AARS1 and AARS2: From Protein Synthesis to Lactylation-Driven Oncogenesis
Abstract
1. Introduction
2. Canonical Functions of AARS1 and AARS2 Concerning Protein Synthesis
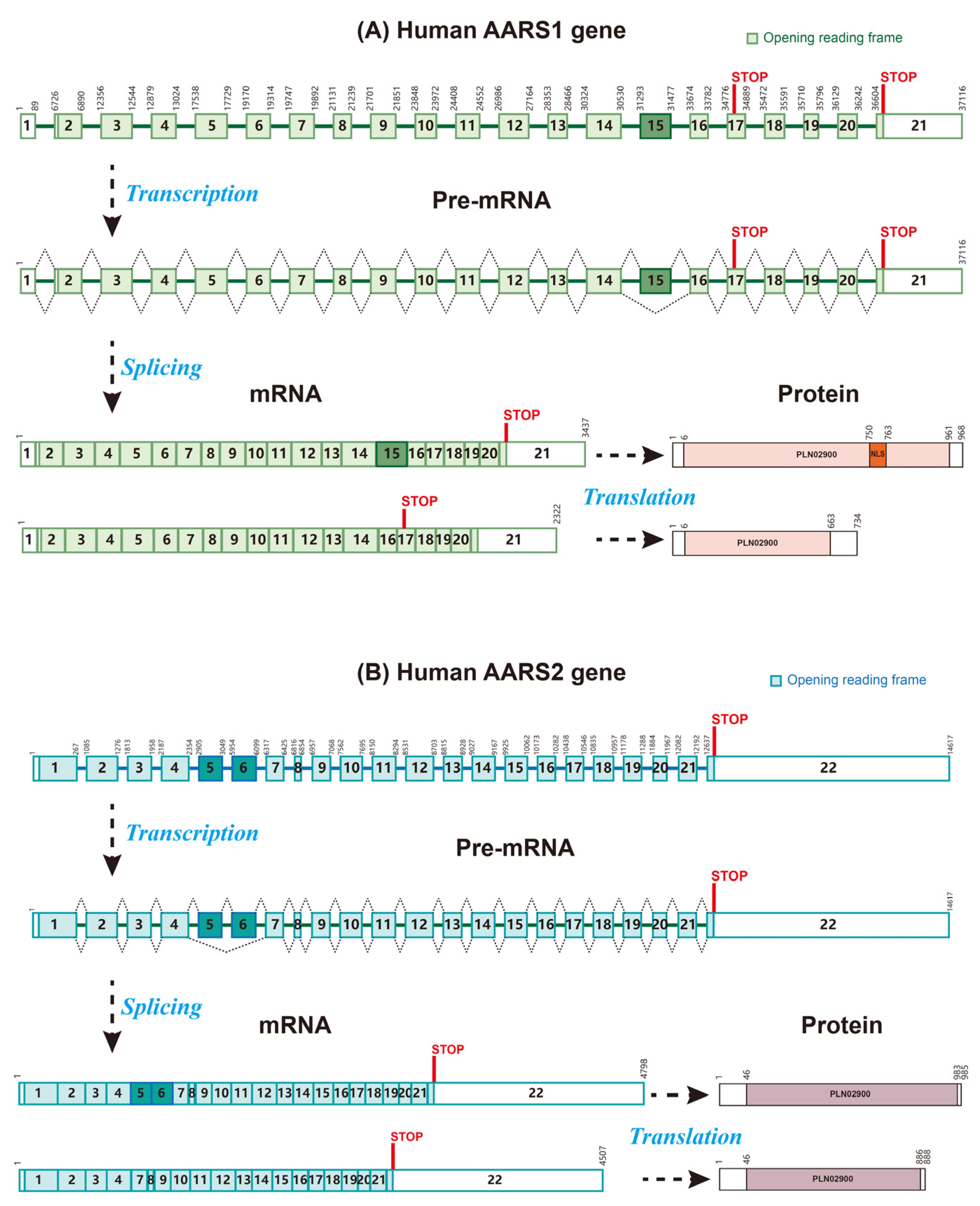
2.1. The Structure and Functional Domains of AARS1 and AARS2
2.2. The Mechanism of Aminoacylation, Including Substrate Specificity (Alanine) and tRNA Charging
2.3. Heterozygous Mutations of AARS1/2 Impair Their Function
3. Lactylation: A Novel Mechanistic Link Between AARS1/AARS2, Metabolism, and Cancer
3.1. Cancer Metabolism and the Warburg Effect
3.2. Introduction of Lactylation and Lactate Sensing
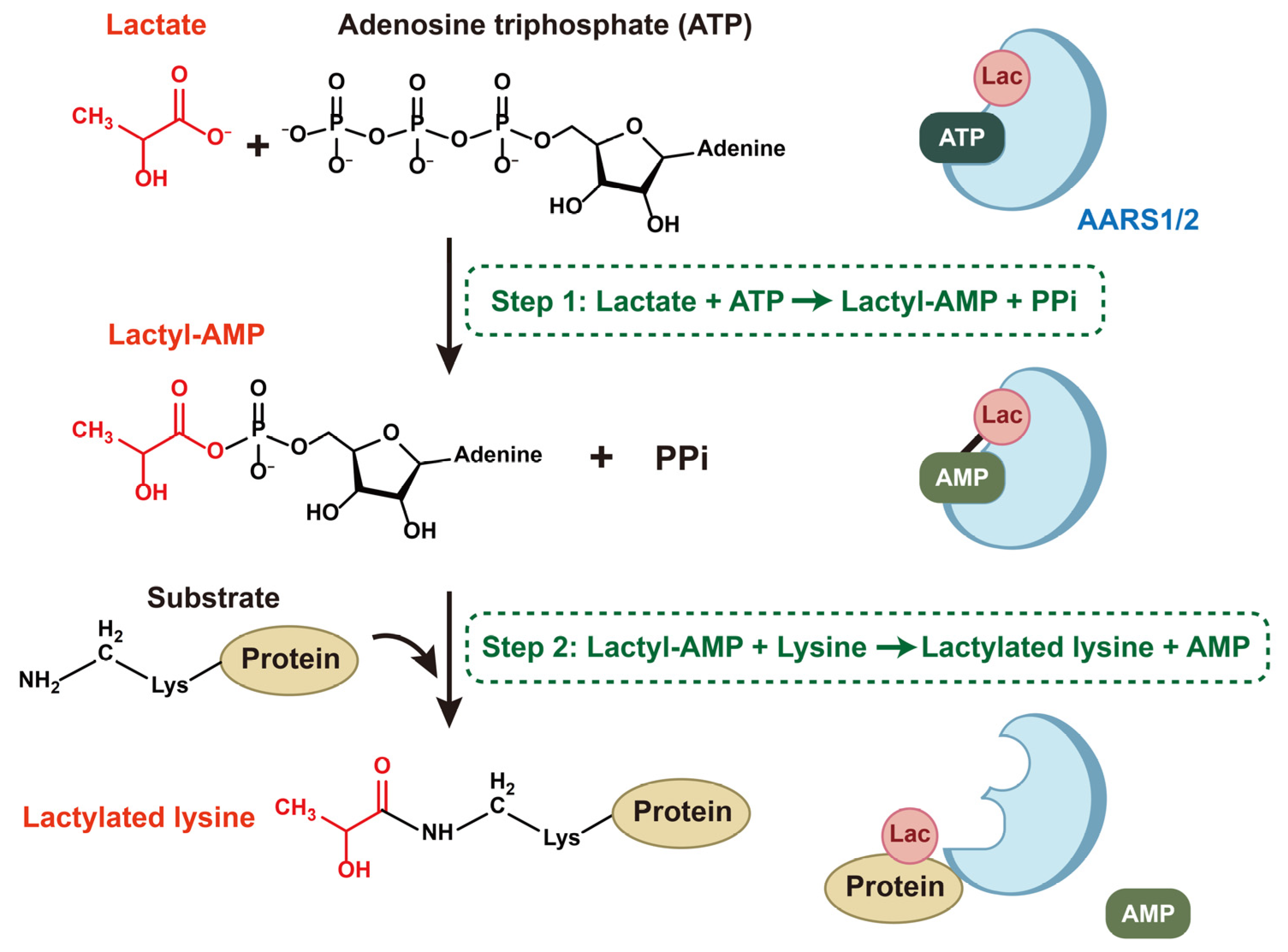
3.3. AARS1 and AARS2 Sense Lactate and Mediate Lactylation
3.3.1. The Recognition of Lactate by AARS1 and AARS2
3.3.2. Lactylation Mediated by AARS1 and AARS2
4. The Expression and Roles of AARS1 and AARS2 in Cancers
4.1. Expression of AARS1/2 in Cancers
4.2. AARS1/2 and Cancer Cell Proliferation and Migration
4.3. AARS1/2 and the Cancer Microenvironment
4.4. AARS2 and Mitochondrial Respiration
4.5. AARS1/2 and Cancer Therapy Resistance
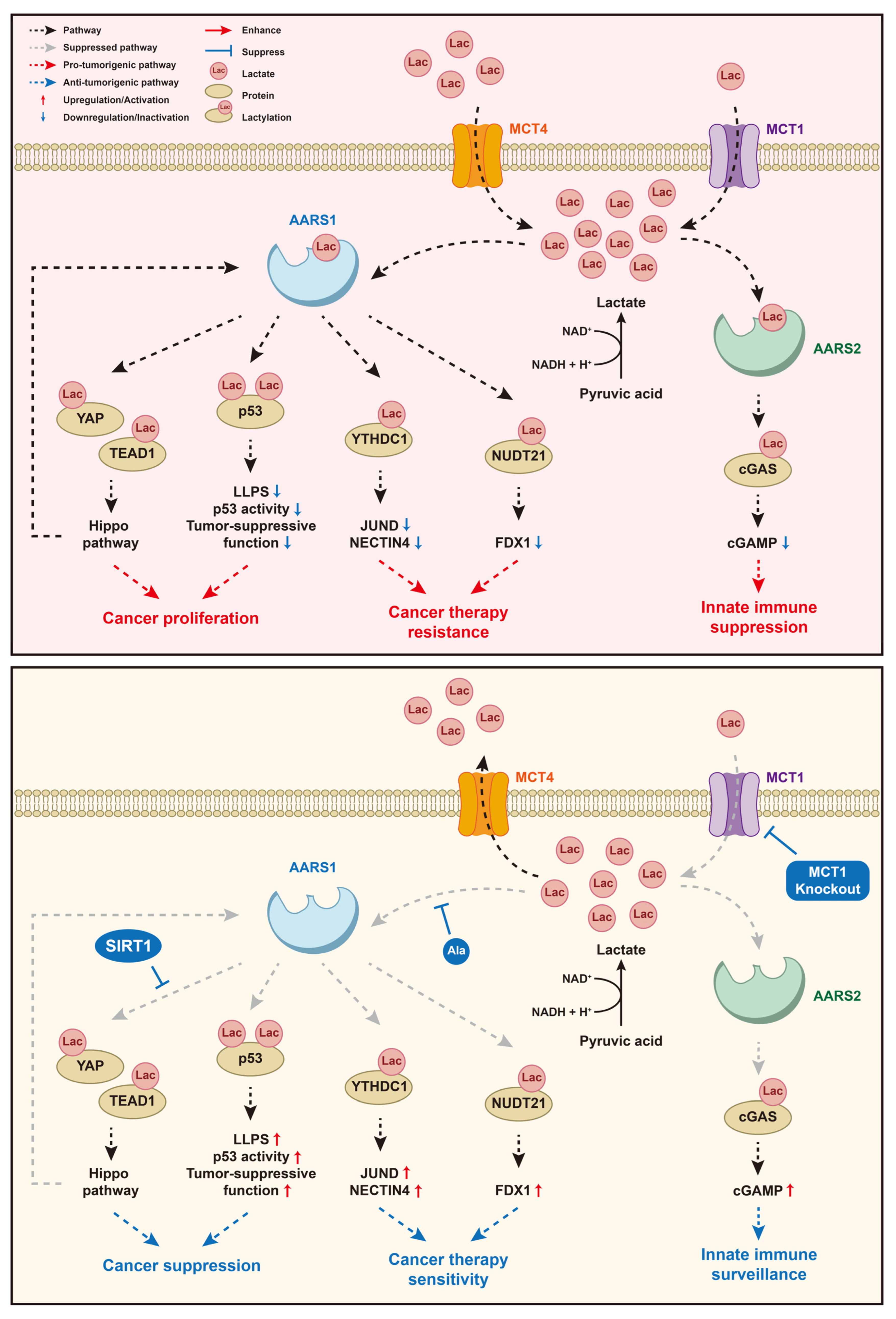
5. The Signaling Pathways of Lactate and AARS1/2 in Cancers
5.1. Lactate/AARS1/p53
5.2. Lactate/AARS1/YAP&TEAD1
5.3. Lactate/AARS1/YTHDC1
5.4. Lactate/AARS1/NUDT21
5.5. Lactate/AARS2/cGAS
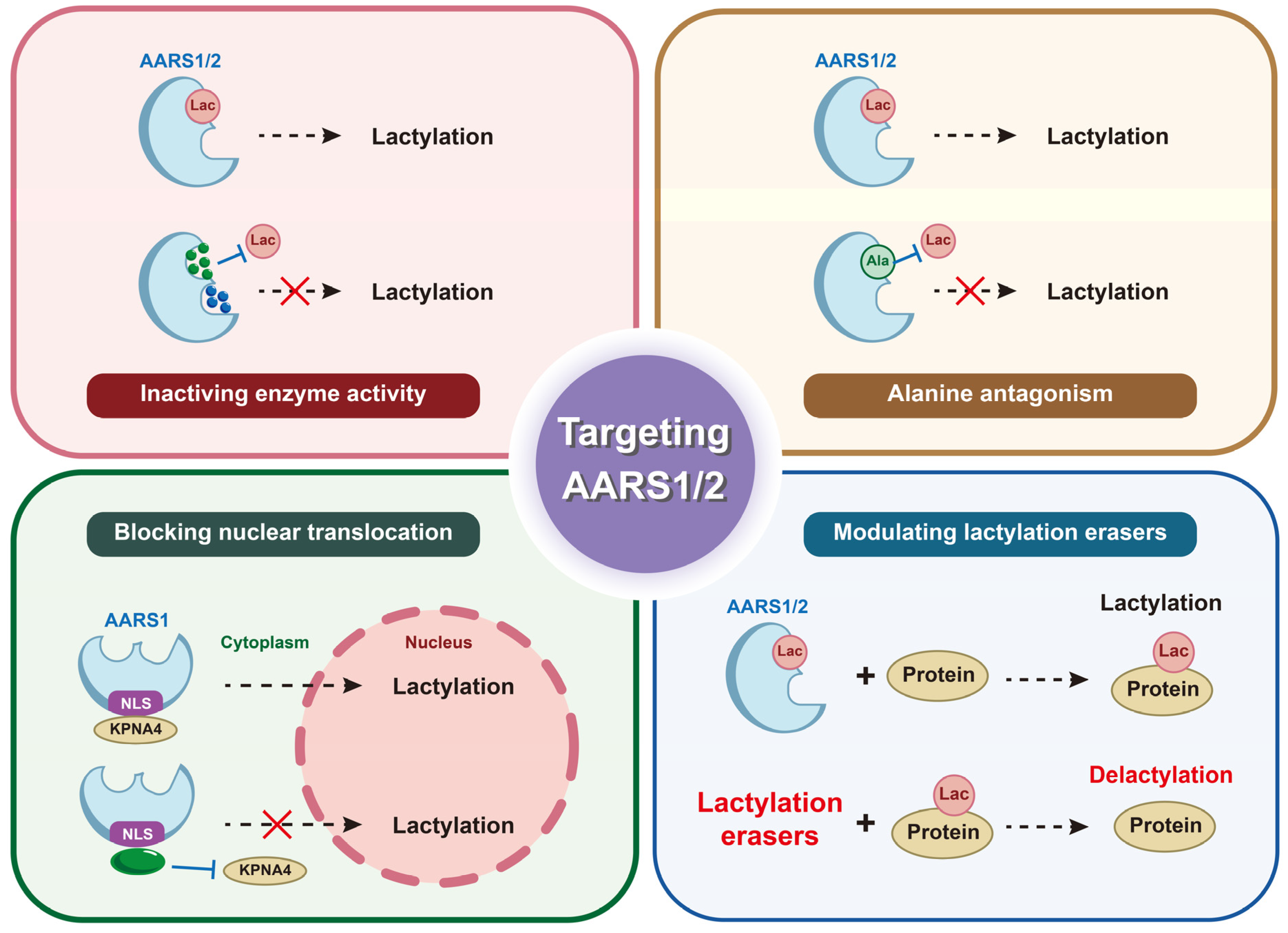
6. AARS1 and AARS2 as Potential Therapeutic Targets for Cancers
6.1. Small-Molecule Inhibitors of Catalytic Activity
6.2. Antagonizing Protein–Protein Interactions Involved in Lactylation
6.3. Targeting Subcellular Localization
6.4. Modulating Lactylation Erasers to Suppress Oncogenic Lactylation
7. Discussion
7.1. Profiling the Full Spectrum of Lactylation Targets of AARS1/2
7.2. Identifying Tissue/Cancer-Type Specificity of AARS1/AARS2 Functions and Lactylation
7.3. Crosstalk Between Lactylation and Other PTMs via AARSs
7.4. Depicting the Lactylation Profile in Tumor Immunity
7.5. Highlighting the Role of Lactate Sensors in Tumorigenesis
7.6. Modulating Lactate Sensing via Lactate Production and Transportation
7.7. Exploring the Regulatory Mechanism of AARS1/2 and Developing Isoform-Specific and Function-Specific Inhibitors
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AARS | Aminoacyl-tRNA synthetase |
| Acetyl-CoA | Acetyl-coenzyme A |
| Acyl-CoA | Acyl-coenzyme A |
| AlaRS | Alanyl-tRNA synthetase |
| ACSL4 | Acyl-CoA synthetase long-chain family member 4 |
| AREG | Pro-angiogenic mediator amphiregulin |
| ARS | Aminoacyl-tRNA synthetase |
| ATP | Adenosine triphosphate |
| BC | Bladder cancer |
| cGAS | Cyclic GMP–AMP synthase |
| CNPY3 | Canopy FGF signaling regulator 3 |
| COAD | Colon adenocarcinoma |
| CPSF6 | Cleavage and polyadenylation specific factor 6 |
| CPT2 | Carnitine palmitoyltransferase 2 |
| CRC | Colorectal cancer |
| CREB | Akt-cAMP response element binding protein |
| DC | Duodenal cancer |
| dPAS | Distal polyadenylation site |
| ESCC | Esophageal squamous cell carcinoma |
| EV | Enfortumab vedotin |
| FDX1 | Ferredoxin 1 |
| FSH | Follicle-stimulating hormone |
| HDAC2 | Histone deacetylase 2 |
| GC | Gastric cancer |
| GPR | G-protein-coupled receptor |
| HCC | Hepatocellular carcinoma |
| HMGB1 | High mobility group box 1 |
| JUND | JunD proto-oncogene |
| K-AA | Lysine aminoacylation |
| K-Ala | Lysine alanylation |
| KPNA4 | Karyopherin subunit alpha 4 |
| Lactyl-CoA | Lactyl-coenzyme A |
| LDHA | Lactate dehydrogenase |
| LLPS | Liquid–liquid phase separation |
| MCT | Monocarboxylate transporter protein |
| NECTIN4 | Nectin cell adhesion molecule 4 |
| NLS | Nuclear localization signal |
| NLRP3 | NLR family pyrin domain containing 3 |
| NPS-TTD | Unsolved non-photosensitive trichothiodystrophy |
| NUDT21 | Nudix hydrolase 21 |
| Osx | Osterix |
| PARP1 | Poly (ADP-ribose) polymerase 1 |
| Pcbp1 | Poly(rC) binding protein 1 |
| PDAC | Pancreatic ductal adenocarcinoma |
| PDHA1 | Pyruvate dehydrogenase A1 |
| PKM2 | Pyruvate kinase M2 |
| PI3K | Phosphoinositide 3-OH kinase |
| PPARγ | Peroxisome proliferator-activated receptor γ |
| PPi | Inorganic pyrophosphate |
| PTM | Post-translational modification |
| RNF183 | Ring finger protein 183 |
| RS | Risk score |
| SIRT1 | Sirtuin 1 |
| TAM | Tumor-associated macrophage |
| TEAD1 | TEA domain transcription factor 1 |
| TFAM | Mitochondrial transcription factor A |
| TGFβ | Transforming growth factor β |
| TME | Tumor microenvironment |
| WDR5 | WD repeat domain 5 |
| YAP | Yes1-associated transcriptional regulator |
| YTHDC1 | YTH N6-methyladenosine RNA binding protein C1 |
References
- Ibba, M.; Soll, D. Aminoacyl-tRNA synthesis. Annu. Rev. Biochem. 2000, 69, 617–650. [Google Scholar] [CrossRef]
- Cusack, S. Aminoacyl-tRNA synthetases. Curr. Opin. Struct. Biol. 1997, 7, 881–889. [Google Scholar] [CrossRef]
- Eriani, G.; Delarue, M.; Poch, O.; Gangloff, J.; Moras, D. Partition of tRNA synthetases into two classes based on mutually exclusive sets of sequence motifs. Nature 1990, 347, 203–206. [Google Scholar] [CrossRef]
- Ludmerer, S.W.; Schimmel, P. Construction and analysis of deletions in the amino-terminal extension of glutamine tRNA synthetase of Saccharomyces cerevisiae. J. Biol. Chem. 1987, 262, 10807–10813. [Google Scholar] [CrossRef]
- Cusack, S.; Härtlein, M.; Leberman, R. Sequence, structural and evolutionary relationships between class 2 aminoacyl-tRNA synthetases. Nucleic Acids Res. 1991, 19, 3489–3498. [Google Scholar] [CrossRef]
- Douglas, J.; Cui, H.; Perona, J.J.; Vargas-Rodriguez, O.; Tyynismaa, H.; Carreño, C.A.; Ling, J.; Ribas de Pouplana, L.; Yang, X.-L.; Ibba, M.; et al. AARS Online: A collaborative database on the structure, function, and evolution of the aminoacyl-tRNA synthetases. IUBMB Life 2024, 76, 1091–1105. [Google Scholar] [CrossRef] [PubMed]
- Nian, F.; Guo, L.; Fei, Z.; Yang, M.; Chen, L.; Zhang, Y.; Zhang, B.; Qian, Y.; Zhang, Z. AARS1-mediated Osterix lactylation promotes its transcriptional activity during osteoblast differentiation. Acta Histochem. 2025, 127, 152273. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Huang, S.-X.; Qin, M.-L.; Pan, Z. Mitochondrial alanyl-tRNA synthetase 2 mediates histone lactylation to promote ferroptosis in intestinal ischemia-reperfusion injury. World J. Gastrointest. Surg. 2025, 17, 106777. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-L.; Ren, S.-T.; Yang, W.-J.; Xu, X.-W.; Zhao, S.-M.; Fang, K.-F.; Lin, Y.; Yuan, Y.-Y.; Zhang, X.-J.; Chen, Y.-Q.; et al. AARS2-catalyzed lactylation induces follicle development and premature ovarian insufficiency. Cell Death Discov. 2025, 11, 209. [Google Scholar] [CrossRef]
- Zhang, Z.; Zheng, L.; Chen, Y.; Chen, Y.; Hou, J.; Xiao, C.; Zhu, X.; Zhao, S.-M.; Xiong, J.-W. AARS2 ameliorates myocardial ischemia via fine-tuning PKM2-mediated metabolism. elife 2025, 13, RP99670. [Google Scholar] [CrossRef]
- He, X.-D.; Gong, W.; Zhang, J.-N.; Nie, J.; Yao, C.-F.; Guo, F.-S.; Lin, Y.; Wu, X.-H.; Li, F.; Li, J.; et al. Sensing and Transmitting Intracellular Amino Acid Signals through Reversible Lysine Aminoacylations. Cell Metab. 2018, 27, 151–166.e6. [Google Scholar] [CrossRef]
- Sung, Y.; Yoon, I.; Han, J.M.; Kim, S. Functional and pathologic association of aminoacyl-tRNA synthetases with cancer. Exp. Mol. Med. 2022, 54, 553–566. [Google Scholar] [CrossRef]
- Wang, J.; Vallee, I.; Dutta, A.; Wang, Y.; Mo, Z.; Liu, Z.; Cui, H.; Su, A.I.; Yang, X.-L. Multi-Omics Database Analysis of Aminoacyl-tRNA Synthetases in Cancer. Genes 2020, 11, 1384. [Google Scholar] [CrossRef]
- Sonkin, D.; Thomas, A.; Teicher, B.A. Cancer treatments: Past, present, and future. Cancer Genet. 2024, 286–287, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Zong, Z.; Xie, F.; Wang, S.; Wu, X.; Zhang, Z.; Yang, B.; Zhou, F. Alanyl-tRNA synthetase, AARS1, is a lactate sensor and lactyltransferase that lactylates p53 and contributes to tumorigenesis. Cell 2024, 187, 2375–2392.e33. [Google Scholar] [CrossRef]
- Ju, J.; Zhang, H.; Lin, M.; Yan, Z.; An, L.; Cao, Z.; Geng, D.; Yue, J.; Tang, Y.; Tian, L.; et al. The alanyl-tRNA synthetase AARS1 moonlights as a lactyltransferase to promote YAP signaling in gastric cancer. J. Clin. Investig. 2024, 134, e174587. [Google Scholar] [CrossRef] [PubMed]
- Schwaederle, M.; Zhao, M.; Lee, J.J.; Eggermont, A.M.; Schilsky, R.L.; Mendelsohn, J.; Lazar, V.; Kurzrock, R. Impact of Precision Medicine in Diverse Cancers: A Meta-Analysis of Phase II Clinical Trials. J. Clin. Oncol. 2015, 33, 3817–3825. [Google Scholar] [CrossRef]
- Meyer-Schuman, R.; Marte, S.; Smith, T.J.; Feely, S.M.E.; Kennerson, M.; Nicholson, G.; Shy, M.E.; Koutmou, K.S.; Antonellis, A. A humanized yeast model reveals dominant-negative properties of neuropathy-associated alanyl-tRNA synthetase mutations. Hum. Mol. Genet. 2023, 32, 2177–2191. [Google Scholar] [CrossRef] [PubMed]
- Lo, W.-S.; Gardiner, E.; Xu, Z.; Lau, C.-F.; Wang, F.; Zhou, J.J.; Mendlein, J.D.; Nangle, L.A.; Chiang, K.P.; Yang, X.-L.; et al. Human tRNA synthetase catalytic nulls with diverse functions. Science 2014, 345, 328–332. [Google Scholar] [CrossRef]
- Perona, J.J.; Gruic-Sovulj, I. Synthetic and Editing Mechanisms of Aminoacyl-tRNA Synthetases. In Aminoacyl-tRNA Synthetases in Biology and Medicine; Kim, S., Ed.; Springer: Dordrecht, The Netherlands, 2014; pp. 1–41. ISBN 978-94-017-8701-7. [Google Scholar]
- Tsui, W.C.; Fersht, A.R. Probing the principles of amino acid selection using the alanyl-tRNA synthetase from Escherichia coli. Nucleic Acids Res. 1981, 9, 4627–4637. [Google Scholar] [CrossRef]
- Ibba, M.; Kast, P.; Hennecke, H. Substrate specificity is determined by amino acid binding pocket size in Escherichia coli phenylalanyl-tRNA synthetase. Biochemistry 1994, 33, 7107–7112. [Google Scholar] [CrossRef] [PubMed]
- Botta, E.; Theil, A.F.; Raams, A.; Caligiuri, G.; Giachetti, S.; Bione, S.; Accadia, M.; Lombardi, A.; Smith, D.E.C.; Mendes, M.I.; et al. Protein instability associated with AARS1 and MARS1 mutations causes trichothiodystrophy. Hum. Mol. Genet. 2021, 30, 1711–1720. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Liu, T.; Zhang, B.; Liu, C.; Luan, X.; Cao, L. An AARS1 variant identified to cause adult-onset leukoencephalopathy with neuroaxonal spheroids and pigmented glia. Transl. Neurodegener. 2023, 12, 19. [Google Scholar] [CrossRef]
- Marten, L.M.; Brinkert, F.; Smith, D.E.C.; Prokisch, H.; Hempel, M.; Santer, R. Recurrent acute liver failure in alanyl-tRNA synthetase-1 (AARS1) deficiency. Mol. Genet. Metab. Rep. 2020, 25, 100681. [Google Scholar] [CrossRef]
- Lu, Y.W.; Liang, Z.; Guo, H.; Fernandes, T.; Espinoza-Lewis, R.A.; Wang, T.; Li, K.; Li, X.; Singh, G.B.; Wang, Y.; et al. PCBP1 regulates alternative splicing of AARS2 in congenital cardiomyopathy. bioRxiv 2023. [Google Scholar] [CrossRef] [PubMed]
- Wilson, W.R.; Hay, M.P. Targeting hypoxia in cancer therapy. Nat. Rev. Cancer 2011, 11, 393–410. [Google Scholar] [CrossRef]
- McKeown, S.R. Defining normoxia, physoxia and hypoxia in tumours—Implications for treatment response. Br. J. Radiol. 2014, 87, 20130676. [Google Scholar] [CrossRef]
- Hardee, M.; Dewhirst, M.; Agarwal, N.; Sorg, B. Novel Imaging Provides New Insights into Mechanisms of Oxygen Transport in Tumors. Curr. Mol. Med. 2009, 9, 435–441. [Google Scholar] [CrossRef]
- Pandkar, M.R.; Dhamdhere, S.G.; Shukla, S. Oxygen gradient and tumor heterogeneity: The chronicle of a toxic relationship. Biochim. Biophys. Acta (BBA)—Rev. Cancer 2021, 1876, 188553. [Google Scholar] [CrossRef]
- Sonveaux, P.; Végran, F.; Schroeder, T.; Wergin, M.C.; Verrax, J.; Rabbani, Z.N.; De Saedeleer, C.J.; Kennedy, K.M.; Diepart, C.; Jordan, B.F.; et al. Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. J. Clin. Investig. 2008, 118, 3930–3942. [Google Scholar] [CrossRef]
- Kennedy, K.M.; Dewhirst, M.W. Tumor metabolism of lactate: The influence and therapeutic potential for MCT and CD147 regulation. Future Oncol. 2010, 6, 127–148. [Google Scholar] [CrossRef] [PubMed]
- Warburg, O. On the Origin of Cancer Cells. Science 1956, 123, 309–314. [Google Scholar] [CrossRef]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef]
- Certo, M.; Llibre, A.; Lee, W.; Mauro, C. Understanding lactate sensing and signalling. Trends Endocrinol. Metab. 2022, 33, 722–735. [Google Scholar] [CrossRef]
- Li, X.; Yang, Y.; Zhang, B.; Lin, X.; Fu, X.; An, Y.; Zou, Y.; Wang, J.-X.; Wang, Z.; Yu, T. Lactate metabolism in human health and disease. Signal Transduct. Target. Ther. 2022, 7, 305. [Google Scholar] [CrossRef]
- Certo, M.; Tsai, C.-H.; Pucino, V.; Ho, P.-C.; Mauro, C. Lactate modulation of immune responses in inflammatory versus tumour microenvironments. Nat. Rev. Immunol. 2021, 21, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Tang, Z.; Huang, H.; Zhou, G.; Cui, C.; Weng, Y.; Liu, W.; Kim, S.; Lee, S.; Perez-Neut, M.; et al. Metabolic regulation of gene expression by histone lactylation. Nature 2019, 574, 575–580. [Google Scholar] [CrossRef]
- Wan, N.; Wang, N.; Yu, S.; Zhang, H.; Tang, S.; Wang, D.; Lu, W.; Li, H.; Delafield, D.G.; Kong, Y.; et al. Cyclic immonium ion of lactyllysine reveals widespread lactylation in the human proteome. Nat. Methods 2022, 19, 854–864. [Google Scholar] [CrossRef] [PubMed]
- Jing, F. Multi-omics reveals lactylation-driven regulatory mechanisms promoting tumor progression in oral squamous cell carcinoma. Genome Biol. 2024, 25, 272. [Google Scholar] [CrossRef]
- Yang, Z.; Yan, C.; Ma, J.; Peng, P.; Ren, X.; Cai, S.; Shen, X.; Wu, Y.; Zhang, S.; Wang, X.; et al. Lactylome analysis suggests lactylation-dependent mechanisms of metabolic adaptation in hepatocellular carcinoma. Nat. Metab. 2023, 5, 61–79. [Google Scholar] [CrossRef]
- Jiang, J.; Huang, D.; Jiang, Y.; Hou, J.; Tian, M.; Li, J.; Sun, L.; Zhang, Y.; Zhang, T.; Li, Z.; et al. Lactate Modulates Cellular Metabolism Through Histone Lactylation-Mediated Gene Expression in Non-Small Cell Lung Cancer. Front. Oncol. 2021, 11, 647559. [Google Scholar] [CrossRef]
- Varner, E.L.; Trefely, S.; Bartee, D.; von Krusenstiern, E.; Izzo, L.; Bekeova, C.; O’Connor, R.S.; Seifert, E.L.; Wellen, K.E.; Meier, J.L.; et al. Quantification of lactoyl-CoA (lactyl-CoA) by liquid chromatography mass spectrometry in mammalian cells and tissues. Open Biol. 2020, 10, 200187. [Google Scholar] [CrossRef]
- Mao, Y.; Zhang, J.; Zhou, Q.; He, X.; Zheng, Z.; Wei, Y.; Zhou, K.; Lin, Y.; Yu, H.; Zhang, H.; et al. Hypoxia induces mitochondrial protein lactylation to limit oxidative phosphorylation. Cell Res. 2024, 34, 13–30. [Google Scholar] [CrossRef]
- Li, H.; Liu, C.; Li, R.; Zhou, L.; Ran, Y.; Yang, Q.; Huang, H.; Lu, H.; Song, H.; Yang, B.; et al. AARS1 and AARS2 sense l-lactate to regulate cGAS as global lysine lactyltransferases. Nature 2024, 634, 1229–1237. [Google Scholar] [CrossRef] [PubMed]
- Brooks, G.A. Lactate as a fulcrum of metabolism. Redox Biol. 2020, 35, 101454. [Google Scholar] [CrossRef]
- Li, L.; Jiang, D.; Liu, H.; Guo, C.; Zhao, R.; Zhang, Q.; Xu, C.; Qin, Z.; Feng, J.; Liu, Y.; et al. Comprehensive proteogenomic characterization of early duodenal cancer reveals the carcinogenesis tracks of different subtypes. Nat. Commun. 2023, 14, 1751. [Google Scholar] [CrossRef] [PubMed]
- Ruan, G.-T.; Xie, H.-L.; Zhu, L.-C.; Ge, Y.-Z.; Yan, L.; Liao, C.; Gong, Y.-Z.; Shi, H.-P. Immune ULBP1 is Elevated in Colon Adenocarcinoma and Predicts Prognosis. Front. Genet. 2022, 13, 762514. [Google Scholar] [CrossRef]
- Chen, Z.; Mei, K.; Xiao, Y.; Xiong, Y.; Long, W.; Wang, Q.; Zhong, J.; Di, D.; Ge, Y.; Luo, Y.; et al. Prognostic Assessment of Oxidative Stress-Related Genes in Colorectal Cancer and New Insights into Tumor Immunity. Oxidative Med. Cell Longev. 2022, 2022, 2518340. [Google Scholar] [CrossRef]
- Liu, L.; Gao, J.; Liu, X.; Zhang, F.; Hu, B.; Zhang, H.; Wang, Z.; Tang, H.; Shi, J.H.; Zhang, S. AARS2 as a novel biomarker for prognosis and its molecular characterization in pan-cancer. Cancer Med. 2023, 12, 21531–21544. [Google Scholar] [CrossRef]
- Zhu, Z. A novel mitochondria-related gene signature for controlling colon cancer cell mitochondrial respiration and proliferation. Hum. Cell 2022, 35, 1126–1139. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.; Huang, Y.; Li, Q.; Wu, Y.; Zheng, L. A Comprehensive Prognostic Model for Colon Adenocarcinoma Depending on Nuclear-Mitochondrial-Related Genes. Technol. Cancer Res. Treat. 2024, 23, 15330338241258570. [Google Scholar] [CrossRef]
- Baghban, R.; Roshangar, L.; Jahanban-Esfahlan, R.; Seidi, K.; Ebrahimi-Kalan, A.; Jaymand, M.; Kolahian, S.; Javaheri, T.; Zare, P. Tumor microenvironment complexity and therapeutic implications at a glance. Cell Commun. Signal. 2020, 18, 59. [Google Scholar] [CrossRef]
- Binnewies, M.; Roberts, E.W.; Kersten, K.; Chan, V.; Fearon, D.F.; Merad, M.; Coussens, L.M.; Gabrilovich, D.I.; Ostrand-Rosenberg, S.; Hedrick, C.C.; et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat. Med. 2018, 24, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Han, F.; Du, Y.; Shi, H.; Zhou, W. Hypoxic microenvironment in cancer: Molecular mechanisms and therapeutic interventions. Signal Transduct. Target. Ther. 2023, 8, 70. [Google Scholar] [CrossRef] [PubMed]
- Bristow, R.G.; Hill, R.P. Hypoxia, DNA repair and genetic instability. Nat. Rev. Cancer 2008, 8, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Xing, Z.; Yang, T.; Li, X.; Xu, H.; Hong, Y.; Shao, S.; Li, T.; Ye, L.; Li, Y.; Jin, X.; et al. High-glucose-associated YTHDC1 lactylation reduces the sensitivity of bladder cancer to enfortumab vedotin therapy. Cell Rep. 2025, 44, 115545. [Google Scholar] [CrossRef]
- Lin, J.; Yin, Y.; Cao, J.; Zhang, Y.; Chen, J.; Chen, R.; Zou, B.; Huang, C.; Lv, Y.; Xu, S.; et al. NUDT21 lactylation reprograms alternative polyadenylation to promote cuproptosis resistance. Cell Discov. 2025, 11, 52. [Google Scholar] [CrossRef]
- Safa, A.R. Drug and apoptosis resistance in cancer stem cells (CSCs): A puzzle with many pieces. Cancer Drug Resist. 2022, 5, 850–872. [Google Scholar] [CrossRef]
- Nussinov, R.; Tsai, C.-J.; Jang, H. Anticancer drug resistance: An update and perspective. Drug Resist. Updates 2021, 59, 100796. [Google Scholar] [CrossRef]
- Shalem, O.; Sanjana, N.E.; Zhang, F. High-throughput functional genomics using CRISPR-Cas9. Nat. Rev. Genet. 2015, 16, 299–311. [Google Scholar] [CrossRef]
- Liu, H.; Wang, P. CRISPR screening and cell line IC50 data reveal novel key genes for trametinib resistance. Clin. Exp. Med. 2024, 25, 21. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; An, R.; Umanah, G.K.; Park, H.; Nambiar, K.; Eacker, S.M.; Kim, B.; Bao, L.; Harraz, M.M.; Chang, C.; et al. A nuclease that mediates cell death induced by DNA damage and poly(ADP-ribose) polymerase-1. Science 2016, 354, aad6872. [Google Scholar] [CrossRef]
- Olivier, M.; Hollstein, M.; Hainaut, P. TP53 Mutations in Human Cancers: Origins, Consequences, and Clinical Use. Cold Spring Harb. Perspect. Biol. 2010, 2, a001008. [Google Scholar] [CrossRef]
- Kandoth, C.; McLellan, M.D.; Vandin, F.; Ye, K.; Niu, B.; Lu, C.; Xie, M.; Zhang, Q.; McMichael, J.F.; Wyczalkowski, M.A.; et al. Mutational landscape and significance across 12 major cancer types. Nature 2013, 502, 333–339. [Google Scholar] [CrossRef]
- Colegio, O.R.; Chu, N.-Q.; Szabo, A.L.; Chu, T.; Rhebergen, A.M.; Jairam, V.; Cyrus, N.; Brokowski, C.E.; Eisenbarth, S.C.; Phillips, G.M.; et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature 2014, 513, 559–563. [Google Scholar] [CrossRef]
- Faubert, B.; Li, K.Y.; Cai, L.; Hensley, C.T.; Kim, J.; Zacharias, L.G.; Yang, C.; Do, Q.N.; Doucette, S.; Burguete, D.; et al. Lactate Metabolism in Human Lung Tumors. Cell 2017, 171, 358–371.e9. [Google Scholar] [CrossRef]
- Zhang, X.-W.; Li, L.; Liao, M.; Liu, D.; Rehman, A.; Liu, Y.; Liu, Z.-P.; Tu, P.-F.; Zeng, K.-W. Thermal Proteome Profiling Strategy Identifies CNPY3 as a Cellular Target of Gambogic Acid for Inducing Prostate Cancer Pyroptosis. J. Med. Chem. 2024, 67, 10005–10011. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Liu, R.; Wang, B.; Xiong, R.; Cui, L.; Liao, Y.; Ruan, Y.; Fang, L.; Lu, X.; Yu, X.; et al. Inhibition of HDAC2 sensitises antitumour therapy by promoting NLRP3/GSDMD-mediated pyroptosis in colorectal cancer. Clin. Transl. Med. 2024, 14, e1692. [Google Scholar] [CrossRef]
- Krauß, L.; Urban, B.C.; Hastreiter, S.; Schneider, C.; Wenzel, P.; Hassan, Z.; Wirth, M.; Lankes, K.; Terrasi, A.; Klement, C.; et al. HDAC2 Facilitates Pancreatic Cancer Metastasis. Cancer Res. 2022, 82, 695–707. [Google Scholar] [CrossRef] [PubMed]
- Qiao, X.; Wu, X.; Chen, S.; Niu, M.-M.; Hua, H.; Zhang, Y. Discovery of novel and potent dual-targeting AXL/HDAC2 inhibitors for colorectal cancer treatment via structure-based pharmacophore modelling, virtual screening, and molecular docking, molecular dynamics simulation studies, and biological evaluation. J. Enzym. Inhib. Med. Chem. 2024, 39, 2295241. [Google Scholar] [CrossRef]
- Feng, F.; Wu, J.; Chi, Q.; Wang, S.; Liu, W.; Yang, L.; Song, G.; Pan, L.; Xu, K.; Wang, C. Lactylome Analysis Unveils Lactylation-Dependent Mechanisms of Stemness Remodeling in the Liver Cancer Stem Cells. Adv. Sci. 2024, 11, e2405975. [Google Scholar] [CrossRef]
- Song, F.; Hou, C.; Huang, Y.; Liang, J.; Cai, H.; Tian, G.; Jiang, Y.; Wang, Z.; Hou, J. Lactylome analyses suggest systematic lysine-lactylated substrates in oral squamous cell carcinoma under normoxia and hypoxia. Cell. Signal. 2024, 120, 111228. [Google Scholar] [CrossRef]
- Yang, D.; Yin, J.; Shan, L.; Yi, X.; Zhang, W.; Ding, Y. Identification of lysine-lactylated substrates in gastric cancer cells. iScience 2022, 25, 104630. [Google Scholar] [CrossRef]
- Duan, Y.; Zhan, H.; Wang, Q.; Li, B.; Gao, H.; Liu, D.; Xu, Q.; Gao, X.; Liu, Z.; Gao, P.; et al. Integrated Lactylome Characterization Reveals the Molecular Dynamics of Protein Regulation in Gastrointestinal Cancers. Adv. Sci. 2024, 11, e2400227. [Google Scholar] [CrossRef]
- Narita, T.; Weinert, B.T.; Choudhary, C. Functions and mechanisms of non-histone protein acetylation. Nat. Rev. Mol. Cell Biol. 2019, 20, 156–174. [Google Scholar] [CrossRef]
- Tang, Y.; Luo, J.; Zhang, W.; Gu, W. Tip60-Dependent Acetylation of p53 Modulates the Decision between Cell-Cycle Arrest and Apoptosis. Mol. Cell 2006, 24, 827–839. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Tan, Y.; Zhang, C.; Zhang, Y.; Zhang, L.; Ren, P.; Deng, H.; Luo, J.; Ke, Y.; Du, X. NAT10 regulates p53 activation through acetylating p53 at K120 and ubiquitinating Mdm2. EMBO Rep. 2016, 17, 349–366. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Song, T.; Ning, J.; Wang, Z.; Yin, Z.; Jiang, P.; Yuan, Q.; Yu, W.; Cheng, F. Lactylation in cancer: Mechanisms in tumour biology and therapeutic potentials. Clin. Transl. Med. 2024, 14, e70070. [Google Scholar] [CrossRef]
- Wang, L.; Li, S.; Luo, H.; Lu, Q.; Yu, S. PCSK9 promotes the progression and metastasis of colon cancer cells through regulation of EMT and PI3K/AKT signaling in tumor cells and phenotypic polarization of macrophages. J. Exp. Clin. Cancer Res. 2022, 41, 303. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-H.; Zhang, P.; Peng, W.-B.; Ye, L.-L.; Xiang, X.; Wei, X.-S.; Niu, Y.-R.; Zhang, S.-Y.; Xue, Q.-Q.; Wang, H.-L.; et al. Altered phenotypic and metabolic characteristics of FOXP3+CD3+CD56+ natural killer T (NKT)-like cells in human malignant pleural effusion. OncoImmunology 2023, 12, 2160558. [Google Scholar] [CrossRef]
- Xiong, J.; He, J.; Zhu, J.; Pan, J.; Liao, W.; Ye, H.; Wang, H.; Song, Y.; Du, Y.; Cui, B.; et al. Lactylation-driven METTL3-mediated RNA m6A modification promotes immunosuppression of tumor-infiltrating myeloid cells. Mol. Cell 2022, 82, 1660–1677.e10. [Google Scholar] [CrossRef] [PubMed]
- Christofk, H.R.; Vander Heiden, M.G.; Harris, M.H.; Ramanathan, A.; Gerszten, R.E.; Wei, R.; Fleming, M.D.; Schreiber, S.L.; Cantley, L.C. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature 2008, 452, 230–233. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, D.; Robay, D.; Hindupur, S.K.; Pohlmann, J.; Colombi, M.; El-Shemerly, M.Y.; Maira, S.-M.; Moroni, C.; Lane, H.A.; Hall, M.N. Dual Inhibition of the Lactate Transporters MCT1 and MCT4 Is Synthetic Lethal with Metformin due to NAD+ Depletion in Cancer Cells. Cell Rep. 2018, 25, 3047–3058.e4. [Google Scholar] [CrossRef]
- Doherty, J.R.; Yang, C.; Scott, K.E.N.; Cameron, M.D.; Fallahi, M.; Li, W.; Hall, M.A.; Amelio, A.L.; Mishra, J.K.; Li, F.; et al. Blocking Lactate Export by Inhibiting the Myc Target MCT1 Disables Glycolysis and Glutathione Synthesis. Cancer Res. 2014, 74, 908–920. [Google Scholar] [CrossRef] [PubMed]
| Protein | Types of Cancer | Expression in Cancer | Survival and Prognosis | Mechanism and Modification Site | Signaling Pathway | Roles in Cancer | Ref. |
|---|---|---|---|---|---|---|---|
| AARS1 | Bladder cancer | N/A | N/A | K82 lactylation on YTHDC1 protein | YTHDC1-RNF183-JUND-NECTIN4-EV therapy | Reducing cancer cell sensitivity to EV therapy | [57] |
| AARS1 | Breast cancer | Overexpression | Negatively associated | K120 and K139 lactylation on p53 protein | p53 pathway | Promoting cancer cell proliferation | [15] |
| AARS1 | Duodenal cancer | Overexpression | N/A | K621 alanylation on PARP1 protein | DNA damage and cell apoptosis | Suppressing cancer cell apoptosis | [47,63] |
| AARS1 | Esophageal squamous cell carcinoma | N/A | N/A | K23 lactylation on NUDT21 protein | NUDT21-CPSF6-FDX1-cuproptosis | Reducing cancer cells sensitivity to cuproptosis | [58] |
| AARS1 | Gastric cancer | Overexpression | Negatively associated | K90 lactylation on YAP protein, K108 lactylation on TEAD1 protein | Hippo pathway | Promoting cancer cell proliferation | [16] |
| AARS2 | Colon adenocarcinoma | Overexpression | Negatively associated | Regulating mitochondrial respiration | N/A | Promoting cell proliferation | [51] |
| AARS2 | Colorectal cancer | Overexpression | Positively associated | N/A | N/A | N/A | [49] |
| AARS2 | Hepatocellular carcinoma | Overexpression | Negatively associated | Regulating cell cycle | mTOR signaling pathway | Promoting cancer cell proliferation and migration | [50] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, L.; Guo, J.; Jia, R. AARS1 and AARS2: From Protein Synthesis to Lactylation-Driven Oncogenesis. Biomolecules 2025, 15, 1323. https://doi.org/10.3390/biom15091323
Gao L, Guo J, Jia R. AARS1 and AARS2: From Protein Synthesis to Lactylation-Driven Oncogenesis. Biomolecules. 2025; 15(9):1323. https://doi.org/10.3390/biom15091323
Chicago/Turabian StyleGao, Lingyue, Jihua Guo, and Rong Jia. 2025. "AARS1 and AARS2: From Protein Synthesis to Lactylation-Driven Oncogenesis" Biomolecules 15, no. 9: 1323. https://doi.org/10.3390/biom15091323
APA StyleGao, L., Guo, J., & Jia, R. (2025). AARS1 and AARS2: From Protein Synthesis to Lactylation-Driven Oncogenesis. Biomolecules, 15(9), 1323. https://doi.org/10.3390/biom15091323






