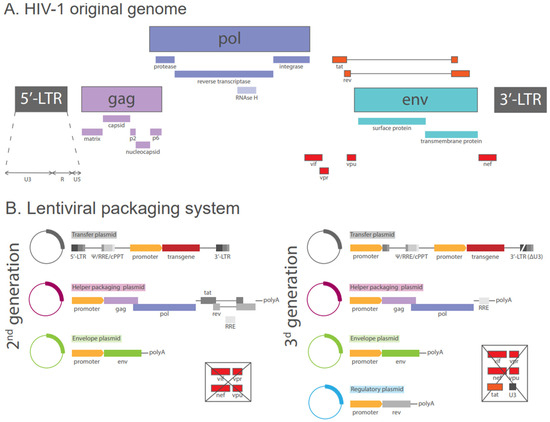
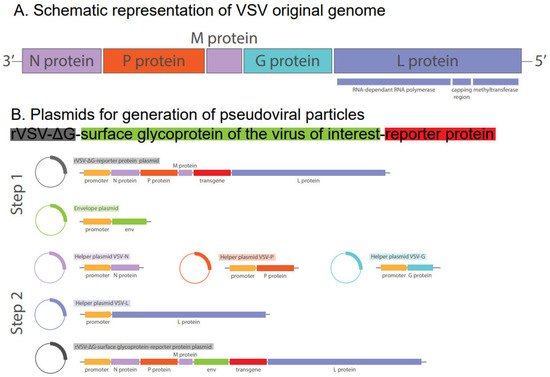
Abstract
The study of pathogenic viruses has always posed significant biosafety challenges. In particular, the study of highly pathogenic viruses requires methods with low biological risk but relatively high sensitivity and convenience in detection. In recent years, pseudoviruses, which consist of a backbone of one virus and envelope proteins of another virus, have become one of the most widely used tools for exploring the mechanisms of viruses binding to cells, membrane fusion and viral entry, as well as for screening the libraries of antiviral substances, evaluating the potential of neutralizing monoclonal antibodies, developing neutralization tests, and therapeutic platforms. During the outbreak of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), pseudotyped virus-based assays played a pivotal role in advancing our understanding of virus–cell interactions and the role of its proteins in disease pathogenesis. Such tools facilitated the search for potential therapeutic agents and accelerated epidemiological studies on post-infection and post-vaccination humoral immunity. This review focuses on the use of pseudoviruses as a model for large-scale applications to study enveloped viruses.
1. Introduction
Viruses are known to replicate only within living cells. In vitro models are frequently employed to examine the efficacy of antiviral agents and the functionality of the immune response, including vaccine-induced responses. In lieu of in vivo assays, the use of in vitro assays can facilitate the acquisition of comprehensive data on the ability of a virus to infect and persist in different cell types [1,2,3,4,5,6]. Moreover, this approach may prove invaluable in investigating the potency and efficacy of antiviral drugs and vaccines. The plaque assay and endpoint dilution assay offer the means of measuring the number of viral particles capable of replicating within a specific cell culture [7]. In a plaque assay, a serially diluted contaminated sample is applied to susceptible and permissive cells. The objective is to infect a cell with a single virion, which will replicate and propagate within the cell, resulting in the production of second-generation virions. These virions will then infect and kill surrounding cells, leading to the formation of a visible hole in the cell monolayer. The apparent concentration of the virus is often defined as plaque-forming units per volume of measure. This methodology is limited to viruses that induce cell lysis or death and, thus far, has remained the gold standard for quantifying the concentration of replication-competent lytic virions [8]. In the event that cells are not killed by the virus, but rather undergo morphological changes and form evaluable groups, focus-forming units are counted. In an endpoint dilution assay, the viral titer can be determined by calculating the tissue culture infectious dose (TCID) based on the presence of cytopathic effects (CPE). In the event that a virus does not result in the formation of CPE, an immunofluorescence assay is conducted in conjunction with the endpoint dilution assay, with the immunofluorescence being monitored instead of CPE. The most reproducible number is expressed as the dilution of the virus that produces pathological changes in half of the inoculated cells, which is referred to as the TCID50 [9].
Virus neutralization assays are typically conducted in conjunction with infectivity assays and are designed to detect antibodies (Abs) that reduce the infectivity of the virus or to assess the efficacy of specifically developed antiviral therapeutic agents [10,11]. There are numerous methods currently used in modern laboratory practice to evaluate the ability of neutralizing Abs (nAbs) or viral inhibitors to decline virus replication and propagation in the examined cells. One such method is the plaque reduction neutralization test (PRNT), which, like traditional approaches to virus research, is considered the gold standard for evaluating nAbs [12,13]. The fundamental configuration of the PRNT experiment entails the formation of virus–agent complexes, the introduction of a mixture to susceptible cells, incubation, and subsequent observation of emerging plaques [14]. An alternative assay has been developed to address the limitations of the PRNT. The focus-reduction neutralization test (FRNT) is analogous to the PRNT, employing a live virus strain and diluting the antibody or a serum of interest to varying concentrations before inoculation into a cell monolayer, with subsequent monitoring of the cytopathic effect (CPE) [15].
The study of highly pathogenic viruses is typically conducted in laboratories that adhere to rigorous biosafety standards, predominantly at levels 3 and 4, as recommended [16].
This obstacle limits the number of organizations able to work with these agents, thereby impeding the pace of academic research and therapeutics development in the field of virology. In light of these limitations, it is important to pursue alternative approaches that can overcome these constraints.
The pseudovirus neutralization test employs packaging-convenient and replication-defective pseudotyped viruses (pseudoviral particles, PVPs) that display the target surface protein of the virus of interest. A comparison of pseudovirus assays with those conducted using live viruses reveals a high degree of convergence in the resulting data [17,18]. Moreover, pseudotyped virus-based assays can be conducted in biosafety level 2 (BSL-2) laboratory facilities. This approach enables both qualitative and quantitative evaluation and is suitable for large-scale detection [19].
3. Pseudotyped Virus-Based Assay Application for Study COVID-19
3.1. Study of Cellular Susceptibility and the Mechanism of Viral Entry
PVP has been employed extensively in the study of the interaction between the virus and host cells. This was employed to examine the interaction between SARS-CoV and SARS-CoV-2 and host cellular receptors [52,64]. Yang and colleagues utilized wild-type, XBB.1.61, or B.1.617.2 lentiviral-based pseudotyped SARS-CoV-2 particles with reporter genes of GFP (green fluorescent protein) and luciferase to demonstrate that PVPs can significantly infect hepatic Huh-7 and HepG2 cells. However, the simultaneous knockout of ACE2 and asialoglycoprotein receptor 1 (ASGR1) prevented SARS-CoV-2 pseudovirus infection, suggesting that ASGR1 promotes SARS-CoV-2 entry into human hepatocytes [65]. Moreover, the presence of the cholesterol uptake receptor Niemann-Pick type C1-like 1, an endosomal membrane protein that regulates intracellular cholesterol trafficking and exhibits high expression levels in several organs, including the kidneys, has been demonstrated to facilitate SARS-CoV-2 entry in vitro using a PVP-based assay [66]. Melano et al. demonstrated the relationship between Wnt3a, a powerful activator of the Wnt/β-catenin signalling pathway, and facilitated ACE2-mediated virus infection. Using lentiviral-based PVPs with a luciferase reporter gene, it was demonstrated that SARS-CoV-2 pseudotyped virus infection with Wuhan, Delta and Omicron variants activates the Wnt/β-catenin signalling pathway, which subsequently stimulates ACE2 transcription [67]. Pellegrini et al. investigated the neurotropic potential of SARS-CoV-2 by challenging human pluripotent stem cell-derived brain organoids with lentiviral-based pseudotyped particles expressing GFP reporter gene. It was found that SARS-CoV-2 infection results in a damage to the choroid plexus epithelium, which subsequently leads to fluid leakage across the blood-cerebrospinal fluid barrier [68]. It has been demonstrated that GRP78 acts as a receptor for SARS-CoV-2 spike protein to mediate ACE2-independent virus entry into monocytes which express low or no ACE2 [69]. Furthermore, the utilization of the pseudovirus-based surrogate test in BSL-2 facilities has demonstrated that a plasma membrane and an appropriate protease are sufficient to facilitate SARS-CoV-2 pseudovirus fusion and the subsequent subcellular location after the entry. This process is likely dependent on the differential activity of airway, cell surface, and endosomal proteases, yet all of them are capable of supporting infection [70].
As the SARS-CoV-2 pandemic progresses, the receptor binding capacity of newly emerging variants of the virus is being investigated using PVP-based assays. The design of PVP carrying the S protein from different viral variants, and in some cases, carrying specific amino acid mutations, has enabled several groups to study the entry efficacy of different SARS-CoV-2 variants and to investigate the evolutionary path of the virus [71,72]. Furthermore, the diverse host factors that may impact viral entry can be investigated through the use of PVP assays. Recently, it was demonstrated that SH3 domain-binding protein 4 regulates the entry of PVP-carrying S protein from the Wuhan, Delta, and Omicron BA.2 variants in an integrin- and clathrin-dependent manner [73].
3.2. Entry Inhibitors Screening, Identification and Evaluation
Since PVP-based assays provide a great deal of insight into the entry mechanism used by SARS-CoV-2, the same platforms could be used to screen for potential entry inhibitors or to preclinically evaluate the selected one. The PVP-based technology is employed as a conformational assay for viral entry inhibitors following in silico analysis of the candidate and subsequent screening methods [74,75].
Palla and colleagues devised and synthesized a class of pyrrolidinones with the objective of reversibly inhibiting of transmembrane protease serine 2 (TMPRSS2) and furin. The use of SARS-CoV-2 PVPs enabled the confirmation of the blocking effect of the most potent inhibitor on virus ACE2-dependent entry, thereby demonstrating the potential of the compound for further preclinical investigation [76]. In a study by Kazakova and colleagues, the antiviral activity of a series of triterpene A-ring hydroxymethylene amino derivatives was investigated. The compounds were evaluated for their potential to inhibit SARS-CoV-2 PVPs, and they demonstrated notable inhibitory activity [77]. Mei et al. proposed that a dual blockade of conservative host receptors, ACE2 and neuropilin-1, would constitute an advantageous pan-inhibition strategy. By employing structure-based virtual screening, the researchers were able to identify a dual-targeting peptide, AP-1, which demonstrated remarkable inhibitory activity against SARS-CoV-2 VOCs in a pseudovirus infectivity assay [78]. Razi et al. employed systematic evolution of ligands by exponential enrichment (SELEX) to identify fluoro-arabino nucleic acid aptamer R8-9, which was found to interfere with the interaction between the SARS-CoV-2 RBD and ACE2. Moreover, it was demonstrated that the selected aptamer inhibits the uptake of spike-bearing VSV-based PVPs by cells [79]. Other researchers demonstrated that the ACE2 receptor facilitated, but was not a prerequisite for, SARS-CoV-2 membrane fusion. In order to test the hypothesis that DNA-lipid tethering could serve as a synthetic attachment factor in place of ACE2, experiments were conducted using two viral particle types: HIV-based pseudoviruses and virus-like particles created by co-expressing S, E, M, and N proteins [80]. The possibility of using the ACE2 peptide for prophilaxis of SARS-CoV-2 infection was also evaluated [81,82].
In addition, the wide range of naturally derived plant-based virus inhibitors used in traditional medicine have been evaluated using the PVP-based system [83,84,85,86]. PVP pseudotyped with S protein from different viral variants, including recently emerging ones, gave the scientific community an insight into the breadth and depth of inhibitor efficacy.
Furthermore, the PVP-based platforms facilitate the investigation of the efficacy of entry inhibitors for co-infections of different viruses, including co-infections with SARS-CoV-2 and influenza. Additionally, the analysis can be extended to encompass different variants of both viruses, utilizing particles pseudotyped with a range of variants of the SARS-CoV-2 S protein and influenza A haemagglutinin [87].
Moreover, K18-hACE2 mice infected with SARS-CoV-2 spike pseudovirus were employed as an in vivo model for the assessment of the efficacy of TMPRSS2 and cathepsin L inhibitors [88]. The scientists established a panel of nanocarriers coupled with anti-hACE2 Abs, which were loaded with TMPRSS2 or/and cathepsin L inhibitors (Camostat or/and E-64d, respectively). In an in vitro model, it was demonstrated that a combination of TMPRSS2 and cathepsin L inhibitors more effectively inhibited the entry of SARS-CoV-2 spike pseudovirus into HEK-hACE2 cells. Treatment of mice infected with luciferase-labelled SARS-CoV-2 spike pseudovirus with that combination of protease inhibitors resulted in significantly reduced luminescence in the infected lung area compared to uninfected controls. Moreover, the implementation of a combination of TMPRSS2 and cathepsin L inhibitors not only hindered infection, as evidenced by a decrease in or absence of luminescence signal from the lung area on Day 2, but also prevented major organ lesions.
3.3. Therapeutic Antibodies Evaluation
A pseudoviral neutralization assay was incorporated into the pipeline for in vitro evaluation of monoclonal antibodies (mAbs) against wild-type SARS-CoV-2 and its variants. Wang et al. constructed an immunoglobulin-like bispecific antibody, designated BI31, by fusing two parental mAbs that target the S protein RBD. In the PVP test, BI31 demonstrated neutralizing effects against SARS-CoV-2, SARS-CoV, and Middle East respiratory syndrome coronavirus, suggesting it has the potential to become a broad-spectrum therapeutic antibody [89]. The pseudoviral neutralization assays facilitate the point the virus variants that are targeted by mAbs. Li Ko et al. identified a potent SARS-CoV-2 antibody, designated SKAI-DS84, through the use of phage display technology. The authors subsequently confirmed the antibody’s capacity to neutralize wild-type, B.1.617.2, and B.1.1.529 subvariants of SARS-CoV-2 using a lentivirus-based nano-luciferase expressing SARS-CoV-2 pseudoviruses [90]. Gao et al. generated a novel monospecific tetravalent IgG1-(scFv)2 version of mAb 553-15 by fusing two forms of 553-15-derived single-chain variable fragments to the C-terminus of the human immunoglobulin G1 Fc fragment. In comparison to the original monoclonal antibody, the enhanced neutralization observed in PVPs assays against VOCs is noteworthy [91]. AZD3152 mAb neutralized a broad panel of pseudovirus variants, including the once dominant Omicron variant JN.1 but reduced potency against XBB subvariants containing F456L [92]. The assistance of pseudoviral-based assays, which utilized the SARS-CoV-2 S proteins from Alpha, Beta, Delta and Omicron (B.1.1.529, BA.2, XBB, and XBB.1.5) variants, enabled the study of the nanobodies which had previously demonstrated neutralizing activities against the Wuhan strain. The nanobodies were obtained through llama immunization with recombinant SARS-CoV-2 S-2P and RBD proteins, subsequently leading to the identification of specific antibody sequences. An analysis focused on 29 nanobodies, exhibiting notably higher levels of expression. However, only 15 of those nanobodies have been shown to possess the capacity to neutralize SARS-CoV-2 pseudovirus infection in vitro, in a model derived from the Wuhan variant. Moreover, the cocktail of nanobodies that was able to neutralize the new variants of the virus was also identified [93].
3.4. Pseudovirus Neutralization Assay for Detection Neutralizing Antibody
Since the onset of the SARS-CoV-2 pandemic, there has been a continuous development of validated pseudovirus-based assays with PVPs bearing the S protein of different SARS-CoV-2 variants [94,95,96]. They were employed extensively in clinical trials designed to assess post-vaccination humoral immunity which allow to study how the effectiveness of post-vaccination immunity changes against different variants of the virus, to evaluate and compare the effectiveness of various vaccines and the duration of post-vaccination immunity [97,98,99,100,101]. The use of PVPs carrying the spike protein of distinct virus variants allowed to evaluate the cross-reactivity of these variants and predict the effectiveness of post-vaccination immunity [102,103,104].
Furthermore, PVPs were employed in regional studies investigating herd immunity to SARS-CoV-2, including protective immunity against the recently emerged variants [66,105]. Pseudovirus tests were used in preclinical trials of new vaccine variants to assess efficacy against both circulating and “historical” variants [106,107]. It was shown that antibodies produced in response to infection with different SARS-CoV-2 variants were characterized by different cross-reactivity to other viral variants. Thus, SARS-CoV-2 Omicron, BA.4/5 infection triggered highly cross-reactive functional antibodies compared to BA.1 strain [108]. Using the panel of pseudoviruses carrying artificially mutated S protein it was revealed that the strongest escape mutations are located in the RBD at sites 357, 420, 440, 456 and 473, but the mutations that significantly affected ACE2 binding are located outside RBD of SARS-CoV-2 [109].
The use of pseudotyped viruses in animal research revealed the existence of five distinct serotypes. For that purpose, VSV-ΔG-G-GFP pseudotyped viruses of 23 corresponding SARS-CoV-2 variants were employed in a virus neutralization assay to assess the cross-neutralization capacity of serially diluted antisera obtained from mice immunized with mRNA vaccines based on the RBD of 23 SARS-CoV-2 variants [110]. Pseudotyped lentiviral viruses bearing the S protein of the SARS-CoV-2 ancestral strain and VOCs (B.1 lineage, Alpha, Beta, Gamma, Delta, and Omicron BA.1) were employed to analyse cat serum samples in the veterinary study of SARS-CoV-2 prevalence and variant surveillance among cats in the USA [111].
The results of PVP neutralization tests demonstrated that a key deletion, designated DelS31, within the S protein of JN.1 is responsible for the virus’s capacity to evade the immune system [112].
Currently, a majority of the global population has developed immunity to SARS-CoV-2, either naturally as a result of infection or as a result of vaccination. In some cases, this immunity has been achieved through a combination of natural infection and vaccination. This raises the question of whether the presence of antibodies may enhance the risk of infection. In a study conducted by Connir et al., a panel of PVP pseudotyped with S protein variants, including Delta, Beta, and Omicron (BA.1 and BA.2), was used to demonstrate that some NTD-S1 protein-specific antibodies derived from convalescent donors exhibited infection-enhancing activities [113].
4. Pseudotyped Virus-Based Assay in Regard to Other RNA Viruses
The development of pseudotyped viruses allowed for the study of a multitude of highly pathogenic and infectious viruses with minimal safety risks.
Pseudotyped viruses that carry the envelope protein of the influenza virus (HA), which is the principal protein for attachment and fusion with the cell, are widely used for the evaluation of continually redesigned vaccines and new developed anti-influenza agents [114].
Prior to the global pandemic caused by SARS-CoV-2, pseudo-viral systems were employed to investigate the cellular tropism of viruses. This resulted in the discovery that SARS-CoV is capable of infecting cells with increased expression of the ACE2 receptor [65], as well as the description of the function of the human foamy virus Bet protein [115]. A comprehensive examination of the function of the human marker for late endosomes/lysosomes, which was shown to serve as a specific receptor for Lassa virus, LAMP1, was conducted by Zhang et al. [116]. Yu et al. demonstrated the impact of a combined substitution in the V3 loop and C4 region of the HIV-1 envelope on co-receptor switching from CCR5 to CXCR4. The impact of these mutations on viral pathogenesis and disease progression was investigated by using two panels of HIV-1-based chimeric pseudoviruses [117].
The use of pseudoviral particles enables the study of the biological characteristics and target cell penetration rates of novel animal viruses with unknown pathogenesis [118,119]. The current focus is on bat SARS-like coronaviruses, which are considered a potential candidate for the next spillover of coronaviruses. The use of pseudotyped viruses allows for the characterization of the interaction of the virus of interest with target cell receptors from different species and its capabilities to infect target cell lines, including human cells [120,121]. Recombinant VSV particles were pseudotyped with a protein derived from the porcine epidemic diarrhoea virus (PEDV) in order to study the process of cellular entry of PEDV and evaluate the potential of viral inhibitors [122].
In order to conduct preclinical trials of pharmacological agents against highly pathogenic viruses such as HIV, Ebola, hepatitis C and others, pseudotyped virus systems were developed [123,124,125,126]. A motif on the hepatitis C virus envelope protein 2 was identified as the agent responsible for suppressing T cell activation [127]. Hu et al. developed a lentiviral pseudovirus-based entry assay utilizing particles bearing the fusion protein of either type A or B respiratory syncytial virus [128]. The pharmacological activity of the promising inhibitors of Lassa virus was evaluated using HIV-1-based pseudotyped viruses [129]. Liang J. et al. employed HIV-1 Env pseudoviruses, generated through co-transfection of a HIV-1 rev/env expression plasmid and an env-deficient HIV-1 backbone plasmid, to investigate and characterize three new trispecific antibodies using a neutralization assay to evaluate their potency against HIV-1 [130]. Luo et al. developed an HIV-based pseudoviral neutralization assay for Nipah virus. The particles were constructed to bear the fusion protein and glycoprotein of the virus of interest [131]. The particles pseudotyped by F or G glycoproteins of Nipah virus, which are involved in the processes of cell attachment and fusion, were employed to validate the role of specific host proteins in the entry process [132].
Furthermore, the detection of nAbs against crucial viral proteins is employed to assess the efficacy of vaccines and to inform the development of post-infection immunity. Xu et al. quantified the levels of polyclonal nAbs present in the serum of individuals who recovered from severe fever with thrombocytopenia syndrome bunya-virus infection using a VSV-based pseudovirus neutralization assay. This local epidemiological study made a significant contribution to the identification of endemic strains and estimation of cross-nAbs levels among the samples [133]. Huttner et al. presented the findings of the study on the long-term clinical efficacy of vaccine rVSV-ZEBOV, a live-attenuated recombinant VSV expressing the Zaire ebolavirus glycoprotein. The series of laboratory tests conducted on the collected serum samples included a VSV-based pseudovirus neutralization assay targeting the Ebola Zaire 95 glycoprotein and the Ebola Zaire Guinea 2014 glycoprotein [134]. In a separate study, Bi et al. demonstrated that three recombinant rabies virus-vectored vaccines encoding glycoproteins of Marburg virus lineages were capable of inducing nAbs in an animal model. The neutralizing activity of mouse sera was measured following vaccination using lentiviral-based pseudoviruses carrying a luciferase reporter gene [135].
5. Conclusions and Future Outlook
The integration of PVP technology into this field presents many advantages, including the capacity to examine highly contagious enveloped viruses under biosafety level 2 conditions. The evolution of this technology has enabled the development of a method for producing recombinant viral particles that are deficient in their core and envelope proteins. This technological development enabled researchers to circumvent mutations in the viral particles, ensuring they would not revert to their wild-type state. The directed infection of these particles to specific target cells has also been facilitated, thereby allowing for precise manipulation of the infection process. A notable property of these particles is their capacity for replication, which is limited to a single round. This significantly expands the number of research groups that can participate in such studies globally, as some regions and countries have limited access to BSL-3 and BSL-4 facilities. Consequently, the biology of new emerging viruses can be understood within a relatively short timeframe. It was noteworthy at the outset of the global pandemic caused by the SARS-CoV-2 virus that the scientific community was able to rapidly gain insights into the viral life cycle and its evolution by employing molecular biology, genetics, and PVP technology. The long-term study of viral infections and pandemics provides insights into viral variability, the formation of virus serotypes, and the possibility of different viral variants circulating simultaneously. PVP-based assays have become a valuable tool for assessing population immunity and screening for different viral inhibitors against these viral variants, offering an alternative to live virus-based assays. Moreover, the high-throughput assays are of great importance for the rapid and robust evaluation of immunological parameters among healthy individuals and patients across the globe, as well as a valuable tool for screening viral inhibitors [136].
While the expanded use of the pseudoviral system offers significant benefits to the scientific community, it also presents a number of limitations. PVPs can only be constructed for enveloped viruses, which excludes such epidemiologically significant viruses as rotavirus and poliovirus. Furthermore, the distribution of surface proteins on viruses is not always accurately reflected due to the diverse shapes that viruses can adopt, including spherical, bullet-like, and other forms. Compared to live viruses, PVPs facilitate simulation of the entry process, but do not accurately reflect intracellular processes such as proliferation and the release of newly synthesized viral particles. Furthermore, the calculation of the titers for pseudoviruses remains challenging due to the use of disparate methodologies across different laboratories. For instance, p24 antigen quantification by ELISA or the detection of a specific number of pseudoviral genomes in a defined volume of solution by reverse transcription fluorescence quantitative PCR are two such methods. In the event when a comparison of the results is required, it is recommended to validate the data using the universal standards, namely live virus-based assays [137]. The development of PVP assays for the study of neutralizing immunity against viruses and the screening of potential viral inhibitors has been conducted by different research groups using a variety of protocols and packaging vectors. Several studies have demonstrated that PVP-based assays utilizing different packaging vectors and live virus-based assays exhibit a high degree of correlation with one another [138,139]. However, there is a paucity of studies that directly compare live virus-based and PVP-based assays.
Notwithstanding the aforementioned disadvantages, pseudovirus-based assays are anticipated to emerge as a highly effective and convenient immunological tool with widespread applications in the future.
Author Contributions
Writing—original draft preparation, S.N.R. and A.E.E.; writing—review and editing, I.V.A.; visualization, S.N.R.; supervision, I.V.A.; funding acquisition, I.V.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Russian Science Foundation, grant number 24-25-20139.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
This review did not report any new data.
Acknowledgments
Authors would like to extend their gratitude to Nedospasov S.A. for his valuable comments and to Bulekova E. for her editorial assistance in refining the text.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Rajan, A.; Piedra, F.A.; Aideyan, L.; McBride, T.; Robertson, M.; Johnson, H.L.; Aloisio, G.M.; Henke, D.; Coarfa, C.; Stossi, F.; et al. Multiple Respiratory Syncytial Virus (RSV) Strains Infecting HEp-2 and A549 Cells Reveal Cell Line-Dependent Differences in Resistance to RSV Infection. J. Virol. 2022, 96, e01904-21. [Google Scholar] [CrossRef] [PubMed]
- Gschwender, H.H.; Brummund, M.; Lehmann-Grube, F. Lymphocytic Choriomeningitis virus. I. Concentration and purification of the infectious virus. J. Virol. 1975, 15, 1317–1322. [Google Scholar] [CrossRef]
- Keep, S.; Stevenson-Leggett, P.; Webb, I.; Fones, A.; Kirk, J.; Britton, P.; Bickerton, E. The spike protein of the apathogenic Beaudette strain of avian coronavirus can elicit a protective immune response against a virulent M41 challenge. PLOS ONE 2024, 19, e0297516. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Ying, Z.; Cai, W.; Wang, J.; Zhou, J.; Yang, H.; Gao, J.; Zhao, Z.; Liu, J.; Ouyang, S.; et al. Immune persistence of an inactivated poliovirus vaccine derived from the Sabin strain: A 10-year follow-up of a phase 3 study. eClinicalMedicine 2023, 64, 102151. [Google Scholar] [CrossRef]
- Ziyaeifar, F.; Soleimani, S. Characterization of the BHK-21 C5 cell line, and determination of cellular sensitivity to rubella virus compared to the routine cell RK13. Arch. Razi Inst. 2020, 76, 461–469. [Google Scholar] [CrossRef]
- Caposio, P.; Van Den Worm, S.; Crawford, L.; Perez, W.; Kreklywich, C.; Gilbride, R.M.; Hughes, C.M.; Ventura, A.B.; Ratts, R.; Marshall, E.E.; et al. Characterization of a live-attenuated HCMV-based vaccine platform. Sci. Rep. 2019, 9, 19236. [Google Scholar] [CrossRef] [PubMed]
- Knudson, D.L.; Tinsley, T.W. Replication of a Nuclear Polyhedrosis Virus in a Continuous Cell Culture of Spodoptera frugiperda: Purification, Assay of Infectivity, and Growth Characteristics of the Virus. J. Virol. 1974, 14, 934–944. [Google Scholar] [CrossRef] [PubMed]
- Juarez, D.; Long, K.C.; Aguilar, P.; Kochel, T.J.; Halsey, E.S. Assessment of plaque assay methods for alphaviruses. J. Virol. Methods 2013, 187, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Jo, E.; Kim, H.; König, A.; Yang, J.; Yoon, S.K.; Windisch, M.P. Determination of infectious hepatitis B virus particles by an end-point dilution assay identifies a novel class of inhibitors. Antivir. Res. 2021, 196, 105195. [Google Scholar] [CrossRef] [PubMed]
- Rubin, H. An analysis of the apparent neutralization of rous sarcoma virus with antiserum to normal chick tissues. Virology 1956, 2, 545–558. [Google Scholar] [CrossRef] [PubMed]
- Durand, D.P. Virus Neutralization Test in a Capillary Tube. Science 1962, 138, 448. [Google Scholar] [CrossRef] [PubMed]
- Tselis, A.C.; Booss, J. Neurovirology; Elsevier: Edinburgh, Scotland, 2014. [Google Scholar]
- Bewley, K.R.; Coombes, N.S.; Gagnon, L.; McInroy, L.; Baker, N.; Shaik, I.; St-Jean, J.R.; St-Amant, N.; Buttigieg, K.R.; Humphries, H.E.; et al. Quantification of SARS-CoV-2 neutralizing antibody by wild-type plaque reduction neutralization, microneutralization and pseudotyped virus neutralization assays. Nat. Protoc. 2021, 16, 3114–3140. [Google Scholar] [CrossRef] [PubMed]
- Roehrig, J.T.; Hombach, J.; Barrett, A.D.T. Guidelines for Plaque-Reduction Neutralization Testing of Human Antibodies to Dengue Viruses. Viral Immunol. 2008, 21, 123–132. [Google Scholar] [CrossRef]
- Vanderheiden, A.; Edara, V.V.; Floyd, K.; Kauffman, R.C.; Mantus, G.; Anderson, E.; Rouphael, N.; Edupuganti, S.; Shi, P.Y.; Menachery, V.D.; et al. Development of a Rapid Focus Reduction Neutralization Test Assay for Measuring SARS-CoV-2 Neutralizing Antibodies. Curr. Protoc. Immunol. 2020, 131, e116. [Google Scholar] [CrossRef]
- Artika, I.M.; Ma’roef, C.N. Laboratory biosafety for handling emerging viruses. Asian Pac. J. Trop. Biomed. 2017, 7, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Chung, W.-C.; Hwang, K.Y.; Kang, S.-J.; Kim, J.-O.; Song, M.J. Development of a neutralization assay based on the pseudotyped chikungunya virus of a Korean isolate. J. Microbiol. 2019, 58, 46–53. [Google Scholar] [CrossRef]
- Teramichi, T.; Fukushi, S.; Hachiya, Y.; Melaku, S.K.; Oguma, K.; Sentsui, H. Evaluation of serological assays available in a biosafety level 2 laboratory and their application for survey of Middle East respiratory syndrome coronavirus among livestock in Ethiopia. J. Veter- Med Sci. 2019, 81, 1887–1891. [Google Scholar] [CrossRef] [PubMed]
- Cantoni, D.; Mayora-Neto, M.; Temperton, N. The role of pseudotype neutralization assays in understanding SARS CoV-2. Oxf. Open Immunol. 2021, 2, iqab005. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.B.; Lee, M.K.; Han, D.P.; Cho, M.W. Development of a Safe and Rapid Neutralization Assay Using Murine Leukemia Virus Pseudotyped with HIV Type 1 Envelope Glycoprotein Lacking the Cytoplasmic Domain. AIDS Res. Hum. Retroviruses 2001, 17, 1715–1724. [Google Scholar] [CrossRef]
- Wei, C.M.; Gibson, M.; Spear, P.G.; Scolnick, E.M. Construction and isolation of a transmissible retrovirus containing the src gene of Harvey murine sarcoma virus and the thymidine kinase gene of herpes simplex virus type 1. J. Virol. 1981, 39, 935–944. [Google Scholar] [CrossRef] [PubMed]
- Rigby, P.W.J. Cloning Vectors Derived from Animal Viruses. J. Gen. Virol. 1983, 64, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Li, W.; Feng, J.; Da Silva, S.R.; Ju, E.; Zhang, H.; Chang, Y.; Moore, P.S.; Guo, H.; Gao, S. SARS-CoV-2 pseudovirus infectivity and expression of viral entry-related factors ACE2, TMPRSS2, Kim-1, and NRP-1 in human cells from the respiratory, urinary, digestive, reproductive, and immune systems. J. Med Virol. 2021, 93, 6671–6685. [Google Scholar] [CrossRef] [PubMed]
- Khatri, R.; Lohiya, B.; Kaur, G.; Maithil, V.; Goswami, A.; Sarmadhikari, D.; Asthana, S.; Samal, S. Understanding the role of conserved proline and serine residues in the SARS-CoV-2 spike cleavage sites in the virus entry, fusion, and infectivity. 3 Biotech 2023, 13, 323. [Google Scholar] [CrossRef] [PubMed]
- Guenthoer, J.; Lilly, M.; Starr, T.N.; Dadonaite, B.; Lovendahl, K.N.; Croft, J.T.; Stoddard, C.I.; Chohan, V.; Ding, S.; Ruiz, F.; et al. Identification of broad, potent antibodies to functionally constrained regions of SARS-CoV-2 spike following a breakthrough infection. Proc. Natl. Acad. Sci. USA 2023, 120, e2220948120. [Google Scholar] [CrossRef]
- Chen, S.; Haam, J.; Walker, M.; Scappini, E.; Naughton, J.; Martin, N.P. Recombinant Viral Vectors as Neuroscience Tools. Curr. Protoc. Neurosci. 2019, 87, e67. [Google Scholar] [CrossRef]
- Cockrell, A.S.; Kafri, T. Gene delivery by lentivirus vectors. Mol. Biotechnol. 2007, 36, 184–204. [Google Scholar] [CrossRef]
- Naldini, L.; Blömer, U.; Gallay, P.; Ory, D.; Mulligan, R.; Gage, F.H.; Verma, I.M.; Trono, D. In Vivo Gene Delivery and Stable Transduction of Nondividing Cells by a Lentiviral Vector. Science 1996, 272, 263–267. [Google Scholar] [CrossRef]
- Miyauchi, K.; Kim, Y.; Latinovic, O.; Morozov, V.; Melikyan, G.B. HIV Enters Cells via Endocytosis and Dynamin-Dependent Fusion with Endosomes. Cell 2009, 137, 433–444. [Google Scholar] [CrossRef]
- German Advisory Committee Blood (Arbeitskreis Blut). Subgroup ‘Assessment of Pathogens Transmissible by Blood’, Human Immunodeficiency Virus (HIV). Transfus. Med. Hemotherapy 2016, 43, 203–222. [Google Scholar] [CrossRef]
- Bleul, C.C.; Wu, L.; Hoxie, J.A.; Springer, T.A.; Mackay, C.R. The HIV coreceptors CXCR4 and CCR5 are differentially expressed and regulated on human T lymphocytes. Proc. Natl. Acad. Sci. USA 1997, 94, 1925–1930. [Google Scholar] [CrossRef] [PubMed]
- Naldini, L. Lentiviruses as gene transfer agents for delivery to non-dividing cells. Curr. Opin. Biotechnol. 1998, 9, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Dull, T.; Zufferey, R.; Kelly, M.; Mandel, R.J.; Nguyen, M.; Trono, D.; Naldini, L. A third-generation lentivirus vector with a conditional packaging system. J. Virol. 1998, 72, 8463–8471. [Google Scholar] [CrossRef] [PubMed]
- Ortinski, P.I.; O’Donovan, B.; Dong, X.; Kantor, B. Integrase-Deficient Lentiviral Vector as an All-in-One Platform for Highly Efficient CRISPR/Cas9-Mediated Gene Editing. Mol. Ther. Methods Clin. Dev. 2017, 5, 153–164. [Google Scholar] [CrossRef]
- Bona, R.; Michelini, Z.; Mazzei, C.; Gallinaro, A.; Canitano, A.; Borghi, M.; Vescio, M.F.; Di Virgilio, A.; Pirillo, M.F.; Klotman, M.E.; et al. Safety and efficiency modifications of SIV-based integrase-defective lentiviral vectors for immunization. Mol. Ther. Methods Clin. Dev. 2021, 23, 263–275. [Google Scholar] [CrossRef]
- Grinshpun, A.; Condiotti, R.; Waddington, S.N.; Peer, M.; Zeig, E.; Peretz, S.; Simerzin, A.; Chou, J.; Pann, C.-J.; Giladi, H.; et al. Neonatal Gene Therapy of Glycogen Storage Disease Type Ia Using a Feline Immunodeficiency Virus–based Vector. Mol. Ther. 2010, 18, 1592–1598. [Google Scholar] [CrossRef]
- Bulcha, J.T.; Wang, Y.; Ma, H.; Tai, P.W.L.; Gao, G. Viral vector platforms within the gene therapy landscape. Signal Transduct. Target. Ther. 2021, 6, 53. [Google Scholar] [CrossRef] [PubMed]
- Russell, T.M.; Santo, E.E.; Golebiewski, L.; Haseley, N.S.; Ferran, M.C. Near-Complete Genome Sequences of Vesicular Stomatitis Virus Indiana Laboratory Strains HR and T1026R1 and Plaque Isolates 22-20 and 22-25. Genome Announc. 2019, 8, e00012-19. [Google Scholar] [CrossRef]
- Cureton, D.K.; Massol, R.H.; Whelan, S.P.J.; Kirchhausen, T. The Length of Vesicular Stomatitis Virus Particles Dictates a Need for Actin Assembly during Clathrin-Dependent Endocytosis. PLOS Pathog. 2010, 6, e1001127. [Google Scholar] [CrossRef]
- Nikolic, J.; Belot, L.; Raux, H.; Legrand, P.; Gaudin, Y.; Albertini, A.A. Structural basis for the recognition of LDL-receptor family members by VSV glycoprotein. Nat. Commun. 2018, 9, 1029. [Google Scholar] [CrossRef]
- Barr, J.N.; Whelan, S.P.J.; Wertz, G.W. Transcriptional control of the RNA-dependent RNA polymerase of vesicular stomatitis virus. Biochim. et Biophys. Acta (BBA) Gene Struct. Expr. 2002, 1577, 337–353. [Google Scholar] [CrossRef]
- Lyles, D.S. Assembly and Budding of Negative-Strand RNA Viruses. In Advances in Virus Research; Elsevier: Amsterdam, The Netherlands, 2013; pp. 57–90. [Google Scholar] [CrossRef]
- Závada, J.; Rosenbergová, M. Phenotypic mixing of vesicular stomatitis virus with fowl plague virus. Acta Virol. 1972, 16, 103–114. [Google Scholar] [PubMed]
- Jayakar, H.R.; Jeetendra, E.; Whitt, M.A. Rhabdovirus assembly and budding. Virus Res. 2004, 106, 117–132. [Google Scholar] [CrossRef] [PubMed]
- Schnell, M.J.; Mebatsion, T.; Conzelmann, K.K. Infectious rabies viruses from cloned cDNA. EMBO J. 1994, 13, 4195–4203. [Google Scholar] [CrossRef]
- Lawson, N.D.; Stillman, E.A.; Whitt, M.A.; Rose, J.K. Recombinant vesicular stomatitis viruses from DNA. Proc. Natl. Acad. Sci. USA 1995, 92, 4477–4481. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, M.E.; Munir, M. Protocol for constructing and characterizing recombinant vectored vaccines for rabies virus. STAR Protoc. 2024, 5, 103392. [Google Scholar] [CrossRef] [PubMed]
- Yahalom-Ronen, Y.; Tamir, H.; Melamed, S.; Politi, B.; Shifman, O.; Achdout, H.; Vitner, E.B.; Israeli, O.; Milrot, E.; Stein, D.; et al. A single dose of recombinant VSV-∆G-spike vaccine provides protection against SARS-CoV-2 challenge. Nat. Commun. 2020, 11, 6402. [Google Scholar] [CrossRef] [PubMed]
- Schnell, M.J.; Buonocore, L.; Kretzschmar, E.; Johnson, E.; Rose, J.K. Foreign glycoproteins expressed from recombinant vesicular stomatitis viruses are incorporated efficiently into virus particles. Proc. Natl. Acad. Sci. USA 1996, 93, 11359–11365. [Google Scholar] [CrossRef]
- Rehman, S.; Bishnoi, S.; Roy, R.; Kumari, A.; Jayakumar, H.; Gupta, S.; Kar, P.; Pattnaik, A.K.; Nayak, D. Emerging Biomedical Applications of the Vesicular Stomatitis Virus Glycoprotein. ACS Omega 2022, 7, 32840–32848. [Google Scholar] [CrossRef] [PubMed]
- Tani, H.; Morikawa, S.; Matsuura, Y. Development and Applications of VSV Vectors Based on Cell Tropism. Front. Microbiol. 2012, 2, 272. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef]
- Fujioka, Y.; Kashiwagi, S.; Yoshida, A.; Satoh, A.O.; Fujioka, M.; Amano, M.; Yamauchi, Y.; Ohba, Y. A method for the generation of pseudovirus particles bearing SARS coronavirus spike protein in high yields. Cell Struct. Funct. 2022, 47, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Maetzig, T.; Galla, M.; Baum, C.; Schambach, A. Gammaretroviral Vectors: Biology, Technology and Application. Viruses 2011, 3, 677–713. [Google Scholar] [CrossRef]
- Rein, A. Murine Leukemia Viruses: Objects and Organisms. Adv. Virol. 2011, 2011, 1–14. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, V.; Melamed, J.; Habib, D.; Pullen, K.; Wallace, K.; Summers, M.F. Identification of a high affinity nucleocapsid protein binding element within the moloney murine leukemia virus Ψ-RNA packaging signal: Implications for genome recognition. J. Mol. Biol. 2001, 314, 217–232. [Google Scholar] [CrossRef] [PubMed]
- Vu, H.N.; Ramsey, J.D.; Pack, D.W. Engineering of a Stable Retroviral Gene Delivery Vector by Directed Evolution. Mol. Ther. 2008, 16, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Ciuculescu, M.F.; Brendel, C.; Harris, C.E.; Williams, D.A. Retroviral Transduction of Murine and Human Hematopoietic Progenitors and Stem Cells. In Hematopoietic Stem Cell Protocols; Bunting, K.D., Qu, C.-K., Eds.; Springer: New York, NY, USA, 2014; pp. 287–309. [Google Scholar] [CrossRef]
- Soneoka, Y.; Cannon, P.M.; Ramsdale, E.E.; Griffiths, J.C.; Romano, G.; Kingsman, S.M.; Kingsman, A.J. A transient three-plasmid expression system for the production of high titer retroviral vectors. Nucleic Acids Res. 1995, 23, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Panda, B.R.; Kingsman, S.M.; Kingsman, A.J. Mutational Analysis of the Putative Receptor-Binding Domain of Moloney Murine Leukemia Virus Glycoprotein gp70. Virology 2000, 273, 90–100. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Morgan, M.A.; Galla, M.; Grez, M.; Fehse, B.; Schambach, A. Retroviral gene therapy in Germany with a view on previous experience and future perspectives. Gene Ther. 2021, 28, 494–512. [Google Scholar] [CrossRef] [PubMed]
- Millet, J.; Whittaker, G. Murine Leukemia Virus (MLV)-based Coronavirus Spike-pseudotyped Particle Production and Infection. Bio-Protocol 2016, 6, e2035. [Google Scholar] [CrossRef]
- Van Heuvel, Y.; Schatz, S.; Hein, M.; Dogra, T.; Kazenmaier, D.; Tschorn, N.; Genzel, Y.; Stitz, J. Novel suspension retroviral packaging cells generated by transposition using transposase encoding mRNA advance vector yields and enable production in bioreactors. Front. Bioeng. Biotechnol. 2023, 11, 1076524. [Google Scholar] [CrossRef]
- Nie, Y.; Wang, P.; Shi, X.; Wang, G.; Chen, J.; Zheng, A.; Wang, W.; Wang, Z.; Qu, X.; Luo, M.; et al. Highly infectious SARS-CoV pseudotyped virus reveals the cell tropism and its correlation with receptor expression. Biochem. Biophys. Res. Commun. 2004, 321, 994–1000. [Google Scholar] [CrossRef]
- Yang, X.; Zheng, X.; Zhu, Y.; Zhao, X.; Liu, J.; Xun, J.; Yuan, S.; Chen, J.; Pan, H.; Yang, J.; et al. Asialoglycoprotein receptor 1 promotes SARS-CoV-2 infection of human normal hepatocytes. Signal Transduct. Target. Ther. 2024, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Elste, J.; Cast, N.; Udawatte, S.; Adhikari, K.; Payen, S.H.; Verma, S.C.; Shukla, D.; Swanson-Mungerson, M.; Tiwari, V. Co-Expression of Niemann-Pick Type C1-Like1 (NPC1L1) with ACE2 Receptor Synergistically Enhances SARS-CoV-2 Entry and Fusion. Biomedicines 2024, 12, 821. [Google Scholar] [CrossRef] [PubMed]
- Elste, J.; Cast, N.; Udawatte, S.; Adhikari, K.; Payen, S.H.; Verma, S.C.; Shukla, D.; Swanson-Mungerson, M.; Tiwari, V. Wnt3a Facilitates SARS-CoV-2 Pseudovirus Entry into Cells. Int. J. Mol. Sci. 2023, 25, 127. [Google Scholar] [CrossRef]
- Pellegrini, L.; Albecka, A.; Mallery, D.L.; Kellner, M.J.; Paul, D.; Carter, A.P.; James, L.C.; Lancaster, M.A. SARS-CoV-2 Infects the Brain Choroid Plexus and Disrupts the Blood-CSF Barrier in Human Brain Organoids. Cell Stem Cell 2020, 27, 951–961.e5. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Lv, Y.; Moser, D.; Zhou, X.; Woehrle, T.; Han, L.; Osterman, A.; Rudelius, M.; Choukér, A.; Lei, P. ACE2-independent SARS-CoV-2 virus entry through cell surface GRP78 on monocytes – evidence from a translational clinical and experimental approach. EBioMedicine 2023, 98, 104869. [Google Scholar] [CrossRef] [PubMed]
- Sengar, A.; Cervantes, M.; Bondalapati, S.T.; Hess, T.; Kasson, P.M. Single-Virus Fusion Measurements Reveal Multiple Mechanistically Equivalent Pathways for SARS-CoV-2 Entry. J. Virol. 2023, 97, e01992-22. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, Y.; Yang, C.; Ren, W.; Qiu, C.; Fan, S.; Ding, Q.; Lan, J. Receptor binding mechanism and immune evasion capacity of SARS-CoV-2 BQ.1.1 lineage. Virology 2024, 600, 110241. [Google Scholar] [CrossRef]
- Zhang, L.; Dopfer-Jablonka, A.; Cossmann, A.; Stankov, M.V.; Graichen, L.; Moldenhauer, A.-S.; Fichter, C.; Aggarwal, A.; Turville, S.G.; Behrens, G.M.N.; et al. Rapid spread of the SARS-CoV-2 JN.1 lineage is associated with increased neutralization evasion. iScience 2024, 27, 109904. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Liu, Y.; Wang, J.; Long, T.; Mok, B.W.Y.; Huang, Y.; Peng, Z.; Jia, Q.; Liu, C.; So, P.-K.; et al. Identifications of novel host cell factors that interact with the receptor-binding domain of the SARS-CoV-2 spike protein. J. Biol. Chem. 2024, 300, 107390. [Google Scholar] [CrossRef]
- Zhang, D.Q.; Ma, Q.H.; Yang, M.C.; Belyakova, Y.Y.; Yang, Z.F.; Radulov, P.S.; Chen, R.H.; Yang, L.J.; Wei, J.Y.; Peng, Y.T.; et al. Peroxide derivatives as SARS-CoV-2 entry inhibitors. Virus Res. 2023, 340, 199295. [Google Scholar] [CrossRef] [PubMed]
- Romeo, A.; Cappelli, G.; Iacovelli, F.; Colizzi, V.; Falconi, M. Computational and experimental validation of phthalocyanine and hypericin as effective SARS-CoV-2 fusion inhibitors. J. Biomol. Struct. Dyn. 2023, 42, 3920–3934. [Google Scholar] [CrossRef] [PubMed]
- Palla, S.R.; Li, C.-W.; Chao, T.-L.; Lo, H.-L.V.; Liu, J.-J.; Pan, M.Y.-C.; Chiu, Y.-T.; Lin, W.-C.; Hu, C.-W.; Yang, C.-M.; et al. Synthesis, evaluation, and mechanism of 1-(4-(arylethylenylcarbonyl)phenyl)-4-carboxy-2-pyrrolidinones as potent reversible SARS-CoV-2 entry inhibitors. Antivir. Res. 2023, 219, 105735. [Google Scholar] [CrossRef]
- Kazakova, O.; Ma, X.; Tretyakova, E.; Smirnova, I.; Slita, A.; Sinegubova, E.; Zarubaev, V.; Jin, H.; Zhou, D.; Xiao, S. Evaluation of A-ring hydroxymethylene-amino- triterpenoids as inhibitors of SARS-CoV-2 spike pseudovirus and influenza H1N1. J. Antibiot. 2023, 77, 39–49. [Google Scholar] [CrossRef]
- Mei, S.; Zou, Y.; Jiang, S.; Xue, L.; Wang, Y.; Jing, H.; Yang, P.; Niu, M.-M.; Li, J.; Yuan, K.; et al. Highly potent dual-targeting angiotensin-converting enzyme 2 (ACE2) and Neuropilin-1 (NRP1) peptides: A promising broad-spectrum therapeutic strategy against SARS-CoV-2 infection. Eur. J. Med. Chem. 2023, 263, 115908. [Google Scholar] [CrossRef]
- Razi, N.; Li, W.; Ignacio, M.A.; Loube, J.M.; Agostino, E.L.; Zhu, X.; Scull, M.A.; DeStefano, J.J. Inhibition of SARS-CoV-2 infection in human airway epithelium with a xeno-nucleic acid aptamer. Respir. Res. 2023, 24, 272. [Google Scholar] [CrossRef]
- Cervantes, M.; Hess, T.; Morbioli, G.G.; Sengar, A.; Kasson, P.M. The ACE2 receptor accelerates but is not biochemically required for SARS-CoV-2 membrane fusion. Chem. Sci. 2023, 14, 6997–7004. [Google Scholar] [CrossRef] [PubMed]
- Curreli, F.; Victor, S.M.B.; Ahmed, S.; Drelich, A.; Tong, X.; Tseng, C.-T.K.; Hillyer, C.D.; Debnath, A.K. Stapled Peptides Based on Human Angiotensin-Converting Enzyme 2 (ACE2) Potently Inhibit SARS-CoV-2 Infection In Vitro. mBio 2020, 11, e02451-20. [Google Scholar] [CrossRef] [PubMed]
- Astrakhantseva, I.V.; Ershova, A.E.; Chuvpilo, S.A.; Kruglova, N.A.; Ishmukhametov, A.A.; Drutskaya, M.S.; Kozlovskaya, L.I.; Nedospasov, S.A. SARS-CoV-2 Binding and Neutralization Properties of Peptides Derived from N-Terminus of Human ACE2. Int. J. Mol. Sci. 2023, 24, 8269. [Google Scholar] [CrossRef]
- Huang, Y.; Luo, R.; Tian, C.; Zu, D.; Yang, J.; Chen, W.; Huang, D.; Duan, S.; Yan, S.; Yuan, Y.; et al. Dual Assay Validation of Rosmarinus officinalis Extract as an Inhibitor of SARS-CoV-2 Spike Protein: Combining Pseudovirus Testing, Yeast Two-Hybrid, and UPLC-Q Exactive Orbitrap-MS Profiling. Phytochem. Anal. 2024. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Q.; Chen, X.; Li, B.; Zhang, Z.; Yao, L.; Liu, X.; Zhang, R. A Natural Bioactive Peptide from Pinctada fucata Pearls Can Be Used as a Potential Inhibitor of the Interaction between SARS-CoV-2 and ACE2 against COVID-19. Int. J. Mol. Sci. 2024, 25, 7902. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, F.; Wu, F.; Su, X.; Zhang, L.; Zhao, X.; Shang, C.; Han, L.; Zhang, Y.; Xiao, Z.; et al. Inhibition of multiple SARS-CoV-2 variants entry by Lycium barbarum L. polysaccharides through disruption of spike protein-ACE2 interaction. Int. J. Biol. Macromol. 2024, 261, 129785. [Google Scholar] [CrossRef]
- Maen, A.; Yavuz, B.G.; Mohamed, Y.I.; Esmail, A.; Lu, J.; Mohamed, A.; Azmi, A.S.; Kaseb, M.; Kasseb, O.; Li, D.; et al. Individual ingredients of NP-101 (Thymoquinone formula) inhibit SARS-CoV-2 pseudovirus infection. Front. Pharmacol. 2024, 15, 1291212. [Google Scholar] [CrossRef] [PubMed]
- Cao, N.; Cai, Y.; Huang, X.; Jiang, H.; Huang, Z.; Xing, L.; Lu, L.; Jiang, S.; Xu, W. Inhibition of influenza A virus and SARS-CoV-2 infection or co-infection by griffithsin and griffithsin-based bivalent entry inhibitor. mBio 2024, 15, e00741-24. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhou, J.; Zhang, X.; Ling, D.; Sun, Y.; Li, C.; Zhou, Q.; Shi, G.; Wang, S.; Lin, X.; et al. Nanoengineered Red Blood Cells Loaded with TMPRSS2 and Cathepsin L Inhibitors Block SARS-CoV-2 Pseudovirus Entry into Lung ACE2+ Cells. Adv. Mater. 2024, 36, 2310306. [Google Scholar] [CrossRef]
- Wang, R.; Guo, J.; Lu, J.; Du, P.; Zhang, J.; Yu, Y.; Chen, L.; Xiong, Z.; Xiang, Y.; Ni, X.; et al. A potential broad-spectrum neutralizing antibody against Betacoronavirus. J. Med Virol. 2023, 95, e29252. [Google Scholar] [CrossRef] [PubMed]
- Ko, H.L.; Lee, D.; Kim, Y.; Jang, H.J.; Lee, Y.W.; Lee, H.-Y.; Seok, S.-H.; Park, J.W.; Limb, J.-K.; On, D.I.; et al. Development of a neutralization monoclonal antibody with a broad neutralizing effect against SARS-CoV-2 variants. Virol. J. 2023, 20, 285. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Jiao, J.; Zhou, Y.; Qi, J.; Zhu, S.; Xu, J.; Nie, L.; Wang, H. A novel monospecific tetravalent IgG1-(scFv)2 version shown enhanced neutralizing and Fc-mediated effector functions against SARS-CoV-2. 3 Biotech 2023, 13, 283. [Google Scholar] [CrossRef]
- Cai, Y.; Diallo, S.; Rosenthal, K.; Ren, K.; Flores, D.J.; Dippel, A.; Oganesyan, V.; Van Dyk, N.; Chen, X.; Cantu, E.; et al. AZD3152 neutralizes SARS-CoV-2 historical and contemporary variants and is protective in hamsters and well tolerated in adults. Sci. Transl. Med. 2024, 16, eado2817. [Google Scholar] [CrossRef]
- Pavan, M.; Bok, M.; Juan, R.B.S.; Malito, J.; Marcoppido, G.; Franco, D.; Militello, D.; Schammas, J.; Bari, S.; Stone, W.; et al. SARS-CoV-2 Specific Nanobodies Neutralize Different Variants of Concern and Reduce Virus Load in the Brain of h-ACE2 Transgenic Mice. Viruses 2024, 16, 185. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Kalkeri, R.; Wang, M.; Haner, B.; Dent, D.; Osman, B.; Skonieczny, P.; Ross, J.; Feng, S.-L.; Cai, R.; et al. Validation of a Pseudovirus Neutralization Assay for Severe Acute Respiratory Syndrome Coronavirus 2: A High-Throughput Method for the Evaluation of Vaccine Immunogenicity. Microorganisms 2024, 12, 1201. [Google Scholar] [CrossRef]
- Manu, A.A.; Owusu, I.A.; Oyawoye, F.O.; Languon, S.; Barikisu, I.A.; Tawiah-Eshun, S.; Quaye, O.; Donkor, K.J.; Paemka, L.; Amegatcher, G.A.; et al. Development and utility of a SARS-CoV-2 pseudovirus assay for compound screening and antibody neutralization assays. Heliyon 2024, 10, e31392. [Google Scholar] [CrossRef] [PubMed]
- Kruglova, N.; Siniavin, A.; Gushchin, V.; Mazurov, D. Different Neutralization Sensitivity of SARS-CoV-2 Cell-to-Cell and Cell-Free Modes of Infection to Convalescent Sera. Viruses 2021, 13, 1133. [Google Scholar] [CrossRef]
- Garay; Whelan, S.P.J.; DuBois, R.M.; O’Rourke, S.M.; Salgado-Escobar, A.E.; Muñoz-Medina, J.E.; Arias, C.F.; López, S. Immune response to SARS-CoV-2 variants after immunization with different vaccines in Mexico. Epidemiology Infect. 2024, 152, e30. [Google Scholar] [CrossRef]
- Radion, E.I.; Mukhin, V.E.; Kholodova, A.V.; Vladimirov, I.S.; Alsaeva, D.Y.; Zhdanova, A.S.; Ulasova, N.Y.; Bulanova, N.V.; Makarov, V.V.; Keskinov, A.A.; et al. Functional Characteristics of Serum Anti-SARS-CoV-2 Antibodies against Delta and Omicron Variants after Vaccination with Sputnik V. Viruses 2023, 15, 1349. [Google Scholar] [CrossRef]
- Ikegame, S.; Siddiquey, M.N.A.; Hung, C.-T.; Haas, G.; Brambilla, L.; Oguntuyo, K.Y.; Kowdle, S.; Chiu, H.-P.; Stevens, C.S.; Vilardo, A.E.; et al. Neutralizing activity of Sputnik V vaccine sera against SARS-CoV-2 variants. Nat. Commun. 2021, 12, 4598. [Google Scholar] [CrossRef]
- Fazli, S.; Thomas, A.; Estrada, A.E.; Ross, H.A.P.; Lee, D.X.; Kazmierczak, S.; Slifka, M.K.; Montefiori, D.; Messer, W.B.; Curlin, M.E. Contralateral second dose improves antibody responses to a 2-dose mRNA vaccination regimen. J. Clin. Investig. 2024, 134, e176411. [Google Scholar] [CrossRef] [PubMed]
- Muangnoicharoen, S.; Wiangcharoen, R.; Lawpoolsri, S.; Nanthapisal, S.; Jongkaewwattana, A.; Duangdee, C.; Kamolratanakul, S.; Luvira, V.; Thanthamnu, N.; Chantratita, N.; et al. Heterologous Ad26.COV2.S booster after primary BBIBP-CorV vaccination against SARS-CoV-2 infection: 1-year follow-up of a phase 1/2 open-label trial. Vaccine 2024, 42, 3999–4010. [Google Scholar] [CrossRef] [PubMed]
- Song, X.-D.; Yang, G.-J.; Shi, C.; Jiang, X.-L.; Wang, X.-J.; Zhang, Y.-W.; Wu, J.; Zhao, L.-X.; Wang, M.-M.; Chen, R.-R.; et al. Finite immune imprinting on neutralizing antibody responses to Omicron subvariants by repeated vaccinations. Int. J. Infect. Dis. 2024, 147, 107198. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Mellis, I.A.; Ho, J.; Bowen, A.; Kowalski-Dobson, T.; Valdez, R.; Katsamba, P.S.; Wu, M.; Lee, C.; Shapiro, L.; et al. Recurrent SARS-CoV-2 spike mutations confer growth advantages to select JN.1 sublineages. Emerg. Microbes Infect. 2024, 13, 2402880. [Google Scholar] [CrossRef] [PubMed]
- Astrakhantseva, I.V.; Krut’, V.G.; Chuvpilo, S.A.; Shevyrev, D.V.; Shumeev, A.N.; Rybtsov, S.A.; Nedospasov, S.A. On Immunological Studies at Sirius University of Science and Technology. Mol. Biol. 2023, 57, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Nkinda; Barabona, G.; Ngare, I.; Nkuwi, E.; Kamori, D.; Msafiri, F.; Kunambi, P.P.; Osati, E.; Kidenya, B.R.; Chuwa, H.; et al. Evaluation of cross-neutralizing immunity following COVID-19 primary series vaccination during the Omicron surge in Tanzania. J. Med Virol. 2024, 96, e29822. [Google Scholar] [CrossRef]
- Du, P.; Li, N.; Tang, S.; Zhou, Z.; Liu, Z.; Wang, T.; Li, J.; Zeng, S.; Chen, J. Development and evaluation of vaccination strategies for addressing the continuous evolution SARS-CoV-2 based on recombinant trimeric protein technology: Potential for cross-neutralizing activity and broad coronavirus response. Heliyon 2024, 10, e34492. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Xue, R.-Y.; Li, G.-C.; Zhang, Y.; Wu, W.-Y.; Liu, J.-Y.; Feng, R.; Jin, Z.; Deng, Y.; Jin, Z.-L.; et al. pGM-CSF as an adjuvant in DNA vaccination against SARS-CoV-2. Int. J. Biol. Macromol. 2024, 264, 130660. [Google Scholar] [CrossRef] [PubMed]
- Richardson, S.I.; Mzindle, N.; Motlou, T.; Manamela, N.P.; Van Der Mescht, M.A.; Lambson, B.E.; Everatt, J.; Amoako, D.G.; Balla, S.; Von Gottberg, A.; et al. SARS-CoV-2 BA.4/5 infection triggers more cross-reactive FcγRIIIa signaling and neutralization than BA.1, in the context of hybrid immunity. J. Virol. 2024, 98, e00678-24. [Google Scholar] [CrossRef] [PubMed]
- Dadonaite, B.; Brown, J.; McMahon, T.E.; Farrell, A.G.; Figgins, M.D.; Asarnow, D.; Stewart, C.; Lee, J.; Logue, J.; Bedford, T.; et al. Spike deep mutational scanning helps predict success of SARS-CoV-2 clades. Nature 2024, 631, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Wu, C.; Wu, X.; Ma, X.; Shu, C.; Chen, Q.; Zheng, A.; Yang, H.; Lu, J.; Du, P.; et al. Classification of five SARS-CoV-2 serotypes based on RBD antigenicities. Sci. Bull. 2023, 68, 3003–3012. [Google Scholar] [CrossRef]
- Ramasamy, S.; Gontu, A.; Neerukonda, S.; Ruggiero, D.; Morrow, B.; Gupta, S.; Amirthalingam, S.; Hardham, J.M.; Lizer, J.T.; Yon, M.; et al. SARS-CoV-2 Prevalence and Variant Surveillance among Cats in Pittsburgh, Pennsylvania, USA. Viruses 2023, 15, 1493. [Google Scholar] [CrossRef]
- Li, P.; Faraone, J.N.; Hsu, C.C.; Chamblee, M.; Liu, Y.; Zheng, Y.-M.; Xu, Y.; Carlin, C.; Horowitz, J.C.; Mallampalli, R.K.; et al. Neutralization and Stability of JN.1-derived LB.1, KP.2.3, KP.3 and KP.3.1.1 Subvariants. bioRxiv 2024. [Google Scholar] [CrossRef]
- Connor, R.; Sakharkar, M.; Rappazzo, G.; Kaku, C.; Curtis, N.; Shin, S.; Wieland-Alter, W.; Wentworth, J.; Mielcarz, D.; Weiner, J.; et al. Characteristics and Functions of Infection-enhancing Antibodies to the N-terminal Domain of SARS-CoV-2. Pathog. Immun. 2024, 9, 1–24. [Google Scholar] [CrossRef]
- Carnell, G.W.; Ferrara, F.; Grehan, K.; Thompson, C.P.; Temperton, N.J. Pseudotype-Based Neutralization Assays for Influenza: A Systematic Analysis. Front. Immunol. 2015, 6, 161. [Google Scholar] [CrossRef] [PubMed]
- Bock; Heinkelein, M.; Lindemann, D.; Rethwilm, A. Cells Expressing the Human Foamy Virus (HFV) Accessory Bet Protein Are Resistant to Productive HFV Superinfection. Virology 1998, 250, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; De La Torre, J.C.; Melikyan, G.B. Human LAMP1 accelerates Lassa virus fusion and potently promotes fusion pore dilation upon forcing viral fusion with non-endosomal membrane. PLOS Pathog. 2022, 18, e1010625. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Feng, Y.; Zhou, Z.; Li, K.; Hu, X.; Liao, L.; Xing, H.; Shao, Y. Substitution of gp120 C4 region compensates for V3 loss-of-fitness mutations in HIV-1 CRF01_AE co-receptor switching. Emerg. Microbes Infect. 2023, 12, e2169196. [Google Scholar] [CrossRef]
- Maruyama, J.; Nao, N.; Miyamoto, H.; Maeda, K.; Ogawa, H.; Yoshida, R.; Igarashi, M.; Takada, A. Characterization of the glycoproteins of bat-derived influenza viruses. Virology 2016, 488, 43–50. [Google Scholar] [CrossRef]
- Giotis, E.S.; Carnell, G.; Young, E.F.; Ghanny, S.; Soteropoulos, P.; Wang, L.-F.; Barclay, W.S.; Skinner, M.A.; Temperton, N. Entry of the bat influenza H17N10 virus into mammalian cells is enabled by the MHC class II HLA-DR receptor. Nat. Microbiol. 2019, 4, 2035–2038. [Google Scholar] [CrossRef]
- Qiao, S.; Wang, X. Structural determinants of spike infectivity in bat SARS-like coronaviruses RsSHC014 and WIV1. J. Virol. 2024, 98, e00342-24. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Liu, C.; Xiong, Q.; Gu, M.; Shi, L.; Wang, C.; Si, J.; Tong, F.; Liu, P.; Huang, M.; et al. Broad host tropism of ACE2-using MERS-related coronaviruses and determinants restricting viral recognition. Cell Discov. 2023, 9, 57. [Google Scholar] [CrossRef]
- Luo, H.; Lv, L.; Yi, J.; Zhou, Y.; Liu, C. Establishment of Replication Deficient Vesicular Stomatitis Virus for Studies of PEDV Spike-Mediated Cell Entry and Its Inhibition. Microorganisms 2023, 11, 2075. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, S.; Wang, Y. Pseudotyped Viruses for Marburgvirus and Ebolavirus. In Pseudotyped Viruses; Wang, Y., Ed.; Springer Nature Singapore: Singapore, 2023; pp. 105–132. [Google Scholar] [CrossRef]
- Marnata, C.; Saulnier, A.; Mompelat, D.; Krey, T.; Cohen, L.; Boukadida, C.; Warter, L.; Fresquet, J.; Vasiliauskaite, I.; Escriou, N.; et al. Determinants Involved in Hepatitis C Virus and GB Virus B Primate Host Restriction. J. Virol. 2015, 89, 12131–12144. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yang, Y.; Cheng, H.; Yan, H.; Wang, P.-Z.; Rong, R.; Zhang, Y.-Y.; Zhang, C.-B.; Du, R.; Rong, L.-J. A cell-based high-throughput protocol to screen entry inhibitors of highly pathogenic viruses with Traditional Chinese Medicines: Screening Entry Inhibitors of Highly Pathogenic Viruses With TCM. J. Med Virol. 2016, 89, 908–916. [Google Scholar] [CrossRef]
- Wang, J.; Cheng, H.; Ratia, K.; Varhegyi, E.; Hendrickson, W.G.; Li, J.; Rong, L. A Comparative High-Throughput Screening Protocol to Identify Entry Inhibitors of Enveloped Viruses. SLAS Discov. Adv. Sci. Drug Discov. 2014, 19, 100–107. [Google Scholar] [CrossRef]
- Bhattarai, N.; McLinden, J.H.; Xiang, J.; Kaufman, T.M.; Stapleton, J.T. Conserved Motifs within Hepatitis C Virus Envelope (E2) RNA and Protein Independently Inhibit T Cell Activation. PLOS Pathog. 2015, 11, e1005183. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Jiang, J.; Tang, Y.; Mei, L.; Wu, L.; Li, L.; Chen, H.; Long, F.; Xiao, J.; Peng, T. A Pseudovirus-Based Entry Assay to Evaluate Neutralizing Activity against Respiratory Syncytial Virus. Viruses 2023, 15, 1548. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xu, L.; Wang, B.; Zhang, D.; Zhao, L.; Bei, Z.; Song, Y. Design, Synthesis, and Biological Evaluation of Benzimidazole Derivatives as Potential Lassa Virus Inhibitors. Molecules 2023, 28, 1579. [Google Scholar] [CrossRef]
- Liang, J.; Zhai, L.; Liang, Z.; Chen, X.; Jiang, Y.; Lin, Y.; Feng, S.; Liu, Y.; Zhao, W.; Wang, F. Rational Design and Characterization of Trispecific Antibodies Targeting the HIV-1 Receptor and Envelope Glycoprotein. Vaccines 2023, 12, 19. [Google Scholar] [CrossRef]
- Luo, X.; Wang, C.; Huang, Y.; Cong, S.; Tan, J.; Hou, W.; Ma, F.; Zheng, L. Establishment of a neutralization assay for Nipah virus using a high-titer pseudovirus system. Biotechnol. Lett. 2023, 45, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Hao, P.; Jin, C.; Xu, W.; Liu, Y.; Li, L.; Du, S.; Shang, L.; Jin, X.; Jin, N.; et al. Interaction of Nipah Virus F and G with the Cellular Protein Cortactin Discovered by a Proximity Interactome Assay. Int. J. Mol. Sci. 2024, 25, 4112. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Liu, Y.; Zhang, F.; Wang, X.; Huang, W.; Wu, Y.; Li, B.; Zhuang, J.; Bing, Y.; Wang, Y.; et al. Analysis of cross neutralizing activity of antibodies from sera of severe fever with thrombocytopenia syndrome patients to deal with different genotype strains. Front. Microbiol. 2022, 13, 1020545. [Google Scholar] [CrossRef]
- Huttner, A.; Agnandji, S.T.; Engler, O.; Hooper, J.W.; Kwilas, S.; Ricks, K.; Clements, T.L.; Jonsdottir, H.R.; Nakka, S.S.; Rothenberger, S.; et al. Antibody responses to recombinant vesicular stomatitis virus-Zaire Ebolavirus vaccination for Ebola virus disease across doses and continents: 5-year durability. Clin. Microbiol. Infect. 2023, 29, 1587–1594. [Google Scholar] [CrossRef]
- Bi, J.; Wang, H.; Han, Q.; Pei, H.; Wang, H.; Jin, H.; Jin, S.; Chi, H.; Yang, S.; Zhao, Y.; et al. A rabies virus-vectored vaccine expressing two copies of the Marburg virus glycoprotein gene induced neutralizing antibodies against Marburg virus in humanized mice. Emerg. Microbes Infect. 2022, 12, 2149351. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Huang, Z.; Xiao, J.; Du, S.; Bu, Q.; Guo, H.; Ye, J.; Chen, S.; Gao, J.; Li, Z.; et al. A quadri-fluorescence SARS-CoV-2 pseudovirus system for efficient antigenic characterization of multiple circulating variants. Cell Rep. Methods 2024, 4, 100856. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Q.; Li, L.; Wu, J.; Tian, M.; Fu, Y. Application of pseudovirus system in the development of vaccine, antiviral-drugs, and neutralizing antibodies. Microbiol. Res. 2022, 258, 126993. [Google Scholar] [CrossRef] [PubMed]
- Sholukh, A.M.; Fiore-Gartland, A.; Ford, E.S.; Miner, M.D.; Hou, Y.J.; Tse, L.V.; Kaiser, H.; Zhu, H.; Lu, J.; Madarampalli, B.; et al. Evaluation of Cell-Based and Surrogate SARS-CoV-2 Neutralization Assays. J. Clin. Microbiol. 2021, 59, e00527-21. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Wu, X.; Wu, J.; Liu, S.; Tong, J.; Li, T.; Yu, Y.; Zhang, L.; Zhao, C.; Lu, Q.; et al. Development of an automated, high-throughput SARS-CoV-2 neutralization assay based on a pseudotyped virus using a vesicular stomatitis virus (VSV) vector. Emerg. Microbes Infect. 2023, 12, e2261566. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).