1. Introduction
To date, hospital-acquired infections account for an estimated 1.7 million infections and 99,000 deaths annually [
1]. While many pathogens originate from patients, an estimated 20–40% of nosocomial infections are caused by cross-contamination from the hands of healthcare workers [
2]. This cross-contamination likely results from healthcare personnel touching contaminated surfaces that are sanitized with agents that lack adequate antimicrobial activity [
3]. Indeed, the current SARS-CoV-2 pandemic has drawn renewed attention to the environmental control of microorganisms and the role contaminated surfaces play in the spread of infectious diseases. One study demonstrated that SARS-CoV-2 can survive at room temperature on hard surfaces for at least 28 days [
4] and on hands for approximately nine hours [
5]. Of particular importance is the control of SARS-CoV-2 contamination in hospitals.
There is a growing body of evidence that current disinfection solutions may be ineffective at reducing hard surface contamination and nosocomial infection in healthcare environments. Despite strict disinfectant regimens, common hospital pathogens, such as
Clostridium difficile,
Acinetobacter baumanni, methicillin-resistant
Staphylococcus aureus (MRSA), and vancomycin-resistant
Enterococcus (VRE), frequently colonize surfaces for extended periods, leading to direct patient infection [
6,
7,
8,
9]. Currently, many disinfectants utilized across multiple healthcare and industrial sectors rely on quaternary ammonium compounds (quats) as their active ingredients [
10]. Quats have numerous advantages: they are inexpensive and have broad activity against bacteria, fungi, and enveloped viruses. However, they have poor activity against
C. difficile spores,
Mycobacterium tuberculosis, and non-enveloped viruses, and their activity is adversely affected by organic matter or soil [
11,
12]. Furthermore, questions have recently been raised as to the safety of quats, where quats in rodents have been shown to affect neural tube formation, reproduction, and respiration and are genotoxic to cells, as well as ecotoxic to aquatic life [
13,
14,
15,
16,
17,
18]. In humans, continuous exposure has been demonstrated to produce contact dermatitis and, in certain cases, induce asthma [
19,
20].
Another significant issue associated with quats is the induction of cross-resistance to antibiotics, where exposure to the common quat benzalkonium chloride (BAC) was shown to increase the resistance of MRSA to beta-lactams [
21] and increase the resistance of enterohemorrhagic
Escherichia coli to six different, commonly used antibiotics [
22]. In addition to the development of cross-resistance, the use of quats also produces bacterial resistance to the disinfectant itself through the upregulation of efflux pumps, decreased porins related to BAC transport, and increased expression of spermidine and
pmrB resulting in a reduction in the surface membrane negative charge (since BAC is cationic) [
23]. Based on the current rate of nosocomial infection coupled with the safety and performance issues associated with quats, there is a growing need to develop safe alternatives that are capable of effectively controlling pathogenic microorganisms within the environment.
Antimicrobial fatty acids represent a known but relatively underutilized resource in the fight against infective organisms. A wide range of fatty acids has been found to have broad-spectrum antibacterial activity [
24]. There are several advantages to utilizing fatty acids in the environmental control of microbes: they do not appear to develop resistance [
25]; they are a naturally occurring component in the human innate immune response to pathogens, particularly on skin [
26,
27]; they have extremely low toxicity risk associated with their use (LD
50 in rats fed capric acid exceeded 10 g/kg [
28]); and they rapidly undergo biodegradation in the body and are utilized as an energy source. Capric acid (also known as decanoic acid), found naturally in coconut oil (~10%), palm kernel oil, and goat milk, is rapidly metabolized to ketones through beta-oxidation and utilized in the mitochondria for energy [
29].
While there is renewed interest in fatty acids as effective antibacterial agents, their stability and solubility have seen limited development. Physically, fatty acids are almost completely water-insoluble and must be combined with solubilizing agents, dissolved in organic solvents, or chemically modified (e.g., methyl esterification) to be utilized in practical applications. However, these modifications can reduce the efficacy of fatty acids when compared to their unmodified forms [
30]. Capric acid, for example, is highly soluble in alcohol, ether, DMSO, and acetone but relatively insoluble in water [
31]. Overcoming this challenge of presenting fatty acids in a safe and soluble form would present a novel agent for use as an environmental antimicrobial and sanitizer.
The present study details the development of a novel antimicrobial agent containing a naturally occurring 10-carbon fatty acid, capric acid, combined with L-arginine to produce a water-soluble ammonium carboxylate salt. The further addition of a 10-carbon monoterpenoid, thymol, provides fast-acting, broad-spectrum antimicrobial and sanitizing activity against multidrug-resistant hospital pathogens in both in vitro and real-world settings.
2. Materials and Methods
2.1. Nuclear Magnetic Resonance (NMR)
An aliquot (250 μL) of GS-2 was diluted with 20 mL MilliQ water, adjusted to pH 4, and extracted 3 times with 40 mL heptane. The aqueous fraction was freeze-dried and reconstituted with D2O for 1H and 13C analysis. The combined heptane fractions were dried under vacuum and taken into CDCl3 for 1H and 13C analysis.
2.2. Gas Chromatorgraphy Mass Spectrometry (GCMS)
GS-2 was analyzed as a mixture, followed by analyses of both the aqueous and heptane-extracted fractions. All detected peaks were matched against the National Institute of Standards and Technology (NIST) database for annotation. Following whole formulation analysis, 250 μL of GS-2 was diluted with 20 mL MilliQ water and extracted 3 times with 40 mL heptane (Sigma-Aldrich, St. Louis, MO, USA). The aqueous fraction was freeze-dried; derivatized with n, o-bis (trimethylsilyl) trifluoroacetamide; and resuspended in methanol (Sigma-Aldrich, St. Louis, MO, USA) for trace analysis. The combined heptane fractions were dried under vacuum, resuspended in n-hexane, and analyzed separately.
2.3. Determining Activity of GS-2 against Bacterial and Fungal Pathogens
The minimum inhibitory concentration (MIC), minimum bactericidal concentration (MBC), and minimum fungicidal concentration (MFC) of both GS-2 and GS-2 with thymol were determined using a broth dilution method under Clinical Laboratory Standards Institute (CLSI) M26-A guidelines [
32].
C. difficile (American Type Culture Collection (ATCC), Manassas, VA, USA) was cultured on BHI-YHT agar for 48 h prior to testing.
Candida auris (Centers for Disease Control and Prevention (CDC) AR Bank 0381, Atlanta, GA, USA) was cultured on Sabouraud dextrose agar for 24 h prior to testing.
Clostridium butyricum (ATCC 19398),
S. aureus (ATCC 6538),
Pseudomonas aeruginosa (ATCC 15442), and
Klebsiella pneumoniae (ATCC 700603) were cultured on tryptic soy agar (TSA) with 5% sheep’s blood for 24 h prior to testing.
Bacillus atrophaeus (ATCC 9372) and
E. coli (ATCC 25922) were cultured on TSA plates for 24 h prior to testing.
Organism suspensions were prepared at 1 105 CFU/mL in appropriate culture media as follows: S. aureus, E. coli, K. pneumoniae, and P. aeruginosa in Luria–Bertani broth; C. auris in Sabouraud dextrose broth; C. butyricum in reinforced clostridium medium; B. atrophaeus in Mueller–Hinton broth; C. difficile in brain–heart infusion broth. GS-2 and brain–heart infusion broth were prepared 24 h prior to testing to allow pre-reduction in an anaerobic chamber overnight for the growth of C. difficile.
Serial dilutions (1:1) of both GS-2 and GS-2 with thymol were prepared in culture media specified above in triplicate for each organism in a 96-well plate. Bacterial and fungal cultures (100 µL) were then added to the diluted GS-2 tests. Plates were then incubated for 24–48 h, either at 30 °C (C. auris), at 36 °C in anaerobic conditions (C. butyricum and C. difficile), or 36 °C in aerobic conditions (S. aureus, B. atrophaeus, E. coli, K. pneumoniae, and P. aeruginosa). MIC, MBC, and MFC were determined via visual assessment.
2.4. Determining Activity of GS-2 against Clinically Isolated Bacterial Pathogens
GS-2 activity was tested against four clinically isolated pathogens: MRSA,
Streptococcus pyogenes, and the carbapenem-resistant enterobacteriaceae (CRE)
E. coli and
K. pneumoniae (samples generously donated from Kalispell Regional Medical Center, Montana) using a 96-well microplate format under CLSI M26-A guidelines [
32]. A serial dilution in Oxoid Sensitest medium of GS-2 was combined with bacteria to determine the MIC and MBC of GS-2 after 24 h of incubation. MIC was determined by visual assessment with no turbidity. MBC was determined by combining technical replicates and centrifuging to pellet bacteria. The supernatant was removed, and the pellet was rehydrated with 1 mL sterile saline. One hundred microliters of this solution was plated onto 5% sheep blood agar (SBA) plates and incubated for 24 h at 37 °C. MBC was the concentration of GS-2 that produced a 99.99% reduction from the average starting inoculum. This value was calculated by a manual dilution series of the inoculum on 5% SBA plates (the starting inoculum was diluted, plated, incubated for 24 h, and counted to obtain an accurate starting concentration for the percent reduction calculation). For growth and contamination controls, media-only and water controls were set up on the 96-well plate and processed consistently with experimental samples to ensure sterility and quality control. Positive growth control wells containing bacteria, media, and sterile water were diluted, plated, and counted to determine the amount of growth from the starting inoculum and to ensure robust growth against the test organism.
2.5. Testing GS-2 and GS-2 with Thymol under Regulatory Disinfectant Standards
MRSA and
E. coli (clinical isolates) were tested against GS-2 and GS-2 with thymol according to the Therapeutic Goods Administration (TGA) Option C household/commercial-grade disinfectant guidelines [
33]. Briefly, bacteria were grown to log-phase in Oxoid Sensitest broth at 37 °C under constant rotation for 6 h. Three milliliters of either 15 mg/mL GS-2, DMSO, 5 mg/mL thymol in DMSO, 30 mg/mL GS-2 in DMSO, or 30 mg/mL GS-2 with 5 mg/mL thymol was combined with 9
10
8 CFU/mL bacteria in 1 mL and incubated for 8 min at room temperature. After 8 min, samples were vortexed, and 20 µL was placed in 5 mL Oxoid Sensitest medium and incubated for 48 h. A pass result required 2 or more of the 5 tubes to be clear (indicating no growth) after 48 h.
2.6. Antibacterial Time-kill Studies with GS-2 against Potential Bioterrorism Pathogen Surrogates
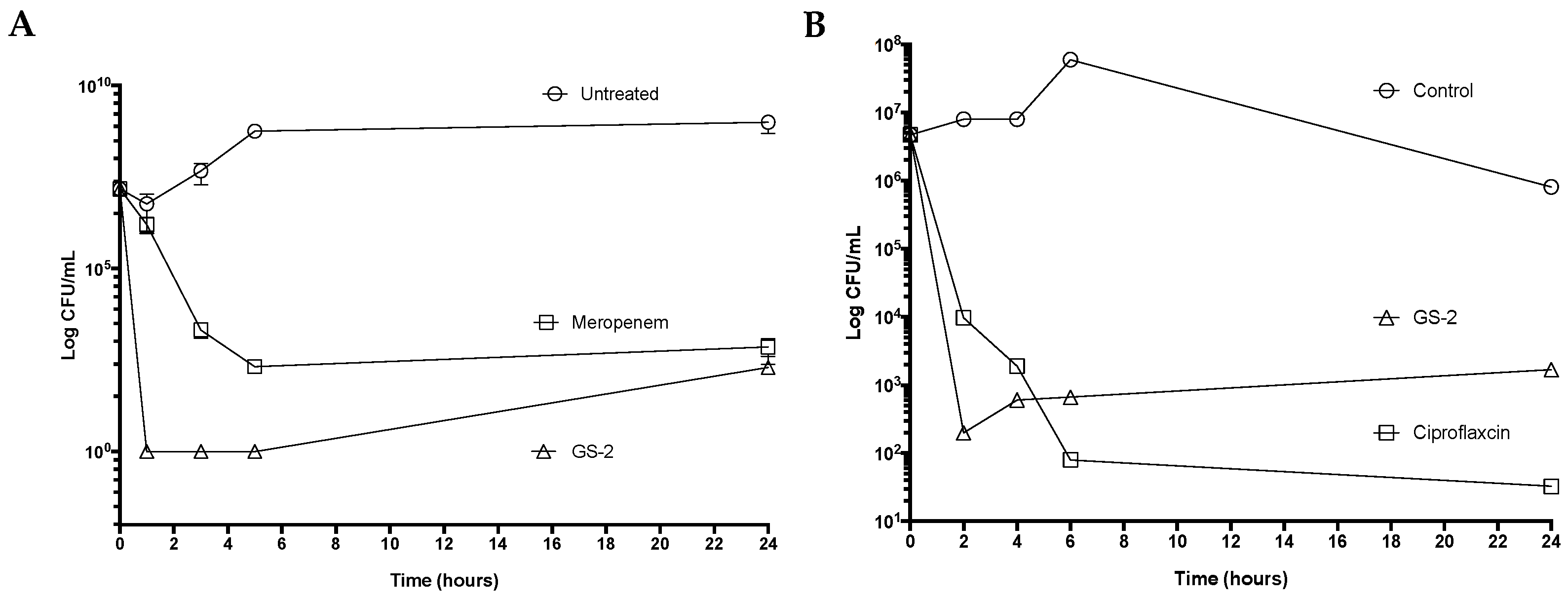
Cultures of Burkholderia thailandensis (ATCC 700388), a surrogate for Burkholderia pseudomallei, and Bacillus anthracis Sterne 34F2 (Fort Dodge Australia Pty Ltd., Colorado Serum Company, Denver, CO, USA) were prepared in nutrient broth and incubated overnight at 37 °C with shaking. Cultures were diluted to an OD600 equivalent of 2 106 CFU/mL in fresh broth and incubated further for 1 h with shaking to obtain a log-phase culture. A final bacterial inoculum of 1 106 CFU/mL in 0.5 mL of the log-phase culture was added to 0.5 mL of broth containing two-fold-concentrated GS-2 (final concentration 4.76 mg/mL), ciprofloxacin (final concentration 0.01 mg/mL), or meropenem (final concentration 0.10 mg/mL; Sigma-Aldrich, St. Louis, MO, USA). Cultures were incubated for 1 h, 3 h, 5 h, and 24 h (B. thailandensis) or 2 h, 4 h, 6 h, and 24 h (B. anthracis). Following each time-point, samples were washed in PBS, serially diluted, and plated onto nutrient agar plates. The plates were incubated at 37 °C for 24–48 h, and CFU/mL was calculated to determine time-kill kinetics.
2.7. Determining Antiviral Activity of GS-2 and GS-2 with Thymol
GS-2 was tested against Murine Hepatitis Virus Strain 1 (MHV-1; ATCC/VR-261, the severe acute respiratory syndrome-associated coronavirus (SARS-CoV) surrogate), Herpes Simplex Virus-1 (HSV-1; ATCC/VR-733), and poliovirus (ATCC/VR-192) according to TMCV 006 ASTM International Standard E1053 [
34]. Briefly, viral suspensions were prepared in 5% FBS, spread over the surface of a glass petri dish, and allowed to dry. Two milliliters of GS-2 (15 mg/mL) or GS-2 (30 mg/mL) with thymol (5 mg/mL) was then applied to the viral film and incubated for 10 min at RT. Following incubation, viruses were harvested from test and control slides with a cell scraper, and GS-2 or GS-2 with thymol was neutralized with 2% FBS in minimum essential medium (MEM). Separately, 2 mL GS-2 or GS-2 with thymol was also combined with 2% FBS in MM and then either combined with viral suspension to ensure neutralization (neutralization control) or set aside for the following step (cytotoxicity control).
The neutralized harvested test suspensions, neutralization control, and cytotoxicity control were serially diluted and plated in quadruplicate onto a 96-well plate containing a monolayer of either A9 cells (ATCC/CCL-1.4; for MHV-1) or Vero cells (ATCC/CCL-81; for HSV-1 and poliovirus). Four wells containing cells only served as controls. Media was added to all wells and incubated for 7–10 days. Then, each well was examined for the presence of cytopathic effects of infection, such as cell rounding, sloughing, and monolayer degradation. Cytotoxicity control wells were examined for damage caused by the test product. The Reed and Muench LD50 method was used to determine the viral titer endpoint.
2.8. GS-2 Surface Sanitizer Trial
Two commercial high-rise buildings with near-identical layouts (built in the same year within 0.25 km of each other and monitored for daily traffic in the building with no significant differences in work population over the two-week test period) were sanitized for two weeks. Building A was sanitized with GS-2 at 15 mg/mL (1.5%), and building B was sanitized with a hospital-grade disinfectant containing 0.42% BAC according to product label instructions. After two weeks of treatment, swabs were taken in both buildings of five high-touch-point surfaces: loading dock bathroom door handles, office bathroom door handles for one level, office door handles into the loading dock, elevator buttons, and elevator touch screens (identical in both buildings). Swabs were taken 90 min before the morning treatment, 90 min after the morning treatment, and again at 4 h after treatment. Swabs were then cultured on 5% sheep blood agar (SBA) plates (Thermo Fisher Scientific, Scoresby, Australia) for 24 h at 37 °C, and the number of colonies was counted.
2.9. Toxicology Studies with GS-2
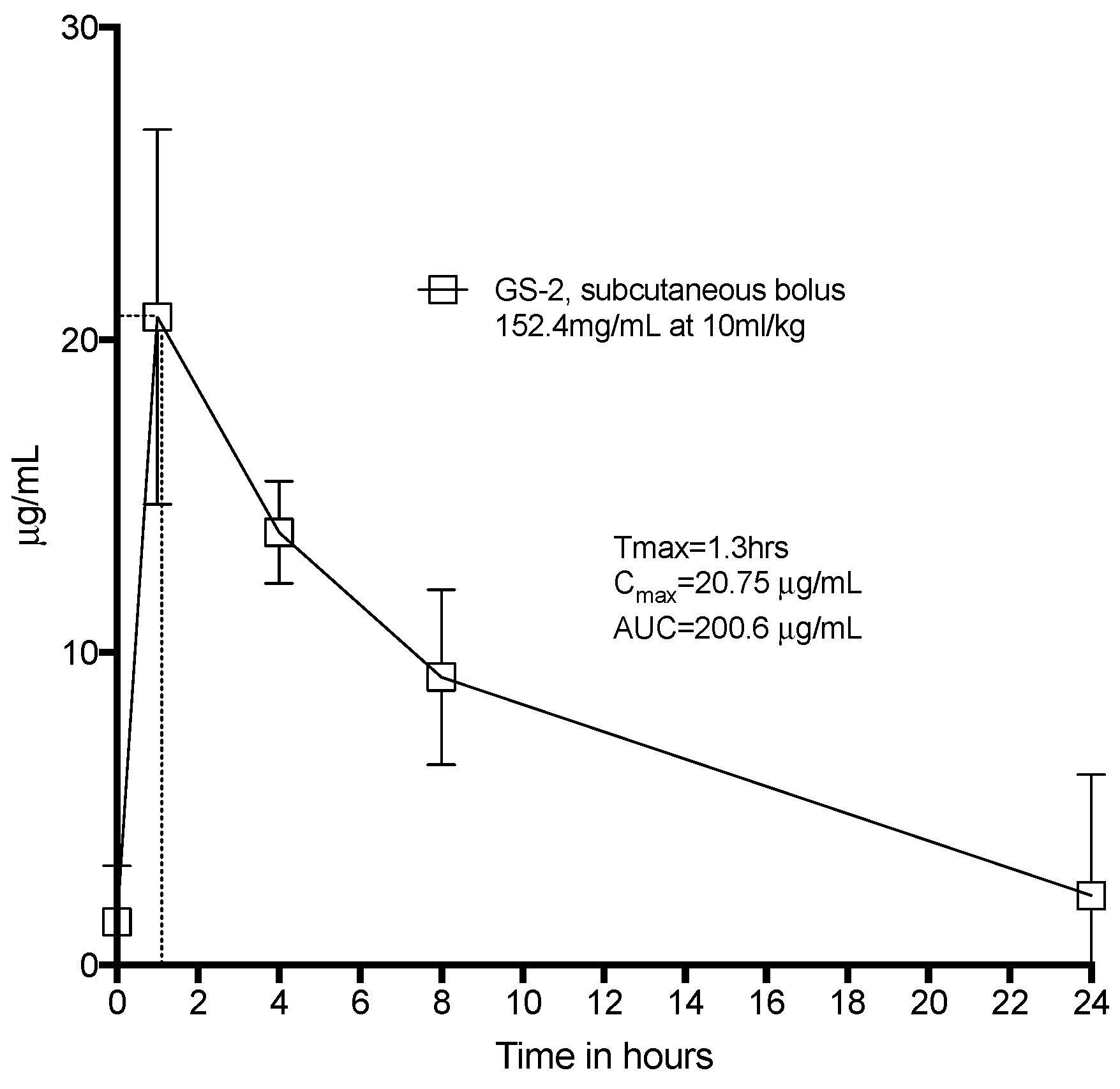
All animal handling and treatment was approved by the University of Melbourne Animal Ethics Committee. All animals were given free access to food, water, and enrichment. They were co-housed for a 72 h acclimation period prior to the beginning of experiments and then housed singly during the dosing protocol. Eight Sprague-Dawley rats were given a subcutaneous bolus dose of GS-2 at 10 mL/kg under the skin between the shoulder blades. Tail vein blood was drawn, processed, and assayed for capric acid at 1 h, 4 h, 8 h, and 24 h via LC-MS. During this period, the rats were observed for any adverse events or abnormal behavior. At 24 h, the animals were euthanized.
2.10. Hospital-Grade Disinfectant Testing EN13727
The GS-2 and thymol formulation (15.0 mg/mL GS-2 with 7.5 mg/mL thymol) was tested for activity against
S. aureus,
Enterococcus hirae (
E. hirae; ATCC 10541), and
P. aeruginosa according to European Standard (EN) 13727:2012 + A2:2015 [
35]: hospital-grade disinfectant with 5 min exposure under dirty conditions. Briefly, bacterial cultures were prepared and diluted to 1.5–5 × 10
8 CFU/mL. Each bacterial culture (1 mL) was combined with 1 mL interfering substance (3 g/L BSA, 3 mL/L erythrocytes) (THermo Fisher, Scoresby, Australia) and incubated at 20 °C for 2 min. Then, 8 mL GS-2 and thymol formulation was added and incubated for 5 min. Following incubation, 1 mL was transferred to a tube containing 8 mL neutralizer (polysorbate 80 (30 g/L) lecithin (3 g/L)) (Sigma-Aldrich, St Louis, MO, USA) and 1 mL water, mixed, incubated at 20 °C for 10 s, inoculated onto TSA plates in duplicate, and incubated overnight at 37 °C. Plates containing no colonies (i.e., ≥5-log reduction in growth) were considered a pass. Plates displaying one colony or more were considered a fail.
2.11. Testing Residual Surface Activity of GS-2 with Thymol
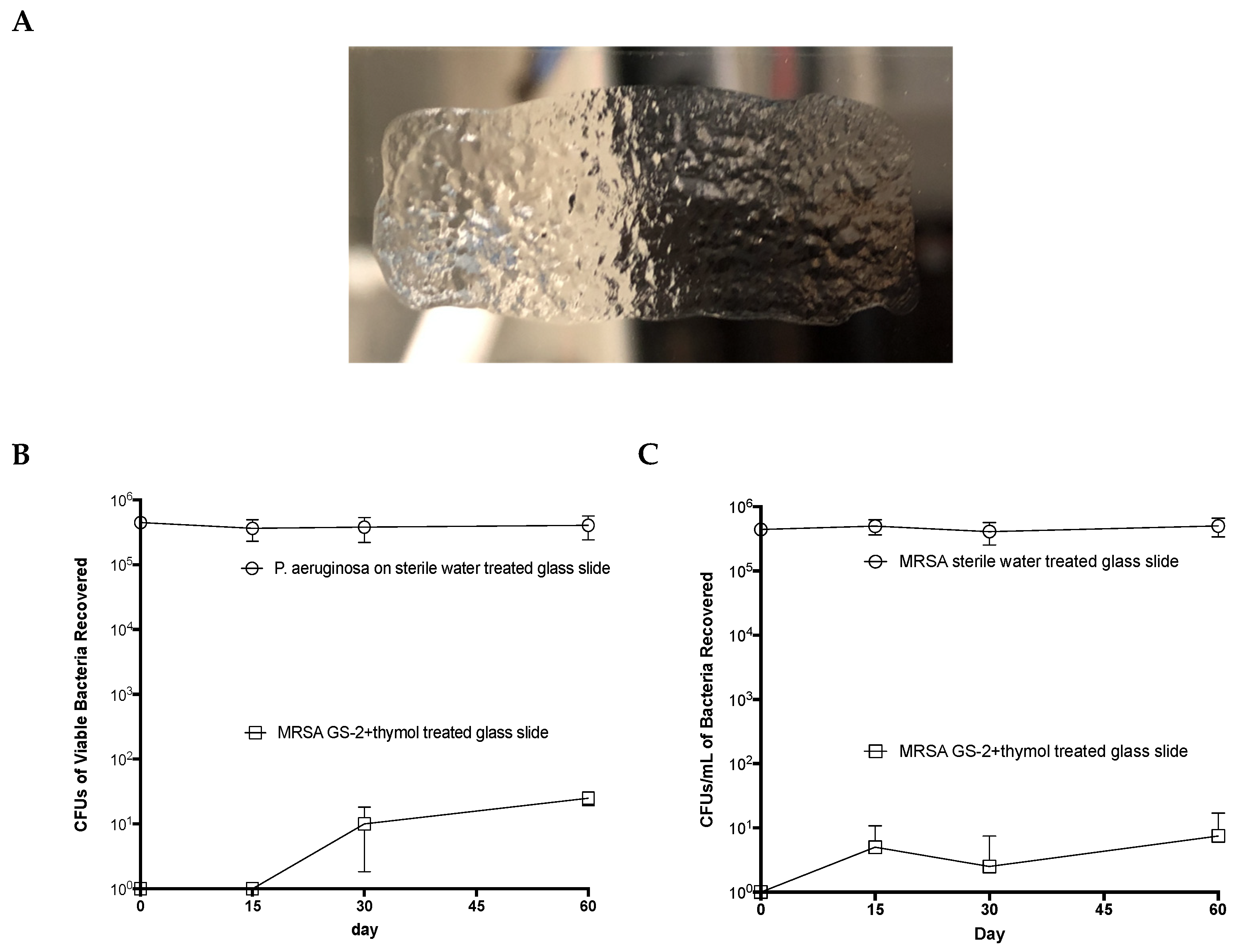
Sterile glass slides were coated with either 200 μL GS-2 with thymol or 200 μL sterile water and allowed to dry. On days 0, 15, 30, and 60, slides were inoculated with 1 106 CFUs MRSA or P. aeruginosa and incubated for 60 min, uncovered, at room temperature. Viable bacteria were then extracted from the slides, diluted, plated onto 5% SBA plates, incubated overnight, and colonies were counted. Enumeration was performed as per NCCLS/CLSI guideline M26-A.
2.12. Statistics
Statistical analyses (Student’s unpaired two-tailed t-test) were performed with GraphPad Prism 9.0 software (GraphPad Software, San Diego, CA, USA).
4. Discussion
The present study described the scientific development of an antimicrobial, moving from initial characterization to standard antimicrobial testing for efficacy and completing with the development of a novel surface disinfectant. The antimicrobial activity of fatty acids is well-known, with multiple studies examining their mechanisms of action and possible utility in controlling microbial contamination. However, a primary issue with fatty acids is very low solubility in water [
44].
In this study, we sought to overcome these issues by combining capric acid with L-arginine to produce a highly water-soluble fatty acid formulation that was effective against a range of bacteria, fungi, and viruses. In real-world testing, GS-2 demonstrated surface sanitizing efficacy over a current hospital-grade disinfectant. With the addition of thymol to GS-2, a rapid, robust antimicrobial was created that was active against bacteria and viruses in vitro and retained antibacterial properties on surfaces for at least two months.
Low fatty acid solubility in water has previously limited the use of fatty acids as antimicrobials. By adding L-arginine, the solubility of capric acid could be increased to 300 mg/mL. We proposed that capric acid, when combined with L-arginine, formed an ammonium carboxylate salt and that this reaction was what allowed for the high solubility in water. We demonstrated that no secondary compounds were formed upon combining these two molecules. This was not surprising since, for an amide bond to form between arginine and capric acid, the components need to be heated above 100 °C to drive off water or require a coupling reagent [
45], processes which were not performed during the compounding of GS-2 components. Furthermore, reducing the pH of GS-2 in the solution resulted in the separation of the components, further supporting the formation of a soluble salt.
The mechanism of action for antibacterial fatty acids has been defined in previous studies to be heavily dependent on three critical processes: (1) increased membrane permeability due to pore formation, leading to cell lysis; (2) attack of bacterial cell membranes via the electron transport chain, decreasing the production of ATP; and (3) inhibition of membrane enzymatic activities, such as glucosyltransferase, that inhibit nutrient uptake [
46,
47,
48,
49]. Interestingly, the results presented here showed that GS-2 was up to ten times more potent against GPCs (MRSA and
S. pyogenes) than GNRs (
E. coli and
K. pneumoniae). However, the required exposure time to show activity against GPCs was much longer (24 h) compared to GNRs. This finding is unusual as it is in direct contradiction with Kabara et al. (1980), where fatty acids with more than eight carbons were found not to inhibit GNR growth [
50]. It is possible that the effect against GNRs observed here was due to the chemical structure of the fatty acid in the solution and a lack of required excipients needed to solubilize the fatty acid that may have improved fatty acid activity in this study. Nonetheless, GS-2 still showed good potency against hardy environmental bacteria, as well as potential bioterrorism agent surrogates
B. anthracis Sterne and
B. thailandensis.
During developmental testing, increasing the concentration of GS-2 in the solution did not significantly improve the time-kill against GPCs, suggesting that the mechanism of action against GPCs was relatively independent of the concentration. This effect may be due to wall teichoic acids (WTAs) that attach to the peptidoglycan cell wall of
S. aureus, which as a result, may require more time for fatty acids to act [
51]. Although the cell walls of GNRs are considerably more complex than GPCs, WTAs are not typically found on GNR cell walls [
51], which could explain why, at equivalent concentrations, GNRs were more susceptible than GPCs over shorter timespans.
The addition of thymol to GS-2 significantly improved the activity of GS-2 against GPCs. GS-2 with thymol demonstrated superior time-kill activity against
S. aureus compared to GS-2 alone but did not have a remarkable improvement on the efficacy or time-kill effect for
E. coli or
K. pneumoniae. Thymol was previously shown to have bacteriostatic effects against
S. aureus at 0.31 mg/mL (MIC) and
E. coli at 5.0 mg/mL (MIC) when exposed for longer periods of time (18–24 h) [
52]. However, in disinfectant testing procedures where exposures are relatively short, thymol at 5.0 mg/mL (in DMSO) was unable to achieve a bactericidal effect. However, when 5.0 mg/mL thymol was combined with GS-2, it was able to kill both
S. aureus and
E. coli in timeframes acceptable for use as a surface disinfectant. This suggests that thymol may act synergistically with GS-2 to deliver bactericidal activity after just 8 min of exposure; however, further studies are required to validate this effect and the mechanism that produces it.
Like the effect against GNRs, GS-2 with thymol only showed a modest improvement in efficacy compared with GS-2 against the SARS-CoV-2 surrogate MHV-1 (from 4.92- to 5.42-log reduction of viral titer after treatment). Doubling the concentration of GS-2 and adding thymol did not produce a clear synergistic effect against the virus but rather a modest additive effect. Whilst thymol has been described as having antiviral activity, high concentrations are required, or cells need to be pre-treated with thymol before infection to observe an effect [
53,
54]. Thus, it is not surprising that only a modest improvement in the antiviral activity of GS-2 was observed with the addition of thymol here. Nevertheless, GS-2 with thymol still demonstrated activity against three different viruses, including non-enveloped poliovirus, which is commonly resistant to other disinfectants. This indicated that GS-2 with thymol may be a suitable antiviral agent over a broad range of virus types.
The testing performed with GS-2 and, subsequently, GS-2 with thymol supported several possible advantages of this fatty acid formulation as a multipurpose antimicrobial sanitizer, particularly over quat-based products. Firstly, GS-2 displayed activity against
C. auris, an emerging pathogen that is considered extremely difficult to eradicate with current disinfectant products [
55]. In this study, GS-2 and GS-2 with thymol also demonstrated efficacy against
C. difficile,
P. aeruginosa, and poliovirus, all of which are considered problematic for quats [
55,
56,
57]. It is also worth noting that the concentrations of GS-2 (capric acid; 15–30 mg/mL) and thymol (5–7.5 mg/mL) used in this study are comparable to the concentrations of BAC used in commercial disinfectants (1–30 mg/mL) [
56], suggesting that GS-2 with thymol would be an acceptable alternative to quats.
Additionally, the results from the comparative commercial building study and the glass slide experiment suggested that GS-2 remained active on surfaces for up to 60 days. Considering the structure of the ammonium carboxylate salt formed, it was not surprising that GS-2 remained unreactive to degradation. Furthermore, given the fatty acid structure, GS-2 may be less sensitive to organic material found on high-touch-point surfaces than BAC; it has been thoroughly established that quats such as BAC lose efficacy on soiled surfaces [
11,
12], which may account for the higher number of viable bacteria recovered from surfaces sanitized with BAC seen here. A recent study testing GS-2 in a pork abattoir also found GS-2 to have superior antibacterial activity on surfaces compared to a quat-based disinfectant [
58], further supporting its capability in a real-world setting. Together, these findings suggest that GS-2 may have broader bactericidal and virucidal activity against a range of pathogens when compared to currently available disinfectants.
Another advantage of GS-2 over other sanitizers is the safety profile; even high systemic doses of capric acid did not induce any toxicities in vivo. Like L-arginine and thymol, other fatty acids also have favorable safety profiles. Together, these results suggest utilizing amino acids to increase the water solubility of fatty acids may provide a platform for exploring, combining, and developing the antimicrobial activities of other fatty acids.
The current study presented scientific evidence regarding the development and testing of a novel antimicrobial ammonium carboxylate salt derived from a fatty acid and amino acid combination. Further evidence was presented that the addition of thymol, a 10-carbon monoterpenoid, to GS-2 enhanced the antimicrobial activity of GS-2, providing improved antimicrobial and disinfectant effects. Based on these data, GS-2 and GS-2 with thymol may have significant utility in the cleaning and disinfecting of surfaces, particularly in high-risk environments where drug-resistant pathogens pose a significant threat to human health. Finally, this platform of soluble fatty acids provides novel opportunities for the development of effective and safe broad-spectrum environmental antimicrobials.