Lipid Self-Assemblies and Nanostructured Emulsions for Cosmetic Formulations
Abstract
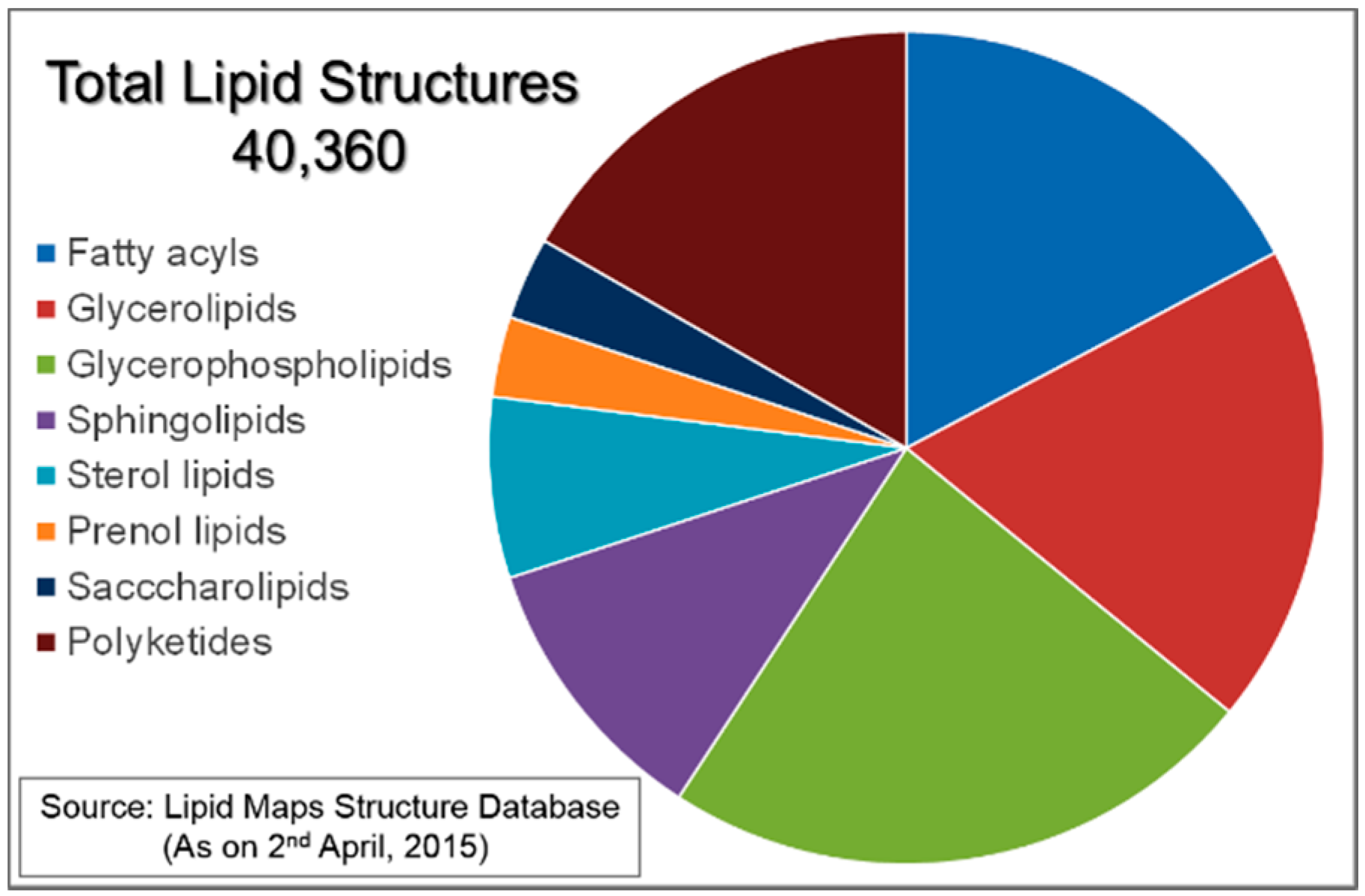
:1. Introduction
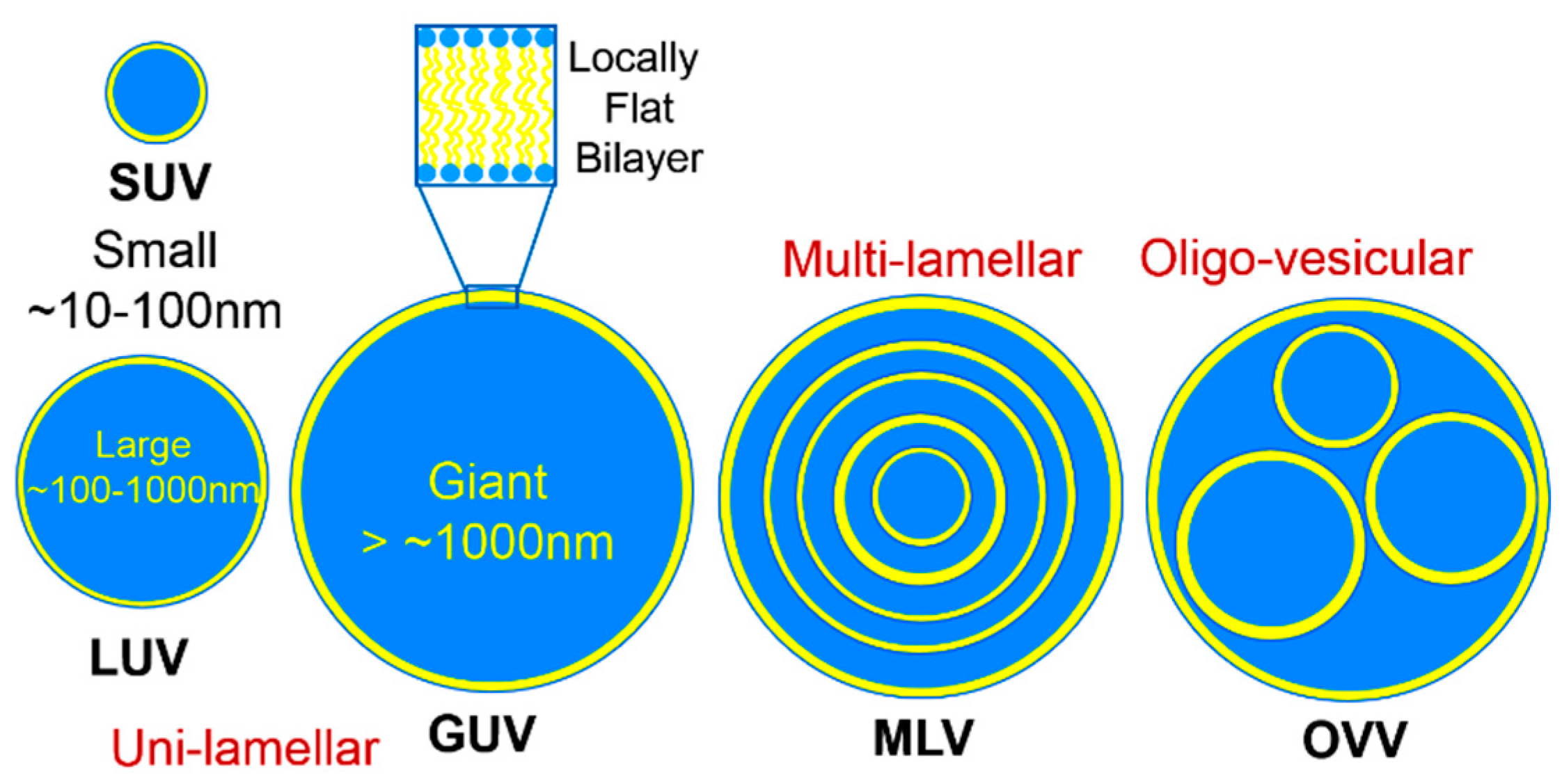
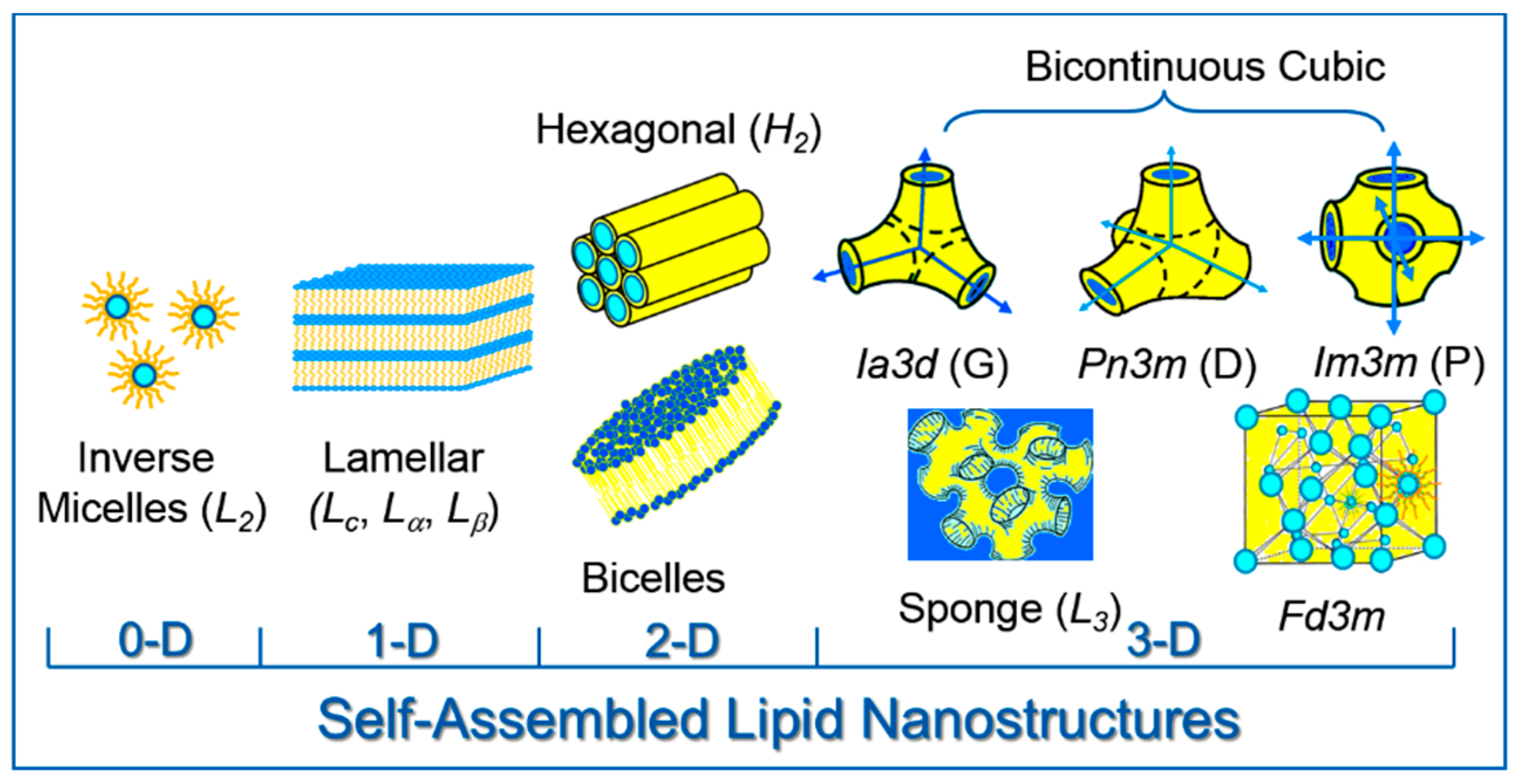
2. Lipid Self-Assembly: Simple and Elegant Nanostructures
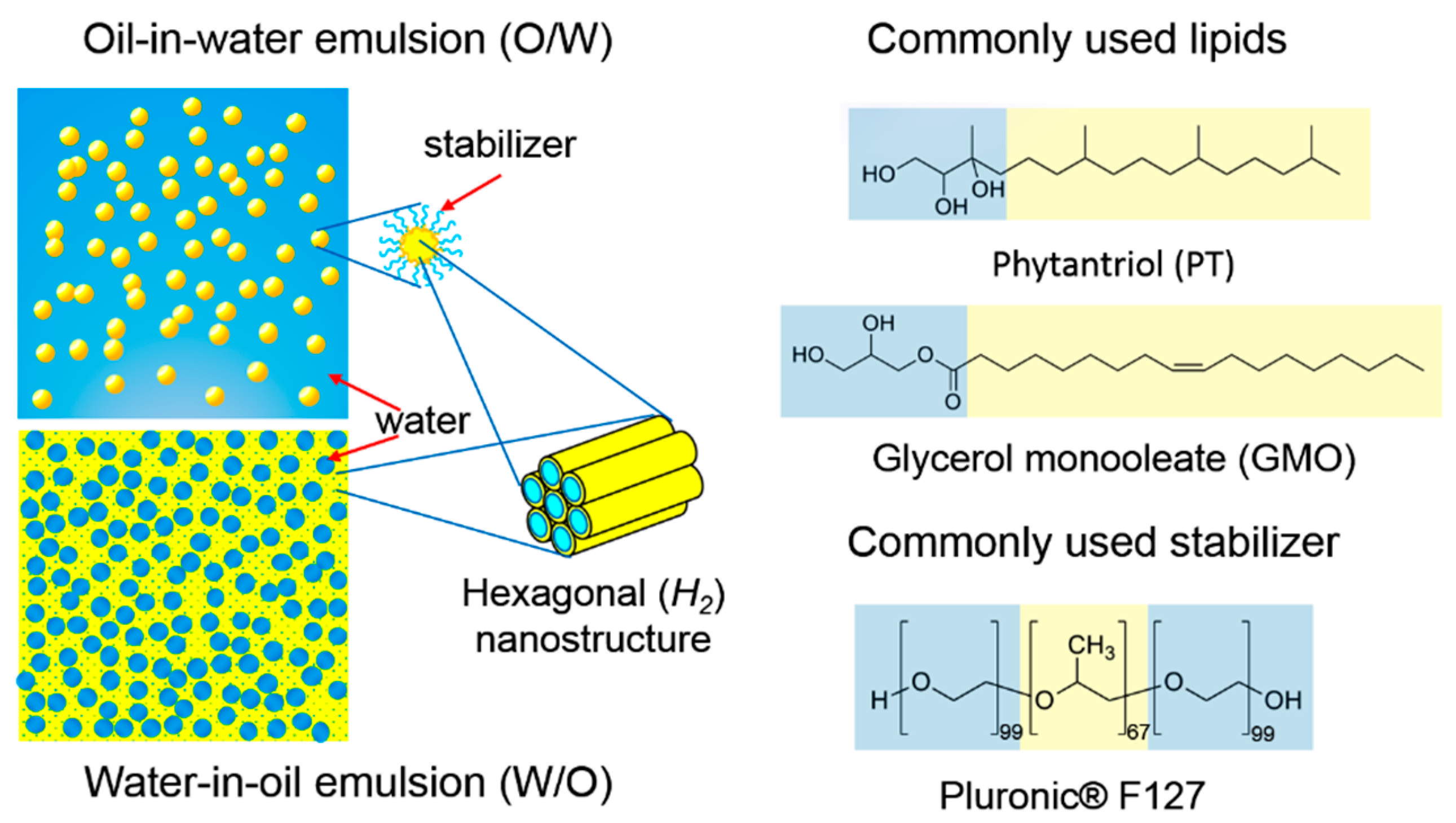
3. Lipid Nanostructured Emulsions: Next Level of Structural Hierarchy
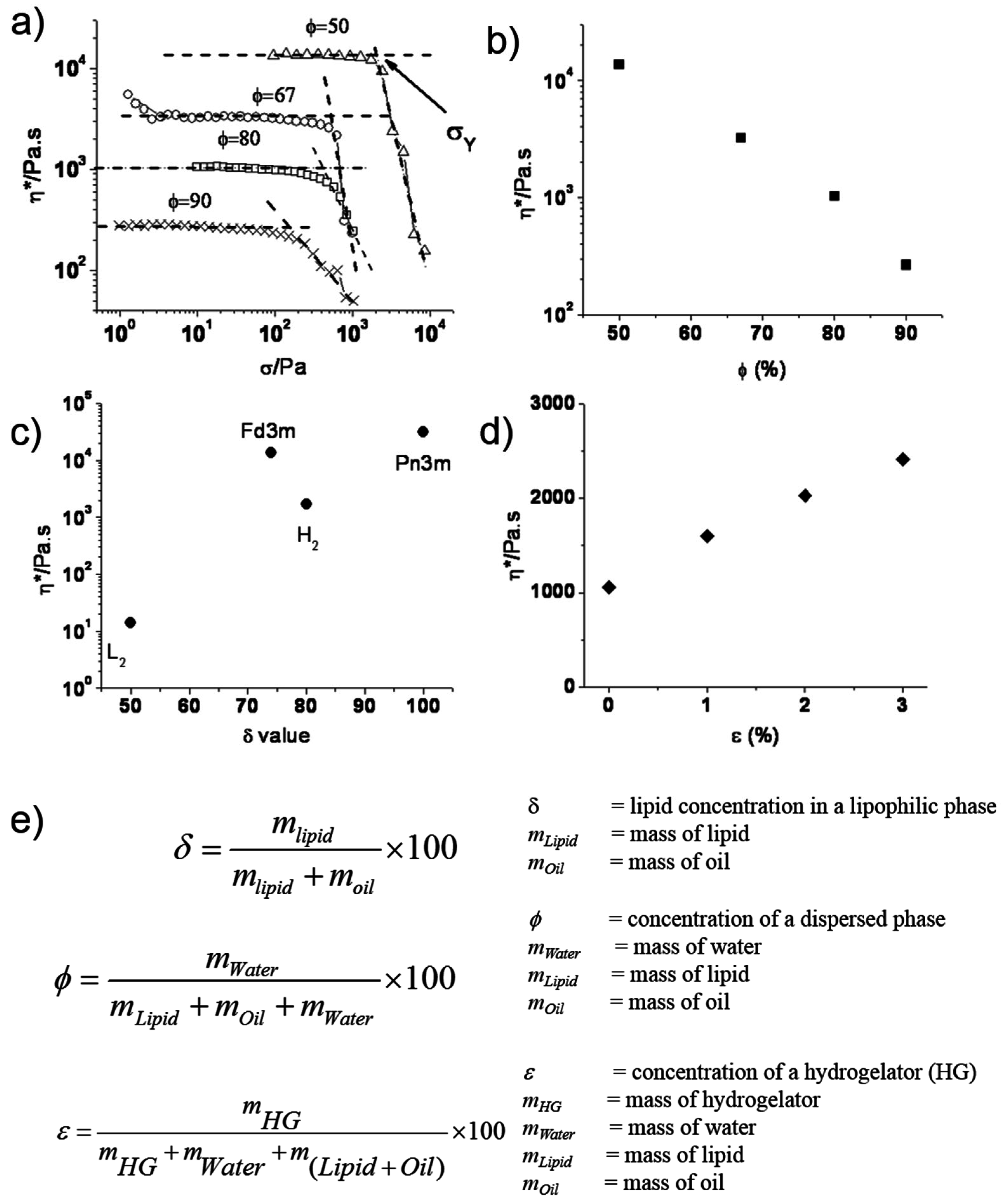
4. Modulating Structure and Properties of Lipid Systems
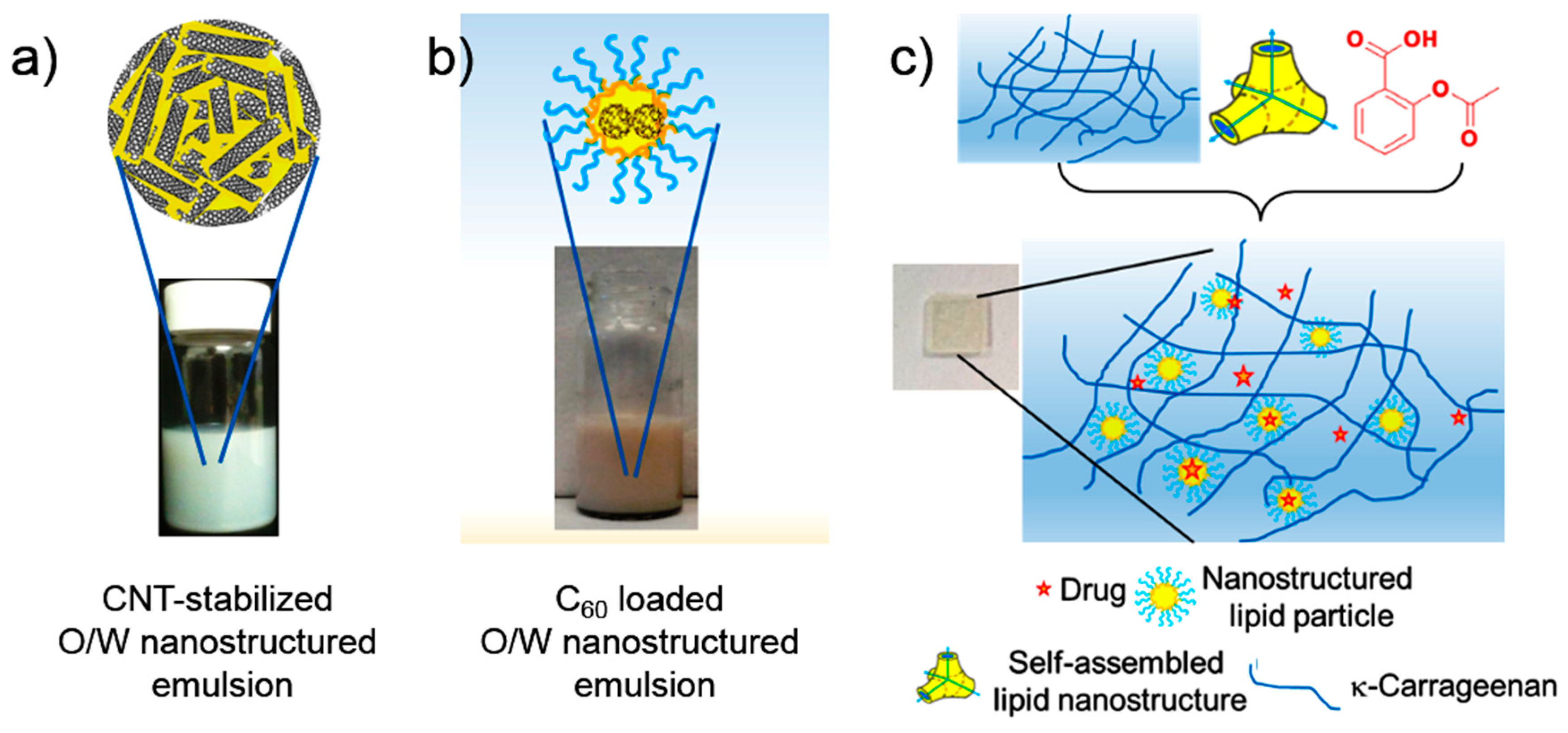
5. Nano-Hybrid Systems Based on Lipid Nanostructures
6. Conclusions and Perspectives
Conflicts of Interest
References
- EU-Regulation. Regulation (ec) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on Cosmetic Products (Recast); The European Parliament and the Council: Brussels, Belgium, 2009; Volume 1223.
- Ingredients-Inventory. Ingredients/Fragrance Inventory (Cosing 2); European Commission: Brussels, Belgium, 2016. [Google Scholar]
- Lautenschläger, H. Lipophilic substances—Oils and lipids in cosmetic products. Kosmet. Int. 2004, 4, 46–48. [Google Scholar]
- Hernandez, E. Pharmaceutical and cosmetic use of lipids. In Bailey's Industrial Oil and Fat Products; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005. [Google Scholar]
- Hernandez, E. Lipids, pharmaceutical and cosmetic use. In Kirk-Othmer Encyclopedia of Chemical Technology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2000. [Google Scholar]
- Alvarez, A.M.R.; Rodríguez, M.L.G. Lipids in pharmaceutical and cosmetic preparations. Grasas Aceites 2000, 51, 74–96. [Google Scholar]
- Lipid Maps Structure Database (LMSD). Lipidomics Gateway. 2015. Available online: www.lipidmaps.org (accessed on 28 October 2016).
- Luzzati, V.; Tardieu, A. Lipid phases: Structure and structural transitions. Annu. Rev. Phys. Chem. 1974, 25, 79–94. [Google Scholar] [CrossRef]
- Larsson, K.; Fontell, K.; Krog, N. Structural relationships between lamellar, cubic and hexagonal phases in monoglyceride-water systems. Possibility of cubic structures in biological systems. Chem. Phys. Lipids 1980, 27, 321–328. [Google Scholar] [CrossRef]
- Seddon, J.M. Structure of the inverted hexagonal (HII) phase, and non-lamellar phase transitions of lipids. Biochim. Biophys. Acta 1990, 1031, 1–69. [Google Scholar] [CrossRef]
- Israelachvili, J. Intermolecular and Surface Forces; Academic Press: London, UK, 1991. [Google Scholar]
- Kumar, V.V. Complementary molecular shapes and additivity of the packing parameter of lipids. Proc. Natl. Acad. Sci. USA 1991, 88, 444–448. [Google Scholar] [CrossRef] [PubMed]
- Israelachvili, J.N.; Mitchell, D.J.; Ninham, B.W. Theory of self-assembly of lipid bilayers and vesicles. Biochim. Biophys. Acta 1977, 470, 185–201. [Google Scholar] [CrossRef]
- Tresset, G. The multiple faces of self-assembled lipidic systems. PMC Biophys. 2009, 2, 3. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, C.V. Lipid crystallization: From self-assembly to hierarchical and biological ordering. Nanoscale 2012, 4, 5779–5791. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.-H.M.; Boxer, S.G. Model membrane systems and their applications. Curr. Opin. Chem. Biol. 2007, 11, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Angelov, B.; Angelova, A.; Drechsler, M.; Garamus, V.M.; Mutafchieva, R.; Lesieur, S. Identification of large channels in cationic pegylated cubosome nanoparticles by synchrotron radiation saxs and cryo-tem imaging. Soft Matter 2015, 11, 3686–3692. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.; Caffrey, M. Interlamellar transition mechanism in model membranes. J. Phys. Chem. B 1996, 100, 5608–5610. [Google Scholar] [CrossRef]
- Rhein, L.D.; Schlossman, M.; O’Lenick, A.; Somasundaran, P. Surfactants in Personal Care Products and Decorative Cosmetics, 3rd ed.; CRC- Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Rye, G.G.; Litwinenko, J.W.; Marangoni, A.G. Fat crystal networks. In Bailey's Industrial Oil and Fat Products; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005. [Google Scholar]
- Garti, N.; Sato, K. Crystallization Processes in Fats and Lipid Systems; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Sanders, C.R.; Prosser, R.S. Bicelles: A model membrane system for all seasons? Structure 1998, 6, 1227–1234. [Google Scholar] [CrossRef]
- Seddon, J.M.; Templer, R.H. Cubic phases of self-assembled amphiphilic aggregates. Philos. Trans. Math. Phys. Eng. Sci. 1993, 344, 377–401. [Google Scholar] [CrossRef]
- Larsson, K. Cubic lipid-water phases: Structures and biomembrane aspects. J. Phys. Chem. 1989, 93, 7304–7314. [Google Scholar] [CrossRef]
- Cates, M.E. The isotropic (“sponge”) to lamellar transition in dilute surfactant systems. Phys. A Stat. Theor. Phys. 1991, 176, 187–199. [Google Scholar] [CrossRef]
- Seddon, J.M.; Templer, R.H. Polymorphism of lipid-water systems. In Handbook of Biological Physics; Lipowsky, R., Sackmann, E., Eds.; Elsevier Science B.V.: Amsterdam, The Netherlands, 1995; Volume 1, pp. 97–160. [Google Scholar]
- Engström, S.; Alfons, K.; Rasmusson, M.; Ljusberg-Wahren, H. Solvent-induced sponge (L3) phases in the solvent-monoolein-water system. In The Colloid Science of Lipids; Lindmann, B., Ninham, B.W., Eds.; Steinkopff-Verlag: Darmstadt, Germany, 1998; pp. 93–98. [Google Scholar]
- Faham, S.; Bowie, J.U. Bicelle crystallization: A new method for crystallizing membrane proteins yields a monomeric bacteriorhodopsin structure. J. Mol. Biol. 2002, 316, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Landau, E.M.; Rosenbusch, J.P. Lipidic cubic phases: A novel concept for the crystallization of membrane proteins. Proc. Natl. Acad. Sci. USA 1996, 93, 14532–14535. [Google Scholar] [CrossRef] [PubMed]
- Wadsten, P.; Woehri, A.B.; Snijder, A.; Katona, G.; Gardiner, A.T.; Cogdell, R.J.; Neutze, R.; Engstroem, S. Lipidic sponge phase crystallization of membrane proteins. J. Mol. Biol. 2006, 364, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Almsherqi, Z.A.; Landh, T.; Kohlwein, S.D.; Deng, Y. Chapter 6 cubic membranes: The missing dimension of cell membrane organization. In International Review of Cell and Molecular Biology; Kwang, W.J., Ed.; Academic Press: London, UK, 2009; Volume 274, pp. 275–342. [Google Scholar]
- Tomas, L. From entangled membranes to eclectic morphologies: Cubic membranes as subcellular space organizers. FEBS Lett. 1995, 369, 13–17. [Google Scholar]
- Sakya, P.; Seddon, J.M.; Templer, R.H.; Mirkin, R.J.; Tiddy, G.J.T. Micellar cubic phases and their structural relationships: The nonionic surfactant system C12EO12/water. Langmuir 1997, 13, 3706–3714. [Google Scholar] [CrossRef]
- Seddon, J.M.; Robins, J.; Gulik-Krzywicki, T.; Delacroix, H. Inverse micellar phases of phospholipids and glycolipids. Phys. Chem. Chem. Phys. 2000, 2, 4485–4493. [Google Scholar] [CrossRef]
- Seddon, J.M.; Zeb, N.; Templer, R.H.; McElhaney, R.N.; Mannock, D.A. An Fd3m lyotropic cubic phase in a binary glycolipid/water system. Langmuir 1996, 12, 5250–5253. [Google Scholar] [CrossRef]
- Siegel, D.P. Inverted micellar intermediates and the transitions between lamellar, cubic, and inverted hexagonal amphiphile phases. 3. Isotropic and inverted cubic state formation via intermediates in transitions between l-alpha and h-ii phases. Chem. Phys. Lipids 1986, 42, 279–301. [Google Scholar]
- Kozlovsky, Y.; Chernomordik, L.V.; Kozlov, M.M. Lipid intermediates in membrane fusion: Formation, structure, and decay of hemifusion diaphragm. Biophys. J. 2002, 83, 2634–2651. [Google Scholar] [CrossRef]
- Mulet, X.; Gong, X.; Waddington, L.J.; Drummond, C.J. Observation of intermediates in lamellar to cubic phase transformations of lipid nanoparticles. Biophys. J. 2010, 98, 286a–287a. [Google Scholar] [CrossRef]
- Shearman, G.C.; Tyler, A.I.I.; Brooks, N.J.; Templer, R.H.; Ces, O.; Law, R.V.; Seddon, J.M. A 3-d hexagonal inverse micellar lyotropic phase. J. Am. Chem. Soc. 2009, 131, 1678–1679. [Google Scholar] [CrossRef] [PubMed]
- Bender, J.; Simonsson, C.; Smedh, M.; Engstrom, S.; Ericson, M.B. Lipid cubic phases in topical drug delivery: Visualization of skin distribution using two-photon microscopy. J. Control. Release 2008, 129, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Lopes, L.B.; Lopes, J.L.; Oliveira, D.C.; Thomazini, J.A.; Garcia, M.T.; Fantini, M.C.; Collett, J.H.; Bentley, M.V. Liquid crystalline phases of monoolein and water for topical delivery of cyclosporin a: Characterization and study of in vitro and in vivo delivery. Eur. J. Pharm. Biopharm. 2006, 63, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Bender, J.; Ericson, M.B.; Merclin, N.; Iani, V.; Rosen, A.; Engstrom, S.; Moan, J. Lipid cubic phases for improved topical drug delivery in photodynamic therapy. J. Control. Release 2005, 106, 350–360. [Google Scholar] [CrossRef] [PubMed]
- Turchiello, R.F.; Vena, F.C.; Maillard, P.; Souza, C.S.; Bentley, M.V.; Tedesco, A.C. Cubic phase gel as a drug delivery system for topical application of 5-ALA, its ester derivatives and m-THPC in photodynamic therapy (PDT). J. Photochem. Photobiol. 2003, 70, 1–6. [Google Scholar] [CrossRef]
- Esposito, E.; Eblovi, N.; Rasi, S.; Drechsler, M.; Di Gregorio, G.M.; Menegatti, E.; Cortesi, R. Lipid-based supramolecular systems for topical application: A preformulatory study. AAPS PharmSci 2003, 5, 62–76. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, C.V.; Wachter, W.; Iglesias, G.R.; Engelskirchen, S.; Ahualli, S. Monoolein: A magic lipid? Phys. Chem. Chem. Phys. 2011, 13, 3004–3021. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, C.V. Nanostructural studies on monoelaidin-water systems at low temperatures. Langmuir 2011, 27, 11790–11800. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, C.V.; Tang, T.Y.; Seddon, A.M.; Seddon, J.M.; Ces, O.; Templer, R.H. Engineering bicontinuous cubic structures at the nanoscale—The role of chain splay. Soft Matter 2010, 6, 3191–3194. [Google Scholar] [CrossRef]
- Spicer, P.; Lynch, M.; Visscher, M.; Hoath, S. Bicontinuous cubic liquid crystalline phase and cubosome personal care delivery systems. In Personal Care Delivery Systems and Formulations; Rosen, M., Ed.; Noyes Publishing: New York, NY, USA, 2003. [Google Scholar]
- Yaghmur, A.; Glatter, O. Characterization and potential applications of nanostructured aqueous dispersions. Adv. Colloid Interface Sci. 2009, 147–148, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Nazaruk, E.; Miszta, P.; Filipek, S.; Górecka, E.; Landau, E.M.; Bilewicz, R. Lyotropic cubic phases for drug delivery: Diffusion and sustained release from the mesophase evaluated by electrochemical methods. Langmuir 2015, 31, 12753–12761. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ma, P.; Gui, S. Cubic and hexagonal liquid crystals as drug delivery systems. BioMed Res. Int. 2014, 2014, 815981. [Google Scholar] [CrossRef] [PubMed]
- Negrini, R.; Mezzenga, R. Diffusion, molecular separation, and drug delivery from lipid mesophases with tunable water channels. Langmuir 2012, 28, 16455–16462. [Google Scholar] [CrossRef] [PubMed]
- Sagalowicz, L.; Leser, M.E.; Watzke, H.J.; Michel, M. Monoglyceride self-assembly structures as delivery vehicles. Trends Food Sci. Technol. 2006, 17, 204–214. [Google Scholar] [CrossRef]
- Makai, M.; Csanyi, E.; Nemeth, Z.; Palinkas, J.; Eros, I. Structure and drug release of lamellar liquid crystals containing glycerol. Int. J. Pharm. 2003, 256, 95–107. [Google Scholar] [CrossRef]
- Shah, J.; Sadhale, Y.; Chilukuri, D.M. Cubic phase gels as drug delivery systems. Adv. Drug Deliv. Rev. 2001, 47, 229–250. [Google Scholar] [CrossRef]
- Engström, S. Drug delivery from cubic and other lipid-water phases. Lipid Technol. 1990, 2, 42–45. [Google Scholar]
- Chemelli, A.; Maurer, M.; Geier, R.; Glatter, O. Optimized loading and sustained release of hydrophilic proteins from internally nanostructured particles. Langmuir 2012, 28, 16788–16797. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, S.B.; Boyd, B.J.; Rades, T.; Hook, S. Bicontinuous cubic liquid crystals as sustained delivery systems for peptides and proteins. Expert Opin. Drug Deliv. 2010, 7, 1133–1144. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.W.Y.; Nguyen, T.H.; Hanley, T.; Boyd, B.J. Nanostructure of liquid crystalline matrix determines in vitro sustained release and in vivo oral absorption kinetics for hydrophilic model drugs. Int. J. Pharm. 2009, 365, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Boyd, B.J.; Whittaker, D.V.; Khoo, S.M.; Davey, G. Lyotropic liquid crystalline phases formed from glycerate surfactants as sustained release drug delivery systems. Int. J. Pharm. 2006, 309, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Roux, D.; Nallet, F.; Diat, O. Rheology of lyotropic lamellar phases. Europhys. Lett. 1993, 24, 53–58. [Google Scholar] [CrossRef]
- Berni, M.G.; Lawrence, C.J.; Machin, D. A review of the rheology of the lamellar phase in surfactant systems. Adv. Colloid Interface Sci. 2002, 98, 217–243. [Google Scholar] [CrossRef]
- Rodriguez-Abreu, C.; Garcia-Roman, M.; Kunieda, H. Rheology and dynamics of micellar cubic phases and related emulsions. Langmuir 2004, 20, 5235–5240. [Google Scholar] [CrossRef] [PubMed]
- Mezzenga, R.; Meyer, C.; Servais, C.; Romoscanu, A.I.; Sagalowicz, L.; Hayward, R.C. Shear rheology of lyotropic liquid crystals: A case study. Langmuir 2005, 21, 3322–3333. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, J.; Ljusberg-Wahren, H.; Almgren, M.; Larsson, K. Cubic lipid–water phase dispersed into submicron particles. Langmuir 1996, 12, 4611–4613. [Google Scholar] [CrossRef]
- Gustafsson, J.; Ljusberg-Wahren, H.; Almgren, M.; Larsson, K. Submicron particles of reversed lipid phases in water stabilized by a nonionic amphiphilic polymer. Langmuir 1997, 13, 6964–6971. [Google Scholar] [CrossRef]
- Kulkarni, C.V.; Mezzenga, R.; Glatter, O. Water-in-oil nanostructured emulsions: Towards the structural hierarchy of liquid crystalline materials. Soft Matter 2010, 6, 5615–5624. [Google Scholar] [CrossRef]
- Yaghmur, A.; de Campo, L.; Sagalowicz, L.; Leser, M.E.; Glatter, O. Emulsified microemulsions and oil-containing liquid crystalline phases. Langmuir 2005, 21, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Boyd, B.J.; Whittaker, D.V.; Khoo, S.M.; Davey, G. Hexosomes formed from glycerate surfactants—Formulation as a colloidal carrier for irinotecan. Int. J. Pharm. 2006, 318, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Mclain, V.C. Final report on the safety assessment of phytantriol. Int. J. Toxicol. 2007, 26 (Suppl. 1), 107–114. [Google Scholar]
- Salonen, A.; Muller, F.O.; Glatter, O. Internally self-assembled submicrometer emulsions stabilized by spherical nanocolloids: Finding the free nanoparticles in the aqueous continuous phase. Langmuir 2010, 26, 7981–7987. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.H.; Hanley, T.; Porter, C.J.; Larson, I.; Boyd, B.J. Phytantriol and glyceryl monooleate cubic liquid crystalline phases as sustained-release oral drug delivery systems for poorly water soluble drugs I. Phase behaviour in physiologically-relevant media. J. Pharm. Pharmacol. 2010, 62, 844–855. [Google Scholar] [PubMed]
- Chong, J.Y.T.; Mulet, X.; Boyd, B.J.; Drummond, C.J. Chapter five—Steric stabilizers for cubic phase lyotropic liquid crystal nanodispersions (cubosomes). In Advances in Planar Lipid Bilayers and Liposomes; Aleš Iglič, C.V.K., Michael, R., Eds.; Academic Press: London, UK, 2015; Volume 21, pp. 131–187. [Google Scholar]
- Yaghmur, A.; Laggner, P.; Almgren, M.; Rappolt, M. Self-assembly in monoelaidin aqueous dispersions: Direct vesicles to cubosomes transition. PLoS ONE 2008, 3, e3747. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, C.V.; Glatter, O. Hierarchically organized systems based on liquid crystalline phases. In Self-Assembled Supramolecular Architectures: Lyotropic Liquid Crystals; Garti, N., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012. [Google Scholar]
- Kulkarni, C.V. Hierarchically structured lipid systems. In Encyclopaedia of Biophysics; Roberts, G.C., Ed.; Springer: Berlin, Germany, 2012. [Google Scholar]
- Kulkarni, C.V. Ultrasonic processing of butter oil (Ghee) into oil-in-water emulsions. J. Food Process. Preserv. 2016, in press. [Google Scholar]
- Dulle, M.; Glatter, O. Internally self-assembled submicrometer emulsions stabilized with a charged polymer or with silica particles. Langmuir 2011, 28, 1136–1141. [Google Scholar] [CrossRef] [PubMed]
- Muller, F.; Salonen, A.; Glatter, O. Phase behavior of phytantriol/water bicontinuous cubic Pn3m cubosomes stabilized by laponite disc-like particles. J. Colloid Interface Sci. 2010, 342, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Patil-Sen, Y.; Sadeghpour, A.; Rappolt, M.; Kulkarni, C.V. Facile preparation of internally self-assembled lipid particles stabilized by carbon nanotubes. J. Vis. Exp. 2016, 108, e53489. [Google Scholar] [CrossRef] [PubMed]
- Gaunt, N.P.; Patil-Sen, Y.; Baker, M.J.; Kulkarni, C.V. Carbon nanotubes for stabilization of nanostructured lipid particles. Nanoscale 2015, 7, 1090–1095. [Google Scholar] [CrossRef] [PubMed]
- Tilley, A.J.; Drummond, C.J.; Boyd, B.J. Disposition and association of the steric stabilizer pluronic® f127 in lyotropic liquid crystalline nanostructured particle dispersions. J. Colloid Interface Sci. 2013, 392, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Spicer, P. Cubosome processing—Industrial nanoparticle technology development. Chem. Eng. Res. Des. 2005, 83, 1283–1286. [Google Scholar] [CrossRef]
- Spicer, P.T. Cubosomes: Bicontinuous cubic liquid crystalline nanostructured particles. In Encyclopedia of Nanoscience and Nanotechnology; Marcel Dekker: New York, NY, USA, 2004. [Google Scholar]
- Garg, G.; Saraf, S.; Saraf, S. Cubosomes: An overview. Biol. Pharm. Bull. 2007, 30, 350–353. [Google Scholar] [CrossRef] [PubMed]
- Angelova, A.; Angelov, B.; Mutafchieva, R.; Lesieur, S.; Couvreur, P. Self-assembled multicompartment liquid crystalline lipid carriers for protein, peptide, and nucleic acid drug delivery. Acc. Chem. Res. 2011, 44, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Barauskas, J.; Cervin, C.; Jankunec, M.; Špandyreva, M.; Ribokaitė, K.; Tiberg, F.; Johnsson, M. Interactions of lipid-based liquid crystalline nanoparticles with model and cell membranes. Int. J. Pharm. 2010, 391, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Bode, J.C.; Kuntsche, J.; Funari, S.S.; Bunjes, H. Interaction of dispersed cubic phases with blood components. Int. J. Pharm. 2013, 448, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Caldero, G.; Llinas, M.; Garcia-Celma, M.J.; Solans, C. Studies on controlled release of hydrophilic drugs from W/O high internal phase ratio emulsions. J. Pharm. Sci. 2009, 99, 701–711. [Google Scholar] [CrossRef] [PubMed]
- Solans, C.; Esquena, J. Highly concentrated (gel) emulsions as reaction media for the preparation of advanced materials. In Highlights in Colloid Science; Platikanov, D., Exerowa, D., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2008; pp. 291–297. [Google Scholar]
- Solans, C.; Esquena, J.; Azemar, N. Highly concentrated (gel) emulsions, versatile reaction media. Curr. Opin. Colloid Interface Sci. 2003, 8, 156–163. [Google Scholar] [CrossRef]
- Solans, C.; Pinazo, A.; Calderó, G.; Infante, M.R. Highly concentrated emulsions as novel reaction media. Colloids Surf. A 2001, 176, 101–108. [Google Scholar] [CrossRef]
- Salentinig, S.; Yaghmur, A.; Guillot, S.; Glatter, O. Preparation of highly concentrated nanostructured dispersions of controlled size. J. Colloid Interface Sci. 2008, 326, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Paruta-Tuarez, E.; Fersadou, H.; Sadtler, V.; Marchal, P.; Choplin, L.; Baravian, C.; Castel, C. Highly concentrated emulsions: 1. Average drop size determination by analysis of incoherent polarized steady light transport. J. Colloid Interface Sci. 2010, 346, 136–142. [Google Scholar] [PubMed]
- Cameron, N.R. High internal phase emulsion templating as a route to well-defined porous polymers. Polymer 2005, 46, 1439–1449. [Google Scholar] [CrossRef]
- Pons, R.; Ravey, J.C.; Sauvage, S.; Stébé, M.J.; Erra, P.; Solans, C. Structural studies on gel emulsions. Colloids Surf. A 1993, 76, 171–177. [Google Scholar] [CrossRef]
- Kunieda, H.; Evans, D.F.; Solans, C.; Yoshida, M. The structure of gel-emulsions in a water/nonionic surfactant/oil system. Colloids Surf. 1990, 47, 35–43. [Google Scholar] [CrossRef]
- Hunter, T.N.; Pugh, R.J.; Franks, G.V.; Jameson, G.J. The role of particles in stabilising foams and emulsions. Adv. Colloid Interface Sci. 2008, 137, 57–81. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, C.V.; Iglesias, G.R.; Glatter, O. Nanostructured water in oil emulsions with high water content formed without stabilizer. In Proceedings of the International Soft Matter Conference 2010, Granada, Spain, 5–8 July 2010.
- Glatter, O.; Glatter, I. Water-in-Oil Emulsions and Methods for Their Preparation. US4,380,503 A, 19 April 1983. [Google Scholar]
- Sergienko, A.Y.; Tai, H.; Narkis, M.; Silverstein, M.S. Polymerized high internal-phase emulsions: Properties and interaction with water. J. Appl. Polym. Sci. 2002, 84, 2018–2027. [Google Scholar] [CrossRef]
- Forney, B.S.; Guymon, C.A. Nanostructure evolution during photopolymerization in lyotropic liquid crystal templates. Macromolecules 2010, 43, 8502–8510. [Google Scholar] [CrossRef]
- Srisiri, W.; Benedicto, A.; O'Brien, D.F.; Trouard, T.P.; Orädd, G.; Persson, S.; Lindblom, G. Stabilization of a bicontinuous cubic phase from polymerizable monoacylglycerol and diacylglycerol. Langmuir 1998, 14, 1921–1926. [Google Scholar] [CrossRef]
- Yang, D.; O’Brien, D.F.; Marder, S.R. Polymerized bicontinuous cubic nanoparticles (cubosomes) from a reactive monoacylglycerol. J. Am. Chem. Soc. 2002, 124, 13388–13389. [Google Scholar] [CrossRef] [PubMed]
- Armitage, B.; Bennett, D.; Lamparski, H.; O’Brien, D. Polymerization and domain formation in lipid assemblies. In Biopolymers Liquid Crystalline Polymers Phase Emulsion; Springer: Berlin, Germany, 1996; Volume 126, pp. 53–84. [Google Scholar]
- Cates, M.E.; Clegg, P.S. Bijels: A new class of soft materials. Soft Matter 2008, 4, 2132–2138. [Google Scholar] [CrossRef]
- Ohm, C.; Brehmer, M.; Zentel, R. Liquid crystalline elastomers as actuators and sensors. Adv. Mater. 2010, 22, 3366–3387. [Google Scholar] [CrossRef] [PubMed]
- Muller, R.H.; Radtke, M.; Wissing, S.A. Nanostructured lipid matrices for improved microencapsulation of drugs. Int. J. Pharm. 2002, 242, 121–128. [Google Scholar] [CrossRef]
- Decher, G. Layer-by-layer assembly (putting molecules to work). In Multilayer Thin Films; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2012; pp. 1–21. [Google Scholar]
- Guillot, S.; Salentinig, S.; Chemelli, A.; Sagalowicz, L.; Leser, M.E.; Glatter, O. Influence of the stabilizer concentration on the internal liquid crystalline order and the size of oil-loaded monolinolein-based dispersions. Langmuir 2010, 26, 6222–6229. [Google Scholar] [CrossRef] [PubMed]
- Yaghmur, A.; de Campo, L.; Sagalowicz, L.; Leser, M.E.; Glatter, O. Control of the internal structure of mlo-based isasomes by the addition of diglycerol monooleate and soybean phosphatidylcholine. Langmuir 2006, 22, 9919–9927. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.D.; Tilley, A.J.; Larson, I.; Lawrence, M.J.; Amenitsch, H.; Rappolt, M.; Hanley, T.; Boyd, B.J. Nonequilibrium effects in self-assembled mesophase materials: Unexpected supercooling effects for cubosomes and hexosomes. Langmuir 2010, 26, 9000–9010. [Google Scholar] [CrossRef] [PubMed]
- Mezzenga, R.; Schurtenberger, P.; Burbidge, A.; Michel, M. Understanding foods as soft materials. Nat. Mater. 2005, 4, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Sagalowicz, L.; Mezzenga, R.; Leser, M.E. Investigating reversed liquid crystalline mesophases. Curr. Opin. Colloid Interface Sci. 2006, 11, 224–229. [Google Scholar] [CrossRef]
- Engelskirchen, S.; Maurer, R.; Glatter, O. Effect of glycerol addition on the internal structure and thermal stability of hexosomes prepared from phytantriol. Colloids Surf. A 2011, 391, 95–100. [Google Scholar] [CrossRef]
- Ubbink, J.; Burbidge, A.; Mezzenga, R. Food structure and functionality—A soft matter perspective. Soft Matter 2008, 4, 1569–1581. [Google Scholar] [CrossRef]
- Mezzenga, R.; Grigorov, M.; Zhang, Z.; Servais, C.; Sagalowicz, L.; Romoscanu, A.I.; Khanna, V.; Meyer, C. Polysaccharide-induced order-to-order transitions in lyotropic liquid crystals. Langmuir 2005, 21, 6165–6169. [Google Scholar] [CrossRef] [PubMed]
- Yaghmur, A.; Kriechbaum, M.; Amenitsch, H.; Steinhart, M.; Laggner, P.; Rappolt, M. Effects of pressure and temperature on the self-assembled fully hydrated nanostructures of monoolein-oil systems. Langmuir 2010, 26, 1177–1185. [Google Scholar] [CrossRef] [PubMed]
- Guillot, S.; Moitzi, C.; Salentinig, S.; Sagalowicz, L.; Leser, M.E.; Glatter, O. Direct and indirect thermal transitions from hexosomes to emulsified micro-emulsions in oil-loaded monoglyceride-based particles. Colloids Surf. A 2006, 291, 78–84. [Google Scholar] [CrossRef]
- Salonen, A.; Muller, F.O.; Glatter, O. Dispersions of internally liquid crystalline systems stabilized by charged disklike particles as pickering emulsions: Basic properties and time-resolved behavior. Langmuir 2008, 24, 5306–5314. [Google Scholar] [CrossRef] [PubMed]
- Salentinig, S.; Sagalowicz, L.; Glatter, O. Self-assembled structures and pka value of oleic acid in systems of biological relevance. Langmuir 2010, 26, 11670–11679. [Google Scholar] [CrossRef] [PubMed]
- Al-Bawab, A.; Friberg, S.E. Some pertinent factors in skin care emulsion. Adv. Colloid Interface Sci. 2006, 123–126, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.M.; Mezzenga, R. Particle tracking microrheology of lyotropic liquid crystals. Langmuir 2011, 27, 6171–6178. [Google Scholar] [CrossRef] [PubMed]
- Tomsic, M.; Guillot, S.; Sagalowicz, L.; Leser, M.E.; Glatter, O. Internally self-assembled thermoreversible gelling emulsions: Isasomes in methylcellulose, kappa-carrageenan, and mixed hydrogels. Langmuir 2009, 25, 9525–9534. [Google Scholar] [CrossRef] [PubMed]
- Guillot, S.; Tomsic, M.; Sagalowicz, L.; Leser, M.E.; Glatter, O. Internally self-assembled particles entrapped in thermoreversible hydrogels. J. Colloid Interface Sci. 2009, 330, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, C.V.; Tomšič, M.; Glatter, O. Immobilization of nanostructured lipid particles in polysaccharide films. Langmuir 2011, 27, 9541–9550. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, C.V.; Moinuddin, Z.; Patil-Sen, Y.; Littlefield, R.; Hood, M. Lipid-hydrogel films for sustained drug release. Int. J. Pharm. 2015, 479, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, C.V.; Moinuddin, Z.; Agarwal, Y. Effect of fullerene on the dispersibility of nanostructured lipid particles and encapsulation in sterically stabilized emulsions. J. Colloid Interface Sci. 2016, 480, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Muller, F.; Salonen, A.; Glatter, O. Monoglyceride-based cubosomes stabilized by laponite: Separating the effects of stabilizer, ph and temperature. Colloids Surf. A 2010, 358, 50–56. [Google Scholar] [CrossRef]
- Guillot, S.; Bergaya, F.; de Azevedo, C.; Warmont, F.; Tranchant, J.F. Internally structured pickering emulsions stabilized by clay mineral particles. J. Colloid Interface Sci. 2009, 333, 563–569. [Google Scholar] [CrossRef] [PubMed]


© 2016 by the author; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulkarni, C.V. Lipid Self-Assemblies and Nanostructured Emulsions for Cosmetic Formulations. Cosmetics 2016, 3, 37. https://doi.org/10.3390/cosmetics3040037
Kulkarni CV. Lipid Self-Assemblies and Nanostructured Emulsions for Cosmetic Formulations. Cosmetics. 2016; 3(4):37. https://doi.org/10.3390/cosmetics3040037
Chicago/Turabian StyleKulkarni, Chandrashekhar V. 2016. "Lipid Self-Assemblies and Nanostructured Emulsions for Cosmetic Formulations" Cosmetics 3, no. 4: 37. https://doi.org/10.3390/cosmetics3040037
APA StyleKulkarni, C. V. (2016). Lipid Self-Assemblies and Nanostructured Emulsions for Cosmetic Formulations. Cosmetics, 3(4), 37. https://doi.org/10.3390/cosmetics3040037





