Research Advances in Cancer-Associated Fibroblasts in Prostate Cancer Progression
Abstract
1. Introduction
2. Impact of CAFs on PCa Progression
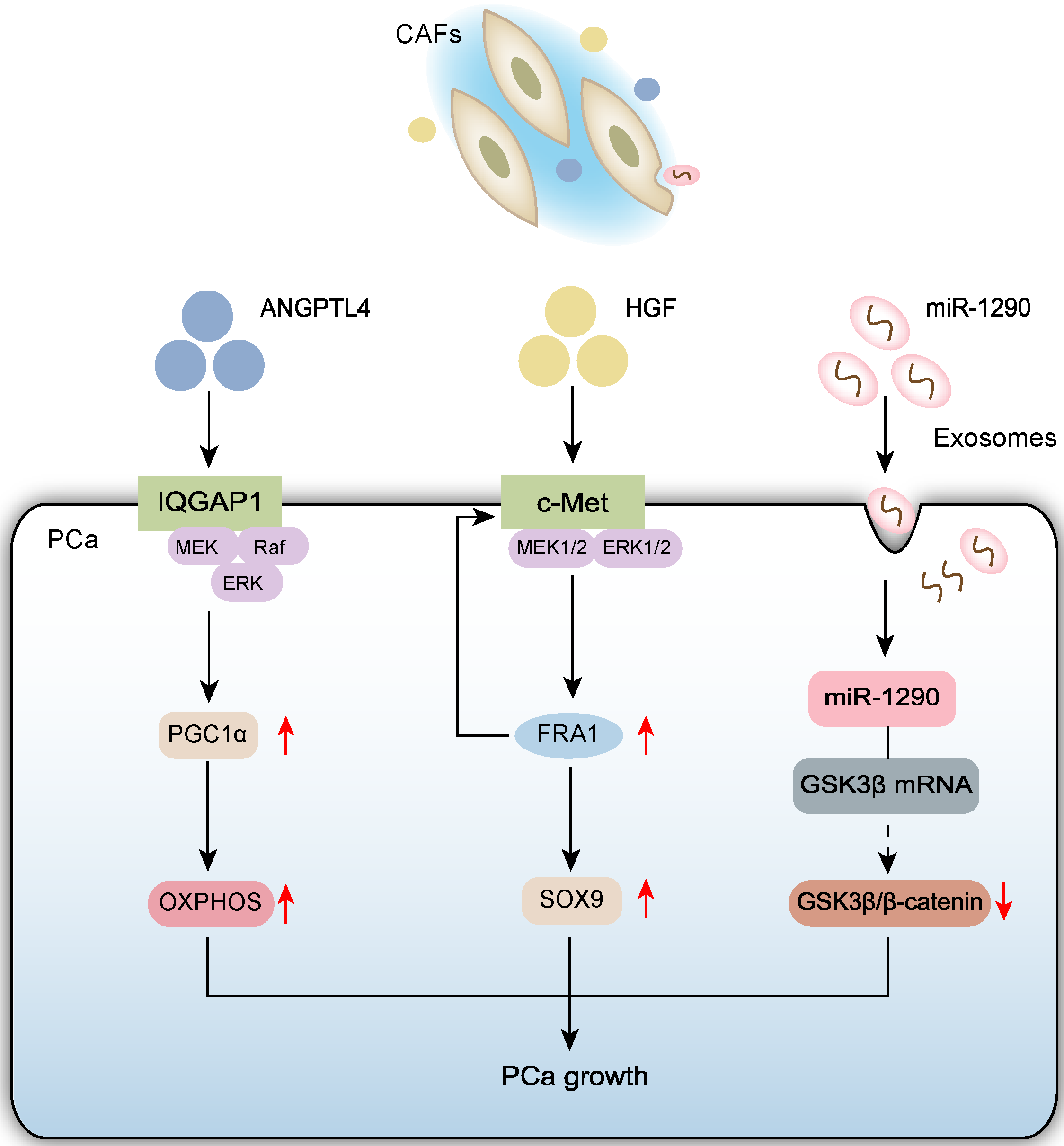
2.1. CAFs Promote PCa Cell Growth
2.1.1. CAFs Promote PCa Cell Proliferation via Paracrine Signaling
2.1.2. CAF-Derived Exosomes Promote PCa Cell Growth
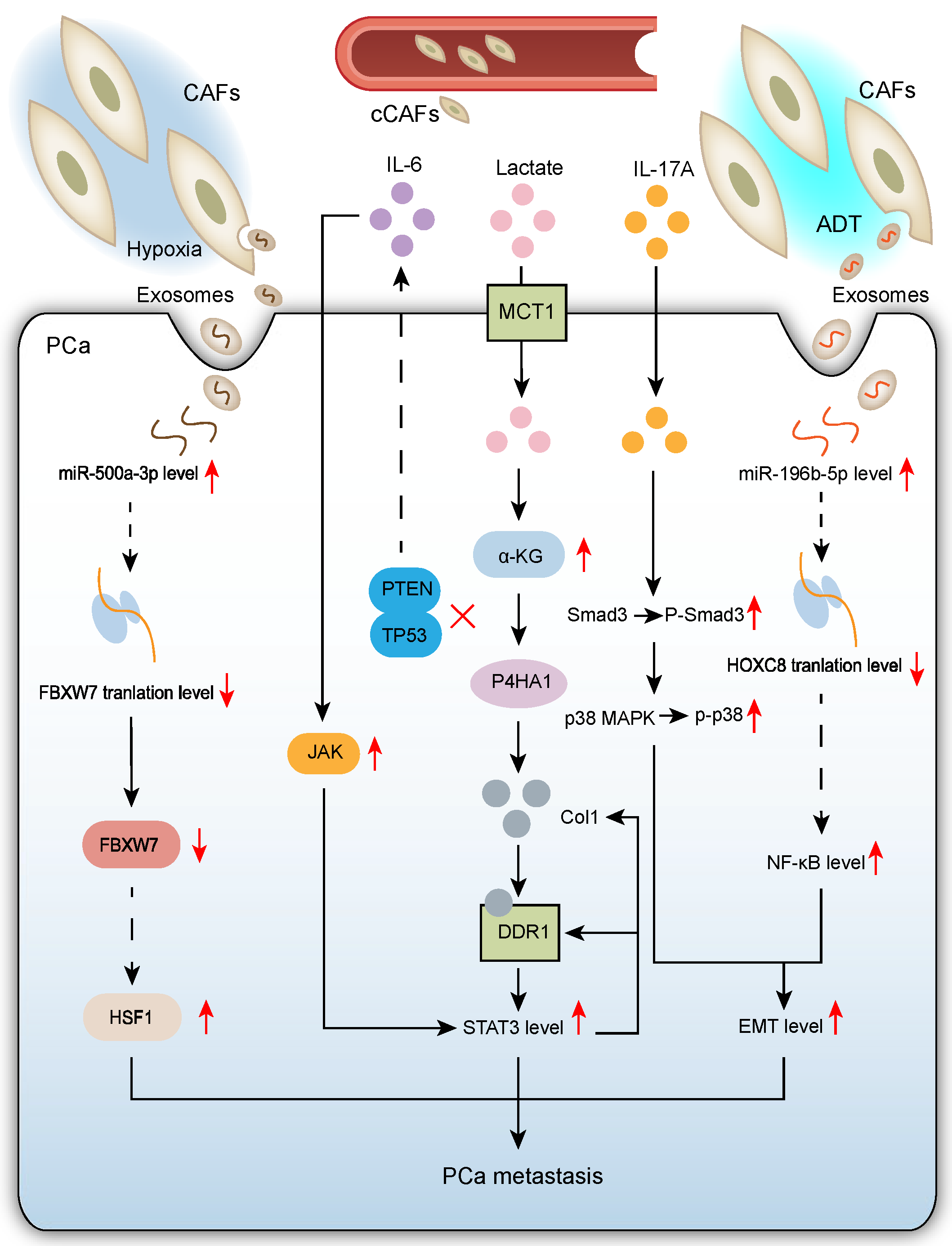
2.2. CAFs Promote PCa Metastasis
2.2.1. CAF-Mediated Mechanisms Promoting PCa Metastasis in Hypoxic TME
2.2.2. CAFs Enhance PCa Cell Epithelial–Mesenchymal Transition (EMT) to Promote Migration and Invasion
2.2.3. Pro-Tumor Crosstalk Between Tumor Suppressor-Deficient Prostate Epithelial Cells and CAFs Drives PCa Metastasis
2.2.4. Circulating CAFs (cCAFs) Emerge as a Novel Mechanism Promoting Cancer Progression and Metastasis
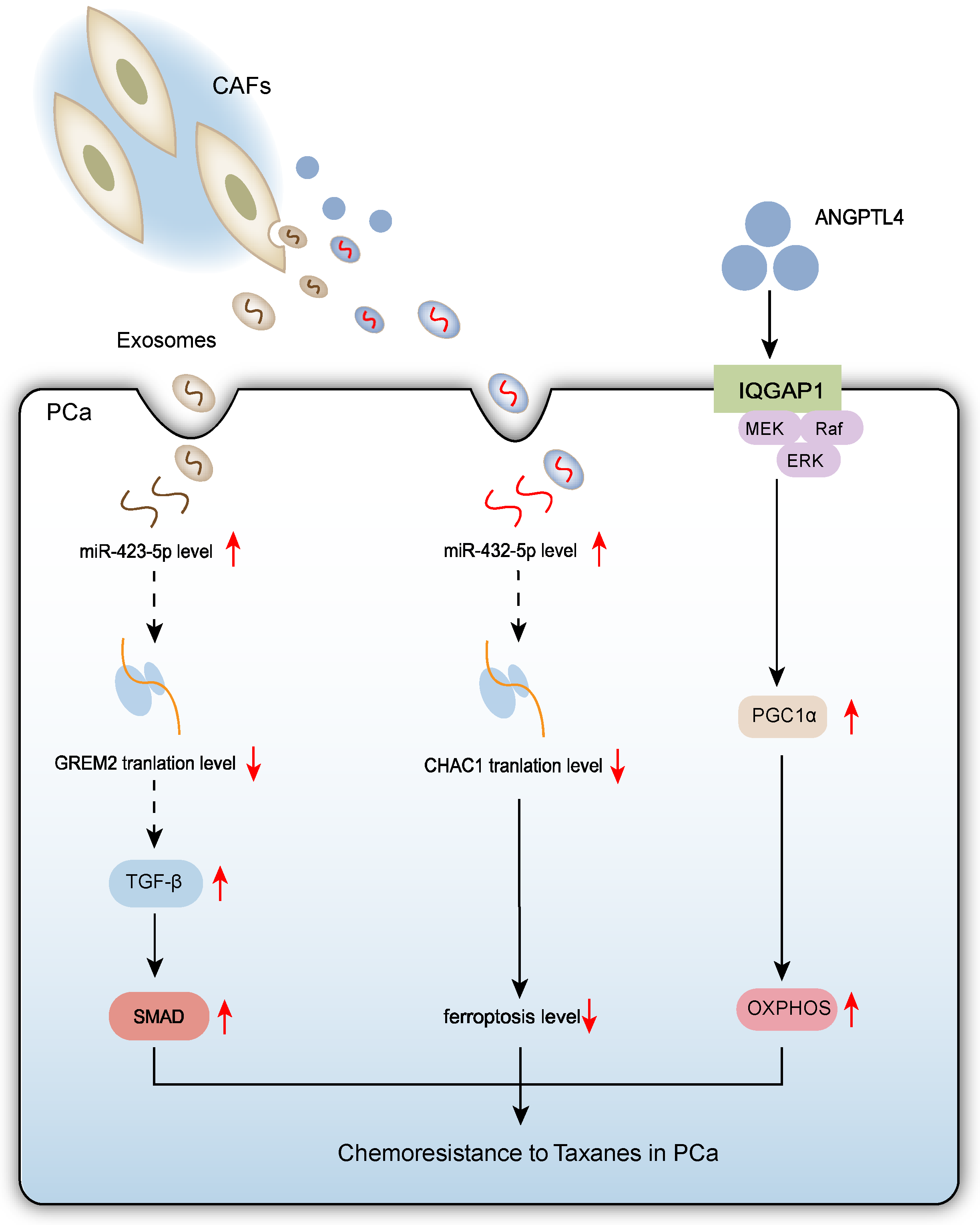
2.3. CAFs Promote Chemoresistance to Taxanes in PCa Cells
3. CAFs Promote the Development and Progression of CRPC
3.1. Different CAF Subtypes Promote CRPC
3.2. CAFs Promote CRPC via Paracrine Mechanisms
4. Anti-Tumor Effects of CAFs in PCa
5. Predicting PCa Patient Prognosis Using CAF-Associated Genes and Expression Products
6. Novel CAF-Targeting Therapeutic Strategies for PCa
7. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schafer, E.J.; Laversanne, M.; Sung, H.; Soerjomataram, I.; Briganti, A.; Dahut, W.; Bray, F.; Jemal, A. Recent Patterns and Trends in Global Prostate Cancer Incidence and Mortality: An Update. Eur. Urol. 2025, 87, 302–313. [Google Scholar] [CrossRef]
- Takayama, K.I. Splicing Factors Have an Essential Role in Prostate Cancer Progression and Androgen Receptor Signaling. Biomolecules 2019, 9, 131. [Google Scholar] [CrossRef]
- Bach, C.; Pisipati, S.; Daneshwar, D.; Wright, M.; Rowe, E.; Gillatt, D.; Persad, R.; Koupparis, A. The status of surgery in the management of high-risk prostate cancer. Nat. Rev. Urol. 2014, 11, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, D.; Yan, W.; Yang, D.; Shen, B. Translational bioinformatics for diagnostic and prognostic prediction of prostate cancer in the next-generation sequencing era. Biomed. Res. Int. 2013, 2013, 901578. [Google Scholar] [CrossRef]
- Harris, W.P.; Mostaghel, E.A.; Nelson, P.S.; Montgomery, B. Androgen deprivation therapy: Progress in understanding mechanisms of resistance and optimizing androgen depletion. Nat. Clin. Pract. Urol. 2009, 6, 76–85. [Google Scholar] [CrossRef]
- Chandrasekar, T.; Yang, J.C.; Gao, A.C.; Evans, C.P. Mechanisms of resistance in castration-resistant prostate cancer (CRPC). Transl. Androl. Urol. 2015, 4, 365–380. [Google Scholar] [CrossRef]
- Wei, R.; Liu, S.; Zhang, S.; Min, L.; Zhu, S. Cellular and Extracellular Components in Tumor Microenvironment and Their Application in Early Diagnosis of Cancers. Anal. Cell Pathol. 2020, 2020, 6283796. [Google Scholar] [CrossRef]
- Giraldo, N.A.; Sanchez-Salas, R.; Peske, J.D.; Vano, Y.; Becht, E.; Petitprez, F.; Validire, P.; Ingels, A.; Cathelineau, X.; Fridman, W.H.; et al. The clinical role of the TME in solid cancer. Br. J. Cancer 2019, 120, 45–53. [Google Scholar] [CrossRef]
- Darby, I.A.; Hewitson, T.D. Fibroblast differentiation in wound healing and fibrosis. Int. Rev. Cytol. 2007, 257, 143–179. [Google Scholar] [CrossRef]
- Zhang, H.; Yue, X.; Chen, Z.; Liu, C.; Wu, W.; Zhang, N.; Liu, Z.; Yang, L.; Jiang, Q.; Cheng, Q.; et al. Define cancer-associated fibroblasts (CAFs) in the tumor microenvironment: New opportunities in cancer immunotherapy and advances in clinical trials. Mol. Cancer 2023, 22, 159. [Google Scholar] [CrossRef]
- Scaglia, N.; Frontini-López, Y.R.; Zadra, G. Prostate Cancer Progression: As a Matter of Fats. Front. Oncol. 2021, 11, 719865. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Zhu, Y.; Shao, X.; Cai, A.; Dong, B.; Xue, W.; Gao, H. Distinct Metabolic Signatures of Hormone-Sensitive and Castration-Resistant Prostate Cancer Revealed by a (1)H NMR-Based Metabolomics of Biopsy Tissue. J. Proteome Res. 2020, 19, 3741–3749. [Google Scholar] [CrossRef] [PubMed]
- Fiaschi, T.; Marini, A.; Giannoni, E.; Taddei, M.L.; Gandellini, P.; De Donatis, A.; Lanciotti, M.; Serni, S.; Cirri, P.; Chiarugi, P. Reciprocal metabolic reprogramming through lactate shuttle coordinately influences tumor-stroma interplay. Cancer Res. 2012, 72, 5130–5140. [Google Scholar] [CrossRef]
- Owen, J.S.; Clayton, A.; Pearson, H.B. Cancer-Associated Fibroblast Heterogeneity, Activation and Function: Implications for Prostate Cancer. Biomolecules 2022, 13, 67. [Google Scholar] [CrossRef]
- Pistore, C.; Giannoni, E.; Colangelo, T.; Rizzo, F.; Magnani, E.; Muccillo, L.; Giurato, G.; Mancini, M.; Rizzo, S.; Riccardi, M.; et al. DNA methylation variations are required for epithelial-to-mesenchymal transition induced by cancer-associated fibroblasts in prostate cancer cells. Oncogene 2017, 36, 5551–5566. [Google Scholar] [CrossRef]
- Barron, D.A.; Rowley, D.R. The reactive stroma microenvironment and prostate cancer progression. Endocr. Relat. Cancer 2012, 19, R187–R204. [Google Scholar] [CrossRef]
- Xiong, Z.; Zhuang, R.L.; Yu, S.L.; Xie, Z.X.; Peng, S.R.; Li, Z.A.; Li, B.H.; Xie, J.J.; Li, Y.N.; Li, K.W.; et al. Cancer-associated fibroblasts regulate mitochondrial metabolism and inhibit chemosensitivity via ANGPTL4-IQGAP1 axis in prostate cancer. J. Adv. Res. 2024, 75, 663–678. [Google Scholar] [CrossRef]
- Qin, H.; Yang, Y.; Jiang, B.; Pan, C.; Chen, W.; Diao, W.; Ding, M.; Cao, W.; Zhang, Z.; Chen, M.; et al. SOX9 in prostate cancer is upregulated by cancer-associated fibroblasts to promote tumor progression through HGF/c-Met-FRA1 signaling. FEBS J. 2021, 288, 5406–5429. [Google Scholar] [CrossRef]
- Wang, S.; Du, P.; Cao, Y.; Ma, J.; Yang, X.; Yu, Z.; Yang, Y. Cancer associated fibroblasts secreted exosomal miR-1290 contributes to prostate cancer cell growth and metastasis via targeting GSK3β. Cell Death Discov. 2022, 8, 371. [Google Scholar] [CrossRef]
- Fernández-Hernando, C.; Suárez, Y. ANGPTL4: A multifunctional protein involved in metabolism and vascular homeostasis. Curr. Opin. Hematol. 2020, 27, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Hurley, P.J.; Simons, B.W.; Marchionni, L.; Berman, D.M.; Ross, A.E.; Schaeffer, E.M. Sox9 is required for prostate development and prostate cancer initiation. Oncotarget 2012, 3, 651–663. [Google Scholar] [CrossRef]
- Liu, J.; Ren, L.; Li, S.; Li, W.; Zheng, X.; Yang, Y.; Fu, W.; Yi, J.; Wang, J.; Du, G. The biology, function, and applications of exosomes in cancer. Acta Pharm. Sin. B 2021, 11, 2783–2797. [Google Scholar] [CrossRef]
- Morin, R.D.; O’Connor, M.D.; Griffith, M.; Kuchenbauer, F.; Delaney, A.; Prabhu, A.L.; Zhao, Y.; McDonald, H.; Zeng, T.; Hirst, M.; et al. Application of massively parallel sequencing to microRNA profiling and discovery in human embryonic stem cells. Genome Res. 2008, 18, 610–621. [Google Scholar] [CrossRef]
- Kalhori, M.R.; Soleimani, M.; Arefian, E.; Alizadeh, A.M.; Mansouri, K.; Echeverria, J. The potential role of miR-1290 in cancer progression, diagnosis, prognosis, and treatment: An oncomiR or onco-suppressor microRNA? J. Cell Biochem. 2022, 123, 506–531. [Google Scholar] [CrossRef]
- Lin, J.; Song, T.; Li, C.; Mao, W. GSK-3β in DNA repair, apoptosis, and resistance of chemotherapy, radiotherapy of cancer. Biochim. Biophys. Acta Mol. Cell Res. 2020, 1867, 118659. [Google Scholar] [CrossRef]
- Liu, Z.; Lin, Z.; Jiang, M.; Zhu, G.; Xiong, T.; Cao, F.; Cui, Y.; Niu, Y.N. Cancer-associated fibroblast exosomes promote prostate cancer metastasis through miR-500a-3p/FBXW7/HSF1 axis under hypoxic microenvironment. Cancer Gene Ther. 2024, 31, 698–709. [Google Scholar] [CrossRef]
- Ippolito, L.; Duatti, A.; Iozzo, M.; Comito, G.; Pardella, E.; Lorito, N.; Bacci, M.; Pranzini, E.; Santi, A.; Sandrini, G.; et al. Lactate supports cell-autonomous ECM production to sustain metastatic behavior in prostate cancer. EMBO Rep. 2024, 25, 3506–3531. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Chen, X.; Li, X.; Deng, C.; Luo, P. The Pro-Migratory and Pro-Invasive Roles of Cancer-Associated Fibroblasts Secreted IL-17A in Prostate Cancer. J. Biochem. Mol. Toxicol. 2025, 39, e70047. [Google Scholar] [CrossRef]
- Song, X.; Li, T.; Zhou, W.; Feng, C.; Zhou, Z.; Chen, Y.; Li, D.; Chen, L.; Zhao, J.; Zhang, Y.; et al. CAF-derived exosomal miR-196b-5p after androgen deprivation therapy promotes epithelial-mesenchymal transition in prostate cancer cells through HOXC8/NF-κB signaling pathway. Biol. Direct 2025, 20, 80. [Google Scholar] [CrossRef]
- Yanushko, D.; German Falcon, B.; El Bizri, R.; Pervizou, D.; Dolgos, R.; Keime, C.; Ye, T.; Thibault-Carpentier, C.; Le Magnen, C.; Henri, S.; et al. p53-loss induced prostatic epithelial cell plasticity and invasion is driven by a crosstalk with the tumor microenvironment. Cell Death Dis. 2025, 16, 46. [Google Scholar] [CrossRef] [PubMed]
- Booijink, R.; Terstappen, L.; Dathathri, E.; Isebia, K.; Kraan, J.; Martens, J.; Bansal, R. Identification of functional and diverse circulating cancer-associated fibroblasts in metastatic castration-naïve prostate cancer patients. Mol. Oncol. 2025, 19, 2074–2091. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Lafuente, N.; Alcaraz-García, M.J.; Sebastián-Ruiz, S.; García-Serna, A.M.; Gómez-Espuch, J.; Moraleda, J.M.; Minguela, A.; García-Alonso, A.M.; Parrado, A. IL-4 Up-Regulates MiR-21 and the MiRNAs Hosted in the CLCN5 Gene in Chronic Lymphocytic Leukemia. PLoS ONE 2015, 10, e0124936. [Google Scholar] [CrossRef]
- Kim, Y.S.; Park, S.J.; Lee, Y.S.; Kong, H.K.; Park, J.H. miRNAs involved in LY6K and estrogen receptor α contribute to tamoxifen-susceptibility in breast cancer. Oncotarget 2016, 7, 42261–42273. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Long, J.; Liu, B.; Xu, M.; Wang, W.; Xie, X.; Wang, X.; Kuang, M. miR-500a-3p promotes cancer stem cells properties via STAT3 pathway in human hepatocellular carcinoma. J. Exp. Clin. Cancer Res. 2017, 36, 99. [Google Scholar] [CrossRef]
- Lin, H.; Zhang, L.; Zhang, C.; Liu, P. Exosomal MiR-500a-3p promotes cisplatin resistance and stemness via negatively regulating FBXW7 in gastric cancer. J. Cell Mol. Med. 2020, 24, 8930–8941. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhang, L.; Wan, D.; Zhou, L.; Zheng, S.; Lin, S.; Qiao, Y. Extracellular matrix and its therapeutic potential for cancer treatment. Signal Transduct. Target. Ther. 2021, 6, 153. [Google Scholar] [CrossRef] [PubMed]
- Psychogios, N.; Hau, D.D.; Peng, J.; Guo, A.C.; Mandal, R.; Bouatra, S.; Sinelnikov, I.; Krishnamurthy, R.; Eisner, R.; Gautam, B.; et al. The human serum metabolome. PLoS ONE 2011, 6, e16957. [Google Scholar] [CrossRef]
- Rabinowitz, J.D.; Enerbäck, S. Lactate: The ugly duckling of energy metabolism. Nat. Metab. 2020, 2, 566–571. [Google Scholar] [CrossRef]
- Tian, Y.; Bai, F.; Zhang, D. New target DDR1: A “double-edged sword” in solid tumors. Biochim. Biophys. Acta Rev. Cancer 2023, 1878, 188829. [Google Scholar] [CrossRef]
- Castellón, E.A.; Indo, S.; Contreras, H.R. Cancer Stemness/Epithelial-Mesenchymal Transition Axis Influences Metastasis and Castration Resistance in Prostate Cancer: Potential Therapeutic Target. Int. J. Mol. Sci. 2022, 23, 14917. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, J.; Ding, M.; Su, Y.; Cui, D.; Jiang, C.; Zhao, S.; Jia, G.; Wang, X.; Ruan, Y.; et al. Loss of exosomal miR-146a-5p from cancer-associated fibroblasts after androgen deprivation therapy contributes to prostate cancer metastasis. J. Exp. Clin. Cancer Res. 2020, 39, 282. [Google Scholar] [CrossRef]
- Liang, G.; Meng, W.; Huang, X.; Zhu, W.; Yin, C.; Wang, C.; Fassan, M.; Yu, Y.; Kudo, M.; Xiao, S.; et al. miR-196b-5p-mediated downregulation of TSPAN12 and GATA6 promotes tumor progression in non-small cell lung cancer. Proc. Natl. Acad. Sci. USA 2020, 117, 4347–4357. [Google Scholar] [CrossRef] [PubMed]
- Stiegelbauer, V.; Vychytilova-Faltejskova, P.; Karbiener, M.; Pehserl, A.M.; Reicher, A.; Resel, M.; Heitzer, E.; Ivan, C.; Bullock, M.; Ling, H.; et al. miR-196b-5p Regulates Colorectal Cancer Cell Migration and Metastases through Interaction with HOXB7 and GALNT5. Clin. Cancer Res. 2017, 23, 5255–5266. [Google Scholar] [CrossRef]
- Özdemir, B.C.; Pentcheva-Hoang, T.; Carstens, J.L.; Zheng, X.; Wu, C.C.; Simpson, T.R.; Laklai, H.; Sugimoto, H.; Kahlert, C.; Novitskiy, S.V.; et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell 2014, 25, 719–734. [Google Scholar] [CrossRef]
- Calon, A.; Lonardo, E.; Berenguer-Llergo, A.; Espinet, E.; Hernando-Momblona, X.; Iglesias, M.; Sevillano, M.; Palomo-Ponce, S.; Tauriello, D.V.; Byrom, D.; et al. Stromal gene expression defines poor-prognosis subtypes in colorectal cancer. Nat. Genet. 2015, 47, 320–329. [Google Scholar] [CrossRef]
- Giannoni, E.; Bianchini, F.; Masieri, L.; Serni, S.; Torre, E.; Calorini, L.; Chiarugi, P. Reciprocal activation of prostate cancer cells and cancer-associated fibroblasts stimulates epithelial-mesenchymal transition and cancer stemness. Cancer Res. 2010, 70, 6945–6956. [Google Scholar] [CrossRef] [PubMed]
- Hamid, A.A.; Gray, K.P.; Shaw, G.; MacConaill, L.E.; Evan, C.; Bernard, B.; Loda, M.; Corcoran, N.M.; Van Allen, E.M.; Choudhury, A.D.; et al. Compound Genomic Alterations of TP53, PTEN, and RB1 Tumor Suppressors in Localized and Metastatic Prostate Cancer. Eur. Urol. 2019, 76, 89–97. [Google Scholar] [CrossRef]
- Lu, T.; Oomens, L.; Terstappen, L.; Prakash, J. In Vivo Detection of Circulating Cancer-Associated Fibroblasts in Breast Tumor Mouse Xenograft: Impact of Tumor Stroma and Chemotherapy. Cancers 2023, 15, 1127. [Google Scholar] [CrossRef] [PubMed]
- Ao, Z.; Shah, S.H.; Machlin, L.M.; Parajuli, R.; Miller, P.C.; Rawal, S.; Williams, A.J.; Cote, R.J.; Lippman, M.E.; Datar, R.H.; et al. Identification of Cancer-Associated Fibroblasts in Circulating Blood from Patients with Metastatic Breast Cancer. Cancer Res. 2015, 75, 4681–4687. [Google Scholar] [CrossRef]
- Cornford, P.; van den Bergh, R.C.N.; Briers, E.; Van den Broeck, T.; Cumberbatch, M.G.; De Santis, M.; Fanti, S.; Fossati, N.; Gandaglia, G.; Gillessen, S.; et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer. Part II-2020 Update: Treatment of Relapsing and Metastatic Prostate Cancer. Eur. Urol. 2021, 79, 263–282. [Google Scholar] [CrossRef]
- Li, S.; Zeng, A.; Hu, Q.; Yan, W.; Liu, Y.; You, Y. miR-423-5p contributes to a malignant phenotype and temozolomide chemoresistance in glioblastomas. Neuro Oncol. 2017, 19, 55–65. [Google Scholar] [CrossRef]
- Lin, J.; Huang, S.; Wu, S.; Ding, J.; Zhao, Y.; Liang, L.; Tian, Q.; Zha, R.; Zhan, R.; He, X. MicroRNA-423 promotes cell growth and regulates G(1)/S transition by targeting p21Cip1/Waf1 in hepatocellular carcinoma. Carcinogenesis 2011, 32, 1641–1647. [Google Scholar] [CrossRef]
- Sun, G.; Ding, X.; Bi, N.; Wu, L.; Wang, J.; Zhang, W.; Dong, X.; Lv, N.; Song, Y.; Zhan, Q.; et al. MiR-423-5p in brain metastasis: Potential role in diagnostics and molecular biology. Cell Death Dis. 2018, 9, 936. [Google Scholar] [CrossRef]
- Shan, G.; Gu, J.; Zhou, D.; Li, L.; Cheng, W.; Wang, Y.; Tang, T.; Wang, X. Cancer-associated fibroblast-secreted exosomal miR-423-5p promotes chemotherapy resistance in prostate cancer by targeting GREM2 through the TGF-β signaling pathway. Exp. Mol. Med. 2020, 52, 1809–1822. [Google Scholar] [CrossRef]
- Mou, Y.; Wang, J.; Wu, J.; He, D.; Zhang, C.; Duan, C.; Li, B. Ferroptosis, a new form of cell death: Opportunities and challenges in cancer. J. Hematol. Oncol. 2019, 12, 34. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Zhao, J.; Shen, J.; Mao, L.; Yang, T.; Liu, J.; Hongbin, S. Cancer associated fibroblast secreted miR-432-5p targets CHAC1 to inhibit ferroptosis and promote acquired chemoresistance in prostate cancer. Oncogene 2024, 43, 2104–2114. [Google Scholar] [CrossRef]
- Desai, K.; McManus, J.M.; Sharifi, N. Hormonal Therapy for Prostate Cancer. Endocr. Rev. 2021, 42, 354–373. [Google Scholar] [CrossRef]
- Avery, D.; Govindaraju, P.; Jacob, M.; Todd, L.; Monslow, J.; Puré, E. Extracellular matrix directs phenotypic heterogeneity of activated fibroblasts. Matrix Biol. 2018, 67, 90–106. [Google Scholar] [CrossRef]
- Dvorak, H.F. Tumors: Wounds that do not heal-redux. Cancer Immunol. Res. 2015, 3, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Fan, A.; Li, Y.; Liu, Z.; Yu, L.; Guo, J.; Hou, J.; Li, X.; Chen, W. Single-cell RNA sequencing reveals that HSD17B2 in cancer-associated fibroblasts promotes the development and progression of castration-resistant prostate cancer. Cancer Lett. 2023, 566, 216244. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, S.; Yang, J.; Cui, C.; Yu, M.; Zhang, Y. ITGBL1 promotes EMT, invasion and migration by activating NF-κB signaling pathway in prostate cancer. Onco Targets Ther. 2019, 12, 3753–3763. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, N.; Liu, Q.; Guo, J.; Pan, Q.; Cheng, B.; Xu, J.; Dong, B.; Yang, G.; Yang, B.; et al. Antiandrogen treatment induces stromal cell reprogramming to promote castration resistance in prostate cancer. Cancer Cell 2023, 41, 1345–1362.e1349. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, Y.Y.; Li, D.; Wang, C.; Wang, S.Y.; Shao, S.H.; Zhu, Z.Y.; Zhao, J.; Zhang, Y.; Ruan, Y.; et al. LMO2 upregulation due to AR deactivation in cancer-associated fibroblasts induces non-cell-autonomous growth of prostate cancer after androgen deprivation. Cancer Lett. 2021, 503, 138–150. [Google Scholar] [CrossRef]
- Armstrong, A.J.; Szmulewitz, R.Z.; Petrylak, D.P.; Holzbeierlein, J.; Villers, A.; Azad, A.; Alcaraz, A.; Alekseev, B.; Iguchi, T.; Shore, N.D.; et al. ARCHES: A Randomized, Phase III Study of Androgen Deprivation Therapy With Enzalutamide or Placebo in Men With Metastatic Hormone-Sensitive Prostate Cancer. J. Clin. Oncol. 2019, 37, 2974–2986. [Google Scholar] [CrossRef]
- Gao, L.; Zhang, W.; Zhang, J.; Liu, J.; Sun, F.; Liu, H.; Hu, J.; Wang, X.; Wang, X.; Su, P.; et al. KIF15-Mediated Stabilization of AR and AR-V7 Contributes to Enzalutamide Resistance in Prostate Cancer. Cancer Res. 2021, 81, 1026–1039. [Google Scholar] [CrossRef]
- Xiong, Z.; Yu, S.L.; Xie, Z.X.; Zhuang, R.L.; Peng, S.R.; Wang, Q.; Gao, Z.; Li, B.H.; Xie, J.J.; Huang, H.; et al. Cancer-associated fibroblasts promote enzalutamide resistance and PD-L1 expression in prostate cancer through CCL5-CCR5 paracrine axis. iScience 2024, 27, 109674. [Google Scholar] [CrossRef]
- Rhim, A.D.; Oberstein, P.E.; Thomas, D.H.; Mirek, E.T.; Palermo, C.F.; Sastra, S.A.; Dekleva, E.N.; Saunders, T.; Becerra, C.P.; Tattersall, I.W.; et al. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell 2014, 25, 735–747. [Google Scholar] [CrossRef]
- Merika, E.E.; Syrigos, K.N.; Saif, M.W. Desmoplasia in pancreatic cancer. Can we fight it? Gastroenterol. Res. Pract. 2012, 2012, 781765. [Google Scholar] [CrossRef]
- Kieffer, Y.; Hocine, H.R.; Gentric, G.; Pelon, F.; Bernard, C.; Bourachot, B.; Lameiras, S.; Albergante, L.; Bonneau, C.; Guyard, A.; et al. Single-Cell Analysis Reveals Fibroblast Clusters Linked to Immunotherapy Resistance in Cancer. Cancer Discov. 2020, 10, 1330–1351. [Google Scholar] [CrossRef]
- Mishra, P.J.; Mishra, P.J.; Humeniuk, R.; Medina, D.J.; Alexe, G.; Mesirov, J.P.; Ganesan, S.; Glod, J.W.; Banerjee, D. Carcinoma-associated fibroblast-like differentiation of human mesenchymal stem cells. Cancer Res. 2008, 68, 4331–4339. [Google Scholar] [CrossRef]
- Song, H.; Lu, T.; Han, D.; Zhang, J.; Gan, L.; Xu, C.; Liu, S.; Li, P.; Zhang, K.; Hu, Z.; et al. YAP1 Inhibition Induces Phenotype Switching of Cancer-Associated Fibroblasts to Tumor Suppressive in Prostate Cancer. Cancer Res. 2024, 84, 3728–3742. [Google Scholar] [CrossRef]
- Lamb, A.D.; Massie, C.E.; Neal, D.E. The transcriptional programme of the androgen receptor (AR) in prostate cancer. BJU Int. 2014, 113, 358–366. [Google Scholar] [CrossRef]
- Sahai, E.; Astsaturov, I.; Cukierman, E.; DeNardo, D.G.; Egeblad, M.; Evans, R.M.; Fearon, D.; Greten, F.R.; Hingorani, S.R.; Hunter, T.; et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat. Rev. Cancer 2020, 20, 174–186. [Google Scholar] [CrossRef]
- Talia, M.; Cesario, E.; Cirillo, F.; Scordamaglia, D.; Di Dio, M.; Zicarelli, A.; Mondino, A.A.; Occhiuzzi, M.A.; De Francesco, E.M.; Belfiore, A.; et al. Cancer-associated fibroblasts (CAFs) gene signatures predict outcomes in breast and prostate tumor patients. J. Transl. Med. 2024, 22, 597. [Google Scholar] [CrossRef]
- Wang, W.; Li, T.; Xie, Z.; Zhao, J.; Zhang, Y.; Ruan, Y.; Han, B. Integrating single-cell and bulk RNA sequencing data unveils antigen presentation and process-related CAFS and establishes a predictive signature in prostate cancer. J. Transl. Med. 2024, 22, 57. [Google Scholar] [CrossRef]
- Elyada, E.; Bolisetty, M.; Laise, P.; Flynn, W.F.; Courtois, E.T.; Burkhart, R.A.; Teinor, J.A.; Belleau, P.; Biffi, G.; Lucito, M.S.; et al. Cross-Species Single-Cell Analysis of Pancreatic Ductal Adenocarcinoma Reveals Antigen-Presenting Cancer-Associated Fibroblasts. Cancer Discov. 2019, 9, 1102–1123. [Google Scholar] [CrossRef] [PubMed]
- Weaver, A.; Bossaer, J.B. Fibroblast growth factor receptor (FGFR) inhibitors: A review of a novel therapeutic class. J. Oncol. Pharm. Pract. 2021, 27, 702–710. [Google Scholar] [CrossRef]
- Ji, X.; Qin, X.; Huang, X.; Wang, W.; Li, H.; Zheng, C.; Huang, Y. S100A11: A Potential Carcinogen and Prognostic Marker That Correlates with the Immunosuppressive Microenvironment in Pan-Cancer. J. Cancer 2023, 14, 88–98. [Google Scholar] [CrossRef]
- Han, D.; Guo, C.; Cheng, H.; Lu, J.; Hou, Z.; Zhang, X.; Luo, Y.; Zhang, B.; Zhao, W.; Shang, P. Downregulation of S100A11 promotes T cell infiltration by regulating cancer-associated fibroblasts in prostate cancer. Int. Immunopharmacol. 2024, 128, 111323. [Google Scholar] [CrossRef]
- Doldi, V.; Callari, M.; Giannoni, E.; D’Aiuto, F.; Maffezzini, M.; Valdagni, R.; Chiarugi, P.; Gandellini, P.; Zaffaroni, N. Integrated gene and miRNA expression analysis of prostate cancer associated fibroblasts supports a prominent role for interleukin-6 in fibroblast activation. Oncotarget 2015, 6, 31441–31460. [Google Scholar] [CrossRef]
- Lang, F.; Hoffmann, E.K. Role of ion transport in control of apoptotic cell death. Compr. Physiol. 2012, 2, 2037–2061. [Google Scholar] [CrossRef]
- Doldi, V.; Tortoreto, M.; Colecchia, M.; Maffezzini, M.; Percio, S.; Giammello, F.; Brandalise, F.; Gandellini, P.; Zaffaroni, N. Repositioning of antiarrhythmics for prostate cancer treatment: A novel strategy to reprogram cancer-associated fibroblasts towards a tumor-suppressive phenotype. J. Exp. Clin. Cancer Res. 2024, 43, 161. [Google Scholar] [CrossRef]
- Xin, L.; Gao, J.; Zheng, Z.; Chen, Y.; Lv, S.; Zhao, Z.; Yu, C.; Yang, X.; Zhang, R. Fibroblast Activation Protein-α as a Target in the Bench-to-Bedside Diagnosis and Treatment of Tumors: A Narrative Review. Front. Oncol. 2021, 11, 648187. [Google Scholar] [CrossRef]
- Chhabra, Y.; Weeraratna, A.T. Fibroblasts in cancer: Unity in heterogeneity. Cell 2023, 186, 1580–1609. [Google Scholar] [CrossRef]
- Eiro, N.; González, L.; Martínez-Ordoñez, A.; Fernandez-Garcia, B.; González, L.O.; Cid, S.; Dominguez, F.; Perez-Fernandez, R.; Vizoso, F.J. Cancer-associated fibroblasts affect breast cancer cell gene expression, invasion and angiogenesis. Cell Oncol. 2018, 41, 369–378. [Google Scholar] [CrossRef]
- Hensbergen, P.J.; Wijnands, P.G.; Schreurs, M.W.; Scheper, R.J.; Willemze, R.; Tensen, C.P. The CXCR3 targeting chemokine CXCL11 has potent antitumor activity in vivo involving attraction of CD8+ T lymphocytes but not inhibition of angiogenesis. J. Immunother. 2005, 28, 343–351. [Google Scholar] [CrossRef]
- Xu, Y.; Song, G.; Xie, S.; Jiang, W.; Chen, X.; Chu, M.; Hu, X.; Wang, Z.W. The roles of PD-1/PD-L1 in the prognosis and immunotherapy of prostate cancer. Mol. Ther. 2021, 29, 1958–1969. [Google Scholar] [CrossRef]
- Gallant, J.P.; Hintz, H.M.; Gunaratne, G.S.; Breneman, M.T.; Recchia, E.E.; West, J.L.; Ott, K.L.; Heninger, E.; Jackson, A.E.; Luo, N.Y.; et al. Mechanistic Characterization of Cancer-associated Fibroblast Depletion via an Antibody-Drug Conjugate Targeting Fibroblast Activation Protein. Cancer Res. Commun. 2024, 4, 1481–1494. [Google Scholar] [CrossRef]
- Xu, Y.; Ma, J.; Zheng, Q.; Wang, Y.; Hu, M.; Ma, F.; Qin, Z.; Lei, N.; Tao, N. MPSSS impairs the immunosuppressive function of cancer-associated fibroblasts via the TLR4-NF-κB pathway. Biosci. Rep. 2019, 39, BSR20182171. [Google Scholar] [CrossRef]
- Ma, J.; Xu, Y.; Zheng, Q.; Wang, Y.; Hu, M.; Ma, F.; Long, H.; Qin, Z.; Tao, N. Ligustilide inhibits the activation of cancer-associated fibroblasts. Life Sci. 2019, 218, 58–64. [Google Scholar] [CrossRef]
- Wong, C.; Stylianopoulos, T.; Cui, J.; Martin, J.; Chauhan, V.P.; Jiang, W.; Popovic, Z.; Jain, R.K.; Bawendi, M.G.; Fukumura, D. Multistage nanoparticle delivery system for deep penetration into tumor tissue. Proc. Natl. Acad. Sci. USA 2011, 108, 2426–2431. [Google Scholar] [CrossRef]
- Kim, J.; Jo, C.; Lim, W.G.; Jung, S.; Lee, Y.M.; Lim, J.; Lee, H.; Lee, J.; Kim, W.J. Programmed Nanoparticle-Loaded Nanoparticles for Deep-Penetrating 3D Cancer Therapy. Adv. Mater. 2018, 30, e1707557. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Chen, D.; Hao, L.; Tian, C.; Yan, Y.; Zhu, L.; Zhang, H.; Zhang, Y.; Zhang, Z. Transformable nanoparticles triggered by cancer-associated fibroblasts for improving drug permeability and efficacy in desmoplastic tumors. Nanoscale 2019, 11, 20030–20044. [Google Scholar] [CrossRef] [PubMed]
| Target | Inhibitor(s) | Effects on CAFs/TME | Reference |
|---|---|---|---|
| ANGPTL4-IQGAP1 | QGGP | Inhibits CAF-induced chemoresistance, enhances docetaxel sensitivity, promotes IQGAP1 degradation | [17] |
| HGF/c-Met | Capmatinib | Blocks CAF-induced SOX9 upregulation, inhibits tumor migration, invasion and stemness, reverses CAF pro-tumor effects | [18] |
| MEK1/2, ERK1/2 | U0126 (MEK1/2 Inhibitor), SCH772984 (ERK1/2 Inhibitor) | Inhibits HGF/CAF-induced SOX9 expression, reduces tumor invasion | [18] |
| miR-1290 | miR-1290 antagomir | Inhibits PCa cell migration, invasion, stemness and EMT, reverses CAF exosome pro-metastatic effect, activates GSK3β/β-catenin signaling | [19] |
| Exosome secretion | GW4869 | Blocks internalization of CAF exosomes by PCa cells, inhibits CAF exosome-mediated promotion of migration and invasion | [26] |
| MCT1 | AR-C155858 | Blocks lactate uptake from CAF secretion, inhibits P4HA1 activity and collagen deposition | [27] |
| P4HA1 | DHB | Inhibits collagen hydroxylation, reduces tumor cell invasion and lung colonization capacity | [27] |
| DDR1 | 7rh | Blocks collagen-DDR1 signaling axis, inhibits STAT3 phosphorylation, stemness and transendothelial migration | [27] |
| STAT3 | Stattic | Inhibits STAT3 phosphorylation, disrupts lactate-P4HA1-COL1-DDR1 positive feedback loop, reduces invasion and prostasphere formation | [27] |
| p38 MAPK | SB203580 | Inhibits p38 phosphorylation, reverses EMT and cell invasion | [28] |
| NF-κB | BAY 11-7082 | Blocks P65 nuclear translocation, reverses miR-196b-5p-induced EMT | [29] |
| JAK | Ruxolitinib | Inhibits STAT3 phosphorylation, blocks CAF-induced EMT | [30] |
| TGF-β | LY2109761 | Partially reverses CAF exosome-induced chemoresistance, inhibits PCa cell proliferation/colony formation, promotes apoptosis | [54] |
| ERK | GDC-0994 | Inhibits ERK downstream phosphorylation of p90RSK, blocks SPP1-mediated paracrine resistance, delays CRPC progression | [63] |
| IL-11RA | BMPP-11 | Targets IL11RA, inhibits PCa tumor growth | [64] |
| CCR5 | Maraviroc | Blocks CCL5-CCR5 axis, reduces AR/PD-L1 expression, enhances enzalutamide efficacy, inhibits tumor growth, reverses immune evasion | [67] |
| FGFR | Erdafitinib | Inhibits PI3K/AKT pathway, reduces CAF activity, combined with S100A11 knockdown enhances T-cell infiltration, reduces tumor volume | [80] |
| IL-8, GIF | Flecainide/Nifedipine | Reduces secretion of pro-metastatic factors IL-8 and GIF, reverses CAF-induced PCa cell migration and EMT | [83] |
| α-SMA | Ligustilide/MPSSS | Downregulates α-SMA expression, reduces CAF activity, reverses immunosuppressive phenotype | [90,91] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Z.; Shen, S.; Gu, C.; Li, S.; Qin, Y.; Mi, Y. Research Advances in Cancer-Associated Fibroblasts in Prostate Cancer Progression. Biomolecules 2025, 15, 1369. https://doi.org/10.3390/biom15101369
Tang Z, Shen S, Gu C, Li S, Qin Y, Mi Y. Research Advances in Cancer-Associated Fibroblasts in Prostate Cancer Progression. Biomolecules. 2025; 15(10):1369. https://doi.org/10.3390/biom15101369
Chicago/Turabian StyleTang, Zhonghao, Si Shen, Chenwei Gu, Sixin Li, Yan Qin, and Yuanyuan Mi. 2025. "Research Advances in Cancer-Associated Fibroblasts in Prostate Cancer Progression" Biomolecules 15, no. 10: 1369. https://doi.org/10.3390/biom15101369
APA StyleTang, Z., Shen, S., Gu, C., Li, S., Qin, Y., & Mi, Y. (2025). Research Advances in Cancer-Associated Fibroblasts in Prostate Cancer Progression. Biomolecules, 15(10), 1369. https://doi.org/10.3390/biom15101369







