Abstract
Background: Fat embolism and fat embolism syndrome are rare but well-known consequences of long bone fractures and orthopedic surgeries. These sources support the mechanical theory of their development. On the other hand, as an alternative pathway suggested by the biochemical theory, lipase activation and fat breakdown are also a possible background for lipid droplets appearing in the vasculature. According to Hulman’s theory, elevated C-reactive protein levels can facilitate calcium-dependent agglutination of very low-density proteins and chylomicrons forming fat globules. The level of this acute-phase protein can increase mainly in advanced-stage cancers but also has predictive or indicative value in treatment success. Methods: This study focused on strictly selected patients with different histological types and origins of cancer, as well as advanced cancer in approximately 90% of the deceased. After collecting the tissue samples, the frozen sections were stained with Oil Red O to detect fat emboli. Results: Less than 50% of the cases showed punctiform, non-clinically relevant pulmonary fat embolism, and fat embolism syndrome was identified in none of the cases. In one, non-advanced cancer case, punctiform kidney fat embolism was observed. Conclusions: The end-of-life anergic state of patients may influence the procedure. In the case of osseous metastases, since the intramedullary sinuses are affected, both the mechanical and the biochemical backgrounds may prevail and mediate fat embolism formation.
1. Introduction
Traumatic fat embolism and fat embolism syndrome are well-known conditions in traumatology, orthopedics, and forensic pathology practice. Soft tissue damage (mechanical or heat) and mainly long or sometimes pelvic bone fractures or orthopedic surgery (e.g., arthroplasty) are common causes of the formation of fat embolism [1,2]. In addition to those mentioned above, there are other non-traumatic reasons, including corticosteroid therapy, osteomyelitis, parenteral lipid infusion, and sickle cell disease, underlining its heterogeneous origins (Table 1) [1,3,4,5,6,7,8,9,10,11,12,13,14].

Table 1.
Possible causes of fat embolism.
Fat globules enter the circulation and cause isolated pulmonary fat embolism or affect multiple organs. According to the mechanical theory, fat enters the venous circulation via intramedullary sinuses. An alternative explanation is the biochemical theory, in which fat breaks down due to lipase activation to free fatty acids and glycerol, initiating an inflammatory response and causing endothelial damage [15,16,17]. Fat embolism is the presence of fat in the lungs and peripheral microcirculation, with or without clinical consequences. Fat embolism syndrome (FES) is a potentially fatal manifestation of fat embolism with certain clinical and laboratory signs and symptoms, although the diagnosis is challenging [18,19,20]. The classical clinical triad of FES syndrome includes respiratory distress, neurological dysfunctions, and petechial rash [9]. The data on this condition’s mortality rate varies from 5.8%–10% to 30.2% [21,22,23]. Zenker described FES in 1861 in human patients [24]. There are different scoring and criteria systems: for example, Schonfeld’s scoring system, Gurd and Wilson’s criteria system, and Lindeque’s criteria (Table 2) [5,15].

Table 2.
Schonfeld’s criteria system for FES (for diagnosis scores more than 5).
The lungs, kidneys, and central nervous system are the most frequently involved organs, and pulmonary symptoms are the most common within 24 to 72 h after trauma.
C-reactive protein (CRP) is a well-known, atypical immunochemical marker associated with various pathological conditions, such as bacterial infections, sepsis, autoimmune disorders, malignancies, and myocardial infarction. It is produced primarily by hepatocytes through the regulation of Interleukin-6 (IL-6) and is a useful indicator in clinical practice [25]. CRP may be a diagnostic and prognostic index for cancer, an acute-phase protein that is not specific to cancer type. Malignancy can promote inflammatory responses, initiate tumor progression, and increase disease severity [26,27]. It seems that CRP and its isoforms have predictive value, not only in cases of de novo tumorigenesis but in also more advanced stages and metastases [28,29]. According to Hulman, CRP triggers calcium-dependent agglutination of very low-density lipoproteins (VLDL) and chylomicrons, causing non-traumatic fat embolism [30]. This study aimed to prove or exclude the presence of fat embolism and fat embolism syndrome in the deceased patients with malignancy and determine a possible role in the cause of death.
2. Materials and Methods
We saved tissue samples from cadavers who had been diagnosed with cancer and excluded all other possible origins of fat embolism or fat embolism syndrome, independently of gender. Thus, any diagnosis or condition listed in Table 1 was excluded, with the exception of bronchopneumonia and attempts at cardiopulmonary resuscitation [31]. Age was not a limiting factor during patient inclusion or exclusion. The sample collection period was September 2024 to March 2025. Over 600 autopsies were conducted, and 11 deceased individuals met the criteria for inclusion in this study. A total of 12 pieces of tissue samples were collected from each patient (1.5 × 1.5 × 1 cm maximum size): 5 pieces of the lungs (1 from each lobe), 4 pieces of the brain (1 from the right frontal lobe, 1 from corpus callosum, 1 from right basal ganglia, and 1 from left occipital lobe), 2 pieces of the kidneys (1 from each side middle section), and 1 piece of heart (left ventricle anterior wall). The autopsies took place 36–190 h after death. In five cases, frozen slides were performed immediately after autopsies, and in the other cases after 24–196 h. For the latter, the tissue samples were fixed on a cryostat chuck, dipped in petrol ether chilled by dry ice to −70 °C, and embedded with Epredia cryomatrix embedding resin (Fisher Scientific, Pittsburgh, PA, USA, product code: 12542716). These samples were stored in a freezer (−18 °C) until processing. Frozen sections of 5–8 μm were made using a cryotome (Thermo Scientific, Pittsburg, PA, USA). After 24 h of drying, the slides were immersed in 70% isopropanol. A quantity of 0.2–0.5 g solid Oil Red O (Sigma-Aldrich, Burlington, MA, USA, catalog number: O0625) was dissolved in 10 mL isopropanol (Molar Chemicals, Halásztelek, Hungary, 2-PROPANOL (IZOPROPANOL), catalog number: 00390-526-206), and 4 mL distilled water was added to 6 mL of the stock solution, which was then filtered before use. The Oil Red O solution was strained through filter paper and dispensed on the slides. After 10 min of staining with Oil Red O, the solution was drained off, and the sections were merged in 70% isopropanol and rinsed with distilled water for 1–2 min. After 1 min of staining with Mayer’s Alum Hematoxylin (Molar Chemicals, catalog number: 42514), tap water bluing was administered. The slides were then covered with a mounting medium. Two forensic experts/pathologists evaluated the slides separately under a light microscope (Leica, Wetzlar, Germany, 2500 DM).
Lung fat embolism was evaluated as per Falzi et al., modified by the Janssen score system (Table 3).

Table 3.
Falzi et al. modified by the Janssen pulmonary fat embolism scoring system, grades, and criteria.
Another set of tissue samples was also harvested from the above-mentioned organs, as well as from the suspected malignancies and possible metastases for usual Hematoxylin and Eosin (H&E) staining after a maximum of 5 days of formalin fixation (VWR Chemicals, Radnor, PA, USA, Formaldehyde 4% aqueous solution, buffered, catalog number: FOR010LAF59001).
We determined the details if we had no relevant or sufficient clinical or pathological data regarding the cancer type, grading, staging, and histological type (hematoxylin-eosin staining, immunohistochemical reactions) of the tumor. However, when we had data, we compared the autopsy results with clinical findings (Figure 1).
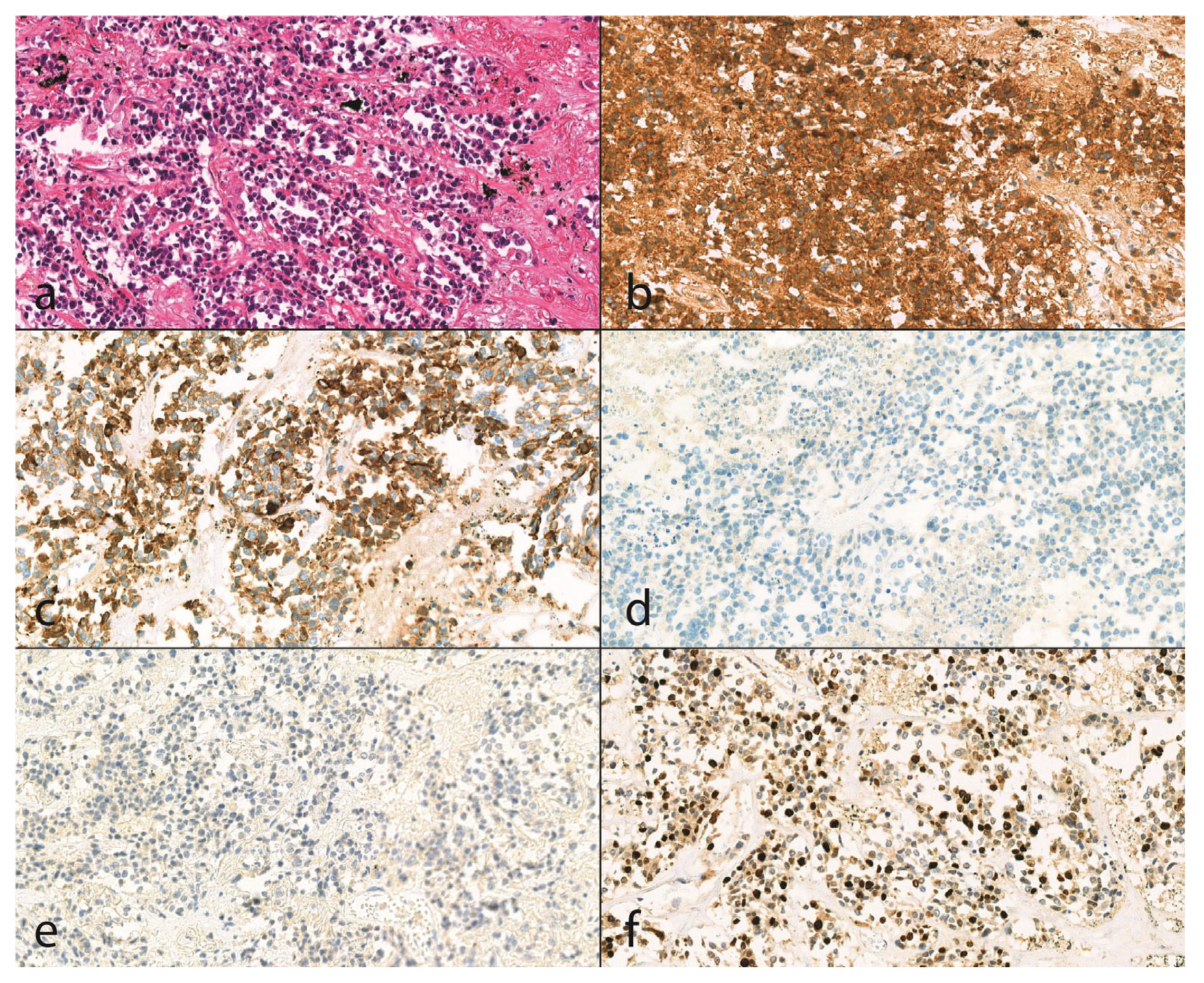
Figure 1.
Histologic findings of an unknown malignancy case (20X magnification). (a): With hematoxylin and eosin staining (H&E), small cell neuroendocrine carcinoma is seen in biopsy material with an infiltrative growth pattern. The tumor consists of small cells with round to oval or elongated nuclei with a smooth nuclear membrane, finely dispersed chromatin, and no prominent nucleoli, and scant eosinophilic cytoplasm. The tumor cells form bundles or small clusters, and the tumor stroma is thin and fibrovascular. In some places, we can see individual cell necrosis. Positive immunoreactivity for (b) CD56 (Dako, CD56, clone: 123C3, catalog number: M7304, (1:200)) and (c) CK7 (Dako, Cytokeratin7, clone: OV-TL 12/31, catalog number: M7018, (1:1500)) with negative staining for (d) p63 (Dako, p63 protein, catalog number: M7317, (1:200)) and (e) WT1 (Leica Biosystems, Novoscastra Liquid Mouse Monoclonal Antibody Wilms’ tumor, product code: NCL-L-WT1-562, (1:25)) support the diagnosis of small cell carcinoma. Mitotic rate is high (f) Ki-67 (Dako, Ki-67 antigen, catalog number: M7240, (1:200)), proliferation index is 50%.
3. Results
Of 621 autopsies (including pathological and forensic autopsies), 15 seemed appropriate for sample collection, and only 11 matched our preset criteria (Table 4).

Table 4.
Synopsis of strictly selected deceased of the study, including age, gender, cause of death, autopsy findings, relevant clinical data, cancer type, grade, TNM (tumor, node, metastases) classification, cancer stage, and the result of Oil Red O lipid staining with the pulmonary fat embolism grade of the individuals.
Nine of the specimens were male and two were female. The average and median ages were 66.4 years and 64 years, respectively. There were various types of cancer in different parts of the gastrointestinal tract (esophagus, stomach, colon, and rectum), prostate, liver, thymus, and lungs. The subepicardial fatty tissue was used as a positive internal control for Oil Red O lipid staining. In 5 out of 11 autopsies (45%), sporadic lung fat embolism was observed. In one of the five cases, there was sporadic pulmonary fat embolism in addition to the kidney (Figure 2). The latter case was one with no advanced-stage cancer.
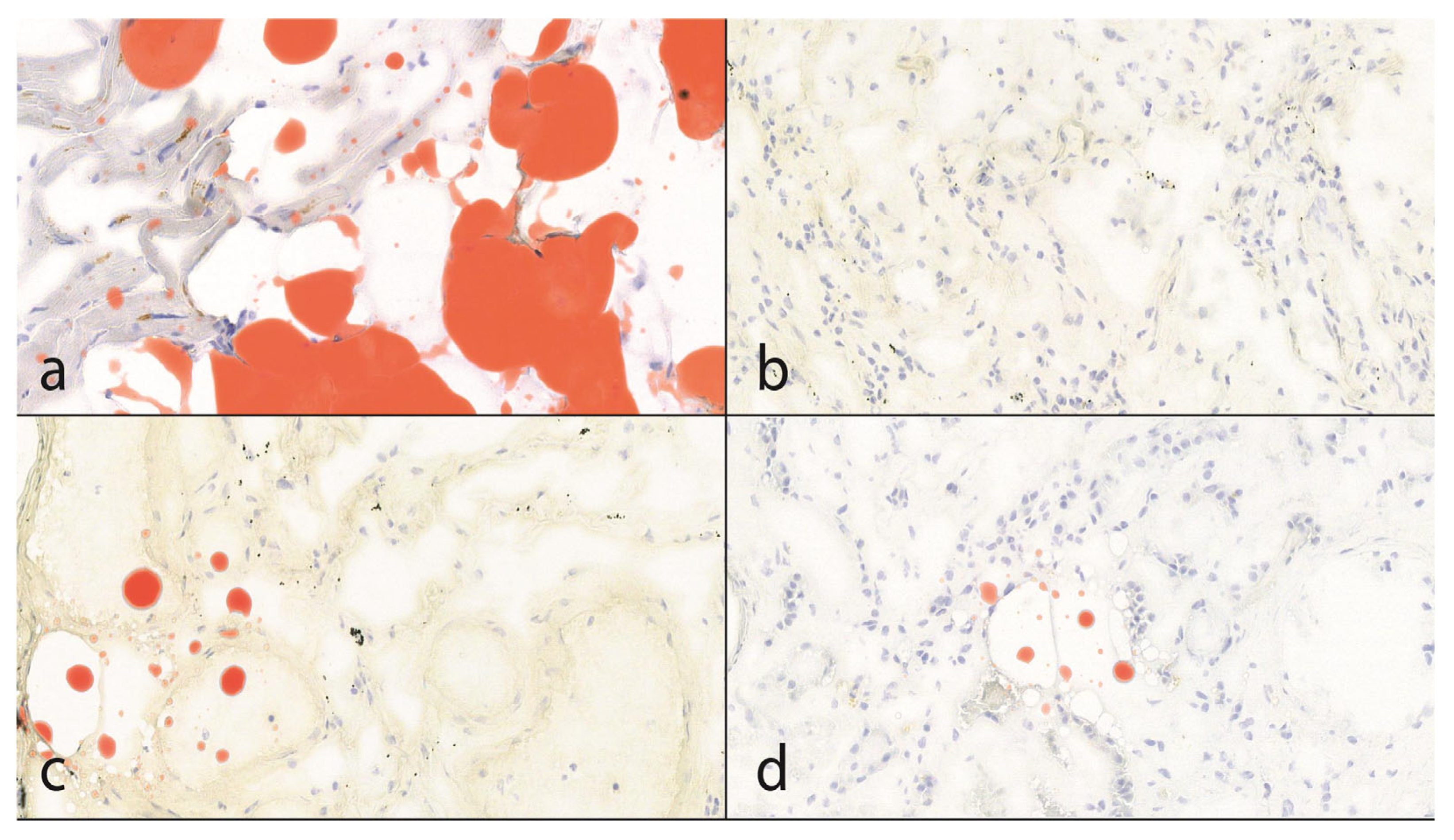
Figure 2.
The results of lipid staining (25× magnification). (a): The subepicardial fatty tissue is positive with Oil Red O lipid staining. This is also useful as an internal control. In the myocytes, lipofuscin formation can also be seen as evidence of former oxidative stress. (b,c): In 55% of the cases, there was no evidence of fat embolism; in 45%, sporadic, punctiform pulmonary fat embolism was detected. According to the Falzi et al. modified by the Janssen score system, the grade of the fat embolism was 0. (d): In 1 case, punctiform, fat embolism of the kidney was observed.
Lung metastases were observed in three cases, liver metastases in two cases, and both lung and liver metastases in one case. In addition, osseous and pleural metastases were seen in one case each, and both metastases were seen in one case, while the adrenal gland and peritoneum were affected in one case each. The antemortem C-reactive protein level was observed in two cases. Cachexia was observed in all of the advanced stages of cancer. In one case, the detected malignancy was the third primary cancer.
There were only two cases with osseous metastases, and one of these two cases showed sporadic lung fat embolism. In our opinion, it is worth expanding the research from this point of view; however, in these cases, the mechanical background of the fat embolism, such as the interference of intramedullary expanding tumor, vasculature, and intramedullary fat, is also possible in addition to the biochemical form.
None of these patients had fat embolism as a lethal condition, and no fat embolism syndrome was observed.
In the four remaining cases (one female, three males, average and median age 62.5 years and 52 years, respectively), besides the cancerous primary disease, bacterial purulent bronchopneumonia was observed. Three patients had locally advanced transitional cell carcinoma of the bladder, well-differentiated squamous cell carcinoma of the tongue, and poorly differentiated squamous cell carcinoma of the esophagus, and one had advanced anaplastic cancer of the lung. Two of the four cases expressed punctiform, sporadic fat embolism of the lung, and in one case, in some fields of view, it was more disseminated and teardrop-like, but not in every field of vision at 25× magnification. In these cases, inflammatory disease could also increase CRP levels (Table 5).

Table 5.
Summary of the deceased individuals who were excluded from the target group because of the coincidence of cancerous disease and purulent bronchopneumonia as a mutual background of CRP level increasing.
4. Discussion
Although the mechanical theory of fat embolism formation, which leads to vessel occlusion and subsequent local organ damage, has been elucidated, the biochemical theory remains ambiguous. Trauma may result in elevated plasma lipase levels or, due to biochemical alterations, fat globules may enter the vasculature and decompose into free fatty acids and glycerol. This process potentially triggers a microvascular inflammatory response, which is a plausible hypothesis [1].
The increased presence and role of CRP in malignancies have been extensively studied. This immunochemical marker is one of the most important, and its potential role from diagnostic, biochemical, and pathophysiological points of view is incontestable [25]. CRP has different forms and can switch between various types, indicating evidence of various functions [32]. CRP ligand affinity is partly calcium-dependent, as in the case of pathogens, histones, glycans, etc., but without calcium, it can bind myelin essential proteins or poly-L-arginine [33,34]. In two of our autopsy cases, the antemortem CRP level was known within 24 h before death. These levels were significantly increased, at 149.6 mg/L and 103.37 mg/L. However, no evidence of fat embolism was found. Furthermore, the number of cases was insufficient for valid statistical analysis. The postmortem measurement of CRP levels may be useful. According to the literature, the pre- and postmortem levels of blood CRP depend on the analysis method (immunoturbidimetric or immunometric). The average reduction was 42–35%, and individually, the highest decrease was 74% [35]. CRP is a pre-diagnostic marker of lung cancer in current smokers and in small cell lung cancers. It correlates with size and staging, indicating a poor prognosis, whereas in adenocarcinomas, it indicates the paucity of treatment response with or without epidermal growth factor receptor (EGFR) in advanced stages [36,37,38]. In other solid tumors (e.g., esophageal, stomach, gynecological, pancreas, head and neck squamous cell carcinoma), the sometimes-notable CRP increase can indicate poor survival or the presence of metastases, determine treatment outcomes or tumor recurrence, and advanced stages, and correlates well with tumor size and disease progression. Preoperative CRP increase can also be linked to worse outcomes [25,26,39,40,41,42,43]. It also seems to be a promising immunohistochemical marker to differentiate between intrahepatic cholangiocarcinoma and other adenocarcinomas [42]. In addition, in leukaemias and lymphomas, it has been reported as a prognostic marker [44,45]. In 82% of our cases, when there was an advanced or locally advanced stage of cancer, we presumed an increased CRP level. However, the possible anergic state of end-stage patients can influence the immunological status and responses; therefore, we had to deal with the downregulation. This may be one of the answers to our study’s low number of pulmonary fat embolisms and the lack of fat embolism syndrome. Although the number of proper cases was low, we also raised the possibility that the type of primary cancer somehow regulates the appearance of fat embolism.
The strategies for the prevention of fat embolism in the case of a cancerous patient are limited or almost impossible, as the elevated CRP level is characteristic of the malignancies. However, the long-term application of corticosteroids increases the possibility of fat embolism, but when used as a prophylactic treatment, it seems effective in decreasing the free fatty acid level, stabilizing membranes. It can also inhibit the complement-mediated leukocyte aggregation in patients with long-bone fractures [15]. Steroids are widely used in oncology because of their anti-inflammatory, anti-swelling, and angiogenesis inhibition effects, both as a therapeutic treatment and for supportive care [46].
Fat embolism, particularly that leading to Fat Embolism Syndrome (FES), has significant implications for cancer patients due to several factors that can increase their risk and complicate their clinical course. While the clinical presentation poses a challenge for diagnosis through multi-organ dysfunctions, it also carries the potential for severe morbidity and mortality. Providing respiratory and hemodynamic support, along with close monitoring, is essential for accurate diagnosis. High awareness and prompt supportive care are critical for improving outcomes. Our findings indicate a need to increase the number of cases by extending the study period and including patients from diverse racial backgrounds, while also broadening the types of cancer studied and the ages of patients in our study group. By enhancing the understanding of distinct mechanisms of the disease, the unique physiological changes in cancer alter the course of presentation and severity of fat embolism. If certain types of cancer or treatment heighten the risk of fat embolism, this allows for better risk stratification and proactive measures. Interdisciplinary approaches can lead to improved preventive care and enhanced supportive care, while also mitigating inflammatory responses and promoting fat globule breakdown, which are critical in improving outcomes for cancer patients with fat embolism. In summary, this study enhances our understanding of fat embolism in cancer patients by exploring the unique aspects of this population.
Limitations
The current study is limited by unintended bias during data collection, which stems from strict patient criteria, a consecutive limited number of cases, and time frame restrictions. These factors contribute to limitations in the female-to-male ratio, the spectrum of cancer types, and the representation of a younger population.
Author Contributions
Conceptualization: B.Á.B. and T.T.; methodology: B.Á.B.; software: B.Á.B. and Z.H; validation: B.Á.B., K.K. and T.T.; formal analysis: B.Á.B., B.D.H. and K.K.; investigation: B.Á.B., Z.H., K.K. and T.T.; resources: B.Á.B. and P.A.G.; data curation: B.D.H. and R.K.P.; writing—original draft preparation: B.Á.B. and T.T. writing—review and editing: R.K.P. and K.K.; visualization: B.Á.B., Z.H., K.K. and T.T.; supervision: R.K.P. and P.A.G.; project administration: B.D.H. and P.A.G.; funding acquisition: P.A.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Regional Institutional Research Ethics Committee, Clinical Center, University of Debrecen (conclusion of 30 October 2024, protocol identifier: 6996-2024).
Informed Consent Statement
Patient consent was waived due to the following: the anonymized data from the autopsy can be utilized freely for scientific and educational purposes without informed consent or ethical permission, according to the 40. § (3) of the Hungarian Act of Forensic Experts (2016.XXIX) and 220. § (1) of the Hungarian Healthcare Act of 1997.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article.
Acknowledgments
The authors wish to thank Marcell Domokos, Attila Takács, and Zsolt Pongor for their technical assistance.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| FES | Fat Embolism Syndrome |
| CRP | C-reactive protein |
| VLDL | Very low-density lipoprotein |
| H&E | Hematoxylin and Eosin |
| EGFR | Epidermal growth factor receptor |
References
- Milroy, C.M.; Parai, J.L. Fat Embolism, Fat Embolism Syndrome and the Autopsy. Acad. Forensic Pathol. 2019, 9, 136–154. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, E.A.; Pellegrini, D.C.; Vanderkolk, W.E.; Minshall, C.T.; Fakhry, S.M.; Cohle, S.D. Incidence of Pulmonary Fat Embolism at Autopsy: An Undiagnosed Epidemic. J. Trauma Inj. Infect. Crit. Care 2011, 71, 312–315. [Google Scholar] [CrossRef] [PubMed]
- Adeyinka, A.; Pierre, L. Fat Embolism. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Turkmen Samdanci, E.; Celik, M.R.; Pehlivan, S.; Celbis, O.; Turkkan, D.; Ozdemir Kara, D.; Pamukcu, E. Histopathological Evaluation of Autopsy Cases with Isolated Pulmonary Fat Embolism (IPFE): Is Cardiopulmonary Resuscitation a Main Cause of Death in IPFE? Open Access Emerg. Med. 2019, 11, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Goyal, R.; Baghel, P.; Sharma, V. Fat Embolism Syndrome: A Comprehensive Review and Update. J. Orthop. Allied Sci. 2018, 6, 56. [Google Scholar] [CrossRef]
- Kim, H.; Seo, B.F.; Evans, G.R.D. Fat Embolism Syndrome: A Review in Cosmetic Surgery. Kosin Med. J. 2024, 39, 169–178. [Google Scholar] [CrossRef]
- Yan, X.; Wu, S.; Zeng, W.; Kong, J. Fat Embolism Syndrome Caused by Fracture or Liposuction: A Retrospective Case Series of Nine Patients. Ann. Med. 2025, 57, 2447427. [Google Scholar] [CrossRef]
- Castiglioni, C.; Carminati, A.; Fracasso, T. Fat Embolism after Intraosseous Catheters in Pediatric Forensic Autopsies. Int. J. Leg. Med. 2023, 137, 787–791. [Google Scholar] [CrossRef]
- Schulz, F.; Trübner, K.; Hilderbrand, E. Fatal Fat Embolism in Acute Hepatic Necrosis with Associated Fatty Liver. Am. J. Forensic Med. Pathol. 1996, 17, 264–268. [Google Scholar] [CrossRef]
- Kosova, E.; Bergmark, B.; Piazza, G. Fat Embolism Syndrome. Circulation 2015, 131, 317–320. [Google Scholar] [CrossRef]
- Babunovska, M.; Jovanovski, A.; Boskovski, B.; Foteva, M.; Kuzmanovski, I.; Trencevska, G.K.; Cvetkovska, E. Fractures in People with Epilepsy: A Nationwide Population-based Cohort Study. Epilepsia Open 2023, 8, 1028–1037. [Google Scholar] [CrossRef]
- Ajzan, A.; Modine, T.; Punjabi, P.; Ganeshalingam, K.; Philips, G.; Gourlay, T. Quantification of Fat Mobilization in Patients Undergoing Coronary Artery Revascularization Using Off-Pump and On-Pump Techniques. J. Extra Corpor. Technol. 2006, 38, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Young, A.E.; Thomas, M.L.; Browse, N.L. Intraosseous Phlebography and Fat Embolism. BMJ 1976, 2, 89–90. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tsitsikas, D.A.; Bristowe, J.; Abukar, J. Fat Embolism Syndrome in Sickle Cell Disease. J. Clin. Med. 2020, 9, 3601. [Google Scholar] [CrossRef] [PubMed]
- Kwiatt, M.; Seamon, M. Fat Embolism Syndrome. Int. J. Crit. Illn. Inj. Sci. 2013, 3, 64. [Google Scholar] [CrossRef]
- Rothberg, D.L.; Makarewich, C.A. Fat Embolism and Fat Embolism Syndrome. J. Am. Acad. Orthop. Surg. 2019, 27, e346–e355. [Google Scholar] [CrossRef]
- Newbigin, K.; Souza, C.A.; Torres, C.; Marchiori, E.; Gupta, A.; Inacio, J.; Armstrong, M.; Peña, E. Fat Embolism Syndrome: State-of-the-Art Review Focused on Pulmonary Imaging Findings. Respir. Med. 2016, 113, 93–100. [Google Scholar] [CrossRef]
- Tzioupis, C.C.; Giannoudis, P.V. Fat Embolism Syndrome: What Have We Learned over the Years? Trauma 2011, 13, 259–281. [Google Scholar] [CrossRef]
- Gupta, B.; Kaur, M.; D’souza, N.; Dey, C.K.; Shende, S.; Kumar, A.; Gamangatti, S. Cerebral Fat Embolism: A Diagnostic Challenge. Saudi J. Anaesth. 2011, 5, 348–352. [Google Scholar] [CrossRef]
- Tsai, S.H.L.; Chen, C.-H.; Tischler, E.H.; Kurian, S.J.; Lin, T.-Y.; Su, C.-Y.; Osgood, G.M.; Mehmood, A.; Fu, T.-S. Fat Embolism Syndrome and In-Hospital Mortality Rates According to Patient Age: A Large Nationwide Retrospective Study. Clin. Epidemiol. 2022, 14, 985–996. [Google Scholar] [CrossRef]
- He, Z.; Shi, Z.; Li, C.; Ni, L.; Sun, Y.; Arioli, F.; Wang, Y.; Ammirati, E.; Wang, D.W. Single-Case Metanalysis of Fat Embolism Syndrome. Int. J. Cardiol. 2021, 345, 111–117. [Google Scholar] [CrossRef]
- Kainoh, T.; Iriyama, H.; Komori, A.; Saitoh, D.; Naito, T.; Abe, T. Risk Factors of Fat Embolism Syndrome After Trauma: A Nested Case-Control Study With the Use of a Nationwide Trauma Registry in Japan. Chest 2021, 159, 1064–1071. [Google Scholar] [CrossRef] [PubMed]
- Bulger, E.M.; Smith, D.G.; Maier, R.V.; Jurkovich, G.J. Fat Embolism Syndrome. A 10-Year Review. Arch. Surg. 1997, 132, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Benevento, M.; Carravetta, F.; Caterino, C.; Nicolì, S.; Ambrosi, L.; Ferorelli, D.; Solarino, B. Fat Embolism Syndrome in a Patient with Osteogenesis Imperfecta: A Case Report. Forensic Sci. 2024, 4, 234–242. [Google Scholar] [CrossRef]
- Mouliou, D.S. C-Reactive Protein: Pathophysiology, Diagnosis, False Test Results and a Novel Diagnostic Algorithm for Clinicians. Diseases 2023, 11, 132. [Google Scholar] [CrossRef]
- Hart, P.C.; Rajab, I.M.; Alebraheem, M.; Potempa, L.A. C-Reactive Protein and Cancer—Diagnostic and Therapeutic Insights. Front. Immunol. 2020, 11, 595835. [Google Scholar] [CrossRef]
- Pastorino, U.; Morelli, D.; Leuzzi, G.; Gisabella, M.; Suatoni, P.; Taverna, F.; Bertocchi, E.; Boeri, M.; Sozzi, G.; Cantarutti, A.; et al. Baseline and Postoperative C-Reactive Protein Levels Predict Mortality in Operable Lung Cancer. Eur. J. Cancer 2017, 79, 90–97. [Google Scholar] [CrossRef]
- Zhou, B.; Shu, B.; Yang, J.; Liu, J.; Xi, T.; Xing, Y. C-Reactive Protein, Interleukin-6 and the Risk of Colorectal Cancer: A Meta-Analysis. Cancer Causes Control 2014, 25, 1397–1405. [Google Scholar] [CrossRef]
- Ananthula, A.; Konda, M.; Bimali, M.; Safar, A.M.; Govindarajan, R. C-Reactive Protein (CRP) as a Predictive Biomarker in Lung Cancer Patients Receiving Immune Checkpoint Inhibitors (ICI). J. Clin. Oncol. 2020, 38, e21645. [Google Scholar] [CrossRef]
- Hulman, G. PATHOGENESIS OF NON-TRAUMATIC FAT EMBOLISM. Lancet 1988, 331, 1366–1367. [Google Scholar] [CrossRef]
- Ihnát Rudinská, L.; Delongová, P.; Vaculová, J.; Farkašová Iannaccone, S.; Tulinský, L.; Ihnát, P. Pulmonary Fat Embolism in Non-Survivors after Cardiopulmonary Resuscitation. Forensic Sci. Int. 2024, 357, 112002. [Google Scholar] [CrossRef]
- Wang, H.-W.; Wu, Y.; Chen, Y.; Sui, S.-F. Polymorphism of Structural Forms of C-Reactive Protein. Int. J. Mol. Med. 2002, 9, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Ngwa, D.N.; Singh, S.K.; Agrawal, A. C-Reactive Protein-Based Strategy to Reduce Antibiotic Dosing for the Treatment of Pneumococcal Infection. Front. Immunol. 2020, 11, 620784. [Google Scholar] [CrossRef] [PubMed]
- Du Clos, T.W. Pentraxins: Structure, Function, and Role in Inflammation. ISRN Inflamm. 2013, 2013, 379040. [Google Scholar] [CrossRef] [PubMed]
- Uhlin-Hansen, L. C-Reactive Protein (CRP), a Comparison of Pre- and Post-Mortem Blood Levels. Forensic Sci. Int. 2001, 124, 32–35. [Google Scholar] [CrossRef]
- Chaturvedi, A.K.; Caporaso, N.E.; Katki, H.A.; Wong, H.-L.; Chatterjee, N.; Pine, S.R.; Chanock, S.J.; Goedert, J.J.; Engels, E.A. C-Reactive Protein and Risk of Lung Cancer. J. Clin. Oncol. 2010, 28, 2719–2726. [Google Scholar] [CrossRef]
- Hong, S.; Kang, Y.A.; Cho, B.C.; Kim, D.J. Elevated Serum C-Reactive Protein as a Prognostic Marker in Small Cell Lung Cancer. Yonsei Med. J. 2012, 53, 111–117. [Google Scholar] [CrossRef]
- Hotta, T.; Nakashima, K.; Hata, K.; Tsubata, Y.; Isobe, T. High Serum C-Reactive Protein Levels Predict Survival in Patients with Treated Advanced Lung Adenocarcinoma. J. Thorac. Dis. 2021, 13, 1476–1484. [Google Scholar] [CrossRef]
- Huang, Y.; Feng, J.-F.; Liu, J.-S.; Chen, Q.-X. Prognostic Role of Serum C-Reactive Protein in Esophageal Cancer: A Systematic Review and Meta-Analysis. Ther. Clin. Risk Manag. 2015, 11, 89–94. [Google Scholar] [CrossRef]
- Baba, H.; Kuwabara, K.; Ishiguro, T.; Hatano, S.; Matsuzawa, T.; Fukuchi, M.; Kumagai, Y.; Ishibashi, K.; Mochiki, E.; Ishida, H. C-Reactive Protein as a Significant Prognostic Factor for Stage IV Gastric Cancer Patients. Anticancer. Res. 2013, 33, 5591–5595. [Google Scholar]
- Nishigaya, Y.; Kobayashi, Y.; Matsuzawa, Y.; Hasegawa, K.; Fukasawa, I.; Watanabe, Y.; Tokunaga, H.; Yaegashi, N.; Iwashita, M. Diagnostic Value of Combination Serum Assay of Lactate Dehydrogenase, D-Dimer, and C-Reactive Protein for Uterine Leiomyosarcoma. J. Obs. Gynaecol. Res. 2019, 45, 189–194. [Google Scholar] [CrossRef]
- Tani, M.; Iida, H.; Maehira, H.; Mori, H.; Miyake, T.; Kaida, S. A High C-Reactive Protein Level on Postoperative Day 7 Is Associated With Poor Survival of Patients with Pancreatic Ductal Adenocarcinoma After Resection. Am. Surg. 2022, 88, 2024–2029. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Cong, R.; Ji, C.; Ruan, W. The Prognostic Role of C-Reactive Protein in Patients with Head and Neck Squamous Cell Carcinoma: A Meta-Analysis. Cancer Med. 2020, 9, 9541–9553. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, C.; Mao, L.; Wang, J.; Li, C.; Qian, W. Clinical Characteristics of Angioimmunoblastic T-Cell Lymphoma in China and C-Reactive Protein as an Independent Prognostic Factor. Medicine 2017, 96, e8091. [Google Scholar] [CrossRef] [PubMed]
- Herishanu, Y.; Polliack, A.; Shenhar-Tsarfaty, S.; Weinberger, R.; Gelman, R.; Ziv-Baran, T.; Zeltser, D.; Shapira, I.; Berliner, S.; Rogowski, O. Increased Serum C-Reactive Protein Levels Are Associated with Shorter Survival and Development of Second Cancers in Chronic Lymphocytic Leukemia. Ann. Med. 2017, 49, 75–82. [Google Scholar] [CrossRef]
- Lossignol, D. A Little Help from Steroids in Oncology. J. Transl. Intern. Med. 2016, 4, 52–54. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).