Abstract
Background: We evaluated the outcome of PCI of de novo stenosis with drug-coated balloons (DCB) versus drug-eluting stents (DES) in patients with insulin-treated diabetes mellitus (ITDM) versus non-insulin-treated diabetes mellitus (NITDM). Methods: Patients were randomized in the BASKET-SMALL 2 trial to DCB or DES and followed over 3 years for MACE (cardiac death, non-fatal myocardial infarction [MI], and target vessel revascularization [TVR]). Outcome in the diabetic subgroup (n = 252) was analyzed with respect to ITDM or NITDM. Results: In NITDM patients (n = 157), rates of MACE (16.7% vs. 21.9%, hazard ratio [HR] 0.68, 95% confidence interval [CI] 0.29–1.58, p = 0.37), death, non-fatal MI, and TVR (8.4% vs. 14.5%, HR 0.30, 95% CI 0.09–1.03, p = 0.057) were similar between DCB and DES. In ITDM patients (n = 95), rates of MACE (DCB 23.4% vs. DES 22.7%, HR 1.12, 95% CI 0.46–2.74, p = 0.81), death, non-fatal MI, and TVR (10.1% vs. 15.7%, HR 0.64, 95% CI 0.18–2.27, p = 0.49) were similar between DCB and DES. TVR was significantly lower with DCB versus DES in all diabetic patients (HR 0.41, 95% CI 0.18–0.95, p = 0.038). Conclusions: DCB compared to DES for treatment of de novo coronary lesions in diabetic patients was associated with similar rates of MACE and numerically lower need for TVR both for ITDM and NITDM patients.
1. Introduction
Patients with diabetes mellitus and coronary artery disease often present with a combination of diffuse coronary lesions and small vessel disease associated with multiple comorbidities increasing the bleeding risk, which inadvertently impacts overall outcomes after percutaneous coronary intervention (PCI). Lesions in diabetic patients consist of lipid-laden plaques with thin fibrous capsules and high calcium content, which increases their vulnerability for rupture and repeat thromboembolic events. In addition, vascular resistance based on alterations of the molecular interaction in smooth muscle cells limits the action of anti-proliferative agents eluted by coronary stents [1]. Hence, treatment with drug-eluting stents (DES) may be associated with technical difficulties in stent delivery and expansion in those diffusely diseased calcified lesions in small vessels and is associated with an increased risk of stent thrombosis and the need for revascularization.
For de novo lesions in small coronary arteries, several trials have shown similar results with the use of drug-coated balloons (DCB) compared to DES [2,3,4]. A possible advantage with the use of DCBs is an antirestenotic efficacy which is associated with the potential for late lumen enlargement in the absence of a metallic stent [5]. Since there is no permanent vascular implant, the risk of late or very late stent thrombosis is eliminated and the need for dual antiplatelet therapy (DAPT) in stable patients can be limited to 4 weeks [2], reducing the bleeding risk.
However, the risk of restenosis, myocardial infarction and stent thrombosis is increased in diabetic compared to non-diabetic patients, both for treatment with DES and DCB [6,7,8,9]. PCI with DES in patients with insulin-treated diabetes mellitus (ITDM) is associated with a significantly higher target lesion failure rate and higher need for revascularization compared to PCI in patients with non-insulin-treated diabetes mellitus (NITDM) [10,11,12,13,14]. Whether the outcome of PCI of de novo coronary artery disease using DCB or DES in patients with ITDM or NITDM is different has not been studied so far.
We analyzed the impact of insulin-treated compared to non-insulin-treated diabetes mellitus in patients undergoing PCI of a de novo lesion on the outcome of drug-coated balloons versus drug-eluting stents in the diabetic population of the randomized BASKET-SMALL 2 trial.
2. Materials and Methods
2.1. Study Design
BASKET-SMALL 2 [15] is an investigator-initiated, randomized, open-label, non-inferiority trial demonstrating a similar efficacy and safety for DCB compared with DES for 3 years in 758 patients with de novo lesions in coronary vessels < 3 mm [2,3]. This subgroup analysis compares the efficacy and safety within 1, 2 and 3 years between patients with NITDM and ITDM.
2.2. Study Population and Randomization
Patients were eligible for the study when they had an indication for PCI and a suitable angiographic anatomy in a small coronary vessel with a diameter between 2 and 3 mm. Successful predilatation of the lesion, i.e., absence of higher grade dissections (National Heart, Lung, and Blood Institute grade C to F), decreased blood flow (thrombolysis in myocardial infarction score ≤ 2), or residual stenosis > 30% was mandatory [16]. At the time of randomization, diabetes mellitus was defined as history of the disease including ITDM status based on insulin treatment. Exclusion criteria included a concomitant PCI of lesions ≥ 3 mm in diameter in the same epicardial coronary artery, PCI of in-stent restenosis, life expectancy of <12 months, pregnancy, enrollment in another randomized trial, or inability to give informed consent. Patients were randomized 1:1 to be treated by either DCB or DES.
2.3. Procedures
Patients randomized to DCB were treated with the paclitaxel-coated SeQuent Please or SeQuent Please Neo balloon (B. Braun Melsungen AG, Melsungen, Germany), while patients randomized to DES were treated with either the everolimus-eluting Xience stent (Abbott Vascular, Santa Clara, CA, USA) or the paclitaxel-eluting Taxus Element stent (Boston Scientific, Natick, MA, USA) (3, 4, 15). The strut thickness of both DES was 81 μm. The DCB needed to be 2 to 3 mm longer on each side than the predilatation balloon to avoid geographical mismatch, and they were inflated at a nominal pressure for at least 30 s, as recommended in the consensus documents [16]. When there were flow-limiting dissections after DCB treatment despite an acceptable result after lesion preparation, stent implantation was performed. After PCI, DAPT was given using acetylsalicylic acid (100 mg per day) and either clopidogrel (75 mg per day), prasugrel (10 mg per day), or ticagrelor (90 mg twice per day); DAPT was continued in stable patients for 4 weeks for DCB or 6 months for DES and in patients with acute coronary syndromes for 12 months. Follow-up was conducted after 12, 24 and 36 months with structured clinical questionnaires or phone calls to assess clinical events and medication. Patients were followed for a median of 3 years.
2.4. Outcomes
The primary endpoint is major adverse cardiac events (MACE) defined as the composite of cardiac death, non-fatal myocardial infarction, and target vessel revascularization (TVR). Cardiac death was defined as any death without a clear cardiac reason, and myocardial infarction was defined according to guidelines [17]. Secondary endpoints are the single components of the primary endpoint according to the Academic Research Consortium definition [18]. An independent critical events committee adjudicated all endpoints.
2.5. Statistical Analysis
All statistical analyses were performed according to the intention-to-treat principle, i.e., all patients were analyzed on the basis of the treatment they were randomly allocated to. All analyses were conducted with the statistical software package R, using “two-sided” statistical tests and confidence intervals, without correction for multiple testing. Categorical data are presented as frequencies and percentages with the difference between study arms analyzed by Pearson’s chi-squared test. For numerical variables, the mean and standard deviation, or the median and interquartile range, are presented as appropriate, with the difference between study arms analyzed using Student’s t-test or the Wilcoxon–Mann–Whitney test, respectively. For each endpoint, treatment effects on the times to event were tested by Cox regressions (with study center as a stratifying factor to account for differences in baseline hazards between study centers). The Kaplan–Meier estimates of the event rates in both study arms are reported along with the corresponding hazard ratios (HR) and 95% confidence intervals (CI). The proportional hazards assumption of the Cox models and the homogeneity of the treatment effects among study centers were checked by testing the correlation of the scaled Schoenfeld residuals with time and the interaction of the stratifying factor study center with treatment in the Cox models, respectively. Endpoints of patients not experiencing an event were considered as censored on the last observation date.
3. Results
Out of the 758 randomized patients, 252 (33.2%) were diabetic and 506 (66.8%) non-diabetic. In the diabetic subgroup, there were 95 (37.7%) patients with ITDM and 157 (62.3%) patients with NITDM. TVR was significantly lower with DCB versus DES in all diabetic patients (HR 0.41, 95% CI 0.18–0.95, p = 0.038).
Baseline characteristics between patients with ITDM or NITDM are depicted in Table 1. ITDM patients compared to NITDM patients had a significantly higher body mass index and a significantly higher frequency of renal dysfunction, while other parameters such as hypercholesterolemia, hypertension, previous MI, previous PCI, or antiplatelet therapy were well balanced between the groups.

Table 1.
Baseline Characteristics.
Table 2 shows the Kaplan–Meier estimates of event rates between the two study arms (DES versus DCB) within the subgroup of patients with ITDM and NITDM for MACE and each single endpoint at one, two, and three years of follow-up. In patients with ITDM, rates of MACE, cardiac death, non-fatal MI, TVR, and all-cause death were statistically not different between patients treated with DCB or DES for up to three years of follow-up. Event rates for TVR were lower in patients treated with DCB compared to DES at all follow-up timepoints. Based on the 3-year TVR rate, the number needed to treat to prevent one additional TVR would be 18 in ITDM.

Table 2.
Comparison of event numbers and Kaplan–Meier estimates of event rates between the two study arms within each subgroup for all endpoints.
In the population with NITDM, the rates of MACE, cardiac death, non-fatal MI, TVR, and all-cause death were statistically not different between patients treated with DCB or DES at one, two and three years of follow-up. In NITDM patients, there was a trend toward a lower TVR rate in patients treated with DCB compared to patients treated with DES at two years (DES versus DCB 11.6% vs. 4.5%, p = 0.063) and three years (DES versus DCB 14.5% vs. 8.4%, p = 0.057) of follow-up. Based on the 3-year TVR rate, the number needed to treat to prevent one additional TVR would be 16 in NITDM.
Figure 1 details the Kaplan–Meier estimates of the cumulative probabilities of MACE (panel A), non-fatal MI (panel B), TVR (panel C), and all-cause death (panel D) during three years in the four combinations of subgroup (ITDM or NITDM) and study arm (DCB or DES). MACE occurred more often in ITDM compared to NITDM patients.
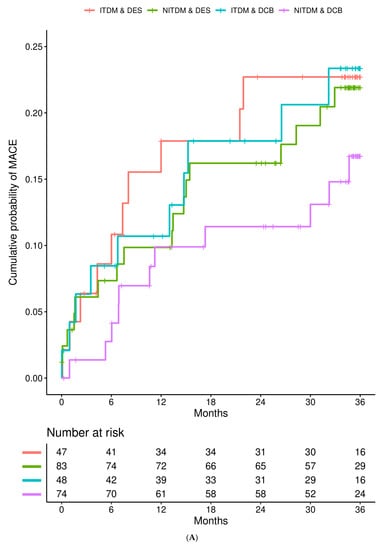
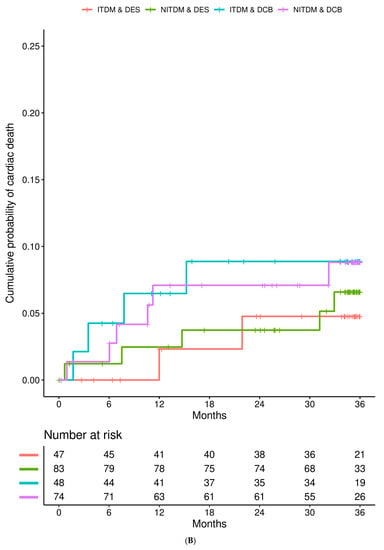
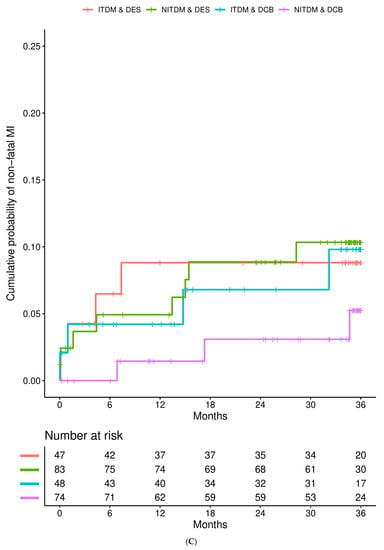
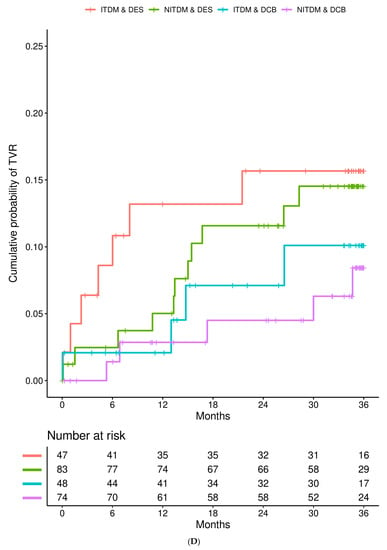
Figure 1.
Interaction is presented for diabetes mellitus and randomized treatment strategy. (A) Kaplan–Meier estimates of the cumulative probabilities of major adverse cardiac events (MACE) during three years in the four combinations of subgroup and study arm (DES = drug eluting stent, DCB = drug coated balloon, NITDM = non-insulin-treated diabetes mellitus, ITDM = insulin-treated diabetes mellitus); (B) Kaplan–Meier estimates of the cumulative probabilities of non-fatal myocardial infarction during three years in the four combinations of subgroup and study arm (DES = drug eluting stent, DCB = drug coated balloon, NITDM = non-insulin-treated diabetes mellitus, ITDM = insulin-treated diabetes mellitus); (C) Kaplan–Meier estimates of the cumulative probabilities of target vessel revascularization (TVR) during three years in the four combinations of subgroup and study arm (DES = drug eluting stent, DCB = drug coated balloon, NITDM = non-insulin-treated diabetes mellitus, ITDM = insulin-treated diabetes mellitus); (D) Kaplan–Meier estimates of the cumulative probabilities of all-cause death during three years in the four combinations of subgroup and study arm (DES = drug eluting stent, DCB = drug coated balloon, NITDM = non-insulin-treated diabetes mellitus, ITDM = insulin-treated diabetes mellitus).
Cox regression analysis stratified by study center with interaction of treatment showed that there was no interaction between ITDM and NITDM and randomized treatment (DCB or DES) with respect to MACE, cardiac death, non-fatal MI, TVR, and all-cause death for all follow-up timepoints (Table 3). In addition, the risk of MACE, non-fatal MI, TVR, and all-cause death was (statistically not significant) lower for patients with NITDM compared to ITDM patients. Risk of TVR was significantly lower with the use of DCB compared to the use of DES after two (0.36 [0.14, 0.94], p = 0.037) and three (0.41 [0.18–0.95], p = 0.038) years of follow-up.

Table 3.
Cox regressions stratified by study center with and without interaction with treatment.
4. Discussion
In this subgroup analysis of the randomized BASKET-SMALL 2 trial, we were able to demonstrate that (1) the risk of MACE, death, non-fatal MI, and TVR is higher in patients with ITDM compared to NITDM for up to three years of follow-up; (2) in patients with ITDM compared to patients with NITDM, rates of MACE, cardiac death, non-fatal MI, TVR, and all-cause death were similar between DCB and DES; and (3) TVR rate was numerically lower with DCB compared to DES both in ITDM and NITDM patients for up to three years of follow-up.
Diabetes, particularly in patients taking insulin, has consistently been shown to be an independent predictor of adverse outcomes after DES. The event rates for diabetic patients on insulin have progressively worse clinical outcomes than the rates for diabetic patients not taking insulin [10,11,12,13,14,19]. Thus, the overall results in analysis of patients with diabetes mellitus strongly depend on the percentage of patients with diabetes on insulin. There is a substantial amount of data comparing the outcome after DES implantation in patients with NITDM versus ITDM, whereas this topic has not been studied in the context of DCB.
For patients treated with DES, a systematic review and meta-analysis of 21,759 ITDM and 15,509 NITDM patients reports significantly higher short and long-term adverse cardiovascular outcomes after PCI in ITDM patients compared with NITDM patients [20]. It is well studied for PCI with DES in diabetic patients that treatment with insulin is associated with a significantly higher target lesion failure rate and higher need for revascularization compared to PCI in diabetic patients with NITDM [10,11,12,13,14,21,22]. At present, there are no data comparing the outcome of DCB versus DES in diabetic patients with de novo coronary artery disease differentiated in NITDM and ITDM. Studies comparing DCB with DES in de novo coronary artery disease in small vessels showed similar results with the use of DCB compared to DES [4,23,24]. In the RESTORE Small Vessel Disease China trial [4], 230 patients with de novo coronary artery disease within a 2.25- and 2.75-mm reference diameter were randomized to DCB or DES in a 1:1 ratio. After 2 years of clinical follow-up, the rate of target lesion failure and target lesion revascularization was similar between the DCB and DES population. Diabetes mellitus was present in 41% of the population, underscoring the importance of diabetes mellitus in patients with de novo lesions in small vessels. Results were not separately given for patients with diabetes mellitus and for patients needing insulin. In a propensity score analysis of 1156 matched patients undergoing PCI with DCB, patients without diabetes mellitus had a significantly lower need for target lesion revascularization during the mean of 366 days of follow-up compared to patients with diabetes mellitus [9]. No results were given with respect to treatment with insulin. In a large registry including 978 patients treated with DCB for de novo coronary artery disease, the presence of diabetes mellitus was associated with a 3.36-fold increased risk of target lesion failure (p < 0.002) [25], but results for ITDM or NITDM were not given.
There are several possible advantages with DCB for the treatment of de novo coronary artery disease compared to DES in patients with diabetes mellitus. With DCB, there is no permanent metallic frame or polymer inducing inflammation, neo-atherosclerosis, or triggering neointimal proliferation. There is no long-term risk of stent thrombosis since with DCB nothing is left behind, allowing late lumen enlargement. In addition, patients with diabetes mellitus have an increased risk of bleeding. Use of DCB allows us to shorten the dual antiplatelet treatment compared to DES. We were able to show in the BASKET-SMALL 2 trial that the need for TVR was significantly lower in diabetic patients with the use of DCB compared to DES. In addition, rates of MACE in patients with NITDM and in patients with ITDM were similar with DCB and DES for up to three years of follow-up. The need for TVR was lower with the use of DCB compared to DES both for ITDM and NITDM patients. However, this difference did not reach statistical significance due to the limited number of patients.
Small vessel coronary disease is an independent predictor for poor outcomes after PCI [26]. The use of DES in such lesions is associated with higher rates of stent failure, restenosis, and repeat revascularization [26,27]. Metallic DES prohibit late lumen enlargement and limit the full restoration of vessels’ endothelial functions [28,29]. Since several randomized trials have shown similar results for DCB versus DES in de novo lesions of small coronary arteries, DCB are an effective and safe alternative treatment strategy in those lesions. Similar to the use of DES, the use of DCB in patients with diabetes mellitus is associated with higher rates of adverse events compared to patients without diabetes mellitus. Of note, preliminary data point out that in diabetic patients the use of DCB compared to DES may be beneficial with respect to a lower need for repeat TLR. Similar to the use of DES, the need for insulin in the setting of de novo coronary artery disease in small vessels in diabetic patients treated with DCB is associated with higher adverse events compared to NITDM patients.
5. Limitations
In this subgroup analysis of the randomized BASKET-SMALL 2 trial, the number of patients in the ITDM and NITDM populations is limited and does not confer enough power to draw definitive conclusions regarding clinical endpoints. Due to the limited number of patients, potential concerns arising from beta error cannot be ruled out. Our results should be interpreted as hypothesis generating and should be confirmed in a randomized trial including patients with ITDM and NITDM. The use of intracoronary imaging was not integrated in the study protocol. Since patients in the study received treatment with paclitaxel-iopromide-coated DCB, these long-term results can only be extrapolated to those who received this type of DCB since there is no class effect. Data regarding duration of diabetes mellitus, dosage of insulin or other medication, or values of hemoglobin A1C were not captured in the BASKET-SMALL 2 trial.
6. Conclusions
Based on the randomized BASKET SMALL 2 trial, the rates of MACE, non-fatal MI, and cardiac death are similar between DCB and DES for patients with ITDM and NITDM. The study demonstrates the sustained efficacy and safety of DCB in diabetic patients with de novo lesions of small coronary vessels for up to 3 years compared to DES both for ITDM and NITDM.
Author Contributions
Conceptualization, J.W., B.S. and R.J.; Methodology, J.W., B.S., A.F., M.-A.O., N.M., S.M.-W., P.R., N.G. and R.J.; Software, S.M.-W., D.W., G.S., G.L.and M.C.; Validation, R.J.; Formal analysis, J.S., J.W., S.M.-W., D.W. and M.C.; Investigation, J.S., J.W., B.S., A.F., M.-A.O., N.M., G.S., G.L., P.R., C.K. and R.J.; Resources, B.S., A.F., P.R. and N.G.; Data curation, J.S. and M.C.; Writing—original draft, J.S. and J.W.; Writing—review & editing, A.F., M.-A.O., N.M., S.M.-W., D.W., G.S., G.L.and P.R.; Visualization, J.S. and M.-A.O.; Supervision, J.W., N.M., M.C., N.G., C.K. and R.J.; Project administration, B.S., N.G., C.K. and R.J.; Funding acquisition, C.K. and R.J. All authors have read and agreed to the published version of the manuscript.
Funding
Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung, Basel Cardiovascular Research Foundation, and B Braun Medical AG.
Institutional Review Board Statement
The trial was performed in 14 centers in Switzerland, Austria, and Germany in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines and was approved by the ethics committees in all participating centers.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Not applicable.
Conflicts of Interest
RVJ has received lecture honoraria and travel support from B Braun and lecture honoraria from Cardionovum and Nipro. M-AO has received proctoring honoraria and travel support from Biosensors and research support from Terumo. NM has received personal fees from Edwards Lifesciences, Medtronic, Biotronik, Novartis, Sanofi Genzyme, AstraZeneca, Pfizer, Bayer, Abbott, Abiomed, and Boston Scientific outside the submitted work. GL is a medical user advisory board member for REVA Medical and has relationships with drug and device companies, including Terumo, Acrostak, Bionsensors, Boston Scientific, Abbott Vascular, Impuls Medical, and Orbus Neich. NG has received travel support from B Braun. BS is a shareholder of InnoRa GmbH and was named as co-inventor on patent applications submitted by Charité University Hospital, Berlin, Germany. All other authors declare no conflicts of interest.
References
- Levine, G.N.; Bates, E.R.; Blankenship, J.C.; Bailey, S.R.; Bittl, J.A.; Cercek, B.; Chambers, C.E.; Ellis, S.G.; Guyton, R.A.; Hollenberg, S.M.; et al. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. J. Am. Coll. Cardiol. 2011, 58, e44–e122. [Google Scholar] [CrossRef]
- Jeger, R.V.; Farah, A.; Ohlow, M.A.; Mangner, N.; Möbius-Winkler, S.; Weilenmann, D.; Wöhrle, J.; Stachel, G.; Markovic, S.; Leibundgut, G.; et al. Long-term efficacy and safety of drug-coated balloons versus drug-eluting stents for small coronary artery disease (BASKET-SMALL 2): 3-year follow-up of a randomised, non-inferiority trial. Lancet 2020, 396, 1504–1510. [Google Scholar] [CrossRef]
- Jeger, R.V.; Farah, A.; Ohlow, M.A.; Mangner, N.; Möbius-Winkler, S.; Leibundgut, G.; Weilenmann, D.; Wöhrle, J.; Richter, S.; Schreiber, M.; et al. Drug-coated balloons for small coronary artery disease (BASKET-SMALL 2): An open-label randomised non-inferiority trial. Lancet 2018, 392, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Qiao, S.; Su, X.; Chen, Y.; Jin, Z.; Chen, H.; Xu, B.; Kong, X.; Pang, W.; Liu, Y.; et al. Drug-Coated Balloon Versus Drug-Eluting Stent for Small-Vessel Disease: The RESTORE SVD China Randomized Trial. JACC Cardiovasc. Interv. 2018, 11, 2381–2392. [Google Scholar] [CrossRef] [PubMed]
- Kleber, F.X.; Schulz, A.; Waliszewski, M.; Hauschild, T.; Böhm, M.; Dietz, U.; Cremers, B.; Scheller, B.; Clever, Y.P. Local paclitaxel induces late lumen enlargement in coronary arteries after balloon angioplasty. Clin. Res. Cardiol. 2015, 104, 217–225. [Google Scholar] [CrossRef]
- Megaly, M.; Ali, A.; Abraham, B.; Khalil, C.; Zordok, M.; Shaker, M.; Tawadros, M.; Hennawy, B.S.; Elbadawi, A.; Omer, M.; et al. Outcomes with Drug-Coated Balloons in Percutaneous Coronary Intervention in Diabetic Patients. Cardiovasc. Revasc. Med. 2020, 21, 78–85. [Google Scholar] [CrossRef]
- Van Belle, E.; Périé, M.; Braune, D.; Chmaït, A.; Meurice, T.; Abolmaali, K.; McFadden, E.P.; Bauters, C.; Lablanche, J.M.; Bertrand, M.E. Effects of coronary stenting on vessel patency and long-term clinical outcome after percutaneous coronary revascularization in diabetic patients. J. Am. Coll. Cardiol. 2002, 40, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Wöhrle, J.; Scheller, B.; Seeger, J.; Farah, A.; Ohlow, M.A.; Mangner, N.; Möbius-Winkler, S.; Weilenmann, D.; Stachel, G.; Leibundgut, G.; et al. Impact of Diabetes on Outcome with Drug-Coated Balloons Versus Drug-Eluting Stents: The BASKET-SMALL 2 Trial. JACC Cardiovasc. Interv. 2021, 14, 1789–1798. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Lu, W.; Han, Z.; Pan, S.; Wang, X.; Shan, Y.; Wang, X.; Zheng, X.; Li, R.; Zhou, Y.; et al. Clinical Outcomes of Drug-Coated Balloon in Coronary Patients with and without Diabetes Mellitus: A Multicenter, Propensity Score Study. J. Diabetes Res. 2021, 2021, 5495219. [Google Scholar] [CrossRef]
- Silber, S.; Serruys, P.W.; Leon, M.B.; Meredith, I.T.; Windecker, S.; Neumann, F.J.; Belardi, J.; Widimsky, P.; Massaro, J.; Novack, V.; et al. Clinical outcome of patients with and without diabetes mellitus after percutaneous coronary intervention with the resolute zotarolimus-eluting stent: 2-year results from the prospectively pooled analysis of the international global RESOLUTE program. JACC Cardiovasc. Interv. 2013, 6, 357–368. [Google Scholar] [CrossRef]
- Chandrasekhar, J.; Dangas, G.; Baber, U.; Sartori, S.; Qadeer, A.; Aquino, M.; Vogel, B.; Faggioni, M.; Vijay, P.; Claessen, B.E.; et al. Impact of insulin treated and non-insulin-treated diabetes compared to patients without diabetes on 1-year outcomes following contemporary PCI. Catheter Cardiovasc. Interv. 2020, 96, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Pi, S.H.; Rhee, T.M.; Lee, J.M.; Hwang, D.; Park, J.; Park, T.K.; Yang, J.H.; Song, Y.B.; Choi, J.H.; Hahn, J.Y.; et al. Outcomes in Patients with Diabetes Mellitus According to Insulin Treatment After Percutaneous Coronary Intervention in the Second-Generation Drug-Eluting Stent Era. Am. J. Cardiol. 2018, 121, 1505–1511. [Google Scholar] [CrossRef]
- Kalkman, D.N.; Woudstra, P.; den Heijer, P.; Menown, I.B.; Erglis, A.; Suryapranata, H.; Arkenbout, K.E.; Iñiguez, A.; van ‘t Hof, A.W.; Muller, P.; et al. One year clinical outcomes in patients with insulin-treated diabetes mellitus and non-insulin-treated diabetes mellitus compared to non-diabetics after deployment of the bio-engineered COMBO stent. Int. J. Cardiol. 2017, 226, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Voudris, V.; Karyofillis, P.; Thomopoulou, S.; Doulaptsis, C.; Manginas, A.; Pavlides, G.; Cokkinos, D.V. Long-term results after drug-eluting stent implantation in diabetic patients according to diabetic treatment. Hell. J. Cardiol. 2011, 52, 15–22. [Google Scholar]
- Gilgen, N.; Farah, A.; Scheller, B.; Ohlow, M.A.; Mangner, N.; Weilenmann, D.; Wöhrle, J.; Jamshidi, P.; Leibundgut, G.; Möbius-Winkler, S.; et al. Drug-coated balloons for de novo lesions in small coronary arteries: Rationale and design of BASKET-SMALL 2. Clin. Cardiol. 2018, 41, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Jeger, R.V.; Eccleshall, S.; Wan Ahmad, W.A.; Ge, J.; Poerner, T.C.; Shin, E.S.; Alfonso, F.; Latib, A.; Ong, P.J.; Rissanen, T.T.; et al. Drug-Coated Balloons for Coronary Artery Disease: Third Report of the International DCB Consensus Group. JACC Cardiovasc. Interv. 2020, 13, 1391–1402. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Simoons, M.L.; Chaitman, B.R.; White, H.D.; Joint ESC/ACCF/AHA/WHF Task Force for Universal Definition of Myocardial Infarction; Authors/Task Force Members Chairpersons; Thygesen, K.; Alpert, J.S.; et al. Third universal definition of myocardial infarction. J. Am. Coll. Cardiol. 2012, 60, 1581–1598. [Google Scholar] [CrossRef] [PubMed]
- Cutlip, D.E.; Windecker, S.; Mehran, R.; Boam, A.; Cohen, D.J.; van Es, G.A.; Steg, P.G.; Morel, M.A.; Mauri, L.; Vranckx, P.; et al. Clinical end points in coronary stent trials: A case for standardized definitions. Circulation 2007, 115, 2344–2351. [Google Scholar] [CrossRef] [PubMed]
- Abizaid, A.; Kornowski, R.; Mintz, G.S.; Hong, M.K.; Abizaid, A.S.; Mehran, R.; Pichard, A.D.; Kent, K.M.; Satler, L.F.; Wu, H.; et al. The influence of diabetes mellitus on acute and late clinical outcomes following coronary stent implantation. J. Am. Coll. Cardiol. 1998, 32, 584–589. [Google Scholar] [CrossRef] [PubMed]
- Bundhun, P.K.; Li, N.; Chen, M.H. Adverse cardiovascular outcomes between insulin-treated and non-insulin treated diabetic patients after percutaneous coronary intervention: A systematic review and meta-analysis. Cardiovasc. Diabetol. 2015, 14, 135. [Google Scholar] [CrossRef]
- Akin, I.; Bufe, A.; Eckardt, L.; Reinecke, H.; Senges, J.; Richardt, G.; Kuck, K.H.; Schneider, S.; Nienaber, C.A.; DES.DE Study Group. Comparison of outcomes in patients with insulin-dependent versus non-insulin dependent diabetes mellitus receiving drug-eluting stents (from the first phase of the prospective multicenter German DES.DE registry). Am. J. Cardiol. 2010, 106, 1201–1207. [Google Scholar] [CrossRef] [PubMed]
- Dangas, G.D.; Farkouh, M.E.; Sleeper, L.A.; Yang, M.; Schoos, M.M.; Macaya, C.; Abizaid, A.; Buller, C.E.; Devlin, G.; Rodriguez, A.E.; et al. Long-term outcome of PCI versus CABG in insulin and non-insulin-treated diabetic patients: Results from the FREEDOM trial. J. Am. Coll. Cardiol. 2014, 64, 1189–1197. [Google Scholar] [CrossRef] [PubMed]
- Latib, A.; Ruparelia, N.; Menozzi, A.; Castriota, F.; Micari, A.; Cremonesi, A.; De Felice, F.; Marchese, A.; Tespili, M.; Presbitero, P.; et al. 3-Year Follow-Up of the Balloon Elution and Late Loss Optimization Study (BELLO). JACC Cardiovasc. Interv. 2015, 8, 1132–1134. [Google Scholar] [CrossRef]
- Cortese, B.; Di Palma, G.; Guimaraes, M.G.; Piraino, D.; Orrego, P.S.; Buccheri, D.; Rivero, F.; Perotto, A.; Zambelli, G.; Alfonso, F. Drug-Coated Balloon Versus Drug-Eluting Stent for Small Coronary Vessel Disease: PICCOLETO II Randomized Clinical Trial. JACC Cardiovasc. Interv. 2020, 13, 2840–2849. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Cho, Y.K.; Kim, S.W.; Hong, Y.J.; Koo, B.K.; Bae, J.W.; Lee, S.H.; Yang, T.H.; Park, H.S.; Choi, S.W.; et al. Clinical Results of Drug-Coated Balloon Treatment in a Large-Scale Multicenter Korean Registry Study. Korean Circ. J. 2022, 52, 444–454. [Google Scholar] [CrossRef]
- Nestelberger, T.; Jeger, R. Drug-coated Balloons for Small Coronary Vessel Interventions: A Literature Review. Interv. Cardiol. 2019, 14, 131–136. [Google Scholar] [CrossRef]
- Sinaga, D.A.; Ho, H.H.; Watson, T.J.; Sim, A.; Nyein, T.T.; Jafary, F.H.; Loh, J.K.; Ooi, Y.W.; Tan, J.K.; Ong, P.J. Drug-Coated Balloons: A Safe and Effective Alternative to Drug-Eluting Stents in Small Vessel Coronary Artery Disease. J. Interv. Cardiol. 2016, 29, 454–460. [Google Scholar] [CrossRef]
- Glagov, S.; Weisenberg, E.; Zarins, C.K.; Stankunavicius, R.; Kolettis, G.J. Compensatory enlargement of human ath- erosclerotic coronary arteries. N. Engl. J. Med. 1987, 316, 1371–1375. [Google Scholar] [CrossRef]
- Picard, F.; Doucet, S.; Asgar, A.W. Contemporary use of drug-coated balloons in coronary artery disease: Where are we now? Arch. Cardiovasc. Dis. 2017, 110, 259–272. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).