Abstract
The incidence of brain pathologies has increased during last decades. Better diagnosis (autism spectrum disorders) and longer life expectancy (Parkinson’s disease, Alzheimer’s disease) partly explain this increase, while emerging data suggest pollutant exposures as a possible but still underestimated cause of major brain disorders. Taking into account that the brain parenchyma is rich in gap junctions and that most pollutants inhibit their function; brain disorders might be the consequence of gap-junctional alterations due to long-term exposures to pollutants. In this article, this hypothesis is addressed through three complementary aspects: (1) the gap-junctional organization and connexin expression in brain parenchyma and their function; (2) the effect of major pollutants (pesticides, bisphenol A, phthalates, heavy metals, airborne particles, etc.) on gap-junctional and connexin functions; (3) a description of the major brain disorders categorized as neurodevelopmental (autism spectrum disorders, attention deficit hyperactivity disorders, epilepsy), neurobehavioral (migraines, major depressive disorders), neurodegenerative (Parkinson’s and Alzheimer’s diseases) and cancers (glioma), in which both connexin dysfunction and pollutant involvement have been described. Based on these different aspects, the possible involvement of pollutant-inhibited gap junctions in brain disorders is discussed for prenatal and postnatal exposures.
1. Introduction
In the last few decades, brain pathologies have become more prevalent in our societies (Table 1) [1]. For some of these pathologies, the increase in their prevalence is probably the consequence of a better diagnosis [autism spectrum disorders, (ASD)] while others, considered as degenerative, are more commonly associated with aging [Parkinson’s (PD) and Alzheimer’s diseases (AD)] [1]. For some of these degenerative pathologies, the incidence increase is most prevalent in high-income countries where life expectancy is the highest. For instance, in the US, the annual incidence rate of AD increases from 1% among people aged 65 years to 8% for people aged 85 years and older. Based on the projected ageing of the population, these numbers will triple in the next 30 years resulting in an increase of nearly 80% in total societal costs per adult [2]. This situation has reached a point where the proportion of deaths related to AD went up by 146.2% between 2000 and 2018, while the overall death rate from stroke, HIV and cardio-vascular diseases decreased. Globally, direct and indirect costs for healthcare related to AD are estimated at nearly USD 500 billion annually [2,3]. Taken together, all brain pathologies, therefore, represent a strong challenge for next few years, in terms of health care and social impact.

Table 1.
Categories, diagnosis, prevalence, symptoms and causes of brain pathologies discussed in this review.
Depending on their etiology, diagnosis and evolution, these brain pathologies can be classified into three major categories of disorders (Table 1), as neurodevelopmental [ASD, attention deficit hyperactivity disorders (ADHD), epilepsy], neurodegenerative (PD and AD) and neurobehavioral [migraines, major depressive disorders (MDD)]. Brain cancer can be included in this list as a 4th category since it cannot be classified as either a neurodevelopmental, neurodegenerative or neurobehavioral disorder (Table 1). Even if still rare (less than 2% of cancer cases), brain cancer incidence has increased in high income countries similar to other brain pathologies [4]. The recent rise in incidence of these various brain pathologies [1,4] leads one to question their possible common etiology.
Part of the answer to this question might be in distinct characteristics of the brain. Contrary to other organs, human brain development still progresses for almost two decades after birth, especially during adolescent years. Even later in life, contrary to earlier held views, the brain is not a “static” organ. This brain plasticity permits humans to adapt their behavior to their environment throughout their life [5]. Therefore, during intra-uterine and postnatal periods of life (childhood, adolescence), neuronal connections are modulated under environmental cues and stimulation (education, psychological traumatisms, etc.) and are maintained during the rest of life. Such connections are the consequences of cellular plasticity, which depends on synaptic activity and on the presence of stem cells able to replace damaged brain cells including neurons, astrocytes, and oligodendrocytes. Due to its multiple functions crucial for survival of the entire organism, the brain is highly protected against mechanical and chemical trauma by the skull externally and the blood–brain barrier (BBB) internally. However, despite this high level of protection, the brain can be reached and exposed to compounds able to cross the BBB. This leads, for instance, to drug addictions and possibly to the higher prevalence of brain pathologies affecting humans from birth (ASD, ADHD, epilepsy) to old age (PD and AD).
In order to assure coordinated brain activity, major brain cells (neurons, astrocytes, oligodendrocytes) are tightly connected through a dense intercellular network. Among such intercellular interactions, gap junctions (GJs) play important roles necessary to brain activity by permitting an efficient transfer of information between neurons through electrical synapses, neurotransmitter and ionic buffering of chemical synapses, axonal myelination, neuronal metabolism through astrocytes, etc. Consequently, the brain is one of the organs abundantly supplied by diversity of GJ-proteins [connexins (Cxs)]. Therefore, considering the sensitivity of Cxs and GJ functions to a wide range of chemical compounds, it is possible that chronic exposures to BBB-permeable pollutants disturb the gap-junctional intercellular communication (GJIC) network in brain. Such GJIC disturbances could then perturb brain physiology (synaptic plasticity, memory storage, behavior, etc.) and explain the rising incidence of brain pathologies.
The aim of the present review is to evaluate whether chemical pollutants can induce neuropathologies through their actions on GJIC and Cxs. In order to argue about such a possibility, this review is made of three major parts reviewing (1) the physiological roles of GJs and Cxs in the brain, (2) the effect of chemical pollutants on Cx functions and, (3) the Cx and environmental involvements in major brain pathologies cited above (Table 1).
2. Gap Junction and Connexin Roles in Brain
In the context of animal evolution, the brain was formed through the cephalisation process. As the anterior part of the central nervous system (CNS), it results from both an increased number of neurons and a particular cellular organization. The adult human brain weighs approximately 1.5 kg and, proportional to the body size, its volume is the highest of all animal species. As a complex organ, the brain is rich in GJs and probably exhibits the widest range of Cx expression in the body with most of the 21 Cx isoforms present (Cx26, Cx29, Cx30, Cx30.2, Cx31.1, Cx31.9, Cx32, Cx36, Cx37, Cx40, Cx43, Cx45, Cx46, Cx47, Cx57) [18,19,20,21,22,23,24]. Paradoxically, while the brain is highly complex in its functional capacities and tissue organization, it only contains a few different cell types expressing, for most of them, several Cx isoforms. Before being stabilized in each differentiated cell type, the Cx expression profile varies during embryogenesis and reveals that Cxs are involved both in brain formation and in many physiological aspects needed for its functions (Table 2).

Table 2.
Gap-junctional organization, connexin expression and functions in human adult brain. Stem cells are not included. Abbreviations: Astro., Astrocytes; BBB, Blood-brain barrier; CMT1X, X-linked Charcot-Marie-Tooth disease type 1; Compart., Compartments; Cx, Connexin; Ependym., Ependymocytes; Endo., Endothelial; GJ, Gap junction; GJIC, Gap-junctional intercellular communication; HC, Hemichannel; KO, Knock out mice; Neuroinflam., Neuroinflammation; NPC, Neuronal progenitor cells; ODDD, Oculodentodigital dysplasia; Oligo., Oligodendrocytes. References are indicated between brackets. In some cases, connexin function as GJIC and/or HC is questioned (?) because not proven yet [25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98].
2.1. Brain Development and Connexin Expression
The brain is the result of an embryonic process starting from the 3rd gestational week to undergo the four following principal phases of development: (1) dorsal induction (primary and secondary neurulations), (2) ventral induction (forebrain patterning), (3) neuronal proliferation and migration, and (4) myelination.
Thus, during the 3rd week of embryogenesis, initiation of the CNS evolves with the development of the notochordal process (neuroectoderm induction). This derivative of primitive ectoderm rapidly grows in length to be converted, within 20 days, from a hollow tube to the notochord. Then, the notochord, in relation with the axial mesoderm, induces the neural plate (primary neurulation). At this stage, the neuroepithelium of the neural plate begins the formation of the brain and spinal cord by appearing at the cranial end of the embryo to differentiate craniocaudally. At the beginning of the 4th week, the neural plate is composed of a broad cranial portion (foetal brain) and a narrow caudal portion (spinal cord). Then, the foetal brain pursues its own particular and complex evolution, which corresponds, for the next 5 weeks, to the proencephalic and hemispheric formation.
From the 10th week, neuronal proliferation continues for at least 10 weeks. Soon (12th week), neuronal migration takes place concomitant with neuronal proliferation up to the 24th week. This neuronal proliferation (neurogenesis) within the cerebral hemispheres arises from neural precursor cells (NPCs) which are either “type 1 stem-like cells” (nestin+, GFAP+) or “type 2a progenitors” (nestin+, GFAP-). These self-renewing and multipotential cells are able to differentiate either into neurons or glial cells. Neurogenesis starts when those cells differentiate in “type 2b progenitor” cells (nestin+, doublecortin+) to become “type 3 neuroblasts” (doublecortin+) capable of migrating to form the cortical layers [24,99,100,101,102,103,104]. Thereafter, those post-mitotic neurons complete their differentiation by extending axon and dendrites from their cell body. Neuronal proliferation and migration continue after birth for several months (postnatal neurogenesis) or even years. Synaptogenesis accompanies this phenomenon up to puberty, and even probably later. Apparently, NPCs, which are at the origin of neurogenesis, are differently located during embryogenesis. For instance, those from the ventricular zone (VZ) are at the site of origin of cortical glutamatergic pyramidal neurons, while those from the ganglionic eminence form GABAergic neurons. After neuronal proliferation and differentiation, programmed neuronal cell death also takes place from the 28th week up to at least the first postnatal week. During all this neuronal organization, gliogenesis (from the 20–24th weeks) and myelination (from the 26–28th weeks) complete the cellular brain organization up to 2–3 postnatal years and maybe more. Finally, as brain tissue grows and differentiates, angiogenesis forms the particular blood vessel network necessary for food and oxygen supply to neurons and other brain cells. Importantly, at the time of birth, the BBB is not finalized due to the incomplete coverage of blood vessels by astrocytic endfeet. Such a process seems to be completed at postnatal day 10 (P10) and the BBB is considered being mature at P20 [105].
Cxs and GJIC play important roles in organs during development [106]; this is also the case for brain formation and cortical development (Figure 1). In rodents, at least 9 Cx isoforms are highly expressed in the embryonic cerebral cortex (Cx26, Cx29, Cx31.1, Cx32, Cx36, Cx37, Cx43, Cx45 and Cx47) [24,107,108], and during cortical development, each Cx isoform has a distinct spatial and temporal pattern of expression [107,109,110,111], which might have functional significance as each Cx isoform has distinct permeability and regulation properties [112]. Cx26, Cx37 and Cx45 are largely distributed from the VZ to the cortical plate, whereas Cx36 and Cx43 are highly expressed in the VZ and less in the cortical plate [108,113]. Cx43 is widely expressed in embryonic brain before being restricted mainly to astrocytes as the CNS matures. While Cx43 is a negative modulator of neuronal differentiation through its carboxyl terminus (C-ter), Cx36 activates this process [114,115]. This is consistent with Cx36 expression in the VZ during the first wave of neurogenesis with, to a lesser extent, Cx26 [108]. Cx36 expression is maintained during the period of neuronal differentiation before being dramatically reduced during postnatal maturation. It is then restricted to only a few neuron populations (interneurons of hippocampus, thalamus, and sparcely in neocortex) where it forms electrical synapses, while most other neurons are connected by chemical synapses [108,109,116,117,118]. Interestingly, two other Cxs (Cx26 and Cx43) are involved in neocortical neuronal migration, which seems to be mainly the consequence of mechanisms driven by the C-ter part of Cx43, independently from its channel function, but depending on its phosphorylation status [113,119,120,121]. In this process, astrocytes participate in the migration of neurons by orientating them [113], and become involved in synaptogenesis, contributing eventually to the formation of tripartite synapses. The differentiation of oligodendrocytes is accompanied by the expression of specific Cxs (Cx29, Cx32, Cx47), which are involved in the postnatal myelination process [63,69,122].
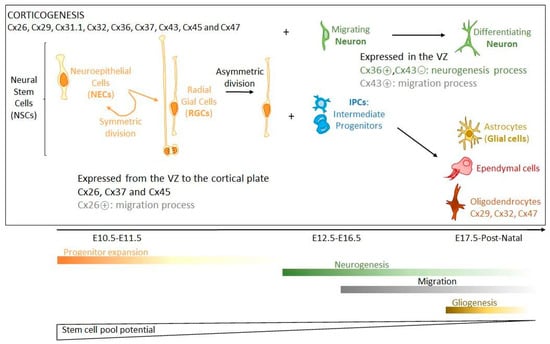
Figure 1.
Corticogenesis. During early cortical development, neural stem cells (NSCs) predominantly undergo symmetric divisions to expand the NSC pool (Progenitor expansion phase). This pool includes neuroepithelial, radial glial and intermediate progenitor cells. Neuroepithelial cells (NECs) become radial glial cells (RGCs), which by asymmetric divisions generate RGC and intermediate progenitors (IPCs) or neurons, both migrating and differentiating (Neurogenesis phase). At later stages of development, NSCs generate the other cell types of the brain including astrocytes, oligodendrocytes, or ependymal cells (Gliogenesis phase). During cortical development, Cx37 and Cx45 are largely distributed from the VZ to the cortical plate, whereas Cx36 and Cx43 are highly expressed in the VZ and less in the cortical plate. While Cx43 is a negative modulator of neuronal differentiation, Cx36 activates this process (in green). Cx26 and Cx43 are involved in neocortical neuronal migration (in grey). The differentiation of oligodendrocytes is accompanied by the expression of specific Cxs (Cx29, Cx32, Cx47) that are involved in the postnatal myelination process. The time scale of these phases of corticogenesis (E: Embryogenesis days) is from mouse [136].
During brain development, the presence of Cxs leads to the establishment of GJIC, which is important to coordinate differentiation of NPC clusters. This is particularly true in the rodent embryonic CNS where spatio-temporal patterns necessary for formation of functional domains have to be synchronized in their behaviour [106,123,124]. Moreover, GJIC probably permits NPCs to exchange signaling messages with adjacent cells to direct them towards a particular differentiation lineage (neuronal or glial) [24,125]. Such GJIC compartmentalization is influenced further by changes of Cx expression over time depending on the differentiation process. In parallel to the establishment of GJIC, Cxs are also involved in brain development via their hemichannel (HC) activity. For instance, during corticogenesis, HC-mediated Ca2+ waves increase in number, amplitude, and distance [126]. Moreover, in the early postnatal neocortex, dendritic GJs mediate the propagation of IP3 and Ca2+ waves. This process is thought to be at the origin of functional regionalization by dividing the immature neocortex into columnar patches of coordinated activity [123,127,128,129].
In summary, Cxs are actively involved in brain formation, which largely extends the classical period of embryogenesis. The numerous Cx isoforms are expressed differently, in a spatio-temporal pattern modulating cell proliferation, differentiation and migration. All these processes are mediated differently by Cxs, which establish either GJIC-forming communicating domains directed towards particular differentiation lineages, paracrine/autocrine communication through HCs or particular intracellular protein interactions. Activated P2X7 receptors are involved in apoptosis and in the clearance of apoptotic cells during neurogenesis [130,131,132,133,134]. Even if not completely defined yet, as pannexin (Panx) channels, Cx HCs could participate in such activation by releasing ATP [24]. Finally, Cx protein interactions can take place either outside the cells like extracellular parts of Cx43 interacting with astrocytes during neuronal migration, or inside the cells by controlling particular signaling pathways. Knowing that several Cxs can be expressed in the same cell type, the events can be controlled in a very subtle way depending on their respective abundance, since different Cxs may have opposite effects in a same cell type (eg., Cx43 and Cx36 in neuronal differentiation) [24,119,135].
2.2. Adult Brain Organization and Connexin Involvement
At term, adult brain tissue is made of a few cell types contributing to different functional compartments such as neuronal, glial, and vascular compartments. The neuronal compartment is only made of neurons interacting between each other either through abundant chemical or less frequent electrical synapses. This compartment is complex with regard to different types of neurons (projection neurons, interneurons, etc.) presenting a high variety in spatial and temporal neurotransmitter release and receptor activation. The glial compartment is the most complex in terms of cell types and organization. Even if glial cells are smaller than neurons, they are 10 times more numerous and constitute at least 50% of the total brain cells [137,138]. The glial compartment is composed of macroglia (astrocytes, oligodendrocytes, ependymocytes) and microglia. The most abundant glial cells are astrocytes whose ramifications are, either, in contact with neuronal synapses (tripartite synapses), oligodendrocytes or cover environing blood capillaries by their endfeet, which are part of the BBB. Astrocytes control the extracellular interstitial space composition by buffering K+ ions and clearing neurotransmitters secreted by neuronal activity. They also secrete gliotransmitters, which can modulate neuronal activity [139]. Oligodendrocytes are myelinating cells whose ramifications surround axons of neurons by forming myelin, which facilitates the propagation of action potentials along axons; myelinated axons constitute the white matter of the CNS. Ependymocytes line the ventricular cavities of brain. They constitute a permeable barrier between the cerebrospinal fluid (CSF) and the interstitial liquid surrounding cells of the CNS. Some of these cells are ciliated and permit the circulation of the CSF [140,141,142,143]. Microglia control inflammation and can differentiate into macrophagocytes in the presence of microorganisms or damaged neurons to phagocytose them. Their role is to protect brain tissue since cells of the immune system cannot normally access the CNS. The vascular compartment is made of endothelial cells, which line brain capillaries. The apical face of endothelial cells is in contact with blood whereas their basal face is in contact, for some of them, with pericytes whose contracting capacity regulates blood flow. The vascular compartment is separated physically from the glial compartment by the basal membrane. Therefore, anatomically, the glial compartment is localized between vascular and neuronal compartments.
Due to the limited extracellular space (20%) of CNS, these different cell types are at high density and extensively interconnected between themselves. Such density and interconnection facilitate neuron function by stabilizing their immediate extracellular environment (astrocytes, oligodendrocytes), bringing nutriments (astrocytes), protecting them (microglia) and increasing the speed of their communication (oligodendrocytes). Most of these very different actions have to be highly coordinated and, therefore, all cells are highly communicating between each other through direct (GJs) or short distance (autocrine/paracrine) (HCs) communication. Interestingly, both types of intercellular communication (direct or autocrine/paracrine), are mediated by the presence of Cxs. This explains why Cxs are so abundant in the brain parenchyma (Table 2).
2.2.1. Gap-Junction Compartments in Brain
Interestingly, the functional compartments presented above can be viewed as each corresponding to a GJ compartment. Indeed, each of these functional compartments can be defined as a directly communicating network in which GJs permit the free diffusion of ions and small hydrophilic molecules between cytosols of adjacent cells belonging to the same functional compartment. Therefore, GJIC defines at least three different GJ compartments (or GJ-connected networks) in a normal brain. One of these compartments (or “networks”) is the neuronal compartment in which neurons communicate between themselves through GJs (electrical synapses), mostly made of Cx36 [25,26,27]. A second one, the vascular compartment is constituted by capillary endothelial cells (endothelium), which are connected between themselves and with pericytes via Cx37, Cx40, and Cx43. The 3rd GJ compartment is localized between the two previous ones and constitutes a panglial compartment (“panglial syncytium”). In this panglial syncytium, the cells expressing Cxs include astrocytes (Cx43, Cx30 and possibly Cx26) and oligodendrocytes (Cx32, Cx29, Cx47), resulting in the formation of GJs directly connecting astrocytes, oligodendrocytes and ependymocytes (Cx43) [41,46,63,144,145,146,147]. Other crucial cell types for brain function, including microglia and stem cells, do not seem to constitute such firmly established GJ compartments. However, since these cell types express Cxs and are able to make GJs, at least occasionally, it is possible to consider them as members of “putative” GJ compartments. As presented below, the spatial organization of “well-established” or “putative” GJ compartments have functional consequences (Figure 2).
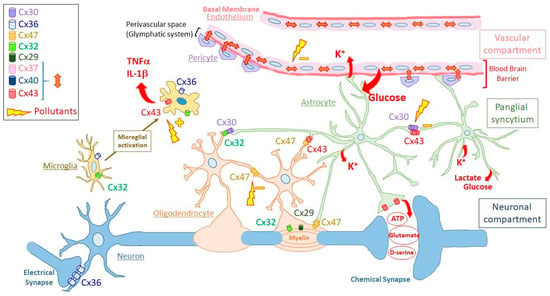
Figure 2.
Schematic representation of the organization and connexin composition of the gap-junction compartments in the human adult brain. The different gap-junction compartments are indicated on the right side of the figure (vascular compartment, panglial syncytium, neuronal compartment). Principal cell types are presented for each gap-junction compartment (vascular compartment: endothelial cells or endothelium with pericytes; panglial syncytium: astrocytes in green and oligodendrocytes with myelin surrounding axons of neurons; neuronal compartment: neurons connected by chemical or electrical synapses). For clarity, pericytes are not enveloped by basement membrane. Microglia are located apart since they do not seem to establish permanent gap junctions between microglial cells or with cells of the different gap-junction compartments. Microglial cells are presented in non-activated and activated states through microglial activation. The connexin isoforms expressed in the different cell types are indicated according to the code color inserted in the left side of the figure. For clarity, connexin hemichannels and gap junctions are both presented as cylinders. Cylinders present between cells are gap junctions made of the indicated connexins, while those located in nonintercellular areas are hemichannels. Cylinders inside myelin represent reflexive gap junctions between the concentrical layers of myelin around neuronal axons. The red double arrows in the vascular compartment represent gap junctions made of either Cx43, Cx37 or Cx40 between endothelial cells and between endothelial cells and pericytes. Single red arrows represent ions (K+) and molecules passing through connexin channels, which are either gap junctions (transfer of K+ and glucose uptaken from extracellular space to be released in blood or close to neurons, respectively) or hemichannels (release of pro-inflammatory cytokines like TNF-α and IL-1β by activated microglia; ATP, D-serine or glutamate released by astrocytes). Yellow lightning bolts represents possible effects of pollutants as either, inhibitors (−) of gap-junctional intercellular communication or indirect activators (+) of connexin hemichannels (See Discussion part for details). For clarity, ependymocytes and stem cell niches are not shown and only two astrocytic endfeet are presented as constituents of the blood–brain barrier. At the level of the blood-brain barrier, the perivascular space delimited by the astrocytic endfeet is part of the so-called glymphatic system involved in draining interstitial fluid and removing metabolic wastes.
The Neuronal Gap-Junction Compartment
The simplest GJ compartment in the brain is the neuronal compartment, which is only made of neurons connected by electrical synapses (Figure 2). This neuronal compartment is quite extended in the embryonic CNS where electrical synapses are numerous. Then, electrical synapses become limited during development [22,24,25,148,149,150,151,152] up to being restricted, in adults, in particular brain areas like the cerebral cortex (inhibitory GABAergic interneurons) [153,154,155,156,157], hippocampus (synchronous activity among inhibitory interneurons), reticular thalamic nucleus (synchronization of burst-firing), suprachiasmic nucleus (circadian behavior) [158,159], hypothalamus (pulsatile oxytocin release) and brainstem (control of respiratory modulation) [27].
All these electrical synapses are mostly made of Cx36, even if Cx31.1, Cx45, Cx57 and others have been found in murine neurons of some brain regions such as the retina [30,31,32,33,34,35]. Therefore, consistent with the role of neurons in which Cx36 is expressed, Cx36 GJs mostly promote the synchronization of interconnected neurons [118,160]. If such a synchronization is required, for example, for circadian rhythm, the presence of inhibitory networks coupled by Cx36 GJs permits resistance to the development of epileptiform synchronization. In relation to this, it is interesting to note that mutations in the regulatory promoter of the Cx36 gene (GJD2) are linked to juvenile myoclonic epilepsy (Table 2) [38,39]. In addition to their function in limiting excitability, neuronal GJs also play a major role in learning and memory. Indeed, Cx36-KO mice exhibit impaired short- and long-term memory as well as reduced motor learning (Table 2) [36,37]. Cx31.1, which is expressed in dopaminergic neurons of the substantia nigra pars compacta [161] and striatal output neurons [29], seems to play a role in exploration of novel environments and object recognition since these functions are either elevated or impaired, respectively, in Cx31.1 KO mice [23,162].
A general property of these electrical synapses is their high degree of plasticity, which makes them capable of modifying their coupling strength under various physiological conditions [27]. Cx36 has also been reported to act not only as a GJ protein but also as HC components releasing ATP during depolarization in cortical spreading depression [28]. Further studies could verify this.
The Panglial Syncytium
As a GJ compartment, the panglial syncytium is made of astrocytes, oligodendrocytes and ependymocytes. Because of their abundance and roles, only the first two cell types will be considered (Figure 2).
Astrocytes are the most numerous cells in the panglial syncytium. They represent an intermediate layer between neurons and blood vessels since they project, for some of them, extensions to synapses (tripartite synapses) as well as to capillaries they surround by their endfeet [163]. Astrocytes are coupled extensively by GJs mostly made of Cx43 but also Cx30, which seems to be present more in grey matter [40] where there seem to be mainly homotypic/homomeric Cx43/Cx43 or Cx30/Cx30 [164]. Contrary to Cx30, whose expression is delayed during brain maturation, Cx43 is more homogeneously present in the astrocyte network of white matter, becoming the most abundant Cx in CNS [40,41,165,166,167,168]. Expression of Cx26 has also been reported in rodent astrocytes after birth, where it was observed in GJs between astrocytes and with oligodendrocytes [41]. However, this expression of Cx26 in astrocytes is still debated as deletion of both Cx43 and Cx30 in astrocytes causes pathological conditions [60], whereas lacZ under the Cx26 promoter is not expressed in astrocytes [43].
As the other important part of the panglial syncytium, oligodendrocytes form few GJs with astrocytes, mainly to those they depend on for homeostatic and nutrient support [169]. In oligodendrocytes, 3 Cx isoforms are expressed (Cx29, Cx32, Cx47) exhibiting different subcellular localizations. Cx47 colocalizes with Cx32 in GJs present in the soma connected to astrocytes (heterologous GJs) while Cx29 and Cx32 are concentrated in reflexive GJs connecting the concentric myelin layers. Cx47 is also present in small GJs linking myelin to astrocytes but not in deeper layers of myelin [62,65,66]. Apparently, oligodendrocytes also communicate, but to a much lesser extent, through homotypic Cx47 GJs, with other oligodendrocytes and their precursors in white matter [67]. Therefore, the only way most oligodendrocytes can communicate between each other is indirectly through GJs with astrocytic «intermediaries» [170,171].
This particular GJ network, in which at least 5 Cx isoforms are involved, plays fundamental roles for neuron survival and activity. Since neurons are distant from blood capillaries, their energy supply (glucose and lactate) cannot be transmitted directly to them but has to pass through astrocytes whose endfeet surround the basal membrane of the vessels [172]. Glucose molecules enter from blood to astrocytic endfeet by specific transporters and then traffic from astrocytes to astrocytes through Cx43/Cx30 GJs to be released by transporters to the immediate proximity of neurons, where uptake occurs. Apparently, in astrocytes, glucose is transformed through a glutamate-induced glycolytic degradation pathway into lactate, which is transferred to neurons via monocarboxylate transporters [45,173]. Moreover, glucose diffusion passing through astrocytes is not only directed to supply neurons, but is also distributed, through GJs, to oligodendrocytes and ependymocytes.
This energy supply in neurons is essential for the generation of action potentials. Due to neuronal activity, these action potentials increase extracellular K+ concentrations, which could be deleterious for neuronal function. Extracellular K+ ions are taken up by astrocytes and redistributed though astrocyte network following their gradient before being released in the interstitial space. Interestingly, GJIC (ionic coupling) of the astrocytic networks plays a particular role in this phenomenon by equalizing the astrocytic membrane potential (VM) to levels comparable to its neighbours [174]. This minimizes VM depolarization due to elevated local [K+]e and thereby maintains a sustained driving force for highly efficient K+ uptake. Thus, GJIC permits to maintain a constant extracellular K+ microenvironment by achieving isopotentiality in astrocytic networks [174]. Astrocyte endfeet contacting capillaries can also release excess K+ ions and associated osmotic water via Kir4.1 potassium channels [175] and AQP4 water channels that are concentrated in the endfoot plasma membrane [176,177]. Involved in the redistribution of extracellular ions and water, Cx43-mediated GJIC has been reported to regulate astroglial volume [48,60,178]. Because astrocytes occupy 50% of the brain volume [179], changes in astrocytic volume classically lead to opposite variations in extracellular space volume, which can affect neuronal activity by altering the concentration and diffusion of extracellular ions and neurotransmitters. Oligodendrocytes also participate in this K+ buffering through their myelin sheath all along the axon of neurons [70]. After entering the innermost cytoplasmic layer of myelin, K+ ions pass through successive paranodal loops through Cx32/Cx29-containing reflexive GJs to the outermost layer of myelin, then into the astrocytes through heterotypic GJs (Cx47/Cx32: oligodendrocyte side; Cx43/Cx30: astrocyte side). Therefore, through a complex and unique communicating network combining a succession of dense autologous, heterologous, and then homologous GJs, both astrocytes and oligodendrocytes participate to K+ siphoning, avoiding the excess of extracellular K+ ions, which do not reenter into neurons by Na+/K+ ATPase pump activity [63]. Failure to remove sufficient K+ results in changes in resting membrane potential allowing spontaneous action potentials.
Moreover, if reflexive GJs permit the diffusion of K+ ions through the myelin layers of oligodendrocytes, they also permit ionic homeostasis necessary for myelin integrity. Any lack of function due to mutation of Cx47 or Cx32 may cause myelin vacuolation or dismyelination [70]. This results in loss of velocity of action potentials along neuronal axons as it is the case in the Pelizaeus–Merzbacher-like disease [Cx47 gene (GJC2) mutation] [77,78] or Charcot–Mary-Tooth disease [Cx32 gene (GJB1) mutation] [75,76]. Astrocytes also contribute to this function by relaying to their network this ionic homeostasis. Any disturbance of astrocytic GJIC may then provoke dismyelination among oligodendrocytes [60]. Moreover, GJIC-deficient astrocytes display a reduced threshold for the generation of epileptiform events [47,60]. Therefore, the panglial syncytium entirely contributes to the efficient propagation of action potentials along the axons by maintaining myelin integrity.
Neurotransmitter buffering is necessary to maintain efficient synaptic transmission. Such a buffering is due to astrocytes present in the so-called «tripartite synapses». By adequate membrane transporters, astrocytes uptake neurotransmitters present in the synaptic space and enable them to diffuse through the astrocytic network before their eventual recycling to the neurons. This process has been documented for glutamate [45,173]. This excitatory amino acid is cleared from the neuronal synapses by astrocytes via glutamate transporters, and is converted into glutamine, which is released and in turn taken up by neurons. Metabotropic glutamate receptor activation on astrocytes triggers a variety of responses via increases in cytosolic Ca2+, such as Ca2+-dependent glutamate release from the astrocytes, which modulates the activity of both excitatory and inhibitory synapses. In vivo studies have identified the astrocytic endfoot processes enveloping the vessel walls as the center for astrocytic Ca2+ signaling and it is possible that Ca2+ signaling events in the cellular component of the BBB are instrumental in modulation of local blood flow as well as substrate transport.
By facilitating K+ and neurotransmitter buffering, the maintenance of an efficient panglial syncytium is therefore necessary to keep the immediate environment of neurons compatible with their function and survival.
The Vascular Gap-Junction Compartment
Although the human brain accounts for only 2% of total body weight, it demands 20% of the overall energy produced by the body. Since the brain lacks the capacity to store energy, a vast vascular compartment responds to the continuous demand of oxygen and glucose (and waste exit) necessary for neuronal survival and activity and for other cells of the CNS [180]. In an adult brain, it is estimated, by stereological approach, that such energy supply and waste exit are carried out by a network of 600 km of capillaries (7 µm diameter) representing an exchange surface of approximately 15–25 m2 [180,181,182,183]. Such a high density of capillaries places each neuron within 20 μm from a capillary. As seen above, neurons require a tightly regulated extracellular environment to function appropriately, and the 20 μm proximity between neurons and capillaries makes them susceptible to possible chemical fluctuations (ions, hormones, neurotransmitters, amino acids, etc.). Causes of such fluctuations are numerous and may even include circulating neurotoxic substances from external origin (drugs, pollutants, etc.). As a protection, the exchange of substances between blood and brain is tightly controlled by the BBB, which, thus, plays fundamental roles such as regulating ion concentrations, facilitating nutrient transport, and preventing toxic substances [184].
Anatomically, BBB is therefore constituted by a 600 km syncytium of vascular endothelial cells (endothelium) lining the lumen of the capillaries (Figure 2). A thin layer of basal membrane separates endothelial cells from less numbered pericytes (ratio 1:3). Despite this thin basal membrane, endothelial cells and pericytes are nevertheless in contact from place to place through GJs [185,186]. Such GJIC and also paracrine communication are involved in pericyte contraction, which contributes to the local regulation of blood flow by controlling capillary diameter [83,86]. Endothelial cells and pericytes are enveloped by the basal membrane of the capillary, which is almost entirely enclosed by astrocyte endfeet (Figure 2). Importantly, the perivascular space delimited by the astrocytic endfeet is part of the so-called glymphatic system involved in draining interstitial fluid and removing metabolic wastes [187,188,189].
Substances can cross the BBB only via paracellular and transcellular pathways restricted by the presence of adherens and tight junctions between endothelial cells. The transcellular trafficking of endocytic vesicles carrying substances from the apical membrane through the cytoplasm before exiting the basal membrane is more limited than in the rest of the organism [180,190]. Endothelial cells extensively communicate between each other and with pericytes through GJs made of Cx37, Cx40 and Cx43 (Figure 2) [80]. This particular GJ compartment is implicated in BBB function since the presence of functional Cxs is necessary for its maintenance [81]. Moreover, expression of Cx30 and Cx43 at the astrocytic endfeet during development coincides with postnatal maturation of BBB. The absence of these Cxs in the astrocyte endfeet weakens the BBB when hydrostatic vascular pressure is increased [49]. Similarly, the presence of Cx43 in astrocytes helps keep the endothelial barrier closed for immune cell infiltration [191]. However, Cx43 on the endothelial side would have an opposite effect on BBB permeability as observed in a murine model of the familial cerebral cavernous malformations type III (fCCM3). This disease is caused by loss-of-function mutations in CCM3 that result in dilated capillary beds, which are susceptible to hemorrhage because of BBB disruption. Interestingly, Cx43 appears to be up-regulated in developing CCM3 lesions and through its interactions with ZO-1 would favor BBB breakdown by limiting tight junction formation [192]. Similarly, the impairment of pericyte-endothelial interaction increases BBB permeability [193].
The situation is probably more complex because GJ channels and Cx HCs of BBB appear to play opposite roles in the regulation of its permeability. GJIC contributes to maintaining BBB integrity, while HCs are associated with ATP signaling release and necessary to generate Ca2+ oscillations linked to BBB disruption induced by proinflammatory signals [194]. Moreover, it is quite possible that Cx HCs then affect the BBB transcellular pathway by modifying [Ca2+]i, which is involved in the vesicular pathway [194]. Such a situation would depend then on the neuroinflammation status since the release of gliotransmitters or cytokines by activated astroglial and microglial cells affects GJIC and activates Cx HCs [195]. In this situation, it would be interesting to determine whether Panxs, which share many common functions with Cx HCs, might also be involved. This is important because disruption of the BBB is associated with many diseases of the CNS associated with neuroinflammation such as neurodegenerative diseases like AD [196,197,198,199,200]. BBB disruption has been also observed in PD [198,201,202,203], epilepsy, seizures [204,205,206], and brain tumors [207,208,209]. Moreover, it is also possible that BBB disruption affects the glymphatic-draining function by disturbing the perivascular space. Disturbance of such a process seems to be involved in AD by preventing the efficient clearance of amyloid-β deposits [210,211].
Other Putative Gap-Junction Compartments in Brain
The GJ compartments that are functionally described above are well established after birth and for the entire life. The integrity of such an organization is maintained and protected against any cell damage. This protection is assured by cells playing complementary roles by eliminating pathogens and damaged cells (microglia) and by replacing damaged cells (stem and precursor cells). Either scattered inside brain parenchyma (microglia) or concentrated in particular niches (stem cells), these cells do not seem to constitute a well-established and permanent GJ compartment. However, they express Cxs and are therefore able to communicate either directly through GJs or through paracrine communication with other cell types, depending on their activation.
- Stem Cell Niches
After birth, neurogenesis can occur in limited niches of the mammalian brain (Figure 3). In adults, those niches are located in the subventricular zone (SVZ), the subgranular zone (SGZ) of the dendrite gyrus of the hippocampus [24,101,104], and in the hypothalamus (dorsal α1, α2 regions and in the «hypothalamic proliferative region», adjacent to the median eminence, in the β region) [212,213]. The restriction of the neurogenesis areas is the result of a progressive developmental process well described for the SVZ [141,142,214,215,216,217,218,219].
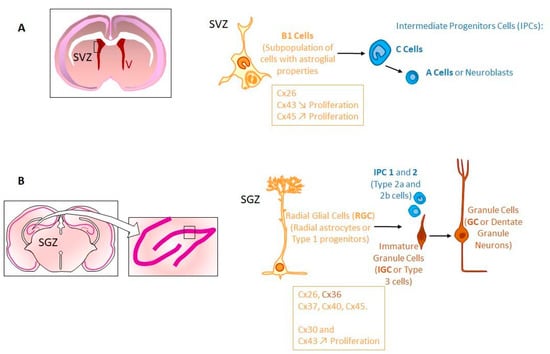
Figure 3.
Stem cell niche and neurogenesis in adult. Neural stem cells (NSCs) are mostly retained in two regions: Sub-ventricular zone (SVZ) and sub-granular zone (SGZ). (A) Frontal cross-section of the adult brain showing the location of the SVZ, in walls of the lateral ventricles (V). In the SVZ, NSCs correspond to type B1 cells. B1 cells express Cx26, Cx43 and Cx45. While Cx43 expression negatively regulates cell proliferation, Cx45 exhibits an opposite role. These B1 cells generate intermediate progenitor cells (IPCs) corresponding to C cells that divide to generate neuroblasts (type A cells). (B) Frontal cross-section of the adult brain showing the hippocampal formation. The insert indicates the location of the dentate gyrus. The adult dentate gyrus contains radial glial cells, which are polarized cells with their cell body in the SGZ. Radial glial cells (RGC or RC, type 1 cells) generate IPCs (IPC1 and IPC2 or type 2a and type 2b cells), which differentiate into immature granule cells (IGCs or type 3 cells) and mature granule cells (GC). In SGZ, Cx26, Cx30, Cx36, Cx37, Cx40, Cx43, Cx45 are expressed in various NPCs. Interestingly, Cx30 and Cx43 seem to be active regulators of hippocampal NPC proliferation while Cx43 itself is a negative regulator of proliferation in SVZ [258].
Once formed, the SVZ lines the walls of the lateral ventricles (LVs). It contains neural stem cells (type B cells) [220] located under the ependymal layer of the ventricle [221]. Those cells have projections towards the CSF in the LV, and also contacts with blood vessels (BVs) of the SVZ plexus [217]. This situation allows type B cells receiving signals from CSF and blood to form transit-amplifying NPCs (type C cells) in asymmetric divisions, which divide to form neuroblasts (type A cells) [220,222,223]. In humans, during the first postnatal months, those neuroblasts migrate to the frontal lobe tangentially close to the walls of LVs and along BVs, through a mechanism probably controlled by astrocyte-released soluble factors [224,225,226]. Then, these neuroblasts individually reach cortical tissue where they differentiate and contribute to inhibitory circuits. These late-arriving inhibitory interneurons could contribute to developmental plasticity since the disruption of their postnatal migration may underlie neurodevelopmental disorders [227,228,229]. Interestingly, such a migration coincides with the such a migration happens during the first months after birth, a critical period for human brain development, when children begin to interact with their environment. This is especially true for the human frontal lobe, which is so important for such interactions and social behavior. Afterwards, neurogenesis and migration decline, disappearing by two years of age [228,229,230,231,232]. This cessation of postnatal neurogenesis in the forebrain seems to be particular to human [233,234]. In addition, SVZ type B cells can also generate oligodendrocyte precursors that contribute to the maintenance of the oligodendrocyte population in the neighboring corpus callosum, striatum and fimbria-fornix [235,236].
Even if it is not well understood yet, cell-cell interactions seem to play an important role during the maturation and maintenance of stem cell niches. Indeed, postnatal NPCs and immature neurons of the SVZ express several types of Cxs (Cx26, Cx43, Cx45) [107,108,237,238,239,240,241,242,243,244]. GJIC has been demonstrated not only between NPCs (radial glial cells) but also between them and astrocytes or microglia [237,239,240,241,245]. Apparently, Cx43 expression increases with postnatal age in SVZ NPCs negatively regulating cell proliferation, contrary to its promoting role during embryogenesis [242,243,246,247]. In contrast, in those cells, Cx45 exhibits an opposite role in NPCs by inducing cell cycle reentry via ATP signaling [243]. Moreover, it has been demonstrated that GJIC mediated by those Cxs is involved in the migration of NPCs within the SVZ [238]. In the postnatal hippocampus, almost the same Cxs are expressed (Cx26, Cx30, Cx37, Cx40, Cx43, Cx45) in various NPCs. However, the expression changes during differentiation (Cx36 appearing in immature neurons). Interestingly, Cx30 and Cx43 seem to be active regulators of hippocampal NPC proliferation while Cx43 itself is a negative regulator of proliferation in SVZ [150,248,249,250,251]. Although the majority of Cxs are docked to function as GJs, unapposed HCs have also been documented on the surface of different cell types [252,253].
By the presence of stem cell niches, the postnatal brain exhibits more plasticity than previously thought and this has implications for memory, learning, and the pathogenesis of neurodegenerative diseases [254,255,256]. For instance, in humans, hippocampal neurogenesis persists during very late decades of life while it is significantly declined in AD patients [257]. The fact that NPCs persist in the adult mammalian brain and can integrate into brain circuity is proof of adult structural plasticity at the cellular level [5]. Interestingly, adult neurogenesis can be regulated by several behavioral factors like running, for instance, which, contrary to stress, induces such a phenomenon. Other behaviors, like learning, seem to have more complex effects, suppressing neurogenesis at some stages (proliferation of progenitor cells), and increasing it at others (differentiation and survival) [5].
In conclusion, any disruption of proliferation and differentiation of neural stem cells and migration of their progeny may contribute to neurodevelopmental and neurocognitive deficits such as autism [227,228,229]. If GJIC is important for such proliferation, differentiation, and survival of neural progenitor cells, any inhibition could indeed have deleterious effects.
- ii.
- Microglia
While they belong to the glial cell types (5–20% of glial cells) with macroglia (astrocytes and oligodendrocytes), microglia are not part of the panglial syncytium from which they differ in their embryonic origin [259,260]. So far, despite the fact they do express Cxs, there is no evidence that microglia establish permanent GJs with astrocytes and/or oligodendrocytes. Therefore, microglia can be considered as distinct from macroglia.
As the resident immune cells in the CNS, microglial cells play important roles in health and disease. In the healthy CNS, they exhibit a ramified morphology with multiple fine processes that survey their surrounding microenvironment. If a pathological stimulus appears, as a result of any type of injury or disease, microglial cells acquire an activated phenotype in which their morphology changes towards a hypertrophic or ameboid-like appearance and their functional behavior is altered [261,262,263,264]. Along with these features, mounting evidence suggests that microglia continually extend and retract their cell processes toward and from synapses, being part of a new spectrum of unexplored capabilities, such as synaptic pruning, maturation and remodeling, as well as modulation of synaptic transmission and plasticity [265,266,267]. Of note, when severe or chronic brain injury occurs, microglia become activated, triggering a widespread release of inflammatory molecules, facilitating the engagement of non-resident brain cells implicated in the innate and adaptive immune function [268].
The main function attributed to activated microglia is the phagocytosis of pathogens, degenerating cells or debris. However, they perform other functions in the activated state, such as removal of dysfunctional synapses (synaptic stripping) [269] or regulation of synaptic plasticity [270]. As part of their activation, microglial cells secrete reactive oxygen species, cytokines and growth factors that influence the pathological process and the subsequent tissue regeneration [261,262,263,271]. Therefore, it is well accepted that surveillant microglia, in addition to their monitoring role in the healthy CNS, can also influence neuronal structure and function contributing to the maintenance of neural circuits [272,273].
Microglial cells are widespread in brain parenchyma but also present, at high density, in stem cells niches. Expressing Cxs (Cx32, Cx36, and when activated, Cx43) [88,89,90,274,275], they can potentially communicate through GJIC with other cell types such as neurons [88] or NPCs in co-cultures [237,276]. In vitro, they form GJs in response to inflammatory stimuli such as cytokines or bacterial pathogens [90,91]. However, a study performed in vivo could not demonstrate the presence of GJIC between microglial cells or between microglia and neurons [277]. Among the microglial Cxs, Cx43 seems to be responsible for the formation of functional GJs [90,278]. Despite the fact that biological significance of microglial coupling remains elusive, it has been hypothesized that GJs are crucial for ruling dynamic changes in microglial phenotype, the exchange of antigen peptides between activated microglia or the cross-presentation of antigens to T cells [98]. Some studies showed that TNF-α-mediated upregulation of Cx HCs contributes to the exacerbated release of glutamate and subsequent neuronal beading and death [279]. Moreover, different inflammatory agents [Aβ, lipopolysaccharide (LPS) and ATP] have been described to increase the opening of HCs formed by Cx43 and Cx32 [280].
In the particular case of stem cell niches, the ability of NPCs to form functional GJs with microglia can be physiologically and pathologically relevant. In healthy conditions, close proximity between those cell types has been documented in the SVZ niche [93,94,95]. Indeed, microglia, which are denser in SVZ, establish a bilateral cross-talk with NPCs that affects both microglial activation state and NPC proliferation and differentiation [93,94,95,96,97]. The inflammatory response triggered by host microglial cells is known to preserve implanted NPCs in an undifferentiated state [281]. Interestingly, microglia in the SVZ display a unique phenotype characterized by specific morphology, low expression of purinergic receptors and microglial markers such as Iba1 and CD68 [282,283] and the release of a distinct set of cytokines [282,284]. Therefore, microglia in the SVZ may influence NPCs behavior through particular characteristics, which may confer on them specific properties and putative roles in the control of SVZ neurogenesis. In line with this assumption, soluble factors released from microglial cells direct the migration of SVZ NPCs and increase the proportion of new neurons in SVZ embryonic and adult cultures [97,285,286,287]. It is interesting to note that the effect of microglia in the promotion of neurogenesis is produced in the early postnatal period, when microglia display mainly an amoeboid morphology and reach their maximum levels before decreasing to adult numbers and adopting a resting ramified morphology [97]. Moreover, it has been shown that microglia are also involved in hippocampal neurogenesis through the phacocytosis of newborn cells that do not integrate the existing circuits and become apoptotic [288]. To our knowledge, it has not been confirmed whether GJIC is involved in this phenomenon.
2.2.2. Connexin Paracrine/Autocrine Functions in Brain
During recent years, the involvement of Cxs in brain physiology has been shown to not be limited to GJ formation. Indeed, Cxs can play important physiological (and pathological) roles through their HC activity, which is responsible for paracrine/autocrine communication. Interestingly, this Cx-mediated paracrine communication seems to be involved in communication pathways between the GJ compartments defined above.
Such physiological Cx-HC activity (opening) has been mostly described in the panglial syncytium and particularly among astrocytes. So far, at least two physiological phenomena have been described in wich Cx HCs are involved. One is in relation with the activity of the neuronal compartment (intercompartment paracrine communication) and the other takes place with microglia, which is not a GJ compartment, in the context of neuroinflammation.
Connexin Paracrine/Autocrine Communication and Neuronal Activity
Both decreased [Ca2+]e [52,53] and increased K+ may trigger the opening of astrocytic Cx43 HCs [54]. In the case of [Ca2+]e, a transient drop in the range of 0.4–0.6 mM is sufficient to induce such an opening. Once open, Cx43 HCs permit ATP release [289], which increases cytosolic Ca2+ in the same astrocytes (autocrine effect) or in the surrounding astrocytes (paracrine effect) through the activation of metabotropic purinergic P2Y1 receptors [290]. In parallel, this paracrine propagation is intensified among the astrocytic network by direct intercellular propagation of IP3 and Ca2+ waves passing across Cx30/Cx43 GJs [48,55]. Therefore, mobilization of intracellular Ca2+ stores represents the primary pathway by which astrocytes respond to neural activity. Finally, intercellular Ca2+ wave propagation progresses through intra- and extracellular signaling pathways, which mutually influence each other in synergistic ways. In turn, increased cytosolic Ca2+ is essential for the release of gliotransmitters. A consequence of the opening of Cx43 HCs is the extracellular release of glutamate, which activates postsynaptic neurons through NMDA channels. It seems that Cx43 modulates presynaptic glutamate vesicular pools by mechanisms which are not completely elucidated [56]. Astrocytic HCs are involved therefore in the modulation of neuronal activity, and more precisely of synaptic strength and plasticity [44].
Thus, such neuron/astrocyte pathways acting through Cx HCs have probably important physiological consequences on brain function, which are not completely analyzed yet. Some studies appear to reveal only the emerging part of the iceberg as, for instance, the release of gliotransmitters through astrocytic Cx43 HCs, which seems to be necessary for fear memory consolidation in the basolateral amygdala [291]. Thus, purinergic signaling molecules released by Cx HCs acting on neuronal and glial P2X and P2Y receptors comprise a fundamental component of synaptic plasticity [21]. On the pathological side, comparable decreases in [Ca2+]e, necessary to activate astrocytic HC pathway, have been noted during epileptic activity and also in ischemia [290,292].
Connexin Paracrine/Autocrine Communication and Microglia Activation
Cx HCs can be activated by IL-1β or TNF-α pro-inflammatory cytokines, which are secreted by microglia during the neuroinflammation process [50,293]. Neuroinflammation is a response of the brain to a pathological situation. This process is associated with reactive gliosis characterized by microglial activation [262] and astrocyte reactivity [294]. For example, the endotoxin LPS, which is present in the external membrane of Gram-negative bacteria, is recognized by TLR4 of microglia and activates the secretion of pro-inflammatory cytokines (IL-1β and TNF-α) that activate [295], in turn, astrocytic Cx43 HC [50,51]. The opening of astrocytic Cx43 HCs results in increased [Ca2+]i and extracellular glutamate concentrations associated with a decrease (50%) in excitatory synaptic transmission in the CA1 area of the hippocampus [51]. This illustrates that activated microglia may modify neuronal activity [51,87,90,296,297,298]. Microglia respond to inflammatory cytokines by upregulation of glutaminase and glutamate release due to HC activation [87], which induces downregulation of the astrocytic glutamate aspartate transporter, GLAST [298]. Therefore, microglial HCs alter extracellular glutamate concentration and astrocyte-dependent glutamate buffering in inflammation. Inflammation-induced dysregulation of GJs not only impacts glutamatergic neurotransmission [51,87,297,298], but also underlies disorders of excitation, such as epilepsy [145]. Therefore, activation of microglia may induce deleterious effects. This is shown in the case of Staphylococcus aureus infection, which leads to alteration in GJIC and HC activity of astrocytes [299], and in the case of AD, in which amyloid-β peptide activates microglial cells and leads to neuronal death through a mechanism involving astrocytic Cx43 HCs [92].
It would also be important to check carefully whether what is attributed to Cx HCs is not performed by Panx-made channels.
2.3. Possible Consequences of Connexin Dysfunction in Brain
As shown above, there is increased evidence indicating that Cxs are strongly involved in brain physiology either as GJs or as HCs. With the exception of microglia, GJIC defines three permanent functional compartments able to communicate between themselves via HC paracrine communication. Therefore, in the neuronal compartment, for instance, Cx36 GJs of the GABAergic interneurons allow for rapid and expansive inhibition via electrically coupled inhibitory syncytia that are calibrated to depolarizing activity [118,156,300]. More generally, several studies indicate that Cxs are critical to maintaining physiologic neuronal excitability, resistance to seizure, and may be central to hippocampus and amygdala based learning [144,156,291].
In the panglial syncytium, the presence of GJs supports physiological resting membrane potential through spatial buffering of K+ and constrains epileptiform bursting [46,63,144,145,146,147]. Astrocytes are central to the ability of the panglial syncytium to carry out this function and recent evidence illustrates that dysregulation of astrocyte Cx43 GJs may drive the pathogenesis of some human temporal lobe epilepsy. Without efficient GJs, astrocytes could not optimize the interstitial space for synaptic transmission by tight control of water and ionic homeostasis. Astrocytes are active participants in the tripartite synapse and modulate synaptic activity in hippocampus, cortex, and hypothalamus. Therefore, astrocytes include both supportive functions as well as active modulation of neuronal output activity [301]. It is clear that multitask functions of astrocytes are essential for higher brain function and Cxs play an essential role in all these functions. Lack of GJIC could lead to movement of water from the vasculature into the parenchyma across an intact BBB, leading to astrocyte swelling and decreased extracellular space. Lack of GJIC could also lead to unstable VM that would perturbate K+ uptake [174]. The astrocytic GJ network then appears to play an important role in both ionic and water regulation in the brain. On the other side, purinergic and glutamatergic gliotransmission, which can modify neuronal activity, relies heavily on autocrine and paracrine signaling by Cx HCs [21].
The fundamental roles of the other important cellular components of the panglial syncytium, the oligodendrocytes (K+ buffering and myelin integrity), are also a consequence of the presence of functional Cxs. For instance, mutations in oligodendrocyte-expressed Cx genes result in phenotypes indistinguishable from inherited hypomyelinating leukodystrophies, which are characterized by impairment of myelin sheath formation, inflammation, and sensorimotor neurological deficits [146,302,303]. A mutation in the Cx47-gene promoter results in Pelizaeus–Merzbacher-like disease [78], while altered Cx32 expression leads to symptomology closely resembling Charcot–Marie-Tooth disease [76]. Moreover, mice lacking Cx32 and Cx47 exhibit more pronounced myelin pathology, which is accompanied by loss of oligodendrocytes and action tremors progressing into tonic-clonic seizures and mortality by the 6th postnatal week [66,70]. Disruption of GJs between oligodendrocytes and astrocytes leads to myelin defect as shown in Cx47—KO mice in which myelin vacuolation is observed in the optic nerve [66].
Loss and activation of astrocytes, oligodendrocyte loss, myelin vacuolation, inflammation, and invasion of phagocytic cells are also common to GJIC disorders [74,146,303], suggesting a role for an intact panglial syncytium in supporting survival of its members and homeostatic functions. However, while non-channel properties of Cxs have been explored in the context of neuronal differentiation [246,251] and glutamatergic transmission [56], the mechanism whereby Cxs support glial survival remains poorly understood.
Since Cxs are implicated in various functional aspects of brain cells, it is not surprising that many cerebral pathologies (hereditary diseases, injury, inflammation, epilepsy, neurodegenerative pathologies, benign and malignant tumors) have been linked to GJIC and/or Cx dysfunctions. Knowing that Cx function is very sensitive to various stimuli, a fundamental question is whether chronic exposure to chemical pollutants may be involved in the observed increased incidence of some of these pathologies by altering Cx functions.
3. Inhibition of Gap-Junctional Intercellular Communication by Environmental Chemical Pollutants
GJ channels, and in particular those composed of Cx43, the predominant Cx in astrocytes, are emerging as major targets of an increasing number of environmental chemical pollutants. Among such pollutants are pesticides, bisphenol A (BPA), phthalates, polychlorinated biphenyls (PCBs), polybrominated biphenyls (PBBs), dicumylperoxide (DCP), perfluorodecanoic acid (PFDA), perfluorooctanoic acid (PFOA), perfluorooctane sulfonic acid (PFOS), 1-monolaurin, polycyclic aromatic hydrocarbons (PAH), 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin (TCDD), fine particulate matter with aerodynamic diameters less than 2.5 µm (PM2.5) and toxic heavy metals (Table 3). The capacity to inhibit GJIC of some of these chemical compounds, as well as others, was known for long and reviewed decades ago [304,305].

Table 3.
Effects of environmental chemical pollutants on gap-junctional intercellular communication of various mammalian cell types. Abbreviations: GJIC, gap-junctional intercellular communication; I, inhibition of GJIC; A, activation of GJIC. The names of the cell lines tested are indicated between parentheses.
Because of their lipophilic properties, some of these environmental chemical pollutants are able to cross the BBB and reach the CNS [306,307]. Although such pollutants are able to modulate GJIC, several underlying direct or indirect mechanism(s) have been proposed. For some of them, an insertion into the membrane lipidic bilayer, with resulting modification in the microenvironment of the membrane channels and a non-specific change in the membrane biophysical properties can be proposed [308]. Another potential mechanism of action is an inhibition of GJIC by physically blocking the GJ channel pore [309,310,311]. Changes in the Cx gene expression, endocytosis and degradation were also observed in response to several pollutants [312,313,314,315,316,317,318,319,320,321]. The last potential mechanisms of action are through non-classical estrogen receptors (ERs) [322,323] or through a toxic action on the aryl hydrocarbon receptor (AhR) [312]. Moreover, several intracellular signaling pathways were found to be involved in GJIC dysregulation including phosphatidylcholine-specific phospholipase C (PC-PLC) and the MAPK-kinase MEK [324,325,326,327,328,329,330].
3.1. Pesticides
Pesticides comprise substances from many different chemical groups with potent biological activity and a large number of different mechanisms of action. Pesticides include herbicides, which are the most common, insecticides, antiparasitics and fungicides. Many of them act as endocrine disruptors and are GJIC modulators.
3.1.1. Insecticides
Organochlorine Insecticides
DDT (1,1,1-trichloro-2,2-bis(4-chlorophenyl) ethane) is a persistent organochlorine chemical that can still be widely found in soils, in aquatic environments, in sediment and in various animal species decades after its use has been discontinued. DDT was the most used organochlorine insecticide in the world since the 1940s for agricultural purposes and for vector-borne disease control such as malaria and typhus, until its banishment in most countries, except in some developing countries in Africa and Asia. After application to soils, DDT may be lost through both volatilization and biodegradation. In the environment, dichlorodiphenyldichloroethylene (DDE) and dichlorodiphenyldichloroethane (DDD) are the major degradation products of DDT. Human exposure to DDT can occur by several routes, including ingestion of contaminated foods, the main source, skin absorption or through respiration and accidental contamination [352]. DDT and its derivatives are recognized as endocrine-disrupting chemicals [353]. Various studies have also shown that DDT and its metabolites (DDE and DDD) can inhibit GJIC in certain types of mammalian cell cultures (Table 3) [305,312,313,331]. In normal human breast epithelial cells, DDT seems to alter GJIC at the post-translational level. At non-cytotoxic concentrations of DTT that inhibit GJIC, a reduction in the number of GJ plaques and a reduction in the Cx43 phosphorylation level is observed. The same results are obtained after treatment with dieldrin, toxaphene, and PCB and PBB congeners. Moreover, there is no change in the steady-state levels of Cx43 mRNA after treatment with these chemicals. Interestingly, a “cocktail effect” is revealed in this study since specific mixtures of two of these chemicals showed that, while each alone do not inhibit GJIC at a given concentration, the mixtures do [313]. DDT inhibits GJIC between rat liver epithelial cells through a phosphatidylcholine-phospholipase C (PC-PLC) pathway independent of MEK [324].
Dieldrin is an organochlorine pesticide developed as an alternative to DDT and used extensively in the USA from the late 1940s until the 1970s, before being removed from the market in 1987. Unfortunately, dieldrin has a long environmental persistence and continues to be a common contaminant in soil. Dielbrin is an endocrine disruptor that displays only very weak binding to ER-α. Low dieldrin concentrations have the ability to inhibit GJIC in certain types of human and other mammalian cell cultures (Table 3) [305,332].
Chlordane (Octachloro-4, 7-methanohydroindane) was introduced in 1945 as the first chlorinated cyclodiene insecticide and became widely used as a soil insecticide against many agricultural pests and was also a widely used termiticide until it was banned in 1988 in the USA. Chlordane is persistent in soils and can volatilize. Chlordane, and other organochlorine pesticides like endosulfan, heptachlor and aldrin, markedly inhibit GJIC between cultured mammalian liver cells (Table 3) [305,333,334]. In the Chinese hamster cell line (V79), derived originally from lung tissue, chlordane does not inhibit GJIC as significantly as DDT or lindane [312].
Methoxychlor (1,1,1-Trichloro-2,2-bis(4-methoxyphenyl)ethane) is an organochlorine pesticide with known estrogenic activities in vitro and in vivo [354]. Methoxychlor inhibits GJIC in oviductal cells in monolayer culture after 1–5 h exposure to noncytotoxic concentrations of 16–64 µM [335]. Incubation of the rat liver epithelial cell line WB-F344 with 25 µM methoxychlor for 30 min almost causes complete inhibition of GJIC without affecting Cx43 phosphorylation status and its intracellular localization (Table 3). Moreover, this rapid inhibition of GJIC is mediated through MAPK ERK1/2, p38 and PC-PLC independently of ER or androgen receptor signaling [325].
Lindane (γ-hexachlorocyclohexane (γ-HCH)) was commercialized as an agricultural insecticide and is now largely banned due to its toxicity. In the environment, lindane was found in air, water, soil and dust, and the general population is also contaminated through diet [355]. This organochlorine has been reported to rapidly inhibit GJIC in different cell lines and under chronic exposure, induces a loss of GJ plaques and Cx43 endocytosis (Table 3) [312,314,315,316,317]. Moreover, the addition of lindane to open Cx43 HCs appears to have no effect on conductance [356]. It was further demonstrated that lindane regulates GJIC through a MEK1/2-dependent mechanism that is independent of PC-PLC [324].
Chlordecone (decachlorooctahydro- 1, 2, 4 -metheno-2H-cyclobuta (cd) pentalene-2 -one, kepone) is an estrogen-like organochlorinated pesticide with low agonist affinity for ER-α. Chlordecone is a known endocrine disruptor that is potentially carcinogenic and exhibits in vivo neurotoxicity, and perturbation of the serotonergic system. Following the chemical disaster in Hopewell, Virginia, in 1975, workers of the kepone manufacturer developed severe neurological disorders after exposure to high doses of the pesticide (motor disorders, mood, speech, immediate memory) [357]. In the French Antilles, it was intensively used from 1973 until 1993 as a substitute for lindane to control the banana root borer. Its persistence in the environment has resulted in a widespread contamination of Guadeloupean soils, water sources, animals and foodstuff leading to 95% of the population of Guadeloupe and 92% of that of Martinique being contaminated. Epidemiological studies showed that chronic low exposure to chlordecone is correlated with neurotoxicity and impaired neurobehavioral development (IQ point loss) in young children, characterized by a decrease in fine motor skills statistically significant only in boys [358,359,360]. However, there are no quantitative data for neurotoxic effects in adulthood in the French Antilles. Non-cytotoxic concentrations (4 µg/mL of kepone and 12 µg/mL of mirex for 3 days) of chlordecone and of its fully chlorinated structural analog, mirex (dodecachlorooctahydro1,3,4- metheno-2H-cyclobuta(cd)-pentalene), inhibit in vitro GJIC in Chinese hamster V79 cells, derived originally from lung tissue (Table 3) [337]. Moreover, in human embryonic palatal mesenchymal cells, chlordecone inhibits GJIC [338].
Toxaphene
Toxaphene is a complex mixture consisting primarily of chlorinated bornanes with lesser amounts of chlorinated camphenes, dihydrocamphenes, bornenes and bornadienes. Toxaphene was widely applied as an insecticide on cotton, soybeans, and corn, with more minor use as an acaricide. Although this compound is no longer produced commercially, it is still frequently found in the environment [361]. In normal human breast epithelial cells, toxaphene (10 µg/mL for 90 min) significantly inhibits GJIC (Table 3) and induces a reduction in the number of GJ plaques and in the Cx43 phosphorylation level [313].
3.1.2. Herbicides
Ioxynil (4-hydroxy-3,5-diiodobenzonitrile) is a benzonitrile herbicide that has been used actively worldwide to control weeds in agriculture since the 1970s. Studies addressing the fate of benzonitrile herbicides in the environment show that some metabolites of these herbicides are very persistent. It was reported to have endocrine disrupting properties [362]. In rat liver epithelial cell line IAR20, which express Cx43, phenolic and octanoic ioxynil are potent inhibitors of Cx43-GJs (Table 3) and induce degradation of Cx43 [318]. Another herbicide, alachlor, inhibits GJIC in WB-F344 rat liver epithelial cells in an MEK1/2 and PC-PLC-independent pathway [324].
3.1.3. Fungicides
The fungicide vinclozolin (3-(3,5-dichlorophenyl)-5-ethenyl-5-methyl-2,4-oxazolidinedione) has been documented for its environmental endocrine disrupting activity [363]. Incubation of rat liver epithelial cells with 250 µM vinclozolin for 30 min causes almost complete inhibition of GJIC (Table 3). Moreover, vinclozolin induces rapid hyperphosphorylation and internalization of Cx43. The rapid inhibition of GJIC by the fungicide is mediated through MAPK ERK1/2, p38 and PC-PLC independently of ER or androgen receptor signaling [325]. Another fungicide, pentachlorophenol (PCP), inhibits GJIC in those cells in a MEK1/2 and PC-PLC-independent pathway [324].
3.2. Bisphenol A
BPA [2,2-bis(4-hydroxy-phenyl)propane] is a ubiquitous synthetic estrogen without a steroid structure widely employed in a variety of consumer products in industrialized countries. Therefore, most people have been exposed almost continuously to sources of BPA through predominantly diet, inhalation, or dermal absorption. BPA has been found in the urine of almost all adults and children tested, in the saliva and serum of pregnant women, in foetuses, placenta, amniotic and follicular fluids and breast milk [322]. The unconjugated BPA level in human blood is 1.3–19.3 nM, and the human foetus exposure level is 4.4–13.2 nM [364]. Moreover, because of its lipophilic properties BPA has been demonstrated to undergo transfer to the CNS via the BBB [306]. While adult animals tolerate acute and high doses of BPA (e.g., >2–50 mg/kg b.w./day at multiple doses), harmful effects were further described in rodents exposed at a BPA concentration below the lowest observed adverse effect level also called “low-dose” (LOAEL, of 50 μg/kg/day for in vivo studies or 50 ng/mL) [310]. Then, in 2014, the European Food Safety Authority reduced this daily dose to 4 μg/kg, lower than that from dietary exposure. Although BPA has been found to have a lower affinity for classical nuclear ERs relative to 17-β estradiol (E2), it can also bind to non-nuclear ERs with estrogenic potency equal to E2 [365]. BPA can also act at low, environmentally relevant, doses, with estrogen-independent effects that can be transmitted by seven-transmembrane ER GPR30 or by estrogen-related receptor γ [322,323].
In several in vivo and in vitro systems, studies have demonstrated that BPA targets GJIC and interferes with Cxs expression, in particular Cx43 (Table 3). Western blot and immunofluorescence analyses showed that BPA exposure (45 µM, 24 h) reduced the level of Cx43 and delocalized it from the plasma membrane to the cytoplasmic compartment in SerW3 Sertoli cells [366]. An in vivo study showed a significant reduction in the expression of Cx43 in the testes of rats exposed neonatally to doses of BPA that were capable of impairing male fertility (0.6 to 10 µg/rat, or 100–1600 µg/kg body weight) [320]. When immature 20-days-old rats were treated by gavage for 5 or 6 consecutive days with a regimen that mimicked acute human short-term or accidental exposure to BPA (~2.5–4 μg/person/day) the integrity of the blood–testis barrier was reversibly perturbed. This observation was confirmed using primary Sertoli cells cultured in vitro and the disruption of Sertoli cell tight junction barrier by BPA was associated with a decline in the level of Cx43 [367]. In mouse immature oocytes, the entry of external Ca2+, probably through GJs, contributes to spontaneous Ca2+ oscillations, which are a marker of competence for fertilization. E2 and BPA at a 10,000-fold higher concentration (100 µM for a short-term exposure of 1 h) exert similar inhibitory action on spontaneous Ca2+ oscillations through unresolved mechanisms that could involve GJs [368]. The in vitro short-term exposure of rat cumulus cell-oocyte complexes (COCs) to BPA (20 ng/mL for 25 h) results in an overall increase in GJ transfer rate, which is coincident with higher Cx43 mRNA, and not Cx43 protein levels suggesting that BPA may cause a change in the recruitment of Cx43 into lipid rafts [339]. In contrast, in another study, treatment of COCs isolated from mouse preovulatory follicles with BPA (2.2 and 2200 nM BPA for 2 h) decreases GJIC in the COCs and do not modify GJIC gene (Cx43 and Cx37) and protein (Cx43) expression. These contradictory results may be explained by the different developmental stages of the follicles [340].
In the rat epithelium-derived BICRM1Rk cell line, which expresses Cx43, BPA inhibits GJIC in an irreversible and dose-dependent manner (from 0.1 to 3.2 µM, for 1 h incubation). Moreover, BPA inhibits GJIC through a modulation of the gating of GJ channels and not through a modulation of Cx43 gene expression [322]. An electrophysiological study showed that BPA reversibly inhibits (10 nM, up to 18% of control) the outward currents of Cx46 HCs expressed in Xenopus oocytes and it was proposed that the endocrine disruptor occupies pore regions of the HC and thus retards current flows [309]. BPA (0.01–1 µM for 48 h) significantly inhibits GJIC between human skin HaCaT cells and significantly downregulates Cx26 mRNA expression (0.1 µM BPA) partially through the ER pathway. In contrast, BPA does not influence Cx43 mRNA, Cx26 and Cx43 protein expression levels and Cx locations in HaCaT cells [319]. Interestingly, an analysis of the Cx43 structure showed the presence of a docking ‘pocket’ in Cx43-based connexon for estradiol-17b and BPA. These interactions with channel putative docking domains can possibly lead to the blockage of Cx43-based GJIC [310,311].
3.3. Phthalates
Phthalates are dialkyl or alkyl aryl ester derivatives of phthalic acid (1,2-benzenedicarboxylic acid). They are nonsteroidal endocrine-disrupting chemicals that have estrogenic activity and thus deleterious effects on hormonal balance [369]. They are used in a variety of products including cosmetic products and lotions, aerosol delivery agents, plasticizers and adhesives, flooring and medical tubing [370]. Humans can be exposed to phthalates through dermal absorption, ingestion, or inhalation of contaminated products [371]. Phthalates are lipid soluble, have less than 24 h half-lives, and do not accumulate appreciably in the body [372]. Phthalate metabolite levels can be measured in the urine, blood, breast milk, and meconium. A series of branched chain phthalate monoesters that contained an ethylalkyl moiety (8 h treatment at sublethal concentrations) inhibit GJIC between male B6C3F1 mouse hepatocytes whereas straight chain phthalates have no effect (Table 3) [341]. Di-isononyl phthalate (DINP) and related C7-C11 dialkyl phthalates were shown to inhibit GJIC in the liver of F344 male rats or B6C3F1 male mice [343]. A further study using 8 phthalate monoesters [mono-2-ethylhexyl phthalate (MEHP), mono-n-octyl phthalate (MNOP), mono-isononyl phthalate (MINP, 3 types, -1, -2, and -3), mono-isoheptyl phthalate (MIHP), mono-isodecyl phthalate (MIDP), and mono-(heptyl, nonyl, undecyl) phthalate (M711P)] provides evidence that the effects of phthalate esters on GJIC are species specific and may not be relevant for human beings. All eight monoesters significantly reduce GJIC in cultures of primary hepatocytes from rat and mouse but not from hamster, cynomolgus macaque and human [344]. A recent work shows that among 20 different phthalates, those with a medium-length side chain (3–6C) were the most potent inhibitors of GJIC and activators of MAPK1/2 in rat liver WB-F344 cells [326].
3.4. Others
Polychlorinated biphenyls (PCBs) and polybrominated biphenyls (PBBs) are industrial chemical mixtures widely used commercially as hydraulic fluids, plasticizers, adhesives, heat transfer fluids, wax extenders, dedusting agents, organic dilutents/extenders, lubricants and as dielectric fluids in capacitors and transformers (PCB), and as a flame retardant (PCB and PBB). These compounds are believed to be endocrine-disrupting chemicals in humans and animals. PCB and PBB are persistent organic pollutants that have been widely detected in the environment including air, water, fish, wildlife, and also in human tissues [373]. The nonplanar PCB 153 (2, 2, 4, 4, 5, 5-hexachlorobiphenyl at 100 µM for 30 min exposure) completely inhibits GJIC in WB-F344 rat liver epithelial stem-like cells (Table 3) through the PC-PLC or sphingomyelinase and Src signaling pathways [327]. PCB 153 has no effect on Cx43 mRNA levels but induces Cx43 hyperphosphorylation, internalization of GJ plaques and degradation in rat liver epithelial cells [321]. PCB 153 inhibits GJIC through PC-PLC independent of Mek [324]. It was further demonstrated that PCB153 regulates GJIC and Cx43 expression in WB-F344 cells by SphK1/S1P axis [sphingosine kinase (SphK), the enzyme responsible for S1P (the bioactive sphingolipid (SL) sphingosine 1-phosphate (S1P) synthesis] [328]. Moreover, various derivatives of hydroxylated PCB inhibit GJIC in WB-F344 rat epithelial cells and have estrogenic activity [344]. PBB (7.5 µg/for 48 h) and several of its congeners significantly inhibit GJIC in V79 Chinese hamster lung cells in vitro [345,346]. As reported previously, at the non-cytotoxic concentrations of PCB and PBB congeners that inhibit GJIC, a reduction in the number of GJ plaques and in the Cx43 phosphorylation level were observed [313]. DCP (used in the manufacture of polymers and elastomers) and PFDA (used in the manufacture of Teflon and Gore-Tex) inhibit GJIC through PC-PLC independent of Mek whereas PFOA (used as surfactant and for the manufacture of polymers) requires both MEK1/2 and PC-PLC. In contrast, PFOS (a surfactant used to coat a wide range of consumer goods, specifically those designed to be waterproof) and 1-monolaurin (used as surfactant in cosmetics and as food additive) require neither MEK1/2 nor PC-PLC [324]. Several PAHs, a broad class of ubiquitous environmental pollutants, transiently inhibit in vitro GJIC between WB-F344 rat liver epithelial cells in the µM range concentrations [347]. Polycyclic aromatic hydrocarbons (PAHs) inhibit GJIC (Table 3) through PC-PLC independent of Mek [324]. Among them, 1-methylanthracene, a major component of the PAH fraction of cigarette smoke, inhibits GJIC in WB-F344 rat liver epithelial cells through a PC-PLC-dependent mechanism, and releases arachidonic acid [329]. TCDD, a dioxin released into the environment in the Seveso disaster, regulates the activity of GJIC in several cells (Table 3) [330,348,374]. TCDD decreases GJIC by phosphorylating Cx43 via PKC α signaling pathway in MCF-7 human breast cancer cells and by affecting the localization of Cx43 in human mammary epithelial cells (HMEC) [330]. AhR is a ligand-activated transcription factor activated by both endogenous and exogenous ligands including PCBs and PAHs. In epithelial WB-F344 cells, TCDD downregulates GJIC (Table 3), significantly decreases the amount of GJ plaques and reduces Cx43 protein levels, possibly via enhanced proteasomal degradation, in an AhR dependent manner [348]. Fine particles (PM2.5) refer to the airborne particles with aerodynamic diameters less than 2.5 µm, whose impact on public health has become of great concern in industrial countries [375]. PM2.5 collected during the dust and non-dust periods significantly inhibits in a dose-dependent manner GJIC between human lung fibroblasts (Table 3) [349]. In an RNA microarray assay using endothelial cells, PM2.5 induced a dysregulation in transcripts involved in GJ signaling pathways [376]. So far, no effect of airborne ultrafine particles (PM0.1; 1–100 nm diameter) has been reported on GJIC. However, the fact that such particles induce neurodevelopmental disorders in mice suggest that studies examining possible effects of ultrafine particles on GJIC in the brain context should be done [377]. A number of toxic metals, which are major environmental pollutants (e.g., mercury, lead, arsenic, cadmium and aluminum) have been shown to interfere with GJs. It was proposed that GJIC inhibition (Table 3) is a mechanism of toxicity of these metals involved in the onset of many diseases [350,351,378].
4. Pathologies
A dramatic example of developmental neurotoxicity due to pollution was the severe methylmercury poisoning which occurred in Minamata and neighboring communities in Japan during the 1950s and 1960s. Afterwards, a considerable number of children were born with conditions resembling cerebral palsy, later known as congenital Minamata disease [379]. This was an acute example showing that environmental pollution can affect CNS development. From then, the prevalence of increasing brain pathologies over the last decade might also be the consequence, at least partly, of environmental pollution (Table 4). Recent evidence suggest air pollution as a source of neurodevelopmental toxicants [380,381]. Interestingly, it seems that these effects may depend on the type of exposure. This is what tends to be revealed in a PubMed search, which found associations between short-term air pollution exposure with AD, epilepsy, and migraine, while long-term air pollution exposure was associated with AD, whereas evidence on the link between air pollution and PD was inconsistent [382]. Despite conflicting results, children exposed to air pollutants (i.e., ambient or traffic-related air pollution, PAHs, and black carbon) during prenatal and postnatal periods seem to be at greater risk of cognitive deficits, behavioral disorders, and psychiatric disorders [383,384,385,386,387,388,389,390,391]. Children are indeed particularly vulnerable to exposure, prenatally and in early life when organ systems, including the brain, are under development. Therefore, for example, ASD now affects up to 1 in 45 children in the USA [392]. A total of 11% of children are affected by ADHD, another neurodevelopmental pathology, and a decline in child intelligence quotient was observed amongst Western nations [393]. PM2.5 appears to be an especially harmful pollutant because it enters the circulation and reaches the brain directly via cerebral blood supply. Prenatal exposure to PM2.5 has been associated with reductions in brain white matter volume, cognitive impairments, and increases in ADHD, migraine and MDD [394,395,396]. Moreover, air pollution exposure in urban residency was associated with adolescent psychotic experiences [397]. Acute and subclinical pesticide poisoning that occur during childhood might lead to lasting neurobehavioural deficits [398]. Environmental exposures during adult life can also increase the risk of neuropathies. This is particularly the case for occupation-related exposures as farmers or even workers exposed to welding fumes (WFs), which comprise a complex mixture of metallic oxides, silicates, and fluorides, resulting in different health effects. Inhalation of WFs in large quantities over long periods may pose a risk of developing neurodegenerative diseases (PD, AD, and epilepsy) by dysregulating gene expression [399].

Table 4.
Major brain disorders. Putative environmental causes and connexin characteristics/involvements. References are indicated between brackets.
Emerging evidence suggested that alterations in normal astrocyte function contributes to the etiology and progression of varied neurological disorders including migraine with aura, neurodegenerative disorders, schizophrenia, epilepsy, and ASD. Therefore, targeting astrocyte dysfunction may lead to new therapeutic strategies for these disorders [450,451]. On the other hand, GJs and in particular Cx43 GJs, the predominant Cx in astrocytes, have emerged as a target for many environmental pollutants. One open question is whether pollutants by acting on Cx43 GJs in the astroglial network, or other Cxs of other brain GJ compartments, can induce cerebral neuropathies and cancer.
4.1. Neurodevelopmental Disorders
4.1.1. Autism Spectrum Disorders
ASD is a neurodevelopmental disorder characterized by deficient social interaction, impaired communication as well as repetitive behaviors. ASD subjects present connectivity and neuroplasticity disturbances associated with morphological alterations in axons, dendrites, and dendritic spines. The etiology of ASD is believed to be multifactorial and to involve genetic, epigenetic, and environmental components [398]. Environmental chemical exposures are increasingly understood to be important in causing neurotoxicity in foetuses and newborns [452,453]. Studies using conditional Cx43 KO mice show that Cx43 is important for neurodevelopment [135]. However, few data are available about the precise involvement of GJs and HCs in ASD (Table 4) [406,454].
Neuroanatomical studies have revealed extensive structural abnormalities in subjects with autism as well as evidence of neuroinflammation. In post-mortem brain tissues, Cx43 protein expression (western blot) was increased significantly in superior frontal cortex (Broadmann’s area 9) [406], an area where structural abnormalities have been revealed in subjects with autism [455]. Dysfunction in this region may be responsible for deficiencies in cognition, language, and social function [456]. Even if non-significant (54%), increased Cx43 was also observed in the parietal brain region (Broadmann’s area 40) [406]. This increase with those of other astrocytic markers (aquaporin 4 and GFAP) may potentially contribute to dysfunctions observed in subjects with autism such as language and visuospatial integration [457,458]. Such increase in Cx43 expression in the brain may significantly increase glial-neuronal signaling, which is potentially responsible for GJIC enhancement in frontal lobe, an area for executive function. This may help to explain defects in sensory processing observed in subjects with autism [459,460].
It has been said that there is a strong correlation of general gastrointestinal disturbances with autism severity [461]. Therefore, it was hypothesized that loss of Cx43 expression in enteric glial cells contributes to brain inflammation in ASD by inducing disturbances in the gut-brain axis [462]. In another respect, acupuncture intervention of «Changqiang» (GV 1) improves learning-memory ability in autism rats, which may be related to its effects in up regulating expression levels as determined through immunostaining of Cx43, Cx32 and Cx36 in the frontal cortex [463].
It was suggested that perinatal mastocyte activation by infectious, stress-related, environmental, or allergic triggers, with a subsequent release of pro-inflammatory and neurotoxic molecules, can contribute to brain inflammation in ASD pathophysiology. Exposure to aberrant environmental conditions and to chemical contaminants may be a potential risk factor for ASD and is the subject of considerable current epidemiological studies and speculation [400,401,402,403]. Thus, autism susceptibility genes are selectively targeted by numerous environmental chemical pollutants (BPA, pesticides, heavy metals, PM, phthalates, etc.) and by many compounds used in food, cosmetics, or household products [404].
4.1.2. Attention Deficit Hyperactivity Disorders
According to the National Institute of Mental Health, ADHD is a disorder marked by an ongoing pattern of inattention and/or hyperactivity-impulsivity that interferes with functioning or development. Frequently, patients have difficulty sustaining focus and are disorganized (inattention), move constantly, including in situations in which it is not appropriate (hyperactivity). Some people with ADHD only have problems with one of the behaviors, while others have both inattention and hyperactivity-impulsivity. Most children have the combined type of ADHD. However, in preschool, the most common ADHD symptom is hyperactivity. All these symptoms interfere with or reduce the quality of how they function socially. ADHD symptoms can appear as early as between the ages of 3 and 6 and can continue through adolescence and adulthood. ADHD symptoms can change over time as a person ages. In young children with ADHD, hyperactivity-impulsivity is the most predominant symptom. As a child reaches elementary school, the symptom of inattention may become more prominent and cause the child to struggle academically. In adolescence, hyperactivity seems to lessen but inattention, restlessness, and impulsivity tend to persist into adulthood. Factors contributing to ADHD are not well known (Table 4) but various exposures during pregnancy have been mentioned (cigarette smoking, environmental toxins, alcohol, or drug use), or exposure to high levels of lead at a young age [407,408,464]. Several epidemiological studies have demonstrated the linkage between exposure to high levels of air pollution and ADHD [409,410,411]. This assumption was even confirmed recently in childhood [412]. Additional studies also reported a particular role of exposure to the agricultural and combustion pollutant, nitrous oxide (N2O), which might be a primary environmental trigger in the onset of not only ADHD but also of other neurodevelopmental disorders, such as ASD. On this particular aspect of agricultural pollutants, glyphosate could also be a factor responsible for ADHD [413].
Genetic causes have also been suggested for ADHD, which seem to implicate candidate genes involved in processes like neuronal migration, cell adhesion and proliferation [414]. One of the possible genetic causes is 1q21.1 recurrent microdeletions linked to developmental delay, with 10–25% of carriers being ADHD patients. Among other brain-related symptoms, intellectual disability was observed in 25–50% of cases, mild-moderate developmental delay (speech and motor delays) in 50–75% of cases and autism less than 10% [465,466,467]. This chromosomal portion carries several genes (PRKAB2, CHD1L, BCL9) along with two Cx genes (GJA5 and GJA8) encoding Cx40 and Cx50. Those Cxs are known to be involved in eye disorders (cataracts, microophtalmia, glaucoma, …) for Cx50 and cardiac defects (atrial fibrillation and structural defects) for Cx40 but not in brain disorders so far (Table 4) [468,469,470,471,472,473,474].
4.1.3. Epilepsy
Epilepsy is a neurological disorder affecting anyone, at any age (even if it is the most common neurological disease in children), and in which abnormal brain activity causes seizures (periods) of unusual behavior, sensations, and sometimes loss of awareness. Seizure symptoms vary widely from staring blankly for a few seconds to violent shakings and twitching of the body. Recently, seizures have been divided into focal, generalized, unknown onset, with subcategories of motor, non-motor, with retained or impaired awareness for focal seizures [475]. Those symptoms are the consequence of abnormal excessive or synchronous neuronal activity in the brain. Recurrent seizures can result in hippocampal damage and seriously impair learning and memory functions in children. In a rat model, this phenomenon was related to neuronal apoptosis [476]. In the past years, several studies showed that epilepsy could be associated with either short-term or long-term chemical environmental exposures occurring during pregnancy or any time after birth (Table 4).
If old studies reported that environmental chemical odors was associated with increased rates of particular forms of epilepsy [477], it became evident, more recently, that air pollution (gaseous and fine particulate air pollution) can be a risk factor for epilepsy as shown in various urban areas in Chile [415] or in China. In the latter country, severe air pollution (PM10) episodes were clearly associated with increased epilepsy rates [416,417] even, if curiously, cumulative exposure with NO2 and CO appeared protective [417]. However, another study, performed in the city of Xi’an, showed that NO2 and SO2 were positively associated with epilepsy [418]. In support of such observations, a growing number of studies have shown that exposures to air pollutants can activate cellular mechanisms involved in epilepsy [478].
Such correlations have been also observed in cases of chemical poisoning and intoxication. For instance, in Japan, domoic acid of diatoms poisoned people after consumption of contaminated mussels; some individuals had seizures such as temporal lobe epilepsy a year after the event [479]. Reports indicate that intoxication of humans with organotin compounds (Tributyltin, TBT) could also be associated with neurological symptoms such as epilepsy and amnesia, perhaps by affecting synaptogenesis and neuronal survival, particularly in early development [480]. Pollutants such as perfluorooctane sulfonate (PFOS) induce tonic convulsion in rodents when ultrasonic stimulus is applied [481,482]. In humans, developmental PCB exposure is known to increase the susceptibility to audiogenic seizures in adulthood [419,420]. The risk of epilepsy and certain types of cancer (cervical cancer) may be increased among adults who were exposed to perchloroethylene-contaminated drinking water during gestation and early childhood [421].
Apparently, lack of GJIC between astrocytes seems to be relevant to epileptogenesis (Table 4) [145,423] even if an increased presence of Cx43 has been observed in hippocampus of epileptic patients [424]. A consequence of such a situation might be the reduction of spatial buffering of coupled astrocytes leaving neurons more susceptible to microenvironmental changes, resulting in spontaneous epileptiform activity and reduced threshold for evoking seizure activity [47,48]. The inhibition of astrocyte coupling during the early phase of epileptogenesis is not caused by reduced amounts of Cxs, but rather, associated with changes in the phosphorylation status of Cx43.
4.2. Neurodegenerative Disorders
Causes of both PD and AD have not been elucidated and several endogenous (genetic) and exogenous (environment) factors contribute to the onset and/or development of these illnesses (Table 1).
4.2.1. Parkinson’s Disease
PD is a chronic and progressive disabling neurodegenerative disorder characterized by the selective loss of dopaminergic neurons of the substantia nigra pars compacta, resulting in marked impairment of motor control, and the presence of cytoplasmic protein aggregates, known as Lewy bodies, in dopaminergic nerve cells. Few studies have linked GJs and Cxs to PD pathophysiology (for review: [483]). Cx43 upregulation has been identified in the striatum of rodent models of PD and in cultured astrocytes stimulated with rotenone [484]. Moreover, gastrodin, a constituent of a Chinese herbal medicine, ameliorates PD by downregulating astrocytic Cx43 [485]. α-synuclein, a component of Lewy bodies, enhances the opening of Cx43 HCs and Panx1 channels in mouse cortical astrocytes which might constitute a novel mechanism involved in the pathogenesis and progression of different α-synucleinopathies including PD [486].
The current view regarding PD etiology is that a genetic basis interacts with environmental factors, causing the disease (Table 1 and Table 4). A systematic review and meta-analysis up to 2018 found weak evidence for an association between air pollution, mostly originating from traffic, and PD [433]. Several studies have also suggested a relationship between BPA exposure and PD pathophysiology [435]. Similarly, epidemiological and experimental studies have emphasized the association between neurodevelopmental and neurodegenerative disorders and pre- and post-natal and chronic exposure to the pesticides [401]. Due to occupational exposure to pesticides, farmers and chemical workers are at increased risk of PD occurrence (long-term brain damage, cognitive dysfunction and some serious behavioral and neurodegenerative disorders). The exposure to neurotoxic metals (such as Pb, Hg, Al, Cd, Mn and As), to PCBs, and solvents have also been implicated in PD etiology [434,436]. Glutamate excitotoxicity is considered to be involved in neurodegenerative diseases such as PD and AD. A recent study suggested that Cx43 GJs are involved in glutamate excitotoxicity induced by Mn exposure. Elevated Mn exposure increases astrocyte apoptosis and significantly disruptes glutamate homeostasis, accompanied by GJIC inhibition and decreased expression of Cx43 [438].
4.2.2. Alzheimer’s Disease
AD is the leading cause of dementia in USA and afflicted more than 5.7 million Americans in 2018 [487]. It is an age-related dementia whose progressive histopathological hallmarks are amyloid-β (Aβ) deposition, gliosis, neuronal death, and synaptic loss in brain areas like cortical and hippocampal regions (Table 1) [488]. The progression of the disease leads to loss of memory and language ability, as well as increased anxiety. Epidemiological reports also indicate that obesity and type 2 diabetes are important risk factors but pollution could also be involved [439,440,489]. Stroke history may also increase risk of developing AD, as it was observed in rats in which stroke is induced by striatal injection of endothelin-1 [490]. Deposition of Aβ (mainly Aβ 40 and 42) aggregates up to forming amyloid plaques associated with a reactive gliosis [488]. In those areas, activated microglia are recruited and reactive astrocytes exhibit a morphofunctional remodeling modifying their interactions with neurons [491]. Interestingly, this sequence of cellular events has been associated precisely with Cx43 expression and/or function in post-mortem brains of AD patients [492,493,494], and by the use of various models such as genetically-modified mice [441,495,496,497] or chemically-treated rats [498].
The early step of activated microglia leading to neuroinflammation seems to first involve mast cells (MCs). Mainly located in meningeal layers and in cerebral parenchyma lying between the BBB and astrocyte endfeet [499], these cells migrate towards Aβ deposits [500] even before plaque formation [496]. Aβ peptides appear to activate Cx43 HCs (and also Panx1) of MCs which mediates Ca2+ influx required for releasing proinflammatory mediators (histamine, ATP, cytokines) and recruiting other cells to the neuroinflammatory response [442,501,502]. Therefore, MCs by acting as early sensors of amyloid peptide would play a critical role in the onset of AD [442,503]. Then, the presence of activated microglia is a source of proinflammatory cytokines known to inhibit GJIC but activate Cx43 HCs in astrocytes [50,51,92,299,441]. Such activation leads to ATP and glutamate release with neurotoxic effects [443,444,503]. However, it has been shown that Aβ oligomers also contribute to Cx43 HC activation by dysregulating Ca2+ homeostasis in astrocytes, especially through increased expression of key components of Ca2+ signaling (mGluR5 receptors and IP3R1) [504,505]. [Ca2+]i increase induces Cx43 HC activity which also provide a pathway for Ca2+ entry in astrocytes. Therefore, a vicious cycle may take place in astrocytes in which high [Ca2+]i triggers Cx43 HC openings that allow Ca2+ entry contributing to maintain elevated [Ca2+]i. One consequence of this process is a chronic Cx43 HC activation leading to release of glutamate and ATP [441,506,507,508]. Such release even amplifies this circle by stimulating in an autocrine manner purinergic and glutamatergic metabotropic receptors that contribute also to maintain high [Ca2+]i in astrocytes [509]. Release of gliotransmitters (ATP/glutamate) through Cx43 HCs results in opening of Cx36 HCs and Panx1 channels in neurons [92,496]. Consequent neuronal Ca2+ overload leads to numerous deleterious consequences including structural neuritic alterations and increased oxidative stress [510]. This stress triggers nearby amyloid plaques and propagates into the cell body leading to caspase-dependent death in some vulnerable cortical neurons [511]. In in vitro assays, the acute application of amyloid-β25–35 (Aβ25–35) increases the activity of Cx36 along with Panx1 channels leading to potential neuronal death [92].
It is interesting to note that monomeric Aβ42 (mAβ) can stimulate the release of the major antioxidant glutathione (GSH) from cultured astrocytes through expression of the ABCC1 transporter. The fact that aggregated Aβ (not mAβ) reduces induction of ABCC1 expression supports the hypothesis that in the early stage of AD, less aggregated Aβ increases GSH release from astrocytes (via ABCC1 transporters and Cx43 HCs) providing temporary protection from oxidative stress that promotes AD development [512]. In line with this observation, several studies indicate that Cx43 is first neuroprotective [441,513,514,515,516]. Therefore, the upregulation of Cx43, which is observed in AD brains, may start as a neuroprotective response to amyloid plaques and explain clinical studies showing increased neuronal activity in the hippocampus and cortex in non-demented individuals at high risk for AD or at presymptomatic stage of familial AD [517,518].
Recent transcriptomic analysis made from post-mortem brain samples showed Cx43 as a major regulator of the expression of AD risk factor genes [519]. From these studies, it appears that Cx43 may even contribute to opposing neuroprotective and neurotoxic phenotypes as shown in previous studies [441,497,513,514,515,516,520]. Indeed, apparently, Cx43 initially supports neuronal functions by elevating gene expression signatures for the glial neurosupportive functions. This is in agreement with enhanced neuronal activity, which is found at the early stage of AD [517,518,521]. However, chronic Cx43 gain of function could lead to detrimental effects on neurons [522,523,524,525]. The molecular mechanisms permitting Cx43 to control gene expression are not known yet but could be monitored through interacting proteins such as drebrin, for instance, whose expression is decreased in brain of AD patients [526].
Taken together, these studies demonstrate that Cx43 HCs are involved, in different cell types, during all progressive steps of the disease. Therefore, strategies that target astroglial Cx43 HC activity seem to be an interesting option as suggested by Cx43 deficiency or pharmacological blockade in mouse models of AD [441,497,527]. Supporting such a hypothesis, the well-known Cx inhibitor, carbenoxolone, is able to reverse Aβ-induced alterations in rat brains [528] but it lacks specificity since it is a general blocker of HC activity but also of GJIC, independent of the Cx/Panx type. More specific blockers of Cx43 HCs are emerging, such as boldine, an alkaloid from the boldo tree, which specifically inhibits HC activity in astrocytes and microglia and decreases neuronal damage in mice model of AD [497]. Synthetic and endogenous cannabinoids (WIN, 2-AG, methanandamide) also reduce the opening of astrocytic Cx43 HCs, abolish Aβ-induced release of glutamate and ATP, and reduce neuronal damage in hippocampal slices exposed to Aβ [529]. Of particular interest, all types of Cx43 blockade (pharmacological or by gene depletion) were also able to diminish AD-related gene expression which is controlled by Cx43 [519].
4.3. Neurobehavioral Disorders
4.3.1. Migraines
A recent study contributed to the limited available evidence, showing that short-term exposure to five air pollutants from traffic combustion sources, including PM2.5, may trigger migraine (Table 4). This association is especially pronounced on high temperature days [425]. Cortical Spreading Depression (CSD), a transient disturbance in electroencephalographic activity characterized by a slow wave of neuronal and glial depolarization that self-propagates across the brain cortex or other brain areas, was suggested to trigger migraine aura and to be responsible for activation of the trigeminovascular system and possibly migraine headache [530]. GJ-mediated propagation of Ca2+ waves and Cx-HCs in astrocytes have been proposed to be involved in CSD propagation. Thus, GJ and HC blockade would represent a possible therapeutic strategy [426,531,532].
4.3.2. Major Depressive Disorders
Despite progress in understanding the etiology and pathophysiology of depression, no established mechanism can currently explain all facets of the disease and many questions remain unresolved. The etiology of depression seems to be determined by multiple influences, including genetic, social, and environmental (Table 1) [394]. However, it is interesting to note that studies using animal models of depression as well as post-mortem analysis of brains of patients with MDD suggest GJIC dysfunction by astrocytic GJ and/or Cx43 HCs while antidepressants would correct these molecular abnormalities. GJs containing Cx43 decrease the propagation of spreading depression, which is a pathological event leading to neuronal inactivation, by facilitation of uptake of glutamate and K+ [57]. These data suggest a link between the expression/function of Cx43 and depressive disorders, thus paving the way for a better understanding of its physiopathology and new therapeutic targets (Table 4) [432,533]. Some environmental pollutants (BPA, phthalates, heavy metals, pesticides, airborne pollutants emitted from vehicles, industries, etc.) are endocrine disrupting substances, which may increase the risk of depression, especially among susceptible individuals. Several studies suggest that prolonged exposure to BPA would have neurotoxic effects and would impact human brain development. Such exposures would then contribute to an increased prevalence of behavioral disorders and neuropathies (Table 4). A relation between prenatal and childhood BPA exposure and depressive symptoms among children, with a more pronounced effects among boys, was proposed in several studies [427,430]. In rodent models, offspring gestationally and/or lactationally exposed to BPA have also been reported to exhibit comparable behavioral deficits [534]. As adults, F1 male offspring of mouse dams exposed to BPA doses representative of human exposure levels, from preconception until lactation, exhibit increased depressive-like behavior [535]. Interestingly, developmental exposure of mice to BPA is associated with impaired synaptogenesis and spinogenesis, two estrogen-mediated processes that can persist into adulthood [536]. Recent epidemiological studies show associations between urinary phthalate levels and depressive symptoms among adult and elderly populations [395,431]. Some epidemiological studies also show an association between urinary heavy metals (Mn, Sn), PAHs, and depressive symptoms in adult population [431]. Exposure to heavy metals can occur through, for example, diet, traffic, and industrial emissions, including dust in industrial and urban areas. Other studies have suggested an association between Cd, Pb, Hg exposure and increased risk of depression [394]. Moreover, an increasing number of studies suggests an association between prenatal and adult pesticide exposure and risk of depression [394,401]. A positive association between the exposure to air pollution and an increase in the risk of depression was also demonstrated [394]. Among these studies, a recent exploratory analyse conducted on 284 London-based children concludes that children who grow up in a highly polluted environment are three to four times more likely to develop MDD by the age of 18. Such an observation suggests that PM2.5 enters the blood circulation, crosses the BBB, and induce brain inflammation, which is linked to depression [396].
4.4. Glioma
The most common brain tumours are gliomas in which the Cx situation is particularly complex (Table 4). Apparently, Cx expression globally decreases during the progression of these tumors. This is the case of Cx43, which has been the most studied Cx due to its high expression in astrocytes from which gliomas are thought to derive [537]. Experimentally, it has been shown that Cx43 expression is inversely correlated to cell proliferation [538,539] probably through its interaction with src [540]. However, glioma cells still expressing Cx43 have been shown to be more invasive either by interacting with surrounding astrocytes [541,542], establishing GJIC with other glioma cells [543] or through its C-terminal able to interact with actin cytoskeleton [544,545,546]. Therefore, Cx43 expression would be involved in glioma invasion. Nevertheless, the situation is even more complicated by the cellular heterogeneity of gliomas during their progression in which cancer stem cells were shown to express Cx46, which is also involved in the aggressive phenotype of those tumorigenic cells [547]. Globally, the Cx involvement in glioma is complex and is not simply a consequence of lack of expression of Cx43 [548]. The expression of Cx changes during progression and, according to the cancer cell type (stem or not), has different effects on proliferation and/or invasion [549]. At this time, it is not known why and how these regulations of Cx expression do occur during glioma progression.
The incidence of CNS tumor is increased in some European countries. Several environmental exposures have been investigated as potential risk factors, but for most, scientific evidence is still lacking. The role of pesticides in CNS tumor risk, especially gliomas and meningiomas, was first investigated by studies of mortality rates in farmers in the USA or Europe but results do not all converge [445]. Previous reviews have concluded that there was a weak-positive association between brain cancers and farming [446]. In 2013, a meta-analysis supports an association between parental occupational exposure to pesticides or residential exposures to pesticides and childhood brain tumors [447,448]. However, a meta-analysis in 2015 indicated that pesticide exposure and the risk of adult glioma have no association [550]. Interestingly, a study shows that endocrine disrupters like endosulfan (an organochlorine insecticide) or 2, 4, 5 hexachlorobiphenyl (a PCB) inhibit GJIC and Cx43 expression in a dose-dependent manner in neuronal stem cells (Table 4). Knowing that those cells are involved in tissue homeostasis, such a disruption may act as a starting point of carcinogenesis [449].
5. Discussion
This review attempts to establish a link between the higher incidence of brain pathologies [1] and dysfunctions with the chronic exposure to pollutants. We hypothesize that the common link would be Cx function (GJIC and HC) which is altered both in brain disorders (Table 4) and by a wide range of pollutants (Table 3).
During the last decades, the brain has become a main focus for research motivated by the prevalence of a large emerging spectrum of brain pathologies [1]. These brain disorders are quite heterogeneous in their symptoms and the age of the affected patients. Some of them, affecting children, are probably the consequence of cerebral developmental defects while others, because of degenerative processes, affect elder people. Depending on the age at diagnosis and their etiology, these brain pathologies can be categorized as neurodevelopmental (ASD, ADHD, epilepsy), neurobehavioral (migraines, MDD) and neurodegenerative (PD, AD) disorders. Brain cancers, mostly represented by tumours of glial origin, can be considered as an extra category. In addition to the increased incidence of brain pathologies, some data report a general decrease in the intellectual quotient among children [393,551].
On the other hand, these last decades have also been characterized by an unprecedented accumulation of pollutants (pesticides, heavy metals, airborne particles, etc.) in the environment due to agricultural, industrial, and urban activities. Such an accumulation started from the 1950s and mostly concerns stable chemical compounds, which persist as permanent pollutants contaminating soils, water, and air. All around the world, for years and even through generations, such a large environmental pollution exposed wide parts of populations in large cities, industrial suburbs, and intensive agricultural areas. Even if health consequences of such long-term exposures are still probably underestimated, some recent studies reported that brain disorders could have, at least in part, environmental causes (Table 1 and Table 4). These observations are in line with the detection of environmental pollutants (pesticides, BPA, …) in human tissues [552,553] and biological fluids such as blood [554,555,556], urine [554,555,556,557,558,559] and maternal milk [560]. The brain is a putative target of chemical pollutants whose lipophilic properties permit them to cross the BBB [306,307] and, thus, reach the brain parenchyma (Figure 2). BPA, for instance, has been detected in human adult brains (0.91 µg/kg) [561] and amniotic fluid (8.3 ng/mL) [556] and urine from premature infants [562]. Knowing the possible deleterious effects of such pollutants, they are in a position to affect brain development and function, as has been recently observed through the impact on intellectual capacities of exposed children [563,564,565].
All of this leads to a crucial question, which is: what would be the effects of the presence of such pollutants on brain functions during prenatal development and from childhood up to adult life? Answering to this question is not a simple task because the deleterious consequences of chemical pollutants may go through a variety of pathways depending on their structure and physical properties. However, taking into account that GJs are both very present in brain (Figure 2, Table 2) and highly sensitive to a wide range of xenobiotics and carcinogens (Table 3), these channels could be, at least partly, a possible answer to the question. Indeed, many pollutants have long been reported to be inhibitors of GJIC [304,305] up to the point that GJIC assays were postulated to be used for screening such xenobiotics [566]. In vitro, almost all the pollutants inhibit GJIC suggesting a non-specific effect through a modification of membrane fluidity acting on the GJ gating. However, for several pollutants, an action through non-classical ERs and several intracellular signaling pathways were found to be involved in GJIC dysregulation. A seductive hypothesis, in view of the emerging role of Cx channels as major targets of pollutants, is that these channels are sensors of environmental and body contamination.
Considering the prenatal situation, exposure of developmental brain to pollutants can significantly induce damaging effects before the completion of BBB maturation, which only happens during the second postnatal year. Because BBB is immature, chemical pollutants are thought to easily enter the brain parenchyma and directly exert their toxic effects on neural tissue [307]. Indeed, many studies highlight the susceptibility of neurodevelopment to environmental exposures and its potential link to the genesis of cerebral pathologies. This hypothesis is known as the “developmental origin of health and disease” (DOHaD) hypothesis [567]. The first 1000 days of life between conception and the second postnatal year are a vulnerable period of human development [568]. This represents a window of very rapid growth and development and, in turn, of greater susceptibility to infection and sensitivity to chemical pollutants. During this period, the human brain undergoes significant changes involving cell proliferation and migration, synaptogenesis, and synaptic pruning [569]. Any exposure to environmental pollutants, interfering with GJIC during this window, could therefore be the source of a silent pandemic of neurodevelopmental toxicity. For instance, such exposures could interfere not only with the brain GJ compartments in formation but also with differentiation processes in which Cxs are involved (myelination, cell migration, etc.) (Figure 1, Figure 2 and Figure 3). Moreover, in late development, neuronal activity is susceptible to be highly affected because of their connections, which are mostly due to Cx36-made electrical synapses before birth. Any GJIC disturbance at this developmental period could then possibly induce alterations at the origin of brain developmental disorders as it has been observed when GJs are inhibited during embryogenesis [106].
From childhood up to adult life, it is conceivable that chemical pollutants may have deleterious effects on brain physiology by disturbing GJIC inside the different GJ compartments (neuronal, panglial syncytium and vascular; Figure 2; Table 2). As shown in Table 3, most studies reporting pollutants as GJIC inhibitors were carried out on cell models expressing Cx43 which is typically the most largely expressed Cx in brain, mostly by astrocytes and endothelial cells (Table 2; Figure 2). Therefore, based on these extensive in vitro data, Cx43 could be a major target of chemical pollutants depending on the condition that sufficient doses would be reached to inhibit its channel function. The pollutants in the blood circulation first impact Cx43 GJs of endothelial cells. Even if the precise role of Cx43 in BBB permeability is not completely defined yet, it is possible that disturbance of Cx43-mediated GJIC between endothelial cells would perturb BBB permeability. An immediate effect of such a permeability disturbance would be then a higher accessibility and exposure of brain parenchyma to circulating pollutants. Consequently, the panglial syncytium appears as a second putative target whose Cx43-mediated GJIC could be affected by the increased permeability to pollutants (Figure 2). Such a disturbance would lead to altered glucose feeding of neurons and other cells of brain parenchyma (distant astrocytes, oligodendrocytes, microglia, ependymocytes). Alteration of K+ siphoning from the extracellular space would also be an additional consequence of the affected panglial syncytium. Then, such alterations could perturb, in turn, neuronal activity, which is very sensitive to both glucose and extracellular K+ concentration variations. Deduced from the GJ compartmental organization, another possible effect of GJIC disturbance inside the panglial syncytium would be myelination defects affecting oligodendrocytes (Figure 2) and, thus, decreased velocity of action potentials along axons. Theoretically, all these GJIC effects are likely to have deleterious consequences on brain functions locally and could explain the emergence of disorders described in this review. Therefore, the scenario of any accumulation of pollutants occurring inside brain parenchyma could explain the higher risk of diagnosis of disorders among old populations. This is in line with studies showing a link between pollutant exposures and the apparition of degenerative disorders like PD and AD (Table 4). Keeping in mind that stem cell niches are very close to blood capillaries and that GJs are involved in their proliferation and differentiation, any disturbance at this level might interfere with maintenance of the brain parenchyma, behavioral adaptation, neuron repair, and, in turn, might play a role in degenerative processes. In addition to the direct adverse effects on GJIC that are mentioned above, there are also possible indirect effects on Cx function that pollutants could cause. A situation during which such an indirect effect could happen is “sterile neuro-inflammation”, when exogenous chemicals or particles induce neuroinflammation through a pathogen-free activation of microglia [570]. Such an activation would then increase expression of Cx43 in microglia favoring the release of pro-inflammatory cytokines (IL-1β, TNF-α) through Cx43 HCs, which affects astrocytic GJIC, and, in turn, synaptic activity. Afterwards, neuroinflammation, as a progressive phenomenon, may lead to a loss of structure and/or functions of neurons. Of note, prior to neuron cell death, some events occur in glia, specifically “reactive gliosis” characterized by activated microglia and reactive astrocytes in which Cx HCs participate. Neuroinflammation is found in most brain pathologies and is indeed associated with Cx changes of expression and/or function [454]. This aspect is important to consider since, in brain disorders, comorbidities are frequently described and can be partially explained by a common etiology like neuroinflammation.
The fact that a correlation between GJIC inhibition by chemical pollutants and the rising incidence of some brain pathologies is not obvious could be explained by the Cx HC involvement. For example, in MDD, Cx43-mediated GJIC and Cx-HC activity exert different effects in their pathophysiology [533]. In agreement with this hypothesis is the reverse relationship that has been observed in astrocytes between GJIC establishment and HC activity [50]. Moreover, despite numerous clues, such a link is not definitively proved yet mostly because of a lack of appropriate models. For instance, the inhibition of GJIC by pollutants has been tested frequently on cell lines that are not from brain origin or even from human origin. More investigations are needed, based on human materials, to definitively focus on Cx function as a target of pollutants and as a possible cause of brain disorders. An obvious point would be to estimate the presence of pollutants in the cerebrospinal fluid and verify their effects on brain cell cultures. For this aspect, more adapted in vitro models are necessary through the reconstitution, for example, of brain parenchyma or as brain organoid cultures. The use of in vivo models may not be useful because of possible differences existing between rodents and human in brain development and organization in terms of Cx expression. However, even if the involvement of Cxs would be proved in the etiology of brain disorders, this statement could not bring currently any obvious therapeutical option. Indeed, there is no real possibility so far to increase GJIC in order to counteract its inhibition by chemical pollutants. Some peptides are known to interfere with Cx43 HCs, which are activated in microglia during neuroinflammation. However, so far, it is probable that those peptides would not act precisely enough in such a sensitive environment as brain parenchyma. Moreover, this review is focused on Cxs and not on Panxs, which have similar functions as Cx HCs. Targeting Cx HCs without targeting Panxs might have a weak effect. Concerning the etiology of brain disorders, it is probably important to consider also Panxs when Cx HCs are involved. To be complete, some studies have revealed cells inside brain parenchyma may communicate through alternative mechanisms. For instance, exosomes, microparticles or apoptotic bodies allow the transfer of gliotransmitters, organelles, DNA/RNA, proteins and pathogens between glial cells and neurons [571]. On the other hand, direct glia-to-neuron signaling not only takes place via GJs as it has been observed in a few cases [88,572,573] but also through long intercellular processes termed tunneling nanotubes (TNTs) [574]. The real involvement of such alternative intercellular communication pathways in brain disorders and its possible links with Cxs is an open field of investigation.
To conclude, if, in the future, Cxs are established as an important etiological part of brain disorders, they might not be a therapeutical target. Without such a targeted therapeutical solution, and in absence of limiting the presence of persisting pollutants in the environment, pre- and post-natal human brains risk being susceptible to still-increased incidence of brain disorders. However, a limitation of exposure can be applied, at least, to air pollution whose control makes it as a preventable risk for human health. In several countries, political actions aiming to reduce air pollution had rapid and dramatic repercussions on health outcomes and all-causes of mortality [575]. Unfortunately, despite preventable air pollution, the use of certain pesticides that have been banned for many years still persist in the soil and continue to pose a threat to public health. This is the case everywhere, even if some studies tend to show a decreased level of exposure in the last decade; for instance, in Denmark [576]. In some areas of the world, pollution-related brain disorders can be even further increased by malnutrition since several studies show that diabetes alters the BBB permeability [577]. Thus, it is probable that the incidence of brain disorders and other chronic diseases will increase for the next decades, especially if effective healthy alternatives for use on crops are not yet available.
Funding
This research is funded by Ligue Nationale contre le Cancer (Comité de Charente-Maritime).
Acknowledgments
MM and ND’s research is funded by Ligue Nationale contre le Cancer (Comité de Charente-Maritime).
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
Aβ: Amyloid β; AD: Alzheimer’s disease; ADHD: attention deficit hyperactivity disorders; AhR: aryl hydrocarbon receptor; ASD: autism spectrum disorders; BBB: blood brain barrier; BPA: bisphenol A; BV: blood vessel; C-ter: carboxyl terminus; [Ca2+]e: extracellular calcium concentration; [Ca2+]i: cytosolic calcium concentration; CNS: central nervous system; COCs: cumulus cell-oocyte complexes; CSD: Cortical Spreading Depression; CSF: cerebrospinal fluid; Cx: connexin; DCP: dicumylperoxide; DDD: dichlorodiphenyldichloroethane; DDE: dichlorodiphenyldichloroethylene; DDT: 1,1,1-trichloro-2,2-bis(4-chlorophenyl) ethane; DINP: di-isononyl phthalate; E2: 17-beta estradiol; ER: estrogen receptors; GJ: gap junction; GJIC: gap junctional intercellular coupling; HC: hemichannel; JC: junctional coupling; KO: knock out; LV: lateral ventricle; M711P: mono-(heptyl, nonyl, undecyl) phthalate; MCs: mast cells; MDD: major depressive disorders; MEHP: mono-2-ethylhexyl phthalate; MIDP: mono-isodecyl phthalate; MIHP: mono-isoheptyl phthalate; MINP: mono-isononyl phthalate; MNOP: mono-n-octyl phthalate; NPC: neural precursor cell; PAH: polycyclic aromatic hydrocarbons; Panx: Pannexin; PBB: polybrominated biphenyls; PCB: polychlorinated biphenyls; PC-PLC: phosphatidylcholine-specific phospholipase C; PD: Parkinson’s disease; PFDA: perfluorodecanoic acid; PFOA: perfluorooctanoic acid; PFOS: perfluorooctane sulfonic acid; PM2.5: fine particulate matter with aerodynamic diameters less than 2.5 µm; S1P: sphingosine 1-phosphate; SGZ: subgranular zone; SphK: sphingosine kinase; SVZ: subventricular zone; TCDD: 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin; VM, membrane potential; VZ: ventricular zone, WF: welding fumes.
References
- GBD 2016 Neurology Collaborators. Global, regional, and national burden of neurological disorders, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 459–480. [Google Scholar] [CrossRef]
- Alzheimer’s Association. 2020 Alzheimer’s disease facts and figures. Alzheimers Dement. 2020, 16, 391–460. [Google Scholar] [CrossRef]
- Weller, J.; Budson, A. Current understanding of Alzheimer’s disease diagnosis and treatment. F1000Research 2018, 7, 1161. [Google Scholar] [CrossRef]
- Kleihues, P.; Barnholtz-Sloan, J.; Ohgaki, H. Tumours of the nervous system. In World Cancer Report 2014; Stewart, B.W., Wild, C.P., Eds.; International Agency for Research on Cancer: Lyon, France, 2014; pp. 511–521. [Google Scholar]
- Gage, F.H. Adult neurogenesis in mammals. Science 2019, 364, 827–828. [Google Scholar] [CrossRef]
- Luo, Y.; Weibman, D.; Halperin, J.M.; Li, X. A Review of Heterogeneity in Attention Deficit/Hyperactivity Disorder (ADHD). Front Hum Neurosci. 2019, 13, 42. [Google Scholar] [CrossRef]
- Polanczyk, G.V.; Salum, G.A.; Sugaya, L.S.; Caye, A.; Rohde, L.A. Annual research review: A meta-analysis of the worldwide prevalence of mental disorders in children and adolescents. J. Child Psychol. Psychiatry 2015, 56, 345–365. [Google Scholar] [CrossRef]
- Bhandari, R.; Paliwal, J.K.; Kuhad, A. Neuropsychopathology of Autism Spectrum Disorder: Complex Interplay of Genetic, Epigenetic, and Environmental Factors. Adv. Neurobiol. 2020, 24, 97–141. [Google Scholar]
- Lyall, K.; Croen, L.; Daniels, J.; Fallin, M.D.; Ladd-Acosta, C.; Lee, B.K.; Park, B.Y.; Snyder, N.W.; Schendel, D.; Volk, H.; et al. The Changing Epidemiology of Autism Spectrum Disorders. Annu. Rev. Public Health 2017, 20, 81–102. [Google Scholar] [CrossRef]
- Elsabbagh, M.; Divan, G.; Koh, Y.J.; Kim, Y.S.; Kauchali, S.; Marcín, C.; Montiel-Nava, C.; Patel, V.; Paula, C.; Wang, C.; et al. Global Prevalence of Autism and Other Pervasive Developmental Disorders. Autism Res. 2012, 5, 160–179. [Google Scholar] [CrossRef]
- Epilepsy. Available online: https://www.who.int/news-room/fact-sheets/detail/epilepsy (accessed on 20 June 2019).
- Hurd, M.D.; Martorell, P.; Delavande, A.; Mullen, K.J.; Langa, K.M. Monetary costs of dementia in the United States. N. Engl. J. Med. 2013, 368, 1326–1334. [Google Scholar] [CrossRef]
- Tysnes, O.B.; Storstein, A. Epidemiology of Parkinson’s disease. J. Neural. Transm. 2017, 124, 901–905. [Google Scholar] [CrossRef] [PubMed]
- Beitz, J.M. Parkinson’s disease: A review. Front. Biosci. 2014, 6, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Vandeleur, C.L.; Fassassi, S.; Castelao, E.; Glaus, J.; Strippoli, M.F.; Lasserre, A.M.; Rudaz, D.; Gebreab, S.; Pistis, G.; Aubry, J.M.; et al. Prevalence and correlates of DSM-5 major depressive and related disorders in the community. Psychiatry Res. 2017, 250, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Walker, E.R.; Druss, B.G. Rate and Predictors of Persistent Major Depressive Disorder in a Nationally Representative Sample. Community Ment. Health J. 2015, 51, 701–707. [Google Scholar] [CrossRef]
- Leso, V.; Gervetti, P.; Mauro, S.; Macrini, M.C.; Ercolano, M.L.; Iavicoli, I. Shift work and migraine: A systematic review. J. Occup. Health 2020, 62, e12116. [Google Scholar] [CrossRef]
- Willecke, K.; Eiberger, J.; Degen, J.; Eckardt, D.; Romualdi, A.; Güldenagel, M.; Deutsch, U.; Söhl, G. Structural and functional diversity of connexin genes in the mouse and human genome. Biol. Chem. 2002, 383, 725–737. [Google Scholar] [CrossRef]
- Nakase, T.; Naus, C.C. Gap junctions and neurological disorders of the central nervous system. Biochim. Biophys. Acta 2004, 1662, 149–158. [Google Scholar] [CrossRef]
- Bruzzone, R. Learning the language of cell-cell communication through connexin channels. Genome Biol. 2001, 2, REPORTS4027. [Google Scholar] [CrossRef]
- Thompson, R.J.; Macvicar, B.A. Connexin and pannexin hemichannels of neurons and astrocytes. Channels 2008, 2, 81–86. [Google Scholar] [CrossRef]
- Kreuzberg, M.M.; Deuchars, J.; Weiss, E.; Schober, A.; Sonntag, S.; Wellershaus, K.; Draguhn, A.; Willecke, K. Expression of connexin30.2 in interneurons of the central nervous system in the mouse. Mol. Cell. Neurosci. 2008, 37, 119–134. [Google Scholar] [CrossRef]
- Dere, E.; Zheng-Fischhöfer, Q.; Viggiano, D.; Gironi Carnevale, U.A.; Ruocco, L.A.; Zlomuzica, A.; Schnichels, M.; Willecke, K.; Huston, J.P.; Sadile, A.G. Connexin31.1 deficiency in the mouse impairs object memory and modulates open-field exploration, acetylcholine esterase levels in the striatum, and cAMP response element-binding protein levels in the striatum and piriform cortex. Neuroscience 2008, 153, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Swayne, L.A.; Bennett, S.A.L. Connexins and pannexins in neuronal development and adult neurogenesis. BMC Cell Biol. 2016, 17 (Suppl. 1), 10. [Google Scholar] [CrossRef] [PubMed]
- Belluardo, N.; Mudò, G.; Trovato-Salinaro, A.; Le Gurun, S.; Charollais, A.; Serre-Beinier, V.; Amato, G.; Haefliger, J.A.; Meda, P.; Condorelli, D.F. Expression of connexin36 in the adult and developing rat brain. Brain Res. 2000, 865, 121–138. [Google Scholar] [CrossRef]
- Condorelli, D.F.; Belluardo, N.; Trovato-Salinaro, A.; Mudò, G. Expression of Cx36 in mammalian neurons. Brain Res. Rev. 2000, 32, 72–85. [Google Scholar] [CrossRef]
- Nagy, J.I.; Pereda, A.E.; Rash, J.E. Electrical synapses in mammalian CNS: Past eras, present focus and future directions. Biochim. Biophys. Acta Biomembr. 2018, 1860, 102–123. [Google Scholar] [CrossRef] [PubMed]
- Schock, S.C.; Leblanc, D.; Hakim, A.M.; Thompson, C.S. ATP release by way of connexin 36 hemichannels mediates ischemic tolerance in vitro. Biochem. Biophys. Res. Commun. 2008, 368, 138–144. [Google Scholar] [CrossRef]
- Venance, L.; Glowinski, J.; Giaume, C. Electrical and chemical transmission between striatal GABAergic output neurones in rat brain slices. J. Physiol. 2004, 559, 215–230. [Google Scholar] [CrossRef]
- Dedek, K.; Schultz, K.; Pieper, M.; Dirks, P.; Maxeiner, S.; Willecke, K.; Weiler, R.; Janssen-Bienhold, U. Localization of heterotypic gap junctions composed of connexin45 and connexin36 in the rod pathway of the mouse retina. Eur. J. Neurosci. 2006, 24, 1675–1686. [Google Scholar] [CrossRef]
- Schubert, T.; Maxeiner, S.; Krüger, O.; Willecke, K.; Weiler, R. Connexin45 mediates gap junctional coupling of bistratified ganglion cells in the mouse retina. J. Comp. Neurol. 2005, 490, 29–39. [Google Scholar] [CrossRef]
- Van Der Giessen, R.S.; Maxeiner, S.; French, P.J.; Willecke, K.; De Zeeuw, C.I. Spatiotemporal distribution of Connexin45 in the olivocerebellar system. J. Comp. Neurol. 2006, 495, 173–184. [Google Scholar] [CrossRef]
- Hombach, S.; Janssen-Bienhold, U.; Söhl, G.; Schubert, T.; Büssow, H.; Ott, T.; Weiler, R.; Willecke, K. Functional expression of connexin57 in horizontal cells of the mouse retina. Eur. J. Neurosci. 2004, 19, 2633–2640. [Google Scholar] [CrossRef] [PubMed]
- Palacios-Prado, N.; Sonntag, S.; Skeberdis, V.A.; Willecke, K.; Bukauskas, F.F. Gating, permselectivity and pH-dependent modulation of channels formed by connexin57, a major connexin of horizontal cells in the mouse retina. J. Physiol. 2009, 587, 3251–3269. [Google Scholar] [CrossRef] [PubMed]
- Ciolofan, C.; Lynn, B.; Wellershaus, K.; Willecke, K.; Nagy, J.I. Spatial relationships of connexin36, connexin57 and zonula occludens-1 in the outer plexiform layer of mouse retina. Neuroscience 2007, 148, 473–488. [Google Scholar] [CrossRef] [PubMed]
- Frisch, C.; De Souza-Silva, M.A.; Söhl, G.; Güldenagel, M.; Willecke, K.; Huston, J.P.; Dere, E. Stimulus complexity dependent memory impairment and changes in motor performance after deletion of the neuronal gap junction protein connexin36 in mice. Behav. Brain Res. 2005, 157, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Belousov, A.B. Deletion of neuronal gap junction protein connexin 36 impairs hippocampal LTP. Neurosci. Lett. 2011, 502, 30–32. [Google Scholar] [CrossRef]
- Hempelmann, A.; Heils, A.; Sander, T. Confirmatory evidence for an association of the connexin-36 gene with juvenile myoclonic epilepsy. Epilepsy Res. 2006, 71, 223–228. [Google Scholar] [CrossRef]
- Mas, C.; Taske, N.; Deutsch, S.; Guipponi, M.; Thomas, P.; Covanis, A.; Friis, M.; Kjeldsen, M.J.; Pizzolato, G.P.; Villemure, J.G.; et al. Association of the connexin36 gene with juvenile myoclonic epilepsy. J. Med. Genet. 2004, 41, e93. [Google Scholar] [CrossRef]
- Nagy, J.I.; Patel, D.; Ochalski, P.A.; Stelmack, J.L. Connexin30 in rodent, cat and human brain: Selective expression in gray matter astrocytes, co-localization with connexin43 at gap junctions and late developmental appearance. Neuroscience 1999, 88, 447–468. [Google Scholar] [CrossRef]
- Nagy, J.I.; Li, X.; Rempel, J.; Stelmack, G.; Patel, D.; Staines, W.A.; Yasumura, T.; Rash, J.E. Cell-specific expression of connexins and evidence of restricted gap junctional coupling between glial cells and between neurons. J. Neurosci. 2001, 21, 1983–2000. [Google Scholar]
- Houades, V.; Koulakoff, A.; Ezan, P.; Seif, I.; Giaume, C. Gap junction-mediated astrocytic networks in the mouse barrel cortex. J. Neurosci. 2008, 28, 5207–5217. [Google Scholar] [CrossRef]
- Filippov, M.A.; Hormuzdi, S.G.; Fuchs, E.C.; Monyer, H. A reporter allele for investigating connexin 26 gene expression in the mouse brain. Eur. J. Neurosci. 2003, 18, 3183–3192. [Google Scholar] [CrossRef] [PubMed]
- Montero, T.D.; Orellana, J.A. Hemichannels: New pathways for gliotransmitter release. Neuroscience 2015, 286, 45–59. [Google Scholar] [CrossRef] [PubMed]
- Pellerin, L.; Magistretti, P.J. Sweet sixteen for ANLS. J. Cereb. Blood Flow. Metab. 2012, 32, 1152–1166. [Google Scholar] [CrossRef]
- Kofuji, P.; Newman, E.A. Potassium buffering in the central nervous system. Neurosci. 2004, 129, 1045–1056. [Google Scholar] [CrossRef] [PubMed]
- Wallraff, A.; Köhling, R.; Heinemann, U.; Theis, M.; Willecke, K.; Steinhäuser, C. The impact of astrocytic gap junctional coupling on potassium buffering in the hippocampus. J. Neurosci. 2006, 26, 5438–5447. [Google Scholar] [CrossRef]
- Pannasch, U.; Vargová, L.; Reingruber, J.; Ezan, P.; Holcman, D.; Giaume, C.; Syková, E.; Rouach, N. Astroglial networks scale synaptic activity and plasticity. Proc. Natl. Acad. Sci. USA 2011, 108, 8467–8472. [Google Scholar] [CrossRef]
- Ezan, P.; André, P.; Cisternino, S.; Saubaméa, B.; Boulay, A.C.; Doutremer, S.; Thomas, M.A.; Quenech’du, N.; Giaume, C.; Cohen-Salmon, M. Deletion of astroglial connexins weakens the blood-brain barrier. J. Cereb. Blood Flow. Metab. 2012, 32, 1457–1467. [Google Scholar] [CrossRef]
- Retamal, M.A.; Froger, N.; Palacios-Prado, N.; Ezan, P.; Sáez, P.J.; Sáez, J.C.; Giaume, C. Cx43 hemichannels and gap junction channels in astrocytes are regulated oppositely by proinflammatory cytokines released from activated microglia. J. Neurosci. 2007, 27, 13781–13792. [Google Scholar] [CrossRef]
- Abudara, V.; Roux, L.; Dallérac, G.; Matias, I.; Dulong, J.; Mothet, J.P.; Rouach, N.; Giaume, C. Activated microglia impairs neuroglial interaction by opening Cx43 hemichannels in hippocampal astrocytes. Glia 2015, 63, 795–811. [Google Scholar] [CrossRef]
- Rusakov, D.A. Depletion of extracellular Ca2+ prompts astroglia to moderate synaptic network activity. Sci. Signal. 2012, 5, pe4. [Google Scholar] [CrossRef]
- Contreras, J.E.; Sáez, J.C.; Bukauskas, F.F.; Bennett, M.V. Gating and regulation of connexin 43 (Cx43) hemichannels. Proc. Natl. Acad. Sci. USA 2003, 100, 11388–11393. [Google Scholar] [CrossRef] [PubMed]
- Rovegno, M.; Sáez, J.C. Role of astrocyte connexin hemichannels in cortical spreading depression. Biochim. Biophys. Acta Biomembr. 2018, 1860, 216–223. [Google Scholar] [CrossRef] [PubMed]
- De Bock, M.; Decrock, E.; Wang, N.; Bol, M.; Vinken, M.; Bultynck, G.; Leybaert, L. The dual face of connexin-based astroglial Ca(2+) communication: A key player in brain physiology and a prime target in pathology. Biochim. Biophys. Acta 2014, 1843, 2211–2232. [Google Scholar] [CrossRef] [PubMed]
- Chever, O.; Pannasch, U.; Ezan, P.; Rouach, N. Astroglial connexin 43 sustains glutamatergic synaptic efficacy. Philos. Trans. R Soc. Lond. B Biol. Sci. 2014, 369, 20130596. [Google Scholar] [CrossRef] [PubMed]
- Theis, M.; Jauch, R.; Zhuo, L.; Speidel, D.; Wallraff, A.; Doring, B.; Frisch, C.; Sohl, G.; Teubner, B.; Euwens, C.; et al. Accelerated hippocampal spreading depression and enhanced locomotory activity in mice with astrocyte-directed inactivation of connexin43. J. Neurosci. 2003, 23, 766–776. [Google Scholar] [CrossRef]
- Frisch, C.; Theis, M.; De Souza Silva, M.A.; Dere, E.; Söhl, G.; Teubner, B.; Namestkova, K.; Willecke, K.; Huston, J.P. Mice with astrocyte-directed inactivation of connexin43 exhibit increased exploratory behaviour, impaired motor capacities, and changes in brain acetylcholine levels. Eur. J. Neurosci. 2003, 18, 2313–2318. [Google Scholar] [CrossRef]
- Dere, E.; De Souza-Silva, M.A.; Frisch, C.; Teubner, B.; Söhl, G.; Willecke, K.; Huston, J.P. Connexin30-deficient mice show increased emotionality and decreased rearing activity in the open-field along with neurochemical changes. Eur. J. Neurosci. 2003, 18, 629–638. [Google Scholar] [CrossRef]
- Lutz, S.E.; Zhao, Y.; Gulinello, M.; Lee, S.C.; Raine, C.S.; Brosnan, C.F. Deletion of astrocyte connexins 43 and 30 leads to a dysmyelinating phenotype and hippocampal CA1 vacuolation. J. Neurosci. 2009, 29, 7743–7752. [Google Scholar] [CrossRef]
- De Bock, M.; Kerrebrouck, M.; Wang, N.; Leybaert, L. Neurological manifestations of oculodentodigital dysplasia: A Cx43 channelopathy of the central nervous system? Front. Pharm. 2013, 4, 120. [Google Scholar] [CrossRef]
- Li, X.; Ionescu, A.V.; Lynn, B.D.; Lu, S.; Kamasawa, N.; Morita, M.; Davidson, K.G.; Yasumura, T.; Rash, J.E.; Nagy, J.I. Connexin47, connexin29 and connexin32 co-expression in oligodendrocytes and Cx47 association with zonula occludens-1 (ZO-1) in mouse brain. Neuroscience 2004, 126, 611–630. [Google Scholar] [CrossRef]
- Kamasawa, N.; Sik, A.; Morita, M.; Yasumura, T.; Davidson, K.G.; Nagy, J.I.; Rash, J.E. Connexin-47 and connexin-32 in gap junctions of oligodendrocyte somata, myelin sheaths, paranodal loops and Schmidt-Lanterman incisures: Implications for ionic homeostasis and potassium siphoning. Neuroscience 2005, 136, 65–86. [Google Scholar] [CrossRef] [PubMed]
- Kagiava, A.; Theophilidis, G.; Sargiannidou, I.; Kyriacou, K.; Kleopa, K.A. Oxaliplatin-induced neurotoxicity is mediated through gap junction channels and hemichannels and can be prevented by octanol. Neuropharmacology 2015, 97, 289–305. [Google Scholar] [CrossRef] [PubMed]
- Nagy, J.I.; Ionescu, A.V.; Lynn, B.D.; Rash, J.E. Coupling of astrocyte connexins Cx26, Cx30, Cx43 to oligodendrocyte Cx29, Cx32, Cx47: Implications from normal and connexin32 knockout mice. Glia 2003, 44, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Odermatt, B.; Wellershaus, K.; Wallraff, A.; Seifert, G.; Degen, J.; Euwens, C.; Fuss, B.; Büssow, H.; Schilling, K.; Steinhäuser, C.; et al. Connexin 47 (Cx47)-deficient mice with enhanced green fluorescent protein reporter gene reveal predominant oligodendrocytic expression of Cx47 and display vacuolized myelin in the CNS. J. Neurosci. 2003, 23, 4549–4559. [Google Scholar] [CrossRef] [PubMed]
- Maglione, M.; Tress, O.; Haas, B.; Karram, K.; Trotter, J.; Willecke, K.; Kettenmann, H. Oligodendrocytes in mouse corpus callosum are coupled via gap junction channels formed by connexin47 and connexin32. Glia 2010, 58, 1104–1117. [Google Scholar] [CrossRef] [PubMed]
- Söhl, G.; Hombach, S.; Degen, J.; Odermatt, B. The oligodendroglial precursor cell line Oli-neu represents a cell culture system to examine functional expression of the mouse gap junction gene connexin29 (Cx29). Front. Pharmacol. 2013, 4, 83. [Google Scholar] [CrossRef]
- Basu, R.; Sarma, J.D. Connexin 43/47 channels are important for astrocyte/ oligodendrocyte cross-talk in myelination and demyelination. J. Biosci. 2018, 43, 1055–1068. [Google Scholar] [CrossRef]
- Menichella, D.M.; Majdan, M.; Awatramani, R.; Goodenough, D.A.; Sirkowski, E.; Scherer, S.S.; Paul, D.L. Genetic and physiological evidence that oligodendrocyte gap junctions contribute to spatial buffering of potassium released during neuronal activity. J. Neurosci. 2006, 26, 10984–10991. [Google Scholar] [CrossRef]
- Eiberger, J.; Kibschull, M.; Strenzke, N.; Schober, A.; Büssow, H.; Wessig, C.; Djahed, S.; Reucher, H.; Koch, D.A.; Lautermann, J.; et al. Expression pattern and functional characterization of connexin29 in transgenic mice. Glia 2006, 53, 601–611. [Google Scholar] [CrossRef]
- Sutor, B.; Schmolke, C.; Teubner, B.; Schirmer, C.; Willecke, K. Myelination defects and neuronal hyperexcitability in the neocortex of connexin32-deficient mice. Cereb. Cortex. 2000, 10, 684–697. [Google Scholar] [CrossRef]
- Menichella, D.M.; Goodenough, D.A.; Sirkowski, E.; Scherer, S.S.; Paul, D.L. Connexins are critical for normal myelination in the CNS. J. Neurosci. 2003, 23, 5963–5973. [Google Scholar] [CrossRef] [PubMed]
- Tress, O.; Maglione, M.; May, D.; Pivneva, T.; Richter, N.; Seyfarth, J.; Binder, S.; Zlomuzica, A.; Seifert, G.; Theis, M.; et al. Panglial gap junctional communication is essential for maintenance of myelin in the CNS. J. Neurosci. 2012, 32, 7499–7518. [Google Scholar] [CrossRef] [PubMed]
- Bergoffen, J.; Scherer, S.S.; Wang, S.; Scott, M.O.; Bone, L.J.; Paul, D.L.; Chen, K.; Lensch, M.W.; Chance, P.F.; Fischbeck, K.H. Connexin mutations in X-linked Charcot-Marie-Tooth disease. Science 1993, 262, 2039–2042. [Google Scholar] [CrossRef]
- Sargiannidou, I.; Kim, G.H.; Kyriakoudi, S.; Eun, B.L.; Kleopa, K.A. A start codon CMT1X mutation associated with transient encephalomyelitis causes complete loss of Cx32. Neurogenetics 2015, 16, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Orthmann-Murphy, J.L.; Enriquez, A.D.; Abrams, C.K.; Scherer, S.S. Loss-of-function GJA12/Connexin47 mutations cause Pelizaeus-Merzbacher-like disease. Mol. Cell. Neurosci. 2007, 34, 629–641. [Google Scholar] [CrossRef]
- Gotoh, L.; Inoue, K.; Helman, G.; Mora, S.; Maski, K.; Soul, J.S.; Bloom, M.; Evans, S.H.; Goto, Y.I.; Caldovic, L.; et al. GJC2 promoter mutations causing Pelizaeus-Merzbacher-like disease. Mol. Genet. Metab. 2014, 111, 393–398. [Google Scholar] [CrossRef]
- Rash, J.E.; Yasumura, T.; Dudek, F.E. Ultrastructure, histological distribution, and freeze-fracture immunocytochemistry of gap junctions in rat brain and spinal cord. Cell Biol. Int. 1998, 22, 731–749. [Google Scholar] [CrossRef]
- De Bock, M.; Vandenbroucke, R.E.; Decrock, E.; Culot, M.; Cecchelli, R.; Leybaert, L. A new angle on blood-CNS interfaces: A role for connexins? FEBS Lett. 2014, 588, 1259–1270. [Google Scholar] [CrossRef]
- Nagasawa, K.; Chiba, H.; Fujita, H.; Kojima, T.; Saito, T.; Endo, T.; Sawada, N. Possible involvement of gap junctions in the barrier function of tight junctions of brain and lung endothelial cells. J. Cell Physiol. 2006, 208, 123–132. [Google Scholar] [CrossRef]
- Li, H.; Yu, S.; Wang, R.; Sun, Z.; Zhou, X.; Zheng, L.; Yin, Z.; Sun, Y. Polymorphism of CONNEXIN37 gene is a risk factor for ischemic stroke in Han Chinese population. Lipids Health Dis. 2018, 17, 72. [Google Scholar] [CrossRef]
- Hammes, H.P.; Lin, J.; Renner, O.; Shani, M.; Lundqvist, A.; Betsholtz, C.; Brownlee, M.; Deutsch, U. Pericytes and the pathogenesis of diabetic retinopathy. Diabetes 2002, 51, 3107–3112. [Google Scholar] [CrossRef] [PubMed]
- Peppiatt, C.M.; Howarth, C.; Mobbs, P.; Attwell, D. Bidirectional control of CNS capillary diameter by pericytes. Nature 2006, 443, 700–704. [Google Scholar] [CrossRef]
- Hamilton, N.B.; Attwell, D.; Hall, C.N. Pericyte-mediated regulation of capillary diameter: A component of neurovascular coupling in health and disease. Front. Neuroenergetics 2010, 2, 5. [Google Scholar] [CrossRef]
- Dalkara, T.; Gursoy-Ozdemir, Y.; Yemisci, M. Brain microvascular pericytes in health and disease. Acta Neuropathol. 2011, 122, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, H.; Jin, S.; Wang, J.; Zhang, G.; Kawanokuchi, J.; Kuno, R.; Sonobe, Y.; Mizuno, T.; Suzumura, A. Tumor necrosis factor-alpha induces neurotoxicity via glutamate release from hemichannels of activated microglia in an autocrine manner. J. Biol. Chem. 2006, 281, 21362–21368. [Google Scholar] [CrossRef]
- Dobrenis, K.; Chang, H.Y.; Pina-Benabou, M.H.; Woodroffe, A.; Lee, S.C.; Rozental, R.; Spray, D.C.; Scemes, E. Human and mouse microglia express connexin36, and functional gap junctions are formed between rodent microglia and neurons. J. Neurosci. Res. 2005, 82, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, S.B.; Uy, B.; Perera, A.; Nicholson, L.F. AGEs-RAGE mediated up-regulation of connexin43 in activated human microglial CHME-5 cells. Neurochem. Int. 2012, 60, 640–651. [Google Scholar] [CrossRef]
- Eugenín, E.A.; Eckardt, D.; Theis, M.; Willecke, K.; Bennett, M.V.; Saez, J.C. Microglia at brain stab wounds express connexin 43 and in vitro form functional gap junctions after treatment with interferon-gamma and tumor necrosis factor-alpha. Proc. Natl. Acad. Sci. USA 2001, 98, 4190–4195. [Google Scholar] [CrossRef]
- Garg, S.; Syed, M.; Kielian, T. Staphylococcus aureus-derived peptidoglycan induces Cx43 expression and functional gap junction intercellular communication in microglia. J. Neurochem. 2005, 95, 475–483. [Google Scholar] [CrossRef]
- Orellana, J.A.; Shoji, K.F.; Abudara, V.; Ezan, P.; Amigou, E.; Sáez, P.J.; Jiang, J.X.; Naus, C.C.; Sáez, J.C.; Giaume, C. Amyloid β-induced death in neurons involves glial and neuronal hemichannels. J. Neurosci. 2011, 31, 4962–4977. [Google Scholar] [CrossRef]
- Goings, G.E.; Kozlowski, D.A.; Szele, F.G. Differential activation of microglia in neurogenic versus non-neurogenic regions of the forebrain. Glia 2006, 54, 329–342. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Perez, O.; Gutierrez-Fernandez, F.; Lopez-Virgen, V.; Collas-Aguilar, J.; Quinones-Hinojosa, A.; Garcia-Verdugo, J.M. Immunological regulation of neurogenic niches in the adult brain. Neuroscience 2012, 226, 270–281. [Google Scholar] [CrossRef] [PubMed]
- Mosher, K.I.; Andres, R.H.; Fukuhara, T.; Bieri, G.; Hasegawa-Moriyama, M.; He, Y.; Guzman, R.; Wyss-Coray, T. Neural progenitor cells regulate microglia functions and activity. Nat. Neurosci. 2012, 15, 1485–1487. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, P.; Battista, D.; Depino, A.; Roca, V.; Graciarena, M.; Pitossi, F. The more you have, the less you get: The functional role of inflammation on neuronal differentiation of endogenous and transplanted neural stem cells in the adult brain. J. Neurochem. 2010, 112, 1368–1385. [Google Scholar] [CrossRef]
- Shigemoto-Mogami, Y.; Hoshikawa, K.; Goldman, J.E.; Sekino, Y.; Sato, K. Microglia enhance neurogenesis and oligodendrogenesis in the early postnatal subventricular zone. J. Neurosci. 2014, 34, 2231–2243. [Google Scholar] [CrossRef]
- Pang, B.; Neijssen, J.; Qiao, X.; Janssen, L.; Janssen, H.; Lippuner, C.; Neefjes, J. Direct antigen presentation and gap junction mediated cross-presentation during apoptosis. J. Immunol. 2009, 183, 1083–1090. [Google Scholar] [CrossRef]
- Temple, S. The development of neural stem cells. Nature 2001, 414, 112–117. [Google Scholar] [CrossRef]
- Heng, J.I.; Chariot, A.; Nguyen, L. Molecular layers underlying cytoskeletal remodelling during cortical development. Trends Neurosci. 2010, 33, 38–47. [Google Scholar] [CrossRef]
- Ming, G.L.; Song, H. Adult neurogenesis in the mammalian brain: Significant answers and significant questions. Neuron 2011, 70, 687–702. [Google Scholar] [CrossRef]
- Kempermann, G.; Wiskott, L.; Gage, F.H. Functional significance of adult neurogenesis. Curr. Opin. Neurobiol. 2004, 14, 186–191. [Google Scholar] [CrossRef]
- Hsieh, J. Orchestrating transcriptional control of adult neurogenesis. Genes Dev. 2012, 26, 1010–1021. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Deng, W.; Gage, F.H. Mechanisms and functional implications of adult neurogenesis. Cell 2008, 132, 645–660. [Google Scholar] [CrossRef] [PubMed]
- Saunders, M.; Magnanti, B.L.; Correia Carreira, S.; Yang, A.; Alamo-Hernández, U.; Riojas-Rodriguez, H.; Calamandrei, G.; Koppe, J.G.; Krayer von Krauss, M.; Keune, H.; et al. Chlorpyrifos and neurodevelopmental effects: A literature review and expert elicitation on research and policy. Environ. Health 2012, 11 (Suppl. 1), S5. [Google Scholar] [CrossRef] [PubMed]
- Levin, M. Gap junctional communication in morphogenesis. Prog. Biophys. Mol. Biol. 2007, 94, 186–206. [Google Scholar] [CrossRef]
- Nadarajah, B.; Jones, AM.; Evans, W.H.; Parnavelas, J.G. Differential expression of connexins during neocortical development and neuronal circuit formation. J. Neurosci. 1997, 17, 3096–3111. [Google Scholar] [CrossRef]
- Cina, C.; Bechberger, J.F.; Ozog, M.A.; Naus, C.C. Expression of connexins in embryonic mouse neocortical development. J. Comp. Neurol. 2007, 504, 298–313. [Google Scholar] [CrossRef]
- Gulisano, M.; Parenti, R.; Spinella, F.; Cicirata, F. Cx36 is dynamically expressed during early development of mouse brain and nervous system. Neuroreport 2000, 11, 3823–3828. [Google Scholar] [CrossRef]
- Söhl, G.; Eiberger, J.; Jung, Y.T.; Kozak, C.A.; Willecke, K. The mouse gap junction gene connexin29 is highly expressed in sciatic nerve and regulated during brain development. Biol. Chem. 2001, 382, 973–978. [Google Scholar] [CrossRef]
- Lo, C.W. The role of gap junction membrane channels in development. J. Bioenerg. Biomembr. 1996, 28, 379–385. [Google Scholar] [CrossRef]
- Harris, A.L. Connexin channel permeability to cytoplasmic molecules. Prog. Biophys. Mol. Biol. 2007, 94, 120–143. [Google Scholar] [CrossRef]
- Elias, L.A.; Wang, D.D.; Kriegstein, A.R. Gap junction adhesion is necessary for radial migration in the neocortex. Nature 2007, 448, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Santiago, M.F.; Alcami, P.; Striedinger, K.M.; Spray, D.; Scemes, E. The carboxyl-terminal domain of connexin43 is a negative modulator of neuronal differentiation. J. Biol. Chem. 2010, 285, 11836–11845. [Google Scholar] [CrossRef] [PubMed]
- Hartfield, E.M.; Rinaldi, F.; Glover, CP.; Wong, L.F.; Caldwell, M.A.; Uney, J.B. Connexin 36 expression regulates neuronal differentiation from neural progenitor cells. PLoS ONE 2011, 6, e14746. [Google Scholar] [CrossRef] [PubMed]
- Söhl, G.; Degen, J.; Teubner, B.; Willecke, K. The murine gap junction gene connexin36 is highly expressed in mouse retina and regulated during brain development. FEBS Lett. 1998, 428, 27–31. [Google Scholar] [CrossRef]
- Condorelli, D.F.; Trovato-Salinaro, A.; Mudò, G.; Mirone, M.B.; Belluardo, N. Cellular expression of connexins in the rat brain: Neuronal localization, effects of kainate-induced seizures and expression in apoptotic neuronal cells. Eur. J. Neurosci. 2003, 18, 1807–1827. [Google Scholar] [CrossRef]
- Deans, M.R.; Gibson, J.R.; Sellitto, C.; Connors, B.W.; Paul, D.L. Synchronous activity of inhibitory networks in neocortex requires electrical synapses containing connexin36. Neuron 2001, 31, 477–485. [Google Scholar] [CrossRef]
- Elias, L.A.; Kriegstein, A.R. Gap junctions: Multifaceted regulators of embryonic cortical development. Trends Neurosci. 2008, 31, 243–250. [Google Scholar] [CrossRef]
- Cina, C.; Maass, K.; Theis, M.; Willecke, K.; Bechberger, J.F.; Naus, C.C. Involvement of the cytoplasmic C-terminal domain of connexin43 in neuronal migration. J. Neurosci. 2009, 29, 2009–2021. [Google Scholar] [CrossRef]
- Qi, G.J.; Chen, Q.; Chen, L.J.; Shu, Y.; Bu, L.L.; Shao, X.Y.; Zhang, P.; Jiao, F.J.; Shi, J.; Tian, B. Phosphorylation of Connexin 43 by Cdk5 Modulates Neuronal Migration During Embryonic Brain Development. Mol. Neurobiol. 2016, 53, 2969–2982. [Google Scholar] [CrossRef]
- Vejar, S.; Oyarzún, J.E.; Retamal, M.A.; Ortiz, F.C.; Orellana, J.A. Connexin and Pannexin-Based Channels in Oligodendrocytes: Implications in Brain Health and Disease. Front. Cell. Neurosci. 2019, 13, 3. [Google Scholar] [CrossRef]
- Peinado, A. Immature neocortical neurons exist as extensive syncytial networks linked by dendrodendritic electrical connections. J. Neurophysiol. 2001, 85, 620–629. [Google Scholar] [CrossRef] [PubMed]
- Peinado, A.; Yuste, R.; Katz, L.C. Extensive dye coupling between rat neocortical neurons during the period of circuit formation. Neuron 1993, 10, 103–114. [Google Scholar] [CrossRef]
- Song, H.J.; Stevens, C.F.; Gage, F.H. Neural stem cells from adult hippocampus develop essential properties of functional CNS neurons. Nat. Neurosci. 2002, 5, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Weissman, T.A.; Riquelme, P.A.; Ivic, L.; Flint, A.C.; Kriegstein, A.R. Calcium waves propagate through radial glial cells and modulate proliferation in the developing neocortex. Neuron 2004, 43, 647–661. [Google Scholar] [CrossRef] [PubMed]
- Yuste, R.; Peinado, A.; Katz, L.C. Neuronal domains in developing neocortex. Science 1992, 257, 665–669. [Google Scholar] [CrossRef] [PubMed]
- Yuste, R.; Nelson, D.A.; Rubin, W.W.; Katz, L.C. Neuronal domains in developing neocortex: Mechanisms of coactivation. Neuron 1995, 14, 7–17. [Google Scholar] [CrossRef]
- Kandler, K.; Katz, L.C. Coordination of neuronal activity in developing visual cortex by gap junction-mediated biochemical communication. J. Neurosci. 1998, 18, 1419–1427. [Google Scholar] [CrossRef]
- Zimmermann, H. Purinergic signaling in neural development. Semin. Cell. Dev. Biol. 2011, 22, 194–204. [Google Scholar] [CrossRef]
- Cavaliere, F.; Donno, C.; D’Ambrosi, N. Purinergic signaling: A common pathway for neural and mesenchymal stem cell maintenance and differentiation. Front. Cell. Neurosci. 2015, 9, 211. [Google Scholar] [CrossRef]
- Messemer, N.; Kunert, C.; Grohmann, M.; Sobottka, H.; Nieber, K.; Zimmermann, H.; Franke, H.; Nörenberg, W.; Straub, I.; Schaefer, M.; et al. P2X7 receptors at adult neural progenitor cells of the mouse subventricular zone. Neuropharmacology 2013, 73, 122–137. [Google Scholar] [CrossRef]
- Delarasse, C.; Gonnord, P.; Galante, M.; Auger, R.; Daniel, H.; Motta, I.; Kanellopoulos, J.M. Neural progenitor cell death is induced by extracellular ATP via ligation of P2X7 receptor. J. Neurochem. 2009, 109, 846–857. [Google Scholar] [CrossRef] [PubMed]
- Lovelace, M.D.; Gu, B.J.; Eamegdool, S.S.; Weible, M.W., 2nd.; Wiley, J.S.; Allen, D.G.; Chan-Ling, T. P2X7 receptors mediate innate phagocytosis by human neural precursor cells and neuroblasts. Stem Cells 2015, 33, 526–541. [Google Scholar] [CrossRef] [PubMed]
- Wiencken-Barger, A.E.; Djukic, B.; Casper, K.B.; McCarthy, K.D. A role for Connexin43 during neurodevelopment. Glia 2007, 55, 675–686. [Google Scholar] [CrossRef] [PubMed]
- Pilaz, L.J.; Silver, D.L. Post-transcriptional regulation in corticogenesis: How RNA-binding proteins help build the brain. Wiley Interdiscip. Rev. RNA 2015, 6, 501–515. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, F.A.; Carvalho, L.R.; Grinberg, L.T.; Farfel, J.M.; Ferretti, R.E.; Leite, R.E.; Jacob Filho, W.; Lent, R.; Herculano-Houzel, S. Equal numbers of neuronal and nonneuronal cells make the human brain an isometrically scaled-up primate brain. J. Comp. Neurol. 2009, 513, 532–541. [Google Scholar] [CrossRef]
- Herculano-Houzel, S. The Human Brain in Numbers: A Linearly Scaled-up Primate Brain. Front Hum. Neurosci. 2009, 3, 31. [Google Scholar] [CrossRef]
- Mayorquin, L.C.; Rodriguez, A.V.; Sutachan, J.J.; Albarracín, S.L. Connexin-Mediated Functional and Metabolic Coupling Between Astrocytes and Neurons. Front. Mol. Neurosci. 2018, 11, 118. [Google Scholar] [CrossRef]
- Johanson, C.E.; Stopa, E.G.; McMillan, P.N. The blood-cerebrospinal fluid barrier: Structure and functional significance. Methods Mol. Biol. 2011, 686, 101–131. [Google Scholar]
- Bruni, J.E. Ependymal development, proliferation, and functions: A review. Microsc. Res. Tech. 1998, 41, 2–13. [Google Scholar] [CrossRef]
- Del Bigio, M.R. The ependyma: A protective barrier between brain and cerebrospinal fluid. Glia 1995, 14, 1–13. [Google Scholar] [CrossRef]
- Del Bigio, M.R. Ependymal cells: Biology and pathology. Acta Neuropathol. 2010, 119, 55–73. [Google Scholar] [CrossRef] [PubMed]
- Battefeld, A.; Klooster, J.; Kole, M.H. Myelinating satellite oligodendrocytes are integrated in a glial syncytium constraining neuronal high-frequency activity. Nat. Commun. 2016, 7, 11298. [Google Scholar] [CrossRef] [PubMed]
- Chever, O.; Dossi, E.; Pannasch, U.; Derangeon, M.; Rouach, N. Astroglial networks promote neuronal coordination. Sci Signal. 2016, 9, ra6. [Google Scholar] [CrossRef] [PubMed]
- Magnotti, L.M.; Goodenough, D.A.; Paul, D.L. Functional heterotypic interactions between astrocyte and oligodendrocyte connexins. Glia 2011, 59, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Wasseff, S.K.; Scherer, S.S. Cx32 and Cx47 mediate oligodendrocyte:astrocyte and oligodendrocyte:oligodendrocyte gap junction coupling. Neurobiol. Dis. 2011, 42, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Pereda, A.E. Electrical synapses and their functional interactions with chemical synapses. Nat. Rev. Neurosci. 2014, 15, 250–263. [Google Scholar] [CrossRef] [PubMed]
- Rash, J.E.; Staines, W.A.; Yasumura, T.; Patel, D.; Furman, C.S.; Stelmack, G.L.; Nagy, J.I. Immunogold evidence that neuronal gap junctions in adult rat brain and spinal cord contain connexin-36 but not connexin-32 or connexin-43. Proc. Natl. Acad. Sci. USA 2000, 97, 7573–7578. [Google Scholar] [CrossRef]
- Rozental, R.; Morales, M.; Mehler, M.F.; Urban, M.; Kremer, M.; Dermietzel, R.; Kessler, J.A.; Spray, D.C. Changes in the properties of gap junctions during neuronal differentiation of hippocampal progenitor cells. J. Neurosci. 1998, 18, 1753–1762. [Google Scholar] [CrossRef]
- Vis, J.C.; Nicholson, L.F.; Faull, R.L.; Evans, W.H.; Severs, N.J.; Green, C.R. Connexin expression in Huntington’s diseased human brain. Cell. Biol. Int. 1998, 22, 837–847. [Google Scholar] [CrossRef]
- Weickert, S.; Ray, A.; Zoidl, G.; Dermietzel, R. Expression of neural connexins and pannexin1 in the hippocampus and inferior olive: A quantitative approach. Mol. Brain Res. 2005, 133, 102–109. [Google Scholar] [CrossRef]
- Kosaka, T. Neuronal gap junctions in the polymorph layer of the rat dentate gyrus. Brain Res. 1983, 277, 347–351. [Google Scholar] [CrossRef]
- Fukuda, T.; Kosaka, T. Gap junctions linking the dendritic network of GABAergic interneurons in the hippocampus. J. Neurosci. 2000, 20, 1519–1528. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, T.; Kosaka, T. The dual network of GABAergic interneurons linked by both chemical and electrical synapses: A possible infrastructure of the cerebral cortex. Neurosci. Res. 2000, 38, 123–130. [Google Scholar] [CrossRef]
- Apostolides, P.F.; Trussell, L.O. Regulation of interneuron excitability by gap junction coupling with principal cells. Nat. Neurosci. 2013, 16, 1764–1772. [Google Scholar] [CrossRef] [PubMed]
- Galarreta, M.; Hestrin, S. A network of fast-spiking cells in the neocortex connected by electrical synapses. Nature 1999, 402, 72–75. [Google Scholar] [CrossRef]
- Sevetson, J.; Haas, J.S. Asymmetry and modulation of spike timing in electrically coupled neurons. J. Neurophysiol. 2015, 113, 1743–1751. [Google Scholar] [CrossRef]
- Wang, M.H.; Chen, N.; Wang, J.H. The coupling features of electrical synapses modulate neuronal synchrony in hypothalamic superachiasmatic nucleus. Brain Res. 2014, 1550, 9–17. [Google Scholar] [CrossRef]
- Landisman, C.E.; Long, M.A.; Beierlein, M.; Deans, M.R.; Paul, D.L.; Connors, B.W. Electrical synapses in the thalamic reticular nucleus. J. Neurosci. 2002, 22, 1002–1009. [Google Scholar] [CrossRef]
- Vandecasteele, M.; Glowinski, J.; Venance, L. Connexin mRNA expression in single dopaminergic neurons of substantia nigra pars compacta. Neurosci. Res. 2006, 56, 419–426. [Google Scholar] [CrossRef]
- Leussis, M.P.; Bolivar, V.J. Habituation in rodents: A review of behavior, neurobiology, and genetics. Neurosci. Biobehav. Rev. 2006, 30, 1045–1064. [Google Scholar] [CrossRef]
- Pannasch, U.; Rouach, N. Emerging role for astroglial networks in information processing: From synapse to behavior. Trends Neurosci. 2013, 36, 405–417. [Google Scholar] [CrossRef] [PubMed]
- Orthmann-Murphy, J.L.; Freidin, M.; Fischer, E.; Scherer, S.S.; Abrams, C.K. Two Distinct Heterotypic Channels Mediate Gap Junction Coupling between Astrocyte and Oligodendrocyte Connexins. J. Neurosci. 2007, 27, 13949. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Ochalski, A.; Hertzberg, E.L.; Nagy, J.I. LM and EM immunolocalization of the gap junctional protein connexin 43 in rat brain. Brain Res. 1990, 508, 313–319. [Google Scholar] [CrossRef]
- Yamamoto, T.; Ochalski, A.; Hertzberg, E.L.; Nagy, J.I. On the organization of astrocytic gap junctions in rat brain as suggested by LM and EM immunohistochemistry of connexin43 expression. J. Comp. Neurol. 1990, 302, 853–883. [Google Scholar] [CrossRef]
- Giaume, C.; McCarthy, K.D. Control of gap-junctional communication in astrocytic networks. Trends Neurosci. 1996, 19, 319–325. [Google Scholar] [CrossRef]
- Iacobas, D.A.; Urban-Maldonado, M.; Iacobas, S.; Scemes, E.; Spray, D.C. Array analysis of gene expression in connexin-43 null astrocytes. Physiol. Genom. 2003, 15, 177–190. [Google Scholar] [CrossRef]
- Griemsmann, S.; Höft, S.P.; Bedner, P.; Zhang, J.; von Staden, E.; Beinhauer, A.; Degen, J.; Dublin, P.; Cope, D.W.; Richter, N.; et al. Characterization of Panglial Gap Junction Networks in the Thalamus, Neocortex, and Hippocampus Reveals a Unique Population of Glial Cells. Cereb. Cortex 2015, 25, 3420–3433. [Google Scholar] [CrossRef]
- Wolburg, H.; Rohlmann, A. Structure—Function relationships in gap junctions. Int. Rev. Cytol. 1995, 157, 315–373. [Google Scholar]
- Rash, J.E.; Duffy, H.S.; Dudek, F.E.; Bilhartz, B.L.; Whalen, L.R.; Yasumura, T. Grid-mapped freeze-fracture analysis of gap junctions in gray and white matter of adult rat central nervous system, with evidence for a “panglial syncytium” that is not coupled to neurons. J. Comp. Neurol. 1997, 388, 265–292. [Google Scholar] [CrossRef]
- Rouach, N.; Koulakoff, A.; Abudara, V.; Willecke, K.; Giaume, C. Astroglial metabolic networks sustain hippocampal synaptic transmission. Science 2008, 322, 1551–1555. [Google Scholar] [CrossRef]
- Pellerin, L.; Magistretti, P.J. Glutamate uptake into astrocytes stimulates aerobic glycolysis: A mechanism coupling neuronal activity to glucose utilization. Proc. Natl. Acad. Sci. USA 1994, 91, 10625–10629. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Buckalew, R.; Du, Y.; Kiyoshi, C.M.; Alford, C.C.; Wang, W.; McTigue, D.M.; Enyeart, J.J.; Terman, D.; Zhou, M. Gap junction coupling confers isopotentiality on astrocyte syncytium. Glia 2016, 64, 214–226. [Google Scholar] [CrossRef] [PubMed]
- Hibino, H.; Fujita, A.; Iwai, K.; Yamada, M.; Kurachi, Y. Differential assembly of inwardly rectifying K+ channel subunits, Kir4.1 and Kir5.1, in brain astrocytes. J. Biol. Chem. 2004, 279, 44065–44073. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.; Nagelhus, E.A.; Amiry-Moghaddam, M.; Bourque, C.; Agre, P.; Ottersen, O.P. Specialized membrane domains for water transport in glial cells: High-resolution immunogold cytochemistry of aquaporin-4 in rat brain. J. Neurosci. 1997, 17, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Rash, J.E.; Davidson, K.G.; Yasumura, T.; Furman, C.S. Freeze-fracture and immunogold analysis of aquaporin-4 (AQP4) square arrays, with models of AQP4 lattice assembly. Neuroscience 2004, 129, 915–934. [Google Scholar] [CrossRef]
- Benfenati, V.; Caprini, M.; Nicchia, G.P.; Rossi, A.; Dovizio, M.; Cervetto, C.; Nobile, M.; Ferroni, S. Carbenoxolone inhibits volume-regulated anion conductance in cultured rat cortical astroglia. Channels 2009, 3, 323–336. [Google Scholar] [CrossRef]
- Ventura, R.; Harris, K.M. Three-dimensional relationships between hippocampal synapses and astrocytes. J. Neurosci. 1999, 19, 6897–6906. [Google Scholar] [CrossRef]
- Wong, A.D.; Ye, M.; Levy, A.F.; Rothstein, J.D.; Bergles, D.E.; Searson, P.C. The blood-brain barrier: An engineering perspective. Front. Neuroeng. 2013, 6, 7. [Google Scholar] [CrossRef]
- Kreczmanski, P.; Heinsen, H.; Mantua, V.; Woltersdorf, F.; Masson, T.; Ulfig, N.; Schmidt-Kastner, R.; Korr, H.; Steinbusch, H.W.M.; Hof, P.R.; et al. Microvessel lengthdensity, total length, and length perneuron in five subcortical regionsin schizophrenia. Acta Neuropathol. 2009, 117, 409–421. [Google Scholar] [CrossRef] [PubMed]
- Kreczmanski, P.; Schmidt-Kastner, R.; Heinsen, H.; Steinbusch, H.W.M.; Hof, P.R.; Schmitz, C. Stereological studies of capillarylength density in the frontal cortexof schizophrenics. Acta Neuropathol. 2005, 109, 510–518. [Google Scholar] [CrossRef]
- Zlokovic, B.V. Neurovascular mechanisms of Alzheimer’s neurodegeneration. Trends Neurosci. 2005, 28, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, B.T.; Davis, T.P. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol. Rev. 2005, 57, 173–185. [Google Scholar] [CrossRef]
- Shepro, D.; Morel, N.M. Pericyte physiology. FASEB J. 1993, 7, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Bonkowski, D.; Katyshev, V.; Balabanov, R.D.; Borisov, A.; Dore-Duffy, P. The CNS microvascular pericyte: Pericyte-astrocyte crosstalk in the regulation of tissue survival. Fluids Barriers CNS 2011, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- Aspelund, A.; Antila, S.; Proulx, S.T.; Karlsen, T.V.; Karaman, S.; Detmar, M.; Wiig, H.; Alitalo, K. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J. Exp. Med. 2015, 212, 991–999. [Google Scholar] [CrossRef]
- Jessen, N.A.; Munk, A.S.F.; Lundgaard, I.; Nedergaard, M. The Glymphatic System: A Beginner’s Guide. Neurochem. Res. 2015, 40, 2583–2599. [Google Scholar] [CrossRef]
- Louveau, A.; Smirnov, I.; Keyes, T.J.; Eccles, J.D.; Rouhani, S.J.; Peske, J.D.; Derecki, N.C.; Castle, D.; Mandell, J.W.; Lee, K.S.; et al. Structural and functional features of central nervous system lymphatic vessels. Nature 2015, 523, 337–341. [Google Scholar] [CrossRef]
- Tajes, M.; Ramos-Fernández, E.; Weng-Jiang, X.; Bosch-Morató, M.; Guivernau, B.; Eraso-Pichot, A.; Salvador, B.; Fernàndez-Busquets, X.; Roquer, J.; Muñoz, F.J. The blood-brain barrier: Structure, function and therapeutic approaches to cross it. Mol. Membr. Biol. 2014, 31, 152–167. [Google Scholar] [CrossRef]
- Boulay, A.C.; Cisternino, S.; Cohen-Salmon, M. Immunoregulation at the gliovascular unit in the healthy brain: A focus on Connexin 43. Brain Behav. Immun. 2016, 56, 1–9. [Google Scholar] [CrossRef]
- Johnson, A.M.; Roach, J.P.; Hu, A.; Stamatovic, S.M.; Zochowski, M.R.; Keep, R.F.; Andjelkovic, A.V. Connexin 43 gap junctions contribute to brain endothelial barrier hyperpermeability in familial cerebral cavernous malformations type III by modulating tight junction structure. FASEB J. 2018, 32, 2615–2629. [Google Scholar] [CrossRef]
- Bhowmick, S.; D’Mello, V.; Caruso, D.; Wallerstein, A.; Abdul-Muneer, P.M. Impairment of pericyte-endothelium crosstalk leads to blood-brain barrier dysfunction following traumatic brain injury. Exp. Neurol. 2019, 317, 260–270. [Google Scholar] [CrossRef] [PubMed]
- De Bock, M.; Van Haver, V.; Vandenbroucke, R.E.; Decrock, E.; Wang, N.; Leybaert, L. Into rather unexplored terrain-transcellular transport across the blood-brain barrier. Glia 2016, 64, 1097–1123. [Google Scholar] [CrossRef] [PubMed]
- Osipova, E.D.; Semyachkina-Glushkovskaya, O.V.; Morgun, A.V.; Pisareva, N.V.; Malinovskaya, N.A.; Boitsova, E.B.; Pozhilenkova, E.A.; Belova, O.A.; Salmin, V.V.; Taranushenko, T.E.; et al. Gliotransmitters and cytokines in the control of blood-brain barrier permeability. Rev. Neurosci. 2018, 29, 567–591. [Google Scholar] [CrossRef] [PubMed]
- Kalaria, R.N. The blood-brain barrier and cerebrovascular pathology in Alzheimer’s disease. Ann. N. Y. Acad. Sci. 1999, 893, 113–125. [Google Scholar] [CrossRef]
- Zlokovic, B.V.; Deane, R.; Sallstrom, J.; Chow, N.; Miano, J.M. Neurovascular pathways and Alzheimer amyloid beta-peptide. Brain Pathol. 2005, 15, 78–83. [Google Scholar] [CrossRef]
- Desai, B.S.; Monahan, A.J.; Carvey, P.M.; Hendey, B. Blood-brain barrier pathology in Alzheimer’s and Parkinson’s disease: Implications for drug therapy. Cell Transplant. 2007, 16, 285–299. [Google Scholar] [CrossRef]
- Zipser, B.D.; Johanson, C.E.; Gonzalez, L.; Berzin, T.M.; Tavares, R.; Hulette, C.M.; Vitek, M.P.; Hovanesian, V.; Stopa, E.G. Microvascular injury and blood-brain barrier leakage in Alzheimer’s disease. Neurobiol. Aging 2007, 28, 977–986. [Google Scholar] [CrossRef]
- Meyer, E.P.; Ulmann-Schuler, A.; Staufenbiel, M.; Krucker, T. Altered morphology and 3D architecture of brain vasculature in a mouse model for Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2008, 105, 3587–3592. [Google Scholar] [CrossRef]
- Kortekaas, R.; Leenders, K.L.; van Oostrom, J.C.; Vaalburg, W.; Bart, J.; Willemsen, A.T.; Hendrikse, N.H. Blood-brain barrier dysfunction in parkinsonian midbrain in vivo. Ann. Neurol. 2005, 57, 176–179. [Google Scholar] [CrossRef]
- Lee, H.; Pienaar, I.S. Disruption of the blood-brain barrier in Parkinson’s disease: Curse or route to a cure? Front. Biosci. 2014, 19, 272–280. [Google Scholar] [CrossRef]
- Bartels, A.L.; Willemsen, A.T.; Kortekaas, R.; de Jong, B.M.; de Vries, R.; de Klerk, O.; van Oostrom, J.C.; Portman, A.; Leenders, K.L. Decreased blood-brain barrier P-glycoprotein function in the progression of Parkinson’s disease, PSP and MSA. J. Neural. Transm. 2008, 115, 1001–1009. [Google Scholar] [CrossRef] [PubMed]
- Seiffert, E.; Dreier, J.P.; Ivens, S.; Bechmann, I.; Tomkins, O.; Heinemann, U.; Friedman, A. Lasting blood-brain barrier disruption induces epileptic focus in the rat somatosensory cortex. J. Neurosci. 2004, 24, 7829–7836. [Google Scholar] [CrossRef] [PubMed]
- Oby, E.; Janigro, D. The blood-brain barrier and epilepsy. Epilepsia 2006, 47, 1761–1774. [Google Scholar] [CrossRef] [PubMed]
- Remy, S.; Beck, H. Molecular and cellular mechanisms of pharmacoresistance in epilepsy. Brain 2006, 129, 18–35. [Google Scholar] [CrossRef]
- Davies, D.C. Blood-brain barrier breakdown in septic encephalopathy and brain tumours. J. Anat. 2002, 200, 639–646. [Google Scholar] [CrossRef]
- Papadopoulos, M.C.; Saadoun, S.; Binder, D.K.; Manley, G.T.; Krishna, S.; Verkman, A.S. Molecular mechanisms of brain tumor edema. Neuroscience 2004, 129, 1011–1020. [Google Scholar] [CrossRef]
- Bronger, H.; König, J.; Kopplow, K.; Steiner, H.H.; Ahmadi, R.; Herold-Mende, C.; Keppler, D.; Nies, T. ABCC drug efflux pumps and organic anion uptake transporters in human gliomas and the blood-tumor barrier. Cancer Res. 2005, 65, 11419–11428. [Google Scholar] [CrossRef]
- Reddy, O.C.; van der Werf, Y.D. The Sleeping Brain: Harnessing the Power of the Glymphatic System through Lifestyle Choices. Brain Sci. 2020, 10, 868. [Google Scholar] [CrossRef]
- Watanabe, C.; Imaizumi, T.; Kawai, H.; Suda, K.; Honma, Y.; Ichihashi, M.; Ema, M.; Mizutani, K.I. Aging of the Vascular System and Neural Diseases. Front. Aging Neurosci. 2020, 12, 557384. [Google Scholar] [CrossRef]
- Andreotti, J.P.; Silva, W.N.; Costa, A.C.; Picoli, C.C.; Bitencourt, F.C.O.; Coimbra-Campos, L.M.C.; Resende, R.R.; Magno, L.A.V.; Romano-Silva, M.A.; Mintz, A.; et al. Neural stem cell niche heterogeneity. Semin. Cell Dev. Biol. 2019, 95, 42–53. [Google Scholar] [CrossRef]
- Yoo, S.; Blackshaw, S. Regulation and function of neurogenesis in the adult mammalian hypothalamus. Prog. Neurobiol. 2018, 170, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Bruni, J.E.; Del Bigio, M.R.; Clattenburg, R.E. Ependyma: Normal and pathological. A review of the literature. Brain Res. 1985, 356, 1–19. [Google Scholar] [CrossRef]
- Jacquet, B.V.; Patel, M.; Iyengar, M.; Liang, H.; Therit, B.; Salinas-Mondragon, R.; Lai, C.; Olsen, J.C.; Anton, E.S.; Ghashghaei, H.T. Analysis of neuronal proliferation, migration and differentiation in the postnatal brain using equine infectious anemia virus-based lentiviral vectors. Gene Ther. 2009, 16, 1021–1033. [Google Scholar] [CrossRef]
- Kyrousi, C.; Arbi, M.; Pilz, G.A.; Pefani, D.E.; Lalioti, M.E.; Ninkovic, J.; Götz, M.; Lygerou, Z.; Taraviras, S. Mcidas and GemC1 are key regulators for the generation of multiciliated ependymal cells in the adult neurogenic niche. Development 2015, 142, 3661–3674. [Google Scholar] [CrossRef] [PubMed]
- Mirzadeh, Z.; Merkle, F.T.; Soriano-Navarro, M.; Garcia-Verdugo, J.M.; Alvarez-Buylla, A. Neural stem cells confer unique pinwheel architecture to the ventricular surface in neurogenic regions of the adult brain. Cell Stem Cell 2008, 3, 265–278. [Google Scholar] [CrossRef]
- Paez-Gonzalez, P.; Abdi, K.; Luciano, D.; Liu, Y.; Soriano-Navarro, M.; Rawlins, E.; Bennett, V.; Garcia-Verdugo, J.M.; Kuo, C.T. Ank3-dependent SVZ niche assembly is required for the continued production of new neurons. Neuron 2011, 71, 61–75. [Google Scholar] [CrossRef]
- Spassky, N.; Merkle, F.T.; Flames, N.; Tramontin, A.D.; García-Verdugo, J.M.; Alvarez-Buylla, A. Adult ependymal cells are postmitotic and are derived from radial glial cells during embryogenesis. J. Neurosci. 2005, 25, 10–18. [Google Scholar] [CrossRef]
- Doetsch, F.; Caillé, I.; Lim, D.A.; García-Verdugo, J.M.; Alvarez-Buylla, A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell 1999, 97, 703–716. [Google Scholar] [CrossRef]
- Kriegstein, A.; Alvarez-Buylla, A. The glial nature of embryonic and adult neural stem cells. Annu. Rev. Neurosci. 2009, 32, 149–184. [Google Scholar] [CrossRef]
- Doetsch, F.; García-Verdugo, J.M.; Alvarez-Buylla, A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J. Neurosci. 1997, 17, 5046–5061. [Google Scholar] [CrossRef]
- García-Verdugo, J.M.; Doetsch, F.; Wichterle, H.; Lim, D.A.; Alvarez-Buylla, A. Architecture and cell types of the adult subventricular zone: In search of the stem cells. J. Neurobiol. 1998, 36, 234–248. [Google Scholar] [CrossRef]
- Bozoyan, L.; Khlghatyan, J.; Saghatelyan, A. Astrocytes control the development of the migration-promoting vasculature scaffold in the postnatal brain via VEGF signaling. J. Neurosci. 2012, 32, 1687–1704. [Google Scholar] [CrossRef]
- Platel, J.C.; Dave, K.A.; Gordon, V.; Lacar, B.; Rubio, M.E.; Bordey, A. NMDA receptors activated by subventricular zone astrocytic glutamate are critical for neuroblast survival prior to entering a synaptic network. Neuron 2010, 65, 859–872. [Google Scholar] [CrossRef]
- Mason, H.A.; Ito, S.; Corfas, G. Extracellular signals that regulate the tangential migration of olfactory bulb neuronal precursors: Inducers, inhibitors, and repellents. J. Neurosci. 2001, 21, 7654–7663. [Google Scholar] [CrossRef]
- Arshad, A.; Vose, L.R.; Vinukonda, G.; Hu, F.; Yoshikawa, K.; Csiszar, A.; Brumberg, J.C.; Ballabh, P. Extended Production of Cortical Interneurons into the Third Trimester of Human Gestation. Cereb. Cortex 2016, 26, 2242–2256. [Google Scholar] [CrossRef]
- Paredes, M.F.; James, D.; Gil-Perotin, S.; Kim, H.; Cotter, J.A.; Ng, C.; Sandoval, K.; Rowitch, D.H.; Xu, D.; McQuillen, P.S.; et al. Extensive migration of young neurons into the infant human frontal lobe. Science 2016, 354, aaf7073. [Google Scholar] [CrossRef]
- Sanai, N.; Nguyen, T.; Ihrie, R.A.; Mirzadeh, Z.; Tsai, H.H.; Wong, M.; Gupta, N.; Berger, M.S.; Huang, E.; Garcia-Verdugo, J.M.; et al. Corridors of migrating neurons in the human brain and their decline during infancy. Nat. Cell Biol. 2011, 478, 382–386. [Google Scholar] [CrossRef]
- Bergmann, O.; Liebl, J.; Bernard, S.; Alkass, K.; Yeung, M.S.; Steier, P.; Kutschera, W.; Johnson, L.; Landén, M.; Druid, H.; et al. The age of olfactory bulb neurons in humans. Neuron 2012, 74, 634–639. [Google Scholar] [CrossRef]
- Quiñones-Hinojosa, A.; Sanai, N.; Soriano-Navarro, M.; Gonzalez-Perez, O.; Mirzadeh, Z.; Gil-Perotin, S.; Romero-Rodriguez, R.; Berger, M.S.; Garcia-Verdugo, J.M.; Alvarez-Buylla, A. Cellular composition and cytoarchitecture of the adult human subventricular zone: A niche of neural stem cells. J. Comp. Neurol. 2006, 494, 415–434. [Google Scholar] [CrossRef]
- Wang, C.; Liu, F.; Liu, Y.Y.; Zhao, C.H.; You, Y.; Wang, L.; Zhang, J.; Wei, B.; Ma, T.; Zhang, Q.; et al. Identification and characterization of neuroblasts in the subventricular zone and rostral migratory stream of the adult human brain. Cell Res. 2011, 21, 1534–1550. [Google Scholar] [CrossRef]
- LaMonica, B.E.; Lui, J.H.; Wang, X.; Kriegstein, A.R. OSVZ progenitors in the human cortex: An updated perspective on neurodevelopmental disease. Curr. Opin. Neurobiol. 2012, 22, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Lui, J.H.; Hansen, D.V.; Kriegstein, A.R. Development and evolution of the human neocortex. Cell 2011, 146, 18–36. [Google Scholar] [CrossRef] [PubMed]
- Menn, B.; Garcia-Verdugo, J.M.; Yaschine, C.; Gonzalez-Perez, O.; Rowitch, D.; Alvarez-Buylla, A. Origin of oligodendrocytes in the subventricular zone of the adult brain. J. Neurosci. 2006, 26, 7907–7918. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Perez, O.; Romero-Rodriguez, R.; Soriano-Navarro, M.; Garcia-Verdugo, J.M.; Alvarez-Buylla, A. Epidermal growth factor induces the progeny of subventricular zone type B cells to migrate and differentiate into oligodendrocytes. Stem Cells 2009, 27, 2032–2043. [Google Scholar] [CrossRef]
- Talaverón, R.; Fernández, P.; Escamilla, B.; Pastor, A.M.; Matarredona, E.R.; Sáez, J.C. Neural progenitor cells isolated from the subventricular zone present hemichannel activity and form functional gap junctions with glial cells. Front. Cell. Neurosci. 2015, 9, 411. [Google Scholar] [CrossRef]
- Marins, M.; Xavier, A.L.R.; Viana, N.B.; Fortes, F.S.A.; Fróes, M.M.; Menezes, J.R.L. Gap junctions are involved in cell migration in the early postnatal subventricular zone. Dev. Neurobiol. 2009, 69, 715–730. [Google Scholar] [CrossRef]
- Freitas, A.S.; Xavier, A.L.R.; Furtado, C.M.; Hedin-Pereira, C.; Fróes, M.M.; Menezes, J.R.L. Dye coupling and connexin expression by cortical radial glia in the early postnatal subventricular zone. Dev. Neurobiol. 2012, 72, 1482–1497. [Google Scholar] [CrossRef]
- Lacar, B.; Young, S.Z.; Platel, J.C.; Bordey, A. Gap junction-mediated calcium waves define communication networks among murine postnatal neural progenitor cells. Eur. J. Neurosci. 2011, 34, 1895–1905. [Google Scholar] [CrossRef]
- Liu, X.; Bolteus, A.J.; Balkin, D.M.; Henschel, O.; Bordey, A. GFAP-expressing cells in the postnatal subventricular zone display a unique glial phenotype intermediate between radial glia and astrocytes. Glia 2006, 54, 394–410. [Google Scholar] [CrossRef]
- Miragall, F.; Albiez, P.; Bartels, H.; de Vries, U.; Dermietzel, R. Expression of the gap junction protein connexin43 in the subependymal layer and the rostral migratory stream of the mouse: Evidence for an inverse correlation between intensity of connexin43 expression and cell proliferation activity. Cell. Tissue Res. 1997, 287, 243–253. [Google Scholar]
- Khodosevich, K.; Zuccotti, A.; Kreuzberg, M.M.; Le Magueresse, C.; Frank, M.; Willecke, K.; Monyer, H. Connexin45 modulates the proliferation of transit-amplifying precursor cells in the mouse subventricular zone. Proc. Natl. Acad. Sci. USA 2012, 109, 20107–20112. [Google Scholar] [CrossRef] [PubMed]
- Duval, N.; Gomès, D.; Calaora, V.; Calabrese, A.; Meda, P.; Bruzzone, R. Cell coupling and Cx43 expression in embryonic mouse neural progenitor cells. J. Cell Sci. 2002, 115, 3241–3251. [Google Scholar] [PubMed]
- Talaverón, R.; Matarredona, E.R.; de la Cruz, R.R.; Macías, D.; Gálvez, V.; Pastor, A.M. Implanted neural progenitor cells regulate glial reaction to brain injury and establish gap junctions with host glial cells. Glia 2014, 62, 623–638. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.; Tang, H.; Cai, J.; Zhu, M.; Zhang, X.; Rao, M.; Mattson, M.P. Gap junctional communication is required to maintain mouse cortical neural progenitor cells in a proliferative state. Dev. Biol. 2004, 272, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Lemcke, H.; Kuznetsov, S.A. Involvement of connexin43 in the EGF/EGFR signalling during self-renewal and differentiation of neural progenitor cells. Cell. Signal. 2013, 25, 2676–2684. [Google Scholar] [CrossRef]
- Imbeault, S.; Gauvin, L.G.; Toeg, H.D.; Pettit, A.; Sorbara, C.D.; Migahed, L.; DesRoches, R.; Menzies, A.S.; Nishii, K.; Paul, D.L.; et al. The extracellular matrix controls gap junction protein expression and function in postnatal hippocampal neural progenitor cells. BMC Neurosci. 2009, 10, 13. [Google Scholar] [CrossRef]
- Rozental, R.; Mehler, M.F.; Morales, M.; Andrade-Rozental, A.F.; Kessler, J.A.; Spray, D.C. Differentiation of hippocampal progenitor cells in vitro: Temporal expression of intercellular coupling and voltage- and ligand-gated responses. Dev. Biol. 1995, 167, 350–362. [Google Scholar] [CrossRef]
- Rozental, R.; Srinivas, M.; Gökhan, S.; Urban, M.; Dermietzel, R.; Kessler, J.A.; Spray, D.C.; Mehler, M.F. Temporal expression of neuronal connexins during hippocampal ontogeny. Brain Res. Rev. 2000, 32, 57–71. [Google Scholar] [CrossRef]
- Kunze, A.; Congreso, M.R.; Hartmann, C.; Wallraff-Beck, A.; Hüttmann, K.; Bedner, P.; Requardt, R.; Seifert, G.; Redecker, C.; Willecke, K.; et al. Connexin expression by radial glia-like cells is required for neurogenesis in the adult dentate gyrus. Proc. Natl. Acad. Sci. USA 2009, 106, 11336–11341. [Google Scholar] [CrossRef]
- Chen, Y.; Deng, Y.; Bao, X.; Reuss, L.; Altenberg, G.A. Mechanism of the defect in gap-junctional communication by expression of a connexin 26 mutant associated with dominant deafness. FASEB J. 2005, 19, 1516–1518. [Google Scholar] [CrossRef]
- Retamal, M.A.; Cortés, C.J.; Reuss, L.; Bennett, M.V.L.; Sáez, J.C. S-nitrosylation and permeation through connexin 43 hemichannels in astrocytes: Induction by oxidant stress and reversal by reducing agents. Proc. Natl. Acad. Sci. USA 2006, 103, 4475–4480. [Google Scholar] [CrossRef] [PubMed]
- Altman, J.; Das, G.D. Post-natal origin of microneurones in the rat brain. Nat. Cell Biol. 1965, 207, 953–956. [Google Scholar] [CrossRef]
- Bauer, S.; Patterson, P.H. The cell cycle-apoptosis connection revisited in the adult brain. J. Cell Biol. 2005, 171, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Rakic, P. Neurogenesis in adult primates. Prog. Brain Res. 2002, 138, 3–14. [Google Scholar] [PubMed]
- Moreno-Jiménez, E.P.; Flor-García, M.; Terreros-Roncal, J.; Rábano, A.; Cafini, F.; Pallas-Bazarra, N.; Ávila, J.; Llorens-Martín, M. Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer’s disease. Nat. Med. 2019, 25, 554–560. [Google Scholar] [CrossRef]
- Fuentealba, L.C.; Obernier, K.; Alvarez-Buylla, A. Adult neural stem cells bridge their niche. Cell Stem Cell 2012, 10, 698–708. [Google Scholar] [CrossRef]
- Ginhoux, F.; Greter, M.; Leboeuf, M.; Nandi, S.; See, P.; Gokhan, S.; Mehler, M.F.; Conway, S.J.; Ng, L.G.; Stanley, E.R.; et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 2010, 330, 841–845. [Google Scholar] [CrossRef]
- Ginhoux, F.; Lim, S.; Hoeffel, G.; Low, D.; Huber, T. Origin and differentiation of microglia. Front. Cell. Neurosci. 2013, 7, 45. [Google Scholar] [CrossRef]
- Kreutzberg, G.W. Microglia: A sensor for pathological events in the CNS. Trends Neurosci. 1996, 19, 312–318. [Google Scholar] [CrossRef]
- Hanisch, U.K.; Kettenmann, H. Microglia: Active sensor and versatile effector cells in the normal and pathologic brain. Nat. Neurosci. 2007, 10, 1387–1394. [Google Scholar] [CrossRef]
- Ransohoff, R.M.; Perry, V.H. Microglial physiology: Unique stimuli, specialized responses. Annu. Rev. Immunol. 2009, 27, 119–145. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Barres, B.A. Microglia and macrophages in brain homeostasis and disease. Nat. Rev. Immunol. 2018, 18, 225–242. [Google Scholar] [CrossRef] [PubMed]
- Schafer, D.P.; Lehrman, E.K.; Stevens, B. The “quad-partite” synapse: Microglia-synapse interactions in the developing and mature CNS. Glia 2013, 61, 24–36. [Google Scholar] [CrossRef] [PubMed]
- Wake, H.; Moorhouse, A.J.; Miyamoto, A.; Nabekura, J. Microglia: Actively surveying and shaping neuronal circuit structure and function. Trends Neurosci. 2013, 36, 209–217. [Google Scholar] [CrossRef]
- Wu, Y.; Dissing-Olesen, L.; MacVicar, B.A.; Stevens, B. Microglia: Dynamic Mediators of Synapse Development and Plasticity. Trends Immunol. 2015, 36, 605–613. [Google Scholar] [CrossRef]
- Salter, M.W.; Stevens, B. Microglia emerge as central players in brain disease. Nat. Med. 2017, 23, 1018–1027. [Google Scholar] [CrossRef]
- Kettenmann, H.; Kirchhoff, F.; Verkhratsky, A. Microglia: New roles for the synaptic stripper. Neuron 2013, 77, 10–18. [Google Scholar] [CrossRef]
- Stellwagen, D.; Malenka, R.C. Synaptic scaling mediated by glial TNF-alpha. Nature 2006, 440, 1054–1059. [Google Scholar] [CrossRef]
- Bessis, A.; Béchade, C.; Bernard, D.; Roumier, A. Microglial control of neuronal death and synaptic properties. Glia 2007, 55, 233–238. [Google Scholar] [CrossRef]
- Wake, H.; Moorhouse, A.J.; Jinno, S.; Kohsaka, S.; Nabekura, J. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J. Neurosci. 2009, 29, 3974–3980. [Google Scholar] [CrossRef]
- Béchade, C.; Cantaut-Belarif, Y.; Bessis, A. Microglial control of neuronal activity. Front. Cell. Neurosci. 2013, 7, 32. [Google Scholar] [CrossRef]
- Parenti, R.; Campisi, A.; Vanella, A.; Cicirata, F. Immunocytochemical and RT-PCR analysis of connexin36 in cultures of mammalian glial cells. Arch. Ital. Biol. 2002, 140, 101–108. [Google Scholar] [PubMed]
- Cepeda, C.; Chang, J.W.; Owens, G.C.; Huynh, M.N.; Chen, J.Y.; Tran, C.; Vinters, H.; Levine, M.S.; Mathern, G.W. In Rasmussen encephalitis, hemichannels associated with microglial activation are linked to cortical pyramidal neuron coupling: A possible mechanism for cellular hyperexcitability. CNS Neurosci. Ther. 2015, 21, 152–163. [Google Scholar] [CrossRef]
- Matarredona, E.R.; Talaverón, R.; Pastor, A.M. Interactions Between Neural Progenitor Cells and Microglia in the Subventricular Zone: Physiological Implications in the Neurogenic Niche and After Implantation in the Injured Brain. Front. Cell. Neurosci. 2018, 12, 268. [Google Scholar] [CrossRef]
- Wasseff, S.K.; Scherer, S.S. Activated microglia do not form functional gap junctions in vivo. J. Neuroimmunol. 2014, 269, 90–93. [Google Scholar] [CrossRef] [PubMed]
- Martínez, A.D.; Eugenín, E.A.; Brañes, M.C.; Bennett, M.V.L.; Sáez, J.C. Identification of second messengers that induce expression of functional gap junctions in microglia cultured from newborn rats. Brain Res. 2002, 943, 191–201. [Google Scholar] [CrossRef]
- Maezawa, I.; Jin, L.W. Rett syndrome microglia damage dendrites and synapses by the elevated release of glutamate. J. Neurosci. 2010, 30, 5346–5356. [Google Scholar] [CrossRef]
- Sáez, P.J.; Shoji, K.F.; Retamal, M.A.; Harcha, P.A.; Ramírez, G.; Jiang, J.X.; von Bernhardi, R.; Sáez, J.C. ATP is required and advances cytokine-induced gap junction formation in microglia in vitro. Mediat. Inflamm. 2013, 2013, 216402. [Google Scholar] [CrossRef]
- Martino, G.; Pluchino, S. The therapeutic potential of neural stem cells. Nat. Rev. Neurosci. 2006, 7, 395–406. [Google Scholar] [CrossRef]
- Ribeiro Xavier, A.; Kress, B.T.; Goldman, S.A.; Lacerda de Menezes, J.R.; Nedergaard, M. A Distinct Population of Microglia Supports Adult Neurogenesis in the Subventricular Zone. J. Neurosci. 2015, 35, 11848–11861. [Google Scholar] [CrossRef]
- Xavier, A.L.; Lima, F.R.S.; Nedergaard, M.; Menezes, J.R.L. Ontogeny of CX3CR1-EGFP expressing cells unveil microglia as an integral component of the postnatal subventricular zone. Front. Cell. Neurosci. 2015, 9, 37. [Google Scholar] [CrossRef] [PubMed]
- Solano Fonseca, R.; Mahesula, S.; Apple, D.M.; Raghunathan, R.; Dugan, A.; Cardona, A.; O’Connor, J.; Kokovay, E. Neurogenic Niche Microglia Undergo Positional Remodeling and Progressive Activation Contributing to Age-Associated Reductions in Neurogenesis. Stem Cells Dev. 2016, 25, 542–555. [Google Scholar] [CrossRef] [PubMed]
- Aarum, J.; Sandberg, K.; Haeberlein, S.L.; Persson, M.A. Migration and differentiation of neural precursor cells can be directed by microglia. Proc. Natl. Acad. Sci. USA 2003, 100, 15983–15988. [Google Scholar] [CrossRef] [PubMed]
- Walton, N.M.; Sutter, B.M.; Laywell, E.D.; Levkoff, L.H.; Kearns, S.M.; Marshall, G.P.; Scheffler, B.; Steindler, D.A. Microglia instruct subventricular zone neurogenesis. Glia 2006, 54, 815–825. [Google Scholar] [CrossRef] [PubMed]
- Hurtado-Chong, A.; Yusta-Boyo, M.J.; Vergaño-Vera, E.; Bulfone, A.; de Pablo, F.; Vicario-Abejón, C. IGF-I promotes neuronal migration and positioning in the olfactory bulb and the exit of neuroblasts from the subventricular zone. Eur. J. Neurosci. 2009, 30, 742–755. [Google Scholar] [CrossRef] [PubMed]
- Sierra, A.; Encinas, J.M.; Deudero, J.J.; Chancey, J.H.; Enikolopov, G.; Overstreet-Wadiche, L.S.; Tsirka, S.E.; Maletic-Savatic, M. Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell Stem Cell 2010, 7, 483–495. [Google Scholar] [CrossRef]
- Hansen, D.B.; Braunstein, T.H.; Nielsen, M.S.; MacAulay, N. Distinct permeation profiles of the connexin 30 and 43 hemichannels. FEBS Lett. 2014, 588, 1446–1457. [Google Scholar] [CrossRef]
- Medina-Ceja, L.; Salazar-Sánchez, J.C.; Ortega-Ibarra, J.; Morales-Villagrán, A. Connexins-Based Hemichannels/Channels and Their Relationship with Inflammation, Seizures and Epilepsy. Int. J. Mol. Sci. 2019, 20, 5976. [Google Scholar] [CrossRef]
- Stehberg, J.; Moraga-Amaro, R.; Salazar, C.; Becerra, A.; Echeverría, C.; Orellana, J.A.; Bultynck, G.; Ponsaerts, R.; Leybaert, L.; Simon, F.; et al. Release of gliotransmitters through astroglial connexin 43 hemichannels is necessary for fear memory consolidation in the basolateral amygdala. FASEB J. 2012, 26, 3649–3657. [Google Scholar] [CrossRef]
- Kim, Y.; Davidson, J.O.; Green, C.R.; Nicholson, L.F.B.; O’Carroll, S.J.; Zhang, J. Connexins and Pannexins in cerebral ischemia. Biochim. Biophys. Acta Biomembr. 2018, 1860, 224–236. [Google Scholar] [CrossRef]
- Froger, N.; Orellana, J.A.; Calvo, C.F.; Amigou, E.; Kozoriz, M.G.; Naus, C.C.; Sáez, J.C.; Giaume, C. Inhibition of cytokine-induced connexin43 hemichannel activity in astrocytes is neuroprotective. Mol. Cell. Neurosci. 2010, 45, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Sofroniew, M.V. Reactive astrocytes in neural repair and protection. Neuroscientist 2005, 11, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Akira, S.; Takeda, K. Toll-like receptor signalling. Nat. Rev. Immunol. 2004, 4, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Marinelli, S.; Basilico, B.; Marrone, M.C.; Ragozzino, D. Microglia-neuron crosstalk: Signaling mechanism and control of synaptic transmission. Semin Cell Dev Biol. 2019, 94, 138–151. [Google Scholar] [CrossRef]
- Mandolesi, G.; Musella, A.; Gentile, A.; Grasselli, G.; Haji, N.; Sepman, H.; Fresegna, D.; Bullitta, S.; De Vito, F.; Musumeci, G.; et al. Interleukin-1β alters glutamate transmission at purkinje cell synapses in a mouse model of multiple sclerosis. J. Neurosci. 2013, 33, 12105–12121. [Google Scholar] [CrossRef]
- Takaki, J.; Fujimori, K.; Miura, M.; Suzuki, T.; Sekino, Y.; Sato, K. L-glutamate released from activated microglia downregulates astrocytic L-glutamate transporter expression in neuroinflammation: The ‘collusion’ hypothesis for increased extracellular L-glutamate concentration in neuroinflammation. J. Neuroinflammation 2012, 9, 275. [Google Scholar] [CrossRef]
- Karpuk, N.; Burkovetskaya, M.; Fritz, T.; Angle, A.; Kielian, T. Neuroinflammation leads to region-dependent alterations in astrocyte gap junction communication and hemichannel activity. J. Neurosci. 2011, 31, 414–425. [Google Scholar] [CrossRef]
- Turecek, J.; Yuen, G.S.; Han, V.Z.; Zeng, X.H.; Bayer, K.U.; Welsh, J.P. NMDA receptor activation strengthens weak electrical coupling in mammalian brain. Neuron 2014, 81, 1375–1388. [Google Scholar] [CrossRef][Green Version]
- Simard, M.; Nedergaard, M. The neurobiology of glia in the context of water and ion homeostasis. Neuroscience 2004, 129, 877–896. [Google Scholar] [CrossRef]
- Schiza, N.; Sargiannidou, I.; Kagiava, A.; Karaiskos, C.; Nearchou, M.; Kleopa, K.A. Transgenic replacement of Cx32 in gap junction-deficient oligodendrocytes rescues the phenotype of a hypomyelinating leukodystrophy model. Hum. Mol. Genet. 2015, 24, 2049–2064. [Google Scholar] [CrossRef][Green Version]
- May, D.; Tress, O.; Seifert, G.; Willecke, K. Connexin47 protein phosphorylation and stability in oligodendrocytes depend on expression of Connexin43 protein in astrocytes. J. Neurosci. 2013, 33, 7985–7996. [Google Scholar] [CrossRef]
- Trosko, J.E.; Yotti, L.P.; Warren, S.T.; Tsushimoto, G.; Chang, C. Inhibition of cell-cell communication by tumour promoters. Carcinog. Compr. Surv. 1982, 7, 565–585. [Google Scholar]
- Budunova, I.V.; Williams, G.M. Cell culture assays for chemicals with tumour-promoting or tumour-inhibiting activity based on the modulation of intercellular communication. Cell. Biol. Toxicol. 1994, 10, 71–116. [Google Scholar] [CrossRef]
- Sun, Y.; Nakashima, M.N.; Takahashi, M.; Kuroda, N.; Nakashima, K. Determination of bisphenol A in rat brain by microdialysis and column switching high-performance liquid chromatography with fluorescence detection. Biomed. Chromatogr. 2002, 16, 319–326. [Google Scholar] [CrossRef]
- Miyazaki, W.; Fujiwara, Y.; Katoh, T. The effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin on the development and function of the blood-brain barrier. Neurotoxicology 2016, 52, 64–71. [Google Scholar] [CrossRef]
- Defamie, N.; Mesnil, M. The modulation of gap-junctional intercellular communication by lipid rafts. Biochim. Biophys. Acta 2012, 1818, 1866–1869. [Google Scholar] [CrossRef]
- Oh, S. Bisphenol A and 4-tert-Octylphenol Inhibit Cx46 Hemichannel. Currents Korean J. Physiol. Pharmacol. 2015, 19, 73–79. [Google Scholar] [CrossRef][Green Version]
- Cheng, C.Y.; Wong, E.W.; Lie, P.P.; Li, M.W.; Su, L.; Siu, E.R.; Yan, H.; Mannu, J.; Mathur, P.P.; Bonanomi, M.; et al. Environmental toxicants and male reproductive function. Spermatogenesis 2011, 1, 2–13. [Google Scholar] [CrossRef]
- Cheng, C.Y.; Wong, E.W.; Lie, P.P.; Li, M.W.; Mruk, D.D.; Yan, H.H.; Mok, K.W.; Mannu, J.; Mathur, P.P.; Lui, W.Y.; et al. Regulation of blood-testis barrier dynamics by desmosome, gap junction, hemidesmosome and polarity proteins: An unexpected turn of events. Spermatogenesis 2011, 1, 105–115. [Google Scholar] [CrossRef]
- Tsushimoto, G.; Chang, C.C.; Trosko, J.E.; Matsumura, E. Cytotoxic, Mutagenic, and Cell-Cell Communication Inhibitory Properties of DDT, Lindane, and Chlordane on Chinese Hamster Cells in vitro. Arch. Environ. Contain. Toxicol. 1983, 12, 721–730. [Google Scholar] [CrossRef]
- Kang, K.S.; Wilson, M.R.; Hayashi, T.; Chang, C.C.; Trosko, J.E. Inhibition of gap junctional intercellular communication in normal human breast epithelial cells after treatment with pesticides, PCBs, and PBBs, alone or in mixtures. Environ. Health Persp. 1996, 104, 192–200. [Google Scholar]
- Ruch, R.J.; Klaunig, J.E. Effects of tumor promoters, genotoxic carcinogens and hepatocytotoxins on mouse hepatocyte intercellular communication. Cell. Biol. Toxicol. 1986, 2, 469–483. [Google Scholar] [CrossRef] [PubMed]
- Criswell, K.A.; Loch-Caruso, R.; Stuenkel, E.L. Lindane inhibition of gap junctional communication in myometrial myocytes is partially dependent on phosphoinositide-generated second messengers. Toxicol. Appl. Pharmacol. 1995, 130, 280–293. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Bonney, W.J.; Ruch, R.J. Changes in gap junction permeability, gap junction number, and connexin43 expression in lindane-treated rat liver epithelial cells. Toxicol. Appl. Pharmacol. 1995, 130, 79–86. [Google Scholar] [CrossRef]
- Defamie, N.; Mograbi, B.; Roger, C.; Cronier, L.; Malassine, A.; Brucker-Davis, F.; Fenichel, P.; Segretain, D.; Pointis, G. Disruption of gap junctional intercellular communication by lindane is associated with aberrant localization of connexin43 and zonula occludens-1 in 42GPA9 Sertoli cells. Carcinogenesis 2001, 22, 1537–1542. [Google Scholar] [CrossRef]
- Leithe, E.; Kjenseth, A.; Bruun, J.; Sirnes, S.; Rivedal, E. Inhibition of connexin43 gap junction channels by the endocrine disruptor ioxynil. Toxicol. Appl. Pharmacol. 2010, 247, 10–17. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, S.; Liu, L.; Hou, X.; Jiang, J.; Wei, X.; Hao, W. Effects of bisphenol A on gap junctions in HaCaT cells as mediated by the estrogen receptor pathway. J. Appl. Toxicol. 2019, 39, 271–281. [Google Scholar] [CrossRef]
- Salian, S.; Doshi, T.; Vanage, G. Neonatal exposure of male rats to Bisphenol A impairs fertility and expression of sertoli cell junctional proteins in the testis. Toxicology 2009, 265, 56–67. [Google Scholar] [CrossRef]
- Simecková, P.; Vondrácek, J.; Andrysík, Z.; Zatloukalová, J.; Krcmár, P.; Kozubík, A.; Machala, M. The 2,2′,4,4′,5,5′-hexachlorobiphenyl-enhanced degradation of connexin 43 involves both proteasomal and lysosomal activities. Toxicol. Sci. 2009, 107, 9–18. [Google Scholar] [CrossRef]
- Lee, I.K.; Rhee, S.K. Inhibitory effect of bisphenol A on gap junctional intercellular communication in an epithelial cell line of rat mammary tissue. Arch Pharm Res. 2007, 30, 337–343. [Google Scholar] [CrossRef]
- Rochester, J.R. Bisphenol A and human health: A review of the literature. Reprod. Toxicol. 2013, 42, 132–155. [Google Scholar] [CrossRef] [PubMed]
- Sovadinova, I.; Babica, P.; Böke, H.; Kumar, E.; Wilke, A.; Park, J.S.; Trosko, J.E.; Upham, B.L. Phosphatidylcholine Specific PLC-Induced Dysregulation of Gap Junctions, a Robust Cellular Response to Environmental Toxicants, and Prevention by Resveratrol in a Rat Liver Cell Model. PLoS ONE 2015, 10, e0124454. [Google Scholar] [CrossRef]
- Babica, P.; Zurabian, R.; Kumar, E.R.; Chopra, R.; Mianecki, M.J.; Park, J.S.; Jaša, L.; Trosko, J.E.; Upham, B.L. Methoxychlor and Vinclozolin Induce Rapid Changes in Intercellular and Intracellular Signaling in Liver Progenitor Cells. Toxicol. Sci. 2016, 153, 174–185. [Google Scholar] [CrossRef] [PubMed]
- Čtveráčková, L.; Jančula, D.; Raška, J.; Babica, P.; Sovadinová, I. Structure-Dependent Effects of Phthalates on Intercellular and Intracellular Communication in Liver Oval Cells. Int. J. Mol. Sci. 2020, 21, E6069. [Google Scholar] [CrossRef] [PubMed]
- Machala, M.; Bláha, L.; Vondrácek, J.; Trosko, J.E.; Scott, J.; Upham, B.L. Inhibition of gap junctional intercellular communication by noncoplanar polychlorinated biphenyls: Inhibitory potencies and screening for potential mode(s) of action. Toxicol. Sci. 2003, 76, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Pierucci, F.; Frati, A.; Squecco, R.; Lenci, E.; Vicenti, C.; Slavik, J.; Francini, F.; Machala, M.; Meacci, E. Non-dioxin-like organic toxicant PCB153 modulates sphingolipid metabolism in liver progenitor cells: Its role in Cx43-formed gap junction impairment. Arch. Toxicol. 2017, 91, 749–760. [Google Scholar] [CrossRef] [PubMed]
- Upham, B.L.; Bláha, L.; Babica, P.; Park, J.S.; Sovadinova, I.; Pudrith, C.; Rummel, A.M.; Weis, L.M.; Sai, K.; Tithof, P.K.; et al. Tumor promoting properties of a cigarette smoke prevalent polycyclic aromatic hydrocarbon as indicated by the inhibition of gap junctional intercellular communication via phosphatidylcholine-specific phospholipase C. Cancer Sci. 2008, 99, 696–705. [Google Scholar] [CrossRef]
- Gakhar, G.; Schrempp, D.; Nguyen, T.A. Regulation of gap junctional intercellular communication by TCDD in HMEC and MCF-7 breast cancer cells. Toxicol. Appl. Pharm. 2009, 235, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.M.; Telang, S.; Tong, C. Inhibition of intercellular communication between liver cells by the liver tumor promoter l,l,1-trichloro-2,2-bis(p-chlorophenyl) ethane. Cancer Lett. 1981, 11, 339–344. [Google Scholar] [CrossRef]
- Stern, A.H. Hazard identification of the potential for dieldrin carcinogenicity to humans. Environ. Res. 2014, 131, 188–214. [Google Scholar] [CrossRef]
- Telang, S.; Tong, C.; Williams, G.M. Epigenetic membrane effects of a possible tumor promoting type on cultured liver cells by the non-genotoxic organochlorine pesticides chlordane and heptachlor. Carcinogenesis 1982, 3, 1175–1178. [Google Scholar] [CrossRef] [PubMed]
- Ruch, R.J.; Fransson, R.; Flodstrom, S.; Warngard, L.; Klaunig, J.E. Inhibition of hepatocyte gap junctional intercellular communication by endosulfan, chlordane and heptachlor. Carcinogenesis 1990, 11, 1097–1101. [Google Scholar] [CrossRef] [PubMed]
- Tiemann, U.; Pohland, R. Inhibitory effects of organochlorine pesticides on intercellular transfer of lucifer yellow in cultured bovine oviductal cells. Reprod. Toxicol. 1999, 13, 123–130. [Google Scholar] [CrossRef]
- Mograbi, B.; Corcelle, E.; Defamie, N.; Samson, M.; Nebout, M.; Segretain, D.; Fénichel, P.; Pointis, G. Aberrant Connexin 43 endocytosis by the carcinogen lindane involves activation of the ERK/mitogen-activated protein kinase pathway. Carcinogenesis 2003, 24, 1415–1423. [Google Scholar] [CrossRef]
- Tsushimoto, G.; Trosko, J.E.; Chang, C.C.; Matsumura, F. Inhibition of intercellular communication by chlordecone (kepone) and mirex in Chinese hamster V79 cells in vitro. Toxicol. Appl. Pharm. 1982, 64, 550–556. [Google Scholar] [CrossRef]
- Caldwell, V.; Loch-Caruso, R. Chlordecone rapidly and reversibly inhibits gap junctional communication in human embryonic palatal mesenchyme cells. In Vitro Toxicol. 1992, 5, 113–122. [Google Scholar]
- Campen, K.A.; McNatty, K.P.; Pitman, J.L. A protective role of cumulus cells after short-term exposure of rat cumulus cell-oocyte complexes to lifestyle or environmental contaminants. Reprod. Toxicol. 2017, 69, 19–33. [Google Scholar] [CrossRef]
- Acuña-Hernández, D.G.; Arreola-Mendoza, L.; Santacruz-Márquez, R.; García-Zepeda, S.P.; Parra-Forero, L.Y.; Olivares-Reyes, J.A.; Hernández-Ochoa, I. Bisphenol A alters oocyte maturation by prematurely closing gap junctions in the cumulus cell-oocyte complex. Toxicol. Appl. Pharmacol. 2018, 344, 13–22. [Google Scholar] [CrossRef]
- Klaunig, J.E.; Ruch, R.J.; DeAngelo, A.B.; Kaylor, W.H. Inhibition of mouse hepatocyte intercellular communication by phthalate monoesters. Cancer Lett. 1988, 43, 65–71. [Google Scholar] [CrossRef]
- Smith, J.H.; Isenberg, J.S.; Pugh, G., Jr.; Kamendulis, L.M.; Ackley, D.; Lington, A.W.; Klaunig, J.E. Comparative in vivo hepatic effects of Di-isononyl phthalate (DINP) and related C7-C11 dialkyl phthalates on gap junctional intercellular communication (GJIC), peroxisomal beta-oxidation (PBOX), and DNA synthesis in rat and mouse liver. Toxicol. Sci. 2000, 54, 312–321. [Google Scholar] [CrossRef]
- Kamendulis, L.M.; Isenberg, J.S.; Smith, J.H.; Pugh, G.J.; Lington, A.W.; Klaunig, J.E. Comparative effects of phthalate monoesters on gap junctional intercellular communication and peroxisome proliferation in rodent and primate hepatocytes. J. Toxicol. Environ. Health A 2002, 65, 569–588. [Google Scholar] [CrossRef] [PubMed]
- Machala, M.; Bláha, L.; Lehmler, H.J.; Plísková, M.; Májková, Z.; Kapplová, P.; Sovadinová, I.; Vondrácek, J.; Malmberg, T.; Robertson, L.W. Toxicity of hydroxylated and quinoid PCB metabolites: Inhibition of gap junctional intercellular communication and activation of aryl hydrocarbon and estrogen receptors in hepatic and mammary cells. Chem. Res. Toxicol. 2004, 17, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Trosko, J.E.; Dawson, B.; Chang, C.C. PBB inhibits metabolic cooperation in Chinese hamster cells in vitro: Its potential as a tumor promoter. Environ. Health Perspect. 1981, 37, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Tsushimoto, G.; Trosko, J.E.; Chang, C.C.; Aust, S.D. Inhibition of metabolic cooperation in Chinese hamster V79 cells in culture by various polybrominated biphenyl (PBB) congeners. Carcinogenesis 1982, 3, 181–185. [Google Scholar] [CrossRef]
- Bláha, L.; Kapplová, P.; Vondrácek, J.; Upham, B.; Machala, M. Inhibition of gap-junctional intercellular communication by environmentally occurring polycyclic aromatic hydrocarbons. Toxicol. Sci. 2002, 65, 43–51. [Google Scholar] [CrossRef]
- Andrysík, Z.; Procházková, J.; Kabátková, M.; Umannová, L.; Simečková, P.; Kohoutek, J.; Kozubík, A.; Machala, M.; Vondráček, J. Aryl hydrocarbon receptor-mediated disruption of contact inhibition is associated with connexin43 downregulation and inhibition of gap junctional intercellular communication. Arch. Toxicol. 2013, 87, 491–503. [Google Scholar] [CrossRef]
- Wang, F.F.; Zheng, C.J.; Guo, X.B. Effect of PM2.5 collected during the dust and non-dust periods on the viability and gap junctional intercellular communication in human lung fibroblasts. Wei Sheng Yan Jiu 2006, 35, 26–30. [Google Scholar]
- Theiss, C.; Meller, K. Aluminum impairs gap junctional intercellular communication between astroglial cells in vitro. Cell Tissue Res. 2002, 310, 143–154. [Google Scholar] [CrossRef]
- Yoshida, M.; Kujiraoka, T.; Hara, M.; Nakazawa, H.; Sumi, Y. Methylmercury inhibits gap junctional intercellular communication in primary cultures of rat proximal tubular cells. Arch. Toxicol. 1998, 72, 192–196. [Google Scholar] [CrossRef]
- Mansouri, A.; Cregut, M.; Abbes, C.; Durand, M.J.; Landoulsi, A.; Thouand, G. The Environmental Issues of DDT Pollution and Bioremediation: A Multidisciplinary Review. Appl. Biochem. Biotechnol. 2017, 181, 309–339. [Google Scholar] [CrossRef]
- Matsushima, A. A Novel Action of Endocrine-Disrupting Chemicals on Wildlife; DDT and Its Derivatives Have Remained in the Environment. Int. J. Mol. Sci. 2018, 19, 1377. [Google Scholar] [CrossRef]
- Cummings, A.M. Methoxychlor as a model for environmental estrogens. Crit. Rev. Toxicol. 1997, 27, 367–379. [Google Scholar] [CrossRef]
- IARC Working Group on the Evaluation of Carcinogenic Risk to Humans. DDT, Lindane, and 2,4-D. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; World Health Organization: Lyon, France, 2018; Volume 113. [Google Scholar]
- Brokamp, C.; Todd, J.; Montemagno, C.; Wendell, D. Electrophysiology of single and aggregate Cx43 hemichannels. PLoS ONE 2012, 7, e47775. [Google Scholar] [CrossRef]
- Epstein, S.S. Kepone—Hazard evaluation. Sci. Total Environ. 1978, 9, 1–62. [Google Scholar] [CrossRef]
- Dallaire, R.; Muckle, G.; Rouget, F.; Kadhel, P.; Bataille, H.; Guldner, L.; Seurin, S.; Chajès, V.; Monfort, C.; Boucher, O.; et al. Cognitive, visual, and motor development of 7-month-old Guadeloupean infants exposed to chlordecone. Environ. Res. 2012, 118, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Boucher, O.; Simard, M.N.; Muckle, G.; Rouget, F.; Kadhel, P.; Bataille, H.; Chajès, V.; Dallaire, R.; Monfort, C.; Thomé, J.P.; et al. Exposure to an organochlorine pesticide (chlordecone) and development of 18-month-old infants. Neurotoxicology 2013, 35, 162–168. [Google Scholar] [CrossRef]
- Nedellec, V.; Rabl, A.; Dab, W. Public health and chronic low chlordecone exposure in Guadeloupe, Part 1: Hazards, exposure-response functions, and exposures. Environ. Health 2016, 15, 75. [Google Scholar] [CrossRef]
- Kucklick, J.R.; Helm, P.A. Advances in the environmental analysis of polychlorinated naphthalenes and toxaphene. Anal. Bioanal. Chem. 2006, 386, 819–836. [Google Scholar] [CrossRef]
- McKinlay, R.; Plant, J.A.; Bell, J.N.; Voulvoulis, N. Endocrine disrupting pesticides: Implications for risk assessment. Environ. Int. 2008, 34, 168–183. [Google Scholar] [CrossRef] [PubMed]
- Van Ravenzwaay, B.; Kolle, S.N.; Ramirez, T.; Kamp, H.G. Vinclozolin: A case study on the identification of endocrine active substances in the past and a future perspective. Toxicol. Lett. 2013, 223, 271–279. [Google Scholar] [CrossRef]
- Vandenberg, L.N.; Hauser, R.; Marcus, M.; Olea, N.; Welshons, W.V. Human exposure to bisphenol A (BPA). Reprod. Toxicol. 2007, 24, 139–177. [Google Scholar] [CrossRef] [PubMed]
- Vinas, R.; Jeng, Y.J.; Watson, C.S. Non-genomic effects of xenoestrogen mixtures. Int. J. Environ. Res. Public Health 2012, 9, 2694–2714. [Google Scholar] [CrossRef] [PubMed]
- Fiorini, C.; Tilloy-Ellul, A.; Chevalier, S.; Charuel, C.; Pointis, G. Sertoli cell junctional proteins as early targets for different classes of reproductive toxicants. Reprod. Toxicol. 2004, 18, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Li, M.W.; Mruk, D.D.; Lee, W.M.; Cheng, C.Y. Disruption of the blood-testis barrier integrity by bisphenol A in vitro: Is this a suitable model for studying blood-testis barrier dynamics? Int. J. Biochem Cell Biol. 2009, 41, 2302–2314. [Google Scholar] [CrossRef]
- Mohri, T.; Yoshida, S. Estrogen and bisphenol A disrupt spontaneous [Ca(2+)](i) oscillations in mouse oocytes. Biochem. Biophys. Res. Commun. 2005, 326, 166–173. [Google Scholar] [CrossRef]
- Zarean, M.; Keikha, M.; Poursafa, P.; Khalighinejad, P.; Amin, M.; Kelishadi, R. A systematic review on the adverse health effects of di-2-ethylhexyl phthalate. Environ. Sci. Pollut. Res. Int. 2016, 23, 24642–24693. [Google Scholar] [CrossRef]
- Kimber, I.; Dearman, R.J. An assessment of the ability of phthalates to influence immune and allergic responses. Toxicology 2010, 271, 73–82. [Google Scholar] [CrossRef]
- North, M.L.; Takaro, T.K.; Diamond, M.L.; Ellis, A.K. Effects of phthalates on the development and expression of allergic disease and asthma. Ann. Allergy Asthma Immunol. 2014, 112, 496–502. [Google Scholar] [CrossRef]
- Koch, H.M.; Preuss, R.; Angerer, J. Di(2-ethylhexyl)phthalate (DEHP): Human. Metabolism and internal exposure—An update and latest results. Int. J. Androl. 2006, 29, 155–165. [Google Scholar] [CrossRef]
- Safe, S. Polychlorinated biphenyls (PCBs) and polybrominated biphenyls (PBBs): Biochemistry, toxicology, and mechanism of action. Crit. Rev. Toxicol. 1984, 13, 319–395. [Google Scholar] [CrossRef]
- Baker, T.K.; Kwiatkowski, A.P.; Madhukar, B.V.; Klaunig, J.E. Inhibition of gap junctional intercellular communication by 2,3,78-tetrachlorodibenzo-p-dioxin (TCDD) in rat hepatocytes. Carcinogenesis 1995, 16, 2321–2326. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Gao, D.; Liao, F.; Zhou, F.; Wang, X. The health effects of ambient PM2.5 and potential mechanisms. Ecotoxicol. Environ. Saf. 2016, 128, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, T.; Zou, L.; Xiong, L.; Zhang, T.; Kong, L.; Xue, Y.; Tang, M. Genome-wide identification and functional analysis of long non-coding RNAs in human endothelial cell line after incubation with PM2.5. Chemosphere 2019, 216, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Klocke, C.; Allen, J.L.; Sobolewski, M.; Mayer-Pröschel, M.; Blum, J.L.; Lauterstein, D.; Zelikoff, J.T.; Cory-Slechta, D.A. Neuropathological Consequences of Gestational Exposure to Concentrated Ambient Fine and Ultrafine Particles in the Mouse. Toxicol. Sci. 2017, 156, 492–508. [Google Scholar] [CrossRef]
- Loch-Caruso, R.; Corcos, I.A.; Trosko, J.E. Inhibition of metabolic coupling by metals. J. Toxicol. Environ. Health 1991, 32, 33–48. [Google Scholar] [CrossRef]
- Yorifuji, T.; Takaoka, S.; Grandjean, P. Accelerated functional losses in ageing congenital Minamata disease patients. Neurotoxicol. Teratol. 2018, 69, 49–53. [Google Scholar] [CrossRef]
- Guxens, M.; Sunyer, J. A review of epidemiological studies on neuropsychological effects of air pollution. Swiss Med Wkly. 2012, 141, 13322. [Google Scholar]
- Dominici, F.; Peng, R.D.; Bell, M.L.; Pham, L.; McDermott, A.; Zeger, S.L.; Samet, J.M. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. JAMA 2006, 295, 1127–1134. [Google Scholar] [CrossRef]
- Rocha, I.I.; Narasimhalu, K.; De Silva, D.A. Impact of Air Pollution and Seasonal Haze on Neurological Conditions. Ann. Acad. Med. Singap. 2020, 49, 26–36. [Google Scholar] [CrossRef]
- Chen, J.C.; Schwartz, J. Neurobehavioral effects of ambient air pollution on cognitive performance in US adults. Neurotoxicology 2009, 30, 231–239. [Google Scholar] [CrossRef]
- Harris, M.H.; Gold, D.R.; Rifas-Shiman, S.L.; Melly, S.J.; Zanobetti, A.; Coull, B.A.; Schwartz, J.D.; Gryparis, A.; Kloog, I.; Koutrakis, P.; et al. Prenatal and Childhood Traffic-Related Pollution Exposure and Childhood Cognition in the Project Viva Cohort (Massachusetts, USA). Environ. Health Perspect. 2015, 123, 1072–1078. [Google Scholar] [CrossRef] [PubMed]
- Perera, F.; Li, T.Y.; Lin, C.; Tang, D. Effects of prenatal polycyclic aromatic hydrocarbon exposure and environmental tobacco smoke on child IQ in a Chinese cohort. Environ. Res. 2012, 114, 40–46. [Google Scholar] [CrossRef]
- Oudin, A.; Forsberg, B.; Adolfsson, A.N.; Lind, N.; Modig, L.; Nordin, M.; Nordin, S.; Adolfsson, R.; Nilsson, L.G. Traffic-Related Air Pollution and Dementia Incidence in Northern Sweden: A Longitudinal Study. Environ. Health Perspect. 2016, 124, 306–312. [Google Scholar] [CrossRef]
- Oudin, A.; Bråbäck, L.; Åström, D.O.; Strömgren, M.; Forsberg, B. Association between neighbourhood air pollution concentrations and dispensed medication for psychiatric disorders in a large longitudinal cohort of Swedish children and adolescents. BMJ Open 2016, 6, e010004. [Google Scholar] [CrossRef] [PubMed]
- Porta, D.; Narduzzi, S.; Badaloni, C.; Bucci, S.; Cesaroni, G.; Colelli, V.; Davoli, M.; Sunyer, J.; Zirro, E.; Schwartz, J.; et al. Air Pollution and Cognitive Development at Age 7 in a Prospective Italian Birth Cohort. Epidemiology 2016, 27, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Suglia, S.F.; Gryparis, A.; Wright, R.O.; Schwartz, J.; Wright, R.J. Association of black carbon with cognition among children in a prospective birth cohort study. Am. J. Epidemiol. 2008, 167, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Volk, H.E.; Lurmann, F.; Penfold, B.; Hertz-Picciotto, I.; McConnell, R. Traffic-related air pollution, particulate matter, and autism. JAMA Psychiatry 2013, 70, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, J.; Zeng, X.; Zeng, Y.; Wang, S.; Chen, S. Association of traffic-related air pollution with children’s neurobehavioral functions in Quanzhou, China. Environ. Health Perspect. 2009, 117, 1612–1618. [Google Scholar] [CrossRef] [PubMed]
- Zablotsky, B.; Black, L.I.; Maenner, M.J.; Schieve, L.A.; Blumberg, S.J. Estimated Prevalence of Autism and Other Developmental Disabilities Following Questionnaire Changes in the 2014 National Health Interview Survey. Natl. Health Stat Rep. 2015, 87, 1–20. [Google Scholar]
- Woodley Of Menie, M.A.; Dunkel, C.S. In France, are secular IQ losses biologically caused? A comment on Dutton and Lynn. Intelligence 2015, 53, 81–85. [Google Scholar] [CrossRef]
- Van den Bosch, M.; Meyer-Lindenberg, A. Environmental Exposures and Depression: Biological Mechanisms and Epidemiological Evidence. Annu. Rev. Public Health 2019, 40, 239–259. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.S.; Lim, Y.H.; Kim, K.N.; Choi, Y.H.; Hong, Y.C.; Lee, N. Urinary phthalate metabolites concentrations and symptoms of depression in an elderly population. Sci. Total Environ. 2018, 625, 1191–1197. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S.; Arseneault, L.; Barratt, B.; Beevers, S.; Danese, A.; Odgers, C.L.; Moffitt, T.E.; Reubeng, A.; Kelly, F.J.; Fisher, H.L. Exploration of NO2 and PM2.5 air pollution and mental health problems using high-resolution data in London-based children from a UK longitudinal cohort study. Psychiatry Res. 2019, 272, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Newbury, J.B.; Arseneault, L.; Beevers, S.; Kitwiroon, N.; Roberts, S.; Pariante, C.M.; Kelly, F.J.; Fisher, H.L. Association of Air Pollution Exposure with Psychotic Experiences During Adolescence. JAMA Psychiatry 2019, 76, 614–623. [Google Scholar] [CrossRef] [PubMed]
- Grandjean, P.; Landrigan, P.J. Neurobehavioural effects of developmental toxicity. Lancet Neurol. 2014, 13, 330–338. [Google Scholar] [CrossRef]
- Rana, H.K.; Akhtar, M.R.; Ahmed, M.B.; Liò, P.; Quinn, J.M.W.; Huq, F.; Moni, M.A. Genetic effects of welding fumes on the progression of neurodegenerative diseases. Neurotoxicology 2019, 71, 93–101. [Google Scholar] [CrossRef]
- Ng, M.; de Montigny, J.G.; Ofner, M.; Do, M.T. Environmental factors associated with autism spectrum disorder: A scoping review for the years 2003–2013. Health Promot. Chronic Dis. Prev. Can. 2017, 37, 1–23. [Google Scholar] [CrossRef]
- Saeedi Saravi, S.S.; Dehpour, A.R. Potential role of organochlorine pesticides in the pathogenesis of neurodevelopmental, neurodegenerative, and neurobehavioral disorders: A review. Life Sci. 2016, 145, 255–264. [Google Scholar] [CrossRef]
- Ye, B.S.; Leung, A.O.W.; Wong, M.H. The association of environmental toxicants and autism spectrum disorders in children. Environ. Pollut. 2017, 227, 234–242. [Google Scholar] [CrossRef]
- Vrijheid, M.; Casas, M.; Gascon, M.; Valvi, D.; Nieuwenhuijsen, M. Environmental pollutants and child health-A review of recent concerns. Int. J. Hyg. Environ. Health 2016, 219, 331–342. [Google Scholar] [CrossRef]
- Carter, C.J.; Blizard, R.A. Autism genes are selectively targeted by environmental pollutants including pesticides, heavy metals, bisphenol A, phthalates and many others in food, cosmetics or household products. Neurochem. Int. 2016, 186, 30197–30198. [Google Scholar] [CrossRef] [PubMed]
- Fluegge, K.; Fluegge, K. Exposure to ambient PM10 and nitrogen dioxide and ADHD risk: A reply to Min & Min (2017). Environ. Int. 2017, 103, 109–110. [Google Scholar] [PubMed]
- Fatemi, S.H.; Folsom, T.D.; Reutiman, T.J.; Lee, S. Expression of astrocytic markers aquaporin 4 and connexin 43 is altered in brains of subjects with autism. Synapse 2008, 62, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, T.D.; Middleton, F.; Faraone, S.V. Environmental risk factors for attention-deficit hyperactivity disorder. Acta Paediatr. 2007, 96, 1269–1274. [Google Scholar] [CrossRef]
- Froehlich, T.E.; Anixt, J.S.; Loe, I.M.; Chirdkiatgumchai, V.; Kuan, L.; Gilman, R.C. Update on environmental risk factors for attention-deficit/hyperactivity disorder. Curr. Psychiatry Rep. 2011, 13, 333–344. [Google Scholar] [CrossRef]
- Chiu, Y.H.; Bellinger, D.C.; Coull, B.A.; Anderson, S.; Barber, R.; Wright, R.O.; Wright, R.J. Associations between traffic-related black carbon exposure and attention in a prospective birth cohort of urban children. Environ. Health Perspect. 2013, 121, 859–864. [Google Scholar] [CrossRef]
- Newman, N.C.; Ryan, P.; Lemasters, G.; Levin, L.; Bernstein, D.; Hershey, G.K.; Lockey, J.E.; Villareal, M.; Reponen, T.; Grinshpun, S.; et al. Traffic-related air pollution exposure in the first year of life and behavioral scores at 7 years of age. Environ. Health Perspect. 2013, 121, 731–736. [Google Scholar] [CrossRef]
- Sunyer, J.; Esnaola, M.; Alvarez-Pedrerol, M.; Forns, J.; Rivas, I.; López-Vicente, M.; Suades-González, E.; Foraster, M.; Garcia-Esteban, R.; Basagaña, X.; et al. Association between traffic-related air pollution in schools and cognitive development in primary school children: A prospective cohort study. PLoS Med. 2015, 12, e1001792. [Google Scholar] [CrossRef]
- Min, J.Y.; Min, K.B. Exposure to ambient PM 10 and NO 2 and the incidence of attention-deficit hyperactivity disorder in childhood. Environ. Int. 2017, 99, 221–227. [Google Scholar] [CrossRef]
- Fluegge, K.R.; Fluegge, K.R. Glyphosate Use Predicts ADHD Hospital Discharges in the Healthcare Cost and Utilization Project Net (HCUPnet): A Two-Way Fixed-Effects Analysis. PLoS ONE 2015, 10, e0133525. [Google Scholar] [CrossRef]
- Banaschewski, T.; Becker, K.; Scherag, S.; Franke, B.; Coghill, D. Molecular genetics of attention-deficit/hyperactivity disorder: An overview. Eur. Child Adolesc. Psychiatry 2010, 19, 237–257. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, S.; Dales, R.E.; Vidal, C.B. Air pollution and hospitalization for epilepsy in Chile. Environ. Int. 2010, 36, 501–505. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Conway, G.A.; Jin, L.; Zhou, J.; Zhang, J.; Li, X.; Zhou, L.; Li, T.; Zhang, J. Increase in Medical Emergency Calls and Calls for Central Nervous System Symptoms During a Severe Air Pollution Event, January 2013, Jinan City, China. Epidemiology 2017, 28, S67–S73. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Tian, X.; Yang, C.; Li, Y.; Hu, Y. Association between ambient air pollution and hospital admission for epilepsy in Eastern China. Epilepsy Res. 2019, 152, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Fan, Y.N.; Kan, H.D.; Chen, R.J.; Liu, J.H.; Li, Y.F.; Zhang, Y.; Ji, A.L.; Cai, T.J. The Novel Relationship between Urban Air Pollution and Epilepsy: A Time Series Study. PLoS ONE 2016, 11, e0161992. [Google Scholar] [CrossRef]
- Poon, E.; Bandara, S.B.; Allen, J.B.; Sadowski, R.N.; Schantz, S.L. Developmental PCB exposure increases susceptibility to audiogenic seizures in adulthood. Neurotoxicology 2015, 46, 117–124. [Google Scholar] [CrossRef]
- Bandara, S.B.; Eubig, P.A.; Sadowski, R.N.; Schantz, S.L. Developmental PCB Exposure Increases Audiogenic Seizures and Decreases Glutamic Acid Decarboxylase in the Inferior Colliculus. Toxicol. Sci. 2016, 149, 335–345. [Google Scholar] [CrossRef][Green Version]
- Aschengrau, A.; Winter, M.R.; Vieira, V.M.; Webster, T.F.; Janulewicz, P.A.; Gallagher, L.G.; Weinberg, J.; Ozonoff, D.M. Long-term health effects of early life exposure to tetrachloroethylene (PCE)-contaminated drinking water: A retrospective cohort study. Environ. Health 2015, 14, 36. [Google Scholar] [CrossRef]
- Bedner, P.; Dupper, A.; Hüttmann, K.; Müller, J.; Herde, M.K.; Dublin, P.; Deshpande, T.; Schramm, J.; Häussler, U.; Haas, C.A.; et al. Astrocyte uncoupling as a cause of human temporal lobe epilepsy. Brain 2015, 138, 1208–1222. [Google Scholar] [CrossRef]
- Khan, D.; Dupper, A.; Deshpande, T.; Graan, P.N.; Steinhäuser, C.; Bedner, P. Experimental febrile seizures impair interastrocytic gap junction coupling in juvenile mice. J. Neurosci. Res. 2016, 94, 804–813. [Google Scholar] [CrossRef]
- Collignon, F.; Wetjen, N.M.; Cohen-Gadol, A.A.; Cascino, G.D.; Parisi, J.; Meyer, F.B.; Marsh, W.R.; Roche, P.; Weigand, S.D. Altered expression of connexin subtypes in mesial temporal lobe epilepsy in humans. J. Neurosurg. 2006, 105, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Myung, W.; Cheong, H.K.; Yi, S.M.; Hong, Y.C.; Cho, S.I.; Kim, H. Ambient air pollution exposure and risk of migraine: Synergistic effect with high temperature. Environ. Int. 2018, 121, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Martins-Ferreira, H.; Nedergaard, M.; Nicholson, C. Perspectives on spreading depression. Brain Res. Rev. 2000, 32, 215–234. [Google Scholar] [CrossRef]
- Mustieles, V.; Pérez-Lobato, R.; Olea, N.; Fernández, M.F. Bisphenol A: Human exposure and neurobehavior. Neurotoxicology 2015, 49, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Perera, F.; Vishnevetsky, J.; Herbstman, J.B.; Calafat, A.M.; Xiong, W.; Rauh, V.; Wang, S. Prenatal bisphenol a exposure and child behavior in an inner-city cohort. Environ. Health Perspect. 2012, 120, 1190–1194. [Google Scholar] [CrossRef]
- Perera, F.; Nolte, E.L.R.; Wang, Y.; Margolis, A.E.; Calafat, A.M.; Wang, S.; Garcia, W.; Hoepner, L.A.; Peterson, B.S.; Rauh, V.; et al. Bisphenol A exposure and symptoms of anxiety and depression among inner city children at 10–12 years of age. Environ. Res. 2016, 151, 195–202. [Google Scholar] [CrossRef]
- Ejaredar, M.; Lee, Y.; Roberts, D.J.; Sauve, R.; Dewey, D. Bisphenol A exposure and children’s behavior: A systematic review. J. Expo. Sci. Environ. Epidemiol. 2017, 27, 175–183. [Google Scholar] [CrossRef]
- Shiue, I. Urinary heavy metals, phthalates and polyaromatic hydrocarbons independent of health events are associated with adult depression: USA NHANES, 2011–2012. Environ. Sci. Pollut. Res 2015, 22, 17095–17103. [Google Scholar] [CrossRef]
- Quesseveur, G.; Portal, B.; Basile, J.A.; Ezan, P.; Mathou, A.; Halley, H.; Leloup, C.; Fioramonti, X.; Déglon, N.; Giaume, C.; et al. Attenuated Levels of Hippocampal Connexin 43 and its Phosphorylation Correlate with Antidepressant- and Anxiolytic-Like Activities in Mice. Front. Cell. Neurosci. 2015, 9, 490. [Google Scholar] [CrossRef]
- Kasdagli, M.I.; Katsouyanni, K.; Dimakopoulou, K.; Samoli, E. Air pollution and Parkinson’s disease: A systematic review and meta-analysis up to 2018. Int. J. Hyg. Environ. Health 2019, 222, 402–409. [Google Scholar] [CrossRef]
- Goldman, S.M. Environmental toxins and Parkinson’s disease. Annu. Rev. Pharm. Toxicol. 2014, 54, 141–164. [Google Scholar] [CrossRef] [PubMed]
- Landolfi, A.; Troisi, J.; Savanelli, M.C.; Vitale, C.; Barone, P.; Amboni, M. Bisphenol A glucuronidation in patients with Parkinson’s disease. Neurotoxicology 2017, 63, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Chin-Chan, M.; Navarro-Yepes, J.; Quintanilla-Vega, B. Environmental pollutants as risk factors for neurodegenerative disorders: Alzheimer and Parkinson diseases. Front. Cell. Neurosci. 2015, 9, 124. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.Y.; Cui, Y.; Deng, F.; Feng, J.C. Connexin: A potential novel target for protecting the central nervous system? Neural Regen. Res. 2015, 10, 659–666. [Google Scholar] [PubMed]
- Lu, C.; Meng, Z.; He, Y.; Xiao, D.; Cai, H.; Xu, Y.; Liu, X.; Wang, X.; Mo, L.; Liang, Z.; et al. Involvement of gap junctions in astrocyte impairment induced by manganese exposure. Brain Res. Bull. 2018, 140, 107–113. [Google Scholar] [CrossRef]
- Wang, J.; Ma, T.; Ma, D.; Li, H.; Hua, L.; He, Q.; Deng, X. The Impact of Air Pollution on Neurodegenerative Diseases. Ther. Drug Monit. 2020. [Google Scholar] [CrossRef]
- Kilian, J.; Kitazawa, M. The emerging risk of exposure to air pollution on cognitive decline and Alzheimer’s disease—Evidence from epidemiological and animal studies. Biomed. J. 2018, 41, 141–162. [Google Scholar] [CrossRef]
- Yi, C.; Mei, X.; Ezan, P.; Mato, S.; Matias, I.; Giaume, C.; Koulakoff, A. Astroglial connexin43 contributes to neuronal suffering in a mouse model of Alzheimer’s disease. Cell Death Differ. 2016, 23, 1691–1701. [Google Scholar] [CrossRef]
- Harcha, P.A.; Vargas, A.; Yi, C.; Koulakoff, A.A.; Giaume, C.; Sáez, J.C. Hemichannels Are Required for Amyloid β-Peptide-Induced Degranulation and Are Activated in Brain Mast Cells of APPswe/PS1dE9 Mice. J. Neurosci. 2015, 35, 9526–9538. [Google Scholar] [CrossRef][Green Version]
- Delekate, A.; Füchtemeier, M.; Schumacher, T.; Ulbrich, C.; Foddis, M.; Petzold, G.C. Metabotropic P2Y1 receptor signalling mediates astrocytic hyperactivity in vivo in an Alzheimer’s disease mouse model. Nat. Commun. 2014, 5, 5422. [Google Scholar] [CrossRef]
- Meyer-Luehmann, M.; Spires-Jones, T.L.; Prada, C.; Garcia-Alloza, M.; de Calignon, A.; Rozkalne, A.; Koenigsknecht-Talboo, J.; Holtzman, D.M.; Bacskai, B.J.; Hyman, B.T. Rapid appearance and local toxicity of amyloid-beta plaques in a mouse model of Alzheimer’s disease. Nature 2008, 451, 720–724. [Google Scholar] [CrossRef] [PubMed]
- Vienne-Jumeau, A.; Tafani, C.; Ricard, D. Environmental risk factors of primary brain tumors: A review. Rev. Neurol. 2019, 175, 664–678. [Google Scholar] [CrossRef] [PubMed]
- Khuder, S.A.; Mutgi, A.B.; Schaub, E.A. Meta-analyses of brain cancer and farming. Am. J. Ind. Med. 1998, 34, 252–260. [Google Scholar] [CrossRef]
- Van Maele-Fabry, G.; Hoet, P.; Lison, D. Parental occupational exposure to pesticides as risk factor for brain tumors in children and young adults: A systematic review and meta-analysis. Environ. Int. 2013, 56, 19–31. [Google Scholar] [CrossRef]
- Van Maele-Fabry, G.; Gamet-Payrastre, L.; Lison, D. Residential exposure to pesticides as risk factor for childhood and young adult brain tumors: A systematic review and meta-analysis. Environ. Int. 2017, 106, 69–90. [Google Scholar] [CrossRef]
- Kang, K.S.; Park, J.E.; Ryu, D.Y.; Lee, Y.S. Effects and neuro-toxic mechanisms of 2, 2′, 4, 4′, 5, 5′-hexachlorobiphenyl and endosulfan in neuronal stem cells. J. Vet. Med Sci. 2001, 63, 1183–1190. [Google Scholar] [CrossRef]
- Seifert, G.; Schilling, K.; Steinhäuser, C. Astrocyte dysfunction in neurological disorders: A molecular perspective. Nat. Rev. Neurosci. 2006, 7, 194–206. [Google Scholar] [CrossRef]
- John Lin, C.C.; Deneen, B. Astrocytes: The missing link in neurologic disease? Semin Pediatr. Neurol. 2013, 20, 236–241. [Google Scholar] [CrossRef][Green Version]
- Herbert, M.R. Contributions of the environment and environmentally vulnerable physiology to autism spectrum disorders. Curr. Opin. Neurol. 2010, 23, 103–110. [Google Scholar] [CrossRef]
- Stamou, M.; Streifel, K.M.; Goines, P.E.; Lein, P.J. Neuronal connectivity as a convergent target of gene × environment interactions that confer risk for Autism Spectrum Disorders. Neurotoxicol. Teratol. 2013, 36, 3–16. [Google Scholar] [CrossRef]
- Sarrouilhe, D.; Dejean, C.; Mesnil, M. Connexin43- and Pannexin-Based Channels in Neuroinflammation and Cerebral Neuropathies. Front. Mol. Neurosci. 2017, 10, 320. [Google Scholar] [CrossRef] [PubMed]
- Bauman, M.L.; Kemper, T.L. Neuroanatomic observations of the brain in autism: A review and future directions. Int. J. Dev. Neurosci. 2005, 23, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Courchesne, E.; Pierce, K. Brain overgrowth in autism during a critical time in development: Implications for frontal pyramidal neuron and interneuron development and connectivity. Int. J. Dev. Neurosci. 2005, 23, 153–170. [Google Scholar] [CrossRef] [PubMed]
- Haas, R.H.; Townsend, J.; Courchesne, E.; Lincoln, A.J.; Schreibman, L.; Yeung-Courchesne, R. Neurologic abnormalities in infantile autism. J. Child Neurol. 1996, 11, 84–92. [Google Scholar] [CrossRef]
- Townsend, J.; Harris, N.S.; Courchesne, E. Visual attention abnormalities in autism: Delayed orienting to location. J. Int. Neuropsychol. Soc. 1996, 2, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Kern, J.K.; Trivedi, M.H.; Garver, C.R.; Grannemann, B.D.; Andrews, A.A.; Savla, J.S.; Johnson, D.G.; Mehta, J.A.; Schroeder, J.L. The pattern of sensory processing abnormalities in autism. Autism 2006, 10, 480–494. [Google Scholar] [CrossRef] [PubMed]
- Kern, J.K.; Trivedi, M.H.; Grannemann, B.D.; Garver, C.R.; Johnson, D.G.; Andrews, A.A.; Savla, J.S.; Mehta, J.A.; Schroeder, J.L. Sensory correlations in autism. Autism 2007, 11, 123–134. [Google Scholar] [CrossRef]
- Adams, J.B.; Johansen, L.J.; Powell, L.D.; Quig, D.; Rubin, R.A. Gastrointestinal flora and gastrointestinal status in children with autism—Comparisons to typical children and correlation with autism severity. BMC Gastroenterol. 2011, 11, 22. [Google Scholar] [CrossRef]
- Grubišc, V.; Parpura, V. The second brain in autism spectrum disorder: Could connexin43 expressed in enteric glial cells play a role? Front. Cell. Neurosci. 2015, 9, 242. [Google Scholar]
- Hong, Y.Z.; Zhang, X.J.; Hong, L.; Huang, Q.R.; Wu, Q. Influence of acupuncture of “Changqiang” (GV 1) on learning-memory ability and gap junction-related protein expression in the prefrontal cortex in autism rats. Zhen Ci Yan Jiu 2014, 39, 173–179. [Google Scholar]
- Dickerson, F.; Adamos, M.B.; Katsafanas, E.; Khushalani, S.; Origoni, A.; Savage, C.L.G.; Schroeder, J.; Schweinfurth, L.A.B.; Stallings, C.; Sweeney, K.; et al. The association among smoking, HSV-1 exposure, and cognitive functioning in schizophrenia, bipolar disorder, and non-psychiatric controls. Schizophr. Res. 2016, 176, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Mefford, H.C.; Sharp, A.J.; Baker, C.; Itsara, A.; Jiang, Z.; Buysse, K.; Huang, S.; Maloney, V.K.; Crolla, J.A.; Baralle, D.; et al. Recurrent rearrangements of chromosome 1q21.1 and variable pediatric phenotypes. N. Engl. J. Med. 2008, 359, 1685–1699. [Google Scholar] [CrossRef] [PubMed]
- Brunetti-Pierri, N.; Berg, J.S.; Scaglia, F.; Belmont, J.; Bacino, C.A.; Sahoo, T.; Lalani, S.R.; Graham, B.; Lee, B.; Shinawi, M.; et al. Recurrent reciprocal 1q21.1 deletions and duplications associated with microcephaly or macrocephaly and developmental and behavioral abnormalities. Nat. Genet. 2008, 40, 1466–1471. [Google Scholar] [CrossRef] [PubMed]
- Bernier, R.; Steinman, K.J.; Reilly, B.; Wallace, A.S.; Sherr, E.H.; Pojman, N.; Mefford, H.C.; Gerdts, J.; Earl, R.; Hanson, E.; et al. Clinical phenotype of the recurrent 1q21.1 copy-number variant. Genet. Med. 2016, 18, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Gollob, M.H.; Jones, D.L.; Krahn, A.D.; Danis, L.; Gong, X.Q.; Shao, Q.; Liu, X.; Veinot, J.P.; Tang, A.S.; Stewart, A.F.; et al. Somatic mutations in the connexin 40 gene (GJA5) in atrial fibrillation. N. Engl. J. Med. 2006, 354, 2677–2688. [Google Scholar] [CrossRef]
- Christiansen, J.; Dyck, J.D.; Elyas, B.G.; Lilley, M.; Bamforth, J.S.; Hicks, M.; Sprysak, K.A.; Tomaszewski, R.; Haase, S.M.; Vicen-Wyhony, L.M.; et al. Chromosome 1q21.1 contiguous gene deletion is associated with congenital heart disease. Circ. Res. 2004, 94, 1429–1435. [Google Scholar] [CrossRef]
- Soemedi, R.; Topf, A.; Wilson, I.J.; Darlay, R.; Rahman, T.; Glen, E.; Hall, D.; Huang, N.; Bentham, J.; Bhattacharya, S.; et al. Phenotype-specific effect of chromosome 1q21.1 rearrangements and GJA5 duplications in 2436 congenital heart disease patients and 6760 controls. Hum. Mol. Genet. 2012, 21, 1513–1520. [Google Scholar] [CrossRef]
- Guida, V.; Ferese, R.; Rocchetti, M.; Bonetti, M.; Sarkozy, A.; Cecchetti, S.; Gelmetti, V.; Lepri, F.; Copetti, M.; Lamorte, G.; et al. A variant in the carboxyl-terminus of connexin 40 alters GAP junctions and increases risk for tetralogy of Fallot. Eur. J. Hum. Genet. 2013, 21, 69–75. [Google Scholar] [CrossRef]
- Mackay, D.S.; Bennett, T.M.; Culican, S.M.; Shiels, A. Exome sequencing identifies novel and recurrent mutations in GJA8 and CRYGD associated with inherited cataract. Hum. Genom. 2014, 8, 19. [Google Scholar] [CrossRef]
- Huang, X.; Xiao, X.; Jia, X.; Li, S.; Li, M.; Guo, X.; Liu, X.; Zhang, Q. Mutation analysis of the genes associated with anterior segment dysgenesis, microcornea and microphthalmia in 257 patients with glaucoma. Int. J. Mol. Med. 2015, 36, 1111–1117. [Google Scholar] [CrossRef][Green Version]
- Prokudin, I.; Simons, C.; Grigg, J.R.; Storen, R.; Kumar, V.; Phua, Z.Y.; Smith, J.; Flaherty, M.; Davila, S.; Jamieson, R.V. Exome sequencing in developmental eye disease leads to identification of causal variants in GJA8, CRYGC, PAX6 and CYP1B1. Eur. J. Hum. Genet. 2014, 22, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Fisher, R.S. The New Classification of Seizures by the International League Against Epilepsy 2017. Curr. Neurol. Neurosci. Rep. 2017, 17, 48. [Google Scholar] [CrossRef] [PubMed]
- Niu, M.; Han, Y.; Li, Q.; Zhang, J. Endogenous sulfur dioxide regulates hippocampal neuron apoptosis in developing epileptic rats and is associated with the PERK signaling pathway. Neurosci. Lett. 2018, 665, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Bell, I.R.; Hardin, E.E.; Baldwin, C.M.; Schwartz, G.E. Increased limbic system symptomatology and sensitizability of young adults with chemical and noise sensitivities. Environ. Res. 1995, 70, 84–97. [Google Scholar] [CrossRef]
- Fernandes, M.J.S.; Carletti, C.O.; Sierra de Araújo, L.F.; Santos, R.C.; Reis, J. Respiratory gases, air pollution and epilepsy. Rev. Neurol. 2019, 175, 604–613. [Google Scholar] [CrossRef]
- Ramsdell, J.S. Neurological disease rises from ocean to bring model for human epilepsy to life. Toxins 2010, 2, 1646–1675. [Google Scholar] [CrossRef]
- Yamada, J.; Inoue, K.; Furukawa, T.; Fukuda, A. Low-concentration tributyltin perturbs inhibitory synaptogenesis and induces neuronal death in immature but not mature neurons. Toxicol. Lett. 2010, 198, 282–288. [Google Scholar] [CrossRef]
- Sato, I.; Kawamoto, K.; Nishikawa, Y.; Tsuda, S.; Yoshida, M.; Yaegashi, K.; Saito, N.; Liu, W.; Jin, Y. Neurotoxicity of perfluorooctane sulfonate (PFOS) in rats and mice after single oral exposure. J. Toxicol. Sci. 2009, 34, 569–574. [Google Scholar] [CrossRef]
- Kawamoto, K.; Sato, I.; Tsuda, S.; Yoshida, M.; Yaegashi, K.; Saito, N.; Liu, W.; Jin, Y. Ultrasonic-induced tonic convulsion in rats after subchronic exposure to perfluorooctane sulfonate (PFOS). J. Toxicol. Sci. 2011, 36, 55–62. [Google Scholar] [CrossRef][Green Version]
- Ahmadian, E.; Eftekhari, A.; Samiei, M.; Maleki Dizaj, S.; Vinken, M. The role and therapeutic potential of connexins, pannexins and their channels in Parkinson’s disease. Cell. Signal. 2019, 58, 111–118. [Google Scholar] [CrossRef]
- Kawasaki, A.; Hayashi, T.; Nakachi, K.; Trosko, J.; Sugihara, K.; Kotake, Y.; Ohta, S. Modulation of connexin 43 in rotenone-induced model of Parkinson’s disease. Neuroscience 2009, 160, 61–68. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Z.; Liu, X.; Fu, Q. Gastrodin ameliorates Parkinson’s disease by downregulating connexin 43. Mol. Med. Rep. 2013, 8, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Díaz, E.F.; Labra, V.C.; Alvear, T.F.; Mellado, L.A.; Inostroza, C.A.; Oyarzún, J.E.; Salgado, N.; Quintanilla, R.A.; Orellana, J.A. Connexin 43 hemichannels and pannexin-1 channels contribute to the α-synuclein-induced dysfunction and death of astrocytes. Glia 2019, 67, 1598–1619. [Google Scholar] [CrossRef] [PubMed]
- Eid, A.; Mhatre, I.; Richardson, J.R. Gene-environment interactions in Alzheimer’s disease: A potential path to precision medicine. Pharmacol. Ther. 2019, 199, 173–187. [Google Scholar] [CrossRef] [PubMed]
- Selkoe, D.J. Alzheimer’s disease beyond 1994: The path to therapeutics. Neurobiol. Aging 1994, 15, S131–S133. [Google Scholar] [CrossRef]
- Fajardo, V.A.; Fajardo, V.A.; LeBlanc, P.J. MacPherson REK. Examining the Relationship between Trace Lithium in Drinking Water and the Rising Rates of Age-Adjusted Alzheimer’s Disease Mortality in Texas. J. Alzheimers Dis. 2018, 61, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Amtul, Z.; Nikolova, S.; Gao, L.; Keeley, R.J.; Bechberger, J.F.; Fisher, A.L.; Bartha, R.; Munoz, D.G.; McDonald, R.J.; Naus, C.C.; et al. Comorbid Aβ toxicity and stroke: Hippocampal atrophy, pathology, and cognitive deficit. Neurobiol. Aging 2014, 35, 1605–1614. [Google Scholar] [CrossRef]
- Sofroniew, M.V.; Vinters, H.V. Astrocytes: Biology and pathology. Acta Neuropathol. 2010, 119, 7–35. [Google Scholar] [CrossRef]
- Nagy, J.I.; Li, W.; Hertzberg, E.L.; Marotta, C.A. Elevated connexin43 immunoreactivity at sites of amyloid plaques in Alzheimer’s disease. Brain Res. 1996, 717, 173–178. [Google Scholar] [CrossRef]
- Begcevic, I.; Kosanam, H.; Martínez-Morillo, E.; Dimitromanolakis, A.; Diamandis, P.; Kuzmanov, U.; Hazrati, L.N.; Diamandis, E.P. Semiquantitative proteomic analysis of human hippocampal tissues from Alzheimer’s disease and age-matched control brains. Clin. Proteom. 2013, 10, 5. [Google Scholar] [CrossRef]
- Hokama, M.; Oka, S.; Leon, J.; Ninomiya, T.; Honda, H.; Sasaki, K.; Iwaki, T.; Ohara, T.; Sasaki, T.; LaFerla, F.M.; et al. Altered expression of diabetes-related genes in Alzheimer’s disease brains: The Hisayama study. Cereb. Cortex 2014, 24, 2476–2488. [Google Scholar] [CrossRef] [PubMed]
- Mei, X.; Ezan, P.; Giaume, C.; Koulakoff, A. Astroglial connexin immunoreactivity is specifically altered at β-amyloid plaques in β-amyloid precursor protein/presenilin1 mice. Neuroscience 2010, 171, 92–105. [Google Scholar] [CrossRef] [PubMed]
- Koulakoff, A.; Mei, X.; Orellana, J.A.; Sáez, J.C.; Giaume, C. Glial connexin expression and function in the context of Alzheimer’s disease. Biochim. Biophys. Acta 2012, 1818, 2048–2057. [Google Scholar] [CrossRef] [PubMed]
- Yi, C.; Ezan, P.; Fernández, P.; Schmitt, J.; Sáez, J.C.; Giaume, C.; Koulakoff, A. Inhibition of glial hemichannels by boldine treatment reduces neuronal suffering in a murine model of Alzheimer’s disease. Glia 2017, 65, 1607–1625. [Google Scholar] [CrossRef] [PubMed]
- Biswas, J.; Gupta, S.; Verma, D.K.; Gupta, P.; Singh, A.; Tiwari, S.; Goswami, P.; Sharma, S.; Singh, S. Involvement of glucose related energy crisis and endoplasmic reticulum stress: Insinuation of streptozotocin induced Alzheimer’s like pathology. Cell. Signal. 2018, 42, 211–226. [Google Scholar] [CrossRef]
- Khalil, M.; Ronda, J.; Weintraub, M.; Jain, K.; Silver, R.; Silverman, A.J. Brain mast cell relationship to neurovasculature during development. Brain Res. 2007, 1171, 18–29. [Google Scholar] [CrossRef]
- Maslinska, D.; Laure-Kamionowska, M.; Maslinski, K.T.; Gujski, M.; Maslinski, S. Distribution of tryptase-containing mast cells and metallothionein reactive astrocytes in human brains with amyloid deposits. Inflamm. Res. 2007, 56, S17–S18. [Google Scholar] [CrossRef]
- Metcalfe, D.D.; Baram, D.; Mekori, Y.A. Mast cells. Physiol. Rev. 1997, 77, 1033–1079. [Google Scholar] [CrossRef]
- Niederhoffer, N.; Levy, R.; Sick, E.; Andre, P.; Coupin, G.; Lombard, Y.; Gies, J.P. Amyloid beta peptides trigger CD47-dependent mast cell secretory and phagocytic responses. Int. J. Immunopathol Pharm. 2009, 22, 473–483. [Google Scholar] [CrossRef]
- Orellana, J.A.; Figueroa, X.F.; Sánchez, H.A.; Contreras-Duarte, S.; Velarde, V.; Sáez, J.C. Hemichannels in the neurovascular unit and white matter under normal and inflamed conditions. CNS Neurol. Disord. Drug Targets 2011, 10, 404–414. [Google Scholar] [CrossRef]
- Alberdi, E.; Wyssenbach, A.; Alberdi, M.; Sánchez-Gómez, M.V.; Cavaliere, F.; Rodríguez, J.J.; Verkhratsky, A.; Matute, C. Ca(2+) -dependent endoplasmic reticulum stress correlates with astrogliosis in oligomeric amyloid β-treated astrocytes and in a model of Alzheimer’s disease. Aging Cell. 2013, 12, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Grolla, A.A.; Sim, J.A.; Lim, D.; Rodriguez, J.J.; Genazzani, A.A.; Verkhratsky, A. Amyloid-β and Alzheimer’s disease type pathology differentially affects the calcium signalling toolkit in astrocytes from different brain regions. Cell Death Dis. 2013, 4, e623. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.C.; Wyeth, M.S.; Baltan-Tekkok, S.; Ransom, B.R. Functional hemichannels in astrocytes: A novel mechanism of glutamate release. J. Neurosci. 2003, 23, 3588–3596. [Google Scholar] [CrossRef] [PubMed]
- Stridh, M.H.; Tranberg, M.; Weber, S.G.; Blomstrand, F.; Sandberg, M. Stimulated efflux of amino acids and glutathione from cultured hippocampal slices by omission of extracellular calcium: Likely involvement of connexin hemichannels. J. Biol. Chem. 2008, 283, 10347–10356. [Google Scholar] [CrossRef]
- Kang, J.; Kang, N.; Lovatt, D.; Torres, A.; Zhao, Z.; Lin, J.; Nedergaard, M. Connexin 43 hemichannels are permeable to ATP. J. Neurosci. 2008, 28, 4702–4711. [Google Scholar] [CrossRef]
- Kuchibhotla, K.V.; Lattarulo, C.R.; Hyman, B.T.; Bacskai, B.J. Synchronous hyperactivity and intercellular calcium waves in astrocytes in Alzheimer mice. Science 2009, 323, 1211–1215. [Google Scholar] [CrossRef]
- Kuchibhotla, K.V.; Goldman, S.T.; Lattarulo, C.R.; Wu, H.Y.; Hyman, B.T.; Bacskai, B.J. Abeta plaques lead to aberrant regulation of calcium homeostasis in vivo resulting in structural and functional disruption of neuronal networks. Neuron 2008, 59, 214–225. [Google Scholar] [CrossRef]
- Xie, H.; Guan, J.; Borrelli, L.A.; Xu, J.; Serrano-Pozo, A.; Bacskai, B.J. Mitochondrial alterations near amyloid plaques in an Alzheimer’s disease mouse model. J. Neurosci. 2013, 33, 17042–17051. [Google Scholar] [CrossRef]
- Ye, B.; Shen, H.; Zhang, J.; Zhu, Y.G.; Ransom, B.R.; Chen, X.C.; Ye, Z.C. Dual pathways mediate β-amyloid stimulated glutathione release from astrocytes. Glia 2015, 63, 2208–2219. [Google Scholar] [CrossRef]
- Kozoriz, M.G.; Bechberger, J.F.; Bechberger, G.R.; Suen, M.W.; Moreno, A.P.; Maass, K.; Willecke, K.; Naus, C.C. The connexin43 C-terminal region mediates neuroprotection during stroke. J. Neuropathol. Exp. Neurol. 2010, 69, 196–206. [Google Scholar] [CrossRef]
- Le, H.T.; Sin, W.C.; Lozinsky, S.; Bechberger, J.; Vega, J.L.; Guo, X.Q.; Sáez, J.C.; Naus, C.C. Gap junction intercellular communication mediated by connexin43 in astrocytes is essential for their resistance to oxidative stress. J. Biol. Chem. 2014, 289, 1345–1354. [Google Scholar] [CrossRef] [PubMed]
- Nakase, T.; Fushiki, S.; Söhl, G.; Theis, M.; Willecke, K.; Naus, C.C. Neuroprotective role of astrocytic gap junctions in ischemic stroke. Cell. Commun. Adhes. 2003, 10, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Nakase, T.; Söhl, G.; Theis, M.; Willecke, K.; Naus, C.C. Increased apoptosis and inflammation after focal brain ischemia in mice lacking connexin43 in astrocytes. Am. J. Pathol. 2004, 164, 2067–2075. [Google Scholar] [CrossRef]
- Bookheimer, S.Y.; Strojwas, M.H.; Cohen, M.S.; Saunders, A.M.; Pericak-Vance, M.A.; Mazziotta, J.C.; Small, G.W. Patterns of brain activation in people at risk for Alzheimer’s disease. N. Engl. J. Med. 2000, 343, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Quiroz, Y.T.; Budson, A.E.; Celone, K.; Ruiz, A.; Newmark, R.; Castrillón, G.; Lopera, F.; Stern, C.E. Hippocampal hyperactivation in presymptomatic familial Alzheimer’s disease. Ann. Neurol. 2010, 68, 865–875. [Google Scholar] [CrossRef]
- Kajiwara, Y.; Wang, E.; Wang, M.; Sin, W.C.; Brennand, K.J.; Schadt, E.; Naus, C.C.; Buxbaum, J.; Zhang, B. GJA1 (connexin43) is a key regulator of Alzheimer’s disease pathogenesis. Acta Neuropathol. Commun. 2018, 6, 144. [Google Scholar] [CrossRef]
- Theodoric, N.; Bechberger, J.F.; Naus, C.C.; Sin, W.C. Role of gap junction protein connexin43 in astrogliosis induced by brain injury. PLoS ONE 2012, 7, e47311. [Google Scholar] [CrossRef]
- Bakker, A.; Krauss, G.L.; Albert, M.S.; Speck, C.L.; Jones, L.R.; Stark, C.E.; Yassa, M.A.; Bassett, S.S.; Shelton, A.L.; Gallagher, M. Reduction of hippocampal hyperactivity improves cognition in amnestic mild cognitive impairment. Neuron 2012, 74, 467–474. [Google Scholar] [CrossRef]
- Esposito, Z.; Belli, L.; Toniolo, S.; Sancesario, G.; Bianconi, C.; Martorana, A. Amyloid β, glutamate, excitotoxicity in Alzheimer’s disease: Are we on the right track? CNS Neurosci. Ther. 2013, 19, 549–555. [Google Scholar] [CrossRef]
- Hynd, M.R.; Scott, H.L.; Dodd, P.R. Glutamate-mediated excitotoxicity and neurodegeneration in Alzheimer’s disease. Neurochem. Int. 2004, 45, 583–595. [Google Scholar] [CrossRef]
- Lewerenz, J.; Maher, P. Chronic Glutamate Toxicity in Neurodegenerative Diseases-What is the Evidence? Front. Neurosci. 2015, 9, 469. [Google Scholar] [CrossRef] [PubMed]
- Ong, W.Y.; Tanaka, K.; Dawe, G.S.; Ittner, L.M.; Farooqui, A.A. Slow excitotoxicity in Alzheimer’s disease. J. Alzheimers Dis. 2013, 35, 643–668. [Google Scholar] [CrossRef]
- Majoul, I.V.; Ernesti, J.S.; Butkevich, E.V.; Duden, R. Drebrins and Connexins: A Biomedical Perspective. Adv. Exp. Med. Biol. 2017, 1006, 225–247. [Google Scholar]
- Ren, R.; Zhang, L.; Wang, M. Specific deletion connexin43 in astrocyte ameliorates cognitive dysfunction in APP/PS1 mice. Life Sci. 2018, 208, 175–191. [Google Scholar] [CrossRef]
- Sharma, S.; Sharma, N.; Saini, A.; Nehru, B. Carbenoxolone Reverses the Amyloid Beta 1–42 Oligomer-Induced Oxidative Damage and Anxiety-Related Behavior in Rats. Neurotox. Res. 2019, 35, 654–667. [Google Scholar] [CrossRef] [PubMed]
- Gajardo-Gómez, R.; Labra, V.C.; Maturana, C.J.; Shoji, K.F.; Santibañez, C.A.; Sáez, J.C.; Giaume, C.; Orellana, J.A. Cannabinoids prevent the amyloid β-induced activation of astroglial hemichannels: A neuroprotective mechanism. Glia 2017, 65, 122–137. [Google Scholar] [CrossRef] [PubMed]
- Charles, A.C.; Baca, S.M. Cortical spreading depression and migraine. Nat. Rev. Neurol. 2013, 9, 637–644. [Google Scholar] [CrossRef]
- Costa, C.; Tozzi, A.; Rainero, I.; Cupini, L.M.; Calabresi, P.; Ayata, C.; Sarchielli, P. Cortical spreading depression as a target for anti-migraine agents. J. Headache Pain 2013, 14, 62. [Google Scholar] [CrossRef]
- Sarrouilhe, D.; Dejean, C.; Mesnil, M. Involvement of gap junction channels in the pathophysiology of migraine with aura. Front. Physiol. 2014, 5, 78. [Google Scholar] [CrossRef]
- Sarrouilhe, D.; Mesnil, M.; Dejean, C. Targeting Gap Junctions: New Insights into the Treatment of Major Depressive Disorder. Curr. Med. Chem. 2019, 26, 3775–3791. [Google Scholar] [CrossRef]
- Palanza, P.; Nagel, S.C.; Parmigiani, S.; Vom Saal, F.S. Perinatal exposure to endocrine disruptors: Sex, timing and behavioral endpoints. Curr. Opin. Behav. Sci. 2016, 7, 69–75. [Google Scholar] [CrossRef]
- Xin, F.; Fischer, E.; Krapp, C.; Krizman, E.N.; Lan, Y.; Mesaros, C.; Snyder, N.W.; Bansal, A.; Robinson, M.B.; Simmons, R.A.; et al. Mice exposed to bisphenol A exhibit depressive-like behavior with neurotransmitter and neuroactive steroid dysfunction. Horm. Behav. 2018, 102, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Kimura, E.; Matsuyoshi, C.; Miyazaki, W.; Benner, S.; Hosokawa, M.; Yokoyama, K.; Kakeyama, M.; Tohyama, C. Prenatal exposure to bisphenol A impacts neuronal morphology in the hippocampal CA1 region in developing and aged mice. Arch. Toxicol. 2016, 90, 691–700. [Google Scholar] [CrossRef] [PubMed]
- Crespin, S.; Fromont, G.; Wager, M.; Levillain, P.; Cronier, L.; Monvoisin, A.; Defamie, N.; Mesnil, M. Expression of a gap junction protein, connexin43, in a large panel of human gliomas: New insights. Cancer Med. 2016, 5, 1742–1752. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.P.; Hossain, M.Z.; Sehgal, A.; Boynton, A.L. Reduced connexin43 expression in high-grade human brain glioma cells. J. Surg. Oncol. 1999, 70, 21–24. [Google Scholar] [CrossRef]
- Soroceanu, L.; Manning, T.J., Jr.; Sontheimer, H. Reduced expression of connexin-43 and functional gap junction coupling in human gliomas. Glia 2001, 33, 107–117. [Google Scholar] [CrossRef]
- Herrero-González, S.; Gangoso, E.; Giaume, C.; Naus, C.C.; Medina, J.M.; Tabernero, A. Connexin43 inhibits the oncogenic activity of c-Src in C6 glioma cells. Oncogene 2010, 29, 5712–5723. [Google Scholar] [CrossRef]
- Sin, W.C.; Aftab, Q.; Bechberger, J.F.; Leung, J.H.; Chen, H.; Naus, C.C. Astrocytes promote glioma invasion via the gap junction protein connexin43. Oncogene 2016, 35, 1504–1516. [Google Scholar] [CrossRef]
- Strale, P.O.; Clarhaut, J.; Lamiche, C.; Cronier, L.; Mesnil, M.; Defamie, N. Down-regulation of Connexin43 expression reveals the involvement of caveolin-1 containing lipid rafts in human U251 glioblastoma cell invasion. Mol. Carcinog. 2012, 51, 845–860. [Google Scholar] [CrossRef]
- Aftab, Q.; Sin, W.C.; Naus, C.C. Reduction in gap junction intercellular communication promotes glioma migration. Oncotarget 2015, 6, 11447–11464. [Google Scholar] [CrossRef]
- Bates, D.C.; Sin, W.C.; Aftab, Q.; Naus, C.C. Connexin43 enhances glioma invasion by a mechanism involving the carboxy terminus. Glia 2007, 55, 1554–1564. [Google Scholar] [CrossRef] [PubMed]
- Crespin, S.; Bechberger, J.; Mesnil, M.; Naus, C.C.; Sin, W.C. The carboxy-terminal tail of connexin43 gap junction protein is sufficient to mediate cytoskeleton changes in human glioma cells. J. Cell. Biochem. 2010, 110, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Chepied, A.; Daoud-Omar, Z.; Meunier-Balandre, A.C.; Laird, D.W.; Mesnil, M.; Defamie, N. Involvement of the Gap Junction Protein, Connexin43, in the Formation and Function of Invadopodia in the Human U251 Glioblastoma Cell Line. Cells 2020, 9, 117. [Google Scholar] [CrossRef] [PubMed]
- Hitomi, M.; Deleyrolle, L.P.; Mulkearns-Hubert, E.E.; Jarrar, A.; Li, M.; Sinyuk, M.; Otvos, B.; Brunet, S.; Flavahan, W.A.; Hubert, C.G.; et al. Differential connexin function enhances self-renewal in glioblastoma. Cell Rep. 2015, 11, 1031–1042. [Google Scholar] [CrossRef]
- Sin, W.C.; Crespin, S.; Mesnil, M. Opposing roles of connexin43 in glioma progression. Biochim. Biophys. Acta 2012, 1818, 2058–2067. [Google Scholar] [CrossRef]
- Aasen, T.; Mesnil, M.; Naus, C.C.; Lampe, P.D.; Laird, D.W. Gap junctions and cancer: Communicating for 50 years. Nat. Rev. Cancer 2016, 16, 775–788. [Google Scholar] [CrossRef]
- Li, H.X.; Meng, H.Y.; Peng, X.X.; Zong, Q.; Zhang, K.; Han, G.L. A Meta-Analysis of Association Between Pesticides Exposure and Glioma Risk in Adults. J. Craniofac. Surg. 2015, 26, e672–e673. [Google Scholar]
- Woodley, M.A.; Te Nijenhuis, J.; Murphy, R. Were the Victorians cleverer than us? The decline in general intelligence estimated from a meta-analysis of the slowing of simple reaction time. Intelligence 2013, 41, 843–850. [Google Scholar] [CrossRef]
- Schönfelder, G.; Wittfoht, W.; Hopp, H.; Talsness, C.E.; Paul, M.; Chahoud, I. Parent bisphenol a accumulation in the human maternal-fetal-placental unit. Environ. Health Perspect. 2002, 110, A703–A707. [Google Scholar] [CrossRef]
- Fernandez, M.F.; Arrebola, J.P.; Taoufiki, J.; Navalón, A.; Ballesteros, O.; Pulgar, R.; Vilchez, J.L.; Olea, N. Bisphenol-A and chlorinated derivatives in adipose tissue of women. Reprod. Toxicol. 2007, 24, 259–264. [Google Scholar] [CrossRef]
- Koch, H.M.; Kolossa-Gehring, M.; Schröter-Kermani, C.; Angerer, J.; Brüning, T. Bisphenol A in 24 h urine and plasma samples of the German Environmental Specimen Bank from 1995 to 2009: A retrospective exposure evaluation. J. Expo. Sci. Environ. Epidemiol. 2012, 22, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Genuis, S.J.; Beesoon, S.; Lobo, R.A.; Birkholz, D. Human elimination of phthalate compounds: Blood, urine, and sweat (BUS) study. Sci. World J. 2012, 2012, 615068. [Google Scholar] [CrossRef] [PubMed]
- Ikezuki, Y.; Tsutsumi, O.; Takai, Y.; Kamei, Y.; Taketani, Y. Determination of bisphenol A concentrations in human biological fluids reveals significant early prenatal exposure. Hum. Reprod. 2002, 17, 2839–2841. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Pierik, F.H.; Angerer, J.; Meltzer, H.M.; Jaddoe, V.W.; Tiemeier, H.; Hoppin, J.A.; Longnecker, M.P. Levels of metabolites of organophosphate pesticides, phthalates, and bisphenol A in pooled urine specimens from pregnant women participating in the Norwegian Mother and Child Cohort Study (MoBa). Int. J. Hyg. Environ. Health 2009, 212, 481–491. [Google Scholar] [CrossRef]
- Pirard, C.; Sagot, C.; Deville, M.; Dubois, N.; Charlier, C. Urinary levels of bisphenol A, triclosan and 4-nonylphenol in a general Belgian population. Environ. Int. 2012, 48, 78–83. [Google Scholar] [CrossRef]
- Calafat, A.M.; Ye, X.; Wong, L.Y.; Reidy, J.A.; Needham, L.L. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003–2004. Environ. Health Perspect. 2008, 116, 39–44. [Google Scholar] [CrossRef]
- Ye, X.; Kuklenyik, Z.; Needham, L.L.; Calafat, A.M. Measuring environmental phenols and chlorinated organic chemicals in breast milk using automated on-line column-switching-high performance liquid chromatography-isotope dilution tandem mass spectrometry. Analyt. Technol. Biomed. Life Sci. 2006, 831, 110–115. [Google Scholar] [CrossRef]
- Geens, T.; Neels, H.; Covaci, A. Distribution of bisphenol-A, triclosan and n-nonylphenol in human adipose tissue, liver and brain. Chemosphere 2012, 87, 796–802. [Google Scholar] [CrossRef]
- Calafat, A.M.; Weuve, J.; Ye, X.; Jia, L.T.; Hu, H.; Ringer, S.; Huttner, K.; Hauser, R. Exposure to bisphenol A and other phenols in neonatal intensive care unit premature infants. Environ. Health Perspect. 2009, 117, 639–644. [Google Scholar] [CrossRef]
- Guo, J.; Wu, C.; Zhang, J.; Qi, X.; Lv, S.; Jiang, S.; Zhou, T.; Lu, D.; Feng, C.; Chang, X.; et al. Prenatal exposure to mixture of heavy metals, pesticides and phenols and IQ in children at 7 years of age: The SMBCS study. Environ. Int. 2020, 139, 105692. [Google Scholar] [CrossRef]
- Tatsuta, N.; Nakai, K.; Kasanuma, Y.; Iwai-Shimada, M.; Sakamoto, M.; Murata, K.; Satoh, H. Prenatal and postnatal lead exposures and intellectual development among 12-year-old Japanese children. Environ. Res. 2020, 189, 109844. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.X.; Wang, Z.H.; Cheng, X.T.; Li, J.; Sang, Z.P.; Zhang, X.D.; Han, L.L.; Qiao, X.Y.; Wu, Z.M.; Wang, Z.Q. Arsenic and fluoride exposure in drinking water: children’s IQ and growth in Shanyin county, Shanxi province, China. Environ. Health Perspect. 2007, 115, 643–647. [Google Scholar] [CrossRef] [PubMed]
- Maes, M.; Yanguas, S.C.; Willebrords, J.; Vinken, M. Models and methods for in vitro testing of hepatic gap junctional communication. Toxicol In Vitro 2015, 30, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Mandy, M.; Nyirenda, M. Developmental Origins of Health and Disease: The relevance to developing nations. Int. Health 2018, 10, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Martorell, R. Improved nutrition in the first 1000 days and adult human capital and health. Am. J. Hum. Biol. 2017, 29. [Google Scholar] [CrossRef] [PubMed]
- Casey, B.J.; Galvan, A.; Hare, T.A. Changes in cerebral functional organization during cognitive development. Curr. Opin. Neurobiol. 2005, 15, 239–244. [Google Scholar] [CrossRef]
- Rubartelli, A.; Lotze, M.T.; Latz, E.; Manfredi, A. Mechanisms of sterile inflammation. Front. Immunol. 2013, 4, 398. [Google Scholar] [CrossRef]
- Frühbeis, C.; Fröhlich, D.; Kuo, W.P.; Krämer-Albers, E.M. Extracellular vesicles as mediators of neuron-glia communication. Front. Cell. Neurosci. 2013, 7, 182. [Google Scholar] [CrossRef]
- Fróes, M.M.; Correia, A.H.; Garcia-Abreu, J.; Spray, D.C.; Campos de Carvalho, A.C.; Neto, M.V. Gap-junctional coupling between neurons and astrocytes in primary central nervous system cultures. Proc. Natl. Acad. Sci. USA 1999, 96, 7541–7546. [Google Scholar] [CrossRef]
- Rozental, R.; Andrade-Rozental, A.F.; Zheng, X.; Urban, M.; Spray, D.C.; Chiu, F.C. Gap junction-mediated bidirectional signaling between human fetal hippocampal neurons and astrocytes. Dev. Neurosci. 2001, 23, 420–431. [Google Scholar] [CrossRef]
- Wang, X.; Bukoreshtliev, N.V.; Gerdes, H.H. Developing neurons form transient nanotubes facilitating electrical coupling and calcium signaling with distant astrocytes. PLoS ONE 2012, 7, e47429. [Google Scholar] [CrossRef] [PubMed]
- Schraufnagel, D.E.; Balmes, J.R.; De Matteis, S.; Hoffman, B.; Kim, W.J.; Perez-Padilla, R.; Rice, M.; Sood, A.; Vanker, A.; Wuebbles, D.J. Health Benefits of Air Pollution Reduction. Ann. Am. Thorac. Soc. 2019, 16, 1478–1487. [Google Scholar] [CrossRef] [PubMed]
- Frederiksen, H.; Nielsen, O.; Koch, H.M.; Skakkebaek, N.E.; Juul, A.; Jørgensen, N.; Andersson, A.M. Changes in urinary excretion of phthalates, phthalate substitutes, bisphenols and other polychlorinated and phenolic substances in young Danish men; 2009–2017. Int. J. Hyg. Environ. Health 2020, 223, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Banks, W.A. The Blood-Brain Barrier Interface in Diabetes Mellitus: Dysfunctions, Mechanisms and Approaches to Treatment. Curr. Pharm. Des. 2020, 26, 1438–1447. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).