Regulation of Cell Adhesion and Migration via Microtubule Cytoskeleton Organization, Cell Polarity, and Phosphoinositide Signaling
Abstract
1. Introduction
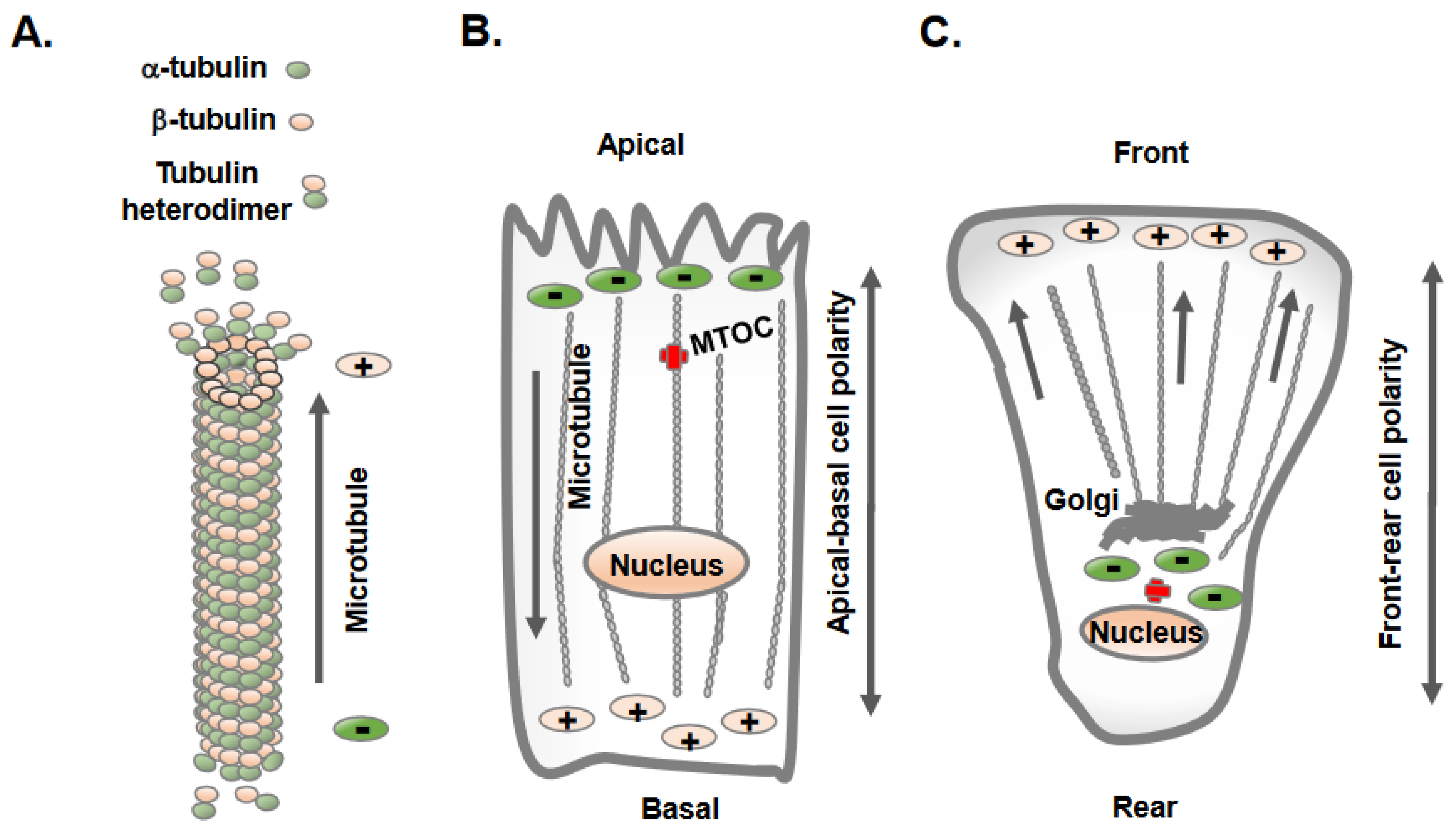
2. Distinct Orientation and Organization of Microtubules in Epithelial vs. Mesenchymal Cells
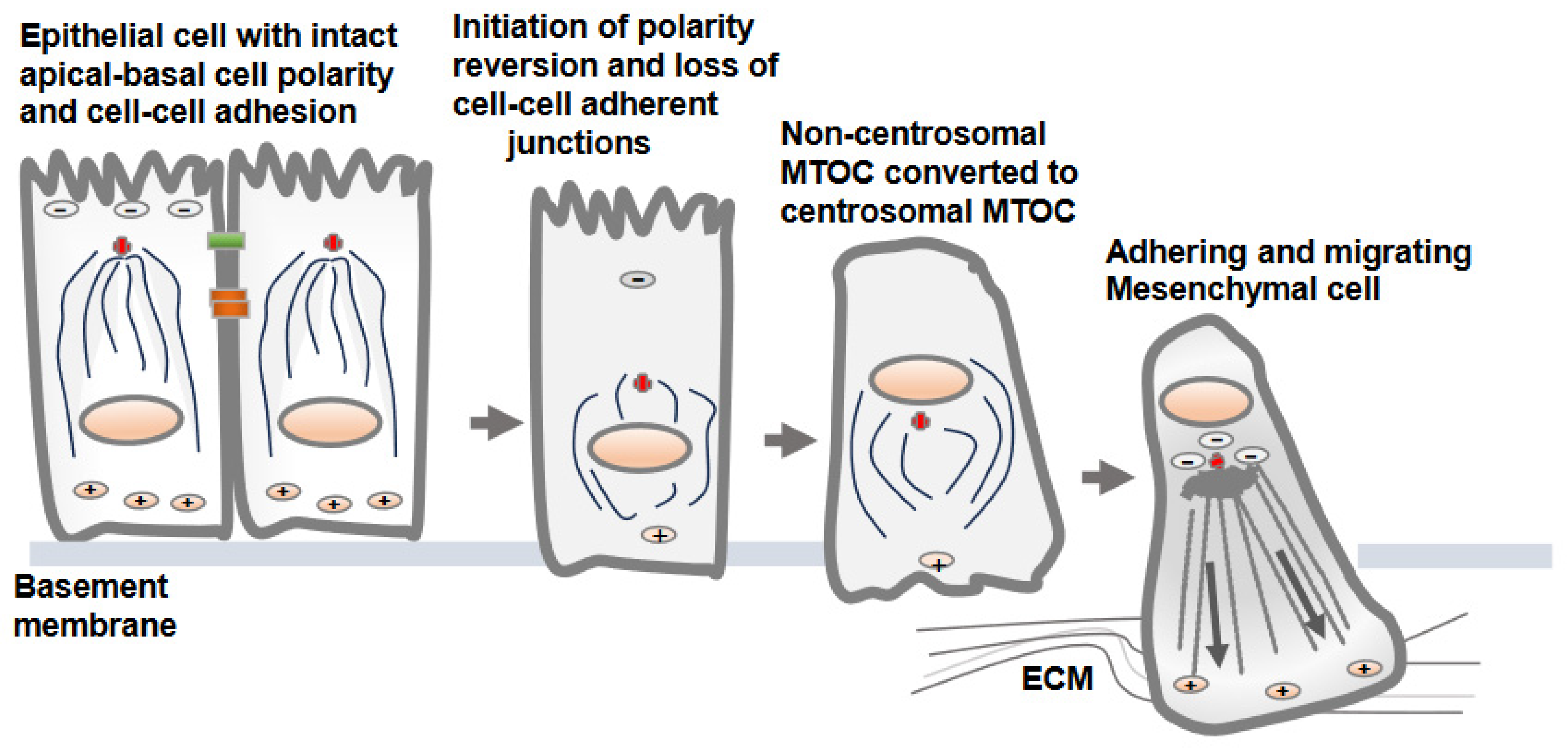
3. Reversion of MTOC from Non-Centrosomal to Centrosomal Positioning in Epithelial Cells Transitioning to Mesenchymal Cells
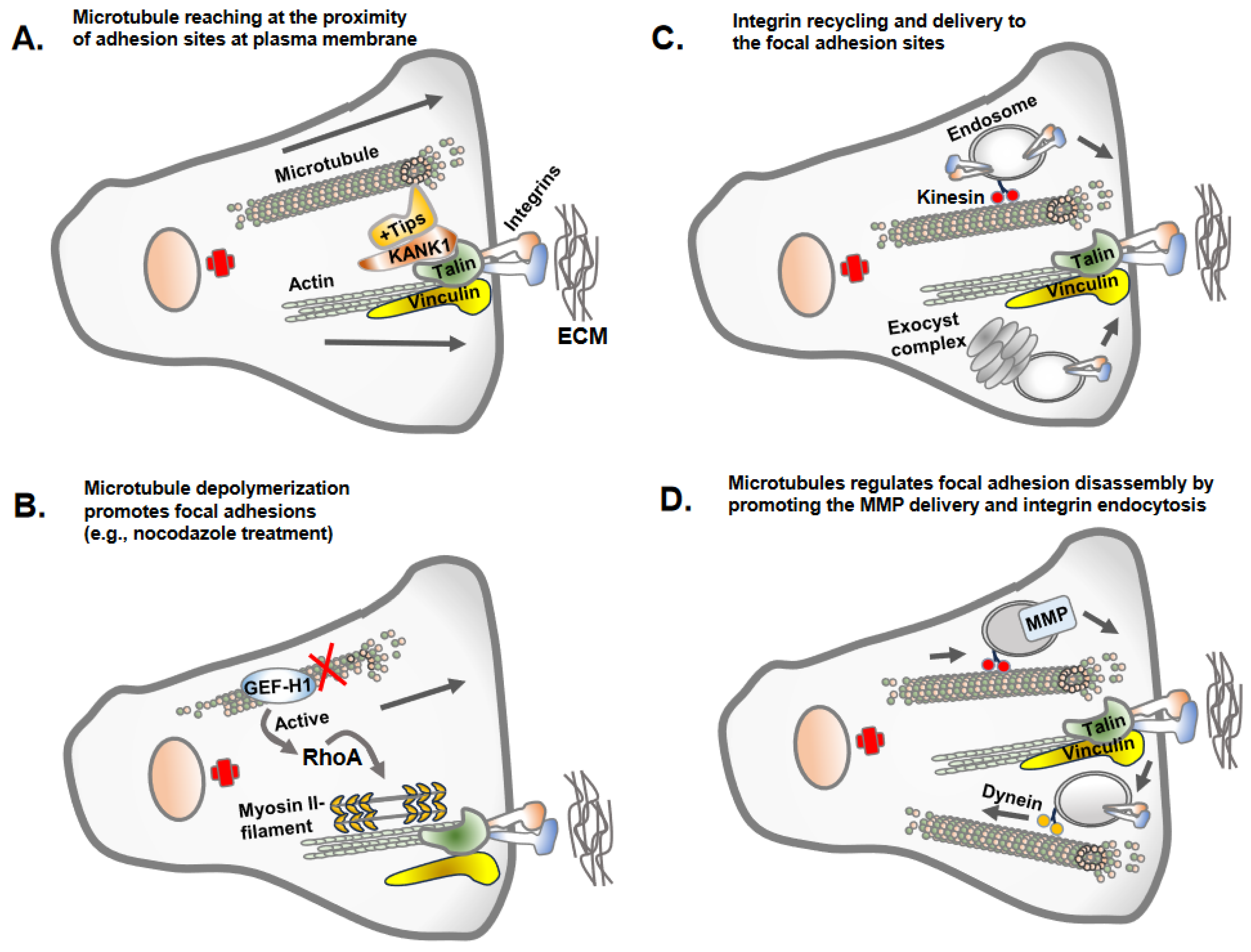
4. Microtubule Assembly and Its Inverse Relation with Focal Adhesion Assembly: Coordinated Interplay in Cell Adhesion and Cell Migration
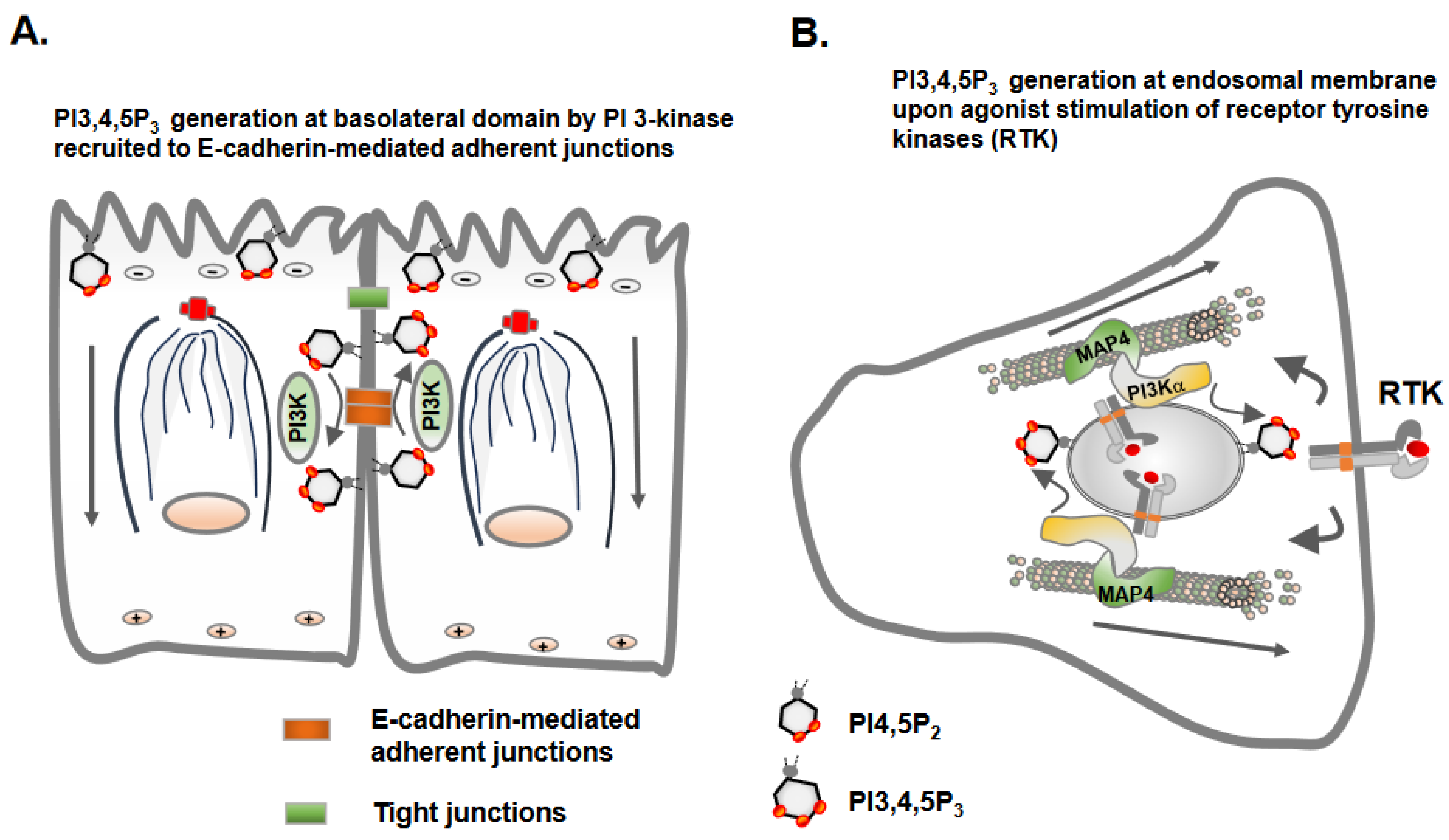
5. Microtubules Link Cell Polarity and Spatial Phosphoinositide Signaling
6. Therapeutic Targeting of Microtubules in Cancer and the Effects on Focal Adhesion Signaling
7. Conclusions and Future Direction
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lu, W.; Kang, Y. Epithelial-Mesenchymal Plasticity in Cancer Progression and Metastasis. Dev. Cell 2019, 49, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Martin-Belmonte, F.; Perez-Moreno, M. Epithelial cell polarity, stem cells and cancer. Nat. Rev. Cancer 2011, 12, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Hamidi, H.; Ivaska, J. Every step of the way: Integrins in cancer progression and metastasis. Nat. Rev. Cancer 2018, 18, 533–548. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.Y.; Fattet, L.; Tsai, J.H.; Kajimoto, T.; Chang, Q.; Newton, A.C.; Yang, J. Apical-basal polarity inhibits epithelial-mesenchymal transition and tumour metastasis by PAR-complex-mediated SNAI1 degradation. Nat. Cell Biol. 2019, 21, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Janiszewska, M.; Primi, M.C.; Izard, T. Cell adhesion in cancer: Beyond the migration of single cells. J. Biol. Chem. 2020, 295, 2495–2505. [Google Scholar] [CrossRef] [PubMed]
- Akhmanova, A.; Kapitein, L.C. Mechanisms of microtubule organization in differentiated animal cells. Nat. Rev. Mol. Cell Biol. 2022, 23, 541–558. [Google Scholar] [CrossRef]
- Burute, M.; Prioux, M.; Blin, G.; Truchet, S.; Letort, G.; Tseng, Q.; Bessy, T.; Lowell, S.; Young, J.; Filhol, O.; et al. Polarity Reversal by Centrosome Repositioning Primes Cell Scattering during Epithelial-to-Mesenchymal Transition. Dev. Cell 2017, 40, 168–184. [Google Scholar] [CrossRef]
- Thyagarajan, P.; Feng, C.; Lee, D.; Shorey, M.; Rolls, M.M. Microtubule polarity is instructive for many aspects of neuronal polarity. Dev. Biol. 2022, 486, 56–70. [Google Scholar] [CrossRef]
- Moorhouse, K.S.; Gudejko, H.F.; McDougall, A.; Burgess, D.R. Influence of cell polarity on early development of the sea urchin embryo. Dev. Dyn. 2015, 244, 1469–1484. [Google Scholar] [CrossRef]
- Ezratty, E.J.; Partridge, M.A.; Gundersen, G.G. Microtubule-induced focal adhesion disassembly is mediated by dynamin and focal adhesion kinase. Nat. Cell Biol. 2005, 7, 581–590. [Google Scholar] [CrossRef]
- Wordeman, L.; Vicente, J.J. Microtubule Targeting Agents in Disease: Classic Drugs, Novel Roles. Cancers 2021, 13, 5650. [Google Scholar] [CrossRef] [PubMed]
- Brouhard, G.J.; Rice, L.M. Microtubule dynamics: An interplay of biochemistry and mechanics. Nat. Rev. Mol. Cell Biol. 2018, 19, 451–463. [Google Scholar] [CrossRef] [PubMed]
- Bellett, G.; Carter, J.M.; Keynton, J.; Goldspink, D.; James, C.; Moss, D.K.; Mogensen, M.M. Microtubule plus-end and minus-end capture at adherens junctions is involved in the assembly of apico-basal arrays in polarised epithelial cells. Cell Motil. Cytoskelet. 2009, 66, 893–908. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, A.D.; Feldman, J.L. Microtubule-organizing centers: From the centrosome to non-centrosomal sites. Curr. Opin. Cell Biol. 2017, 44, 93–101. [Google Scholar] [CrossRef]
- Russell, D.G.; Burns, R.G. The polar ring of coccidian sporozoites: A unique microtubule-organizing centre. J. Cell Sci. 1984, 65, 193–207. [Google Scholar] [CrossRef]
- Zhou, P.; Yang, G.; Xie, W. Organization of cortical microtubules in differentiated cells. J. Cell Physiol. 2023, 238, 1141–1147. [Google Scholar] [CrossRef]
- Schatten, H. Transitions from Centrosomal to Non-centrosomal Microtubule Organization During Cellular Polarization. Adv. Anat. Embryol. Cell Biol. 2022, 235, 75–79. [Google Scholar]
- Schmoranzer, J.; Fawcett, J.P.; Segura, M.; Tan, S.; Vallee, R.B.; Pawson, T.; Gundersen, G.G. Par3 and dynein associate to regulate local microtubule dynamics and centrosome orientation during migration. Curr. Biol. 2009, 19, 1065–1074. [Google Scholar] [CrossRef]
- Zheng, Y.; Buchwalter, R.A.; Zheng, C.; Wight, E.M.; Chen, J.V.; Megraw, T.L. A perinuclear microtubule-organizing centre controls nuclear positioning and basement membrane secretion. Nat. Cell Biol. 2020, 22, 297–309. [Google Scholar] [CrossRef]
- Burakov, A.V.; Nadezhdina, E.S. Centering and Shifting of Centrosomes in Cells. Cells 2020, 9, 1351. [Google Scholar] [CrossRef]
- Xie, B.; Pu, Y.; Yang, F.; Chen, W.; Yue, W.; Ma, J.; Zhang, N.; Jiang, Y.; Wu, J.; Lin, Y.; et al. Proteomic Mapping and Targeting of Mitotic Pericentriolar Material in Tumors Bearing Centrosome Amplification. Cancer Res. 2022, 82, 2576–2592. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, P.; Xiao, G.Y.; Tan, X.; Zheng, V.J.; Shi, L.; Rabassedas, M.N.B.; Guo, H.F.; Liu, X.; Yu, J.; Diao, L.; et al. The EMT activator ZEB1 accelerates endosomal trafficking to establish a polarity axis in lung adenocarcinoma cells. Nat. Commun. 2021, 12, 6354. [Google Scholar] [CrossRef] [PubMed]
- Carney, P.R.; Couve, E. Cell polarity changes and migration during early development of the avian peripheral auditory system. Anat. Rec. 1989, 225, 156–164. [Google Scholar] [CrossRef]
- Nelson, W.J. Remodeling epithelial cell organization: Transitions between front-rear and apical-basal polarity. Cold Spring Harb. Perspect. Biol. 2009, 1, a000513. [Google Scholar] [CrossRef] [PubMed]
- Godde, N.J.; Galea, R.C.; Elsum, I.A.; Humbert, P.O. Cell polarity in motion: Redefining mammary tissue organization through EMT and cell polarity transitions. J. Mammary Gland. Biol. Neoplasia 2010, 15, 149–168. [Google Scholar] [CrossRef]
- Bakir, B.; Chiarella, A.M.; Pitarresi, J.R.; Rustgi, A.K. EMT, MET, Plasticity, and Tumor Metastasis. Trends Cell Biol. 2020, 30, 764–776. [Google Scholar] [CrossRef]
- Seetharaman, S.; Etienne-Manneville, S. Microtubules at focal adhesions—A double-edged sword. J. Cell Sci. 2019, 132, jcs232843. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Y.; Sarkar, A.; Wang, X. Keratocytes Generate High Integrin Tension at the Trailing Edge to Mediate Rear De-adhesion during Rapid Cell Migration. iScience 2018, 9, 502–512. [Google Scholar] [CrossRef]
- Mavrakis, M.; Juanes, M.A. The compass to follow: Focal adhesion turnover. Curr. Opin. Cell Biol. 2023, 80, 102152. [Google Scholar] [CrossRef]
- Seetharaman, S.; Etienne-Manneville, S. Cytoskeletal Crosstalk in Cell Migration. Trends Cell Biol. 2020, 30, 720–735. [Google Scholar] [CrossRef]
- Broussard, J.A.; Webb, D.J.; Kaverina, I. Asymmetric focal adhesion disassembly in motile cells. Curr. Opin. Cell Biol. 2008, 20, 85–90. [Google Scholar] [CrossRef]
- Zhao, A.J.; Montes-Laing, J.; Perry, W.M.; Shiratori, M.; Merfeld, E.; Rogers, S.L.; Applewhite, D.A. The Drosophila spectraplakin Short stop regulates focal adhesion dynamics by cross-linking microtubules and actin. Mol. Biol. Cell 2022, 33, ar19. [Google Scholar] [CrossRef]
- Li, X.; Goult, B.T.; Ballestrem, C.; Zacharchenko, T. The structural basis of the talin-KANK1 interaction that coordinates the actin and microtubule cytoskeletons at focal adhesions. Open Biol. 2023, 13, 230058. [Google Scholar] [CrossRef]
- Moon, H.H.; Kreis, N.N.; Friemel, A.; Roth, S.; Schulte, D.; Solbach, C.; Louwen, F.; Yuan, J.; Ritter, A. Mitotic Centromere-Associated Kinesin (MCAK/KIF2C) Regulates Cell Migration and Invasion by Modulating Microtubule Dynamics and Focal Adhesion Turnover. Cancers 2021, 13, 5673. [Google Scholar] [CrossRef]
- Kenific, C.M.; Wittmann, T.; Debnath, J. Autophagy in adhesion and migration. J. Cell Sci. 2016, 129, 3685–3693. [Google Scholar] [CrossRef] [PubMed]
- Garcin, C.; Straube, A. Microtubules in cell migration. Essays Biochem. 2019, 63, 509–520. [Google Scholar]
- Rafiq, N.B.M.; Nishimura, Y.; Plotnikov, S.V.; Thiagarajan, V.; Zhang, Z.; Shi, S.; Natarajan, M.; Viasnoff, V.; Kanchanawong, P.; Jones, G.E.; et al. A mechano-signalling network linking microtubules, myosin IIA filaments and integrin-based adhesions. Nat. Mater. 2019, 18, 638–649. [Google Scholar] [CrossRef] [PubMed]
- Zeitz, M.; Kierfeld, J. Feedback mechanism for microtubule length regulation by stathmin gradients. Biophys. J. 2014, 107, 2860–2871. [Google Scholar] [CrossRef][Green Version]
- Liu, H.; Yue, J.; Huang, H.; Gou, X.; Chen, S.Y.; Zhao, Y.; Wu, X. Regulation of Focal Adhesion Dynamics and Cell Motility by the EB2 and Hax1 Protein Complex. J. Biol. Chem. 2015, 290, 30771–30782. [Google Scholar] [CrossRef] [PubMed]
- Drabek, K.; van Ham, M.; Stepanova, T.; Draegestein, K.; van Horssen, R.; Sayas, C.L.; Akhmanova, A.; Ten Hagen, T.; Smits, R.; Fodde, R.; et al. Role of CLASP2 in microtubule stabilization and the regulation of persistent motility. Curr. Biol. 2006, 16, 2259–2264. [Google Scholar] [CrossRef]
- Ezratty, E.J.; Bertaux, C.; Marcantonio, E.E.; Gundersen, G.G. Clathrin mediates integrin endocytosis for focal adhesion disassembly in migrating cells. J. Cell Biol. 2009, 187, 733–747. [Google Scholar] [CrossRef] [PubMed]
- Dupin, I.; Camand, E.; Etienne-Manneville, S. Classical cadherins control nucleus and centrosome position and cell polarity. J. Cell Biol. 2009, 185, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Stehbens, S.J.; Paszek, M.; Pemble, H.; Ettinger, A.; Gierke, S.; Wittmann, T. CLASPs link focal-adhesion-associated microtubule capture to localized exocytosis and adhesion site turnover. Nat. Cell Biol. 2014, 16, 561–573. [Google Scholar] [CrossRef]
- Mendoza, P.A.; Silva, P.; Díaz, J.; Arriagada, C.; Canales, J.; Cerda, O.; Torres, V.A. Calpain2 mediates Rab5-driven focal adhesion disassembly and cell migration. Cell Adhes. Migr. 2018, 12, 185–194. [Google Scholar] [CrossRef]
- Ling, K.; Doughman, R.L.; Firestone, A.J.; Bunce, M.W.; Anderson, R.A. Type I gamma phosphatidylinositol phosphate kinase targets and regulates focal adhesions. Nature 2002, 420, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Ling, K.; Doughman, R.L.; Iyer, V.V.; Firestone, A.J.; Bairstow, S.F.; Mosher, D.F.; Schaller, M.D.; Anderson, R.A. Tyrosine phosphorylation of type Igamma phosphatidylinositol phosphate kinase by Src regulates an integrin-talin switch. J. Cell Biol. 2003, 163, 1339–1349. [Google Scholar] [CrossRef]
- Maritzen, T.; Schachtner, H.; Legler, D.F. On the move: Endocytic trafficking in cell migration. Cell Mol. Life Sci. 2015, 72, 2119–2134. [Google Scholar] [CrossRef]
- Thapa, N.; Sun, Y.; Schramp, M.; Choi, S.; Ling, K.; Anderson, R.A. Phosphoinositide signaling regulates the exocyst complex and polarized integrin trafficking in directionally migrating cells. Dev. Cell 2012, 22, 116–130. [Google Scholar] [CrossRef]
- Beri, P.; Popravko, A.; Yeoman, B.; Kumar, A.; Chen, K.; Hodzic, E.; Chiang, A.; Banisadr, A.; Placone, J.K.; Carter, H.; et al. Cell Adhesiveness Serves as a Biophysical Marker for Metastatic Potential. Cancer Res. 2020, 80, 901–911. [Google Scholar] [CrossRef]
- Ling, K.; Bairstow, S.F.; Carbonara, C.; Turbin, D.A.; Huntsman, D.G.; Anderson, R.A. Type I gamma phosphatidylinositol phosphate kinase modulates adherens junction and E-cadherin trafficking via a direct interaction with mu 1B adaptin. J. Cell Biol. 2007, 176, 343–353. [Google Scholar] [CrossRef]
- Martin-Belmonte, F.; Gassama, A.; Datta, A.; Yu, W.; Rescher, U.; Gerke, V.; Mostov, K. PTEN-mediated apical segregation of phosphoinositides controls epithelial morphogenesis through Cdc42. Cell 2007, 128, 383–397. [Google Scholar] [CrossRef] [PubMed]
- Gassama-Diagne, A.; Yu, W.; Ter Beest, M.; Martin-Belmonte, F.; Kierbel, A.; Engel, J.; Mostov, K. Phosphatidylinositol-3,4,5-trisphosphate regulates the formation of the basolateral plasma membrane in epithelial cells. Nat. Cell Biol. 2006, 8, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Gervais, L.; Claret, S.; Januschke, J.; Roth, S.; Guichet, A. PIP5K-dependent production of PIP2 sustains microtubule organization to establish polarized transport in the Drosophila oocyte. Development 2008, 135, 3829–3838. [Google Scholar] [CrossRef]
- Roman-Fernandez, A.; Roignot, J.; Sandilands, E.; Nacke, M.; Mansour, M.A.; McGarry, L.; Shanks, E.; Mostov, K.E.; Bryant, D.M. The phospholipid PI(3,4)P(2) is an apical identity determinant. Nat. Commun. 2018, 9, 5041. [Google Scholar] [CrossRef]
- Halet, G.; Viard, P.; Carroll, J. Constitutive PtdIns(3,4,5)P3 synthesis promotes the development and survival of early mammalian embryos. Development 2008, 135, 425–429. [Google Scholar] [CrossRef] [PubMed]
- Thapa, N.; Chen, M.; Horn, H.T.; Choi, S.; Wen, T.; Anderson, R.A. Phosphatidylinositol-3-OH kinase signalling is spatially organized at endosomal compartments by microtubule-associated protein 4. Nat. Cell Biol. 2020, 22, 1357–1370. [Google Scholar] [CrossRef]
- Reddy, P.; Liu, L.; Ren, C.; Lindgren, P.; Boman, K.; Shen, Y.; Lundin, E.; Ottander, U.; Rytinki, M.; Liu, K. Formation of E-cadherin-mediated cell-cell adhesion activates AKT and mitogen activated protein kinase via phosphatidylinositol 3 kinase and ligand-independent activation of epidermal growth factor receptor in ovarian cancer cells. Mol. Endocrinol. 2005, 19, 2564–2578. [Google Scholar] [CrossRef]
- Pece, S.; Chiariello, M.; Murga, C.; Gutkind, J.S. Activation of the protein kinase Akt/PKB by the formation of E-cadherin-mediated cell-cell junctions. Evidence for the association of phosphatidylinositol 3-kinase with the E-cadherin adhesion complex. J. Biol. Chem. 1999, 274, 19347–19351. [Google Scholar] [CrossRef]
- Choi, S.; Hedman, A.C.; Sayedyahossein, S.; Thapa, N.; Sacks, D.B.; Anderson, R.A. Agonist-stimulated phosphatidylinositol-3,4,5-trisphosphate generation by scaffolded phosphoinositide kinases. Nat. Cell Biol. 2016, 18, 1324–1335. [Google Scholar] [CrossRef]
- Thapa, N.; Choi, S.; Tan, X.; Wise, T.; Anderson, R.A. Phosphatidylinositol Phosphate 5-Kinase Igamma and Phosphoinositide 3-Kinase/Akt Signaling Couple to Promote Oncogenic Growth. J. Biol. Chem. 2015, 290, 18843–18854. [Google Scholar] [CrossRef]
- Nishioka, T.; Aoki, K.; Hikake, K.; Yoshizaki, H.; Kiyokawa, E.; Matsuda, M. Rapid turnover rate of phosphoinositides at the front of migrating MDCK cells. Mol. Biol. Cell 2008, 19, 4213–4223. [Google Scholar] [CrossRef]
- Saito, K.; Mori, M.; Kambara, N.; Ohta, Y. FilGAP, a GAP protein for Rac, regulates front-rear polarity and tumor cell migration through the ECM. FASEB J. 2021, 35, e21508. [Google Scholar] [CrossRef]
- Feng, Z.; Yu, C.H. PI(3,4)P(2)-mediated membrane tubulation promotes integrin trafficking and invasive cell migration. Proc. Natl. Acad. Sci. USA 2021, 118, e2017645118. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, H.; Yoshida, S.; Muroi, E.; Yoshida, N.; Kawamura, M.; Kouchi, Z.; Nakamura, Y.; Sakai, R.; Fukami, K. Phosphoinositide 3-kinase signaling pathway mediated by p110alpha regulates invadopodia formation. J. Cell Biol. 2011, 193, 1275–1288. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.; Blowes, L.M.; Laly, A.C.; Connelly, J.T. Regulation of collective cell polarity and migration using dynamically adhesive micropatterned substrates. Acta Biomater. 2021, 126, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues-Ferreira, S.; Moindjie, H.; Haykal, M.M.; Nahmias, C. Predicting and Overcoming Taxane Chemoresistance. Trends Mol. Med. 2021, 27, 138–151. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Mao, W.; Rodriguez-Aguayo, C.; Mangala, L.S.; Bartholomeusz, G.; Iles, L.R.; Jennings, N.B.; Ahmed, A.A.; Sood, A.K.; Lopez-Berestein, G.; et al. Paclitaxel Sensitivity of Ovarian Cancer Can be Enhanced by Knocking Down Pairs of Kinases that Regulate MAP4 Phosphorylation and Microtubule Stability. Clin. Cancer Res. 2018, 24, 5072–5084. [Google Scholar] [CrossRef] [PubMed]
- Das, T.; Anand, U.; Pandey, S.K.; Ashby, C.R., Jr.; Assaraf, Y.G.; Chen, Z.S.; Dey, A. Therapeutic strategies to overcome taxane resistance in cancer. Drug Resist. Updat. 2021, 55, 100754. [Google Scholar] [CrossRef]
- Chang, Y.C.; Nalbant, P.; Birkenfeld, J.; Chang, Z.F.; Bokoch, G.M. GEF-H1 couples nocodazole-induced microtubule disassembly to cell contractility via RhoA. Mol. Biol. Cell 2008, 19, 2147–2153. [Google Scholar] [CrossRef]
- Zheng, Y.B.; Gong, J.H.; Zhen, Y.S. Focal adhesion kinase is activated by microtubule-depolymerizing agents and regulates membrane blebbing in human endothelial cells. J. Cell Mol. Med. 2020, 24, 7228–7238. [Google Scholar] [CrossRef]
- Ng, D.H.; Humphries, J.D.; Byron, A.; Millon-Fremillon, A.; Humphries, M.J. Microtubule-dependent modulation of adhesion complex composition. PLoS ONE 2014, 9, e115213. [Google Scholar] [CrossRef] [PubMed]
- Parker, A.L.; Teo, W.S.; McCarroll, J.A.; Kavallaris, M. An Emerging Role for Tubulin Isotypes in Modulating Cancer Biology and Chemotherapy Resistance. Int. J. Mol. Sci. 2017, 18, 1434. [Google Scholar] [CrossRef] [PubMed]
- Janke, C.; Magiera, M.M. The tubulin code and its role in controlling microtubule properties and functions. Nat. Rev. Mol. Cell Biol. 2020, 21, 307–326. [Google Scholar] [CrossRef]
- Remo, A.; Li, X.; Schiebel, E.; Pancione, M. The Centrosome Linker and Its Role in Cancer and Genetic Disorders. Trends Mol. Med. 2020, 26, 380–393. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.M.; Sixt, M. Mechanisms of 3D cell migration. Nat. Rev. Mol. Cell Biol. 2019, 20, 738–752. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thapa, N.; Wen, T.; Cryns, V.L.; Anderson, R.A. Regulation of Cell Adhesion and Migration via Microtubule Cytoskeleton Organization, Cell Polarity, and Phosphoinositide Signaling. Biomolecules 2023, 13, 1430. https://doi.org/10.3390/biom13101430
Thapa N, Wen T, Cryns VL, Anderson RA. Regulation of Cell Adhesion and Migration via Microtubule Cytoskeleton Organization, Cell Polarity, and Phosphoinositide Signaling. Biomolecules. 2023; 13(10):1430. https://doi.org/10.3390/biom13101430
Chicago/Turabian StyleThapa, Narendra, Tianmu Wen, Vincent L. Cryns, and Richard A. Anderson. 2023. "Regulation of Cell Adhesion and Migration via Microtubule Cytoskeleton Organization, Cell Polarity, and Phosphoinositide Signaling" Biomolecules 13, no. 10: 1430. https://doi.org/10.3390/biom13101430
APA StyleThapa, N., Wen, T., Cryns, V. L., & Anderson, R. A. (2023). Regulation of Cell Adhesion and Migration via Microtubule Cytoskeleton Organization, Cell Polarity, and Phosphoinositide Signaling. Biomolecules, 13(10), 1430. https://doi.org/10.3390/biom13101430






