Abstract
According to United Nations Environment Programme (UNEP), ensuring a clean and healthy environment will provide multiple benefits to society and economy. Sustainable production, followed by appropriate management of industrial and agricultural waste, will protect and enhance biodiversity and ecosystem services. To achieve this objective, specific policies must be put in place and specific actions performed for making a low-carbon and resource-efficient economy with reduced production of petrol-derived goods. The aim of the study has been to produce effective and safe anti-age beauty masks made of non-woven tissues based on the use of chitin nanofibril (CN) and nanolignin (LG), obtained from crustaceans and plant biomass, respectively. To this purpose, nanoparticles and electrospun fibres have been characterized by Dynamic Light Scattering and SEM, while the safeness and effectiveness of the obtained tissues was verified in vitro on a culture of keratinocytes and fibroblasts, and controlled in vivo by expert dermatologists on 30 volunteer photo-aged women, by subjective and objective bioengineered methods. The in vitro results have shown that the beauty masks have no toxic effects on the viability of keratinocytes and fibroblasts treated by the Dimethyl Tetrazole (MTT) method, and exhibit a decreased expression of cytokines, playing a central role in the regulation of immune and inflammatory responses in premature aging and environmental assaults. The reparative and antiaging effectiveness of these innovative beauty masks have been also verified on the release of Metallo Proteinase I (MMP-1) and the increased synthesis of collagen type I, reduced in skin aging. The first preliminary in vivo results, obtained by engineering methods, have confirmed the protective and rejuvenating activity shown by the in vitro study conducted on 30 voluntary women exhibiting signs of photoaging. The raw materials used are of natural origin being also respectful of the environment, according to the Organization for Economic Cooperation and Development (EOCD) and EU programmes.
1. Introduction
According to the National Science Foundation and the World Technology Evaluation Center (WTEC) Panel Report on nanotechnology, future techno-economical challenges could create a trillion-dollar industry in a short period while maintaining sustainable development based on the use of alternative sources of raw materials, processed by innovative industrial methodologies and with low consumption of water and energy [1,2].
By using waste materials and other post-harvest losses, the supply of food and goods can be increased and sustained, simply by the combined use of nanotechnologies and increasing energy and water efficiency. At present, marine capture fisheries yield 110–130 million tons of seafood annually, producing 30 million tonnes of discards [3], while 140 billion metric tonnes of biomass is generated every year from agriculture [4,5]. Thus according to United Nations Environment Programme (UNEP), “ensuring a clean and healthy environment, through an effective environment management, will provide multiple benefits to society and economy, while a sustainable production together with an appropriate fishery and forest management, will protect and enhance biodiversity and ecosystem services” [6].
The EU, therefore, has set the objective of becoming a smart, sustainable, and inclusive economy by 2020. To achieve this objective, specific policies must be put in place and specific actions performed for making a low-carbon and resource-efficient economy with reduced production of petrol-derived goods. This is the long-term goal for 2050 [7]. In addition, recently green economy has emerged as a strategic priority for governments and intergovernmental organizations [8,9,10]. To this end, in recent years natural nanoparticles and non-woven tissues have been widely investigated as a promising biomaterial, also because they are easily available and relatively inexpensive. Among them, chitin-derived compounds and lignin represent an important class of polymers suitable for use in the cosmetic and biomedical fields [11,12].
Chitin nanofibril (CN), industrially produced by a green patented technology [13,14], is an interesting crystalline polymer derived from crustacean waste, which could be used for the sustainable production of goods [15,16]. It has a mean dimension of 240 nm × 7 nm × 5 nm with a mean weight of 0.074 × 106 Dalton, while each crystallite has about 15,000 -NH2 groups and a degree of deacetylation of about 50% with respect to amorphous chitin. As a natural ingredient CN may be considered, therefore, an ideal compound to substitute for petrol-derived polymers for producing cosmetic and healthy products for aged and sensitive skin [17,18,19,20,21,22]. It has been shown, in fact, that this natural polymer is a non-toxic, skin-friendly, and environmentally friendly compound that, due to its chemical structure, is easily metabolized from the environmental chitinases and chitotriosidases, also produced by the human body [23]. Additionally, while CN has been shown to bond many molecules of water (at least 3 trillion for 1 ml!), it possesses a high moisturizing effectiveness, also having the capacity to load as carrier different active ingredients. Finally, it is an adaptable engineering material useful for making nanocomposites for producing non-woven tissue beauty masks by electrospinning or casting technology [17,20,24,25].
Just like the chitin polymer, lignin (LG) is another bio-based waste material, available in large quantity as a by-product of the manufacturing of cellulose pulp and bioethanol from lignocellulosic biomass [26,27]. Purified biolignin, with a mean dimension of 163 nm, contains many phenolic polymers with beneficial properties to human health, including antioxidant and photoprotective activities, but is also involved in cell-to-cell signalling [28].
On the other hand, CN-LG nanoparticles, as bivalent carriers composed of positively charged CN and negatively charged LG, are capable of entrapping many different active ingredients into their molecular structure, leading and releasing them at the right skin site, at the right dose, and the right time [29]. Thus, this innovative complex may act as a carrier and active ingredient, useful to reinforce the moisturizing and defensive networks of the skin.
It is interesting to underline that the beauty mask market represents an increasing cosmetic category that has shown, in 2013, the fastest growth among all the personal care products, ranging from $1.8 billion in China to $1.1 billion and $1.4 billion in the USA and Europe, respectively [30].
Beauty masks are considered an effective means of completing routine skin care treatments and are an excellent alternative to the expensive professional Beauty Centre. They provide, in fact, a formal, intimate skin care experience that consumers can have at home. Like cosmetic emulsions, the majority of tissue masks made of synthetic tissues produced by petrol-derived compounds soaked with active ingredients, require the use of emulsifiers, preservatives, and other chemicals, which may cause photo-allergy and sensitization.
Following our previous technical experiences [15,16,17,18,19,20,21,22], the aim of the study has been to produce effective and safe beauty masks made by non-woven tissues based on the use of CN-lignin block co-polymeric nanoparticles, electrospun with other natural and biocompatible polymers. These non-woven tissues may be considered innovative because they are made of natural fibre, and able to trap different active ingredients before the electrospinning process. Additionally, the fibres are produced by the use of by-products obtained from the biomass. Moreover, these bio-masks have interesting characteristics, being free of emulsifiers, preservatives, and other chemicals. For these reasons they may be considered auto-preserving dry tissues, capable of releasing entrapped ingredients only when in direct contact with wet skin, for a period of about 20 min.
The network of active ingredients used, entrapped into CN-lignin by the gelation method, were: sodium ascorbyl phosphate (vitamin C derived), melatonin, and beta-glucan known for their antioxidant, immunomodulant, and blanching activity, also previously shown by other studies of our group [16,17,18,19,20,21]; and nicotinamide for its effectiveness at balancing and re-establishing the continuous turnover of Natural Moisturizing Factor (NMF) at the level of the skin lipid lamellae, and repairing the DNA damage induced by Ultraviolet (UV) rays [31,32,33]. Melatonin, which is able to counteract oxidative stress and suppress damaged cells’ proliferation, has been shown to provide cellular protection against UVR damage when topically applied [34]. Moreover, all the other ingredients used to make the non-woven tissue of the beauty mask, such as CN, LG, Chitosan, Polyethylene Oxide (PEOX), and the polypeptides, contributed to reinforce the anti-inflammatory, immunomodulating, and re-epithelialisation activity of the active ingredients selected, as reported in our previous studies [17,18,19,20,21].
2. Experimental Section
The beauty masks were controlled for their safeness and effectiveness both in vitro on keratinocyte and fibroblast cultures and in vivo on 30 volunteer women showing signs of premature skin aging (photoaging) for a period of 30 days.
2.1. Materials and Methods
2.1.1. Materials
Chitin nanofibrils in the form of a 2% water suspension were kindly supplied by MAVI sud Srl (Aprilia, Italy), bio-lignin™ by CIMV (Neully-Sur-Seine, France), polypeptides from Roussel Sas (Puteaux, France), while PEOX was purchased from Amerchol (Dow Italia, Milano, Italy), Chitosan from Giusto Faravelli Spa (Milano, Italy), sodium ascorbyl phosphate and nicotinamide from DSM Ltd. (Basel, Switzerland), melatonin from Agrar (Roma, Italy), and betaglucan from Mibelle AG Biochemistry (Boks, Switzerland).
2.1.2. Methods
Chitin Nanofibrils/bio-lignin non-woven tissues, as reported elsewhere [17], were electrospun with different active ingredients by the NS Lab 500 (Elmarco, Liberec, Czech Republic) on a substrate of polypropylene of pharmaceutical grade to produce innovative beauty masks [17,18].
Before continuing the tissue production by electrospinning, CN-biolignin™ aggregated polymeric nanoparticles were made by using the spray-dryer DF 500 B9 (JCF, Milano, Italy).
These nanoparticles were dissolved in water to obtain a gel mixture, which was then used as starting material for an electrospun material. The sol-gel mixture prepared for the electrospinning tests was obtained mixing the CN-bio-lignin™ complex with deionised water at 15 °C for a few minutes. Then PEOX was added to the solution and stirred until completely dissolved. This last step took 24 h to obtain a homogeneous gel without agglomerations.
Electrospinning
The electrospinning process was performed by using an NS LAB 500 based on nozzle-less technology. The proof of concept of this technique is that a rotating drum is dipped into a bath of the liquid solution. A thin layer of the solution is carried out on the drum surface and exposed to a high-voltage electric field. If the voltage exceeds the critical value, a number of electrospinning jets are generated. The jets are distributed over the electrode surface with periodicity. This is one of the main advantages of nozzle-less electrospinning: the number and location of the jets is set up naturally in their optimal positions.
The setting parameters of the machine were:
- Voltage: 45–75 kV
- Collecting electrode (CE): cylinder
- Spinning electrode (SE): cylinder
- Distance SE/CE: 10–16 cm
- CE rotation: 2–8 rpm
- Substrate material: Spunbond, 30 gsm, polypropylene 100% with antistatic treatment.
2.2. Measurements
All the in vivo evaluations have been performed after a 30 min acclimatization period in a room at 22 °C and 50% humidity, by the 3C System device, previously used in other studies by our group [35,36].
2.2.1. Skin Hydration
The hydration of the horny layer was assessed by measuring the electrical capacitance of the skin surface by means of the 3C System (Dermotech, Roma, Italy). When the probe of this apparatus was applied on the skin (recording time 0.5 s), the capacitance has displayed digitally in arbitrary 3C units.
The obtained results, expressed as mean values of the measurements performed on five different skin sites (cheeks, forehead, chin, and nose) have been shown as percent values.
2.2.2. Transepidermal Water Loss (TEWL)
Water evaporating from the skin surface was measured quantitatively by the 3C methodology. The reported values are expressed in g/h·m2. The 3C System probe consists of a cylindrical open chamber measuring system with diameter 14 mm, height 10 mm, and distance from the skin area of 0.95 cm2. Two sensor units, containing thin capacitative film transducers, are placed in the probe at 3 and 7 mm distance from the skin surface. TEWL is calculate digitally in g/m2·h and expressed in percent.
2.2.3. Skin Surface Lipids
The skin surface lipids level was measured by the 3C system. The evaluation is based on photometric measurements of light transmission through a surface imprint, obtained by applying a frosted plastic foil to the designed skin area. It allows adherence of skin lipids in a 1 cm2 area, the transparency of which is calculated digitally in μg/cm2. The obtained results are reported as % of lipid increase.
2.2.4. Skin Colour
Age spots were determined by the use of Chromameter CR-300 (Minolta, Japan), according to the methodology previously reported [21] .The meter’s double-beam feedback system detects any slight deviations in the xenon light’s spectral distribution, and the microcomputer compensates for them, ensuring the utmost accuracy in measurements. This apparatus is a right weight and compact tristimulus colour analyzer for measuring reflected object colour reported as a* mode.
2.2.5. Characterization of Nanoparticles and Non-Woven Tissue
The average size distribution and polydispersity of the CN-LG nanoparticles were estimated by Dynamic Light Scattering using a Malvern Zetasizer (Malvern Instrument Ltd., Malvern, Worcertershire, UK) and by FESEM, as reported elsewhere [37]. The surface morphology of electrospun nanofibers was characterized by a field emission electron microscope—FESEM Auriga Zeiss, including microanalysis EDS 123 Mn-Ka eV (Zeiss-Bruker, Milano-Italy) and EBL –7 nm resolution (Raith). Samples cut from the electrospun material mounted on aluminium stubs were coated by an ultrathin layer of platinum for better conductivity during imaging. The samples were observed at magnifications between 100 and 40,000 times their original sizes to visually evaluate the electrospinnability and existence of beads.
Fibre diameters were also determined using Image-J image processing software. For each electrospun material, at least 100 fibres were considered from three different images to calculate the average diameter.
2.2.6. Cytotoxicity Assay and Collagen I Synthesis
The cytotoxicity of the active tissue, in comparison with the control tissue and the untreated control, was performed by the modified MTT assay that measured the metabolic activity of living cells [38].
Keratinocytes and fibroblasts, isolated from the skin samples taken from volunteers of the study group, were cultured in 9BM medium (Cambrex Co, Charles City, IA, USA) with 10% foetal bovine serum at 37 °C and 5% CO2, according to our previous experience [39]. At cell confluence, the beauty masks, suspended in sterilized distilled water, were introduced to a fresh culture medium at a concentration of 20 μg/mL to determine by the MTT method the viability, cytotoxicity, and proliferation of keratinocytes and fibroblasts in comparison with the control, untreated, and non-woven tissues free of active ingredients. This colorimetric assay is an indirect method for assessing cell growth and proliferation. It gives a yellowish aqueous solution, which on reduction with reducing agents present in metabolically active cells yields a violet-blue water-insoluble compound, known as formazan. Formazan, extracted with organic solvents, has been controlled spectrophotometrically at 570 nm. The keratinocyte and fibroblast functionality in the control tissue and active tissue has been calculated as the ratio between the absorbance of the treated sample and the control tissue/untreated sample, expressed as percentage.
For the collagen type I synthesis, fibroblast cells were seeded in 96-well plates, cultured until the cells reached 70% confluence, then exposed to 20 μg/mL of control mask, active mask, and Vitamin C, and analysed with ELISA after 24 h. The procedures were controlled in triplicate and the data are expressed as percentage of untreated sample.
2.2.7. Reduction of UV Induces IL-8 and TNF-Alpha Release
UV light directly and indirectly influences the viability and energy levels of skin cells in a dose-dependent manner [40]. It induces a dramatic decrease of energy levels in keratinocytes together with inflammation and a consequential production of TNF-alpha and IL-8. This is the reason we irradiate the cells after pre-treatment with the non-woven tissue.
At cell confluence the keratinocytes were irradiated with UV light (2 J/cm2 UVA; 0.2 J/cm2 UVB) before and after pre-treatment with a water suspension containing 20 μg of the beauty mask. The release of IL-8 and TNF-α obtained by the irradiated cell, treated or not (control) with the beauty mask control (control tissue), and the active tissue, was verified in triplicate by the Luminescence ELISA method and reported as percentage reduction vs. untreated.
2.2.8. Metalloproteinase Release
The metalloproteinase release was verified by the use of aged fibroblasts. To achieve the accelerated senescence of fibroblasts, their culture has been treated by H2O2, normally used to induce formation of ROS.
ROS, not only induces a reduction in the collagen synthesis, but also increases the secretion of MMPs. In particular, the release of MMP1, responsible for fragmenting type 1 collagen, has been verified.
Thus, fibroblasts have been seeded in sterile 250-mL flasks with DMEM medium and 10% FBS, according to our previous study [39]. After 24 h incubation, the medium with and without 600 μg of H2O2 and cells were incubated for a further 2 h and soon afterwards removed and replaced with a fresh medium. Maintaining the culture for a further 144 h and substituting the medium after 70 h, both aged and normal fibroblasts were detached with trypsin, seeded in 96-well microplates, and cultured for 24 h, replacing the culture medium with DMEM containing the beta Transforming Growth Factor (β-TGF) and the beauty mask with and without the active ingredients (control tissue) (20 μg/mL). The MMP-1 release, evaluated in triplicate, was performed by an ELISA kit and reported as percentage reduction vs. untreated.
2.2.9. Collagenase Inhibition
Hydroxyproline represents about 10% of the total content of amino acids in collagen. Determining its quantity in a culture of fibroblasts added with the collagenase enzyme, it is possible to indirectly establish the efficacy of the beauty mask tissues as inhibitors of this process. Thus, fibroblast cultures were incubated with a collagenase enzyme (Sigma-Aldrich, Milano, Italy) and with suspensions of the tissues. After hydrolysis and oxygenation, the red colour obtained through the liberated hydroxyproline and the Erhlich’s solution added was quantified by a spectrophotometer reading at 569 nm for each culture, according to our previous study [40].
2.2.10. In Vivo Experimental Procedure
To evaluate the safety, tolerability, and effectiveness of the mask, a preliminary in vivo study was done. This study, organized for a period of one month at a plastic surgery office with 30 women volunteers (mean age ± 53 years) with evident signs of photoaging, was evaluated by an expert dermatologist. The criterion for entry in the study, conducted according to the Declaration of Helsinki as revised successively in Seoul, was the presence of one or more signs of photoaging affecting the face of the subjects, such as wrinkling around the eyes, crease lines around the mouth and cheeks, etc., corresponding to the 3–5 degree scale described by Larnier et al. [41].
The non-woven tissues were cut to the dimensions of 15 cm × 20 cm to form a beauty mask put into an aluminium-sealed envelope and sterilized by gamma rays. The beauty masks were applied on skin that was made wet by distilled water, spraying other water on the tissues soon after their application. After 20 min and their removal by an expert beautician, the in vivo effectiveness was verified 30 min later by expert dermatologists and controlled by bioengineered methods. After the beauty mask application, all the subjects have treated their skin, every day for one month, with the assigned cosmetic products using a procedure suggested to them by the dermatologists, according to our previous study [39].
The obtained results were controlled both by bioengineered methods and a score method from expert dermatologists, according to a visual analogical score, following the reported parameters: 0 = no reduction of the wrinkles’ depth, number of fine lines, black colour intensity of the aged spots, and skin irritation phenomena; 5 = satisfactory reduction of the skin wrinkling depth, number of fine lines, black colour intensity of the aged spots, and skin irritation phenomena; 10 = Evident reduction of the skin wrinkling depth, number of fine lines, black colour intensity of the aged spots, and no presence of skin irritation phenomena.
In addition, subjects were asked to provide a self-assessment of the results obtained after the end of the treatment, at seven days and a month, relative to the baseline for fine lines, skin brightness, and skin smoothness. These variables were scored on a scale of 1–4 indicating total (4), strong (3), moderate (2), slight (1), and none (0).
The mean sum of the obtained results is reported in % with respect to the baseline values.
2.2.11. Statistical Analysis
Each measurement at a follow-up visit was compared with the baseline score using paired t-tests. The Wilcoxon signed rank tests were applied to study variations between measurements obtained on the first and last days of the study and paired T-tests between measurements of the active mask and the tissue-vehicle at 20 min, one week, and one month, compared to untreated skin. All the analyses were two-tailed and Pearson’s correlation analysis was used to control the relationships between the biophysical measurements. Results achieving two-tailed p value less than 0.05 were considered to be statistically significant. Calculations were performed with SAS software, version 9.1 (SAS Institute Inc., Cary, NC, USA).
3. Results and Discussion
3.1. In Vitro
The in vitro results have shown that both beauty mask (active tissue) and control tissue, not only has no toxic effects on the viability of keratinocytes and fibroblasts treated by the MTT method, but increases their growth (Figure 1 and Figure 2) while causing a decreased expression of cytokines such as IL-8 and TNF-alpha (Figure 3 and Figure 4), which play a central role in the regulation of immune and inflammatory responses to premature aging and environmental aggressions [42]. TNF-alpha (Tumour Necrosis Factor) production seems triggered, in fact, by UVB-induced photodamage to the DNA of keratinocytes, also inducing the production of other inflammatory mediators such as IL-8 [43,44,45].
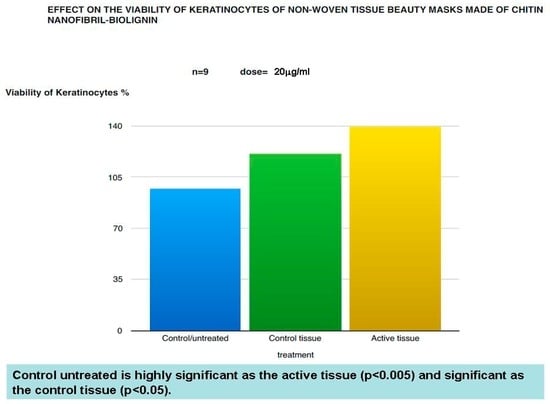
Figure 1.
Viability of keratinocytes treated by the mask determined by the MTT method.
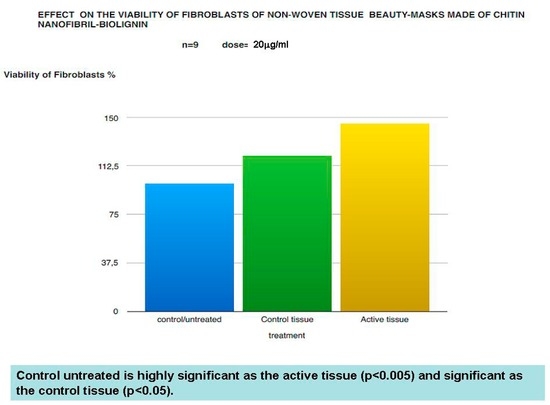
Figure 2.
Viability of fibroblasts treated by the mask determined by the MTT method.
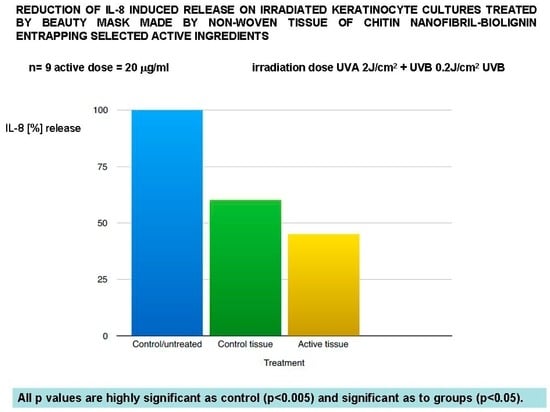
Figure 3.
Anti-inflammatory effectiveness of the mask verified on the IL-8 release.
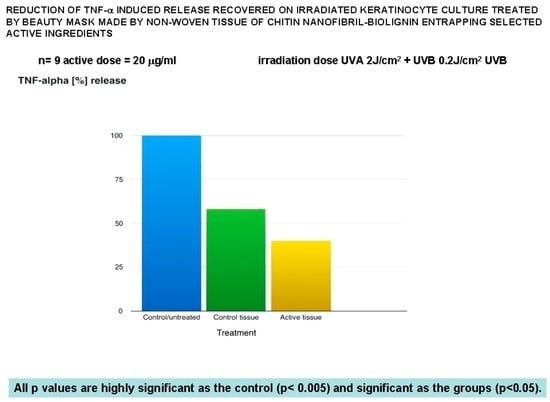
Figure 4.
Anti-inflammatory effectiveness of the mask verified by the TNF-alpha release.
On the other hand, while IL-8 recruits neutrophils from the bloodstream into the area of inflammation, neutrophils play a dual role [46,47], phagocytizing the inflamed cells and at the same time amplifying the inflammation process. Reducing the production of both TNF-α and IL-8 should be one of the key to slow down the erythema correlated to the amount of DNA damage present in the skin of aged and photoaged people [46,47]. Moreover, these phenomena are associated with the activation of various proteases such as MMPs and collagenase, which digests the ECM matrix normally repaired by the leukocytes’ mobilization [48,49,50].
This is the reason why the reparative and antiaging effectiveness of these innovative non-woven tissues has been verified on the release of MMP-1, the synthesis of collagen type I, as the major collagen in the dermis, and on the modulation of the collagenase activity.
As a result, the beauty mask treatment seems effective to significantly reduce the release of both IL-8 (Figure 3) and TNF-alpha (Figure 4), as well as to inhibit MMP1 (Figure 5) and collagenase activity (Figure 7), at the same time increasing the synthesis of collagen type I (Figure 6).
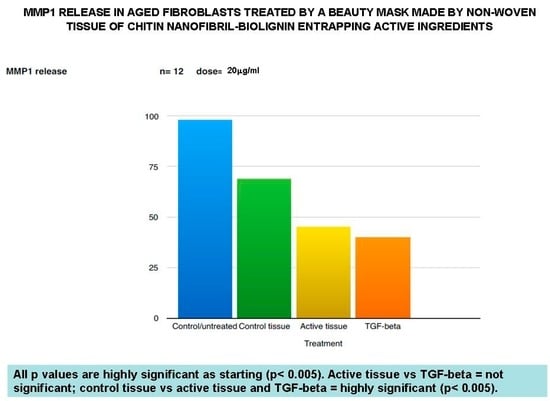
Figure 5.
Anti-aging effectiveness of the in mask verified by the reduced release of MMP-1.
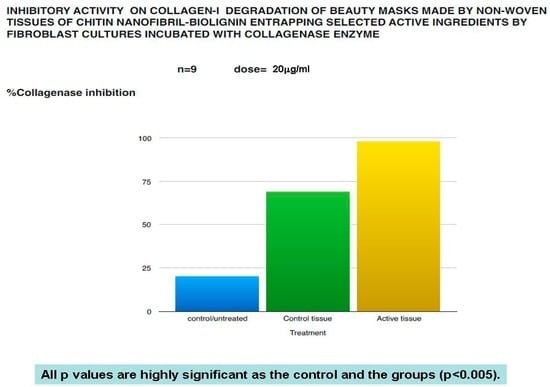
Figure 7.
Anti-aging effectiveness of the mask verified by the collagenase inhibition.
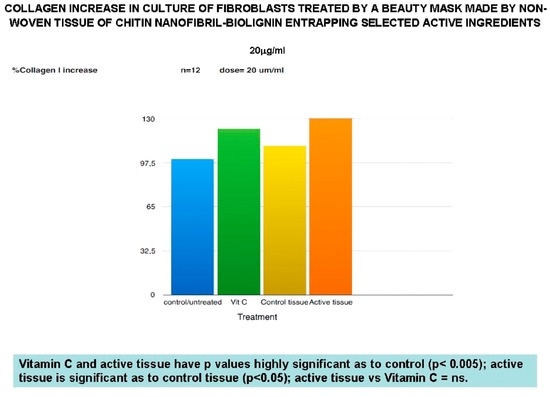
Figure 6.
Anti-aging effectiveness of the mask verified by the increased production of collagen-1.
Additionally, the non-woven tissue alone (control tissue) has also been shown to be active in reducing TNF-alpha, as well as MMP-1 release, significantly reduced from the active tissue in the same way as beta-Transforming Growth Factor activity. Moreover, the control tissue (CN-lignin carrier) has shown its own effectiveness in increasing the collagen type I synthesis, while the active tissue seems to have the same effectiveness as vitamin C, used as an active control (Figure 6).
It is important to remember that both intrinsic aging and extrinsic photoaging increase the secretion of several interleukins such as IL-8 and TNF-alpha, amplifying the expression of genes that propagate the inflammatory response with an increased expression of MMPs.
As a consequence, the speed of degradation of skin components by collagenase becomes greater than the speed of their synthesis. This is one of the phenomena that affect the general skin structure through the appearance of wrinkling and fine lines. As a consequence, in young skin new collagen is able to repair MMP-1-mediated damage, while in older skin, especially if photoaged, this repairing activity disappears because of its reduced synthesis by fibroblasts.
However, it is interesting to underline that the non-woven tissue carrier, used as a basic material to produce beauty masks, has shown its own effectiveness, probably due to the hydrating and reparative activity of CN, which bonds many water molecules thanks to a backbone that is similar to hyaluronic acid. In addition, it seems able to strengthen the antioxidant, photoprotective, and bacteriostatic activity of the biolignin™, thereby increasing the final effectiveness of the beauty masks. Obviously the antioxidant network made of the selected active ingredients has increased the antiaging effectiveness of the final non-woven CN-LG tissue used for this study.
The presence of niacinamide, in fact, reinforces the moisturizing and protective activity of the non-woven tissue for its capacity to promote the synthesis of filaggrine, as a precursor of the natural moisturizing factor (NMF) at the level of the stratum corneum [31], as a modulator of cytokines such as IL-8 and TNF [32], and as an enhancer of DNA repairing activity on irradiated skin [33] (all ingredients used and characterized previously by our group) [50]. Moreover, melatonin [34], vitamin C, and beta-glucan increase its antioxidant, immunomodulant, and protective effectiveness, as reported by our previous studies [51,52,53,54].
3.2. In Vivo
The in vivo results have confirmed the protective and rejuvenation activity shown by the in vitro studies. As shown in Figure 8, Figure 9, Figure 10 and Figure 11, this cosmetic treatment has given the best result after 20 min of activity, notably increasing skin hydration (Figure 8) and surface skin lipids (Figure 9) and re-establishing the skin’s normality with an evident reduction of the TEWL (Figure 10) and black spots (Figure 11) provoked by the physiological aging process and increased by UV rays and previous environmental exposure of the in-study subjects. However, it is interesting to underline that the skin amelioration remained for a further month after the cosmetic treatment.
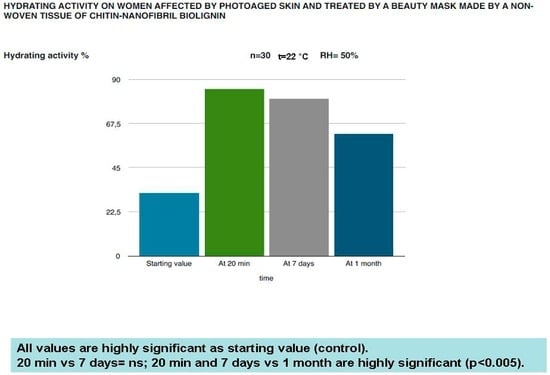
Figure 8.
Moisturizing effectiveness of the in-study mask on the skin of photoaged women.
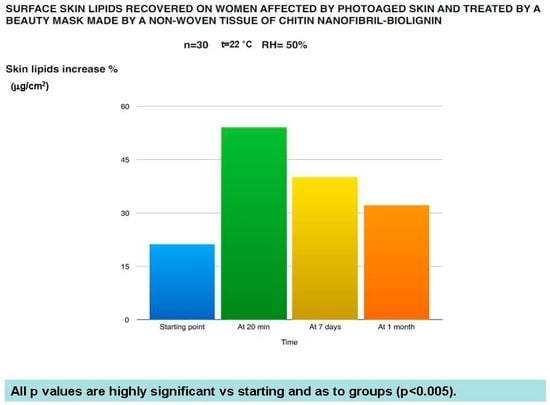
Figure 9.
Effectiveness of the mask on the skin lipids balance of photoaged women.
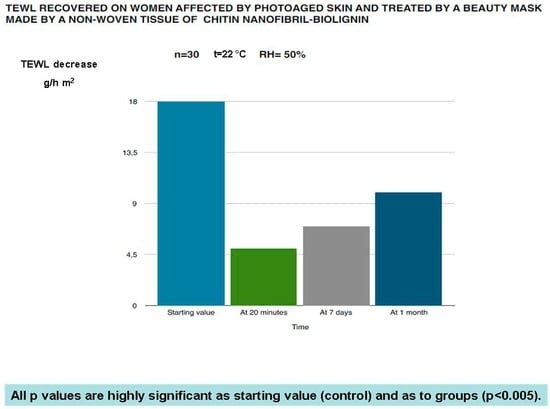
Figure 10.
Effectiveness of the in-study mask on the TEWL balance of photoaged women.
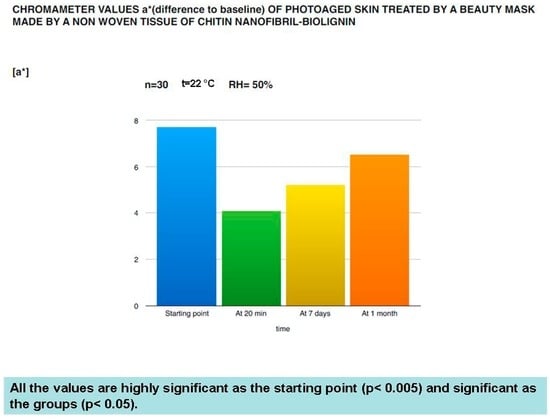
Figure 11.
Anti-aging effectiveness of the mask on the black spots of photoaged women.
The interesting results obtained one month later suggest that the use of the mask treatment at least one time a week and not only one time a month has a significant effect in terms of reinforcing normal cosmetic treatments. However, in our opinion the effectiveness shown by this innovative mask treatment is due also to the contemporary activity of the CN-Bio-lignin™ tissue used as a carrier and the selected active ingredients trapped in the CN fibres before the electrospinning process.
CN, in fact, was metabolized by the chitotriosidase enzymes to obtain glucosamine and acetylglucosamine, which probably could be used to produce the glycosaminoglycans necessary for the dermis structure. Moreover, it could also modulate the glycation process, or produce glucose used as energy from the skin cells [55]. On the other hand, bio-lignin™, rich in polyphenolic units, could have antimicrobial, antioxidant, and photoprotective effectiveness [28,56]. These combined activities could reinforce the physiological effectiveness of the antioxidant and immunomodulant network, normally entrapped into the Extra Cellular Matrix (ECM). The in vivo effectiveness, shown by the bioengineered methods, has been confirmed from the visual and direct control of dermatologists and by patient satisfaction, as reported in Figure 12 and Figure 13.
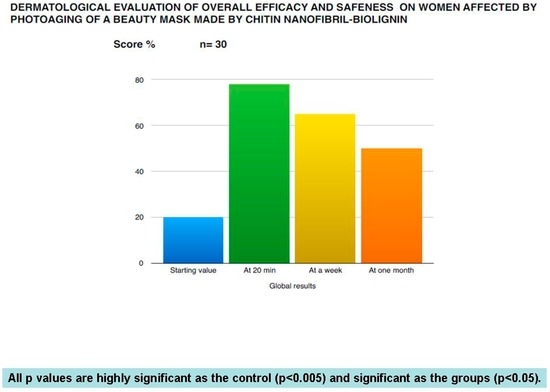
Figure 12.
Dermatological subjective evaluation of the mask on the skin rejuvenation of photoaged women.
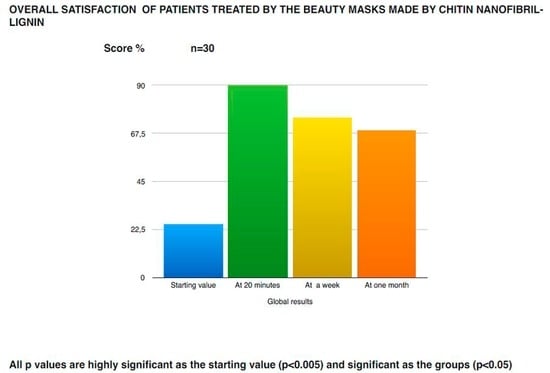
Figure 13.
Patient evaluation of the mask’s rejuvenation effectiveness on their own skin.
The high effectiveness shown by these beauty masks is probably due to the activity of the ingredients, which remain stable and active because they are “entrapped” into a dry support represented by the natural non-woven tissues used. The reparative and antiaging effectiveness shown in vivo after only one treatment with the beauty mask application has surely been increased thanks to the combined use of a kit of cosmetic products suggested to the women by the plastic surgeon, based on the previously obtained results reported in other studies [51,52,53,54].
It is important to remember that these beauty masks are safe for humans and for the environment because of the use of natural ingredients obtained from waste material, such as CN from crustacean by-products, and lignin from plant biomass. In addition, this innovative treatment has been shown to modulate the skin microbioma, also reducing skin inflammation, probably because of the contemporary antibacterial activity of chitosan, CN, and lignin (data not shown).
Finally, this innovative beauty mask is 100% biodegradable as well as environmentally friendly because it is free of preservatives, emulsifiers, and chemicals [57].
4. Conclusions
According to the EOCD and EU programmes for a greener economy, it has been shown that it is possible to use industrial and agricultural by-products such as CN and lignin to produce green nanocomposites for making innovative and effective beauty masks. The non-woven tissues obtained from these natural polymers have been shown to be useful in producing beauty masks that are not only effective on aged and sensitive skin, but also very safe and stable for a long period of time because they are free of water.
In conclusion, the regular use of these innovative beauty masks seems to be useful to highlight the effectiveness of normal cosmetic treatments in slowing down the skin premature aging, contemporary saving the environment.
Due to these results, we are going on with our studies to verify the possibility of using these safe and effective non-woven tissues as an active topical means of soothing itching, for example, the itch phenomena, or treating various skin disorders such as acne and rosacea, and reducing the formation of actinic keratoses by accelerating their regression.
Acknowledgments
We would like to thank MAVI sud s.rl. for the samples and the financial support given to our study.
Author Contributions
Marco Palombo and Maria Cardillo contributed to the in vivo studies; Angelo Chianese controlled the physical-chemical characteristics of the non-woven tissues and the other researchers were involved into the in vivo experiments.
Conflicts of Interest
Pierfrancesco Morganti, Francesco Carezzi, Maria Luisa Nunziata and Gianluca Morganti works at MAVI sud s.r.l. The other authors declare no conflict of interest.
References and Notes
- Roco, M.C.; Mirkin, C.A.; Hersan, M.C. WTEC Panel Report on Nanotechnology Research Direction for Societal Needs in 2020: Retrospective and Outlook. Available online: http://www.wtec.org/nano2/Nanotechnology_Research_Directions_to_2020 (accessed on 15 November 2016).
- National Science Foundation, FY 2015 Strategic Sustainability Performance Plan. Available online: https://www.nsf.gov/pubs/2016/nsf16025/nsf16025 (accessed on 15 November 2016).
- Nellmann, C.; MacDevette, M.; Manders, T.; Eickhout, B.; Svihus, B.; Prins, A.G.; Kaltenborn, B.P. The Environmental Food Crisis; UNEP, Birkeland Trykkeri AG: Oslo, Norway, 2009. [Google Scholar]
- The United Nations Environment Programme. Converting Waste Agricultural Biomass into a Resource; UNEP: Osaka, Japan; Shiga, Japan, 2009. [Google Scholar]
- Lipinski, B.; Hanson, C.; Lomax, J.; Kitinoja, L. Creating Food Loss and Waste; UNEP Working Paper, June; UNEP: Osaka, Japan; Shiga, Japan, 2013. [Google Scholar]
- The United Nations Environment Programme. Human Health and the Environment. Post Note 3. 2015. Available online: www.unep.org/post (accessed on 15 November 2016).
- European Commission. General Union Environment. Living Well, within the Limits of Our Planet; Publications Office of the European Union: Luxemburg, 2014. [Google Scholar]
- SOER. The European Environment-State and Outlook 2015-European Briefings—Green Economy; European Environment Agency: Copenhagen, Denmark, 2015. [Google Scholar]
- The United Nations Environment Programme. Towards a Green Economy: Pathways to Sustainable Development and Poverty Eradication; United Environment Programme: New York, NY, USA, 2011. [Google Scholar]
- Organisation for Economic Co-operation and Development. Towards Green Growth; Organization for Economic Cooperation and Development: Paris, France, 2011. [Google Scholar]
- Santis, M.R.E.; Fonseca, A.C.; Mendonca, P.V.; Branco, R.; Serra, A.C.; Morais, P.V.; Coelho, J.F.J. Recent Developments in Antimicrobial Polymers: A Review. Materials 2016, 9, 599. [Google Scholar] [CrossRef]
- Morganti, P. New Horizon in Cosmetic Dermatology. J. Appl. Cosmetol. 2016, 34, 9–18. [Google Scholar]
- Morganti, P.; Muzzarelli, C. Spray-Dried Chitin Nanofibrils, Method for Production Uses Thereof. U.S. Patent 8,552,164, 8 October 2013. [Google Scholar]
- Muzzarelli, C.; Morganti, P. Preparation of Chitin and Derivatives Thereof for Cosmetic and Therapeutic Use. U.S. Patent 8,383,157, 26 February 2013. [Google Scholar]
- Morganti, P.; Muzzarelli, R.A.A.; Muzzarelli, C. Multifunctional use of Innovative Chitin Nanofibrils for Skin Care. J. Appl. Cosmetol. 2006, 24, 105–114. [Google Scholar]
- Morganti, P.; Del Ciotto, P.; Fabrizi, G.; Guarneri, F.; Cardillo, A.; Palombo, M.; Morganti, G. Safety and Tolerability of Chitin Nanofibrils-Hyaluronic acid nanoparticles entrapping Lutein. Note 1: Nanoparticles Characterization and Bioavailability. SOFW J. 2013, 139, 12–23. [Google Scholar]
- Morganti, P.; Tishchenko, G.; Palombo, M.; Kelnar, L.; Brozova, L.; Spirkova, M.; Pavlova, E.; Kobera, L.; Carezzi, F. Chitin nanofibrils for biomimetic products: Nanoparticles and nanocomposite chitosan films in health-care. In Marine Biomaterials: Isolation, Characterization and Application; Kim, S.-K., Ed.; CRC-Press: New York, NY, USA, 2013; pp. 681–715. [Google Scholar]
- Morganti, P.; Chen, H.D.; Gao, X.H.; Del Ciotto, P.; Carezzi, F.; Morganti, G. Nanoparticles of Chitin Nanofibril Hyaluronan block polymer entrapping Lutein as UVA protective compound. In Carotenoids: Food Source, Production and Health benefits; Yamaguchi, M., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2013; pp. 237–259. [Google Scholar]
- Morganti, P.; Carezzi, F.; Morganti, G.; Fabrizi, G. Chitin-Hyaluronan Nanoparticles to Deliver Anti-aging Ingredients through the Skin. Cosmetics 2014, 1, 140–158. [Google Scholar] [CrossRef]
- Morganti, P.; Carezzi, F.; Del Ciotto, P.; Tishchenco, G.; Chianese, A. A Green Multifunctional Polymer from Discarded Material: Chitin Nanofibril. Br. J. Appl. Sci. Technol. 2014, 4, 4175–4190. [Google Scholar] [CrossRef]
- Morganti, P.; Del Ciotto, P.; Carezzi, F.; Guarneri, F.; Yeo, Y.J. Skin Lightening Efficacy of New Formulations Enhanced by Chitin Nanoparticles Delivery System. Note 1. J. Appl. Cosmetol. 2014, 32, 57–71. [Google Scholar]
- Morganti, P. The meaning of nanodimension involving the Cosmetic Chemist from lab to the Industrial Process. J. Sci. Res. Rep. 2014, 4, 79–100. [Google Scholar]
- Eide, K.B.; Norberg, A.L.; Heggset, E.B.; Lindbom, A.R.; Varum, K.M.; Eijsink, V.G.H.; Sorlie, M. Human Chitotriosidase-Catalyzed Hydrolysis of Chitosan. Biochemistry 2012, 51, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Morganti, P. Green economy and bionanotechnology to transform waste materials in useful goods: Results of EU projects. Eurocosmetics 2015, 22, 12–16. [Google Scholar]
- Mikesiva, J.; Haseka, J.; Tishchenko, G.; Morganti, P. Rheological study of Chitosan acetate solutions containing chitin nanofibrils. Carbohydr. Polym. 2014, 112, 753–757. [Google Scholar] [CrossRef] [PubMed]
- Vanholme, R.; Denedts, B.; Morreel, K.; Ralph, J.; Boerjan, W. Lignin biosynthesis and structure. Plant Physiol. 2010, 153, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Ten, E.; Vermerris, W. Recent Developments in polymers derived from industrial lignin. J. Appl. Polym. Sci. 2015, 132, 1–13. [Google Scholar] [CrossRef]
- Èspinosa-Acosta, J.L.; Torres-Chavez, P.I.; Ramirez-Wong, B.; Lopez-Saiz, C.M.; Montano-Leyva, B. Antioxidant, Antimicrobial, and Antimutagenic Properties of Technical Lignins and Their Applications. BioResources 2016, 11, 1–30. [Google Scholar]
- Morganti, P. Green Ingredients in Cosmetic Dermatology. Molecular Aspects of Ingredients and Carriers. J. Appl. Cosmetol. 2016, 34, 65–79. [Google Scholar]
- Morganti, P. Innovative and Natural Beauty Masks Eco-compatible and Skin-friendly. Report presented at University of Shenyang and University of Jilin, China, 14 January 2015. [Google Scholar]
- Matts, P.J.; Rawlings, A.V. The effects of niacinamide-containing moisturizers. In Skin Moisturization, 2nd ed.; Rawlings, A.V., Leyden, J.J., Eds.; Informa: New York, NY, USA, 2009; pp. 232–333. [Google Scholar]
- Surjana, D.; Halliday, G.M.; Damian, D.L. Role of nicotinamide in DNA damage, mutagenesis, and DNA repair. J. Nucleic Acids 2010. [Google Scholar] [CrossRef] [PubMed]
- Susja, A.D.; Halliday, G.M.; Damian, D.L. Nicotinamide enhances repair of ultraviolet radiation-induced DNA damage in human keratinocytes and ex vivo skin. Carcinogenesis 2013, 34, 1144–1149. [Google Scholar]
- Klesriczynski, K.; Fischer, T.W. Melatonin and human skin aging. Dermatoendocrinology 2012, 4, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Cardillo, A.; Morganti, P. Fast and non-invasive method for assessing skin hydration. J. Appl. Cosmetol. 1994, 12, 11–16. [Google Scholar]
- Morganti, P.; Fabrizi, G.; Palombo, P.; Palombo, M.; Ruocco, E.; Cardillo, A.; Morganti, G. Chitin-Nanofibrils: A New Active Cosmetic Carrier. J. Appl. Cosmetol. 2008, 26, 113–128. [Google Scholar]
- Morganti, P.; Del Ciotto, P.; Stoller, M.; Chianese, A. Antibacterial and Anti-inflammatory Green Nanocomposites. Chem. Eng. Trans. 2016, 47, 61–66. [Google Scholar]
- Modann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and citotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar]
- Morganti, P.; Palombo, M.; Fabrizi, G.; Guarneri, G.; Slovacchia, F.; Cardillo, A.; Del Ciotto, P.; Carezzi, F.; Morganti, G. New Insight on Anti-aging Activity of Chitin Nanofibril-Hyaluronan Block Copolymers Entrapping Active Ingredients: In vitro and in vivo study. J. Appl. Cosmetol. 2013, 31, 1–29. [Google Scholar]
- Krutmann, J.; Schroeder, P. Role of mitochondria in photoaging of human skin-the defective powerhouse model. J. Invest. Dermatol. Symp. Proc. 2009, 14, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Larnier, C.; Ortonne, J.P.; Venot, A.; Faivre, B.; Beani, J.C.; Thomas, P.; Brown, T.C.; Sendagorta, E. Evaluation of Cutaneous photodamages using a photographic scale. Br. J. Dermatol. 1994, 130, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Curfs, J.H.A.J.; Meis, J.F.G.M.; Hoogkamp-Kostanje, J.A.A. A primer on cytokines: Sources, receptors, effects and inducers. Clin. Microbiol. Rev. 1997, 10, 742–780. [Google Scholar] [PubMed]
- Walter, S.L.; Young, A.R. An action spectrum (299–320 nm) for TNA-alpha protein in human skin in vivo suggests that basal layer Epidermal DNA is the chromophore. PNAS 2007, 104, 19051–19054. [Google Scholar]
- Young, A.R.; Chadwick, C.A.; Harrison, G.I.; Nikaido, O.; Ramsden, J.; Potter, C.S. The similarity of action spectra of thymine dimers in human epidermis and erythema suggests that DNA is the chromophore of erythema. J. Invest. Dermatol. 1998, 111, 982–988. [Google Scholar] [CrossRef] [PubMed]
- Schottelius, A.J.G.; Moldawer, L.L.; Dinarello, C.A.; Asadullah, K.; Sterry, W.; Edwards, C.K. Biology of tumour necrosis Factor-alpha-implications of psoriasis. Exp. Dermatol. 2004, 13, 193–222. [Google Scholar] [CrossRef] [PubMed]
- Da Bara, A.; Sekido, N.; Akahoshi, T.; Wada, T.; Mukaida, N.; Matsushima, K. Essential involvement of interleukine-8 in acute inflammation. J. Leukoc. Biol. 1994, 56, 559–564. [Google Scholar]
- Rijken, F.; Bruijnzeel, P.L.B. The pathogenesis of photoaging: The role of neutrophils and neutrophil-derived enzymes. J. Invest. Dermatol. Symp. Proc. 2009, 14, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Quan, T.; Qin, Z.; Xia, W.; Shao, Y.; Voorthees, J.J.; Fisher, G.J. Matrix-degrading metalloproteinases in photoaging. J. Invest. Dermatol. Symp. Proc. 2009, 14, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Pillai, S.; Oresajo, C.; Hayward, J. Ultraviolet radiation and skin aging: Roles of reactive oxygen species, inflammation and protease activation, and strategies for prevention of inflammation-induced matrix degradation-a review. Int. J. Cosmet. Sci. 2005, 27, 17–34. [Google Scholar] [CrossRef] [PubMed]
- Antonicelli, F.; Bellon, G.; Debelle, L.; Homebeck, W. Elastin-elastase and inflamm-aging. Rev. Curr. Top Dev. Biol. 2007, 79, 99–155. [Google Scholar]
- Morganti, P.; Del Ciotto, P.; Fabien-Soule, V. Application of chitin nanofibrils and collagen of marine origin as bioactive ingredients. In Marine Cosmeceuticals: Latest Trends and Prospects; Kim, S.K., Ed.; CRC Press: New York, NY, USA, 2011; pp. 267–290. [Google Scholar]
- Morganti, P. Reflections on Cosmetics, Cosmeceuticals, and Nutraceuticals. Clin. Dermatol. 2008, 26, 318–320. [Google Scholar] [CrossRef] [PubMed]
- Morganti, P.; Palombo, P.; Palombo, M.; Fabrizi, G.; Cardillo, A.; Carezzi, F.; Morganti, G.; Ruocco, E.; Dziergowski, S. Cosmetic in skin aging: Achieving the efficacy by the chitin nano-structured crystallites. SOFW J. 2010, 136, 14–25. [Google Scholar]
- Morganti, P.; Fabrizi, G.; Palombo, P.; Palombo, M.; Guarneri, F.; Cardillo, A.; Morganti, G. New Chitin Complexes and their Anti-Aging Activity from Inside Out. JNHA 2012, 16, 242–245. [Google Scholar] [CrossRef]
- Morganti, P. Use of Chitin Nanofibrils from biomass for an Innovative Bioeconomy. In Nanofabrication Using Nanomaterials; Ebothe, J., Ahmed, W., Eds.; One Central Press: London, UK, 2016; pp. 1–22. [Google Scholar]
- Quian, Y.; Qiu, X.; Zhu, S. Lignin: A nature-inspired sun blocker for broad-spectrum sunscreens. Green Chem. 2015, 17, 320–324. [Google Scholar] [CrossRef]
- Morganti, P. The Easy Biodegradability of an Innovative Non-woven Tissue. Unpublished Data. 2016. [Google Scholar]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).