Abstract
Benzimidazoles are considered a promising class of bioactive heterocyclic compounds that show a wide variety of useful biological properties due to their structural similarities to nucleotides such as purines. Among these properties, great attention has been given to the antibacterial activity exhibited by molecules containing a benzimidazole nucleus in their structure since recent research results have shown the potential of such molecules as alternatives in the fight against bacterial resistance. When these compounds have phenylsubstituted groups in the 2-position of the imidazole ring, a series of molecules can be obtained with generally improved pharmacological activity. These types of compounds are suitable for the formation of stable complexes with several transition metals, including nickel and copper; such compounds have also exhibited many biological properties in different reports. Accordingly, this brief review focuses on recent work on the synthesis and characterization of metal complexes of Ni(II) and Cu(II) with ligands derived from 2-(phenylsubstituted) benzimidazole that were subsequently evaluated for antibacterial activity.
1. Introduction
Antibacterial resistance, which is the ability of bacteria to mutate and exchange genetic information to produce immune responses to antibiotic activity, has been increasing in recent decades to the point of becoming a public health problem; this widespread antibacterial resistance has led to a loss of efficacy in the action of first-line antibiotics against these microorganisms, thus hindering the treatment of infectious diseases and possibly leading to death [1,2]. For this reason, there is considerable interest in controlling bacterial resistance. Medicinal chemistry is one of the fields that has assumed the challenge of developing new alternatives to overcome the relevant difficulties and implications. Research and development in this area are based on the design and synthesis of new molecules with pharmacological potential and corresponding studies of biological activity that generally include heterocyclic compounds [3].
Organic compounds with N-heterocyclic ring systems have demonstrated a wide range of applications, including pharmacological applications [3,4,5], as supported by various studies that have explored their antimicrobial [6,7,8,9], anticancer [10], antioxidative [11], and antiparasitic activity [12]. These compounds have become target molecules that could play an important role in the fight against problems associated with the most common diseases, including those related to bacterial resistance. Within this group of compounds are benzimidazoles, which were first synthesized in 1872 by Hoebrecker, 1875 by Ladenberg, and 1878 by Wund [13]. However, it was not until 1944 that Wolley speculated that benzimidazoles could act like purines, given their remarkable similarity, and therefore cause some biological responses [14]. Today there are several drugs whose structures are based on benzimidazoles (Figure 1), such as omeprazole (proton pump inhibitor), pimobendan (vasodilator), and mebendazole (anthelmintic).
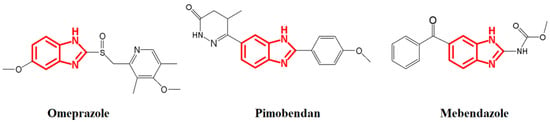
Figure 1.
Some commercial drugs derived from benzimidazoles.
The cores of benzimidazoles arise from the fusion of benzene at positions 4 and 5 of the imidazole ring, from which different derived molecules have been developed with biological activity such as anti-inflammatory [15], analgesic [16], antitumor [17], antifungal [18], and antibacterial [19] (Figure 2). Benzimidazoles are characterized by acid-base properties, solubility, the potential presence of electron density donor atoms, the ability to form salts, and marked stability. Because of these features, benzimidazoles are pharmacophores [20]. They have the right steric and electronic properties to make sure they interact optimally with a specific biological target and either start or stop its biological response [21].
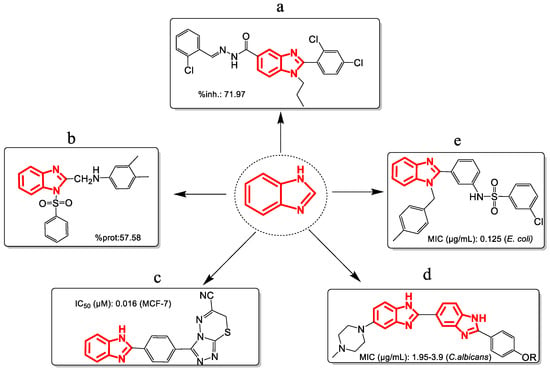
Figure 2.
Benzimidazole derivatives with activity (a) anti-inflammatory, (b) analgesic, (c) antiproliferative, (d) antifungal, (e) antibacterial. Note: %inh = Paw edema inhibition; %prot = percentage of protection; IC50 = 50% inhibitory concentration; MIC = minimum inhibitory concentration.
Benzimidazole derivatives can be obtained by different methods (Figure 3), including the condensation of phenylenediamines and aldehydes to produce Schiff bases that are subsequently subjected to oxidative cyclodehydrogenation [22]; the use of aromatic or heteroaromatic 2-nitroamines and formic acid in the presence of Fe and NH4Cl [23]; the use of N-methyl-1,2-phenylenediamines, carbonitriles and sodium hydride [24]; or the high-temperature condensation of phenylenediamines and aromatic carboxylic acids under strongly acidic conditions (polyphosphoric acid (PPA), 180 °C) to obtain 2-(phenylsubstituted) benzimidazoles [22,25,26].
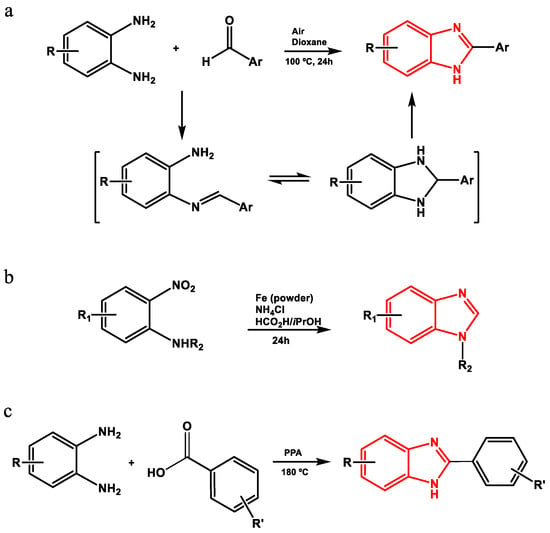
Figure 3.
Method of synthesis of 2-(phenylsubstituted) benzimidazoles by (a) oxidative cyclodehydrogenation using air, (b) from 2-nitrosamines, (c) condensation with PPA.
Among benzimidazole derivatives, multiple biological activities have been reported [15,16,27,28], especially antitumor activities. A possible mechanism of action has been proposed to be related to interactions of these with DNA (covalent bonds, intercalation, etc.) [27,29], similar to previously existing medications for the treatment of various types of cancer, such as cisplatin, carboplatin, and oxaliplatin. Although these drugs have shown a broad spectrum of cytotoxicity against various cell lines, their use or clinical application on humans has been limited by their neurotoxic, nephrotoxic, ototoxic, nausea, vomiting, and tumor cell resistance effects [27,30,31,32,33].
Regarding antifungal responses, benomyl (the first fungicide registered in 1968, subsequently introduced to the market in 1971 by the Du Pont company), thiabendazole, and carbendazim (commercially used fungicides that act as metabolic inhibitors of the electron transport chain, enzymes, nucleic acid metabolism, protein synthesis, and sterol synthesis) contain a benzimidazole core in their structure; as a result, this type of molecule has become a focus of widespread research in the search for new compounds that contribute to minimizing resistance to drugs for the treatment of infections caused by fungi [34,35,36].
Because antibiotic resistance and multidrug resistance imply an imminent risk of bacterial propagation, which limits the efficacy of the treatment of diseases caused by such pathogens, the antibacterial activity of benzimidazole derivatives has also been evaluated [33,37,38,39,40,41]. In the search for possible new bactericidal agents, some 2-phenyl-substituted benzimidazoles are noteworthy, such as 2-(4-aminophenyl)benzimidazole derivatives that showed moderate antibacterial activity (MIC: 25 µg∙mL−1) against P. aeruginosa [42]; derivatives of 2-{4-[(4-substituted piperazine/piperidine-1-yl)carbonyl]phenyl}-1H-benzimidazole that exhibited potent antibacterial activity (MIC: 12.5 µg∙mL−1) against S. aureus [43]; and derivatives of 1-(4-(6-chloro-1H-benzimidazol-2-yl)phenyl)-3-(4-chlorophenyl)urea and 4-(2-(4-(6-methyl-1H-benzimidazol-2-yl)phenyl)hydrazono)pyrazolidine-3,5-dione that demonstrated potent antibacterial action (IC50: 0.61 and 0.58 µM) against E. coli and S. aureus, respectively [44].
Although organic molecules are generally the first option for the development of new drugs in medicinal chemistry, with the discovery of the anticancer properties of cisplatin, metal complexes have become highly studied compounds of interest as possible therapeutic agents [45,46,47,48]. Compounds that have a benzimidazole nucleus are among the most widely used organic ligands for the formation of metal complexes since they are suitable coordinating agents of metal ions due to their conjugated pi system and the azomethine nitrogen [49,50,51], which allow them to bind to the metal center in a monodentate manner or act as a chelate ligand.
Complexes derived from benzimidazole with lanthanide ions and different d-block metals have demonstrated efficient biological activity in a variety of studies [49,50,52,53,54,55,56]. The antibacterial activity of these complexes has been attributed to various factors, such as complex-DNA interactions (intercalating, nonintercalating, cleavable, etc.), the blocking of metal binding sites in enzymes, disturbances in the respiratory process, and blocking of protein synthesis, among others. This antimicrobial activity is believed to be closely related to an increase in the lipophilicity of the metal complex during adduct formation, which usually improves the bioactivity of free ligands [57]. An example of this mechanism is the compound [diodo-di(2-phenylbenzimidazole)platinum(II)], which showed higher antibacterial activity (MIC: 0.14 µM) against B. subtilis and K. pneumoniae than the free organic ligand did (MIC: 1.28 µM) [58]. Another study showed that the antibacterial activity of a Schiff base derived from 2-(3-aminophenyl)benzimidazole against S. aureus (MIC: 500 µg∙mL−1) improves when it forms a metal complex with lanthanum ions (MIC: 125 µg∙mL−1) [56]. In recent years, attention has been paid to nickel and copper complexes with benzimidazole ligands or derivatives because Cu(II) and Ni(II) metal ions are cheaper than analogous complexes with metal centers such as Ag(I) and Pt(II) and also exhibit biological properties as anticancer and antibacterial agents [59,60,61,62,63].
Monodentate 2-(phenylsubstituted) benzimidazoles, 2-(hydroxyphenyl)benzimidazoles, and Schiff bases derived from 2-(aminophenyl)benzimidazole are reconognized important ligands on account of their extensive coordination chemistry, ease of functionalization, and growing interest in the design of bioactive and catalytically active metal complexes [60,61]. The phenyl substitutions modulate electronic properties and steric hindrance, thereby influencing metal–ligand interactions. Moreover, the hydroxyphenyl derivatives offer additional donor sites through phenolic oxygen atoms, enhancing chelating ability. Schiff bases, with their imine functionality, enable multidentate coordination and structural diversity. In such a manner, these ligands provide a versatile platform to explore structure–activity relationships in coordination compounds.
This review describes the characterization and evaluation of the antibacterial activity of metal complexes of Ni(II) and Cu(II) with ligands derived from 2-(phenylsubstituted) benzimidazole, highlighting their importance as possible antibacterial agents that could contribute to the fight against antibiotic resistance. It is important to note that, in the antibacterial activity studies addressed in this review, the complexes are dissolved directly in culture medium or using dimethyl sulfoxide (DMSO) as a vehicle to subsequently improve solubility in the culture medium for the microdilution method. For the disk diffusion method, the complexes are usually dissolved in DMSO, and pure DMSO is used as the control solvent. In this study, the Scopus database was chosen for the bibliographic search, it has bibliometric tools to track, analyze, and visualize research [64]. The search equation used was TITLE-ABS-KEY (antibacterial AND benzimidazoles AND derivatives) AND PUBYEAR > 2008 AND PUBYEAR < 2026 AND (LIMIT-TO (DOCTYPE, “ar”) OR LIMIT-TO (DOCTYPE, “re”) OR LIMIT-TO (DOCTYPE, “ch”) OR LIMIT-TO (DOCTYPE, “cp”) OR LIMIT-TO (DOCTYPE, “cr”)) AND (LIMIT-TO (LANGUAGE, “English”)) which applies basic filters to ensure that the final sample analyzed presents documents that exclusively address topics around the antibacterial activity of benzimidazole derivatives published between the period from 2009 to 2025. Documents that did not meet these criteria were excluded. The VOSviewer software (www.vosviewer.com, (accessed on 10 December 2024)); version 1.6.18, Leiden University, Leiden, The Netherlands) was applied to visualize the preliminary results and create a bibliographic map (Figure 4). Each node symbolizes a specific keyword, and its size indicates the importance of the keyword within the existing literature. The connections between the nodes illustrate the co-occurrence relationships that exist between the keywords. Additionally, keywords are distinguished by color coding to reflect their respective clusters, so keywords from the same cluster show a greater propensity to appear simultaneously in the literature.
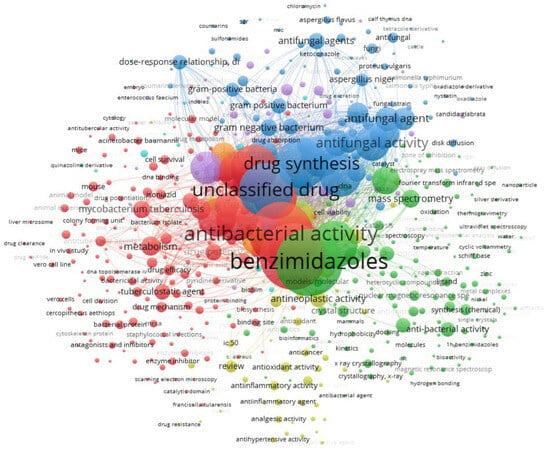
Figure 4.
Co-occurrence network map of keywords (seven clusters). The size of the nodes indicates the number of publications that include the keywords. The proximity of two nodes indicates the relationship of their co-occurrence link.
2. Metal Complexes of Ni(II) and Cu(II) with Monodentate 2-(Phenylsubstituted) Benzimidazole Derivatives
Nickel and copper are essential elements for biological systems [65]. Both have different oxidation states, with 2+ being the most stable, which makes it easy for these elements to form complexes with different coordination numbers [66]. It should be noted that nickel is present in the active sites of several important metalloproteinases, such as urease and Ni/Fe hydrogenase. In addition, different studies have shown the pharmacological potential of nickel and copper complexes [67,68,69,70].
In the literature, interesting results have been found regarding the antibacterial activity of copper and nickel metal complexes with 2-(phenylsubstituted) benzimidazole ligands [71,72,73,74], with scarce reports of complexes with monodentate 2-(phenylsubstituted) benzimidazole derivatives. Complexes 1–4 (Figure 5) were synthesized from salts of Cu(II) and Ni(II) to which the ligands BZa and BZb were added under constant agitation. Once the reaction was complete, the mixtures were refluxed for 48 h at 50 °C. Characterization of the metal complexes indicated that monodentate ligand binding to the metal occurred through a coordinated bond with the nitrogen atom (azomethine) [71].
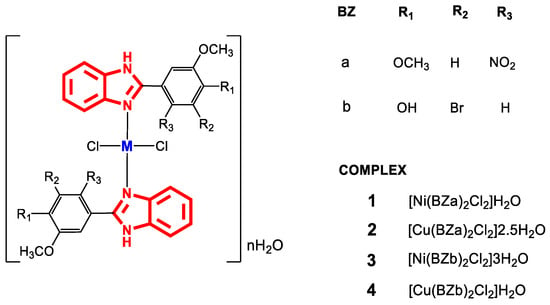
Figure 5.
Structure of complexes 1–4.
The electronic spectra of the Ni(II) complexes (1 and 3) showed the transitions 3T1 (F)→ 3T2 (P) at 580 nm, 3T1 (F) → 3A2 at 650–725 nm, and 3T1 (F)→ 3T1 (P) at 700–775 nm. These transitions indicated a tetrahedral geometry around the Ni(II) ion, which was confirmed by magnetic moment values of 4.03 and 3.53 BM for complexes 1 and 3, respectively. Similarly, in Cu complexes (2 and 4), transitions of 2Eg → 2A1g at 540–570 nm, 2Eg→ 2B2g at 617–670 nm, and 2Eg→ 2B1g at 705–755 nm and magnetic moment values of 1.75 and 1.71 BM were obtained, which correspond to planar square geometry. Additionally, Fourier transform infrared (FT-IR) spectroscopy showed the bands that correspond to the vibrations of the compounds that were obtained, and thermogravimetric analysis confirmed that water molecules were present (Table 1).

Table 1.
FT-IR and TGA results for complexes 1–4.
The antibacterial activity was determined by the broth microdilution method against the Gram-positive (+) strains S. aureus and S. pyogenes and Gram-negative (-) strains E. coli and P. aeruginosa, and the results are compared with those of Ciprofloxacin. The results revealed that 1 and 2 were more effective against E. coli and S. aureus (MIC: 100 and 250 µg∙mL−1, respectively), while complex 3 showed very good activity against S. aureus and E. coli (MIC: 50 and 6.25 µg∙mL−1, respectively). In addition, 4 exhibited moderate antimicrobial activity against S. pyogenes, E. coli and P. aeruginosa (MIC: 500 µg∙mL−1 for the three strains) [71]; however, none exceeded the minimum inhibitory concentration (MIC) of the reference antibiotic. In this study, it was demonstrated that the metal complexes presented better results of biological activity compared to the free ligands, generally with lower MIC for the nickel complexes, which shows a direct relationship between the activity and the nature of the metal, as well as the dependence on the number of hydration waters in the complexes. These types of compounds were more active with the presence of the BZb ligand, which could be attributed to the presence of substituents such as -OH on the 2-phenyl ring, which provides greater hydrophilicity and therefore greater solubility in biological media.
Complex 5 (Figure 6) was synthesized by mixing methanolic solutions of Ni(ClO4)2 and m-aminophenylbenzimidazole (m-APB) and refluxing for a period of 4–6 h [68]. The formation of 5 was confirmed by FT-IR spectroscopy. Vibrations were observed corresponding to C=N bonds within the range from 1600 to 1632 cm−1 (displaced with respect to the free ligand), NH(amino) at 3300 cm−1, NH(benzimidazole) at 3050 cm−1 and ClO4− between 1100 and 620 cm−1 for the coordinated ligand. The electrolytic behavior was verified by molar conductivity measurements (76 S∙cm2∙mol−1). Additionally, 1H-NMR signals attributable to amino protons (NH2) and benzimidazole (NH) were observed as singlets at 5.78 and 16.0 ppm, respectively, while the aromatic protons showed multiplets between 6.98 and 7.66 ppm. The planar square geometry was corroborated by UV-vis spectroscopy, where bands at 368 and 570 nm were observed for the 1A1g → 1B1g and 1A1g → 1A2g transitions, respectively) [75,76].
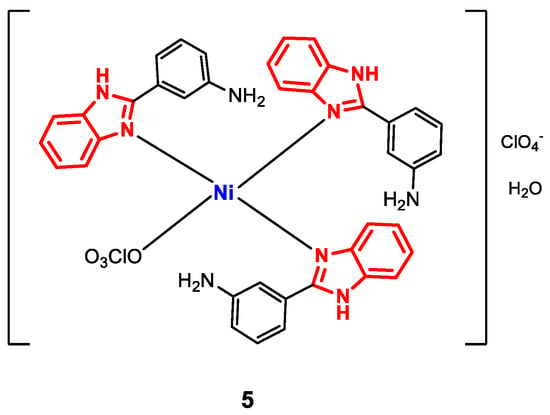
Figure 6.
Structure of complex 5.
Antibacterial activity of complex 5 was evaluated by the agar diffusion method; the zones of inhibition caused by the bacterial strains E. coli (-), K. pneumoniae (-), S. aureus (+), and B. subtilis (+) were measured. The results showed that there was no specificity of the activity against Gram-positive or Gram-negative strains (6 mm for S. aureus and E. coli, 7 mm for B. subtilis, and 9 mm for K. pneumoniae), and that compared to the values of the standard drug (ampicillin), the complex could be considered moderately active, generally observing better antibacterial activity in comparison with the ligand, confirming that the presence of the metal influences the biological activity, since the increase in activity after complexation can increase the lipophilicity and inertia of the metal-ligand bonds [77].
3. Metal Complexes of Ni(II) and Cu(II) with 2-(Hydroxyphenyl)benzimidazole Ligands
The stability of metal complexes improves with the presence of ligands that contain different coordination points (multidentate ligands), a property that several 2-(phenylsubstituted) benzimidazole derivatives possess. For example, 2-(hydroxyphenyl)benzimidazole ligands contain a hydroxy group in the phenyl, which can be deprotonated to form a strong O-M covalent bond. For the complexes [NiL(H2O)3(AcO)]H2O (6) and [CuL(H2O)(AcO)] (7) (Figure 7), which were synthesized from a ligand L and M(AcO)2nH2O, both their structure and the geometry were optimized using Hyperchem 8.03 software to obtain the bond lengths around the central ion (Table 2). For the determination and confirmation of the structure of complexes 6 and 7, FT-IR was used, which revealed main bands of C=N at 1572 and 1586 cm−1, CO at 1280–1301 cm−1, M-O between 569 and 633 cm−1, M-N between 459 and 514 cm−1 and the water molecules in the network at 3479 and 3455 cm−1, as well as bands for acetate groups between 1352 and 1558 cm−1. In addition, EI-MS analysis revealed molecular weights of 399.01 and 349.83 g∙mol−1, corresponding to the formulas C15H20N2NiO7 and C15H14CuN2O4, respectively, and a metal/ligand coordination ratio of 1:1.
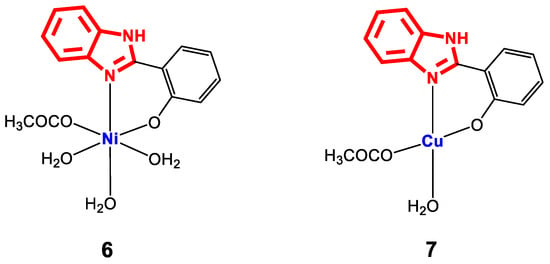
Figure 7.
Structure of complexes 6 and 7.

Table 2.
Optimized bond lengths of complexes 6 and 7.
The antibacterial activity of 6 and 7 against the Gram-negative (-) strains P. aeruginosa, E. coli, Acinetobacter, and Klebsiella was evaluated by the well diffusion method. Stock solutions of the chelates were prepared in DMSO, and the diameters of the inhibition zones were measured. The results showed high responses for all strains except for E. coli and Klebsiella (complex 6: 16 and 20 mm against Acinetobacter and P. aeruginosa, respectively; complex 7: 15 and 17 mm against P. aeruginosa) [78]. When comparing the results of activity against P. aeruginosa, it is observed that the nickel complex is more active than the copper complex, which could be explained from a structural point of view if we take into account that complex 6 presents a greater number of coordination and hydration waters than 7, which is related to an increase in the solubility of the molecule in the biological medium. In the general explanation of antimicrobial activity, the authors refer to Overton’s concept, whereby the high activity of metal chelates is related to the non-selective passage of liposoluble materials through the lipid membrane, regardless of the structural differences between the membranes of Gram-positive and Gram-negative bacteria. Additionally, it has been reported that some metal complexes with heterocyclic ligands, such as benzimidazole, can exert effects on the integrity of the bacterial membrane without completely penetrating it. In these cases, the antibacterial mechanism of action includes electrostatic or hydrophobic interaction with surface components of the membrane, inducing its destabilization or permeabilization [56]. These dual mechanisms, one dependent on penetration and the other based on surface interactions, might help to explain the antibacterial non-specificity observed with some metal complexes.
Tavman et al. evaluated the antibacterial activity of a series of complexes with 2-(1H-benzimidazol-2-yl)-1,4-phenylsubstituted ligands (Figure 8) [79,80]. In general, these coordination compounds were synthesized using ethanolic solutions of the corresponding ligand and metal salts.
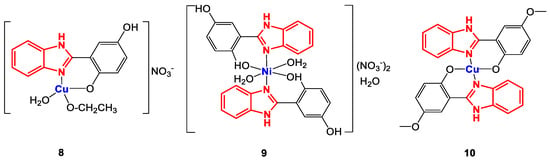
Figure 8.
Structure of complexes 8–10.
The formation of the metal complexes and their structure were characterized through FT-IR, magnetic moment measurements, and ESI-MS (except for 10). FT-IR spectroscopy revealed bands attributable to the characteristic vibrations of C=N bonds between 1600 and 1630 cm−1, CO between 1200 and 1260 cm−1 [81], OH above 3400 cm−1, M-O at approximately 500 cm−1 [81,82], NO3− between 1385 and 1389 cm−1, NH between 3230 and 3450 cm−1 and OCH3 between 2918 and 2971 cm−1. Additionally, ESI-MS spectrometry yielded the fragments [M]+ and [M-2H2O]+ with m/z 415.0 and 673.7 for 8 and 9, respectively. Additionally, some physical and analytical properties were measured, confirming the geometry of complexes 8–10 through their magnetic moments (Table 3).

Table 3.
Physical and analytical properties of complexes 8–10.
The antibacterial activity of complexes 8–10 was evaluated by the microdilution method in Mueller–Hinton broth against the strains S. aureus (+), B. cereus (+), B. subtilis (+), S. epidermidis (+), E. coli (-), K. pneumoniae (-), P. aeruginosa (-), S. enteritidis (-), and P. mirabilis (-). The results showed that all the complexes were more effective against Gram-positive (+) than Gram-negative (-) strains, so it could be concluded that the activity will be related to the mechanisms depending on the composition of the bacterial cell wall. For the case of copper complexes (8 and 10), the number of ligands plays an important role when evaluating biological activity, since the complex with a metal/ligand ratio of 1:2 (10) was more active than complex 8 (metal/ligand ratio 1:1). On the other hand, when comparing the results between the copper complexes and the nickel complex, it is observed that generally there is greater activity for Ni(II); even 9 exhibited potent antibacterial activity (39 μg∙mL−1) against S. epidermidis, which exceeded that of the reference drug ciprofloxacin (156 µg∙mL−1), as indicated in Table 4 [79,80].

Table 4.
In vitro antibacterial activity of complexes 8–10 (MIC, µg∙mL−1).
Complex 11 (Figure 9) was synthesized by direct reaction of Cu(NO3)2·3H2O and the corresponding ligand in methanol, with refluxing for 4 h [83].
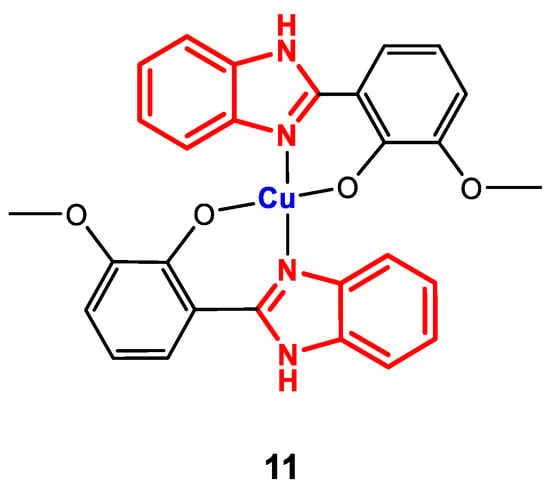
Figure 9.
Structure of complex 11.
The formation of complex 11 was confirmed by FT-IR spectroscopy. Bands corresponding to the vibration of M-O bonds at 500 cm−1, OCH3 between 800 and 1500 cm−1 and C=N at approximately 1600–1630 cm−1 were obtained. The planar square geometry was corroborated through UV-Vis spectroscopy, where bands were observed at 357, 456 and 645 nm, corresponding to metal-ligand charge transfer and 2B1g → 2A1g and 2B1g → 2Eg transitions, respectively. Additionally, the molar conductivity was measured, which confirmed the nonelectrolytic behavior of the complex (12 S∙cm2∙mol−1).
The antibacterial activity against the strains B. subtilis (+), S. aureus (+), E. coli (-), K. pneumoniae (-), S. epidermidis (+), P. aeruginosa (-), S. enteriditis (-), and B. cereus (+) was determined by the agar disk diffusion method by measuring the inhibition zone and the macro-dilution broth method for the detection of the antibacterial effect quantitatively. The results showed that 11 showed activity against only S. aureus (zone of inhibition: 8 mm, MIC: 532 µg∙mL−1) and S. epidermidis (zone of inhibition: 8 mm, MIC > 532 µg∙mL−1) [83], the presence of the metal improves the biological activity compared to the ligand that did not form a zone of inhibition in the qualitative experiment. This study also allows us to suggest the effect of the position of the methoxy group in the phenolic fragment of the ligand, since when comparing the results of complexes 10 and 11 against the same bacterial strains evaluated in the two studies (S. aureus and S. epidermidis), it is found that when the methoxy substituent is in the para-position to -OH, it is more active compared to the analog with the methoxy substituent in the ortho-position to -OH.
Padalkar et al. synthesized complexes 12 and 13 from the ligand 5-(diethylamino)-2-(5-nitro-1H-benzimidazol-2-yl)phenol and copper and nickel salts (Figure 10) for subsequent determination of their antibacterial activity against the strains S. aureus (+) and E. coli (-) [84].
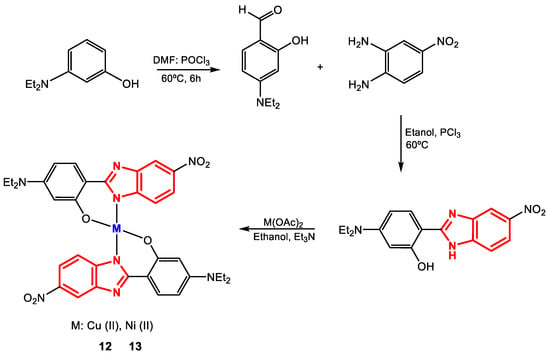
Figure 10.
Synthesis of complexes 12 and 13.
The proposed structure of the ligand was confirmed by 1H-NMR, where singlets were observed at 12.43 and 8.17 ppm for OH and NH protons, respectively; multiplets between 7.85 and 6.27 ppm for aromatic protons; and multiplets at 3.42 and 1.21 for CH2 and CH3 protons, respectively. The formation of complexes 12 and 13 was evaluated by atomic absorption spectroscopy (AAS), where the concentrations of the metals obtained experimentally were remarkably close to those predicted theoretically. In addition, thermogravimetric analysis (TGA) indicated that both complexes are thermally stable up to 250 °C and that once this temperature is exceeded, the stability of 12 is greater than that of 13. Furthermore, antibacterial evaluation revealed that both complexes are more active than the free ligand, so the activity would be improved with the presence of metal ions (Table 5). The results also show that the complexes exhibit similar activity (close MIC values) or better than the reference drug (streptomycin) and that 12 is more effective against E. coli than against S. aureus, while 13 presents the same MIC values against Gram-positive and Gram-negative bacteria (~62.5 µg∙mL−1) [84].

Table 5.
In vitro antibacterial activity of complexes 12 and 13 (MIC, µg∙mL−1).
In general, in studies against E. coli and S. aureus strains, complexes 12 and 13 showed greater antibacterial activity than complexes 9–11 (with similar structure and metal/ligand ratio 1:2), which could depend on the substituent groups present in the phenyl rings of the 2-(phenylsubstituted) benzimidazoles ligands, as well as the position of these groups in the phenolic ring, suggesting that the diethylamino group in the meta-position to -OH and the presence of the nitro group in the benzimidazole ring favors the activity of this type of ligand.
From methanolic solutions of the ligands 2-(5-nitro-1H-benzimidazol-2-yl)-4-bromophenol, 3-(5-chloro-1H-benzimidazol-2-yl)-benzene-1,2-diol and 2-(o-sulfamoylphenyl)benzimidazole, copper and nickel complexes 14–16 were synthesized [85,86] (Figure 11).
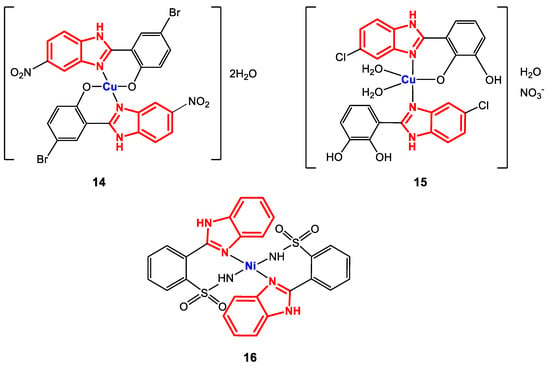
Figure 11.
Structure of complexes 14–16.
The structure of complexes 14–16 was confirmed through elemental analysis, where a metal/ligand ratio of 1:2 was obtained. Additionally, FT-IR spectroscopy revealed bands corresponding to the vibrations of the following bonds for complex 14: CO at 1280 cm−1, C-Br between 500 and 550 cm−1 and C-NO2 between 1305 and 1532 cm−1. For complex 15, bands were observed corresponding to M-OC at 500 cm−1, NH at 3400 cm−1, C=N at 1600 cm−1 and NO3− at 1385 cm−1, while for 16, the main visualized bands were NHsulf between 1431 and 1488 cm−1 and C=N between 1531 and 1562 cm−1. In addition, for 14 and 16, magnetic moment values of 1.65 BM and 1.86 BM, respectively, supported the prediction of planar square geometry, while ESI-MS revealed isotopic fragmentation patterns corresponding to the isotopes of bromine, chlorine, and copper.
The antibacterial activity of complexes 14–16 was evaluated using broth microdilution and agar disk diffusion techniques against the bacterial strains S. aureus (+), S. epidermidis (+), E. coli (-), K. pneumoniae (-), P. aeruginosa (-), P. mirabilis (-), and B. bronchiseptica (-). The results showed that 14 and 15 are selective against Gram-positive strains, since they only showed activity against S. aureus and S. epidermidis (MIC values: 19.5 and 39.0 µg∙mL−1, respectively, for 14 and 625 µg∙mL−1 against both strains for 15) [85,86]. In addition, 14 has a greater activity than the reference drug ciprofloxacin against S. epidermidis (156 µg∙mL−1). Complex 16 exhibited potent activity against B. bronchiseptica with an inhibition zone of 17 mm, similar to that of the reference drug cefixime (24 mm) [73]. These results continue to show that the substituents on the benzene rings of the ligands are fundamental in the structure–activity relationship, since with the presence of the nitro group on the benzimidazole ring and nucleophilic substituents on the 2-(phenylsubstituted), the antibacterial activity improves for these species.
4. Ni(II) and Cu(II) Metal Complexes with Schiff Bases Obtained from 2-(Aminophenyl)benzimidazole
Schiff bases are considered ligands with great advantages because they are easy to obtain and can form multidentate complexes with several metal centers due to the presence of donor heteroatoms, which generally appear in their structures as nitrogen and oxygen molecules [87,88]. Schiff bases derived from benzimidazoles are quite attractive for applications in medicinal chemistry due to their broad biological activity [56], especially those based on (2-aminophenyl)benzimidazole that have been studied for their anticancer activity [89,90,91]. Ni(II) and Cu(II) complexes (17 and 18) with the Schiff base (E)-2-((4-(1H-benzimidazol-2-yl)phenylimino)methyl)-4-bromophenol were synthesized (Figure 12) to determine their antibacterial activity [72]. Once obtained, the complexes were characterized to confirm their proposed structure. Through X-ray diffraction (XRD), the arrangements of the atoms that make up the ligand were obtained (Figure 13), and some bond lengths and angles were obtained (Table 6). The 1H-NMR spectrum of 17 revealed multiplet signals between 6.79 and 8.79 ppm attributable to aromatic protons, a singlet at 9.08 ppm corresponding to the azomethine proton (CH = N) and a singlet at 13.17 ppm due to the NH proton. 13C-NMR revealed signals between 115 and 136 ppm for carbons in the aromatic ring, at approximately 170 ppm for azomethine carbon, and at 151 ppm for C=N imidazole. Likewise, for complex 18, FT-IR revealed signals corresponding to the vibrations of NH bonds at 3349 cm−1, azomethine C=N at 1595 cm−1, imidazole C=N at 1515 cm−1, O-M at 580 cm−1 and M-N at 420 cm−1.
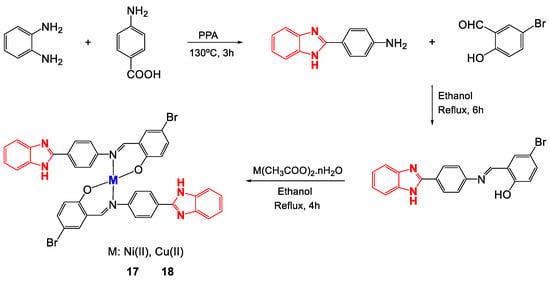
Figure 12.
Synthesis of Ni(II) and Cu(II) complexes with the ligand-((4-(1H-benzimidazol-2-yl)phenylimino)methyl)-4-bromophenol.
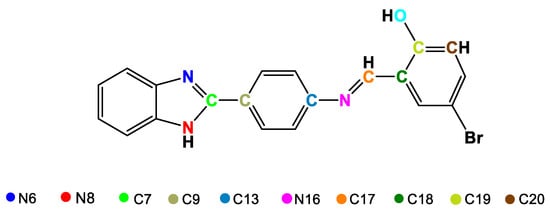
Figure 13.
Distribution and enumeration of atoms in the ligand 2-((4-(1H-benzimidazol-2-yl)phenylimino)methyl)-4-bromophenol.

Table 6.
Bond lengths and angles of the ligand 2-((4-(1H-benzimidazol-2-yl)phenylimino)methyl-4-bromophenol.
The antibacterial activity against the strains M. luteus (+), E. aerogenes (-), and E. coli (-) was determined by the disk diffusion method, measurement of the diameters of the inhibition zones, and subsequent determination of the MIC. The results revealed high antibacterial activity for 17 (high inhibition zone values and low MIC values) compared to compound 18, as shown in Table 7, which can be correlated with the DNA binding exhibited by this complex (intercalation binding, ∆Tm: 3.2 °C and Kb: 9.5 × 104 M−1), which was studied by thermal denaturation and UV-Vis spectroscopy [72].

Table 7.
Antibacterial activity of Ni(II) and Cu(II) complexes with the ligand-((4-(1H-benzimidazol-2-yl)phenylimino)methyl)-4-bromophenol.
Cu(II) and Ni(II) complexes have also been synthesized with other Schiff bases, such as BMEP derived from 2-(2-(aminophenyl)benzimidazole) (Figure 14) [57].
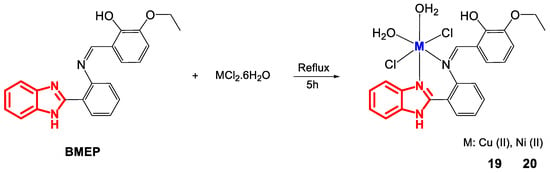
Figure 14.
Synthesis of complexes 19 and 20.
Characterization by elemental analysis and molar conductivity measurements (Ω) (Table 8) showed a metal-ligand ratio of 1:1 and a nonelectrolytic nature for 19 and 20. Their octahedral geometry was confirmed by UV-Vis spectroscopy and magnetic moment measurements. The UV-Vis spectrum of 19 revealed a broad band at 14,556 cm−1 due to the transition 2Eg → 2T2g and a magnetic moment of 1.67 BM, while 20 exhibited three bands at 22,371, 30,303 and 32,894 cm−1 associated with the electronic transitions 3A2g → 3T2g(F), 3A2g → 3T1g(F) y 3T2g → 3T1g(P) and a magnetic moment of 3.10 BM. IR spectroscopy indicated bands of CH = N at 1604 and 1616 cm−1, M-N at 435 and 447 cm−1 and M-OH and 1336 and 3390 cm−1 for the complexes, respectively.

Table 8.
Physical and analytical properties of the complexes 19 and 20.
The antibacterial activity against the strains S. aureus (+), B. subtilis (+), E. coli (-), and P. fluorescences (-) was evaluated by the disk diffusion method and measurement of the zones of inhibition. Streptomycin was used as a standard (value not reported), and DMSO was used as a solvent control. Thus, it was shown that both 19 and 20 were more potent against E. coli and S. aureus than the other strains (Table 9), with better results for the Cu(II) complex in comparison to the Ni(II) complex [57].

Table 9.
Bacterial growth inhibition zones (mm) for complexes 19 and 20.
The results of the antibacterial activity showed that the complexes generally present higher values of the inhibition zones compared to the Schiff base ligand, which confirms the synergistic effect for these species when the metal ion is present. The authors attribute this finding to the possible lipophilic behavior of the complexes based on the overtone concept of cell permeability and to the Tweedy chelation theory, where after chelation there is a metal-ligand charge exchange so that the polarity of the metal ion is reduced and the delocalization of pi electrons in the aromatic system of the ligand increases, thus increasing the lipophilicity of the complex [57].
5. Conclusions
The need to find new alternatives for the control of infectious diseases caused by bacteria has led to the design and development of new biologically active molecules to overcome the great public health inconvenience of bacterial resistance. In this sense, the study of metallopharmaceuticals continues to be an option due to the large number of research reports that demonstrate the synergistic effect of the presence of metal ions in organic systems, such as N-heterocyclic ligands. It has been frequently found that metal complexes are more effective than free ligands since complexation usually improves lipophilicity and biological activity. Accordingly, copper and nickel complexes with ligands derived from 2-(phenylsubstituted) benzimidazole have been demonstrated to have antibacterial potential; some such complexes even exhibit greater activity than the reference drugs, making them compounds of interest for the design of new drugs. Functional groups on the benzimidazole core have been shown to greatly affect solubility, playing an important role in its antibacterial activity. It is important to mention that the chelate ligands in these systems generally improve the antibacterial potential compared to that of monodentate ligands, especially if they are Schiff base ligands containing the azomethine functional group (which is of great biological interest). However, the lack of knowledge of the different mechanisms of action involved in the antibacterial activity of these compounds has generated the need for additional studies, such as analyses of quantitative structure–activity relationships (QSAR), cytotoxicity, and interaction with biomolecules (DNA, proteins, and lipids, among others). Finally, the results presented in this review can serve as a valuable source of information for the rational development of new precursors with antibacterial activity.
Author Contributions
Conceptualization, A.A.-M. and D.P.-C.; methodology, I.V.M.-D.; formal analysis and investigation, I.V.M.-D. and A.A.-M.; resources, A.A.-M. and D.P.-C.; writing—original draft preparation, I.V.M.-D., A.A.-M. and D.P.-C.; writing—review and editing, A.A.-M. and D.P.-C.; visualization, A.A.-M.; supervision and project administration, D.P.-C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Universidad del Valle and Universidad del Magdalena, VIN2024203 project (Fonciencias).
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Tacconelli, E.; Pezzani, M.D. Public Health Burden of Antimicrobial Resistance in Europe. Lancet Infect. Dis. 2019, 19, 4–6. [Google Scholar] [CrossRef] [PubMed]
- López-Pueyo, M.J.; Barcenilla-Gaite, F.; Amaya-Villar, R.; Garnacho-Montero, J. Multirresistencia Antibiotica En Unidades de Criticos. Med. Intensiv. 2011, 35, 41–53. [Google Scholar] [CrossRef]
- Jampilek, J. Heterocycles in Medicinal Chemistry. Molecules 2019, 24, 3839. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wu, Y.; Zhao, Y.; Zhao, Y.; Wang, H.; Wang, H.; Zhang, F.; Zhang, F.; Li, R.; Li, R.; et al. Ambient Reductive Synthesis of N-Heterocyclic Compounds over Cellulose-Derived Carbon Supported Pt Nanocatalyst under H2atmosphere. Green Chem. 2020, 22, 3820–3826. [Google Scholar] [CrossRef]
- Kaur, N. Synthesis of Five-Membered N,N,N-and N,N,N,N-Heterocyclic Compounds: Applications of Microwaves. Synth. Commun. 2015, 45, 1711–1742. [Google Scholar] [CrossRef]
- Lasmari, S.; Ikhlef, S.; Boulcina, R.; Mokrani, E.H.; Bensouici, C.; Gürbüz, N.; Dündar, M.; Karcı, H.; Özdemir, İ.; Koç, A.; et al. New Silver N–Heterocyclic Carbenes Complexes: Synthesis, Molecular Docking Study and Biological Activities Evaluation as Cholinesterase Inhibitors and Antimicrobials. J. Mol. Struct. 2021, 1238, 130399. [Google Scholar] [CrossRef]
- Shakurova, E.R.; Parfenova, L.V. Synthesis of N-Heterocyclic Analogues of 28-O-Methyl Betulinate, and Their Antibacterial and Antifungal Properties. Molbank 2020, 2020, M1100. [Google Scholar] [CrossRef]
- Furdui, B.; Parfene, G.; Ghinea, I.O.; Dinica, R.M.; Bahrim, G.; Demeunynck, M. Synthesis and in Vitro Antimicrobial Evaluation of New N-Heterocyclic Diquaternary Pyridinium Compounds. Molecules 2014, 19, 11572–11585. [Google Scholar] [CrossRef]
- Stachowicz, J.; Krajewska-Kulak, E.; Lukaszuk, C.; Niewiadomy, A. Relationship between Antifungal Activity against Candida Albicans and Electron Parameters of Selected N-Heterocyclic Thioamides. Indian J. Pharm. Sci. 2014, 76, 287–298. [Google Scholar]
- Zou, T.; Lok, C.N.; Wan, P.K.; Zhang, Z.F.; Fung, S.K.; Che, C.M. Anticancer Metal-N-Heterocyclic Carbene Complexes of Gold, Platinum and Palladium. Curr. Opin. Chem. Biol. 2018, 43, 30–36. [Google Scholar] [CrossRef]
- Yaqoob, M.; Gul, S.; Zubair, N.F.; Iqbal, J.; Iqbal, M.A. Theoretical Calculation of Selenium N-Heterocyclic Carbene Compounds through DFT Studies: Synthesis, Characterization and Biological Potential. J. Mol. Struct. 2020, 1204, 127462. [Google Scholar] [CrossRef]
- Soto-Sánchez, J.; Ospina-Villa, J.D. Current Status of Quinoxaline and Quinoxaline 1,4-Di-N-Oxides Derivatives as Potential Antiparasitic Agents. Chem. Biol. Drug Des. 2021, 98, 683–699. [Google Scholar] [CrossRef] [PubMed]
- Keri, R.S.; Hiremathad, A.; Budagumpi, S.; Nagaraja, B.M. Comprehensive Review in Current Developments of Benzimidazole-Based Medicinal Chemistry. Chem. Biol. Drug Des. 2015, 86, 799–845. [Google Scholar] [CrossRef] [PubMed]
- Woolley, D.W. Some Biological Effects Produced By Benzimidazole and Their Reversal By Purines. J. Biol. Chem. 1944, 152, 225–232. [Google Scholar] [CrossRef]
- Vasantha, K.; Basavarajaswamy, G.; Vaishali Rai, M.; Boja, P.; Pai, V.R.; Shruthi, N.; Bhat, M. Rapid ‘One-Pot’ Synthesis of a Novel Benzimidazole-5-Carboxylate and Its Hydrazone Derivatives as Potential Anti-Inflammatory and Antimicrobial Agents. Bioorg. Med. Chem. Lett. 2015, 25, 1420–1426. [Google Scholar] [CrossRef]
- Gaba, M.; Gaba, P.; Uppal, D.; Dhingra, N.; Bahia, M.S.; Silakari, O.; Mohan, C. Benzimidazole Derivatives: Search for GI-Friendly Anti-Inflammatory Analgesic Agents. Acta Pharm. Sin. B 2015, 5, 337–342. [Google Scholar] [CrossRef]
- Acar Çevik, U.; Kaya Çavuşoğlu, B.; Sağlık, B.N.; Osmaniye, D.; Levent, S.; Ilgın, S.; Özkay, Y.; Kaplancıklı, Z.A. Synthesis, Docking Studies and Biological Activity of New Benzimidazole-Triazolothiadiazine Derivatives as Aromatase Inhibitor. Molecules 2020, 25, 1642. [Google Scholar] [CrossRef]
- Thamban Chandrika, N.; Shrestha, S.K.; Ranjan, N.; Sharma, A.; Arya, D.P.; Garneau-Tsodikova, S. New Application of Neomycin B–Bisbenzimidazole Hybrids as Antifungal Agents. ACS Infect. Dis. 2018, 4, 196–207. [Google Scholar] [CrossRef]
- Dokla, E.M.E.; Abutaleb, N.S.; Milik, S.N.; Kandil, E.A.; Qassem, O.M.; Elgammal, Y.; Nasr, M.; McPhillie, M.J.; Abouzid, K.A.M.; Seleem, M.N.; et al. SAR Investigation and Optimization of Benzimidazole-Based Derivatives as Antimicrobial Agents against Gram-Negative Bacteria. Eur. J. Med. Chem. 2023, 247, 115040. [Google Scholar] [CrossRef]
- Gaba, M.; Singh, S.; Mohan, C. Benzimidazole: An Emerging Scaffold for Analgesic and Anti-Inflammatory Agents. Eur. J. Med. Chem. 2014, 76, 494–505. [Google Scholar] [CrossRef]
- Prieto-Martínez, F.D.; Peña-Castillo, A.; Méndez-Lucio, O.; Fernández-de Gortari, E.; Medina-Franco, J.L. Molecular Modeling and Chemoinformatics to Advance the Development of Modulators of Epigenetic Targets. In Advances in Protein Chemistry and Structural Biology; Elsevier: Amsterdam, The Netherlands, 2016; pp. 1–26. [Google Scholar]
- Lin, S.; Yang, L. A Simple and Efficient Procedure for the Synthesis of Benzimidazoles Using Air as the Oxidant. Tetrahedron Lett. 2005, 46, 4315–4319. [Google Scholar] [CrossRef]
- Yadav, G.; Ganguly, S. Structure Activity Relationship (SAR) Study of Benzimidazole Scaffold for Different Biological Activities: A Mini-Review. Eur. J. Med. Chem. 2015, 97, 419–443. [Google Scholar] [CrossRef]
- Sluiter, J.; Christoffers, J. Synthesis of 1-Methylbenzimidazoles from Carbonitriles. Synlett 2009, 2009, 63–66. [Google Scholar] [CrossRef]
- Lu, J.; Yang, B.; Bai, Y. Microwave Irradiation Synthesis of 2-Substituted Benzimidazoles Using PPA as a Catalyst under Solvent-Free Conditions. Synth. Commun. 2002, 32, 3703–3709. [Google Scholar] [CrossRef]
- El-All, A.S.A.; Ragab, F.A.F.; El-Din, A.A.M.; Abdalla, M.M.; El-Hefnawi, M.M.; El-Rashedy, A.A. Design, Synthesis and Anticancer Evaluation of Some Selected Schiff Bases Derived from Benzimidazole Derivative. Glob. J. Pharmacol. 2013, 7, 143–152. [Google Scholar] [CrossRef]
- Tarı, Ö.; Gümüş, F.; Açık, L.; Aydın, B. Synthesis, Characterization and DNA Binding Studies of Platinum(II) Complexes with Benzimidazole Derivative Ligands. Bioorg. Chem. 2017, 74, 272–283. [Google Scholar] [CrossRef] [PubMed]
- Desai, N.C.; Pandya, D.; Vaja, D. Synthesis and Antimicrobial Activity of Some Heterocyclic Compounds Bearing Benzimidazole and Pyrazoline Motifs. Med. Chem. Res. 2018, 27, 52–60. [Google Scholar] [CrossRef]
- Pages, B.J.; Ang, D.L.; Wright, E.P.; Aldrich-Wright, J.R. Metal Complex Interactions with DNA. Dalton Trans. 2015, 44, 3505–3526. [Google Scholar] [CrossRef]
- Giaccone, G. Clinical Perspectives on Platinum Resistance. Drugs 2000, 59, 9–17. [Google Scholar] [CrossRef]
- Çevik, U.A.; Osmaniye, D.; Çavuşoğlu, B.K.; Sağlik, B.N.; Levent, S.; Ilgin, S.; Can, N.Ö.; Özkay, Y.; Kaplancikli, Z.A. Synthesis of Novel Benzimidazole–Oxadiazole Derivatives as Potent Anticancer Activity. Med. Chem. Res. 2019, 28, 2252–2261. [Google Scholar] [CrossRef]
- El-Gohary, N.S.; Shaaban, M.I. Synthesis and Biological Evaluation of a New Series of Benzimidazole Derivatives as Antimicrobial, Antiquorum-Sensing and Antitumor Agents. Eur. J. Med. Chem. 2017, 131, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Narasimhan, B.; Lim, S.M.; Ramasamy, K.; Vasudevan, M.; Shah, S.A.A.; Mathur, A. Synthesis and Evaluation of Antimicrobial, Antitubercular and Anticancer Activities of Benzimidazole Derivatives. Egypt. J. Basic Appl. Sci. 2018, 5, 100–109. [Google Scholar] [CrossRef]
- Chandrika, N.T.; Shrestha, S.K.; Ngo, H.X.; Garneau-Tsodikova, S. Synthesis and Investigation of Novel Benzimidazole Derivatives as Antifungal Agents. Bioorg. Med. Chem. 2016, 24, 3680–3686. [Google Scholar] [CrossRef]
- Dane, F.; Dalgiç, Ö. The Effects of Fungicide Benomyl (Benlate) on Growth and Mitosis in Onion (Allium Cepa L.) Root Apical Meristem. Acta Biol. Hung. 2005, 56, 119–128. [Google Scholar] [CrossRef]
- Si, W.J.; Wang, X.B.; Chen, M.; Wang, M.Q.; Lu, A.M.; Yang, C.L. Design, Synthesis, Antifungal Activity and 3D-QSAR Study of Novel Pyrazole Carboxamide and Niacinamide Derivatives Containing Benzimidazole Moiety. New J. Chem. 2019, 43, 3000–3010. [Google Scholar] [CrossRef]
- Gullapelli, K.; Brahmeshwari, G.; Ravichander, M.; Kusuma, U. Synthesis, Antibacterial and Molecular Docking Studies of New Benzimidazole Derivatives. Egypt. J. Basic Appl. Sci. 2017, 4, 303–309. [Google Scholar] [CrossRef]
- Küçükbay, H.; Durmazt, R.; Sirecitt, N.; Günalt, S. Synthesis of Some Benzimidazole Derivatives and Their Antibacterial and Antifungal Activities. Asian J. Chem. 2009, 21, 6181–6189. [Google Scholar]
- Wen, J.; Luo, Y.L.; Zhang, H.Z.; Zhao, H.H.; Zhou, C.H.; Cai, G.X. A Green and Convenient Approach toward Benzimidazole Derivatives and Their Antimicrobial Activity. Chin. Chem. Lett. 2016, 27, 391–394. [Google Scholar] [CrossRef]
- Gowda, J.; Khader, A.M.A.; Kalluraya, B.; Hidayathulla, S. Synthesis, Characterization and Antibacterial Activity of Benzimidazole Derivatives Carrying Quinoline Moiety. Indian J. Chem. Sect. B 2011, 50, 1491–1495. [Google Scholar] [CrossRef]
- Mishra, V.R.; Ghanavatkar, C.W.; Mali, S.N.; Qureshi, S.I.; Chaudhari, H.K.; Sekar, N. Design, Synthesis, Antimicrobial Activity and Computational Studies of Novel Azo Linked Substituted Benzimidazole, Benzoxazole and Benzothiazole Derivatives. Comput. Biol. Chem. 2019, 78, 330–337. [Google Scholar] [CrossRef]
- Chhonker, Y.S.; Veenu, B.; Hasim, S.R.; Kaushik, N.; Kumar, D.; Kumar, P. Synthesis and Pharmacological Evaluation of Some New 2-Phenyl Benzimidazoles Derivatives and Their Schiff’s Bases. J. Chem. 2009, 6, 342–347. [Google Scholar] [CrossRef][Green Version]
- Kuş, C.; Sözüdönmez, F.; Altanlar, N. Synthesis and Antimicrobial Activity of Some Novel 2-[4-(Substituted Piperazin-/Piperidin-1-Ylcarbonyl)Phenyl]- 1 H-Benzimidazole Derivatives. Arch. Pharm. 2009, 342, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Saleh, O.R.; Shaldum, M.A.; Goda, R.M.; Shehata, I.A.; El-Ashmawy, M.B. Synthesis and Antibacterial Evaluation of New2-Phenylbenzimidazole Derivatives. ChemistrySelect 2019, 4, 10307–10315. [Google Scholar] [CrossRef]
- Frei, A.; Zuegg, J.; Elliott, A.G.; Baker, M.; Braese, S.; Brown, C.; Chen, F.; Dowson, C.G.; Dujardin, G.; Jung, N.; et al. Metal Complexes as a Promising Source for New Antibiotics. Chem. Sci. 2020, 11, 2627–2639. [Google Scholar] [CrossRef] [PubMed]
- Deo, K.M.; Ang, D.L.; McGhie, B.; Rajamanickam, A.; Dhiman, A.; Khoury, A.; Holland, J.; Bjelosevic, A.; Pages, B.; Gordon, C.; et al. Platinum Coordination Compounds with Potent Anticancer Activity. Coord. Chem. Rev. 2018, 375, 148–163. [Google Scholar] [CrossRef]
- Kenny, R.G.; Marmion, C.J. Toward Multi-Targeted Platinum and Ruthenium Drugs—A New Paradigm in Cancer Drug Treatment Regimens? Chem. Rev. 2019, 119, 1058–1137. [Google Scholar] [CrossRef]
- Paprocka, R.; Wiese-Szadkowska, M.; Janciauskiene, S.; Kosmalski, T.; Kulik, M.; Helmin-Basa, A. Latest Developments in Metal Complexes as Anticancer Agents. Coord. Chem. Rev. 2022, 452, 214307. [Google Scholar] [CrossRef]
- Aragón-Muriel, A.; Liscano-Martínez, Y.; Rufino-Felipe, E.; Morales-Morales, D.; Oñate-Garzón, J.; Polo-Cerón, D. Synthesis, Biological Evaluation and Model Membrane Studies on Metal Complexes Containing Aromatic N,O-Chelate Ligands. Heliyon 2020, 6, e04126. [Google Scholar] [CrossRef]
- Sabithakala, T.; Chittireddy, V.R.R. DNA Binding and in Vitro Anticancer Activity of 2-((1H-Benzimidazol-2-Yl)Methylamino)Acetic Acid and Its Copper(II) Mixed-Polypyridyl Complexes: Synthesis and Crystal Structure. Appl. Organomet. Chem. 2018, 32, e4550. [Google Scholar] [CrossRef]
- Wu, Y.C.; You, J.Y.; Jiang, K.; Wu, H.Q.; Xiong, J.F.; Wang, Z.Y. Novel Benzimidazole-Based Ratiometric Fluorescent Probes for Acidic PH. Dye. Pigment. 2018, 149, 1–7. [Google Scholar] [CrossRef]
- Sharaby, C.M.; Amine, M.F.; Hamed, A.A. Synthesis, Structure Characterization and Biological Activity of Selected Metal Complexes of Sulfonamide Schiff Base as a Primary Ligand and Some Mixed Ligand Complexes with Glycine as a Secondary Ligand. J. Mol. Struct. 2017, 1134, 208–216. [Google Scholar] [CrossRef]
- Kalarani, R.; Sankarganesh, M.; Kumar, G.G.V.; Kalanithi, M. Synthesis, Spectral, DFT Calculation, Sensor, Antimicrobial and DNA Binding Studies of Co(II), Cu(II) and Zn(II) Metal Complexes with 2-Amino Benzimidazole Schiff Base. J. Mol. Struct. 2020, 1206, 127725. [Google Scholar] [CrossRef]
- Ishak, N.N.M.; Jamsari, J.; Ismail, A.Z.; Tahir, M.I.M.; Tiekink, E.R.T.; Veerakumarasivam, A.; Ravoof, T.B.S.A. Synthesis, Characterisation and Biological Studies of Mixed-Ligand Nickel (II) Complexes Containing Imidazole Derivatives and Thiosemicarbazide Schiff Bases. J. Mol. Struct. 2019, 1198, 126888. [Google Scholar] [CrossRef]
- Apohan, E.; Yilmaz, U.; Yilmaz, O.; Serindag, A.; Küçükbay, H.; Yesilada, O.; Baran, Y. Synthesis, Cytotoxic and Antimicrobial Activities of Novel Cobalt and Zinc Complexes of Benzimidazole Derivatives. J. Organomet. Chem. 2017, 828, 52–58. [Google Scholar] [CrossRef]
- Aragón-Muriel, A.; Liscano, Y.; Upegui, Y.; Robledo, S.M.; Ramírez-Apan, M.T.; Morales-Morales, D.; Oñate-Garzón, J.; Polo-Cerón, D. In Vitro Evaluation of the Potential Pharmacological Activity and Molecular Targets of New Benzimidazole-Based Schiff Base Metal Complexes. Antibiotics 2021, 10, 728. [Google Scholar] [CrossRef]
- Sunitha, M.; Jogi, P.; Ushaiah, B.; Kumari, C.G. Synthesis, Characterization and Antimicrobial Activity of Transition Metal Complexes of Schiff Base Ligand Derived from 3-Ethoxy Salicylaldehyde and 2-(2-Aminophenyl) 1-H-Benzimidazole. J. Chem. 2012, 9, 2516–2523. [Google Scholar] [CrossRef]
- Utku, S.; Topal, M.; Dogen, A.; Serin, M.S. Synthesis, Characterization, Antibacterial and Antifungal Evaluation of Some New Platinum(II) Complexes of 2-Phenylbenzimidazole Ligands. Turk. J. Chem. 2010, 34, 427–436. [Google Scholar] [CrossRef]
- Daisylet, B.S.; Raphael, S.J.; Kumar, P.; Mohan, A.; Dasan, A. Exploring Photocatalytic, Antimicrobial, and Biofilm Inhibition Properties of Cuminaldehyde Derived Benzimidazole Metal Complexes. J. Mol. Struct. 2025, 1328, 141311. [Google Scholar] [CrossRef]
- Masaryk, L.; Tesarova, B.; Choquesillo-Lazarte, D.; Milosavljevic, V.; Heger, Z.; Kopel, P. Structural and Biological Characterization of Anticancer Nickel(II) Bis(Benzimidazole) Complex. J. Inorg. Biochem. 2021, 217, 111395. [Google Scholar] [CrossRef]
- G, P.; Revanasiddappa, H.D.; B, J.; T, P.B.; Shivamallu, C.; Viswanath, P.M.; Achar, R.R.; Silina, E.; Stupin, V.; Manturova, N.; et al. Novel Benzimidazole Derived Imine Ligand and Its Co(III) and Cu(II) Complexes as Anticancer Agents: Chemical Synthesis, DFT Studies, In Vitro and In Vivo Biological Investigations. Pharmaceuticals 2023, 16, 125. [Google Scholar] [CrossRef]
- Ajibola, A.A.; Perveen, F.; Jan, K.; Anibijuwon, I.I.; Shaibu, S.E.; Sieroń, L.; Maniukiewicz, W. A Five-Coordinate Copper(II) Complex Constructed from Sterically Hindered 4-Chlorobenzoate and Benzimidazole: Synthesis, Crystal Structure, Hirshfeld Surface Analysis, DFT, Docking Studies and Antibacterial Activity. Crystals 2020, 10, 991. [Google Scholar] [CrossRef]
- Nguyen, V.-T.; Huynh, T.-K.-C.; Ho, G.-T.-T.; Nguyen, T.-H.-A.; Le Anh Nguyen, T.; Dao, D.Q.; Mai, T.V.T.; Huynh, L.K.; Hoang, T.-K.-D. Metal Complexes of Benzimidazole-Derived as Potential Anti-Cancer Agents: Synthesis, Characterization, Combined Experimental and Computational Studies. R. Soc. Open Sci. 2022, 9, 220659. [Google Scholar] [CrossRef] [PubMed]
- Baas, J.; Schotten, M.; Plume, A.; Côté, G.; Karimi, R. Scopus as a Curated, High-Quality Bibliometric Data Source for Academic Research in Quantitative Science Studies. Quant. Sci. Stud. 2020, 1, 377–386. [Google Scholar] [CrossRef]
- Maret, W. The Metals in the Biological Periodic System of the Elements: Concepts and Conjectures. Int. J. Mol. Sci. 2016, 17, 66. [Google Scholar] [CrossRef]
- Tao, W.; Zhu, C.; Xu, Q.; Li, S.; Xiong, X.; Cheng, H.; Zou, X.; Lu, X. Electronic Structure and Oxidation Mechanism of Nickel–Copper Converter Matte from First-Principles Calculations. ACS Omega 2020, 5, 20090–20099. [Google Scholar] [CrossRef] [PubMed]
- Vijayan, N.; Princess, R.; Raja, S.J.; Joseph, J. Synthesis, Structural Elucidation, Pharmacological and Molecular Docking Studies of Terpolymer Transition Metal Complexes. J. Mol. Struct. 2021, 1227, 129424. [Google Scholar] [CrossRef]
- Selvaganapathy, M.; Raman, N. Pharmacological Activity of a Few Transition Metal Complexes: A Short Review. J. Chem. Biol. Ther. 2016, 01, 1000108. [Google Scholar] [CrossRef]
- Sahoo, S.C.; Kataria, R.; Mehta, S.K. Copper and Its Complexes: A Pharmaceutical Perspective. In Chemical Drug Design; De Gruyter: Berlin, Germany, 2016; pp. 215–241. [Google Scholar] [CrossRef]
- Haribabu, J.; Jeyalakshmi, K.; Arun, Y.; Bhuvanesh, N.S.P.; Perumal, P.T.; Karvembu, R. Synthesis, DNA/Protein Binding, Molecular Docking, DNA Cleavage and in Vitro Anticancer Activity of Nickel(Ii) Bis(Thiosemicarbazone) Complexes. RSC Adv. 2015, 5, 46031–46049. [Google Scholar] [CrossRef]
- Maru, M.S.; Shah, M.K. Synthesis, Characterization and Antimicrobial Evaluation of Transition Metal Complexes of Monodentate 2-(Substituted Phenyl) -1H-Benzo[d]Imidazoles. Chiang Mai J. Sci. 2015, 42, 217. [Google Scholar]
- Mahmood, K.; Hashmi, W.; Ismail, H.; Mirza, B.; Twamley, B.; Akhter, Z.; Rozas, I.; Baker, R.J. Synthesis, DNA Binding and Antibacterial Activity of Metal(II) Complexes of a Benzimidazole Schiff Base. Polyhedron 2019, 157, 326–334. [Google Scholar] [CrossRef]
- Ashraf, A.; Siddiqui, W.A.; Akbar, J.; Mustafa, G.; Krautscheid, H.; Ullah, N.; Mirza, B.; Sher, F.; Hanif, M.; Hartinger, C.G. Metal Complexes of Benzimidazole Derived Sulfonamide: Synthesis, Molecular Structures and Antimicrobial Activity. Inorganica Chim. Acta 2016, 443, 179–185. [Google Scholar] [CrossRef]
- Lewis, A.; McDonald, M.; Scharbach, S.; Hamaway, S.; Plooster, M.; Peters, K.; Fox, K.M.; Cassimeris, L.; Tanski, J.M.; Tyler, L.A. The Chemical Biology of Cu(II) Complexes with Imidazole or Thiazole Containing Ligands: Synthesis, Crystal Structures and Comparative Biological Activity. J. Inorg. Biochem. 2016, 157, 52–61. [Google Scholar] [CrossRef]
- Kilic, A.; Tas, E.; Yilmaz, I. Synthesis, Spectroscopic and Redox Properties of the Mononuclear. J. Chem. Sci. 2009, 121, 43–56. [Google Scholar] [CrossRef]
- Abd-Elzaher, M.M. Synthesis, Characterization, and Antimicrobial Activity of Cobalt(II), Nickel(II), Copper(II) and Zinc(II) Complexes with Ferrocenyl Schiff Bases Containing a Phenol Moiety. Appl. Organomet. Chem. 2004, 18, 149–155. [Google Scholar] [CrossRef]
- Roopashree, B.; Gayathri, V.; Mukund, H. Synthesis, Characterization, and Biological Activities of Zinc, Cadmium, Copper, and Nickel Complexes Containing Meta -Aminophenyl Benzimidazole. J. Coord. Chem. 2012, 65, 1354–1370. [Google Scholar] [CrossRef]
- El-Sayed, Y.S.; Gaber, M.; El-Nahass, M.N. Structural Elucidation, Spectroscopic, and Metalochromic Studies of 2-(2-Hydroxy Phenyl)-1-H–Benzimidazole Complexes: Metal Ions Sensing, DNA Binding, and Antimicrobial Activity Evaluation. J. Mol. Struct. 2021, 1229, 129809. [Google Scholar] [CrossRef]
- Tavman, A.; Ikiz, S.; Baǧcigil, A.F.; Özgür, N.Y.; AK, S. Synthesis, Characterization, and Antibacterial Effect of 4-Methoxy-2-(5-H/Me/Cl/NO2 -1H-Benzimidazol-2-Yl)-Phenols and Some Transition Metal Complexes. Turk. J. Chem. 2009, 33, 321–331. [Google Scholar] [CrossRef]
- Tavman, A.; Çinarli, A.; Gürbüz, D.; Birteksöz, A.S. Synthesis, Characterization and Antimicrobial Activity of 2-(5-H/ Me/F/Cl/NO2-1H-Benzimidazol-2-Yl)-Benzene-1,4-Diols and Some Transition Metal Complexes. J. Iran. Chem. Soc. 2012, 9, 815–825. [Google Scholar] [CrossRef]
- Tavman, A.; Agh-Atabay, N.M.; Neshat, A.; Gucin, F.; Dulger, B.; Haciu, D. Structural Characterization and Antimicrobial Activity of 2-(5-H/Methyl-1H-Benzimidazol-2-Yl)-4-Bromo/Nitro-Phenol Ligands and Their Fe(NO3)3 Complexes. Transit. Met. Chem. 2006, 31, 194–200. [Google Scholar] [CrossRef]
- Leovac, V.M.; Jovanović, L.S.; Češljević, V.I.; Bjelica, L.J.; Arion, V.B.; Gerbeleu, N.V. Transition metal complexes with the thiosemicarbazide-based ligands—XXIII. Synthesis, physicochemical properties and voltammetric characterization of iron(III) complexes with terdentate and quadridentate thiosemicarbazide derivatives. Polyhedron 1994, 13, 3005–3014. [Google Scholar] [CrossRef]
- Tavman, A.; Ikiz, S.; Bagcigil, A.F.; Özgür, N.Y.; Ak, S. Preparation, Characterization and Antibacterial Effect of 2-Methoxy-6-(5-H/Me/Cl/NO2-1H-Benzimidazol-2-Yl)Phenols and Some Transition Metal Complexes. J. Serbian Chem. Soc. 2009, 74, 537–548. [Google Scholar] [CrossRef]
- Padalkar, V.S.; Patil, V.S.; Gupta, V.D.; Phatangare, K.R.; Umape, P.G.; Sekar, N. Synthesis, Characterization, Thermal Properties, and Antimicrobial Activities of 5-(Diethylamino)-2-(5-Nitro-1 H-Benzimidazol-2-Yl)Phenol and Its Transition Metal Complexes. Int. Sch. Res. Not. Org. Chem. 2011, 2011, 738361. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tavman, A.; Boz, I.; Seher Birteksöz, A.; Cinarli, A. Spectral Characterization and Antimicrobial Activity of Cu(II) and Fe(III) Complexes of 2-(5-Cl/NO2-1H-Benzimidazol-2-Yl)-4-Br/NO2-Phenols. J. Coord. Chem. 2010, 63, 1398–1410. [Google Scholar] [CrossRef]
- Tavman, A.; Seher Birteksoz, A.; Oksuzomer, F. Spectral and Thermal Characterization and Antimicrobial Effect of 3-(5-H/Me/Cl/NO2-1H-Benzimidazol-2-Yl)-Benzene-1,2-Diols and Some Transition Metal Complexes. S. Afr. J. Chem. 2012, 65, 150–158. [Google Scholar]
- Kumar, J.; Rai, A.; Raj, V. A Comprehensive Review on the Pharmacological Activity of Schiff Base Containing Derivatives. Org. Med. Chem. 2017, 1, 88–102. [Google Scholar] [CrossRef]
- Liu, X.; Hamon, J.R. Recent Developments in Penta-, Hexa- and Heptadentate Schiff Base Ligands and Their Metal Complexes. Coord. Chem. Rev. 2019, 389, 94–118. [Google Scholar] [CrossRef]
- Li, H.C.; Zheng, Y.F.; Wang, M.Z.; Geng, C.C.; Hou, M.; Liu, C.Q.; Li, W.G. Synthesis, Crystal Structures and Anticancer Studies of Ni (II) and Co (III) Complexes Based on 2-(2-Aminophenyl)-1H-Benzimidazole Schiff Base Derivatives. Inorganica Chim. Acta 2025, 577, 122506. [Google Scholar] [CrossRef]
- Hou, M.; Li, H.C.; An, N.; Li, W.G.; Tong, J. Synthesis, Structure and Anticancer Studies of Cu (II), Ni (II) and Co (II) Complexes Based on 2,3-Dihydroxybenzaldehyde-2-(2-Aminophenyl)Benzimidazole Schiff Base. Arab. J. Chem. 2023, 16, 105144. [Google Scholar] [CrossRef]
- Hou, M.; Li, H.C.; An, N.; Pang, S.Y.; Li, W.G.; Tong, J. Synthesis, Crystal Structures and Anticancer Studies of Ni (II), Co (III) and Zn (II) Complexes Based on 5-Bromosalicylaldehyde-2-(2-Aminophenyl)Benzimidazole Schiff Base. J. Mol. Struct. 2023, 1294, 136500. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Österreichische Pharmazeutische Gesellschaft. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).