Abstract
Diabetes mellitus (DM) encompasses a group of metabolic diseases characterised by abnormal glucose levels. The pathophysiology of DM involves intricate disruptions in glucose metabolism and immune regulation. The gut microbiome is known to play a crucial role in human health and disease, and changes in its composition have been reported in numerous conditions, including DM. In this review, we discuss recent findings on the intricate relationship between the gut microbiome and DM, including its complications. We highlight the involvement of gut microorganisms in inflammation and metabolic processes, and we summarise current evidence on how antidiabetic therapies influence microbiome composition and activity. Finally, we explore the potential role of microbiome monitoring in predicting treatment response.
1. Introduction
Diabetes mellitus (DM) is a broad term that encompasses various metabolic conditions associated with elevated glucose levels, including type 1 DM (T1D), type 2 DM (T2D), and gestational DM (GDM), among others. The defining feature of these conditions is abnormal glucose regulation, arising from different pathophysiological mechanisms involving insulin activity or its presence. Yet the pathophysiology of these metabolic disorders is considerably more complex, involving immune responses and inflammation [1,2]. In addition, DM and its complications are linked to alterations in the gut microbiome. It is well established that intestinal microorganisms—including bacteria, fungi, and viruses—exert profound effects on human health and disease. Over the years, researchers have examined and identified connections between the gut microbiome and seemingly distant organs, such as the lungs [3], brain [4], and the skin [5], among others. Notably, studies have also demonstrated the role of the gut microbiome in metabolic alterations associated with DM, such as insulin resistance, a major mechanism underlying the development of T2D [6]. This review discusses recent findings on the relationship between DM and the intestinal microbiome.
2. Intestinal Bacteria and the Pathogenesis of Diabetes
2.1. Gut Microbiome in Patients with Diabetes
In mammals, the gut microbiome is dominated by four major bacterial phyla: Actinobacteria, Bacteroidetes, Firmicutes, and Proteobacteria [7]. The gut microbiome can influence the development of DM by modulating inflammation, insulin resistance, and the production of metabolites such as short-chain fatty acids (SCFAs), which play a role in regulating glucose metabolism and insulin sensitivity [8]
The profile of the microbiome depends on numerous factors, including general health, diet, medications, geographical location, and cultural background [9,10]. It has been shown that patients with DM exhibit significant alterations in microbiome diversity. However, more recent studies suggest that such associations may not always be present. In a recent meta-analysis by Machado et al. [11], the authors reviewed studies comparing microbiome diversity in patients with T2D and healthy controls using 16S sequencing. Thirteen articles were included, but the pooled analyses did not show a significant difference in alpha diversity. However, such findings should be interpreted in a broader context. First, differences in microbiome diversity may be less pronounced but still biologically relevant. Second, the method of analysis—alpha versus beta diversity—can influence the results. For example, Cui et al. [12] examined the microbiome in patients with GDM, analysing 718 bacterial species. They did not find significant differences in beta diversity between patients with GDM and those with normal glucose tolerance. Nonetheless, their analysis of enterotypes dominated by Firmicutes, Bacteroides, or Prevotella showed that the latter was more prevalent in patients with the metabolic condition, suggesting an altered microbiome profile. The same study also assessed the effects of nutritional management in patients with GDM. The foods high in fiber may influence relationship between microbial characteristics and glucose metabolism, with microbial fiber fermentation capacity showing a negative correlation with gestational glucose levels. The intervention increased the abundance of Bifidobacterium, underscoring the link between lifestyle, DM control, and microbiome composition—a theme explored in greater detail in the following sections.
Individuals with T2D can exhibit an increased abundance of opportunistic pathogens such as Ruminococcus, Fusobacterium, and Blautia while experiencing a decline in populations of SCFA-producing bacteria, including Bifidobacterium, Bacteroides, Faecalibacterium, Akkermansia muciniphila, and Roseburia [8,13]. Analysis of the gut microbiome in women with both normal weight and overweight/obesity revealed significant differences between patients with T2D and healthy individuals. A reduced abundance of Firmicutes and Clostridium species was observed in the small intestine of patients with T2D. Additionally, the Bacteroidetes to Firmicutes ratio was positively correlated with plasma glucose levels, suggesting a potential link between microbiome composition and the regulation of carbohydrate metabolism [14,15].
Other studies indicate that butyrate-producing bacteria are significantly reduced in T2D. These include Subdoligranulum, Eubacterium rectale, Faecalibacterium prausnitzii, Roseburia intestinalis, Roseburia inulinivorans, as well as Ruminococcus [16,17]. Butyrate mainly exerts significant anti-inflammatory effects, which are associated with reduced release of reactive oxygen species, thereby helping to maintain homeostasis of the colorectal epithelium [9,18]. Faecalibacterium, in addition to being strictly anaerobic, is highly sensitive to oxygen [19]. It is likely that bacteria of this genus, besides producing SCFAs, also play a stabilising role in the gut microbiome; that is, their abundance may be negatively correlated with higher intra-individual diversity in microbiome composition [20]. In the case of the genus Roseburia, which is anaerobic, the most common species inhabiting the human intestine is R. intestinalis. This bacterium is mainly responsible for regulating metabolism, influencing immune stimulation, and supporting maintenance of the intestinal barrier [21]. Because of its ability to produce butyrate, R. intestinalis also contributes to energy homeostasis, including glucose-related regulation [22].
Bifidobacterium have also been shown to decrease in abundance. This is an unfavourable phenomenon because this genus plays an important role in maintaining proper intestinal permeability, improving glucose tolerance, and supporting insulin release [23]. Bifidobacterium appear in the human intestine within the first weeks of life, mobilised by the bifidogenic properties of specific oligosaccharides in breast milk, known as human milk oligosaccharides. Metagenomic studies have shown that the gut is typically populated by these bacteria in large numbers, though with relatively low species diversity. The most common are B. bifidum, B. adolescentis, B. breve, and B. longum [24]. With age, the abundance of Bifidobacterium in the intestines declines, and their composition also varies significantly between individuals, shaped largely by both inter- and intra-individual variability [25]. Bifidobacterium adolescentis is among the first to colonise the newborn intestine. These bacteria do not produce spores and are immobile. Their role is mainly in preventing intestinal inflammation, which in turn has a positive effect on the overall condition of the host [26,27]. Bifidobacterium bifidum is considered the dominant species inhabiting the human intestine [26]. Its genome contains approximately 3000 genes, primarily encoding carbohydrate-active enzymes that enable glycan metabolism [25,28].
In addition, the level of Lactobacillus was found to be significantly elevated because it is positively correlated with glycosylated haemoglobin and fasting blood glucose levels. This could indicate that level of Lactobacillus is positively correlated with glycemic markers and the developments of diabetes [29]. However Lactobacillus can stimulate release of incretine hormones such as GLP-1, which increase insulin secretion [30]. Lactobacillus, often referred to collectively as lactobacilli, are well adapted to conditions in the human digestive tract. They are catalase-negative and lack the ability to produce spores. The genus Lactobacillus in the gut is represented by many species, including L. casei, L. lactis, L. gasseri, and L. salivarius, among others [31]. These bacteria contribute to maintaining the integrity of the intestinal epithelial barrier [32]. Certain Lactobacillus species can regulate the expression of mucin genes, thereby influencing mucus production, which is indirectly linked to gut immune responses [33]. Moreover, Lactobacillus species exhibit antibacterial properties through the production of metabolites such as organic acids, SCFAs, and bacteriocins. They also play a role in modulating the immune response via signalling between the gastrointestinal tract and distant organs. Collectively, these mechanisms enable Lactobacillus to trigger anti-inflammatory effects that may help alleviate symptoms associated with DM [34].
Increased amounts of Blautia, Coprococcus, Collinsella, Sporobacterium, and Peptostreptococcus have been reported in patients with T2D [9]. Blautia is associated not only with the reduction in metabolic symptoms but also with selective antibacterial properties, allowing interaction with other microorganisms. Members of the genus Blautia are anaerobic and non-motile, and many do not produce spores. Adequate levels of these bacteria in the intestine have a beneficial effect on glucose and lipid homeostasis [35,36]. However, Blautia also produces acetic acid, which can impair insulin signalling [37]. Furthermore, changes have been observed in the abundance of Ruminococcus and Lactobacillus (e.g., L. lactis), as well as Streptococcus thermophilus [38,39]. Other studies confirm a reduced abundance of Bifidobacterium, along with decreases in Roseburia and Faecalibacterium [39]. Patients diagnosed with prediabetes are characterised by reduced levels of Clostridium and elevated levels of Ruminococcus and Streptococcus [40] (Figure 1).
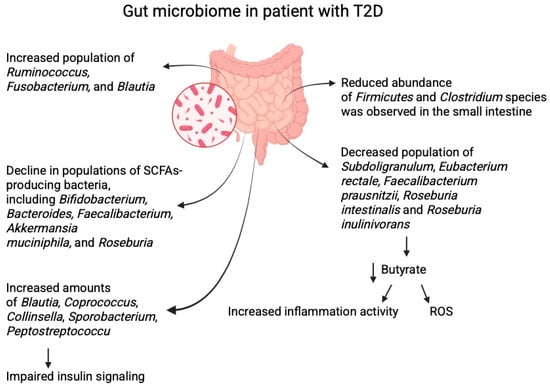
Figure 1.
The effects of diabetes on the composition of gut microbiome. Created in BioRender. Physiology, D. (2025) Available online: https://BioRender.com/e6l8dch.
2.2. Mechanisms of Microbiome in Diabetes Pathophysiology
Growing evidence highlights the role of the gut microbiome in regulating immune and metabolic pathways, with microbial dysbiosis and metabolic endotoxaemia implicated in T2D development. Chronic low-grade inflammation—marked by elevated production of pro-inflammatory cytokines such as interleukin (IL)-6, IL-1β, and tumour necrosis factor-α (TNF-α)—is a typical feature of patients with DM [41]. Gut dysbiosis, characterised by a reduction in beneficial anti-inflammatory taxa and an expansion of inflammation-promoting bacteria, contributes to persistent metabolic disturbances. This can be illustrated by examining levels of circulating lipopolysaccharide (LPS) or LPS-binding protein, the latter of which is elevated in animal models of T2D [42]. Bacteria positively associated with higher levels of pro-inflammatory molecules include Bacteroides, Parabacteroides, Parasutterella, and Ruminococcus gnavus, among others [42].
The immunomodulatory properties of the microbiome are further demonstrated in studies involving faecal microbiome transplantation (FMT). Mice that received FMT from patients with GDM rapidly developed altered intestinal microbiome profiles, together with changes in immune status, including elevated IL-6 and IL-10. These microbial alterations included a reduced presence of Prevotella copri, consistent with findings in women with GDM [43]. Recently, Zhou et al. [44] published a large meta-analysis examining therapies aimed at modulating the gut microbiome in T2D. The analysis included 54 studies involving 3390 patients. Interventions included synbiotics, prebiotics, probiotics, and FMT. These therapies produced significant effects on inflammatory markers: alongside reduced high-sensitivity C-reactive protein, there was a decrease in TNF-α and LPS and an increase in IL-10. Together, these findings emphasise the role of DM in shaping the intestinal microbiome and, in turn, influencing systemic inflammation. Microbiome-targeted therapies show potential in mitigating inflammatory processes.
Disruptions in intestinal barrier integrity are among the key pathophysiological mechanisms linked to T2D, leading to increased intestinal permeability and the translocation of bacterial components into the bloodstream. This process results in metabolic endotoxaemia, which can exacerbate chronic inflammation and contribute to disease progression [45,46,47].
SCFAs, particularly butyrate, may play an additional role in regulating intestinal permeability. Butyrate interacts with serotonin transporters and the peroxisome proliferator-activated receptor-γ (PPAR-γ) signalling pathway, potentially improving epithelial function and reducing barrier permeability [8,48,49,50]. Increased intestinal permeability is also implicated in the pathogenesis of T1D because it allows the translocation of exogenous antigens and bacterial toxins that damage pancreatic β-cells. Some bacterial toxins, such as those produced by Streptomyces, can directly induce β-cell destruction and impair glucose tolerance. In NOD mice, the absence of the MyD88 protein has been shown to prevent DM development, while elimination of the gut microbiome increases susceptibility to the disease—highlighting the protective role of the microbiome in regulating immune responses [51,52].
The gut microbiome influences glucose homeostasis and insulin resistance, both of which are key factors in disease development. Alterations in microbiome composition can lead to metabolic disturbances in major organs involved in metabolism, such as the liver, skeletal muscle, and adipose tissue. The microbiome also modulates carbohydrate digestion, gut hormone production, and enzymatic activity, directly impacting glucose regulation and insulin sensitivity
Certain probiotic bacteria exert beneficial effects in the context of T2D. For example, Bifidobacterium lactis enhances glycogen synthesis in the liver and reduces the expression of genes involved in gluconeogenesis, thereby improving glycaemic control [53]. In addition, B. lactis promotes the translocation of glucose transporter type 4 (GLUT4) to the cell membrane, facilitating glucose uptake in response to insulin and enhancing insulin sensitivity. Another example is Lactobacillus gasseri BNR17, which also increases GLUT4 expression in muscle, potentially supporting glucose regulation in insulin-resistant individuals. Furthermore, bacteria such as Akkermansia muciniphila and Lactobacillus plantarum can downregulate the expression of flavin-containing monooxygenase 3 (FMO3), an enzyme involved in xenobiotic metabolism. Excessive FMO3 activity has been linked to hyperglycaemia and hyperlipidaemia, while its suppression is associated with improved insulin sensitivity in insulin-resistant mice [48,54,55]. Another probiotic, Lactobacillus casei, has been shown to enhance glucose metabolism by increasing the expression of genes involved in insulin signalling, such as phosphatidylinositol-3-kinase, insulin receptor substrate 2, AMP-activated protein kinase, and protein kinase B. Lactobacillus casei also contributes to hepatic glycogen synthesis and regulates bile acid metabolism by modulating bile acid–chloride exchange, further supporting glucose control mechanisms [55,56,57].
SCFAs interact with G protein–coupled receptors (GPR41 and GPR43) which induce a variety of cellular responses. Activation of GPR43 suppresses cAMP production and stimulates mitogen activating protein kinase (MAPK), one of the most crucial signalling pathways in human cells. Moreover, by interacting with their receptors, SCFAs influence metabolism activity. One of the examples involves GLP-1, as experiments involving GRP-deficient mouse showed reduce release of GLP-1 [58,59]. GLP-1, an incretin hormone, enhances insulin secretion in response to food intake, inhibits glucagon release, improves insulin sensitivity, and promotes weight loss by increasing satiety. These effects explain growing interest in the use of GLP-1 agonists in the treatment of T2D. In addition, SCFAs can modulate inflammatory responses by reducing chronic low-grade inflammation, a common feature of T2D. This effect may be related to bacterial translocation from the gut to other tissues, such as mesenteric adipose tissue, where it triggers inflammatory mechanisms. Dysregulation of SCFAs production due to an unfavourable gut microbiome composition can impair glucose and insulin metabolism, thereby contributing to the progression of T2D [60,61,62].
Furthermore, bacteria such as Bifidobacterium and Lactobacillus produce bile salt hydrolases, which convert primary bile acids into their free forms, allowing further transformation into secondary bile acids. These secondary bile acids activate the TGR5 receptor, which in turn stimulates GLP-1 secretion and improves carbohydrate metabolism [8,60].
A diet rich in animal products, particularly red meat, provides large amounts of L-carnitine, choline, and betaine, which are metabolised by the gut microbiome into trimethylamine. This compound is subsequently oxidised by FMO3 [55] in the liver, forming trimethylamine N-oxide (TMAO), which is then excreted by the kidneys [8,63]. Human studies have demonstrated a correlation between serum TMAO levels (and its precursors) with the incidence of cardiovascular disease and T2D [29,64,65] (Figure 2). The breakdown of TMAO is essential for maintaining glucose homeostasis in the host. Elevated serum TMAO levels have been observed in patients with DM, showing a positive correlation with inflammatory factors such as IL-6 and TNF-α. Conversely, dietary changes that reduce TMAO levels have been associated with improved insulin sensitivity in diabetic individuals [66].
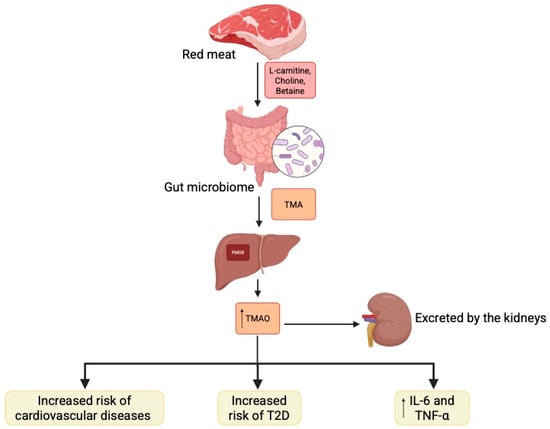
Figure 2.
Gut microbiome is involved in the formation of trimethylamine which is then metabolized into trimethylamine N-oxide. Its altered expression is linked with pathophysiology of diabetes Created in BioRender. Physiology, D. (2025) Available online: https://BioRender.com/w73u5i9.
Enhancing fatty acid oxidation and energy expenditure while reducing fatty acid synthesis can alleviate obesity and T2D. Studies have shown that Akkermansia muciniphila, Bacteroides acidifaciens, Lactobacillus gasseri, and SCFAs promote fatty acid oxidation in adipose tissue. For instance, A. muciniphila increases levels of 2-oleoylglycerol, 2-palmitoylglycerol, and 2-acylglycerol in adipose tissue, thereby enhancing fatty acid oxidation and adipocyte differentiation [67]. Additionally, B. acidifaciens improves fatty acid oxidation in adipose tissue through the TGR5–PPAR-α pathway [68].
Similarly, butyrate promotes fatty acid oxidation and thermogenesis by inhibiting histone deacetylation in muscle, thereby increasing mitochondrial activity and energy expenditure. In the liver and adipose tissue, butyrate—along with propionate and acetate—reduces PPAR-γ expression, which in turn enhances fatty acid oxidation and lipid catabolism [8,69]. Butyrate acts as a ligand for GPCR41 and GPCR43 in the intestine, stimulating the secretion of GLP-1 and peptide YY, hormones that regulate glucose metabolism and satiety [69,70,71]. In addition, Bifidobacterium lactis and Lactobacillus gasseri positively influence liver metabolism by downregulating genes responsible for gluconeogenesis, while promoting glycogen synthesis and facilitating GLUT4 translocation in muscle. These effects improve glucose uptake and contribute to reducing hyperglycaemia [72].
Regarding the modulation of lipid metabolism by gut bacteria, Lactobacillus gasseri contributes to weight reduction by upregulating genes involved in fatty acid β-oxidation while simultaneously inhibiting lipid biosynthesis pathways [73]. In animal models, Akkermansia muciniphila and Lactobacillus casei have also been shown to reduce serum malondialdehyde levels, a marker of oxidative lipid damage, which may be relevant to T2D pathogenesis [74]. Despite the association between gut dysbiosis and T2D development, numerous studies indicate that certain Gram-positive bacteria may exert protective and therapeutic effects. These mechanisms include the production of SCFAs, modulation of immune responses, and regulation of glucose and lipid metabolism. Bacteria from the genera Lactobacillus and Bifidobacterium, through dietary fibre fermentation, increase SCFAs concentrations (mainly butyrate, propionate, and acetate), which improve insulin sensitivity and reduce inflammation [75].
Another mechanism through which bacteria can improve metabolic function is their impact on signalling pathways in adipocytes. For example, Lactobacillus rhamnosus increases adiponectin levels in adipose tissue, leading to improved insulin sensitivity and a reduced risk of insulin resistance [76]. Additionally, some Gram-positive bacteria can inhibit intestinal enzymes such as α-glucosidase, delaying carbohydrate digestion and limiting postprandial hyperglycaemia [77]. Therefore, appropriate modulation of the gut microbiome through supplementation with specific Gram-positive bacteria may represent a potential therapeutic strategy for managing T2D and other metabolic disorders.
2.3. Diabetic Complications
In individuals with T2D, a decrease in the abundance of Faecalibacterium and Roseburia, together with reduced SCFAs production, has been associated with heightened inflammatory processes. These processes are recognised as a major contributing factor to the development of DM complications, including vascular damage, neuropathy, and retinopathy [78]. For example, R. intestinalis has been shown to stimulate the production of IL-22, an anti-inflammatory cytokine that improves insulin sensitivity and alleviates symptoms associated with DM [79,80]. Conversely, a reduction in the activity of SCFA-producing bacteria disrupts immune balance, thereby promoting chronic inflammation—a hallmark of DM [81].
Ruminococcus and Blautia are implicated in fibre fermentation, a process that promotes the production of SCFAs, with butyric acid being a notable constituent [82]. Butyric acid plays an important role in regulating glucose metabolism by improving tissue sensitivity to insulin and modulating the expression of genes involved in glucose regulation [83]. Several studies have shown that a decrease in the levels of these bacteria within the gastrointestinal tract can result in impaired glycaemic control, which in turn may lead to insulin resistance—a key pathogenic mechanism underpinning the development of T2D [84]. An imbalance of the gut microbiome, characterised by a predominance of pro-inflammatory bacteria and a concurrent reduction in SCFA-producing bacteria such as Faecalibacterium and Roseburia, has been associated with an escalation of DM complications [85]. The strongest association has been observed for Ruminococcus gnavus, which is directly linked to percentage body fat. This finding suggests that an abnormal gut microbiome composition may influence metabolic processes such as lipogenesis, thereby increasing the risk of metabolic syndrome and, consequently, the development of DM and its complications [86].
Furthermore, a decrease in Bifidobacterium and Lactobacillus populations—both known to help maintain microbiome balance and regulate the immune response—has been linked to a higher risk of infections and metabolic complications [87]. A meta-analysis of 12 randomised trials showed that in 10 of these, Lactobacillus strains significantly reduced HbA1c, fasting insulin, and the HOMA-IR insulin resistance index in individuals with T2D [88]. Animal studies have supported these findings. For instance, L. casei CCFM419 improved glycaemic control and reduced insulin resistance in murine models of DM [89]. Another study found that L. acidophilus reduced blood glucose levels in patients with T2D [90]. In a diet-induced obesity model, Lactobacillus improved glucose tolerance, likely by reducing endoplasmic reticulum stress in muscle, inhibiting macrophage activation, and increasing GLUT4 expression [91,92]. In addition, these bacteria have been shown to promote insulin secretion by regulating autonomic nervous system neurotransmitters, reducing insulin-degrading enzyme activity, and slowing insulin degradation, thereby stabilising blood glucose levels [93]. A reduction in Lactobacillus within the gut microbiome may therefore be associated with impaired glycaemic control, increased insulin resistance, and chronic inflammation, which together heighten the risk of DM complications [94]. Moreover, gut dysbiosis has been shown to promote obesity and increase oxidative stress, both of which exacerbate inflammatory processes and contribute to further deterioration of insulin sensitivity [95,96].
Patients with DM are more susceptible to infections because of impaired immune responses, neuropathy, and microcirculatory disturbances [97]. One of the most serious complications is diabetic foot syndrome, which leads to the development of chronic wounds that are difficult to heal [98]. The presence of diabetic neuropathy often results in unrecognised mechanical trauma, which, when combined with impaired circulation, promotes ulcer formation [99]. These wounds provide an ideal environment for colonisation and infection by Gram-positive bacteria such as Staphylococcus aureus, Streptococcus spp., Enterococcus spp., and intestinal commensals like Ruminococcus, which can act opportunistically under conditions of dysbiosis [100]. It is estimated that 40–80% of patients with DM develop wound infections, significantly increasing the risk of severe complications such as necrotising tissue inflammation, osteomyelitis, or even limb amputation [101,102,103].
Mendelian randomization and 16S rDNA sequencing studies were conducted in both patients and animal models with diabetic neuropathy which demonstrated links between gut microbiota species such as Firmicutes and Bacteroides and the onset of diabetic peripheral neuropathy, indicating that and imbalance in gut microbiota plays a role in its development. The study demonstrated that patients with diabetic neuropathy presented higher abundance of Firmicutes and lower abundance of Bacteroides [104]. The other study demonstrated that HbA1c levels had negative correlation with the abundance of Ruminococcus 1, while fasting plasma glucose (FPG) levels were positively correlated with the presence of Bacteroides and Dialister [78]. Study by Yang et al. presented that changes in microbial diversity, including elevated levels of Actinobacteria and Firmicutes and decreased Bacteroidetes, are linked to diabetic neuropathy and may affect insulin resistance, thereby playing a role in peripheral nerve damage and chronic pain [105].
Staphylococcus aureus is one of the most prevalent pathogens isolated from diabetic wounds [102]. It can form biofilms, which hinder microbial elimination and increase antibiotic resistance. Moreover, certain strains such as methicillin-resistant S. aureus, detected in approximately 15–18% of infected wounds, are highly virulent and resistant to treatment, making these infections especially difficult to manage [103,106]. By contrast, Enterococcus spp.—particularly E. faecalis and E. faecium—are also frequently isolated from chronic diabetic wounds [107]. These bacteria exhibit intrinsic resistance to many antibiotics, including vancomycin-resistant enterococci, complicating treatment and predisposing patients to septic complications [108,109].
2.4. Antidiabetic Drugs and Gut Microbiome
2.4.1. Metformin
Treatment of DM can modulate the composition of the gut microbiome by influencing bacterial metabolism and colonisation capacity. Metformin, the most common first-line drug for patients with DM, exerts a variety of off-target effects, including alterations in the gut microbiome. Increasing evidence suggests that metformin helps regulate glucose levels partly through its effects on the microbiome.
Metformin has been shown to shift the gut microbial profile towards one resembling that of healthy subjects in both mice and humans [17]. It promotes the growth of beneficial bacteria such as Akkermansia muciniphila and Bifidobacterium, thereby strengthening the intestinal barrier and exerting anti-inflammatory effects [75,110]. In overweight and obese individuals, metformin altered the microbiome profile and increased SCFAs concentrations, particularly acetate and butyrate, after 6 months of treatment [111]. In animal models, metformin has also been found to stimulate GLP-1 secretion [112]. These findings add to the evidence of the broad pleiotropic effects of metformin. Enhanced SCFAs production may improve fasting insulin levels and HOMA-IR, thereby reducing insulin resistance [113]. Drug-induced microbiome alterations are also functionally significant. For example, metformin treatment reduces the abundance of Firmicutes, a phylum positively correlated with fasting plasma glucose and HbA1c [114]. Changes in the composition of bacteria from the Proteobacteria and Firmicutes phyla have also been linked to improved cognition [115]. Furthermore, alterations in the microbiome lead to changes in microbiome-associated metabolites—including amino acids, peptides, benzenoids, bile acids, carbohydrates, fatty and organic acids, as well as phenylpropanoids and polyketides—as shown in detailed studies using mouse models of DM [116] (Figure 3).
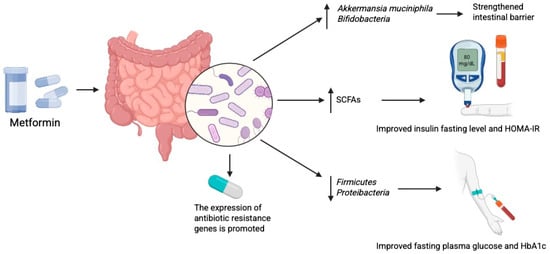
Figure 3.
Metformin modulates gut microbiome which exerts anti diabetic effects. Created in BioRender. Physiology, D. (2025) Available online: https://BioRender.com/7ecg3bf.
Importantly, Kim et al. [117] recently reported that metformin treatment may also exert negative effects on the microbiome. Specifically, the authors found that metformin promoted the expression of antibiotic resistance genes in Escherichia coli. This highlights the need to consider potential implications of metformin therapy in the context of antibiotic resistance. To minimise such adverse effects, it may be valuable to identify patients most likely to respond positively to metformin. It has been suggested that baseline microbiome profiling could help distinguish future responders from non-responders to treatment [118,119].
2.4.2. SGLT2 Inhibitors
SGLT-2 inhibitors represent another group of oral antidiabetic drugs recommended for patients with DM, particularly those with concomitant cardiovascular or renal disease, as demonstrated by clinical trial results [120,121,122]. Research indicates that SGLT-2 inhibitors influence the gut microbiome and the levels of microbial metabolites. In mice with diabetic nephropathy, analysis of the gut microbiome showed an increased abundance of Firmicutes and a decreased abundance of Bacteroidetes [123]. In T2D rat models, dapagliflozin was found to increase the presence of the Proteobacteria phylum [124]. Diabetic nephropathy was also associated with reduced levels of Akkermansia, Bifidobacterium, Muribaculum, and Muribaculaceae. Treatment with dapagliflozin reversed these phylum-level shifts and promoted the presence of beneficial bacteria [123]. In a clinical study, treatment with empagliflozin was linked to an increased abundance of Faecalibacterium, Lachnospiraceae, Eubacterium, and Eggerthellaceae, while simultaneously decreasing Bilophila, Hungatella, and Escherichia-Shigella [125]. Additionally, empagliflozin was shown to increase Roseburia spp. and Faecalibacterium, both known SCFAs producers, thereby supporting improved insulin sensitivity [126].
Conversely, the administration of insulin does not directly influence the composition of the gut microbiome. However, by stabilising glucose levels, it has been shown to reduce the growth of potentially pathogenic Gram-positive bacteria such as Staphylococcus aureus [127]. Improved glycemic control through insulin therapy may also contribute to a reduction in chronic inflammation [128,129].
2.4.3. Probiotics and Prebiotics
Probiotic treatments and dietary interventions aimed at restoring beneficial gut flora hold promise for the management of DM and its complications. Supplementation with Lactobacillus and Bifidobacterium strains has demonstrated potential benefits in improving insulin sensitivity, reducing inflammation, and preventing infections in patients with DM [130,131]. The mechanism of action of probiotics involves the fermentation of fibre to SCFAs, which support glucose metabolism and reduce inflammation [58]. A diet enriched with soluble fibre and prebiotics has been shown to stimulate the growth of beneficial bacteria such as Roseburia and Faecalibacterium, thereby enhancing glucose metabolism and strengthening intestinal barrier function, which in turn can reduce the risk of diabetic complications [81,132]. Blautia wexlerae supplementation has been inversely associated with obesity and T2D. Its administration to mice reduced these conditions by modulating the gut microbiome. The beneficial effects of B. wexlerae are attributed to its anti-inflammatory properties and its influence on lipid metabolism and the intestinal environment [133].
In the TEDDY over 10,000 stool samples were analyzed from children with genetic predisposition to T1D. Microbiome was monitored from 3 month of age until the development of pancreatic islet autoimmunity or diabetes. Studies found that healthy children had higher number of genes in their microbiomes responsible for synthesis of short-chin fatty acids (e.g., acetate, propionate) and fermentation. The presence of transketolase and lactate dehydrogenase, which are enzymes specific to fermentation processes, is linked to development of beneficial microbiome in children, as these enzymes contribute to the formation of stronger immunological barrier. Changes in microbiome including deficiency of SCFA-producing bacteria and predominance of Bacteroides were associated with risk of T1D development. Genetic analyses have shown that specific host gene variants influence the configuration of microbiome and the immune response and eventually the risk of T1D. Breastfeeding and antibiotic use in the first months of life may significantly influence the development of the microbiome and expression of bacterial genes responsive to breast milk products. Genetic predisposition to T1D is strongly associated with variants in the HLA system, particularly HLA-DQ and HLA-DR. A mutation in the IFIH1 gene, which plays a role in immune response, can increase the risk of autoimmune diseases and may also influence how the host interacts with its microbiome by altering the recognition of bacterial antigens and the resulting inflammatory reaction. This gene encodes a receptor involved in detecting viral infections and activating immune responses, thereby potentially affecting host microbiota interactions. Other genes related to immune functions and the epithelial barrier, such as INS and PTPN2, may similarly impact these processes [134].
Food choices play major role in the risk, onset and the control of diabetes, especially in T2D. Heavy processes foods, red meat, sugary items and refined carbs arise the likelihood and severity of the disease, whereas eating patterns rich in fruits, vegetables, whole grains and lean protein help protect against it [135]. Study by Szczerba et al. demonstrated that diet plays complex role in T2D. Calories reduction and dietary pattern such as Mediterranean, plant-based, high-protein and low-carbohydrate (below 26% of total energy) diets could offer health improvements related to heart and metabolism in individuals with T2D [136]. About 24% of deaths and disability adjusted life years (DALYs) linked to T2D in older adults are attributed to poor dietary habits, including sugary drinks and high intake of processed meats and low consumption of vegetables, fruits and whole grains [137]. Studies indicate that following a plant-based diet significantly reduces the risk of developing DM [138]. Moreover, habits such as skipping breakfast, eating to fast and frequently eating alone have been linked to an increased risk of DM [139]. Balanced dietary patterns and healthy eating habits play crucial role in preventing and managing T2D, reducing risk and associated health burdens.
Bacteria have yet to be widely adopted as standard clinical biomarkers; however, mounting evidence from numerous studies suggests their potential value in the diagnosis and monitoring of DM and its complications. In half of the T2D microbiome studies, at least one of four phylogenetically distant bacterial genera (Bifidobacterium, Roseburia, Faecalibacterium, and Akkermansia) showed a decline, highlighting their importance not only as biomarkers but also as modulators of disease. While these bacteria have been investigated as probiotics in murine models, human studies remain limited [140,141]. Further clinical trials are required to confirm these findings and to determine the optimal dosage and application of probiotics in the management of DM. Nevertheless, the results to date suggest that probiotics may provide significant benefits, including the modulation of gut microbiome and the reduction in bacterial infections [132].
3. Gut Virome
Although the population of gut viruses surpasses that of bacteria in number, their composition and function remain underexplored [142]. This is largely due to the difficulty of isolating and identifying viruses within the complex intestinal environment, as well as the dynamic nature of the gut virome, which shifts with age, diet, and geographical region [143]. With the advent of new metagenomic methods, some of these challenges have been overcome, leading to rapid growth in gut virome research. As a result, considerable work has been devoted to investigating links between the human viral profile and both health and disease. The gut virome has been implicated as a potential factor in the pathogenesis of colorectal cancer [144], inflammatory bowel disease [145], liver diseases [146], obesity [147], and diabetes.
3.1. Type 1 Diabetes Mellitus
Theories linking T1D to viral infections have circulated for nearly a century, but only recently have new virus-detection methods—particularly next-generation sequencing—enabled deeper investigation of the gut virome’s role in the disease [148]. Strong evidence supports an association between Enterovirus infection and T1D. A meta-analysis of 38 case–control studies showed that Enterovirus infection was nearly eight times more likely in the T1D group than in controls. However, this finding was based on blood and tissue samples; no significant correlation was observed in stool samples [149]. Interestingly, one study reported a higher abundance of Circoviridae-related sequences in healthy participants than in patients with T1D, suggesting a possible protective influence of this viral family [150]. Similarly, Mastadenovirus infection has been proposed to reduce the risk of T1D development [151]. More broadly, research has suggested that T1D is associated with altered abundance of certain gut viruses [143,144], reduced viral diversity, and changes in some phage populations [150]. Nonetheless, these findings remain contested because numerous studies have reported no significant associations between gut virome composition and T1D [152,153,154,155] (Table 1). Many of the discrepancies may be explained by differences in study populations, sample preparation, and virus detection methods. A large, multicentre trial using standardised methodology is therefore needed to draw stronger conclusions.

Table 1.
Summary of recent studies investigating the virome in association with T1DM.
3.2. Type 2 Diabetes Mellitus
The relationship between the gut virome and T2D appears equally complex. Several studies have shown that patients with T2D are characterised by decreased viral diversity, alterations in taxonomic composition, and disrupted virus–bacteria interactions [158]. The gut virome may even serve as a diagnostic marker for T2D, especially when combined with bacteriome profiles. Indeed, as many as 81 viral species have been identified as distinguishing patients with T2D from healthy individuals [159]. One of the starkest contrasts between these groups concerns phage richness. One study found that T2D is associated with an increased number of gut phages, seven of which were specific to the disease [160]. It has also been suggested that Enterobacteriaceae phage abundance may be particularly elevated in T2D [161]. By contrast, other studies indicate a widespread decline in viral numbers, including most—but not all—phages in patients with T2D [147,159] (Table 2). Subtle shifts in the abundance of different phage groups nonetheless appear to exert a major influence on shaping the T2D microbiome, though the mechanisms remain unclear.

Table 2.
Studies investigating the role of the microbiome in diabetes type 2.
Although direct evidence for a causal relationship between gut virome dysbiosis and T2D is still lacking, recent research has advanced our understanding of their complex interplay. One study identified significant differences between the viromes of obese patients with and without T2D, with greater dysbiosis in those with T2D. These findings imply that gut virome alterations are not merely by-products of T2D risk factors but instead correlate with disease onset [147]. Viral profile abnormalities have also been linked with diabetic nephropathy, suggesting a possible role in T2D complications [159]. In line with this, faecal virome transplantation has recently been shown to normalise blood glucose parameters in obese mice [162]. This example not only underscores the gut virome’s role in T2D pathogenesis but also raises hopes for the development of novel virus-based therapies for metabolic diseases [163].
The gut virome has been hypothesised to influence the development of DM through a variety of mechanisms. First, it plays an important role in shaping human immunity, which is particularly relevant to T1D pathogenesis. In the intestine, viral infection can be detected by TLR3 or TLR7, triggering an immune response characterised by interferon-β production and inflammation [164]. Moreover, many viruses—including phages—can cross from the gut into the bloodstream via intestinal epithelial cell transport. Although usually cleared rapidly [165], they may still affect immune activity by modulating T- and B-cell function or regulating cytokine release [143]. These mechanisms are especially important in the case of Enterovirus, which contributes to T1D not only through direct cytolysis of β-cells, but also by activating pre-existing autoreactive T cells and through molecular mimicry [148]. Second, viruses can increase intestinal permeability, thereby contributing to DM and other inflammatory diseases [166,167]. This occurs through antiviral cytokines and viral proteins, which impair the intestinal barrier, as well as through viral nucleic acids that bind to TLRs on enterocytes and further disrupt barrier function [148]. Third, the phage population shapes the gut bacteriome and its metabolism [168,169], both of which play a profound role in DM, as discussed in previous sections. Phages regulate bacterial populations through classical predator–prey interactions, but they may also mediate horizontal gene transfer [170], disseminate virulence factors, or alter bacterial metabolic pathways [171]. Given this immense complexity, the mechanistic relationship between the gut virome and DM remains largely a terra incognita—a fascinating and still developing field of research (Table 2).
In T1D, viral infections especially those caused by enteroviruses are believed to initiate autoimmune attacks on insulin-producing beta-cells. This occurs through mechanisms like infection of islets, interferon-inducted stress and immune system activation [172]. In T2D virome primary affects disease progression in bacteriophage population that interact with gut microbiome. Described changes include reduced viral alpha diversity and phage-mediated modifications, resulting in complications such as nephropathy. The virome further contributes to inflammation and immune regulation, thereby influencing metabolic stability and inulin sensitivity [158]. Recent animal research demonstrated that fecal virome transplantation (FVT)-the transfer of viral components from healthy donors can reduce symptoms of T2D and obesity [147], which may be a promising future treatment approach. In T1D, antiviral drugs that target specific viruses which are associated with disease presented potential in maintaining beta-cell function after diagnosis, which provides promising intervention. Examining viral markers in the gut and oral virome could lead to new ways of prevention, diagnosis and treatment for patients with T1D and T2D, although further research needed to be conducted for verification. The complex interaction between viral and bacterial communities may enable personalized microbiome-based therapies which incorporate viral population dynamics to improve diabetes care.
4. Conclusions and Future Perspectives
Current evidence highlights the complex relationship between the gut microbiome, microbiome-associated metabolites, metabolic processes, and DM. While debate continues regarding the precise impact of DM on microbiome diversity, available data consistently point to alterations in microbial composition in patients with this metabolic disorder. These changes affect the profile of microbiome-associated metabolites, the levels of which differ in individuals with DM. Shifts in intestinal microorganisms in DM are associated with inflammatory responses, which can be tracked through cytokines, LPS, and acute-phase proteins. In addition, bacteria are directly involved in glucose metabolism, with certain species regulating GLUT expression, gluconeogenesis activity, and insulin signalling cascades. Moreover, bacteria and their metabolites regulate lipid metabolism, thereby influencing energy expenditure and insulin resistance.
Recent findings also demonstrate that antidiabetic drugs modify the gut microbiome, further confirming the role of bacteria in DM-related mechanisms. Metformin and SGLT-2 inhibitors, in particular, exert profound effects on bacterial abundance at the phylum level, leading to functional changes. With an increasing number of studies investigating the role of intestinal viruses in the pathophysiology of DM, further research is needed to clarify their involvement to a degree comparable to what is now known about bacteria. Nevertheless, current evidence suggests that intestinal viruses may serve as potential biomarkers in patients with DM.
Future research exploring the role of the gut microbiome in DM pathophysiology should prioritize several key directions to enhance clinical translation. Further studies should evaluate how manipulation of gut microbiome including symbiotic, probiotics and prebiotics affects glycemic control and affects complications associated with DM. Interaction of microbiome profiling into algorithms could enable personalized monitoring and optimization of antidiabetic treatments. Moreover, algorithms cloud help in the identification of patients who would benefit from specific therapies regarding metformin or SGLT-2 inhibitors. Future research will allow to address existing knowledge gaps and develop better therapeutic strategies and diagnostic tools for patients with diabetes. In addition, studies continue to investigate activation mechanism of SCFAs receptors [173,174,175], with potential development of clinically relevant agonists in the future.
Author Contributions
Conceptualization, A.P.; writing—original draft preparation, K.K., P.P., J.Z., P.S., A.J., E.B. and A.P.; writing—review and editing, K.K., P.P., J.Z., P.S., A.J., E.B. and A.P.; supervision, A.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| DM | Diabetes mellitus |
| T1D | Type 1 diabetes mellitus |
| T2D | Type 2 diabetes mellitus |
| GDM | Gestational diabetes mellitus |
| SCFAs | Short chain fatty acid |
| ROS | Reactive oxygen species |
| IL-1β | Interleukin-1β |
| TNF-α | Tumor necrosis factor α |
| LPS | Lipopolysaccharide |
| LBP | LPS-binding protein |
| FMT | Fecal microbiome transplantation |
| CRP | C-reactive protein |
| PPAR-γ | Peroxisome Proliferator-Activated Receptor γ |
| GLUT | Glucose transporter |
| FMO3 | Flavin-containing monooxygenase 3 |
| PI3K | Phosphatidylinositol-3-kinase |
| IRS2 | Insulin receptor substrate 2 |
| AMPK | AMP-activated protein kinase |
| GPCR | G protein-coupled receptors |
| TMAO | Trimethylamine N-oxide |
| GLP-1 | Glucagon-like peptide-1 |
References
- Rohm, T.V.; Meier, D.T.; Olefsky, J.M.; Donath, M.Y. Inflammation in obesity, diabetes, and related disorders. Immunity 2022, 55, 31–55. [Google Scholar] [CrossRef]
- Blagov, A.V.; Summerhill, V.I.; Sukhorukov, V.N.; Popov, M.A.; Grechko, A.V.; Orekhov, A.N. Type 1 diabetes mellitus: Inflammation, mitophagy, and mitochondrial function. Mitochondrion 2023, 72, 11–21. [Google Scholar] [CrossRef]
- Ma, P.J.; Wang, M.M.; Wang, Y. Gut microbiota: A new insight into lung diseases. Biomed. Pharmacother. 2022, 155, 113810. [Google Scholar] [CrossRef]
- Barrio, C.; Arias-Sanchez, S.; Martin-Monzon, I. The gut microbiota-brain axis, psychobiotics and its influence on brain and behaviour: A systematic review. Psychoneuroendocrinology 2022, 137, 105640. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, M.R.; Akter, S.; Tamanna, S.K.; Mazumder, L.; Esti, I.Z.; Banerjee, S.; Akter, S.; Hasan, M.R.; Acharjee, M.; Hossain, M.S.; et al. Impact of gut microbiome on skin health: Gut-skin axis observed through the lenses of therapeutics and skin diseases. Gut Microbes 2022, 14, 2096995. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, T.; Kubota, T.; Nakanishi, Y.; Tsugawa, H.; Suda, W.; Kwon, A.T.; Yazaki, J.; Ikeda, K.; Nemoto, S.; Mochizuki, Y.; et al. Gut microbial carbohydrate metabolism contributes to insulin resistance. Nature 2023, 621, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Aw, W.; Fukuda, S. Understanding the role of the gut ecosystem in diabetes mellitus. J. Diabetes Investig. 2018, 9, 5–12. [Google Scholar] [CrossRef]
- Gurung, M.; Li, Z.; You, H.; Rodrigues, R.; Jump, D.B.; Morgun, A.; Shulzhenko, N. Role of gut microbiota in type 2 diabetes pathophysiology. EBioMedicine 2020, 51, 102590. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, A.L.; Stephens, J.W.; Harris, D.A. Gut microbiota influence in type 2 diabetes mellitus (T2DM). Gut Pathog. 2021, 13, 50. [Google Scholar] [CrossRef]
- Darwitz, B.P.; Genito, C.J.; Thurlow, L.R. Triple threat: How diabetes results in worsened bacterial infections. Infect. Immun. 2024, 92, e0050923. [Google Scholar] [CrossRef]
- Liu, T.; Cao, Y.; Liang, N.; Ma, X.Q.; Fang, J.A.; Zhang, X.D. Investigating the causal association between gut microbiota and type 2 diabetes: A meta-analysis and Mendelian randomization. Front. Public Health 2024, 12, 1342313. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Wang, S.; Niu, J.; Ma, J.; Yang, H. Bifidobacterium species serve as key gut microbiome regulators after intervention in gestational diabetes mellitus. BMC Microbiol. 2024, 24, 520. [Google Scholar] [CrossRef]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef]
- Larsen, N.; Vogensen, F.K.; van den Berg, F.W.; Nielsen, D.S.; Andreasen, A.S.; Pedersen, B.K.; Al-Soud, W.A.; Sørensen, S.J.; Hansen, L.H.; Jakobsen, M. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS ONE 2010, 5, e9085. [Google Scholar] [CrossRef]
- Hung, W.C.; Hung, W.W.; Tsai, H.J.; Chang, C.C.; Chiu, Y.W.; Hwang, S.J.; Kuo, M.C.; Chen, S.C.; Dai, C.Y.; Tsai, Y.C. The Association of Targeted Gut Microbiota with Body Composition in Type 2 Diabetes Mellitus. Int. J. Med. Sci. 2021, 18, 511–519. [Google Scholar] [CrossRef]
- Qin, J.; Li, Y.; Cai, Z.; Li, S.; Zhu, J.; Zhang, F.; Liang, S.; Zhang, W.; Guan, Y.; Shen, D.; et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 2012, 490, 55–60. [Google Scholar] [CrossRef]
- Forslund, K.; Hildebrand, F.; Nielsen, T.; Falony, G.; Le Chatelier, E.; Sunagawa, S.; Prifti, E.; Vieira-Silva, S.; Gudmundsdottir, V.; Pedersen, H.K.; et al. Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature 2015, 528, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Hamer, H.M.; Jonkers, D.; Venema, K.; Vanhoutvin, S.; Troost, F.J.; Brummer, R.J. Review article: The role of butyrate on colonic function. Aliment. Pharmacol. Ther. 2008, 27, 104–119. [Google Scholar] [CrossRef]
- Martín, R.; Rios-Covian, D.; Huillet, E.; Auger, S.; Khazaal, S.; Bermúdez-Humarán, L.G.; Sokol, H.; Chatel, J.M.; Langella, P. Faecalibacterium: A bacterial genus with promising human health applications. FEMS Microbiol. Rev. 2023, 47, fuad039. [Google Scholar] [CrossRef] [PubMed]
- Olsson, L.M.; Boulund, F.; Nilsson, S.; Khan, M.T.; Gummesson, A.; Fagerberg, L.; Engstrand, L.; Perkins, R.; Uhlén, M.; Bergström, G.; et al. Dynamics of the normal gut microbiota: A longitudinal one-year population study in Sweden. Cell Host Microbe 2022, 30, 726–739.e3. [Google Scholar] [CrossRef]
- Nie, K.; Ma, K.; Luo, W.; Shen, Z.; Yang, Z.; Xiao, M.; Tong, T.; Yang, Y.; Wang, X. Roseburia intestinalis: A Beneficial Gut Organism From the Discoveries in Genus and Species. Front. Cell Infect. Microbiol. 2021, 11, 757718. [Google Scholar] [CrossRef] [PubMed]
- Canfora, E.E.; Jocken, J.W.; Blaak, E.E. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat. Rev. Endocrinol. 2015, 11, 577–591. [Google Scholar] [CrossRef]
- Sedighi, M.; Razavi, S.; Navab-Moghadam, F.; Khamseh, M.E.; Alaei-Shahmiri, F.; Mehrtash, A.; Amirmozafari, N. Comparison of gut microbiota in adult patients with type 2 diabetes and healthy individuals. Microb. Pathog. 2017, 111, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Ventura, M.; Turroni, F.; Lugli, G.A.; van Sinderen, D. Bifidobacteria and humans: Our special friends, from ecological to genomics perspectives. J. Sci. Food Agric. 2014, 94, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Turroni, F.; Duranti, S.; Milani, C.; Lugli, G.A.; van Sinderen, D.; Ventura, M. Bifidobacterium bifidum: A Key Member of the Early Human Gut Microbiota. Microorganisms 2019, 7, 544. [Google Scholar] [CrossRef]
- Al-Ishaq, R.K.; Samuel, S.M.; Büsselberg, D. The Influence of Gut Microbial Species on Diabetes Mellitus. Int. J. Mol. Sci. 2023, 24, 8118. [Google Scholar] [CrossRef]
- Turroni, F.; Peano, C.; Pass, D.A.; Foroni, E.; Severgnini, M.; Claesson, M.J.; Kerr, C.; Hourihane, J.; Murray, D.; Fuligni, F.; et al. Diversity of bifidobacteria within the infant gut microbiota. PLoS ONE 2012, 7, e36957. [Google Scholar] [CrossRef]
- Turroni, F.; Bottacini, F.; Foroni, E.; Mulder, I.; Kim, J.H.; Zomer, A.; Sánchez, B.; Bidossi, A.; Ferrarini, A.; Giubellini, V.; et al. Genome analysis of Bifidobacterium bifidum PRL2010 reveals metabolic pathways for host-derived glycan foraging. Proc. Natl. Acad. Sci. USA 2010, 107, 19514–19519. [Google Scholar] [CrossRef]
- Karlsson, F.H.; Tremaroli, V.; Nookaew, I.; Bergström, G.; Behre, C.J.; Fagerberg, B.; Nielsen, J.; Bäckhed, F. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature 2013, 498, 99–103. [Google Scholar] [CrossRef]
- Pintarič, M.; Langerholc, T. Probiotic Mechanisms Affecting Glucose Homeostasis: A Scoping Review. Life 2022, 12, 1187. [Google Scholar] [CrossRef]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.A.P.; Harris, H.M.B.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef] [PubMed]
- Kocot, A.M.; Jarocka-Cyrta, E.; Drabińska, N. Overview of the Importance of Biotics in Gut Barrier Integrity. Int. J. Mol. Sci. 2022, 23, 2896. [Google Scholar] [CrossRef]
- Martín, R.; Chamignon, C.; Mhedbi-Hajri, N.; Chain, F.; Derrien, M.; Escribano-Vázquez, U.; Garault, P.; Cotillard, A.; Pham, H.P.; Chervaux, C.; et al. The potential probiotic Lactobacillus rhamnosus CNCM I-3690 strain protects the intestinal barrier by stimulating both mucus production and cytoprotective response. Sci. Rep. 2019, 9, 5398. [Google Scholar] [CrossRef]
- Rastogi, S.; Singh, A. Gut microbiome and human health: Exploring how the probiotic genus Lactobacillus modulate immune responses. Front. Pharmacol. 2022, 13, 1042189. [Google Scholar] [CrossRef]
- Liu, X.; Mao, B.; Gu, J.; Wu, J.; Cui, S.; Wang, G.; Zhao, J.; Zhang, H.; Chen, W. Blautia—A new functional genus with potential probiotic properties? Gut Microbes 2021, 13, 1–21. [Google Scholar] [CrossRef]
- Tong, X.; Xu, J.; Lian, F.; Yu, X.; Zhao, Y.; Xu, L.; Zhang, M.; Zhao, X.; Shen, J.; Wu, S.; et al. Structural Alteration of Gut Microbiota during the Amelioration of Human Type 2 Diabetes with Hyperlipidemia by Metformin and a Traditional Chinese Herbal Formula: A Multicenter, Randomized, Open Label Clinical Trial. mBio 2018, 9, e02392-17. [Google Scholar] [CrossRef] [PubMed]
- Kimura, I.; Ozawa, K.; Inoue, D.; Imamura, T.; Kimura, K.; Maeda, T.; Terasawa, K.; Kashihara, D.; Hirano, K.; Tani, T.; et al. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat. Commun. 2013, 4, 1829. [Google Scholar] [CrossRef] [PubMed]
- Qi, C.J.; Zhang, Q.; Yu, M.; Xu, J.P.; Zheng, J.; Wang, T.; Xiao, X.H. Imbalance of Fecal Microbiota at Newly Diagnosed Type 1 Diabetes in Chinese Children. Chin. Med. J. Engl. 2016, 129, 1298–1304. [Google Scholar] [CrossRef]
- Leiva-Gea, I.; Sánchez-Alcoholado, L.; Martín-Tejedor, B.; Castellano-Castillo, D.; Moreno-Indias, I.; Urda-Cardona, A.; Tinahones, F.J.; Fernández-García, J.C.; Queipo-Ortuño, M.I. Gut Microbiota Differs in Composition and Functionality Between Children with Type 1 Diabetes and MODY2 and Healthy Control Subjects: A Case-Control Study. Diabetes Care 2018, 41, 2385–2395. [Google Scholar] [CrossRef]
- Allin, K.H.; Tremaroli, V.; Caesar, R.; Jensen, B.A.H.; Damgaard, M.T.F.; Bahl, M.I.; Licht, T.R.; Hansen, T.H.; Nielsen, T.; Dantoft, T.M.; et al. Aberrant intestinal microbiota in individuals with prediabetes. Diabetologia 2018, 61, 810–820. [Google Scholar] [CrossRef]
- Candela, M.; Biagi, E.; Soverini, M.; Consolandi, C.; Quercia, S.; Severgnini, M.; Peano, C.; Turroni, S.; Rampelli, S.; Pozzilli, P.; et al. Modulation of gut microbiota dysbioses in type 2 diabetic patients by macrobiotic Ma-Pi 2 diet. Br. J. Nutr. 2016, 116, 80–93. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, T.; Sun, J.; Huang, Y.; Liu, T.; Ye, Z.; Hu, J.; Zhang, G.; Chen, H.; Ye, Z.; et al. Calorie restriction ameliorates hyperglycemia, modulates the disordered gut microbiota, and mitigates metabolic endotoxemia and inflammation in type 2 diabetic rats. J. Endocrinol. Investig. 2023, 46, 699–711. [Google Scholar] [CrossRef] [PubMed]
- Pinto, Y.; Frishman, S.; Turjeman, S.; Eshel, A.; Nuriel-Ohayon, M.; Shrossel, O.; Ziv, O.; Walters, W.; Parsonnet, J.; Ley, C.; et al. Gestational diabetes is driven by microbiota-induced inflammation months before diagnosis. Gut 2023, 72, 918–928. [Google Scholar] [CrossRef]
- Zhou, X.; Zheng, W.; Kong, W.; Zhang, J.; Liao, Y.; Min, J.; Zeng, T. Glucose Parameters, Inflammation Markers, and Gut Microbiota Changes of Gut Microbiome-Targeted Therapies in Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Clin. Endocrinol. Metab. 2025, 110, 2980–3008. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, N.; Emoto, T.; Yamashita, T.; Watanabe, H.; Hayashi, T.; Tabata, T.; Hoshi, N.; Hatano, N.; Ozawa, G.; Sasaki, N.; et al. Bacteroides vulgatus and Bacteroides dorei Reduce Gut Microbial Lipopolysaccharide Production and Inhibit Atherosclerosis. Circulation 2018, 138, 2486–2498. [Google Scholar] [CrossRef]
- Chelakkot, C.; Choi, Y.; Kim, D.K.; Park, H.T.; Ghim, J.; Kwon, Y.; Jeon, J.; Kim, M.S.; Jee, Y.K.; Gho, Y.S.; et al. Akkermansia muciniphila-derived extracellular vesicles influence gut permeability through the regulation of tight junctions. Exp. Mol. Med. 2018, 50, e450. [Google Scholar] [CrossRef]
- Plovier, H.; Everard, A.; Druart, C.; Depommier, C.; Van Hul, M.; Geurts, L.; Chilloux, J.; Ottman, N.; Duparc, T.; Lichtenstein, L.; et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat. Med. 2017, 23, 107–113. [Google Scholar] [CrossRef]
- Shen, Z.; Zhu, C.; Quan, Y.; Yang, J.; Yuan, W.; Yang, Z.; Wu, S.; Luo, W.; Tan, B.; Wang, X. Insights into Roseburia intestinalis which alleviates experimental colitis pathology by inducing anti-inflammatory responses. J. Gastroenterol. Hepatol. 2018, 33, 1751–1760. [Google Scholar] [CrossRef]
- Kinoshita, M.; Suzuki, Y.; Saito, Y. Butyrate reduces colonic paracellular permeability by enhancing PPARgamma activation. Biochem. Biophys. Res. Commun. 2002, 293, 827–831. [Google Scholar] [CrossRef]
- Concannon, P.; Rich, S.S.; Nepom, G.T. Genetics of type 1A diabetes. N. Engl. J. Med. 2009, 360, 1646–1654. [Google Scholar] [CrossRef]
- Myers, M.A.; Hettiarachchi, K.D.; Ludeman, J.P.; Wilson, A.J.; Wilson, C.R.; Zimmet, P.Z. Dietary microbial toxins and type 1 diabetes. Ann. N. Y Acad. Sci. 2003, 1005, 418–422. [Google Scholar] [CrossRef]
- Kim, S.H.; Huh, C.S.; Choi, I.D.; Jeong, J.W.; Ku, H.K.; Ra, J.H.; Kim, T.Y.; Kim, G.B.; Sim, J.H.; Ahn, Y.T. The anti-diabetic activity of Bifidobacterium lactis HY8101 in vitro and in vivo. J. Appl. Microbiol. 2014, 117, 834–845. [Google Scholar] [CrossRef]
- Liu, W.C.; Yang, M.C.; Wu, Y.Y.; Chen, P.H.; Hsu, C.M.; Chen, L.W. Lactobacillus plantarum reverse diabetes-induced Fmo3 and ICAM expression in mice through enteric dysbiosis-related c-Jun NH2-terminal kinase pathways. PLoS ONE 2018, 13, e0196511. [Google Scholar] [CrossRef]
- Miao, J.; Ling, A.V.; Manthena, P.V.; Gearing, M.E.; Graham, M.J.; Crooke, R.M.; Croce, K.J.; Esquejo, R.M.; Clish, C.B.; Vicent, D.; et al. Flavin-containing monooxygenase 3 as a potential player in diabetes-associated atherosclerosis. Nat. Commun. 2015, 6, 6498. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, E.; Yin, B.; Fang, D.; Chen, P.; Wang, G.; Zhao, J.; Zhang, H.; Chen, W. Effects of Lactobacillus casei CCFM419 on insulin resistance and gut microbiota in type 2 diabetic mice. Benef. Microbes 2017, 8, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Guo, X.; Guo, J.; He, Q.; Li, H.; Song, Y.; Zhang, H. Lactobacillus casei reduces susceptibility to type 2 diabetes via microbiota-mediated body chloride ion influx. Sci. Rep. 2014, 4, 5654. [Google Scholar] [CrossRef] [PubMed]
- Remely, M.; Aumueller, E.; Merold, C.; Dworzak, S.; Hippe, B.; Zanner, J.; Pointner, A.; Brath, H.; Haslberger, A.G. Effects of short chain fatty acid producing bacteria on epigenetic regulation of FFAR3 in type 2 diabetes and obesity. Gene 2014, 537, 85–92. [Google Scholar] [CrossRef]
- Kimura, I.; Inoue, D.; Hirano, K.; Tsujimoto, G. The SCFA Receptor GPR43 and Energy Metabolism. Front. Endocrinol. 2014, 5, 85. [Google Scholar] [CrossRef]
- Allin, K.H.; Nielsen, T.; Pedersen, O. Mechanisms in endocrinology: Gut microbiota in patients with type 2 diabetes mellitus. Eur. J. Endocrinol. 2015, 172, R167–R177. [Google Scholar] [CrossRef]
- Arora, T.; Bäckhed, F. The gut microbiota and metabolic disease: Current understanding and future perspectives. J. Intern. Med. 2016, 280, 339–349. [Google Scholar] [CrossRef]
- Amar, J.; Chabo, C.; Waget, A.; Klopp, P.; Vachoux, C.; Bermúdez-Humarán, L.G.; Smirnova, N.; Bergé, M.; Sulpice, T.; Lahtinen, S.; et al. Intestinal mucosal adherence and translocation of commensal bacteria at the early onset of type 2 diabetes: Molecular mechanisms and probiotic treatment. EMBO Mol. Med. 2011, 3, 559–572. [Google Scholar] [CrossRef]
- Mohamadshahi, M.; Veissi, M.; Haidari, F.; Shahbazian, H.; Kaydani, G.A.; Mohammadi, F. Effects of probiotic yogurt consumption on inflammatory biomarkers in patients with type 2 diabetes. Bioimpacts 2014, 4, 83–88. [Google Scholar] [CrossRef]
- Brahe, L.K.; Le Chatelier, E.; Prifti, E.; Pons, N.; Kennedy, S.; Blædel, T.; Håkansson, J.; Dalsgaard, T.K.; Hansen, T.; Pedersen, O.; et al. Dietary modulation of the gut microbiota--a randomised controlled trial in obese postmenopausal women. Br. J. Nutr. 2015, 114, 406–417. [Google Scholar] [CrossRef]
- Karlsson Videhult, F.; Öhlund, I.; Stenlund, H.; Hernell, O.; West, C.E. Probiotics during weaning: A follow-up study on effects on body composition and metabolic markers at school age. Eur. J. Nutr. 2015, 54, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Hütt, P.; Songisepp, E.; Rätsep, M.; Mahlapuu, R.; Kilk, K.; Mikelsaar, M. Impact of probiotic Lactobacillus plantarum TENSIA in different dairy products on anthropometric and blood biochemical indices of healthy adults. Benef. Microbes 2015, 6, 233–243. [Google Scholar] [CrossRef]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M.; et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.Y.; Lee, Y.S.; Kim, Y.; Lee, S.H.; Ryu, S.; Fukuda, S.; Hase, K.; Yang, C.S.; Lim, H.S.; Kim, M.S.; et al. Gut commensal Bacteroides acidifaciens prevents obesity and improves insulin sensitivity in mice. Mucosal Immunol. 2017, 10, 104–116. [Google Scholar] [CrossRef]
- den Besten, G.; Bleeker, A.; Gerding, A.; van Eunen, K.; Havinga, R.; van Dijk, T.H.; Oosterveer, M.H.; Jonker, J.W.; Groen, A.K.; Reijngoud, D.J.; et al. Short-Chain Fatty Acids Protect Against High-Fat Diet-Induced Obesity via a PPARγ-Dependent Switch From Lipogenesis to Fat Oxidation. Diabetes 2015, 64, 2398–2408. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Yin, J.; Zhang, J.; Ward, R.E.; Martin, R.J.; Lefevre, M.; Cefalu, W.T.; Ye, J. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes 2009, 58, 1509–1517. [Google Scholar] [CrossRef]
- Barengolts, E.; Green, S.J.; Eisenberg, Y.; Akbar, A.; Reddivari, B.; Layden, B.T.; Dugas, L.; Chlipala, G. Gut microbiota varies by opioid use, circulating leptin and oxytocin in African American men with diabetes and high burden of chronic disease. PLoS ONE 2018, 13, e0194171. [Google Scholar] [CrossRef]
- Xu, J.; Lian, F.; Zhao, L.; Zhao, Y.; Chen, X.; Zhang, X.; Guo, Y.; Zhang, C.; Zhou, Q.; Xue, Z.; et al. Structural modulation of gut microbiota during alleviation of type 2 diabetes with a Chinese herbal formula. ISME J. 2015, 9, 552–562. [Google Scholar] [CrossRef]
- Kang, J.H.; Yun, S.I.; Park, M.H.; Park, J.H.; Jeong, S.Y.; Park, H.O. Anti-obesity effect of Lactobacillus gasseri BNR17 in high-sucrose diet-induced obese mice. PLoS ONE 2013, 8, e54617. [Google Scholar] [CrossRef]
- Zhang, L.; Qin, Q.; Liu, M.; Zhang, X.; He, F.; Wang, G. Akkermansia muciniphila can reduce the damage of gluco/lipotoxicity, oxidative stress and inflammation, and normalize intestine microbiota in streptozotocin-induced diabetic rats. Pathog. Dis. 2018, 76, fty028. [Google Scholar] [CrossRef]
- Wu, H.; Esteve, E.; Tremaroli, V.; Khan, M.T.; Caesar, R.; Mannerås-Holm, L.; Ståhlman, M.; Olsson, L.M.; Serino, M.; Planas-Fèlix, M.; et al. Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat. Med. 2017, 23, 850–858. [Google Scholar] [CrossRef]
- Pedersen, C.; Gallagher, E.; Horton, F.; Ellis, R.J.; Ijaz, U.Z.; Wu, H.; Jaiyeola, E.; Diribe, O.; Duparc, T.; Cani, P.D.; et al. Host-microbiome interactions in human type 2 diabetes following prebiotic fibre (galacto-oligosaccharide) intake. Br. J. Nutr. 2016, 116, 1869–1877. [Google Scholar] [CrossRef]
- Sasaki, M.; Ogasawara, N.; Funaki, Y.; Mizuno, M.; Iida, A.; Goto, C.; Koikeda, S.; Kasugai, K.; Joh, T. Transglucosidase improves the gut microbiota profile of type 2 diabetes mellitus patients: A randomized double-blind, placebo-controlled study. BMC Gastroenterol. 2013, 13, 81. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ye, X.; Ding, D.; Lu, Y. Characteristics of the intestinal flora in patients with peripheral neuropathy associated with type 2 diabetes. J. Int. Med. Res. 2020, 48, 300060520936806. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ota, N.; Manzanillo, P.; Kates, L.; Zavala-Solorio, J.; Eidenschenk, C.; Zhang, J.; Lesch, J.; Lee, W.P.; Ross, J.; et al. Interleukin-22 alleviates metabolic disorders and restores mucosal immunity in diabetes. Nature 2014, 514, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, T.W.; Pham, H.P.; Bridonneau, C.; Aubry, C.; Lamas, B.; Martin-Gallausiaux, C.; Moroldo, M.; Rainteau, D.; Lapaque, N.; Six, A.; et al. Microorganisms linked to inflammatory bowel disease-associated dysbiosis differentially impact host physiology in gnotobiotic mice. ISME J. 2016, 10, 460–477. [Google Scholar] [CrossRef]
- Zhu, C.; Song, K.; Shen, Z.; Quan, Y.; Tan, B.; Luo, W.; Wu, S.; Tang, K.; Yang, Z.; Wang, X. Roseburia intestinalis inhibits interleukin-17 excretion and promotes regulatory T cells differentiation in colitis. Mol. Med. Rep. 2018, 17, 7567–7574. [Google Scholar] [CrossRef]
- Valles-Colomer, M.; Falony, G.; Darzi, Y.; Tigchelaar, E.F.; Wang, J.; Tito, R.Y.; Schiweck, C.; Kurilshikov, A.; Joossens, M.; Wijmenga, C.; et al. The neuroactive potential of the human gut microbiota in quality of life and depression. Nat. Microbiol. 2019, 4, 623–632. [Google Scholar] [CrossRef]
- Traisaeng, S.; Batsukh, A.; Chuang, T.H.; Herr, D.R.; Huang, Y.F.; Chimeddorj, B.; Huang, C.M. Leuconostoc mesenteroides fermentation produces butyric acid and mediates Ffar2 to regulate blood glucose and insulin in type 1 diabetic mice. Sci. Rep. 2020, 10, 7928. [Google Scholar] [CrossRef] [PubMed]
- Zhai, L.; Xiao, H.; Lin, C.; Wong, H.L.X.; Lam, Y.Y.; Gong, M.; Wu, G.; Ning, Z.; Huang, C.; Zhang, Y.; et al. Gut microbiota-derived tryptamine and phenethylamine impair insulin sensitivity in metabolic syndrome and irritable bowel syndrome. Nat. Commun. 2023, 14, 4986. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, P.; Devkumar, P.; Chattopadhyay, I. Could dysbiosis of inflammatory and anti-inflammatory gut bacteria have an implications in the development of type 2 diabetes? A pilot investigation. BMC Res. Notes 2021, 14, 52. [Google Scholar] [CrossRef]
- Grahnemo, L.; Nethander, M.; Coward, E.; Gabrielsen, M.E.; Sree, S.; Billod, J.M.; Engstrand, L.; Abrahamsson, S.; Langhammer, A.; Hveem, K.; et al. Cross-sectional associations between the gut microbe Ruminococcus gnavus and features of the metabolic syndrome. Lancet Diabetes Endocrinol. 2022, 10, 481–483. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Si, Q.; Lin, G.; Zhu, M.; Lu, J.; Zhang, H.; Wang, G.; Chen, W. Is Effective in Relieving Type 2 Diabetes and May Be Related to Its Dominant Core Genome and Gut Microbiota Modulation Capacity. Nutrients 2022, 14, 2479. [Google Scholar] [CrossRef]
- Yao, K.; Zeng, L.; He, Q.; Wang, W.; Lei, J.; Zou, X. Effect of Probiotics on Glucose and Lipid Metabolism in Type 2 Diabetes Mellitus: A Meta-Analysis of 12 Randomized Controlled Trials. Med. Sci. Monit. 2017, 23, 3044–3053. [Google Scholar] [CrossRef]
- Wang, G.; Li, X.; Zhao, J.; Zhang, H.; Chen, W. Lactobacillus casei CCFM419 attenuates type 2 diabetes via a gut microbiota dependent mechanism. Food Funct. 2017, 8, 3155–3164. [Google Scholar] [CrossRef]
- Moroti, C.; Souza Magri, L.F.; de Rezende Costa, M.; Cavallini, D.C.; Sivieri, K. Effect of the consumption of a new symbiotic shake on glycemia and cholesterol levels in elderly people with type 2 diabetes mellitus. Lipids Health Dis. 2012, 11, 29. [Google Scholar] [CrossRef]
- Honda, K.; Moto, M.; Uchida, N.; He, F.; Hashizume, N. Anti-diabetic effects of lactic acid bacteria in normal and type 2 diabetic mice. J. Clin. Biochem. Nutr. 2012, 51, 96–101. [Google Scholar] [CrossRef]
- Tabuchi, M.; Ozaki, M.; Tamura, A.; Yamada, N.; Ishida, T.; Hosoda, M.; Hosono, A. Antidiabetic effect of Lactobacillus GG in streptozotocin-induced diabetic rats. Biosci. Biotechnol. Biochem. 2003, 67, 1421–1424. [Google Scholar] [CrossRef]
- Yadav, H.; Jain, S.; Sinha, P.R. Oral administration of dahi containing probiotic Lactobacillus acidophilus and Lactobacillus casei delayed the progression of streptozotocin-induced diabetes in rats. J. Dairy. Res. 2008, 75, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.Y.; Korivi, M.; Tsai, C.H.; Yang, J.H.; Tsai, Y.C. Supplementation of Lactobacillus plantarum K68 and Fruit-Vegetable Ferment along with High Fat-Fructose Diet Attenuates Metabolic Syndrome in Rats with Insulin Resistance. Evid. Based Complement. Altern. Med. 2013, 2013, 943020. [Google Scholar] [CrossRef] [PubMed]
- Han, C.Y. Roles of Reactive Oxygen Species on Insulin Resistance in Adipose Tissue. Diabetes Metab. J. 2016, 40, 272–279. [Google Scholar] [CrossRef]
- Zeng, Z.; Yuan, Q.; Yu, R.; Zhang, J.; Ma, H.; Chen, S. Ameliorative Effects of Probiotic Lactobacillus paracasei NL41 on Insulin Sensitivity, Oxidative Stress, and Beta-Cell Function in a Type 2 Diabetes Mellitus Rat Model. Mol. Nutr. Food Res. 2019, 63, e1900457. [Google Scholar] [CrossRef] [PubMed]
- Volmer-Thole, M.; Lobmann, R. Neuropathy and Diabetic Foot Syndrome. Int. J. Mol. Sci. 2016, 17, 917. [Google Scholar] [CrossRef]
- Hurlow, J.J.; Humphreys, G.J.; Bowling, F.L.; McBain, A.J. Diabetic foot infection: A critical complication. Int. Wound J. 2018, 15, 814–821. [Google Scholar] [CrossRef]
- Lipsky, B.A.; Berendt, A.R.; Cornia, P.B.; Pile, J.C.; Peters, E.J.; Armstrong, D.G.; Deery, H.G.; Embil, J.M.; Joseph, W.S.; Karchmer, A.W.; et al. 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin. Infect. Dis. 2012, 54, e132–e173. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, L.; He, M.; Zhao, Y. Sequence analysis of microbiota in clinical human cases with diabetic foot ulcers from China. Heliyon 2024, 10, e34368. [Google Scholar] [CrossRef]
- Prompers, L.; Huijberts, M.; Schaper, N.; Apelqvist, J.; Bakker, K.; Edmonds, M.; Holstein, P.; Jude, E.; Jirkovska, A.; Mauricio, D.; et al. Resource utilisation and costs associated with the treatment of diabetic foot ulcers. Prospective data from the Eurodiale Study. Diabetologia 2008, 51, 1826–1834. [Google Scholar] [CrossRef]
- Dunyach-Remy, C.; Ngba Essebe, C.; Sotto, A.; Lavigne, J.P. Staphylococcus aureus Toxins and Diabetic Foot Ulcers: Role in Pathogenesis and Interest in Diagnosis. Toxins 2016, 8, 209. [Google Scholar] [CrossRef]
- Matheson, E.M.; Bragg, S.W.; Blackwelder, R.S. Diabetes-Related Foot Infections: Diagnosis and Treatment. Am. Fam. Physician 2021, 104, 386–394. [Google Scholar]
- Hu, Y.; Liang, Y.; Lv, Y. Causal relationship between gut microbiota and diabetic neuropathy: A Mendelian randomization and 16S rRNA sequencing analysis. Front. Endocrinol. 2025, 16, 1632406. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhao, B.; Wang, Y.; Lan, H.; Liu, X.; Hu, Y.; Cao, P. Diabetic neuropathy: Cutting-edge research and future directions. Signal Transduct. Target. Ther. 2025, 10, 132. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, K.E.; Boeckh, S.; Stacey, H.J.; Jones, J.D. The microbiology of diabetic foot infections: A meta-analysis. BMC Infect. Dis. 2021, 21, 770. [Google Scholar] [CrossRef]
- Ioannou, P.; Chatzisymeon, D.; Maraki, S.; Samonis, G.; Kofteridis, D.P. Mixed Skin and Soft Tissue Infection Caused by. Maedica 2022, 17, 211–214. [Google Scholar] [CrossRef]
- Du, F.; Ma, J.; Gong, H.; Bista, R.; Zha, P.; Ren, Y.; Gao, Y.; Chen, D.; Ran, X.; Wang, C. Microbial Infection and Antibiotic Susceptibility of Diabetic Foot Ulcer in China: Literature Review. Front. Endocrinol. 2022, 13, 881659. [Google Scholar] [CrossRef] [PubMed]
- Abi Frem, J.; Ghanem, M.; Doumat, G.; Kanafani, Z.A. Clinical manifestations, characteristics, and outcome of infections caused by vancomycin-resistant enterococci at a tertiary care center in Lebanon: A case-case-control study. J. Infect. Public Health 2023, 16, 741–745. [Google Scholar] [CrossRef]
- Shin, N.R.; Lee, J.C.; Lee, H.Y.; Kim, M.S.; Whon, T.W.; Lee, M.S.; Bae, J.W. An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut 2014, 63, 727–735. [Google Scholar] [CrossRef]
- Mueller, N.T.; Differding, M.K.; Zhang, M.; Maruthur, N.M.; Juraschek, S.P.; Miller, E.R., 3rd; Appel, L.J.; Yeh, H.C. Metformin Affects Gut Microbiome Composition and Function and Circulating Short-Chain Fatty Acids: A Randomized Trial. Diabetes Care 2021, 44, 1462–1471. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Lou, X.; Jiang, C.; Ji, X.; Tao, X.; Sun, J.; Bao, Z. Gut microbiota is correlated with gastrointestinal adverse events of metformin in patients with type 2 diabetes. Front. Endocrinol. 2022, 13, 1044030. [Google Scholar] [CrossRef] [PubMed]
- Pham, N.H.T.; Joglekar, M.V.; Wong, W.K.M.; Nassif, N.T.; Simpson, A.M.; Hardikar, A.A. Short-chain fatty acids and insulin sensitivity: A systematic review and meta-analysis. Nutr. Rev. 2024, 82, 193–209. [Google Scholar] [CrossRef]
- Gao, Y.; Zhao, T.; Lv, N.; Liu, S.; Yuan, T.; Fu, Y.; Zhao, W.; Zhu, B. Metformin-induced changes of the gut microbiota in patients with type 2 diabetes mellitus: Results from a prospective cohort study. Endocrine 2024, 85, 1178–1192. [Google Scholar] [CrossRef]
- Rosell-Diaz, M.; Petit-Gay, A.; Molas-Prat, C.; Gallardo-Nuell, L.; Ramio-Torrenta, L.; Garre-Olmo, J.; Perez-Brocal, V.; Moya, A.; Jove, M.; Pamplona, R.; et al. Metformin-induced changes in the gut microbiome and plasma metabolome are associated with cognition in men. Metabolism 2024, 157, 155941. [Google Scholar] [CrossRef]
- Cheng, M.; Jia, X.; Ren, L.; Chen, S.; Wang, W.; Wang, J.; Cong, B. Region-Specific Effects of Metformin on Gut Microbiome and Metabolome in High-Fat Diet-Induced Type 2 Diabetes Mouse Model. Int. J. Mol. Sci. 2024, 25, 7250. [Google Scholar] [CrossRef]
- Kim, H.B.; Cho, Y.J.; Choi, S.S. Metformin increases gut multidrug resistance genes in type 2 diabetes, potentially linked to Escherichia coli. Sci. Rep. 2024, 14, 21480. [Google Scholar] [CrossRef]
- Elbere, I.; Silamikelis, I.; Dindune, I.I.; Kalnina, I.; Briviba, M.; Zaharenko, L.; Silamikele, L.; Rovite, V.; Gudra, D.; Konrade, I.; et al. Baseline gut microbiome composition predicts metformin therapy short-term efficacy in newly diagnosed type 2 diabetes patients. PLoS ONE 2020, 15, e0241338. [Google Scholar] [CrossRef]
- Elbere, I.; Orlovskis, Z.; Ansone, L.; Silamikelis, I.; Jagare, L.; Birzniece, L.; Megnis, K.; Leskovskis, K.; Vaska, A.; Turks, M.; et al. Gut microbiome encoded purine and amino acid pathways present prospective biomarkers for predicting metformin therapy efficacy in newly diagnosed T2D patients. Gut Microbes 2024, 16, 2361491. [Google Scholar] [CrossRef] [PubMed]
- Voors, A.A.; Angermann, C.E.; Teerlink, J.R.; Collins, S.P.; Kosiborod, M.; Biegus, J.; Ferreira, J.P.; Nassif, M.E.; Psotka, M.A.; Tromp, J.; et al. The SGLT2 inhibitor empagliflozin in patients hospitalized for acute heart failure: A multinational randomized trial. Nat. Med. 2022, 28, 568–574. [Google Scholar] [CrossRef]
- Nassif, M.E.; Windsor, S.L.; Borlaug, B.A.; Kitzman, D.W.; Shah, S.J.; Tang, F.; Khariton, Y.; Malik, A.O.; Khumri, T.; Umpierrez, G.; et al. The SGLT2 inhibitor dapagliflozin in heart failure with preserved ejection fraction: A multicenter randomized trial. Nat. Med. 2021, 27, 1954–1960. [Google Scholar] [CrossRef]
- Heerspink, H.J.L.; Stefansson, B.V.; Correa-Rotter, R.; Chertow, G.M.; Greene, T.; Hou, F.F.; Mann, J.F.E.; McMurray, J.J.V.; Lindberg, M.; Rossing, P.; et al. Dapagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2020, 383, 1436–1446. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Du, H.; Ke, L.; Zheng, L.; Nan, S.; Ni, L.; Pan, Y.; Fu, Z.; He, Q.; Jin, J. Gut-kidney interaction reinforces dapagliflozin-mediated alleviation in diabetic nephropathy. Am. J. Physiol. Cell Physiol. 2025, 328, C452–C466. [Google Scholar] [CrossRef]
- Yang, M.; Shi, F.H.; Liu, W.; Zhang, M.C.; Feng, R.L.; Qian, C.; Liu, W.; Ma, J. Dapagliflozin Modulates the Fecal Microbiota in a Type 2 Diabetic Rat Model. Front. Endocrinol. 2020, 11, 635. [Google Scholar] [CrossRef]
- Deng, X.; Zhang, C.; Wang, P.; Wei, W.; Shi, X.; Wang, P.; Yang, J.; Wang, L.; Tang, S.; Fang, Y.; et al. Cardiovascular Benefits of Empagliflozin Are Associated with Gut Microbiota and Plasma Metabolites in Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2022, 107, 1888–1896. [Google Scholar] [CrossRef]
- Ng, H.Y.; Zhang, L.; Tan, J.T.; Hui, R.W.H.; Yuen, M.F.; Seto, W.K.; Leung, W.K.; Cheung, K.S. Gut Microbiota Predicts Treatment Response to Empagliflozin Among MASLD Patients without Diabetes Mellitus. Liver Int. 2025, 45, e70023. [Google Scholar] [CrossRef] [PubMed]
- de Souza, P.R.K.; Ferreira, S.S.; Nunes, F.P.B.; Casagrande, F.B.; Tessaro, F.H.G.; Silva, M.C.F.; Cruz, J.W.M.C.; Mamizuka, E.M.; Martins, J.O. Cytokine and Adhesion Molecule Expression Induced by Different Strains of Staphylococcus aureus in Type 1 Diabetic Rats: Role of Insulin. Front. Immunol. 2018, 9, 3165. [Google Scholar] [CrossRef] [PubMed]
- Antar, S.A.; Ashour, N.A.; Sharaky, M.; Khattab, M.; Zaid, R.T.; Roh, E.J.; Elkamhawy, A.; Al-Karmalawy, A.A. Diabetes mellitus: Classification, mediators, and complications; A gate to identify potential targets for the development of new effective treatments. Biomed. Pharmacother. 2023, 168, 115734. [Google Scholar] [CrossRef]
- Duncan, B.B.; Schmidt, M.I. Chronic activation of the innate immune system may underlie the metabolic syndrome. Sao Paulo Med. J. 2001, 119, 122–127. [Google Scholar] [CrossRef]
- Zhang, Y.; Gu, Y.; Ren, H.; Wang, S.; Zhong, H.; Zhao, X.; Ma, J.; Gu, X.; Xue, Y.; Huang, S.; et al. Gut microbiome-related effects of berberine and probiotics on type 2 diabetes (the PREMOTE study). Nat. Commun. 2020, 11, 5015. [Google Scholar] [CrossRef]
- Lee, E.; Jung, S.R.; Lee, S.Y.; Lee, N.K.; Paik, H.D.; Lim, S.I. Strain Ln4 Attenuates Diet-Induced Obesity, Insulin Resistance, and Changes in Hepatic mRNA Levels Associated with Glucose and Lipid Metabolism. Nutrients 2018, 10, 643. [Google Scholar] [CrossRef]
- Al-Fakhrany, O.M.; Elekhnawy, E. Next-generation probiotics: The upcoming biotherapeutics. Mol. Biol. Rep. 2024, 51, 505. [Google Scholar] [CrossRef]
- Hosomi, K.; Saito, M.; Park, J.; Murakami, H.; Shibata, N.; Ando, M.; Nagatake, T.; Konishi, K.; Ohno, H.; Tanisawa, K.; et al. Oral administration of Blautia wexlerae ameliorates obesity and type 2 diabetes via metabolic remodeling of the gut microbiota. Nat. Commun. 2022, 13, 4477. [Google Scholar] [CrossRef]
- Vatanen, T.; Franzosa, E.A.; Schwager, R.; Tripathi, S.; Arthur, T.D.; Vehik, K.; Lernmark, A.; Hagopian, W.A.; Rewers, M.J.; She, J.X.; et al. The human gut microbiome in early-onset type 1 diabetes from the TEDDY study. Nature 2018, 562, 589–594. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Hoffmann, G.; Lampousi, A.M.; Knuppel, S.; Iqbal, K.; Schwedhelm, C.; Bechthold, A.; Schlesinger, S.; Boeing, H. Food groups and risk of type 2 diabetes mellitus: A systematic review and meta-analysis of prospective studies. Eur. J. Epidemiol. 2017, 32, 363–375. [Google Scholar] [CrossRef]
- Szczerba, E.; Barbaresko, J.; Schiemann, T.; Stahl-Pehe, A.; Schwingshackl, L.; Schlesinger, S. Diet in the management of type 2 diabetes: Umbrella review of systematic reviews with meta-analyses of randomised controlled trials. BMJ Med. 2023, 2, e000664. [Google Scholar] [CrossRef]
- Tang, Y.; Chen, Y.; Zhou, Y.; Wu, B.; Zhang, S.; Gong, Y.; Ni, Q. Global burden of Type 2 Diabetes Mellitus attributable to dietary risks in elderly adults: Insights from the Global Burden of Disease study 2021. Front. Nutr. 2025, 12, 1557923. [Google Scholar] [CrossRef] [PubMed]
- Heidarzadeh-Esfahani, N.; Darbandi, M.; Khamoushi, F.; Najafi, F.; Soleimani, D.; Moradi, M.; Shakiba, E.; Pasdar, Y. Association of plant-based dietary patterns with the risk of type 2 diabetes mellitus using cross-sectional results from RaNCD cohort. Sci. Rep. 2024, 14, 3814. [Google Scholar] [CrossRef] [PubMed]
- Kalandarova, M.; Ahmad, I.; Aung, T.N.N.; Moolphate, S.; Shirayama, Y.; Okamoto, M.; Aung, M.N.; Yuasa, M. Association Between Dietary Habits and Type 2 Diabetes Mellitus in Thai Adults: A Case-Control Study. Diabetes Metab. Syndr. Obes. 2024, 17, 1143–1155. [Google Scholar] [CrossRef]
- Moya-Pérez, A.; Neef, A.; Sanz, Y. Bifidobacterium pseudocatenulatum CECT 7765 Reduces Obesity-Associated Inflammation by Restoring the Lymphocyte-Macrophage Balance and Gut Microbiota Structure in High-Fat Diet-Fed Mice. PLoS ONE 2015, 10, e0126976. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tang, H.; Zhang, C.; Zhao, Y.; Derrien, M.; Rocher, E.; van-Hylckama Vlieg, J.E.; Strissel, K.; Zhao, L.; Obin, M.; et al. Modulation of gut microbiota during probiotic-mediated attenuation of metabolic syndrome in high fat diet-fed mice. ISME J. 2015, 9, 1–15. [Google Scholar] [CrossRef]
- Gregory, A.C.; Zablocki, O.; Zayed, A.A.; Howell, A.; Bolduc, B.; Sullivan, M.B. The Gut Virome Database Reveals Age-Dependent Patterns of Virome Diversity in the Human Gut. Cell Host Microbe 2020, 28, 724–740.e728. [Google Scholar] [CrossRef]
- Cao, Z.; Sugimura, N.; Burgermeister, E.; Ebert, M.P.; Zuo, T.; Lan, P. The gut virome: A new microbiome component in health and disease. EBioMedicine 2022, 81, 104113. [Google Scholar] [CrossRef]
- Ho, S.X.; Law, J.H.; Png, C.W.; Alberts, R.; Zhang, Y.; Chu, J.J.H.; Tan, K.K. Alterations in colorectal cancer virome and its persistence after surgery. Sci. Rep. 2024, 14, 2819. [Google Scholar] [CrossRef]
- Tun, H.M.; Peng, Y.; Massimino, L.; Sin, Z.Y.; Parigi, T.L.; Facoetti, A.; Rahman, S.; Danese, S.; Ungaro, F. Gut virome in inflammatory bowel disease and beyond. Gut 2024, 73, 350–360. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.L.; Duan, Y.; Fouts, D.E.; Schnabl, B. Intestinal virome and therapeutic potential of bacteriophages in liver disease. J. Hepatol. 2021, 75, 1465–1475. [Google Scholar] [CrossRef]
- Yang, K.; Niu, J.; Zuo, T.; Sun, Y.; Xu, Z.; Tang, W.; Liu, Q.; Zhang, J.; Ng, E.K.W.; Wong, S.K.H.; et al. Alterations in the Gut Virome in Obesity and Type 2 Diabetes Mellitus. Gastroenterology 2021, 161, 1257–1269.e13. [Google Scholar] [CrossRef] [PubMed]
- Isaacs, S.R.; Foskett, D.B.; Maxwell, A.J.; Ward, E.J.; Faulkner, C.L.; Luo, J.Y.X.; Rawlinson, W.D.; Craig, M.E.; Kim, K.W. Viruses and Type 1 Diabetes: From Enteroviruses to the Virome. Microorganisms 2021, 9, 1519. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Ye, F.; Chen, Y.; Xu, J.; Zhao, Y.; Wang, Y.; Lan, T. Association Between Enterovirus Infection and Type 1 Diabetes Risk: A Meta-Analysis of 38 Case-Control Studies. Front. Endocrinol. 2021, 12, 706964. [Google Scholar] [CrossRef]
- Zhao, G.; Vatanen, T.; Droit, L.; Park, A.; Kostic, A.D.; Poon, T.W.; Vlamakis, H.; Siljander, H.; Härkönen, T.; Hämäläinen, A.M.; et al. Intestinal virome changes precede autoimmunity in type I diabetes-susceptible children. Proc. Natl. Acad. Sci. USA 2017, 114, E6166–E6175. [Google Scholar] [CrossRef]
- Gavin, P.G.; Kim, K.W.; Craig, M.E.; Hill, M.M.; Hamilton-Williams, E.E. Multi-omic interactions in the gut of children at the onset of islet autoimmunity. Microbiome 2022, 10, 230. [Google Scholar] [CrossRef] [PubMed]
- Kramná, L.; Kolářová, K.; Oikarinen, S.; Pursiheimo, J.P.; Ilonen, J.; Simell, O.; Knip, M.; Veijola, R.; Hyöty, H.; Cinek, O. Gut virome sequencing in children with early islet autoimmunity. Diabetes Care 2015, 38, 930–933. [Google Scholar] [CrossRef] [PubMed]
- Cinek, O.; Kramna, L.; Odeh, R.; Alassaf, A.; Ibekwe, M.A.U.; Ahmadov, G.; Elmahi, B.M.E.; Mekki, H.; Lebl, J.; Abdullah, M.A. Eukaryotic viruses in the fecal virome at the onset of type 1 diabetes: A study from four geographically distant African and Asian countries. Pediatr. Diabetes 2021, 22, 558–566. [Google Scholar] [CrossRef]
- Lee, H.S.; Briese, T.; Winkler, C.; Rewers, M.; Bonifacio, E.; Hyoty, H.; Pflueger, M.; Simell, O.; She, J.X.; Hagopian, W.; et al. Next-generation sequencing for viruses in children with rapid-onset type 1 diabetes. Diabetologia 2013, 56, 1705–1711. [Google Scholar] [CrossRef]
- Cinek, O.; Kramna, L.; Lin, J.; Oikarinen, S.; Kolarova, K.; Ilonen, J.; Simell, O.; Veijola, R.; Autio, R.; Hyöty, H. Imbalance of bacteriome profiles within the Finnish Diabetes Prediction and Prevention study: Parallel use of 16S profiling and virome sequencing in stool samples from children with islet autoimmunity and matched controls. Pediatr. Diabetes 2017, 18, 588–598. [Google Scholar] [CrossRef]
- Kim, K.W.; Horton, J.L.; Pang, C.N.I.; Jain, K.; Leung, P.; Isaacs, S.R.; Bull, R.A.; Luciani, F.; Wilkins, M.R.; Catteau, J.; et al. Higher abundance of enterovirus A species in the gut of children with islet autoimmunity. Sci. Rep. 2019, 9, 1749. [Google Scholar] [CrossRef]
- Wook Kim, K.; Allen, D.W.; Briese, T.; Couper, J.J.; Barry, S.C.; Colman, P.G.; Cotterill, A.M.; Davis, E.A.; Giles, L.C.; Harrison, L.C.; et al. Distinct Gut Virome Profile of Pregnant Women with Type 1 Diabetes in the ENDIA Study. Open Forum Infect. Dis. 2019, 6, ofz025. [Google Scholar] [CrossRef]
- Fang, L.; Ning, J. Gut virome and diabetes: Discovering links, exploring therapies. Arch. Microbiol. 2024, 206, 346. [Google Scholar] [CrossRef] [PubMed]
- Fan, G.; Cao, F.; Kuang, T.; Yi, H.; Zhao, C.; Wang, L.; Peng, J.; Zhuang, Z.; Xu, T.; Luo, Y.; et al. Alterations in the gut virome are associated with type 2 diabetes and diabetic nephropathy. Gut Microbes 2023, 15, 2226925. [Google Scholar] [CrossRef]
- Ma, Y.; You, X.; Mai, G.; Tokuyasu, T.; Liu, C. A human gut phage catalog correlates the gut phageome with type 2 diabetes. Microbiome 2018, 6, 24. [Google Scholar] [CrossRef]
- Chen, Q.; Ma, X.; Li, C.; Shen, Y.; Zhu, W.; Zhang, Y.; Guo, X.; Zhou, J.; Liu, C. Enteric Phageome Alterations in Patients with Type 2 Diabetes. Front. Cell Infect. Microbiol. 2020, 10, 575084. [Google Scholar] [CrossRef]
- Rasmussen, T.S.; Mentzel, C.M.J.; Kot, W.; Castro-Mejía, J.L.; Zuffa, S.; Swann, J.R.; Hansen, L.H.; Vogensen, F.K.; Hansen, A.K.; Nielsen, D.S. Faecal virome transplantation decreases symptoms of type 2 diabetes and obesity in a murine model. Gut 2020, 69, 2122–2130. [Google Scholar] [CrossRef]
- Zeng, C.; Wan, S.R.; Guo, M.; Tan, X.Z.; Zeng, Y.; Wu, Q.; Xie, J.J.; Yan, P.; Long, Y.; Zheng, L.; et al. Fecal virome transplantation: A promising strategy for the treatment of metabolic diseases. Biomed. Pharmacother. 2024, 177, 117065. [Google Scholar] [CrossRef]
- Yang, J.Y.; Kim, M.S.; Kim, E.; Cheon, J.H.; Lee, Y.S.; Kim, Y.; Lee, S.H.; Seo, S.U.; Shin, S.H.; Choi, S.S.; et al. Enteric Viruses Ameliorate Gut Inflammation via Toll-like Receptor 3 and Toll-like Receptor 7-Mediated Interferon-β Production. Immunity 2016, 44, 889–900. [Google Scholar] [CrossRef]
- Popescu, M.; Van Belleghem, J.D.; Khosravi, A.; Bollyky, P.L. Bacteriophages and the Immune System. Annu. Rev. Virol. 2021, 8, 415–435. [Google Scholar] [CrossRef]
- Tetz, G.; Tetz, V. Bacteriophage infections of microbiota can lead to leaky gut in an experimental rodent model. Gut Pathog. 2016, 8, 33. [Google Scholar] [CrossRef]
- Tetz, G.V.; Ruggles, K.V.; Zhou, H.; Heguy, A.; Tsirigos, A.; Tetz, V. Bacteriophages as potential new mammalian pathogens. Sci. Rep. 2017, 7, 7043. [Google Scholar] [CrossRef] [PubMed]
- Federici, S.; Nobs, S.P.; Elinav, E. Phages and their potential to modulate the microbiome and immunity. Cell Mol. Immunol. 2021, 18, 889–904. [Google Scholar] [CrossRef]
- Łusiak-Szelachowska, M.; Weber-Dąbrowska, B.; Żaczek, M.; Borysowski, J.; Górski, A. The Presence of Bacteriophages in the Human Body: Good, Bad or Neutral? Microorganisms 2020, 8, 2012. [Google Scholar] [CrossRef] [PubMed]
- Borodovich, T.; Shkoporov, A.N.; Ross, R.P.; Hill, C. Phage-mediated horizontal gene transfer and its implications for the human gut microbiome. Gastroenterol. Rep. 2022, 10, goac012. [Google Scholar] [CrossRef] [PubMed]
- Shuwen, H.; Kefeng, D. Intestinal phages interact with bacteria and are involved in human diseases. Gut Microbes 2022, 14, 2113717. [Google Scholar] [CrossRef] [PubMed]
- Lemos, J.R.N.; Hirani, K.; von Herrath, M. Immunological and virological triggers of type 1 diabetes: Insights and implications. Front. Immunol. 2023, 14, 1326711. [Google Scholar] [CrossRef] [PubMed]
- Ke, Y.; Huang, Y.; Yi, C.; Ma, L.; Chu, X.; Wu, B.; Zhao, Q.; Han, S. Structural insights into endogenous ligand selectivity and activation mechanisms of FFAR1 and FFAR2. Cell Rep. 2024, 43, 115024. [Google Scholar] [CrossRef]
- Zhang, X.; Guseinov, A.A.; Jenkins, L.; Valentini, A.; Marsango, S.; Schultz-Knudsen, K.; Ulven, T.; Rexen Ulven, E.; Tikhonova, I.G.; Milligan, G.; et al. Allosteric modulation and biased signalling at free fatty acid receptor 2. Nature 2025, 643, 1428–1438. [Google Scholar] [CrossRef]
- Kugawa, M.; Kawakami, K.; Kise, R.; Suomivuori, C.M.; Tsujimura, M.; Kobayashi, K.; Kojima, A.; Inoue, W.J.; Fukuda, M.; Matsui, T.E.; et al. Structural insights into lipid chain-length selectivity and allosteric regulation of FFA2. Nat. Commun. 2025, 16, 2809. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).