Comparative LC-ESIMS-Based Metabolite Profiling of Senna italica with Senna alexandrina and Evaluating Their Hepatotoxicity
Abstract
1. Introduction
2. Materials and Methods
2.1. Solvents and Chemicals
2.2. Plant Materials
2.3. Extraction
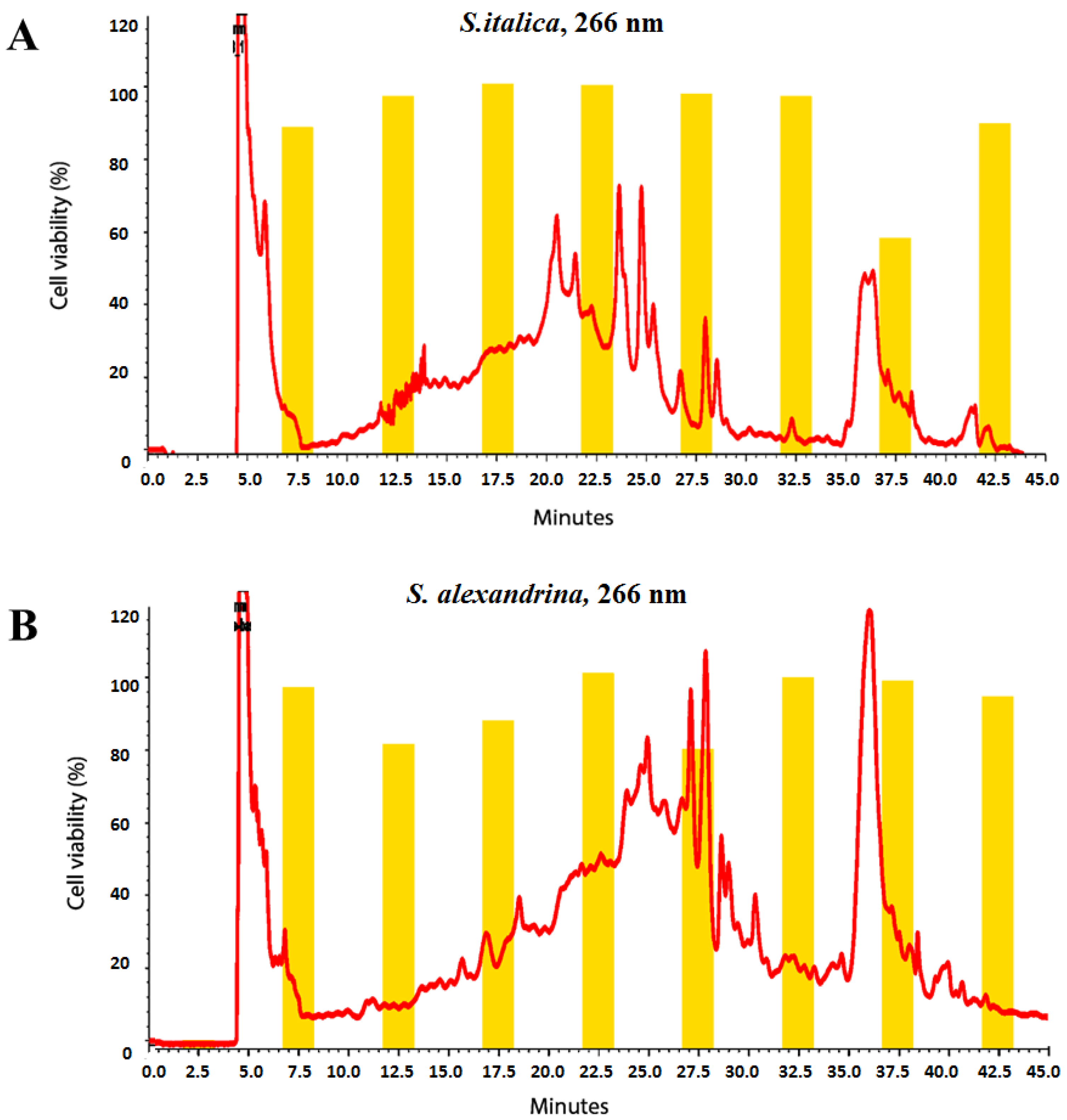
2.4. Microfractionation for Activity Profiling
2.5. HPLC-ESI-QqTOFMS and HPLC-ESI-QqTOFMS/MS Analyzes
2.6. Determination of Sennoside A and Sennoside B
2.7. MZmine Preprocessing
2.8. Data Clustering
2.9. Identification of Compounds
2.10. Cell Culture
2.11. Cell Viability
3. Results
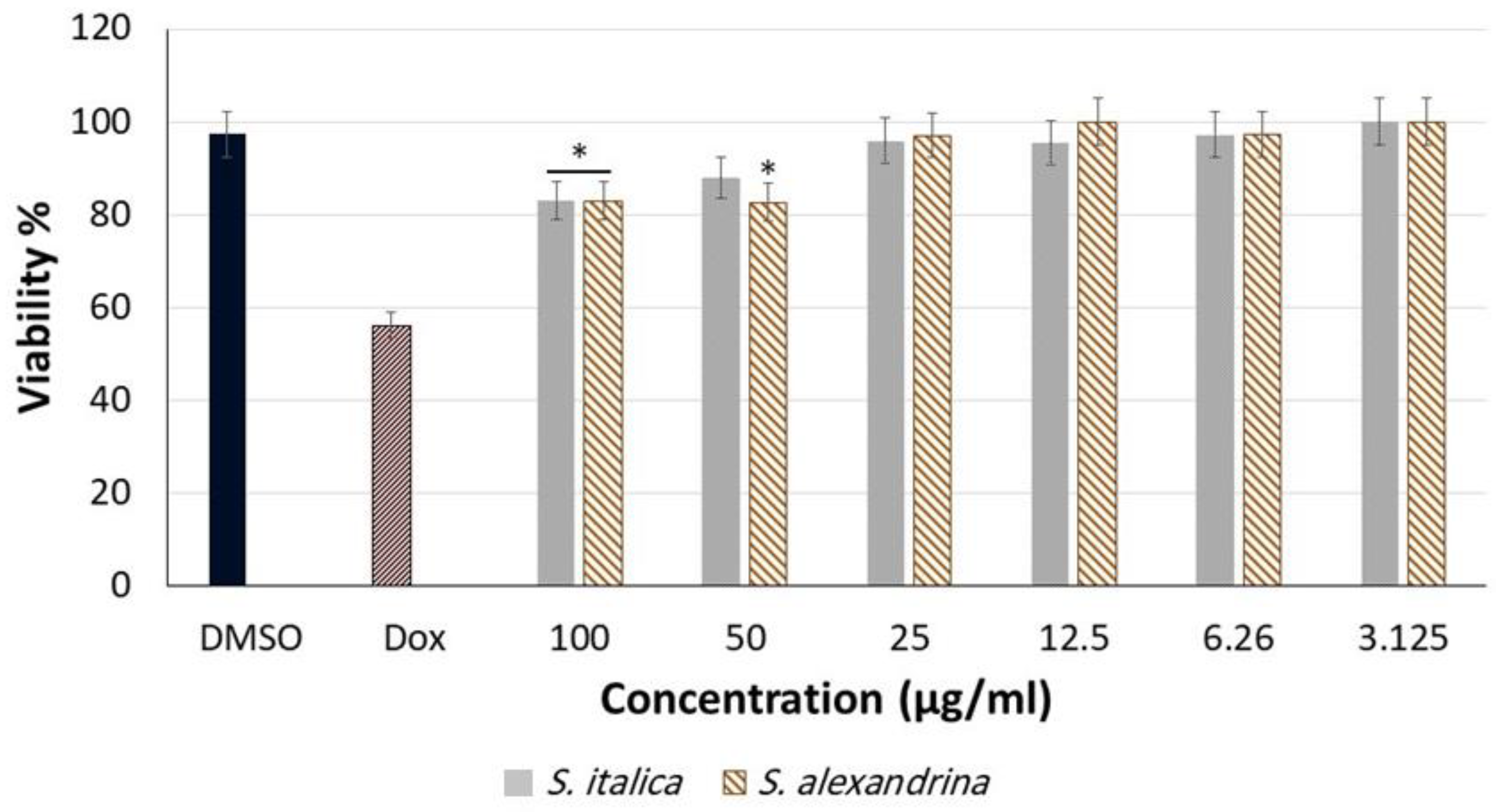
3.1. Cytotoxicity
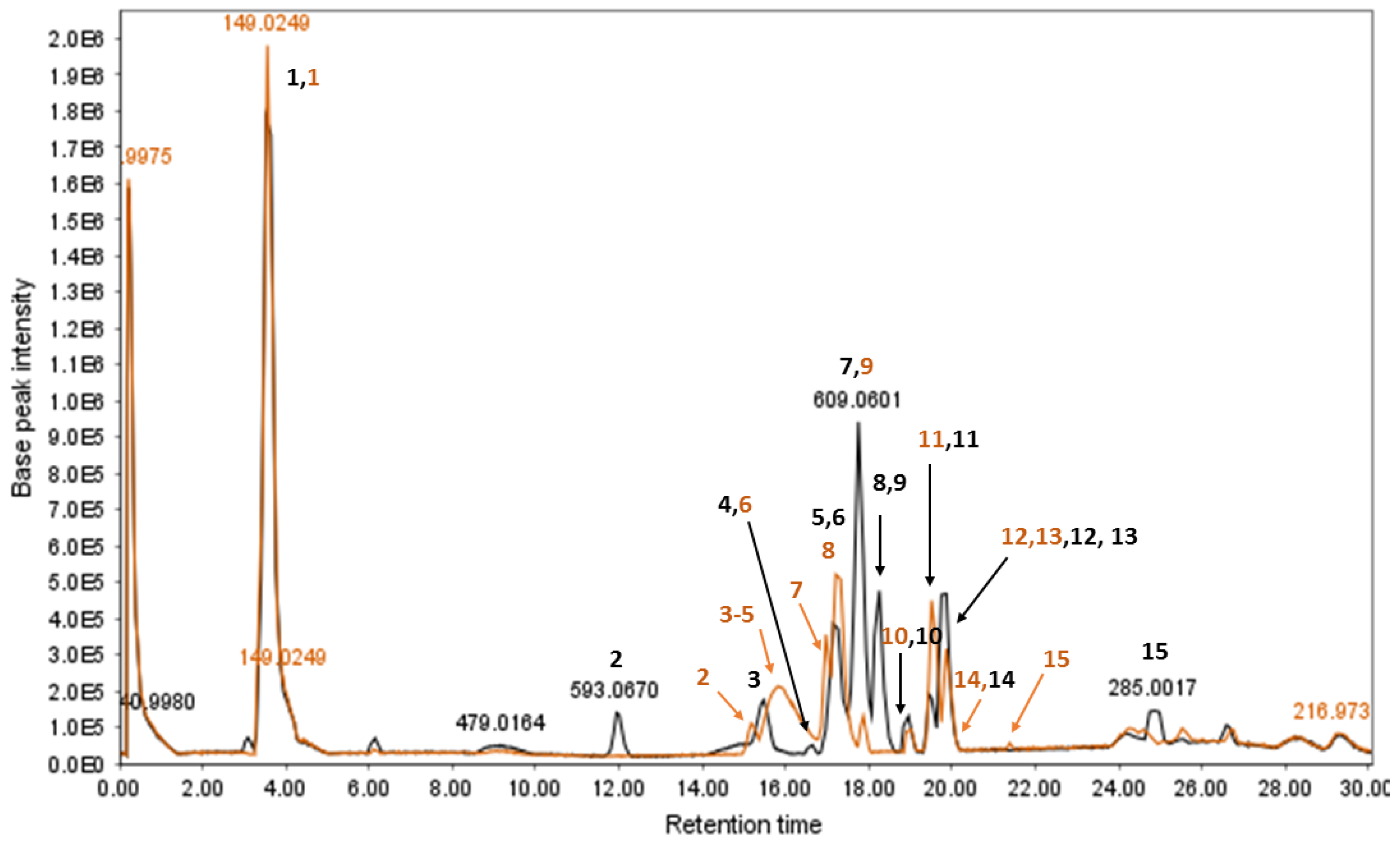
3.2. HPLC-ESIMS and MS/MS Analysis
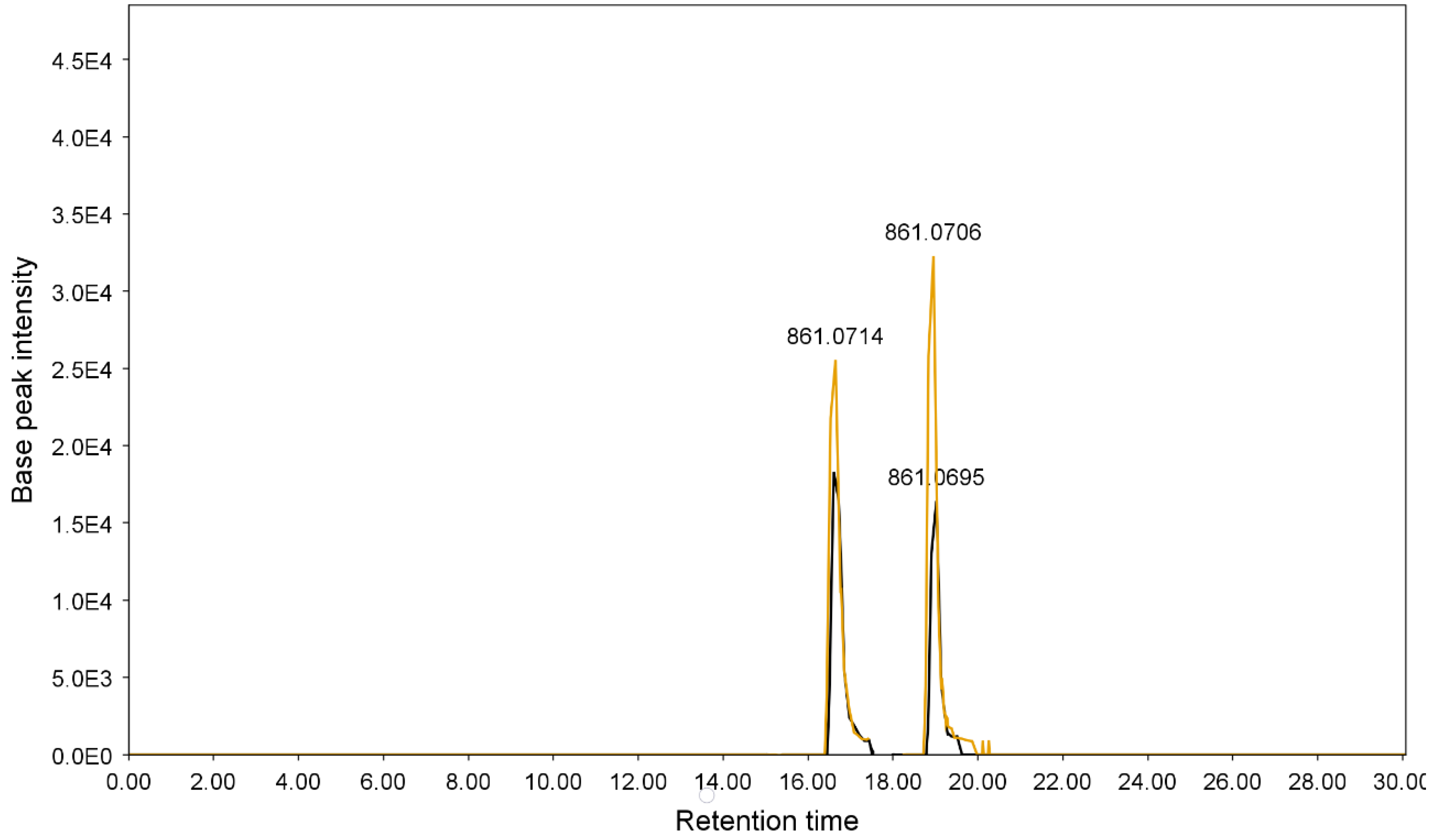
3.2.1. Determination of Sennosides A and B
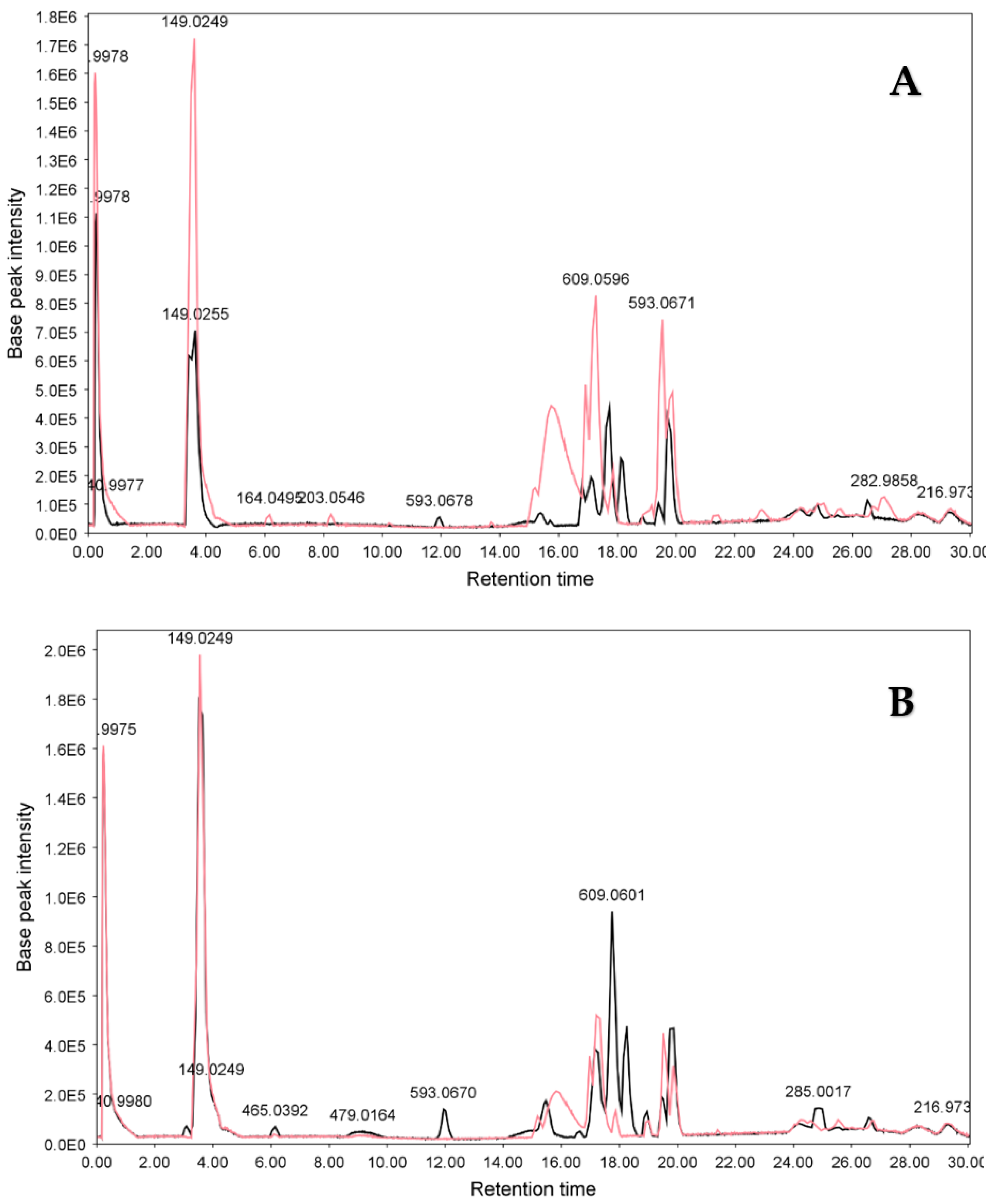
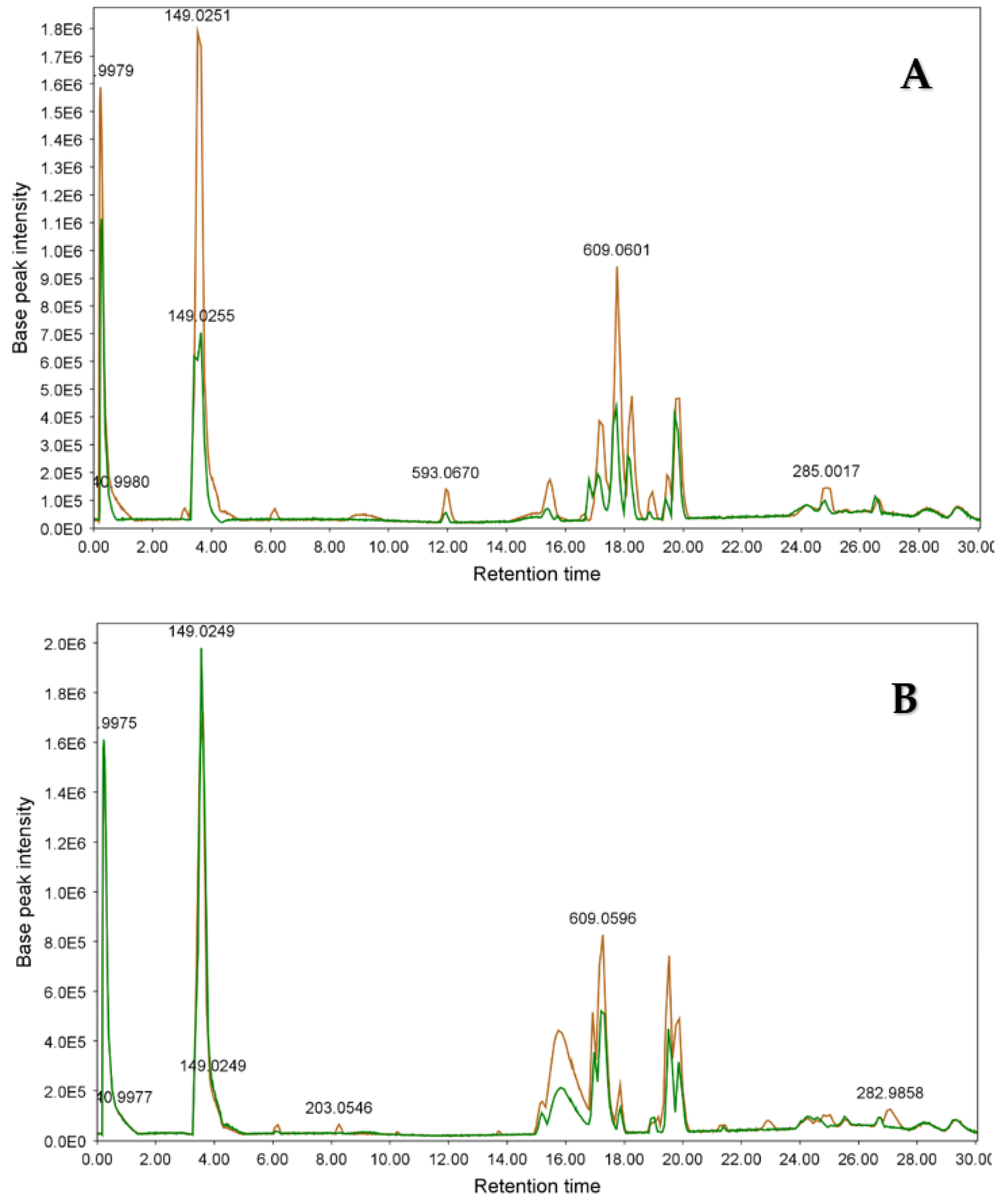
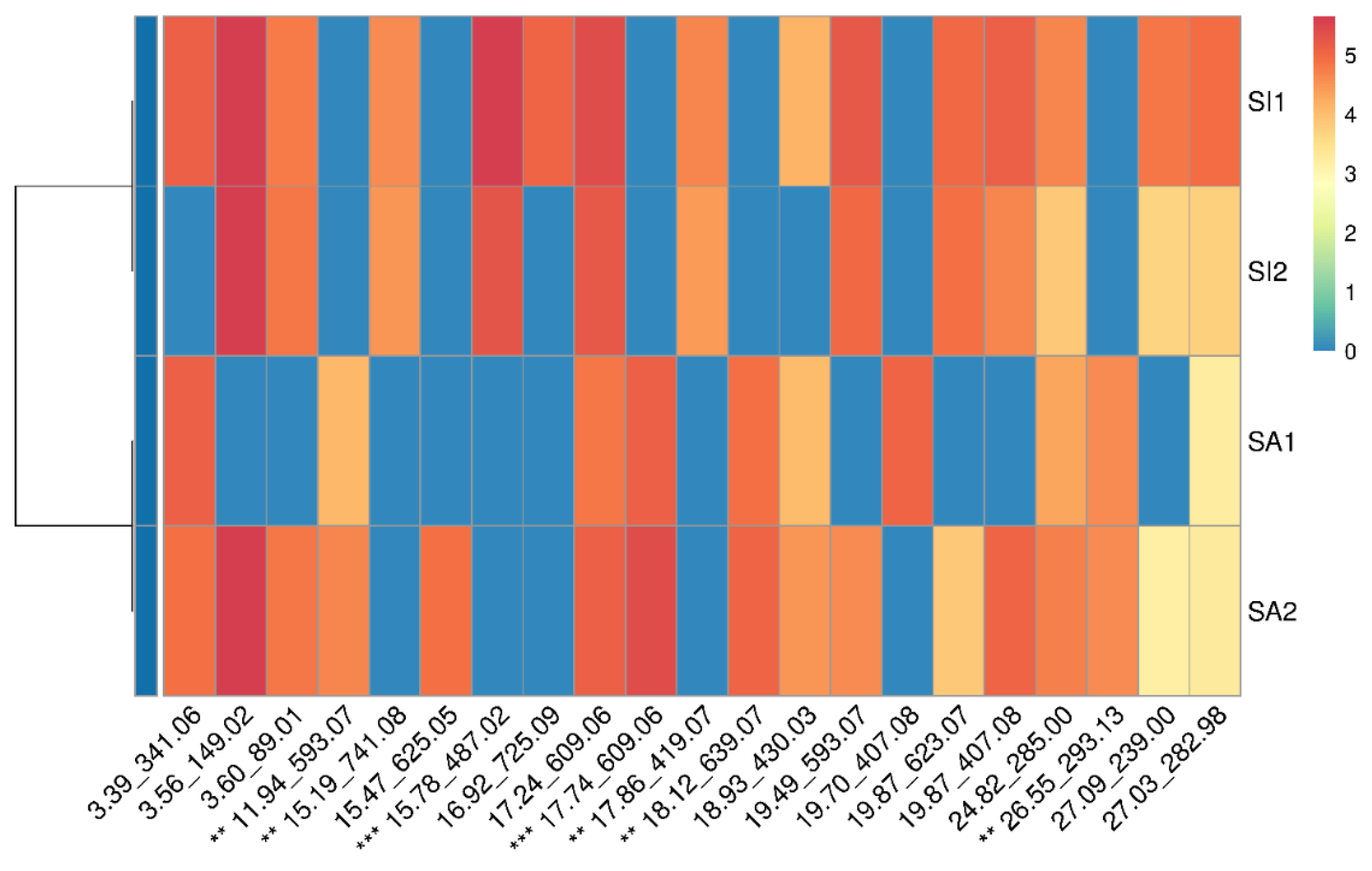
3.2.2. LC-ESIMS and LC-ESIMS/MS Profiling
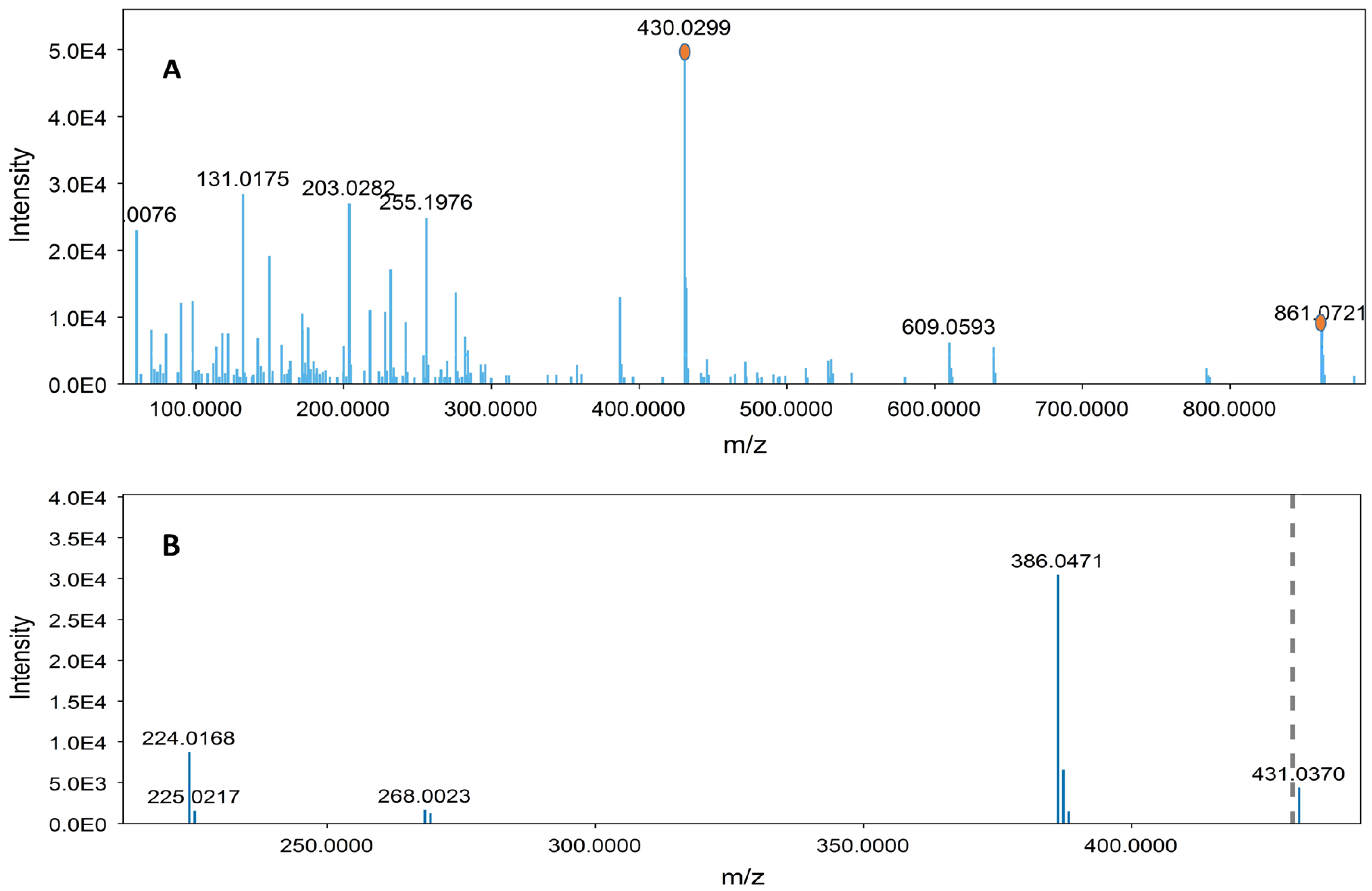
3.2.3. Identification of Compounds
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marazzi, B.; Endress, P.K.; De Queiroz, L.P.; Conti, E. Phylogenetic relationships within Senna (Leguminosae, Cassiinae) based on three chloroplast DNA regions: Patterns in the evolution of floral symmetry and extrafloral nectaries. Am. J. Bot. 2006, 93, 288–303. [Google Scholar] [CrossRef] [PubMed]
- Rama Reddy, N.R.; Mehta, R.H.; Soni, P.H.; Makasana, J.; Gajbhiye, N.A.; Ponnuchamy, M.; Kumar, J. Next generation sequencing and transcriptome analysis predicts biosynthetic pathway of sennosides from Senna (Cassia angustifolia Vahl.), a non-model plant with potent laxative properties. PLoS ONE 2015, 10, e0129422. [Google Scholar] [CrossRef] [PubMed]
- Agarkar, S.; Jadge, D. Phytochemical and pharmacological investigations of genus Cassia: A review. Asian J. Chem. 1999, 11, 295–299. [Google Scholar]
- Naz, H.; Nawaz, H.; Hanif, M.A.; Ayub, M.A.; Khatun, S. Indian Senna. In Medicinal Plants of South Asia; Elsevier: Amsterdam, The Netherlands, 2020; pp. 439–449. [Google Scholar]
- POWO. Plants of the World Online. 2022. Available online: http://www.plantsoftheworldonline.org/ (accessed on 1 September 2022).
- Ramchander, P.J.; Middha, A. Recent advances on senna as a laxative: A comprehensive review. J. Pharmacogn. Phytochem. 2017, 6, 349–353. [Google Scholar]
- Leung, L.; Riutta, T.; Kotecha, J.; Rosser, W. Chronic constipation: An evidence-based review. J. Am. Board Fam. Med. 2011, 24, 436–451. [Google Scholar] [CrossRef]
- Akbar, S. Senna alexandrina Mill.(Fabaceae/Leguminosae). In Handbook of 200 Medicinal Plants; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1629–1637. [Google Scholar]
- Sreejith, G.; Latha, P.G.; Shine, V.J.; Anuja, G.I.; Suja, S.R.; Sini, S.; Shyama, S.; Pradeep, S.; Shikha, P.; Rajasekharan, S. Anti-allergic, anti-inflammatory and anti-lipidperoxidant effects of Cassia occidentalis Linn. Indian J. Exp. Biol. 2010, 48, 494–498. [Google Scholar] [PubMed]
- Oladeji, O.S.; Adelowo, F.E.; Oluyori, A.P. The genus Senna (Fabaceae): A review on its traditional uses, botany, phytochemistry, pharmacology and toxicology. S. Afr. J. Bot. 2021, 138, 1–32. [Google Scholar] [CrossRef]
- Srivastava, M.; Srivastava, S.; Rawat, A. Chemical standardization of Cassia angustifolia Vahl seed. Pharmacogn. J. 2010, 2, 554–560. [Google Scholar] [CrossRef]
- Nkantchoua, G.C.N.; Njapdounke, J.S.K.; Fifen, J.J.; Taiwe, G.S.; Ojong, L.J.; Kandeda, A.K.; Bum, E.N. Anticonvulsant effects of Senna spectabilis on seizures induced by chemicals and maximal electroshock. J. Ethnopharmacol. 2018, 212, 18–28. [Google Scholar] [CrossRef]
- Silva, C.; Monteiro, M.; Rocha, H.; Ribeiro, A.; Caldeira-de-Araujo, A.; Leitão, A.; Bezerra, R.; Pádula, M. Assessment of antimutagenic and genotoxic potential of senna (Cassia angustifolia Vahl.) aqueous extract using in vitro assays. Toxicol. In Vitro 2008, 22, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Takido, M. Studies on the Constituents of the Seeds of Cassia obtusifolia L.I. The Structure of Obtusifolin. Chem. Pharm. Bull. 1958, 6, 397–400. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.; Nishida, Y.; Yamazaki, M.; Nakahara, K.; Michalska-Hartwich, M.; Furmanowa, M.; Leistner, E.; Yoshida, T. Chrysophanol glycosides from callus cultures of monocotyledonous Kniphofia spp. (Asphodelaceae). Chem. Pharm. Bull. 2004, 52, 1262–1264. [Google Scholar] [CrossRef] [PubMed]
- Adedayo, O.; Anderson, W.; Moo-Young, M.; Snieckus, V.; Patil, P.; Kolawole, D. Kinetics of antibacterial activity and physicochemical damage caused by the extracts of Senna alata flowers. Pharm. Biol. 2002, 40, 461–465. [Google Scholar] [CrossRef]
- Dave, H.; Ledwani, L. A review on anthraquinones isolated from Cassia species and their applications. Indian J. Nat. Prod. Resour. 2012, 3, 291–319. [Google Scholar]
- Rai, M. A review on some antidiabetic plants of India. Anc. Sci. Life 1995, 14, 168. [Google Scholar]
- Chauhan, D.; Chauhan, J.; Siddiqui, I.; Singh, J. Two new anthraquinone glycosides from the leaves of Cassia occidentalis. Indian J. Chem. 2001, 40B, 860–863. [Google Scholar]
- Majid, U.; Siddiqi, T.O.; Aref, I.M.; Iqbal, M. Quantitative changes in proteins, pigments and sennosides of Cassia angustifolia vahl treated with mancozeb. Pak. J. Bot. 2013, 45, 1509–1514. [Google Scholar]
- Ayo, R. Phytochemical constituents and bioactivities of the extracts of Cassia nigricans Vahl: A review. J. Med. Plant Res. 2010, 4, 1339–1348. [Google Scholar]
- Adelowo, F.; Oladeji, O. An overview of the phytochemical analysis of bioactive compounds in Senna alata. Adv. Biochem. 2017, 5, 102–109. [Google Scholar] [CrossRef]
- Laghari, A.Q.; Memon, S.; Nelofar, A.; Laghari, A.H. Extraction, identification and antioxidative properties of the flavonoid-rich fractions from leaves and flowers of Cassia Angustifolia. Am. J. Anal. Chem. 2011, 2, 871. [Google Scholar] [CrossRef]
- Ahmed, S.I.; Hayat, M.Q.; Tahir, M.; Mansoor, Q.; Ismail, M.; Keck, K.; Bates, R.B. Pharmacologically active flavonoids from the anticancer, antioxidant and antimicrobial extracts of Cassia angustifolia Vahl. BMC Complement. Altern. Med. 2016, 16, 460. [Google Scholar] [CrossRef]
- Jani, D.K.; Goswami, S. Antidiabetic activity of Cassia angustifolia Vahl. and Raphanus sativus Linn. leaf extracts. J. Tradit. Complement. Med. 2020, 10, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Cuellar, M.; Giner, R.; Recio, M.; Manez, S.; Rıos, J. Topical anti-inflammatory activity of some Asian medicinal plants used in dermatological disorders. Fitoterapia 2001, 72, 221–229. [Google Scholar] [CrossRef]
- Aggarwal, B.; Prasad, S.; Reuter, S.; Kannappan, R.; Yadav, V.; Park, B.; Hye Kim, J.; Gupta, S.; Phromnoi, K.; Sundaram, C. Identification of novel anti-inflammatory agents from Ayurvedic medicine for prevention of chronic diseases:“reverse pharmacology” and “bedside to bench” approach. Curr. Drug Targets 2011, 12, 1595–1653. [Google Scholar] [CrossRef]
- VijayaSekhar, V.; Prasad, M.S.; Joshi, D.; Narendra, K.; Satya, A.K.; Rao, K. Assessment of phytochemical evaluation and in-vitro antimicrobial activity of Cassia angustifolia. Int. J. Pharmacogn. Phytochem. Res. 2016, 8, 305–312. [Google Scholar]
- Singanboina, K.; Chinna, V.; Kumar Ratnampally, S.; Karnakar Rao, K. Antibacterial activity of Cassia angustifolia (Vahl.) leaf extracts grown in three different soil treatments. Int. J. Pharm. Life Sci. 2014, 5, 3631–3633. [Google Scholar]
- Lin, L.T.; Liu, L.T.; Chiang, L.C.; Lin, C.C. In vitro anti-hepatoma activity of fifteen natural medicines from Canada. Phytother. Res. 2002, 16, 440–444. [Google Scholar] [CrossRef] [PubMed]
- Safa, O.; Soltanipoor, M.A.; Rastegar, S.; Kazemi, M.; Dehkordi, K.N.; Ghannadi, A. An ethnobotanical survey on hormozgan province, Iran. Avicenna J. Phytomed. 2013, 3, 64. [Google Scholar]
- Abdisa, T. Review on traditional medicinal plant and its extract effect on tick control in Ethiopia. J. Vet. Med. Res. 2017, 4, 102. [Google Scholar]
- El-Sayed, N.H.; Dooh, A.A.; El-Khrisy, S.; Mabry, T.J. Flavonoids of Cassia Ital. Phytochemistry 1992, 31, 2187. [Google Scholar] [CrossRef]
- Jothi, R.S.; Bharathy, V.; Uthayakumari, F. Antioxidant potential of aerial part of Senna italica sub species micrantha Mill. J. Pharm. Sci. Res. 2015, 7, 621. [Google Scholar]
- Kuete, V.; Wiench, B.; Alsaid, M.S.; Alyahya, M.A.; Fankam, A.G.; Shahat, A.A.; Efferth, T. Cytotoxicity, mode of action and antibacterial activities of selected Saudi Arabian medicinal plants. BMC Complement. Alternat. Med. 2013, 13, 354. [Google Scholar] [CrossRef] [PubMed]
- Masoko, P.; Gololo, S.S.; Mokgotho, M.P.; Eloff, J.N.; Howard, R.; Mampuru, L. Evaluation of the antioxidant, antibacterial, and antiproliferative activities of the acetone extract of the roots of Senna italica (Fabaceae). Afric. J. Tradit. Complement. Altern. Med. 2010, 7, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Dabai, Y.; Kawo, A.; Aliyu, R. Phytochemical screening and antibacterial activity of the leaf and root extracts of Senna italica. Afr. J. Pharm. Pharmacol. 2012, 6, 914–918. [Google Scholar] [CrossRef]
- Malematja, R.; Bagla, V.; Njanje, I.; Mbazima, V.; Poopedi, K.; Mampuru, L.; Mokgotho, M. Potential hypoglycaemic and antiobesity effects of Senna italica leaf acetone extract. Evid.-Based Complement. Altern. Med. 2018, 2018, 5101656. [Google Scholar] [CrossRef]
- Sermakkani, M.; Thangapandian, V. Anti-Inflammatory Potential of Cassia italica (Mill) Lam Leaves. Int. J. Pharm. Pharm. Sci. 2013, 5, 18–22. [Google Scholar]
- Jain, S.; Jain, R.; Sharma, R.; Capasso, F. Pharmacological investigation of Cassia italica. J. Ethnopharmacol. 1997, 58, 135–142. [Google Scholar] [CrossRef]
- Saravanapriya, P.; Devi, K.P. Plant Extracts with Putative Hepatotoxicity Activity. In Influence of Nutrients, Bioactive Compounds, and Plant Extracts in Liver Diseases; Elsevier: Amsterdam, The Netherlands, 2021; pp. 259–287. [Google Scholar]
- van Gorkom, B.A.; Karrenbeld, A.; van der Sluis, T.; Zwart, N.; de Vries, E.G.; Kleibeuker, J.H. Apoptosis induction by sennoside laxatives in man; escape from a protective mechanism during chronic sennoside use. J. Pathol. 2001, 194, 493–499. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kim, W.; Lee, Y.G.; Kang, H.J.; Lee, S.H.; Park, S.Y.; Min, J.K.; Lee, S.R.; Chung, S.J. Identification of sennoside A as a novel inhibitor of the Slingshot (SSH) family proteins related to cancer metastasis. Pharmacol. Res. 2017, 119, 422–430. [Google Scholar] [CrossRef]
- Akaberi, M.; Danton, O.; Tayarani-Najaran, Z.; Asili, J.; Iranshahi, M.; Emami, S.A.; Hamburger, M. HPLC-based activity profiling for antiprotozoal compounds in the endemic Iranian medicinal plant Helichrysum oocephalum. J. Nat. Prod. 2019, 82, 958–969. [Google Scholar] [CrossRef]
- Nesměrák, K.; Kudláček, K.; Čambal, P.; Štícha, M.; Kozlík, P.; Červený, V. Authentication of senna extract from the eighteenth century and study of its composition by HPLC–MS. Mon. Chem. Chem. Mon. 2020, 151, 1241–1248. [Google Scholar] [CrossRef]
- Pluskal, T.; Castillo, S.; Villar-Briones, A.; Orešič, M. MZmine 2: Modular framework for processing, visualizing, and analyzing mass spectrometry-based molecular profile data. BMC Bioinform. 2010, 11, 395. [Google Scholar] [CrossRef]
- Božičević, A.; Dobrzyński, M.; De Bie, H.; Gafner, F.; Garo, E.; Hamburger, M. Automated comparative metabolite profiling of large LC-ESIMS data sets in an ACD/MS workbook suite add-in, and data clustering on a new open-source web platform FreeClust. Anal. Chem. 2017, 89, 12682–12689. [Google Scholar] [CrossRef] [PubMed]
- Akaberi, M.; Najaran, Z.T.; Azizi, N.; Emami, S.A. Metabolite profiling and antiprotozoal activity of three endemic Iranian Helichrysum species. Ind. Crops Prod. 2021, 174, 114196. [Google Scholar] [CrossRef]
- Akaberi, M.; Emami, S.A.; Vatani, M.; Tayarani-Najaran, Z. Evaluation of antioxidant and anti-melanogenic activity of different extracts of aerial parts of N. Sintenisii in murine melanoma B16F10 cells. Iran. J. Pharm. Res. 2018, 17, 225–235. [Google Scholar] [PubMed]
- Farag, M.A.; El Senousy, A.S.; El-Ahmady, S.H.; Porzel, A.; Wessjohann, L.A. Comparative metabolome-based classification of Senna drugs: A prospect for phyto-equivalency of its different commercial products. Metabolomics 2019, 15, 80. [Google Scholar] [CrossRef]
- Omer, H.A.A.; Caprioli, G.; Abouelenein, D.; Mustafa, A.M.; Uba, A.I.; Ak, G.; Ozturk, R.B.; Zengin, G.; Yagi, S. Phenolic Profile, Antioxidant and Enzyme Inhibitory Activities of Leaves from Two Cassia and Two Senna Species. Molecules 2022, 27, 5590. [Google Scholar] [CrossRef]
- Coetzee, J.; McIteka, L.; Malan, E.; Ferreira, D. Structure and synthesis of the first procassinidin dimers based on epicatechin, and gallo- and epigallo-catechin. Phytochemistry 2000, 53, 795–804. [Google Scholar] [CrossRef]
- Zhou, Z.-h.; Fang, Z.; Jin, H.; Chen, Y.; He, L. Selective Monomethylation of Quercetin. Synthesis 2010, 2010, 3980–3986. [Google Scholar]
- Hatano, T.; Uebayashi, H.; Ito, H.; Shiota, S.; Tsuchiya, T.; Yoshida, T. Phenolic constituents of cassia seeds and antibacterial effect of some naphthalenes and anthraquinones on methicillin-resistant Staphylococcus aureus. Chem. Pharm. Bull. 1999, 47, 1121–1127. [Google Scholar] [CrossRef] [PubMed]
- Madkour, H.M.; Ghareeb, M.A.; Abdel-Aziz, M.S.; Khalaf, O.M.; Saad, A.M.; El-Ziaty, A.K.; Abdel-Mogib, M. Gas chromatography-mass spectrometry analysis, antimicrobial, anticancer and antioxidant activities of n-hexane and methylene chloride extracts of Senna Ital. J. Appl. Pharm. Sci. 2017, 7, 23–32. [Google Scholar]
- Wald, A. Constipation: Advances in Diagnosis and Treatment. JAMA 2016, 315, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Yamaguchi, T.; Odaka, T.; Nakamura, T.; Tsuchiya, S.; Yokosuka, O.; Yano, S. Regionally differential effects of sennoside A on spontaneous contractions of colon in mice. Basic Clin. Pharmacol. Toxicol. 2007, 101, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Kon, R.; Ikarashi, N.; Nagoya, C.; Takayama, T.; Kusunoki, Y.; Ishii, M.; Ueda, H.; Ochiai, W.; Machida, Y.; Sugita, K.; et al. Rheinanthrone, a metabolite of sennoside A, triggers macrophage activation to decrease aquaporin-3 expression in the colon, causing the laxative effect of rhubarb extract. J. Ethnopharmacol. 2014, 152, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Mncube, S.; Gololo, S.; Mogale, M. Seasonal variations of phytochemical content and antioxidant activity of Senna italica leaves. Asian J. Chem. 2020, 32, 2371–2374. [Google Scholar] [CrossRef]
- Le, J.; Ji, H.; Zhou, X.; Wei, X.; Chen, Y.; Fu, Y.; Ma, Y.; Han, Q.; Sun, Y.; Gao, Y.; et al. Pharmacology, Toxicology, and Metabolism of Sennoside A, A Medicinal Plant-Derived Natural Compound. Front. Pharmacol. 2021, 12, 714586. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.L.; Jiang, H.B.; Liu, B.Y.; Li, W.D.; Du, A.L.; Luo, X.Q.; Li, X.Q. Effects of rhein on intestinal transmission, colonic electromyography and expression of aquaporin-3 by colonic epithelium cells in constipated mice. Int. J. Clin. Exp. Pathol. 2018, 11, 614–623. [Google Scholar] [PubMed]
- Zhang, B.; Huo, M.; Chen, Z.; Gao, F.; Liu, Y.; Zhou, X. Long-Term Administration of Anthraquinone Rhein on Induction of Constipation in Sprague-Dawley Rats via SCF/c-Kit Signaling Pathways. Can. J. Gastroenterol. Hepatol. 2021, 2021, 6649199. [Google Scholar] [CrossRef]
- Zheng, Y.F.; Liu, C.F.; Lai, W.F.; Xiang, Q.; Li, Z.F.; Wang, H.; Lin, N. The laxative effect of emodin is attributable to increased aquaporin 3 expression in the colon of mice and HT-29 cells. Fitoterapia 2014, 96, 25–32. [Google Scholar] [CrossRef]

| Senna italica | Senna alexandrina | |
|---|---|---|
| Plant | an annual or perennial erect to prostrate herb or subshrub | a subshrub, shrub, or tree |
| Distribution | seasonally dry tropical biome | subtropical biome |
| Leaves | 3–12 cm long, eglandular | 5–15 cm long, eglandular |
| Stipules | triangular to ovate-triangular, 3–9 mm long | subulate, linear or narrowly triangular, 3–5 mm long |
| Leaflets | 4–6 pairs, obovate-elliptic to obovate-oblong, minutely appressed puberulous | 4–8 pairs, lanceolate to elliptic, appressed puberulous or pubescent |
| Racemes | 2–25 cm long | 5–30 cm long |
| Bracts | broadly ovate or elliptic, shortly acuminate, 3–5 mm long, caducous | elliptic to obovate, obtuse to shortly acuminate, 5–11 mm long |
| Sepals | usually blackish or brownish except for hyaline margins | greenish or hyaline |
| Petals | yellowish-white to bright yellow, 0.8–2 cm long | yellow or orange-yellow, 0.7–1.7 cm long |
| Stamens | arranged, 9–10: 2 anthers large, 4–5 medium-sized, 3 small | 10: 2 anthers large, 5 medium-sized, 3 small |
| Pods | shortly oblong-falcate, flattened, 3–6 × 1.3–2 cm, transversely venose, with a ridge of raised crests along middle of each valve | shortly oblong, slightly curved to almost straight, flattened, dehiscent, transversely septate within |
| Seeds | transversely arranged, compressed, oblong-ovate, apiculate near hilum, often emarginate at opposite end, reticulate, with a small areole on each face | transversely arranged, compressed, oblong or oblong-ovate, apiculate near hilum, often emarginate at opposite end, reticulate or rugose, with a small areole on each face |
| Native range | Algeria, Angola, Botswana, Burkina, Cameroon, Cape Provinces, Cape Verde, Central African Republic, Chad, Djibouti, Egypt, Eritrea, Ethiopia, Free State, Gambia, Gulf States, India, Iran, Iraq, Kenya, KwaZulu-Natal, Lebanon–Syria, Libya, Mali, Mauritania, Morocco, Mozambique, Namibia, Niger, Nigeria, Northern Provinces, Oman, Pakistan, Palestine, Saudi Arabia, Senegal, Sinai, Somalia, Sudan, Swaziland, Tanzania, Uganda, West Himalaya, Western Sahara, Yemen, and Zimbabwe | Algeria, Central African Republic, Chad, Djibouti, Egypt, Eritrea, Ethiopia, Gulf of Guinea Island, India, Kenya, Mali, Mauritania, Niger, Nigeria, Oman, Pakistan, Palestine, Saudi Arabia, Sinai, Socotra, Somalia, Sri Lanka, Sudan, and Yemen |
| Sample | Content % | |
|---|---|---|
| Sennoside A 1 | Sennoside B | |
| SA1 | 1.85 ± 0.095 | 0.41 ± 0.12 |
| SA2 | 1.61 ± 0.38 | 0.24 ± 0.13 |
| SI1 | 1.00 ± 0.38 | 0.32 ± 0.17 |
| SI2 | 0.61 ± 0.34 | 0.18 ± 0.14 |
| Peak No. | tr 1 (min) | MS− | Formula | MS/MS | Identification | Ref. |
|---|---|---|---|---|---|---|
| 1 | 3.52 2 | 149.0249 | - | 89.0131 | Unresolved | - |
| 2 | 12.01 | 593.0678 | - | 503.0483, 473.0416, 383.0238, 353.0172 | Unresolved | - |
| 3 | 15.47 | 625.0513 | C27H30O17 | 300.9938 | 1. Quercetin-O-di-hexoside 2. Hydroxykaempferol di-hexoside | [50] |
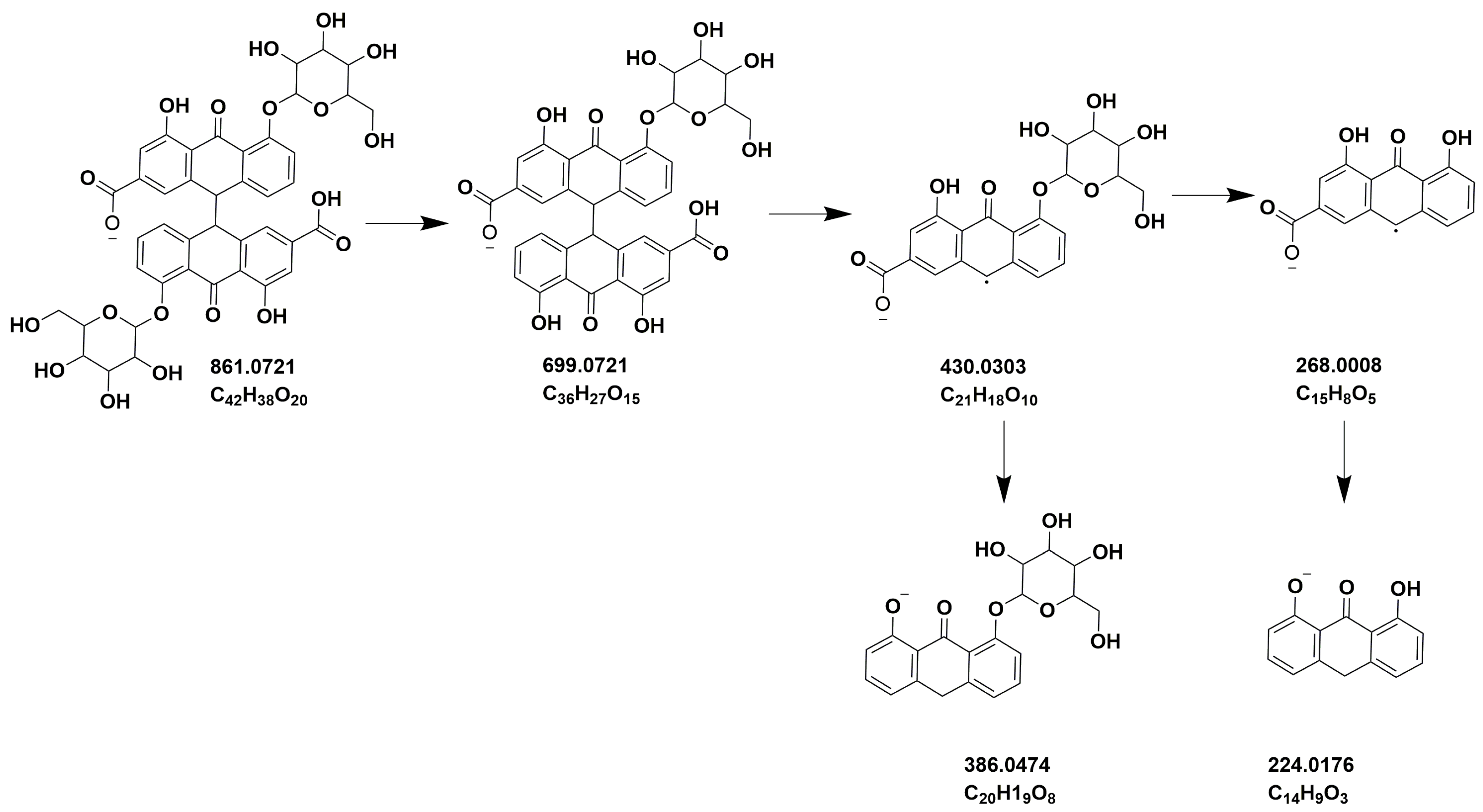
| 4 | 16.61 | 861.0703 | C42H38O20 | 699.0378, 386.0472, 224.0159, 430.0312 | Sennoside B | [45] |
| 5 | 17.17 | 609.0591 | C27H30O16 | 300.9933 | Rutin | [45,50] |
| 6 | 17.32 | 463.0223 | C21H20O12 | 299.9863, 300.9929 | Isoquercitrin/hyperoside | [51] |
| 7 | 17.75 | 609.0586 | C27H30O16 | 285.0010 | Kaempferol-O-di-glycoside | [45] |
| 8 | 18.25 | 639.0654 | C28H32O17 | 315.0075 | Isorhamnetin-O-di-hexoside | [50] |
| 9 | 18.42 | 529.0751 | C30H26O9 | 289.0320 | Cassiaflavan-epicatechin | [52] |
| 10 | 19.02 | 861.0729 | C42H38O20 | 430.0303, 386.0474, 224.0176 | Sennoside A | [45] |
| 11 | 19.44 | 593.0671 | C27H30O15 | 447.0313, 285.0010 | Kaempferol-O-hexoside-pentoside | [45] |
| 12 | 19.65 | 447.0308 | C21H20O11 | 283.9939 | 2-Hydroxyemodin glucoside | [50] |
| 13 | 19.86 | 407.0770 | C20H24O9 | 245.0477 | Tinnevellin-O-glucoside Torachrysone/Isotorachrysone O-glucoside | [45,50] |
| 14 | 20.0 | 623.0706 | C28H32O16 | 315.0070 | 1. Rhamnetin/isorhamnetin 3-neohesperidoside 2. Nepetin di-hexoside 3. 2′,3′,4′,6,7-Pentahydroxyflavone di-hexoside | [53] |
| 15 | 24.94 | 245.0484 | C14H14O4 | 230.0267, 215.0054 | Torachrysone/isotorachrysone/tinnevellin | [50] |
| Peak No. | tr1 (min) | MS− | Formula | MS/MS | Identification | Ref. |
|---|---|---|---|---|---|---|
| 1 | 3.52 2 | 149.0249 | - | 89.0131 | Unresolved | - |
| 2 | 15.16 | 741.0855 | - | 299.9895 | Unresolved | - |
| 3 | 15.53 | 581.1065 | C26H30O15 | 563.0961, 257.0457, 239.0385 | Norrubrofusarin gentiobioside | [54] |
| 4 | 15.80 | 487.0218 | - | 240.9692 | Unresolved | - |
| 5 | 15.85 | 431.0377 | C21H20O10 | 269.0079 240.0096 | Aloe-emodin glucoside | [50] |
| 6 | 16.61 | 861.0293 | C42H38O20 | 430.0293, 386.0465, 224.0171 | Sennoside B | [45] |
| 7 | 16.96 | 445.0155 | C21H18O11 | 282.9854, 239.0021 | Cassic acid (rhein) glucoside | [50] |
| 8 | 17.17 | 609.0604 | C27H30O16 | 300.9929 | Rutin | [45,50] |
| 9 | 17.75 | 419.0768 | C20H20O10 | 401.0677, 257.0465, 239.0390 | De-methyl-toralactone hexoside | [54] |
| 10 | 19.02 | 861.0695 | C42H38O20 | 430.0298, 386.0461, 224.0164 | Sennoside A | [45] |
| 11 | 19.44 | 593.0665 | C27H30O15 | 447.0316, 285.0000 | Kaempferol-O-hexoside-pentoside | [45] |
| 12 | 19.65 | 447.0301 | C21H20O11 | 283.9939 | 2-Hydroxyemodin glucoside | [50] |
| 13 | 19.86 | 407.0768 | C20H24O9 | 245.0476 | Tinnevellin-O-glucoside Torachrysone/Isotorachrysone O-glucoside | [45,50] |
| 14 | 20.0 | 623.0743 | C28H32O16 | 315.0068 | 1. Rhamnetin/isorhamnetin 3-neohesperidoside 2. Nepetin di-hexoside 3. 2’,3’,4’,6,7-Pentahydroxyflavone di-hexoside | [53] |
| 15 | 21.38 | 245.0476 | C14H14O4 | 230.0260, 215.0048 | Torachrysone/isotorachrysone/tinnevellin | [45,50] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zibaee, E.; Akaberi, M.; Tayarani-Najaran, Z.; Nesměrák, K.; Štícha, M.; Shahraki, N.; Javadi, B.; Emami, S.A. Comparative LC-ESIMS-Based Metabolite Profiling of Senna italica with Senna alexandrina and Evaluating Their Hepatotoxicity. Metabolites 2023, 13, 559. https://doi.org/10.3390/metabo13040559
Zibaee E, Akaberi M, Tayarani-Najaran Z, Nesměrák K, Štícha M, Shahraki N, Javadi B, Emami SA. Comparative LC-ESIMS-Based Metabolite Profiling of Senna italica with Senna alexandrina and Evaluating Their Hepatotoxicity. Metabolites. 2023; 13(4):559. https://doi.org/10.3390/metabo13040559
Chicago/Turabian StyleZibaee, Elaheh, Maryam Akaberi, Zahra Tayarani-Najaran, Karel Nesměrák, Martin Štícha, Naghmeh Shahraki, Behjat Javadi, and Seyed Ahmad Emami. 2023. "Comparative LC-ESIMS-Based Metabolite Profiling of Senna italica with Senna alexandrina and Evaluating Their Hepatotoxicity" Metabolites 13, no. 4: 559. https://doi.org/10.3390/metabo13040559
APA StyleZibaee, E., Akaberi, M., Tayarani-Najaran, Z., Nesměrák, K., Štícha, M., Shahraki, N., Javadi, B., & Emami, S. A. (2023). Comparative LC-ESIMS-Based Metabolite Profiling of Senna italica with Senna alexandrina and Evaluating Their Hepatotoxicity. Metabolites, 13(4), 559. https://doi.org/10.3390/metabo13040559








