A Randomized Study to Determine the Sun Protection Factor of Natural Pterostilbene from Pterocarpus Marsupium
Abstract
1. Introduction
2. Material and Methods
2.1. Test Material
2.2. DPPH (2,2-diphenyl-1-picrylhydrazyl) Free Radical Scavenging Activity
2.3. Oxygen Radical Absorption Capacity (ORAC)Assay
2.4. Hydroxyl Radical Antioxidant Capacity (HORAC) Assay
2.5. Reactive Oxygen Species Scavenging Activity (ROS)
2.6. In Vitro Evaluation of the Sun Protection Factor by UV Spectrophotometry
2.7. Ethics
2.8. Primary Skin Irritation Test
2.9. In vivo Determination of Sun Protection Factor (SPF)
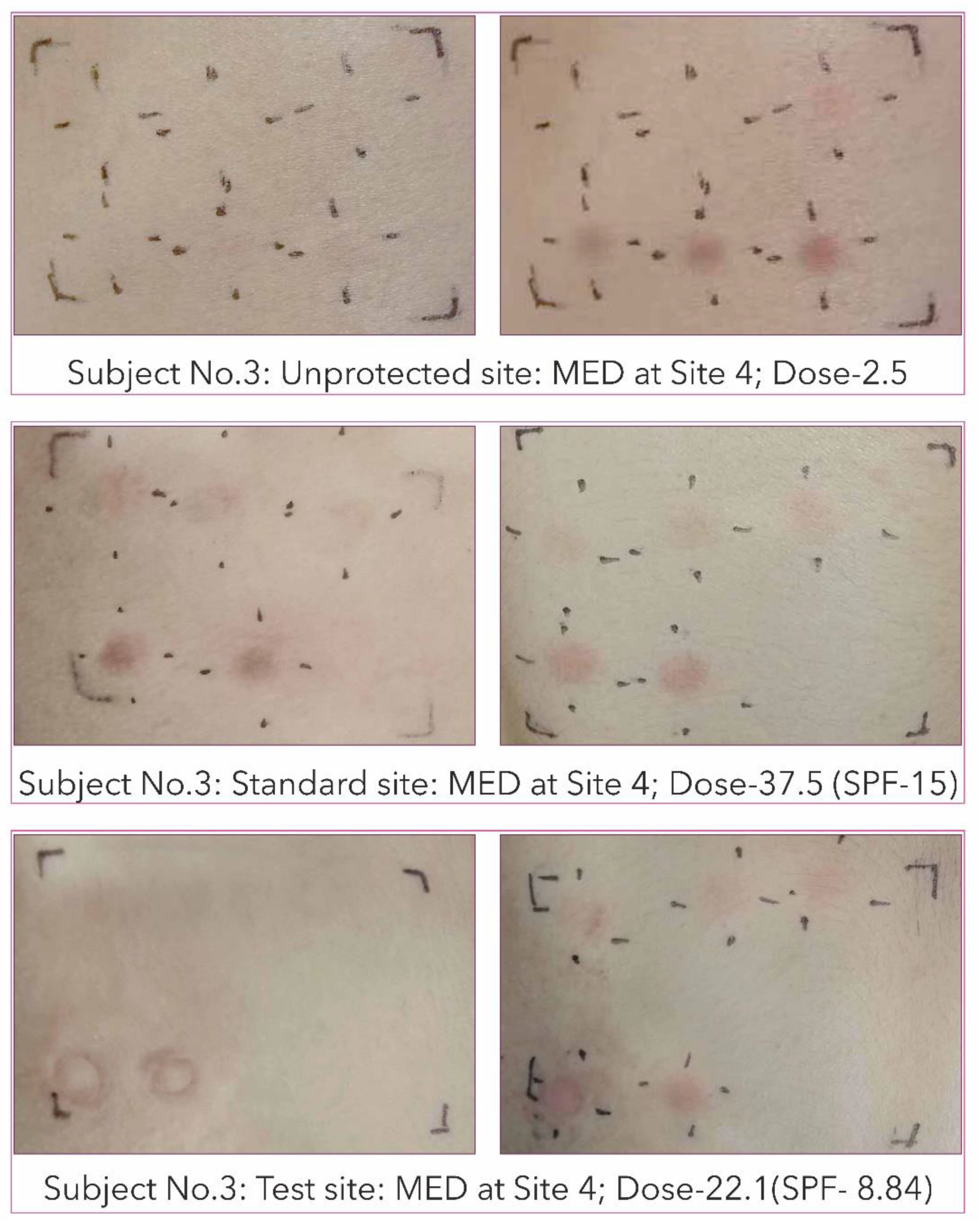
2.10. Minimal Erythema Dose (MED) Determination
2.11. Mean SPF Calculation
2.12. Statistical Analysis
3. Results
3.1. Antioxidant Activity of Pterostilbene
3.2. Reactive Oxygen Species Scavenging Capacity of Pterostilbene in Vitro
3.3. In Vitro SPF of Pterostilbene Cream Formulation of 0.4%Pterostilbene
3.4. In Vivo Primary Skin Irritation Test of Pterostilbene Cream Formulation
3.5. In Vivo Determination of Sun Protection Factor (SPF) of Pterostilbene Cream Formulation
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- McKenzie, R.L.; Björn, L.O.; Bais, A.; Ilyas, M. Changes in biologically active ultraviolet radiation reaching the Earth’s surface. Photochem. Photobiol. Sci. 2003, 2, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Korać, R.; Khambholja, K. Potential of herbs in skin protection from ultraviolet radiation. Pharmacogn. Rev. 2011, 5, 164. [Google Scholar] [CrossRef] [PubMed]
- Moan, J.D. Visible light and uv radiation, in brune. In Radiation at Home, Outdoors and in the Workplace; Hellborg, R., Persson, B.R.R., Pääkkönen, R., Eds.; Scandinavian Science Publisher: Oslo, Norway, 2001; pp. 69–86. [Google Scholar]
- Ichihashi, M.; Ando, H.; Yoshida, M.; Niki, Y.; Matsui, M. Photoaging of the skin. Anti Aging Med. 2009, 6, 46–59. [Google Scholar] [CrossRef]
- Wondrak, G.T.; Jacobson, M.K.; Jacobson, E.L. Endogenous UVA-photosensitizers: Mediators of skin photodamage and novel targets for skin photoprotection. Photochem. Photobiol. Sci. 2006, 5, 215–237. [Google Scholar] [CrossRef]
- Afaq, F.; Adhami, V.M.; Ahmad, N.; Mukhtar, H. Botanical antioxidants for chemoprevention of photocarcinogenesis. Front. Biosci. 2002, 7, d784–d792. [Google Scholar] [CrossRef]
- De Gruijl, F.R. Photocarcinogenesis: UVA vs UVB. Methods Enzymol. 2000, 319, 359–366. [Google Scholar]
- Mbanga, L.; Mulenga, M.; Mpiana, P.T.; Bokolo, K.; Mumbwa, M.; Mvingu, K. Determination of Sun Protection Factor (SPF) of some body creams and lotions marketed in kinshasa by ultraviolet spectrophotometry. Int. J. Adv. Res. Chem. Sci. 2014, 1, 7–13. [Google Scholar]
- D’Orazio, J.; Jarrett, S.; Amaro-Ortiz, A.; Scott, T. UV radiation and the skin. Int. J. Mol. Sci. 2013, 14, 12222–12248. [Google Scholar] [CrossRef]
- Matsumura, Y.; Ananthaswamy, H.N. Toxic effects of ultraviolet radiation on the skin. Toxicol. Appl. Pharmacol. 2004, 195, 298–308. [Google Scholar] [CrossRef]
- Kaidbey, K.H.; Agin, P.P.; Sayre, R.M.; Kligman, A.M. Photoprotection by melanin—A comparison of black and Caucasian skin. J. Am. Acad. Dermatol. 1979, 1, 249–260. [Google Scholar] [CrossRef]
- Fajuyigbe, D.; Young, A.R. The impact of skin colour on human photobiological responses. Pigment Cell Melanoma Res. 2016, 29, 607–618. [Google Scholar] [CrossRef]
- Santos, B.A.; da Silva, A.C.; Bello, M.L.; Gonçalves, A.S.; Gouvêa, T.A.; Rodrigues, R.F.; Cabral, L.M.; Rodrigues, C.R. Molecular modeling for the investigation of UV absorbers for sunscreens: Triazine and benzotriazole derivatives. J. Photochem. Photobiol. A Chem. 2018, 356, 219–229. [Google Scholar]
- Losantos, R.; Ardoiz, I.F.; Aguilera, J.; Herrera-Ceballos, E.; García-Iriepa, C.; Campos, P.J.; Sampedro, D. Rational design and synthesis of efficient sunscreens to boost the solar protection factor. Angew. Chem. Int. Ed. Engl. 2017, 129, 2632–2635. [Google Scholar] [CrossRef]
- Garcia, E.B.; Machado, T.S.C.; Ferraris, F.K.; Amendoeira, F.C. Ferraris Contaminação ambiental e da cadeia alimentar com filtros solares: Um potencial risco à saúde humana. Rev. Anal. 2015, 13, 45–54. [Google Scholar]
- Fastelli, P.; Renzi, M. Exposure of key marine species to sunscreens: Changing ecotoxicity as a possible indirect effect of global warming. Mar. Pollut. Bull. 2019, 149, 110517. [Google Scholar] [CrossRef]
- Foltête, A.; Masfaraud, J.; Bigorgne, E.; Nahmani, J.; Chaurand, P.; Botta, C.; Labille, J.; Rose, J.; Férard, J.; Cotelle, S. Environmental impact of sunscreen nanomaterials: Ecotoxicity and genotoxicity of altered TiO2 nanocomposites on Vicia faba. Environ. Pollut. 2011, 159, 2515–2522. [Google Scholar] [CrossRef]
- Ruszkiewicz, J.A.; Pinkas, A.; Ferrer, B.; Peres, T.V.; Tsatsakis, A.; Aschner, M. Neurotoxic effect of active ingredients in sunscreen products, a contemporary review. Toxicol. Rep. 2017, 4, 245–259. [Google Scholar] [CrossRef]
- Goswami, P.K.; Samant, M.; Srivastava, R. Natural sunscreen agents: A review. Sch. Acad. J. Pharm. 2013, 2, 458–463. [Google Scholar]
- Lawrence, K.P.; Long, P.F.; Young, A.R. Mycosporine-like amino acids for skin photoprotection. Curr. Med. Chem. 2017, 24, 5512–5527. [Google Scholar] [CrossRef]
- Lawrence, K.P.; Gacesa, R.; Long, P.; Young, A. (Molecular photoprotection of human keratinocytes In Vitro by the naturally occurring mycosporine-like amino acid palythine. Br. J. Derm. 2018, 178, 1353–1363. [Google Scholar] [CrossRef]
- Gacesa, R.; Lawrence, K.P.; Georgakopoulos, N.D.; Yabe, K.; Dunlap, W.C.; Barlow, D.J.; Wells, G.; Young, A.R.; Long, P.F. The mycosporine-like amino acids porphyra-334 and shinorine are antioxidants and direct antagonists of Keap1-Nrf2 binding. Biochimie 2018, 154, 35–44. [Google Scholar] [CrossRef]
- Tao, S.; Justiniano, R.; Zhang, D.; Wondrak, G.T. The Nrf2-inducers tanshinone I and dihydrotanshinone protect human skin cells and reconstructed human skin against solar simulated UV. Redox Biol. 2013, 1, 532–541. [Google Scholar] [CrossRef]
- Mann, T.; Eggers, K.; Rippke, F.; Tesch, M.; Buerger, A.; Darvin, M.E.; Schanzer, S.; Meinke, M.C.; Lademann, K.; Kolbe, L. High energy visible light at ambient doses and intensities induces oxidative stress of skin—Protective effects of the antioxidant and Nrf2 inducer licochalcone a In Vitro and In Vivo. Photodermatol. Photoimmunol. Photomed. 2019, 00, 1–10. Available online: https://onlinelibrary.wiley.com/doi/pdf/10.1111/phpp.12523 (accessed on 24 February 2020). [CrossRef] [PubMed]
- Kageyama, H.; Rungaroon, W.S. Antioxidative, anti-inflammatory and anti-aging properties of mycosporine-like amino acids: Molecular and cellular mechanisms in the protection of skin-aging. Mar. Drugs 2019, 17, 222. [Google Scholar] [CrossRef]
- McCormack, D.; McFadden, D. A review of pterostilbene antioxidant activity and disease modification. Oxid. Med. Cell. Longev. 2013, 2013, 575482. [Google Scholar] [CrossRef]
- Langcake, P.; Cornford, C.A.; Pryce, R.J. Identification of pterostilbene as a phytoalexin from Vitis vinifera leaves. Phytochemistry 1979, 18, 1025–1027. [Google Scholar] [CrossRef]
- Langcake, P.; Pryce, R.J. A new class of phytoalexins from grapevines. Experientia 1977, 33, 151–152. [Google Scholar] [CrossRef]
- Manickam, M.; Ramanathan, M.; Jahromi, M.A.; Chansouria, J.P.; Ray, A.B. Antihyperglycemic activity of phenolics from Pterocarpus marsupium. J. Nat. Prod. 1997, 60, 609–610. [Google Scholar] [CrossRef]
- Paul, B.; Masih, I.; Deopujari, J.; Charpentier, C. Occurrence of resveratrol and pterostilbene in age-old darakchasava, an ayurvedic medicine from India. J. Ethnopharmacol. 1999, 68, 71–76. [Google Scholar] [CrossRef]
- Rimando, A.; Kalt, M.; Magee, J.B.; Dewey, J.; Ballington, J.R. Resveratrol, pterostilbene, and piceatannol in vaccinium berries. J. Agric. Food Chem. 2004, 52, 4713–4719. [Google Scholar] [CrossRef]
- Majeed, M.; Bhat, B.; Gangadharan, G.K.; Anand, S. Melanogenesis Inhibition by 3,5-Dimethoxy-4′-Hydroxystilbenes and Cosmeceutical Compositions Thereof. US Patent Application No. 12/408,808, 23 September 2010. [Google Scholar]
- Schalka, S.; Dos Reis, V.M.S. Sun protection factor: Meaning and controversies. Fator de proteção solar: Significado e controvérsia. An Bras Derm. 2011, 86, 507–515. [Google Scholar] [CrossRef]
- Yang, S.I.; Liu, S.; Brooks, G.J.; Lanctot, Y.; Gruber, J.V. Reliable and simple spectrophotometric determination of sun protection factor: A case study using organic UV filter-based sunscreen products. J. Cosmet. Derm. 2018, 17, 518–522. [Google Scholar] [CrossRef]
- Herald, T.J.; Gadgil, P.; Tilley, M. High-throughput microplate assays for screening flavonoid content and DPPH-scavenging activity in sorghum bran and flour. J. Sci. Food Agric. 2012, 92, 2326–2331. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Hampsch-Woodill, M.; Flanagan, J.; Prior, R.L. High-Throughput assay of oxygen radical absorbance capacity (ORAC) using a multichannel liquid handling system coupled with a microplate fluorescence reader in 96-well format. J. Agric. Food Chem. 2002, 50, 4437–4444. [Google Scholar] [CrossRef]
- Ou, B.; Hampsch-Woodill, M.; Flanagan, J.; Deemer, E.K.; Prior, R.L.; Huang, D. Novel fluorometric assay for hydroxyl radical prevention capacity using fluorescein as the probe. J. Agric. Food Chem. 2002, 50, 2772–2777. [Google Scholar] [CrossRef]
- LeBel, C.P.; Ischiropoulos, H.; Bondy, S.C. Evaluation of the probe 2′, 7′-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem. Res. Toxicol. 1992, 5, 227–231. [Google Scholar] [CrossRef]
- Mansur, J.S.; Breder, M.N.R.; Mansur, M.C.A.; Azulay, R.D. Determinação do fator de proteção solar por espectrofotometria. Open J. Appl. Sci. 1986, 61, 121–124. [Google Scholar]
- Sayre, R.M.; Agin, P.P.; LeVee, G.J.; Marlowe, E.A. comparison of In Vivo and In Vitro testing of sunscreening formulas. Photochem. Photobiol. 1979, 29, 559–566. [Google Scholar] [CrossRef]
- BIS. 4011:2018 Methods of Test for Safety Evaluation of Cosmetics; Third revision (ICS 71.100.40); BIS: Basel, Switzerland, 2018. [Google Scholar]
- ISO. 24444:2010, Cosmetics—Sun Protection Test Methods—In Vivo Determination of the Sun Protection Factor (SPF); ISO: Geneva, Switzerland, 2010. [Google Scholar]
- Singh, M.; Sharma, V. Spectrophotometric determination of sun protection factor and antioxidant potential of an herbal mixture. Br. Biotechnol. J. 2016, 10, 1–8. [Google Scholar] [CrossRef]
- Rajnochová Svobodová, A.; Gabrielová, E.; Michaelides, L.; Kosina, P.; Ryšavá, A.; Ulrichová, J.; Zálešák, B.; Vostálová, J. UVA-photoprotective potential of silymarin and silybin. Arch. Dermatol. Res. 2018, 310, 413–424. [Google Scholar] [CrossRef]
- Sklar, L.R.; Almutawa, F.; Lim, H.W.; Hamzavi, I. Effects of ultraviolet radiation, visible light, and infrared radiation on erythema and pigmentation: A review. Photochem. PhotoBiol. Sci. 2013, 12, 54–64. [Google Scholar] [CrossRef]
- Tyrrell, R.M. Ultraviolet radiation and free radical damage to skin. Biochem. Soc. Symp. 1995, 61, 47–53. [Google Scholar]
- Sirerol, J.A.; Feddi, F.; Mena, S.; Rodriguez, M.L.; Sirera, P.; Aupí, M.; Pérez, S.; Asensi, M.; Ortega, A.; Estrela, J.M. Topical treatment with pterostilbene, a natural phytoalexin, effectively protects hairless mice against UVB radiation-induced skin damage and carcinogenesis. Free Radic. Biol. Med. 2015, 85, 1–11. [Google Scholar] [CrossRef]
- United States: Environmental Protection Agency. Health Effects of UV Radiation; United States: Environmental Protection Agency: Washington, WA, USA, 2017.
- Damian, D.L.; Halliday, G.M.; StC.Barnetson, R. Sun protection factor measurement of sunscreens is dependent on minimal erythema dose. Br. J. Dermatol. 1999, 141, 502–507. [Google Scholar] [CrossRef]
- Ray, P.D.; Huang, B.W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef]
- Fisher, G.J. The pathophysiology of photoaging of the skin. Cutis 2005, 75, 5–9. [Google Scholar]
- Masaki, H. Role of antioxidants in the skin: Anti-aging effects. J. Dermatol. Sci. 2010, 58, 85–90. [Google Scholar] [CrossRef]
- Sato, K.; Taguchi, H.; Maeda, T.; Minami, H.; Asada, Y.; Watanbe, Y.; Yoshikawa, K. The primary cytotoxicity in Ultravoilet-A-irradaited Riboflavin solution is derived from Hydrogen peroxide. J. Investig. Dermatol. 1995, 105, 608–612. [Google Scholar] [CrossRef]
- Yoshimoto, S.; Yoshida, M.; Ando, H.; Ichihashi, M. Establishment of photoaging In Vitro by repetitive UVA irradiation: Induction of characteristic markers of senescence and its prevention b paplal with potent catalase activity. Photochem Photobiol. 2018, 94, 438–444. [Google Scholar] [CrossRef]
- Wood, C.; Murphy, E. Sunscreens efficacy. Glob. Cosmet. Ind. Duluth 2000, 167, 38–44. [Google Scholar]
- Majeed, M.; Majeed, S.; Jain, R.; Mundkur, L.; Rajalakshmi, H.R.; Lad, P.S.; Neupane, P. An open-label single-arm, monocentric study assessing the efficacy and safety of natural pterostilbene (Pterocarpus marsupium) for skin brightening and antiaging effects clinical. Cosmet. Investig. Dermatol. 2020, 13, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Pissavini, M.; Ferrero, L.; Alaro, V.; Heinrich, U.; Tronnier, H.; Kockott, D.; Lutz, D.; Tournier, V.; Zambonin, M.; Meloni, M. Determination of the In Vitro SPF. Cosmet. Toilet. 2003, 118, 63–72. [Google Scholar]
- Walters, C.; Keeney, A.; Wigal, C.T.; Johnston, C.R.; Cornelius, R.D. The spectrophotometric analysis and modeling of sunscreens. J. Chem. Educ. 1997, 74, 99. [Google Scholar] [CrossRef]
- Michael, W.A.; Bain, G. Measuring the Sun Protection Factor of Sunscreens; Application Note: 51463; Thermo Fisher Scientific: Madison, WI, USA, 2007. [Google Scholar]
- Agrapidis-Paloympis, L.; Nash, R.; Shaath, N. The effect of solvents on the ultraviolet absorbance of sunscreens. J. Soc. Cosmet. Chem. 1987, 38, 209–221. [Google Scholar]
- Punam, N.S.; Ukirade, P.S.; Salunkhe, S.D.; Sutar, S.T.; Magdum, C.S.; Mohite, S.K.; Lokapure, S.G.; Metri, S.M. In Vitro evaluation of sun protection factor of fruit extract of Carica papaya L. as a lotion formulation. Euro. J. Exp. Bio. 2014, 4, 44–47. [Google Scholar]
- Jangde, R.; Daharwal, S.J. Herbal sunscreen: An overview. Res. J. Top. Cosmet. Sci. 2011, 2, 2. [Google Scholar]
- Sirerol, J.A.; Rodríguez, M.L.; Mena, S.; Asensi, M.A.; Estrela, J.M.; Ortega, A.L. Role of natural stilbenes in the prevention of cancer. Oxid. Med. Cell. Longev. 2016, 2016, 3128951. [Google Scholar] [CrossRef]
- Deng, H.; Li, H.; Ho, Z.Y.; Dai, X.Y.; Chen, Q.; Li, R.; Liang, B.; Zhu, H. Pterostilbene’s protective effects against photodamage caused by UVA/UVB irradiation. Pharmazie 2018, 73, 651–658. [Google Scholar] [CrossRef]
- Li, H.; Jiang, N.; Liang, B.; Liu, Q.; Zhang, E.; Peng, L.; Deng, H.; Li, R.; Li, Z.; Zhu, H. Pterostilbene protects against UVB-induced photo-damage through a phosphatidylinositol-3-kinase-dependent Nrf2/ARE pathway in human keratinocytes. Redox Rep. 2017, 22, 501–507. [Google Scholar] [CrossRef]

| Wavelength λ (nm) | Absorbance (λ) | EE (λ) × I (λ) (Constant) | EE (λ) × I (λ) × ABS (λ) |
|---|---|---|---|
| 290 | 2.047 ± 0.006 | 0.015 | 0.030 ± 0.0001 |
| 295 | 2.175 ± 0.012 | 0.0817 | 0.177 ± 0.0009 |
| 300 | 2.192 ± 0.009 | 0.2874 | 0.629 ± 0.002 |
| 305 | 2.211 ± 0.010 | 0.3278 | 0.724 ± 0.003 |
| 310 | 2.126± 0.008 | 0.1864 | 0.396 ± 0.001 |
| 315 | 2.096 ± 0.007 | 0.0839 | 0.175 ± 0.0006 |
| 320 | 2.138 ± 0.021 | 0.018 | 0.038 ± 0.0003 |
| ∑ EE(λ) × I (λ) × ABS (λ) | 2.173 ± 0.006 | ||
| SPF = CF × ∑ EE(λ) × I (λ) × ABS (λ) | 21.73 ± 0.06 | ||
| Wavelength λ (nm) | Cream Base | 0.4% of 90% Pterostilbene Cream Formulation | ||
|---|---|---|---|---|
| Absorbance (λ) | EE(λ) × I (λ) × ABS (λ) | Absorbance (λ) | EE(λ) × I (λ) × ABS (λ) | |
| 290 | 0.04 ± 0.005 | 0.0006 | 0.744 ± 0.002 | 0.011 ± 0.005 |
| 295 | 0.011 ± 0.001 | 0.0009 | 0.816 ± 0.003 | 0.067 ± 0.036 |
| 300 | 0.005 ± 0.0001 | 0.0014 | 0.873 ± 0.001 | 0.251 ± 0.144 |
| 305 | 0.002 ± 0.0001 | 0.0006 | 0.913 ± 0.002 | 0.299 ± 0.172 |
| 310 | 0.001 ± 0.0001 | 0.00018 | 0.891 ± 0.002 | 0.166 ± 0.095 |
| 315 | 0.001 ± 0.0001 | 0.000083 | 0.886 ± 0.002 | 0.074 ± 0.042 |
| 320 | 0.002 ± 0.0005 | 0.000036 | 0.892 ± 0.002 | 0.016 ± 0.008 |
| ∑ EE(λ) × I (λ) × ABS (λ) | 0.003765 ± 0.0001 | 0.884 ± 0.0005 | ||
| SPF = CF × ∑ EE(λ) × I (λ) × ABS (λ) | 0.037651 ± 0.001 | 8.84364 ± 0.0005 | ||
| Investigational Products | Mean Irritation Score-0 Hrs | Irritancy Assessment | Mean Irritation Score-24 Hrs | Irritancy Assessment | Mean Irritation Score-7 Days | Irritancy Assessment |
|---|---|---|---|---|---|---|
| Pterostilbene cream (CS/1118/ML163A) | 0.08 | Non-Irritant | 0 | Non-Irritant | 0 | Non-Irritant |
| Positive control SLS (sodium lauryl sulphate) | 2.29 | Irritant | 2.33 | Irritant | 0.5 | Non-irritant |
| Parameter | Standard Site (Bayer’s P3) | Test Product Site Pterostilbene Cream |
|---|---|---|
| Mean SPF (n = 10) | 15.10 | 6.20 |
| SD | 0.81 | 1.30 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Majeed, M.; Majeed, S.; Jain, R.; Mundkur, L.; Rajalakshmi, H.R.; Lad, P.; Neupane, P. A Randomized Study to Determine the Sun Protection Factor of Natural Pterostilbene from Pterocarpus Marsupium. Cosmetics 2020, 7, 16. https://doi.org/10.3390/cosmetics7010016
Majeed M, Majeed S, Jain R, Mundkur L, Rajalakshmi HR, Lad P, Neupane P. A Randomized Study to Determine the Sun Protection Factor of Natural Pterostilbene from Pterocarpus Marsupium. Cosmetics. 2020; 7(1):16. https://doi.org/10.3390/cosmetics7010016
Chicago/Turabian StyleMajeed, Muhammed, Shaheen Majeed, Renuka Jain, Lakshmi Mundkur, H. R. Rajalakshmi, Prachi Lad, and Prakriti Neupane. 2020. "A Randomized Study to Determine the Sun Protection Factor of Natural Pterostilbene from Pterocarpus Marsupium" Cosmetics 7, no. 1: 16. https://doi.org/10.3390/cosmetics7010016
APA StyleMajeed, M., Majeed, S., Jain, R., Mundkur, L., Rajalakshmi, H. R., Lad, P., & Neupane, P. (2020). A Randomized Study to Determine the Sun Protection Factor of Natural Pterostilbene from Pterocarpus Marsupium. Cosmetics, 7(1), 16. https://doi.org/10.3390/cosmetics7010016







