards advanced flame retardant organic coatings: Expecting a new function from polyaniline. Prog. Org. Coat. 2019, 130, 144–148. [Google Scholar] [CrossRef]
- Ahmadi, Z.; Chauhan, N.P.S.; Zarrintaj, P.; Khiabani, A.B.; Saeb, M.R.; Mozafari, M. Chapter 13—Experimental procedures for assessing electrical and thermal conductivity of polyaniline. In Fundamentals and Emerging Applications of Polyaniline; Mozafari, M., Chauhan, N.P.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 227–258. [Google Scholar]
- Zarrintaj, P.; Saeb, M.R. Chapter 5—Synthetic route of polyaniline (IV): Irradiation path. In Fundamentals and Emerging Applications of Polyaniline; Mozafari, M., Chauhan, N.P.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 91–103. [Google Scholar]
- Yazdi, M.K.; Saeidi, H.; Zarrintaj, P.; Saeb, M.R.; Mozafari, M. Chapter 9—PANI-CNT nanocomposites. In Fundamentals and Emerging Applications of Polyaniline; Mozafari, M., Chauhan, N.P.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 143–163. [Google Scholar]
- Li, L.; Ge, J.; Guo, B.; Ma, P.X. In situ forming biodegradable electroactive hydrogels. Polym. Chem. 2014, 5, 2880–2890. [Google Scholar] [CrossRef]
- Rahman, N.A.; Feisst, V.; Dickinson, M.E.; Malmström, J.; Dunbar, P.R.; Travas-Sejdic, J. Functional polyaniline nanofibre mats for human adipose-derived stem cell proliferation and adhesion. Mater. Chem. Phys. 2013, 138, 333–341. [Google Scholar] [CrossRef]
- Zarrintaj, P.; Vahabi, H.; Saeb, M.R.; Mozafari, M. Chapter 14—Application of polyaniline and its derivatives. In Fundamentals and Emerging Applications of Polyaniline; Mozafari, M., Chauhan, N.P.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 259–272. [Google Scholar]
- Zhao, X.; Li, P.; Guo, B.; Ma, P.X. Antibacterial and conductive injectable hydrogels based on quaternized chitosan-graft-polyaniline/oxidized dextran for tissue engineering. Acta Biomater. 2015, 26, 236–248. [Google Scholar] [CrossRef]
- Gizdavic-Nikolaidis, M.; Ray, S.; Bennett, J.R.; Easteal, A.J.; Cooney, R.P. Electrospun Functionalized Polyaniline Copolymer-Based Nanofibers with Potential Application in Tissue Engineering. Macromol. Biosci. 2010, 10, 1424–1431. [Google Scholar] [CrossRef]
- Hafshejani, T.M.; Zamanian, A.; Venugopal, J.R.; Rezvani, Z.; Sefat, F.; Saeb, M.R.; Vahabi, H.; Zarrintaj, P.; Mozafari, M. Antibacterial glass-ionomer cement restorative materials: A critical review on the current status of extended release formulations. J. Control. Release 2017, 262, 317–328. [Google Scholar] [CrossRef]
- McKeon-Fischer, K.; Freeman, J. Addition of conductive elements to polymeric scaffolds for muscle tissue engineering. Nano Life 2012, 2, 1230011. [Google Scholar] [CrossRef]
- Baheiraei, N.; Yeganeh, H.; Ai, J.; Gharibi, R.; Azami, M.; Faghihi, F. Synthesis, characterization and antioxidant activity of a novel electroactive and biodegradable polyurethane for cardiac tissue engineering application. Mater. Sci. Eng. C 2014, 44, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Choi, J.H.; Kim, S.; Jang, I.; Jeong, S.; Lee, J.Y. Micropatterned conductive hydrogels as multifunctional muscle-mimicking biomaterials: Graphene-incorporated hydrogels directly patterned with femtosecond laser ablation. Acta Biomater. 2019. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Qu, J.; Zhao, X.; Zhang, M. Degradable conductive self-healing hydrogels based on dextran-graft-tetraaniline and N-carboxyethyl chitosan as injectable carriers for myoblast cell therapy and muscle regeneration. Acta Biomater. 2019, 84, 180–193. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Dong, R.; Guo, B.; Ma, P.X. Dopamine-incorporated dual bioactive electroactive shape memory polyurethane elastomers with physiological shape recovery temperature, high stretchability, and enhanced C2C12 myogenic differentiation. ACS Appl. Mater. Interfaces 2017, 9, 29595–29611. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.I.; Jun, I.D.; Choi, M.J.; Nho, Y.C.; Lee, Y.M.; Shin, H. Development of Electroactive and Elastic Nanofibers that contain Polyaniline and Poly (L-lactide-co-ε-caprolactone) for the Control of Cell Adhesion. Macromol. Biosci. 2008, 8, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Bouten, C.V.C.; Dankers, P.Y.W.; Driessen-Mol, A.; Pedron, S.; Brizard, A.M.A.; Baaijens, F.P.T. Substrates for cardiovascular tissue engineering. Adv. Drug Deliv. Rev. 2011, 63, 221–241. [Google Scholar] [CrossRef]
- Guo, B.; Finne-Wistrand, A.; Albertsson, A.-C. Facile synthesis of degradable and electrically conductive polysaccharide hydrogels. Biomacromolecules 2011, 12, 2601–2609. [Google Scholar] [CrossRef]
- Guo, B.; Finne-Wistrand, A.; Albertsson, A.-C. Electroactive Hydrophilic Polylactide Surface by Covalent Modification with Tetraaniline. Macromolecules 2012, 45, 652–659. [Google Scholar] [CrossRef]
- Zhang, X.; Qi, H.; Wang, S.; Feng, L.; Ji, Y.; Tao, L.; Li, S.; Wei, Y. Cellular responses of aniline oligomers: A preliminary study. Toxicol. Res. 2012, 1, 201–205. [Google Scholar] [CrossRef]
- Qi, H.; Liu, M.; Xu, L.; Feng, L.; Tao, L.; Ji, Y.; Zhang, X.; Wei, Y. Biocompatibility evaluation of aniline oligomers with different end-functional groups. Toxicol. Res. 2013, 2, 427–433. [Google Scholar] [CrossRef]
- Xu, C.; Huang, Y.; Yepez, G.; Wei, Z.; Liu, F.; Bugarin, A.; Tang, L.; Hong, Y. Development of dopant-free conductive bioelastomers. Sci. Rep. 2016, 6, 34451. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Ge, J.; Li, Y.; Guo, B.; Ma, P.X. Nanofibrous electroactive scaffolds from a chitosan-grafted-aniline tetramer by electrospinning for tissue engineering. RSC Adv. 2014, 4, 13652–13661. [Google Scholar] [CrossRef]
- Kim, H.; Jeong, S.-M.; Park, J.-W. Electrical switching between vesicles and micelles via redox-responsive self-assembly of amphiphilic rod− coils. J. Am. Chem. Soc. 2011, 133, 5206–5209. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Yu, M.; Chen, X.; Nie, S.; Lai, W.Y.; Su, W.; Cui, Z.; Huang, W. Ito-Free Flexible Electronics: Screen-Printed Poly (3, 4-Ethylenedioxythiophene): Poly (Styrenesulfonate) Grids as ITO-Free Anodes for Flexible Organic Light-Emitting Diodes (Adv. Funct. Mater. 11/2018). Adv. Funct. Mater. 2018, 28, 1870072. [Google Scholar] [CrossRef]
- Povlich, L.K.; Feldman, K.E.; Shim, B.S.; Martin, D.C. 1.130-Electroactive Polymeric Biomaterials. In Comprehensive Biomaterials; Ducheyne, P., Ed.; Elsevier: Oxford, UK, 2011; pp. 547–561. [Google Scholar]
- Niu, X.; Rouabhia, M.; Chiffot, N.; King, M.W.; Zhang, Z. An electrically conductive 3D scaffold based on a nonwoven web of poly (l-lactic acid) and conductive poly (3, 4-ethylenedioxythiophene). J. Biomed. Mater. Res. Part A 2015, 103, 2635–2644. [Google Scholar] [CrossRef] [PubMed]
- Abidian, M.R.; Corey, J.M.; Kipke, D.R.; Martin, D.C. Conducting-polymer nanotubes improve electrical properties, mechanical adhesion, neural attachment, and neurite outgrowth of neural electrodes. Small 2010, 6, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Malliaras, G.; Abidian, M.R. Organic bioelectronic materials and devices. Adv. Mater. 2015, 27, 7492. [Google Scholar] [CrossRef] [PubMed]
- Mehmood, U.; Al-Ahmed, A.; Hussein, I.A. Review on recent advances in polythiophene based photovoltaic devices. Renew. Sustain. Energy Rev. 2016, 57, 550–561. [Google Scholar] [CrossRef]
- Schopf, G.; Kossmehl, G. Polythiophenes-Electrically Conductive Polymers; Springer: Berlin, Germany, 1997. [Google Scholar]
- Iyoda, M.; Shimizu, H. Multifunctional π-expanded oligothiophene macrocycles. Chem. Soc. Rev. 2015, 44, 6411–6424. [Google Scholar] [CrossRef]
- Hussain, S.T.; Abbas, F.; Kausar, A.; Khan, M.R. New polyaniline/polypyrrole/polythiophene and functionalized multiwalled carbon nanotube-based nanocomposites: Layer-by-layer in situ polymerization. High Perform. Polym. 2013, 25, 70–78. [Google Scholar] [CrossRef]
- Bitounis, D.; Ali-Boucetta, H.; Hong, B.H.; Min, D.H.; Kostarelos, K. Prospects and challenges of graphene in biomedical applications. Adv. Mater. 2013, 25, 2258–2268. [Google Scholar] [CrossRef]
- Goodarzi, S.; Da Ros, T.; Conde, J.; Sefat, F.; Mozafari, M. Fullerene: Biomedical engineers get to revisit an old friend. Mater. Today 2017, 20, 460–480. [Google Scholar] [CrossRef]
- Goenka, S.; Sant, V.; Sant, S. Graphene-based nanomaterials for drug delivery and tissue engineering. J. Control. Release 2014, 173, 75–88. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Guillot, S.; Dabboue, H.; Tranchant, J.-F.; Salvetat, J.-P. Carbon nanotubes as structural nanofibers for hyaluronic acid hydrogel scaffolds. Biomacromolecules 2008, 9, 505–509. [Google Scholar] [CrossRef]
- Lau, C.; Cooney, M.J.; Atanassov, P. Conductive macroporous composite chitosan− carbon nanotube scaffolds. Langmuir 2008, 24, 7004–7010. [Google Scholar] [CrossRef]
- Alasv, N.; Mozafari, M. Graphene-Proceed with Caution: What We Know, what We don’t. J. Clin. Toxicol. 2015, 5, E122. [Google Scholar]
- Li, Y.; Samad, Y.A.; Polychronopoulou, K.; Alhassan, S.M.; Liao, K. Highly Electrically Conductive Nanocomposites Based on PolymerInfused Graphene Sponges. Sci. Rep. 2014, 4, 4652. [Google Scholar] [CrossRef]
- Menaa, F.; Abdelghani, A.; Menaa, B. Graphene nanomaterials as biocompatible and conductive scaffolds for stem cells: Impact for tissue engineering and regenerative medicine. J. Tissue Eng. Regen. Med. 2015, 9, 1321–1338. [Google Scholar] [CrossRef]
- Song, H.S.; Kwon, O.S.; Kim, J.-H.; Conde, J.; Artzi, N. 3D hydrogel scaffold doped with 2D graphene materials for biosensors and bioelectronics. Biosens. Bioelectron. 2017, 89, 187–200. [Google Scholar] [CrossRef]
- Tran, P.A.; Zhang, L.; Webster, T.J. Carbon nanofibers and carbon nanotubes in regenerative medicine. Adv. Drug Deliv. Rev. 2009, 61, 1097–1114. [Google Scholar] [CrossRef]
- Edwards, S.L.; Werkmeister, J.A.; Ramshaw, J.A. Carbon nanotubes in scaffolds for tissue engineering. Expert Rev. Med. Devices 2009, 6, 499–505. [Google Scholar] [CrossRef]
- Mozafari, M. The Critical Impact of Controlled Drug Delivery in the Future of Tissue Engineering. Trends Biomater. Artif. Organs. 2014, 28. [Google Scholar]
- Hopley, E.L.; Salmasi, S.; Kalaskar, D.M.; Seifalian, A.M. Carbon nanotubes leading the way forward in new generation 3D tissue engineering. Biotechnol. Adv. 2014, 32, 1000–1014. [Google Scholar] [CrossRef]
- MacDonald, R.A.; Voge, C.M.; Kariolis, M.; Stegemann, J.P. Carbon nanotubes increase the electrical conductivity of fibroblast-seeded collagen hydrogels. Acta Biomater. 2008, 4, 1583–1592. [Google Scholar] [CrossRef]
- Upadhyayula, V.K.K.; Gadhamshetty, V. Appreciating the role of carbon nanotube composites in preventing biofouling and promoting biofilms on material surfaces in environmental engineering: A review. Biotechnol. Adv. 2010, 28, 802–816. [Google Scholar] [CrossRef]
- Edwards, S.L.; Church, J.S.; Werkmeister, J.A.; Ramshaw, J.A. Tubular micro-scale multiwalled carbon nanotube-based scaffolds for tissue engineering. Biomaterials 2009, 30, 1725–1731. [Google Scholar] [CrossRef]
- Cardiel, J.J.; Zhao, Y.; Kim, J.-H.; Chung, J.-H.; Shen, A.Q. Electro-conductive porous scaffold with single-walled carbon nanotubes in wormlike micellar networks. Carbon 2014, 80, 203–212. [Google Scholar] [CrossRef]
- Abarrategi, A.; Gutiérrez, M.C.; Moreno-Vicente, C.; Hortigüela, M.J.; Ramos, V.; López-Lacomba, J.L.; Ferrer, M.L.; del Monte, F. Multiwall carbon nanotube scaffolds for tissue engineering purposes. Biomaterials 2008, 29, 94–102. [Google Scholar] [CrossRef]
- Correa-Duarte, M.A.; Wagner, N.; Rojas-Chapana, J.; Morsczeck, C.; Thie, M.; Giersig, M. Fabrication and biocompatibility of carbon nanotube-based 3D networks as scaffolds for cell seeding and growth. Nano Lett. 2004, 4, 2233–2236. [Google Scholar] [CrossRef]
- Liao, H.; Qi, R.; Shen, M.; Cao, X.; Guo, R.; Zhang, Y.; Shi, X. Improved cellular response on multiwalled carbon nanotube-incorporated electrospun polyvinyl alcohol/chitosan nanofibrous scaffolds. Colloids Surf. B Biointerfaces 2011, 84, 528–535. [Google Scholar] [CrossRef]
- Mackle, J.N.; Blond, D.J.P.; Mooney, E.; McDonnell, C.; Blau, W.J.; Shaw, G.; Barry, F.P.; Murphy, J.M.; Barron, V. In vitro Characterization of an Electroactive Carbon-Nanotube-Based Nanofiber Scaffold for Tissue Engineering. Macromol. Biosci. 2011, 11, 1272–1282. [Google Scholar] [CrossRef]
- Vardharajula, S.; Ali, S.Z.; Tiwari, P.M.; Eroğlu, E.; Vig, K.; Dennis, V.A.; Singh, S.R. Functionalized carbon nanotubes: Biomedical applications. Int. J. Nanomed. 2012, 7, 5361. [Google Scholar]
- Serrano, M.C.; Gutiérrez, M.C.; del Monte, F. Role of polymers in the design of 3D carbon nanotube-based scaffolds for biomedical applications. Prog. Polym. Sci. 2014, 39, 1448–1471. [Google Scholar] [CrossRef]
- Hermant, M.-C.; van der Schoot, P.; Klumperman, B.; Koning, C.E. Probing the Cooperative Nature of the Conductive Components in Polystyrene/Poly (3, 4-ethylenedioxythiophene): Poly (styrene sulfonate)− Single-Walled Carbon Nanotube Composites. Acs Nano 2010, 4, 2242–2248. [Google Scholar] [CrossRef]
- Garipcan, B.; Odabas, S.; Demirel, G.; Burger, J.; Nonnenmann, S.S.; Coster, M.T.; Gallo, E.M.; Nabet, B.; Spanier, J.E.; Piskin, E. In Vitro Biocompatibility of n-Type and Undoped Silicon Nanowires. Adv. Eng. Mater. 2011, 13, B3–B9. [Google Scholar] [CrossRef]
- Tölli, M.A.; Ferreira, M.P.; Kinnunen, S.M.; Rysä, J.; Mäkilä, E.M.; Szabó, Z.; Serpi, R.E.; Ohukainen, P.J.; Välimäki, M.J.; Correia, A.M. In vivo biocompatibility of porous silicon biomaterials for drug delivery to the heart. Biomaterials 2014, 35, 8394–8405. [Google Scholar] [CrossRef]
- Tan, Y.; Richards, D.; Xu, R.; Stewart-Clark, S.; Mani, S.K.; Borg, T.K.; Menick, D.R.; Tian, B.; Mei, Y. Silicon Nanowire-Induced Maturation of Cardiomyocytes Derived from Human Induced Pluripotent Stem Cells. Nano Lett. 2015, 15, 2765–2772. [Google Scholar] [CrossRef]
- Jiang, K.; Fan, D.; Belabassi, Y.; Akkaraju, G.; Montchamp, J.-L.; Coffer, J.L. Medicinal surface modification of silicon nanowires: Impact on calcification and stromal cell proliferation. Acs Appl. Mater. Interfaces 2008, 1, 266–269. [Google Scholar] [CrossRef]
- Conde, J.; Dias, J.T.; Grazú, V.; Moros, M.; Baptista, P.V.; Jesus, M. Revisiting 30 years of biofunctionalization and surface chemistry of inorganic nanoparticles for nanomedicine. Front. Chem. 2014, 2, 48. [Google Scholar] [CrossRef]
- Sperling, R.A.; Parak, W. Surface modification, functionalization and bioconjugation of colloidal inorganic nanoparticles. Philos. Trans. R. Soc. A 2010, 368, 1333–1383. [Google Scholar] [CrossRef]
- Ravichandran, R.; Sridhar, R.; Venugopal, J.R.; Sundarrajan, S.; Mukherjee, S.; Ramakrishna, S. Gold Nanoparticle Loaded Hybrid Nanofibers for Cardiogenic Differentiation of Stem Cells for Infarcted Myocardium Regeneration. Macromol. Biosci. 2014, 14, 515–525. [Google Scholar] [CrossRef]
- McKeon-Fischer, K.D.; Freeman, J.W. Characterization of electrospun poly(L-lactide) and gold nanoparticle composite scaffolds for skeletal muscle tissue engineering. J. Tissue Eng. Regen. Med. 2011, 5, 560–568. [Google Scholar] [CrossRef]
- Sperling, R.A.; Gil, P.R.; Zhang, F.; Zanella, M.; Parak, W.J. Biological applications of gold nanoparticles. Chem. Soc. Rev. 2008, 37, 1896–1908. [Google Scholar] [CrossRef]
- Cohen-Karni, T.; Jeong, K.J.; Tsui, J.H.; Reznor, G.; Mustata, M.; Wanunu, M.; Graham, A.; Marks, C.; Bell, D.C.; Langer, R. Nanocomposite gold-silk nanofibers. Nano Lett. 2012, 12, 5403–5406. [Google Scholar] [CrossRef]
- Conde, J.; Oliva, N.; Artzi, N. Implantable hydrogel embedded dark-gold nanoswitch as a theranostic probe to sense and overcome cancer multidrug resistance. Proc. Natl. Acad. Sci. USA 2015, 112, E1278–E1287. [Google Scholar] [CrossRef]
- Conde, J.; Bao, C.; Cui, D.; Baptista, P.V.; Tian, F. Antibody–drug gold nanoantennas with Raman spectroscopic fingerprints for in vivo tumour theranostics. J. Control. Release 2014, 183, 87–93. [Google Scholar] [CrossRef]
- Gilam, A.; Conde, J.; Weissglas-Volkov, D.; Oliva, N.; Friedman, E.; Artzi, N.; Shomron, N. Local microRNA delivery targets Palladin and prevents metastatic breast cancer. Nat. Commun. 2016, 7, 12868. [Google Scholar] [CrossRef]
- Conde, J.; Oliva, N.; Zhang, Y.; Artzi, N. Local triple-combination therapy results in tumour regression and prevents recurrence in a colon cancer model. Nat. Mater. 2016, 15, 1128–1138. [Google Scholar] [CrossRef]
- Riley, P. Melanin. Int. J. Biochem. Cell Biol. 1997, 29, 1235–1239. [Google Scholar] [CrossRef]
- Kai, D.; Prabhakaran, M.P.; Jin, G.; Ramakrishna, S. Biocompatibility evaluation of electrically conductive nanofibrous scaffolds for cardiac tissue engineering. J. Mater. Chem. B 2013, 1, 2305–2314. [Google Scholar] [CrossRef]
- Bernsmann, F.; Frisch, B.; Ringwald, C.; Ball, V. Protein adsorption on dopamine–melanin films: Role of electrostatic interactions inferred from ζ-potential measurements versus chemisorption. J. Colloid Interface Sci. 2010, 344, 54–60. [Google Scholar] [CrossRef]
- Bernsmann, F.; Ersen, O.; Voegel, J.C.; Jan, E.; Kotov, N.A.; Ball, V. Melanin-Containing Films: Growth from Dopamine Solutions versus Layer-by-Layer Deposition. ChemPhysChem 2010, 11, 3299–3305. [Google Scholar] [CrossRef]
- Balachandran, U.; Odekirk, B.; Eror, N.G. Electrical conductivity in calcium titanate. J. Solid State Chem. 1982, 41, 185–194. [Google Scholar] [CrossRef]
- Figueiredo, F.M.; Kharton, V.V.; Waerenborgh, J.C.; Viskup, A.P.; Naumovich, E.N.; Frade, J.R. Influence of Microstructure on the Electrical Properties of Iron-Substituted Calcium Titanate Ceramics. J. Am. Ceram. Soc. 2004, 87, 2252–2261. [Google Scholar] [CrossRef]
- Thrivikraman, G.; Mallik, P.K.; Basu, B. Substrate conductivity dependent modulation of cell proliferation and differentiation in vitro. Biomaterials 2013, 34, 7073–7085. [Google Scholar] [CrossRef]
- Farokhi, M.; Mottaghitalab, F.; Shokrgozar, M.A.; Kaplan, D.L.; Kim, H.-W.; Kundu, S.C. Prospects of peripheral nerve tissue engineering using nerve guide conduits based on silk fibroin protein and other biopolymers. Int. Mater. Rev. 2017, 62, 367–391. [Google Scholar] [CrossRef]
- Subramanian, A.; Krishnan, U.M.; Sethuraman, S. Development of biomaterial scaffold for nerve tissue engineering: Biomaterial mediated neural regeneration. J. Biomed. Sci. 2009, 16, 108. [Google Scholar] [CrossRef]
- Ahn, H.-S.; Hwang, J.-Y.; Kim, M.S.; Lee, J.-Y.; Kim, J.-W.; Kim, H.-S.; Shin, U.S.; Knowles, J.C.; Kim, H.-W.; Hyun, J.K. Carbon-nanotube-interfaced glass fiber scaffold for regeneration of transected sciatic nerve. Acta Biomater. 2015, 13, 324–334. [Google Scholar] [CrossRef]
- Huang, J.; Hu, X.; Lu, L.; Ye, Z.; Zhang, Q.; Luo, Z. Electrical regulation of Schwann cells using conductive polypyrrole/chitosan polymers. J. Biomed. Mater. Res. A 2010, 93, 164–174. [Google Scholar] [CrossRef]
- Qi, F.; Wang, Y.; Ma, T.; Zhu, S.; Zeng, W.; Hu, X.; Liu, Z.; Huang, J.; Luo, Z. Electrical regulation of olfactory ensheathing using conductive polypyrrole/chitosan polymers. Biomaterials 2013, 34, 1799–1809. [Google Scholar] [CrossRef]
- Lee, J.Y.; Bashur, C.A.; Goldstein, A.S.; Schmidt, C.E. Polypyrrole-coated electrospun PLGA nanofibers for neural tissue applications. Biomaterials 2009, 30, 4325–4335. [Google Scholar] [CrossRef]
- Thrivikraman, G.; Madras, G.; Basu, B. Intermittent electrical stimuli for guidance of human mesenchymal stem cell lineage commitment towards neural-like cells on electroconductive substrates. Biomaterials 2014, 35, 6219–6235. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, L.; Hu, T.; Ma, P.X.; Guo, B. Conductive micropatterned polyurethane films as tissue engineering scaffolds for Schwann cells and PC12 cells. J. Colloid Interface Sci. 2018, 518, 252–262. [Google Scholar] [CrossRef]
- Guarino, V.; Alvarez-Perez, M.A.; Borriello, A.; Napolitano, T.; Ambrosio, L. Conductive PANi/PEGDA macroporous hydrogels for nerve regeneration. Adv. Healthc. Mater. 2013, 2, 218–227. [Google Scholar] [CrossRef]
- Bagher, Z.; Atoufi, Z.; Alizadeh, R.; Farhadi, M.; Zarrintaj, P.; Moroni, L.; Setayeshmehr, M.; Komeili, A.; Kamrava, S.K. Conductive hydrogel based on chitosan-aniline pentamer/gelatin/agarose significantly promoted motor neuron-like cells differentiation of human olfactory ecto-mesenchymal stem cells. Mater. Sci. Eng. C 2019, 101, 243–253. [Google Scholar] [CrossRef]
- Manouchehri, S.; Bagheri, B.; Rad, S.H.; Nezhad, M.N.; Kim, Y.C.; Park, O.O.; Farokhi, M.; Jouyandeh, M.; Ganjali, M.R.; Yazdi, M.K. Electroactive bio-epoxy incorporated chitosan-oligoaniline as an advanced hydrogel coating for neural interfaces. Prog. Org. Coat. 2019, 131, 389–396. [Google Scholar] [CrossRef]
- Zarrintaj, P.; Bakhshandeh, B.; Rezaeian, I.; Heshmatian, B.; Ganjali, M.R. A novel electroactive agarose-aniline pentamer platform as a potential candidate for neural tissue engineering. Sci. Rep. 2017, 7, 17187. [Google Scholar] [CrossRef]
- Zarrintaj, P.; Rezaeian, I.; Bakhshandeh, B.; Heshmatian, B.; Ganjali, M.R. Bio-conductive scaffold based on agarose-polyaniline for tissue engineering. J. Skin Stem Cell 2017, 4. [Google Scholar] [CrossRef]
- Zarrintaj, P.; Urbanska, A.M.; Gholizadeh, S.S.; Goodarzi, V.; Saeb, M.R.; Mozafari, M. A facile route to the synthesis of anilinic electroactive colloidal hydrogels for neural tissue engineering applications. J. Colloid Interface Sci. 2018, 516, 57–66. [Google Scholar] [CrossRef]
- Atoufi, Z.; Zarrintaj, P.; Motlagh, G.H.; Amiri, A.; Bagher, Z.; Kamrava, S.K. A novel bio electro active alginate-aniline tetramer/agarose scaffold for tissue engineering: Synthesis, characterization, drug release and cell culture study. J. Biomater. Sci. Polym. Ed. 2017, 28, 1617–1638. [Google Scholar] [CrossRef]
- Alizadeh, R.; Zarrintaj, P.; Kamrava, S.K.; Bagher, Z.; Farhadi, M.; Heidari, F.; Komeili, A.; Gutiérrez, T.J.; Saeb, M.R. Conductive agarose/alginate/chitosan-based hydrogels for neural disorder therapy. Carbohydr. Polym. 2019, 115161. [Google Scholar] [CrossRef]
- Mattson, M.P.; Haddon, R.C.; Rao, A.M. Molecular functionalization of carbon nanotubes and use as substrates for neuronal growth. J. Mol. Neurosci. 2000, 14, 175–182. [Google Scholar] [CrossRef]
- Kabiri, M.; Soleimani, M.; Shabani, I.; Futrega, K.; Ghaemi, N.; Ahvaz, H.H.; Elahi, E.; Doran, M.R. Neural differentiation of mouse embryonic stem cells on conductive nanofiber scaffolds. Biotechnol. Lett. 2012, 34, 1357–1365. [Google Scholar] [CrossRef]
- Li, N.; Zhang, Q.; Gao, S.; Song, Q.; Huang, R.; Wang, L.; Liu, L.; Dai, J.; Tang, M.; Cheng, G. Three-dimensional graphene foam as a biocompatible and conductive scaffold for neural stem cells. Sci. Rep. 2013, 3, 1604. [Google Scholar] [CrossRef]
- Lee, J.Y.; Lee, J.-W.; Schmidt, C.E. Neuroactive conducting scaffolds: Nerve growth factor conjugation on active ester-functionalized polypyrrole. J. R. Soc. Interface 2008, 6. [Google Scholar] [CrossRef]
- Huang, L.; Zhuang, X.; Hu, J.; Lang, L.; Zhang, P.; Wang, Y.; Chen, X.; Wei, Y.; Jing, X. Synthesis of biodegradable and electroactive multiblock polylactide and aniline pentamer copolymer for tissue engineering applications. Biomacromolecules 2008, 9, 850–858. [Google Scholar] [CrossRef]
- Richardson-Burns, S.M.; Hendricks, J.L.; Foster, B.; Povlich, L.K.; Kim, D.-H.; Martin, D.C. Polymerization of the conducting polymer poly (3, 4-ethylenedioxythiophene)(PEDOT) around living neural cells. Biomaterials 2007, 28, 1539–1552. [Google Scholar] [CrossRef]
- Stewart, E.; Kobayashi, N.R.; Higgins, M.J.; Quigley, A.F.; Jamali, S.; Moulton, S.E.; Kapsa, R.M.; Wallace, G.G.; Crook, J.M. Electrical stimulation using conductive polymer polypyrrole promotes differentiation of human neural stem cells: A biocompatible platform for translational neural tissue engineering. Tissue Eng. Part C Methods 2014, 21, 385–393. [Google Scholar] [CrossRef]
- Xie, J.; MacEwan, M.R.; Willerth, S.M.; Li, X.; Moran, D.W.; Sakiyama-Elbert, S.E.; Xia, Y. Conductive core–sheath nanofibers and their potential application in neural tissue engineering. Adv. Funct. Mater. 2009, 19, 2312–2318. [Google Scholar] [CrossRef]
- Cui, Z.; Ni, N.C.; Wu, J.; Du, G.Q.; He, S.; Yau, T.M.; Weisel, R.D.; Sung, H.W.; Li, R.K. Polypyrrole-chitosan conductive biomaterial synchronizes cardiomyocyte contraction and improves myocardial electrical impulse propagation. Theranostics 2018, 8, 2752–2764. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Lu, L.; Zhang, J.; Hu, X.; Zhang, Y.; Liang, W.; Wu, S.; Luo, Z. Electrical Stimulation to Conductive Scaffold Promotes Axonal Regeneration and Remyelination in a Rat Model of Large Nerve Defect. PLoS ONE 2012, 7, e39526. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Holzwarth, J.M.; Yan, Y.; Xu, P.; Zheng, H.; Yin, Y.; Li, S.; Ma, P.X. Conductive PPY/PDLLA conduit for peripheral nerve regeneration. Biomaterials 2014, 35, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.G.; Khaing, Z.Z.; Xin, S.; Tien, L.W.; Ghezzi, C.E.; Mouser, D.J.; Sukhavasi, R.C.; Preda, R.C.; Gil, E.S.; Kaplan, D.L. Into the groove: Instructive silk-polypyrrole films with topographical guidance cues direct DRG neurite outgrowth. J. Biomater. Sci. Polym. Ed. 2015, 26, 1327–1342. [Google Scholar] [CrossRef] [PubMed]
- Abidian, M.R.; Daneshvar, E.D.; Egeland, B.M.; Kipke, D.R.; Cederna, P.S.; Urbanchek, M.G. Hybrid conducting polymer–hydrogel conduits for axonal growth and neural tissue engineering. Adv. Healthc. Mater. 2012, 1, 762–767. [Google Scholar] [CrossRef]
- Zeng, J.; Huang, Z.; Yin, G.; Qin, J.; Chen, X.; Gu, J. Fabrication of conductive NGF-conjugated polypyrrole–poly (l-lactic acid) fibers and their effect on neurite outgrowth. Colloids Surf. B Biointerfaces 2013, 110, 450–457. [Google Scholar] [CrossRef]
- Ghasemi-Mobarakeh, L.; Prabhakaran, M.P.; Morshed, M.; Nasr-Esfahani, M.H.; Baharvand, H.; Kiani, S.; Al-Deyab, S.S.; Ramakrishna, S. Application of conductive polymers, scaffolds and electrical stimulation for nerve tissue engineering. J. Tissue Eng. Regen. Med. 2011, 5, e17–e35. [Google Scholar] [CrossRef]
- Chiono, V.; Tonda-Turo, C. Trends in the design of nerve guidance channels in peripheral nerve tissue engineering. Prog. Neurobiol. 2015, 131, 87–104. [Google Scholar] [CrossRef]
- Sudwilai, T.; Ng, J.J.; Boonkrai, C.; Israsena, N.; Chuangchote, S.; Supaphol, P. Polypyrrole-coated electrospun poly (lactic acid) fibrous scaffold: Effects of coating on electrical conductivity and neural cell growth. J. Biomater. Sci. Polym. Ed. 2014, 25, 1240–1252. [Google Scholar] [CrossRef]
- Lee, W.; Parpura, V. Carbon nanotubes as substrates/scaffolds for neural cell growth. Prog. Brain Res. 2009, 180, 110–125. [Google Scholar]
- Zhou, K.; Thouas, G.A.; Bernard, C.C.; Nisbet, D.R.; Finkelstein, D.I.; Li, D.; Forsythe, J.S. Method to impart electro-and biofunctionality to neural scaffolds using graphene–polyelectrolyte multilayers. ACS Appl. Mater. Interfaces 2012, 4, 4524–4531. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Gao, H.; Zhu, G.; Cao, X.; Shi, X.; Wang, Y. The preparation and characterization of polycaprolactone/graphene oxide biocomposite nanofiber scaffolds and their application for directing cell behaviors. Carbon 2015, 95, 1039–1050. [Google Scholar] [CrossRef]
- Ribeiro, J.; Pereira, T.; Caseiro, A.R.; Armada-da-Silva, P.; Pires, I.; Prada, J.; Amorim, I.; Amado, S.; França, M.; Gonçalves, C. Evaluation of biodegradable electric conductive tube-guides and mesenchymal stem cells. World J. Stem Cells 2015, 7, 956. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tavangarian, F.; Li, Y. Carbon nanostructures as nerve scaffolds for repairing large gaps in severed nerves. Ceram. Int. 2012, 38, 6075–6090. [Google Scholar] [CrossRef]
- Fabbro, A.; Sucapane, A.; Toma, F.M.; Calura, E.; Rizzetto, L.; Carrieri, C.; Roncaglia, P.; Martinelli, V.; Scaini, D.; Masten, L. Adhesion to carbon nanotube conductive scaffolds forces action-potential appearance in immature rat spinal neurons. PLoS ONE 2013, 8, e73621. [Google Scholar] [CrossRef] [PubMed]
- Kabiri, M.; Oraee-Yazdani, S.; Shafiee, A.; Hanaee-Ahvaz, H.; Dodel, M.; Vaseei, M.; Soleimani, M. Neuroregenerative effects of olfactory ensheathing cells transplanted in a multi-layered conductive nanofibrous conduit in peripheral nerve repair in rats. J. Biomed. Sci. 2015, 22, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.Q.; Wang, T.; Zhao, B.; Li, J.; Jin, L. Soft Graphene Nanofibers Designed for the Acceleration of Nerve Growth and Development. Adv. Mater. 2015, 27, 6462–6468. [Google Scholar] [CrossRef] [PubMed]
- Behan, B.L.; DeWitt, D.G.; Bogdanowicz, D.R.; Koppes, A.N.; Bale, S.S.; Thompson, D.M. Single-walled carbon nanotubes alter Schwann cell behavior differentially within 2D and 3D environments. J. Biomed. Mater. Res. Part A 2011, 96, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Tosun, Z.; McFetridge, P. A composite SWNT–collagen matrix: Characterization and preliminary assessment as a conductive peripheral nerve regeneration matrix. J. Neural Eng. 2010, 7, 066002. [Google Scholar] [CrossRef] [PubMed]
- Aregueta-Robles, U.A.; Woolley, A.J.; Poole-Warren, L.A.; Lovell, N.H.; Green, R.A. Organic electrode coatings for next-generation neural interfaces. Front. Neuoeng. 2014. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi-Mobarakeh, L.; Prabhakaran, M.P.; Morshed, M.; Nasr-Esfahani, M.H.; Ramakrishna, S. Electrical stimulation of nerve cells using conductive nanofibrous scaffolds for nerve tissue engineering. Tissue Eng. Part A 2009, 15, 3605–3619. [Google Scholar] [CrossRef] [PubMed]
- Bhang, S.H.; Jeong, S.I.; Lee, T.J.; Jun, I.; Lee, Y.B.; Kim, B.S.; Shin, H. Electroactive Electrospun Polyaniline/Poly [(L-lactide)-co-(ε-caprolactone)] Fibers for Control of Neural Cell Function. Macromol. Biosci. 2012, 12, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Sun, Y.; Finne-Wistrand, A.; Mustafa, K.; Albertsson, A.-C. Electroactive porous tubular scaffolds with degradability and non-cytotoxicity for neural tissue regeneration. Acta Biomater. 2012, 8, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Sirivisoot, S.; Pareta, R.; Harrison, B.S. Protocol and cell responses in three-dimensional conductive collagen gel scaffolds with conductive polymer nanofibres for tissue regeneration. Interface Focus 2014, 4, 20130050. [Google Scholar] [CrossRef] [PubMed]
- Baniasadi, H.; SA, A.R.; Mashayekhan, S. Fabrication and characterization of conductive chitosan/gelatin-based scaffolds for nerve tissue engineering. Int. J. Biol. Macromol. 2015, 74, 360–366. [Google Scholar] [CrossRef]
- Urbanchek, M.; Shim, B.; Baghmanli, Z.; Wei, B.; Schroeder, K.; Langhals, N.; Miriani, R.; Egeland, B.; Kipke, D.; Martin, D. Conduction properties of decellularized nerve biomaterials. In Proceedings of the 26th Southern Biomedical Engineering Conference SBEC 2010, College Park, MD, USA, 30 April–2 May 2010; pp. 430–433. [Google Scholar]
- Meng, X.; Stout, D.A.; Sun, L.; Beingessner, R.L.; Fenniri, H.; Webster, T.J. Novel injectable biomimetic hydrogels with carbon nanofibers and self assembled rosette nanotubes for myocardial applications. J. Biomed. Mater. Res. Part A 2013, 101, 1095–1102. [Google Scholar] [CrossRef]
- Chen, Q.-Z.; Harding, S.E.; Ali, N.N.; Lyon, A.R.; Boccaccini, A.R. Biomaterials in cardiac tissue engineering: Ten years of research survey. Mater. Sci. Eng. R. Rep. 2008, 59, 1–37. [Google Scholar] [CrossRef]
- Stout, D.A.; Basu, B.; Webster, T.J. Poly (lactic–co-glycolic acid): Carbon nanofiber composites for myocardial tissue engineering applications. Acta Biomater. 2011, 7, 3101–3112. [Google Scholar] [CrossRef]
- Zia, S.; Mozafari, M.; Natasha, G.; Tan, A.; Cui, Z.; Seifalian, A.M. Hearts beating through decellularized scaffolds: Whole-organ engineering for cardiac regeneration and transplantation. Crit. Rev. Biotechnol. 2015, 1–11. [Google Scholar] [CrossRef]
- Kai, D.; Prabhakaran, M.P.; Jin, G.; Ramakrishna, S. Polypyrrole-contained electrospun conductive nanofibrous membranes for cardiac tissue engineering. J. Biomed. Mater. Res. Part A 2011, 99, 376–385. [Google Scholar] [CrossRef]
- Mukherjee, S.; Venugopal, J.R.; Ravichandran, R.; Ramakrishna, S.; Raghunath, M. Multimodal biomaterial strategies for regeneration of infarcted myocardium. J. Mater. Chem. 2010, 20, 8819–8831. [Google Scholar] [CrossRef]
- Moura, R.M.; de Queiroz, A.A.A. Dendronized polyaniline nanotubes for cardiac tissue engineering. Artif. Organs 2011, 35, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Sepantafar, M.; Maheronnaghsh, R.; Mohammadi, H.; Rajabi-Zeleti, S.; Annabi, N.; Aghdami, N.; Baharvand, H. Stem cells and injectable hydrogels: Synergistic therapeutics in myocardial repair. Biotechnol. Adv. 2016, 34, 362–379. [Google Scholar] [CrossRef] [PubMed]
- Kharaziha, M.; Shin, S.R.; Nikkhah, M.; Topkaya, S.N.; Masoumi, N.; Annabi, N.; Dokmeci, M.R.; Khademhosseini, A. Tough and flexible CNT–polymeric hybrid scaffolds for engineering cardiac constructs. Biomaterials 2014, 35, 7346–7354. [Google Scholar] [CrossRef] [PubMed]
- Gálvez-Montón, C.; Prat-Vidal, C.; Roura, S.; Soler-Botija, C.; Bayes-Genis, A. Cardiac tissue engineering and the bioartificial heart. Rev. Española De Cardiol. 2013, 66, 391–399. [Google Scholar] [CrossRef]
- Tandon, V.; Zhang, B.; Radisic, M.; Murthy, S.K. Generation of tissue constructs for cardiovascular regenerative medicine: From cell procurement to scaffold design. Biotechnol. Adv. 2013, 31, 722–735. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.R.; Jung, S.M.; Zalabany, M.; Kim, K.; Zorlutuna, P.; Kim, S.B.; Nikkhah, M.; Khabiry, M.; Azize, M.; Kong, J. Carbon-nanotube-embedded hydrogel sheets for engineering cardiac constructs and bioactuators. ACS Nano 2013, 7, 2369–2380. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, L.; Guo, B.; Ma, P.X. Interwoven aligned conductive nanofiber yarn/hydrogel composite scaffolds for engineered 3D cardiac anisotropy. ACS Nano 2017, 11, 5646–5659. [Google Scholar] [CrossRef]
- Spearman, B.S.; Hodge, A.J.; Porter, J.L.; Hardy, J.G.; Davis, Z.D.; Xu, T.; Zhang, X.; Schmidt, C.E.; Hamilton, M.C.; Lipke, E.A. Conductive interpenetrating networks of polypyrrole and polycaprolactone encourage electrophysiological development of cardiac cells. Acta Biomater. 2015, 28, 109–120. [Google Scholar] [CrossRef]
- Liang, S.; Zhang, Y.; Wang, H.; Xu, Z.; Chen, J.; Bao, R.; Tan, B.; Cui, Y.; Fan, G.; Wang, W. Paintable and Rapidly Bondable Conductive Hydrogels as Therapeutic Cardiac Patches. Adv. Mater. 2018, 30, 1704235. [Google Scholar] [CrossRef] [PubMed]
- Borriello, A.; Guarino, V.; Schiavo, L.; Alvarez-Perez, M.; Ambrosio, L. Optimizing PANi doped electroactive substrates as patches for the regeneration of cardiac muscle. J. Mater. Sci. Mater. Med. 2011, 22, 1053–1062. [Google Scholar] [CrossRef] [PubMed]
- Crowder, S.W.; Liang, Y.; Rath, R.; Park, A.M.; Maltais, S.; Pintauro, P.N.; Hofmeister, W.; Lim, C.C.; Wang, X.; Sung, H.-J. Poly (ε-caprolactone)-carbon nanotube composite scaffolds for enhanced cardiac differentiation of human mesenchymal stem cells. Nanomedicine 2013, 8, 1763–1776. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, V.; Cellot, G.; Toma, F.M.; Long, C.S.; Caldwell, J.H.; Zentilin, L.; Giacca, M.; Turco, A.; Prato, M.; Ballerini, L. Carbon nanotubes promote growth and spontaneous electrical activity in cultured cardiac myocytes. Nano Lett. 2012, 12, 1831–1838. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, V.; Cellot, G.; Toma, F.M.; Long, C.S.; Caldwell, J.H.; Zentilin, L.; Giacca, M.; Turco, A.; Prato, M.; Ballerini, L. Carbon nanotubes instruct physiological growth and functionally mature syncytia: Nongenetic engineering of cardiac myocytes. ACS Nano 2013, 7, 5746–5756. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhou, J.; Liu, Z.; Chen, J.; Lü, S.; Sun, H.; Li, J.; Lin, Q.; Yang, B.; Duan, C. A PNIPAAm-based thermosensitive hydrogel containing SWCNTs for stem cell transplantation in myocardial repair. Biomaterials 2014, 35, 5679–5688. [Google Scholar] [CrossRef] [PubMed]
- Stout, D.A.; Raimondo, E.; Marostica, G.; Webster, T.J. Growth characteristics of different heart cells on novel nanopatch substrate during electrical stimulation. Bio-Med Mater. Eng. 2014, 24, 2101–2107. [Google Scholar]
- Hsiao, C.-W.; Bai, M.-Y.; Chang, Y.; Chung, M.-F.; Lee, T.-Y.; Wu, C.-T.; Maiti, B.; Liao, Z.-X.; Li, R.-K.; Sung, H.-W. Electrical coupling of isolated cardiomyocyte clusters grown on aligned conductive nanofibrous meshes for their synchronized beating. Biomaterials 2013, 34, 1063–1072. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Lü, S.; Jiang, X.-X.; Li, X.; Li, H.; Lin, Q.; Mou, Y.; Zhao, Y.; Han, Y.; Zhou, J.; et al. Carbon nanotubes enhance intercalated disc assembly in cardiac myocytes via the β1-integrin-mediated signaling pathway. Biomaterials 2015, 55, 84–95. [Google Scholar] [CrossRef]
- Dvir, T.; Timko, B.P.; Brigham, M.D.; Naik, S.R.; Karajanagi, S.S.; Levy, O.; Jin, H.; Parker, K.K.; Langer, R.; Kohane, D.S. Nanowired three-dimensional cardiac patches. Nat. Nanotechnol. 2011, 6, 720–725. [Google Scholar] [CrossRef]
- Zhou, J.; Chen, J.; Sun, H.; Qiu, X.; Mou, Y.; Liu, Z.; Zhao, Y.; Li, X.; Han, Y.; Duan, C. Engineering the heart: Evaluation of conductive nanomaterials for improving implant integration and cardiac function. Sci. Rep. 2014, 4. [Google Scholar] [CrossRef]
- Noorman, M.; van der Heyden, M.A.; van Veen, T.A.; Cox, M.G.; Hauer, R.N.; de Bakker, J.M.; van Rijen, H.V. Cardiac cell–cell junctions in health and disease: Electrical versus mechanical coupling. J. Mol. Cell. Cardiol. 2009, 47, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Yazdanpanah, A.; Amoabediny, G.; Shariatpanahi, P.; Nourmohammadi, J.; Tahmasbi, M.; Mozafari, M. Synthesis and Characterization of Polylactic Acid Tubular Scaffolds with Improved Mechanical Properties for Vascular Tissue Engineering. Trends Biomater. Artif. Organs 2014, 28, 99–105. [Google Scholar]
- Rowlands, A.S.; Cooper-White, J.J. Directing phenotype of vascular smooth muscle cells using electrically stimulated conducting polymer. Biomaterials 2008, 29, 4510–4520. [Google Scholar] [CrossRef] [PubMed]
- Mihardja, S.S.; Sievers, R.E.; Lee, R.J. The effect of polypyrrole on arteriogenesis in an acute rat infarct model. Biomaterials 2008, 29, 4205–4210. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-W.; Serna, F.; Schmidt, C.E. Carboxy-endcapped conductive polypyrrole: Biomimetic conducting polymer for cell scaffolds and electrodes. Langmuir 2006, 22, 9816–9819. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lee, J.-W. Porous-structured Conductive Polypyrrole Cell Scaffolds. Notes 2014, 35, 293. [Google Scholar] [CrossRef]
- Bidez, P.R.; Li, S.; MacDiarmid, A.G.; Venancio, E.C.; Wei, Y.; Lelkes, P.I. Polyaniline, an electroactive polymer, supports adhesion and proliferation of cardiac myoblasts. J. Biomater. Sci. Polym. Ed. 2006, 17, 199–212. [Google Scholar] [CrossRef] [PubMed]
- Qazi, T.H.; Rai, R.; Dippold, D.; Roether, J.E.; Schubert, D.W.; Rosellini, E.; Barbani, N.; Boccaccini, A.R. Development and characterization of novel electrically conductive PANI–PGS composites for cardiac tissue engineering applications. Acta Biomater. 2014, 10, 2434–2445. [Google Scholar] [CrossRef] [PubMed]
- Baheiraei, N.; Yeganeh, H.; Ai, J.; Gharibi, R.; Ebrahimi-Barough, S.; Azami, M.; Vahdat, S.; Baharvand, H. Preparation of a porous conductive scaffold from aniline pentamer-modified polyurethane/PCL blend for cardiac tissue engineering. J. Biomed. Mater. Res. Part A 2015, 103, 3179–3187. [Google Scholar] [CrossRef]
- Stout, D.A.; Yoo, J.; Webster, T.J. Poly Lactic-Co-Glycolic Acid Carbon Nanofiber Composite for Enhancing Cardiomyocyte Function. MRS Online Proc. Libr. Arch. 2011, mrsf10-1316-qq1306-1333. [Google Scholar] [CrossRef]
- Han, Z.; Kong, H.; Meng, J.; Wang, C.; Xie, S.; Xu, H. Electrospun aligned nanofibrous scaffold of carbon nanotubes-polyurethane composite for endothelial cells. J. Nanosci. Nanotechnol. 2009, 9, 1400–1402. [Google Scholar] [CrossRef]
- Mooney, E.; Mackle, J.N.; Blond, D.J.P.; O’Cearbhaill, E.; Shaw, G.; Blau, W.J.; Barry, F.P.; Barron, V.; Murphy, J.M. The electrical stimulation of carbon nanotubes to provide a cardiomimetic cue to MSCs. Biomaterials 2012, 33, 6132–6139. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.M.; Eng, G.; Caridade, S.G.; Mano, J.F.; Reis, R.L.; Vunjak-Novakovic, G. Electrically Conductive Chitosan/Carbon Scaffolds for Cardiac Tissue Engineering. Biomacromolecules 2014, 15, 635–643. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, R.A.; Laurenzi, B.F.; Viswanathan, G.; Ajayan, P.M.; Stegemann, J.P. Collagen–carbon nanotube composite materials as scaffolds in tissue engineering. J. Biomed. Mater. Res. Part A 2005, 74, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Pok, S.; Vitale, F.; Eichmann, S.L.; Benavides, O.M.; Pasquali, M.; Jacot, J.G. Biocompatible Carbon Nanotube–Chitosan Scaffold Matching the Electrical Conductivity of the Heart. ACS Nano 2014, 8, 9822–9832. [Google Scholar] [CrossRef] [PubMed]
- Wan, A.M.-D.; Inal, S.; Williams, T.; Wang, K.; Leleux, P.; Estevez, L.; Giannelis, E.P.; Fischbach, C.; Malliaras, G.G.; Gourdon, D. 3D Conducting Polymer Platforms for Electrical Control of Protein Conformation and Cellular Functions. J. Mater. Chem. B 2015, 3, 5040–5048. [Google Scholar] [CrossRef]
- Petrisor, B.; Lau, J.T.C. Electrical Bone Stimulation: An Overview and its Use in High Risk and Charcot Foot and Ankle Reconstructions. Foot Ankle Clin. 2005, 10, 609–620. [Google Scholar] [CrossRef]
- Björninen, M.; Siljander, A.; Pelto, J.; Hyttinen, J.; Kellomäki, M.; Miettinen, S.; Seppänen, R.; Haimi, S. Comparison of chondroitin sulfate and hyaluronic acid doped conductive polypyrrole films for adipose stem cells. Ann. Biomed. Eng. 2014, 42, 1889–1900. [Google Scholar] [CrossRef]
- Hamlehkhan, A.; Mozafari, M.; Nezafati, N.; Azami, M.; Samadikuchaksaraei, A. Novel bioactive poly (ε-caprolactone)-gelatin-hydroxyapatite nanocomposite scaffolds for bone regeneration. Key Eng. Mater. 2012, 493–494, 909–915. [Google Scholar] [CrossRef]
- Cheng, C.W.; Solorio, L.D.; Alsberg, E. Decellularized tissue and cell-derived extracellular matrices as scaffolds for orthopaedic tissue engineering. Biotechnol. Adv. 2014, 32, 462–484. [Google Scholar] [CrossRef]
- Henkel, J.; Woodruff, M.A.; Epari, D.R.; Steck, R.; Glatt, V.; Dickinson, I.C.; Choong, P.F.M.; Schuetz, M.A.; Hutmacher, D.W. Bone Regeneration Based on Tissue Engineering Conceptions—A 21st Century Perspective. Bone Res. 2013, 1, 216. [Google Scholar] [CrossRef] [PubMed]
- Baxter, F.R.; Bowen, C.R.; Turner, I.G.; Dent, A.C. Electrically active bioceramics: A review of interfacial responses. Ann. Biomed. Eng. 2010, 38, 2079–2092. [Google Scholar] [CrossRef] [PubMed]
- Griffin, M.; Bayat, A. Electrical stimulation in bone healing: Critical analysis by evaluating levels of evidence. Eplasty 2011, 11, e34. [Google Scholar]
- Cui, H.; Wang, Y.; Cui, L.; Zhang, P.; Wang, X.; Wei, Y.; Chen, X. In Vitro Studies on Regulation of Osteogenic Activities by Electrical Stimulus on Biodegradable Electroactive Polyelectrolyte Multilayers. Biomacromolecules 2014, 15, 3146–3157. [Google Scholar] [CrossRef] [PubMed]
- Shahini, A.; Yazdimamaghani, M.; Walker, K.J.; Eastman, M.A.; Hatami-Marbini, H.; Smith, B.J.; Ricci, J.L.; Madihally, S.V.; Vashaee, D.; Tayebi, L. 3D conductive nanocomposite scaffold for bone tissue engineering. Int. J. Nanomed. 2014, 9, 167. [Google Scholar]
- Liu, Y.; Cui, H.; Zhuang, X.; Wei, Y.; Chen, X. Electrospinning of aniline pentamer-graft-gelatin/PLLA nanofibers for bone tissue engineering. Acta Biomater. 2014, 10, 5074–5080. [Google Scholar] [CrossRef]
- Lyu, C.-Q.; Lu, J.-Y.; Cao, C.-H.; Luo, D.; Fu, Y.-X.; He, Y.-S.; Zou, D.-R. Induction of Osteogenic Differentiation of Human Adipose-Derived Stem Cells by a Novel Self-Supporting Graphene Hydrogel Film and the Possible Underlying Mechanism. ACS Appl. Mater. Interfaces 2015, 7, 20245–20254. [Google Scholar] [CrossRef]
- Meng, S.; Zhang, Z.; Rouabhia, M. Accelerated osteoblast mineralization on a conductive substrate by multiple electrical stimulation. J. Bone Miner. Metab. 2011, 29, 535–544. [Google Scholar] [CrossRef]
- Meng, S.; Rouabhia, M.; Zhang, Z. Electrical stimulation modulates osteoblast proliferation and bone protein production through heparin-bioactivated conductive scaffolds. Bioelectromagnetics 2013, 34, 189–199. [Google Scholar] [CrossRef]
- Whitehead, M.A.; Fan, D.; Akkaraju, G.R.; Canham, L.T.; Coffer, J.L. Accelerated calcification in electrically conductive polymer composites comprised of poly (ε-caprolactone), polyaniline, and bioactive mesoporous silicon. J. Biomed. Mater. Res. Part A 2007, 83, 225–234. [Google Scholar] [CrossRef]
- Runge, M.B.; Dadsetan, M.; Baltrusaitis, J.; Yaszemski, M.J. Electrically conductive surface modifications of three-dimensional polypropylene fumarate scaffolds. J. Biol. Regul. Homeost. Agents 2011, 25, S15. [Google Scholar] [PubMed]
- Pelto, J.; Björninen, M.; Pälli, A.; Talvitie, E.; Hyttinen, J.; Mannerström, B.; Suuronen Seppanen, R.; Kellomäki, M.; Miettinen, S.; Haimi, S. Novel polypyrrole-coated polylactide scaffolds enhance adipose stem cell proliferation and early osteogenic differentiation. Tissue Eng. Part A 2013, 19, 882–892. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Liu, Y.; Deng, M.; Pang, X.; Zhang, P.; Wang, X.; Chen, X.; Wei, Y. Synthesis of biodegradable and electroactive tetraaniline grafted poly (ester amide) copolymers for bone tissue engineering. Biomacromolecules 2012, 13, 2881–2889. [Google Scholar] [CrossRef] [PubMed]
- Newman, P.; Minett, A.; Ellis-Behnke, R.; Zreiqat, H. Carbon nanotubes: Their potential and pitfalls for bone tissue regeneration and engineering. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 1139–1158. [Google Scholar] [CrossRef] [PubMed]
- Nishida, E.; Miyaji, H.; Takita, H.; Kanayama, I.; Tsuji, M.; Akasaka, T.; Sugaya, T.; Sakagami, R.; Kawanami, M. Graphene oxide coating facilitates the bioactivity of scaffold material for tissue engineering. Jpn. J. Appl. Phys. 2014, 53, 06JD04. [Google Scholar] [CrossRef]
- Xie, X.; Hu, K.; Fang, D.; Shang, L.; Tran, S.D.; Cerruti, M. Graphene and hydroxyapatite self-assemble into homogeneous, free standing nanocomposite hydrogels for bone tissue engineering. Nanoscale 2015, 7, 7992–8002. [Google Scholar] [CrossRef] [PubMed]
- Elkhenany, H.; Amelse, L.; Lafont, A.; Bourdo, S.; Caldwell, M.; Neilsen, N.; Dervishi, E.; Derek, O.; Biris, A.S.; Anderson, D. Graphene supports in vitro proliferation and osteogenic differentiation of goat adult mesenchymal stem cells: Potential for bone tissue engineering. J. Appl. Toxicol. 2015, 35, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Sayyar, S.; Murray, E.; Thompson, B.; Chung, J.; Officer, D.L.; Gambhir, S.; Spinks, G.M.; Wallace, G.G. Processable conducting graphene/chitosan hydrogels for tissue engineering. J. Mater. Chem. B 2015, 3, 481–490. [Google Scholar] [CrossRef]
- Rajzer, I.; Rom, M.; Menaszek, E.; Pasierb, P. Conductive PANI patterns on electrospun PCL/gelatin scaffolds modified with bioactive particles for bone tissue engineering. Mater. Lett. 2015, 138, 60–63. [Google Scholar] [CrossRef]
- Sirivisoot, S.; Harrison, B.S. Skeletal myotube formation enhanced by electrospun polyurethane carbon nanotube scaffolds. Int. J. Nanomed. 2011, 6, 2483. [Google Scholar] [CrossRef]
- Bach, A.; Beier, J.; Stern-Staeter, J.; Horch, R. Skeletal muscle tissue engineering. J. Cell. Mol. Med. 2004, 8, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Bian, W.; Bursac, N. Tissue engineering of functional skeletal muscle: Challenges and recent advances. IEEE Eng. Med. Biol. Mag. 2008, 27, 109–113. [Google Scholar] [PubMed]
- Kawahara, Y.; Yamaoka, K.; Iwata, M.; Fujimura, M.; Kajiume, T.; Magaki, T.; Takeda, M.; Ide, T.; Kataoka, K.; Asashima, M. Novel electrical stimulation sets the cultured myoblast contractile function to ‘on’. Pathobiology 2007, 73, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Gilmore, K.J.; Kita, M.; Han, Y.; Gelmi, A.; Higgins, M.J.; Moulton, S.E.; Clark, G.M.; Kapsa, R.; Wallace, G.G. Skeletal muscle cell proliferation and differentiation on polypyrrole substrates doped with extracellular matrix components. Biomaterials 2009, 30, 5292–5304. [Google Scholar] [CrossRef] [PubMed]
- Stern-Straeter, J.; Bach, A.; Stangenberg, L.; Foerster, V.; Horch, R.; Stark, G.; Beier, J. Impact of electrical stimulation on three-dimensional myoblast cultures-a real-time RT-PCR study. J. Cell. Mol. Med. 2005, 9, 883–892. [Google Scholar] [CrossRef]
- Jun, I.; Jeong, S.; Shin, H. The stimulation of myoblast differentiation by electrically conductive sub-micron fibers. Biomaterials 2009, 30, 2038–2047. [Google Scholar] [CrossRef]
- Wang, L.; Wu, Y.; Guo, B.; Ma, P.X. Nanofiber yarn/hydrogel core–shell scaffolds mimicking native skeletal muscle tissue for guiding 3D myoblast alignment, elongation, and differentiation. ACS Nano 2015, 9, 9167–9179. [Google Scholar] [CrossRef]
- Quigley, A.F.; Razal, J.M.; Kita, M.; Jalili, R.; Gelmi, A.; Penington, A.; Ovalle-Robles, R.; Baughman, R.H.; Clark, G.M.; Wallace, G.G. Electrical stimulation of myoblast proliferation and differentiation on aligned nanostructured conductive polymer platforms. Adv. Healthc. Mater. 2012, 1, 801–808. [Google Scholar] [CrossRef]
- Chen, M.-C.; Sun, Y.-C.; Chen, Y.-H. Electrically conductive nanofibers with highly oriented structures and their potential application in skeletal muscle tissue engineering. Acta Biomater. 2013, 9, 5562–5572. [Google Scholar] [CrossRef]
- Ku, S.H.; Lee, S.H.; Park, C.B. Synergic effects of nanofiber alignment and electroactivity on myoblast differentiation. Biomaterials 2012, 33, 6098–6104. [Google Scholar] [CrossRef]
- Li, L.; Ge, J.; Wang, L.; Guo, B.; Ma, P.X. Electroactive nanofibrous biomimetic scaffolds by thermally induced phase separation. J. Mater. Chem. B 2014, 2, 6119–6130. [Google Scholar] [CrossRef]
- Ribeiro, S.; Costa, P.; Ribeiro, C.; Sencadas, V.; Botelho, G.; Lanceros-Méndez, S. Electrospun styrene–butadiene–styrene elastomer copolymers for tissue engineering applications: Effect of butadiene/styrene ratio, block structure, hydrogenation and carbon nanotube loading on physical properties and cytotoxicity. Compos. Part B Eng. 2014, 67, 30–38. [Google Scholar] [CrossRef]
- Wang, Q.; He, W.; Huang, J.; Liu, S.; Wu, G.; Teng, W.; Wang, Q.; Dong, Y. Synthesis of water soluble, biodegradable, and electroactive polysaccharide crosslinker with aldehyde and carboxylic groups for biomedical applications. Macromol. Biosci. 2011, 11, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.G.; Lee, J.Y.; Schmidt, C.E. Biomimetic conducting polymer-based tissue scaffolds. Curr. Opin. Biotechnol. 2013, 24, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Zarrintaj, P.; Mostafapoor, F.; Milan, P.B.; Saeb, M.R. Theranostic Platforms Proposed for Cancerous Stem Cells: A Review. Curr. Stem Cell Res. Ther. 2019, 14, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Tahmasbi Rad, A.; Ali, N.; Kotturi, H.S.R.; Yazdimamaghani, M.; Smay, J.; Vashaee, D.; Tayebi, L. Conducting scaffolds for liver tissue engineering. J. Biomed. Mater. Res. Part A 2014, 102, 4169–4181. [Google Scholar] [CrossRef] [PubMed]
- Mohebbi, S.; Nezhad, M.N.; Zarrintaj, P.; Jafari, S.H.; Gholizadeh, S.S.; Saeb, M.R.; Mozafari, M. Chitosan in biomedical engineering: A critical review. Curr. Stem Cell Res. Ther. 2019, 14, 93–116. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Liu, J.; Dvir, T.; Jin, L.; Tsui, J.H.; Qing, Q.; Suo, Z.; Langer, R.; Kohane, D.S.; Lieber, C.M. Macroporous nanowire nanoelectronic scaffolds for synthetic tissues. Nat. Mater. 2012, 11, 986–994. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).