Abstract
Osteoarthritis (OA) is one of the most common diseases leading to physical disability, with age being the main risk factor, and degeneration of articular cartilage is the main focus for the pathogenesis of OA. Autophagy is a crucial intracellular homeostasis system recycling flawed macromolecules and cellular organelles to sustain the metabolism of cells. Growing evidences have revealed that autophagy is chondroprotective by regulating apoptosis and repairing the function of damaged chondrocytes. Then, OA is related to autophagy depending on different stages and models. In this review, we discuss the character of autophagy in OA and the process of the autophagy pathway, which can be modulated by some drugs, key molecules and non-coding RNAs (microRNAs, long non-coding RNAs and circular RNAs). More in-depth investigations of autophagy are needed to find therapeutic targets or diagnostic biomarkers through in vitro and in vivo situations, making autophagy a more effective way for OA treatment in the future. The aim of this review is to introduce the concept of autophagy and make readers realize its impact on OA. The database we searched in is PubMed and we used the keywords listed below to find appropriate article resources.
1. Introduction
1.1. Osteoarthritis
Osteoarthritis (OA) is a very common degenerative joint disease influencing many people by causing pain, stiffness and disability, especially for the elderly [1]. About one-third of people aged more than 65 are in pain with OA [2]; it costs a great deal of socioeconomic burden owing to the increasing prevalence caused by complicated and multifactorial situation including occupation, food habits, hormones, and heredity [3].
The global prevalence of knee OA is around 3.8% [4]. There is a report revealing that OA is the second-most cause, around 7.1%, of musculoskeletal diseases’ burden, and the burden of OA considerably raised 63.1% from 1990 to 2007 [5]. Besides, OA is one of the most challenging diseases to remedy since there are no blood vessels and nerve supplies involved in mature articular cartilage. The regeneration ability is weak and usually, fibrous cartilage is formed [6].
Symptomatic OA requires medical intervention. Nevertheless, the therapeutic choice is still limited and no disease-modifying drug is available. The degenerative process of osteoarthritic cartilage in aging patients is not able to be completely delayed [7,8]. Furthermore, surgery like total knee arthroplasty is risk and costly. Hence, an alternative approach has been revealed. In addition to the application in cancer, autophagy may be a surrogate for OA, which numerous pieces of research are focusing on [9].
1.2. Pathogenesis of Osteoarthritis
OA is recognized as a whole joint disease involved in cartilage, infrapatellar fat pad, subchondral bone, ligaments, meniscus and synovial membrane, which eventually causes joint failure [10]. There are several risk factors to increase the peril of developing OA. Diabetes elevating inflammatory response, causing atherosclerosis to affect blood flow of subchondral bone is thought to be contributory to OA [11]. Post-menopausal women have raised their risk of OA due to lower estrogen level and lack of ability to protect cartilage from oxidative stress [12]. Obesity which overloads the weight-bearing system of our body is also a crucial part of inducing OA [10]. Besides, about 12% of OA can be caused by trauma immediately or chronically which forms alterations in biomechanics whether it is structural damage or joint instability [13,14].
However, the pathogenesis of OA is mostly about aging. The imbalance of catabolism and anabolism in the cartilage matrix is related to aging because the more vulnerable joint cannot afford damage from outside [15]. Hemostasis could not be maintained by increasing catabolic along with decreasing the anabolic pathway of chondrocytes which leads to progressive degradation of cartilage [16]. In histology, the main pathogenesis of OA is the terminal differentiation of chondrocytes and the subsequent apoptosis. Chondrocyte apoptosis is also an important issue in OA. Chondrocyte apoptosis is associated with OA in the feature of cartilage and extracellular matrix devastation, which was proved by electron microscopy to show a few characterized cellular morphology in the sample of osteoarthritic cartilage, such as loss of nuclear volume, apoptotic bodies, cell-surface blebs and even the alteration of nuclear shape [17,18].
We can outline OA as early and late stages [1]. Not only growth of the cellular size but also congregate chondrocytes are found in the early stage, but the compensation is not enough for the cell matrix as the amount of glycosaminoglycan (GAG) starts to diminish. However, in the late stage, situations become more severe along with chondrocyte death, hypo-cellularity and lacunar emptying [1,19].
Besides, the situation of autophagy (macro-autophagy) and apoptosis in cartilage may change depending on the stage of OA. Early stage of OA demonstrated both elevated markers of autophagy and apoptosis in the superficial zone, indicating that autophagy is an adaptive response to apoptotic cell death, while in late stage of OA, in the deep zone, more apoptosis with the absence of autophagy represented the chondrocyte calcification [20]. Then, there is a type of chondrocyte death called chondroptosis, also related to autophagy proved by the existence of autophagic vacuoles [21]. Almonte-Becerill proposed that this cell death is like a mixture of autophagy and classical apoptosis [20]; it is the concept appearing in the late stage of OA as both apoptosis and autophagy are activated at this time in chondrocytes to show a different way of cellular demise [20].
2. Autophagy
2.1. Definition, Classification and Mechanism
2.1.1. Definition
Cellular components are constantly recycled and remodeled in humans. There are two major systems of degradation in eukaryotic cells; one is proteasomal degradation which selectively identifies ubiquitinated substrates, and the other is lysosomal degradation [22]. In lysosomal degradation, protein, organelle, cytosolic components and even extracellular substances would be transferred to lysosomes through autophagic activity wherever they are in animals, plants or yeast cells [22,23]. Thought to be nonselective in general. However, autophagy has been noticed to selectively remove possibly detrimental or unnecessary cytoplasmic material lately. Aggrephagy means eliminating accumulated proteins; meanwhile, mitophagy is the way to take off mitochondria (damaged organelle) [24,25].
We can find the different functions of autophagy in previous studies. Hunger response is the process that promotes autophagy and decomposes large molecules to intermediate metabolites used in anabolism or katabolism, for example, the amino acid deficiency in the liver [26]. When it comes to regulating cell activity, autophagy is responsible for degrading normal proteins and recombining cells during aging, differentiation and metamorphosis among animals [27,28].
2.1.2. Classification
Autophagy could be categorized as three types by physiological functions: chaperone-mediated autophagy, microautophagy, and macro-autophagy [29,30], and macro-autophagy is mostly discussed in the field of physiology, cytology and pathology. Besides, macro-autophagy utilized at the beginning to take away unemployed proteins and damaged organelles is the main pathway of mammalian autophagy. Microautophagy functions as maintaining organelle size and membrane homeostasis by engulfing small cytoplasmic components [31], while chaperone-mediated autophagy degrades proteins in a more selective way, such as pentapeptide recognized by hsc70 (heat-shock cognate protein of 70 kDa) in the amino acid sequence [32].
2.1.3. Mechanism
There are a few key steps involved in macro-autophagy. First is the elongation of phagophores to produce autophagosome (Figure 1). The formation of autophagosomes, cytosolic double-membrane vesicles segregating part of the cytoplasm is crucial in macro-autophagy [33,34]. There are mainly two membrane sources autophagosomes are from. In yeast, the membrane is from different organelles while another one is the omega-shaped structure from omegasome, the endoplasmic reticulum subdomain enriched in phosphatidylinositol-3-phosphate (PtdIns3P) [35]. The origin of the autophagosome is a phagosome that also needs the help of autophagy-related gene (Atg) proteins joining into a phagophore assembly site (PAS) [36].
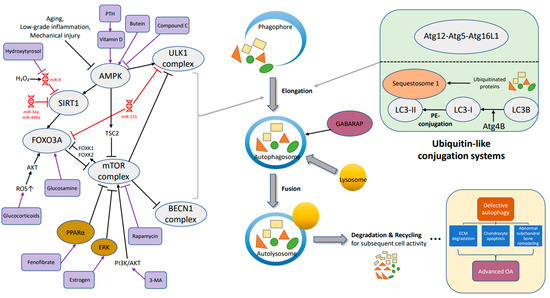
Figure 1.
The mechanism of autophagy (key molecules and pathways) in osteoarthritis includes the following main steps: elongation, fusion, degradation and recycling. Defective autophagy could lead to extracellular matrix degradation, chondrocyte apoptosis and abnormal subchondral bone remodeling, resulting in osteoarthritis. →, promote; ┤, inhibit.
The process from phagophore to autophagosome depends on the activity of two ubiquitin-like conjugation systems (Figure 1), the Atg5–Atg12 system and the microtubule-associated protein-1 lightchain 3 (LC3, Atg8) system [37]; the ubiquitin chain, also known as the “eat-me” signal label, is identified by the autophagic receptor. In Atg5–Atg12 system, the ubiquitin-like protein of Atg12 attaches to Atg5 with a covalent bond, and combines with Atg16L1 to form the group: Atg12–Atg5–Atg16L1, which is indispensable for the elongation of the pre-autophagosomal membrane [38,39].
The other axis is microtubule-associated protein-1 lightchain 3 system related to lightchain 3 (LC3), also known as Atg8. The precursor form, LC3B, is cleaved by protease Atg4B at its C-term, and turns into the cytosolic free-form (LC3-I). Then, the alteration from LC3-I to LC3-II (phosphatidylethanolamine-conjugated) is the beginning of autophagy [40,41,42,43].
Second, fusion: autophagosomes fuse with endosomes and lysosomes to become an autolysosome which is controlled by the GABARAP subfamily (Figure 1). The GABARAP subfamily helps recruit Pleckstrin homology domain-containing protein family member 1 (PLEKHM1), which acts on the homotypic fusion and protein sorting (HOPS) complex and its contained LIR (LC3-interacting region), stimulating the fusion of endosomes and autophagosomes with lysosomes as a consequence [44]. By the way, LC3-Ⅱcontinues to target to the elongating autophagosome membrane until its fusion with lysosomes, and changes into autolysosomes breaking down the materials contained within [40].
There is evidence that AMP-activated protein kinase (AMPK) also promotes autophagosome and lysosomal fusion [45]. Jang (2018) and colleagues mentioned using trehalose or compound C to treat cells knocked out of AMPKα1 mostly inducing the stimulation of autophagosomes instead of autolysosomes, suggesting that the fusion necessitates AMPKα1.
After the integration, there are degradation and recycling; encapsulated large molecules divided into lipids, amino acids, energy and nucleotides are prepared for subsequent cellular renovation, homeostasis or response to the cell stress [46,47].
2.2. Role of Autophagy in Osteoarthritis
OA is linked to chondrocyte apoptosis. Autophagy is thought to be in balance with apoptosis when there is increased chondrocyte apoptosis occurring with lower expression of autophagy regulators in OA joints of human beings and rodents [48]. Autophagy seems to be a protective mechanism for stabilized microenvironment in articular cartilage and less autophagic activity is observed in aging or osteoarthritic joints [49].
Autophagy in OA can be seen in the aspect of autophagy genes. Initially, autophagy mRNA of Beclin-1 (BECN1) and LC3 is increased which is considered a compensation against cellular stress. In mild OA, difference start to happen in the superficial zone of articular cartilage: protein expression levels of BECN1, LC3 and ULK1(an autophagy-initiating kinase) become lower, thought to be defective autophagy. Then, there is an all-layered (including middle and deep zones) autophagic genetic change not only reduced ULK1 (Unc-51-like kinase 1), LC3 and BECN1 but also fewer ATG genes (ATG3, ATG5, ATG12) in advanced OA [48,50,51,52]; furthermore, chondrocyte apoptosis arise as markers like PARP and p85 elevating.
In mouse OA, loss of proteoglycan, one of the principal contents to the extracellular matrix, was found to be associated with reduction of the autophagic markers [48,53]. Some knockout research also proved the connection between OA and autophagy with ablation of ATG5, ATG7 [54,55].
3. Signaling Pathway Related to Autophagy in Osteoarthritis
3.1. AMPK-SIRT1-FOXO3A
AMPK is a heterotrimeric complex comprising catalytic α subunit and regulatory β-, γ-subunits [56]; it is also an important energy sensor balancing energy in control of protein, glucose or lipid metabolism. In addition, AMPK promotes autophagy and has its own role in OA (chondroprotection) thought to be related to redox regulation and mitochondrial function (mitophagy) [57,58,59]. AMPK can stimulate mitophagy for the removal of damaged mitochondria which is blocked by mTORC1 [60,61].
There are reports showing that AMPK could phosphorylate and activate the downstream targets such as sirtuin1 (SIRT1) and Forkhead box class O 3a (FOXO3a) (Figure 1), then restraining the injury and gene expression of inflammation [59], while FOXK1 and FOXK2 (members of the FOX family) phosphorylated by mTOR in high nutrient condition would work against FOXO3 to suppress autophagic gene activity [62].
SIRT1 and FOXO3a are mentioned to promote autophagy-related protein expression (BECN1, LC3), deacetylate autophagic proteins (ATG5, ATG7, and ATG8) and help assembly of autophagosome [63]. For example, hydroxytyrosol, which is found in olive oil and shows antioxidant properties, encourages the transfer of SIRT 1 to the nucleus. SIRT 1 into the nucleus is speculated to catalyze deacylation of LC3-II, and LC3-II is back to cytosol for autophagy initiation. Thus, hydroxytyrosol is thought to be beneficial when chondrocytes face oxidative stress [64,65]. In the SIRT family, SIRT 1 is most relevant to OA through autophagy, mitochondrial biogenesis and other response. 17β-Estradiol is also proved to promote chondrocyte protection by mitophagy through the AMPK/SIRT1/mTOR pathway [66].
Promotion of autophagic gene expression by FOXOs has occurred in neurons, muscle and even hematopoietic stem cells [67,68], and there is FOXO3 which elicit autophagy in chondrocytes of mouse and human [69]. Glucosamine, which is a common dietary supplement, also promotes autophagy in cartilage through activating FOXO3 (dephosphorylation); in contrast, glucocorticoids can stimulate FOXO3 and activate autophagy through a higher level of ROS [70,71].
3.2. AMPK-ULK1
ULK1, homologue of yeast ATG1, is a serine/threonine kinase in mammals; Among five members of ULK1 homologues, there are ULK1 and ULK2 participating in autophagy and ULK1 is thought to be an inducer of the process [72]. ULK activates BECN1 by phosphorylation and combines to form a protein complex, relocating to site of the phagophore to initiate the composition of the autophagosome. Besides, there is interaction between AMPK and Ulk1 when the level of glucose is changed (Figure 1). Active AMPK promote Ulk1 via phosphorylation of serine 317 and serine 777, strengthening autophagy under nutrient deprivation [73]. On the other hand, AMPK can also inhibit mTOR by phosphorylation through the intermediate regulator TSC2 [74]. Besides, there is report indicating the association between ULK1 mutants and the accumulation of defective mitochondria which implies the effect of ULK1 on mitophagy, a core event in OA as well [75].
Some instances are described through the AMPK/ULK1 axis. Ansari et al. observed that butein, an organic compound belongs to the polyphenol family (found to be useful in OA management), triggers autophagic flux through phosphorylation of AMPKα threonine-172 and ULK1 serine-317, and suppresses mTOR in OA-like chondrocytes [76]. Then, there is one research about PLCγ1 (Phospholipase C gamma 1) speaking about the indirect reaction of AMPK and ULK1 with the utilization of AMPK activator metformin decreasing the level of p-PLCγ1 and blocking mTOR/ULK1 axis, eventually encouraging the synthesis of cartilage extracellular matrix [77].
4. Non-Coding RNA and Autophagy in Osteoarthritis
4.1. Introduction of Non-Coding RNA
Nucleic acids were discovered in 1869. Then, after quite a long time, more than a hundred years, regulatory roles of non-coding transcripts start to be identified and discussed [78]. The very first regulatory non-coding RNA (ncRNA) was found from E. coli discovered in 1984 by Masayuki Inouye which was a bacterial small RNA (sRNA) [79].
98% of the genome is known to be transcribed, but most of the transcripts do not encode proteins and they are called ncRNAs [80]. Lots of studies have revealed ncRNAs to be associated with regulating autophagy in various diseases, for example, diabetes, cancer, myocardial infarction and so on [81].
There are many types of ncRNA, regulatory ncRNAs such as microRNA (one of the small ncRNAs), long non-coding RNA (lncRNA) and circular RNA (circRNA) which belongs to the lncRNAs; all these three types of ncRNAs are commonly involved in the process of autophagy in OA, and genes can be modulated at different stages including epigenetic, transcriptional, and post-transcriptional levels [82,83]. In addition, there is a complicated chain called the competing endogenous RNA (ceRNA) network adopting microarrays to analyze and display reactions between ncRNAs (microRNA, lncRNA and circRNA) and mRNA which can help us to detect the detailed molecular mechanism and uncover more OA-related genes [84,85].
4.2. MicroRNAs and Autophagy in Osteoarthritis
MicroRNA (miRNA) is a posttranscriptional modulator that has been involved in cartilage homeostasis and regulated biological processes of chondrocyte autophagy by targeting genes; especially for its widespread target genes, miRNA have an impact on lots of diseases, such as cancer, inflammatory bowel disease, myocardial infarction and so on [81,86,87,88,89].
Conserved sequences have been the key point making miRNAs an interesting topic for researchers. The details of miRNA composition originate from primary RNA (pri-miRNA) which is cleaved into a hairpin around 70 nucleotides and then become pre-miRNA. Pre-miRNA is transported by Exportin-5 from the cell nucleus to cytoplasm; after that, with an RNase III called Dicer changing the structure into a double-stranded miRNA about 22 nucleotides left. Consequently, a biological complex named miRNA-induced silencing complex (mRISC) was formed by the composition of mature miRNA and proteins of the argonaute (AGO) family [90,91,92,93,94].
This complex identifies certain genes and binds to the 3′-UTR of targeted mRNAs. Furthermore, the extent of complementary base pairing decides the intensity of regulation. Inhibition of mRNA translation is elicited by partial complementary base pairing; on the other hand, cleavage and degradation of mRNA occur when there is perfect complementarity [94,95].
MiRNAs have been found to modulate SIRT1 and influence OA progression. By decreasing SIRT1 expression, miR-9, miR-34a, or miR-449a possibly cause cartilage destruction; hence, inhibiting these miRNAs may be a therapeutic target for OA [96,97,98]. Furthermore, D’Adamo (2016) and their colleagues mentioned miR-155 is quite crucial in autophagy in OA, thought to suppress the autophagic activity through targeting ULK1, FOXO3 (a key transcription factor of ATG genes) and ATG14 [99].
Several miRNAs have also been engaged in OA with other mechanisms. MiR-27a enhanced autophagy in IL-1β-treated chondrocytes via downregulating PI3K [100]. MiR-107 was unveiled with the promotion of autophagy by targeting TRAF3; miR-411 with the target of HIF-1alpha mRNA showed the ability to strengthen autophagy in OA models [101,102]. Contrarily, miR-375 was elevated in the samples from osteoarthritic cartilage possibly through inhibition of ATG2B and restraining autophagy along with more cellular stress [103]. MiR-378 aggravated OA via suppressing ATG2A and the subsequent autophagic activity [104].
In addition to the miRNAs mentioned above, still lots of miRNAs are being investigated. Many possible candidates emerge due to their differential expressions in OA cartilage, and involvement in autophagic activity in non-cartilage tissues [105]. Other reasons may be their high cartilage specificity or sharing the same target with the miRNA which has been confirmed to mediate autophagy in chondrocytes. Nevertheless, their precise pathway or mechanism, and those associated proteins, genes or cytokines must be elucidated.
4.3. lncRNAs and Autophagy in Osteoarthritis
LncRNAs were investigated in the late 1980s with more than 200 nucleotides. H19 was the first eukaryote lncRNA gene localized to mouse chromosome 7 which has a key role in embryonic development [106], and changed our concept for the biological relevance of lncRNAs. Then, in the 2000s, a worldwide effort to lncRNAs began.
LncRNAs are produced by RNA polymerase II and III, capable of modulating protein-coding genes in positive and negative directions [107]. The regulatory mechanism of long noncoding RNAs is intricate and related to chromatin, histone, mRNA and so on [108,109]. There are also connections between lncRNAs and miRNAs; for instance, they can combine to form regulatory networks which indirectly affect gene regulation of miRNAs; on the other hand, lncRNAs may serve as miRNA precursors transforming into miRNAs through RNases [110,111].
A few lncRNAs have been noticed to participate in OA through autophagy. Song (2014) et al. led the way to find GAS5 (growth arrest-specific 5) which already had a role in apoptosis [112,113]. GAS5 suppressed miR-21 which controlled the expression of type II collagen, aggrecan and MMP13, and decreased autophagic activity in osteoarthritic chondrocytes. Ji (2021) et al. furthermore mentioned the level of GAS5 in the late stage of OA is superior to the one in the early stage, and silencing lncRNA GAS5 help the autophagy of chondrocytes. The reason may be the binding ability of GAS5 to miR-144 which modulates mTOR expression [114].
Another lncRNA being discussed is HOTAIR (HOX transcript antisense RNA); it is known to enhance proliferation, invasion and metastasis in preclinical studies about cancer [115,116], and highly expressed in cancer, such as acute myeloid leukemia [117]. Moreover, HOTAIR is relevant to autophagy and OA, thought to be associated with chondrocyte apoptosis in higher level of caspase 3 and less Bcl-2 expression. The mechanism of autophagy inhibition by HOTAIR is mediated by sponging miR-130a-3p which also suppress cell growth [118]. Later, Chinese medicine used to make knee OA therapy is found to protect the joint via chondrocyte autophagy in an animal study. Cangxitongbi (CXTB) promote autophagy, reduce HOTAIR expression, and ameliorate the cartilage structure [119]. Other lncRNAs regulating autophagy in OA are listed in Table 1 and Figure 2.

Table 1.
LncRNA regulation of autophagy in OA.
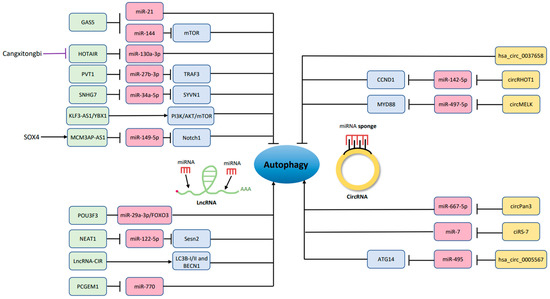
Figure 2.
LncRNAs and circRNAs that promote or inhibit chondrocyte autophagy. The green color blocks (lncRNAs) and the yellow color blocks (circRNAs) modulate the pink color blocks (miRNAs) and the purple color blocks (other regulatory molecules) which result in altering the autophagic activity of chondrocytes. →, promote; ┤, inhibit.
4.4. circRNAs and Autophagy in Osteoarthritis
CircRNA, which is not influenced by RNA exonuclease, was found to be a mediator of autophagy usually through targeting miRNA. Precisely, it performs as a miRNA sponge owing to abundant binding sites with higher stability and disease specificity, modulating those miRNAs through the site called MRE (miRNA response element) [128,129,130,131].
There is circRNA exacerbating tumor progression of cancer via enhancing the autophagic level [132]. CircRNAs have also been involved in OA microenvironment for control of autophagy to perturb the situation of inflammation [133,134]. Many more surveys about circRNAs in OA have emerged since 2015 [129], described below (Table 2) and showed in Figure 2.
4.4.1. hsa_circ_0005567 (hsa_circ_0005567/miR-495/ATG14 Axis)
Zhang et al. found circ_0005567 sponged miR-495 to reduce ATG14 expression. Autophagy-related markers, LC3 and BECN1, and the ratio of LC3-II/LC3-I was upregulated by circ_0005567. Then, 3-methyladenine (3-MA), an autophagy inhibitor, overturned the promotion of autophagy mediated by circ_0005567 expression. In conclusion, the overexpression of circ_0005567 improved autophagy deficiency in OA chondrocytes and debilitated IL-1β-mediated chondrocyte apoptosis [135].
4.4.2. ciRS-7 (ciRS-7/miR-7 Axis)
In the research of Zhou (2020), they first found the level of ciRS-7 and miR-7 was not normal in OA. Then, in vitro study revealed autophagy inhibition and cartilage degradation mediated by IL-1β with down-regulation of ciRS-7 and upregulation of miR-7, which was thought to be an axis involved in the reaction by PI3K/AKT/mTOR activation. Furthermore, miR-7 mimics deteriorated destruction of cartilage in the animal model [136].
4.4.3. circPan3 (circPan3/miR-667-5p Axis)
CircPan3 has its role in autophagy via targeting miR-667-5p; they show contrary validity towards chondrocytes which was proved in rat models. Lower expression of circPan3 was showed in the OA condition, and Zeng and colleagues demonstrated that circPan3 enhanced BECN1, LC3-II and Col2a1 expression and decreased the level of ADAMTS-5 and MMP13 [137].
4.4.4. hsa_circ_0037658 (-)
The link between hsa_circ_0037658 and autophagy was noticed in chondrocyte cell line, CHON-001 [138]. There is more hsa_circ_0037658 appearance in OA [139], and hsa_circ_0037658 small hairpin RNAs (shRNA) have the ability to decelerate apoptosis rate, overturn the increased MMP13 and decreased aggrecan, type II collagen-induced by IL-1β. Additionally, when hsa_circ_0037658 is knocked down, there is induction of autophagy: the same as hsa_circ_0037658 shRNAs reverse the reduction of LC3, ATG5 and BECN1.
4.4.5. circRHOT1 (-) (circRHOT1/miR-142-5p/CCND1 Axis)
In the condition of OA, there is activated Cyclin D1 (CCND1), circRHOT1 and less miR-142-5p expression. miR-142-5p is negatively associated with CCND1 and circRHOT1 since circRHOT1 acted as miR-142-5p sponge to promote CCND1 in chondrocytes. Then, when circRHOT1 was knocked down, cartilage degeneration in OA model mitigated with raising level of aggrecan and type II collagen; even the autophagic marker: BECN1 and LC3 were elevated [140].
4.4.6. circMELK (-) (circMELK/miR-497-5p/MYD88/NF-κB Axis)
In the study of Zhang (2022), it was constructed by using in vitro model to test circMELK, one of the most upregulated circRNAs in post-menopausal osteoporosis patients, for its effect on chondrocytes and see whether it will be a target for OA therapy. Autophagy was evaluated by LC3 and BECN1 expression. In sum, circMELK as a sponge of miR-497-5p modulated the action of the MYD88/NF-κB pathway, enhancing apoptosis and restraining autophagy of OA chondrocytes. However, the authors also mentioned the limitations of lacking enough sample size and in vivo experiments [141].

Table 2.
CircRNA regulation of autophagy in OA.
Table 2.
CircRNA regulation of autophagy in OA.
| First Author, Year | Experimental Design and Treatments | Results |
|---|---|---|
| Zhang, 2020 [135] | Model: human primary chondrocytes were added with recombinant human IL-1β (10 ng/mL) to stimulate degeneration for 24 h. 3-MA was used to pretreat chondrocytes. Treatments: Transfection with circ_0005567 overexpression vector | hsa_circ_0005567 Target/signaling pathway: miR-495/ATG14; LC3 ↑, BECN1 ↑, LC3-II/LC3-I ↑ Effects on chondrocytes: Promoting autophagy and inhibiting chondrocyte apoptosis |
| Sui, 2020 [138] | Model: In vitro-CHON-001 cells treated with IL-1β for 24 h; In vivo-15 Wistar rats (12-week-old) were randomly divided into three groups: control (n = 5), OA (n = 5) and OA + hsa_circ_0037658 shRNA1 (n = 5). Treatments: 24 h after surgery, rats in OA + hsa_circ_0037658 shRNA1 group were injected with lenti-hsa_circ_0037658 shRNA1 through the joint cavity twice a week. | hsa_circ_0037658 Target/signaling pathway: LC3 ↓, ATG5 ↓, BECN1 ↓, p62 ↑, AIF ↑ Effects on chondrocytes: Inhibiting autophagy; Knockdown of hsa_circ_0037658 alleviated the symptom of OA in vivo; Hsa_circ_0037658 shRNA reversed IL-1β-induced cell growth inhibition via inducing cell autophagy. |
| Zhou, 2020 [136] | Model: rats with DMM; C28/I2 chondrocytes stimulated with IL-1β for 24 h; OA articular cartilage samples and healthy cartilage samples from trauma patients without OA were collected. Treatments: The mixture of circRNA and miRNA was added to the cells for a 24 h transfection. | ciRS-7 Target/signaling pathway: miR-7; caspase 9 ↓, Bax ↓, Bcl-2 ↑ Effects on chondrocytes: Promoting autophagy and ameliorating cartilage degradation |
| Zeng, 2021 [137] | Model: IL-1β-induced rat chondrocytes and cartilage tissues of OA rats Treatments: Cell transfection | circPan3 Target/signaling pathway: miR-667-5p; ↑ BECN1, LC3-II, Col2a1, Acan; ↓ ADAMTS-5, MMP13 Effects on chondrocytes: Promoting autophagy |
| Man, 2022 [140] | Model: clinical OA samples, OA rats, chondrocytes Treatments: circRHOT1 shRNA | circRHOT1 (−) Target/signaling pathway: miR-142-5p/CCND1; ↓ BECN1, LC3, aggrecan and collagen II; ↑ COMP, CTX-II Effects on chondrocytes: Inhibiting autophagy and promoting chondrocyte proliferation, Knockdown of circRHOT1 could repress cartilage degeneration. |
| Zhang, 2022 [141] | Model: OA human cartilage tissue Treatments: sh-circMELK, cell transfection | circMELK (−) Target/signaling pathway: miR-497-5p/MYD88/NF-κB Effects on chondrocytes: Inhibiting autophagy and promoting chondrocyte apoptosis in OA |
The relationship between ncRNA and its character for the autophagic activity in OA can be so intricate and need to be elucidated, yet it means the likelihood of keeping broadening the search for OA-related ncRNAs. Hopefully, they can serve as more comprehensive therapeutic targets or diagnostic biomarkers.
5. Prospective of Treatment via Autophagy in Osteoarthritis
There are wide variety of therapeutic options via autophagy in OA or for chondroprotection whether it is a hormone, antineoplastic agents and even supplements considered a novel strategy, for example, glucosamine could inhibit the mTOR pathway and trigger autophagy (Figure 1), thus helping to maintain cell homeostasis [142]. There is research showing that glucosamine has functions of anabolism and anti-inflammation for chondrocytes by controlling the molecular pathway of autophagy which is useful in animal study [70]. Growth of LC3 turnover and increment of LC3-II enhance the autophagic activity. Glucosamine also modulates cartilage cell apoptosis or survival, depending on time. Activation of autophagy is shown in short-term exposure of glucosamine; meanwhile, inhibition of autophagy is observed in the long period usage of glucosamine [143].
When it comes to parathyroid hormone (PTH), Chen and his partners found that PTH-(1-34) could improve the development of OA in an animal model by autophagy (Figure 1). In virtue of diminishing mTOR, reinforcing BECN1 and LC3, chondrocyte apoptosis may be weakened [144]. Vitamin D, a necessity for bone health, was also proved to alleviate inflammatory events of OA through AMPK-mTOR pathway [145]. Besides, the LC3-II: I ratio and level of BECN1 arose by administration of vitamin D, while its therapeutic effect was overturned by 3-MA, an autophagy inhibitor. Furthermore, Kong mentioned that vitamin D could be crucial in autophagosome aggregating in OA condition [145].
17β-Estradiol as the major female sex hormone have been found to take part in chondrocyte autophagy through ERK signaling pathway [146] (Figure 1). Ge et al. (2019) noticed a decreasing level of LC3 in the cartilage of the mice accepting ovariectomy. More LC3 conversion was observed and the inhibitory effect of compound C (AMPK inhibitor) would be offset when 17β-estradiol was used. Fenofibrate (FN), a drug for dyslipidemia, also shows the possibility for OA therapy. Lower expression of PPARα was found in OA mice and knees of OA patients. As a PPARα agonist, FN strengthened autophagic activity, preventing cartilage from degradation, and there is a retrospective study revealing the effect of fibrate to clinically ameliorating OA situation [147].
Rapamycin, also known as Sirolimus, is a drug with numerous effects which is often used in renal allograft rejection; its derivatives were approved for the treatment of advanced renal cancer carcinoma [148]. In addition, rapamycin showed its possibility in OA therapy by autophagy through lessening mTOR, stimulating LC3 and finally alleviating cartilage destruction. Local intra-articular administration of rapamycin in rodents seems to be effective [149], and decreased MMP13 and Col10a1 were identified after rapamycin treatment. Nowadays, multiple techniques combined to test therapeutic potential become more common. Xue et al. created a drug delivery system for cartilage exhibiting that via laser irradiation, rapamycin released from mesoporous polydopamine (MPDA) core could help chondrocyte preservation through activating autophagy [150]; this nanoplatform not only presents positive outcomes in the animal study but also implies plenty of opportunities for the progress of autophagy-related treatment.
6. Conclusions
OA is a public health issue with multiple mechanisms (inflammation, apoptosis, proliferation…) involved in articular cartilage. Autophagy is a characteristic process controlling the balance of the intracellular microenvironment, without an exception in chondrocytes. Then, this topic attracts thoroughly hard work and various aspects of comprehension which forms a molecular network added in many more genes and therapeutic agents; nonetheless, autophagy is not the only factor to decide the progress of OA. Crosstalk effects and multitargeting relationships should be taken into consideration. Comprehensive research of osteoarthritic cartilage function and successive clinical trials are necessities. Hopefully, there will be a disease-modifying strategy combined with different new medical techniques to be verified and treat OA fundamentally or those findings extend their utilized message into fields of inflammatory, degenerative diseases.
Author Contributions
Conceptualization, W.-C.K., J.-C.C., P.-C.L., C.-C.L., S.-C.W., C.-W.W. and C.-H.C.; methodology, W.-C.K., J.-C.C., P.-C.L., C.-C.L., S.-C.C., S.-C.W., S.-Y.L. and M.-J.L.; software, S.-C.C., S.-C.W., L.-h.C., M.-J.L. and C.-D.Y.; validation, C.-C.L., S.-C.W., T.-C.L., S.-Y.L., M.-J.L., C.-D.Y., Y.-C.W., J.-Y.L., P.-C.L., C.-W.W. and C.-H.C.; data curation, W.-C.K., J.-C.C., P.-C.L.,C.-C.L., S.-Y.L., S.-C.C., S.-C.W., L.-h.C., M.-J.L. and C.-D.Y.; writing—original draft preparation, W.-C.K., J.-C.C. and P.-C.L., writing—review and editing, C.-W.W. and C.-H.C.; project administration, C.-W.W. and C.-H.C.; funding acquisition, C.-H.C. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported in part by the National Health Research Institute (NHRI-EX101-9935EI) of Taiwan, Kaohsiung Medical University (NPUST-KMU-109-P002, NCTUKMU108-BIO-04, KMU-TC108A02-1, KMU-DK105009, KMU-DK(B) 110002 and KMU-111-P005), Kaohsiung Municipal Ta-Tung Hospital (KMTTH-109-R014, KMTTH-DK(B) 110002-1 and KMTTH-110-R008), and the Minister of Science and Technology of Taiwan (MOST 110-2314-B-006-037-MY3, 108-2314-B-037-059-MY3, 110-2314-B-037-029-MY3 and 111-2314-B-037-106-). The funders had no role in the study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We appreciate the support from members of our orthopedic research center, especially Yi-Shan Lin, Kaohsiung Medical University, Kaohsiung City, Taiwan.
Conflicts of Interest
The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Hwang, H.S.; Kim, H.A. Chondrocyte Apoptosis in the Pathogenesis of Osteoarthritis. Int. J. Mol. Sci. 2015, 16, 26035–26054. [Google Scholar] [CrossRef] [PubMed]
- Hawker, G.A. Osteoarthritis is a serious disease. Clin. Exp. Rheumatol. 2019 37, 3–6.
- Lorenz, H.; Richter, W. Osteoarthritis: Cellular and molecular changes in degenerating cartilage. Prog. Histochem. Cytochem. 2006, 40, 135–163. [Google Scholar] [CrossRef]
- Cross, M.; Smith, E.; Hoy, D.; Nolte, S.; Ackerman, I.; Fransen, M.; Bridgett, L.; Williams, S.; Guillemin, F.; Hill, C.L.; et al. The global burden of hip and knee osteoarthritis: Estimates from the global burden of disease 2010 study. Ann. Rheum. Dis. 2014, 73, 1323–1330. [Google Scholar] [CrossRef]
- Vos, T.; Abajobir, A.A.; Abate, K.H.; Abbafati, C.; Abbas, K.M.; Abd-Allah, F.; Abdulkader, R.S.; Abdulle, A.M.; Abebo, T.A.; Abera, S.F.; et al. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1211–1259. [Google Scholar] [CrossRef]
- Rim, Y.A.; Ju, J.H. The Role of Fibrosis in Osteoarthritis Progression. Life 2020, 11, 3. [Google Scholar] [CrossRef] [PubMed]
- Makris, E.A.; Gomoll, A.H.; Malizos, K.N.; Hu, J.C.; Athanasiou, K.A. Repair and tissue engineering techniques for articular cartilage. Nat. Rev. Rheumatol. 2015, 11, 21–34. [Google Scholar] [CrossRef]
- Anandacoomarasamy, A.; March, L. Current evidence for osteoarthritis treatments. Ther. Adv. Musculoskelet. Dis. 2010, 2, 17–28. [Google Scholar] [CrossRef]
- Onorati, A.V.; Dyczynski, M.; Ojha, R.; Amaravadi, R.K. Targeting autophagy in cancer. Cancer 2018, 124, 3307–3318. [Google Scholar] [CrossRef]
- Martel-Pelletier, J.; Barr, A.J.; Cicuttini, F.M.; Conaghan, P.G.; Cooper, C.; Goldring, M.B.; Goldring, S.R.; Jones, G.; Teichtahl, A.J.; Pelletier, J.P. Osteoarthritis. Nat. Rev. Dis. Prim. 2016, 2, 16072. [Google Scholar] [CrossRef]
- Rehling, T.; Bjorkman, A.D.; Andersen, M.B.; Ekholm, O.; Molsted, S. Diabetes Is Associated with Musculoskeletal Pain, Osteoarthritis, Osteoporosis, and Rheumatoid Arthritis. J. Diabetes Res. 2019, 2019, 6324348. [Google Scholar] [CrossRef] [PubMed]
- Watt, F.E. Hand osteoarthritis, menopause and menopausal hormone therapy. Maturitas 2016, 83, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Punzi, L.; Galozzi, P.; Luisetto, R.; Favero, M.; Ramonda, R.; Oliviero, F.; Scanu, A. Post-traumatic arthritis: Overview on pathogenic mechanisms and role of inflammation. RMD Open 2016, 2, e000279. [Google Scholar] [CrossRef]
- Rupasov, A.; Cain, U.; Montoya, S.; Blickman, J.G. Imaging of Posttraumatic Arthritis, Avascular Necrosis, Septic Arthritis, Complex Regional Pain Syndrome, and Cancer Mimicking Arthritis. Radiol. Clin. N. Am. 2017, 55, 1111–1130. [Google Scholar] [CrossRef]
- Rahmati, M.; Nalesso, G.; Mobasheri, A.; Mozafari, M. Aging and osteoarthritis: Central role of the extracellular matrix. Ageing. Res. Rev. 2017, 40, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Shane Anderson, A.; Loeser, R.F. Why is osteoarthritis an age-related disease? Best Pract. Res. Clin. Rheumatol. 2010, 24, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, G.; Castrogiovanni, P.; Trovato, F.M.; Weinberg, A.M.; Al-Wasiyah, M.K.; Alqahtani, M.H.; Mobasheri, A. Biomarkers of Chondrocyte Apoptosis and Autophagy in Osteoarthritis. Int. J. Mol. Sci. 2015, 16, 20560–20575. [Google Scholar] [CrossRef] [PubMed]
- Zamli, Z.; Sharif, M. Chondrocyte apoptosis: A cause or consequence of osteoarthritis? Int. J. Rheum. Dis. 2011, 14, 159–166. [Google Scholar] [CrossRef]
- Kim, H.A.; Blanco, F.J. Cell death and apoptosis in osteoarthritic cartilage. Curr. Drug Targets 2007, 8, 333–345. [Google Scholar] [CrossRef]
- Almonte-Becerril, M.; Navarro-Garcia, F.; Gonzalez-Robles, A.; Vega-Lopez, M.A.; Lavalle, C.; Kouri, J.B. Cell death of chondrocytes is a combination between apoptosis and autophagy during the pathogenesis of Osteoarthritis within an experimental model. Apoptosis 2010, 15, 631–638. [Google Scholar] [CrossRef]
- Salucci, S.; Falcieri, E.; Battistelli, M. Chondrocyte death involvement in osteoarthritis. Cell Tissue Res. 2022, 389, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N.; Komatsu, M. Autophagy: Renovation of cells and tissues. Cell 2011, 147, 728–741. [Google Scholar] [CrossRef] [PubMed]
- Rajawat, Y.S.; Hilioti, Z.; Bossis, I. Aging: Central role for autophagy and the lysosomal degradative system. Ageing Res. Rev. 2009, 8, 199–213. [Google Scholar] [CrossRef]
- Mizumura, K.; Choi, A.M.; Ryter, S.W. Emerging role of selective autophagy in human diseases. Front. Pharmacol. 2014, 5, 244. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N.; Yoshimori, T.; Ohsumi, Y. The role of Atg proteins in autophagosome formation. Annu. Rev. Cell Dev. Biol. 2011, 27, 107–132. [Google Scholar] [CrossRef]
- Mizushima, N.; Klionsky, D.J. Protein turnover via autophagy: Implications for metabolism. Annu. Rev. Nutr. 2007, 27, 19–40. [Google Scholar] [CrossRef]
- Jaber, F.A.; Khan, N.M.; Ansari, M.Y.; Al-Adlaan, A.A.; Hussein, N.J.; Safadi, F.F. Autophagy plays an essential role in bone homeostasis. J. Cell. Physiol. 2019, 234, 12105–12115. [Google Scholar] [CrossRef]
- Mizushima, N.; Levine, B. Autophagy in mammalian development and differentiation. Nat. Cell Biol. 2010, 12, 823–830. [Google Scholar] [CrossRef]
- Glick, D.; Barth, S.; Macleod, K.F. Autophagy: Cellular and molecular mechanisms. J. Pathol. 2010, 221, 3–12. [Google Scholar] [CrossRef]
- Pallauf, K.; Rimbach, G. Autophagy, polyphenols and healthy ageing. Ageing Res. Rev. 2013, 12, 237–252. [Google Scholar] [CrossRef]
- Jeon, H.; Im, G.I. Autophagy in osteoarthritis. Connect. Tissue Res. 2017, 58, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Chiang, H.L.; Dice, J.F. Peptide sequences that target proteins for enhanced degradation during serum withdrawal. J. Biol. Chem. 1988, 263, 6797–6805. [Google Scholar] [CrossRef]
- Levine, B.; Mizushima, N.; Virgin, H.W. Autophagy in immunity and inflammation. Nature 2011, 469, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N.; Ohsumi, Y.; Yoshimori, T. Autophagosome formation in mammalian cells. Cell Struct. Funct. 2002, 27, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; He, D.; Yao, Z.; Klionsky, D.J. The machinery of macroautophagy. Cell Res. 2014, 24, 24–41. [Google Scholar] [CrossRef]
- Kotani, T.; Kirisako, H.; Koizumi, M.; Ohsumi, Y.; Nakatogawa, H. The Atg2-Atg18 complex tethers pre-autophagosomal membranes to the endoplasmic reticulum for autophagosome formation. Proc. Natl. Acad. Sci. USA 2018, 115, 10363–10368. [Google Scholar] [CrossRef]
- Ohsumi, Y.; Mizushima, N. Two ubiquitin-like conjugation systems essential for autophagy. Semin. Cell Dev. Biol. 2004, 15, 231–236. [Google Scholar] [CrossRef]
- Mizushima, N.; Sugita, H.; Yoshimori, T.; Ohsumi, Y. A new protein conjugation system in human. The counterpart of the yeast Apg12p conjugation system essential for autophagy. J. Biol. Chem. 1998, 273, 33889–33892. [Google Scholar] [CrossRef]
- Mizushima, N.; Kuma, A.; Kobayashi, Y.; Yamamoto, A.; Matsubae, M.; Takao, T.; Natsume, T.; Ohsumi, Y.; Yoshimori, T. Mouse Apg16L, a novel WD-repeat protein, targets to the autophagic isolation membrane with the Apg12-Apg5 conjugate. J. Cell Sci. 2003, 116, 1679–1688. [Google Scholar] [CrossRef]
- Tanida, I.; Sou, Y.S.; Ezaki, J.; Minematsu-Ikeguchi, N.; Ueno, T.; Kominami, E. HsAtg4B/HsApg4B/autophagin-1 cleaves the carboxyl termini of three human Atg8 homologues and delipidates microtubule-associated protein light chain 3- and GABAA receptor-associated protein-phospholipid conjugates. J. Biol. Chem. 2004, 279, 36268–36276. [Google Scholar] [CrossRef]
- Ryter, S.W.; Choi, A.M. Autophagy in lung disease pathogenesis and therapeutics. Redox Biol. 2015, 4, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Ryter, S.W.; Cloonan, S.M.; Choi, A.M. Autophagy: A critical regulator of cellular metabolism and homeostasis. Mol. Cells 2013, 36, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Ravikumar, B.; Sarkar, S.; Davies, J.E.; Futter, M.; Garcia-Arencibia, M.; Green-Thompson, Z.W.; Jimenez-Sanchez, M.; Korolchuk, V.I.; Lichtenberg, M.; Luo, S.; et al. Regulation of mammalian autophagy in physiology and pathophysiology. Physiol. Rev. 2010, 90, 1383–1435. [Google Scholar] [CrossRef] [PubMed]
- McEwan, D.G.; Popovic, D.; Gubas, A.; Terawaki, S.; Suzuki, H.; Stadel, D.; Coxon, F.P.; Miranda de Stegmann, D.; Bhogaraju, S.; Maddi, K.; et al. PLEKHM1 regulates autophagosome-lysosome fusion through HOPS complex and LC3/GABARAP proteins. Mol. Cell 2015, 57, 39–54. [Google Scholar] [CrossRef]
- Jang, M.; Park, R.; Kim, H.; Namkoong, S.; Jo, D.; Huh, Y.H.; Jang, I.S.; Lee, J.I.; Park, J. AMPK contributes to autophagosome maturation and lysosomal fusion. Sci. Rep. 2018, 8, 12637. [Google Scholar] [CrossRef]
- Carames, B.; Taniguchi, N.; Seino, D.; Blanco, F.J.; D’Lima, D.; Lotz, M. Mechanical injury suppresses autophagy regulators and pharmacologic activation of autophagy results in chondroprotection. Arthritis Rheum. 2012, 64, 1182–1192. [Google Scholar] [CrossRef]
- Hansen, M.; Chandra, A.; Mitic, L.L.; Onken, B.; Driscoll, M.; Kenyon, C. A role for autophagy in the extension of lifespan by dietary restriction in C. elegans. PLoS Genet. 2008, 4, e24. [Google Scholar] [CrossRef]
- Carames, B.; Taniguchi, N.; Otsuki, S.; Blanco, F.J.; Lotz, M. Autophagy is a protective mechanism in normal cartilage, and its aging-related loss is linked with cell death and osteoarthritis. Arthritis Rheum. 2010, 62, 791–801. [Google Scholar] [CrossRef]
- Rubinsztein, D.C.; Marino, G.; Kroemer, G. Autophagy and aging. Cell 2011, 146, 682–695. [Google Scholar] [CrossRef]
- Shapiro, I.M.; Layfield, R.; Lotz, M.; Settembre, C.; Whitehouse, C. Boning up on autophagy: The role of autophagy in skeletal biology. Autophagy 2014, 10, 7–19. [Google Scholar] [CrossRef]
- Bhuiyan, M.S.; Pattison, J.S.; Osinska, H.; James, J.; Gulick, J.; McLendon, P.M.; Hill, J.A.; Sadoshima, J.; Robbins, J. Enhanced autophagy ameliorates cardiac proteinopathy. J. Clin. Investig. 2013, 123, 5284–5297. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Vasheghani, F.; Li, Y.H.; Blati, M.; Simeone, K.; Fahmi, H.; Lussier, B.; Roughley, P.; Lagares, D.; Pelletier, J.P.; et al. Cartilage-specific deletion of mTOR upregulates autophagy and protects mice from osteoarthritis. Ann. Rheum. Dis. 2015, 74, 1432–1440. [Google Scholar] [CrossRef] [PubMed]
- Van den Berg, W.B. Osteoarthritis year 2010 in review: Pathomechanisms. Osteoarthr. Cartil. 2011, 19, 338–341. [Google Scholar] [CrossRef] [PubMed]
- Vuppalapati, K.K.; Bouderlique, T.; Newton, P.T.; Kaminskyy, V.O.; Wehtje, H.; Ohlsson, C.; Zhivotovsky, B.; Chagin, A.S. Targeted Deletion of Autophagy Genes Atg5 or Atg7 in the Chondrocytes Promotes Caspase-Dependent Cell Death and Leads to Mild Growth Retardation. J. Bone Miner. Res. 2015, 30, 2249–2261. [Google Scholar] [CrossRef]
- Bouderlique, T.; Vuppalapati, K.K.; Newton, P.T.; Li, L.; Barenius, B.; Chagin, A.S. Targeted deletion of Atg5 in chondrocytes promotes age-related osteoarthritis. Ann. Rheum. Dis. 2016, 75, 627–631. [Google Scholar] [CrossRef]
- Hardie, D.G.; Schaffer, B.E.; Brunet, A. AMPK: An Energy-Sensing Pathway with Multiple Inputs and Outputs. Trends Cell Biol. 2016, 26, 190–201. [Google Scholar] [CrossRef]
- Kim, J.; Guan, K.L. Regulation of the autophagy initiating kinase ULK1 by nutrients: Roles of mTORC1 and AMPK. Cell Cycle 2011, 10, 1337–1338. [Google Scholar] [CrossRef]
- Wang, J.; Li, J.; Song, D.; Ni, J.; Ding, M.; Huang, J.; Yan, M. AMPK: Implications in osteoarthritis and therapeutic targets. Am. J. Transl. Res. 2020, 12, 7670–7681. [Google Scholar]
- Yao, F.; Ji, G.; Zhang, L. AMPK: A novel target controlling inflammation. Sheng Li Xue Bao [Acta Physiol. Sin.] 2012, 64, 341–345. [Google Scholar]
- Vaamonde-Garcia, C.; Lopez-Armada, M.J. Role of mitochondrial dysfunction on rheumatic diseases. Biochem. Pharmacol. 2019, 165, 181–195. [Google Scholar] [CrossRef]
- Garcia, D.; Shaw, R.J. AMPK: Mechanisms of Cellular Energy Sensing and Restoration of Metabolic Balance. Mol. Cell 2017, 66, 789–800. [Google Scholar] [CrossRef] [PubMed]
- Bowman, C.J.; Ayer, D.E.; Dynlacht, B.D. Foxk proteins repress the initiation of starvation-induced atrophy and autophagy programs. Nat. Cell Biol. 2014, 16, 1202–1214. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.H.; Cao, L.; Mostoslavsky, R.; Lombard, D.B.; Liu, J.; Bruns, N.E.; Tsokos, M.; Alt, F.W.; Finkel, T. A role for the NAD-dependent deacetylase Sirt1 in the regulation of autophagy. Proc. Natl. Acad. Sci. USA 2008, 105, 3374–3379. [Google Scholar] [CrossRef] [PubMed]
- Cetrullo, S.; D’Adamo, S.; Guidotti, S.; Borzi, R.M.; Flamigni, F. Hydroxytyrosol prevents chondrocyte death under oxidative stress by inducing autophagy through sirtuin 1-dependent and -independent mechanisms. Biochim. Biophys. Acta 2016, 1860, 1181–1191. [Google Scholar] [CrossRef]
- Chin, K.Y.; Pang, K.L. Therapeutic Effects of Olive and Its Derivatives on Osteoarthritis: From Bench to Bedside. Nutrients 2017, 9, 1060. [Google Scholar] [CrossRef]
- Mei, R.; Lou, P.; You, G.; Jiang, T.; Yu, X.; Guo, L. 17beta-Estradiol Induces Mitophagy Upregulation to Protect Chondrocytes via the SIRT1-Mediated AMPK/mTOR Signaling Pathway. Front. Endocrinol. 2020, 11, 615250. [Google Scholar] [CrossRef]
- Zhao, J.; Brault, J.J.; Schild, A.; Goldberg, A.L. Coordinate activation of autophagy and the proteasome pathway by FoxO transcription factor. Autophagy 2008, 4, 378–380. [Google Scholar] [CrossRef]
- Schaffner, I.; Minakaki, G.; Khan, M.A.; Balta, E.A.; Schlotzer-Schrehardt, U.; Schwarz, T.J.; Beckervordersandforth, R.; Winner, B.; Webb, A.E.; DePinho, R.A.; et al. FoxO Function Is Essential for Maintenance of Autophagic Flux and Neuronal Morphogenesis in Adult Neurogenesis. Neuron 2018, 99, 1188–1203.e6. [Google Scholar] [CrossRef]
- Almeida, M.; Porter, R.M. Sirtuins and FoxOs in osteoporosis and osteoarthritis. Bone 2019, 121, 284–292. [Google Scholar] [CrossRef]
- Carames, B.; Kiosses, W.B.; Akasaki, Y.; Brinson, D.C.; Eap, W.; Koziol, J.; Lotz, M.K. Glucosamine activates autophagy in vitro and in vivo. Arthritis Rheum. 2013, 65, 1843–1852. [Google Scholar] [CrossRef]
- Shen, C.; Cai, G.Q.; Peng, J.P.; Chen, X.D. Autophagy protects chondrocytes from glucocorticoids-induced apoptosis via ROS/Akt/FOXO3 signaling. Osteoarthr. Cartil. 2015, 23, 2279–2287. [Google Scholar] [CrossRef] [PubMed]
- Zachari, M.; Ganley, I.G. The mammalian ULK1 complex and autophagy initiation. Essays Biochem. 2017, 61, 585–596. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kundu, M.; Viollet, B.; Guan, K.L. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 2011, 13, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Inoki, K.; Zhu, T.; Guan, K.L. TSC2 mediates cellular energy response to control cell growth and survival. Cell 2003, 115, 577–590. [Google Scholar] [CrossRef]
- Egan, D.F.; Shackelford, D.B.; Mihaylova, M.M.; Gelino, S.; Kohnz, R.A.; Mair, W.; Vasquez, D.S.; Joshi, A.; Gwinn, D.M.; Taylor, R.; et al. Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science 2011, 331, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.Y.; Ahmad, N.; Haqqi, T.M. Butein Activates Autophagy Through AMPK/TSC2/ULK1/mTOR Pathway to Inhibit IL-6 Expression in IL-1beta Stimulated Human Chondrocytes. Cell. Physiol. Biochem. 2018, 49, 932–946. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Y.; Qu, N.; Zhang, B.; Xia, C. PLCgamma1 inhibition-driven autophagy of IL-1beta-treated chondrocyte confers cartilage protection against osteoarthritis, involving AMPK, Erk and Akt. J. Cell. Mol. Med. 2021, 25, 1531–1545. [Google Scholar] [CrossRef]
- Jarroux, J.; Morillon, A.; Pinskaya, M. History, Discovery, and Classification of lncRNAs. Adv. Exp. Med. Biol. 2017, 1008, 1–46. [Google Scholar] [CrossRef]
- Inouye, M.; Delihas, N. Small RNAs in the prokaryotes: A growing list of diverse roles. Cell 1988, 53, 5–7. [Google Scholar] [CrossRef]
- Zhang, P.; Wu, W.; Chen, Q.; Chen, M. Non-Coding RNAs and their Integrated Networks. J. Integr. Bioinform. 2019, 16. [Google Scholar] [CrossRef]
- Ghafouri-Fard, S.; Shoorei, H.; Mohaqiq, M.; Majidpoor, J.; Moosavi, M.A.; Taheri, M. Exploring the role of non-coding RNAs in autophagy. Autophagy 2022, 18, 949–970. [Google Scholar] [CrossRef] [PubMed]
- Peschansky, V.J.; Wahlestedt, C. Non-coding RNAs as direct and indirect modulators of epigenetic regulation. Epigenetics 2014, 9, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Ponjavic, J.; Ponting, C.P.; Lunter, G. Functionality or transcriptional noise? Evidence for selection within long noncoding RNAs. Genome Res. 2007, 17, 556–565. [Google Scholar] [CrossRef] [PubMed]
- Sen, R.; Ghosal, S.; Das, S.; Balti, S.; Chakrabarti, J. Competing endogenous RNA: The key to posttranscriptional regulation. Sci. J. 2014, 2014, 896206. [Google Scholar] [CrossRef]
- Wu, X.; Bian, B.; Lin, Z.; Wu, C.; Sun, Y.; Pan, Y.; Dai, Y.; Lui, T.H.; Zhuang, T.; Pan, X. Identification of exosomal mRNA, lncRNA and circRNA signatures in an osteoarthritis synovial fluid-exosomal study. Exp. Cell Res. 2022, 410, 112881. [Google Scholar] [CrossRef]
- Wei, L.; Chen, Z.; Cheng, N.; Li, X.; Chen, J.; Wu, D.; Dong, M.; Wu, X. MicroRNA-126 Inhibit Viability of Colorectal Cancer Cell by Repressing mTOR Induced Apoptosis and Autophagy. Onco. Targets Ther. 2020, 13, 2459–2468. [Google Scholar] [CrossRef]
- Fu, X.T.; Shi, Y.H.; Zhou, J.; Peng, Y.F.; Liu, W.R.; Shi, G.M.; Gao, Q.; Wang, X.Y.; Song, K.; Fan, J.; et al. MicroRNA-30a suppresses autophagy-mediated anoikis resistance and metastasis in hepatocellular carcinoma. Cancer Lett. 2018, 412, 108–117. [Google Scholar] [CrossRef]
- Brest, P.; Lapaquette, P.; Souidi, M.; Lebrigand, K.; Cesaro, A.; Vouret-Craviari, V.; Mari, B.; Barbry, P.; Mosnier, J.F.; Hebuterne, X.; et al. A synonymous variant in IRGM alters a binding site for miR-196 and causes deregulation of IRGM-dependent xenophagy in Crohn’s disease. Nat. Genet. 2011, 43, 242–245. [Google Scholar] [CrossRef]
- Zhang, C.; Liang, R.; Gan, X.; Yang, X.; Chen, L.; Jian, J. MicroRNA-384-5p/Beclin-1 As Potential Indicators For Epigallocatechin Gallate Against Cardiomyocytes Ischemia Reperfusion Injury By Inhibiting Autophagy Via PI3K/Akt Pathway. Drug Des. Devel. Ther. 2019, 13, 3607–3623. [Google Scholar] [CrossRef]
- Lund, E.; Guttinger, S.; Calado, A.; Dahlberg, J.E.; Kutay, U. Nuclear export of microRNA precursors. Science 2004, 303, 95–98. [Google Scholar] [CrossRef]
- Hutvágner, G.; McLachlan, J.; Pasquinelli, A.E.; Bálint, E.; Tuschl, T.; Zamore, P.D. A cellular function for the RNA-interference enzyme Dicer in the maturation of the let-7 small temporal RNA. Science 2001, 293, 834–838. [Google Scholar] [CrossRef]
- Hammond, S.M.; Boettcher, S.; Caudy, A.A.; Kobayashi, R.; Hannon, G.J. Argonaute2, a link between genetic and biochemical analyses of RNAi. Science 2001, 293, 1146–1150. [Google Scholar] [CrossRef]
- Lee, Y.; Ahn, C.; Han, J.; Choi, H.; Kim, J.; Yim, J.; Lee, J.; Provost, P.; Rådmark, O.; Kim, S.; et al. The nuclear RNase III Drosha initiates microRNA processing. Nature 2003, 425, 415–419. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Doench, J.G.; Petersen, C.P.; Sharp, P.A. siRNAs can function as miRNAs. Genes Dev. 2003, 17, 438–442. [Google Scholar] [CrossRef] [PubMed]
- Park, K.W.; Lee, K.M.; Yoon, D.S.; Park, K.H.; Choi, W.J.; Lee, J.W.; Kim, S.H. Inhibition of microRNA-449a prevents IL-1beta-induced cartilage destruction via SIRT1. Osteoarthr. Cartil. 2016, 24, 2153–2161. [Google Scholar] [CrossRef]
- Yan, S.; Wang, M.; Zhao, J.; Zhang, H.; Zhou, C.; Jin, L.; Zhang, Y.; Qiu, X.; Ma, B.; Fan, Q. MicroRNA-34a affects chondrocyte apoptosis and proliferation by targeting the SIRT1/p53 signaling pathway during the pathogenesis of osteoarthritis. Int. J. Mol. Med. 2016, 38, 201–209. [Google Scholar] [CrossRef]
- D’Adamo, S.; Cetrullo, S.; Guidotti, S.; Borzi, R.M.; Flamigni, F. Hydroxytyrosol modulates the levels of microRNA-9 and its target sirtuin-1 thereby counteracting oxidative stress-induced chondrocyte death. Osteoarthr. Cartil. 2017, 25, 600–610. [Google Scholar] [CrossRef]
- D’Adamo, S.; Alvarez-Garcia, O.; Muramatsu, Y.; Flamigni, F.; Lotz, M.K. MicroRNA-155 suppresses autophagy in chondrocytes by modulating expression of autophagy proteins. Osteoarthr. Cartil. 2016, 24, 1082–1091. [Google Scholar] [CrossRef]
- Cai, C.; Min, S.; Yan, B.; Liu, W.; Yang, X.; Li, L.; Wang, T.; Jin, A. MiR-27a promotes the autophagy and apoptosis of IL-1β treated-articular chondrocytes in osteoarthritis through PI3K/AKT/mTOR signaling. Aging 2019 11, 6371–6384. [CrossRef]
- Zhao, X.; Li, H.; Wang, L. MicroRNA-107 regulates autophagy and apoptosis of osteoarthritis chondrocytes by targeting TRAF3. Int. Immunopharmacol. 2019, 71, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Huang, R.; Ma, H.; Zhao, X.; Wang, G. miRNA-411 Regulates Chondrocyte Autophagy in Osteoarthritis by Targeting Hypoxia-Inducible Factor 1 alpha (HIF-1alpha). Med. Sci. Monit. 2020, 26, e921155. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, Z.; Pi, Y.; Chen, Y.; Mei, L.; Luo, Y.; Xie, J.; Mao, X. MicroRNA-375 exacerbates knee osteoarthritis through repressing chondrocyte autophagy by targeting ATG2B. Aging 2020, 12, 7248–7261. [Google Scholar] [CrossRef]
- Feng, L.; Yang, Z.; Li, Y.; Pan, Q.; Zhang, X.; Wu, X.; Lo, J.H.T.; Wang, H.; Bai, S.; Lu, X.; et al. MicroRNA-378 contributes to osteoarthritis by regulating chondrocyte autophagy and bone marrow mesenchymal stem cell chondrogenesis. Mol. Ther. Nucleic Acids 2022, 28, 328–341. [Google Scholar] [CrossRef]
- Yu, Y.; Zhao, J. Modulated Autophagy by MicroRNAs in Osteoarthritis Chondrocytes. Biomed. Res. Int. 2019, 2019, 1484152. [Google Scholar] [CrossRef]
- Bartolomei, M.S.; Zemei, S.; Tilghman, S.M. Parental imprinting of the mouse H19 gene. Nature 1991, 351, 153–155. [Google Scholar] [CrossRef] [PubMed]
- Dahariya, S.; Paddibhatla, I.; Kumar, S.; Raghuwanshi, S.; Pallepati, A.; Gutti, R.K. Long non-coding RNA: Classification, biogenesis and functions in blood cells. Mol. Immunol. 2019, 112, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Umlauf, D.; Fraser, P.; Nagano, T. The role of long non-coding RNAs in chromatin structure and gene regulation: Variations on a theme. Biol. Chem. 2008, 389, 323–331. [Google Scholar] [CrossRef]
- Guttman, M.; Amit, I.; Garber, M.; French, C.; Lin, M.F.; Feldser, D.; Huarte, M.; Zuk, O.; Carey, B.W.; Cassady, J.P.; et al. Chromatin signature reveals over a thousand highly conserved large non-coding RNAs in mammals. Nature 2009, 458, 223–227. [Google Scholar] [CrossRef]
- Ebert, M.S.; Neilson, J.R.; Sharp, P.A. MicroRNA sponges: Competitive inhibitors of small RNAs in mammalian cells. Nat. Methods 2007, 4, 721–726. [Google Scholar] [CrossRef]
- Aravin, A.A.; Hannon, G.J.; Brennecke, J. The Piwi-piRNA pathway provides an adaptive defense in the transposon arms race. Science 2007, 318, 761–764. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Ahn, C.; Chun, C.H.; Jin, E.J. A long non-coding RNA, GAS5, plays a critical role in the regulation of miR-21 during osteoarthritis. J. Orthop. Res. 2014, 32, 1628–1635. [Google Scholar] [CrossRef]
- Gasic, V.; Stankovic, B.; Zukic, B.; Janic, D.; Dokmanovic, L.; Krstovski, N.; Lazic, J.; Milosevic, G.; Lucafo, M.; Stocco, G.; et al. Expression Pattern of Long Non-coding RNA Growth Arrest-specific 5 in the Remission Induction Therapy in Childhood Acute Lymphoblastic Leukemia. J. Med. Biochem. 2019, 38, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Ji, Q.; Qiao, X.; Liu, Y.; Wang, D. Expression of long-chain noncoding RNA GAS5 in osteoarthritis and its effect on apoptosis and autophagy of osteoarthritis chondrocytes. Histol. Histopathol. 2021, 36, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Alsager, S.; Zhuo, Y.; Shan, B. HOX transcript antisense RNA (HOTAIR) in cancer. Cancer Lett. 2019, 454, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Li, Z. Long non-coding RNA HOTAIR: A novel oncogene (Review). Mol. Med. Rep. 2015, 12, 5611–5618. [Google Scholar] [CrossRef]
- Wang, S.L.; Huang, Y.; Su, R.; Yu, Y.Y. Silencing long non-coding RNA HOTAIR exerts anti-oncogenic effect on human acute myeloid leukemia via demethylation of HOXA5 by inhibiting Dnmt3b. Cancer Cell Int. 2019, 19, 114. [Google Scholar] [CrossRef]
- He, B.; Jiang, D. HOTAIR-induced apoptosis is mediated by sponging miR-130a-3p to repress chondrocyte autophagy in knee osteoarthritis. Cell Biol. Int. 2020, 44, 524–535. [Google Scholar] [CrossRef]
- Song, X.Y.; Zhao, M.; Zhang, P.; Yang, L.S.; Bi, R.X.; Xie, W.P. Cangxitongbi capsules protect the articular cartilage in the rat knee through the long non-coding RNA HOTAIR/p38MAPK pathway. Ann. Transl. Med. 2022, 10, 23. [Google Scholar] [CrossRef]
- Kang, Y.; Song, J.; Kim, D.; Ahn, C.; Park, S.; Chun, C.H.; Jin, E.J. PCGEM1 stimulates proliferation of osteoarthritic synoviocytes by acting as a sponge for miR-770. J. Orthop. Res. 2016, 34, 412–418. [Google Scholar] [CrossRef]
- Wang, C.L.; Peng, J.P.; Chen, X.D. LncRNA-CIR promotes articular cartilage degeneration in osteoarthritis by regulating autophagy. Biochem. Biophys. Res. Commun. 2018, 505, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Yu, Y.; Yin, F.; Yang, C.; Li, B.; Lin, J.; Yu, H. Knockdown of PVT1 inhibits IL-1beta-induced injury in chondrocytes by regulating miR-27b-3p/TRAF3 axis. Int. Immunopharmacol. 2020, 79, 106052. [Google Scholar] [CrossRef]
- Tian, F.; Wang, J.; Zhang, Z.; Yang, J. LncRNA SNHG7/miR-34a-5p/SYVN1 axis plays a vital role in proliferation, apoptosis and autophagy in osteoarthritis. Biol. Res. 2020, 53, 9. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.; Lin, L.; Zou, R.; Lin, F.; Liu, Y. Mesenchymal stem cell-derived exosome mediated long non-coding RNA KLF3-AS1 represses autophagy and apoptosis of chondrocytes in osteoarthritis. Cell Cycle 2022, 21, 289–303. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Hu, Q.F.; Li, J.; Shi, C.J.; Luo, J.W.; Tian, W.C.; Pan, L.W. SOX4-activated lncRNA MCM3AP-AS1 aggravates osteoarthritis progression by modulating miR-149-5p/Notch1 signaling. Cytokine 2022, 152, 155805. [Google Scholar] [CrossRef]
- Zhang, S.; Jin, Z. Bone Mesenchymal Stem Cell-Derived Extracellular Vesicles Containing Long Noncoding RNA NEAT1 Relieve Osteoarthritis. Oxid. Med. Cell. Longev. 2022, 2022, 5517648. [Google Scholar] [CrossRef]
- Shi, M.; Sun, M.; Wang, C.; Shen, Y.; Wang, Y.; Yan, S. Therapeutic potential of POU3F3, a novel long non-coding RNA alleviates the pathogenesis of osteoarthritis by regulating miR-29a-3p/FOXO3 axis. Curr. Gene. Ther. 2022. [Google Scholar] [CrossRef]
- Hansen, T.B.; Jensen, T.I.; Clausen, B.H.; Bramsen, J.B.; Finsen, B.; Damgaard, C.K.; Kjems, J. Natural RNA circles function as efficient microRNA sponges. Nature 2013, 495, 384–388. [Google Scholar] [CrossRef]
- Mao, X.; Cao, Y.; Guo, Z.; Wang, L.; Xiang, C. Biological roles and therapeutic potential of circular RNAs in osteoarthritis. Mol. Ther. Nucleic Acids 2021, 24, 856–867. [Google Scholar] [CrossRef]
- Memczak, S.; Jens, M.; Elefsinioti, A.; Torti, F.; Krueger, J.; Rybak, A.; Maier, L.; Mackowiak, S.D.; Gregersen, L.H.; Munschauer, M.; et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature 2013, 495, 333–338. [Google Scholar] [CrossRef]
- Zhou, Z.; Du, D.; Chen, A.; Zhu, L. Circular RNA expression profile of articular chondrocytes in an IL-1beta-induced mouse model of osteoarthritis. Gene 2018, 644, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Ling, Y.; Mehrpour, M.; Saw, P.E.; Liu, Z.; Tan, W.; Tian, Z.; Zhong, W.; Lin, W.; Luo, Q.; et al. Autophagy-associated circRNA circCDYL augments autophagy and promotes breast cancer progression. Mol. Cancer 2020, 19, 65. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.S.; Zhang, F.J.; Zeng, C.; Luo, W.; Xiao, W.F.; Gao, S.G.; Lei, G.H. Autophagy in osteoarthritis. Jt. Bone Spine 2016, 83, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Qi, L.; Chen, R.; He, J.; Liu, Z.; Wang, W.; Tu, C.; Li, Z. Circular RNAs in osteoarthritis: Indispensable regulators and novel strategies in clinical implications. Arthritis Res. Ther. 2021, 23, 23. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Cheng, F.; Rong, G.; Tang, Z.; Gui, B. Hsa_circ_0005567 Activates Autophagy and Suppresses IL-1beta-Induced Chondrocyte Apoptosis by Regulating miR-495. Front. Mol. Biosci. 2020, 7, 216. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Li, J.; Zhou, Y.; Yang, Z.; Yang, H.; Li, D.; Zhang, J.; Zhang, Y.; Xu, N.; Huang, Y.; et al. Down-regulated ciRS-7/up-regulated miR-7 axis aggravated cartilage degradation and autophagy defection by PI3K/AKT/mTOR activation mediated by IL-17A in osteoarthritis. Aging 2020, 12, 20163–20183. [Google Scholar] [CrossRef]
- Zeng, J.; Zhang, Z.; Liao, Q.; Lu, Q.; Liu, J.; Yuan, L.; Liu, G. CircPan3 Promotes the Ghrelin System and Chondrocyte Autophagy by Sponging miR-667-5p During Rat Osteoarthritis Pathogenesis. Front. Cell. Dev. Biol. 2021, 9, 719898. [Google Scholar] [CrossRef]
- Sui, C.; Liu, D.; Que, Y.; Xu, S.; Hu, Y. Knockdown of hsa_circ_0037658 inhibits the progression of osteoarthritis via inducing autophagy. Hum. Cell 2021, 34, 76–85. [Google Scholar] [CrossRef]
- Xiang, S.; Li, Z.; Bian, Y.; Weng, X. RNA sequencing reveals the circular RNA expression profiles of osteoarthritic synovium. J. Cell. Biochem. 2019, 120, 18031–18040. [Google Scholar] [CrossRef]
- Man, G.; Yang, H.; Shen, K.; Zhang, D.; Zhang, J.; Wu, H.; Zhang, H.; Wang, J. Circular RNA RHOT1 Regulates miR-142-5p/CCND1 to Participate in Chondrocyte Autophagy and Proliferation in Osteoarthritis. J. Immunol. Res. 2022, 2022, 4370873. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, R.; Huang, X.; Yin, E.; Yang, Y.; Yi, C.; You, H.; Song, X.; Yuan, X. Circular RNA MELK Promotes Chondrocyte Apoptosis and Inhibits Autophagy in Osteoarthritis by Regulating MYD88/NF-kappaB Signaling Axis through MicroRNA-497-5p. Contrast Media Mol. Imaging 2022, 2022, 7614497. [Google Scholar] [CrossRef] [PubMed]
- Wani, W.Y.; Ouyang, X.; Benavides, G.A.; Redmann, M.; Cofield, S.S.; Shacka, J.J.; Chatham, J.C.; Darley-Usmar, V.; Zhang, J. O-GlcNAc regulation of autophagy and alpha-synuclein homeostasis; implications for Parkinson’s disease. Mol. Brain 2017, 10, 32. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.H.; Park, S.; Ahn, C.; Song, J.; Kim, D.; Jin, E.J. Beneficial reward-to-risk action of glucosamine during pathogenesis of osteoarthritis. Eur. J. Med. Res. 2015, 20, 89. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Ho, M.L.; Chang, L.H.; Kang, L.; Lin, Y.S.; Lin, S.Y.; Wu, S.C.; Chang, J.K. Parathyroid hormone-(1–34) ameliorated knee osteoarthritis in rats via autophagy. J. Appl. Physiol. 2018, 124, 1177–1185. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.; Wang, C.; Shi, Y.; Yan, L.; Xu, J.; Qi, W. Active vitamin D activates chondrocyte autophagy to reduce osteoarthritis via mediating the AMPK-mTOR signaling pathway. Biochem. Cell Biol. 2020, 98, 434–442. [Google Scholar] [CrossRef]
- Ge, Y.; Zhou, S.; Li, Y.; Wang, Z.; Chen, S.; Xia, T.; Shen, J.; Teng, H.; Jiang, Q. Estrogen prevents articular cartilage destruction in a mouse model of AMPK deficiency via ERK-mTOR pathway. Ann. Transl. Med. 2019, 7, 336. [Google Scholar] [CrossRef]
- Nogueira-Recalde, U.; Lorenzo-Gomez, I.; Blanco, F.J.; Loza, M.I.; Grassi, D.; Shirinsky, V.; Shirinsky, I.; Lotz, M.; Robbins, P.D.; Dominguez, E.; et al. Fibrates as drugs with senolytic and autophagic activity for osteoarthritis therapy. eBioMedicine 2019, 45, 588–605. [Google Scholar] [CrossRef]
- Li, S.; Kong, Y.; Si, L.; Chi, Z.; Cui, C.; Sheng, X.; Guo, J. Phosphorylation of mTOR and S6RP predicts the efficacy of everolimus in patients with metastatic renal cell carcinoma. BMC Cancer 2014, 14, 376. [Google Scholar] [CrossRef]
- Takayama, K.; Kawakami, Y.; Kobayashi, M.; Greco, N.; Cummins, J.H.; Matsushita, T.; Kuroda, R.; Kurosaka, M.; Fu, F.H.; Huard, J. Local intra-articular injection of rapamycin delays articular cartilage degeneration in a murine model of osteoarthritis. Arthritis Res. Ther. 2014, 16, 482. [Google Scholar] [CrossRef]
- Xue, S.; Zhou, X.; Sang, W.; Wang, C.; Lu, H.; Xu, Y.; Zhong, Y.; Zhu, L.; He, C.; Ma, J. Cartilage-targeting peptide-modified dual-drug delivery nanoplatform with NIR laser response for osteoarthritis therapy. Bioact. Mater. 2021, 6, 2372–2389. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).