Abstract
Glutamate is the major excitatory neurotransmitter in the central nervous system and is intricately linked to learning and memory. Its activity depends on the expression of AMPA and NMDA receptors and excitatory amino transporters on neurons and glial cells. Glutamate transporters prevent the excess accumulation of glutamate in synapses, which can lead to aberrant synaptic signaling, excitotoxicity, or cell death. Neuroinflammation can occur acutely after surgical trauma and contributes to the development of perioperative neurocognitive disorders, which are characterized by impairment in multiple cognitive domains. In this review, we aim to examine how glutamate handling and glutamatergic function are affected by neuroinflammation and their contribution to cognitive impairment. We will first summarize the current data regarding glutamate in neurotransmission, its receptors, and their regulation and trafficking. We will then examine the impact of inflammation on glutamate handling and neurotransmission, focusing on changes in glial cells and the effect of cytokines. Finally, we will discuss these changes in the context of perioperative neuroinflammation and the implications they have for perioperative neurocognitive disorders.
1. Introduction
It is becoming apparent that systemic circulating factors can pass through a dysfunctional blood–brain barrier (BBB) and have a profound impact upon brain homeostasis, aging, and neurodegeneration [1]. The systemic immune response to endogenous or exogenous triggers releases substances that can, in turn, initiate immune and inflammatory reactions in the central nervous system (CNS). Neuroinflammation, oxidative stress, and excitotoxicity are associated with several neurological disorders, such as Alzheimer’s disease (AD) [2,3,4], Parkinson’s disease [5,6,7], and multiple sclerosis [8,9,10]. Neuroinflammation can cause disruptions to synaptic transmission and glial and neuronal dysfunction that contribute to cognitive impairment. Among these changes are alterations to the release, uptake, and clearance of glutamate, as well as changes in the functions and subunit composition of its receptors [11]. Glutamate, being the major excitatory neurotransmitter in the CNS, is primarily released from presynaptic vesicles and acts on different types of postsynaptic glutamate receptors [12,13].
Unlike the development of neurodegenerative diseases that occurs without a single identifiable trigger or event, perioperative neurocognitive disorders (PNDs) are clearly linked to surgery. This group of neurological disorders encompasses a range of conditions from acute delirium to more sustained postoperative cognitive dysfunction. Tissue trauma from surgery causes the release of substances that can overwhelm the immune system and set up an inflammatory response in the brain. Patients are usually exposed to anesthetic agents that also have intrinsic effects on neurotransmitters and neurotransmission. Accumulating evidence indicates that the overactivation of the inflammatory response and the change in glutamate metabolism contributes to the development of PNDs [14,15].
The purpose of this review is to outline how glutamatergic neurotransmission is affected by perioperative neuroinflammation. We will first summarize the current data regarding glutamate receptor configuration and trafficking, and glutamate release and handling in the CNS. We will then provide a summary of the effects of peripheral immune cells and cytokines on glial cells and how they alter normal glutamate regulation. Finally, we will outline the experimental evidence on the effects of commonly used anesthetic agents and surgical trauma on glutamate in the CNS and some potential treatments.
2. Glutamate Receptors
Glutamate receptors can be broadly divided into ionotropic and metabotropic types. Ionotropic receptors are coupled with ion channels to form receptor–channel complexes that mediate fast signal transmission; these include the α-amino -3 hydroxy-5 methyl-4 isoxazole propionic acid receptors (AMPARs), N-methyl-d-aspartate receptors (NMDARs), and the kainate receptors (KARs). Metabotropic glutamate receptors are coupled with G-proteins on cell membranes, and include mGluR1 to mGluR8 types [16,17]. GluA1 to Glu4 AMPARs are tetrameric assemblies of two dimers encoded by the four GRIA (GRIA1 to GRIA4) genes. The four main tetrameric complexes are composed of GluA1/2 and GluA2/3 heteromers and GluA1 homopolymers. GluA1/A2 heteromers are the dominant AMPARs in the CA1 hippocampus, with around 80% of synaptic and more than 95% of somatic extra-synaptic receptors of this type, with the remainder being the GluA2/GluA3 heteromers. GluA4 mainly appears during embryonic development [18,19]. Calcium-permeable AMPARs (mainly consisting of a GluA2-lacking AMPAR) may emerge under some pathological conditions, such as status epilepticus, glaucoma, and neuroinflammation. This GluA2-lacking AMPAR has a linear current/voltage curve and is permeable to Ca2+ [20,21,22,23].
The NMDAR is a heterotetramer composed of NR1 to NR3 subunits, with NR2 having four subtypes (NR2A, NR2B, NR2C, and NR2D) and NR3 having two subtypes. Under normal conditions, NMDARs are blocked by magnesium ions; they are activated after postsynaptic depolarization and the removal of these ions [24]. However, the overactivation of NMDARs can lead to neuronal excitotoxicity, cell apoptosis, and cell death through the activation of calcium ion-mediated intracellular pathways [25].
Metabotropic glutamate receptors exist both in the CNS and peripherally and are mainly expressed in neurons and glial cells in proximity to the synaptic cleft [26,27]. Metabotropic glutamate receptors (mGlus) are a family of G-protein-coupled receptors activated by the glutamate neurotransmitter. The family has eight molecular clones termed metabotropic glutamate receptor 1–8 (mGlu1–8) [28]. mGluRs can be divided into three groups (Group I–III mGlus). Group I includes mGlu1 and mGlu5, Group II includes mGlu2–3, and Group III includes mGlu4 and mGlu6–8 [29]. mGlus play crucial roles in the modulation of neuronal excitability, synaptic plasticity, and the release of neurotransmitters [30]. Most metabotropic glutamate receptors are located presynaptically, except for the Group I (mGluR1 and mGluR5) receptors [31]. These Group I receptors can increase the activity of NMDARs and induce excitotoxicity [32]. In addition, mGluR5 can mediate experience-dependent NMDA subunit switching [33].
3. Glutamate Release and Handling
Glutamate can be released by vesicular or non-vesicular release mechanisms. Under physiological conditions, synaptic release is primarily via the vesicular mode. When an action potential reaches the terminal, an influx of Ca2+ triggers the exocytosis of glutamate vesicles. Ca2+ can then bind to synaptotagmin, causing it to bind to a complex composed of SNARE and Sec1/Munc18-like (SM) proteins that mediate membrane fusion during exocytosis, thus promoting the release of neurotransmitters [34]. The non-vesicular mechanism occurs under pathological conditions and involves anion channel release, the reverse efflux of glutamate, and xC-system release [35]. The anion channels are mainly located on astrocytes and can release glutamate if the astrocytes become swollen, as seen in ischemia-reperfusion injury [36]. The efflux of glutamate is mediated through excitatory amino transporters (EAATs) located on endothelial cells and glial cells. EAATs help with the uptake of glutamate; the BBB can function in efflux mode to selectively move glutamate from the brain to the blood. The glial cells lining the BBB take up the glutamate and release it into the proximity of endothelial cells, promoting efflux. [37]. Glutamate transport in the outward direction is termed reverse transport. The reverse transport of glutamate occurs both in neurons and astrocytes. Glutamate can be released extracellularly by reverse transport when the neurons’ extracellular Na+/intracellular K+ levels decrease or when the intracellular Na+/extracellular K+ levels increase. This process is mediated by the electrochemical gradient of co- and counter-transported ions produced by the glutamate transporter EAAC1 (EAAT3) [38,39]. In astrocytes, reverse transport happens in some extreme situations; for example, after ATP depletion, the membrane gradients collapse, glutamate uptake ceases, and the efflux of glutamate occurs via reverse transport [40]. In addition, astrocytes can release glutamate through other modalities such as exocytosis, hemichannels, anion transporters, and P2X receptors [41]. The system xC- is mainly located in glial cells and is responsible for exchanging glutamate with cystine, a substrate used for the synthesis of the antioxidant molecule glutathione (GSH). In many brain regions, the xC-system acts as the main source of intracellular cystine by exporting glutamate extracellularly. Extracellular glutamate can inhibit the xC-system and contribute to the depletion of GSH, which can lead to oxidative glutamate toxicity. Inflammatory cytokines, including TNF-a and IL-1β, can upregulate the xC-system. Increased xC expression can have neuroprotective effects or excitotoxic side effects in different animal models [42].
Glutamate would accumulate in the brain if not for the transporter proteins that remove it from the extracellular fluid to maintain low extracellular concentrations. Extracellular glutamate is controlled by a family of plasma membrane enzymes called EAATs. EAAT 1–5 are encoded by the SLC1A3, SLC1A2, SLC1A1, SLC1A6, and SLC1A7 genes, respectively [43]. EAAT1/2 are considered glial transporters and are widely found in the cerebellum and forebrain, while EAAT3/4 are considered as neuronal transporters and are widely distributed in the forebrain, spinal cord, and cerebellum [44,45,46]. Glutamate transporters are important for the termination of excitatory signals, glutamate recycling, and the prevention of excitotoxic injury [47]. GLAT1 (EAAT2) is expressed at high levels in brain astrocytes and at lower levels in neurons. Three variants of GLT1 exist (GLT1a, GLT1b, and GLT1c); GLT-1a is the only glutamate transporter subtype identified in axon terminals and contributes significantly to glutamate uptake into excitatory terminals [48,49].
4. Regulation of Glutamatergic Neurotransmission by Glial Cells
4.1. Microglia
Microglia are innate immune cells in the brain parenchyma that have similar actions to circulating macrophages [50]. In addition to their role in mediating immune responses in the CNS, microglia also provide nutrition to neurons and can respond dynamically to changes in neuronal activity [51]. Microglia can prune developing synapses and regulate synaptic plasticity and function. The dysfunction of microglia–synapse interactions can lead to synapse loss and neurodegenerative disease [52]. While in their surveillance state, microglia constantly scan the local microenvironment, but once activated, they can exhibit different morphologies and their functions can vary from being pro-inflammatory to anti-inflammatory [53]. They can become activated in the perioperative period through a series of peripherally initiated processes. Traumatized tissues in the body can release damage-related molecular patterns (DAMPs), such as high molecular group box I protein (HMGB1). When these DAMPs are combined with Toll-like receptors (TLRs) and receptors for advanced glycosylation end products (RAGEs) on the surface of bone marrow-derived monocytes (BMDMs), the nuclear translocation of NF-κB occurs, resulting in the increased expression of cytokines [54,55,56]. The secreted pro-inflammatory cytokines can act on BMDMs through a positive feedback loop to further promote the translocation of NF-κB and the release of cytokines [57]. Pro-inflammatory cytokines can upregulate the cyclooxygenase 2 isozyme (COX-2) [58].
Under normal conditions, the BBB can prevent the entry of harmful substances into the brain. However, the presence of peripheral pro-inflammatory cytokines damages the BBB via the action of COX2 and matrix metalloproteins (MMPs) in the endothelial cells. This allows pro-inflammatory cytokines together with BMDMs to migrate into the CNS, which in turn leads to the activation of microglia [59]. Inflammatory cytokines can promote the release of glutamate [60,61], and the glutamate that is released from activated microglia in turn stimulates glutamate receptors on the microglia to further release cytokines [62]. Thus, activated microglia can display a positive feedback loop to amplify the further release of cytokines and discharge a large amount of glutamate into the extra-synaptic space [63] (Figure 1). The degradation of extracellular ATP alleviates glutamate-induced inhibition of microglial proliferation [64]. Metabotropic glutamate receptors (mGluR2/5) are expressed on microglia, and when the mGluR2 receptor is activated, they can enhance the release of inflammatory cytokines, including TNFα, glutamate, and nitric oxide (NO), leading to neurotoxicity [65,66]. On the other hand, the activation of mGluR5 seems to have an opposite effect on neuroprotection [67].
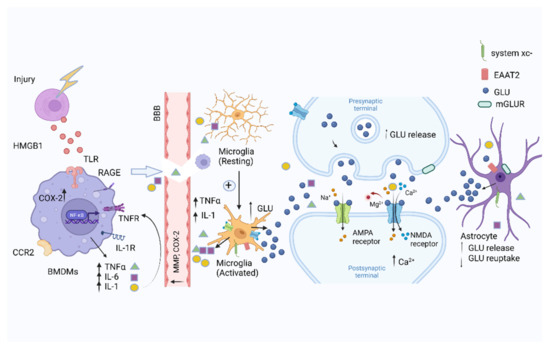
Figure 1.
Systemic immune responses to trauma. Injured cells release DAMPs, including HMGB1, in response to surgical trauma after being combined with the TLR and RAGE, which can activate nuclear factor-kappa B (NF-κB) signaling pathways in BMDMs, promoting the release of pro-inflammatory cytokines, including IL-6 and TNFα, IL-1. The increased expression of COX-2 and MMPs disrupts the integrity of the blood–brain barrier. Pro-inflammatory cytokines activate microglia to further amplify the release of pro-inflammatory cytokines in the brain. Glia activated by the pro-inflammatory cytokines can further stimulate the release of glutamate. The postsynaptic intracellular Ca2+ concentration increases by the overactivation of NMDARs. The ability of astrocytes to clear glutamate is decreased. Figure created with Biorender.com (accessed on 2 March 2022).
4.2. Astrocytes
Astrocytes can provide nutritional support, maintain synaptic homeostasis, regulate synaptic pruning, and participate in neural signal transduction. They play essential roles in oxidative stress and the regulation of glutamate metabolism and cycling [68,69,70]. Microglia can initiate an immune response in the CNS and subsequently activate astrocytes [71]. The increased expression of glial fibrillary acidic protein (GFAP) is a marker of astrocyte activation. Glia cells share some common transcriptional pathways after neuroinflammation occurs, such as the NF-κB pathway. The nuclear translocation of NF-κB is boosted by TNF-α, interleukin (IL)-1b, and IL-17. In addition, sphingolipids, such as sphingosine 1-phosphate (S1P) and lactosylceramide (LacCer), can also trigger NF-κB translocation [72]. The sodium-dependent glutamate transporters EAAT2 are present on astrocytes [73]. Dysfunctional glutamate transporters and increased extracellular glutamate levels can cause neuronal injury. EAAT2 can reduce excess glutamate levels in the synaptic cleft to reduce excitotoxicity [74]. More than 90% of glutamate is cleared by the type 2 EAAT (Figure 1). EAAT2 (termed glutamate transporter I (GLT-1) in rodents) is the major amino transporter of glutamate in the CNS. Glutamate can be converted into glutamine, which is then released and taken up by neurons and transported to synaptic vesicles through glutamate transporters (VGLUT1-3) to complete the cycle of glutamine metabolism [73,75]. Astrocytes express many immune-derived receptors, including those for cytokines, chemokines, and complement proteins, and activation by these factors can recruit macrophages to enter the CNS [76,77]. β-catenin, a transcriptional co-activator in the Wnt/β-catenin pathways expressed in astrocytes, can positively regulate EAAT2 at the transcriptional level in progenitor-derived astrocytes by partnering with T cell factor 1 and 3 [78]. TNFα can increase the release of glutamate and decrease EAAT2 protein expression in astrocytes [79,80].
4.3. Oligodendrocytes
Oligodendrocytes and astrocytes can mutually affect each other during neuroinflammation. Oligodendrocytes secrete pro-inflammatory cytokines to induce NF-κB signaling and pro-inflammatory functions in astrocytes [72,81]. The receptors on oligodendrocytes respond to the inflammatory stimuli secreted by the astrocytes. Activated astrocytes promote the myelination and apoptosis of oligodendrocytes via TNFα, Fas ligand (FasL), and glutamate secretion [82,83,84]. Oligodendrocytes have effects on excitatory neurotransmission. Oligodendrocytes are highly vulnerable to AMPA and kainate receptor-mediated toxicity. AMPA and kainate receptor-mediated excitotoxicity contributes to demyelination and axonal injury in mature oligodendrocytes. Glutamate regulation has a potential neuroprotective strategy, as evidenced by the deletion of GluA4 from mature oligodendrocytes in experimental autoimmune encephalomyelitis (EAE) [85,86]. The overactivation of NMDARs can impair myelin synthesis. Activated microglia release glutamate through the system xc- cystine-glutamate antiporter and block glutamate transporters in oligodendrocytes [87]. In addition, pro-inflammatory cytokines can also impair the clearance of glutamate by the EAATs on oligodendrocytes [88].
5. Regulation of Glutamate Neurotransmission by Peripheral Immune Cells
5.1. Macrophages
Macrophages can be recruited into the CNS by CCL2/CCR2 signals [89]. Many of these macrophages reside in the perivascular area and express glutamate transporters and both metabotropic and ionotropic receptors. Macrophages can secrete glutamate, thus increasing the excitotoxicity of the inflammatory environment [90]. In patients with neurodegenerative diseases such as AD, β-Amyloid protein can enhance the macrophage’s ability to produce more oxygen free radicals and glutamate [91]. In addition, it can induce NMDAR-mediated neurotoxicity by secreting glutamate [90]. The activated macrophage can contribute to spine loss in multiple sclerosis (MS) and EAE by secreting glutamate, inflammatory cytokines, free radicals, and MMPs [92].
5.2. T cells
T cells can balance glutamatergic and GABAergic neurotransmission in the CNS to decrease excitotoxicity and can attenuate astrocyte activity [93]. They express AMPA GluR3 subunits and NMDARs and respond to glutamate in a dose-dependent manner [94]. Low concentrations of glutamate can promote T cell adhesion and migration, whereas higher concentrations can act on AMPARs and NMDARs to stimulate proliferation and metabotropic glutamate (mGluRs) receptors to reduce cell apoptosis [95].
6. The Effects of Pro-Inflammatory Cytokines on Glutamatergic Function
6.1. Interleukin IL-1β
IL-1β is a pro-inflammatory cytokine that can act on NMDARs to increase NMDA receptor-induced intracellular calcium increase, an action that can be abolished by IL-1 antagonists [96]. This cytokine can also inhibit NMDAR-mediated synaptic transmission by depressing the isolated NMDA-EPSP amplitude in the dentate gyrus [97]. IL-1β can also inhibit the uptake of glutamate by astrocytes [98].
6.2. IFN-γ
IFN-γ is produced by T lymphocytes [93] and can change the phenotype of astrocytes [11] to stimulate glutamate clearance [98]. It can also stimulate macrophages to secrete glutamate compounds, such as QUIN, and alter glutamatergic neurotransmission [99]. IFN-γ has been shown to enhance glutamate neurotoxicity through AMPARs; the IFN-γ receptor forms a CP-AMPA receptor complex and phosphorylates GluRl at serine 845 via the JAKT2/STAT1 pathway [100].
6.3. Interleukin 6 (IL-6)
IL-6 is a crucial component of the inflammatory response and has important roles in the immune and hematopoietic systems [101]. Experimentally, it has been shown to protect cultured hippocampal neurons from glutamate-induced cell death [102]. However, chronic IL-6 exposure disrupts neuronal function and may contribute to the pathophysiology associated with many neurological diseases [103]. IL-6 inhibits glutamate neurotransmitter release in the cerebral cortex accompanied by the stimulation of STAT3 tyrosine phosphorylation [104].
6.4. TNFα
Pro-inflammatory cytokines can regulate synaptic strength, and AMPARs play an important role in synaptic plasticity in this regard. TNFα can change the AMPAR subunits to have a significant effect on neurotransmission. [105]. Under physiological conditions, GluR2-containing AMPARs are not permeable to Ca2+ and are resistant to the phosphorylation of the GluR1 Ser831 site. In contrast, GluR2-lacking AMPARs participate in Ca2+-mediated excitotoxicity [106]. When TNFα is applied to hippocampal cell cultures, it can significantly increase the expression of GluR1 and the number of GluR2-lacking AMPARs (Figure 2); this effect can be reduced by sequestering TNFα [107]. TNFα can significantly increase presynaptic glutamate release in cultured neurons [105]. When applied to brain slices from neonatal mice, TNFα can cause dose-dependent neuronal excitotoxicity through increased calpains activity in the Purkinje neurons [108]. TNFα can also inhibit the activity of glutamate transporters and thereby increase neurotoxicity [109].
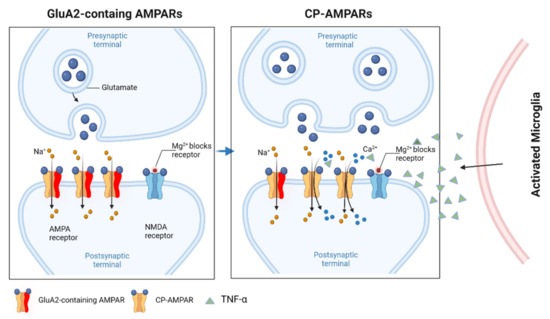
Figure 2.
GluA2-lacking CP-AMPARs in neuroinflammation. GluA2-lacking receptors (CP-AMPARs) are relatively rare in most excitatory neurons in baseline conditions. However, GluA1/2 heteromers are replaced with GluA1 homomers after induction by pro-inflammatory cytokines, such as TNFα. The GluA2 heteromers are Ca2+ impermeable, whereas the GluA1 homomers (i.e., GluA2-lacking AMPARs) permit the passage of both Na+ and Ca2+. Figure created with Biorender.com (accessed on 2 March 2022).
7. Anesthesia, Surgical Trauma, Glutamatergic Transmission, and Cognitive Dysfunction
Perioperative neurocognitive disorders are common in the elderly after surgery, especially in those with pre-existing diseases or frailty [110,111]. It can present as delirium with inattention and changes in the level of consciousness or a more delayed and subtle neurocognitive impairment, previously termed postoperative cognitive dysfunction (POCD) [58]. POCD can manifest as memory impairment, a decline in executive function, changes in mood and personality, or a combination of such [58]. The incidence of POCD in patients older than 60 years is 25.8% at 7 days postoperatively. Three months later, this value is still around 10% [112]. Although the pathogenesis of POCD is not yet clear, it may be related to the central cholinergic system, the excitatory amino acid system, and other neurotransmitters [113]. Animal and human studies suggest that neuroinflammation from surgery and anesthesia is important in its development [114,115].
Neuroinflammation, glutamatergic dysfunction, and cognitive dysfunction are intricately linked. Surgical trauma can incite a series of peripheral immune and inflammatory responses that result in profound peripheral inflammation [116,117], which can trigger neuroinflammation [118]. Among other factors, neuroinflammation contributes to the development of perioperative neurocognitive disorders, manifesting as acute delirium or more subtle delayed postoperative neurocognitive dysfunction. The latter has similar features to neurodegenerative diseases such as AD and may share similar pathophysiological mechanisms. Indeed, those with mild cognitive impairment or of advanced age are at particular risk of developing PNDs, and those who develop PNDs may later manifest more overt elements similar to AD.
Glutamate plays a vital role in long-term potentiation (LTP), a process that is considered to underpin learning and memory. Peripheral inflammation increases the expression of the NMDAR subunit 2B (NR2B) and NR2B receptor-mediated synaptic currents in the anterior cingulate cortex and contributes to pain sensitization [119]. Peripheral inflammation can affect the function of glutamate receptors and transporters and impair cognition. AMPARs are involved in excitotoxicity through the activation of NMDARs. The excessive stimulation of NMDARs or AMPARs can induce neuronal apoptosis [120,121].
In addition to the presence of peripheral and neuroinflammation, the perioperative picture is further complicated as most forms of surgery are usually performed under anesthesia. Volatile anesthetics have a significant impact on the levels of pro-inflammatory cytokines that, in turn, can affect glutamatergic transmission. Different anesthesia agents, anesthesia exposure times, ages, and surgical models have been evaluated and each combination can produce slightly different effects. The two most commonly clinically used volatile anesthetic agents, and hence evaluated in the most detail experimentally, are isoflurane and sevoflurane. The former has been in clinical use for a longer period but is increasingly being replaced by sevoflurane.
7.1. Isoflurane
Volatile agents have intrinsic effects on neurotransmission, especially on the glutamatergic system, either directly or via their effects on inflammatory cytokines. Brief exposure to isoflurane can significantly increase pro-inflammatory TNF-α, IL-6, and IL-1β levels [122]. Isoflurane can abate excitatory transmission by reducing the release and increasing the uptake of glutamate into the presynaptic terminal [123] and can reduce glutamate release in the hippocampus after ischemia [124].
Excitatory amino acid transporters have a significant role in glutamate reuptake at the synapses and, consequently, in cognition. Isoflurane can enhance the expression of EAAT3 on the plasma membrane via a protein kinase C (PKC)-dependent pathway [125,126] and imparts neuroprotective effects by preserving the function of EAAT3 for L-glutamate and L-cysteine uptake [127]. EAAT3 knockout mice have an obvious baseline cognitive impairment, and isoflurane anesthetic does not additionally affect the cognition of the mice [128]. Cao et al. also demonstrated that isoflurane inhibits context-related fear conditioning in EAAT3 knockout mice. In addition, increased GluR1 can be trafficked to the plasma membrane via EAAT3 [126]. The expression of EAAT1 can also be influenced by isoflurane; EAAT1 was shown to be increased in the neonatal rats after exposure to isoflurane anesthetic [129]. Isoflurane inhalation does not affect the activity of wild-type EAAT2 [130].
The effect of isoflurane on the brain is in part affected by the age at which the exposure takes place. During the development period of the brain, anesthetic exposure can interfere with the normal patterns of synaptogenesis and thus may impair the assembly of neural circuits, which in turn could affect cognition [131]. Calpain-2 is a neutral cysteine protease that is highly expressed in the CNS and can be activated by NMDARs [132]. In one study using neonatal mice, isoflurane exposure significantly increased the expression of the NR2B subunit compared with the NR2A subunit and the calpain-2 protease [133]. However, Wang et al. found that the expression of NR2A increased while NR2B decreased in the hippocampus of neonatal rats after the isoflurane exposure [129].
The effect of isoflurane exposure in adult rodents can also be quite variable. In 4 to 5-month-old mice, two hours of isoflurane inhalation can significantly improve cognitive performance and the expression of NR1 and NR2B subunits on the following day; however, this upregulation was only maintained for a relatively short period [134]. Lin et al. and Cao et al. both demonstrated that isoflurane could impair cognitive performance, as assessed by a Barnes maze and fear conditioning tests, in adult rodents after exposure [135,136]. The effect of isoflurane on cognition may be dependent on the dose and the duration of exposure. A shorter duration or lower concentrations of isoflurane induce some improvement in cognitive performance associated with increased NR2B expression. In contrast, a longer duration of exposure decreases NR2B expression and impaired cognition in adult mice [137]. In aged mice, isoflurane anesthesia can also impair cognitive function [135]. Isoflurane has also been shown to diminish learning and memory in older rats, accompanied by increased glutamate levels in the cerebrospinal fluid as well as GLAST expression in the hippocampus [138].
7.2. Sevoflurane
In a similar fashion to isoflurane, sevoflurane can also significantly increase the levels of cytokines that, in turn, can affect glutamatergic transmission. Sevoflurane can increase IL-6, IL-8, and TNFα by decreasing the activation of the PI3K/Akt/mTOR pathway in young rats [139]. Exposure to this drug can also directly reduce calcium-dependent glutamate release in the human brain [140]. In neonatal rats, sevoflurane exposure was shown to change LTP and long-term depression (LTD) by increasing the expression of GluR2-lacking AMPA receptors [141]. Exposure in utero increases the expression of NR2B, decreases the number of pyramidal neurons in the entorhinal cortex (ECT), and leads to abnormal development in the newborn [142]. Exposure during gestation also increases the expression of NR1 and NR2A in neonatal mice; however, in contrast, NR2B is decreased in the hippocampus [143]. Neonatal mice exposed to sevoflurane can have reduced activity of glutamatergic neurons in the amygdala. This leads to a learning deficit in fear conditioning after mature adulthood [144]. Sevoflurane can also induce glial dysfunction in neonatal rats and inhibit the glutamate-aspartate transporter (GLAST) through the Janus kinase/signal transducer and activator of transcription (JAK/STAT) pathway [145].
There appears to be age differences in the glutamatergic and cognitive responses to sevoflurane. A 2 h exposure to sevoflurane at a minimum alveolar concentration can improve cognitive performance and increase the expression of NMDA receptors 1 and 2B in 4–5-month-old mice [146]. On the other hand, two hours of sevoflurane exposure in 24-month-old rats was shown to result in impaired learning and memory [147]. Peng et al. also found that sevoflurane inhalation for 4 h caused cognitive impairment in 24-month-old but not in 3-month-old Sprague Dawley (SD) rats by inhibiting the PPAR-γ pathway. This impairment can be reversed by traditional Chinese medicine cistanches [147]. Sevoflurane exposure can also inhibit mGluRs and impair cognition. The mGluR-LTD in aged mice can increase the expression of small conductance calcium-activated potassium type 2 channels. The memory deficit can be reversed by a potassium channel blocker [148].
7.3. Surgical Trauma
The impact of general anesthesia on the immune system of healthy patients appears to be comparatively less substantial than that from surgical trauma, especially after major surgery [149,150]. Systemic inflammation, including that following trauma, is an evolutionary response to defend the body against infection or trauma [151], and the increased circulating cytokines have an effect on the glial cells and the glutamatergic system. The expression of TNF-α, IL-1β, IL-6, and IFN-γ fluctuates during the perioperative period [152]; for example, the expression of IL-6 is increased after surgery under isoflurane anesthesia [153].
The glutamatergic system also undergoes substantial changes in response to surgery. Both plasma and CSF glutamate levels are significantly increased in neurosurgical patients. Under physiological conditions, the free passage of plasma glutamate is inhibited by the intact blood–brain barrier; however, under pathological conditions, plasma glutamate levels have been shown to passively follow their gradient, traversing the damaged BBB to the cerebral extracellular space [154]. In 24 to 25-month-old rats, the increased expression of NR2 seen after abdominal surgery under isoflurane anesthesia correlated with cognitive impairment that could be attenuated by local or systemic analgesia. These results suggest that postoperative pain may have a role in cognitive impairment. However, this study did not distinguish the NR2 receptors’ subtype, which is a limitation [155].
As it appears that NR2 subunits participate in both pain perception and cognition, Zhang et al. showed that pain can significantly increase the levels of the pro-inflammatory cytokine TNF-α, increase the brain levels of cyclin-dependent kinase 5 (CDK5), and decrease the expression of NR2B in the medial prefrontal cortex without any changes in the hippocampus. In this study using a surgical incision on the paw of 9-month-old mice under isoflurane anesthesia, there was also hippocampus-independent learning impairment; however, all these changes in NR2B were attenuated by a CDK5 inhibitor or a local anesthetic agent. These results indicated that the surgical incision-induced nociception reduced the expression of NR2B by increasing the expression of CDK5 [156]. Riazi et al. used a model of colonic inflammation in SD rats—induced by the intracolonic injection of 2,4,6-trunitrobenzenesulfonic acid under isoflurane anesthesia—to show that the amplitudes of miniature excitatory postsynaptic currents increased without changing the frequencies and paired-pulse ratios, indicating changes in the postsynaptic effect. Furthermore, enhanced AMPA and NMDA mediated currents in the evoked excitatory postsynaptic currents (eEPSCs) in the Schaffer collateral pathway and the rectification index indicated an increased contribution from GluR2-lacking AMPA receptors [157].
Surgery can also alter the composition of AMPAR subunits, which is accompanied by impaired cognition. AMPAR GluR2 subunits were shown to be significantly decreased in the hippocampus after partial hepatic lobectomy under sevoflurane anesthesia in D-galactose-induced aging mice [158]. In addition, the internalization of GluR2 and cognitive impairment can be found after exposure to a high concentration of sevoflurane or propofol in a tibial fracture animal model; the mechanism underlying this effect may be related to a decrease in the activity of the PI3K-GluA2 pathway [159]. However, there exist different results obtained using various techniques. Electrophysiological recording in the CA1 hippocampus revealed a decrease in the AMPA/NMDA receptor ratio without a change in the rectification index in a tibial fracture model under sevoflurane anesthesia, indicating no internalization of the GluR2. This impairment could be reversed by the acetylcholinesterase inhibitor galantamine [160].
8. Potential Therapeutics
The perioperative period associated with major surgery can have profound impacts on the function of glutamate in the CNS. The relationship is particularly complex due to the multiple approaches by which surgery-induced inflammation can affect glutamatergic function, beginning with the assault on the blood–brain barrier. Once an array of peripheral cytokines and immune cells can gain entry to the CNS through the dysfunctional BBB, the ensuing neuroinflammatory response can affect glutamate release and reuptake, glutamate receptor phenotypes, and EAAT functions. Aberrant synaptic function and excitotoxicity in vulnerable regions contribute to cognitive dysfunction. The net effect may be further complicated by the intrinsic effects of volatile anesthetic agents on glutamatergic function that may be age- or duration-dependent.
It is perhaps this complicated interplay between such diverse factors peculiar to the perioperative period that has limited the translational potential of therapies targeting the glutamatergic system. However, there are a few promising experimental examples of agents improving cognitive function by suppressing inflammation that eventually alleviate the adverse changes related to glutamate handling and receptors. Dexmedetomidine is a sedative hypnotic drug that can decrease the level of pro-inflammatory cytokines and decrease the expression of NR2A and NR2B to protect against neuronal injury after exposure to sevoflurane [129,139]. Several herbal compounds have also shown experimental benefits. Senegenin is a component of the root Polygala tenuifolia that can improve cognition and the hippocampal expression of NR2B in sevoflurane-induced cognitive dysfunction [161]. Fingolimod (FTY720), a sphingosine-1-phosphate receptor modulator, can improve learning and memory and increase the expression of GluR2 after a partial hepatic lobectomy [158]. It can reduce T cell- and macrophage/microglia-mediated inflammation and attenuate astrocyte activation [162]. Vitexin is a bioactive compound extracted from hawthorn leaves that has a neuroprotective effect through the TRPV1 and NR2B pathways [163]. Interestingly, the β-lactam antibiotic ceftriaxone used to treat CNS infections can improve cognition by increasing GLT1 expression, thereby reducing neuroinflammation and apoptosis [164].
9. Conclusions
In summary, alterations to glutamate handling and glutamatergic transmission by neuroinflammation play a major role in the manifestation of perioperative neurocognitive disorders. We must build a more comprehensive picture of the differential effects of how each perioperative variable affects this system in order to develop more strategic therapeutic options.
Author Contributions
Y.Z., J.-M.-T.C. and G.-T.-C.W. designed the concept. Y.Z. prepared the draft manuscript. G.-T.-C.W. reviewed and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This was supported by departmental funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pluvinage, J.V.; Wyss-Coray, T. Author Correction: Systemic factors as mediators of brain homeostasis, ageing and neurodegeneration. Nat. Rev. Neurosci. 2020, 21, 298. [Google Scholar] [CrossRef] [Green Version]
- Calsolaro, V.; Edison, P. Neuroinflammation in Alzheimer’s disease: Current evidence and future directions. Alzheimer’s Dement. 2016, 12, 719–732. [Google Scholar] [CrossRef]
- Britschgi, M.; Wyss-Coray, T. Systemic and acquired immune responses in Alzheimer’s disease. Int. Rev. Neurobiol. 2007, 82, 205–233. [Google Scholar]
- Paouri, E.; Georgopoulos, S. Systemic and CNS inflammation crosstalk: Implications for Alzheimer’s disease. Curr. Alzheimer Res. 2019, 16, 559–574. [Google Scholar] [CrossRef]
- Hirsch, E.C.; Hunot, S. Neuroinflammation in Parkinson’s disease: A target for neuroprotection? Lancet. Neurol. 2009, 8, 382–397. [Google Scholar] [CrossRef]
- Collins, L.M.; Toulouse, A.; Connor, T.J.; Nolan, Y.M. Contributions of central and systemic inflammation to the pathophysiology of Parkinson’s disease. Neuropharmacology 2012, 62, 2154–2168. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, C.C.; Tarelli, R. Parkinson’s disease and systemic inflammation. Parkinson’s Dis. 2011, 2011, 436813. [Google Scholar] [CrossRef] [Green Version]
- Grigoriadis, N.; Grigoriadis, S.; Polyzoidou, E.; Milonas, I.; Karussis, D. Neuroinflammation in multiple sclerosis: Evidence for autoimmune dysregulation, not simple autoimmune reaction. Clin. Neurol. Neurosurg. 2006, 108, 241–244. [Google Scholar] [CrossRef]
- Rosenberg, G.A. Matrix metalloproteinases and neuroinflammation in multiple sclerosis. Neuroscientist 2002, 8, 586–595. [Google Scholar] [CrossRef]
- Christensen, J.R.; Börnsen, L.; Ratzer, R.; Piehl, F.; Khademi, M.; Olsson, T.; Sørensen, P.S.; Sellebjerg, F. Systemic inflammation in progressive multiple sclerosis involves follicular T-helper, Th17-and activated B-cells and correlates with progression. PLoS ONE 2013, 8, e57820. [Google Scholar] [CrossRef]
- Tilleux, S.; Hermans, E. Neuroinflammation and regulation of glial glutamate uptake in neurological disorders. J. Neurosci. Res. 2007, 85, 2059–2070. [Google Scholar] [CrossRef]
- Michaelis, E.K. Molecular biology of glutamate receptors in the central nervous system and their role in excitotoxicity, oxidative stress and aging. Prog. Neurobiol. 1998, 54, 369–415. [Google Scholar] [CrossRef]
- Zhou, Y.; Danbolt, N.C. Glutamate as a neurotransmitter in the healthy brain. J. Neural Transm. 2014, 121, 799–817. [Google Scholar] [CrossRef] [Green Version]
- Safavynia, S.A.; Goldstein, P.A. The Role of Neuroinflammation in Postoperative Cognitive Dysfunction: Moving From Hypothesis to Treatment. Front. Psychiatry 2018, 9, 752. [Google Scholar] [CrossRef]
- Kempuraj, D.; Thangavel, R.; Natteru, P.; Selvakumar, G.; Saeed, D.; Zahoor, H.; Zaheer, S.; Iyer, S.; Zaheer, A. Neuroinflammation induces neurodegeneration. J. Neurol. Neurosurg. Spine 2016, 1, 1003. [Google Scholar]
- Traynelis, S.F.; Wollmuth, L.P.; McBain, C.J.; Menniti, F.S.; Vance, K.M.; Ogden, K.K.; Hansen, K.B.; Yuan, H.; Myers, S.J.; Dingledine, R. Glutamate receptor ion channels: Structure, regulation, and function. Pharmacol. Rev. 2010, 62, 405–496. [Google Scholar] [CrossRef] [Green Version]
- Niswender, C.M.; Conn, P.J. Metabotropic glutamate receptors: Physiology, pharmacology, and disease. Annu. Rev. Pharmacol. Toxicol. 2010, 50, 295–322. [Google Scholar] [CrossRef] [Green Version]
- Lu, W.; Shi, Y.; Jackson, A.C.; Bjorgan, K.; During, M.J.; Sprengel, R.; Seeburg, P.H.; Nicoll, R.A. Subunit composition of synaptic AMPA receptors revealed by a single-cell genetic approach. Neuron 2009, 62, 254–268. [Google Scholar] [CrossRef] [Green Version]
- Luchkina, N.V.; Huupponen, J.; Clarke, V.R.; Coleman, S.K.; Keinänen, K.; Taira, T.; Lauri, S.E. Developmental switch in the kinase dependency of long-term potentiation depends on expression of GluA4 subunit-containing AMPA receptors. Proc. Natl. Acad. Sci. USA 2014, 111, 4321–4326. [Google Scholar] [CrossRef] [Green Version]
- Rajasekaran, K.; Todorovic, M.; Kapur, J. Calcium-permeable AMPA receptors are expressed in a rodent model of status epilepticus. Ann. Neurol. 2012, 72, 91–102. [Google Scholar] [CrossRef] [Green Version]
- Konen, L.M.; Wright, A.L.; Royle, G.A.; Morris, G.P.; Lau, B.K.; Seow, P.W.; Zinn, R.; Milham, L.T.; Vaughan, C.W.; Vissel, B. A new mouse line with reduced GluA2 Q/R site RNA editing exhibits loss of dendritic spines, hippocampal CA1-neuron loss, learning and memory impairments and NMDA receptor-independent seizure vulnerability. Mol. Brain 2020, 13, 27. [Google Scholar] [CrossRef] [Green Version]
- Vargas, J.L.C.; Osswald, I.K.; Unsain, N.; Aurousseau, M.R.; Barker, P.A.; Bowie, D.; Di Polo, A. Soluble tumor necrosis factor alpha promotes retinal ganglion cell death in glaucoma via calcium-permeable AMPA receptor activation. J. Neurosci. 2015, 35, 12088–12102. [Google Scholar] [CrossRef] [Green Version]
- Conrad, K.L.; Tseng, K.Y.; Uejima, J.L.; Reimers, J.M.; Heng, L.-J.; Shaham, Y.; Marinelli, M.; Wolf, M.E. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature 2008, 454, 118–121. [Google Scholar] [CrossRef]
- Paoletti, P.; Neyton, J. NMDA receptor subunits: Function and pharmacology. Curr. Opin. Pharmacol. 2007, 7, 39–47. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Reddy, P.H. Role of Glutamate and NMDA Receptors in Alzheimer’s Disease. J. Alzheimer’s Dis. 2017, 57, 1041–1048. [Google Scholar] [CrossRef] [Green Version]
- Fazio, F.; Ulivieri, M.; Volpi, C.; Gargaro, M.; Fallarino, F. Targeting metabotropic glutamate receptors for the treatment of neuroinflammation. Curr. Opin. Pharmacol. 2018, 38, 16–23. [Google Scholar] [CrossRef]
- Julio-Pieper, M.; Flor, P.J.; Dinan, T.G.; Cryan, J.F. Exciting times beyond the brain: Metabotropic glutamate receptors in peripheral and non-neural tissues. Pharmacol. Rev. 2011, 63, 35–58. [Google Scholar] [CrossRef] [Green Version]
- Conn, P.J.; Pin, J.-P. Pharmacology and functions of metabotropic glutamate receptors. Annu. Rev. Pharmacol. Toxicol. 1997, 37, 205–237. [Google Scholar] [CrossRef] [Green Version]
- Swanson, C.J.; Bures, M.; Johnson, M.P.; Linden, A.-M.; Monn, J.A.; Schoepp, D.D. Metabotropic glutamate receptors as novel targets for anxiety and stress disorders. Nat. Rev. Drug Discov. 2005, 4, 131–144. [Google Scholar] [CrossRef]
- Conn, P.J. Physiological roles and therapeutic potential of metabotropic glutamate receptors. Ann. N. Y. Acad. Sci. 2003, 1003, 12–21. [Google Scholar] [CrossRef]
- Shigemoto, R.; Kinoshita, A.; Wada, E.; Nomura, S.; Ohishi, H.; Takada, M.; Flor, P.J.; Neki, A.; Abe, T.; Nakanishi, S.; et al. Differential presynaptic localization of metabotropic glutamate receptor subtypes in the rat hippocampus. J. Neurosci. 1997, 17, 7503–7522. [Google Scholar] [CrossRef] [Green Version]
- Skeberdis, V.A.; Lan, J.; Opitz, T.; Zheng, X.; Bennett, M.V.; Zukin, R.S. mGluR1-mediated potentiation of NMDA receptors involves a rise in intracellular calcium and activation of protein kinase C. Neuropharmacology 2001, 40, 856–865. [Google Scholar] [CrossRef]
- Matta, J.A.; Ashby, M.C.; Sanz-Clemente, A.; Roche, K.W.; Isaac, J.T. mGluR5 and NMDA receptors drive the experience- and activity-dependent NMDA receptor NR2B to NR2A subunit switch. Neuron 2011, 70, 339–351. [Google Scholar] [CrossRef] [Green Version]
- Südhof, T.C. Neurotransmitter release: The last millisecond in the life of a synaptic vesicle. Neuron 2013, 80, 675–690. [Google Scholar] [CrossRef] [Green Version]
- Haroon, E.; Miller, A.H.; Sanacora, G. Inflammation, Glutamate, and Glia: A Trio of Trouble in Mood Disorders. Neuropsychopharmacology 2017, 42, 193–215. [Google Scholar] [CrossRef]
- Seki, Y.; Feustel, P.J.; Keller, R.W., Jr.; Tranmer, B.I.; Kimelberg, H.K. Inhibition of ischemia-induced glutamate release in rat striatum by dihydrokinate and an anion channel blocker. Stroke 1999, 30, 433–440. [Google Scholar] [CrossRef] [Green Version]
- Cohen-Kashi-Malina, K.; Cooper, I.; Teichberg, V.I. Mechanisms of glutamate efflux at the blood-brain barrier: Involvement of glial cells. J. Cereb. Blood Flow Metab. 2012, 32, 177–189. [Google Scholar] [CrossRef]
- Zhang, Z.; Tao, Z.; Gameiro, A.; Barcelona, S.; Braams, S.; Rauen, T.; Grewer, C. Transport direction determines the kinetics of substrate transport by the glutamate transporter EAAC1. Proc. Natl. Acad. Sci. USA 2007, 104, 18025–18030. [Google Scholar] [CrossRef] [Green Version]
- Grewer, C.; Gameiro, A.; Zhang, Z.; Tao, Z.; Braams, S.; Rauen, T. Glutamate forward and reverse transport: From molecular mechanism to transporter-mediated release after ischemia. IUBMB Life 2008, 60, 609–619. [Google Scholar] [CrossRef] [Green Version]
- Anderson, C.M.; Swanson, R.A. Astrocyte glutamate transport: Review of properties, regulation, and physiological functions. Glia 2000, 32, 1–14. [Google Scholar] [CrossRef]
- Haydon, P.G.; Carmignoto, G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiol. Rev. 2006, 86, 1009–1031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewerenz, J.; Hewett, S.J.; Huang, Y.; Lambros, M.; Gout, P.W.; Kalivas, P.W.; Massie, A.; Smolders, I.; Methner, A.; Pergande, M.; et al. The cystine/glutamate antiporter system x(c)(-) in health and disease: From molecular mechanisms to novel therapeutic opportunities. Antioxid. Redox Signal. 2013, 18, 522–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bjørn-Yoshimoto, W.E.; Underhill, S.M. The importance of the excitatory amino acid transporter 3 (EAAT3). Neurochem. Int. 2016, 98, 4–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Šerý, O.; Sultana, N.; Kashem, M.A.; Pow, D.V.; Balcar, V.J. GLAST but not least—Distribution, function, genetics and epigenetics of L-glutamate transport in brain—focus on GLAST/EAAT1. Neurochem. Res. 2015, 40, 2461–2472. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Hassel, B.; Eid, T.; Danbolt, N.C. Axon-terminals expressing EAAT2 (GLT-1; Slc1a2) are common in the forebrain and not limited to the hippocampus. Neurochem. Int. 2019, 123, 101–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bar-Peled, O.; Ben-Hur, H.; Biegon, A.; Groner, Y.; Dewhurst, S.; Furuta, A.; Rothstein, J.D. Distribution of glutamate transporter subtypes during human brain development. J. Neurochem. 1997, 69, 2571–2580. [Google Scholar] [CrossRef] [Green Version]
- Bridges, R.J.; Esslinger, C.S. The excitatory amino acid transporters: Pharmacological insights on substrate and inhibitor specificity of the EAAT subtypes. Pharmacol. Ther. 2005, 107, 271–285. [Google Scholar] [CrossRef]
- Holmseth, S.; Scott, H.A.; Real, K.; Lehre, K.P.; Leergaard, T.B.; Bjaalie, J.G.; Danbolt, N.C. The concentrations and distributions of three C-terminal variants of the GLT1 (EAAT2; slc1a2) glutamate transporter protein in rat brain tissue suggest differential regulation. Neuroscience 2009, 162, 1055–1071. [Google Scholar] [CrossRef]
- Chen, W.; Mahadomrongkul, V.; Berger, U.V.; Bassan, M.; DeSilva, T.; Tanaka, K.; Irwin, N.; Aoki, C.; Rosenberg, P.A. The glutamate transporter GLT1a is expressed in excitatory axon terminals of mature hippocampal neurons. J. Neurosci. 2004, 24, 1136–1148. [Google Scholar] [CrossRef]
- Colton, C.A. Heterogeneity of microglial activation in the innate immune response in the brain. J. Neuroimmune Pharmacol. 2009, 4, 399–418. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Dissing-Olesen, L.; MacVicar, B.A.; Stevens, B. Microglia: Dynamic Mediators of Synapse Development and Plasticity. Trends Immunol. 2015, 36, 605–613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, S.; Dissing-Olesen, L.; Stevens, B. New insights on the role of microglia in synaptic pruning in health and disease. Curr. Opin. Neurobiol. 2016, 36, 128–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michelucci, A.; Heurtaux, T.; Grandbarbe, L.; Morga, E.; Heuschling, P. Characterization of the microglial phenotype under specific pro-inflammatory and anti-inflammatory conditions: Effects of oligomeric and fibrillar amyloid-beta. J. Neuroimmunol. 2009, 210, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Raoof, M.; Chen, Y.; Sumi, Y.; Sursal, T.; Junger, W.; Brohi, K.; Itagaki, K.; Hauser, C.J. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 2010, 464, 104–107. [Google Scholar] [CrossRef] [Green Version]
- Lotze, M.T.; Tracey, K.J. High-mobility group box 1 protein (HMGB1): Nuclear weapon in the immune arsenal. Nat. Rev. Immunol. 2005, 5, 331–342. [Google Scholar] [CrossRef]
- Park, J.S.; Gamboni-Robertson, F.; He, Q.; Svetkauskaite, D.; Kim, J.Y.; Strassheim, D.; Sohn, J.W.; Yamada, S.; Maruyama, I.; Banerjee, A.; et al. High mobility group box 1 protein interacts with multiple Toll-like receptors. Am. J. Physiol. Cell Physiol. 2006, 290, C917–C924. [Google Scholar] [CrossRef]
- Skvarc, D.R.; Berk, M.; Byrne, L.K.; Dean, O.M.; Dodd, S.; Lewis, M.; Marriott, A.; Moore, E.M.; Morris, G.; Page, R.S.; et al. Post-Operative Cognitive Dysfunction: An exploration of the inflammatory hypothesis and novel therapies. Neurosci. Biobehav. Rev. 2018, 84, 116–133. [Google Scholar] [CrossRef]
- Saxena, S.; Maze, M. Impact on the brain of the inflammatory response to surgery. Presse Med. 2018, 47, e73–e81. [Google Scholar] [CrossRef]
- Amantea, D.; Nappi, G.; Bernardi, G.; Bagetta, G.; Corasaniti, M.T. Post-ischemic brain damage: Pathophysiology and role of inflammatory mediators. FEBS J. 2009, 276, 13–26. [Google Scholar] [CrossRef]
- Ye, L.; Huang, Y.; Zhao, L.; Li, Y.; Sun, L.; Zhou, Y.; Qian, G.; Zheng, J.C. IL-1β and TNF-α induce neurotoxicity through glutamate production: A potential role for neuronal glutaminase. J. Neurochem. 2013, 125, 897–908. [Google Scholar] [CrossRef]
- Mascarucci, P.; Perego, C.; Terrazzino, S.; De Simoni, M. Glutamate release in the nucleus tractus solitarius induced by peripheral lipopolysaccharide and interleukin-1β. Neuroscience 1998, 86, 1285–1290. [Google Scholar] [CrossRef]
- Noda, M.; Nakanishi, H.; Nabekura, J.; Akaike, N. AMPA-kainate subtypes of glutamate receptor in rat cerebral microglia. J. Neurosci. 2000, 20, 251–258. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.S.; Joh, T.H. Microglia, major player in the brain inflammation: Their roles in the pathogenesis of Parkinson’s disease. Exp. Mol. Med. 2006, 38, 333–347. [Google Scholar] [CrossRef] [Green Version]
- George, J.; Gonçalves, F.Q.; Cristóvão, G.; Rodrigues, L.; Meyer Fernandes, J.R.; Gonçalves, T.; Cunha, R.A.; Gomes, C.A. Different danger signals differently impact on microglial proliferation through alterations of ATP release and extracellular metabolism. Glia 2015, 63, 1636–1645. [Google Scholar] [CrossRef]
- Taylor, D.L.; Jones, F.; Kubota, E.S.; Pocock, J.M. Stimulation of microglial metabotropic glutamate receptor mGlu2 triggers tumor necrosis factor alpha-induced neurotoxicity in concert with microglial-derived Fas ligand. J. Neurosci. 2005, 25, 2952–2964. [Google Scholar] [CrossRef] [Green Version]
- Pinteaux-Jones, F.; Sevastou, I.G.; Fry, V.A.; Heales, S.; Baker, D.; Pocock, J.M. Myelin-induced microglial neurotoxicity can be controlled by microglial metabotropic glutamate receptors. J. Neurochem. 2008, 106, 442–454. [Google Scholar] [CrossRef]
- Piers, T.M.; Heales, S.J.; Pocock, J.M. Positive allosteric modulation of metabotropic glutamate receptor 5 down-regulates fibrinogen-activated microglia providing neuronal protection. Neurosci. Lett. 2011, 505, 140–145. [Google Scholar] [CrossRef]
- Marina, N.; Turovsky, E.; Christie, I.N.; Hosford, P.S.; Hadjihambi, A.; Korsak, A.; Ang, R.; Mastitskaya, S.; Sheikhbahaei, S.; Theparambil, S.M.; et al. Brain metabolic sensing and metabolic signaling at the level of an astrocyte. Glia 2018, 66, 1185–1199. [Google Scholar] [CrossRef] [Green Version]
- Tasdemir-Yilmaz, O.E.; Freeman, M.R. Astrocytes engage unique molecular programs to engulf pruned neuronal debris from distinct subsets of neurons. Genes Dev. 2014, 28, 20–33. [Google Scholar] [CrossRef] [Green Version]
- Matos, M.; Augusto, E.; Oliveira, C.R.; Agostinho, P. Amyloid-beta peptide decreases glutamate uptake in cultured astrocytes: Involvement of oxidative stress and mitogen-activated protein kinase cascades. Neuroscience 2008, 156, 898–910. [Google Scholar] [CrossRef]
- Zhang, D.; Hu, X.; Qian, L.; O’Callaghan, J.P.; Hong, J.-S. Astrogliosis in CNS pathologies: Is there a role for microglia? Mol. Neurobiol. 2010, 41, 232–241. [Google Scholar] [CrossRef] [Green Version]
- Linnerbauer, M.; Wheeler, M.A.; Quintana, F.J. Astrocyte crosstalk in CNS inflammation. Neuron 2020, 108, 608–622. [Google Scholar] [CrossRef]
- O’Kane, R.L.; Martínez-López, I.; DeJoseph, M.R.; Viña, J.R.; Hawkins, R.A. Na(+)-dependent glutamate transporters (EAAT1, EAAT2, and EAAT3) of the blood-brain barrier. A mechanism for glutamate removal. J. Biol. Chem. 1999, 274, 31891–31895. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.L.; Kong, Q.; Cuny, G.D.; Glicksman, M.A. Glutamate transporter EAAT2: A new target for the treatment of neurodegenerative diseases. Future Med. Chem. 2012, 4, 1689–1700. [Google Scholar] [CrossRef] [Green Version]
- Du, X.; Li, J.; Li, M.; Yang, X.; Qi, Z.; Xu, B.; Liu, W.; Xu, Z.; Deng, Y. Research progress on the role of type I vesicular glutamate transporter (VGLUT1) in nervous system diseases. Cell Biosci. 2020, 10, 26. [Google Scholar] [CrossRef] [Green Version]
- Abbott, N.J.; Rönnbäck, L.; Hansson, E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat. Rev. Neurosci. 2006, 7, 41–53. [Google Scholar] [CrossRef]
- Sofroniew, M.V. Multiple roles for astrocytes as effectors of cytokines and inflammatory mediators. Neuroscientist 2014, 20, 160–172. [Google Scholar] [CrossRef]
- Lutgen, V.; Narasipura, S.D.; Sharma, A.; Min, S.; Al-Harthi, L. β-Catenin signaling positively regulates glutamate uptake and metabolism in astrocytes. J. Neuroinflamm. 2016, 13, 242. [Google Scholar] [CrossRef] [Green Version]
- Vesce, S.; Rossi, D.; Brambilla, L.; Volterra, A. Glutamate release from astrocytes in physiological conditions and in neurodegenerative disorders characterized by neuroinflammation. Int. Rev. Neurobiol. 2007, 82, 57–71. [Google Scholar] [CrossRef]
- Noch, E.; Khalili, K. Molecular mechanisms of necrosis in glioblastoma: The role of glutamate excitotoxicity. Cancer Biol. Ther. 2009, 8, 1791–1797. [Google Scholar] [CrossRef]
- Tzartos, J.S.; Friese, M.A.; Craner, M.J.; Palace, J.; Newcombe, J.; Esiri, M.M.; Fugger, L. Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis. Am. J. Pathol. 2008, 172, 146–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valentin-Torres, A.; Savarin, C.; Barnett, J.; Bergmann, C.C. Blockade of sustained tumor necrosis factor in a transgenic model of progressive autoimmune encephalomyelitis limits oligodendrocyte apoptosis and promotes oligodendrocyte maturation. J. Neuroinflamm. 2018, 15, 121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Maeda, Y.; Ming, X.; Cook, S.; Chapin, J.; Husar, W.; Dowling, P. Apoptotic death following Fas activation in human oligodendrocyte hybrid cultures. J. Neurosci. Res. 2002, 69, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Bezzi, P.; Domercq, M.; Brambilla, L.; Galli, R.; Schols, D.; De Clercq, E.; Vescovi, A.; Bagetta, G.; Kollias, G.; Meldolesi, J. CXCR4-activated astrocyte glutamate release via TNFα: Amplification by microglia triggers neurotoxicity. Nat. Neurosci. 2001, 4, 702–710. [Google Scholar] [CrossRef] [PubMed]
- Evonuk, K.S.; Doyle, R.E.; Moseley, C.E.; Thornell, I.M.; Adler, K.; Bingaman, A.M.; Bevensee, M.O.; Weaver, C.T.; Min, B.; DeSilva, T.M. Reduction of AMPA receptor activity on mature oligodendrocytes attenuates loss of myelinated axons in autoimmune neuroinflammation. Sci. Adv. 2020, 6, eaax5936. [Google Scholar] [CrossRef] [Green Version]
- McDonald, J.W.; Althomsons, S.P.; Hyrc, K.L.; Choi, D.W.; Goldberg, M.P. Oligodendrocytes from forebrain are highly vulnerable to AMPA/kainate receptor-mediated excitotoxicity. Nat. Med. 1998, 4, 291–297. [Google Scholar] [CrossRef]
- Domercq, M.; Sánchez-Gómez, M.V.; Sherwin, C.; Etxebarria, E.; Fern, R.; Matute, C. System xc- and glutamate transporter inhibition mediates microglial toxicity to oligodendrocytes. J. Immunol. 2007, 178, 6549–6556. [Google Scholar] [CrossRef] [Green Version]
- Nutma, E.; van Gent, D.; Amor, S.; Peferoen, L.A.N. Astrocyte and Oligodendrocyte Cross-Talk in the Central Nervous System. Cells 2020, 9, 600. [Google Scholar] [CrossRef] [Green Version]
- Semple, B.D.; Kossmann, T.; Morganti-Kossmann, M.C. Role of chemokines in CNS health and pathology: A focus on the CCL2/CCR2 and CXCL8/CXCR2 networks. J. Cereb. Blood Flow Metab. 2010, 30, 459–473. [Google Scholar] [CrossRef] [Green Version]
- Piani, D.; Frei, K.; Do, K.Q.; Cuénod, M.; Fontana, A. Murine brain macrophages induced NMDA receptor mediated neurotoxicity in vitro by secreting glutamate. Neurosci. Lett. 1991, 133, 159–162. [Google Scholar] [CrossRef]
- Klegeris, A.; McGeer, P.L. beta-amyloid protein enhances macrophage production of oxygen free radicals and glutamate. J. Neurosci. Res. 1997, 49, 229–235. [Google Scholar] [CrossRef]
- Hendriks, J.J.; Teunissen, C.E.; de Vries, H.E.; Dijkstra, C.D. Macrophages and neurodegeneration. Brain Res. Brain Res. Rev. 2005, 48, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Kipnis, J.; Gadani, S.; Derecki, N.C. Pro-cognitive properties of T cells. Nat. Rev. Immunol. 2012, 12, 663–669. [Google Scholar] [CrossRef] [Green Version]
- Ganor, Y.; Grinberg, I.; Reis, A.; Cooper, I.; Goldstein, R.S.; Levite, M. Human T-leukemia and T-lymphoma express glutamate receptor AMPA GluR3, and the neurotransmitter glutamate elevates the cancer-related matrix-metalloproteinases inducer CD147/EMMPRIN, MMP-9 secretion and engraftment of T-leukemia in vivo. Leuk. Lymphoma 2009, 50, 985–997. [Google Scholar] [CrossRef]
- Ganor, Y.; Levite, M. The neurotransmitter glutamate and human T cells: Glutamate receptors and glutamate-induced direct and potent effects on normal human T cells, cancerous human leukemia and lymphoma T cells, and autoimmune human T cells. J. Neural Transm. 2014, 121, 983–1006. [Google Scholar] [CrossRef] [PubMed]
- Viviani, B.; Bartesaghi, S.; Gardoni, F.; Vezzani, A.; Behrens, M.M.; Bartfai, T.; Binaglia, M.; Corsini, E.; Di Luca, M.; Galli, C.L.; et al. Interleukin-1beta enhances NMDA receptor-mediated intracellular calcium increase through activation of the Src family of kinases. J. Neurosci. 2003, 23, 8692–8700. [Google Scholar] [CrossRef]
- Curran, B.P.; Murray, H.J.; O’Connor, J.J. A role for c-Jun N-terminal kinase in the inhibition of long-term potentiation by interleukin-1beta and long-term depression in the rat dentate gyrus in vitro. Neuroscience 2003, 118, 347–357. [Google Scholar] [CrossRef]
- Hu, S.; Sheng, W.S.; Ehrlich, L.C.; Peterson, P.K.; Chao, C.C. Cytokine effects on glutamate uptake by human astrocytes. Neuroimmunomodulation 2000, 7, 153–159. [Google Scholar] [CrossRef]
- Pemberton, L.A.; Kerr, S.J.; Smythe, G.; Brew, B.J. Quinolinic acid production by macrophages stimulated with IFN-gamma, TNF-alpha, and IFN-alpha. J. Interferon Cytokine Res. 1997, 17, 589–595. [Google Scholar] [CrossRef]
- Mizuno, T.; Zhang, G.; Takeuchi, H.; Kawanokuchi, J.; Wang, J.; Sonobe, Y.; Jin, S.; Takada, N.; Komatsu, Y.; Suzumura, A. Interferon-gamma directly induces neurotoxicity through a neuron specific, calcium-permeable complex of IFN-gamma receptor and AMPA GluR1 receptor. FASEB J. 2008, 22, 1797–1806. [Google Scholar] [CrossRef]
- Heinrich, P.C.; Castell, J.V.; Andus, T. Interleukin-6 and the acute phase response. Biochem. J. 1990, 265, 621–636. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Hatanaka, H. Interleukin-6 protects cultured rat hippocampal neurons against glutamate-induced cell death. Brain Res. 1994, 643, 173–180. [Google Scholar] [CrossRef]
- Merrill, J.E. Tumor necrosis factor alpha, interleukin 1 and related cytokines in brain development: Normal and pathological. Dev. Neurosci. 1992, 14, 1–10. [Google Scholar] [CrossRef]
- D’Arcangelo, G.; Tancredi, V.; Onofri, F.; D’Antuono, M.; Giovedì, S.; Benfenati, F. Interleukin-6 inhibits neurotransmitter release and the spread of excitation in the rat cerebral cortex. Eur. J. Neurosci. 2000, 12, 1241–1252. [Google Scholar] [CrossRef]
- Beattie, E.C.; Stellwagen, D.; Morishita, W.; Bresnahan, J.C.; Ha, B.K.; Von Zastrow, M.; Beattie, M.S.; Malenka, R.C. Control of synaptic strength by glial TNFalpha. Science 2002, 295, 2282–2285. [Google Scholar] [CrossRef]
- Derkach, V.A.; Oh, M.C.; Guire, E.S.; Soderling, T.R. Regulatory mechanisms of AMPA receptors in synaptic plasticity. Nat. Rev. Neurosci. 2007, 8, 101–113. [Google Scholar] [CrossRef]
- Ferguson, A.R.; Christensen, R.N.; Gensel, J.C.; Miller, B.A.; Sun, F.; Beattie, E.C.; Bresnahan, J.C.; Beattie, M.S. Cell death after spinal cord injury is exacerbated by rapid TNF alpha-induced trafficking of GluR2-lacking AMPARs to the plasma membrane. J. Neurosci. 2008, 28, 11391–11400. [Google Scholar] [CrossRef]
- Bliss, R.M.; Finckbone, V.L.; Trice, J.; Strahlendorf, H.; Strahlendorf, J. Tumor necrosis factor-α (TNF-α) augments AMPA-induced Purkinje neuron toxicity. Brain Res. 2011, 1386, 1–14. [Google Scholar] [CrossRef]
- Zou, J.Y.; Crews, F.T. TNF alpha potentiates glutamate neurotoxicity by inhibiting glutamate uptake in organotypic brain slice cultures: Neuroprotection by NF kappa B inhibition. Brain Res. 2005, 1034, 11–24. [Google Scholar] [CrossRef]
- Le, Y.; Liu, S.; Peng, M.; Tan, C.; Liao, Q.; Duan, K.; Ouyang, W.; Tong, J. Aging differentially affects the loss of neuronal dendritic spine, neuroinflammation and memory impairment at rats after surgery. PLoS ONE 2014, 9, e106837. [Google Scholar] [CrossRef]
- Tang, Y.; Wang, X.; Zhang, S.; Duan, S.; Qing, W.; Chen, G.; Ye, F.; Le, Y.; Ouyang, W. Pre-existing weakness is critical for the occurrence of postoperative cognitive dysfunction in mice of the same age. PLoS ONE 2017, 12, e0182471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rundshagen, I. Postoperative cognitive dysfunction. Deutsch. Ärztebl. Int. 2014, 111, 119–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moller, J.T.; Cluitmans, P.; Rasmussen, L.S.; Houx, P.; Rasmussen, H.; Canet, J.; Rabbitt, P.; Jolles, J.; Larsen, K.; Hanning, C. Long-term postoperative cognitive dysfunction in the elderly: ISPOCD1 study. Lancet 1998, 351, 857–861. [Google Scholar] [CrossRef]
- Eckenhoff, R.G. The Perioperative Neurocognitive Disorders; Cambridge University Press: Cambridge, UK, 2019. [Google Scholar]
- Subramaniyan, S.; Terrando, N. Narrative Review Article: Neuroinflammation and Perioperative Neurocognitive Disorders. Anesth. Analg. 2019, 128, 781–788. [Google Scholar] [CrossRef]
- Shen, X.; Dong, Y.; Xu, Z.; Wang, H.; Miao, C.; Soriano, S.G.; Sun, D.; Baxter, M.G.; Zhang, Y.; Xie, Z. Selective anesthesia-induced neuroinflammation in developing mouse brain and cognitive impairment. J. Am. Soc. Anesthesiol. 2013, 118, 502–515. [Google Scholar] [CrossRef] [Green Version]
- Lord, J.M.; Midwinter, M.J.; Chen, Y.F.; Belli, A.; Brohi, K.; Kovacs, E.J.; Koenderman, L.; Kubes, P.; Lilford, R.J. The systemic immune response to trauma: An overview of pathophysiology and treatment. Lancet 2014, 384, 1455–1465. [Google Scholar] [CrossRef] [Green Version]
- Toft, P.; Tønnesen, E. The systemic inflammatory response to anaesthesia and surgery. Curr. Anaesth. Crit. Care 2008, 19, 349–353. [Google Scholar] [CrossRef]
- Wu, L.-J.; Toyoda, H.; Zhao, M.-G.; Lee, Y.-S.; Tang, J.; Ko, S.W.; Jia, Y.H.; Shum, F.W.; Zerbinatti, C.V.; Bu, G. Upregulation of forebrain NMDA NR2B receptors contributes to behavioral sensitization after inflammation. J. Neurosci. 2005, 25, 11107–11116. [Google Scholar] [CrossRef]
- Zeron, M.M.; Fernandes, H.B.; Krebs, C.; Shehadeh, J.; Wellington, C.L.; Leavitt, B.R.; Baimbridge, K.G.; Hayden, M.R.; Raymond, L.A. Potentiation of NMDA receptor-mediated excitotoxicity linked with intrinsic apoptotic pathway in YAC transgenic mouse model of Huntington’s disease. Mol. Cell. Neurosci. 2004, 25, 469–479. [Google Scholar] [CrossRef]
- Cheung, N.S.; Beart, P.M.; Pascoe, C.J.; John, C.A.; Bernard, O. Human Bcl-2 protects against AMPA receptor-mediated apoptosis. J. Neurochem. 2000, 74, 1613–1620. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Lu, Y.; Dong, Y.; Zhang, G.; Zhang, Y.; Xu, Z.; Culley, D.J.; Crosby, G.; Marcantonio, E.R.; Tanzi, R.E.; et al. The inhalation anesthetic isoflurane increases levels of proinflammatory TNF-α, IL-6, and IL-1β. Neurobiol. Aging 2012, 33, 1364–1378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larsen, M.; Hegstad, E.; Berg-Johnsen, J.; Langmoen, I.A. Isoflurane increases the uptake of glutamate in synaptosomes from rat cerebral cortex. Br. J. Anaesth. 1997, 78, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.M.; Drummond, J.C.; Cole, D.J.; Goskowicz, R.L. Isoflurane reduces ischemia-induced glutamate release in rats subjected to forebrain ischemia. Anesthesiology 1995, 82, 996–1003. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zuo, Z. Isoflurane induces a protein kinase C alpha-dependent increase in cell-surface protein level and activity of glutamate transporter type 3. Mol. Pharmacol. 2005, 67, 1522–1533. [Google Scholar] [CrossRef] [Green Version]
- Cao, J.; Wang, Z.; Mi, W.; Zuo, Z. Isoflurane unveils a critical role of glutamate transporter type 3 in regulating hippocampal GluR1 trafficking and context-related learning and memory in mice. Neuroscience 2014, 272, 58–64. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.A.; Choi, J.G.; Zuo, Z. Volatile anesthetics attenuate oxidative stress-reduced activity of glutamate transporter type 3. Anesth. Analg. 2009, 109, 1506–1510. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Park, S.H.; Zuo, Z. Effects of isoflurane on learning and memory functions of wild-type and glutamate transporter type 3 knockout mice. J. Pharm. Pharmacol. 2012, 64, 302–307. [Google Scholar] [CrossRef]
- Wang, X.; Shan, Y.; Tang, Z.; Gao, L.; Liu, H. Neuroprotective effects of dexmedetomidine against isoflurane-induced neuronal injury via glutamate regulation in neonatal rats. Drug Des. Dev. Ther. 2019, 13, 153–160. [Google Scholar] [CrossRef] [Green Version]
- Fang, H.; Huang, Y.; Zuo, Z. The different responses of rat glutamate transporter type 2 and its mutant (tyrosine 403 to histidine) activity to volatile anesthetics and activation of protein kinase C. Brain Res. 2002, 953, 255–264. [Google Scholar] [CrossRef]
- Vutskits, L.; Xie, Z. Lasting impact of general anaesthesia on the brain: Mechanisms and relevance. Nat. Rev. Neurosci. 2016, 17, 705–717. [Google Scholar] [CrossRef]
- Saatman, K.E.; Bozyczko-Coyne, D.; Marcy, V.; Siman, R.; McIntosh, T.K. Prolonged calpain-mediated spectrin breakdown occurs regionally following experimental brain injury in the rat. J. Neuropathol. Exp. Neurol. 1996, 55, 850–860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, X.; Zhang, X.; Li, S.; Chi, X.; Luo, A.; Zhao, Y. NR2B receptor- and calpain-mediated KCC2 cleavage resulted in cognitive deficiency exposure to isoflurane. Neurotoxicology 2020, 76, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Rammes, G.; Starker, L.K.; Haseneder, R.; Berkmann, J.; Plack, A.; Zieglgänsberger, W.; Ohl, F.; Kochs, E.F.; Blobner, M. Isoflurane anaesthesia reversibly improves cognitive function and long-term potentiation (LTP) via an up-regulation in NMDA receptor 2B subunit expression. Neuropharmacology 2009, 56, 626–636. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Li, L.; Lin, D.; Zuo, Z. Isoflurane induces learning impairment that is mediated by interleukin 1β in rodents. PLoS ONE 2012, 7, e51431. [Google Scholar] [CrossRef]
- Lin, D.; Zuo, Z. Isoflurane induces hippocampal cell injury and cognitive impairments in adult rats. Neuropharmacology 2011, 61, 1354–1359. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Wang, P.; Zhang, X.; Zhang, W.; Gu, G. Effects of different concentration and duration time of isoflurane on acute and long-term neurocognitive function of young adult C57BL/6 mouse. Int. J. Clin. Exp. Pathol. 2014, 7, 5828–5836. [Google Scholar]
- Qu, X.; Xu, C.; Wang, H.; Xu, J.; Liu, W.; Wang, Y.; Jia, X.; Xie, Z.; Xu, Z.; Ji, C.; et al. Hippocampal glutamate level and glutamate aspartate transporter (GLAST) are up-regulated in senior rat associated with isoflurane-induced spatial learning/memory impairment. Neurochem. Res. 2013, 38, 59–73. [Google Scholar] [CrossRef]
- Wang, N.; Wang, M. Dexmedetomidine suppresses sevoflurane anesthesia-induced neuroinflammation through activation of the PI3K/Akt/mTOR pathway. BMC Anesthesiol. 2019, 19, 134. [Google Scholar] [CrossRef] [Green Version]
- Moe, M.C.; Berg-Johnsen, J.; Larsen, G.A.; Røste, G.K.; Vinje, M.L. Sevoflurane reduces synaptic glutamate release in human synaptosomes. J. Neurosurg. Anesthesiol. 2002, 14, 180–186. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, F.; Shi, J. Neonatal exposure to sevoflurane caused cognitive deficits by dysregulating SK2 channels and GluA2-lacking AMPA receptors in juvenile rat hippocampus. Neuropharmacology 2018, 141, 66–75. [Google Scholar] [CrossRef]
- Gao, Y.; Zhao, T.; Chen, Y.; Sun, Z.; Lu, J.; Shi, Z.; Song, X. Prenatal sevoflurane exposure causes abnormal development of the entorhinal cortex in rat offspring. J. Integr. Neurosci. 2021, 20, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cheng, Y.; Liu, G.; Tian, X.; Tu, X.; Wang, J. Chronic exposure of gestation rat to sevoflurane impairs offspring brain development. Neurol. Sci. 2012, 33, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Satomoto, M.; Sun, Z.; Adachi, Y.U.; Makita, K. Neonatal Sevoflurane Exposure Induces Adulthood Fear-induced Learning Disability and Decreases Glutamatergic Neurons in the Basolateral Amygdala. J. Neurosurg. Anesthesiol. 2018, 30, 59–64. [Google Scholar] [CrossRef]
- Wang, W.; Lu, R.; Feng, D.Y.; Zhang, H. Sevoflurane Inhibits Glutamate-Aspartate Transporter and Glial Fibrillary Acidic Protein Expression in Hippocampal Astrocytes of Neonatal Rats Through the Janus Kinase/Signal Transducer and Activator of Transcription (JAK/STAT) Pathway. Anesth. Analg. 2016, 123, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Haseneder, R.; Starker, L.; Berkmann, J.; Kellermann, K.; Jungwirth, B.; Blobner, M.; Eder, M.; Kochs, E.; Rammes, G. Sevoflurane anesthesia improves cognitive performance in mice, but does not influence in vitro long-term potentation in hippocampus CA1 stratum radiatum. PLoS ONE 2013, 8, e64732. [Google Scholar] [CrossRef] [Green Version]
- Peng, S.; Li, P.; Liu, P.; Yan, H.; Wang, J.; Lu, W.; Liu, C.; Zhou, Y. Cistanches alleviates sevoflurane-induced cognitive dysfunction by regulating PPAR-γ-dependent antioxidant and anti-inflammatory in rats. J. Cell. Mol. Med. 2020, 24, 1345–1359. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, F.; Shi, J. Sevoflurane anesthesia impairs metabotropic glutamate receptor-dependent long-term depression and cognitive functions in senile mice. Geriatr. Gerontol. Int. 2019, 19, 357–362. [Google Scholar] [CrossRef]
- Jafarzadeh, A.; Hadavi, M.; Hassanshahi, G.; Rezaeian, M.; Vazirinejad, R. General anesthetics on immune system cytokines: A narrative review article. Anesth. Pain Med. 2020, 10, e103033. [Google Scholar] [CrossRef]
- Hietbrink, F.; Koenderman, L.; Rijkers, G.; Leenen, L. Trauma: The role of the innate immune system. World J. Emerg. Surg. 2006, 1, 15. [Google Scholar] [CrossRef] [Green Version]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef]
- Helmy, S.A.; Wahby, M.A.; El-Nawaway, M. The effect of anaesthesia and surgery on plasma cytokine production. Anaesthesia 1999, 54, 733–738. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Gu, C.; Mandeville, E.T.; Dong, Y.; Esposito, E.; Zhang, Y.; Yang, G.; Shen, Y.; Fu, X.; Lo, E.H. Anesthesia and surgery impair blood–brain barrier and cognitive function in mice. Front. Immunol. 2017, 8, 902. [Google Scholar] [CrossRef] [PubMed]
- Stover, J.; Kempski, O. Anesthesia increases circulating glutamate in neurosurgical patients. Acta Neurochir. 2005, 147, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Chi, H.; Kawano, T.; Tamura, T.; Iwata, H.; Takahashi, Y.; Eguchi, S.; Yamazaki, F.; Kumagai, N.; Yokoyama, M. Postoperative pain impairs subsequent performance on a spatial memory task via effects on N-methyl-D-aspartate receptor in aged rats. Life Sci. 2013, 93, 986–993. [Google Scholar] [CrossRef]
- Zhang, X.; Xin, X.; Dong, Y.; Zhang, Y.; Yu, B.; Mao, J.; Xie, Z. Surgical incision-induced nociception causes cognitive impairment and reduction in synaptic NMDA receptor 2B in mice. J. Neurosci. 2013, 33, 17737–17748. [Google Scholar] [CrossRef] [Green Version]
- Riazi, K.; Galic, M.A.; Kentner, A.C.; Reid, A.Y.; Sharkey, K.A.; Pittman, Q.J. Microglia-dependent alteration of glutamatergic synaptic transmission and plasticity in the hippocampus during peripheral inflammation. J. Neurosci. 2015, 35, 4942–4952. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Xiao, B.; Li, C.X.; Wang, Y. Fingolimod (FTY720) improves postoperative cognitive dysfunction in mice subjected to D-galactose-induced aging. Neural Regen. Res. 2020, 15, 1308–1315. [Google Scholar] [CrossRef]
- Hu, N.; Wang, M.; Xie, K.; Wang, H.; Wang, C.; Wang, C.; Wang, C.; Li, Y.; Yu, Y.; Wang, G. Internalization of GluA2 and the underlying mechanisms of cognitive decline in aged rats following surgery and prolonged exposure to sevoflurane. Neurotoxicology 2015, 49, 94–103. [Google Scholar] [CrossRef]
- Wang, T.; Zhu, H.; Hou, Y.; Gu, W.; Wu, H.; Luan, Y.; Xiao, C.; Zhou, C. Galantamine reversed early postoperative cognitive deficit via alleviating inflammation and enhancing synaptic transmission in mouse hippocampus. Eur. J. Pharmacol. 2019, 846, 63–72. [Google Scholar] [CrossRef]
- Xie, W.; Yang, Y.; Gu, X.; Zheng, Y.; Sun, Y.E.; Liang, Y.; Bo, J.; Ma, Z. Senegenin attenuates hepatic ischemia-reperfusion induced cognitive dysfunction by increasing hippocampal NR2B expression in rats. PLoS ONE 2012, 7, e45575. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.H.; Seubert, S.; Huhn, K.; Brecht, L.; Rötger, C.; Waschbisch, A.; Schlachetzki, J.; Klausmeyer, A.; Melms, A.; Wiese, S.; et al. Fingolimod effects in neuroinflammation: Regulation of astroglial glutamate transporters? PLoS ONE 2017, 12, e0171552. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhang, B.; Shan, S.; Zhao, X. Neuroprotective effects of vitexin against isoflurane-induced neurotoxicity by targeting the TRPV1 and NR2B signaling pathways. Mol. Med. Rep. 2016, 14, 5607–5613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, S.W.; Su, H.C.; Nyam, T.E.; Chio, C.C.; Kuo, J.R.; Wang, C.C. Ceftriaxone therapy attenuates brain trauma in rats by affecting glutamate transporters and neuroinflammation and not by its antibacterial effects. BMC Neurosci. 2021, 22, 54. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).