Calcium and Silicon Delivery to Artificial and Human Nails from Nail Polish Formulations
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Analysis of the White Portland Cement
2.3. Preparation of Nail Polish Formulations
2.4. Calcium and Silicon Release from Nail Polish
3. Results
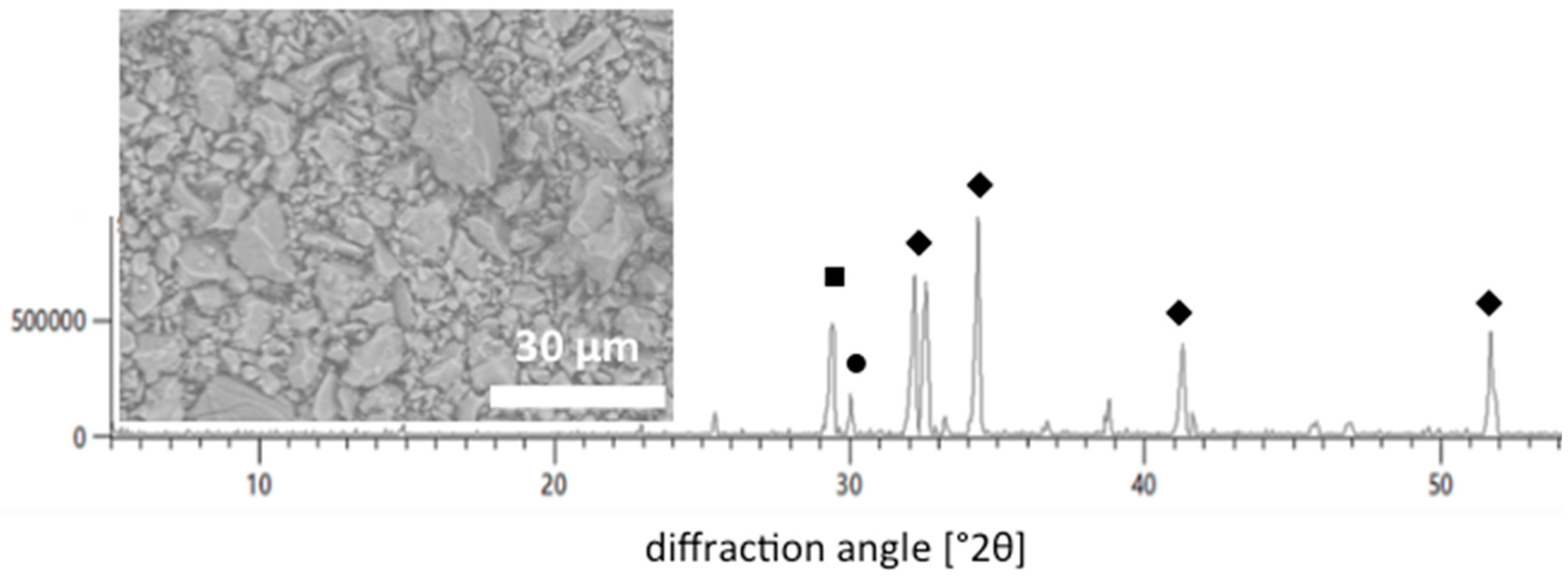
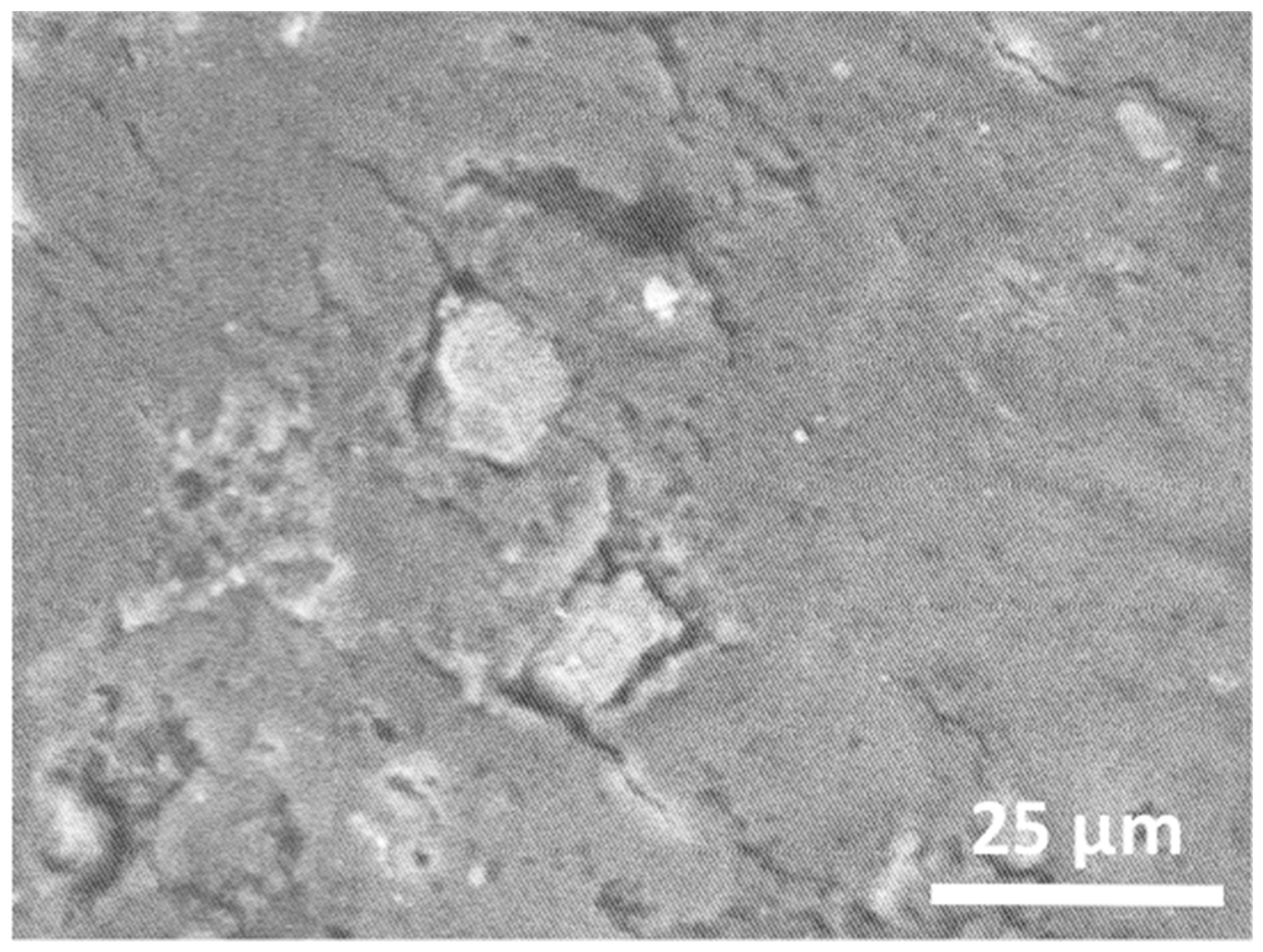
3.1. Analysis of the White Portland Cement
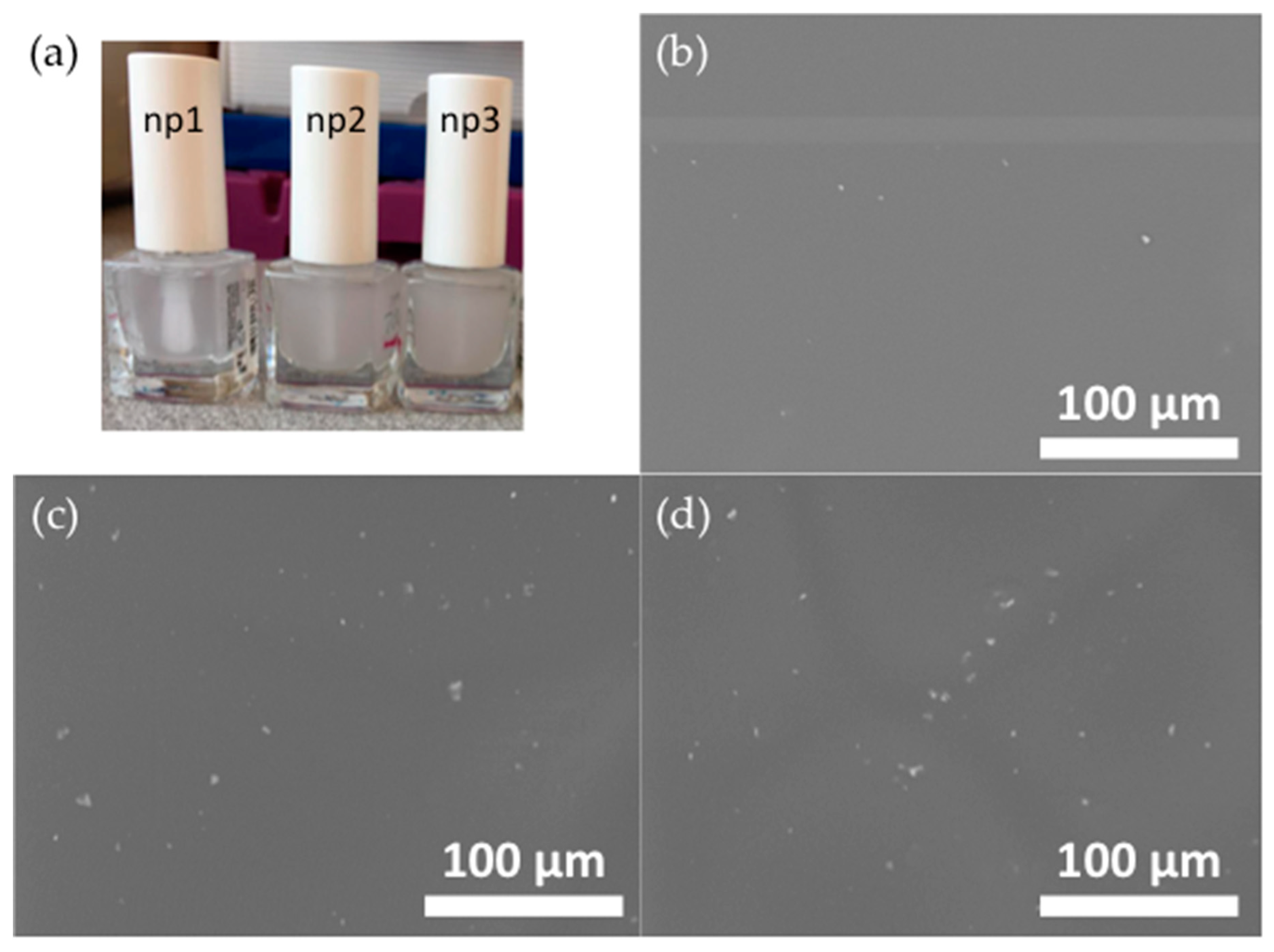
3.2. Analysis of the Modified Nail Polish Formulations
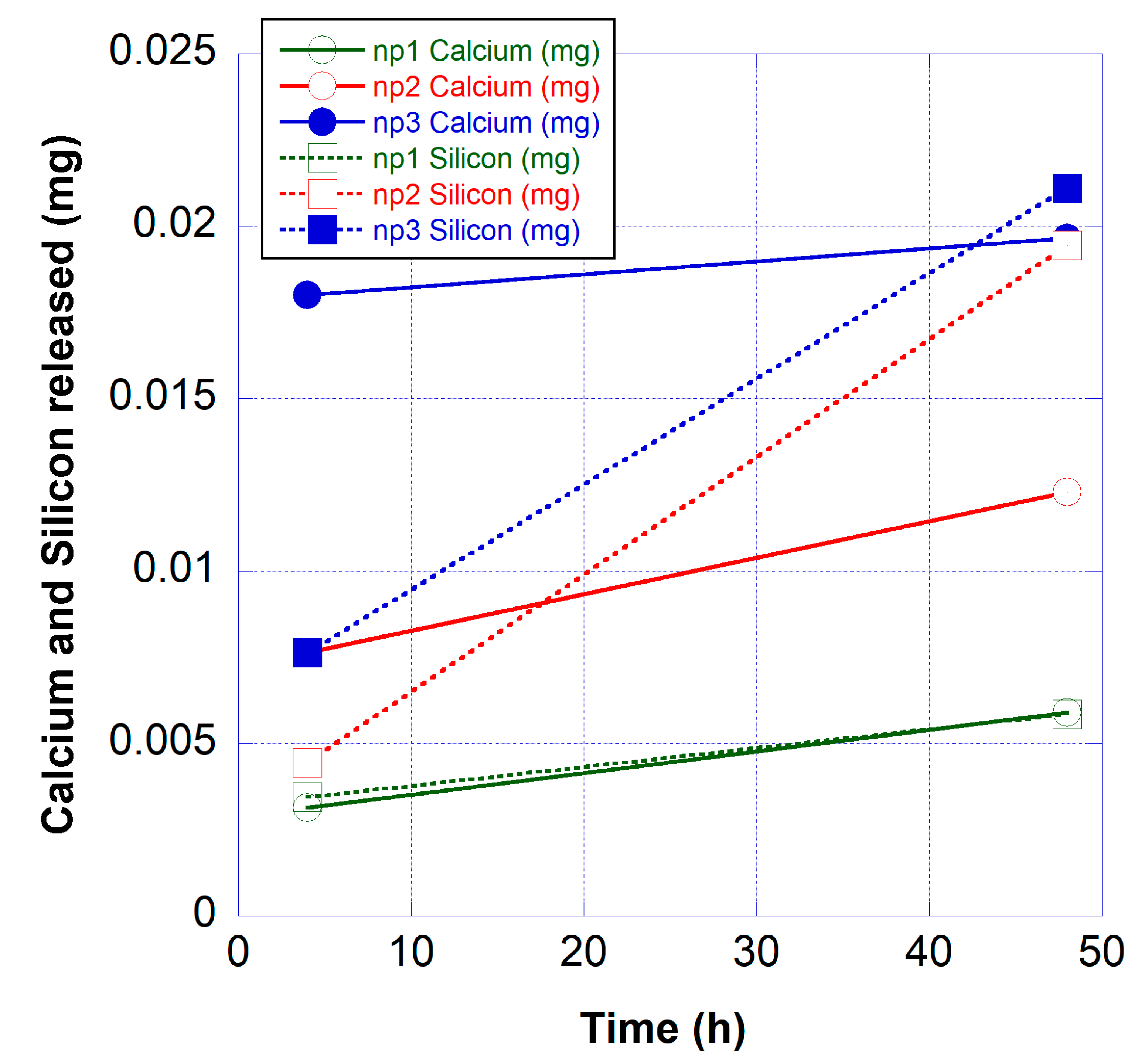
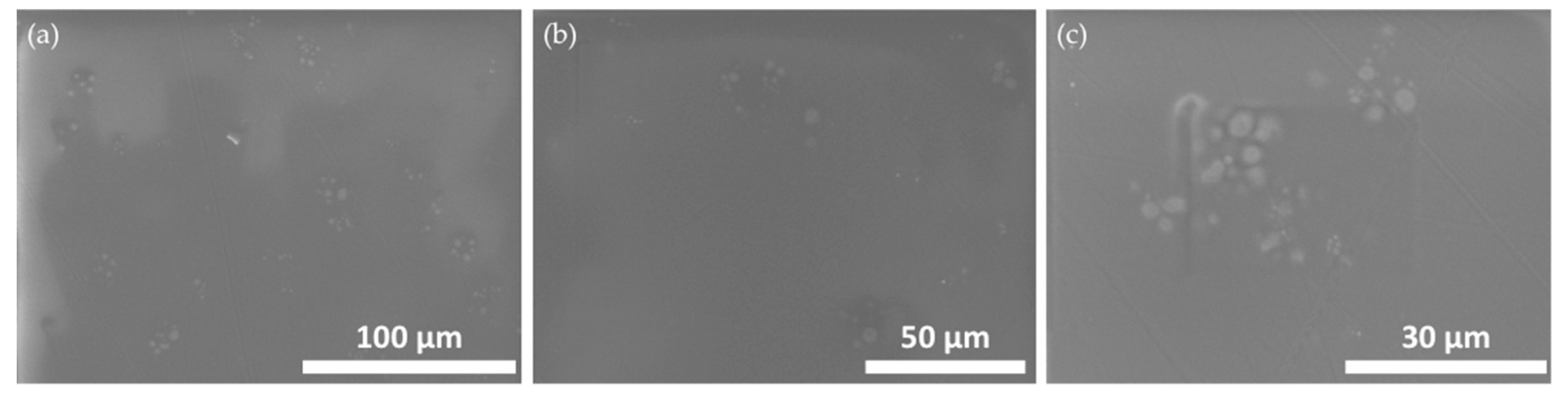
3.3. Calcium and Silicon Release from Nail Polish Formulations
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- International Diabetes Federation. IDF Diabetes Atlas, 9th ed.; International Diabetes Federation: Brussels, Belgium, 2019. [Google Scholar]
- Bristow, I. Non-ulcerative skin pathologies of the diabetic foot. Diabetes-Metab Res. 2008, 24, S84–S89. [Google Scholar] [CrossRef]
- Sihota, P.; Yadav, R.N.; Dhiman, V.; Bhadada, S.K.; Mehandia, V.; Kumar, N. Investigation of diabetic patient’s fingernail quality to monitor type 2 diabetes induced tissue damage. Sci. Rep.-UK 2019, 9, 3193. [Google Scholar] [CrossRef]
- Baswan, S.; Kasting, G.B.; Li, S.K.; Wickett, R.; Adams, B.; Eurich, S.; Schamper, R. Understanding the formidable nail barrier: A review of the nail microstructure, composition and diseases. Mycoses 2017, 60, 284–295. [Google Scholar] [CrossRef]
- Shivakumar, H.N.; Repka, M.A.; Narasimha Murthy, S. Transungual drug delivery: An update. J. Drug Deliv. Sci. Technol. 2014, 24, 301–310. [Google Scholar] [CrossRef]
- Forslind, B.; Wroblewski, R.; Afzelius, B.A. Calcium and sulfur location in human nail. J. Investig. Dermatol. 1976, 67, 273–275. [Google Scholar] [CrossRef]
- Barel, A.; Calomme, M.; Timchenko, A.; Paepe, K.D.; Demeester, N.; Rogiers, V.; Clarys, P.; Vanden Berghe, D. Effect of oral intake of choline-stabilized orthosilicic acid on skin, nails and hair in women with photodamaged skin. Arch. Dermatol. Res. 2005, 297, 147–153. [Google Scholar] [CrossRef]
- Pravina, P.; Sayaji, D.; Avinash, M. Calcium and its role in human body. Int. J. Res. Pharm. Biomed. Sci. 2013, 4, 659–668. [Google Scholar]
- Saeedi, P.; Shavandi, A.; Meredith-Jones, K. Nail Properties and bone health: A review. J. Funct. Biomater. 2018, 9, 31. [Google Scholar] [CrossRef] [PubMed]
- Jugdaohsingh, R. Silicon and bone health. J. Nutr. Health Aging 2007, 11, 99–110. [Google Scholar]
- Hillson, R. Nails in diabetes. Pract Diabetes 2017, 34, 230–231. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Miyamoto, M.; Sugibayashi, K.; Morimoto, Y. Drug permeation through the three layers of the human nail plate. J. Pharm. Pharmacol. 1999, 51, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Laubé, F.; Poupon, A.; Zinck, P.; Müller-Goymann, C.; Reichl, S.; Nardello-Rataj, V. Physicochemical investigations of native nails and synthetic models for a better understanding of surface adhesion of nail lacquers. Eur J. Pharm. Sci. 2019, 131, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Cutrín Gómez, E.; Anguiano Igea, S.; Delgado-Charro, M.B.; Gómez Amoza, J.L.; Otero Espinar, F.J. Microstructural alterations in the onychomycotic and psoriatic nail: Relevance in drug delivery. Eur. J. Pharm. Biopharm. 2018, 128, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Nogueiras-Nieto, L.; Gómez-Amoza, J.L.; Delgado-Charro, M.B.; Otero-Espinar, F.J. Hydration and N-acetyl-l-cysteine alter the microstructure of human nail and bovine hoof: Implications for drug delivery. J. Control. Release 2011, 156, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Walters, K.A.; Abdalghafor, H.M.; Lane, M.E. The human nail—Barrier characterisation and permeation enhancement. Int. J. Pharm. 2012, 435, 10–21. [Google Scholar] [CrossRef]
- Palliyil, B.B.; Li, C.; Owaisat, S.; Lebo, D.B. Lateral drug diffusion in human nails. AAPS PharmSciTech 2014, 15, 1429–1438. [Google Scholar] [CrossRef][Green Version]
- Tsai, M.-T.; Tsai, T.-Y.; Shen, S.-C.; Ng, Y.C.; Lee, Y.-J.; Lee, J.-D.; Yang, C.-H. Evaluation of laser-assisted trans-nail drug delivery with optical coherence tomography. Sensors 2016, 16, 2111. [Google Scholar] [CrossRef]
- Vanstone, S.; Cordery, S.F.; Stone, J.M.; Gordeev, S.N.; Guy, R.H. Precise laser poration to control drug delivery into and through human nail. J. Control. Release 2017, 268, 72–77. [Google Scholar] [CrossRef]
- Monti, D.; Egiziano, E.; Burgalassi, S.; Tampucci, S.; Terreni, E.; Tivegna, S.; Chetoni, P. Influence of a combination of chemical enhancers and iontophoresis on in vitro transungual permeation of nystatin. AAPS PharmSciTech 2018, 19, 1574–1581. [Google Scholar] [CrossRef]
- Kline-Schoder, A.; Le, Z.; Zderic, V. Ultrasound-enhanced drug delivery for treatment of onychomycosis. J. Ultras Med. 2018, 37, 1743–1752. [Google Scholar] [CrossRef]
- Vlahovic, T.; Merchant, T.; Chanda, S.; Zane, L.T.; Coronado, D. In vitro nail penetration of tavaborole topical solution, 5%, through nail polish on ex vivo human fingernails. J. Drugs Dermatol. 2015, 14, 675–678. [Google Scholar] [PubMed]
- Im, Y.-H.; Xiong, Z.; Elg, D.T.; Graves, D.B. Uptake and diffusion of plasma-generated reactive nitrogen species through keratinized membrane. J. Phys. D Appl. Phys. 2019, 52, 195201. [Google Scholar] [CrossRef]
- Chaundri, S.K.; Jain, N.K. History of cosmetics. Asian J. Pharm. 2009, 3, 164–167. [Google Scholar]
- Andrè, J.; Baran, R. Nail cosmetics: Handle of skin care. In Handbook of Cosmetic Science and Technology, 3rd ed.; Bare, A.O., Paye, M., Maibach, H.I., Eds.; CRC Press: Boca Raton, FL, USA, 2014; pp. 745–767. [Google Scholar]
- Pati, N.B.; Dey, B.K.; Sudip, D.; Subhas, S. Nail drug delivery system: A review. J. Adv. Pharm. Educ. Res. 2012, 2, 101–109. [Google Scholar]
- Krishna, G.S.; Kumar, P.P.; Murugan, K.B. Nail as a promising drug delivery system for controlled release. Int. J. Pharm. Sci. 2013, 4, 907–915. [Google Scholar]
- Iorizzo, M.; Piraccini, B.M.; Tosti, A. Nail cosmetics in nail disorders. J. Cosmet. Dermatol. 2007, 6, 53–58. [Google Scholar] [CrossRef]
- Standardization, I.O.f. ISO 10993: Biological Evaluation of Medical Devices. In Part 12: Sample Preparation and Reference Materials; International Organization for Standardization: Geneva, Switzerland, 2002. [Google Scholar]
- Standardization, I.O.f. ISO 10993: Biological Evaluation of Medical Devices. In Part 15: Identification and Quantification of Degradation Products From Metals and Alloys; International Organization for Standardization: Geneva, Switzerland, 2000. [Google Scholar]
- Seshadri, D.; De, D. Nails in nutritional deficiencies. Indian J. Dermatol. Venereol. Leprol. 2012, 78, 237–241. [Google Scholar] [CrossRef]
- Stutzman, P.E.; Feng, P.; Bullard, J.W. Phase analysis of Portland cements by combined quantitative X-ray powder diffraction and scanning electron microscopy. J. Res. Natl. Inst. Stan. 2016, 121, 47–107. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Mertin, D.; Lippold, B.C. In-vitro permeability of the human nail and of a keratin membrane from bovine hooves: Prediction of the penetration rate of antimycotics through the nail plate and their efficacy. J. Pharm. Pharmacol. 1997, 49, 866–872. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Komatsu, T.; Sumi, M.; Numajiri, S.; Miyamoto, M.; Kobayashi, D.; Sugibayashi, K.; Morimoto, Y. In vitro permeation of several drugs through the human nail plate: Relationship between physicochemical properties and nail permeability of drugs. Eur. J. Pharm. Sci. 2004, 21, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.B.; Sammeta, S.M.; Vaka, S.R.; Narasimha Murthy, S. A study on the effect of inorganic salts in transungual drug delivery of terbinafine. J. Pharm. Pharmacol. 2009, 61, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Walters, K.A.; Flynn, G.L.; Marvel, J.R. Physicochemical characterization of the human nail: Solvent effects on the permeation of homologous alcohols. J. Pharm. Pharmacol. 1985, 37, 771–775. [Google Scholar] [CrossRef] [PubMed]
- Baswan, S.M.; Li, S.K.; LaCount, T.D.; Kasting, G.B. Size and charge dependence of ion transport in human nail plate. J. Pharm. Sci. 2016, 105, 1201–1208. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strømme, M.; Engqvist, V.; Burot, L.; López, A. Calcium and Silicon Delivery to Artificial and Human Nails from Nail Polish Formulations. Cosmetics 2020, 7, 15. https://doi.org/10.3390/cosmetics7010015
Strømme M, Engqvist V, Burot L, López A. Calcium and Silicon Delivery to Artificial and Human Nails from Nail Polish Formulations. Cosmetics. 2020; 7(1):15. https://doi.org/10.3390/cosmetics7010015
Chicago/Turabian StyleStrømme, Märta, Viktoria Engqvist, Louise Burot, and Alejandro López. 2020. "Calcium and Silicon Delivery to Artificial and Human Nails from Nail Polish Formulations" Cosmetics 7, no. 1: 15. https://doi.org/10.3390/cosmetics7010015
APA StyleStrømme, M., Engqvist, V., Burot, L., & López, A. (2020). Calcium and Silicon Delivery to Artificial and Human Nails from Nail Polish Formulations. Cosmetics, 7(1), 15. https://doi.org/10.3390/cosmetics7010015




