Abstract
Osteoarthritis (OA) is now widely recognized not merely as a cartilage-centric disease but as a multifactorial disorder affecting the entire joint as an organ, including the articular cartilage, subchondral bone, synovium, ligaments, menisci, and the innervating neural elements. This review explores the complex pathophysiology of OA with a focus on the emerging mechanisms of pain and inflammation that extend beyond articular cartilage degradation. Joint inflammation driven by immune activation in response to cellular stress signals promotes the release of pro-inflammatory mediators and catabolic enzymes. Key signaling pathways such as NF-κB, MAPKs, and JAK/STAT amplify these responses, and pain is sustained through peripheral and central sensitization, contributing to exacerbating symptoms even in the absence of visible joint damage. This review also integrates molecular and cellular mechanisms to highlight innovative therapies aimed at modifying both the structural damage and neurosensory drivers of pain. These approaches offer the potential to not only alleviate symptoms but also alter disease progression, signaling a move toward personalized, mechanism-based treatments. Given the intricate interactions among joint tissues, immune activation, and sensory processing, a comprehensive strategy that targets both structural degeneration and neuroinflammation is essential for the future of OA management. Emphasizing the joint as an integrated organ, we advocate for translational research linking molecular pathology with clinically meaningful outcomes.
Keywords:
osteoarthritis; pain; cartilage; degeneration; inflammation; regeneration; pain management 1. Pathophysiology of OA as a Whole-Joint Disease
Osteoarthritis (OA) is a worldwide joint disorder that plays a major role in the global chronic pain scenario [1,2,3,4]. It progresses through distinct stages, each marked by an increasing severity of symptoms and joint degeneration, leading to pain and disability. The effectiveness of current pain management strategies for OA is limited, as traditional painkillers often do not offer sustained relief and are linked with adverse side effects [5,6,7,8,9,10]. This gap in effective pain management often leads to joint replacement, which accounts for 90% of all hip and knee arthroplasties, imposing a significant economic burden [11,12,13,14]. The annual cost in the United States is estimated at USD 136.8 billion, including both healthcare expenses and indirect costs [15,16,17].
Traditionally regarded as a disease primarily affecting articular cartilage, OA is now widely understood to involve the entire joint as an organ system [18]. This paradigm shift recognizes that OA pathology includes not only cartilage degradation but also synovial inflammation, subchondral bone remodeling, meniscal damage, ligament laxity, and alterations in the periarticular muscles, with each joint tissue playing a distinct role in the pain and dysfunction associated with the disease (Figure 1) [18,19,20,21,22,23].
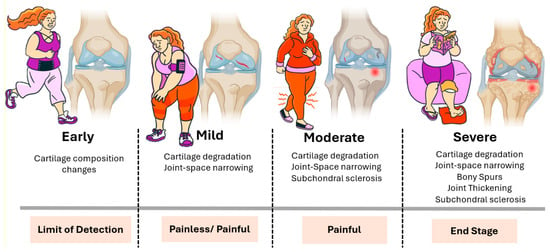
Figure 1.
Clinical and structural progression of osteoarthritis. Illustration of the typical stages of OA progression, both in terms of joint structure and patient experience. In the early stage, subtle biochemical and compositional changes occur in the cartilage, typically without noticeable symptoms and below the threshold of standard detection methods. In the mild stage, cartilage degradation and joint space narrowing become evident, although symptoms may range from absent to intermittent pain. The moderate stage is characterized by more pronounced cartilage loss, subchondral bone sclerosis, and persistent joint pain. In the severe stage, structural damage is extensive, including bony spur formation (osteophytes), joint thickening, and significant subchondral sclerosis. This stage is typically associated with chronic pain and functional impairment and often represents the endpoint of disease progression. The horizontal bars below each stage indicate both the clinical detectability and pain profile, underscoring the disconnect between the radiographic findings and patient-reported symptoms at earlier stages.
An imbalance between anabolic and catabolic activities in chondrocytes leads to the deterioration of articular cartilage, which cushions the ends of bones [24,25], resulting in pain and reduced joint function [18,19,25,26,27]. Changes in the subchondral bone, such as the formation of bone spurs and structural alterations, can further worsen symptoms [27,28,29,30,31,32,33,34]. The inflammation of the synovial membrane contributes to swelling and discomfort, while damage to the surrounding ligaments and muscles can compromise joint stability and function, intensifying OA symptoms [32,33,35,36,37,38,39,40]. These pathological changes seen in OA are due to a combination of mechanical, genetic, and biochemical processes that occur in these joint tissues (Figure 2) [22,25,26,41,42]. Biochemical processes involve the release of enzymes and inflammatory mediators that further damage the joint structure [43]. Inflammation plays a central role in OA, leading to pain, swelling, and further tissue degradation [19,26,43,44,45,46]. Mechanical stress from overuse, injury, or excess weight can lead to cartilage breakdown and changes in the bone, contributing to the development and progression of OA [25,47,48,49,50]. The interaction between these factors leads to a cycle of worsening joint damage and symptoms [25,49].
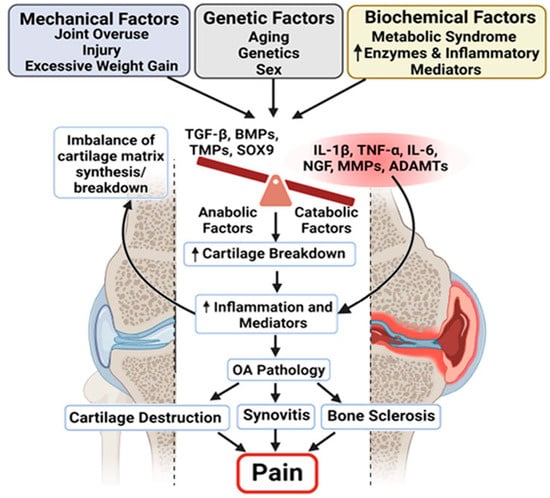
Figure 2.
Multifactorial drivers of osteoarthritis pathology and pain. Schematic illustrating the complex interplay between mechanical, genetic, and biochemical factors in the development and progression of OA. Mechanical stressors (e.g., joint overuse, injury, excess weight), genetic predispositions (e.g., age, sex, inherited traits), and biochemical influences (e.g., metabolic syndrome and inflammatory mediators) converge to disrupt the balance between anabolic and catabolic processes in the joint. This imbalance leads to an increased breakdown of the cartilage matrix and the heightened production of inflammatory mediators. The resulting inflammation contributes to the key pathological features of OA: cartilage destruction, synovitis, and subchondral bone sclerosis. Collectively, these changes lead to chronic joint pain, the hallmark symptom of OA, highlighting the need for therapies that address both structural damage and inflammatory pain pathways. The arrows indicate proposed mechanistic links contributing to the progression of joint tissue degeneration and pain.
Therefore, understanding the individual contributions and the complex, multifactorial interplay of joint tissues in relation to pain is essential not only for comprehending symptomatic OA but also for developing effective treatments that target the multifaceted nature of OA [44,51,52,53]. This broader understanding significantly influences drug development approaches, as effective treatments must reach multiple joint compartments and cell types. Moreover, outcome measures should extend beyond cartilage repair to encompass overall joint health and functional improvement.
2. Pain as Both a Sensory and Emotional Experience
Nociception is the physiological process of detecting harmful stimuli and forms the basis of pain experience [54,55,56]. Upon encountering potentially damaging stimuli, the nociceptors, which are sensory neurons in the tissues, initiate pain transmission [54,55,56,57,58]. This process is intricately linked to aberrant inputs from dorsal root ganglion (DRG) neurons. These pseudo-unipolar cells possess an axonal stalk that branches into two terminals: the peripheral terminal, which innervates peripheral tissues, and the central terminal, which extends to the dorsal horn of the spinal cord [59]. This unique structure enables nociceptors to both receive and transmit signals at each terminal. Triggered by a spectrum of stimuli detected by specialized receptors, they initiate a cascade of voltage-gated sodium and potassium channels, instrumental in the generation and propagation of action potentials [59,60,61]. These action potentials convey distress signals along the nociceptor axons, ultimately transmitting them to higher levels of the nervous system, from the brainstem to the thalamus and cortex [62].
Unlike nociception, pain is shaped by biological, psychological, and social factors, making it a subjective experience not always reflected in nerve activity. Pain also plays a fundamental role in the healing process, serving as an alert mechanism that initiates behaviors promoting recovery following surgery, injury, or illness [63]. The mindset of a patient can significantly impact their healing process. Based on the opinion of Stanford experts, positive attitudes and supportive social environments have been shown to measurably enhance physical healing [64]. This indicates that the perception and internal processing of pain are integral to recovery. Effective pain management can improve quality of life and decrease neuroplastic changes associated with pain [65,66].
3. Exploring Pain in OA: Beyond Sensation to Emotion
Nociceptors are abundant in the joint capsule, ligaments, periosteum, menisci, subchondral bone, and synovium, playing a key role in the complex process of perception [21,67,68,69]. Although cartilage lacks nerve structures and does not directly cause pain, its degradation or damage releases factors that stimulate pain [69,70,71]. Moreover, the role of subchondral bone is increasingly recognized in the pathogenesis of OA pain [23,72]. Changes in bone structure and increased bone turnover can contribute to the pain experienced by OA patients [71,73]. The subchondral bone might become more sensitive due to an influx in nerve fibers or changes in blood supply, leading to an enhanced pain response [28,74,75,76]. However, the precise mechanisms and specific joint tissues responsible remain unclear [34,77]. A comprehensive review by Martel-Pelletier et al. provides an in-depth analysis of OA, including its pathophysiology, structural changes within joints, and the multifactorial nature of pain in OA. The authors discuss the complex interplay between cartilage degradation, inflammation, and the involvement of other joint tissues. This reference is valuable as it offers insights into the pain mechanisms, highlighting the areas where further research is needed to effectively treat this condition [41].
Under normal circumstances, nociceptive responses to painful stimuli are transient. Adding to the complexity of this pain pathway, any disturbances in the biochemical environment of the joint, mediated by factors such as cytokines and neuropeptides, can lower the activation threshold of nociceptors, leading to heightened sensitivity and pain [78,79,80,81]. Therefore, in the context of chronic pathology, pain pathways can undergo significant changes, leading to hypersensitivity, a state known as sensitization. This increased sensitivity can lead to mechanical allodynia, causing pain during joint movement (Figure 3) [58,65,82]. Furthermore, the continued input from pain sensors can induce long-lasting changes in the central nervous system, resulting in central sensitization, characterized by the prolonged hyperexcitability of the pain pathway [57,58,81,83,84].
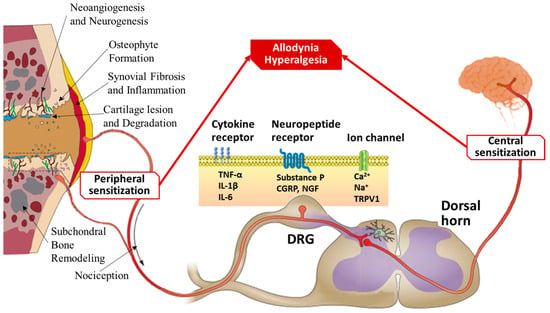
Figure 3.
Mechanisms of pain sensitization in osteoarthritis. Schematic illustrating the molecular and neural pathways contributing to OA-associated pain, with a focus on peripheral and central sensitization processes. Joint degeneration triggers peripheral sensitization through cartilage degradation, subchondral bone remodeling, osteophyte formation, synovial inflammation, and neoangiogenesis, leading to increased nociceptive signaling. Inflammatory mediators such as TNF-α, IL-1β, and IL-6 bind to cytokine receptors on nociceptive neurons. Neuropeptides such as substance P, CGRP, and NGF act on their respective receptors, while ion channels (e.g., TRPV1) facilitate calcium and sodium influx, amplifying pain signals. These changes culminate in allodynia (pain due to non-painful stimuli) and hyperalgesia (increased pain from painful stimuli). Persistent nociceptive input from the periphery to the DRG and dorsal horn of the spinal cord leads to central sensitization, whereby the central nervous system becomes hyperresponsive to sensory input, further contributing to chronic pain in OA.
Researchers are exploring the concept of central sensitization, where the central nervous system becomes more sensitized to pain signals from the joint, amplifying the perception of pain [58,65,83,85,86,87]. Central sensitization may disrupt the correlation between structural joint changes and pain perception, explaining why some individuals with OA experience pain that appears disproportionate to radiographic findings [65,88]. Interestingly, the effects of central sensitization may be reversible after successful joint replacement surgeries, suggesting that ongoing peripheral input is necessary to sustain central nervous system alterations in most patients [65,89].
3.1. The Role of DAMPs and PRRs in the Pathogenesis of Pain in OA
OA is frequently referred to as a “wear and tear” disease [18,19,26,90,91,92,93,94]. When cartilage is damaged, it releases Damage-Associated Molecular Patterns (DAMPs), which are diverse and include extracellular matrix (ECM) breakdown products, such as fibronectin fragments, and cellular alarmins like high-mobility group box 1 (HMGB1), S100A8/9, and HSP [95,96]. These molecules are not merely debris; they actively participate in the disease process, leading to a cascade of molecular responses that perpetuate joint degeneration [19,34,70,97,98,99,100,101]. They function as endogenous danger signals, alerting the body to tissue damage [95,102,103,104,105,106,107].
DAMPs are recognized by pattern-recognition receptors (PRRs), which are expressed by various cells within the joint, including synovial macrophages, fibroblast-like synoviocytes, and chondrocytes (Figure 4) [34,95,108,109,110,111,112,113,114]. Upon DAMP recognition, PRRs trigger intracellular signaling pathways that lead to the production of inflammatory mediators such as cytokines (e.g., IL-1β and TNF-α), chemokines, and other factors that promote inflammation and pain. The binding of alarmins to PRRs initiates a cascade of inflammatory responses that contribute to joint damage [96,115,116]. The activation of PRRs leads to the upregulation of proteolytic enzymes, including matrix metalloproteinases (MMPs) and aggrecanases (ADAMTSs), which further degrade the ECM of the cartilage [44,91,108,115,117,118,119,120]. This production of inflammatory mediators and proteolytic enzymes establishes a feedback loop. As the ECM degrades, more DAMPs are released, which in turn activate more PRRs, leading to further inflammation and tissue damage. This cycle becomes self-amplifying and is a central driver of the progressive nature of OA [18,19,26,96,98,99,121].
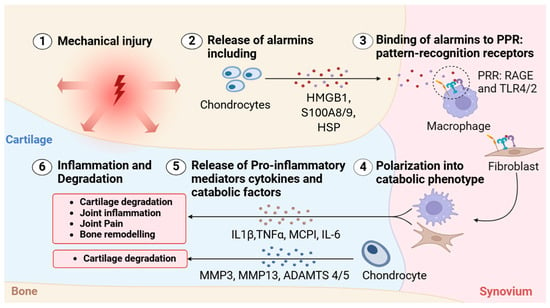
Figure 4.
Alarmin-mediated activation of inflammatory cascades in osteoarthritis. Schematic depicting the sequential molecular events initiated by mechanical injury that contribute to inflammation and tissue degradation in OA. (1) Mechanical injury to joint tissues leads to cellular stress and damage, particularly within cartilage. (2) Chondrocytes respond by releasing DAMPs, also known as alarmins, including high-mobility group box 1 (HMGB1), S100A8/9, and heat shock proteins (HSPs). (3) These alarmins bind to pattern-recognition receptors (PRRs), such as RAGE and Toll-like receptors (TLR2/4), primarily on macrophages. (4) This interaction activates fibroblasts and chondrocytes, polarizing them into a catabolic phenotype. (5) Polarized cells release a range of pro-inflammatory cytokines and catabolic enzymes, including IL-1β, TNF-α, monocyte chemoattractant protein-1 (MCP1), IL-6, matrix metalloproteinases (MMP-3 and MMP-13), and aggrecanases (ADAMTS-4 and -5). (6) These mediators drive a feedback loop of inflammation, cartilage degradation, joint pain, and bone remodeling, perpetuating the progression of OA.
3.2. Pain Markers in Osteoarthritis
Pain in OA is multifactorial, arising from the interplay between cartilage degradation, inflammatory mediators, and nociceptive signaling [34,122]. In response to cartilage damage, chondrocytes attempt to repair the tissue by increasing ECM production [123]. However, this process also triggers the production of catabolic factors like Runx2 and matrix metalloproteinase-13 (MMP-13), which break down ECM even further [120,124]. This leads to the early stages of OA marked by cartilage thinning, type X collagen production, and the formation of surface fibrillation [125,126].
The DAMPs released due to the breakdown of the cartilage trigger inflammatory signaling cascades, with IL-1β and tumor necrosis factor-alpha (TNF-α) as primary pro-inflammatory cytokines implicated in OA [18,96,127]. IL-1 receptor type I (IL-1RI), activated by the binding of IL-1β, promotes the upregulation of aggrecanases, such as A Disintegrin and Metalloproteinase with Thrombospondin Motifs (ADAMTS-4 and ADAMTS-5) and MMPs, which further degrade ECM components [18,120,127,128,129]. Studies have shown that OA chondrocytes express increased levels of IL-1RI compared to normal chondrocytes [130]. Additionally, elevated levels of IL-1β have been detected in OA-affected synovial fluid, cartilage, and subchondral bone [131,132]. Alongside IL-1β, TNF-α plays a synergistic role by activating TNF receptors (TNF-Rs), which further amplify inflammation and ECM degradation [133,134,135].
At the molecular level, IL-1β and TNF-α activate several key signaling pathways that cause cartilage degradation and pain in OA [18]. The mitogen-activated protein kinase (MAPK) pathway is one of them and includes Janus Kinase/Signal Transduction and Activators of Transcription (JNK/STAT), p38, and Extracellular Signal-Regulated Kinase (Figure 5) [18,136,137,138]. These pathways regulate the expression of matrix proteins and catabolic enzymes, such as MMP-1, -3, -9, -13, and ADAMTS-4 and -5, with the release of inflammatory cytokines (IL-1β, IL-6, IL-17, IL-23, CCL2) and pain mediators, including the nerve growth factor (NGF) and substance P (SP) [18,138,139].
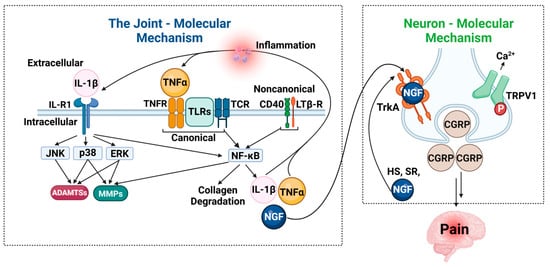
Figure 5.
Molecular mechanisms linking inflammation and pain in osteoarthritis. Schematic outlining the key molecular pathways contributing to inflammation and pain in osteoarthritis (OA), divided into joint-related and neuron-related mechanisms. Left panel—Joint: molecular mechanism: inflammatory cytokines such as IL-1β and TNF-α bind to their respective receptors (IL-1R and TNFR), initiating intracellular signaling cascades through the canonical and non-canonical NF-κB pathways. These cascades activate downstream kinases such as JNK, p38, and ERK, resulting in upregulation of matrix-degrading enzymes, including matrix metalloproteinases (MMPs) and aggrecanases (ADAMTSs). This contributes to extracellular matrix (ECM) degradation, synovial inflammation, and joint degeneration. Additional signaling via TLRs, CD40, and the lymphotoxin-β receptor (LTβR) reinforces the inflammatory response. These processes also stimulate the production of NGF, IL-1β, and TNF-α, further propagating inflammation. Right panel—Neuron: molecular mechanism: NGF binds to its high-affinity receptor TrkA on sensory neurons, triggering retrograde transport and the release of pain mediators such as calcitonin gene-related peptide (CGRP). NGF-TrkA signaling also activates ion channels like TRPV1, increasing calcium influx and neuronal excitability, which contributes to peripheral sensitization and pain perception. Together, these molecular interactions underscore the bidirectional relationship between joint inflammation and neuronal pain mechanisms in OA.
Another important pathway that plays a central role in cartilage degradation and inflammation is the nuclear factor kappa B (NF-κB) [140,141,142,143,144,145]. NF-κB signaling can occur through two main mechanisms: the canonical NF-κB pathway, triggered by Toll-like receptors (TLRs), TNF receptors (TNF-Rs), and IL-1RI, and the non-canonical pathway, driven by CD40, lymphotoxin β (LTβ), and B-cell activating factors [143,144,145]. The exacerbation of these inflammatory mediators further sensitizes nociceptive pathways. IL-1β and TNF-α induce the expression of the nerve growth factor (NGF), which binds to tropomyosin receptor kinase A (TrkA) on sensory neurons [146,147]. This NGF–TrkA complex is retrogradely transported to neuronal cell bodies, upregulating pain-related gene expression, including the synthesis of substance P (SP) and the calcitonin gene-related peptide (CGRP), which enhance pain transmission [146]. Furthermore, NGF binding to TrkA activates ion channels such as transient receptor potential vanilloid 1 (TRPV1) and voltage-gated sodium channels, leading to nociceptor depolarization and sensitization [146,148,149]. Elevated NGF and SP levels have been detected in OA joints, further reinforcing their role in chronic pain development [150,151,152,153].
Another emerging aspect of inflammation in OA pathophysiology is macrophage polarization, which occurs through cellular crosstalk [114,135,154]. In response to microenvironmental stimuli, macrophages differentiate into classically activated (M1) macrophages or alternatively activated (M2) macrophages [155,156]. M1 macrophages play a dominant role in driving inflammation and ECM breakdown, while M2 is involved in tissue remodeling and immunomodulation [127,155,156]. An imbalance favoring M1 macrophages driven by IL-1β and TNF-α leads to the further secretion of pro-inflammatory mediators, resulting in persistent inflammation, worsening cartilage degradation, and chronic pain in OA [127,154,155,156].
The synovial membrane also undergoes chronic inflammatory changes, involving both innate and adaptive immune responses [18,44,154]. The continued release of inflammatory mediators amplifies pain signaling, accelerating the disease progression. Additionally, mast cell activation leads to the secretion of histamine, serotonin, and additional NGF, creating a positive feedback loop that perpetuates inflammation and pain [157,158].
4. Current Treatments for OA
The current treatment options for OA are primarily palliative, as no established gold standard for disease-modifying therapy exists [159]. The non-pharmacological interventions are the foundation of early OA therapy (Figure 6). Weight loss, exercise, and physical therapy can significantly reduce pain and improve mobility in many patients [160].
Furthermore, pharmacological treatments such as Acetaminophen and oral non-steroidal anti-inflammatory drugs (NSAIDs) are commonly used to reduce pain and inflammation [161,162]. However, the long-term use of these medications can cause gastrointestinal, renal, or cardiovascular side effects [162,163]. In addition, topical analgesic therapies offer a non-invasive option with fewer systemic side effects. These include capsaicin cream, which depletes substance P from sensory neurons to reduce pain signaling, and methyl salicylate creams, which produce a counterirritant effect and mild anti-inflammatory action. Such topical agents can be particularly beneficial for patients with localized joint pain or those who cannot tolerate systemic medications [164]. In patients with severe pain, unresponsive to first-line analgesics, the short-term use of opioids or adjuncts like duloxetine may be considered, but these carry significant side effects. While these medications can improve day-to-day comfort, they do not prevent ongoing cartilage degeneration [162,165].
For localized symptomatic relief, intra-articular (IA) injections are another key option (Figure 6). Corticosteroid injections can rapidly reduce joint inflammation and pain, with a temporary effect [165]. However, repeated steroid injections are generally limited due to potential deleterious effects on cartilage, such as chondrocyte toxicity, accelerated cartilage thinning, subchondral bone osteonecrosis, and risks of soft-tissue atrophy or systemic glucocorticoid exposure [165,166,167,168]. Visco supplementation with hyaluronic acid is also used in knee OA to improve lubrication; however, it may cause transient adverse events, including injection-site pain, swelling or effusion, local erythema, allergic reactions, and acute pseudoseptic inflammatory flares [169,170].
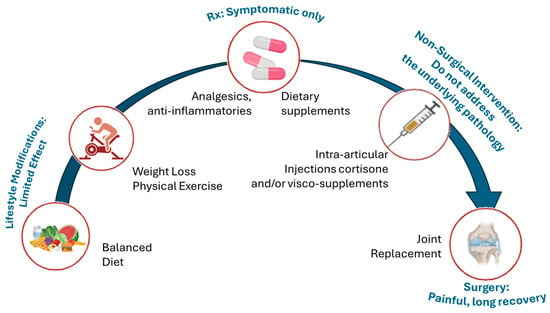
Figure 6.
Current stepwise management of OA and associated limitations. Figure outlining the typical progression of treatment strategies for OA, from early lifestyle modifications to end-stage surgical intervention. The current treatment approach for osteoarthritis (OA) begins with lifestyle changes and non-opioid medications to manage symptoms but does not address the root cause of the disease. As OA progresses, options like intra-articular injections may provide temporary relief, but cartilage degeneration continues. When these methods fail, joint replacement surgery becomes necessary, though it is invasive and costly. This progression underscores the urgent need for therapies that can modify the disease earlier and more effectively.
Surgical interventions such as joint arthroplasties become appropriate in advanced OA (Figure 6) or when conservative measures fail to provide relief [171,172,173]. Although they can reduce pain and restore joint function, it remains an invasive, high-cost surgery typically reserved for late-stage disease [171]. Table 1 summarizes the spectrum of current management options for OA [23,162,165].

Table 1.
Current OA treatments and their therapeutic targets.
5. Regenerative Therapies Targeting Pain Pathways
Given the limitations of conventional therapies, there is a need for alternative approaches that not only slow disease progression but also effectively alleviate pain [164]. Tissue engineering and regenerative medicine have therefore emerged as promising strategies to promote the growth of new cartilage, protect existing tissue, and potentially reverse the pathological joint environment, thereby reducing pain [164,174,175,176]. Since chronic inflammation is a key driver of both joint degeneration and pain, incorporating anti-inflammatory strategies within regenerative approaches is essential [124]. These strategies integrate engineering techniques with biological components, such as cells and bioactive molecules, to rebuild functional tissue and provide more lasting relief from OA-related pain [177,178].
5.1. Orthobiologics in Osteoarthritis: PRP, Cell Therapy, and Tissue Engineering Approaches
A variety of orthobiologic therapies have emerged as promising interventions for OA. Orthobiologics refer to a broad class of biological products used to promote musculoskeletal healing and include platelet-rich plasma (PRP) and its alternatives, bone marrow aspirate concentrate (BMAC), bone grafts and matrix substitutes, bone morphogenic proteins (BMPs), mesenchymal stem cells (MSCs), growth factors, and tissue engineering approaches using natural, synthetic, or composite scaffolds [179]. Among these, PRP and MSCs have garnered substantial attention for their regenerative and anti-inflammatory effects in OA. PRP therapy uses a growth factor-rich biologic to promote tissue repair and modulate inflammation in the joint microenvironment [180]. In contrast, stem cells, particularly MSCs, are undifferentiated cells that, when stimulated, can develop into specialized tissue cells capable of replacing worn or damaged tissue [177]. It is important to distinguish that MSC administration alone constitutes a form of cell therapy rather than tissue engineering, which, by definition, requires the integration of cells, scaffolds, and growth factors [177]. The inclusion of biomaterial scaffolds with MSCs would then appropriately qualify under tissue engineering [178,181]. Despite their different mechanisms, both therapies target both cartilage structural degeneration and chronic inflammation, thereby providing OA-related pain relief [182,183,184].
PRP is a blood-derived component that contains platelet concentrations above the normal level and includes platelet-related growth factors and plasma-derived fibrinogen [185,186]. Platelets are the frontline healing response to injuries, as they release growth factors (e.g., TGF-β and PDGF), cytokines (e.g., IL-1 and IL-6), and adhesion molecules (e.g., fibrin, fibronectin, vitronectin) essential for healing processes such as cell proliferation, angiogenesis, and ECM formation for tissue repair [187,188]. Clinically, PRP has been investigated as a treatment for OA and offers several advantages: it is autologous, relatively safe, easy to prepare, and associated with minimal side effects [189,190]. The IA PRP injections deliver a concentrated dose of these molecules directly to the affected joint, thereby promoting cartilage regeneration and reducing inflammation [177,180]. The treatment primarily targets cartilage and synovium, enhancing extracellular matrix synthesis and modulating synovial inflammation, ultimately improving cartilage integrity and alleviating joint pain [190,191].
An important step in the therapeutic use of PRP is its activation, which triggers platelets to release their growth factors. Activation is commonly achieved by administering calcium chloride and/or thrombin [64]. Once activated, PRP causes the platelets to degranulate, with nearly 100% of the growth factors being released within one hour of activation [192]. A recent meta-analysis demonstrated that exogenously activated PRP is more effective in improving pain and function than non-activated PRP in patients with knee OA [190,193]. However, conflicting evidence suggesting less efficient wound healing has raised questions about whether the rapid delivery of growth factors is ideal [186,188]. It remains unclear whether PRP should be activated, and since its efficacy can vary due to differences in preparation protocols, its regenerative capacity is generally more limited compared to stem cell-based therapies [190]. Despite these limitations, PRP is frequently studied in combination with stem cells to enhance therapeutic outcomes. Continued research is needed to standardize preparation methods and validate their long-term clinical effectiveness [194].
MSCs are multipotent stromal cells capable of differentiating into cartilage-producing chondrocytes [195,196,197]. These cells are gaining attention as potential treatments for OA due to their ability to target both cartilage and subchondral bone, contributing to structural repair and reducing chronic inflammation [195,197,198]. Clinical studies suggest that MSC therapy can improve joint function and delay disease progression, although outcomes vary depending on cell source, dose, and delivery method [199,200].
MSCs, derived from bone marrow, adipose tissue, or umbilical cord, exhibit immunomodulatory and chondrogenic properties [201]. These cells can differentiate into chondrocytes, secrete anti-inflammatory cytokines, and inhibit matrix-degrading enzymes [201]. Notably, MSC-derived exosomes, which are nano-sized extracellular vesicles rich in bioactive molecules, have been shown to reduce pro-inflammatory cytokines and modulate the NF-κB signaling pathway [183,184,185,186]. Additionally, bone marrow-derived MSCs (BMSCs) have been shown to enhance chondrocyte survival and suppress COX-2 expression, thereby limiting inflammatory pain and structural damage [187]. Moreover, various biomaterials such as hydrogels, scaffolds, and nanofibers have been engineered to support MSC survival, promote chondrogenesis, and enable the sustained release of bioactive compounds [202,203]. The synergy between MSCs and biomaterial scaffolds is being explored to enhance cartilage regeneration and restore joint function in preclinical and early clinical studies [203].
5.2. Gene Therapies for OA
Emerging gene therapy strategies aim to modify the expression of pro-inflammatory cytokines and catabolic enzymes involved in OA progression [204]. Viral vectors such as adeno-associated viruses and lentiviruses have been explored to deliver therapeutic genes directly into the joint, targeting molecules like IL-1β, TNF-α, MMP-13, and ADAMTS. For example, IL-1 receptor antagonist (IL-1Ra) gene delivery has shown promise in reducing joint inflammation and cartilage damage in preclinical models [205]. More recently, CRISPR-Cas9-mediated editing has been investigated to selectively disrupt catabolic gene pathways or enhance anabolic factors such as SOX9 and aggrecan [206]. While gene therapy holds great potential for disease modification, challenges remain in achieving targeted, sustained expression with minimal immunogenicity and off-target effects [207].
5.3. Disease-Modifying Osteoarthritis Drugs (DMOADs)
Unlike conventional treatments that focus on symptom relief, DMOADs aim to alter the structural progression of OA [208]. Several DMOAD candidates are currently being evaluated in clinical trials for their potential to delay or reverse structural progression in OA Table 2). The outcomes of these trials will determine the future landscape of OA therapeutics, especially for interventions that move beyond symptom control toward structural modification and long-term disease management [209]. While no DMOAD has yet received full regulatory approval, these agents represent a shift toward mechanism-based interventions aimed at modifying the disease trajectory rather than simply alleviating pain.

Table 2.
Emerging pharmacologic and biologic disease-modifying therapies for OA and their molecular targets.
Table 2.
Emerging pharmacologic and biologic disease-modifying therapies for OA and their molecular targets.
| Treatment | Mode of Action | Target | Benefits |
|---|---|---|---|
| MMP-inhibitor PG-116800 (NCT01919164) | Inhibits cartilage matrix degradation | Cartilage matrix | Limits degradation and slows disease progression |
| Sprifermin (truncated FGF18) | Stimulates chondrocyte proliferation | Cartilage matrix | Improves cartilage thickness [210] |
| BMP-7 or OP-1 (NCT01133613, NCT01111045, NCT00456157) | Promotes chondrogenic differentiation | Cartilage matrix | Enhances cartilage repair and reduces pain [205] |
| AMG 108 (IL-1R1 antibody) (NCT00110942) | Inhibits IL-1β activity | IL-1 receptor | Reduces inflammation and failed to demonstrate significant clinical benefit [211] |
| Adalimumab (TNF inhibitor) (ACTRN 12612000791831) | Blocks TNF-α signaling | TNF-α receptor | Reduces pain and improves physical function |
| Infliximab | Inhibits TNF-alpha | TNFα receptor | Reduced progression of hand OA in recent-onset RA patients [212] |
| Tanezumab (anti-NGF antibody) | Blocks NGF-TrkA interaction | Targets NGF | Improves joint functional and pain scores, safety concerns, and NCT02697773 |
| Trans-capsaicin (CNTX-4975) | Inhibits TRPV1 receptor | TRPV1 | Decreases pain perception |
| Mavatrep (JNJ-39439335) | Inhibits TRPV1 | TRPV1 | Significant pain reduction but dose adjustments needed (EudraCT 2009-010961-21) |
| Selective agonist CR845 | Inhibits opioid receptors | Activates kappa-opioid receptor | Dose-dependent pain reduction, effective in hip OA (NCT02524197 and NCT02944448) |
Additionally, several approved drugs are being investigated as repurposed agents in the treatment of OA, such as liraglutide (anti-diabetic and anti-obesity drug: NCT02905864), Metformin (anti-diabetic drug: NCT04767841 and NCT05034029), and Zoledronic acid (anti-osteoporotic drug: NCT04303026) [164].
In summary, orthobiologic therapies, including PRP, MSC-based cell therapy, and tissue-engineering strategies, offer promising avenues for managing OA-related pain by targeting both inflammatory and degenerative processes within the joint [213,214]. Approaches range from intra-articular injections of bioactive molecules that reduce inflammation and modulate the joint environment to the transplantation of stem cells and biomaterials that promote cartilage repair [64,215]. While many of these therapies are still under investigation, early results indicate their potential to delay OA progression and alleviate chronic pain. Tissue engineering in OA aims not only to regenerate damaged tissues but also to reduce reliance on pharmacologic pain management and delay or eliminate the need for joint replacement.
6. Pain Measurement for Drug Development
Given the complexity of OA pain mechanisms, targeting the pain pathway for treatment is challenging. Various methods are employed to classify and measure evoked pain behaviors, weight-bearing deficits, gait abnormalities, and spontaneous pain behaviors in animals (Table 3). These include assessing mechanical allodynia in the hind paw using von Frey monofilaments, knee hyperalgesia through force application, thermal hypersensitivity on a hot/cold plate, static weight-bearing using a capacitance meter, dynamic weight-bearing through a specialized apparatus, gait analysis using the CatWalk or TreadScan systems, and monitoring spontaneous pain behaviors using video recording, conditioned place preference chambers, and a custom-made burrowing device [215,216,217,218,219].

Table 3.
Pain assessment in animal models.
Moreover, a comprehensive range of assessment tools is utilized for clinical evaluation for joint-related symptoms, function, patient perception, and activity levels. Several of these tools are summarized in Table 4 [196,197,198,199,200,201,202,203,204].

Table 4.
Medical assessment of OA pain in humans.
7. Conclusions
Growing evidence suggests that OA pain is not solely driven by structural joint damage but by a complex interplay between chronic low-grade inflammation, joint tissue degeneration, and alterations in pain processing pathways [21]. Inflammatory mediators sensitize peripheral nociceptors, while ongoing input from damaged joints can lead to central sensitization, amplifying the pain experience [248,249]. As a result, OA pain may persist despite minimal visible joint damage, often becoming chronic and difficult to treat with conventional therapies [250]. As our understanding of OA pathophysiology evolves, current research prioritizes the multi-targeted, mechanism-based treatments that address the interconnected biological and sensory drivers of disease [9]. This highlights the need for new treatment strategies that go beyond pain relief and focus on both inflammation and nerve-related pain, especially for patients who are not responsive to traditional pain medication [251].
Current nanomedicine platforms in clinical trials offer promising advancements in targeted and sustained IA drug delivery by localized therapeutic action with reduced systemic side effects and the direct modulation of the joint environment [252]. However, recent studies have emphasized the biological limitations of IA delivery, including rapid clearance and restricted tissue penetration, highlighting the need for integrative strategies that combine local and systemic therapies [253,254]. Therefore, IA drug delivery should be viewed not as a standalone solution but as one component of a comprehensive, patient-centered treatment plan [255]. Effective OA management must be comprehensive and patient-centered, combining IA therapies with systemic pharmacological agents, biomechanical interventions (e.g., physiotherapy and orthotics), and lifestyle modifications such as weight management and physical activity [254]. Incorporating these complementary approaches can enhance the therapeutic benefit of IA therapies, support long-term joint function, and improve overall patient outcomes [253,254].
Author Contributions
Conceptualization, M.A., L.M.E., J.A. and F.M.; writing—original draft preparation, Section 1, M.A. and L.M.E.; Section 2, M.A., L.M.E. and F.Y.; Section 3, M.A., L.M.E., A.L., F.Y. and M.P.G.; Section 4, M.A., L.M.E., F.Y., N.E., J.B. and F.M.; Section 5: M.A., L.M.E. and J.A.; writing—review and editing, M.A., F.Y., L.M.E., M.P.G., G.M., N.E., J.B., J.A. and F.M.; visualization, M.A. and L.M.E.; supervision, L.M.E., J.B., J.A. and F.M.; project administration, L.M.E.; funding acquisition, F.M. and J.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research is supported by the Canadian Institute of Health Research (CIHR PJT 180252) and Kuwait Foundation for the Advancement of Sciences (KFAS) (CB24-63MM-01).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
Two of the authors (J.A. and F.M.) are co-founders of Trepso Therapeutics Inc. which owns the patent for the use of sLink N, entitled “Methods and Compositions for Treatment of Cartilage and Disc Disorders”, as described in the United States Patent No. US 10,202,420 B2 Date of patent 12 February 2019. F.M., J.A., L.M.E. and M.G. are the inventors of sLink N for OA applications. The remaining authors have no competing interests to declare.
Abbreviations
The following abbreviations are used in this manuscript:
| OA | Osteoarthritis |
| ECM | Extracellular Matrix |
| DRG | Dorsal Root Ganglion |
| ADAMTS | A Disintegrin and Metalloproteinase with Thrombospondin Motifs |
| DAMPs | Damage-Associated Molecular Patterns |
| IL1β | Interleukin 1β |
| IL1R1 | Interleukin 1 Receptor 1 |
| TNF-α | Tumour Necrosis Factor-alpha |
| TrkA | Tropomyosin receptor kinase A |
| LTβ | Lymphotoxin β |
| TLRs | Toll-like receptors |
| NF-κB | Nuclear Factor kappa-light-chain-enhancer of activated B cells |
| NGF | Nerve Growth Factor |
| JAK/STAT | Janus Kinase/Signal Transducer and Activator of Transcription |
| MAPK | Mitogen-Activated Protein Kinase |
| MMP | Matrix Metalloproteinase |
| MSCs | Mesenchymal Stem Cells |
| BMSCs | Bone Marrow-derived Mesenchymal Stem Cells |
| PRP | Platelet-Rich Plasma |
| PRRs | Pattern-Recognition Receptors |
| S100A8/9 | S100 Calcium Binding Proteins A8/A9 |
| IL-1Ra | IL-1 receptor antagonist |
| HMGB1 | High-Mobility Group Box 1 |
| HSPs | Heat Shock Proteins |
| PDGF | Platelet-Derived Growth |
| TGF-β | Transforming Growth Factor Beta |
| HS | Histamine |
| SP | Substance P |
| CSD | Corticosteroid Drugs |
| NRS | Numeric Rating Scale |
| VAS | Visual Analog Scale |
| VDS | Verbal Descriptor Scale |
| MPQ | McGill Pain Questionnaire |
| BPI | Brief Pain Inventory |
| KOOS | Knee Injury and Osteoarthritis Outcome Score |
| HOOS | Hip Disability and Osteoarthritis Outcome Score |
| IKDC | International Knee Documentation Committee |
| CPM | Conditioned Pain Modulation |
| QST | Quantitative Sensory Testing |
References
- Glyn-Jones, S.; Palmer, A.J.; Agricola, R.; Price, A.J.; Vincent, T.L.; Weinans, H.; Carr, A.J. Osteoarthritis. Lancet 2015, 386, 376–387. [Google Scholar] [CrossRef] [PubMed]
- Hunter, D.J.; Bierma-Zeinstra, S. Osteoarthritis. Lancet 2019, 393, 1745–1759. [Google Scholar] [CrossRef] [PubMed]
- Cieza, A.; Causey, K.; Kamenov, K.; Hanson, S.W.; Chatterji, S.; Vos, T. Global estimates of the need for rehabilitation based on the Global Burden of Disease study 2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 2006–2017. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Wu, X.; Tao, C.; Gong, W.; Chen, M.; Qu, M.; Zhong, Y.; He, T.; Chen, S.; Xiao, G. Osteoarthritis: Pathogenic signaling pathways and therapeutic targets. Signal Transduct. Target. Ther. 2023, 8, 56. [Google Scholar] [CrossRef]
- Bannuru, R.R.; Osani, M.C.; Vaysbrot, E.E.; Arden, N.K.; Bennell, K.; Bierma-Zeinstra, S.M.A.; Kraus, V.B.; Lohmander, L.S.; Abbott, J.H.; Bhandari, M.; et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthr. Cartil. 2019, 27, 1578–1589. [Google Scholar] [CrossRef]
- da Costa, B.R.; Reichenbach, S.; Keller, N.; Nartey, L.; Wandel, S.; Jüni, P.; Trelle, S. Effectiveness of non-steroidal anti-inflammatory drugs for the treatment of pain in knee and hip osteoarthritis: A network meta-analysis. Lancet 2017, 390, E21–E33. [Google Scholar] [CrossRef]
- Zhang, W.; Ouyang, H.; Dass, C.R.; Xu, J. Current research on pharmacologic and regenerative therapies for osteoarthritis. Bone Res. 2016, 4, 15040. [Google Scholar] [CrossRef]
- Pelletier, J.P.; Martel-Pelletier, J.; Rannou, F.; Cooper, C. Efficacy and safety of oral NSAIDs and analgesics in the management of osteoarthritis: Evidence from real-life setting trials and surveys. Semin. Arthritis Rheum. 2016, 45, S22–S27. [Google Scholar] [CrossRef]
- Tong, L.; Yu, H.; Huang, X.; Shen, J.; Xiao, G.; Chen, L.; Wang, H.; Xing, L.; Chen, D. Current understanding of osteoarthritis pathogenesis and relevant new approaches. Bone Res. 2022, 10, 60. [Google Scholar] [CrossRef]
- Farinelli, L.; Riccio, M.; Gigante, A.; De Francesco, F. Pain Management Strategies in Osteoarthritis. Biomedicines 2024, 12, 805. [Google Scholar] [CrossRef]
- Skou, S.T.; Roos, E.M. Good Life with osteoArthritis in Denmark (GLA:D™): Evidence-based education and supervised neuromuscular exercise delivered by certified physiotherapists nationwide. BMC Musculoskelet. Disord. 2017, 18, 72. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.E.V.; Murray, C.M.; Stanton, T.R. Patient perspectives of pain and function after knee replacement: A systematic review and meta-synthesis of qualitative studies. Pain Rep. 2022, 7, e1006. [Google Scholar] [CrossRef] [PubMed]
- Marsh, M.; Newman, S. Trends and developments in hip and knee arthroplasty technology. J. Rehabil. Assist. Technol. Eng. 2021, 8, 2055668320952043. [Google Scholar] [CrossRef]
- Goode, V.M.; Morgan, B.; Muckler, V.C.; Cary, M.P., Jr.; Zdeb, C.E.; Zychowicz, M. Multimodal Pain Management for Major Joint Replacement Surgery. Orthop. Nurs. 2019, 38, 150–156. [Google Scholar] [CrossRef]
- Leifer, V.P.; Katz, J.N.; Losina, E. The burden of OA-health services and economics. Osteoarthr. Cartil. 2022, 30, 10–16. [Google Scholar] [CrossRef]
- Fallon, E.A.; Boring, M.A.; Foster, A.L.; Stowe, E.W.; Lites, T.D.; Odom, E.L.; Seth, P. Prevalence of Diagnosed Arthritis—United States, 2019–2021. Morb. Mortal. Wkly. Rep. 2023, 72, 1101–1107. [Google Scholar] [CrossRef] [PubMed]
- Yelin, E.; Weinstein, S.; King, T. The burden of musculoskeletal diseases in the United States. Semin. Arthritis Rheum. 2016, 46, 259–260. [Google Scholar] [CrossRef]
- Loeser, R.F.; Goldring, S.R.; Scanzello, C.R.; Goldring, M.B. Osteoarthritis: A disease of the joint as an organ. Arthritis Rheum. 2012, 64, 1697–1707. [Google Scholar] [CrossRef]
- Goldring, M.B.; Otero, M. Inflammation in osteoarthritis. Curr. Opin. Rheumatol. 2011, 23, 471–478. [Google Scholar] [CrossRef]
- Berenbaum, F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!). Osteoarthr. Cartil. 2013, 21, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Coaccioli, S.; Sarzi-Puttini, P.; Zis, P.; Rinonapoli, G.; Varrassi, G. Osteoarthritis: New Insight on Its Pathophysiology. J. Clin. Med. 2022, 11, 6013. [Google Scholar] [CrossRef]
- Abramoff, B.; Caldera, F.E. Osteoarthritis. Med. Clin. N. Am. 2020, 104, 299–311. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, X.; Wang, S.; Jing, Y.; Su, J. Subchondral bone microenvironment in osteoarthritis and pain. Bone Res. 2021, 9, 20. [Google Scholar] [CrossRef] [PubMed]
- Bačenková, D.; Trebuňová, M.; Demeterová, J.; Živčák, J. Human Chondrocytes, Metabolism of Articular Cartilage, and Strategies for Application to Tissue Engineering. Int. J. Mol. Sci. 2023, 24, 17096. [Google Scholar] [CrossRef]
- Hodgkinson, T.; Kelly, D.C.; Curtin, C.M.; O’Brien, F.J. Mechanosignalling in cartilage: An emerging target for the treatment of osteoarthritis. Nat. Rev. Rheumatol. 2022, 18, 67–84. [Google Scholar] [CrossRef]
- Heinegård, D.; Saxne, T. The role of the cartilage matrix in osteoarthritis. Nat. Rev. Rheumatol. 2011, 7, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Martel-Pelletier, J.; Boileau, C.; Pelletier, J.P.; Roughley, P.J. Cartilage in normal and osteoarthritis conditions. Best Pract. Res. Clin. Rheumatol. 2008, 22, 351–384. [Google Scholar] [CrossRef]
- Burr, D.B.; Gallant, M.A. Bone remodelling in osteoarthritis. Nat. Rev. Rheumatol. 2012, 8, 665–673. [Google Scholar] [CrossRef]
- Hunter, D.J.; Felson, D.T. Osteoarthritis. BMJ 2006, 332, 639–642. [Google Scholar] [CrossRef]
- Findlay, D.M. Vascular pathology and osteoarthritis. Rheumatology 2007, 46, 1763–1768. [Google Scholar] [CrossRef] [PubMed]
- Goldring, S.R.; Goldring, M.B. Changes in the osteochondral unit during osteoarthritis: Structure, function and cartilage–bone crosstalk. Nat. Rev. Rheumatol. 2016, 12, 632–644. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.; Liu, X.; He, Z.; Han, X.; Yan, M.; Qu, X.; Li, X.; Yu, Z. Articular Cartilage Degradation and Aberrant Subchondral Bone Remodeling in Patients with Osteoarthritis and Osteoporosis. J. Bone Miner. Res. 2019, 35, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Chan, Y.T.; Yung, P.S.H.; Tuan, R.S.; Jiang, Y. Subchondral Bone Remodeling: A Therapeutic Target for Osteoarthritis. Front. Cell Dev. Biol. 2021, 8, 607764. [Google Scholar] [CrossRef]
- Neogi, T.; Guermazi, A.; Roemer, F.; Nevitt, M.C.; Scholz, J.; Arendt-Nielsen, L.; Woolf, C.; Niu, J.; Bradley, L.A.; Quinn, E.; et al. Association of Joint Inflammation with Pain Sensitization in Knee Osteoarthritis: The Multicenter Osteoarthritis Study. Arthritis Rheumatol. 2016, 68, 654–661. [Google Scholar] [CrossRef]
- Sharma, L.; Song, J.; Felson, D.T.; Cahue, S.; Shamiyeh, E.; Dunlop, D.D. The role of knee alignment in disease progression and functional decline in knee osteoarthritis. JAMA 2001, 286, 188–195. [Google Scholar] [CrossRef]
- Felson, D.T. Clinical practice. Osteoarthritis of the knee. N. Engl. J. Med. 2006, 354, 841–848. [Google Scholar] [CrossRef]
- Diracoglu, D.; Aydin, R.; Baskent, A.; Celik, A. Effects of kinesthesia and balance exercises in knee osteoarthritis. J. Clin. Rheumatol. 2005, 11, 303–310. [Google Scholar] [CrossRef]
- Buckwalter, J.A.; Anderson, D.D.; Brown, T.D.; Tochigi, Y.; Martin, J.A. The Roles of Mechanical Stresses in the Pathogenesis of Osteoarthritis: Implications for Treatment of Joint Injuries. Cartilage 2013, 4, 286–294. [Google Scholar] [CrossRef]
- Bennell, K.L.; Hunt, M.A.; Wrigley, T.V.; Lim, B.W.; Hinman, R.S. Role of muscle in the genesis and management of knee osteoarthritis. Rheum. Dis. Clin. N. Am. 2008, 34, 731–754. [Google Scholar] [CrossRef]
- Sanchez-Lopez, E.; Coras, R.; Torres, A.; Lane, N.E.; Guma, M. Synovial inflammation in osteoarthritis progression. Nat. Rev. Rheumatol. 2022, 18, 258–275. [Google Scholar] [CrossRef] [PubMed]
- Martel-Pelletier, J.; Barr, A.J.; Cicuttini, F.M.; Conaghan, P.G.; Cooper, C.; Goldring, M.B.; Goldring, S.R.; Jones, G.; Teichtahl, A.J.; Pelletier, J.-P. Osteoarthritis. Nat. Rev. Dis. Primers 2016, 2, 16072. [Google Scholar] [CrossRef] [PubMed]
- Grässel, S.; Zaucke, F.; Madry, H. Osteoarthritis: Novel Molecular Mechanisms Increase Our Understanding of the Disease Pathology. J. Clin. Med. 2021, 10, 1938. [Google Scholar] [CrossRef]
- Chow, Y.Y.; Chin, K.-Y. The Role of Inflammation in the Pathogenesis of Osteoarthritis. Mediat. Inflamm. 2020, 2020, 8293921. [Google Scholar] [CrossRef]
- Scanzello, C.R.; Goldring, S.R. The role of synovitis in osteoarthritis pathogenesis. Bone 2012, 51, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Sokolove, J.; Lepus, C.M. Role of inflammation in the pathogenesis of osteoarthritis: Latest findings and interpretations. Ther. Adv. Musculoskelet. Dis. 2013, 5, 77–94. [Google Scholar] [CrossRef]
- Lotz, M.; Martel-Pelletier, J.; Christiansen, C.; Brandi, M.L.; Bruyère, O.; Chapurlat, R.; Collette, J.; Cooper, C.; Giacovelli, G.; Kanis, J.A.; et al. Value of biomarkers in osteoarthritis: Current status and perspectives. Ann. Rheum. Dis. 2013, 72, 1756–1763. [Google Scholar] [CrossRef]
- Heijink, A.; Gomoll, A.H.; Madry, H.; Drobnič, M.; Filardo, G.; Espregueira-Mendes, J.; Van Dijk, C.N. Biomechanical considerations in the pathogenesis of osteoarthritis of the knee. Knee Surg. Sports Traumatol. Arthrosc. 2012, 20, 423–435. [Google Scholar] [CrossRef]
- Guilak, F. Biomechanical factors in osteoarthritis. Best Pract. Res. Clin. Rheumatol. 2011, 25, 815–823. [Google Scholar] [CrossRef]
- van den Bosch, M.H.J.; Blom, A.B.; van der Kraan, P.M. Inflammation in osteoarthritis: Our view on its presence and involvement in disease development over the years. Osteoarthr. Cartil. 2024, 32, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.; Lee, K.; Ju, J.H. Recent Updates of Diagnosis, Pathophysiology, and Treatment on Osteoarthritis of the Knee. Int. J. Mol. Sci. 2021, 22, 2619. [Google Scholar] [CrossRef]
- Sellam, J.; Berenbaum, F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat. Rev. Rheumatol. 2010, 6, 625–635. [Google Scholar] [CrossRef]
- Mathiessen, A.; Conaghan, P.G. Synovitis in osteoarthritis: Current understanding with therapeutic implications. Arthritis Res. Ther. 2017, 19, 18. [Google Scholar] [CrossRef] [PubMed]
- Ayral, X.; Pickering, E.H.; Woodworth, T.G.; Mackillop, N.; Dougados, M. Synovitis: A potential predictive factor of structural progression of medial tibiofemoral knee osteoarthritis—Results of a 1 year longitudinal arthroscopic study in 422 patients. Osteoarthr. Cartil. 2005, 13, 361–367. [Google Scholar] [CrossRef]
- McKune, C.M. Nociception and pain. In Veterinary Anesthesia and Analgesia: The Fifth Edition of Lumb and Jones; John Wiley & Sons: Hoboken, NJ, USA, 2017; pp. 584–623. [Google Scholar] [CrossRef]
- Armstrong, S.A.; Herr, M.J. Physiology, Nociception; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Schaible, H.G. Mechanisms of Chronic Pain in Osteoarthritis. Curr. Rheumatol. Rep. 2012, 14, 549–556. [Google Scholar] [CrossRef]
- Basbaum, A.I.; Bautista, D.M.; Scherrer, G.; Julius, D. Cellular and molecular mechanisms of pain. Cell 2009, 139, 267–284. [Google Scholar] [CrossRef]
- Wood, M.J.; Miller, R.E.; Malfait, A.-M. The Genesis of Pain in Osteoarthritis: Inflammation as a Mediator of Osteoarthritis Pain. Clin. Geriatr. Med. 2022, 38, 221–238. [Google Scholar] [CrossRef] [PubMed]
- Fu, K.; Robbins, S.R.; McDougall, J.J. Osteoarthritis: The genesis of pain. Rheumatology 2017, 57, iv43–iv50. [Google Scholar] [CrossRef]
- Morgan, M.; Nazemian, V.; Harrington, K.; Ivanusic, J.J. Mini review: The role of sensory innervation to subchondral bone in osteoarthritis pain. Front. Endocrinol. 2022, 13, 1047943. [Google Scholar] [CrossRef]
- Yu, H.; Huang, T.; Lu, W.W.; Tong, L.; Chen, D. Osteoarthritis Pain. Int. J. Mol. Sci. 2022, 23, 4642. [Google Scholar] [CrossRef] [PubMed]
- Perrot, S. Osteoarthritis pain. Best Pract. Res. Clin. Rheumatol. 2015, 29, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.P.; Vase, L.; Hooten, W.M. Chronic pain: An update on burden, best practices, and new advances. Lancet 2021, 397, 2082–2097. [Google Scholar] [CrossRef]
- Bechler, C.J. A Categorical Perspective on Attitudes: Implications for Perceived Change, Persuasive Targeting, and the Attitude-Behavior Relationship. Ph.D. Thesis, Stanford University, Stanford, CA, USA, 2021. [Google Scholar]
- Ohashi, Y.; Uchida, K.; Fukushima, K.; Inoue, G.; Takaso, M. Mechanisms of Peripheral and Central Sensitization in Osteoarthritis Pain. Cureus 2023, 15, e35331. [Google Scholar] [CrossRef]
- Wise, B.L.; Niu, J.; Zhang, Y.; Wang, N.; Jordan, J.M.; Choy, E.; Hunter, D.J. Psychological factors and their relation to osteoarthritis pain. Osteoarthr. Cartil. 2010, 18, 883–887. [Google Scholar] [CrossRef]
- Vincent, T.L. Peripheral pain mechanisms in osteoarthritis. PAIN 2020, 161, S138–S146. [Google Scholar] [CrossRef] [PubMed]
- Alves, C.J.; Couto, M.; Sousa, D.M.; Magalhães, A.; Neto, E.; Leitão, L.; Conceição, F.; Monteiro, A.C.; Ribeiro-da-Silva, M.; Lamghari, M. Nociceptive mechanisms driving pain in a post-traumatic osteoarthritis mouse model. Sci. Rep. 2020, 10, 15271. [Google Scholar] [CrossRef]
- Eitner, A.; Hofmann, G.O.; Schaible, H.-G. Mechanisms of Osteoarthritic Pain. Studies in Humans and Experimental Models. Front. Mol. Neurosci. 2017, 10, 349. [Google Scholar] [CrossRef]
- He, Y.; Li, Z.; Alexander, P.G.; Ocasio-Nieves, B.D.; Yocum, L.; Lin, H.; Tuan, R.S. Pathogenesis of Osteoarthritis: Risk Factors, Regulatory Pathways in Chondrocytes, and Experimental Models. Biology 2020, 9, 194. [Google Scholar] [CrossRef]
- Chen, D.; Shen, J.; Zhao, W.; Wang, T.; Han, L.; Hamilton, J.L.; Im, H.-J. Osteoarthritis: Toward a comprehensive understanding of pathological mechanism. Bone Res. 2017, 5, 16044. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Kim, J.S.; van Wijnen, A.J.; Im, H.J. Osteoarthritic tissues modulate functional properties of sensory neurons associated with symptomatic OA pain. Mol. Biol. Rep. 2011, 38, 5335–5339. [Google Scholar] [CrossRef] [PubMed]
- Ayobami, O.O.; Goldring, S.R.; Goldring, M.B.; Wright, T.M.; van der Meulen, M.C.H. Contribution of joint tissue properties to load-induced osteoarthritis. Bone Rep. 2022, 17, 101602. [Google Scholar] [CrossRef]
- Castañeda, S.; Roman-Blas, J.A.; Largo, R.; Herrero-Beaumont, G. Subchondral bone as a key target for osteoarthritis treatment. Biochem. Pharmacol. 2012, 83, 315–323. [Google Scholar] [CrossRef]
- Findlay, D.M.; Kuliwaba, J.S. Bone-cartilage crosstalk: A conversation for understanding osteoarthritis. Bone Res. 2016, 4, 16028. [Google Scholar] [CrossRef]
- Suri, S.; Walsh, D.A. Osteochondral alterations in osteoarthritis. Bone 2012, 51, 204–211. [Google Scholar] [CrossRef]
- Malfait, A.M.; Schnitzer, T.J. Towards a mechanism-based approach to pain management in osteoarthritis. Nat. Rev. Rheumatol. 2013, 9, 654–664. [Google Scholar] [CrossRef] [PubMed]
- Di Maio, G.; Villano, I.; Ilardi, C.R.; Messina, A.; Monda, V.; Iodice, A.C.; Porro, C.; Panaro, M.A.; Chieffi, S.; Messina, G.; et al. Mechanisms of Transmission and Processing of Pain: A Narrative Review. Int. J. Environ. Res. Public Health 2023, 20, 3064. [Google Scholar] [CrossRef]
- Baral, P.; Udit, S.; Chiu, I.M. Pain and immunity: Implications for host defence. Nat. Rev. Immunol. 2019, 19, 433–447. [Google Scholar] [CrossRef]
- Cao, Y.; Fan, D.; Yin, Y. Pain mechanism in rheumatoid arthritis: From cytokines to central sensitization. Mediat. Inflamm. 2020, 2020, 2076328. [Google Scholar] [CrossRef]
- Miller, R.J.; Malfait, A.M.; Miller, R.E. The innate immune response as a mediator of osteoarthritis pain. Osteoarthr. Cartil. 2020, 28, 562–571. [Google Scholar] [CrossRef]
- Chakrabarti, S. Mechanisms of Peripheral Sensitization in Inflammatory Knee Pain. Ph.D. Thesis, University of Cambridge, Cambridge, UK, 2020. [Google Scholar]
- Woolf, C.J. Central sensitization: Implications for the diagnosis and treatment of pain. PAIN 2011, 152, S2–S15. [Google Scholar] [CrossRef]
- Khan, A.; Khan, S.; Kim, Y.S. Insight into Pain Modulation: Nociceptors Sensitization and Therapeutic Targets. Curr. Drug Targets 2019, 20, 775–788. [Google Scholar] [CrossRef] [PubMed]
- Latremoliere, A.; Woolf, C.J. Central sensitization: A generator of pain hypersensitivity by central neural plasticity. J. Pain 2009, 10, 895–926. [Google Scholar] [CrossRef]
- Lluch Girbés, E.; Nijs, J.; Torres-Cueco, R.; López Cubas, C. Pain treatment for patients with osteoarthritis and central sensitization. Phys. Ther. 2013, 93, 842–851. [Google Scholar] [CrossRef] [PubMed]
- Ohtori, S.; Orita, S.; Yamashita, M.; Ishikawa, T.; Ito, T.; Shigemura, T.; Nishiyama, H.; Konno, S.; Ohta, H.; Takaso, M.; et al. Existence of a neuropathic pain component in patients with osteoarthritis of the knee. Yonsei Med. J. 2012, 53, 801–805. [Google Scholar] [CrossRef] [PubMed]
- Bedson, J.; Croft, P.R. The discordance between clinical and radiographic knee osteoarthritis: A systematic search and summary of the literature. BMC Musculoskelet. Disord. 2008, 9, 116. [Google Scholar] [CrossRef]
- Gwilym, S.E.; Filippini, N.; Douaud, G.; Carr, A.J.; Tracey, I. Thalamic atrophy associated with painful osteoarthritis of the hip is reversible after arthroplasty: A longitudinal voxel-based morphometric study. Arthritis Rheum. 2010, 62, 2930–2940. [Google Scholar] [CrossRef]
- Mobasheri, A.; Rayman, M.P.; Gualillo, O.; Sellam, J.; van der Kraan, P.; Fearon, U. The role of metabolism in the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2017, 13, 302–311. [Google Scholar] [CrossRef]
- Kapoor, M.; Martel-Pelletier, J.; Lajeunesse, D.; Pelletier, J.-P.; Fahmi, H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat. Rev. Rheumatol. 2011, 7, 33–42. [Google Scholar] [CrossRef]
- Felson, D.T. Osteoarthritis as a disease of mechanics. Osteoarthr. Cartil. 2013, 21, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Yue, L.; Berman, J. What Is Osteoarthritis? JAMA 2022, 327, 1300. [Google Scholar] [CrossRef]
- Langworthy, M.; Dasa, V.; Spitzer, A.I. Knee osteoarthritis: Disease burden, available treatments, and emerging options. Ther. Adv. Musculoskelet. Dis. 2024, 16, 1759720X241273009. [Google Scholar] [CrossRef] [PubMed]
- Lambert, C.; Zappia, J.; Sanchez, C.; Florin, A.; Dubuc, J.E.; Henrotin, Y. The Damage-Associated Molecular Patterns (DAMPs) as Potential Targets to Treat Osteoarthritis: Perspectives From a Review of the Literature. Front. Med. 2020, 7, 607186. [Google Scholar] [CrossRef]
- Sengprasert, P.; Kamenkit, O.; Tanavalee, A.; Reantragoon, R. The Immunological Facets of Chondrocytes in Osteoarthritis: A Narrative Review. J. Rheumatol. 2024, 51, 13–24. [Google Scholar] [CrossRef]
- Chen, Y.; Jiang, W.; Yong, H.; He, M.; Yang, Y.; Deng, Z.; Li, Y. Macrophages in osteoarthritis: Pathophysiology and therapeutics. Am. J. Transl. Res. 2020, 12, 261–268. [Google Scholar] [PubMed]
- Scanzello, C.R. Role of low-grade inflammation in osteoarthritis. Curr. Opin. Rheumatol. 2017, 29, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Robinson, W.H.; Lepus, C.M.; Wang, Q.; Raghu, H.; Mao, R.; Lindstrom, T.M.; Sokolove, J. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2016, 12, 580–592. [Google Scholar] [CrossRef]
- Sofat, N. Analysing the role of endogenous matrix molecules in the development of osteoarthritis. Int. J. Exp. Pathol. 2009, 90, 463–479. [Google Scholar] [CrossRef]
- van Lent, P.L.; Blom, A.B.; Schelbergen, R.F.; Slöetjes, A.; Lafeber, F.P.; Lems, W.F.; Cats, H.; Vogl, T.; Roth, J.; van den Berg, W.B. Active involvement of alarmins S100A8 and S100A9 in the regulation of synovial activation and joint destruction during mouse and human osteoarthritis. Arthritis Rheum. 2012, 64, 1466–1476. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, M.E. DAMPs, PAMPs and alarmins: All we need to know about danger. J. Leukoc. Biol. 2007, 81, 1–5. [Google Scholar] [CrossRef]
- Schaefer, L. Complexity of Danger: The Diverse Nature of Damage-associated Molecular Patterns. J. Biol. Chem. 2014, 289, 35237–35245. [Google Scholar] [CrossRef]
- Harris, H.E.; Raucci, A. Alarmin(g) news about danger. EMBO Rep. 2006, 7, 774–778. [Google Scholar] [CrossRef]
- Piccinini, A.M.; Midwood, K.S. DAMPening Inflammation by Modulating TLR Signalling. Mediat. Inflamm. 2010, 2010, 672395. [Google Scholar] [CrossRef] [PubMed]
- Lotze, M.T.; Tracey, K.J. High-mobility group box 1 protein (HMGB1): Nuclear weapon in the immune arsenal. Nat. Rev. Immunol. 2005, 5, 331–342. [Google Scholar] [CrossRef]
- Roh, J.S.; Sohn, D.H. Damage-Associated Molecular Patterns in Inflammatory Diseases. Immune Netw. 2018, 18, e27. [Google Scholar] [CrossRef] [PubMed]
- Burrage, P.S. Matrix Metalloproteinases: Role In Arthritis. Front. Biosci. 2006, 11, 529–543. [Google Scholar] [CrossRef]
- Chen, G.Y.; Nuñez, G. Sterile inflammation: Sensing and reacting to damage. Nat. Rev. Immunol. 2010, 10, 826–837. [Google Scholar] [CrossRef] [PubMed]
- Boulanger, L.M. Immune Proteins in Brain Development and Synaptic Plasticity. Neuron 2009, 64, 93–109. [Google Scholar] [CrossRef] [PubMed]
- Gómez, R.; Villalvilla, A.; Largo, R.; Gualillo, O.; Herrero-Beaumont, G. TLR4 signalling in osteoarthritis—Finding targets for candidate DMOADs. Nat. Rev. Rheumatol. 2015, 11, 159–170. [Google Scholar] [CrossRef]
- Lotz, M.; Loeser, R.F. Effects of aging on articular cartilage homeostasis. Bone 2012, 51, 241–248. [Google Scholar] [CrossRef]
- Kraus, V.B.; Blanco, F.J.; Englund, M.; Karsdal, M.A.; Lohmander, L.S. Call for standardized definitions of osteoarthritis and risk stratification for clinical trials and clinical use. Osteoarthr. Cartil. 2015, 23, 1233–1241. [Google Scholar] [CrossRef]
- Chen, B.; Sun, Y.; Xu, G.; Jiang, J.; Zhang, W.; Wu, C.; Xue, P.; Cui, Z. Role of crosstalk between synovial cells and chondrocytes in osteoarthritis (Review). Exp. Ther. Med. 2024, 27, 201. [Google Scholar] [CrossRef]
- Soares, C.L.R.; Wilairatana, P.; Silva, L.R.; Moreira, P.S.; Vilar Barbosa, N.M.M.; da Silva, P.R.; Coutinho, H.D.M.; de Menezes, I.R.A.; Felipe, C.F.B. Biochemical aspects of the inflammatory process: A narrative review. Biomed. Pharmacother. 2023, 168, 115764. [Google Scholar] [CrossRef]
- Li, D.; Wu, M. Pattern recognition receptors in health and diseases. Signal Transduct. Target. Ther. 2021, 6, 291. [Google Scholar] [CrossRef] [PubMed]
- Rahmati, M.; Mobasheri, A.; Mozafari, M. Inflammatory mediators in osteoarthritis: A critical review of the state-of-the-art, current prospects, and future challenges. Bone 2016, 85, 81–90. [Google Scholar] [CrossRef]
- Goldring, M.B.; Marcu, K.B. Cartilage homeostasis in health and rheumatic diseases. Arthritis Res. Ther. 2009, 11, 224. [Google Scholar] [CrossRef]
- Rock, K.L.; Latz, E.; Ontiveros, F.; Kono, H. The Sterile Inflammatory Response. Annu. Rev. Immunol. 2010, 28, 321–342. [Google Scholar] [CrossRef]
- Mukherjee, A.; Das, B. The role of inflammatory mediators and matrix metalloproteinases (MMPs) in the progression of osteoarthritis. Biomater. Biosyst. 2024, 13, 100090. [Google Scholar] [CrossRef] [PubMed]
- Blaney Davidson, E.N.; Van Caam, A.P.M.; Van Der Kraan, P.M. Osteoarthritis year in review 2016: Biology. Osteoarthr. Cartil. 2017, 25, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Knights, A.J.; Redding, S.J.; Maerz, T. Inflammation in osteoarthritis: The latest progress and ongoing challenges. Curr. Opin. Rheumatol. 2023, 35, 128–134. [Google Scholar] [CrossRef]
- Akkiraju, H.; Nohe, A. Role of Chondrocytes in Cartilage Formation, Progression of Osteoarthritis and Cartilage Regeneration. J. Dev. Biol. 2015, 3, 177–192. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Deng, Z.; Chen, K.; Jian, S.; Zhou, F.; Yang, Y.; Fu, Z.; Xie, H.; Xiong, J.; Zhu, W. Cartilage tissue engineering: From proinflammatory and anti-inflammatory cytokines to osteoarthritis treatments (Review). Mol. Med. Rep. 2022, 25, 99. [Google Scholar] [CrossRef]
- Maldonado, M.; Nam, J. The role of changes in extracellular matrix of cartilage in the presence of inflammation on the pathology of osteoarthritis. Biomed. Res. Int. 2013, 2013, 284873. [Google Scholar] [CrossRef]
- Mononen, M.E.; Tanska, P.; Isaksson, H.; Korhonen, R.K. A Novel Method to Simulate the Progression of Collagen Degeneration of Cartilage in the Knee: Data from the Osteoarthritis Initiative. Sci. Rep. 2016, 6, 21415. [Google Scholar] [CrossRef]
- Terkawi, M.A.; Ebata, T.; Yokota, S.; Takahashi, D.; Endo, T.; Matsumae, G.; Shimizu, T.; Kadoya, K.; Iwasaki, N. Low-Grade Inflammation in the Pathogenesis of Osteoarthritis: Cellular and Molecular Mechanisms and Strategies for Future Therapeutic Intervention. Biomedicines 2022, 10, 1109. [Google Scholar] [CrossRef]
- Molnar, V.; Matišić, V.; Kodvanj, I.; Bjelica, R.; Jeleč, Ž.; Hudetz, D.; Rod, E.; Čukelj, F.; Vrdoljak, T.; Vidović, D.; et al. Cytokines and Chemokines Involved in Osteoarthritis Pathogenesis. Int. J. Mol. Sci. 2021, 22, 9208. [Google Scholar] [CrossRef]
- Jacques, C.; Gosset, M.; Berenbaum, F.; Gabay, C. The Role of IL-1 and IL-1Ra in Joint Inflammation and Cartilage Degradation. In Vitamins & Hormones; Academic Press: Cambridge, MA, USA, 2006; Volume 74, pp. 371–403. [Google Scholar]
- Arend, W.P.; Malyak, M.; Guthridge, C.J.; Gabay, C. Interleukin-1 receptor antagonist: Role in biology. Annu. Rev. Immunol. 1998, 16, 27–55. [Google Scholar] [CrossRef] [PubMed]
- Brandt, K.D.; Dieppe, P.; Radin, E.L. Etiopathogenesis of Osteoarthritis. Rheum. Dis. Clin. N. Am. 2008, 34, 531–559. [Google Scholar] [CrossRef]
- Pelletier, J.P.; Martel-Pelletier, J.; Abramson, S.B. Osteoarthritis, an inflammatory disease: Potential implication for the selection of new therapeutic targets. Arthritis Rheum. 2001, 44, 1237–1247. [Google Scholar] [CrossRef]
- Bhol, N.K.; Bhanjadeo, M.M.; Singh, A.K.; Dash, U.C.; Ojha, R.R.; Majhi, S.; Duttaroy, A.K.; Jena, A.B. The interplay between cytokines, inflammation, and antioxidants: Mechanistic insights and therapeutic potentials of various antioxidants and anti-cytokine compounds. Biomed. Pharmacother. 2024, 178, 117177. [Google Scholar] [CrossRef]
- Henderson, B.; Pettipher, E.R. Arthritogenic actions of recombinant IL-1 and tumour necrosis factor alpha in the rabbit: Evidence for synergistic interactions between cytokines in vivo. Clin. Exp. Immunol. 1989, 75, 306–310. [Google Scholar]
- Chou, C.-H.; Jain, V.; Gibson, J.; Attarian, D.E.; Haraden, C.A.; Yohn, C.B.; Laberge, R.-M.; Gregory, S.; Kraus, V.B. Synovial cell cross-talk with cartilage plays a major role in the pathogenesis of osteoarthritis. Sci. Rep. 2020, 10, 10868. [Google Scholar] [CrossRef] [PubMed]
- Mao, S.; Neu, M.; Germershaus, O.; Merkel, O.; Sitterberg, J.; Bakowsky, U.; Kissel, T. Influence of polyethylene glycol chain length on the physicochemical and biological properties of poly(ethylene imine)-graft-poly(ethylene glycol) block copolymer/SiRNA polyplexes. Bioconjug. Chem. 2006, 17, 1209–1218. [Google Scholar] [CrossRef] [PubMed]
- Loeser, R.F.; Erickson, E.A.; Long, D.L. Mitogen-activated protein kinases as therapeutic targets in osteoarthritis. Curr. Opin. Rheumatol. 2008, 20, 581–586. [Google Scholar] [CrossRef]
- Prasadam, I.; Crawford, R.; Xiao, Y. Aggravation of ADAMTS and matrix metalloproteinase production and role of ERK1/2 pathway in the interaction of osteoarthritic subchondral bone osteoblasts and articular cartilage chondrocytes—Possible pathogenic role in osteoarthritis. J. Rheumatol. 2012, 39, 621–634. [Google Scholar] [CrossRef]
- Poole, A.R.; Kobayashi, M.; Yasuda, T.; Laverty, S.; Mwale, F.; Kojima, T.; Sakai, T.; Wahl, C.; El-Maadawy, S.; Webb, G.; et al. Type II collagen degradation and its regulation in articular cartilage in osteoarthritis. Ann. Rheum. Dis. 2002, 61 (Suppl. 2), II78–II81. [Google Scholar] [CrossRef]
- Marcu, K.B.; Otero, M.; Olivotto, E.; Borzi, R.M.; Goldring, M.B. NF-kappaB signaling: Multiple angles to target OA. Curr. Drug Targets 2010, 11, 599–613. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.; Aguda, B.D.; Rath, B.; Agarwal, S. Biomechanical thresholds regulate inflammation through the NF-kappaB pathway: Experiments and modeling. PLoS ONE 2009, 4, e5262. [Google Scholar] [CrossRef]
- Gu, R.; Liu, N.; Luo, S.; Huang, W.; Zha, Z.; Yang, J. MicroRNA-9 regulates the development of knee osteoarthritis through the NF-kappaB1 pathway in chondrocytes. Medicine 2016, 95, e4315. [Google Scholar] [CrossRef] [PubMed]
- Roman-Blas, J.A.; Jimenez, S.A. NF-kappaB as a potential therapeutic target in osteoarthritis and rheumatoid arthritis. Osteoarthr. Cartil. 2006, 14, 839–848. [Google Scholar] [CrossRef] [PubMed]
- Rigoglou, S.; Papavassiliou, A.G. The NF-κB signalling pathway in osteoarthritis. Int. J. Biochem. Cell Biol. 2013, 45, 2580–2584. [Google Scholar] [CrossRef]
- Choi, M.C.; Jo, J.; Park, J.; Kang, H.K.; Park, Y. NF-κB Signaling Pathways in Osteoarthritic Cartilage Destruction. Cells 2019, 8, 734. [Google Scholar] [CrossRef]
- Malfait, A.M.; Miller, R.E.; Block, J.A. Targeting neurotrophic factors: Novel approaches to musculoskeletal pain. Pharmacol. Ther. 2020, 211, 107553. [Google Scholar] [CrossRef]
- Malfait, A.M.; Miller, R.E.; Miller, R.J. Basic Mechanisms of Pain in Osteoarthritis: Experimental Observations and New Perspectives. Rheum. Dis. Clin. N. Am. 2021, 47, 165–180. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, J.; McNaughton, P.A. NGF rapidly increases membrane expression of TRPV1 heat-gated ion channels. EMBO J. 2005, 24, 4211–4223. [Google Scholar] [CrossRef]
- Schaefer, I.; Prato, V.; Arcourt, A.; Taberner, F.J.; Lechner, S.G. Differential modulation of voltage-gated sodium channels by nerve growth factor in three major subsets of TrkA-expressing nociceptors. Mol. Pain 2018, 14, 1744806918814640. [Google Scholar] [CrossRef]
- Dyck, P.J.; Peroutka, S.; Rask, C.; Burton, E.; Baker, M.K.; Lehman, K.A.; Gillen, D.A.; Hokanson, J.L.; O’Brien, P.C. Intradermal recombinant human nerve growth factor induces pressure allodynia and lowered heat-pain threshold in humans. Neurology 1997, 48, 501–505. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, Y.; Uchida, K.; Fukushima, K.; Satoh, M.; Koyama, T.; Tsuchiya, M.; Saito, H.; Takahira, N.; Inoue, G.; Takaso, M. NGF Expression and Elevation in Hip Osteoarthritis Patients with Pain and Central Sensitization. Biomed. Res. Int. 2021, 2021, 9212585. [Google Scholar] [CrossRef] [PubMed]
- Shang, X.; Wang, Z.; Tao, H. Mechanism and therapeutic effectiveness of nerve growth factor in osteoarthritis pain. Ther. Clin. Risk Manag. 2017, 13, 951–956. [Google Scholar] [CrossRef]
- Lisowska, B.; Lisowski, A.; Siewruk, K. Substance P and Chronic Pain in Patients with Chronic Inflammation of Connective Tissue. PLoS ONE 2015, 10, e0139206. [Google Scholar] [CrossRef]
- Semenistaja, S.; Skuja, S.; Kadisa, A.; Groma, V. Healthy and Osteoarthritis-Affected Joints Facing the Cellular Crosstalk. Int. J. Mol. Sci. 2023, 24, 4120. [Google Scholar] [CrossRef]
- Yin, X.; Wang, Q.; Tang, Y.; Wang, T.; Zhang, Y.; Yu, T. Research progress on macrophage polarization during osteoarthritis disease progression: A review. J. Orthop. Surg. Res. 2024, 19, 584. [Google Scholar] [CrossRef]
- Mushenkova, N.V.; Nikiforov, N.G.; Shakhpazyan, N.K.; Orekhova, V.A.; Sadykhov, N.K.; Orekhov, A.N. Phenotype Diversity of Macrophages in Osteoarthritis: Implications for Development of Macrophage Modulating Therapies. Int. J. Mol. Sci. 2022, 23, 8381. [Google Scholar] [CrossRef] [PubMed]
- Mai, L.; Liu, Q.; Huang, F.; He, H.; Fan, W. Involvement of Mast Cells in the Pathophysiology of Pain. Front. Cell. Neurosci. 2021, 15, 665066. [Google Scholar] [CrossRef]
- Hao, G.; Han, S.; Xiao, Z.; Shen, J.; Zhao, Y.; Hao, Q. Synovial mast cells and osteoarthritis: Current understandings and future perspectives. Heliyon 2024, 10, e41003. [Google Scholar] [CrossRef]
- Jiang, P.; Hu, K.; Jin, L.; Luo, Z. A brief review of current treatment options for osteoarthritis including disease-modifying osteoarthritis drugs (DMOADs) and novel therapeutics. Ann. Med. Surg. 2024, 86, 4042–4048. [Google Scholar] [CrossRef]
- Allen, K.D.; Ambrose, K.R.; Booker, S.Q.; Buck, A.N.; Huffman, K.F. Non-Pharmacological Pain Management for Osteoarthritis: Review Update. Curr. Rheumatol. Rep. 2025, 27, 19. [Google Scholar] [CrossRef] [PubMed]
- Hermann, W.; Lambova, S.; Muller-Ladner, U. Current Treatment Options for Osteoarthritis. Curr. Rheumatol. Rev. 2018, 14, 108–116. [Google Scholar] [CrossRef] [PubMed]
- van Laar, M.; Pergolizzi, J.V., Jr.; Mellinghoff, H.U.; Merchante, I.M.; Nalamachu, S.; O’Brien, J.; Perrot, S.; Raffa, R.B. Pain treatment in arthritis-related pain: Beyond NSAIDs. Open Rheumatol. J. 2012, 6, 320–330. [Google Scholar] [CrossRef]
- Wongrakpanich, S.; Wongrakpanich, A.; Melhado, K.; Rangaswami, J. A Comprehensive Review of Non-Steroidal Anti-Inflammatory Drug Use in The Elderly. Aging Dis. 2018, 9, 143–150. [Google Scholar] [CrossRef]
- Fuggle, N.R.; Cooper, C.; Oreffo, R.O.C.; Price, A.J.; Kaux, J.F.; Maheu, E.; Cutolo, M.; Honvo, G.; Conaghan, P.G.; Berenbaum, F.; et al. Alternative and complementary therapies in osteoarthritis and cartilage repair. Aging Clin. Exp. Res. 2020, 32, 547–560. [Google Scholar] [CrossRef]
- Primorac, D.; Molnar, V.; Matišić, V.; Hudetz, D.; Jeleč, Ž.; Rod, E.; Čukelj, F.; Vidović, D.; Vrdoljak, T.; Dobričić, B.; et al. Comprehensive Review of Knee Osteoarthritis Pharmacological Treatment and the Latest Professional Societies’ Guidelines. Pharmaceuticals 2021, 14, 205. [Google Scholar] [CrossRef] [PubMed]
- Guermazi, A.; Neogi, T.; Katz, J.N.; Kwoh, C.K.; Conaghan, P.G.; Felson, D.T.; Roemer, F.W. Intra-articular Corticosteroid Injections for the Treatment of Hip and Knee Osteoarthritis-related Pain: Considerations and Controversies with a Focus on Imaging-Radiology Scientific Expert Panel. Radiology 2020, 297, 503–512. [Google Scholar] [CrossRef]
- Kompel, A.J.; Roemer, F.W.; Murakami, A.M.; Diaz, L.E.; Crema, M.D.; Guermazi, A. Intra-articular Corticosteroid Injections in the Hip and Knee: Perhaps Not as Safe as We Thought? Radiology 2019, 293, 656–663. [Google Scholar] [CrossRef]
- Boutin, R.D.; Pai, J.; Meehan, J.P.; Newman, J.S.; Yao, L. Rapidly progressive idiopathic arthritis of the hip: Incidence and risk factors in a controlled cohort study of 1471 patients after intra-articular corticosteroid injection. Skelet. Radiol. 2021, 50, 2449–2457. [Google Scholar] [CrossRef]
- Peck, J.; Slovek, A.; Miro, P.; Vij, N.; Traube, B.; Lee, C.; Berger, A.A.; Kassem, H.; Kaye, A.D.; Sherman, W.F.; et al. A Comprehensive Review of Viscosupplementation in Osteoarthritis of the Knee. Orthop. Rev. (Pavia) 2021, 13, 25549. [Google Scholar] [CrossRef]
- Tapasvi, S.; Mohanty, S.S.; Vedavyasa Acharya, K.K.; Bhattacharya, K.; Easwaran, R.; Charugulla, S.N. Viscosupplementation for Management of Knee Osteoarthritis from an Indian Perspective: An Expert Consensus Report. Pain Ther. 2019, 8, 217–231. [Google Scholar] [CrossRef] [PubMed]
- Madry, H. Surgical therapy in osteoarthritis. Osteoarthr. Cartil. 2022, 30, 1019–1034. [Google Scholar] [CrossRef]
- Hawker, G.A.; Wright, J.G.; Coyte, P.C.; Williams, J.I.; Harvey, B.; Glazier, R.; Badley, E.M. Differences between men and women in the rate of use of hip and knee arthroplasty. N. Engl. J. Med. 2000, 342, 1016–1022. [Google Scholar] [CrossRef] [PubMed]
- Malfait, A.M. Modelling pain in post-traumatic osteoarthritis of the knee. PAIN 2012, 153, 257–258. [Google Scholar] [CrossRef]
- Roseti, L.; Desando, G.; Cavallo, C.; Petretta, M.; Grigolo, B. Articular Cartilage Regeneration in Osteoarthritis. Cells 2019, 8, 1305. [Google Scholar] [CrossRef]
- Primorac, D.; Molnar, V.; Tsoukas, D.; Uzieliene, I.; Tremolada, C.; Brlek, P.; Klarić, E.; Vidović, D.; Zekušić, M.; Pachaleva, J.; et al. Tissue engineering and future directions in regenerative medicine for knee cartilage repair: A comprehensive review. Croat. Med. J. 2024, 65, 268–287. [Google Scholar] [CrossRef] [PubMed]
- Luyten, F.P.; Vanlauwe, J. Tissue engineering approaches for osteoarthritis. Bone 2012, 51, 289–296. [Google Scholar] [CrossRef]
- Zhu, C.; Wu, W.; Qu, X. Mesenchymal stem cells in osteoarthritis therapy: A review. Am. J. Transl. Res. 2021, 13, 448–461. [Google Scholar]
- Ganguly, P.; Birch, M. Biomaterial Scaffolds for Mesenchymal Stem Cell Based Therapy Aimed at Tissue Engineering Application for Osteoarthritis. Recent Pat. Nanomed. 2015, 5, 19–32. [Google Scholar] [CrossRef]
- Mavrogenis, A.F.; Karampikas, V.; Zikopoulos, A.; Sioutis, S.; Mastrokalos, D.; Koulalis, D.; Scarlat, M.M.; Hernigou, P. Orthobiologics: A review. Int. Orthop. 2023, 47, 1645–1662. [Google Scholar] [CrossRef]
- Everts, P.; Onishi, K.; Jayaram, P.; Lana, J.F.; Mautner, K. Platelet-Rich Plasma: New Performance Understandings and Therapeutic Considerations in 2020. Int. J. Mol. Sci. 2020, 21, 7794. [Google Scholar] [CrossRef]
- Liang, J.; Liu, P.; Yang, X.; Liu, L.; Zhang, Y.; Wang, Q.; Zhao, H. Biomaterial-based scaffolds in promotion of cartilage regeneration: Recent advances and emerging applications. J. Orthop. Transl. 2023, 41, 54–62. [Google Scholar] [CrossRef]
- Ip, H.L.; Nath, D.K.; Sawleh, S.H.; Kabir, M.H.; Jahan, N. Regenerative Medicine for Knee Osteoarthritis—The Efficacy and Safety of Intra-Articular Platelet-Rich Plasma and Mesenchymal Stem Cells Injections: A Literature Review. Cureus 2020, 12, e10575. [Google Scholar] [CrossRef] [PubMed]
- Tjandra, K.C.; Novriansyah, R.; Sudiasa, I.N.S.; Ar, A.; Rahmawati, N.A.D.; Dilogo, I.H. Modified Mesenchymal stem cell, platelet-rich plasma, and hyaluronic acid intervention in early stage osteoarthritis: A systematic review, meta-analysis, and meta-regression of arthroscopic-guided intra-articular approaches. PLoS ONE 2024, 19, e0295876. [Google Scholar] [CrossRef]
- Zhang, J.-Y.; Xiang, X.-N.; Yu, X.; Liu, Y.; Jiang, H.-Y.; Peng, J.-L.; He, C.-Q.; He, H.-C. Mechanisms and applications of the regenerative capacity of platelets-based therapy in knee osteoarthritis. Biomed. Pharmacother. 2024, 178, 117226. [Google Scholar] [CrossRef]
- Filardo, G.; Di Matteo, B.; Kon, E.; Merli, G.; Marcacci, M. Platelet-rich plasma in tendon-related disorders: Results and indications. Knee Surg. Sports Traumatol. Arthrosc. 2018, 26, 1984–1999. [Google Scholar] [CrossRef] [PubMed]
- Ramaswamy Reddy, S.H.; Reddy, R.; Babu, N.C.; Ashok, G.N. Stem-cell therapy and platelet-rich plasma in regenerative medicines: A review on pros and cons of the technologies. J. Oral Maxillofac. Pathol. 2018, 22, 367–374. [Google Scholar] [CrossRef]
- Seong, G.J.; Hong, S.; Jung, S.A.; Lee, J.J.; Lim, E.; Kim, S.J.; Lee, J.H. TGF-beta-induced interleukin-6 participates in transdifferentiation of human Tenon’s fibroblasts to myofibroblasts. Mol. Vis. 2009, 15, 2123–2128. [Google Scholar]
- Dhillon, R.S.; Schwarz, E.M.; Maloney, M.D. Platelet-rich plasma therapy—Future or trend? Arthritis Res. Ther. 2012, 14, 219. [Google Scholar] [CrossRef]
- Simental-Mendía, M.; Ortega-Mata, D.; Tamez-Mata, Y.; Olivo, C.A.A.; Vilchez-Cavazos, F. Comparison of the clinical effectiveness of activated and non-activated platelet-rich plasma in the treatment of knee osteoarthritis: A systematic review and meta-analysis. Clin. Rheumatol. 2023, 42, 1397–1408. [Google Scholar] [CrossRef] [PubMed]
- Bensa, A.; Previtali, D.; Sangiorgio, A.; Boffa, A.; Salerno, M.; Filardo, G. PRP Injections for the Treatment of Knee Osteoarthritis: The Improvement Is Clinically Significant and Influenced by Platelet Concentration: A Meta-analysis of Randomized Controlled Trials. Am. J. Sports Med. 2025, 53, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, M.I.; Whitney, K.; Evans, T.; LaPrade, R.F. Platelet-Rich Plasma and Cartilage Repair. Curr. Rev. Musculoskelet. Med. 2018, 11, 573–582. [Google Scholar] [CrossRef]
- GBD 2017 DALYs and HALE Collaborators. Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1859–1922. [Google Scholar] [CrossRef] [PubMed]
- Wang-Saegusa, A.; Cugat, R.; Ares, O.; Seijas, R.; Cuscó, X.; Garcia-Balletbó, M. Infiltration of plasma rich in growth factors for osteoarthritis of the knee short-term effects on function and quality of life. Arch. Orthop. Trauma Surg. 2011, 131, 311–317. [Google Scholar] [CrossRef]
- Bansal, H.; Leon, J.; Pont, J.L.; Wilson, D.A.; Bansal, A.; Agarwal, D.; Preoteasa, I. Platelet-rich plasma (PRP) in osteoarthritis (OA) knee: Correct dose critical for long term clinical efficacy. Sci. Rep. 2021, 11, 3971. [Google Scholar] [CrossRef]
- Lin, J.; Huang, J.; Jiao, Z.; Nian, M.; Li, C.; Dai, Y.; Jia, S.; Zhang, X. Mesenchymal stem cells for osteoarthritis: Recent advances in related cell therapy. Bioeng. Transl. Med. 2025, 10, e10701. [Google Scholar] [CrossRef]
- Thoene, M.; Bejer-Olenska, E.; Wojtkiewicz, J. The Current State of Osteoarthritis Treatment Options Using Stem Cells for Regenerative Therapy: A Review. Int. J. Mol. Sci. 2023, 24, 8925. [Google Scholar] [CrossRef]
- Chen, Y.; Cheng, R.-J.; Wu, Y.; Huang, D.; Li, Y.; Liu, Y. Advances in Stem Cell-Based Therapies in the Treatment of Osteoarthritis. Int. J. Mol. Sci. 2024, 25, 394. [Google Scholar] [CrossRef]
- Tian, R.; Su, S.; Yu, Y.; Liang, S.; Ma, C.; Jiao, Y.; Xing, W.; Tian, Z.; Jiang, T.; Wang, J. Revolutionizing osteoarthritis treatment: How mesenchymal stem cells hold the key. Biomed. Pharmacother. 2024, 173, 116458. [Google Scholar] [CrossRef]
- Maleitzke, T.; Elazaly, H.; Festbaum, C.; Eder, C.; Karczewski, D.; Perka, C.; Duda, G.N.; Winkler, T. Mesenchymal Stromal Cell-Based Therapy-An Alternative to Arthroplasty for the Treatment of Osteoarthritis? A State of the Art Review of Clinical Trials. J. Clin. Med. 2020, 9, 2062. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, J.A.; Jones, I.A.; Han, B.; Vangsness, C.T., Jr. Intra-articular Mesenchymal Stem Cell Therapy for the Human Joint: A Systematic Review. Am. J. Sports Med. 2018, 46, 3550–3563. [Google Scholar] [CrossRef]
- Krawczenko, A.; Klimczak, A. Adipose Tissue-Derived Mesenchymal Stem/Stromal Cells and Their Contribution to Angiogenic Processes in Tissue Regeneration. Int. J. Mol. Sci. 2022, 23, 2425. [Google Scholar] [CrossRef]
- Stratton, S.; Shelke, N.B.; Hoshino, K.; Rudraiah, S.; Kumbar, S.G. Bioactive polymeric scaffolds for tissue engineering. Bioact. Mater. 2016, 1, 93–108. [Google Scholar] [CrossRef] [PubMed]
- Stamnitz, S.; Klimczak, A. Mesenchymal Stem Cells, Bioactive Factors, and Scaffolds in Bone Repair: From Research Perspectives to Clinical Practice. Cells 2021, 10, 1925. [Google Scholar] [CrossRef] [PubMed]
- Kohn, D.B.; Chen, Y.Y.; Spencer, M.J. Successes and challenges in clinical gene therapy. Gene Ther. 2023, 30, 738–746. [Google Scholar] [CrossRef]
- Evans, C.H.; Kraus, V.B.; Setton, L.A. Progress in intra-articular therapy. Nat. Rev. Rheumatol. 2014, 10, 11–22. [Google Scholar] [CrossRef]
- Vlashi, R.; Zhang, X.; Li, H.; Chen, G. Potential therapeutic strategies for osteoarthritis via CRISPR/Cas9 mediated gene editing. Rev. Endocr. Metab. Disord. 2024, 25, 339–367. [Google Scholar] [CrossRef]
- Vickram, A.S.; Manikandan, S.; Richard, T.; Lakshmi, S.V.; Chopra, H. Targeted Gene Therapy: Promises and Challenges in Disease Management. J. Bio-X Res. 2024, 07, 81–89. [Google Scholar] [CrossRef]
- Cho, Y.; Jeong, S.; Kim, H.; Kang, D.; Lee, J.; Kang, S.-B.; Kim, J.-H. Disease-modifying therapeutic strategies in osteoarthritis: Current status and future directions. Exp. Mol. Med. 2021, 53, 1689–1696. [Google Scholar] [CrossRef]
- Kim, H.; Seo, J.; Lee, Y.; Park, K.; Perry, T.A.; Arden, N.K.; Mobasheri, A.; Choi, H. The current state of the osteoarthritis drug development pipeline: A comprehensive narrative review of the present challenges and future opportunities. Ther. Adv. Musculoskelet. Dis. 2022, 14, 1759720x221085952. [Google Scholar] [CrossRef] [PubMed]
- Karsdal, M.A.; Michaelis, M.; Ladel, C.; Siebuhr, A.S.; Bihlet, A.R.; Andersen, J.R.; Guehring, H.; Christiansen, C.; Bay-Jensen, A.C.; Kraus, V.B. Disease-modifying treatments for osteoarthritis (DMOADs) of the knee and hip: Lessons learned from failures and opportunities for the future. Osteoarthr. Cartil. 2016, 24, 2013–2021. [Google Scholar] [CrossRef]
- Cohen, S.B.; Proudman, S.; Kivitz, A.J.; Burch, F.X.; Donohue, J.P.; Burstein, D.; Sun, Y.N.; Banfield, C.; Vincent, M.S.; Ni, L.; et al. A randomized, double-blind study of AMG 108 (a fully human monoclonal antibody to IL-1R1) in patients with osteoarthritis of the knee. Arthritis Res. Ther. 2011, 13, R125. [Google Scholar] [CrossRef] [PubMed]
- Loef, M.; Kroon, F.P.B.; Bergstra, S.A.; van der Pol, J.A.; Lems, W.F.; Kerstens, P.; Allaart, C.F.; Kloppenburg, M. TNF inhibitor treatment is associated with a lower risk of hand osteoarthritis progression in rheumatoid arthritis patients after 10 years. Rheumatology 2018, 57, 1917–1924. [Google Scholar] [CrossRef]
- Madry, H.; Cucchiarini, M. Tissue-Engineering Strategies to Repair Joint Tissue in Osteoarthritis: Nonviral Gene-Transfer Approaches. Curr. Rheumatol. Rep. 2014, 16, 450. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Chen, R.; Chen, C.; Yang, F.; Xiao, H.; Geng, B.; Xia, Y. Tissue engineering strategies hold promise for the repair of articular cartilage injury. Biomed. Eng. Online 2024, 23, 92. [Google Scholar] [CrossRef]
- Hargreaves, K.; Dubner, R.; Brown, F.; Flores, C.; Joris, J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. PAIN 1988, 32, 77–88. [Google Scholar] [CrossRef]
- Bozkurt, A.; Deumens, R.; Scheffel, J.; O’Dey, D.M.; Weis, J.; Joosten, E.A.; Führmann, T.; Brook, G.A.; Pallua, N. CatWalk gait analysis in assessment of functional recovery after sciatic nerve injury. J. Neurosci. Methods 2008, 173, 91–98. [Google Scholar] [CrossRef]
- Deuis, J.R.; Dvorakova, L.S.; Vetter, I. Methods Used to Evaluate Pain Behaviors in Rodents. Front. Mol. Neurosci. 2017, 10, 284. [Google Scholar] [CrossRef]
- Guo, T.Z.; Wei, T.; Li, W.W.; Li, X.Q.; Clark, J.D.; Kingery, W.S. Immobilization contributes to exaggerated neuropeptide signaling, inflammatory changes, and nociceptive sensitization after fracture in rats. J. Pain 2014, 15, 1033–1045. [Google Scholar] [CrossRef] [PubMed]
- Tate, C.C.; Tate, M.C.; LaPlaca, M.C. Fibronectin and laminin increase in the mouse brain after controlled cortical impact injury. J. Neurotrauma 2007, 24, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.E.; Malfait, A.M. Osteoarthritis pain: What are we learning from animal models? Best Pract. Res. Clin. Rheumatol. 2017, 31, 676–687. [Google Scholar] [CrossRef] [PubMed]
- Tatem, K.S.; Quinn, J.L.; Phadke, A.; Yu, Q.; Gordish-Dressman, H.; Nagaraju, K. Behavioral and locomotor measurements using an open field activity monitoring system for skeletal muscle diseases. J. Vis. Exp. 2014, 91, e51785. [Google Scholar] [CrossRef]
- Ma, L.; Liu, S.; Yi, M.; Wan, Y. Spontaneous pain as a challenge of research and management in chronic pain. Med. Rev. 2022, 2, 308–319. [Google Scholar] [CrossRef]
- Turner, P.V.; Pang, D.S.; Lofgren, J.L. A Review of Pain Assessment Methods in Laboratory Rodents. Comp. Med. 2019, 69, 451–467. [Google Scholar] [CrossRef]
- Lakes, E.H.; Allen, K.D. Gait analysis methods for rodent models of arthritic disorders: Reviews and recommendations. Osteoarthr. Cartil. 2016, 24, 1837–1849. [Google Scholar] [CrossRef]
- Christiansen, C.L.; Stevens-Lapsley, J.E. Weight-bearing asymmetry in relation to measures of impairment and functional mobility for people with knee osteoarthritis. Arch. Phys. Med. Rehabil. 2010, 91, 1524–1528. [Google Scholar] [CrossRef]
- Malfait, A.M.; Little, C.B.; McDougall, J.J. A commentary on modelling osteoarthritis pain in small animals. Osteoarthr. Cartil. 2013, 21, 1316–1326. [Google Scholar] [CrossRef]
- Zheng, Y.; Lunn, A.; Gao, J.; Chen, H.; Yao, Y. Quantitative evaluation of hindlimb grip strength in mice as a measure of neuromuscular function. MethodsX 2025, 14, 103118. [Google Scholar] [CrossRef] [PubMed]
- Nugent, S.M.; Lovejoy, T.I.; Shull, S.; Dobscha, S.K.; Morasco, B.J. Associations of Pain Numeric Rating Scale Scores Collected during Usual Care with Research Administered Patient Reported Pain Outcomes. Pain Med. 2021, 22, 2235–2241. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Valente, M.A.; Pais-Ribeiro, J.L.; Jensen, M.P. Validity of four pain intensity rating scales. PAIN 2011, 152, 2399–2404. [Google Scholar] [CrossRef] [PubMed]
- Åström, M.; Thet Lwin, Z.M.; Teni, F.S.; Burström, K.; Berg, J. Use of the visual analogue scale for health state valuation: A scoping review. Qual. Life Res. 2023, 32, 2719–2729. [Google Scholar] [CrossRef]
- Tsai, P.F.; Beck, C.; Richards, K.C.; Phillips, L.; Roberson, P.K.; Evans, J. The Pain Behaviors for Osteoarthritis Instrument for Cognitively Impaired Elders (PBOICIE). Res. Gerontol. Nurs. 2008, 1, 116–122. [Google Scholar] [CrossRef]
- Stewart, C. Practical Management of Pain; Mosby: Maryland Heights, MO, USA, 2014; pp. 467–473. [Google Scholar]
- Melzack, R. The McGill Pain Questionnaire: Major properties and scoring methods. PAIN 1975, 1, 277–299. [Google Scholar] [CrossRef]
- Im, D.D.; Jambaulikar, G.D.; Kikut, A.; Gale, J.; Weiner, S.G. Brief Pain Inventory—Short Form: A New Method for Assessing Pain in the Emergency Department. Pain Med. 2020, 21, 3263–3269. [Google Scholar] [CrossRef]
- Harato, K.; Iwama, Y.; Kaneda, K.; Kobayashi, S.; Niki, Y.; Nagura, T. Pain detect questionnaire and pain catastrophizing scale affect gait pattern in patients with knee osteoarthritis. J. Exp. Orthop. 2022, 9, 52. [Google Scholar] [CrossRef]
- Freynhagen, R.; Baron, R.; Gockel, U.; Tölle, T.R. painDETECT: A new screening questionnaire to identify neuropathic components in patients with back pain. Curr. Med. Res. Opin. 2006, 22, 1911–1920. [Google Scholar] [CrossRef]
- Bouhassira, D.; Attal, N.; Alchaar, H.; Boureau, F.; Brochet, B.; Bruxelle, J.; Cunin, G.; Fermanian, J.; Ginies, P.; Grun-Overdyking, A.; et al. Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4). PAIN 2005, 114, 29–36. [Google Scholar] [CrossRef]
- Braaksma, C.; Wolterbeek, N.; Veen, M.R.; Prinsen, C.A.C.; Ostelo, R. Systematic review and meta-analysis of measurement properties of the Hip disability and Osteoarthritis Outcome Score—Physical Function Shortform (HOOS-PS) and the Knee Injury and Osteoarthritis Outcome Score—Physical Function Shortform (KOOS-PS). Osteoarthr. Cartil. 2020, 28, 1525–1538. [Google Scholar] [CrossRef] [PubMed]
- Cook, K.F.; Schalet, B.D.; Kallen, M.A.; Rutsohn, J.P.; Cella, D. Establishing a common metric for self-reported pain: Linking BPI Pain Interference and SF-36 Bodily Pain Subscale scores to the PROMIS Pain Interference metric. Qual. Life Res. 2015, 24, 2305–2318. [Google Scholar] [CrossRef] [PubMed]
- Riddle, D.L.; Perera, R.A. The WOMAC Pain Scale and Crosstalk From Co-occurring Pain Sites in People With Knee Pain: A Causal Modeling Study. Phys. Ther. 2020, 100, 1872–1881. [Google Scholar] [CrossRef]
- Anderson, A.F.; Irrgang, J.J.; Kocher, M.S.; Mann, B.J.; Harrast, J.J. The International Knee Documentation Committee Subjective Knee Evaluation Form: Normative data. Am. J. Sports Med. 2006, 34, 128–135. [Google Scholar] [CrossRef]
- Barber-Westin, S.D.; Noyes, F.R.; McCloskey, J.W. Rigorous statistical reliability, validity, and responsiveness testing of the Cincinnati knee rating system in 350 subjects with uninjured, injured, or anterior cruciate ligament-reconstructed knees. Am. J. Sports Med. 1999, 27, 402–416. [Google Scholar] [CrossRef]
- Briggs, K.K.; Lysholm, J.; Tegner, Y.; Rodkey, W.G.; Kocher, M.S.; Steadman, J.R. The reliability, validity, and responsiveness of the Lysholm score and Tegner activity scale for anterior cruciate ligament injuries of the knee: 25 years later. Am. J. Sports Med. 2009, 37, 890–897. [Google Scholar] [CrossRef]
- Malviya, S.; Voepel-Lewis, T.; Burke, C.; Merkel, S.; Tait, A.R. The revised FLACC observational pain tool: Improved reliability and validity for pain assessment in children with cognitive impairment. Pediatr. Anesth. 2006, 16, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Mogil, J.S.; Pang, D.S.J.; Silva Dutra, G.G.; Chambers, C.T. The development and use of facial grimace scales for pain measurement in animals. Neurosci. Biobehav. Rev. 2020, 116, 480–493. [Google Scholar] [CrossRef]
- Kennedy, D.L.; Kemp, H.I.; Ridout, D.; Yarnitsky, D.; Rice, A.S.C. Reliability of conditioned pain modulation: A systematic review. PAIN 2016, 157, 2410–2419. [Google Scholar] [CrossRef]
- Knazovicky, D.; Helgeson, E.S.; Case, B.; Gruen, M.E.; Maixner, W.; Lascelles, B.D.X. Widespread somatosensory sensitivity in naturally occurring canine model of osteoarthritis. PAIN 2016, 157, 1325–1332. [Google Scholar] [CrossRef]
- Ji, J.; Huh, Y.; Ji, R.-R. Inflammatory Mediators, Nociceptors, and Their Interactions in Pain. In Neuroimmune Interactions in Pain: Mechanisms and Therapeutics; Ji, R.-R., Cheng, J., Ji, J., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 87–119. [Google Scholar]
- Pinho-Ribeiro, F.A.; Verri, W.A.; Chiu, I.M. Nociceptor Sensory Neuron–Immune Interactions in Pain and Inflammation. Trends Immunol. 2017, 38, 5–19. [Google Scholar] [CrossRef] [PubMed]
- Perrot, S.; Anne-Priscille, T. Pain in osteoarthritis from a symptom to a disease. Best Pract. Res. Clin. Rheumatol. 2023, 37, 101825. [Google Scholar] [CrossRef]
- Panchal, Y.; Pardeshi, A.; Singhal, A.; Soni, H.; Singh, G.; Gomathy, G.; Shhroye, N.; Thakur, N.; Thorat, P. Effective Strategies for Managing Chronic Pain in Patients: A Review of the Current Evidence. Universe Int. J. Interdiscip. Res. 2024, 5, 85–96. [Google Scholar]
- Liao, J.; Gu, Q.; Liu, Z.; Wang, H.; Yang, X.; Yan, R.; Zhang, X.; Song, S.; Wen, L.; Wang, Y. Edge advances in nanodrug therapies for osteoarthritis treatment. Front. Pharmacol. 2024, 15, 1402825. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, T. Multimodal approach to intraarticular drug delivery in knee osteoarthritis. Rheumatol. Int. 2020, 40, 1763–1769. [Google Scholar] [CrossRef]
- Veronese, N.; Cooper, C.; Bruyère, O.; Al-Daghri, N.M.; Branco, J.; Cavalier, E.; Cheleschi, S.; da Silva Rosa, M.C.; Conaghan, P.G.; Dennison, E.M.; et al. Multimodal Multidisciplinary Management of Patients with Moderate to Severe Pain in Knee Osteoarthritis: A Need to Meet Patient Expectations. Drugs 2022, 82, 1347–1355. [Google Scholar] [CrossRef]
- Ho, M.J.; Kim, S.R.; Choi, Y.W.; Kang, M.J. Recent advances in intra-articular drug delivery systems to extend drug retention in joint. J. Pharm. Investig. 2019, 49, 9–15. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).