Transcriptomic Profiling Reveals Altered Expression of Genes Involved in Metabolic and Immune Processes in NDV-Infected Chicken Embryos
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Experimental Birds and Sample Collection
2.3. RNA Isolation and Quality Check
2.4. Sequencing and Data Analysis
2.5. Functional Analysis
2.6. Protein–Protein Interaction (PPI)
2.7. Quantitative Real-Time PCR (RT-qPCR) Validation
3. Results
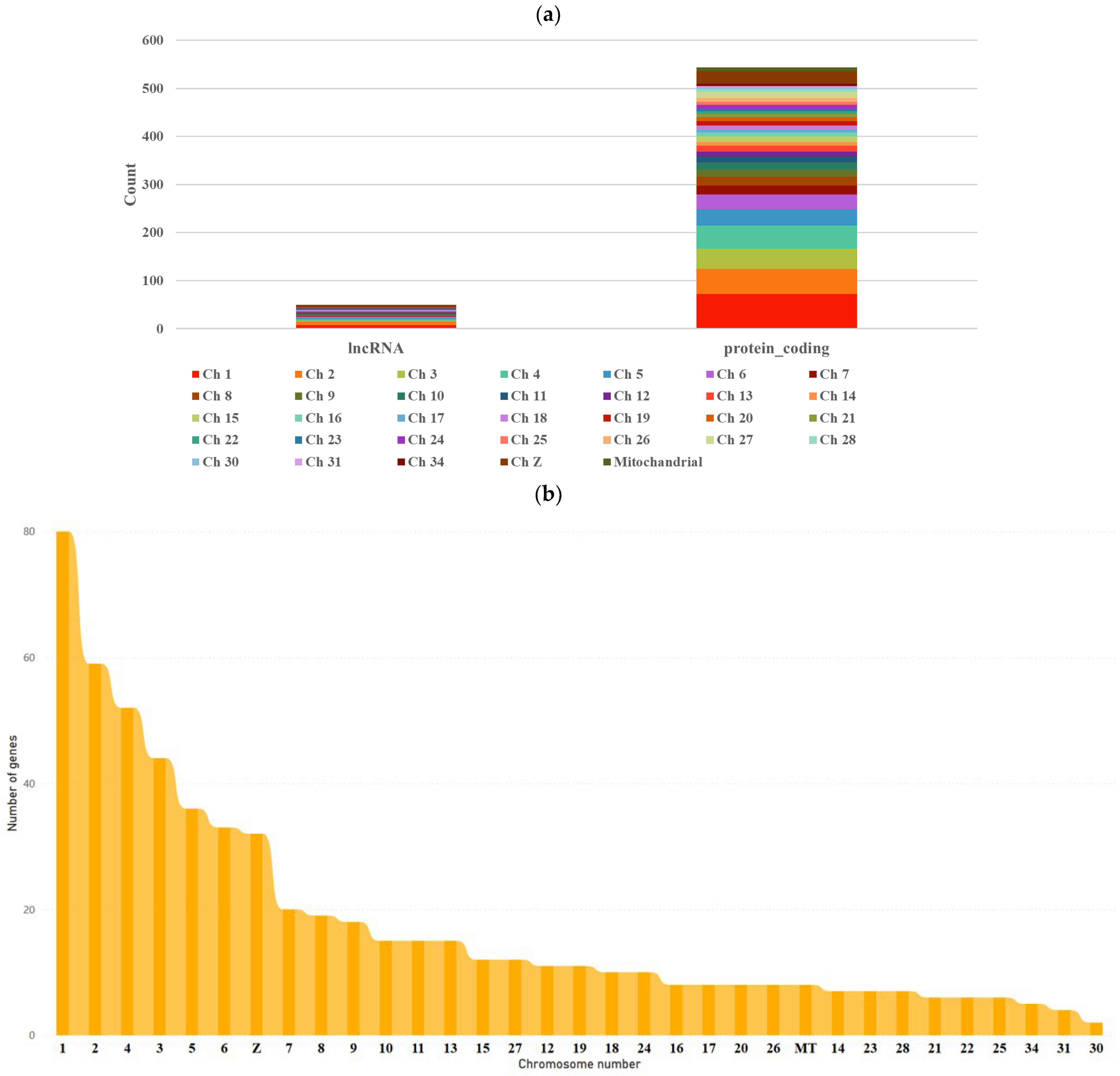
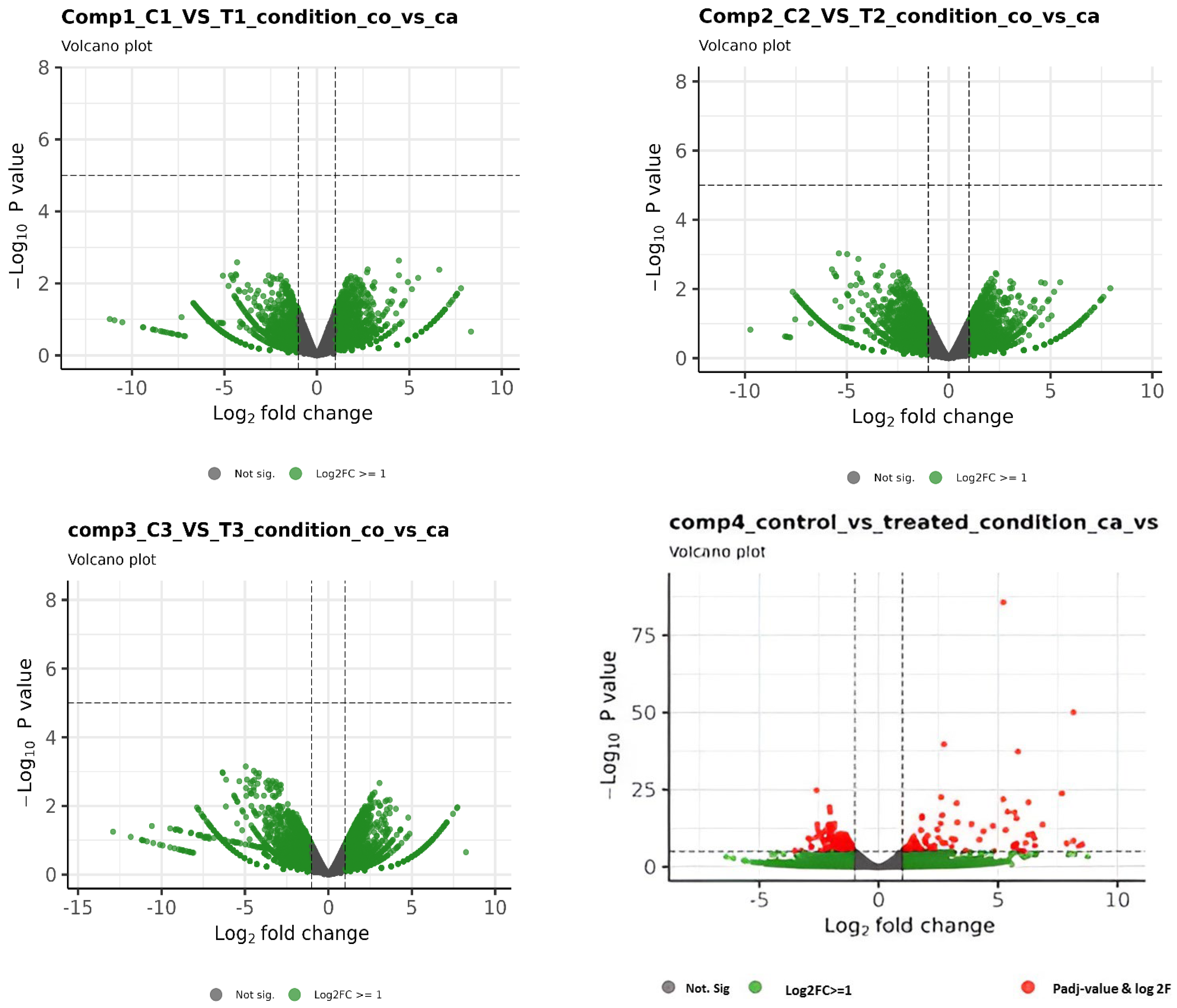
3.1. RNA-Seq Data Analysis
3.2. Principal Component Analysis (PCA)
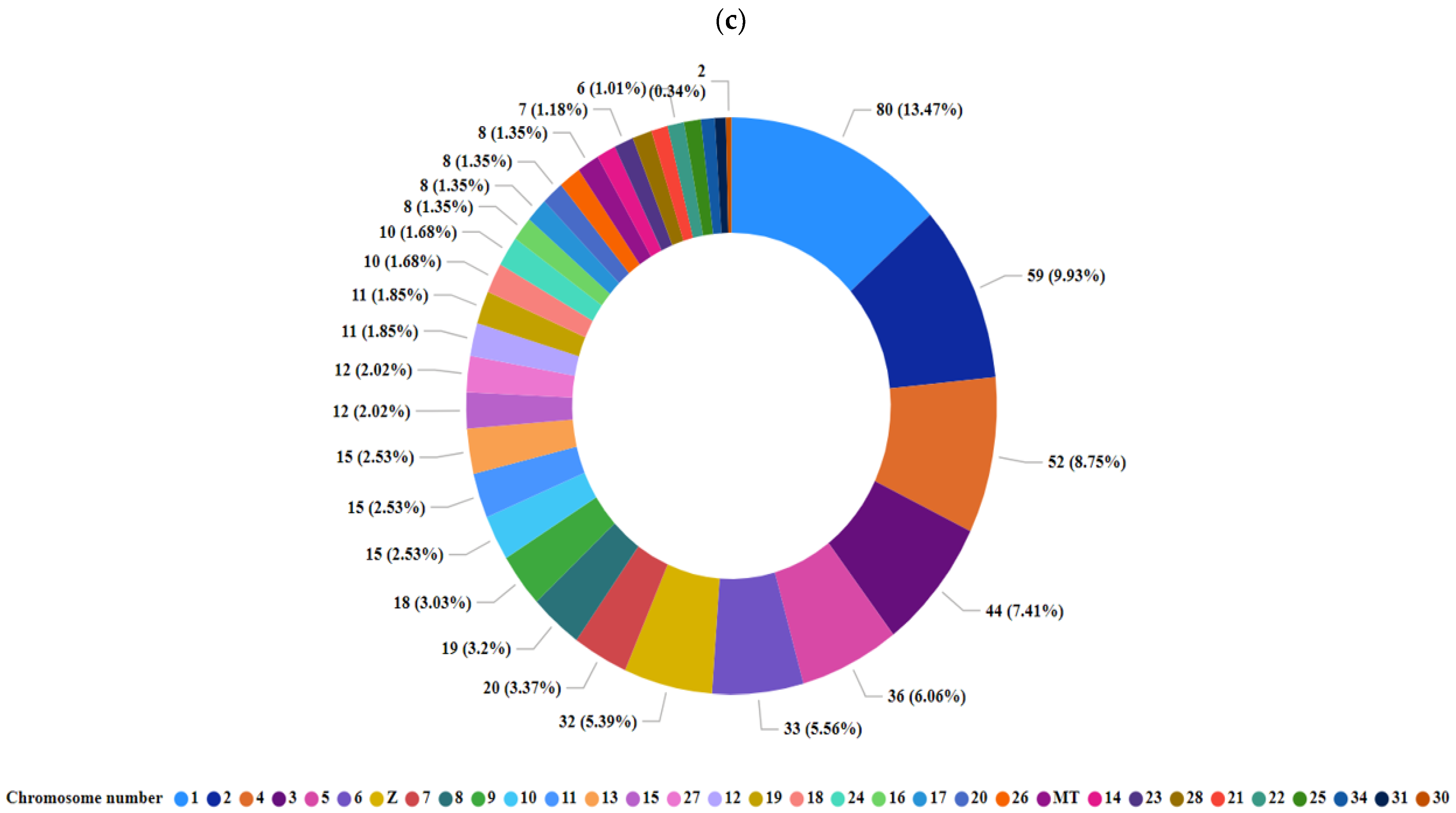
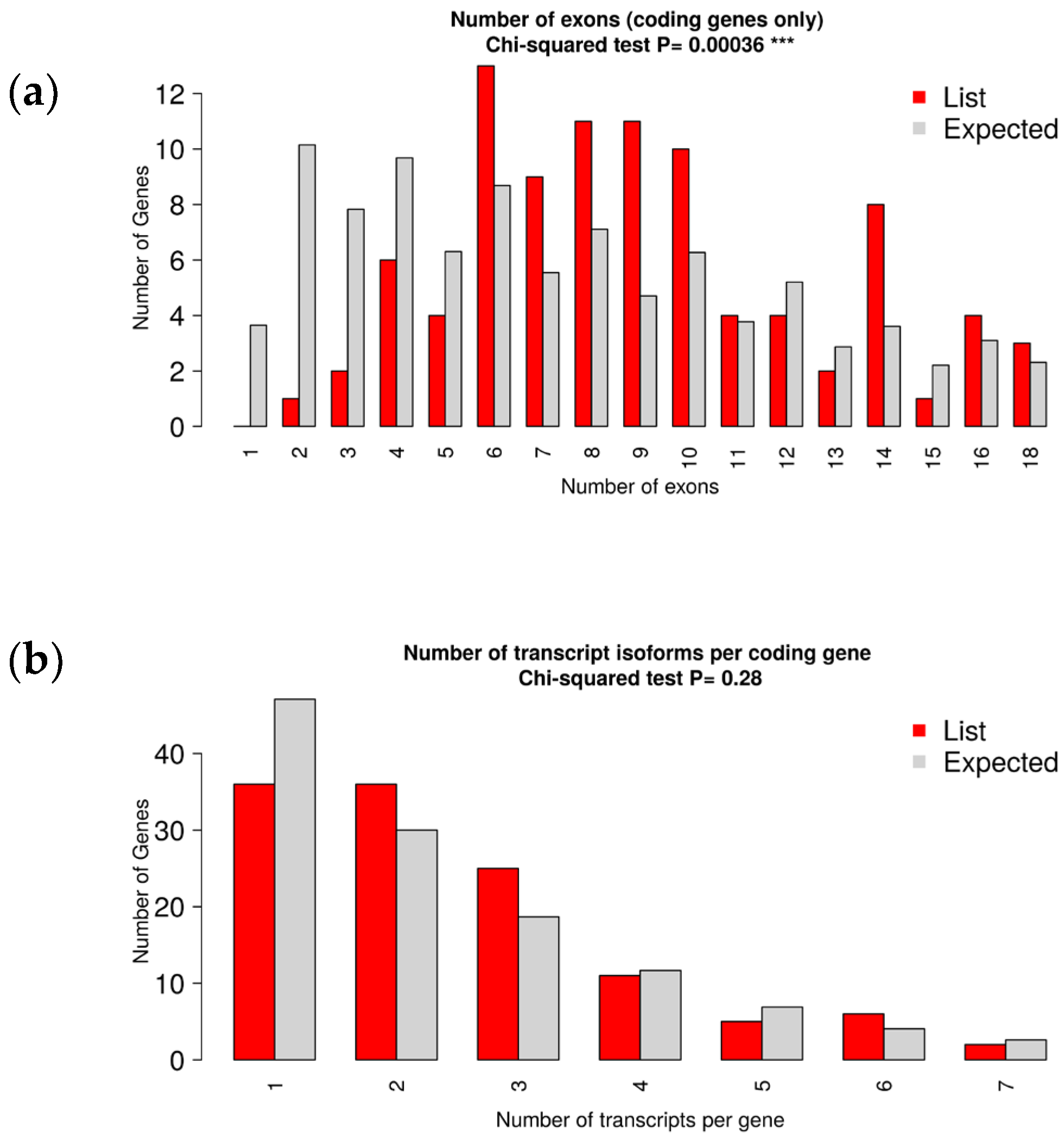
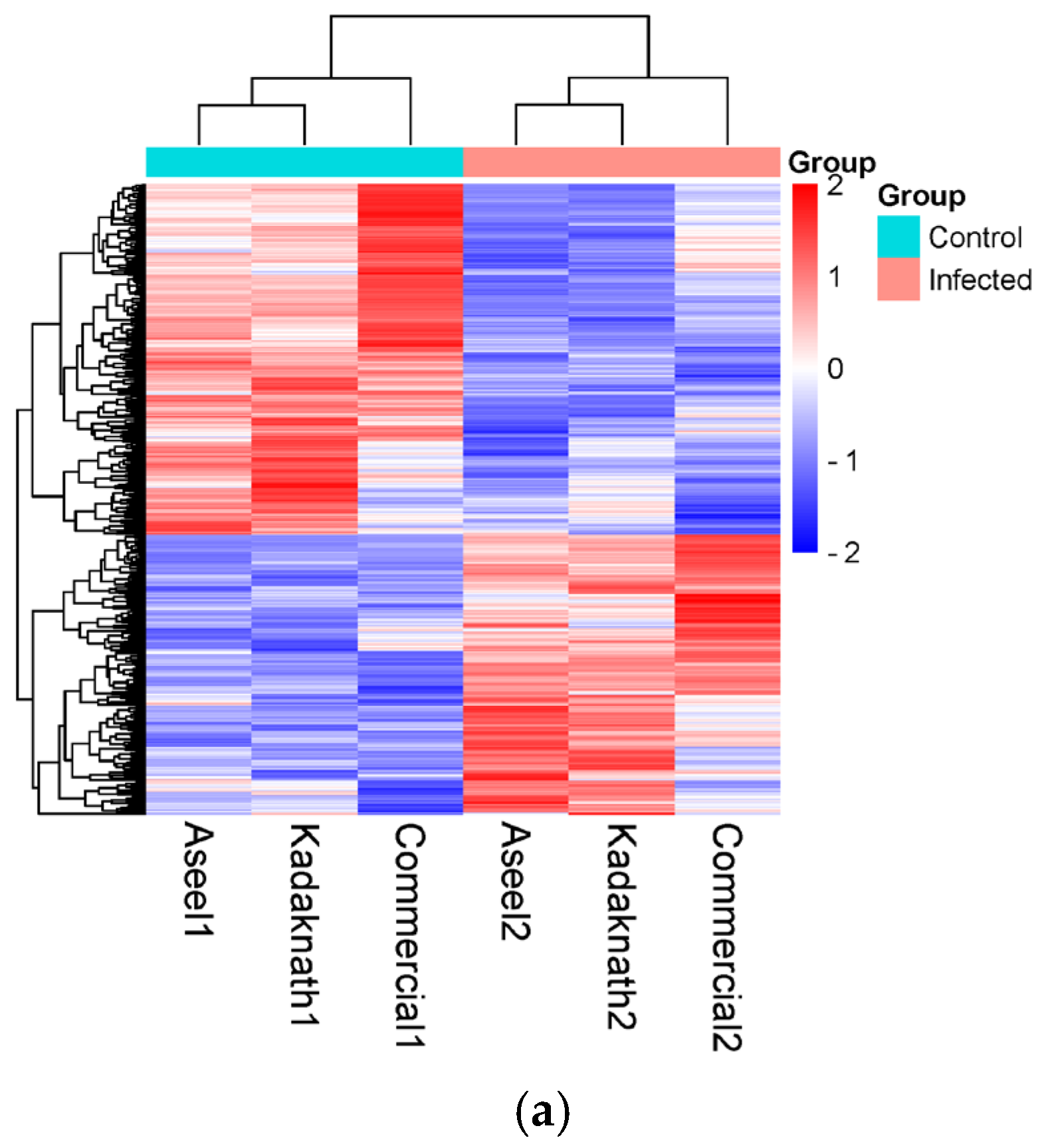
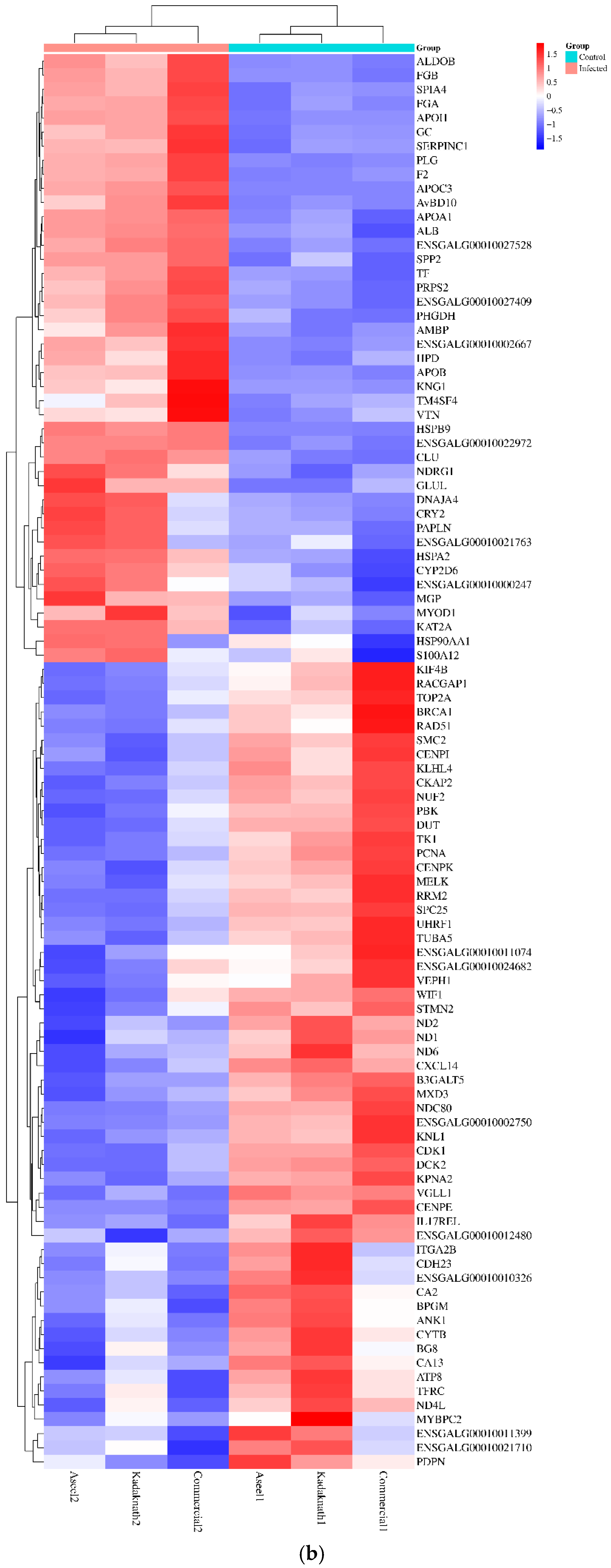
3.3. Identification and Characterization of Transcripts and Differential Expression Analysis
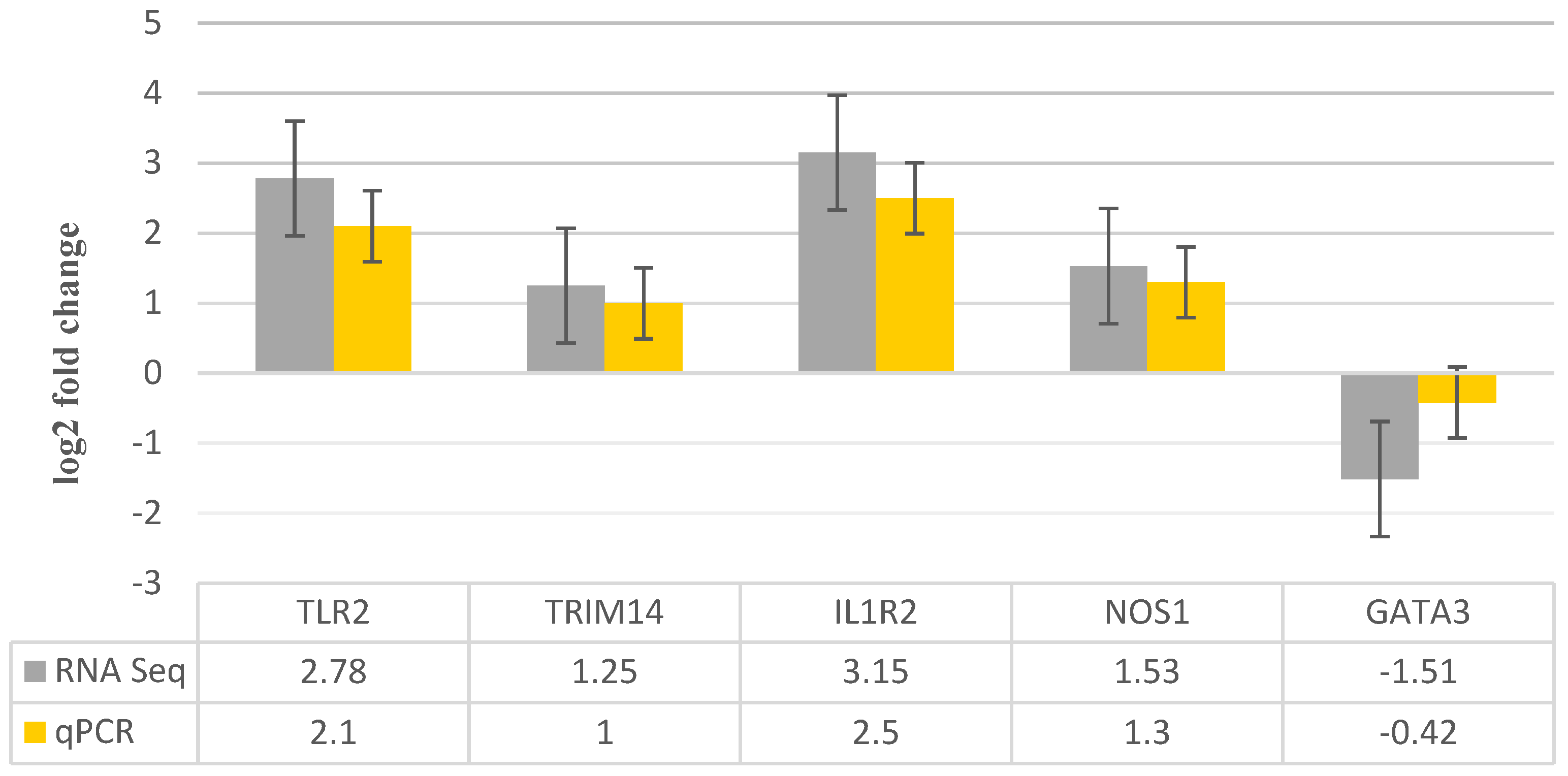
3.4. RNA-Seq Data Validation by RT-qPCR
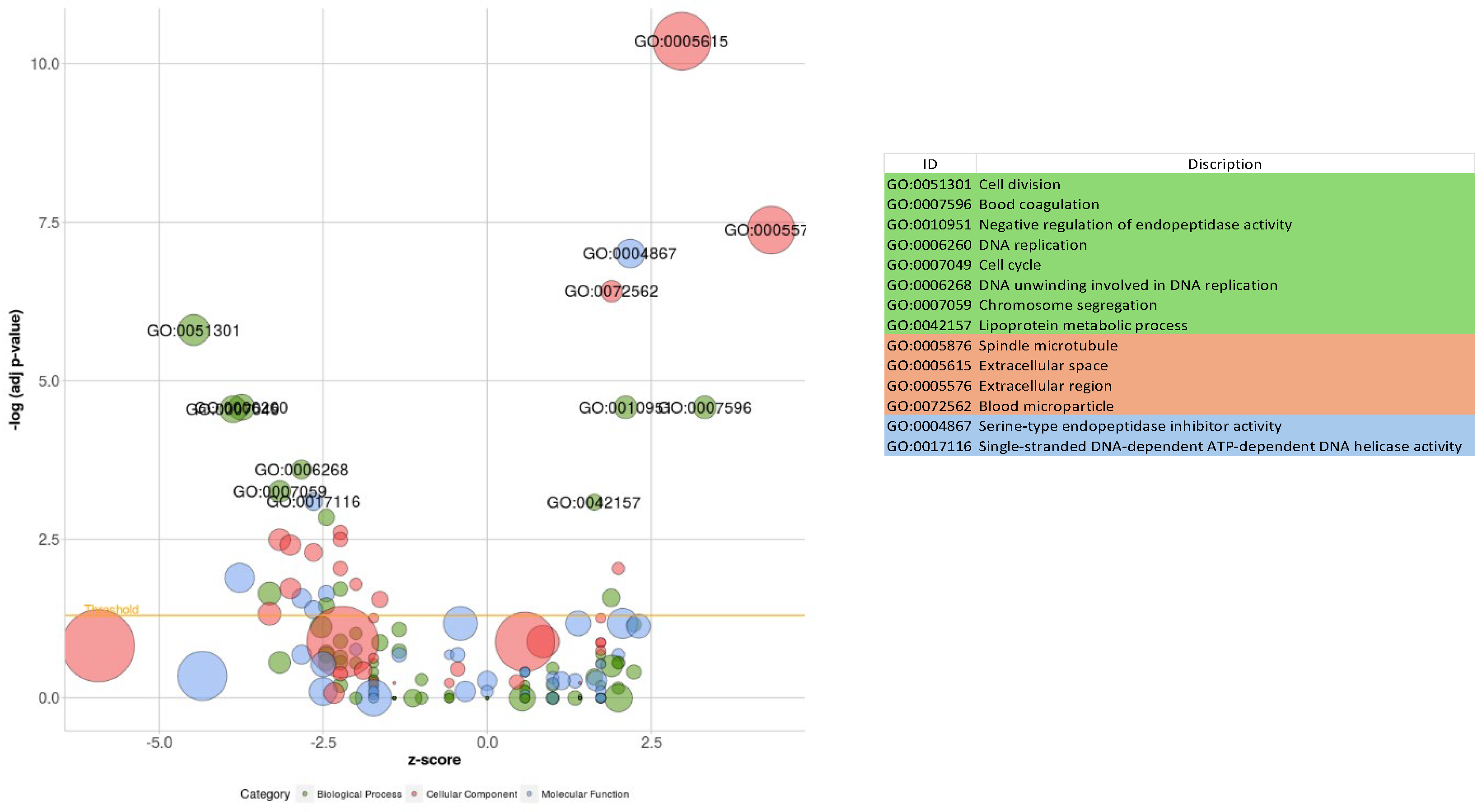
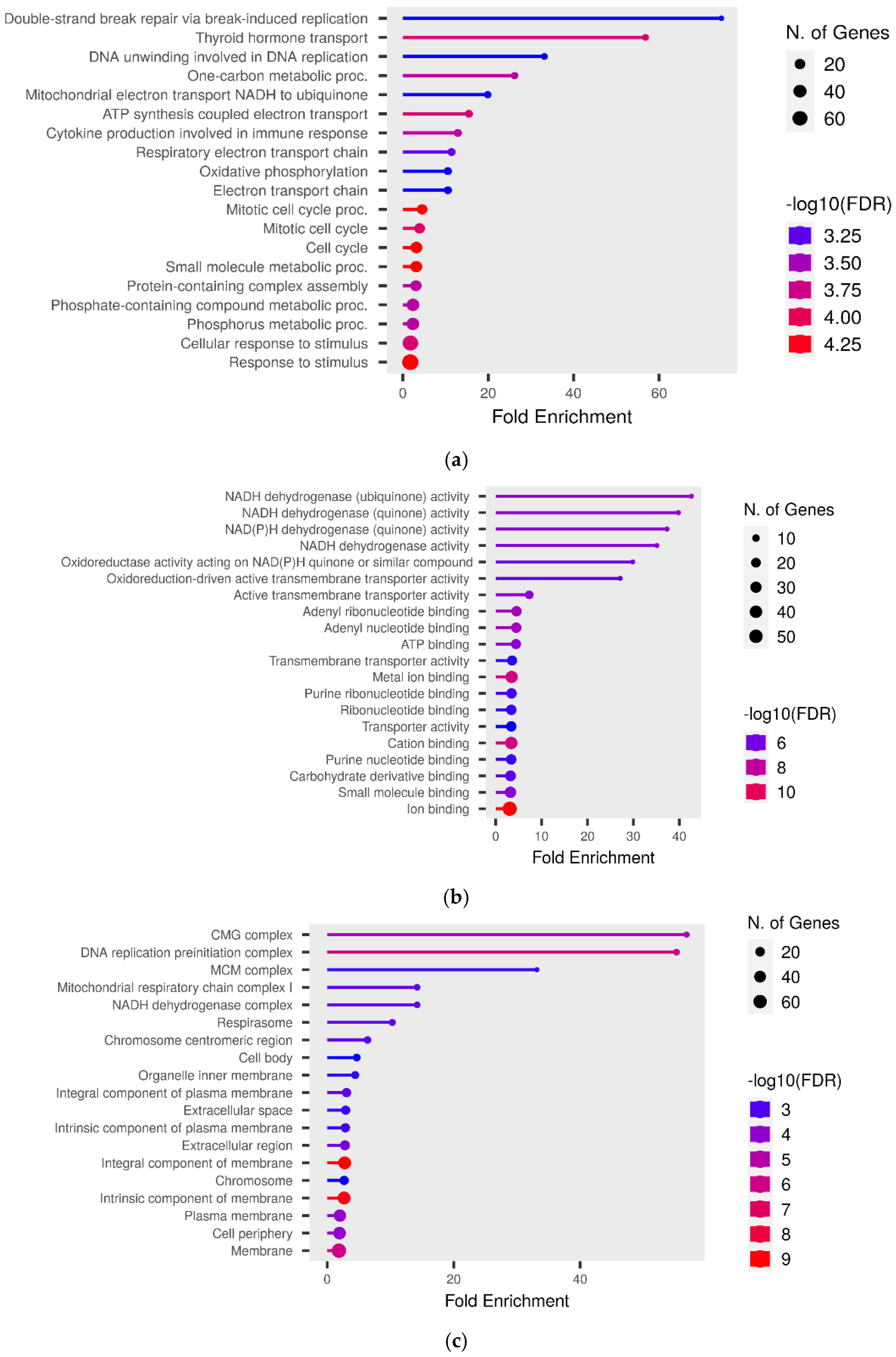
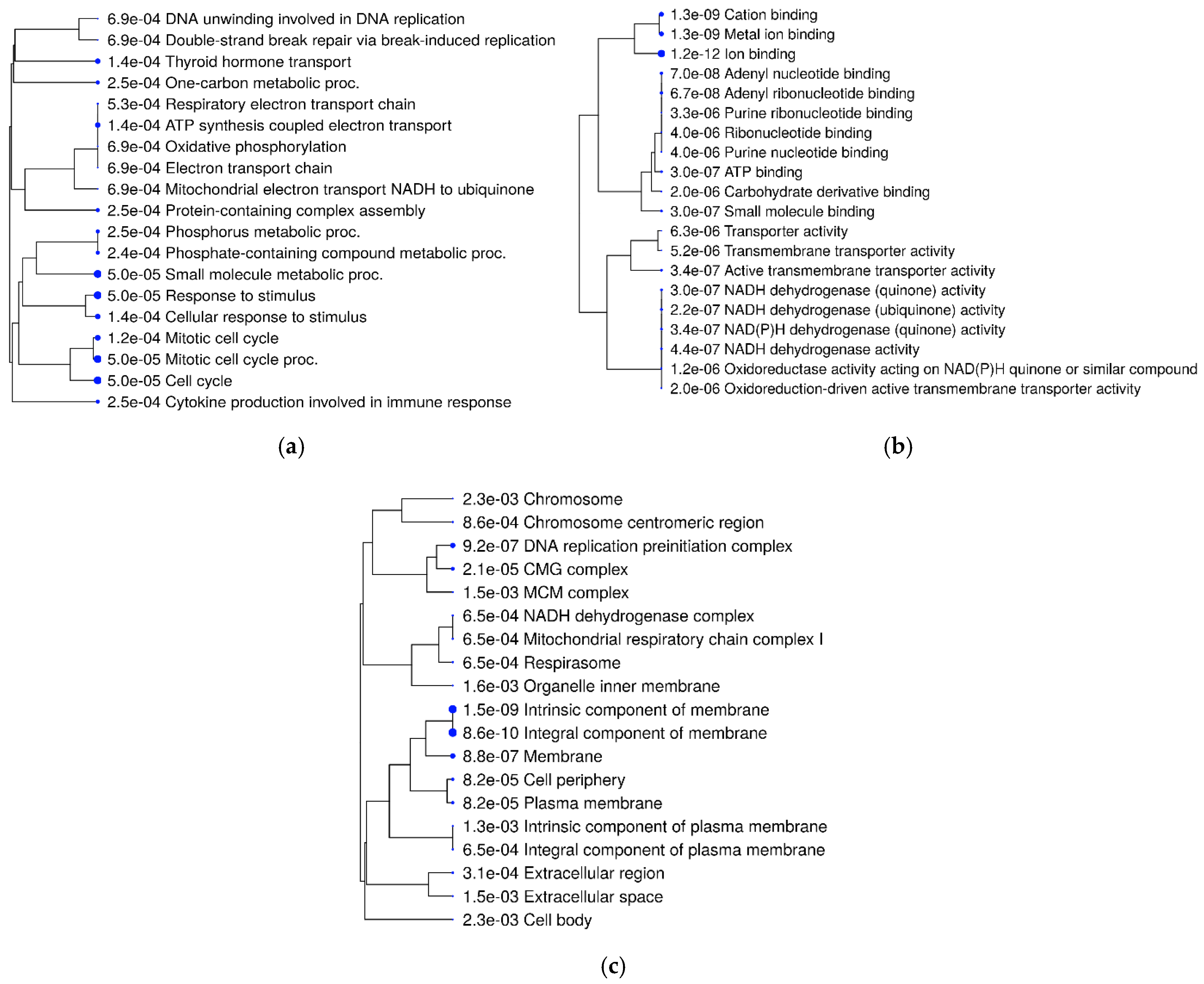
3.5. Functional Analysis
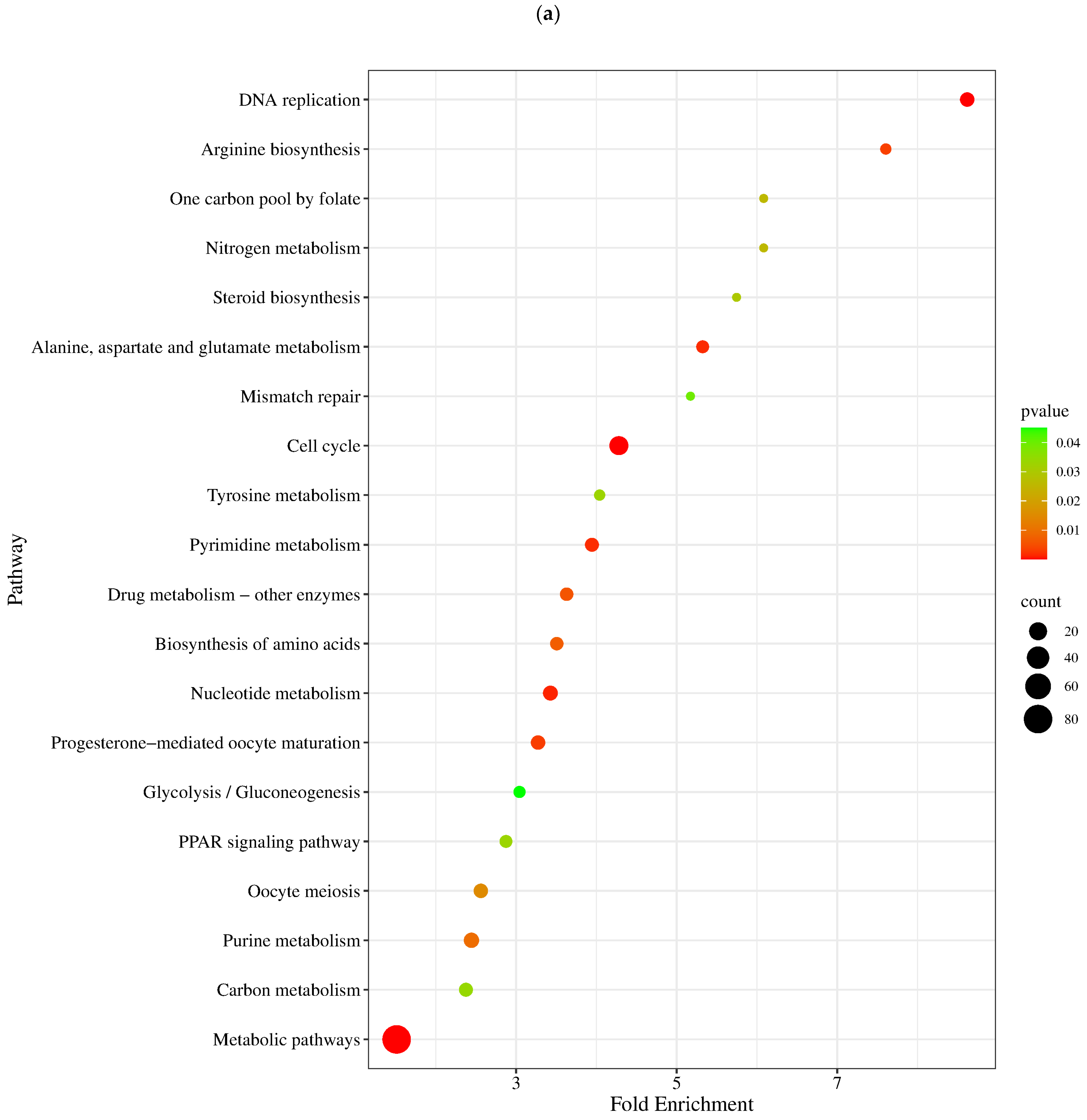
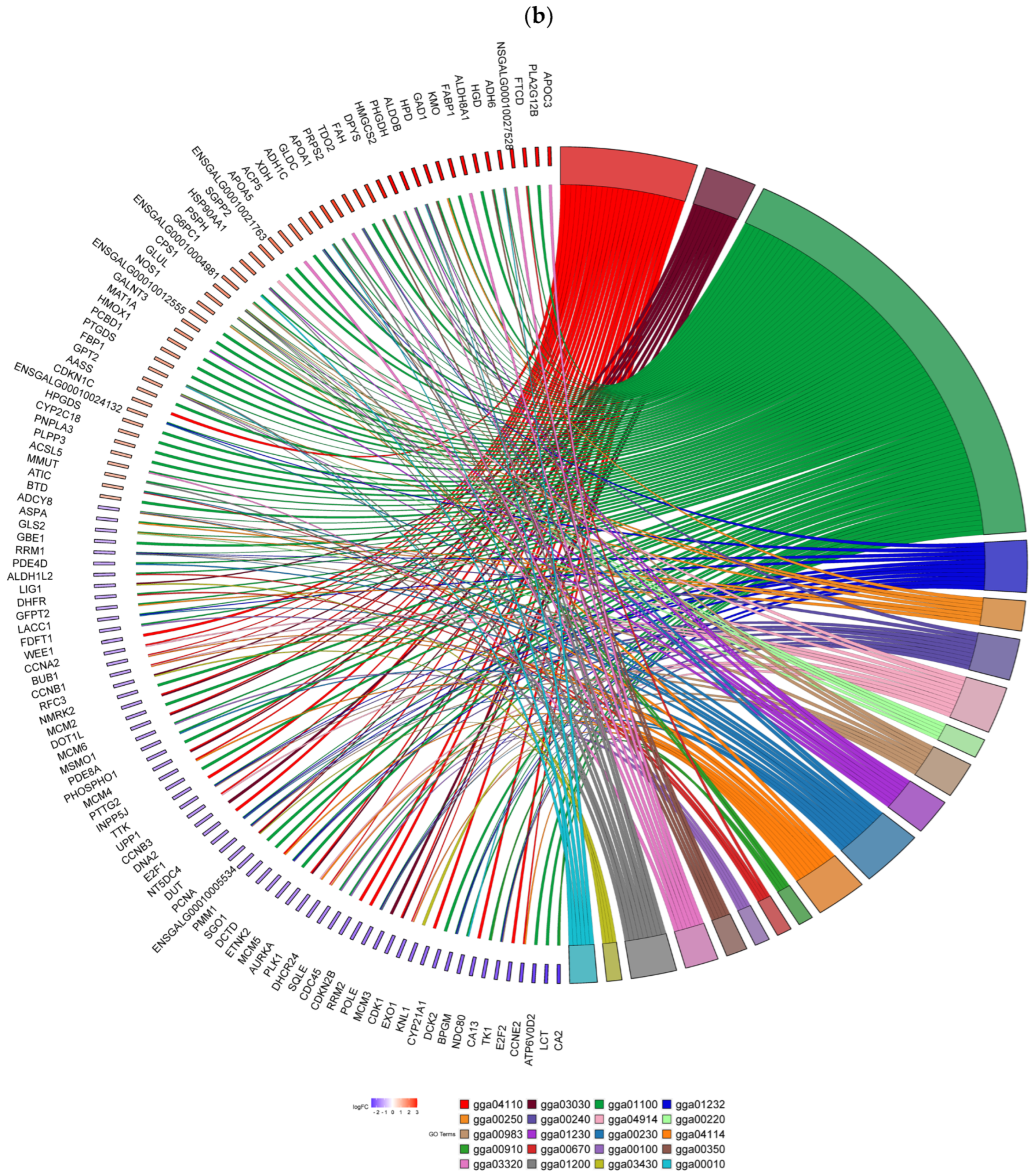
3.6. KEGG Pathway Analysis
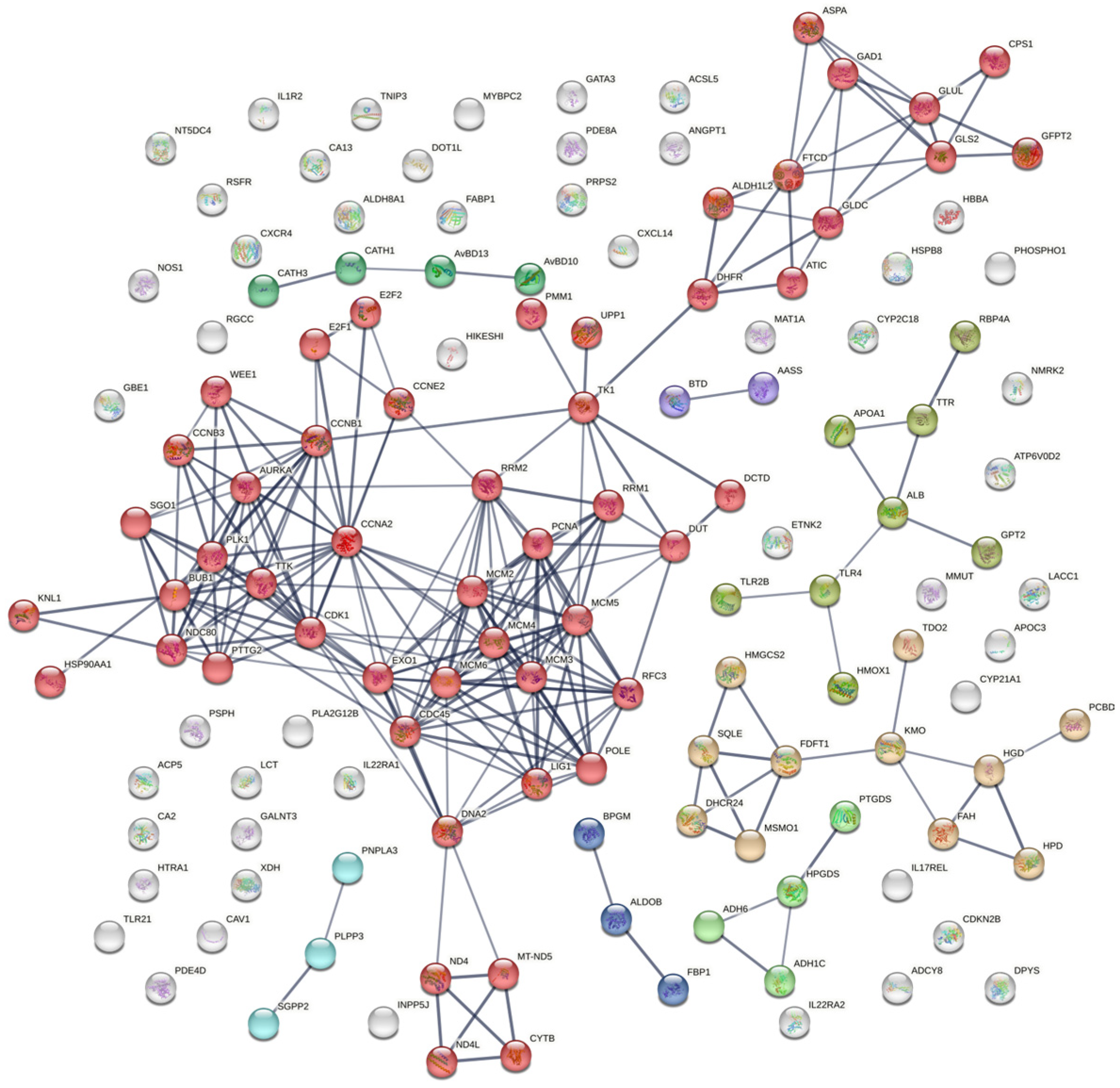
3.7. Protein–Protein Interaction (PPI)
4. Discussion
4.1. Differential Gene Expression Analysis
4.2. GO and KEGG Pathway Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Y.; Saelao, P.; Chanthavixay, G.; Gallardo, R.A.; Wolc, A.; Fulton, J.E.; Dekkers, J.M.; Lamont, S.J.; Kelly, T.R.; Zhou, H. Genomic Regions and Candidate Genes Affecting Response to Heat Stress with Newcastle Virus Infection in Commercial Layer Chicks Using Chicken 600K Single Nucleotide Polymorphism Array. Int. J. Mol. Sci. 2024, 25, 2640. [Google Scholar] [CrossRef] [PubMed]
- DAHD. Animal Husbandry Statistics (AHS). Available online: https://dahd.gov.in/schemes/programmes/animal-husbandry-statistics (accessed on 19 October 2024).
- Food and Agriculture Organization of the United Nations. Production|Gateway to Poultry Production and Products. Available online: https://www.fao.org/poultry-production-products/production/en/ (accessed on 19 October 2024).
- Jaynudin, K.; Joshi, B.; Mathakiya, R.; Prajapati, K.; Sipai, S. Economic Impact of Genotype-Xiii Newcastle Disease Virus Infection on Commercial Vaccinated Layer Farms in India. Int. J. Livest. Res. 2018, 8, 280–288. [Google Scholar] [CrossRef]
- Sharma, R.; Saran, S.; Yadav, A.S.; Kumar, S.; Verma, M.R.; Kumar, D.; Tyagi, J.S. Economic Losses Due to Newcastle Disease in Layers in Subtropical India. Indian J. Anim. Sci. 2023, 93, 422–426. [Google Scholar] [CrossRef]
- Bhadouriya, S.; Kapoor, S.; Krishan, B.; Chhabra, R. Isolation and Characterization of the Newcastle Disease Virus (NDV) of Haryana Region Based on F-Gene Sequence. J. Anim. Res. 2018, 8, 999–1003. [Google Scholar] [CrossRef]
- Narayanan, M.S.; Parthiban, M.; Sathiya, P.; Kumanan, K. Molecular Detection of Newcastle Disease Virus Using Flinders Tehnology Associates-PCR. Vet. Arch. 2010, 80, 51–60. [Google Scholar]
- Prajapati, A.K.K.S.; Bhadaniya, B.B.J.A.R.; Vagh, D.T.F.A.; Padodara, B.J.T.R.J.; Kumbhani, K.N.M.T.R. An Economical Impact of Newcastle Disease Outbreaks in Various Commercial Broiler Chicken Farms During 2020–21 in Gujarat, India. Int. J. Curr. Microbiol. Appl. Sci. 2021, 10, 411–420. [Google Scholar] [CrossRef]
- Charkhkar, S.; Bashizade, M.; Sotoudehnejad, M.; Ghodrati, M.; Bulbuli, F.; Akbarein, H. The Evaluation and Importance of Newcastle Disease’s Economic Loss in Commercial Layer Poultry. J. Poult. Sci. Avian Dis. 2024, 2, 1–4. [Google Scholar] [CrossRef]
- Waweru, K.M.; Omia, D.O.; Kiganane, L.; Miroro, O.; Chemuliti, J.; Nyamongo, I.K.; Bukachi, S.A. Socio-Economic and Structural Barriers in Newcastle Disease Vaccines Uptake by Smallholder Women Farmers in Southeastern Kenya. PLoS ONE 2023, 18, e0283076. [Google Scholar] [CrossRef]
- Ogolla, K.O.; Anyona, D.N.; Chemuliti, J.K.; Kimani, W.W.; King’oo, F.M.; Waweru, K.M.; Omia, D.O.; Nyamongo, I.K.; Bukachi, S.A. Effectiveness of a Community-Centered Newcastle Disease Vaccine Delivery Model Under Paid and Free Vaccination Frameworks in Southeastern Kenya. PLoS ONE 2024, 19, e0308088. [Google Scholar] [CrossRef]
- Saelao, P.; Wang, Y.; Chanthavixay, G.; Yu, V.; Gallardo, R.A.; Dekkers, J.C.M.; Lamont, S.J.; Kelly, T.; Zhou, H. Integrated Proteomic and Transcriptomic Analysis of Differential Expression of Chicken Lung Tissue in Response to NDV Infection during Heat Stress. Genes 2018, 9, 579. [Google Scholar] [CrossRef]
- Radhika, R.; Thiagarajan, D.; Veeramani, P.; Karthickeyan, S.M.K. Aseel, Kadaknath and White Leghorn Chicken Immune Response to Variation in Sheep Red Blood Cell. Int. J. Pure App. Biosci. 2017, 5, 335–340. [Google Scholar] [CrossRef]
- Rout, P.K.; Pani, P.K.; Naithani, S. Genetic Susceptibility of Indigenous Chicks to Subgroup A Rous Sarcoma Virus Inoculated via the Chorioallantoic Membrane. Vet. Immunol. Immunopathol. 1992, 33, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.P.; Kannaki, T.R.; Mahapatra, R.K.; Reddy, M.R.; Paul, S.S.; Bhattacharya, T.K.; Laxmi, N.A.; Jayakumar, S.; Chatterjee, R.N. Immunocompetence Profile of Indian Native vs Exotic Chicken Breeds. Indian J. Anim. Res. 2022, 1, 5. [Google Scholar] [CrossRef]
- Jaiswal, G.; Kumar, S.; Prasad, Y. Immunocompetence Traits and Their Inheritance Pattern in Kadaknath Native Chicken. Indian J. Anim. Res. 2014, 48, 509. [Google Scholar] [CrossRef]
- Muthusamy, M.; Nagarajan, M.; Karuppusamy, S.; Ramasamy, K.T.; Ramasamy, A.; Kalaivanan, R.; Ramasamy, G.K.M.T.; Kannan, T.A. Unveiling the Genetic Symphony: Diversity and Expression of Chicken IFITM Genes in Aseel and Kadaknath Breeds. Heliyon 2024, 10, e37729. [Google Scholar] [CrossRef]
- Malarmathi, M.; Murali, N.; Selvaraju, M.; Sivakumar, K.; Gowthaman, V.; Raghavendran, V.B.; Raja, A.; Peters, S.O.; Thiruvenkadan, A.K. In Vitro Characterization of chIFITMs of Aseel and Kadaknath Chicken Breeds Against Newcastle Disease Virus Infection. Biology 2023, 12, 919. [Google Scholar] [CrossRef]
- Zerjal, T.; Härtle, S.; Gourichon, D.; Guillory, V.; Bruneau, N.; Laloë, D.; Pinard-van der Laan, M.-H.; Trapp, S.; Bed’hom, B.; Quéré, P. Assessment of Trade-Offs Between Feed Efficiency, Growth-Related Traits, and Immune Activity in Experimental Lines of Layer Chickens. Genet. Sel. Evol. 2021, 53, 44. [Google Scholar] [CrossRef]
- Yunis, R.; Ben-David, A.; Heller, E.D.; Cahaner, A. Immunocompetence and Viability under Commercial Conditions of Broiler Groups Differing in Growth Rate and in Antibody Response to Escherichia coli Vaccine. Poult. Sci. 2000, 79, 810–816. [Google Scholar] [CrossRef]
- Blohm, U.; Weigend, S.; Preisinger, R.; Beer, M.; Hoffmann, D. Immunological Competence of Different Domestic Chicken Breeds Against Avian Influenza Infection. Avian Dis. 2016, 60, 262–268. [Google Scholar] [CrossRef]
- Guo, L.; Mu, Z.; Nie, F.; Chang, X.; Duan, H.; Li, H.; Zhang, J.; Zhou, J.; Ji, Y.; Li, M. Thymic Transcriptome Analysis after Newcastle Disease Virus Inoculation in Chickens and the Influence of Host Small RNAs on NDV Replication. Sci. Rep. 2021, 11, 10270. [Google Scholar] [CrossRef]
- Guo, L.-X.; Nie, F.-R.; Huang, A.-Q.; Wang, R.-N.; Li, M.-Y.; Deng, H.-Y.; Zhou, Y.-Z.; Zhou, X.-M.; Huang, Y.-K.; Zhou, J.; et al. Transcriptomic Analysis of Chicken Immune Response to Infection of Different Doses of Newcastle Disease Vaccine. Gene 2021, 766, 145077. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, S.F. Developmental Biology, the Stem Cell of Biological Disciplines. PLoS Biol. 2017, 15, e2003691. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Xu, Z.; Qiu, Y.; Qiu, X.; Tan, L.; Song, C.; Sun, Y.; Liao, Y.; Liu, X.; Ding, C. Single-Cell Transcriptome Atlas of Newcastle Disease Virus in Chickens Both In Vitro and In Vivo. Microbiol. Spectr. 2023, 11, e05121-22. [Google Scholar] [CrossRef] [PubMed]
- Schilling, M.A.; Katani, R.; Memari, S.; Cavanaugh, M.; Buza, J.; Radzio-Basu, J.; Mpenda, F.N.; Deist, M.S.; Lamont, S.J.; Kapur, V. Transcriptional Innate Immune Response of the Developing Chicken Embryo to Newcastle Disease Virus Infection. Front. Genet. 2018, 9, 61. [Google Scholar] [CrossRef]
- Andrews, S. Babraham Bioinformatics-FastQC A Quality Control Tool for High Throughput Sequence Data. Available online: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 19 June 2024).
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. Fastp: An Ultra-Fast All-in-One FASTQ Preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast Universal RNA-Seq Aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. Feature Counts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Yu, G.; Wang, L.-G.; Han, Y.; He, Q.-Y. clusterProfiler: An R Package for Comparing Biological Themes Among Gene Clusters. OMICS J. Integr. Biol. 2012, 16, 284. [Google Scholar] [CrossRef]
- Luo, W.; Brouwer, C. Pathview: An R/Bioconductor Package for Pathway-Based Data Integration and Visualization. Bioinformatics 2013, 29, 1830–1831. [Google Scholar] [CrossRef]
- Walter, W.; Sánchez-Cabo, F.; Ricote, M. GOplot: An R Package for Visually Combining Expression Data with Functional Analysis. Bioinformatics 2015, 31, 2912–2914. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.X.; Jung, D.; Yao, R. ShinyGO: A Graphical Gene-Set Enrichment Tool for Animals and Plants. Bioinformatics 2020, 36, 2628–2629. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Chen, M.; Huang, X.; Zhang, G.; Zeng, L.; Zhang, G.; Wu, S.; Wang, Y. SRplot: A Free Online Platform for Data Visualization and Graphing. PLoS ONE 2023, 18, e0294236. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The STRING Database in 2023: Protein-Protein Association Networks and Functional Enrichment Analyses for Any Sequenced Genome of Interest. Nucleic Acids Res. 2023, 51, D638–D646. [Google Scholar] [CrossRef] [PubMed]
- Pfaf, M.W. A New Mathematical Model for Relative Quantification in Real-Time RT–PCR. Nucleic Acids Res. 2001, 29, 45. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Lee, J.Y.; Song, J.J.; Wooming, A.; Li, X.; Zhou, H.; Bottje, W.G.; Kong, B.-W. Transcriptional Profiling of Host Gene Expression in Chicken Embryo Lung Cells Infected with Laryngotracheitis Virus. BMC Genom. 2010, 11, 445. [Google Scholar] [CrossRef]
- Xie, J.; Zeng, Q.; Wang, M.; Ou, X.; Ma, Y.; Cheng, A.; Zhao, X.-X.; Liu, M.; Zhu, D.; Chen, S.; et al. Transcriptomic Characterization of a Chicken Embryo Model Infected With Duck Hepatitis A Virus Type 1. Front. Immunol. 2018, 9, 1845. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, R.; Qu, G.; Peng, Y.; Xu, L.; Wang, C.; Huang, C.; Wang, Q. Transcriptome Analysis Reveals New Insight of Fowl Adenovirus Serotype 4 Infection. Front. Microbiol. 2020, 11, 146. [Google Scholar] [CrossRef]
- Li, P.; He, F.; Wu, C.; Zhao, G.; Hardwidge, P.R.; Li, N.; Peng, Y. Transcriptomic Analysis of Chicken Lungs Infected with Avian and Bovine Pasteurella Multocida Serotype A. Front. Vet. Sci. 2020, 7, 452. [Google Scholar] [CrossRef]
- Wang, X.P.; Wen, B.; Zhang, X.J.; Ma, L.; Liang, X.L.; Zhang, M.L. Transcriptome Analysis of Genes Responding to Infection of Leghorn Male Hepatocellular Cells With Fowl Adenovirus Serotype 4. Front. Vet. Sci. 2022, 9, 871038. [Google Scholar] [CrossRef] [PubMed]
- Zou, M.; Wang, T.; Wang, Y.; Luo, R.; Sun, Y.; Peng, X. Comparative Transcriptome Analysis Reveals the Innate Immune Response to Mycoplasma Gallisepticum Infection in Chicken Embryos and Newly Hatched Chicks. Animals 2023, 13, 1667. [Google Scholar] [CrossRef] [PubMed]
- Emam, M.; Mehrabani-Yeganeh, H.; Barjesteh, N.; Nikbakht, G.; Thompson-Crispi, K.; Charkhkar, S.; Mallard, B. The Influence of Genetic Background versus Commercial Breeding Programs on Chicken Immunocompetence. Poult. Sci. 2014, 93, 77–84. [Google Scholar] [CrossRef]
- Han, D.; Zhang, Y.; Chen, J.; Hua, G.; Li, J.; Deng, X.; Deng, X. Transcriptome Analyses of Differential Gene Expression in the Bursa of Fabricius between Silky Fowl and White Leghorn. Sci. Rep. 2017, 7, 45959. [Google Scholar] [CrossRef]
- Sadr, A.S.; Nassiri, M.; Ghaderi-Zefrehei, M.; Heidari, M.; Smith, J.; Muhaghegh Dolatabady, M. RNA-Seq Profiling between Commercial and Indigenous Iranian Chickens Highlights Differences in Innate Immune Gene Expression. Genes 2023, 14, 793. [Google Scholar] [CrossRef]
- Huang, Z.; Xu, Z.; Bai, H.; Huang, Y.; Kang, N.; Ding, X.; Liu, J.; Luo, H.; Yang, C.; Chen, W.; et al. Evolutionary Analysis of a Complete Chicken Genome. Proc. Natl. Acad. Sci. USA 2023, 120, e2216641120. [Google Scholar] [CrossRef]
- Ayers, K.L.; Davidson, N.M.; Demiyah, D.; Roeszler, K.N.; Grützner, F.; Sinclair, A.H.; Oshlack, A.; Smith, C.A. RNA Sequencing Reveals Sexually Dimorphic Gene Expression Before Gonadal Differentiation in Chicken and Allows Comprehensive Annotation of the W-Chromosome. Genome Biol. 2013, 14, R26. [Google Scholar] [CrossRef]
- Xu, Z.; Che, T.; Li, F.; Tian, K.; Zhu, Q.; Mishra, S.K.; Dai, Y.; Li, M.; Li, D. The Temporal Expression Patterns of Brain Transcriptome during Chicken Development and Ageing. BMC Genom. 2018, 19, 917. [Google Scholar] [CrossRef]
- Nie, H.; Crooijmans, R.; Bastiaansen, J.; Megens, H.-J.; Groenen, M. Regional Regulation of Transcription in the Chicken Genome. BMC Genom. 2010, 11, 28. [Google Scholar] [CrossRef]
- Hadders, M.A.; Beringer, D.X.; Gros, P. Structure of C8α-MACPF Reveals Mechanism of Membrane Attack in Complement Immune Defense. Science 2007, 317, 1552–1554. [Google Scholar] [CrossRef]
- Monson, M.S.; Van Goor, A.G.; Persia, M.E.; Rothschild, M.F.; Schmidt, C.J.; Lamont, S.J. Genetic Lines Respond Uniquely Within the Chicken Thymic Transcriptome to Acute Heat Stress and Low Dose Lipopolysaccharide. Sci. Rep. 2019, 9, 13649. [Google Scholar] [CrossRef] [PubMed]
- Rabiei, M.; Low, W.Y.; Ren, Y.; Cahyono, M.I.; Doan, P.T.K.; Dharmayanti, I.; Grande, E.D.; Hemmatzadeh, F. Indicators of the Molecular Pathogenesis of Virulent Newcastle Disease Virus in Chickens Revealed by Transcriptomic Profiling of Spleen. Sci. Rep. 2021, 11, 17570. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, Y.; Zheng, S.J. Immune Evasion of Mycoplasma Gallisepticum: An Overview. Int. J. Mol. Sci. 2024, 25, 2824. [Google Scholar] [CrossRef]
- Yu, H.; Mi, C.; Wang, Q.; Dai, G.; Zhang, T.; Zhang, G.; Xie, K.; Zhao, Z. Long Noncoding RNA Profiling Reveals That LncRNA BTN3A2 Inhibits the Host Inflammatory Response to Eimeria tenella Infection in Chickens. Front. Immunol. 2022, 13, 891001. [Google Scholar] [CrossRef]
- Kern, C.; Wang, Y.; Chitwood, J.; Korf, I.; Delany, M.; Cheng, H.; Medrano, J.F.; Van Eenennaam, A.L.; Ernst, C.; Ross, P.; et al. Genome-Wide Identification of Tissue-Specific Long Non-Coding RNA in Three Farm Animal Species. BMC Genom. 2018, 19, 684. [Google Scholar] [CrossRef]
- Karimi, P.; Bakhtiarizadeh, M.R.; Salehi, A.; Izadnia, H.R. Transcriptome Analysis Reveals the Potential Roles of Long Non-Coding RNAs in Feed Efficiency of Chicken. Sci. Rep. 2022, 12, 2558. [Google Scholar] [CrossRef]
- Kosinska-Selbi, B.; Mielczarek, M.; Szyda, J. Review: Long Non-Coding RNA in Livestock. Animal 2020, 14, 2003–2013. [Google Scholar] [CrossRef]
- Lagarrigue, S.; Lorthiois, M.; Degalez, F.; Gilot, D.; Derrien, T. LncRNAs in Domesticated Animals: From Dog to Livestock Species. Mamm. Genome 2022, 33, 248–270. [Google Scholar] [CrossRef]
- Jehl, F.; Muret, K.; Bernard, M.; Boutin, M.; Lagoutte, L.; Désert, C.; Dehais, P.; Esquerré, D.; Acloque, H.; Giuffra, E.; et al. An Integrative Atlas of Chicken Long Non-Coding Genes and Their Annotations Across 25 Tissues. Sci. Rep. 2020, 10, 20457. [Google Scholar] [CrossRef]
- Mi, H.; Ebert, D.; Muruganujan, A.; Mills, C.; Albou, L.-P.; Mushayamaha, T.; Thomas, P.D. PANTHER Version 16: A Revised Family Classification, Tree-Based Classification Tool, Enhancer Regions and Extensive API. Nucleic Acids Res. 2021, 49, D394–D403. [Google Scholar] [CrossRef]
- Ginn, L.; Montagna, M.L.; Wu, Q.; Shi, L. Diverse Roles of Long Non-Coding RNAs in Viral Diseases. Rev. Med. Virol. 2020, 31, e2198. [Google Scholar] [CrossRef]
- Ouyang, J.; Hu, J.; Chen, J.-L. lncRNAs Regulate the Innate Immune Response to Viral Infection. Wiley Interdiscip. Rev. RNA 2015, 7, 129. [Google Scholar] [CrossRef] [PubMed]
- Evans, A.S. Characterizing the Role of BPIFB Proteins During Positive Strand RNA Virus Infection. Available online: http://d-scholarship.pitt.edu/39747/ (accessed on 19 October 2024).
- Evans, A.S.; Lennemann, N.J.; Fan, K.; Coyne, C.B. BPIFB3 Facilitates Flavivirus Infection by Controlling RETREG1-Dependent Reticulophagy. bioRxiv 2018, 333435. [Google Scholar] [CrossRef]
- Yang, W.; Gu, Z.; Zhang, H.; Hu, H. To TRIM the Immunity: From Innate to Adaptive Immunity. Front. Immunol. 2020, 11, 02157. [Google Scholar] [CrossRef]
- Choi, J.; Phelan, J.D.; Wright, G.W.; Häupl, B.; Huang, D.W.; Shaffer, A.L.; Young, R.M.; Wang, Z.; Zhao, H.; Yu, X.; et al. Regulation of B Cell Receptor-Dependent NF-κB Signaling by the Tumor Suppressor KLHL14. Proc. Natl. Acad. Sci. USA 2020, 117, 6092–6102. [Google Scholar] [CrossRef]
- Pal, A.; Pal, A.; Banerjee, S.; Batabyal, S.; Chatterjee, P.N. Mutation in Cytochrome B Gene Causes Debility and Adverse Effects on Health of Sheep. Mitochondrion 2019, 46, 393–404. [Google Scholar] [CrossRef]
- Lee, H.J.; Takemoto, N.; Kurata, H.; Kamogawa, Y.; Miyatake, S.; O’Garra, A.; Arai, N. Gata-3 Induces T Helper Cell Type 2 (Th2) Cytokine Expression and Chromatin Remodeling in Committed Th1 Cells. J. Exp. Med. 2000, 192, 105–116. [Google Scholar] [CrossRef]
- Zheng, W.; Flavell, R.A. The Transcription Factor GATA-3 Is Necessary and Sufficient for Th2 Cytokine Gene Expression in CD4 T Cells. Cell 1997, 89, 587–596. [Google Scholar] [CrossRef]
- Chu, Q.; Ding, Y.; Cai, W.; Liu, L.; Zhang, H.; Song, J. Marek’s Disease Virus Infection Induced Mitochondria Changes in Chickens. Int. J. Mol. Sci. 2019, 20, 3150. [Google Scholar] [CrossRef]
- Beere, H.M. ‘The Stress of Dying’: The Role of Heat Shock Proteins in the Regulation of Apoptosis. J. Cell Sci. 2004, 117, 2641–2651. [Google Scholar] [CrossRef]
- Wang, L.; Yang, S.; Zhao, K.; Han, L. Expression Profiles of the Heat Shock Protein 70 Gene in Response to Heat Stress in Agrotis C-Nigrum (Lepidoptera: Noctuidae). J. Insect Sci. 2015, 15, 9. [Google Scholar] [CrossRef]
- Nawaz, A.H.; Lin, S.; Wang, F.; Zheng, J.; Sun, J.; Zhang, W.; Jiao, Z.; Zhu, Z.; An, L.; Zhang, L. Investigating the Heat Tolerance and Production Performance in Local Chicken Breed Having Normal and Dwarf Size. Animal 2023, 17, 100707. [Google Scholar] [CrossRef] [PubMed]
- Rachman, M.P.; Bamidele, O.; Dessie, T.; Smith, J.; Hanotte, O.; Gheyas, A.A. Genomic Analysis of Nigerian Indigenous Chickens Reveals Their Genetic Diversity and Adaptation to Heat-Stress. Sci. Rep. 2024, 14, 2209. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yu, W. Heat Shock Proteins and Viral Infection. Front. Immunol. 2022, 13, 947789. [Google Scholar] [CrossRef]
- Hauser, H.; Shen, L.; Gu, Q.-L.; Krueger, S.; Chen, S.-Y. Secretory Heat-Shock Protein as a Dendritic Cell-Targeting Molecule: A New Strategy to Enhance the Potency of Genetic Vaccines. Gene Ther. 2004, 11, 924–932. [Google Scholar] [CrossRef]
- Chang, X.; Shi, X.; Zhang, X.; Chen, J.; Fan, X.; Yang, Y.; Wang, L.; Wang, A.; Deng, R.; Zhou, E.; et al. miR-382-5p Promotes Porcine Reproductive and Respiratory Syndrome Virus (PRRSV) Replication by Negatively Regulating the Induction of Type I Interferon. FASEB J. 2020, 34, 4497–4511. [Google Scholar] [CrossRef]
- Huang, W.-L. Ranking Gene Ontology Terms for Predicting Non-Classical Secretory Proteins in Eukaryotes and Prokaryotes. J. Theor. Biol. 2012, 312, 105–113. [Google Scholar] [CrossRef]
- de Lima, C.B.; dos Santos, É.C.; Ispada, J.; Fontes, P.K.; Nogueira, M.F.G.; dos Santos, C.M.D.; Milazzotto, M.P. The Dynamics Between in Vitro Culture and Metabolism: Embryonic Adaptation to Environmental Changes. Sci. Rep. 2020, 10, 15672. [Google Scholar] [CrossRef]
- Van Every, H.A.; Schmidt, C.J. Transcriptomic and Metabolomic Characterization of Post-Hatch Metabolic Reprogramming During Hepatic Development in the Chicken. BMC Genom. 2021, 22, 380. [Google Scholar] [CrossRef]
- Kumar, H.; Choo, H.; Iskender, A.U.; Srikanth, K.; Kim, H.; Zhunushov, A.T.; Jang, G.W.; Lim, Y.; Song, K.-D.; Park, J.-E. RNA Seq Analyses of Chicken Reveals Biological Pathways Involved in Acclimation into Different Geographical Locations. Sci. Rep. 2020, 10, 19288. [Google Scholar] [CrossRef]
- Xiao, Q.; Wang, L.; Wang, J.; Wang, M.; Wang, D.W.; Ding, H. A Novel lncRNA GM47544 Modulates Triglyceride Metabolism by Inducing Ubiquitination-Dependent Protein Degradation of APOC3. Mol. Metab. 2024, 88, 102011. [Google Scholar] [CrossRef] [PubMed]
- Bhale, A.S.; Venkataraman, K. Leveraging Knowledge of HDLs Major Protein ApoA1: Structure, Function, Mutations, and Potential Therapeutics. Biomed. Pharmacother. 2022, 154, 113634. [Google Scholar] [CrossRef]
- Allen, R.G.; Barrett, J.T.; Campbell, B.J. Lipoprotein Inhibitor of Newcastle Disease Virus from Chicken Lung. Appl. Microbiol. 1971, 21, 53–60. [Google Scholar] [CrossRef]
- WU, H.; LI, X.; SHEN, C. Peroxisome Proliferator-Activated Receptor γ in White and Brown Adipocyte Regulation and Differentiation. Physiol. Res. 2020, 69, 759–773. [Google Scholar] [CrossRef]
- Murakami, M.; Taketomi, Y.; Sato, H.; Yamamoto, K. Secreted Phospholipase A2 Revisited. J. Biochem. 2011, 150, 233–255. [Google Scholar] [CrossRef]
- Brownlie, R.; Zhu, J.; Allan, B.; Mutwiri, G.K.; Babiuk, L.A.; Potter, A.; Griebel, P. Chicken TLR21 Acts as a Functional Homologue to Mammalian TLR9 in the Recognition of CpG Oligodeoxynucleotides. Mol. Immunol. 2009, 46, 3163–3170. [Google Scholar] [CrossRef]
- Chuang, Y.-C.; Tseng, J.-C.; Yang, J.-X.; Liu, Y.-L.; Yeh, D.-W.; Lai, C.-Y.; Yu, G.-Y.; Hsu, L.-C.; Huang, C.-M.; Chuang, T.-H. Toll-like Receptor 21 of Chicken and Duck Recognize a Broad Array of Immunostimulatory CpG-Oligodeoxynucleotide Sequences. Vaccines 2020, 8, 639. [Google Scholar] [CrossRef]
- Nawab, A.; An, L.; Wu, J.; Li, G.; Liu, W.; Zhao, Y.; Wu, Q.; Xiao, M. Chicken Toll-like Receptors and Their Significance in Immune Response and Disease Resistance. Int. Rev. Immunol. 2019, 38, 284–306. [Google Scholar] [CrossRef]
- Barnes, B.J.; Somerville, C.C. Modulating Cytokine Production via Select Packaging and Secretion From Extracellular Vesicles. Front. Immunol. 2020, 11, 1040. [Google Scholar] [CrossRef]
- Henry, G.; Garner, W.L. Inflammatory Mediators in Wound Healing. Surg. Clin. N. Am. 2003, 83, 483–507. [Google Scholar] [CrossRef]
- Fitzgerald, W.; Freeman, M.L.; Lederman, M.M.; Vasilieva, E.; Romero, R.; Margolis, L. A System of Cytokines Encapsulated in ExtraCellular Vesicles. Sci. Rep. 2018, 8, 8973. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Zhu, M.; Duan, J.; Wang, H.; Chen, J.; Xiao, Y.; Wang, Y.; Wang, J.; Yu, X.; Yang, H. Comprehensive Analysis of Immune-Related Prognosis of TK1 in Hepatocellular Carcinoma. Front. Oncol. 2022, 11, 786873. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Wang, S.; Du, H.; Fan, L.; Yuan, W.; Xu, Q.; Ren, J.; Lin, Q.; Xiang, B.; Ding, C.; et al. NDV-Induced Autophagy Enhances Inflammation through NLRP3/Caspase-1 Inflammasomes and the P38/MAPK Pathway. Vet. Res. 2023, 54, 43. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Balasubramaniam, S.D.; Lee, Y.J.; Balakrishnan, V.; Oon, C.E. Minichromosome Maintenance Complex (MCM) Genes Profiling and MCM2 Protein Expression in Cervical Cancer Development. Asian Pac. J. Cancer Prev. 2019, 20, 3043–3049. [Google Scholar] [CrossRef]
- Seo, Y.-S.; Kang, Y.-H. The Human Replicative Helicase, the CMG Complex, as a Target for Anti-Cancer Therapy. Front. Mol. Biosci. 2018, 5, 26. [Google Scholar] [CrossRef]
- Czernik, M.; Winiarczyk, D.; Sampino, S.; Gręda, P.; Parillo, S.; Modliński, J.A.; Loi, P. Mitochondrial Function and Intracellular Distribution Is Severely Affected in In Vitro Cultured Mouse Embryos. Sci. Rep. 2022, 12, 16152. [Google Scholar] [CrossRef]
- Maheshwari, A.; Peng, J.; Ramatchandirin, B.; Pearah, A.; He, L. Development and Functions of Mitochondria in Early Life. Newborn 2022, 1, 131–141. [Google Scholar] [CrossRef]
- Kaukonen, J.; Juselius, J.K.; Tiranti, V.; Kyttälä, A.; Zeviani, M.; Comi, G.P.; Keränen, S.; Peltonen, L.; Suomalainen, A. Role of Adenine Nucleotide Translocator 1 in mtDNA Maintenance. Science 2000, 289, 782–785. [Google Scholar] [CrossRef]
- Kornblum, C.; Nicholls, T.J.; Haack, T.B.; Schöler, S.; Peeva, V.; Danhauser, K.; Hallmann, K.; Zsurka, G.; Rorbach, J.; Iuso, A.; et al. Loss-of-Function Mutations in MGME1 Impair mtDNA Replication and Cause Multi-Systemic Mitochondrial Disease. Nat. Genet. 2013, 45, 214–219. [Google Scholar] [CrossRef]
- Ahmed, N.; Ronchi, D.; Comi, G.P. Genes and Pathways Involved in Adult Onset Disorders Featuring Muscle Mitochondrial DNA Instability. Int. J. Mol. Sci. 2015, 16, 18054–18076. [Google Scholar] [CrossRef]
- Thompson, K.; Majd, H.; Dallabona, C.; Reinson, K.; King, M.S.; Alston, C.L.; He, L.; Lodi, T.; Jones, S.A.; Fattal-Valevski, A.; et al. Recurrent De Novo Dominant Mutations in SLC25A4 Cause Severe Early-Onset Mitochondrial Disease and Loss of Mitochondrial DNA Copy Number. Am. J. Hum. Genet. 2016, 99, 860–876. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.-J.; Monson, M.; Kaiser, M.; Lamont, S.J. Induction of Chicken Host Defense Peptides Within Disease-Resistant and -Susceptible Lines. Genes 2020, 11, 1195. [Google Scholar] [CrossRef]
- Cuperus, T.; Coorens, M.; van Dijk, A.; Haagsman, H.P. Avian Host Defense Peptides. Dev. Comp. Immunol. 2013, 41, 352–369. [Google Scholar] [CrossRef] [PubMed]
- Almalki, S.G. The Pathophysiology of the Cell Cycle in Cancer and Treatment Strategies Using Various Cell Cycle Checkpoint Inhibitors. Pathol.-Res. Pract. 2023, 251, 154854. [Google Scholar] [CrossRef] [PubMed]
- Xing, Z.; Wang, X.; Liu, J.; Zhang, M.; Feng, K.; Wang, X. Expression and Prognostic Value of CDK1, CCNA2, and CCNB1 Gene Clusters in Human Breast Cancer. J. Int. Med. Res. 2021, 49, 0300060520980647. [Google Scholar] [CrossRef]
- Kelman, L.M.; Kelman, Z. Replication|DNA Replication Fork, Eukaryotic. In Encyclopedia of Biological Chemistry III, 3rd ed.; Jez, J., Ed.; Elsevier: Oxford, UK, 2013; pp. 63–66. ISBN 978-0-12-822040-5. [Google Scholar]
- Sun, Y.; Cheng, Z.; Liu, S. MCM2 in Human Cancer: Functions, Mechanisms, and Clinical Significance. Mol. Med. 2022, 28, 128. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muthusamy, M.; Ramasamy, K.T.; Peters, S.O.; Palani, S.; Gowthaman, V.; Nagarajan, M.; Karuppusamy, S.; Thangavelu, V.; Aranganoor Kannan, T. Transcriptomic Profiling Reveals Altered Expression of Genes Involved in Metabolic and Immune Processes in NDV-Infected Chicken Embryos. Metabolites 2024, 14, 669. https://doi.org/10.3390/metabo14120669
Muthusamy M, Ramasamy KT, Peters SO, Palani S, Gowthaman V, Nagarajan M, Karuppusamy S, Thangavelu V, Aranganoor Kannan T. Transcriptomic Profiling Reveals Altered Expression of Genes Involved in Metabolic and Immune Processes in NDV-Infected Chicken Embryos. Metabolites. 2024; 14(12):669. https://doi.org/10.3390/metabo14120669
Chicago/Turabian StyleMuthusamy, Malarmathi, Kannaki T. Ramasamy, Sunday Olusola Peters, Srinivasan Palani, Vasudevan Gowthaman, Murali Nagarajan, Sivakumar Karuppusamy, Vasanthakumar Thangavelu, and Thiruvenkadan Aranganoor Kannan. 2024. "Transcriptomic Profiling Reveals Altered Expression of Genes Involved in Metabolic and Immune Processes in NDV-Infected Chicken Embryos" Metabolites 14, no. 12: 669. https://doi.org/10.3390/metabo14120669
APA StyleMuthusamy, M., Ramasamy, K. T., Peters, S. O., Palani, S., Gowthaman, V., Nagarajan, M., Karuppusamy, S., Thangavelu, V., & Aranganoor Kannan, T. (2024). Transcriptomic Profiling Reveals Altered Expression of Genes Involved in Metabolic and Immune Processes in NDV-Infected Chicken Embryos. Metabolites, 14(12), 669. https://doi.org/10.3390/metabo14120669








