Polyphenol and Tannin Nutraceuticals and Their Metabolites: How the Human Gut Microbiota Influences Their Properties
Abstract
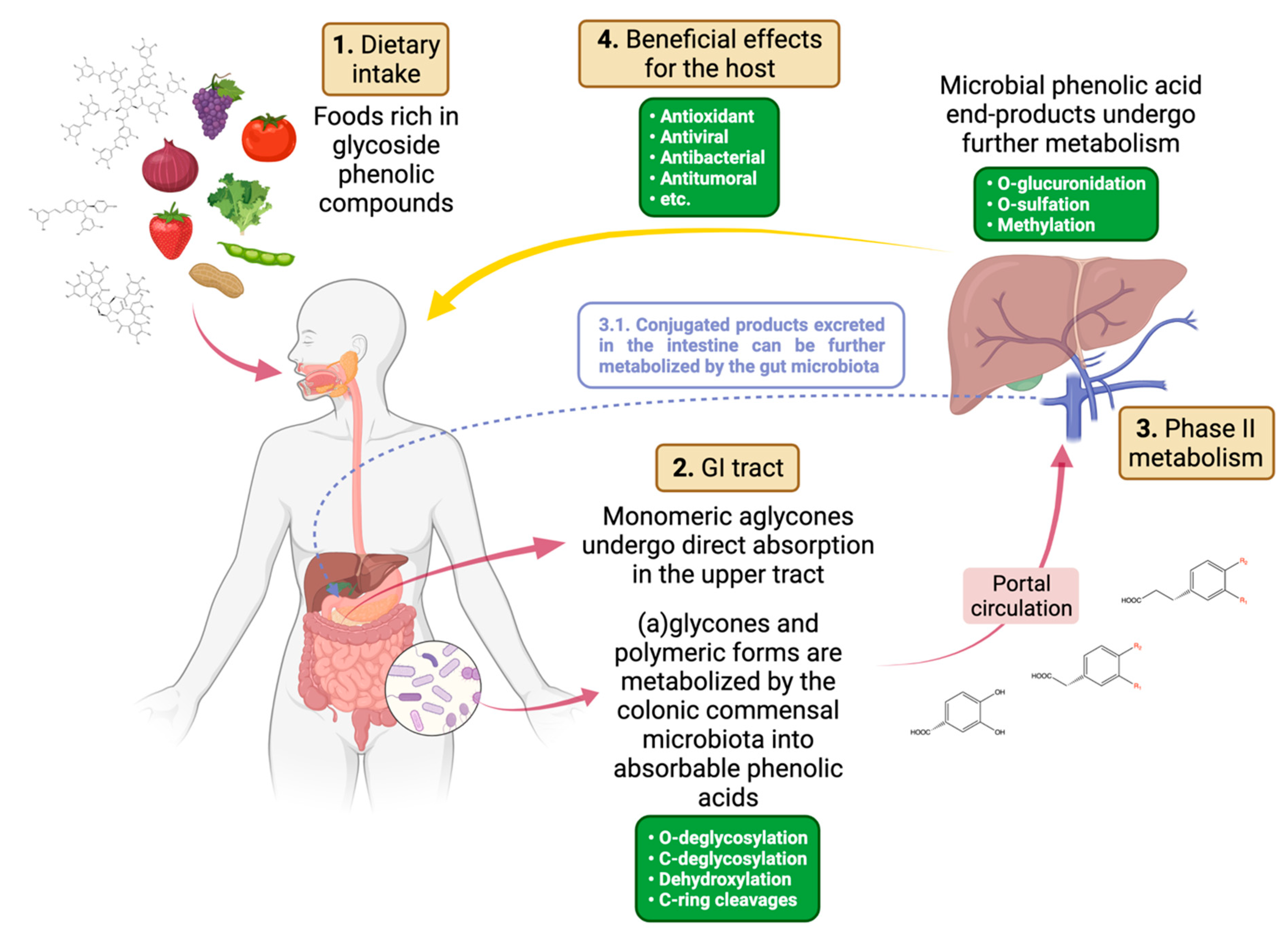
:1. Introduction
2. Polyphenols and Tannins
2.1. Flavonoids
2.1.1. Flavonols
2.1.2. Flavanones
2.1.3. Isoflavones
2.1.4. Flavones
2.1.5. Flavan-3-ols
2.1.6. Anthocyanins
2.2. Stilbenes
2.3. Phenolic Acids
2.4. Tannins
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Santini, A.; Tenore, G.C.; Novellino, E. Nutraceuticals: A paradigm of proactive medicine. Eur. J. Pharm. Sci. 2017, 96, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Soto-Hernandez, M.; Palma-Tenango, M.; del Rosario Garcia-Mateos, M. Phenolic Compounds—Biological Activity; InTech: Rijeka, Croatia, 2017; ISBN 978-953-51-2960-8. [Google Scholar]
- Rothwell, J.A.; Perez-Jimenez, J.; Neveu, V.; Medina-Remón, A.; M’Hiri, N.; García-Lobato, P.; Manach, C.; Knox, C.; Eisner, R.; Wishart, D.S.; et al. Phenol-explorer 3.0: A major update of the phenol-explorer database to incorporate data on the effects of food processing on polyphenol content. Database 2013, 2013, bat070. [Google Scholar] [CrossRef] [PubMed]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef] [PubMed]
- Cosme, P.; Rodríguez, A.B.; Espino, J.; Garrido, M. Plant phenolics: Bioavailability as a key determinant of their potential health-promoting applications. Antioxidants 2020, 9, 1263. [Google Scholar] [CrossRef]
- Tong, Z.; He, W.; Fan, X.; Guo, A. Biological function of plant tannin and its application in animal health. Front. Vet. Sci. 2022, 8, 803657. [Google Scholar] [CrossRef]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and human health: The role of bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef]
- Tena, N.; Martín, J.; Asuero, A.G. State of the art of anthocyanins: Antioxidant activity, sources, bioavailability, and therapeutic effect in human health. Antioxidants 2020, 9, 451. [Google Scholar] [CrossRef]
- Dower, J.I.; Geleijnse, J.M.; Kroon, P.A.; Philo, M.; Mensink, M.; Kromhout, D.; Hollman, P.C.H. Does epicatechin contribute to the acute vascular function effects of dark chocolate? A randomized, crossover study. Mol. Nutr. Food Res. 2016, 60, 2379–2386. [Google Scholar] [CrossRef]
- Smeriglio, A.; Barreca, D.; Bellocco, E.; Trombetta, D. Proanthocyanidins and hydrolysable tannins: Occurrence, dietary intake and pharmacological effects. Br. J. Pharmacol. 2017, 174, 1244–1262. [Google Scholar] [CrossRef] [Green Version]
- Kawabata, K.; Yoshioka, Y.; Terao, J. Role of intestinal microbiota in the bioavailability and physiological functions of dietary polyphenols. Molecules 2019, 24, 370. [Google Scholar] [CrossRef] [Green Version]
- Corrêa, T.A.F.; Rogero, M.M.; Hassimotto, N.M.A.; Lajolo, F.M. The two-way polyphenols-microbiota interactions and their effects on obesity and related metabolic diseases. Front. Nutr. 2019, 6, 188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashemzaei, M.; Far, A.D.; Yari, A.; Heravi, R.E.; Tabrizian, K.; Taghdisi, S.M.; Sadegh, S.E.; Tsarouhas, K.; Kouretas, D.; Tzanakakis, G.; et al. Anticancer and apoptosis-inducing effects of quercetin in vitro and in vivo. Oncol. Rep. 2017, 38, 819–828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leyva-Soto, A.; Alejandra Chavez-Santoscoy, R.; Porras, O.; Hidalgo-Ledesma, M.; Serrano-Medina, A.; Alejandra Ramírez-Rodríguez, A.; Alejandra Castillo-Martinez, N. Epicatechin and quercetin exhibit in vitro antioxidant effect, improve biochemical parameters related to metabolic syndrome, and decrease cellular genotoxicity in humans. Food Res. Int. 2021, 142, 110101. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Salehi, B.; Sharifi-Rad, J.; Gondal, T.A.; Saeed, F.; Imran, A.; Shahbaz, M.; Fokou, P.V.T.; Arshad, M.U.; Khan, H.; et al. Kaempferol: A key emphasis to its anticancer potential. Molecules 2019, 24, 2277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bondonno, N.P.; Dalgaard, F.; Kyrø, C.; Murray, K.; Bondonno, C.P.; Lewis, J.R.; Croft, K.D.; Gislason, G.; Scalbert, A.; Cassidy, A.; et al. Flavonoid intake is associated with lower mortality in the danish diet cancer and health cohort. Nat. Commun. 2019, 10, 3651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menezes, R.; Rodriguez-Mateos, A.; Kaltsatou, A.; González-Sarrías, A.; Greyling, A.; Giannaki, C.; Andres-Lacueva, C.; Milenkovic, D.; Gibney, E.R.; Dumont, J.; et al. Impact of flavonols on cardiometabolic biomarkers: A meta-analysis of randomized controlled human trials to explore the role of inter-individual variability. Nutrients 2017, 9, 117. [Google Scholar] [CrossRef]
- Adamczak, A.; Ożarowski, M.; Karpiński, T.M. Antibacterial activity of some flavonoids and organic acids widely distributed in plants. J. Clin. Med. 2020, 9, 109. [Google Scholar] [CrossRef] [Green Version]
- Mouffouk, C.; Mouffouk, S.; Mouffouk, S.; Hambaba, L.; Haba, H. Flavonols as potential antiviral drugs targeting SARS-CoV-2 proteases (3CLpro and PLpro), spike protein, RNA-dependent RNA polymerase (RdRp) and angiotensin-converting enzyme II receptor (ACE2). Eur. J. Pharmacol. 2021, 891, 173759. [Google Scholar] [CrossRef]
- Zakaryan, H.; Arabyan, E.; Oo, A.; Zandi, K. Flavonoids: Promising Natural Compounds against Viral Infections. Arch. Virol. 2017, 162, 2539–2551. [Google Scholar] [CrossRef]
- Marín, L.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Bioavailability of dietary polyphenols and gut microbiota metabolism: Antimicrobial properties. Biomed. Res. Int. 2015, 2015, 905215. [Google Scholar] [CrossRef] [Green Version]
- Zamora-Ros, R.; Knaze, V.; Rothwell, J.A.; Hémon, B.; Moskal, A.; Overvad, K.; Tjønneland, A.; Kyrø, C.; Fagherazzi, G.; Boutron-Ruault, M.C.; et al. Dietary polyphenol intake in Europe: The European prospective investigation into cancer and nutrition (EPIC) study. Eur. J. Nutr. 2016, 55, 1359–1375. [Google Scholar] [CrossRef] [PubMed]
- Rogozinska, M.; Biesaga, M. Decomposition of flavonols in the presence of saliva. Appl. Sci. 2020, 10, 7511. [Google Scholar] [CrossRef]
- Riva, A.; Kolimár, D.; Spittler, A.; Wisgrill, L.; Herbold, C.W.; Abrankó, L.; Berry, D. Conversion of rutin, a prevalent dietary flavonol, by the human gut microbiota. Front. Microbiol. 2020, 11, 585428. [Google Scholar] [CrossRef] [PubMed]
- Beekwilder, J.; Marcozzi, D.; Vecchi, S.; De Vos, R.; Janssen, P.; Francke, C.; Vlieg, J.V.H.; Hall, R.D. Characterization of rhamnosidases from Lactobacillus plantarum and Lactobacillus acidophilus. Appl. Environ. Microbiol. 2009, 75, 3447–3454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bang, S.H.; Hyun, Y.J.; Shim, J.; Hong, S.W.; Kim, D.H. Metabolism of rutin and poncirin by human intestinal microbiota and cloning of their metabolizing α-L-rhamnosidase from bifidobacterium dentium. J. Microbiol. Biotechnol. 2015, 25, 18–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murota, K.; Terao, J. Antioxidative flavonoid quercetin: Implication of its intestinal absorption and metabolism. Arch. Biochem. Biophys. 2003, 417, 12–17. [Google Scholar] [CrossRef]
- Najmanová, I.; Najmanová, N.; Pourová, J.; Pourová, P.; Vopř Salovásalov´salová, M.; Pilařov, V.; Pilařová, P.; Semeck´y, V.; Semeck´y, S.; Nováková, L.; et al. Flavonoid metabolite 3-(3-Hydroxyphenyl)propionic acid formed by human microflora decreases arterial blood pressure in rats. Mol. Nutr. Food Res. 2016, 60, 981–991. [Google Scholar] [CrossRef] [PubMed]
- Santangelo, R.; Silvestrini, A.; Mancuso, C. Ginsenosides, catechins, quercetin and gut microbiota: Current evidence of challenging interactions. Food Chem. Toxicol. 2019, 123, 42–49. [Google Scholar] [CrossRef]
- Dhuique-Mayer, C.; Caris-Veyrat, C.; Ollitrault, P.; Curk, F.; Amiot, M.J. Varietal and interspecific influence on micronutrient contents in citrus from the Mediterranean area. J. Agric. Food Chem. 2005, 53, 2140–2145. [Google Scholar] [CrossRef]
- Denaro, M.; Smeriglio, A.; Trombetta, D. Antioxidant and anti-inflammatory activity of citrus flavanones mix and its stability after in vitro simulated digestion. Antioxidants 2021, 10, 140. [Google Scholar] [CrossRef]
- Boniface, P.K.; Elizabeth, F.I. Flavones as a privileged scaffold in drug discovery: Current developments. Curr. Org. Synth. 2019, 16, 968–1001. [Google Scholar] [CrossRef] [PubMed]
- Schar, M.Y.; Curtis, P.J.; Hazim, S.; Ostertag, L.M.; Kay, C.D.; Potter, J.F.; Cassidy, A. Orange juice–derived flavanone and phenolic metabolites do not acutely affect cardiovascular risk biomarkers: A randomized, placebo-controlled, crossover trial in men at moderate risk of cardiovascular disease. Am. J. Clin. Nutr. 2015, 101, 931–938. [Google Scholar] [CrossRef] [Green Version]
- Salehi, B.; Fokou, P.V.T.; Sharifi-Rad, M.; Zucca, P.; Pezzani, R.; Martins, N.; Sharifi-Rad, J. The therapeutic potential of naringenin: A review of clinical trials. Pharmaceuticals 2019, 12, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Nie, M.; Liu, C.; Jiang, N.; Liu, C.; Li, D. Citrus flavanones enhance β-carotene uptake in vitro experiment using Caco-2 cell: Structure-activity relationship and molecular mechanisms. J. Agric. Food Chem. 2019, 67, 4280–4288. [Google Scholar] [CrossRef] [PubMed]
- Fareza, M.S.; Syah, Y.M.; Mujahidin, D.; Juliawaty, L.D.; Kurniasih, I. Antibacterial flavanones and dihydrochalcones from macaranga trichocarpa. Z. Nat. C J. Biosci. 2014, 69, 375–380. [Google Scholar] [CrossRef]
- Valdés, E.; González, C.; Díaz, K.; Vásquez-Martínez, Y.; Mascayano, C.; Torrent, C.; Cabezas, F.; Mejias, S.; Montoya, M.; Cortez-San Martín, M.; et al. Biological properties and absolute configuration of flavanones from calceolaria thyrsiflora graham. Front. Pharmacol. 2020, 11, 1125. [Google Scholar] [CrossRef]
- Tutunchi, H.; Naeini, F.; Ostadrahimi, A.; Hosseinzadeh-Attar, M.J. Naringenin, a Flavanone with antiviral and anti-inflammatory effects: A promising treatment strategy against COVID-19. Phyther. Res. 2020, 34, 3137–3147. [Google Scholar] [CrossRef]
- Serra, A.; MacIà, A.; Romero, M.P.; Reguant, J.; Ortega, N.; Motilva, M.J. Metabolic pathways of the colonic metabolism of flavonoids (flavonols, flavones and flavanones) and phenolic acids. Food Chem. 2012, 130, 383–393. [Google Scholar] [CrossRef]
- Ávila-gálvez, M.Á.; Giménez-bastida, J.A.; González-sarrías, A.; Espín, J.C. New insights into the metabolism of the flavanones eriocitrin and hesperidin: A comparative human pharmacokinetic study. Antioxidants 2021, 10, 435. [Google Scholar] [CrossRef]
- Anacleto, S.L.; Milenkovic, D.; Kroon, P.A.; Needs, P.W.; Lajolo, F.M.; Hassimotto, N.M.A. Citrus flavanone metabolites protect pancreatic-β cells under oxidative stress induced by cholesterol. Food Funct. 2020, 11, 8612–8624. [Google Scholar] [CrossRef]
- Braune, A.; Blaut, M. Bacterial species involved in the conversion of dietary flavonoids in the human gut. Gut Microbes 2016, 7, 216–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, J.; Bi, X.; Yu, B.; Chen, D. Isoflavones: Anti-inflammatory benefit and possible caveats. Nutrients 2016, 8, 361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vitale, D.C.; Piazza, C.; Melilli, B.; Drago, F.; Salomone, S. Isoflavones: Estrogenic activity, biological effect and bioavailability. Eur. J. Drug Metab. Pharmacokinet. 2013, 38, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Messina, M.; Mejia, S.B.; Cassidy, A.; Duncan, A.; Kurzer, M.; Nagato, C.; Ronis, M.; Rowland, I.; Sievenpiper, J.; Barnes, S. Neither soyfoods nor isoflavones warrant classification as endocrine disruptors: A technical review of the observational and clinical data. Crit. Rev. Food Sci. Nutr. 2021, 61, 1–57. [Google Scholar] [CrossRef]
- Lambert, M.N.T.; Thybo, C.B.; Lykkeboe, S.; Rasmussen, L.M.; Frette, X.; Christensen, L.P.; Jeppesen, P.B. Combined bioavailable isoflavones and probiotics improve bone status and estrogen metabolism in postmenopausal osteopenic women: A randomized controlled trial. Am. J. Clin. Nutr. 2017, 106, 909–920. [Google Scholar] [CrossRef] [Green Version]
- Albert Dhayakaran, R.P.; Neethirajan, S.; Xue, J.; Shi, J. Characterization of antimicrobial efficacy of soy isoflavones against pathogenic biofilms. LWT Food Sci. Technol. 2015, 63, 859–865. [Google Scholar] [CrossRef]
- Kalli, S.; Araya-Cloutier, C.; Hageman, J.; Vincken, J.P. Insights into the molecular properties underlying antibacterial activity of prenylated (iso)flavonoids against MRSA. Sci. Rep. 2021, 11, 14180. [Google Scholar] [CrossRef]
- Smith, B.N.; Oelschlager, M.L.; Abdul Rasheed, M.S.; Dilger, R.N. Dietary soy isoflavones reduce pathogen-related mortality in growing pigs under porcine reproductive and respiratory syndrome viral challenge. J. Anim. Sci. 2020, 98, skaa024. [Google Scholar] [CrossRef]
- Toro-Funes, N.; Morales-Gutiérrez, F.J.; Veciana-Nogués, M.T.; Vidal-Carou, M.C.; Spencer, J.P.E.; Rodriguez-Mateos, A. The intracellular metabolism of isoflavones in endothelial cells. Food Funct. 2015, 6, 97–107. [Google Scholar] [CrossRef]
- Gaya, P.; Peirotén, Á.; Medina, M.; Landete, J.M. Isoflavone metabolism by a collection of lactic acid bacteria and bifidobacteria with biotechnological interest. Int. J. Food Sci. Nutr. 2016, 67, 117–124. [Google Scholar] [CrossRef]
- Wang, X.-L.L.; Kim, H.-J.J.; Kang, S.-I.; Kim, S.-I.; Hur, H.-G. Production of phytoestrogen s-equol from daidzein in mixed culture of two anaerobic bacteria. Arch. Microbiol. 2007, 187, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Alshehri, M.M.; Sharifi-Rad, J.; Herrera-Bravo, J.; Jara, E.L.; Salazar, L.A.; Kregiel, D.; Uprety, Y.; Akram, M.; Iqbal, M.; Martorell, M.; et al. Therapeutic potential of isoflavones with an emphasis on daidzein. Oxid. Med. Cell. Longev. 2021, 2021, 6331630. [Google Scholar] [CrossRef] [PubMed]
- Sekikawa, A.; Ihara, M.; Lopez, O.; Kakuta, C.; Lopresti, B.; Higashiyama, A.; Aizenstein, H.; Chang, Y.-F.; Mathis, C.; Miyamoto, Y.; et al. Effect of S-equol and soy isoflavones on heart and brain. Curr. Cardiol. Rev. 2018, 15, 114–135. [Google Scholar] [CrossRef]
- Frankenfeld, C.L.; Atkinson, C.; Wähälä, K.; Lampe, J.W. Obesity prevalence in relation to gut microbial environments capable of producing equol or O-desmethylangolensin from the isoflavone daidzein. Eur. J. Clin. Nutr. 2014, 68, 526–530. [Google Scholar] [CrossRef] [PubMed]
- Shoaib, M.; Shah, S.W.A.; Ali, N.; Shah, I.; Naveed Umar, M.; Shafiullah; Ayaz, M.; Tahir, M.N.; Akhtar, S. In Vitro enzyme inhibition potentials and antioxidant activity of synthetic flavone derivatives. J. Chem. 2015, 2015, 516878. [Google Scholar] [CrossRef] [Green Version]
- Raina, R.; Hussain, A.; Sharma, R. Molecular insight into apoptosis mediated by flavones in cancer. World Acad. Sci. J. 2020, 2, 1. [Google Scholar] [CrossRef]
- Lin, C.H.; Chang, C.Y.; Lee, K.R.; Lin, H.J.; Chen, T.H.; Wan, L. Flavones inhibit breast cancer proliferation through the Akt/FOXO3a signaling pathway. BMC Cancer 2015, 15, 958. [Google Scholar] [CrossRef] [Green Version]
- Hostetler, G.L.; Ralston, R.A.; Schwartz, S.J. Flavones: Food Sources, Bioavailability, Metabolism, and Bioactivity. Adv. Nutr. 2017, 8, 423–435. [Google Scholar] [CrossRef] [Green Version]
- Noh, K.; Kang, Y.; Nepal, M.R.; Jeong, K.S.; Oh, D.G.; Kang, M.J.; Lee, S.; Kang, W.; Jeong, H.G.; Jeong, T.C. Role of intestinal microbiota in baicalin-induced drug interaction and its pharmacokinetics. Molecules 2016, 21, 337. [Google Scholar] [CrossRef] [Green Version]
- Braune, A.; Blaut, M. Intestinal bacterium eubacterium cellulosolvens deglycosylates flavonoid C- and o-glucosides. Appl. Environ. Microbiol. 2012, 78, 8151–8153. [Google Scholar] [CrossRef] [Green Version]
- Yang, G.; Hong, S.; Yang, P.; Sun, Y.; Wang, Y.; Zhang, P.; Jiang, W.; Gu, Y. Discovery of an ene-reductase for initiating flavone and flavonol catabolism in gut bacteria. Nat. Commun. 2021, 12, 790. [Google Scholar] [CrossRef] [PubMed]
- Hanske, L.; Loh, G.; Sczesny, S.; Blaut, M.; Braune, A. The Bioavailability of apigenin-7-glucoside is influenced by human intestinal microbiota in rats. J. Nutr. 2009, 139, 1095–1102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, L.; Vance, T.; Kim, B.; Lee, S.G.; Caceres, C.; Wang, Y.; Hubert, P.A.; Lee, J.Y.; Chun, O.K.; Bolling, B.W. Aronia berry polyphenol consumption reduces plasma total and low-density lipoprotein cholesterol in former smokers without lowering biomarkers of inflammation and oxidative stress: A randomized controlled trial. Nutr. Res. 2017, 37, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, Y.; Zhu, T.; Yang, S.; Cao, J.; Li, X.; Wang, L.S.; Sun, C. Beneficial regulatory effects of polymethoxyflavone—rich fraction from ougan (Citrus Reticulata Cv. Suavissima) fruit on gut microbiota and identification of its intestinal metabolites in mice. Antioxidants 2020, 9, 831. [Google Scholar] [CrossRef]
- Guruvayoorappan, C.; Kuttan, G. (+)-Catechin Inhibits tumour angiogenesis and regulates the production of nitric oxide and TNF-α in LPS-stimulated macrophages. Innate Immun. 2008, 14, 160–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Núñez-iglesias, M.J.; Novio, S.; García, C.; Pérez-muñuzuri, M.E.; Martínez, M.C.; Santiago, J.L.; Boso, S.; Gago, P.; Freire-garabal, M. Co-adjuvant therapy efficacy of catechin and procyanidin B2 with docetaxel on hormone-related cancers in vitro. Int. J. Mol. Sci. 2021, 22, 7178. [Google Scholar] [CrossRef]
- Gupta, S.C.; Kannappan, R.; Reuter, S.; Kim, J.H.; Aggarwal, B.B. Chemosensitization of tumors by resveratrol. Ann. N. Y. Acad. Sci. 2011, 1215, 150–160. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.C.; Kim, J.H.; Prasad, S.; Aggarwal, B.B. Regulation of survival, proliferation, invasion, angiogenesis, and metastasis of tumor cells through modulation of inflammatory pathways by nutraceuticals. Cancer Metastasis Rev. 2010, 29, 405–434. [Google Scholar] [CrossRef] [Green Version]
- Lei, L.; Yang, Y.; He, H.; Chen, E.; Du, L.; Dong, J.; Yang, J.; Lei, L.; Yang, Y.; He, H.; et al. Flavan-3-Ols consumption and cancer risk: A meta-analysis of epidemiologic studies. Oncotarget 2016, 7, 73573–73592. [Google Scholar] [CrossRef] [Green Version]
- Rigling, M.; Liu, Z.; Hofele, M.; Prozmann, J.; Zhang, C.; Ni, L.; Fan, R.; Zhang, Y. Aroma and catechin profile and in vitro antioxidant activity of green tea infusion as affected by submerged fermentation with Wolfiporia cocos (Fu Ling). Food Chem. 2021, 361, 130065. [Google Scholar] [CrossRef]
- Nguyen, C.T.; Lee, A.H.; Pham, N.M.; Van Do, V.; Ngu, N.D.; Tran, B.Q.; Binns, C. Habitual tea drinking associated with a lower risk of type 2 diabetes in Vietnamese adults. Asia Pac. J. Clin. Nutr. 2018, 27, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ouyang, Y.Y.; Liu, J.; Zhao, G. Flavonoid intake and risk of CVD: A systematic review and meta-analysis of prospective cohort studies. Br. J. Nutr. 2014, 111, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, J.; Xu, Z.; Zheng, W. A review of the antiviral role of green tea catechins. Molecules 2017, 22, 1337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miklasińska, M.; Kȩpa, M.; Wojtyczka, R.D.; Idzik, D.; Dziedzic, A.; Wąsik, T.J. Catechin hydrate augments the antibacterial action of selected antibiotics against Staphylococcus aureus clinical strains. Molecules 2016, 21, 244. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Venancio, V.P.; Fang, C.; Dupont, A.W.; Talcott, S.T.; Mertens-Talcott, S.U. Mango (Mangifera indica L.) polyphenols reduce IL-8, GRO, and GM-SCF plasma levels and increase lactobacillus species in a pilot study in patients with inflammatory bowel disease. Nutr. Res. 2020, 75, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Monagas, M.; Urpi-Sarda, M.; Sánchez-Patán, F.; Llorach, R.; Garrido, I.; Gómez-Cordovés, C.; Andres-Lacueva, C.; Bartolomé, B. Insights into the metabolism and microbial biotransformation of dietary Flavan-3-Ols and the bioactivity of their metabolites. Food Funct. 2010, 1, 233–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef]
- Liu, Z.; De Bruijn, W.J.C.; Bruins, M.E.; Vincken, J.P. Microbial metabolism of theaflavin-3,3′-digallate and its gut microbiota composition modulatory effects. J. Agric. Food Chem. 2021, 69, 232–245. [Google Scholar] [CrossRef]
- Campos, E.M.; Stehle, P.; Simon, M.C. Microbial metabolites of Flavan-3-Ols and their biological activity. Nutrients 2019, 11, 2260. [Google Scholar] [CrossRef] [Green Version]
- Mena, P.; González de Llano, D.; Brindani, N.; Esteban-Fernández, A.; Curti, C.; Moreno-Arribas, M.V.; Del Rio, D.; Bartolomé, B. 5-(3′,4′-Dihydroxyphenyl)-γ-valerolactone and its sulphate conjugates, representative circulating metabolites of Flavan-3-Ols, exhibit anti-adhesive activity against uropathogenic Escherichia coli in bladder epithelial cells. J. Funct. Foods 2017, 29, 275–280. [Google Scholar] [CrossRef]
- Fu, Z.; Liska, D.A.; Talan, D.; Chung, M. Cranberry reduces the risk of urinary tract infection recurrence in otherwise healthy women: A systematic review and meta-analysis. J. Nutr. 2017, 147, 2282–2288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, T.; Hu, N.; Ding, C.; Zhang, Q.; Li, W.; Suo, Y.; Wang, H.; Bai, B.; Ding, C. In Vitro and In Vivo biological activities of anthocyanins from Nitraria tangutorun bobr. fruits. Food Chem. 2016, 194, 296–303. [Google Scholar] [CrossRef]
- Gaiz, A.; Kundur, A.R.; Colson, N.; Shibeeb, S.; Singh, I. Assessment of in vitro effects of anthocyanins on platelet function. Altern. Ther. Health Med. 2020, 26, 12–17. [Google Scholar] [PubMed]
- Demeilliers, C.; Toufektsian, M.C.; Salen, P.; Laporte, F.; Petroni, K.; de Lorgeril, M. Ethanol drinking, brain mitochondrial DNA, polyunsaturated fatty acids and effects of dietary anthocyanins. Clin. Nutr. Exp. 2017, 12, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Magni, G.; Marinelli, A.; Riccio, D.; Lecca, D.; Tonelli, C.; Abbracchio, M.P.; Petroni, K.; Ceruti, S. Purple corn extract as anti-allodynic treatment for trigeminal pain: Role of microglia. Front. Cell. Neurosci. 2018, 12, 378. [Google Scholar] [CrossRef] [PubMed]
- García-Conesa, M.T.; Chambers, K.; Combet, E.; Pinto, P.; Garcia-Aloy, M.; Andrés-Lacueva, C.; De Pascual-Teresa, S.; Mena, P.; Ristic, A.K.; Hollands, W.J.; et al. Meta-analysis of the effects of foods and derived products containing ellagitannins and anthocyanins on cardiometabolic biomarkers: Analysis of factors influencing variability of the individual responses. Int. J. Mol. Sci. 2018, 19, 694. [Google Scholar] [CrossRef] [Green Version]
- Thompson, K.; Hosking, H.; Pederick, W.; Singh, I.; Santhakumar, A.B. The effect of anthocyanin supplementation in modulating platelet function in sedentary population: A randomised, double-blind, placebo-controlled, cross-over trial. Br. J. Nutr. 2017, 118, 368–374. [Google Scholar] [CrossRef]
- Thompson, K.; Pederick, W.; Singh, I.; Santhakumar, A.B. Anthocyanin supplementation in alleviating thrombogenesis in overweight and obese population: A randomized, double-blind, placebo-controlled study. J. Funct. Foods 2017, 32, 131–138. [Google Scholar] [CrossRef]
- Cassidy, A.; Bertoia, M.; Chiuve, S.; Flint, A.; Forman, J.; Rimm, E.B. Habitual intake of anthocyanins and flavanones and risk of cardiovascular disease in men. Am. J. Clin. Nutr. 2016, 104, 587–594. [Google Scholar] [CrossRef] [Green Version]
- Pour, P.M.; Fakhri, S.; Asgary, S.; Farzaei, M.H.; Echeverría, J. The signaling pathways, and therapeutic targets of antiviral agents: Focusing on the antiviral approaches and clinical perspectives of anthocyanins in the management of viral diseases. Front. Pharmacol. 2019, 10, 1207. [Google Scholar] [CrossRef] [Green Version]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ávila, M.; Hidalgo, M.; Sánchez-Moreno, C.; Pelaez, C.; Requena, T.; Pascual-Teresa, S. de Bioconversion of anthocyanin glycosides by Bifidobacteria and Lactobacillus. Food Res. Int. 2009, 42, 1453–1461. [Google Scholar] [CrossRef]
- Semaming, Y.; Pannengpetch, P.; Chattipakorn, S.C.; Chattipakorn, N. Pharmacological properties of protocatechuic acid and its potential roles as complementary medicine. Evid. Based Complement Altern. Med. 2015, 2015, 593902. [Google Scholar] [CrossRef] [Green Version]
- Shin, S.Y.; Cha, B.K.; Kim, W.S.; Park, J.Y.; Kim, J.W.; Choi, C.H. The effect of phloroglucinol in patients with diarrhea-predominant irritable bowel syndrome: A randomized, double-blind, placebo-controlled trial. J. Neurogastroenterol. Motil. 2020, 26, 117–127. [Google Scholar] [CrossRef] [Green Version]
- Jung, H.I.; Kim, H.J.; Choi, E.S.; Lee, J.Y.; Park, K.S.; Cho, K.B.; Lee, Y.J. Effectiveness of oral phloroglucinol as a premedication for unsedated esophagogastroduodenoscopy: A prospective, double-blinded, placebo-controlled, randomized trial. PLoS ONE 2021, 16, e0255016. [Google Scholar] [CrossRef] [PubMed]
- Kiokias, S.; Oreopoulou, V. A review of the health protective effects of phenolic acids against a range of severe pathologic conditions (including coronavirus-based infections). Molecules 2021, 26, 5405. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Zhang, P.; Zhu, Y.; Lou, Q.; He, S. Antioxidant and prebiotic activity of five peonidin-based anthocyanins extracted from purple sweet potato (Ipomoea Batatas (L.) Lam.). Sci. Rep. 2018, 8, 5018. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Tan, Y.; Chen, G.; Wang, G.; Sun, J.; Ou, S.; Chen, W.; Bai, W. Metabolism of anthocyanins and consequent effects on the gut microbiota. Crit. Rev. Food Sci. Nutr. 2018, 59, 982–991. [Google Scholar] [CrossRef] [PubMed]
- Leporini, L.; Giampietro, L.; Amoroso, R.; Ammazzalorso, A.; Fantacuzzi, M.; Menghini, L.; Maccallini, C.; Ferrante, C.; Brunetti, L.; Orlando, G.; et al. In Vitro protective effects of resveratrol and stilbene alkanoic derivatives on induced oxidative stress on C2C12 and MCF7 cells. J. Biol. Regul. Homeost. Agents 2017, 31, 589–601. [Google Scholar]
- Truong, V.L.; Jun, M.; Jeong, W.S. Role of resveratrol in regulation of cellular defense systems against oxidative stress. BioFactors 2018, 44, 36–49. [Google Scholar] [CrossRef]
- Freyssin, A.; Page, G.; Fauconneau, B.; Rioux Bilan, A. Natural stilbenes effects in animal models of alzheimer’s disease. Neural Regen. Res. 2020, 15, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Rauf, A.; Imran, M.; Sulera, H.A.R.; Ahmad, B.; Peters, D.G.; Mubarak, M.S. A comprehensive review of the health perspectives of resveratrol. Food Funct. 2017, 8, 4284–4305. [Google Scholar] [CrossRef] [PubMed]
- Mattio, L.M.; Catinella, G.; Dallavalle, S.; Pinto, A. Stilbenoids: A natural arsenal against bacterial pathogens. Antibiotics 2020, 9, 336. [Google Scholar] [CrossRef] [PubMed]
- Righi, D.; Huber, R.; Koval, A.; Marcourt, L.; Schnee, S.; Le Floch, A.; Ducret, V.; Perozzo, R.; De Ruvo, C.C.; Lecoultre, N.; et al. Generation of stilbene antimicrobials against multiresistant strains of Staphylococcus aureus through biotransformation by the enzymatic secretome of Botrytis cinerea. J. Nat. Prod. 2020, 83, 2347–2356. [Google Scholar] [CrossRef]
- Goddard, T.N.; Patel, J.; Park, H.B.; Crawford, J.M. Dimeric stilbene antibiotics target the bacterial cell wall in drug-resistant gram-positive pathogens. Biochemistry 2020, 59, 1966–1971. [Google Scholar] [CrossRef]
- Medrano-Padial, C.; Puerto, M.; Richard, T.; Cantos-Villar, E.; Pichardo, S. Protection and reversion role of a pure stilbene extract from grapevine shoot and its major compounds against an induced oxidative stress. J. Funct. Foods 2021, 79, 104393. [Google Scholar] [CrossRef]
- Medrano-Padial, C.; Puerto, M.; Prieto, A.I.; Ayala, N.; Beaumont, P.; Rouger, C.; Krisa, S.; Pichardo, S. In Vivo Genotoxicity Evaluation of a stilbene extract prior to its use as a natural additive: A combination of the micronucleus test and the comet assay. Foods 2021, 10, 439. [Google Scholar] [CrossRef]
- Filardo, S.; Di Pietro, M.; Mastromarino, P.; Sessa, R. Therapeutic potential of resveratrol against emerging respiratory viral infections. Pharmacol. Ther. 2020, 214, 107613. [Google Scholar] [CrossRef]
- Sirerol, J.A.; Rodríguez, M.L.; Mena, S.; Asensi, M.A.; Estrela, J.M.; Ortega, A.L. Role of natural stilbenes in the prevention of cancer. Oxid. Med. Cell. Longev. 2016, 2016, 3128951. [Google Scholar] [CrossRef] [Green Version]
- Giuliani, C.; Marzorati, M.; Innocenti, M.; Vilchez-Vargas, R.; Vital, M.; Pieper, D.H.; Van De Wiele, T.; Mulinacci, N. Dietary Supplement Based on Stilbenes: A focus on gut microbial metabolism by the in vitro simulator M-SHIME®. Food Funct. 2016, 7, 4564–4575. [Google Scholar] [CrossRef]
- Jarosova, V.; Vesely, O.; Marsik, P.; Jaimes, J.D.; Smejkal, K.; Kloucek, P.; Havlik, J. Metabolism of stilbenoids by human faecal microbiota. Molecules 2019, 24, 1155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bode, L.M.; Bunzel, D.; Huch, M.; Cho, G.S.; Ruhland, D.; Bunzel, M.; Bub, A.; Franz, C.M.A.P.; Kulling, S.E. In Vivo and in vitro metabolism of trans-resveratrol by human gut microbiota. Am. J. Clin. Nutr. 2013, 97, 295–309. [Google Scholar] [CrossRef] [PubMed]
- Reinisalo, M.; Kårlund, A.; Koskela, A.; Kaarniranta, K.; Karjalainen, R.O. Polyphenol stilbenes: Molecular mechanisms of defence against oxidative stress and aging-related diseases. Oxid. Med. Cell. Longev. 2015, 2015, 340520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wightman, E.L.; Haskell-Ramsay, C.F.; Reay, J.L.; Williamson, G.; Dew, T.; Zhang, W.; Kennedy, D.O. The effects of chronic trans-resveratrol supplementation on aspects of cognitive function, mood, sleep, health and cerebral blood flow in healthy, young humans. Br. J. Nutr. 2015, 114, 1427–1437. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, D.O.; Wightman, E.L.; Reay, J.L.; Lietz, G.; Okello, E.J.; Wilde, A.; Haskell, C.F. Effects of resveratrol on cerebral blood flow variables and cognitive performance in humans: A double-blind, placebo-controlled, crossover investigation. Am. J. Clin. Nutr. 2010, 91, 1590–1597. [Google Scholar] [CrossRef]
- Shagufta; Ahmad, I. Tamoxifen a pioneering drug: An update on the therapeutic potential of tamoxifen derivatives. Eur. J. Med. Chem. 2018, 143, 515–531. [Google Scholar] [CrossRef]
- Wolfe, C.; Pagano, P.; Pillar, C.M.; Shinabarger, D.L.; Boulos, R.A. Comparison of the in vitro antibacterial activity of ramizol, fidaxomicin, vancomycin, and metronidazole against 100 clinical isolates of clostridium difficile by broth microdilution. Diagn. Microbiol. Infect. Dis. 2018, 92, 250–252. [Google Scholar] [CrossRef] [Green Version]
- Sibley, K.; Chen, J.; Koetzner, L.; Mendes, O.; Kimzey, A.; Lansita, J.; Boulos, R.A. A 14-day repeat dose oral gavage range-finding study of a first-in-class CDI investigational antibiotic, in rats. Sci. Rep. 2019, 9, 158. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.H.; Park, J.K.; Kim, K.M.; Lee, H.J.; Kim, S. In Vitro and in vivo antithrombotic and cytotoxicity effects of ferulic acid. J. Biochem. Mol. Toxicol. 2018, 32, e22004. [Google Scholar] [CrossRef]
- Shen, Y.; Song, X.; Li, L.; Sun, J.; Jaiswal, Y.; Huang, J.; Liu, C.; Yang, W.; Williams, L.; Zhang, H.; et al. Protective effects of p-coumaric acid against oxidant and hyperlipidemia-an in vitro and in vivo evaluation. Biomed. Pharmacother. 2019, 111, 579–587. [Google Scholar] [CrossRef]
- Cechinel-Zanchett, C.C.; Bolda Mariano, L.N.; Schlickmann, F.; Cechinel-Filho, V.; de Souza, P. In Vitro effects of two bioactive compounds, gallic acid and methyl gallate, on urolithiasis. Actas Urológicas Españolas 2021, 45, 604–608. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Zhou, S.; Yang, S. Vanillic acid suppresses HIF-1α Expression via inhibition of MTOR/P70S6K/4E-BP1 and Raf/MEK/ERK pathways in human colon cancer HCT116 cells. Int. J. Mol. Sci. 2019, 20, 465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Du, C.; Beaman, H.T.; Monroe, M.B.B. Characterization of phenolic acid antimicrobial and antioxidant structure–property relationships. Pharmaceutics 2020, 12, 419. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhai, T.; Haider, S.; Liu, Y.; Huang, Z.J. Synergistic effect of chlorogenic acid and caffeic acid with fosfomycin on growth inhibition of a resistant Listeria monocytogenes strain. ACS Omega 2020, 5, 7537–7544. [Google Scholar] [CrossRef] [Green Version]
- Tan, S.; Gao, J.; Li, Q.; Guo, T.; Dong, X.; Bai, X.; Yang, J.; Hao, S.; He, F. Synergistic effect of chlorogenic acid and levofloxacin against Klebsiella pneumonia infection in vitro and in vivo. Sci. Rep. 2020, 10, 20013. [Google Scholar] [CrossRef]
- de Camargo, A.C.; Regitano-d’Arce, M.A.B.; Rasera, G.B.; Canniatti-Brazaca, S.G.; do Prado-Silva, L.; Alvarenga, V.O.; Sant’Ana, A.S.; Shahidi, F. Phenolic acids and flavonoids of peanut by-products: Antioxidant capacity and antimicrobial effects. Food Chem. 2017, 237, 538–544. [Google Scholar] [CrossRef]
- Stalmach, A.; Mullen, W.; Barron, D.; Uchida, K.; Yokota, T.; Cavin, C.; Steiling, H.; Williamson, G.; Crozier, A. Metabolite profiling of hydroxycinnamate derivatives in plasma and urine after the ingestion of coffee by humans: Identification of biomarkers of coffee consumption. Drug Metab. Dispos. 2009, 37, 1749–1758. [Google Scholar] [CrossRef] [Green Version]
- Omar, M.H.; Mullen, W.; Stalmach, A.; Auger, C.; Rouanet, J.M.; Teissedre, P.L.; Caldwell, S.T.; Hartley, R.C.; Crozier, A. Absorption, disposition, metabolism, and excretion of [3-14C]caffeic acid in rats. J. Agric. Food Chem. 2012, 60, 5205–5214. [Google Scholar] [CrossRef]
- Zheng, S.; Zhang, H.; Liu, R.; Huang, C.L.; Li, H.; Deng, Z.Y.; Tsao, R. Do short chain fatty acids and phenolic metabolites of the gut have synergistic anti-inflammatory effects?—New insights from a TNF-α-induced Caco-2 cell model. Food Res. Int. 2021, 139, 109833. [Google Scholar] [CrossRef]
- Tuyen, P.T.; Xuan, T.D.; Khang, D.T.; Ahmad, A.; Van Quan, N.; Anh, T.T.T.; Anh, L.H.; Minh, T.N. Phenolic compositions and antioxidant properties in bark, flower, inner skin, kernel and leaf extracts of Castanea crenata sieb. et Zucc. Antioxidants 2017, 6, 31. [Google Scholar] [CrossRef] [Green Version]
- Roldan, M.B.; Cousins, G.; Muetzel, S.; Zeller, W.E.; Fraser, K.; Salminen, J.P.; Blanc, A.; Kaur, R.; Richardson, K.; Maher, D.; et al. Condensed tannins in white clover (Trifolium Repens) foliar tissues expressing the transcription factor TaMYB14-1 bind to forage protein and reduce ammonia and methane emissions in vitro. Front. Plant Sci. 2022, 12, 777354. [Google Scholar] [CrossRef] [PubMed]
- Cardoso-Gutierrez, E.; Aranda-Aguirre, E.; Robles-Jimenez, L.E.; Castelán-Ortega, O.A.; Chay-Canul, A.J.; Foggi, G.; Angeles-Hernandez, J.C.; Vargas-Bello-Pérez, E.; González-Ronquillo, M. Effect of tannins from tropical plants on methane production from ruminants: A systematic review. Vet. Anim. Sci. 2021, 14, 100214. [Google Scholar] [CrossRef] [PubMed]
- Baer-Dubowska, W.; Szaefer, H.; Majchrzak-Celińska, A.; Krajka-Kuźniak, V. Tannic acid: Specific form of tannins in cancer chemoprevention and therapy-old and new applications. Curr. Pharmacol. Rep. 2020, 6, 28–37. [Google Scholar] [CrossRef] [Green Version]
- Youness, R.A.; Kamel, R.; Elkasabgy, N.A.; Shao, P.; Farag, M.A. Recent advances in tannic acid (gallotannin) anticancer activities and drug delivery systems for efficacy improvement; A comprehensive review. Molecules 2021, 26, 1486. [Google Scholar] [CrossRef]
- Belhaoues, S.; Amri, S.; Bensouilah, M. Major phenolic compounds, antioxidant and antibacterial activities of Anthemis praecox link aerial parts. S. Afr. J. Bot. 2020, 131, 200–205. [Google Scholar] [CrossRef]
- Suzilla, W.Y.; Izzati, A.; Isha, I.; Zalina, A.; Rajaletchumy, V.K. Formulation and evaluation of antimicrobial herbosomal gel from Quercus infectoria extract. IOP Conf. Ser. Mater. Sci. Eng. 2020, 736, 022030. [Google Scholar] [CrossRef] [Green Version]
- Pandey, A.; Negi, P.S. Phytochemical composition, in vitro antioxidant activity and antibacterial mechanisms of Neolamarckia cadamba fruits extracts. Nat. Prod. Res. 2017, 32, 1189–1192. [Google Scholar] [CrossRef]
- Kaczmarek, B. Tannic acid with antiviral and antibacterial activity as a promising component of biomaterials—A minireview. Materials 2020, 13, 3224. [Google Scholar] [CrossRef]
- Wiese, S.; Esatbeyoglu, T.; Winterhalter, P.; Kruse, H.P.; Winkler, S.; Bub, A.; Kulling, S.E. Comparative biokinetics and metabolism of pure monomeric, dimeric, and polymeric Flavan-3-Ols: A randomized cross-over study in humans. Mol. Nutr. Food Res. 2015, 59, 610–621. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Li, D.; Ho, C.T.; Li, J.; Wan, X. The absorption, distribution, metabolism and excretion of procyanidins. Food Funct. 2016, 7, 1273–1281. [Google Scholar] [CrossRef]
- Molino, S.; Lerma-Aguilera, A.; Jiménez-Hernández, N.; Gosalbes, M.J.; Rufián-Henares, J.Á.; Francino, M.P. Enrichment of food with tannin extracts promotes healthy changes in the human gut microbiota. Front. Microbiol. 2021, 12, 625782. [Google Scholar] [CrossRef] [PubMed]
- Sallam, I.E.; Abdelwareth, A.; Attia, H.; Aziz, R.K.; Homsi, M.N.; von Bergen, M.; Farag, M.A. Effect of gut microbiota biotransformation on dietary tannins and human health implications. Microorganisms 2021, 9, 965. [Google Scholar] [CrossRef] [PubMed]
- Cerdá, B.; Tomás-Barberán, F.A.; Espín, J.C. Metabolism of antioxidant and chemopreventive ellagitannins from strawberries, raspberries, walnuts, and oak-aged wine in humans: Identification of biomarkers and individual variability. J. Agric. Food Chem. 2004, 53, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Daza, M.C.; Pulido-Mateos, E.C.; Lupien-Meilleur, J.; Guyonnet, D.; Desjardins, Y.; Roy, D. Polyphenol-mediated gut microbiota modulation: Toward prebiotics and further. Front. Nutr. 2021, 8, 689456. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fabbrini, M.; D’Amico, F.; Barone, M.; Conti, G.; Mengoli, M.; Brigidi, P.; Turroni, S. Polyphenol and Tannin Nutraceuticals and Their Metabolites: How the Human Gut Microbiota Influences Their Properties. Biomolecules 2022, 12, 875. https://doi.org/10.3390/biom12070875
Fabbrini M, D’Amico F, Barone M, Conti G, Mengoli M, Brigidi P, Turroni S. Polyphenol and Tannin Nutraceuticals and Their Metabolites: How the Human Gut Microbiota Influences Their Properties. Biomolecules. 2022; 12(7):875. https://doi.org/10.3390/biom12070875
Chicago/Turabian StyleFabbrini, Marco, Federica D’Amico, Monica Barone, Gabriele Conti, Mariachiara Mengoli, Patrizia Brigidi, and Silvia Turroni. 2022. "Polyphenol and Tannin Nutraceuticals and Their Metabolites: How the Human Gut Microbiota Influences Their Properties" Biomolecules 12, no. 7: 875. https://doi.org/10.3390/biom12070875
APA StyleFabbrini, M., D’Amico, F., Barone, M., Conti, G., Mengoli, M., Brigidi, P., & Turroni, S. (2022). Polyphenol and Tannin Nutraceuticals and Their Metabolites: How the Human Gut Microbiota Influences Their Properties. Biomolecules, 12(7), 875. https://doi.org/10.3390/biom12070875










