Determination of Polycyclic Aromatic Hydrocarbons (PAHs) and Phthalates in Human Placenta by Mixed Hexane/Ether Extraction and Gas Chromatography–Mass Spectrometry/Mass Spectrometry (GC-MS/MS)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population and Specimens
2.2. Laboratory Instruments and Consumables
2.3. Preparation of Storage Solutions
2.4. Internal Standard Liquid
2.5. Mixed Standard Solution (Chemistry)
2.6. Standard Series
2.7. Placenta Sample Pre-Processing Protocol
2.8. Chromatographic Conditions
2.9. Sampling Conditions
2.10. Analysis of Results
2.11. Principle and Quality Control
2.12. Precautions
3. Results
3.1. Comparison of Different Extractants
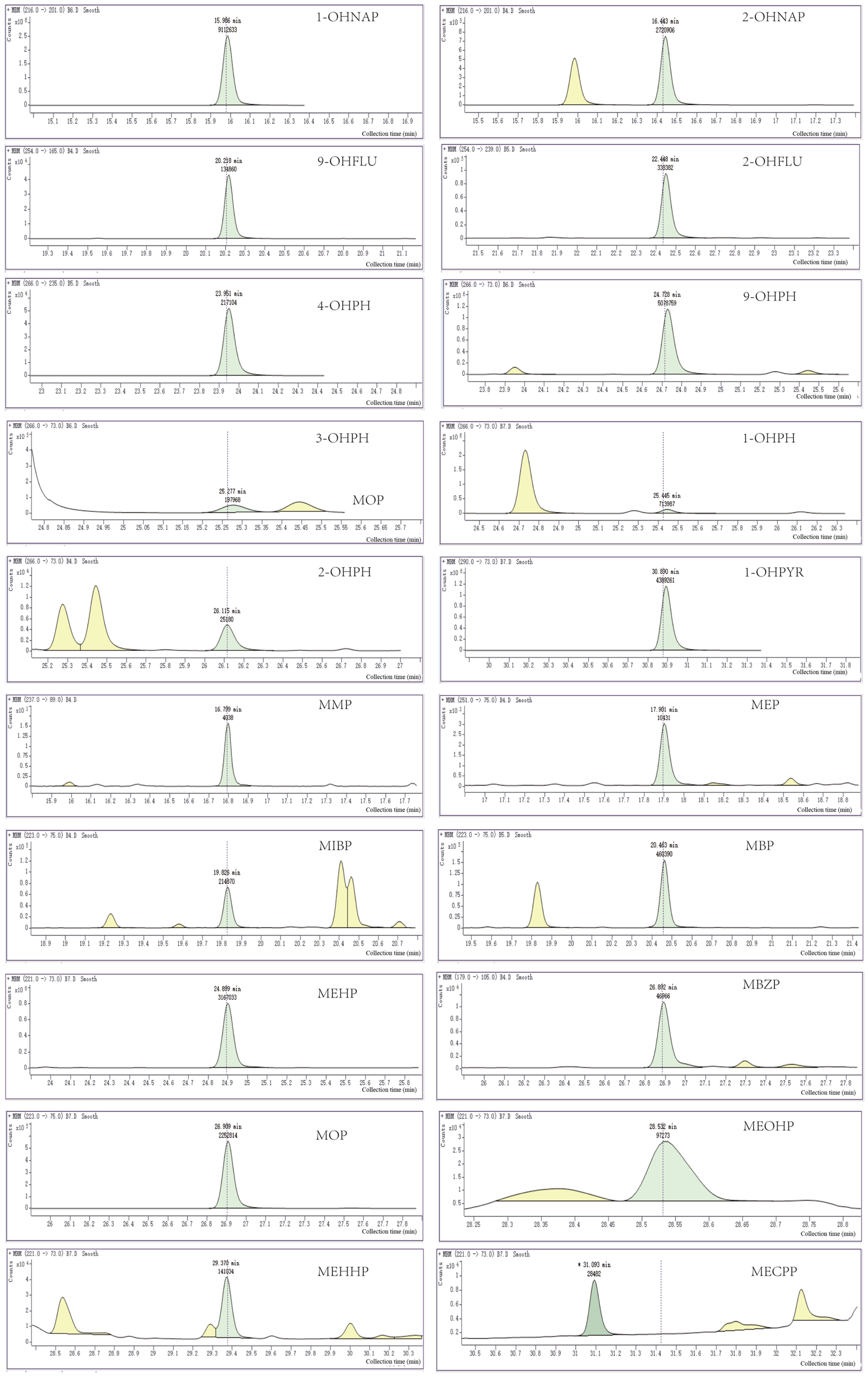
3.2. Sample Chromatogram
3.3. Spiked Recovery Rate
4. Discussion
5. Detection Method Evaluation
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, X.; Xu, X.; Dai, Y.; Cheng, Z.; Zheng, X.; Huo, X. Association of prenatal exposure to PAHs with anti-Müllerian hormone (AMH) levels and birth outcomes of newborns. Sci. Total. Environ. 2020, 723, 138009. [Google Scholar] [CrossRef]
- Zhang, X.; Li, X.; Jing, Y.; Fang, X.; Zhang, X.; Lei, B.; Yu, Y. Transplacental transfer of polycyclic aromatic hydrocarbons in paired samples of maternal serum, umbilical cord serum, and placenta in Shanghai, China. Environ. Pollut. 2017, 222, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Doroodzani, A.K.; Dobaradaran, S.; Akhbarizadeh, R.; Raeisi, A.; Rahmani, E.; Mahmoodi, M.; Nabipour, I.; Keshmiri, S.; Darabi, A.H.; Khamisipour, G.; et al. Diet, exposure to polycyclic aromatic hydrocarbons during pregnancy, and fetal growth: A comparative study of mothers and their fetuses in industrial and urban areas in Southwest Iran. Environ. Pollut. 2021, 276, 116668. [Google Scholar] [CrossRef]
- Jiang, L.; Xiao, Q.; Zhang, J.; Zhao, Y.; Chen, L.; Lu, S. Association between fetal exposure to polycyclic aromatic hydrocarbons and low birth weight: A case-control study in Shenzhen, China. Environ. Sci. Pollut. Res. Int. 2022, 29, 88779–88787. [Google Scholar] [CrossRef]
- Agarwal, P.; Anand, M.; Chakraborty, P.; Singh, L.; Masih, J.; Taneja, A. Placental levels of polycyclic aromatic hydrocarbons (PAHs) and their association with birth weight of infants. Drug Chem. Toxicol. 2022, 45, 868–877. [Google Scholar] [CrossRef]
- Huo, X.; Wu, Y.; Xu, L.; Zeng, X.; Qin, Q.; Xu, X. Maternal urinary metabolites of PAHs and its association with adverse birth outcomes in an intensive e-waste recycling area. Environ. Pollut. 2019, 245, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Strømmen, K.; Lyche, J.L.; Moltu, S.J.; Müller, M.H.; Blakstad, E.W.; Brække, K.; Sakhi, A.K.; Thomsen, C.; Nakstad, B.; Rønnestad, A.E.; et al. Estimated daily intake of phthalates, parabens, and bisphenol A in hospitalised very low birth weight infants. Chemosphere 2022, 309 Pt 1, 136687. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Li, R.; Zhao, Y.; Zhu, Q.; Xia, B.; Chen, S.; Zhang, Y. Evaluating effects of prenatal exposure to phthalates on neonatal birth weight: Structural equation model approaches. Chemosphere 2018, 205, 674–681. [Google Scholar] [CrossRef]
- Guo, X.; Sheng, Y.; Liu, B.; Tang, P.; Liu, R.; Wu, L.; Chen, J.; Huang, D.; Liu, S.; Qiu, X. Exposure to phthalates in early pregnancy and the risk of fetal growth restriction: A nested case-control study in a Zhuang Chinese population. Environ. Sci. Pollut. Res. Int. 2022, 29, 57318–57329. [Google Scholar] [CrossRef]
- Shen, R.; Zhao, L.L.; Yu, Z.; Zhang, C.; Chen, Y.H.; Wang, H.; Zhang, Z.H.; Xu, D.X. Maternal di-(2-ethylhexyl) phthalate exposure during pregnancy causes fetal growth restrictionin a stage-specific but gender-independent manner. Reprod. Toxicol. 2017, 67, 117–124. [Google Scholar] [CrossRef]
- Gao, F.; Hu, W.; Li, Y.; Shen, H.; Hu, J. Mono-2-ethylhexyl phthalate inhibits human extravillous trophoblast invasion via the PPARypathway. Toxicol. Appl. Pharmacol. 2017, 327, 23–29. [Google Scholar] [CrossRef]
- Xu, Y.; Knipp, G.T.; Cook, T.J. Effects of di-(2-ethylhexyl)-phthalate and its metabolites on the lipid profiling in rat HRP-1 trophoblast cells. Arch. Toxicol. 2006, 80, 293–298. [Google Scholar] [CrossRef]
- Myatt, L.; Cui, X. Oxidative stress in the placenta. Histochem. Cell Biol. 2004, 122, 369–382. [Google Scholar] [CrossRef] [PubMed]
- van’t Erve, T.J.; Rosen, E.M.; Barrett, E.S.; Nguyen, R.H.N.; Sathyanarayana, S.; Milne, G.L.; Calafat, A.M.; Swan, S.H.; Ferguson, K.K. Phthalates and phthalate alternatives have diverse associations with oxidative stress and inflammation in pregnant women. Environ. Sci. Technol. 2019, 53, 3258–3267. [Google Scholar] [CrossRef] [PubMed]
- Tetz, L.M.; Cheng, A.A.; Korte, C.S.; Giese, R.W.; Wang, P.; Harris, C.; Meeker, J.D.; Loch-Caruso, R. Mono-2-ethylhexyl phthalate induces oxidative stress responses in human placental cells in vitro. Toxicol. Appl. Pharmacol. 2013, 268, 47–54. [Google Scholar] [CrossRef]
- Shoaito, H.; Petit, J.; Chissey, A. The role of peroxisome proliferatoractivated receptory(PPARy) in mono (2-ethylhexyil) phthalate(MEHP)-mediated cytotrophoblast differentiation. Environ. Health Perspect. 2019, 127, 27003–27017. [Google Scholar] [CrossRef]
- Ahbab, M.A.; Güven, C.; Koçkaya, E.A.; Barlas, N. Comparative developmental toxicity evaluation of di-n-hexyl phthalate and dicyclohexyl phthalate in rats. Toxicol. Ind. Health 2017, 33, 696–716. [Google Scholar] [CrossRef]
- Xu, Y.; Agrawal, S.; Cook, T.J.; Knipp, G.T. Maternal di-(2-ethylhexyl)-phthalate exposure influences essentialfatty acid homeostasis in rat placenta. Placenta 2008, 29, 962–969. [Google Scholar] [CrossRef]
- Adibi, J.J.; Whyatt, R.M.; Hauser, R.; Bhat, H.K.; Davis, B.J.; Calafat, A.M.; Hoepner, L.A.; Perera, F.P.; Tang, D.; Williams, P.L. Transcriptional biomarkers of steroidogenesis and trophoblast differentiation in the placenta in relation to prenatal phthalate exposure. Environ. Health Perspect. 2010, 118, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Petit, J.; Wakx, A.; Gil, S.; Fournier, T.; Auzeil, N.; Rat, P.; Laprévote, O. Lipidome-wide disturbances of human placental JEG-3 cells by the presence of MEHP. Biochimie 2018, 149, 1–8. [Google Scholar] [CrossRef]
- Wang, R.; Wang, W. Benzo[a]pyrene-7,8-dio-9,10-epoxide suppresses the migration and invasion of human extravillous trophoblast HTR-8/SVneo cells by downregulating MMP2 through inhibiion of FAKISRCIPI3KIAKTpathway. Toxicology 2017, 386, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Connor, E.E.; Chegini, N. Modulation by benzo[a]pyrene of epidermal growth factor receptors, cell proliferation, and secretion of human chorionic gonadotropin in human placental cell lines. Biochem. Pharmacol. 1995, 50, 1171–1180. [Google Scholar]
- Zhu, L.W.; Lu, Y.; Zhang, L.Y. Association study of PAHs exposure during pregnancy with placental mitochondrial copy number. Prev. Med. 2022, 34, 248–252. (In Chinese) [Google Scholar]
- Dong, Q.; Hou, H.; Wu, J.; Chen, Y. The Nrf2-ARE pathway is associated with Schisandrin b attenuating benzo (a) pyrenelnduced HTR cells damages in vitro. Environ. Toxicol. 2016, 31, 1439–1449. [Google Scholar] [CrossRef] [PubMed]
- Li, Y. Experimental Study on the Effect of (2-Ethylhexyl) Phthalate Exposure during Pregnancy on the Neurodevelopment of Offspring by Interfering with the Transplacental Transport of Thyroid Hormones. Master’s Thesis, Anhui Medical University, Hefei, China, 2020. (In Chinese). [Google Scholar]
- Yin, S.-S. Load Levels, Health Effects and Mechanisms of Human Exposure to Plasticizers, Organochlorine Pesticides and Polycyclic Aromatic Hydrocarbons. Ph.D. Thesis, Zhejiang University, Zhejiang, China, 2019. (In Chinese). [Google Scholar]
- Zhu, Y. Morphological Study of Urinary Phthalate Metabolites in Pregnant Women and the Association between Phthalate Exposure during Pregnancy and Neonatal Birth Outcomes. Ph.D. Thesis, Huazhong University of Science and Technology, Wuhan, China, 2017. (In Chinese). [Google Scholar]
- Chen, X. Study on the Relationship between Maternal and Fetal Polycyclic Aromatic Hydrocarbon Exposure Dose, Metabolic Enzyme Gene Polymorphism and Cellular Damage in a Population with Hyperemesis. Master’s Thesis, Kunming University of Technology, Kunming, China, 2016. (In Chinese). [Google Scholar]
- Wu, N.; Tao, L.; Tian, K.; Wang, X.; He, C.; An, S.; Tian, Y.; Liu, X.; Chen, W.; Zhang, H.; et al. Risk assessment and environmental determinants of urinary phthalate metabolites in pregnant women in Southwest China. Environ. Sci. Pollut. Res. Int. 2023, 30, 53077–53088. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Fang, D.; Liu, Y.; Xiong, S.; Wang, X.; Tian, Y.; Zhang, H.; An, S.; He, C.; Chen, W.; et al. Exposure characteristics of phthalate metabolites among the Zunyi cohort of pregnant women in Southwest China. Environ. Sci. Pollut. Res. Int. 2022, 29, 58869–58880. [Google Scholar] [CrossRef]
- Wang, X.; He, C.; Wu, N.; Tian, Y.; Wang, L.; Liao, J.; Fang, D.; Liu, X.; An, S.; Chen, W.; et al. Maternal urine phthalate metabolite exposure and miscarriage risk: A nested case-control study of the Zunyi Birth Cohort. Environ. Sci. Pollut. Res. Int. 2023, 30, 23124–23134. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, R.; Liu, X.; Liu, Y.; Xiong, S.; Wang, X.; Zhang, H.; Li, Q.; Liao, J.; Fang, D.; et al. Characteristics of exposure to 10 polycyclic aromatic hydrocarbon metabolites among pregnant women: Cohort of pregnant women in Zunyi, southwest China. Occup. Environ. Med. 2023, 80, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y. A Nested Case-Control Study on the Association between OH-PAHs and Spontaneous Abortion. Master’s Thesis, Zunyi Medical University, Zunyi, China, 2022. (In Chinese). [Google Scholar]
- Wang, X. Development and Validation of a Risk Prediction Model for the Development of Gestational Diabetes Mellitus. Master’s Thesis, Zunyi Medical University, Zunyi, China, 2022. (In Chinese). [Google Scholar]
- HJ/T 92; Technical Specifications for Monitoring Total Water Pollutant Discharges, 2002, China Standards Database (CSD). FAO: Rome, Italy, 2002. (In Chinese)
- Hu, G. Quality control techniques in environmental organics monitoring in the United States. Environ. Monit. Manag. Technol. 2003, 15, 44–46. (In Chinese) [Google Scholar]
- Pinsrithong, S.; Bunkoed, O. Hierarchical porous nanostructured polypyrrole-coated hydrogel beads containing reduced graphene oxide and magnetite nanoparticles for extraction of phthalates in bottled drinks. J. Chromatogr. A 2018, 1570, 19–27. [Google Scholar] [CrossRef]
- Rodríguez-Ramos, R.; Socas-Rodríguez, B.; Santana-Mayor, Á.; Salazar-Carballo, P.Á.; Rodríguez-Delgado, M.Á. Sustainable polypyrrole-based magnetic-microextraction of phthalates from jellies and apple-based beverages prior to tandem mass spectrometry analysis. J. Chromatogr. A 2021, 1637, 461858. [Google Scholar] [CrossRef]
- Santana-Mayor, Á.; Socas-Rodríguez, B.; Afonso, M.D.M.; Palenzuela-López, J.A.; Rodríguez-Delgado, M.Á. Reduced graphene oxide-coated magnetic-nanoparticles as sorbent for the determination of phthalates in environmental samples by micro-dispersive solid-phase extraction followed by ultra-high-performance liquid chromatography tandem mass spectrometry. J. Chromatogr. A 2018, 1565, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, P.; Belka, M.; Bączek, T.; Płotka-Wasylka, J. The presence of polycyclic aromatic hydrocarbons in disposable baby diapers: A facile determination method via salting-out assisted liquid-liquid extraction coupled with gas chromatography-mass spectrometry. J. Chromatogr. A 2023, 1698, 463981. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, X.; Wang, X.; Wang, H.; Zhao, J.; Zhou, Z.; Du, X.; Lu, X. In situ anchor of multi-walled carbon nanotubes into iron-based metal-organic frameworks for enhanced adsorption of polycyclic aromatic hydrocarbons by magnetic solid-phase extraction. J. Chromatogr. A 2022, 1681, 463459. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Chen, B.; Qin, X.; Zhou, Z.; Liang, Y.; He, M.; Hu, B. Porous aromatic framework coated stir bar sorptive extraction coupled with gas chromatography for the analysis of 16 polycyclic aromatic hydrocarbons in atmospheric particles and environmental water samples. J. Chromatogr. A 2022, 1673, 463139. [Google Scholar] [CrossRef] [PubMed]
| Extractants | PAHS | PAEs | Standard Curve (R2 > 0.98) |
|---|---|---|---|
| Acetonitrile | 1-OHNAP, 2-OHFLU, 3-OHPHE, 9-OHPHE, 1-OHPHE, 2-OHNAP, 4-OHPHE, 1-OHPYR | MBZP, MEHP, MIBP | 0.75 |
| Ethyl acetate | 1-OHNAP, 2-OHFLU, 3-OHPHE, 9-OHPHE, 1-OHPHE, 2-OHNAP, 4-OHPHE, 1-OHPYR, 9-OHFLU | MBP, MEHHP, MEP, MOP, MBZP, MEHP, MIBP | 0.90 |
| n-hexane + ether | 1-OHNAP, 2-OHFLU, 3-OHPHE, 9-OHPHE, 1-OHPHE, 2-OHNAP, 4-OHPHE, 1-OHPYR, 2-OHPHE, 9-OHFLU | MEOHP, MMP, MBP, MEHHP, MEP, MOP, MBZP, MEHP, MIBP | 0.99 |
| n-hexane + ether (1 extraction/2 extractions) and ethyl acetate (2 extractions/ extractions) | 1-OHNAP, 2-OHFLU, 3-OHPHE, 9-OHPHE, 1-OHPHE, 2-OHNAP, 4-OHPHE, 1-OHPYR, 9-OHFLU | MMP, MBP, MEHHP, MEP, MOP, MBZP, MEHP, MIBP | 0.90 |
| Compounds | Inner Label | Regression Equation | R2 | LOD (μg/L) | LOQ (μg/L) | Recycling Rate (%) | RSD |
|---|---|---|---|---|---|---|---|
| 1-OHNAP | 1-OHNAP-D7 | Y = 0.0367x + 0.0126 | 0.9998 | 0.0050 | 0.0167 | 107.05 | 1.40 |
| 2-OHNAP | 1-OHNAP-D7 | Y = 0.0509x + 0.0035 | 0.9999 | 0.0027 | 0.0090 | 105.71 | 2.27 |
| 9-OHFLE | 1-OHNAP-D7 | Y = 0.0371x − 0.0011 | 0.9998 | 0.0023 | 0.0078 | 94.15 | 2.85 |
| 2-OHFLE | 1-OHNAP-D7 | Y = 0.0663x − 0.0013 | 0.9998 | 0.0029 | 0.0098 | 91.99 | 3.18 |
| 4-OHPHE | 1-OHNAP-D7 | Y = 0.0409x + 6.1544 | 0.9999 | 0.0167 | 0.0556 | 90.08 | 5.01 |
| 9-OHPHE | 1-OHNAP-D7 | Y = 0.2914x − 0.0055 | 0.9982 | 0.0044 | 0.0147 | 105.01 | 4.99 |
| 1-OHPHE | 1-OHNAP-D7 | Y = 0.0383x − 0.0016 | 0.9998 | 0.0071 | 0.0236 | 81.09 | 4.96 |
| 3-OHPHE | 1-OHNAP-D7 | Y = 0.0188x − 0.0010 | 0.9999 | 0.0095 | 0.0316 | 84.50 | 5.48 |
| 2-OHPHE | 1-OHNAP-D7 | Y = 0.0171x − 4.7902 | 0.9999 | 0.0115 | 0.0385 | 68.19 | 2.41 |
| 1-OHPYR | 1-OHPYR-D9 | Y = 1.770x + 0.0213 | 0.9999 | 0.0003 | 0.0011 | 100.30 | 1.97 |
| MMP | MEHP-C4 | Y = 0.0037x + 5.9620 | 0.9986 | 0.0375 | 0.1250 | 80.45 | 8.37 |
| MEP | MEHP-C4 | Y = 0.9972x + 0.0202 | 0.9972 | 0.0288 | 0.0962 | 102.31 | 6.15 |
| MIBP | MEHP-C4 | Y = 0.1002x + 0.0813 | 0.9988 | 0.0023 | 0.0075 | 99.71 | 4.95 |
| MBP | MEHP-C4 | Y = 0.1681x + 0.1936 | 0.9982 | 0.0015 | 0.0051 | 92.64 | 4.77 |
| MOP | MEHP-C4 | Y = 0.1300x + 0.0021 | 0.9997 | 0.0625 | 0.2083 | 100.00 | 3.15 |
| MEHP | MEHP-C4 | Y = 0.1090x + 0.0234 | 0.9999 | 0.0326 | 0.1087 | 112.59 | 3.59 |
| MEOHP | MEHHP-C4 | Y = 0.0834x − 0.0876 | 0.9986 | 1.0714 | 3.5714 | 105.16 | 4.14 |
| MBZP | MEHP-C4 | Y = 0.0877x + 0.0351 | 0.9976 | 0.0405 | 0.1351 | 92.01 | 5.83 |
| MEHHP | MEHHP-C4 | Y = 0.2029x − 0.0621 | 0.9998 | 0.0103 | 0.1087 | 104.21 | 3.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tao, L.; Tian, Y.; Liao, D.; An, S.; Chen, W.; Liu, X.; Xu, P.; Shen, X.; Zhou, Y. Determination of Polycyclic Aromatic Hydrocarbons (PAHs) and Phthalates in Human Placenta by Mixed Hexane/Ether Extraction and Gas Chromatography–Mass Spectrometry/Mass Spectrometry (GC-MS/MS). Metabolites 2023, 13, 978. https://doi.org/10.3390/metabo13090978
Tao L, Tian Y, Liao D, An S, Chen W, Liu X, Xu P, Shen X, Zhou Y. Determination of Polycyclic Aromatic Hydrocarbons (PAHs) and Phthalates in Human Placenta by Mixed Hexane/Ether Extraction and Gas Chromatography–Mass Spectrometry/Mass Spectrometry (GC-MS/MS). Metabolites. 2023; 13(9):978. https://doi.org/10.3390/metabo13090978
Chicago/Turabian StyleTao, Lin, Yingkuan Tian, Dengqing Liao, Songlin An, Wei Chen, Xiang Liu, Pei Xu, Xubo Shen, and Yuanzhong Zhou. 2023. "Determination of Polycyclic Aromatic Hydrocarbons (PAHs) and Phthalates in Human Placenta by Mixed Hexane/Ether Extraction and Gas Chromatography–Mass Spectrometry/Mass Spectrometry (GC-MS/MS)" Metabolites 13, no. 9: 978. https://doi.org/10.3390/metabo13090978
APA StyleTao, L., Tian, Y., Liao, D., An, S., Chen, W., Liu, X., Xu, P., Shen, X., & Zhou, Y. (2023). Determination of Polycyclic Aromatic Hydrocarbons (PAHs) and Phthalates in Human Placenta by Mixed Hexane/Ether Extraction and Gas Chromatography–Mass Spectrometry/Mass Spectrometry (GC-MS/MS). Metabolites, 13(9), 978. https://doi.org/10.3390/metabo13090978






