Abstract
Background: Cardiovascular disease (CVD) is very widespread in countries with a Western-style diet, representing one of the major causes of morbidity. Genetic factors, obesity, diabetes, dyslipidemia, smoking, and ageing are risk factors for CVD outcomes. From a pathogenic point of view, the condition of low-grade inflammation of the arteries leads to endothelial damage and atherosclerosis development. Nowadays, a broad range of drugs is available to treat CVD, but many of them are associated with side effects. Therefore, alternative therapeutic remedies need to be discovered in combination with conventional drugs. A balanced diet rich in fruits and vegetables, e.g., the Mediterranean diet, has been shown to lower the incidence of CVD. Plant-derived polyphenols are ingested in food, and these compounds can exert beneficial effects on human health, such as antioxidant and anti-inflammatory activities. Objective: In the present review, the cellular and molecular bases of the beneficial effects of polyphenols in the prevention and treatment of CVD will be pointed out. Methods: This review has been conducted on the basis of a literature review spanning mainly the last two decades. Results: We found that an increased dietary intake of polyphenols is associated with a parallel decrease in chronic disease incidence, including CVD. Conclusion: Despite a plethora of preclinical studies, more clinical trials are needed for a more appropriate treatment of CVD with polyphenols.
1. Introduction
Cardiovascular disease represents one of the major causes of morbidity in countries adopting Western lifestyles, with an annual expectation of deaths by 2030 that exceeds 23.6 million [1]. The term CVD encompasses a variety of conditions, such as coronary artery disease (CAD), stroke, peripheral artery disease, hypertension, cerebrovascular disease, and heart failure (HF). Among risk factors of CVD, genetic factors, obesity, diabetes, dyslipidemia, smoking, and ageing account for occurrences of CVD [2,3]. The above conditions lead to endothelial cell dysfunction, oxidative stress, proliferation of smooth muscle cells, and fibroblasts, with conversion of macrophages to foam cells within the artery walls [4]. Furthermore, the condition of vascular low-grade inflammation promotes atherosclerotic plaque formation, ultimately, causing HF [5]. Therapeutically, a broad range of drugs is available for the treatment of CVD, i.e., statins, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, calcium channel blockers, fibrates, and beta-blockers; however, many of them are associated with side effects [6]. Therefore, there is a need to discover and apply innovative therapies in combination with conventional ones for a more appropriate management of CVD [7,8]. It is known that a balanced diet is beneficial for preventing CVD. In fact, consumption of fruits and vegetables has been shown to decrease the incidence of CVD. In this respect, the Mediterranean diet (MD) decreases inflammatory biomarkers, e.g., interleukin (IL)-1 beta, IL-5, and C-Reactive Protein (CRP), thus preventing chronic disease outcomes [7,9,10,11]. In this framework, the PREDIMED study demonstrated that the MD, based on the high consumption of fruits, vegetables, whole grains, and extra virgin olive oil (EVOO), was associated with a reduced risk of CVD [12]. Of note, dietary interventions aimed at reducing low-grade inflammation have led to divergent results due to differences in the tested dietary compounds and chosen inflammatory markers [13,14]. Plant-derived compounds contained in food possess beneficial effects on human health. Among these natural products, polyphenols can be found in fruits, vegetables, seeds, nuts, as well as in red wine, tea, coffee, extra virgin EVOO, and chocolate [15,16,17,18,19]. Nowadays, the human population is more aware of the beneficial effects of polyphenols, and their dietary intake has increased, with a parallel decrease in chronic disease, including CVD [20]. In the present review, the classification, pharmacological activities, and main mechanisms of action of polyphenols will be described. Experimental and clinical evidence of the beneficial effects of these natural compounds on CVD will be discussed.
1.1. Classification and General Properties of Polyphenols
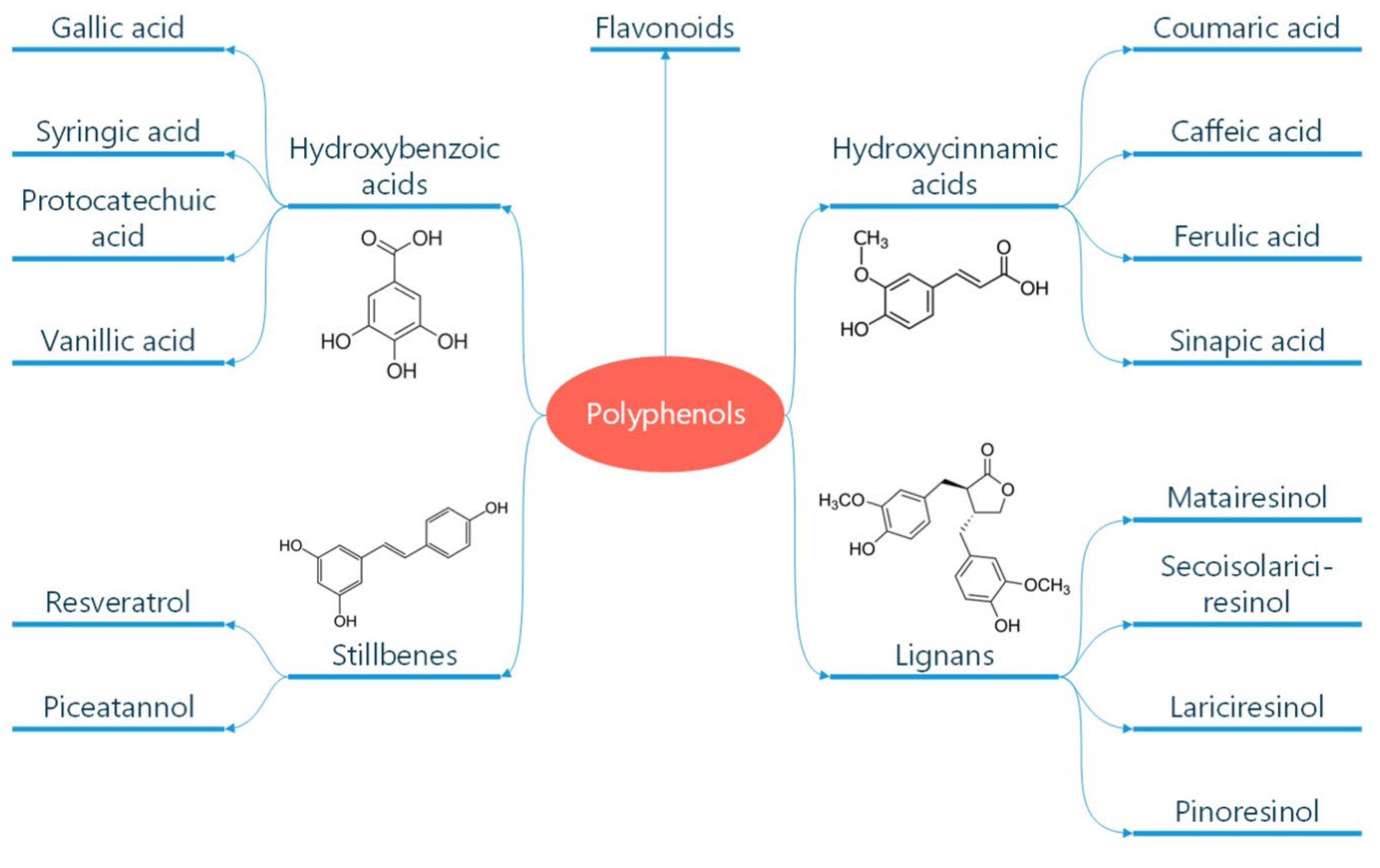
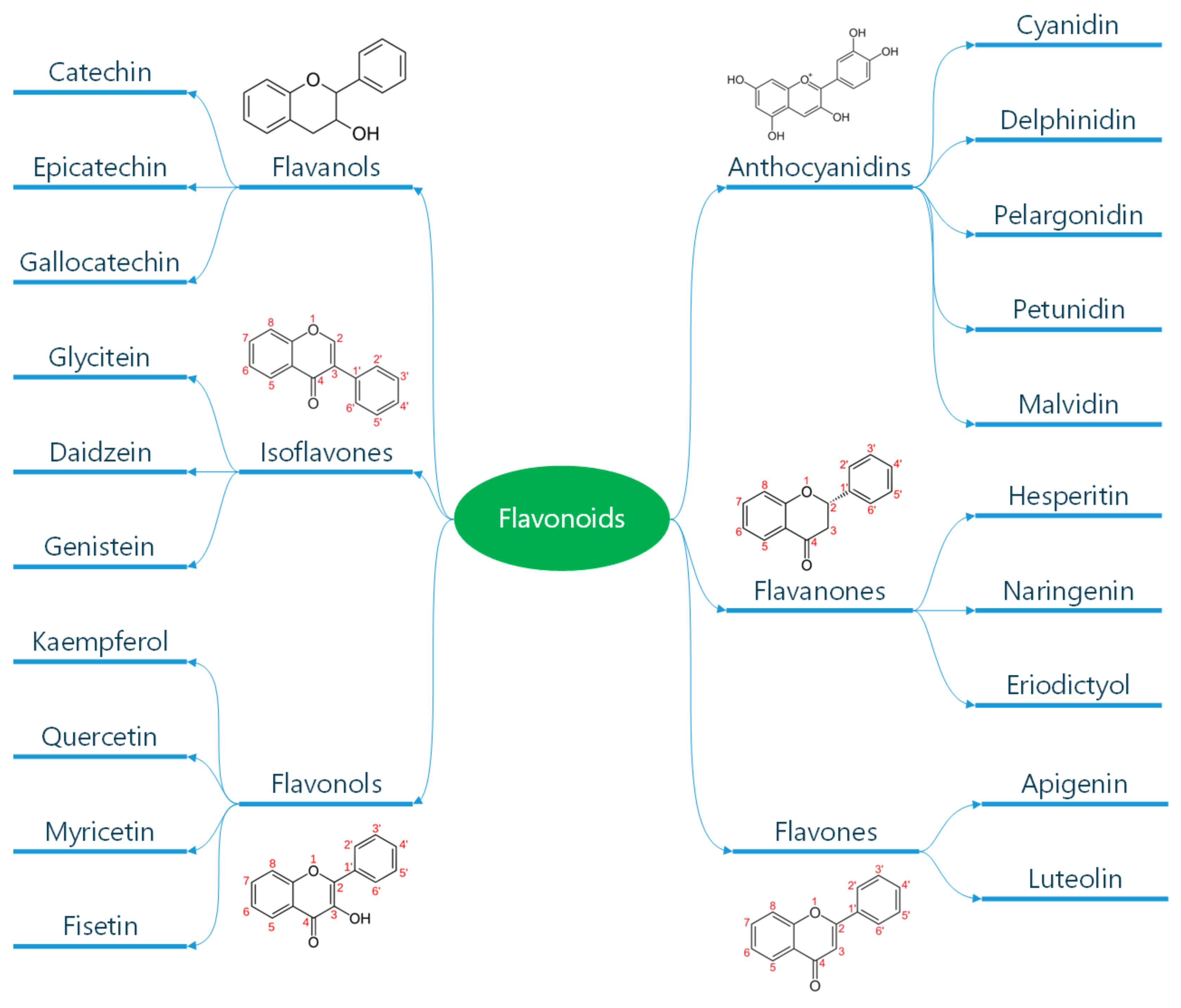
Polyphenols are classified according to the number of phenolic rings and the structural elements they bind [16]. They can be divided into four main classes: flavonoids, non-flavonoid stilbenes, phenolic acids, and lignans (Figure 1) [21]. Flavonoids are naturally occurring compounds that encompass six categories: flavanones, flavones, flavanols, isoflavones, flavan-3-ols, and anthocyanidins (Figure 2) [22]. Structurally, they possess two aromatic rings and a heterocyclic ring with a C6-C3-C6 configuration (Figure 2). They are contained in plants as glycosides and non-glycosylated conjugate compounds, and their type of structure influences bioavailability [23]. Stilbenes, e.g., resveratrol (RES), are composed of two phenyl residues linked by a two-carbon methylene bridge, which can be glycosylated, methylated, or prenylated by specific enzymes (Figure 1) [24]. Among flavonoids, flavonols and flavan-3-ols have been the object of intensive research. The flavonol quercetin exhibits antihypertensive effects by acting on the contraction of smooth muscles in renal blood vessels, producing vasodilation [25]. Among flavan-3-ols, epigallocatechin-3-gallate (EGCG) is present in green tea and is endowed with antioxidant, anti-inflammatory, and antiatherogenic properties [26]. Among stilbenes, RES is the most studied compound for its anti-inflammatory, antioxidant, anti-proliferative, anti-apoptotic, and mitochondrial protective effects [27].
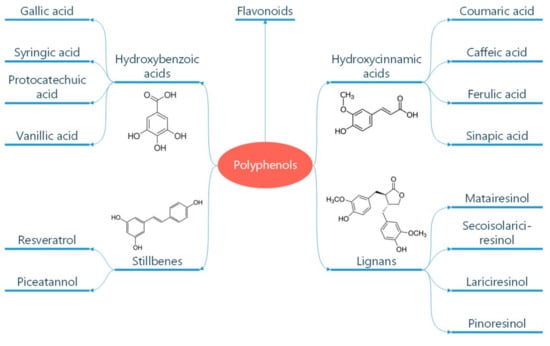
Figure 1.
Classification of polyphenols. Polyphenols are natural compounds found in plant-based foods and beverages. Their classification into different subclasses like phenolic acids, flavonoids, stilbenes, and lignans is reported. The chemical formula of these molecules is also reported. Reproduced with permission from Caiati et al. [7].
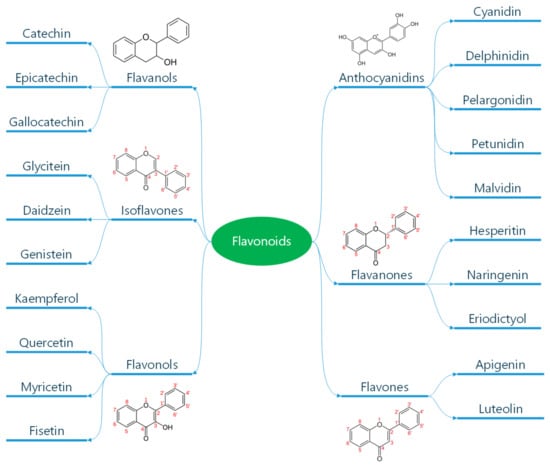
Figure 2.
Classification of flavonoids. Flavonoids are a subclass of polyphenols and can be classified into flavonols, flavones, isoflavones, flavanones, antho-cyanidins, and flavanols based on their ring structure as illustrated here. Flavonoids have diverse biological activities and potential health benefits, including antioxidant and anti-inflammatory effects. Reproduced with permission from Caiati et al. [7].
1.2. Absorption of Polyphenols
In the stomach, polyphenols are digested by pepsin, and peristaltic movements in the presence of a low pH into particles, even less than 500 microns in diameter [28]. The passage of polyphenols from the stomach to the small intestine occurs at a pH around 7, and then, pancreatic and biliary enzymes become activated [29]. From a general point of view, in food, most phenolic compounds are present in glycosylated form (where the sugar group is called “glycone” and the non-sugar polyphenolic component “aglycone”) or in the form of polymers. Both forms are unable to be absorbed (with some exceptions, such as anthocyanins) and must undergo several processes, especially hydrolysis by the microflora of the colon or by some enzymes before being absorbed; the degree of absorption often derives from the chemical structure of these compounds and from the sugar part linked to them [21]. From their contact with the oral cavity, especially with saliva, many phenolic compounds undergo transformations until the formation of glycosides [30]. There are three further possible mechanisms through which glycosides undergo the hydrolyzation process: (i) in the microvilli present at the level of the epithelial cell membranes of the small intestine by the enzyme lactase phloridizin hydrolase (LPH) [29]; (ii) through the β-glucosidase present in the cytoplasm of epithelial cells where the compounds are transported by the active sodium-dependent glucose transporter (SGLT1) [31]; (iii) the polyphenols that are not absorbed in the small intestine; once they reach the colon, they are hydrolyzed by the microflora present in the colon [32]. Thus, after the hydrolyzation process, polyphenols in the form of aglycons enter the intestinal epithelium according to different modalities. For instance, polyphenols with low molecular weight, i.e., phenolic acids, flavonoid aglycon, tea polyphenols, and cocoa polyphenols (epicatechin, procyanidin B2, and catechin) are absorbed by passive diffusion [33]. Another method of polyphenol absorption is sodium/glucose transport through the sodium/glucose-linked transporter 1 (SGLT1) [34]. Accordingly, glycosides may be absorbed by SGTL1 in small amounts, and then re-secreted into the digestive system, or they may be further digested by a cytosolic glucosidase [35]. Thus, polyphenols can undergo transepithelial transport through a monocarboxylic acid transporter, as in the case of caffeic acid and ferulic acid (Figure 1) [36]. Most polyphenols reach the large intestine, where they undergo significant transformations due to intestinal microbiota. In particular, they are digested by bacteria of the gut microbiota via glycosylation, hydroxylation, demethylation, deconjugation, ring cleavage, hydrolysis, epimerization, and chain shortening processes [37]. Therefore, gut microbiota play a crucial role in their metabolism. These microbial transformations generate bioactive metabolites that can influence the gut environment, modulate immune responses, and potentially contribute to disease prevention [38]. In particular, poorly absorbed wine and tea polyphenols given orally to rats were proven to reduce DNA oxidative damage in caecal mucosal cells [39] and reduce the number of tumors in rats treated with azoxymethane [40].
On the other hand, some polyphenols are absorbed into the enterocytes of the small intestine, and before entering circulation, undergo phase II of enzymatic detoxification with the production of sulfates, glucuronides, and methylated derivatives [41]. Polyphenol bioavailability and accumulation in tissues depend on multidrug resistance-associated proteins, which are ATP-dependent efflux transporters, and referred to as phase III metabolism [42]. Then, polyphenols reach the bloodstream mostly coupled to proteins, and the liver via the portal circulation, where they are conjugated to O-sulphate or O-glucuronide forms (a second phase metabolism), and finally, are eliminated through the kidneys [43] (Table 1).
Other factors involved in polyphenol absorption include the presence of specific dietary components that can significantly impact polyphenol absorption and metabolism. The intestinal absorption of polyphenols can be affected by various factors, including the food matrix and other bioactive compounds. These interactions can either enhance or hinder polyphenol bioavailability, which is the extent to which they become available for absorption and exert their effects. Proteins, lipids (fats), and carbohydrates present in food can interact with polyphenols, potentially impacting their solubility, stability, and ability to be absorbed. For example, some proteins may bind to polyphenols, reducing their availability for absorption, while fats might protect polyphenols from degradation or promote their solubility, especially more hydrophobic ones like curcumin, naringenin, and others [44].
Polyphenols can bind to dietary fiber, affecting their release and subsequent absorption [45].
Methods like boiling, frying, or baking can alter polyphenol content and their interactions with other food components [46].
The gut microbiome plays a crucial role in polyphenol metabolism and absorption. Consuming prebiotics and probiotics can support a healthy gut environment, potentially improving polyphenol bioavailability [47].
In conclusion, a healthy diet can maximize polyphenol absorption, enhancing their positive protective effects.

Table 1.
Antioxidant Activity of Polyphenols.
Table 1.
Antioxidant Activity of Polyphenols.
| 1.1. Scavenging activity depends on the donation of an electron or H atom from a hydroxyl group to a free radical [48]. |
| 1.2. A catechol group in the structure of polyphenols is associated with antioxidant activity [49]. |
| 1.3. The phenolic core of quercetin and catechin scavenges reactive oxygen species (ROS), acting as a buffer or collecting electrons [50]. |
| 1.4. Polyphenols inhibit enzymes, such as xanthine oxidase and nicotinamide adenine dinucleotide phosphatase, thus reducing the generation of ROS [51]. |
| 1.5. Quercetin exhibits the best capacity to chelate metal ions [52]. |
1.3. Antioxidant Properties of Polyphenols
Polyphenols behave as potent antioxidant agents thanks to catechol groups and hydroxylation patterns, such as the 3-hydroxyl group in flavanols or electron deficiency in anthocyanins [53]. Using the ferric reducing ability power, it has been demonstrated that the presence of a catechol ring in the structure of polyphenols is associated with their antioxidant activity [49]. Reactive oxygen species (ROS), i.e., superoxide, hydrogen peroxide, and hypochlorous acid, are scavenged by quercetin and catechin through the phenolic core, acting as a buffer or collecting electrons [50]. Furthermore, polyphenols have been shown to inhibit enzymes that generate ROS, such as xanthine oxidase and nicotinamide adenine dinucleotide phosphatase [51]. Among polyphenols, quercetin has the best capacity to chelate metal ions due to its low redox potential, thus preventing the production of ROS [27]. The scavenging activity of polyphenols is connected to their ability to donate an electron or H atom from an aromatic hydroxyl group to a free radical, thus abrogating its effect [48]. The antioxidant capacity of polyphenols in vivo is lower than in vitro, since it can be mimicked by other compounds [54]. For instance, in vivo, the polyphenol-mediated antioxidant activity exerted by apple consumption is mostly due to the metabolic effect of fructose on urate.
1.4. Effects of Polyphenols on the Vascular Endothelium
The major function of endothelial cells (ECs) is to regulate vascular tone [55]. The endothelial (e) nitric oxide (NO) synthase (eNOS) generates NO from L-arginine, which, in turn, acts on the vascular smooth muscles, thus triggering guanyl cyclase, with accumulation of cyclic guanosine monophosphate, which activates the protein kinase G, thus leading to vasorelaxation. Furthermore, the endothelium-derived hyperpolarizing factor causes vasorelaxation, targeting the K+ channels in the vasculature. Additionally, prostacyclin I2 (PGI2), generated during the cyclooxygenase (COX) pathway, leads to vasodilation. On the other hand, endothelial products, such as angiotensin II (ANG II), endothelin-1 (ET-1), and thromboxane (TXA) A2, have vasoconstrictive effects [56]. NO generation accounts for the main effects of polyphenols on the endothelium [57]. In this respect, red wine polyphenols are a potent inducer of serum NO in healthy subjects 30 min after ingestion [58]. In vitro studies have demonstrated that healthy human peripheral blood monocytes are an additional source of NO, thus contributing to vasodilation after ingestion of red wine [16]. In this regard, short-term oral treatment of normotensive rats with red wine polyphenols decreased blood pressure [59]. Such an effect depends on the induction of the gene responsible for inducible NO synthase and COX-2 in the arteries, as well as on the calcium ion-dependent pathway [60]. In this last respect, RES and quercetin have been shown to induce an increase in calcium concentration by opening the potassium channels or inhibiting Ca2+ ATPase within the endoplasmic reticulum of ECs [61]. Evidence has been provided that red wine polyphenols enhance endothelial NO production via the redox-responsive PI3/Akt channel, the increase in intracellular protein-Ca2+, and tyrosine phosphorylation with activation of eNOS [62,63]. Apart from NO generation, polyphenols exhibit other effects on the endothelium via increased release of PGI2 [64]. In fact, in vitro and in vivo studies, using cocoa extracts rich in procyanidins, demonstrated that the ratio of leukotrienes-to-PGI2 was reduced. Moreover, polyphenols can increase endothelial NO by decreasing levels of phosphodiesterases (PDE)-2 and PDE-4 [65] (Table 2).

Table 2.
Effects of Polyphenols on the Vascular Endothelium.
Moving to the clinical arena, strong epidemiologic data support a relationship between higher intake of polyphenolic flavonoids and reduced risk of cardiovascular disease; this seems to be mediated by improved endothelial function and reduced platelet aggregation, as appropriately reviewed [66].
While epidemiological studies and clinical trials can show associations between polyphenol intake and health outcomes, they often lack the detail to explain the underlying mechanisms. Mechanistic studies, especially those using endothelial cell models, can pinpoint the precise molecular pathways and cellular processes that polyphenols affect. We think that the best approach for this purpose is to study the endothelial function in clinical trials by using either the FMD approach, which, however, does not precisely reflect the coronary endothelial function [67], or the direct non-invasive coronary endothelial function test with transthoracic coronary Doppler echocardiography, using the cold pressure test as endothelium-dependent stimulus [68,69,70].
1.5. Anti-Inflammatory Activity of Polyphenols
Inflammation is a response of the body to various stimuli, including pathogens, mechanical insults, and damaged tissue. Pro-inflammatory cytokines, e.g., interleukin (IL)-1 beta, IL-6, IL-8, and tumor necrosis factor (TNF)-alpha, as well as various enzymes, such as COX, lipooxigenase (LOX), and protein kinase, are responsible for the inflammatory response. With special reference to the role of polyphenols, there is evidence that red wine polyphenols can, in vitro, reduce the production of pro-inflammatory cytokines, blocking the activation of the NF-kB pathway [71]. Moreover, red wine polyphenols can interfere with endotoxin binding to Toll-Like Receptor (TLR)-4, thus abrogating the nuclear factor kappa-light chain enhancer of activated B cells (NF-kB) pathway with interruption of pro-inflammatory cytokine release [72]. Additionally, polyphenols contained in fermented grape marc (FGM) induce activation of Foxp3+ T regulatory cells with release of the anti-inflammatory cytokine, IL-10 [73]. In addition, FGM reduces the respiratory burst of neutrophils and basophils in in vitro experiments, playing an antioxidant and anti-inflammatory role [74]. Quercetin has been found to dampen the generation of prostaglandins, leukotrienes, and TAXs, abrogating production of COX and LOX [75,76]. In fact, both COX and LOX mediate the formation of arachidonic acid, which, in turn, fuels inflammation via release of IL-1 beta and IL-8. The nucleotide-binding domain and leucine-rich repeat-containing receptors (NLRs) belong to the family of pattern recognition receptors (PRRs), triggering inflammatory responses upon danger and cell damage signals. Among them, NLRP3 inflammasome is a multiprotein complex that activates inflammatory caspase-1 [77]. Caspase-1 cleaves and maturates the pro-inflammatory cytokines IL-1 beta and IL-18, as well as the protein gasodermin, contributing to the release of the above mediators, thus initiating the cell death pyroptosis [78]. Activation of the NLRP3 inflammasome is involved in CVD, including atherosclerosis, myocardial infarction, and cardiac remodeling [79]. In this framework, in the middle cerebral artery occlusion/reperfusion model, supplementation of various polyphenols decreased levels of NLRP3 [80]. This is associated with the downregulation of IL-1 beta and IL-18 in the serum or brain tissue [81]. In the myocardial ischemia (MI)/reperfusion model, certain polyphenols, i.e., RES and flavone, in vivo reduced levels of caspase-1 and IL-1 beta in the myocardial tissue [82,83]. In all these studies, the decrease in NLRP3 levels was associated with improvement of clinical markers [80]. Clinically, aged male subjects at high cardiovascular risk underwent acute administration of aged wine, with a decrease in Tlr2, Nlrp3, and Il1receptor genes [84] (Table 3).

Table 3.
Anti-Inflammatory Activity of Polyphenols.
These data have been confirmed in clinical trials. In healthy adults, 4 weeks of red wine intake (with high RES content) significantly reduced PCR and IL-6 levels and improved the response of platelet and leukocyte adhesion molecules (sP-selectin and sE-selectin) [85,86,87]. However, considering resveratrol as a supplement, a meta-analysis of 11 randomized controlled trials observed no effect on PCR concentration [88]. As usual, the supplementation, not being a natural intervention, creates problems and appears less effective. Natural products are always better. In fact, short-term RCTs (1–4 weeks) focusing on blueberries, grapes, apples, and pomegranates showed a significant reduction in inflammation markers, including IL-6, PCR, and tumor necrosis factor (TNF-alfa) in healthy or hyperinsulinemic subjects at high cardiometabolic risk [89,90,91,92,93,94,95].
Further research is needed to optimize polyphenol delivery, determine optimal dosages, and understand individual responses to polyphenol interventions.
1.6. Anti-Atherogenic Effects of Polyphenols
Atherosclerosis represents a pathogenic common denominator of various diseases, including CAD, ischemic stroke, and peripheral artery disease [96]. This disease stems from the endothelial damage provoked by several offending factors that then drive the augmentation of ROS in the blood. Those offending factors have been recently reported in [7,97]. In brief, they are largely man-made, such as stress, pollutants of all sorts (especially those contained in food, such as farming chemicals, fertilizers, and pesticides and herbicides like glyphosate), drugs, processed food, tabacco smoking, air pollution, alcohol, cosmetic and cleaning products, heavy metals, chronic infections, electromagnetic radiation (cellular phone, cell-tower emitting radiation), ionizing radiation (in particular those medically derived like computed tomography scan and angiography), and intravascular prosthesis like arterial stents. Diabetes per se induces tissue damage, and it terribly enhances the damaging effects of the previously mentioned atherogenic factors, dramatically enhancing the formation of ROS [7]. From a pathogenic point of view, increased levels of ROS further enhance endothelial damage, with the intervention of neutrophils, macrophages, and platelets [98]. In fact, prolonged contact of ECs with hydrogen peroxide, peroxynitrite, and oxidized low-density lipoproteins (ox-LDL) leads to severe damage of the endothelium [7,96,99]. One of the initial consequences of coronary endothelial dysfunction is the reduction of NO production and the ensuing microvascular vasoconstriction at rest. This kind of derangement can be spotted with positron emission tomography (PET) since it causes myocardial dishomogeneous perfusion at rest [100] and is the mechanism that explains the angiographic slow coronary phenomenon, as recently demonstrated [101].
This strong oxidative drive also involves oxidated LDL microparticles. This causes the first step of atherosclerotic plaque formation, that is, the generation of oxidized LDLs, which pass through the endothelial barrier, eliciting cytotoxic effects and the inflammatory response [102], since ox-LDL microparticles are modified substances that elicit a strong immunologic reaction. Focusing on the molecular biology details, activated ECs express adhesins, i.e., vascular cell adhesion-1 (VCAM-1), intercellular adhesion molecule 1 (ICAM-1), and E-selectins, which allow transmigration of monocytes and T cells into the arterial wall [103]. Particularly, monocytes engulf ox-LDL, becoming foam cells, which accumulate as fatty bands in the artery walls [104]. Then, a stabilized plaque is formed, which can break under prolonged inflammation [105]; this happens only in case of prolonged and uncontrolled exposure to those factors generating ROS with consequent escalating concentration of ox-LDL. Ruptured plaques cause thrombosis, which may lead to heart attacks, ischemic strokes, and peripheral ischemia [106]. There is evidence that polyphenols can exert beneficial effects on atherosclerosis. In cholesterol-fed rabbits, administration of red wine polyphenols decreased neointimal growth, lipid accumulation, and inflammation in the iliac arteries [107]. In hamsters, red wine supplementation reduced neointimal hyperplasia, inhibiting the entry of monocytes into the arterial wall [108]. Clinically, there is evidence that purple grape juice reduced the levels of plasmatic ox-LDL in patients with CAD [109]. This effect has been shown to depend on the production of NO by polyphenols, as also supported by others [110,111] (Table 4). This result has been confirmed in a controlled clinical trial [112]. In this study, the traditional Mediterranean diet with its high content of polyphenols [113] was proved to be beneficial in reducing the extent of LDL oxidation in subjects with a high risk of CAD.

Table 4.
Anti-Atherogenic Effects of Polyphenols.
However, the main radical approach that can stop progression and start regression of atherosclerosis is the elimination of those damaging factors (mentioned before) that create ROS and cause chronic endothelium inflammation and ox-LDL [114].
2. Focus on the Cardiovascular Effects of Relevant Polyphenols
Flavan-3-Ols
Flavan-3-ols (Figure 2) represent the most abundant polyphenols in fruits, vegetables, red wine, green tea, and cocoa [115]. They encompass monomeric, oligomeric, and polymeric compounds. Monomeric forms include catechin, epicatechin, gallocatechin, epigallocatechin, epicatechin-3-O-gallate, and EGCG (Figure 2). Oligomers or polymers are known as proanthocyanidins, while polymers composed of epicatechin or catechin are termed procyanidins. The antioxidant activity of flavan-3-ols is based on their ability to donate an electron or to chelate metal ions, thus stopping ROS production [116]. At the same time, flavan-3-ols maintain mitochondrial activity while enhancing antioxidative enzymes involved in ROS scavenging [117,118]. The anti-inflammatory activity of flavan-3-ols depends on the regulation of gene expression involved in cardiometabolic health. Particularly, they act on endothelial transcription factor GATA-2, the NF-kB p105 subunit, forkhead box C1, and peroxisome proliferator-activated receptor gamma [119]. In addition, flan-3-ols target different miRNAs, regulating cellular pathways involved in cell adhesion, cellular signaling, and immune response [120]. Of note, cocoa flavan-3-ols metabolites enhance ApOAI expression, which represents the major component of high-density lipoproteins, thus exerting antiatherogenic properties [121]. The cardioprotective effects of flavan-3-ols have been attributed to two major microbial-derived metabolites, namely, the hydroxy-phenyl-gamma-valerolactones, and their derived hydroxy-phenyl valeric acids [122]. These metabolites have, in vivo, shown hypotensive activity in rats, and in vitro abrogation of monocyte adhesion to ECs treated with tumor necrosis factor (TNF)-alpha [123]. Another flavan-3-ol metabolite, the protocatechuic acid, diminished diabetic cardiomyopathy in rats, stimulating glucose metabolism, improving oxidative stress, and reducing inflammation [124]. Flavan-3-ols have been shown to act on gut dysbiosis, improving cardiac function. In fact, a metabolite from the gut microbiota, trimethylamine N-oxide (TMAO), has been associated with CVD pathogenesis in terms of increased cholesterol levels and higher risk of atherosclerosis [125]. In this respect, the intake of cocoa and red berry flavan-3-ols reduces TMAO levels, improving cardiovascular markers in healthy aging adults [126]. Various clinical trials have been conducted, using cocoa flavan-3-ols in patients with CVD. In hypertensive individuals, consumption of dark chocolate led to a reduction of systolic blood pressure (SBP) and diastolic blood pressure (DBP) in comparison to baseline [127]. In another study, in essential hypertensive subjects with impaired glucose tolerance receiving 100g/day of chocolate, a reduction in both SBP and DBP and an increase in flow-mediated dilation (FMD) were observed [128]. In patients with CAD receiving dietary high-flavan-3-ol intervention, an increase in brachial artery FMD and a reduction in SBP were recorded [129]. In patients with congestive HF, intake of flavan-3-ol-rich chocolate improved FMD [130]. Other studies have been conducted with green tea catechins in both healthy and unhealthy individuals. In healthy volunteers aged between 21 and 70 years receiving two capsules of Camelia sinensis for 3 months, a reduction in SBP was observed [131]. In another group of healthy adult men aged 18–35 years, administration of 450 mg of sour tea led to a reduction in SBP and DPB [132]. In healthy postmenopausal women, acute ingestion of catechin-rich green tea improved postprandial glucose status while increasing serum thioredoxin levels, but no changes in cardiovascular risk factors were observed [133]. In overweight women aged 19–57 years, receiving a low-calorie diet along with 3 capsules of green tea or placebo capsules, a decrease in SBP and DPB was observed in both groups [134]. Another trial conducted in healthy male volunteers supplemented with an aqueous green tea extract showed no alterations of cardiac risk factors [135]. Additionally, minor effects on cardiovascular risk markers were observed following tea catechin administration to active older people [136]. Taken together, the above data suggest that studies with polyphenols conducted in both healthy and unhealthy individuals led to contrasting results.
3. Resveratrol
Stilbenes, and particularly, RES (Figure 1), despite a low bioavailability, possess a strong antioxidant activity in vitro. RES protects cardiomyocytes and ECS against ROS effects, inhibiting NADPH oxidases while increasing the mitochondrial respiratory chain enzymes [137]. RES acts by upregulating SIRT1, which, in turn, induces deacetylation of NF-kB, and enhancement of superoxide dismutase (SOD), catalase, and glutathione peroxidase 1 [138,139]. Furthermore, RES can reverse eNOS uncoupling, upregulating GCH1 expression in apolipoprotein E knockout mice [140]. Additionally, RES activates Nrf-2, which, in turn, increases cellular antioxidant content in the placenta of sows and piglets [141]. As a potent anti-inflammatory agent, RES can inhibit the expression of pro-inflammatory cytokines, downregulating TLR4 expression and silencing NF-kB activity [142,143]. Moreover, RES can inhibit VCAM-1, ICAM-1, and E-selectin, suppressing the TNF-alpha-induced NF-kB activation [144]. RES inhibits COX-1 and COX-2 enzymes via SIRT1 activation, thus decreasing PGE2 and TAX2, and, consequently, inflammation [145]. In patients with systolic HF, RES administration improved clinical conditions by inhibiting oxidative phosphorylation in leukocytes, gene expression encoding B cell receptors, and leukocyte extravasation signal [52]. ROS-mediated overexpression of MAPKs is involved in cardiac hypertrophy and remodeling [146]. RES can stimulate MKP-1 and downregulate mTOR, thus dampening mitogen-activated protein kinase (MAPK) activity, with a reduction in cardiac and endothelial hypertrophy [147,148]. With special reference to cardiac fibrosis, it has been reported that RES can mitigate cardiac fibroblast activity in rats, downregulating the transforming-growth factor-beta/Smad 2/3 signaling pathway via overexpression of the Smad 7 inhibitor protein and silencing the miR-17 gene [149]. RES can modulate endothelial function, inhibiting overproduction of the vasoconstrictive agent ET-1 and enhancing eNOS phosphorylation, with an increase in NO production [150]. There is evidence that upregulation of ET- and a decrease in NO are involved in the pathogenesis of atherosclerosis [151]. The effects of RES on mitochondrial biogenesis have been documented. In fact, RES activates the AMPK/SIRT1/PGC1 alpha pathway, with enhancement of Nrf-1 and Nrf-2 transcription factors, thus attenuating high-glucose oxidative stress and cardiomyocyte apoptosis in diabetic mice [152]. As far as clinical trials are concerned, the effects of RES have been studied in patients with hypertension. In one study, long-term administration of RES could reduce hypertension along with standard medical therapy [153]. In a meta-analysis, in hypertensive subjects, daily RES consumption reduced SBP, but not DBP [154]. Conversely, in two other studies, the hypotensive effects of RES were not confirmed [52,155]. With special reference to vascular protection, RES long-term administration improved the FMD of the brachial artery in overweight and hypertensive individuals, stable CAD patients, and patients with metabolic syndrome, respectively [156,157,158]. In another study, acute RES administration to hypertensive patients improved FMD without changes in SBP [159]. A systematic review and meta-analysis have provided evidence that RES can modify lipid profile, diabetes, and inflammation associated with atherosclerosis in metabolic syndrome patients [160,161,162]. A few clinical trials have been conducted in patients with HF. In post-MI patients, administration of 10 mg/day of RES for 3 months improved the diastolic function [157]. In patients with angina pectoris, RES supplementation at 20 mg/day for 2 months reduced serum levels of the N-terminal prohormone brain natriuretic peptide (NT-proBNP) [163]. In patients with symptomatic systolic HF, 100 mg/day of RES supplementation improved systolic and diastolic function, as well as serum biomarkers such as NT-proBNP and IL-1 and Il-6 levels [52].
4. Curcumin
Curcumin (diferuloyl methane) is a natural polyphenol extracted from the rhizomes of the turmeric plant (Curcuma longa L.) [164]. Structurally, curcumin possesses a constitutional double bond, thus behaving as an electron donor, which mitigates ROS effects [165]. Furthermore, curcumin exerts anti-inflammatory effects, as well as modulation of lipid metabolism and the immune system [166]. In cadmium-induced hypertensive rats, curcumin administration normalized vascular dysfunction and blood pressure [167]. Similar results were achieved in Sprague rats with lead acetate- and cadmium chlorate-induced hypertension [168]. Furthermore, in spontaneous hypertensive rats, curcumin administration attenuated the coronary artery damage [169]. Additionally, in an ANG-II-induced hypertensive rat model, curcumin administration reduced the ANG-II type-I receptor-mediated vasoconstriction, thus preventing hypertension [170]. A few clinical trials have been conducted in hypertensive patients using curcumin. A group of 14 men and 24 women with an average blood pressure of 121–140/81–90 mm Hg received cavacurmin (500 mg), eicosapentaenoil acid, astaxanthin, and gamma linolenic acid for 4 weeks [171]. A significant decrease in SBP was observed only in women. In refractory or relapsing lupus nephritis patients, administration of curcumin (500 mg) for 3 months led to a significant decrease in SBP [172]. Moreover, a combination of curcumin and galactomannan (500 mg) was administered to obese subjects, with a declining trend in blood pressure and aortic stiffness and an increase in anti-inflammatory cytokines [173]. Conversely, in another study, a 12-week treatment of healthy middle-aged and older adults with 200 mg of curcumin did not modify blood pressure, despite a reduction in oxidative stress and improvement in endothelial function [174]. Previously, evidence has been provided that RES and curcumin in combination could lower oxidative stress, inflammation, and tumor growth [175]. Such a combination improved endothelial function, inhibiting the gene regulatory activity of TNF-alpha and abrogating the NF-kB pathway.
5. Extra Virgin Olive Oil Polyphenols
EVOO represents a food supply endowed with antioxidant and anti-inflammatory activities [176]. EVOO is mainly composed of monosaturated fatty acids, alpha-tocopherol, and polyphenols [177]. The phenylethanoid derivatives hydroxytyrosol (HT) and thyrosol are the major polyphenols contained in EVOO [178]. HT is the most studied EVOO polyphenol in terms of anti-inflammatory activity and CVD prevention. In healthy male Wistar rats, HT administration inhibited collagen-induced platelet aggregation in whole blood [179]. This effect has been attributed to the inhibition of platelet synthesis of TxB2, production of vascular PGI2, and an increase in vascular NO. Additionally, HT alkyl ether derivatives exerted similar effects, thus acting as anti-aggregating agents at the endothelial level [180]. In human clinical trials, HT has been studied for its capacity to attenuate the pathogenesis of atherosclerosis. In 30 hypercholesterolemic volunteers (aged 20–70 years), administration of HT derived from Coratina olives led to a normalization of SBP and lipid profile [181]. Similar results were achieved through supplementation of Body Lipid, containing HT, berberine, coenzyme Q10, and monacolin K to hypercholesterolemic individuals [182]. In another study, administration of HT and punicalagin to an adult population improved dyslipidemia and decreased SBP and DBP [183]. HT and punicalagin increased endothelial capacity and reduced ox-LDL. Furthermore, in 40 healthy volunteers, administration of HT (15 mg/day for 3 weeks) increased in blood samples antioxidant activity, oxidation biomarkers (thiols), and SOD1, while malonedialdehyde (MDA) and NO metabolites were decreased [184]. Conversely, in another study, administration of HT to human volunteers with mild hyperlipidemia did not influence CVD biomarkers, while levels of vitamin C increased [185]. In this framework, a very recent study based on the supplementation of 15 mg HT/day to patients 24 h after stroke for 45 consecutive days led to encouraging results [186]. In fact, a decrease in glycated hemoglobin and DBP and a modulation of the expression of the gene encoding for apolipoproteins were recorded. A limitation of these studies is the possible co-presence of other compounds that can also contribute to the efficacy of the treatment. This is the case of trials conducted with dietary supplementation of EVOO, where the effects of polyphenols cannot be distinguished from those of other components, such as unsaturated fatty acids.
6. Cardiovascular Effects of Wheat Polyphenols
Wheat (Triticum sp.) is widely used all over the world. There is evidence that 2–3 servings/day of whole wheat grains reduce the risk of CVD [187]. Among phenolic acids, ferulic acid is the major component of wheat, and the number of hydroxyl groups correlates with its antioxidant potential [188]. Experimentally, extracts enriched in ferulic, synaptic, and p-coumaric acids downregulated pro-inflammatory cytokines and chemokine/interferon-gamma-inducible protein 10 kDa [189]. Furthermore, fermented wheat germ polyphenols could reduce lipid metabolism in hyperlipidemic rats, activating the AMPK pathway. Clinically, wheat aleurone improved redox status in overweight/obese individuals at higher risk of CVD [190]. Ferulic acid could lower total cholesterol, triglycerides, MAD, and C-RP in hyperlipidemic individuals, thus preventing atherosclerosis outcomes [191]. The role of quercetin, a flavonol contained in whole wheat grain, has been preclinically investigated. Its athero-protective effects have been ascribed to the suppression of inflammation and apoptosis [192]. Quercetin derivatives can induce regression of atheromatous plaques, triggering autophagy and inhibiting the breakdown of elastin, macrophage infiltration, and production of both matrix-metallo-proteinase 9 and adhesion molecules [193,194]. Additionally, quercetin could prevent cardiac/ischemia and/or reperfusion injury through regulation of the PI3K/Akt pathway [195] (Table 5).

Table 5.
Cardiovascular Effects of Polyphenols.
6.1. Adverse Effects of Polyphenols
A few side effects attributed to polyphenol administration have been recorded. For instance, RES administration to humans may lead to emesis, mild hepatic dysfunction, and diarrhea [196,197]. In rats, high oral doses of RES (3g/Kg/day) provoked nephrotoxicity [198]. Additionally, flavonoids can cause mild gastrointestinal symptoms, insomnia, headache, palpitations, and an increase in serum transaminases [199,200]. Other side effects of polyphenol ingestion include a reduced gastrointestinal transport of folic acid, thiamine, and iron [201].
There are other toxicity considerations. Polyphenols’ long-term safety data are still being investigated, particularly for high doses. Some polyphenols can exhibit carcinogenic or genotoxic effects at high concentrations, and interactions with medications or other substances are also possible. In particular, high doses of certain polyphenols like caffeic acid have been linked to tumor development in animal studies [202]. Polyphenols can interfere with iron absorption, potentially leading to iron deficiency in susceptible individuals. They can also interact with medications, potentially affecting their efficacy or increasing side effects [203]. Some polyphenols can act as pro-oxidants, potentially contributing to cellular damage under certain conditions through the formation of quinone, which can react with cysteine in glutathione or proteins. This process generates ROS and can cause DNA damage [204]. High intake of certain polyphenols, particularly from supplements, has been associated with liver toxicity [205]. Important factors to consider include the following:
- -
- The amount of polyphenols consumed is crucial. Natural dietary sources generally provide safe levels, but high-dose supplementation raises concerns [206].
- -
- Metabolic differences between individuals can influence how polyphenols are processed and their effects [207].
- -
- More long-term human studies are needed to fully assess the safety of various polyphenol intakes.
- -
- Polyphenols in fruits, vegetables, and other plant-based foods are generally considered safe due to lower concentrations and the presence of other beneficial compounds [44].
- -
- High-dose polyphenol supplements require careful consideration due to the potential for adverse effects [205].
In summary, while polyphenols offer potential health benefits, especially from dietary sources, it is important to be aware of the potential risks associated with high-dose supplementation and to consider individual factors when assessing polyphenol intake.
6.2. Caveats in Polyphenols
In clinical trials, polyphenols, in particular RES, a compound found in red wine and other sources, have shown promising results in preclinical studies for various health benefits, but clinical trials often yield inconsistent results [208]. This discrepancy can be attributed to factors like varying dosages, individual differences in absorption and metabolism, the complexity of translating findings from animal models to humans, and, finally, associated food in the diet; unhealthy food, in fact, can blunt the positive effect of polyphenols. In particular, diets high in processed foods, saturated fats, sugars, and excessive alcohol can negatively impact the gut microbiome, which is crucial for polyphenol absorption and utilization [209]; these variables could be relatively difficult to control in clinical trials.
In the context of polyphenol consumption patterns, the beneficial effects of red wine, fruit juices, green tea, and chocolate have been reported previously. However, it should be stressed that the health impact of such kinds of food depends not only on polyphenol content, but also on consumption pattern, dose, and dietary context. For example, the culture of drinking red wine with meals, as part of the Mediterranean diet, differs significantly from unhealthy patterns of alcohol consumption [210]. Similarly, frequent intake of fruit juices or sweetened tea may contribute to metabolic disorders due to high fructose content [211]. Therefore, it is essential to emphasize that quantity, context, and overall healthy lifestyle are key factors modulating the effects of polyphenols.
Regarding how polyphenols interact with medications, polyphenols, found in many plant-based foods and supplements, can interact with certain medications, potentially affecting how the body processes and utilizes those drugs. These interactions can alter drug metabolism, absorption, and excretion, possibly leading to reduced drug efficacy or increased toxicity. This can be particularly critical for drugs like direct oral anticoagulants (DOACs) with a narrow therapeutic index; supplements containing polyphenols alongside DOAC therapy carry the risk of bleeding or a reduction in DOAC therapeutic effect. In fact, they have antiplatelet effects, which in conjunction with DOACs can potentially significantly increase the risk of bleeding, as is obviously the case when combining antiplatelet drugs with DOACs [212]. These interactions primarily occur through the modulation of drug metabolism and absorption by affecting enzymes like CYP450 and transporters like P-glycoprotein. Some polyphenols can inhibit or induce these enzymes and transporters, leading to altered drug levels in the body. This is particularly critical for those drugs with a narrow therapeutic index, like the above-mentioned DOAC, but also warfarin, cyclosporine A, and digoxin [213]. However, concerns regarding medication interactions arise only with the promotion of polyphenol consumption at levels far above natural occurrence, that is, when they are assumed as supplements [214].
7. Conclusions and Future Trends
There is a large body of evidence that polyphenols exert antioxidant and anti-inflammatory activities, thus regulating major pathways involved in cellular activation and metabolism. In this respect, polyphenols exert beneficial effects on CVD, such as stroke, hypertension, and HF. For example, the MD is a balanced diet, which promotes human health, including prevention of CVD. However, dietary foods contain many compounds, e.g., vitamins, minerals, polyphenols, and unsaturated fatty acids, all endowed with protective effects in the host. Therefore, in this review, emphasis has been placed on the cardioprotective effects of single polyphenols alone or a combination, to rule out potential effects of other dietary compounds. Undoubtedly, preclinical studies conducted with a variety of polyphenols suggest their beneficial effects on CVD. On the other hand, clinical trials are still few and, sometimes, based on a low number of participants. Therefore, the actual effects of polyphenol intake on healthy and unhealthy human populations need a more robust confirmation with more clinical trials.
Author Contributions
The authors confirm their contribution to the paper. Study conception and design: E.J.; data collection: E.J.; analysis and interpretation of results: E.J. and C.C.; draft manuscript: E.J. and C.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| ANG II | Angiotensin II |
| CAD | Coronary Artery Disease |
| COX | Cyclooxygenase |
| CRP | C-Reactive Protein |
| CVD | Cardiovascular Disease |
| DBP | Diastolic Blood Pressure |
| ECs | Endothelial Cells |
| EGCG | Epigallo-Catechin-Gallate |
| ENOS | Endothelial Nitric Oxide Synthase |
| ET-1 | Endothelin-1 |
| EVO | Extra Virgin Olive Oil |
| FGM | Fermented Grape Marc |
| FMD | Flood-Mediated Dilation |
| HF | Heart Failure |
| ICAM | Intercellular Adhesion Molecule-1 |
| IL | Interleukin |
| LOX | Lipoxigenase |
| MAD | Malondialdehyde |
| MAPK | Mitogen-Activated Protein Kinase |
| MD | Mediterranean Diet |
| MI | Myocardial Ischemia |
| NF-kB | Nuclear Factor Kappa-Light Chain Enhancer of Activated B cells |
| NLRs | Nucleotide-Binding Domain and Leucine-Rich Repeat Containing Receptors |
| NO | Nitric Oxide |
| oxLDL | Oxidized Lipoproteins |
| PDE | Phosphodiesterase |
| PG | Prostaglandin |
| PGI2 | Prostacyclin-I 2 |
| PRR | Pattern Recognition Receptors |
| PVAs | Hydroxy-Phenyl-Valeric Acids |
| PVLs | Hydroxy-Phenyl-Gamma-Valerolactones |
| RES | Resveratrol |
| ROS | Reactive Oxygen Species |
| SBP | Systolic Blood Pressure |
| SGLT1 | Sodium-Glucose-Linked Transporter 1 |
| SOD | Superoxide Dismutase |
| TXA | Thromboxane |
| TMAO | Trimethyl-Amine-Oxide |
| TNF | Tumor Necrosis Factor-alpha |
| VCM | Vascular Cell Adhesion-1 |
References
- Laslett, L.J.; Alagona, P., Jr.; Clark, B.A., 3rd; Drozda, J.P., Jr.; Saldivar, F.; Wilson, S.R.; Poe, C.; Hart, M. The worldwide environment of cardiovascular disease: Prevalence, diagnosis, therapy, and policy issues: A report from the American College of Cardiology. J. Am. Coll. Cardiol. 2012, 60 (Suppl. S25), S1–S49. [Google Scholar] [CrossRef] [PubMed]
- Force, U.S.P.S.T.; Curry, S.J.; Krist, A.H.; Owens, D.K.; Barry, M.J.; Caughey, A.B.; Davidson, K.W.; Doubeni, C.A.; Epling, J.W., Jr.; Kemper, A.R.; et al. Risk Assessment for Cardiovascular Disease with Nontraditional Risk Factors: US Preventive Services Task Force Recommendation Statement. JAMA 2018, 320, 272–280. [Google Scholar] [CrossRef]
- Knowles, J.W.; Ashley, E.A. Cardiovascular disease: The rise of the genetic risk score. PLoS Med. 2018, 15, e1002546. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, X.; Millican, R.; Sherwood, J.; Martin, S.; Jo, H.; Yoon, Y.S.; Brott, B.C.; Jun, H.W. Recent advances in nanomaterials for therapy and diagnosis for atherosclerosis. Adv. Drug Deliv. Rev. 2021, 170, 142–199. [Google Scholar] [CrossRef] [PubMed]
- Steven, S.; Frenis, K.; Oelze, M.; Kalinovic, S.; Kuntic, M.; Bayo Jimenez, M.T.; Vujacic-Mirski, K.; Helmstadter, J.; Kroller-Schon, S.; Munzel, T.; et al. Vascular Inflammation and Oxidative Stress: Major Triggers for Cardiovascular Disease. Oxid. Med. Cell Longev. 2019, 2019, 7092151. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liao, R.; Zhang, S.; Weng, H.; Liu, Y.; Tao, T.; Yu, F.; Li, G.; Wu, J. Promising remedies for cardiovascular disease: Natural polyphenol ellagic acid and its metabolite urolithins. Phytomedicine 2023, 116, 154867. [Google Scholar] [CrossRef] [PubMed]
- Caiati, C.; Stanca, A.; Lepera, M.E. Free Radicals and Obesity-Related Chronic Inflammation Contrasted by Antioxidants: A New Perspective in Coronary Artery Disease. Metabolites 2023, 13, 712. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, I.; Wilairatana, P.; Saqib, F.; Nasir, B.; Wahid, M.; Latif, M.F.; Iqbal, A.; Naz, R.; Mubarak, M.S. Plant Polyphenols and Their Potential Benefits on Cardiovascular Health: A Review. Molecules 2023, 28, 6403. [Google Scholar] [CrossRef] [PubMed]
- Koelman, L.; Egea Rodrigues, C.; Aleksandrova, K. Effects of Dietary Patterns on Biomarkers of Inflammation and Immune Responses: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Adv. Nutr. 2022, 13, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Lisco, G.; Giagulli, V.A.; De Pergola, G.; Guastamacchia, E.; Jirillo, E.; Triggiani, V. Pancreatic Macrophages and their Diabetogenic Effects: Highlight on Several Metabolic Scenarios and Dietary Approach. Endocr. Metab. Immune Disord. Drug Targets 2023, 23, 304–315. [Google Scholar] [CrossRef] [PubMed]
- Soldati, L.; Di Renzo, L.; Jirillo, E.; Ascierto, P.A.; Marincola, F.M.; De Lorenzo, A. The influence of diet on anti-cancer immune responsiveness. J. Transl. Med. 2018, 16, 75. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Arellano, A.; Ramallal, R.; Ruiz-Canela, M.; Salas-Salvado, J.; Corella, D.; Shivappa, N.; Schroder, H.; Hebert, J.R.; Ros, E.; Gomez-Garcia, E.; et al. Dietary Inflammatory Index and Incidence of Cardiovascular Disease in the PREDIMED Study. Nutrients 2015, 7, 4124–4138. [Google Scholar] [CrossRef] [PubMed]
- Rochlani, Y.; Pothineni, N.V.; Kovelamudi, S.; Mehta, J.L. Metabolic syndrome: Pathophysiology, management, and modulation by natural compounds. Ther. Adv. Cardiovasc. Dis. 2017, 11, 215–225. [Google Scholar] [CrossRef] [PubMed]
- van den Brink, W.; van Bilsen, J.; Salic, K.; Hoevenaars, F.P.M.; Verschuren, L.; Kleemann, R.; Bouwman, J.; Ronnett, G.V.; van Ommen, B.; Wopereis, S. Current and Future Nutritional Strategies to Modulate Inflammatory Dynamics in Metabolic Disorders. Front. Nutr. 2019, 6, 129. [Google Scholar] [CrossRef] [PubMed]
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouysegu, L. Plant polyphenols: Chemical properties, biological activities, and synthesis. Angew. Chem. Int. Ed. Engl. 2011, 50, 586–621. [Google Scholar] [CrossRef] [PubMed]
- Magrone, T.; Magrone, M.; Russo, M.A.; Jirillo, E. Recent Advances on the Anti-Inflammatory and Antioxidant Properties of Red Grape Polyphenols: In Vitro and In Vivo Studies. Antioxidants 2019, 9, 35. [Google Scholar] [CrossRef] [PubMed]
- Serreli, G.; Boronat, A.; De la Torre, R.; Rodriguez-Morato, J.; Deiana, M. Cardiovascular and Metabolic Benefits of Extra Virgin Olive Oil Phenolic Compounds: Mechanistic Insights from In Vivo Studies. Cells 2024, 13, 1555. [Google Scholar] [CrossRef] [PubMed]
- Briones-Valdivieso, C.; Briones, F.; Orellana-Urzua, S.; Chichiarelli, S.; Saso, L.; Rodrigo, R. Novel Multi-Antioxidant Approach for Ischemic Stroke Therapy Targeting the Role of Oxidative Stress. Biomedicines 2024, 12, 501. [Google Scholar] [CrossRef] [PubMed]
- Godos, J.; Ferri, R.; Lanza, G.; Caraci, F.; Vistorte, A.O.R.; Yelamos Torres, V.; Grosso, G.; Castellano, S. Mediterranean Diet and Sleep Features: A Systematic Review of Current Evidence. Nutrients 2024, 16, 282. [Google Scholar] [CrossRef] [PubMed]
- Andriantsitohaina, R.; Auger, C.; Chataigneau, T.; Etienne-Selloum, N.; Li, H.; Martinez, M.C.; Schini-Kerth, V.B.; Laher, I. Molecular mechanisms of the cardiovascular protective effects of polyphenols. Br. J. Nutr. 2012, 108, 1532–1549. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Remesy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.L.; Zahradka, P.; Taylor, C.G. Efficacy of flavonoids in the management of high blood pressure. Nutr. Rev. 2015, 73, 799–822. [Google Scholar] [CrossRef] [PubMed]
- Crozier, A.; Del Rio, D.; Clifford, M.N. Bioavailability of dietary flavonoids and phenolic compounds. Mol. Asp. Med. 2010, 31, 446–467. [Google Scholar] [CrossRef] [PubMed]
- Dubrovina, A.S.; Kiselev, K.V. Regulation of stilbene biosynthesis in plants. Planta 2017, 246, 597–623. [Google Scholar] [CrossRef] [PubMed]
- Oyagbemi, A.A.; Omobowale, T.O.; Ola-Davies, O.E.; Asenuga, E.R.; Ajibade, T.O.; Adejumobi, O.A.; Arojojoye, O.A.; Afolabi, J.M.; Ogunpolu, B.S.; Falayi, O.O.; et al. Quercetin attenuates hypertension induced by sodium fluoride via reduction in oxidative stress and modulation of HSP 70/ERK/PPARgamma signaling pathways. Biofactors 2018, 44, 465–479. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.M.; Hernandez Bautista, R.J.; Sandhu, M.A.; Hussein, O.E. Beneficial Effects of Citrus Flavonoids on Cardiovascular and Metabolic Health. Oxid. Med. Cell Longev. 2019, 2019, 5484138. [Google Scholar] [CrossRef] [PubMed]
- Gal, R.; Halmosi, R.; Gallyas, F., Jr.; Tschida, M.; Mutirangura, P.; Toth, K.; Alexy, T.; Czopf, L. Resveratrol and beyond: The Effect of Natural Polyphenols on the Cardiovascular System: A Narrative Review. Biomedicines 2023, 11, 2888. [Google Scholar] [CrossRef] [PubMed]
- Bohn, T. Bioavailability of Non-Provitamin A Carotenoids. Curr. Nutr. Food Sci. 2008, 4, 240–258. [Google Scholar] [CrossRef]
- Day, A.J.; Canada, F.J.; Diaz, J.C.; Kroon, P.A.; McLauchlan, R.; Faulds, C.B.; Plumb, G.W.; Morgan, M.R.; Williamson, G. Dietary flavonoid and isoflavone glycosides are hydrolysed by the lactase site of lactase phlorizin hydrolase. FEBS Lett. 2000, 468, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Bennick, A. Interaction of plant polyphenols with salivary proteins. Crit. Rev. Oral Biol. Med. 2002, 13, 184–196. [Google Scholar] [CrossRef] [PubMed]
- da Silva, V.M.; Sato, J.A.P.; Araujo, J.N.; Squina, F.M.; Muniz, J.R.C.; Riske, K.A.; Garcia, W. Systematic studies of the interactions between a model polyphenol compound and microbial beta-glucosidases. PLoS ONE 2017, 12, e0181629. [Google Scholar] [CrossRef]
- Kawabata, K.; Yoshioka, Y.; Terao, J. Role of Intestinal Microbiota in the Bioavailability and Physiological Functions of Dietary Polyphenols. Molecules 2019, 24, 370. [Google Scholar] [CrossRef] [PubMed]
- Kosinska, A.; Andlauer, W. Cocoa polyphenols are absorbed in Caco-2 cell model of intestinal epithelium. Food Chem. 2012, 135, 999–1005. [Google Scholar] [CrossRef] [PubMed]
- Henry-Vitrac, C.; Desmouliere, A.; Girard, D.; Merillon, J.M.; Krisa, S. Transport, deglycosylation, and metabolism of trans-piceid by small intestinal epithelial cells. Eur. J. Nutr. 2006, 45, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Rahman, B.; Schneider, H.P.; Broer, A.; Deitmer, J.W.; Broer, S. Helix 8 and helix 10 are involved in substrate recognition in the rat monocarboxylate transporter MCT1. Biochemistry 1999, 38, 11577–11584. [Google Scholar] [CrossRef] [PubMed]
- Konishi, Y.; Kobayashi, S.; Shimizu, M. Transepithelial transport of p-coumaric acid and gallic acid in Caco-2 cell monolayers. Biosci. Biotechnol. Biochem. 2003, 67, 2317–2324. [Google Scholar] [CrossRef] [PubMed]
- Selma, M.V.; Espin, J.C.; Tomas-Barberan, F.A. Interaction between phenolics and gut microbiota: Role in human health. J. Agric. Food Chem. 2009, 57, 6485–6501. [Google Scholar] [CrossRef] [PubMed]
- Scalbert, A.; Morand, C.; Manach, C.; Rémésy, C. Absorption and metabolism of polyphenols in the gut and impact on health. Biomed. Pharmacother. 2002, 56, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Giovannelli, L.; Testa, G.; De Filippo, C.; Cheynier, V.; Clifford, M.N.; Dolara, P. Effect of complex polyphenols and tannins from red wine on DNA oxidative damage of rat colon mucosa in vivo. Eur. J. Nutr. 2000, 39, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Caderni, G.; De Filippo, C.; Luceri, C.; Salvadori, M.; Giannini, A.; Biggeri, A.; Remy, S.; Cheynier, V.; Dolara, P. Effects of black tea, green tea and wine extracts on intestinal carcinogenesis induced by azoxymethane in F344 rats. Carcinogenesis 2000, 21, 1965–1969. [Google Scholar] [CrossRef] [PubMed]
- Leonarduzzi, G.; Testa, G.; Sottero, B.; Gamba, P.; Poli, G. Design and development of nanovehicle-based delivery systems for preventive or therapeutic supplementation with flavonoids. Curr. Med. Chem. 2010, 17, 74–95. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.D.; Sang, S.; Yang, C.S. Biotransformation of green tea polyphenols and the biological activities of those metabolites. Mol. Pharm. 2007, 4, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Bohn, T.; Blackwood, M.; Francis, D.; Tian, Q.; Schwartz, S.J.; Clinton, S.K. Bioavailability of phytochemical constituents from a novel soy fortified lycopene rich tomato juice developed for targeted cancer prevention trials. Nutr. Cancer 2013, 65, 919–929. [Google Scholar] [CrossRef] [PubMed]
- Kardum, N.; Glibetic, M. Polyphenols and Their Interactions With Other Dietary Compounds: Implications for Human Health. Adv. Food Nutr. Res. 2018, 84, 103–144. [Google Scholar] [CrossRef] [PubMed]
- Vitali Čepo, D.; Radić, K.; Turčić, P.; Anić, D.; Komar, B.; Šalov, M. Food (Matrix) Effects on Bioaccessibility and Intestinal Permeability of Major Olive Antioxidants. Foods 2020, 9, 1831. [Google Scholar] [CrossRef] [PubMed]
- Sejbuk, M.; Mirończuk-Chodakowska, I.; Karav, S.; Witkowska, A.M. Dietary Polyphenols, Food Processing and Gut Microbiome: Recent Findings on Bioavailability, Bioactivity, and Gut Microbiome Interplay. Antioxidants 2024, 13, 1220. [Google Scholar] [CrossRef] [PubMed]
- Rein, M.J.; Renouf, M.; Cruz-Hernandez, C.; Actis-Goretta, L.; Thakkar, S.K.; da Silva Pinto, M. Bioavailability of bioactive food compounds: A challenging journey to bioefficacy. Br. J. Clin. Pharmacol. 2013, 75, 588–602. [Google Scholar] [CrossRef] [PubMed]
- Pollard, S.E.; Kuhnle, G.G.; Vauzour, D.; Vafeiadou, K.; Tzounis, X.; Whiteman, M.; Rice-Evans, C.; Spencer, J.P. The reaction of flavonoid metabolites with peroxynitrite. Biochem. Biophys. Res. Commun. 2006, 350, 960–968. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive Oxygen Species, Oxidative Damage, and Antioxidative Defense Mechanism in Plants under Stressful Conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Krinsky, N.I. Mechanism of action of biological antioxidants. Proc. Soc. Exp. Biol. Med. 1992, 200, 248–254. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, J.D.; Mallet, A.I.; McAnlis, G.T.; Young, I.S.; Halliwell, B.; Sanders, T.A.; Wiseman, H. Consumption of flavonoids in onions and black tea: Lack of effect on F2-isoprostanes and autoantibodies to oxidized LDL in healthy humans. Am. J. Clin. Nutr. 2001, 73, 1040–1044. [Google Scholar] [CrossRef] [PubMed]
- Gal, R.; Deres, L.; Horvath, O.; Eros, K.; Sandor, B.; Urban, P.; Soos, S.; Marton, Z.; Sumegi, B.; Toth, K.; et al. Resveratrol Improves Heart Function by Moderating Inflammatory Processes in Patients with Systolic Heart Failure. Antioxidants 2020, 9, 1108. [Google Scholar] [CrossRef] [PubMed]
- Kahkonen, M.P.; Heinonen, M. Antioxidant activity of anthocyanins and their aglycons. J. Agric. Food Chem. 2003, 51, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Lotito, S.B.; Frei, B. The increase in human plasma antioxidant capacity after apple consumption is due to the metabolic effect of fructose on urate, not apple-derived antioxidant flavonoids. Free Radic. Biol. Med. 2004, 37, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Cines, D.B.; Pollak, E.S.; Buck, C.A.; Loscalzo, J.; Zimmerman, G.A.; McEver, R.P.; Pober, J.S.; Wick, T.M.; Konkle, B.A.; Schwartz, B.S.; et al. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood 1998, 91, 3527–3561. [Google Scholar] [PubMed]
- Sanches-Silva, A.; Testai, L.; Nabavi, S.F.; Battino, M.; Pandima Devi, K.; Tejada, S.; Sureda, A.; Xu, S.; Yousefi, B.; Majidinia, M.; et al. Therapeutic potential of polyphenols in cardiovascular diseases: Regulation of mTOR signaling pathway. Pharmacol. Res. 2020, 152, 104626. [Google Scholar] [CrossRef] [PubMed]
- Duarte, J.; Andriambeloson, E.; Diebolt, M.; Andriantsitohaina, R. Wine polyphenols stimulate superoxide anion production to promote calcium signaling and endothelial-dependent vasodilatation. Physiol. Res. 2004, 53, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, S.; Nakamura, Y.; Takahashi, M.; Ouchi, Y.; Hosoda, K.; Nozawa, M.; Kinoshita, M. Effect of red wine and ethanol on production of nitric oxide in healthy subjects. Am. J. Cardiol. 2001, 87, 1029–1031. [Google Scholar] [CrossRef] [PubMed]
- Li, H.F.; Chen, S.A.; Wu, S.N. Evidence for the stimulatory effect of resveratrol on Ca(2+)-activated K+ current in vascular endothelial cells. Cardiovasc. Res. 2000, 45, 1035–1045. [Google Scholar] [CrossRef] [PubMed]
- Andriambeloson, E.; Stoclet, J.C.; Andriantsitohaina, R. Mechanism of endothelial nitric oxide-dependent vasorelaxation induced by wine polyphenols in rat thoracic aorta. J. Cardiovasc. Pharmacol. 1999, 33, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.; Andriambeloson, E.; Takeda, K.; Andriantsitohaina, R. Red wine polyphenols increase calcium in bovine aortic endothelial cells: A basis to elucidate signalling pathways leading to nitric oxide production. Br. J. Pharmacol. 2002, 135, 1579–1587. [Google Scholar] [CrossRef] [PubMed]
- Schramm, D.D.; Wang, J.F.; Holt, R.R.; Ensunsa, J.L.; Gonsalves, J.L.; Lazarus, S.A.; Schmitz, H.H.; German, J.B.; Keen, C.L. Chocolate procyanidins decrease the leukotriene-prostacyclin ratio in humans and human aortic endothelial cells. Am. J. Clin. Nutr. 2001, 73, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Ndiaye, M.; Chataigneau, M.; Lobysheva, I.; Chataigneau, T.; Schini-Kerth, V.B. Red wine polyphenol-induced, endothelium-dependent NO-mediated relaxation is due to the redox-sensitive PI3-kinase/Akt-dependent phosphorylation of endothelial NO-synthase in the isolated porcine coronary artery. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2005, 19, 455–457. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Conklin, B.S.; Lin, P.H.; Lumsden, A.B.; Yao, Q.; Chen, C. Red wine prevents homocysteine-induced endothelial dysfunction in porcine coronary arteries. J. Surg. Res. 2003, 115, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Lugnier, C.; Schini, V.B. Characterization of cyclic nucleotide phosphodiesterases from cultured bovine aortic endothelial cells. Biochem. Pharmacol. 1990, 39, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Vita, J.A. Polyphenols and cardiovascular disease: Effects on endothelial and platelet function. Am. J. Clin. Nutr. 2005, 81 (Suppl. S1), 292S–297S. [Google Scholar] [CrossRef] [PubMed]
- Lerman, A.; Zeiher, A.M. Endothelial function: Cardiac events. Circulation 2005, 111, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Kiviniemi, T. Assessment of coronary blood flow and the reactivity of the microcirculation non-invasively with transthoracic echocardiography. Clin. Physiol. Funct. Imaging 2008, 28, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Chandraratna, P.A.; Nimalasuriya, A.R.; Vlachonassios, K.D.; Mathews, S.J.; Kedes, W.; Marwah, O.S.; Saad, M. Usefulness of the response of flow velocity in the left anterior descending coronary artery to the cold pressor test for evaluating endothelium-dependent vascular relaxation in the coronary microvasculature by transesophageal echocardiography in subjects with angiographically normal coronary arteries. Am. J. Cardiol. 1999, 84, 1362–1365. [Google Scholar] [CrossRef] [PubMed]
- Caiati, C.; Pollice, P.; Iacovelli, F.; Sturda, F.; Lepera, M.E. Accelerated stenotic flow in the left anterior descending coronary artery explains the causes of impaired coronary flow reserve: An integrated transthoracic enhanced Doppler study. Front. Cardiovasc. Med. 2023, 10, 1186983. [Google Scholar] [CrossRef] [PubMed]
- Magrone, T.; Jirillo, E. Potential application of dietary polyphenols from red wine to attaining healthy ageing. Curr. Top. Med. Chem. 2011, 11, 1780–1796. [Google Scholar] [CrossRef] [PubMed]
- Magrone, T.; Jirillo, E. Polyphenols from red wine are potent modulators of innate and adaptive immune responsiveness. Proc. Nutr. Soc. 2010, 69, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Marzulli, G.; Magrone, T.; Kawaguchi, K.; Kumazawa, Y.; Jirillo, E. Fermented grape marc (FGM): Immunomodulating properties and its potential exploitation in the treatment of neurodegenerative diseases. Curr. Pharm. Des. 2012, 18, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Marzulli, G.; Magrone, T.; Vonghia, L.; Kaneko, M.; Takimoto, H.; Kumazawa, Y.; Jirillo, E. Immunomodulating and anti-allergic effects of Negroamaro and Koshu Vitis vinifera fermented grape marc (FGM). Curr. Pharm. Des. 2014, 20, 864–868. [Google Scholar] [CrossRef] [PubMed]
- Al-Khayri, J.M.; Sahana, G.R.; Nagella, P.; Joseph, B.V.; Alessa, F.M.; Al-Mssallem, M.Q. Flavonoids as Potential Anti-Inflammatory Molecules: A Review. Molecules 2022, 27, 2901. [Google Scholar] [CrossRef] [PubMed]
- Krauth, V.; Bruno, F.; Pace, S.; Jordan, P.M.; Temml, V.; Preziosa Romano, M.; Khan, H.; Schuster, D.; Rossi, A.; Filosa, R.; et al. Highly potent and selective 5-lipoxygenase inhibition by new, simple heteroaryl-substituted catechols for treatment of inflammation. Biochem. Pharmacol. 2023, 208, 115385. [Google Scholar] [CrossRef] [PubMed]
- Martinon, F.; Burns, K.; Tschopp, J. The inflammasome: A molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol. Cell 2002, 10, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Gaidt, M.M.; Hornung, V. Pore formation by GSDMD is the effector mechanism of pyroptosis. EMBO J. 2016, 35, 2167–2169. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zeng, X.; Li, X.; Mehta, J.L.; Wang, X. Role of NLRP3 inflammasome in the pathogenesis of cardiovascular diseases. Basic Res. Cardiol. 2018, 113, 5. [Google Scholar] [CrossRef] [PubMed]
- Villalva, M.; Martinez-Garcia, J.J.; Jaime, L.; Santoyo, S.; Pelegrin, P.; Perez-Jimenez, J. Polyphenols as NLRP3 inflammasome modulators in cardiometabolic diseases: A review of in vivo studies. Food Funct. 2023, 14, 9534–9553. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Gu, X.; Wang, H.; Ding, S. Resveratrol improves cardiac function and left ventricular fibrosis after myocardial infarction in rats by inhibiting NLRP3 inflammasome activity and the TGF-beta1/SMAD2 signaling pathway. PeerJ 2021, 9, e11501. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Mou, S.Q.; Li, W.J.; Zhang, N.; Zhou, Z.Y.; Ding, W.; Bian, Z.Y.; Liao, H.H. Resveratrol Inhibits Ischemia-Induced Myocardial Senescence Signals and NLRP3 Inflammasome Activation. Oxid. Med. Cell Longev. 2020, 2020, 2647807. [Google Scholar] [CrossRef] [PubMed]
- Lv, D.; Cheng, X.; Tang, L.; Jiang, M. The cardioprotective effect of total flavonoids on myocardial ischemia/reperfusion in rats. Biomed. Pharmacother. 2017, 88, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; RuXian, G. Didymin, a natural flavonoid, relieves the progression of myocardial infarction via inhibiting the NLR family pyrin domain containing 3 inflammasome. Pharm. Biol. 2022, 60, 2319–2327. [Google Scholar] [CrossRef] [PubMed]
- Chiva-Blanch, G.; Urpi-Sarda, M.; Ros, E.; Arranz, S.; Valderas-Martinez, P.; Casas, R.; Sacanella, E.; Llorach, R.; Lamuela-Raventos, R.M.; Andres-Lacueva, C.; et al. Dealcoholized red wine decreases systolic and diastolic blood pressure and increases plasma nitric oxide: Short communication. Circ. Res. 2012, 111, 1065–1068. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Sacanella, E.; Badia, E.; Antunez, E.; Nicolas, J.M.; Fernandez-Sola, J.; Rotilio, D.; de Gaetano, G.; Rubin, E.; Urbano-Marquez, A. Different effects of red wine and gin consumption on inflammatory biomarkers of atherosclerosis: A prospective randomized crossover trial. Effects of wine on inflammatory markers. Atherosclerosis 2004, 175, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Agell, M.; Sacanella, E.; Tobias, E.; Monagas, M.; Antunez, E.; Zamora-Ros, R.; Andres-Lacueva, C.; Lamuela-Raventos, R.M.; Fernandez-Sola, J.; Nicolas, J.M.; et al. Inflammatory markers of atherosclerosis are decreased after moderate consumption of cava (sparkling wine) in men with low cardiovascular risk. J. Nutr. 2007, 137, 2279–2284. [Google Scholar] [CrossRef] [PubMed]
- Sahebkar, A.; Serban, C.; Ursoniu, S.; Wong, N.D.; Muntner, P.; Graham, I.M.; Mikhailidis, D.P.; Rizzo, M.; Rysz, J.; Sperling, L.S.; et al. Lack of efficacy of resveratrol on C-reactive protein and selected cardiovascular risk factors--Results from a systematic review and meta-analysis of randomized controlled trials. Int. J. Cardiol. 2015, 189, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Zhong, R.; Liu, Y.; Jiang, X.; Tang, X.; Li, Z.; Xia, M.; Ling, W. Effects of bayberry juice on inflammatory and apoptotic markers in young adults with features of non-alcoholic fatty liver disease. Nutrition 2014, 30, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, B.; Saedisomeolia, A.; Wood, L.G.; Yaseri, M.; Tavasoli, S. Effects of pomegranate extract supplementation on inflammation in overweight and obese individuals: A randomized controlled clinical trial. Complement. Ther. Clin. Pract. 2016, 22, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Larmo, P.S.; Yang, B.; Hurme, S.A.; Alin, J.A.; Kallio, H.P.; Salminen, E.K.; Tahvonen, R.L. Effect of a low dose of sea buckthorn berries on circulating concentrations of cholesterol, triacylglycerols, and flavonols in healthy adults. Eur. J. Nutr. 2009, 48, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Mathison, B.D.; Kimble, L.L.; Kaspar, K.L.; Khoo, C.; Chew, B.P. Consumption of cranberry beverage improved endogenous antioxidant status and protected against bacteria adhesion in healthy humans: A randomized controlled trial. Nutr. Res. 2014, 34, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Ono-Moore, K.D.; Snodgrass, R.G.; Huang, S.; Singh, S.; Freytag, T.L.; Burnett, D.J.; Bonnel, E.L.; Woodhouse, L.R.; Zunino, S.J.; Peerson, J.M.; et al. Postprandial Inflammatory Responses and Free Fatty Acids in Plasma of Adults Who Consumed a Moderately High-Fat Breakfast with and without Blueberry Powder in a Randomized Placebo-Controlled Trial. J. Nutr. 2016, 146, 1411–1419. [Google Scholar] [CrossRef] [PubMed]
- Zern, T.L.; Wood, R.J.; Greene, C.; West, K.L.; Liu, Y.; Aggarwal, D.; Shachter, N.S.; Fernandez, M.L. Grape polyphenols exert a cardioprotective effect in pre- and postmenopausal women by lowering plasma lipids and reducing oxidative stress. J. Nutr. 2005, 135, 1911–1917. [Google Scholar] [CrossRef] [PubMed]
- Karlsen, A.; Paur, I.; Bohn, S.K.; Sakhi, A.K.; Borge, G.I.; Serafini, M.; Erlund, I.; Laake, P.; Tonstad, S.; Blomhoff, R. Bilberry juice modulates plasma concentration of NF-kappaB related inflammatory markers in subjects at increased risk of CVD. Eur. J. Nutr. 2010, 49, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Falk, E. Pathogenesis of atherosclerosis. J. Am. Coll. Cardiol. 2006, 47, C7–C12. [Google Scholar] [CrossRef] [PubMed]
- Caiati, C.; Pollice, P.; Favale, S.; Lepera, M.E. The Herbicide Glyphosate and Its Apparently Controversial Effect on Human Health: An Updated Clinical Perspective. Endocr. Metab. Immune Disord. Drug Targets 2020, 20, 489–505. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, P.N. Molecular biology of atherosclerosis. Physiol. Rev. 2013, 93, 1317–1542. [Google Scholar] [CrossRef] [PubMed]
- Caiati, C. Contrast-Enhanced Ultrasound Reveals That Lipoprotein Apheresis Improves Myocardial But Not Skeletal Muscle Perfusion. JACC Cardiovasc. Imaging 2019, 12, 1441–1443. [Google Scholar] [CrossRef] [PubMed]
- Gould, K.L.; Ornish, D.; Scherwitz, L.; Brown, S.; Edens, R.P.; Hess, M.J.; Mullani, N.; Bolomey, L.; Dobbs, F.; Armstrong, W.T.; et al. Changes in myocardial perfusion abnormalities by positron emission tomography after long-term, intense risk factor modification. JAMA J. Am. Med. Assoc. 1995, 274, 894–901. [Google Scholar] [CrossRef] [PubMed]
- Caiati, C.; Iacovelli, F.; Mancini, G.; Lepera, M.E. Hidden Coronary Atherosclerosis Assessment but Not Coronary Flow Reserve Helps to Explain the Slow Coronary Flow Phenomenon in Patients with Angiographically Normal Coronary Arteries. Diagnostics 2022, 12, 2173. [Google Scholar] [CrossRef] [PubMed]
- Hansson, G.K.; Libby, P. The immune response in atherosclerosis: A double-edged sword. Nat. Rev. Immunol. 2006, 6, 508–519. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.B.; Mengi, S.A.; Xu, Y.J.; Arneja, A.S.; Dhalla, N.S. Pathogenesis of atherosclerosis: A multifactorial process. Exp. Clin. Cardiol. 2002, 7, 40–53. [Google Scholar] [PubMed]
- Cai, H.; Harrison, D.G. Endothelial dysfunction in cardiovascular diseases: The role of oxidant stress. Circ. Res. 2000, 87, 840–844. [Google Scholar] [CrossRef] [PubMed]
- Jennings, L.K. Mechanisms of platelet activation: Need for new strategies to protect against platelet-mediated atherothrombosis. Thromb. Haemost. 2009, 102, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Furie, B.; Furie, B.C. Mechanisms of thrombus formation. N. Engl. J. Med. 2008, 359, 938–949. [Google Scholar] [CrossRef] [PubMed]
- Feng, A.N.; Chen, Y.L.; Chen, Y.T.; Ding, Y.Z.; Lin, S.J. Red wine inhibits monocyte chemotactic protein-1 expression and modestly reduces neointimal hyperplasia after balloon injury in cholesterol-Fed rabbits. Circulation 1999, 100, 2254–2259. [Google Scholar] [CrossRef] [PubMed]
- Rolnik, A.; Żuchowski, J.; Stochmal, A.; Olas, B. Quercetin and kaempferol derivatives isolated from aerial parts of Lens culinaris Medik as modulators of blood platelet functions. Ind. Crops Prod. 2020, 152, 112536. [Google Scholar] [CrossRef]
- Stein, J.H.; Keevil, J.G.; Wiebe, D.A.; Aeschlimann, S.; Folts, J.D. Purple grape juice improves endothelial function and reduces the susceptibility of LDL cholesterol to oxidation in patients with coronary artery disease. Circulation 1999, 100, 1050–1055. [Google Scholar] [CrossRef] [PubMed]
- Fuhrman, B.; Lavy, A.; Aviram, M. Consumption of red wine with meals reduces the susceptibility of human plasma and low-density lipoprotein to lipid peroxidation. Am. J. Clin. Nutr. 1995, 61, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Ciumarnean, L.; Milaciu, M.V.; Runcan, O.; Vesa, S.C.; Rachisan, A.L.; Negrean, V.; Perne, M.G.; Donca, V.I.; Alexescu, T.G.; Para, I.; et al. The Effects of Flavonoids in Cardiovascular Diseases. Molecules 2020, 25, 4320. [Google Scholar] [CrossRef] [PubMed]
- Fito, M.; Guxens, M.; Corella, D.; Saez, G.; Estruch, R.; de la Torre, R.; Frances, F.; Cabezas, C.; Lopez-Sabater, M.D.C.; Marrugat, J.; et al. Effect of a traditional Mediterranean diet on lipoprotein oxidation: A randomized controlled trial. Arch. Intern. Med. 2007, 167, 1195–1203. [Google Scholar] [CrossRef] [PubMed]
- Castro-Barquero, S.; Lamuela-Raventos, R.M.; Domenech, M.; Estruch, R. Relationship between Mediterranean Dietary Polyphenol Intake and Obesity. Nutrients 2018, 10, 1523. [Google Scholar] [CrossRef] [PubMed]
- Ornish, D.; Brown, S.E.; Scherwitz, L.W.; Billings, J.H.; Armstrong, W.T.; Ports, T.A.; McLanahan, S.M.; Kirkeeide, R.L.; Brand, R.J.; Gould, K.L. Can lifestyle changes reverse coronary heart disease? The Lifestyle Heart Trial. Lancet 1990, 336, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Pinto, P.; Santos, C.N. Worldwide (poly)phenol intake: Assessment methods and identified gaps. Eur. J. Nutr. 2017, 56, 1393–1408. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.Y.; Sang, L.X.; Jiang, M. Catechins and Their Therapeutic Benefits to Inflammatory Bowel Disease. Molecules 2017, 22, 484. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Ramiro, I.; Martin, M.A.; Ramos, S.; Bravo, L.; Goya, L. Comparative effects of dietary flavanols on antioxidant defences and their response to oxidant-induced stress on Caco2 cells. Eur. J. Nutr. 2011, 50, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.G.; Katiyar, S.K.; Agarwal, R.; Mukhtar, H. Enhancement of antioxidant and phase II enzymes by oral feeding of green tea polyphenols in drinking water to SKH-1 hairless mice: Possible role in cancer chemoprevention. Cancer Res. 1992, 52, 4050–4052. [Google Scholar] [PubMed]
- Ruskovska, T.; Massaro, M.; Carluccio, M.A.; Arola-Arnal, A.; Muguerza, B.; Vanden Berghe, W.; Declerck, K.; Bravo, F.I.; Calabriso, N.; Combet, E.; et al. Systematic bioinformatic analysis of nutrigenomic data of flavanols in cell models of cardiometabolic disease. Food Funct. 2020, 11, 5040–5064. [Google Scholar] [CrossRef] [PubMed]
- Godos, J.; Vitale, M.; Micek, A.; Ray, S.; Martini, D.; Del Rio, D.; Riccardi, G.; Galvano, F.; Grosso, G. Dietary Polyphenol Intake, Blood Pressure, and Hypertension: A Systematic Review and Meta-Analysis of Observational Studies. Antioxidants 2019, 8, 152. [Google Scholar] [CrossRef] [PubMed]
- Oleaga, C.; Ciudad, C.J.; Izquierdo-Pulido, M.; Noe, V. Cocoa flavanol metabolites activate HNF-3beta, Sp1, and NFY-mediated transcription of apolipoprotein AI in human cells. Mol. Nutr. Food Res. 2013, 57, 986–995. [Google Scholar] [CrossRef] [PubMed]
- Mena, P.; Bresciani, L.; Brindani, N.; Ludwig, I.A.; Pereira-Caro, G.; Angelino, D.; Llorach, R.; Calani, L.; Brighenti, F.; Clifford, M.N.; et al. Phenyl-gamma-valerolactones and phenylvaleric acids, the main colonic metabolites of flavan-3-ols: Synthesis, analysis, bioavailability, and bioactivity. Nat. Prod. Rep. 2019, 36, 714–752. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.C.; Kim, J.H.; Kim, J.S.; Oh, Y.S.; Han, S.M.; Park, J.H.Y.; Lee, K.W.; Lee, C.Y. 5-(3′,4′-Dihydroxyphenyl-gamma-valerolactone), a Major Microbial Metabolite of Proanthocyanidin, Attenuates THP-1 Monocyte-Endothelial Adhesion. Int. J. Mol. Sci. 2017, 18, 1363. [Google Scholar] [CrossRef]
- Bhattacharjee, N.; Dua, T.K.; Khanra, R.; Joardar, S.; Nandy, A.; Saha, A.; De Feo, V.; Dewanjee, S. Protocatechuic Acid, a Phenolic from Sansevieria roxburghiana Leaves, Suppresses Diabetic Cardiomyopathy via Stimulating Glucose Metabolism, Ameliorating Oxidative Stress, and Inhibiting Inflammation. Front. Pharmacol. 2017, 8, 251. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Islam, F.; Or-Rashid, M.H.; Mamun, A.A.; Rahaman, M.S.; Islam, M.M.; Meem, A.F.K.; Sutradhar, P.R.; Mitra, S.; Mimi, A.A.; et al. The Gut Microbiota (Microbiome) in Cardiovascular Disease and Its Therapeutic Regulation. Front. Cell Infect. Microbiol. 2022, 12, 903570. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Cordero, J.; Mateos, R.; Gonzalez-Ramila, S.; Seguido, M.A.; Sierra-Cinos, J.L.; Sarria, B.; Bravo, L. Dietary Supplements Containing Oat Beta-Glucan and/or Green Coffee (Poly)phenols Showed Limited Effect in Modulating Cardiometabolic Risk Biomarkers in Overweight/Obese Patients without a Lifestyle Intervention. Nutrients 2023, 15, 2223. [Google Scholar] [CrossRef] [PubMed]
- Grassi, D.; Necozione, S.; Lippi, C.; Croce, G.; Valeri, L.; Pasqualetti, P.; Desideri, G.; Blumberg, J.B.; Ferri, C. Cocoa reduces blood pressure and insulin resistance and improves endothelium-dependent vasodilation in hypertensives. Hypertension 2005, 46, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Grassi, D.; Desideri, G.; Necozione, S.; Lippi, C.; Casale, R.; Properzi, G.; Blumberg, J.B.; Ferri, C. Blood pressure is reduced and insulin sensitivity increased in glucose-intolerant, hypertensive subjects after 15 days of consuming high-polyphenol dark chocolate. J. Nutr. 2008, 138, 1671–1676. [Google Scholar] [CrossRef] [PubMed]
- Heiss, C.; Jahn, S.; Taylor, M.; Real, W.M.; Angeli, F.S.; Wong, M.L.; Amabile, N.; Prasad, M.; Rassaf, T.; Ottaviani, J.I.; et al. Improvement of endothelial function with dietary flavanols is associated with mobilization of circulating angiogenic cells in patients with coronary artery disease. J. Am. Coll. Cardiol. 2010, 56, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Flammer, A.J.; Sudano, I.; Wolfrum, M.; Thomas, R.; Enseleit, F.; Periat, D.; Kaiser, P.; Hirt, A.; Hermann, M.; Serafini, M.; et al. Cardiovascular effects of flavanol-rich chocolate in patients with heart failure. Eur. Heart J. 2012, 33, 2172–2180. [Google Scholar] [CrossRef] [PubMed]
- Nantz, M.P.; Rowe, C.A.; Bukowski, J.F.; Percival, S.S. Standardized capsule of Camellia sinensis lowers cardiovascular risk factors in a randomized, double-blind, placebo-controlled study. Nutrition 2009, 25, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Kafeshani, M.; Entezari, M.H.; Karimian, J.; Pourmasoumi, M.; Maracy, M.R.; Amini, M.R.; Hadi, A. A comparative study of the effect of green tea and sour tea on blood pressure and lipid profile in healthy adult men. ARYA Atheroscler. 2017, 13, 109–116. [Google Scholar] [PubMed]
- Takahashi, M.; Miyashita, M.; Suzuki, K.; Bae, S.R.; Kim, H.K.; Wakisaka, T.; Matsui, Y.; Takeshita, M.; Yasunaga, K. Acute ingestion of catechin-rich green tea improves postprandial glucose status and increases serum thioredoxin concentrations in postmenopausal women. Br. J. Nutr. 2014, 112, 1542–1550. [Google Scholar] [CrossRef] [PubMed]
- Diepvens, K.; Kovacs, E.M.; Nijs, I.M.; Vogels, N.; Westerterp-Plantenga, M.S. Effect of green tea on resting energy expenditure and substrate oxidation during weight loss in overweight females. Br. J. Nutr. 2005, 94, 1026–1034. [Google Scholar] [CrossRef] [PubMed]
- Frank, J.; George, T.W.; Lodge, J.K.; Rodriguez-Mateos, A.M.; Spencer, J.P.; Minihane, A.M.; Rimbach, G. Daily consumption of an aqueous green tea extract supplement does not impair liver function or alter cardiovascular disease risk biomarkers in healthy men. J. Nutr. 2009, 139, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, R.; Kotani, K.; Ayabe, M.; Tsuzaki, K.; Shimada, J.; Sakane, N.; Takase, H.; Ichikawa, H.; Yonei, Y.; Ishii, K. Minor effects of green tea catechin supplementation on cardiovascular risk markers in active older people: A randomized controlled trial. Geriatr. Gerontol. Int. 2013, 13, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Xia, N.; Daiber, A.; Forstermann, U.; Li, H. Antioxidant effects of resveratrol in the cardiovascular system. Br. J. Pharmacol. 2017, 174, 1633–1646. [Google Scholar] [CrossRef] [PubMed]
- Bagul, P.K.; Deepthi, N.; Sultana, R.; Banerjee, S.K. Resveratrol ameliorates cardiac oxidative stress in diabetes through deacetylation of NFkB-p65 and histone 3. J. Nutr. Biochem. 2015, 26, 1298–1307. [Google Scholar] [CrossRef] [PubMed]
- Spanier, G.; Xu, H.; Xia, N.; Tobias, S.; Deng, S.; Wojnowski, L.; Forstermann, U.; Li, H. Resveratrol reduces endothelial oxidative stress by modulating the gene expression of superoxide dismutase 1 (SOD1), glutathione peroxidase 1 (GPx1) and NADPH oxidase subunit (Nox4). J. Physiol. Pharmacol. 2009, 60 (Suppl. S4), 111–116. [Google Scholar] [PubMed]
- Xia, N.; Daiber, A.; Habermeier, A.; Closs, E.I.; Thum, T.; Spanier, G.; Lu, Q.; Oelze, M.; Torzewski, M.; Lackner, K.J.; et al. Resveratrol reverses endothelial nitric-oxide synthase uncoupling in apolipoprotein E knockout mice. J. Pharmacol. Exp. Ther. 2010, 335, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Guo, T.; Li, G.; Sun, S.; He, S.; Cheng, B.; Shi, B.; Shan, A. Dietary resveratrol improves antioxidant status of sows and piglets and regulates antioxidant gene expression in placenta by Keap1-Nrf2 pathway and Sirt1. J. Anim. Sci. Biotechnol. 2018, 9, 34. [Google Scholar] [CrossRef] [PubMed]
- Yeung, F.; Hoberg, J.E.; Ramsey, C.S.; Keller, M.D.; Jones, D.R.; Frye, R.A.; Mayo, M.W. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004, 23, 2369–2380. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, S.; Yang, Q.; Shi, Y.; Zheng, M.; Liu, Y.; Chen, F.; Song, G.; Xu, H.; Wan, T.; et al. Resveratrol reduces the proinflammatory effects and lipopolysaccharide- induced expression of HMGB1 and TLR4 in RAW264.7 cells. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2014, 33, 1283–1292. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, H.; Tang, W.; Qiu, Q.; Peng, J. Resveratrol prevents TNF-alpha-induced VCAM-1 and ICAM-1 upregulation in endothelial progenitor cells via reduction of NF-kappaB activation. J. Int. Med. Res. 2020, 48, 300060520945131. [Google Scholar] [CrossRef] [PubMed]
- Meng, T.; Xiao, D.; Muhammed, A.; Deng, J.; Chen, L.; He, J. Anti-Inflammatory Action and Mechanisms of Resveratrol. Molecules 2021, 26, 229. [Google Scholar] [CrossRef] [PubMed]
- Muslin, A.J. MAPK signalling in cardiovascular health and disease: Molecular mechanisms and therapeutic targets. Clin. Sci. 2008, 115, 203–218. [Google Scholar] [CrossRef] [PubMed]
- Kondoh, K.; Nishida, E. Regulation of MAP kinases by MAP kinase phosphatases. Biochim. Biophys. Acta 2007, 1773, 1227–1237. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Huang, Y.; Zheng, W.; Yan, J.; Cheng, M.; Zhao, R.; Chen, L.; Hu, C.; Jia, W. Resveratrol reduces intracellular reactive oxygen species levels by inducing autophagy through the AMPK-mTOR pathway. Front. Med. 2018, 12, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lu, Y.; Ong′achwa, M.J.; Ge, L.; Qian, Y.; Chen, L.; Hu, X.; Li, F.; Wei, H.; Zhang, C.; et al. Resveratrol Inhibits the TGF-beta1-Induced Proliferation of Cardiac Fibroblasts and Collagen Secretion by Downregulating miR-17 in Rat. Biomed Res. Int. 2018, 2018, 8730593. [Google Scholar] [CrossRef] [PubMed]
- Parsamanesh, N.; Asghari, A.; Sardari, S.; Tasbandi, A.; Jamialahmadi, T.; Xu, S.; Sahebkar, A. Resveratrol and endothelial function: A literature review. Pharmacol. Res. 2021, 170, 105725. [Google Scholar] [CrossRef] [PubMed]
- Forstermann, U.; Xia, N.; Li, H. Roles of Vascular Oxidative Stress and Nitric Oxide in the Pathogenesis of Atherosclerosis. Circ. Res. 2017, 120, 713–735. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Feng, J.; Zhang, R.; Chen, J.; Han, D.; Li, X.; Yang, B.; Li, X.; Fan, M.; Li, C.; et al. SIRT1 Activation by Resveratrol Alleviates Cardiac Dysfunction via Mitochondrial Regulation in Diabetic Cardiomyopathy Mice. Oxid. Med. Cell Longev. 2017, 2017, 4602715. [Google Scholar] [CrossRef] [PubMed]
- Theodotou, M.; Fokianos, K.; Mouzouridou, A.; Konstantinou, C.; Aristotelous, A.; Prodromou, D.; Chrysikou, A. The effect of resveratrol on hypertension: A clinical trial. Exp. Ther. Med. 2017, 13, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ma, W.; Zhang, P.; He, S.; Huang, D. Effect of resveratrol on blood pressure: A meta-analysis of randomized controlled trials. Clin. Nutr. 2015, 34, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.M.; Eckardt, P.; Aleman, J.O.; da Rosa, J.C.; Liang, Y.; Iizumi, T.; Etheve, S.; Blaser, M.J.; Breslow, J.L.; Holt, P.R. The effects of trans-resveratrol on insulin resistance, inflammation, and microbiota in men with the metabolic syndrome: A pilot randomized, placebo-controlled clinical trial. J. Clin. Transl. Res. 2019, 4, 122–135. [Google Scholar] [PubMed]
- Wong, R.H.; Howe, P.R.; Buckley, J.D.; Coates, A.M.; Kunz, I.; Berry, N.M. Acute resveratrol supplementation improves flow-mediated dilatation in overweight/obese individuals with mildly elevated blood pressure. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Magyar, K.; Halmosi, R.; Palfi, A.; Feher, G.; Czopf, L.; Fulop, A.; Battyany, I.; Sumegi, B.; Toth, K.; Szabados, E. Cardioprotection by resveratrol: A human clinical trial in patients with stable coronary artery disease. Clin. Hemorheol. Microcirc. 2012, 50, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Fujitaka, K.; Otani, H.; Jo, F.; Jo, H.; Nomura, E.; Iwasaki, M.; Nishikawa, M.; Iwasaka, T.; Das, D.K. Modified resveratrol Longevinex improves endothelial function in adults with metabolic syndrome receiving standard treatment. Nutr. Res. 2011, 31, 842–847. [Google Scholar] [CrossRef] [PubMed]
- Marques, B.; Trindade, M.; Aquino, J.C.F.; Cunha, A.R.; Gismondi, R.O.; Neves, M.F.; Oigman, W. Beneficial effects of acute trans-resveratrol supplementation in treated hypertensive patients with endothelial dysfunction. Clin. Exp. Hypertens. 2018, 40, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Asgary, S.; Karimi, R.; Momtaz, S.; Naseri, R.; Farzaei, M.H. Effect of resveratrol on metabolic syndrome components: A systematic review and meta-analysis. Rev. Endocr. Metab. Disord. 2019, 20, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Hoseini, A.; Namazi, G.; Farrokhian, A.; Reiner, Z.; Aghadavod, E.; Bahmani, F.; Asemi, Z. The effects of resveratrol on metabolic status in patients with type 2 diabetes mellitus and coronary heart disease. Food Funct. 2019, 10, 6042–6051. [Google Scholar] [CrossRef] [PubMed]
- Simental-Mendia, L.E.; Guerrero-Romero, F. Effect of resveratrol supplementation on lipid profile in subjects with dyslipidemia: A randomized double-blind, placebo-controlled trial. Nutrition 2019, 58, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Militaru, C.; Donoiu, I.; Craciun, A.; Scorei, I.D.; Bulearca, A.M.; Scorei, R.I. Oral resveratrol and calcium fructoborate supplementation in subjects with stable angina pectoris: Effects on lipid profiles, inflammation markers, and quality of life. Nutrition 2013, 29, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Goel, A.; Kunnumakkara, A.B.; Aggarwal, B.B. Curcumin as "Curecumin": From kitchen to clinic. Biochem. Pharmacol. 2008, 75, 787–809. [Google Scholar] [CrossRef] [PubMed]
- Mansuri, M.L.; Parihar, P.; Solanki, I.; Parihar, M.S. Flavonoids in modulation of cell survival signalling pathways. Genes Nutr. 2014, 9, 400. [Google Scholar] [CrossRef] [PubMed]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A Review of Its Effects on Human Health. Foods 2017, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Kukongviriyapan, U.; Pannangpetch, P.; Kukongviriyapan, V.; Donpunha, W.; Sompamit, K.; Surawattanawan, P. Curcumin protects against cadmium-induced vascular dysfunction, hypertension and tissue cadmium accumulation in mice. Nutrients 2014, 6, 1194–1208. [Google Scholar] [CrossRef] [PubMed]
- Tubsakul, A.; Sangartit, W.; Pakdeechote, P.; Kukongviriyapan, V.; Apaijit, K.; Kukongviriyapan, U. Curcumin Mitigates Hypertension, Endothelial Dysfunction and Oxidative Stress in Rats with Chronic Exposure to Lead and Cadmium. Tohoku J. Exp. Med. 2021, 253, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Shen, T.; Xie, J.; Wang, S.; He, Y.; Zhu, F. Curcumin modulates covalent histone modification and TIMP1 gene activation to protect against vascular injury in a hypertension rat model. Exp. Ther. Med. 2017, 14, 5896–5902. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Wang, W.; Li, M.; Ren, H.; Chen, C.; Wang, J.; Wang, W.E.; Yang, J.; Zeng, C. Curcumin Exerts its Anti-hypertensive Effect by Down-regulating the AT1 Receptor in Vascular Smooth Muscle Cells. Sci. Rep. 2016, 6, 25579. [Google Scholar] [CrossRef] [PubMed]
- Birudaraju, D.; Cherukuri, L.; Kinninger, A.; Chaganti, B.T.; Shaikh, K.; Hamal, S.; Flores, F.; Roy, S.K.; Budoff, M.J. A combined effect of Cavacurcumin, Eicosapentaenoic acid (Omega-3s), Astaxanthin and Gamma -linoleic acid (Omega-6) (CEAG) in healthy volunteers- a randomized, double-blind, placebo-controlled study. Clin. Nutr. ESPEN 2020, 35, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Khajehdehi, P.; Zanjaninejad, B.; Aflaki, E.; Nazarinia, M.; Azad, F.; Malekmakan, L.; Dehghanzadeh, G.R. Oral supplementation of turmeric decreases proteinuria, hematuria, and systolic blood pressure in patients suffering from relapsing or refractory lupus nephritis: A randomized and placebo-controlled study. J. Ren. Nutr. 2012, 22, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.S.; Fleenor, B.S. The emerging role of curcumin for improving vascular dysfunction: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 2790–2799. [Google Scholar] [CrossRef] [PubMed]
- Santos-Parker, J.R.; Strahler, T.R.; Bassett, C.J.; Bispham, N.Z.; Chonchol, M.B.; Seals, D.R. Curcumin supplementation improves vascular endothelial function in healthy middle-aged and older adults by increasing nitric oxide bioavailability and reducing oxidative stress. Aging 2017, 9, 187–208. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, X.; Si, H. Synergistic anti-inflammatory effects and mechanisms of the combination of resveratrol and curcumin in human vascular endothelial cells and rodent aorta. J. Nutr. Biochem. 2022, 108, 109083. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Lopez, C.; Carpena, M.; Lourenco-Lopes, C.; Gallardo-Gomez, M.; Lorenzo, J.M.; Barba, F.J.; Prieto, M.A.; Simal-Gandara, J. Bioactive Compounds and Quality of Extra Virgin Olive Oil. Foods 2020, 9, 1014. [Google Scholar] [CrossRef] [PubMed]
- Cicerale, S.; Conlan, X.A.; Sinclair, A.J.; Keast, R.S. Chemistry and health of olive oil phenolics. Crit. Rev. Food Sci. Nutr. 2009, 49, 218–236. [Google Scholar] [CrossRef] [PubMed]
- Finicelli, M.; Squillaro, T.; Galderisi, U.; Peluso, G. Polyphenols, the Healthy Brand of Olive Oil: Insights and Perspectives. Nutrients 2021, 13, 3831. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Correa, J.A.; Navas, M.D.; Munoz-Marin, J.; Trujillo, M.; Fernandez-Bolanos, J.; de la Cruz, J.P. Effects of hydroxytyrosol and hydroxytyrosol acetate administration to rats on platelet function compared to acetylsalicylic acid. J. Agric. Food Chem. 2008, 56, 7872–7876. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Marin, J.; De la Cruz, J.P.; Reyes, J.J.; Lopez-Villodres, J.A.; Guerrero, A.; Lopez-Leiva, I.; Espartero, J.L.; Labajos, M.T.; Gonzalez-Correa, J.A. Hydroxytyrosyl alkyl ether derivatives inhibit platelet activation after oral administration to rats. Food Chem. Toxicol. 2013, 58, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.G.; Fogacci, F.; Di Micoli, A.; Veronesi, M.; Grandi, E.; Borghi, C. Hydroxytyrosol-Rich Olive Extract for Plasma Cholesterol Control. Appl. Sci. 2022, 12, 10086. [Google Scholar] [CrossRef]
- D’Addato, S.; Scandiani, L.; Mombelli, G.; Focanti, F.; Pelacchi, F.; Salvatori, E.; Di Loreto, G.; Comandini, A.; Maffioli, P.; Derosa, G. Effect of a food supplement containing berberine, monacolin K, hydroxytyrosol and coenzyme Q(10) on lipid levels: A randomized, double-blind, placebo controlled study. Drug Des. Devel Ther. 2017, 11, 1585–1592. [Google Scholar] [CrossRef] [PubMed]
- Quiros-Fernandez, R.; Lopez-Plaza, B.; Bermejo, L.M.; Palma Milla, S.; Zangara, A.; Candela, C.G. Oral Supplement Containing Hydroxytyrosol and Punicalagin Improves Dyslipidemia in an Adult Population without Co-Adjuvant Treatment: A Randomized, Double-Blind, Controlled and Crossover Trial. Nutrients 2022, 14, 1879. [Google Scholar] [CrossRef] [PubMed]
- Colica, C.; Di Renzo, L.; Trombetta, D.; Smeriglio, A.; Bernardini, S.; Cioccoloni, G.; Costa de Miranda, R.; Gualtieri, P.; Sinibaldi Salimei, P.; De Lorenzo, A. Antioxidant Effects of a Hydroxytyrosol-Based Pharmaceutical Formulation on Body Composition, Metabolic State, and Gene Expression: A Randomized Double-Blinded, Placebo-Controlled Crossover Trial. Oxid. Med. Cell Longev. 2017, 2017, 2473495. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Huertas, E.; Fonolla, J. Hydroxytyrosol supplementation increases vitamin C levels in vivo. A human volunteer trial. Redox Biol. 2017, 11, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Naranjo, A.; Alvarez-Soria, M.J.; Aranda-Villalobos, P.; Martinez-Rodriguez, A.M.; Martinez-Lara, E.; Siles, E. Hydroxytyrosol, a Promising Supplement in the Management of Human Stroke: An Exploratory Study. Int. J. Mol. Sci. 2024, 25, 4799. [Google Scholar] [CrossRef] [PubMed]
- Laddomada, B.; Caretto, S.; Mita, G. Wheat Bran Phenolic Acids: Bioavailability and Stability in Whole Wheat-Based Foods. Molecules 2015, 20, 15666–15685. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Sun, X.Y.; Mariga, A.M.; Yang, Q.; Fang, Y.; Hu, Q.H.; Pei, F. Effects of ferulic acid on the polymerization behavior of gluten protein and its components. Food Hydrocoll. 2024, 147, 109388. [Google Scholar] [CrossRef]
- Calabriso, N.; Massaro, M.; Scoditti, E.; Pasqualone, A.; Laddomada, B.; Carluccio, M.A. Phenolic extracts from whole wheat biofortified bread dampen overwhelming inflammatory response in human endothelial cells and monocytes: Major role of VCAM-1 and CXCL-10. Eur. J. Nutr. 2020, 59, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Costabile, G.; Vitale, M.; Della Pepa, G.; Cipriano, P.; Vetrani, C.; Testa, R.; Mena, P.; Bresciani, L.; Tassotti, M.; Calani, L.; et al. A wheat aleurone-rich diet improves oxidative stress but does not influence glucose metabolism in overweight/obese individuals: Results from a randomized controlled trial. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 715–726. [Google Scholar] [CrossRef] [PubMed]
- Salazar-Lopez, N.J.; Astiazaran-Garcia, H.; Gonzalez-Aguilar, G.A.; Loarca-Pina, G.; Ezquerra-Brauer, J.M.; Dominguez Avila, J.A.; Robles-Sanchez, M. Ferulic Acid on Glucose Dysregulation, Dyslipidemia, and Inflammation in Diet-Induced Obese Rats: An Integrated Study. Nutrients 2017, 9, 675. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.L.; Zhao, C.H.; Yao, X.L.; Zhang, H. Quercetin attenuates high fructose feeding-induced atherosclerosis by suppressing inflammation and apoptosis via ROS-regulated PI3K/AKT signaling pathway. Biomed. Pharmacother. 2017, 85, 658–671. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Jia, Q.; Shen, D.; Yan, L.; Chen, C.; Xing, S. Quercetin has a protective effect on atherosclerosis via enhancement of autophagy in ApoE(-/-) mice. Exp. Ther. Med. 2019, 18, 2451–2458. [Google Scholar] [CrossRef] [PubMed]
- Kondo, M.; Izawa-Ishizawa, Y.; Goda, M.; Hosooka, M.; Kagimoto, Y.; Saito, N.; Matsuoka, R.; Zamami, Y.; Chuma, M.; Yagi, K.; et al. Preventive Effects of Quercetin against the Onset of Atherosclerosis-Related Acute Aortic Syndromes in Mice. Int. J. Mol. Sci. 2020, 21, 7226. [Google Scholar] [CrossRef] [PubMed]
- Ferenczyova, K.; Kalocayova, B.; Bartekova, M. Potential Implications of Quercetin and its Derivatives in Cardioprotection. Int. J. Mol. Sci. 2020, 21, 1585. [Google Scholar] [CrossRef] [PubMed]
- Brown, V.A.; Patel, K.R.; Viskaduraki, M.; Crowell, J.A.; Perloff, M.; Booth, T.D.; Vasilinin, G.; Sen, A.; Schinas, A.M.; Piccirilli, G.; et al. Repeat dose study of the cancer chemopreventive agent resveratrol in healthy volunteers: Safety, pharmacokinetics, and effect on the insulin-like growth factor axis. Cancer Res. 2010, 70, 9003–9011. [Google Scholar] [CrossRef] [PubMed]
- la Porte, C.; Voduc, N.; Zhang, G.; Seguin, I.; Tardiff, D.; Singhal, N.; Cameron, D.W. Steady-State pharmacokinetics and tolerability of trans-resveratrol 2000 mg twice daily with food, quercetin and alcohol (ethanol) in healthy human subjects. Clin. Pharmacokinet. 2010, 49, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Crowell, J.A.; Korytko, P.J.; Morrissey, R.L.; Booth, T.D.; Levine, B.S. Resveratrol-associated renal toxicity. Toxicol. Sci. 2004, 82, 614–619. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Webster, D.; Cao, J.; Shao, A. The safety of green tea and green tea extract consumption in adults—Results of a systematic review. Regul. Toxicol. Pharmacol. 2018, 95, 412–433. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Correa, M.; Shoskes, D.A.; Sanchez, P.; Zhao, R.; Hylind, L.M.; Wexner, S.D.; Giardiello, F.M. Combination treatment with curcumin and quercetin of adenomas in familial adenomatous polyposis. Clin. Gastroenterol. Hepatol. 2006, 4, 1035–1038. [Google Scholar] [CrossRef] [PubMed]
- Hurrell, R.; Egli, I. Iron bioavailability and dietary reference values. Am. J. Clin. Nutr. 2010, 91, 1461S–1467S. [Google Scholar] [CrossRef] [PubMed]
- Hirose, M.; Fukushima, S.; Shirai, T.; Hasegawa, R.; Kato, T.; Tanaka, H.; Asakawa, E.; Ito, N. Stomach Carcinogenicity of Caffeic Acid, Sesamol and Catechol in Rats and Mice. Jpn. J. Cancer Res. 1990, 81, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Zhang, X.; Liu, Y.; Wang, H.; Luo, J.; Luo, Y.; An, P. Effects of dietary polyphenol supplementation on iron status and erythropoiesis: A systematic review and meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2021, 114, 780–793. [Google Scholar] [CrossRef] [PubMed]
- Andrés, C.M.C.; Pérez de la Lastra, J.M.; Juan, C.A.; Plou, F.J.; Pérez-Lebeña, E. Polyphenols as Antioxidant/Pro-Oxidant Compounds and Donors of Reducing Species: Relationship with Human Antioxidant Metabolism. Processes 2023, 11, 2771. [Google Scholar] [CrossRef]
- Martin, K.R.; Appel, C.L. Polyphenols as dietary supplements: A double-edged sword. Nutr. Diet. Suppl. 2009, 2, 1–12. [Google Scholar] [CrossRef]
- Ofosu, F.K.; Daliri, E.B.-M.; Elahi, F.; Chelliah, R.; Lee, B.-H.; Oh, D.-H. New Insights on the Use of Polyphenols as Natural Preservatives and Their Emerging Safety Concerns. Front. Sustain. Food Syst. 2020, 4, 14. [Google Scholar] [CrossRef]
- Favari, C.; Rinaldi de Alvarenga, J.F.; Sánchez-Martínez, L.; Tosi, N.; Mignogna, C.; Cremonini, E.; Manach, C.; Bresciani, L.; Del Rio, D.; Mena, P. Factors driving the inter-individual variability in the metabolism and bioavailability of (poly)phenolic metabolites: A systematic review of human studies. Redox Biol. 2024, 71, 103095. [Google Scholar] [CrossRef] [PubMed]
- Berman, A.Y.; Motechin, R.A.; Wiesenfeld, M.Y.; Holz, M.K. The therapeutic potential of resveratrol: A review of clinical trials. NPJ Precis. Oncol. 2017, 1, 35. [Google Scholar] [CrossRef] [PubMed]
- Rondinella, D.; Raoul, P.C.; Valeriani, E.; Venturini, I.; Cintoni, M.; Severino, A.; Galli, F.S.; Mora, V.; Mele, M.C.; Cammarota, G.; et al. The Detrimental Impact of Ultra-Processed Foods on the Human Gut Microbiome and Gut Barrier. Nutrients 2025, 17, 859. [Google Scholar] [CrossRef] [PubMed]
- Boban, M.; Stockley, C.; Teissedre, P.-L.; Restani, P.; Fradera, U.; Stein-Hammer, C.; Ruf, J.-C. Drinking pattern of wine and effects on human health: Why should we drink moderately and with meals? Food Funct. 2016, 7, 2937–2942. [Google Scholar] [CrossRef] [PubMed]
- Lara-Castor, L.; O’Hearn, M.; Cudhea, F.; Miller, V.; Shi, P.; Zhang, J.; Sharib, J.R.; Cash, S.B.; Barquera, S.; Micha, R.; et al. Burdens of type 2 diabetes and cardiovascular disease attributable to sugar-sweetened beverages in 184 countries. Nat. Med. 2025, 31, 552–564. [Google Scholar] [CrossRef] [PubMed]
- Grzesk, G.; Rogowicz, D.; Wolowiec, L.; Ratajczak, A.; Gilewski, W.; Chudzinska, M.; Sinkiewicz, A.; Banach, J. The Clinical Significance of Drug-Food Interactions of Direct Oral Anticoagulants. Int. J. Mol. Sci. 2021, 22, 8531. [Google Scholar] [CrossRef] [PubMed]
- Duda-Chodak, A.; Tarko, T. Possible Side Effects of Polyphenols and Their Interactions with Medicines. Molecules 2023, 28, 2536. [Google Scholar] [CrossRef] [PubMed]
- Hooper, B.; Frazier, R. Polyphenols in the diet: Friend or foe? Nutr. Bull. 2012, 37, 297–308. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).