Abstract
Since its discovery in the early 1980s, the epidermal growth factor receptor (EGFR) has emerged as a pivotal and multifaceted player in elucidating the intricate mechanisms underlying various human diseases and their associations with cell survival, proliferation, and cellular homeostasis. Recent advancements in research have underscored the profound and multifaceted role of EGFR in viral infections, highlighting its involvement in viral entry, replication, and the subversion of host immune responses. In this regard, the importance of EGFR trafficking has also been highlighted in recent studies. The dynamic relocation of EGFR to diverse intracellular organelles, including endosomes, lysosomes, mitochondria, and even the nucleus, is a central feature of its functionality in diverse contexts. This dynamic intracellular trafficking is not merely a passive process but an orchestrated symphony, facilitating EGFR involvement in various cellular pathways and interactions with viral components. Furthermore, EGFR, which is initially anchored on the plasma membrane, serves as a linchpin orchestrating viral entry processes, a crucial early step in the viral life cycle. The role of EGFR in this context is highly context-dependent and varies among viruses. Here, we present a comprehensive summary of the current state of knowledge regarding the intricate interactions between EGFR and viruses. These interactions are fundamental for successful propagation of a wide array of viral species and affect viral pathogenesis and host responses. Understanding EGFR significance in both normal cellular processes and viral infections may not only help develop innovative antiviral therapies but also provide a deeper understanding of the intricate roles of EGFR signaling in infectious diseases.
1. Introduction
The multifaceted role of the epidermal growth factor receptor (EGFR) is of paramount importance not only in understanding human diseases in relation to cell survival, proliferation, and cellular homeostasis [1,2,3] but also in the intricate realm of viral infections. In a recent study, most evidence supported the roles of EGFR in facilitating viral entry, replication, or escape from the host immune response in viral infections [4].
Viral infections begin with the binding of the viral particles to specific cellular receptors, setting in motion a complex cascade of intracellular events meticulously crafted to manipulate the host cell machinery, thereby facilitating viral replication [5]. The selection of these receptors plays a pivotal role in guiding viruses to their desired tissues and orchestrating successful traversal of cellular barriers, allowing the viral genome to infiltrate host cells and propagate with remarkable precision [6]. Notably, viruses recognize and engage EGFR, a prominent cell surface receptor, as an indispensable component of their strategy for host cell entry [7]. The central role of EGFR in orchestrating viral entry, replication, and the evasion of immune responses has become increasingly evident. In this regard, EGFR trafficking plays a pivotal role in virus–host interactions by allowing the translocation of EGFR into an array of intracellular organelles, including endosomes, lysosomes, mitochondria, and even the nucleus [8].
This review discusses the recent progress in understanding the pivotal role of EGFR in viral entry and its overarching function as a coordinator that governs viral replication. We summarize the current state of knowledge regarding the intricate interactions between EGFR and various viruses, and the interactions that are unequivocally indispensable for the successful propagation of these viral agents. We have also curated data for a spectrum of viruses that were tested with EGFR inhibitors in vitro or in vivo and summarized them in Table 1. Exploring the function of EGFR in viral infections can offer insights into the development of innovative antiviral strategies.

Table 1.
Role of EGFR in viral infection.
2. Structure and Properties of the EGFR Protein
EGFR (ERBB1/HER1; 170 kDa) belongs to the human ErbB family of receptor tyrosine kinases (RTKs), which are transmembrane receptors with three functional domains: an extracellular domain (ECD), a transmembrane domain, and an intracellular domain (ICD). The ECD consists of two ligand-binding domains (L1 and L2) and two cysteine-rich domains (CR1 and CR2). The ligands of EGFR are epidermal growth factor (EGR), amphiregulin (AR), transforming growth factor alpha (TGF-α), epiregulin (EREG), heparin-binding epidermal growth factor (HB-EGF), betacellulin (BTC), and epigen (EPGN); the binding of these ligands with the receptor induces conformational changes to trigger signaling. The ICD has a conserved cytoplasmic catalytic tyrosine kinase domain and multiple tyrosine residues that contain two lobes, an adenosine triphosphate (ATP)-binding site and a substrate-catalysis site [1,42,43]. The intrinsic tyrosine kinase activity of EGFR induces substrate phosphorylation or autophosphorylation to trigger EGFR signaling. EGFR is ubiquitously expressed in the epithelial layers of the lung, gut, and skin, and is related to several cancers that show overexpression and mutations in EGFR, such as non-small cell lung cancer (NSCLC), head and neck squamous cell carcinoma (HNSCC), melanoma, breast and ovarian cancers, and glioblastoma (GBM) [44,45,46,47,48]. EGFR also plays key roles in homeostatic regulation of cellular proliferation, inhibition of apoptosis, increased cell migration and differentiation, mucus production, activation of the inflammatory response, and maintenance of cell survival [49]. EGFR is located on the plasma membrane and is widely expressed in other intracellular organelles such as endosomes, mitochondria, the nucleus, and lysosomes [50].
3. Activation and Regulation of EGFR Signaling
EGFR activation induces dimerization and phosphorylation of the molecule, in which tyrosine-phosphorylated sites promote the activation of downstream signaling cascades and EGFR internalization. In downstream signaling, phosphorylated tyrosine residues of EGFR recruit specific adapters and activate effector proteins to trigger certain signaling molecules such as mitogen-activated protein kinases (MAPKs), Janus kinase (JAK)/signal transducer and activator of transcription (STAT), phospholipase C-gamma 1 (PLCγ1), and phosphatidylinositide 3-kinases (PI3Ks). Phosphorylation of tyrosine (Y) residues 1068, Y1086, Y1101, Y1148, Y703, Y845, and Y974 is responsible for the activation of EGFR functions [51,52]. During EGFR internalization, EGFR moves to endosomes and is subsequently recycled to the cell surface or trafficked to other intracellular organelles and the nucleus [53]. EGFR internalization also induces EGFR endocytosis in a clathrin-dependent or clathrin-independent manner [54,55]. The nuclear portion of EGFR is translocated with importin β1, which has a nuclear localization sequence (NLS) to activate DNA-dependent protein kinase or regulate the transcriptional activity of many important genes for cell proliferation and survival [54,55,56]. EGFR is also translocated into the mitochondria, which is the main source of ATP and reactive oxygen species generation, and subsequently regulates mitochondrial bioenergetics [57]. The overall EGFR signaling network orchestrates cell survival to control cellular homeostasis.
4. EGFR as a Receptor for Viral Entry
Viral infections are a recurring challenge to human health, with various viruses employing diverse strategies to invade host cells. Understanding the mechanisms underlying viral entry is essential for developing effective antiviral strategies. In the following section, we discuss the critical role of EGFR in the entry of both DNA and RNA viruses (Figure 1A).
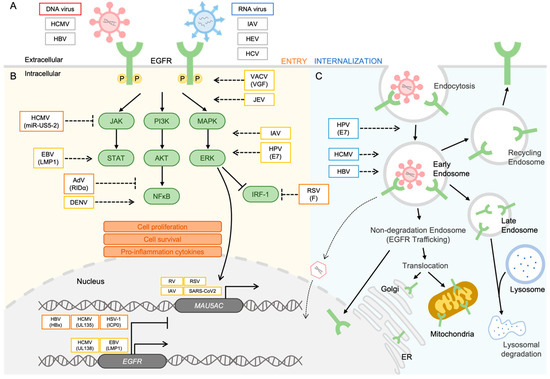
Figure 1.
Viruses modulate the EGFR signaling network. (A) For entry, gB of HCMV binds to EGFR and induces internalization of EGFR and virus particles. HBV-NTCB interacts with EGFR, facilitating the entry process. EGFR acts as a co-factor on the plasma membrane to facilitate host cell entry in IAV, HEV, and HCV infections. (B) For regulation of the EGFR signaling pathway and EGFR expression, accumulation of EGFR is observed in the nucleus. UL138 of HCMV and LMP1 of EBV induce upregulation of EGFR expression. Conversely, HBx of HBV, UL135 of HCMV, and ICP0 of HSV-1 suppress EGFR production. The expression of MUC5AC is increased by respiratory virus infections such as RV, SARS-CoV-2, IAV, and RSV. The phosphorylation of EGFR is triggered by VACV and JEV infection, and the MAPK/ERK pathway is activated by IAV and HPV-6 infection. IRF-1 is suppressed in RSV infection. In the PI3K/Akt pathway, DENV induces NF-κB activation, whereas the RIDα of AdV inhibits NF-κB. The miR-US5-2 of HCMV inhibits the activation of the Jak/STAT pathway, whereas LMP1 of EBV upregulates STAT activation. (C) For EGFR trafficking with viral proteins into intracellular organelles, E7 of HPV promotes the internalization of EGFR, and EGFR trafficking into endosomes is essential for HCMV and HBV replication. This figure was generated by using a drawing tool available in Microsoft PowerPoint version 2023.
4.1. DNA Viruses
Human cytomegalovirus (HCMV) causes severe and fatal diseases in immunocompromised individuals and is associated with atherosclerosis, coronary restenosis, and virus-induced birth defects. HCMV is an enveloped, linear, double-stranded DNA virus [58] that uses EGFR as a receptor to enter host cells and induce critical downstream steps in the viral life cycle. The ErbB family cDNAs transfected into Chinese hamster ovary cells shows critical binding of the HCMV envelope glycoprotein B (gB) to EGFR [9]. HCMV also establishes a latent infection and persistently infects myeloid-lineage cells [59]. CD34+ human progenitor cells (HPCs) are replication-restricted cells that are crucial reservoirs of HCMV latency [60]. EGFR signaling contributes to the successful establishment of viral latency during early events in CD34+ cells. HCMV entry, cellular trafficking, and nuclear translocation are mediated by the activation of downstream EGFR signaling in HPCs. The EGFR inhibitor AG1478 upregulates the expression of HCMV lytic IE1/IE2 mRNA, downregulates latency-associated UL138 mRNA, and alters the expression of cellular hematopoietic interleukin 12 (IL-12). EGFR also acts as a determinant in the selection of hematopoietic cells for HCMV latency tropism and is important in the early stages of successful HCMV infection [10].
Hepatitis B virus (HBV) is an enveloped virus with a circular DNA genome, and sodium taurocholate co-transporting polypeptide (NTCP) is required for HBV entry into cells [61,62]. Among the factors determining the susceptibility of cells to HBV infection, EGFR plays a critical role in inducing the internalization of HBV virions. Molecular interactions between NTCP and EGFR are important for supporting viral infection, and point mutations in NTCP and inactivation of EGFR can disrupt the NTCP–EGFR interaction. On the host cell surface, HBV attaches to EGFR-NTCP to promote the internalization of HBV virions that cross the plasma membrane of cells [11].
4.2. RNA Viruses
Influenza A viruses (IAVs) are enveloped viruses with single-stranded, negative-sense RNA genomes. Sialic acids are receptors for IAVs on cellular surfaces and facilitate the entry of viral particles via endocytic pathways [63]. EGFR also facilitates the uptake of IAVs through multivalent IAV binding to generate clusters of lipid rafts and activate EGFR signaling and facilitate IAV entry [12].
Hepatitis E virus (HEV) is a non-enveloped virus with a single-stranded, positive-sense RNA genome that causes acute viral hepatitis worldwide [64]. EGFR serves as a novel host receptor for HEV entry without affecting the viral RNA replication process to propagate the virus. Ectopic expression of EGFR shows a proviral role in supporting HEV entry into HepaRG cells and primary human hepatocytes [13].
Hepatitis C virus (HCV) is an enveloped virus with a single-stranded, positive-sense RNA genome that causes acute and chronic hepatitis related to hepatocellular carcinoma (HCC) and death [65,66]. A functional RNAi kinase screening assay identified EGFR and ephrin receptor A2 as the host co-factors for HCV infection. EGFR kinase inhibitors have shown antiviral activity against HCV infection in both cell culture and animal model systems [14]. The interaction between HCV and Cd81 induces EGFR activation. The internalization of EGFR facilitates the entry of HCV, which is enhanced by EGFR ligands. EGFR inhibition suppresses EGFR-mediated endocytosis during HCV infection [15].
6. Opposing Regulation by EGFR to Control Viral Infection
EGFR plays a pivotal role in the intricate interplay between viruses and host cells. It plays a dual, multifaceted role, functioning as both an enabler and a suppressor of viral infection, and its activity is often contingent on the specific viral state. These intricate functions rely on the precisely orchestrated regulation of EGFR activation, which can effectively toggle between various viral infection states, such as latency and reactivation, or profoundly affect discrete phases of the viral life cycle.
6.1. Opposite Roles of EGFR in Latency and Reactivation of Viral Infection
EGFR mediates opposing regulatory mechanisms to switch between the viral reactivation and the latent states [112]. HCMV latency and reactivation in CD34+ hematopoietic progenitor cells are regulated by HCMV microRNAs (miRNAs), small RNAs that decrease protein expression [113]. HCMV miR-US5-2 downregulates GAB1, an adapter protein that regulates EGFR-induced MEK/ERK signaling. The transcription factor early growth response gene 1 (EGR1), which is located downstream of EGFR-induced MEK/ERK signaling, also regulates the expression of the HCMV latency determinant UL138. HCMV miR-US5-2 is a critical modulator that targets GAB1, attenuates EGFR signaling pathways, and interferes with EGR1 and HCMV UL138 expression during HCMV infection [114]. Ultimately, attenuation of EGFR signaling induces HCMV reactivation from latency. HCMV coordinates with two viral genes, UL135 and UL138, to reactivate the lytic cycle from a latent state. HCMV UL135 is a critical factor in HCMV reactivation against the suppressive effector HCMV UL138, which performs the opposite function in regulating viral replication. UL135 and UL138 of HCMV target the same receptor, EGFR, to regulate the viral life cycle. Thus, HCMV UL138 induces EGFR expression and activation of the cell surface, while HCMV UL135 reduces EGFR expression on the host cell surface for reactivation. Inhibition of EGFR induces reactivation from latency and viral replication. Thus, the combination of UL135 or UL135 with EGFR represents a molecular switch that regulates the latency or reactivation of HCMV infection [35,112]. HCMV UL135 interacts with Src homology 3 (SH3) domain-containing kinase-binding protein 1 (SH3KBP1) and Abelson-interacting protein-1 (Abi-1), which have SH3 domains as host adapter proteins, and these protein complexes are required for regulating EGFR signaling in HCMV reactivation [115].
6.2. Opposite Roles of EGFR at Different Stages of Viral Infection
Activation of EGFR signaling occurs via virus binding and is immediately inhibited in the early stages of infection. HBV is an enveloped virus with a circular DNA genome, and NTCP is required for HBV entry [61,62]. In assessments of the susceptibility of cells to HBV infection, EGFR has been shown to be critical for inducing the internalization of HBV virions. Molecular interactions between NTCP and EGFR are important for supporting viral infection. Point mutations in NTCP and inactivation of EGFR disrupt the NTCP-EGFR interaction. On the host cell surface, HBV induces attachment to EGFR-NTCP to promote the internalization of HBV virions that cross the plasma membrane of cells [11]. Moreover, NTCP serves as a receptor for HBV entry, and EGFR is also involved in NTCP-mediated entry. Specifically, the EGFR endocytosis machinery is required for the internalization process in HBV entry, which involves the phosphorylation of EGFR and recruitment of adapter-related protein complex 2 subunit alpha 1 (AP2A1) and EGFR pathway substrate 15 (EPS15) as adapter molecules. The factors responsible for EGFR activation, such as the EGFR-sorting machinery, are involved in EGFR ubiquitination, such as the signal-transducing adapter molecule (STAM) and lysosome-associated protein transmembrane 4 beta (LAPTM4B), and promote the localization of HBV preS1 along with EGFR transport from endosomes to lysosomes. In late endosomes, EGFR transport is essential for promoting productive HBV infections [38]. In contrast, after HBV infection, such as the stages after viral entry, EGFR is one of the major targets of HBV-encoded X (HBx) to control cell growth. HCC is associated with HBV infection through the expression of the HBx protein to regulate hepatocarcinogenesis. HBx increases miR-7 expression, which targets EGFR mRNA to decrease the expression of EGFR and induces slow cell growth in HCC. HBx-miR-7-EGFR regulation is critical for controlling the growth rate of HCC cells [39].
Herpes simplex virus type 1 (HSV-1) is a common pathogen causing cold sores that progress to herpes keratitis and herpes simplex encephalitis, and also causes serious disease in transplant recipients. HSV-1 is an enveloped virus that contains double-stranded DNA. HSV-1 establishes latency in sensory neurons and can be reactivated to produce progeny virions in epithelial cells [116,117]. For HSV-1 infection into neuronal cells, reorganization of the actin cytoskeleton is essential to promote the entry of HSV-1 into neuronal cells. F-actin and cofilin regulate the efficacy of HSV-1 entry. The initial activation of the EGFR signaling pathway by binding to HSV-1 induces F-actin polymerization and cofilin phosphorylation. EGFR suppresses the infectivity of HSV-1 without affecting viral binding to the cells [40]. In contrast, infected cell protein 0 (ICP0) of HSV-1 encodes an SH3 domain-binding site, which is essential for the downregulation of EGFR. Both the surface levels and total EGFR expression decrease during HSV-1 infection [41].
EGFR is also downregulated in the early stages of human adenovirus (HAdV) infection. Specifically, E3 of human group C adenoviruses contributes to EGFR downregulation [118,119]. HAdV is a non-enveloped virus with linear double-stranded DNA [120]. In adenovirus pathogenesis, the early transcription region 3 (E3) of the adenovirus, E3-13.7, is an integral membrane protein that associates with EGFR to alter EGFR trafficking. The residues 675–697 of EGFR include lysosomal sorting signals, which are required for E3-13.7-induced downregulation in early endosomes. E3-13.7 and the EGFR complex are located in the early endocytic compartments and then dissociate, promoting the recycling of EGFR and the retention of E3-13.7 in endosomes [121]. Adenovirus infection triggers the stress-induced EGFR trafficking pathway, which activates the host innate immune response. The E3 RIDα protein is encoded by group C adenoviruses, which promotes the downregulation of the EGFR/NFκB signaling pathway. Interestingly, stress-induced pathways of EGFR trafficking are related to severe disease, which depend on specific adenovirus serotypes without conserved RIDα [122].
The expression and functionality of EGFR have also been determined in monocytic leukemic cell lines and macrophage subpopulations [123]. HCMV infects peripheral blood monocytes, which mediate the transfer of virus particles to latency sites such as the bone marrow. Monocytes are critical for understanding HCMV pathogenesis in the host. For productive HCMV propagation, the ability of the virus to cross the cell membrane and translocate viral DNA into the nucleus is crucial for replication of the HCMV genome [124]. HCMV infection promotes the activation of EGFR signaling, which is required for entry into monocytes and stimulates cell movement. The actin nucleator neural Wiskott–Aldrich syndrome protein (N-WASP) normally controls actin growth in leukocytes. However, upon viral infection, activated EGFR induces the stimulation of highly activated N-WASP to promote cell movement [125,126], while N-WASP knockdown inhibits HCMV-induced monocyte motility. The inhibition of EGFR activation can suppress viral entry into monocytes, and EGFR activation plays a key role in mediating viral entry into monocytes and promoting viral spread during HCMV pathogenesis [36]. In the post-entry steps of HCMV infections, EGFR kinase activity regulates the subcellular localization of the viral particles in monocytes. Activation of EGFR signaling by inducing HCMV gB-EGFR internalization is required for the translocation of viral DNA into the host nucleus and productive HCMV infection [37]. However, the role of EGFR in HCMV infection is controversial and depends on the virus strain or cell line specificity [9,127,128]. Downregulation of EGFR expression is a characteristic of HCMV infection. HCMV early gene products are necessary to suppress EGFR expression [128]. HCMV infection mediates the upregulation of Wilms’ tumor 1 (WT1) protein, a transcription factor associated with the negative regulation of EGFR. The binding of WT1 to the EGFR promoter also increases during HCMV infection, while depletion of WT1 suppresses HCMV-induced negative regulation of EGFR. HCMV-induced WT1 acts as a negative regulator to maintain the reduction in EGFR mRNA levels [129].
7. Discussion
We assessed the existing knowledge regarding the interactions between EGFR and various viruses, which play a crucial role in the efficient propagation of these viruses. Additionally, we curated well-defined data regarding the relationships of multiple viral species with EGFR, as depicted in Figure 1, and proposed a schematic representation to elucidate the role of EGFR in viral infections. As summarized in this review, both DNA and RNA viruses harness the EGFR protein in a proviral capacity. For example, the Zaire Ebola virus (ZEBOV) activates the PI3K pathway to facilitate its entry into host cells. ZEBOV is notorious for inducing severe hemorrhagic fever with a 90% mortality rate in humans, representing a formidable public health concern. Thus, these observations underscore the potentially pivotal role of EGFR in severe infections by viruses such as ZEBOV [114,130,131]. The significance of EGFR dynamics is not limited to human viruses but extends to animal viruses as well. Two examples of animal viruses, namely, Bovine Parainfluenza Virus 3 (BPIV3) and Transmissible Gastroenteritis Virus (TGEV), also utilize the EGFR signaling pathways for host cell entry. BPIV3 is a notable respiratory virus in cattle, and its entry into Madin–Darby Bovine kidney (MDBK) cells is mediated by the clathrin-dependent endocytosis pathway, which can be inhibited by an EGFR inhibitor. Activation of EGFR signaling leads to an increase in viral infectivity during the entry process. Furthermore, EGFR inhibition represses the rearrangement of the F-actin cytoskeleton. TGEV is associated with severe diarrhea in newborn piglets, leading to high mortality rates. EGFR interacts with the spike protein of TGEV, facilitating the formation of the EGFR and aminopeptidase N (the receptor for TGEV) complex, which accelerates TGEV entry into host cells [132,133]. Having explored the pivotal role of EGFR in diverse viral infections, we suggest that the growing emphasis on its significance positions EGFR as a promising candidate for future antiviral development.
Author Contributions
S.S.N. and H.J.S. conceptualized and wrote the manuscript. S.S.N. drew the figures and summarized the data in the tables. H.J.S. guided and supervised the study. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Research Fund of the Chungnam National University.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Correction Statement
The article has been published with a minor correction to the references 9-133. This change does not affect the scientific content of the article.
References
- Downward, J.; Yarden, Y.; Mayes, E.; Scrace, G.; Totty, N.; Stockwell, P.; Ullrich, A.; Schlessinger, J.; Waterfield, M.D. Close similarity of epidermal growth factor receptor and v-erb-B oncogene protein sequences. Nature 1984, 307, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Dunn, G.P.; Rinne, M.L.; Wykosky, J.; Genovese, G.; Quayle, S.N.; Dunn, I.F.; Agarwalla, P.K.; Chheda, M.G.; Campos, B.; Wang, A.; et al. Emerging insights into the molecular and cellular basis of glioblastoma. Gene Dev. 2012, 26, 756–784. [Google Scholar] [CrossRef] [PubMed]
- Wykosky, J.; Fenton, T.; Furnari, F.; Cavenee, W.K. Therapeutic targeting of epidermal growth factor receptor in human cancer: Successes and limitations. Chin. J. Cancer 2011, 30, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Kitazato, K.; Wang, Y. Viruses exploit the function of epidermal growth factor receptor. Rev. Med. Virol. 2014, 24, 274–286. [Google Scholar] [CrossRef] [PubMed]
- Grove, J.; Marsh, M. The cell biology of receptor-mediated virus entry. J. Cell Biol. 2011, 195, 1071–1082. [Google Scholar] [CrossRef]
- Marsh, M.; Helenius, A. Virus entry: Open sesame. Cell 2006, 124, 729–740. [Google Scholar] [CrossRef]
- Lai, K.M.; Lee, W.L. The roles of epidermal growth factor receptor in viral infections. Growth Factors 2022, 40, 46–72. [Google Scholar] [CrossRef]
- Li, H.; You, L.; Xie, J.; Pan, H.; Han, W. The roles of subcellularly located EGFR in autophagy. Cell Signal 2017, 35, 223–230. [Google Scholar] [CrossRef]
- Wang, X.; Huong, S.M.; Chiu, M.L.; Raab-Traub, N.; Huang, E.S. Epidermal growth factor receptor is a cellular receptor for human cytomegalovirus. Nature 2003, 424, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Collins-McMillen, D.; Buehler, J.C.; Goodrum, F.D.; Yurochko, A.D. Human Cytomegalovirus Requires Epidermal Growth Factor Receptor Signaling to Enter and Initiate the Early Steps in the Establishment of Latency in CD34(+) Human Progenitor Cells. J. Virol. 2017, 91, e01206-16. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, M.; Saso, W.; Sugiyama, R.; Ishii, K.; Ohki, M.; Nagamori, S.; Suzuki, R.; Aizaki, H.; Ryo, A.; Yun, J.H.; et al. Epidermal growth factor receptor is a host-entry cofactor triggering hepatitis B virus internalization. Proc. Natl. Acad. Sci. USA 2019, 116, 8487–8492. [Google Scholar] [CrossRef]
- Eierhoff, T.; Hrincius, E.R.; Rescher, U.; Ludwig, S.; Ehrhardt, C. The epidermal growth factor receptor (EGFR) promotes uptake of influenza A viruses (IAV) into host cells. PLoS Pathog. 2010, 6, e1001099. [Google Scholar] [CrossRef] [PubMed]
- Schrader, J.A.; Burkard, T.L.; Bruggemann, Y.; Gomer, A.; Meister, T.L.; Fu, R.M.; Mehnert, A.K.; Dao Thi, V.L.; Behrendt, P.; Durantel, D.; et al. EGF receptor modulates HEV entry in human hepatocytes. Hepatology 2023, 77, 2104–2117. [Google Scholar] [CrossRef] [PubMed]
- Lupberger, J.; Zeisel, M.B.; Xiao, F.; Thumann, C.; Fofana, I.; Zona, L.; Davis, C.; Mee, C.J.; Turek, M.; Gorke, S.; et al. EGFR and EphA2 are host factors for hepatitis C virus entry and possible targets for antiviral therapy. Nat. Med. 2011, 17, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Diao, J.; Pantua, H.; Ngu, H.; Komuves, L.; Diehl, L.; Schaefer, G.; Kapadia, S.B. Hepatitis C virus induces epidermal growth factor receptor activation via CD81 binding for viral internalization and entry. J. Virol. 2012, 86, 10935–10949. [Google Scholar] [CrossRef] [PubMed]
- Kung, C.P.; Meckes, D.G., Jr.; Raab-Traub, N. Epstein-Barr virus LMP1 activates EGFR, STAT3, and ERK through effects on PKCdelta. J. Virol. 2011, 85, 4399–4408. [Google Scholar] [CrossRef]
- Xu, Y.; Shi, Y.; Yuan, Q.; Liu, X.; Yan, B.; Chen, L.; Tao, Y.; Cao, Y. Epstein-Barr Virus encoded LMP1 regulates cyclin D1 promoter activity by nuclear EGFR and STAT3 in CNE1 cells. J. Exp. Clin. Cancer Res. 2013, 32, 90. [Google Scholar] [CrossRef]
- Langhammer, S.; Koban, R.; Yue, C.; Ellerbrok, H. Inhibition of poxvirus spreading by the anti-tumor drug Gefitinib (Iressa). Antivir. Res. 2011, 89, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Postigo, A.; Martin, M.C.; Dodding, M.P.; Way, M. Vaccinia-induced epidermal growth factor receptor-MEK signalling and the anti-apoptotic protein F1L synergize to suppress cell death during infection. Cell. Microbiol. 2009, 11, 1208–1218. [Google Scholar] [CrossRef]
- Beerli, C.; Yakimovich, A.; Kilcher, S.; Reynoso, G.V.; Flaschner, G.; Muller, D.J.; Hickman, H.D.; Mercer, J. Vaccinia virus hijacks EGFR signalling to enhance virus spread through rapid and directed infected cell motility. Nat. Microbiol. 2019, 4, 216–225. [Google Scholar] [CrossRef]
- Wang, Q.; Pan, W.; Wang, S.; Pan, C.; Ning, H.; Huang, S.; Chiu, S.H.; Chen, J.L. Protein Tyrosine Phosphatase SHP2 Suppresses Host Innate Immunity against Influenza A Virus by Regulating EGFR-Mediated Signaling. J. Virol. 2021, 95, e02001-20. [Google Scholar] [CrossRef]
- Barbier, D.; Garcia-Verdugo, I.; Pothlichet, J.; Khazen, R.; Descamps, D.; Rousseau, K.; Thornton, D.; Si-Tahar, M.; Touqui, L.; Chignard, M.; et al. Influenza A induces the major secreted airway mucin MUC5AC in a protease-EGFR-extracellular regulated kinase-Sp1-dependent pathway. Am. J. Respir. Cell Mol. Biol. 2012, 47, 149–157. [Google Scholar] [CrossRef]
- Kalinowski, A.; Ueki, I.; Min-Oo, G.; Ballon-Landa, E.; Knoff, D.; Galen, B.; Lanier, L.L.; Nadel, J.A.; Koff, J.L. EGFR activation suppresses respiratory virus-induced IRF1-dependent CXCL10 production. Am. J. Physiol. Lung Cell. Mol. Physiol. 2014, 307, L186–L196. [Google Scholar] [CrossRef]
- Monick, M.M.; Cameron, K.; Staber, J.; Powers, L.S.; Yarovinsky, T.O.; Koland, J.G.; Hunninghake, G.W. Activation of the epidermal growth factor receptor by respiratory syncytial virus results in increased inflammation and delayed apoptosis. J. Biol. Chem. 2005, 280, 2147–2158. [Google Scholar] [CrossRef]
- Kalinowski, A.; Galen, B.T.; Ueki, I.F.; Sun, Y.; Mulenos, A.; Osafo-Addo, A.; Clark, B.; Joerns, J.; Liu, W.; Nadel, J.A.; et al. Respiratory syncytial virus activates epidermal growth factor receptor to suppress interferon regulatory factor 1-dependent interferon-lambda and antiviral defense in airway epithelium. Mucosal Immunol. 2018, 11, 958–967. [Google Scholar] [CrossRef] [PubMed]
- Currier, M.G.; Lee, S.; Stobart, C.C.; Hotard, A.L.; Villenave, R.; Meng, J.; Pretto, C.D.; Shields, M.D.; Nguyen, M.T.; Todd, S.O.; et al. EGFR Interacts with the Fusion Protein of Respiratory Syncytial Virus Strain 2-20 and Mediates Infection and Mucin Expression. PLoS Pathog. 2016, 12, e1005622. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Lee, P.K.; Lee, W.M.; Zhao, Y.; Yu, D.; Chen, Y. Rhinovirus-induced major airway mucin production involves a novel TLR3-EGFR-dependent pathway. Am. J. Respir. Cell Mol. Biol. 2009, 40, 610–619. [Google Scholar] [CrossRef]
- Hewson, C.A.; Haas, J.J.; Bartlett, N.W.; Message, S.D.; Laza-Stanca, V.; Kebadze, T.; Caramori, G.; Zhu, J.; Edbrooke, M.R.; Stanciu, L.A.; et al. Rhinovirus induces MUC5AC in a human infection model and in vitro via NF-kappaB and EGFR pathways. Eur. Respir. J. 2010, 36, 1425–1435. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Gualano, R.C.; Hibbs, M.L.; Anderson, G.P.; Bozinovski, S. Epidermal growth factor receptor signaling to Erk1/2 and STATs control the intensity of the epithelial inflammatory responses to rhinovirus infection. J. Biol. Chem. 2008, 283, 9977–9985. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.G.; Chen, H.W.; Zhang, H.X.; Wang, K.; Su, J.; Chen, Y.R.; Wang, X.R.; Fu, Z.F.; Cui, M. EGFR Activation Impairs Antiviral Activity of Interferon Signaling in Brain Microvascular Endothelial Cells During Japanese Encephalitis Virus Infection. Front. Microbiol. 2022, 13, 894356. [Google Scholar] [CrossRef] [PubMed]
- Venkataraman, T.; Coleman, C.M.; Frieman, M.B. Overactive Epidermal Growth Factor Receptor Signaling Leads to Increased Fibrosis after Severe Acute Respiratory Syndrome Coronavirus Infection. J. Virol. 2017, 91, e00182-17. [Google Scholar] [CrossRef]
- Kato, T.; Asakura, T.; Edwards, C.E.; Dang, H.; Mikami, Y.; Okuda, K.; Chen, G.; Sun, L.; Gilmore, R.C.; Hawkins, P.; et al. Prevalence and Mechanisms of Mucus Accumulation in COVID-19 Lung Disease. Am. J. Respir. Crit. Care Med. 2022, 206, 1336–1352. [Google Scholar] [CrossRef] [PubMed]
- Duran, A.; Valero, N.; Mosquera, J.; Fuenmayor, E.; Alvarez-Mon, M. Gefitinib and pyrrolidine dithiocarbamate decrease viral replication and cytokine production in dengue virus infected human monocyte cultures. Life Sci. 2017, 191, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Chuang, F.K.; Liao, C.L.; Hu, M.K.; Chiu, Y.L.; Lee, A.R.; Huang, S.M.; Chiu, Y.L.; Tsai, P.L.; Su, B.C.; Chang, T.H.; et al. Antiviral Activity of Compound L3 against Dengue and Zika Viruses In Vitro and In Vivo. Int. J. Mol. Sci. 2020, 21, 4050. [Google Scholar] [CrossRef] [PubMed]
- Umashankar, M.; Rak, M.; Bughio, F.; Zagallo, P.; Caviness, K.; Goodrum, F.D. Antagonistic determinants controlling replicative and latent states of human cytomegalovirus infection. J. Virol. 2014, 88, 5987–6002. [Google Scholar] [CrossRef] [PubMed]
- Chan, G.; Nogalski, M.T.; Yurochko, A.D. Activation of EGFR on monocytes is required for human cytomegalovirus entry and mediates cellular motility. Proc. Natl. Acad. Sci. USA 2009, 106, 22369–22374. [Google Scholar] [CrossRef] [PubMed]
- Fulkerson, H.L.; Chesnokova, L.S.; Kim, J.H.; Mahmud, J.; Frazier, L.E.; Chan, G.C.; Yurochko, A.D. HCMV-induced signaling through gB-EGFR engagement is required for viral trafficking and nuclear translocation in primary human monocytes. Proc. Natl. Acad. Sci. USA 2020, 117, 19507–19516. [Google Scholar] [CrossRef]
- Iwamoto, M.; Saso, W.; Nishioka, K.; Ohashi, H.; Sugiyama, R.; Ryo, A.; Ohki, M.; Yun, J.H.; Park, S.Y.; Ohshima, T.; et al. The machinery for endocytosis of epidermal growth factor receptor coordinates the transport of incoming hepatitis B virus to the endosomal network. J. Biol. Chem. 2020, 295, 800–807. [Google Scholar] [CrossRef]
- Chen, Y.J.; Chien, P.H.; Chen, W.S.; Chien, Y.F.; Hsu, Y.Y.; Wang, L.Y.; Chen, J.Y.; Lin, C.W.; Huang, T.C.; Yu, Y.L.; et al. Hepatitis B Virus-Encoded X Protein Downregulates EGFR Expression via Inducing MicroRNA-7 in Hepatocellular Carcinoma Cells. Evid. Based Complement. Alternat. Med. 2013, 2013, 682380. [Google Scholar] [CrossRef]
- Zheng, K.; Xiang, Y.; Wang, X.; Wang, Q.; Zhong, M.; Wang, S.; Wang, X.; Fan, J.; Kitazato, K.; Wang, Y. Epidermal growth factor receptor-PI3K signaling controls cofilin activity to facilitate herpes simplex virus 1 entry into neuronal cells. mBio 2014, 5, e00958-00913. [Google Scholar] [CrossRef]
- Liang, Y.; Kurakin, A.; Roizman, B. Herpes simplex virus 1 infected cell protein 0 forms a complex with CIN85 and Cbl and mediates the degradation of EGF receptor from cell surfaces. Proc. Natl. Acad. Sci. USA 2005, 102, 5838–5843. [Google Scholar] [CrossRef] [PubMed]
- Mitsudomi, T.; Yatabe, Y. Epidermal growth factor receptor in relation to tumor development: EGFR gene and cancer. FEBS J. 2010, 277, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.R.; Wolf, E. The Epidermal Growth Factor Receptor Ligands at a Glance. J. Cell. Physiol. 2009, 218, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Uribe, M.L.; Marrocco, I.; Yarden, Y. EGFR in Cancer: Signaling Mechanisms, Drugs, and Acquired Resistance. Cancers 2021, 13, 2748. [Google Scholar] [CrossRef]
- Seshacharyulu, P.; Ponnusamy, M.P.; Haridas, D.; Jain, M.; Ganti, A.K.; Batra, S.K. Targeting the EGFR signaling pathway in cancer therapy. Expert Opin. Ther. Targets 2012, 16, 15–31. [Google Scholar] [CrossRef]
- Sharma, S.V.; Bell, D.W.; Settleman, J.; Haber, D.A. Epidermal growth factor receptor mutations in lung cancer. Nat. Rev. Cancer 2007, 7, 169–181. [Google Scholar] [CrossRef]
- Sigismund, S.; Avanzato, D.; Lanzetti, L. Emerging functions of the EGFR in cancer. Mol. Oncol. 2018, 12, 3–20. [Google Scholar] [CrossRef]
- Levantini, E.; Maroni, G.; Del Re, M.; Tenen, D.G. EGFR signaling pathway as therapeutic target in human cancers. Semin. Cancer Biol. 2022, 85, 253–275. [Google Scholar] [CrossRef]
- Yarden, Y.; Sliwkowski, M.X. Untangling the ErbB signalling network. Nat. Rev. Mol. Cell Biol. 2001, 2, 127–137. [Google Scholar] [CrossRef]
- Han, W.; Lo, H.W. Landscape of EGFR signaling network in human cancers: Biology and therapeutic response in relation to receptor subcellular locations. Cancer Lett. 2012, 318, 124–134. [Google Scholar] [CrossRef]
- Yewale, C.; Baradia, D.; Vhora, I.; Patil, S.; Misra, A. Epidermal growth factor receptor targeting in cancer: A review of trends and strategies. Biomaterials 2013, 34, 8690–8707. [Google Scholar] [CrossRef] [PubMed]
- Foley, J.; Nickerson, N.K.; Nam, S.; Allen, K.T.; Gilmore, J.L.; Nephew, K.P.; Riese, D.J., 2nd. EGFR signaling in breast cancer: Bad to the bone. Semin. Cell Dev. Biol. 2010, 21, 951–960. [Google Scholar] [CrossRef] [PubMed]
- Eden, E.R.; Huang, F.; Sorkin, A.; Futter, C.E. The role of EGF receptor ubiquitination in regulating its intracellular traffic. Traffic 2012, 13, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.C.; Hung, M.C. Characterization of a novel tripartite nuclear localization sequence in the EGFR family. J. Biol. Chem. 2007, 282, 10432–10440. [Google Scholar] [CrossRef] [PubMed]
- Dittmann, K.; Mayer, C.; Rodemann, H.P. Inhibition of radiation-induced EGFR nuclear import by C225 (Cetuximab) suppresses DNA-PK activity. Radiother. Oncol. 2005, 76, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Jaganathan, S.; Yue, P.; Paladino, D.C.; Bogdanovic, J.; Huo, Q.; Turkson, J. A functional nuclear epidermal growth factor receptor, SRC and Stat3 heteromeric complex in pancreatic cancer cells. PLoS ONE 2011, 6, e19605. [Google Scholar] [CrossRef] [PubMed]
- Bollu, L.R.; Ren, J.; Blessing, A.M.; Katreddy, R.R.; Gao, G.; Xu, L.; Wang, J.; Su, F.; Weihua, Z. Involvement of de novo synthesized palmitate and mitochondrial EGFR in EGF induced mitochondrial fusion of cancer cells. Cell Cycle 2014, 13, 2415–2430. [Google Scholar] [CrossRef]
- Gibson, W. Molecular biology of human cytomegalovirus. In Molecular Aspects of Human Cytomegalovirus Diseases; Springer: Berlin/Heidelberg, Germany, 1993; pp. 303–329. [Google Scholar]
- Goodrum, F.; Caviness, K.; Zagallo, P. Human cytomegalovirus persistence. Cell. Microbiol. 2012, 14, 644–655. [Google Scholar] [CrossRef]
- Mendelson, M.; Monard, S.; Sissons, P.; Sinclair, J. Detection of endogenous human cytomegalovirus in CD34+ bone marrow progenitors. J. Gen. Virol. 1996, 77 Pt 12, 3099–3102. [Google Scholar] [CrossRef]
- Seeger, C.; Mason, W.S. Molecular biology of hepatitis B virus infection. Virology 2015, 479–480, 672–686. [Google Scholar] [CrossRef]
- Le Seyec, J.; Chouteau, P.; Cannie, I.; Guguen-Guillouzo, C.; Gripon, P. Infection process of the hepatitis B virus depends on the presence of a defined sequence in the pre-S1 domain. J. Virol. 1999, 73, 2052–2057. [Google Scholar] [CrossRef] [PubMed]
- Lakadamyali, M.; Rust, M.J.; Zhuang, X. Endocytosis of influenza viruses. Microbes Infect. 2004, 6, 929–936. [Google Scholar] [CrossRef]
- Batts, W.; Yun, S.; Hedrick, R.; Winton, J. A novel member of the family Hepeviridae from cutthroat trout (Oncorhynchus clarkii). Virus Res. 2011, 158, 116–123. [Google Scholar] [CrossRef] [PubMed]
- De Francesco, R.; Tomei, L.; Altamura, S.; Summa, V.; Migliaccio, G. Approaching a new era for hepatitis C virus therapy: Inhibitors of the NS3-4A serine protease and the NS5B RNA-dependent RNA polymerase. Antivir. Res. 2003, 58, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Berenguer, M.; Lopez-Labrador, F.X.; Wright, T.L. Hepatitis C and liver transplantation. J. Hepatol. 2001, 35, 666–678. [Google Scholar] [CrossRef] [PubMed]
- Rickinson, A.B. Epstein-Barr virus in action in vivo. N. Engl. J. Med. 1998, 338, 1461–1463. [Google Scholar] [CrossRef]
- Johannsen, E.; Luftig, M.; Chase, M.R.; Weicksel, S.; Cahir-McFarland, E.; Illanes, D.; Sarracino, D.; Kieff, E. Proteins of purified Epstein-Barr virus. Proc. Natl. Acad. Sci. USA 2004, 101, 16286–16291. [Google Scholar] [CrossRef]
- Kaye, K.M.; Izumi, K.M.; Li, H.; Johannsen, E.; Davidson, D.; Longnecker, R.; Kieff, E. An Epstein-Barr virus that expresses only the first 231 LMP1 amino acids efficiently initiates primary B-lymphocyte growth transformation. J. Virol. 1999, 73, 10525–10530. [Google Scholar] [CrossRef]
- Wang, D.; Liebowitz, D.; Kieff, E. An EBV membrane protein expressed in immortalized lymphocytes transforms established rodent cells. Cell 1985, 43, 831–840. [Google Scholar] [CrossRef]
- Paine, E.; Scheinman, R.I.; Baldwin, A.S., Jr.; Raab-Traub, N. Expression of LMP1 in epithelial cells leads to the activation of a select subset of NF-kappa B/Rel family proteins. J. Virol. 1995, 69, 4572–4576. [Google Scholar] [CrossRef]
- Mainou, B.A.; Everly, D.N., Jr.; Raab-Traub, N. Epstein-Barr virus latent membrane protein 1 CTAR1 mediates rodent and human fibroblast transformation through activation of PI3K. Oncogene 2005, 24, 6917–6924. [Google Scholar] [CrossRef]
- Soni, V.; Cahir-McFarland, E.; Kieff, E. LMP1 TRAFficking activates growth and survival pathways. Adv. Exp. Med. Biol. 2007, 597, 173–187. [Google Scholar] [CrossRef]
- Devergne, O.; Hatzivassiliou, E.; Izumi, K.M.; Kaye, K.M.; Kleijnen, M.F.; Kieff, E.; Mosialos, G. Association of TRAF1, TRAF2, and TRAF3 with an Epstein-Barr virus LMP1 domain important for B-lymphocyte transformation: Role in NF-kappaB activation. Mol. Cell. Biol. 1996, 16, 7098–7108. [Google Scholar] [CrossRef]
- Izumi, K.M.; Kaye, K.M.; Kieff, E.D. The Epstein-Barr virus LMP1 amino acid sequence that engages tumor necrosis factor receptor associated factors is critical for primary B lymphocyte growth transformation. Proc. Natl. Acad. Sci. USA 1997, 94, 1447–1452. [Google Scholar] [CrossRef]
- Zheng, X.; Hu, L.; Chen, F.; Christensson, B. Expression of Ki67 antigen, epidermal growth factor receptor and Epstein-Barr virus-encoded latent membrane protein (LMP1) in nasopharyngeal carcinoma. Eur. J. Cancer B Oral Oncol. 1994, 30B, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Meckes, D.G., Jr.; Shair, K.H.; Marquitz, A.R.; Kung, C.P.; Edwards, R.H.; Raab-Traub, N. Human tumor virus utilizes exosomes for intercellular communication. Proc. Natl. Acad. Sci. USA 2010, 107, 20370–20375. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.G.; Tan, Y.N.; Liu, Y.P.; Song, X.; Zeng, L.; Gu, H.H.; Tang, M.; Li, W.; Yi, W.; Cao, Y. Epstein-Barr virus latent membrane protein 1 modulates epidermal growth factor receptor promoter activity in a nuclear factor kappa B-dependent manner. Cell Signal. 2004, 16, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Kung, C.P.; Raab-Traub, N. Epstein-Barr virus latent membrane protein 1 induces expression of the epidermal growth factor receptor through effects on Bcl-3 and STAT3. J. Virol. 2008, 82, 5486–5493. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Song, X.; Deng, X.; Xie, D.; Lee, L.M.; Liu, Y.; Li, W.; Li, L.; Deng, L.; Wu, Q.; et al. Nuclear accumulation of epidermal growth factor receptor and acceleration of G1/S stage by Epstein-Barr-encoded oncoprotein latent membrane protein 1. Exp. Cell Res. 2005, 303, 240–251. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.; Gupta, A.T.K.; Ghafoor, A.; Akin, D.; Bashir, R. Characterization of vaccinia virus particles using microscale silicon cantilever resonators and atomic force microscopy. Sens. Actuators B-Chem. 2006, 115, 189–197. [Google Scholar] [CrossRef]
- Engelmayer, J.; Larsson, M.; Subklewe, M.; Chahroudi, A.; Cox, W.I.; Steinman, R.M.; Bhardwaj, N. Vaccinia virus inhibits the maturation of human dendritic cells: A novel mechanism of immune evasion. J. Immunol. 1999, 163, 6762–6768. [Google Scholar] [CrossRef]
- Wasilenko, S.T.; Banadyga, L.; Bond, D.; Barry, M. The vaccinia virus F1L protein interacts with the proapoptotic protein Bak and inhibits Bak activation. J. Virol. 2005, 79, 14031–14043. [Google Scholar] [CrossRef]
- King, C.S.; Cooper, J.A.; Moss, B.; Twardzik, D.R. Vaccinia virus growth factor stimulates tyrosine protein kinase activity of A431 cell epidermal growth factor receptors. Mol. Cell. Biol. 1986, 6, 332–336. [Google Scholar] [CrossRef]
- Handa, Y.; Durkin, C.H.; Dodding, M.P.; Way, M. Vaccinia Virus F11 Promotes Viral Spread by Acting as a PDZ-Containing Scaffolding Protein to Bind Myosin-9A and Inhibit RhoA Signaling. Cell Host Microbe 2013, 14, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Doorbar, J.; Foo, C.; Coleman, N.; Medcalf, L.; Hartley, O.; Prospero, T.; Napthine, S.; Sterling, J.; Winter, G.; Griffin, H. Characterization of events during the late stages of HPV16 infection in vivo using high-affinity synthetic Fabs to E4. Virology 1997, 238, 40–52. [Google Scholar] [CrossRef] [PubMed]
- Kombe Kombe, A.J.; Li, B.; Zahid, A.; Mengist, H.M.; Bounda, G.A.; Zhou, Y.; Jin, T. Epidemiology and Burden of Human Papillomavirus and Related Diseases, Molecular Pathogenesis, and Vaccine Evaluation. Front. Public Health 2020, 8, 552028. [Google Scholar] [CrossRef] [PubMed]
- Basukala, O.; Banks, L. The Not-So-Good, the Bad and the Ugly: HPV E5, E6 and E7 Oncoproteins in the Orchestration of Carcinogenesis. Viruses 2021, 13, 1892. [Google Scholar] [CrossRef] [PubMed]
- Basukala, O.; Trejo-Cerro, O.; Myers, M.P.; Pim, D.; Massimi, P.; Thomas, M.; Guarnaccia, C.; Owen, D.; Banks, L. HPV-16 E7 Interacts with the Endocytic Machinery via the AP2 Adaptor mu2 Subunit. mBio 2022, 13, e0230222. [Google Scholar] [CrossRef]
- Li, Y.; Masaki, T.; Yamane, D.; McGivern, D.R.; Lemon, S.M. Competing and noncompeting activities of miR-122 and the 5' exonuclease Xrn1 in regulation of hepatitis C virus replication. Proc. Natl. Acad. Sci. USA 2013, 110, 1881–1886. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, M.; Oshiumi, H.; Matsumoto, M.; Seya, T. DDX60, a DEXD/H box helicase, is a novel antiviral factor promoting RIG-I-like receptor-mediated signaling. Mol. Cell. Biol. 2011, 31, 3802–3819. [Google Scholar] [CrossRef] [PubMed]
- Loo, Y.M.; Gale, M., Jr. Immune signaling by RIG-I-like receptors. Immunity 2011, 34, 680–692. [Google Scholar] [CrossRef]
- Oshiumi, H.; Miyashita, M.; Okamoto, M.; Morioka, Y.; Okabe, M.; Matsumoto, M.; Seya, T. DDX60 Is Involved in RIG-I-Dependent and Independent Antiviral Responses, and Its Function Is Attenuated by Virus-Induced EGFR Activation. Cell Rep. 2015, 11, 1193–1207. [Google Scholar] [CrossRef]
- Taubenberger, J.K.; Kash, J.C. Influenza virus evolution, host adaptation, and pandemic formation. Cell Host Microbe 2010, 7, 440–451. [Google Scholar] [CrossRef] [PubMed]
- Puchelle, E. Airway secretions: New concepts and functions. Eur. Respir. J. 1992, 5, 3–4. [Google Scholar] [CrossRef] [PubMed]
- Mazur, N.I.; Martinon-Torres, F.; Baraldi, E.; Fauroux, B.; Greenough, A.; Heikkinen, T.; Manzoni, P.; Mejias, A.; Nair, H.; Papadopoulos, N.G.; et al. Lower respiratory tract infection caused by respiratory syncytial virus: Current management and new therapeutics. Lancet Respir. Med. 2015, 3, 888–900. [Google Scholar] [CrossRef] [PubMed]
- Walsh, E.E. Respiratory syncytial virus infection in adults. Semin. Respir. Crit. Care Med. 2011, 32, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Monick, M.M.; Cameron, K.; Powers, L.S.; Butler, N.S.; McCoy, D.; Mallampalli, R.K.; Hunninghake, G.W. Sphingosine kinase mediates activation of extracellular signal-related kinase and Akt by respiratory syncytial virus. Am. J. Respir. Cell Mol. Biol. 2004, 30, 844–852. [Google Scholar] [CrossRef]
- Stokes, K.L.; Chi, M.H.; Sakamoto, K.; Newcomb, D.C.; Currier, M.G.; Huckabee, M.M.; Lee, S.; Goleniewska, K.; Pretto, C.; Williams, J.V.; et al. Differential pathogenesis of respiratory syncytial virus clinical isolates in BALB/c mice. J. Virol. 2011, 85, 5782–5793. [Google Scholar] [CrossRef]
- Chi, L.; Shan, Y.; Cui, Z. N-Acetyl-L-Cysteine Protects Airway Epithelial Cells during Respiratory Syncytial Virus Infection against Mucin Synthesis, Oxidative Stress, and Inflammatory Response and Inhibits HSPA6 Expression. Anal. Cell. Pathol. 2022, 2022, 4846336. [Google Scholar] [CrossRef]
- Savolainen, C.; Blomqvist, S.; Hovi, T. Human rhinoviruses. Paediatr. Respir. Rev. 2003, 4, 91–98. [Google Scholar] [CrossRef]
- Makela, M.J.; Puhakka, T.; Ruuskanen, O.; Leinonen, M.; Saikku, P.; Kimpimaki, M.; Blomqvist, S.; Hyypia, T.; Arstila, P. Viruses and bacteria in the etiology of the common cold. J. Clin. Microbiol. 1998, 36, 539–542. [Google Scholar] [CrossRef]
- Temte, J.L. A family physician’s perspective on picornavirus infections in primary care. Arch. Fam. Med. 2000, 9, 921–922. [Google Scholar] [CrossRef]
- Inoue, D.; Yamaya, M.; Kubo, H.; Sasaki, T.; Hosoda, M.; Numasaki, M.; Tomioka, Y.; Yasuda, H.; Sekizawa, K.; Nishimura, H.; et al. Mechanisms of mucin production by rhinovirus infection in cultured human airway epithelial cells. Respir. Physiol. Neurobiol. 2006, 154, 484–499. [Google Scholar] [CrossRef]
- Grunberg, K.; Smits, H.H.; Timmers, M.C.; de Klerk, E.P.; Dolhain, R.J.; Dick, E.C.; Hiemstra, P.S.; Sterk, P.J. Experimental rhinovirus 16 infection. Effects on cell differentials and soluble markers in sputum in asthmatic subjects. Am. J. Respir. Crit. Care Med. 1997, 156, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Li, G. Immune response and blood-brain barrier dysfunction during viral neuroinvasion. Innate Immun. 2021, 27, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Uchil, P.D.; Satchidanandam, V. Phylogenetic analysis of Japanese encephalitis virus: Envelope gene based analysis reveals a fifth genotype, geographic clustering, and multiple introductions of the virus into the Indian subcontinent. Am. J. Trop. Med. Hyg. 2001, 65, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.S.; Zheng, J.P.; Mok, Y.W.; Li, Y.M.; Liu, Y.N.; Chu, C.M.; Ip, M.S. SARS: Prognosis, outcome and sequelae. Respirology 2003, 8, S36–S40. [Google Scholar] [CrossRef]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef]
- Chong, H.Y.; Leow, C.Y.; Abdul Majeed, A.B.; Leow, C.H. Flavivirus infection-A review of immunopathogenesis, immunological response, and immunodiagnosis. Virus Res. 2019, 274, 197770. [Google Scholar] [CrossRef]
- Fernandez-Garcia, M.D.; Mazzon, M.; Jacobs, M.; Amara, A. Pathogenesis of flavivirus infections: Using and abusing the host cell. Cell Host Microbe 2009, 5, 318–328. [Google Scholar] [CrossRef]
- Buehler, J.; Zeltzer, S.; Reitsma, J.; Petrucelli, A.; Umashankar, M.; Rak, M.; Zagallo, P.; Schroeder, J.; Terhune, S.; Goodrum, F. Opposing Regulation of the EGF Receptor: A Molecular Switch Controlling Cytomegalovirus Latency and Replication. PLoS Pathog. 2016, 12, e1005655. [Google Scholar] [CrossRef]
- Lau, B.; Poole, E.; Van Damme, E.; Bunkens, L.; Sowash, M.; King, H.; Murphy, E.; Wills, M.; Van Loock, M.; Sinclair, J. Human cytomegalovirus miR-UL112-1 promotes the down-regulation of viral immediate early-gene expression during latency to prevent T-cell recognition of latently infected cells. J. Gen. Virol. 2016, 97, 2387–2398. [Google Scholar] [CrossRef] [PubMed]
- Hancock, M.H.; Mitchell, J.; Goodrum, F.D.; Nelson, J.A. Human Cytomegalovirus miR-US5-2 Downregulation of GAB1 Regulates Cellular Proliferation and UL138 Expression through Modulation of Epidermal Growth Factor Receptor Signaling Pathways. mSphere 2020, 5, e00582-20. [Google Scholar] [CrossRef]
- Rak, M.A.; Buehler, J.; Zeltzer, S.; Reitsma, J.; Molina, B.; Terhune, S.; Goodrum, F. Human Cytomegalovirus UL135 Interacts with Host Adaptor Proteins to Regulate Epidermal Growth Factor Receptor and Reactivation from Latency. J. Virol. 2018, 92, e00919-18. [Google Scholar] [CrossRef]
- Shukla, D.; Spear, P.G. Herpesviruses and heparan sulfate: An intimate relationship in aid of viral entry. J. Clin. Investig. 2001, 108, 503–510. [Google Scholar] [CrossRef]
- Xiang, Y.; Zheng, K.; Ju, H.; Wang, S.; Pei, Y.; Ding, W.; Chen, Z.; Wang, Q.; Qiu, X.; Zhong, M.; et al. Cofilin 1-mediated biphasic F-actin dynamics of neuronal cells affect herpes simplex virus 1 infection and replication. J. Virol. 2012, 86, 8440–8451. [Google Scholar] [CrossRef] [PubMed]
- Verheijden, G.F.; Moolenaar, W.H.; Ploegh, H.L. Retention of epidermal growth factor receptors in the endoplasmic reticulum of adenovirus-infected cells. Biochem. J. 1992, 282 Pt 1, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, P.; Carlin, C. Adenovirus E3 protein causes constitutively internalized epidermal growth factor receptors to accumulate in a prelysosomal compartment, resulting in enhanced degradation. Mol. Cell. Biol. 1994, 14, 3695–3706. [Google Scholar] [CrossRef][Green Version]
- Benko, M.; Aoki, K.; Arnberg, N.; Davison, A.J.; Echavarria, M.; Hess, M.; Jones, M.S.; Kajan, G.L.; Kajon, A.E.; Mittal, S.K.; et al. ICTV Virus Taxonomy Profile: Adenoviridae 2022. J. Gen. Virol. 2022, 103, 1721. [Google Scholar] [CrossRef]
- Crooks, D.; Kil, S.J.; McCaffery, J.M.; Carlin, C. E3-13.7 integral membrane proteins encoded by human adenoviruses alter epidermal growth factor receptor trafficking by interacting directly with receptors in early endosomes. Mol. Biol. Cell. 2000, 11, 3559–3572. [Google Scholar] [CrossRef]
- Zeng, X.; Carlin, C.R. Adenovirus early region 3 RIDalpha protein limits NFkappaB signaling through stress-activated EGF receptors. PLoS Pathog. 2019, 15, e1008017. [Google Scholar] [CrossRef]
- Dreux, A.C.; Lamb, D.J.; Modjtahedi, H.; Ferns, G.A. The epidermal growth factor receptors and their family of ligands: Their putative role in atherogenesis. Atherosclerosis 2006, 186, 38–53. [Google Scholar] [CrossRef]
- Mocarski, E.S.; Shenk, T.; Griffiths, P.D.; Pass, R.F. Cytomegaloviruses. In Fields Virology, 6th ed.; Wolters Kluwer Health Adis (ESP): Lake Cook Road Riverwoods, IL, USA, 2013. [Google Scholar]
- Suzuki, T.; Mimuro, H.; Suetsugu, S.; Miki, H.; Takenawa, T.; Sasakawa, C. Neural Wiskott-Aldrich syndrome protein (N-WASP) is the specific ligand for Shigella VirG among the WASP family and determines the host cell type allowing actin-based spreading. Cell. Microbiol. 2002, 4, 223–233. [Google Scholar] [CrossRef]
- Badolato, R.; Sozzani, S.; Malacarne, F.; Bresciani, S.; Fiorini, M.; Borsatti, A.; Albertini, A.; Mantovani, A.; Ugazio, A.G.; Notarangelo, L.D. Monocytes from Wiskott-Aldrich patients display reduced chemotaxis and lack of cell polarization in response to monocyte chemoattractant protein-1 and formyl-methionyl-leucyl-phenylalanine. J. Immunol. 1998, 161, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- Isaacson, M.K.; Feire, A.L.; Compton, T. Epidermal growth factor receptor is not required for human cytomegalovirus entry or signaling. J. Virol. 2007, 81, 6241–6247. [Google Scholar] [CrossRef] [PubMed]
- Fairley, J.A.; Baillie, J.; Bain, M.; Sinclair, J.H. Human cytomegalovirus infection inhibits epidermal growth factor (EGF) signalling by targeting EGF receptors. J. Gen. Virol. 2002, 83, 2803–2810. [Google Scholar] [CrossRef] [PubMed]
- Jafferji, I.; Bain, M.; King, C.; Sinclair, J.H. Inhibition of epidermal growth factor receptor (EGFR) expression by human cytomegalovirus correlates with an increase in the expression and binding of Wilms' Tumour 1 protein to the EGFR promoter. J. Gen. Virol. 2009, 90, 1569–1574. [Google Scholar] [CrossRef] [PubMed]
- Hoenen, T.; Groseth, A.; Falzarano, D.; Feldmann, H. Ebola virus: Unravelling pathogenesis to combat a deadly disease. Trends Mol. Med. 2006, 12, 206–215. [Google Scholar] [CrossRef]
- Saeed, M.F.; Kolokoltsov, A.A.; Freiberg, A.N.; Holbrook, M.R.; Davey, R.A. Phosphoinositide-3 kinase-Akt pathway controls cellular entry of Ebola virus. PLoS Pathog. 2008, 4, e1000141. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Xia, Y.; Wang, H.; He, H. Epidermal growth factor receptor (EGFR) promotes uptake of bovine parainfluenza virus type 3 into MDBK cells. Vet. Microbiol. 2022, 271, 109488. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Zhang, S.; Shen, Y.; Yang, Q. Epidermal growth factor receptor is a co-factor for transmissible gastroenteritis virus entry. Virology 2018, 521, 33–43. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).