Abstract
The cosmetic industry is constantly evolving to create new formulations that offer controlled and specific release of active ingredients, as well as greater penetration, duration and stability of the resulting products. To fulfil all these objectives, the use of cyclodextrins in different cosmetic formulations is being considered. CDs are cyclic oligosaccharides with a hydrophilic outer surface and an inner cavity able to encapsulate hydrophobic molecules. This property can be used to form complexes with hydrophobic molecules and solubilise them in aqueous matrices, such as creams or gels. This review analyses the main advantages that these agents provide in cosmetic products, such as protection, administration and controlled release of bioactive ingredients, improved water solubility, reduced fragrance volatility, masking off unpleasant odours, modification of the physicochemical properties of formulations or prevention of ingredient side effects, among others. Formulations of lotions, sunscreens, deodorants, gels or perfumes containing CDs are already on the market, and new ones are being developed. Moreover, the regulations concerning their use, the types of cyclodextrins allowed and the mechanism required to produce CD-guest inclusion complexes are reviewed. Likewise, the use of CDs alone or encapsulating other compounds makes them an extremely versatile nanomaterial for dermofacial and cosmetic formulations.
1. Introduction
The cosmetic industry is constantly growing, evolving and researching to develop new products that satisfy customer expectations. The aim is to formulate products with superior performance, offering controlled and targeted release of active ingredients, longer duration and penetration, and greater stability of the resulting product. In this context, the use of cyclodextrins (CDs) in different cosmetic formulations is being considered.
Cyclodextrins (CDs) were discovered in 1891 by Villiers, but did not become important until the 1980s, with their first uses in chromatography [1]. Nowadays the use of CDs is widely spread as in chemical, pharmaceutical [2], textile [3], food [4], or cosmetic industries [5], among many others.
CDs are truncated-cone-shaped molecules made up of several glucose units linked together by α-1,4-glycosidic bonds. The inner of this hollow cavity is hydrophobic, allowing the inclusion of poorly water-soluble host molecules of the appropriate size. In contrast, the outer layer of the CDs is hydrophilic, allowing its solubilization in aqueous solutions. This duality of cyclodextrins is responsible for one of their main applications, the solubilization of hydrophobic molecules stored within them in aqueous matrices [1,6]. These molecules are naturally produced by bacterial degradation of starch. Bacillus firmus possesses an enzyme called cyclodextringlycosyl transferase (CGTase), which is responsible for starch fragmentation and cyclisation [7]. The action of the enzyme CGTase produces CDs with six, seven or eight glucose units, which are the native CDs and are termed α-CD, β-CD, and γ-CD, respectively (Figure 1). The hydrophobic cavity of native CDs also increases as the number of glucose units rises [8]. Chemical modifications or polymerisation [9] of native cyclodextrins provide a wide variety of derivatives with different physicochemical and biological properties. There are currently more than 1500 types of cyclodextrins, some of them produced by hydroxylation, methylation, or addition of other functional groups to natural cyclodextrins [8].
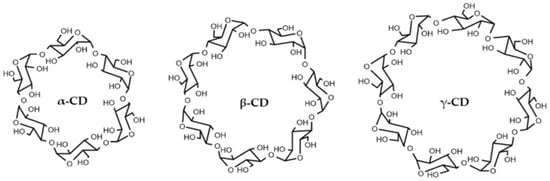
Figure 1.
Structure of natural CDs. Obtained from [10].
CDs and their derivatives are widely used in industry due to their structure, biocompatibility, biodegradability and versatility. They can be used to protect host molecules housed in their interior, which hinders their oxidation, degradation or reaction with other molecules [10]. CDs can also increase the apparent solubility of hydrophobic molecules in aqueous matrices, eliminate undesired odours and tastes [11] or enhance those desired, and improve the handling of substances by transforming oils and liquids into powders or increasing the stability of emulsions [5]. Indeed, the most characteristic feature of CDs is their ability to form inclusion complexes with various molecules through host-guest interactions. These properties make them widely used in numerous industries such as the pharmaceutical, food, textile, and cosmetic industries, in agriculture, in environmental protection and in chemical and biological analysis, among others [1,2,3,4,5,6]. This work will focus on the use of CDs in cosmetic, hygiene and personal care products.
The use of CDs in various cosmetic products is restricted and regulated, depending on the countries in which they are marketed. In the United States, cosmetics are defined by the Federal Food, Drug, and Cosmetic Act (FD&C Act) in section 201(i) as “articles intended to be rubbed, poured, sprinkled, or sprayed on, introduced into, or otherwise applied to the human body…for cleansing, beautifying, promoting attractiveness, or altering the appearance” [12,13]. In addition, in 2022, the Cosmetics Regulation Modernisation Act (MoCRA) was passed, defining a cosmetic product as “a preparation of cosmetic ingredients with a qualitatively and quantitatively set composition for use in a finished product” [14]. Therefore, cosmetics and cosmetic products include hair dyes, perfumes, eye and face make-up, lipsticks, deodorants and cleansing shampoos, among others. For CD to be used in these formulations, the safety of the resulting product and its components must be demonstrated. The safety of CDs has been established by the Cosmetic Ingredient Review Expert Panel, which endorsed the use of native CDs, 2-hydroxypropyl-β-cyclodextrin (HP-β-CD) and hydroxyethyl-β-cyclodextrin (He-β-CD) as safe cosmetic ingredients [8]. Furthermore, within the European Union, cyclodextrins are classified as excipients and their use is subject to regulation by the European Medicines Agency. Studies in human volunteers have demonstrated that CDs are as safe as other substances currently employed in cosmetics and perfumery. Concentrations of up to 0.1% of natural CDs were well tolerated. Although CDs alone display limited transdermal absorption, their permeability increases when co-administered with absorption enhancers. Currently, natural CDs and HP-β-CDs can be used for dermal administration [15,16]. In Japan, cosmetics are regulated under the Pharmaceutical and Medical Devices Law (PMDL), which classifies native cyclodextrins as natural products and permits their use with relatively few restrictions in the development of new cosmetic formulations [17].
This review addresses the use of natural CDs, as well as modified CDs, cyclodextrin-based polymers and cyclodextrin-containing polymers in the cosmetics industry. The diverse applications of these molecules in the cosmetic industry are analysed, including the protection and controlled release of bioactive compounds, fragrance and odour control, formulation stabilisation, and reduction of skin irritancy, among other uses.
2. Cyclodextrin Types and Methods to Encapsulate Bioactive Ingredients
It is well established that CDs can be employed for the encapsulation of bioactive compounds; however, a vast variety of CDs is available on the market, both natural and semi-synthetic derivatives [4], as well as monomeric and polymeric forms [18]. Consequently, the selection of a specific CD and encapsulation method depends on the physicochemical properties of the target molecule.
Inclusion complexes between bioactive compounds and CDs can be generated through different techniques. For example, Fernández-Romero et al. (2021) evaluated several approaches for encapsulating curcumin within epichlorohydrin-β-CD polymers [19] to produce hydrogels for the treatment of inflammatory skin diseases. The methods investigated included physical mixing in a mortar, kneading, co-grinding, co-lyophilisation, and co-evaporation, all employing a drug:CD ratio of 1:10 w/w. Comprehensive characterisation (dynamic light scattering, differential scanning calorimetry, X-ray diffraction, etc.) revealed that co-grinding was the most effective strategy, as it significantly enhanced curcumin solubility in aqueous solution and reduced the particle size more than any other method [19].
Other researchers have prepared inclusion complexes between polymeric CDs and drugs using the solvent evaporation method in dichloromethane [20] with a drug:CD ratio of 1:2 w/w. In general, for binary complexes of drugs or flavour molecules and CDs, the drug:encapsulating agent ratio may vary, but it rarely exceeds 1:10 w/w. This specific ratio has been reported for the encapsulation of curcumin in CD polymers [19], ganciclovir in β-CD [21], and flavour molecules in β-CD [22].
When selecting the most suitable CD for encapsulating a bioactive compound, it is essential to take into account not only the internal cavity diameter and physicochemical characteristics of the CDs (Table 1) but also the potential non-covalent interactions, such as hydrogen bonds and hydrophobic interactions [23,24]. Studies on stilbenes [25,26] have demonstrated the critical role of these interactions on the encapsulation constant (KF) of bioactive compounds. Specifically, the establishment of hydrogen bonds between CDs and guest molecules enhances the stability of the binary complex, resulting in a higher KF [26]. Moreover, derivatisation of natural CDs, such as β-CD, with organic substituents to generate derivatives, such as HP-β-CD and methyl-β-CD (M-β-CD), increases the hydrophobicity of the host environment, thereby improving the encapsulation effectiveness and further elevating the KF value [27].
Investigation of the KF of bioactive compounds is crucial for the development of optimised formulations with the most appropriate CD. The KF can be determined using a variety of analytical techniques, including fluorescence spectroscopy [25,26,28,29,30], UV/Vis spectroscopy [31], HPLC [32,33] or NMR [34,35], among other methods. The choice of method depends primarily on the physicochemical properties of the guest molecule (absorbance, fluorescence, etc.). In recent years, experimental analyses have increasingly been complemented by in silico approaches, such as molecular docking and molecular dynamics [29,36].
To select the optimal cyclodextrin, numerous studies must be carried out, each compound of interest has a different affinity for each cyclodextrin. It is important to note that not all cyclodextrins are the same and their physicochemical properties also differ. For example, natural β-CD has very low water solubility (1.8 mg/100 mL), but this increases when it is derivatised with methyl or hydroxypropyl groups, among others. The toxicity of each cyclodextrin and its possible interactions with other compounds in cosmetic formulations must also be considered.

Table 1.
Types and physicochemical properties of different monomeric CDs.
Table 1.
Types and physicochemical properties of different monomeric CDs.
| Encapsulating Agent | Number of Glucose Units | Internal Cavity Size (Å) | Substituent Groups (Type) | Average Degree of Substitution (SD) * | Molecular Weight (g/mol) ** | Water Solubility (25 °C mg/100 mL) | Reference |
|---|---|---|---|---|---|---|---|
| α-CD | 6 | 4.7–5.2 | None (Natural) | 0 | 972.8 | 12.8 | [6] |
| β-CD | 7 | 6–6.4 | None (Natural) | 0 | 1135 | 1.8 | [6] |
| γ-CD | 8 | 7.5–8.3 | None (Natural) | 0 | 1297 | 25.6 | [6] |
| HP-β-CD | 7 | 6–6.4 | Hydroxypropyl groups (-CH2CH2COOH) | 0.7–4.5 | 1541.5 | 100 | [37,38,39] |
| M-β-CD | 7 | 6–6.4 | Methyl groups (CH3) | 7–14 | 1303.3 | 50 | [40,41] |
| HP-γ-CD | 8 | 7.5–8.3 | Hydroxypropyl groups (-CH2CH2COOH) | 4–6 | 1587.5 | High | [42,43,44] |
| He-β-CD | 7 | 6–6.4 | Hydroxyetil groups | 14 | 1443.3 | 55 | [45] |
| Carboxymetil-β-CD | 7 | 6–6.4 | Carboximetil groups (-CH2COOH) | 5 | 1446 | Higher than β-CD | [46] |
| Sulfobutil eter-β-CD | 7 | 6–6.4 | Sulfobutileter groups (-OCH2CH2CH2CH2SO3−) | 6–7.1 | 2083 | ≥500 mg/mL | [47] |
* The degree of substitution depends on the product and the manufacturer. Typical ranges are specified. ** The molecular weight depends on the average number of substituents (DS). The table provides data from commercial examples.
3. Protection and Delivery of Bioactive Ingredients
Bioactive ingredients are molecules with beneficial properties, such as antioxidant, anti-ageing, anti-inflammatory, or anti-cancer effects, among others [48]. When talking about bioactive ingredients, phenolic compounds (phenylpropanoids, phenolic acids, flavonoids, etc.) are of particular interest due to their strong antioxidant activity [49]. This activity arises from multiple factors, including the presence of functional groups (hydroxyl, carboxyl) within their chemical structures, as well as their ability to modulate gene expression pathways that trigger antioxidant responses. Such mechanisms contribute to protection against the damaging effects of free radicals [50].
Free radicals, particularly reactive oxygen species (ROS) such as singlet oxygen, superoxide anion (O2·−) and hydroxyl radical (·OH), are highly detrimental to living organisms, as they promote the oxidation of cellular structures. This oxidative damage leads to a range of adverse outcomes, including oxidative stress, cancer, and other pathologies [51]. In the skin, ROS also contribute to premature ageing [49], highlighting the importance of developing cosmetics enriched with bioactive ingredients capable of neutralising these species. Indeed, several phenylpropanoids are already employed in cosmetic formulations; for example, cinnamic acid and its derivatives in sunscreens, as well as other phenylpropanoids like ferulic acid incorporated into sunscreens, facial serums, and anti-wrinkle creams, among other products [52].
However, the majority of bioactive ingredients are highly labile and undergo rapid degradation upon UV radiation, atmospheric conditions, or microbial activity [50,53]. Consequently, ensuring both the stability of these compounds and their effective delivery represents one of the primary challenges that must be overcome in the formulation development. CDs offer a promising solution to these issues, as their incorporation into cosmetic formulations can stabilise the product by encapsulating bioactive ingredients [54,55].
3.1. Cyclodextrins and Their Protective Ability
Bioactive ingredients must be protected from degradation to exert their antioxidant activity, and CDs have been widely used to stabilise these compounds, as previously mentioned. A compilation of examples illustrating the use of CDs for the encapsulation of bioactive compounds is presented in Table 2. For instance, regarding phenolic compounds, several studies have demonstrated that stilbenes (resveratrol, piceatannol, oxyresveratrol, etc.) and phenolic acids (neochlorogenic acid) are potent antioxidants [25,53,56,57] that are extremely sensitive to light and temperature, undergoing rapid degradation upon exposure to UV radiation [58]. Stilbenes such as resveratrol are particularly prone to light-induced isomerisation from their biologically active trans form to the less active and less stable cis isomer, ultimately leading to their degradation [58]. Encapsulation of phenolic compounds, such as stilbenes, by CDs has been extensively investigated due to the strong host-guest affinity [50], which safeguards these bioactive ingredients from degradation induced by abiotic stressors [5,50], including light, heat and oxidation, enzymatic hydrolysis [5,59] and other undesired interactions and chemical reactions [5,60].
Other phenylpropanoids, such as ferulic acid, have also been encapsulated in CDs to protect them from oxidation and photodegradation [61]. Ferulic acid is a potent antioxidant and photoprotective compound, making its incorporation into cosmetic formulations, such as sunscreens, particularly valuable. Studies have shown that it can increase the Sun Protection Factor (SPF) of sunscreens by 37% and the UV Protection Factor (UV-PF) by 26% [62]. However, ferulic acid is subjected to the same stability challenges as other phenolic compounds, and its encapsulation in CDs was proposed to overcome these limitations. Thereby, encapsulation studies using a range of CDs demonstrated that α-CD was the most effective at preserving the stability and antioxidant capacity of ferulic acid [61].
While numerous studies have reported the encapsulation of phenylpropanoids like stilbenes [25,63] to prevent degradation, CDs can also be employed to stabilise other labile molecules, such as essential oils [64], which are highly susceptible to both chemical degradation and evaporation, as in the case of citronellal [65]. Additional studies have confirmed that β-CD and HP-β-CD maintain the antioxidant activity of essential oils extracted from Lavandula viridis, Lavandula pedunculata and Thymus lotocephalus [66].
Curcumin is another bioactive compound containing two aromatic rings and is highly photosensitive; its encapsulation in CDs has been shown to enhance stability, thereby enabling its use as an antioxidant and anti-inflammatory agent [19,67].
Among other significant bioactive compounds in the cosmetic industry, coenzyme Q10 (CoQ10) is a potent antioxidant capable of scavenging free radicals [68]. In addition, it acts as a stabilizer of the electron transport chain and cellular respiration, meaning its role extends beyond the elimination of oxidative species to the prevention of their formation [69]. Like many bioactive molecules, CoQ10 is highly susceptible to degradation. Several studies have investigated its encapsulation in CDs [68,69,70], demonstrating that incorporation into β-CD and γ-CD enhances both thermostability and photostability of CoQ10, whereas the unencapsulated compound is highly vulnerable to external conditions and rapidly degraded [69].
Similarly, certain vitamins are of particular interest in the cosmetic industry, notably vitamin E [55], which is a key scavenger of free radicals. Vitamin E has been successfully encapsulated in various CDs, including γ-CD [55], HP-β-CD [71], and large-ring (26 glucose units) CDs [72,73].

Table 2.
CDs used to encapsulate several bioactive ingredients of different natures in the cosmetic industry and effects observed upon encapsulation.
Table 2.
CDs used to encapsulate several bioactive ingredients of different natures in the cosmetic industry and effects observed upon encapsulation.
| Bioactive Ingredient | Encapsulating Agent | Effects Observed upon Encapsulation | Reference |
|---|---|---|---|
| Stilbenes (resveratrol, piceatannol, oxyreseratrol, pinostibene) | α-CD β-CD HP-β-CD M-β-CD | Protection from light and heat degradation. Protection from oxidation and enzymatic hydrolysis | [5,50,59] |
| Ferulic acid | α-CD HP-γ-CD | Protection from light degradation Protection from oxidation Increase solubility Enhance UV absorption capacity | [62] |
| Citronellal oil and other essential oils | β-CD HP-β-CD | Protection from degradation Avoid the evaporation | [65,66] |
| Curcumin | β-CD | Increase in stability Improvement of bioavailability and bioaccessibility Increase transdermal permeation | [67] |
| CoQ10 | β-CD γ-CD | Enhances thermostability and photostability | [68,69,70] |
| Essential oils (vanillin, geraniol, jasmon, rose oil, apple fragrance, rosemary oil) | β-CD | Improve stability and solubility Control of odour release | [74,75,76,77,78,79] |
Novel materials such as polymer-free electrospun cyclodextrin-based membranes have also been used for the encapsulation of many of these bioactive ingredients of cosmetic interest, especially essential oils. Geraniol, for example, was included in electrospun HP-γ-CD membranes, while limonene, vanillin and menthol were encapsulated in electrospun HP-β-CD membranes. The use of these delivery systems increases the shelf life of the compounds, as well as their solubility, stability and availability, while also controlling their release. However, these promising systems for the development of new commercial products require further study to improve their industrialisation and employability [80].
3.2. Cyclodextrins and Their Delivery and Release Ability
When applying bioactive compounds topically, it is important to consider that the skin functions as a natural barrier, preventing the absorption of most compounds and limiting penetration through the stratum corneum [81]. The bioavailability and permeability of many bioactive compounds are therefore very low; some are highly volatile, and their limited solubility further hampers their effective incorporation into cosmetic formulations. To overcome this obstacle, CDs are valuable tools, not only for protecting guest molecules from degradation but also for enhancing their physicochemical properties [82]. Many relevant plant secondary metabolites, including terpenoids, phenylpropanoids, and alkaloids, are of a hydrophobic nature [83], and these compounds constitute the primary source of bioactive ingredients in the food and cosmetic industries [52].
CDs increase the penetration and permeation of bioactive compounds by forming hydrophilic inclusion complexes capable of crossing the stratum corneum via transappendageal diffusion [8]. The formation of these inclusion complexes is transient and reversible, allowing the guest molecule to dissociate from the binary complex and, due to its low molecular weight, diffuse through the skin, while the binary complex acts as a reservoir [8]. This mechanism facilitates controlled release and gradual absorption of the bioactive compound.
There are additional advantages to using CDs as transdermal delivery vehicles for bioactive ingredients, as they typically do not interfere with the skin and, owing to their high molecular weight, are poorly absorbed, with the majority remaining on the skin surface [8]. However, it should be noted that modified CDs, such as randomly methylated β-CDs, exhibit more lipophilic behaviour and can interact more closely with skin components, potentially affecting skin permeability [84].
Using curcumin as an example, due to its anti-inflammatory and antioxidant activities, in vivo studies often report limited effects. This is primarily due to the poor bioavailability of curcuminoids, which are poorly absorbed and rapidly metabolised and excreted [85]. CDs can address both the stability and bioavailability challenges, enhancing the bioaccessibility of curcuminoids, including curcumin, demethoxycurcumin, and bisdemethoxycurcumin [86]. Further studies demonstrated that binary complexes of curcumin and HP-β-CD at a drug:CD ratio of 1:2 (w/w) increased transdermal permeation in rats, thereby improving in vivo efficacy [87].
Ferulic acid has previously been described as a photoprotective compound employed in sunscreens, and its encapsulation in α-CD has also been mentioned [61]. Further studies have investigated the encapsulation of ferulic acid and other natural UV absorbers to enhance their water solubility, as the solubility of natural UV absorbers is generally much lower than that of synthetic molecules such as octylmethoxycinnamate and avobenzone [88]. In this context, a range of natural UV absorbers (ferulic acid, alloxazine, luteolin, rutin, apigenin and rhoifolin) was tested for encapsulation in α-CD, M-β-CD, HP-β-CD, γ-CD and HP-γ-CD. The results demonstrated that ferulic acid, alloxazine, and rutin were able to form inclusion complexes with some of the CDs examined [88]. The same study further showed that HP-γ-CD formed inclusion complexes with ferulic acid in a drug:CD ratio of 2:1, and that encapsulation not only increased the solubility of the compound but also induced a bathochromic shift, enhancing its UV absorption capacity.
3.3. Polymeric Cyclodextrins and Their Benefits
In recent years, cyclodextrin-based polymers and cyclodextrin-containing polymers have opened the door to new strategies for the administration, protection, and controlled release of bioactive ingredients in cosmetics [89,90]. These systems combine the host-guest complexation capabilities of CDs with the structural and functional properties of additional polymers, resulting in enhanced performance and consumer benefits in formulations such as creams, gels, and serums.
The three-dimensional structure of cyclodextrin-based polymers confers several advantages over monomeric CDs [89,91]:
- Increased loading capacity: the polymer network can encapsulate larger quantities of bioactive compounds compared to individual CDs.
- Prolonged release profile: encapsulation within a polymer matrix facilitates sustained release, ensuring extended efficacy.
- Improved solubility and stability: the polymer matrix enhances the solubility of hydrophobic molecules and protects them from degradation caused by light, heat, or oxidation.
On the other hand, CD compositions combined with other polymers, such as hyaluronic acid, xanthan gum, or chitosan, exploit the complementary properties of CDs and the added polymers, providing greater stability, enhanced permeability, and synergistic effects arising from the combination of different materials [92,93].
Several strategies involving CD-based polymers have been reported in recent years. One example is the use of cyclodextrin polypseudorotaxanes for nail lacquers [94], in which β-CD is combined with poloxamer to form a polypseudorotaxane for the delivery of ciclopirox olamine and clobetasol propionate. Moreover, hydro-ethanolic mixtures containing over 50% ethanol, in combination with lower concentrations of poloxamer, accelerated lacquer drying by reducing stickiness and promoting efficient film formation on the nail surface. Interestingly, hydro-ethanolic vehicles also enhanced the penetration of both drugs. A second example is the transdermal delivery of carvedilol using cyclodextrin-based poly(pseudo)rotaxanes. Carvedilol was dispersed in (i) Soluplus® (polyvinyl caprolactam-polyvinyl acetate-polyethylene glycol graft copolymer (PCL-PVAc-PEG), molar mass 115,000 g/mol; and (ii) inSolutol® HS 15, a mixture of PEG15 mono- and di-esters of 12-hydroxystearic acid and free PEG in 70/30 ratio; in combination with α-CD or HPβ-CD. The last one, was able to form complexes with the drug and modulate the release when the complex was prepared by spray-drying [95]. Another notable case is the use of a β-CD nanosponge hydrogel for the delivery of imiquimod. Although this was a preliminary study without a complete cosmetic formulation, the addition of the polymer resulted in a slower release compared to commercial CDs [96].
Recently, the same research group developed a melatonin-based cream incorporating imprinted and non-imprinted nanosponges (MIP-NS, a polymer with a designed cavity to host melatonin). The formulation consisted of an oil-in-water emulsion, into which both polymer types containing melatonin were introduced after cream preparation at room temperature under vigorous mixing. As anticipated, the release from imprinted nanosponges was more efficient compared to conventional nanosponges. All formulations exhibited stability during centrifugation tests, showing no signs of phase separation, precipitation, or syneresis. Regarding spreadability, the cream containing MIP-NSs displayed reduced spreadability due to its higher viscosity, which may slightly hinder ease of application. The MIP-NS formulation also demonstrated the highest viscosity overall. While this could benefit the controlled release of the drug, it might compromise the user’s application experience [97].
Several examples of composite systems have been reported in the literature. One study investigated the complexation of pterostilbene/HP-βCD complexes dispersed in pullulan fibres by electrospinning to develop a facial mask [98]. The results demonstrated improved release and water retention capacities, with preliminary indications of biosafety. Another study reported the formation of a nanogel for the delivery of bioactive ingredients in cosmetics using γ-CD/chitosan/fucoidan formulation [99]. The results indicated enhanced skin hydration and protection of the bioactive compound through the formation of an inclusion complex with cyclodextrin.
Curcumin is one of the molecules whose encapsulation and controlled release in CD polymers has also been investigated. This compound has been incorporated into epichlorohydrin-β-CD polymers to develop curcumin-containing hydrogels [19], resulting in formulations that significantly increase the water solubility of curcumin, besides reducing the particle size, potentially facilitating skin permeation. Furthermore, the incorporation of curcumin into CD coordination frameworks with metal ions (CD-MOFs) has also been explored, demonstrating that CD-MOFs markedly enhance curcumin solubility and extend its release profile [100]. CD-MOFs have also been used to encapsulate resveratrol, specifically within CD-MOFs/chitosan nanocapsules [101]. Encapsulation within these nanocapsules extends the resveratrol release profile, with maximum release observed at 24 h, whereas most free resveratrol is released within 6 h. Additionally, the nanocapsules substantially increase the solubility of resveratrol and reduce particle size, further improving its delivery properties.
Recently, an important real-world example of the application of CD-MOFs in cosmetics is provided by Noble Panacea®, the luxury skincare brand co-founded by Sir Fraser Stoddart (Nobel Laureate in Chemistry). This brand uses a proprietary formulation based on γ-cyclodextrin metal–organic frameworks (γ-CD-MOFs)—in which γ-cyclodextrin acts as the organic ligand forming a porous framework around alkali-metal nodes (as first described by Stoddart and co-workers) [90].
In the context of the cosmetic product, the CD component functions both as a host cavity for entrapping key bio-active molecules (enabling increased solubility, protection and controlled release) and as part of the structural MOF network which enhances delivery into the skin environment and prolongs the effect. The commercial name of the term is Organic Super Molecular Vessel™ (OSMV™) [102]. Publicly available information indicates that the MOF technology is integrated into the brand’s formulations, though exact concentrations are not fully disclosed in peer-reviewed literature. Although, in their patent we can find the next quote in line 171: <<Typical loading ratios range from about 0.1 mg to about 0.6 mg of the active ingredient per 1mg of OSMVTM >> [102].
The inclusion of such CD-MOF technology in a commercial cosmetic line underscores the translational relevance of polymeric cyclodextrin and cyclodextrin-MOF systems: bridging advanced host–guest and porous material chemistry with consumer-oriented formulations.
4. Permanence of Fragrances and Off-Odours Masking
Fragrances, or aromatic substances, are organic molecules characterised by distinctive and generally pleasant odours. They are extensively used in perfumes, scented cosmetics, gels, shampoos, and other household products, where fragrances can also serve to mask unpleasant odours [103].
CDs can be employed to enhance the longevity of fragrances and improve odour masking in cosmetic formulations. They can increase the solubility of odour molecules, prevent their degradation, reduce their high volatility [8], and mask undesirable odours from other formulation components or from the body itself [10,54]. Several examples of these applications are detailed below:
4.1. Complexation of Essential Oils to Enhance Their Aroma
Essential oils are mixtures of volatile secondary metabolites synthesised by a wide variety of aromatic plants. They possess distinctive odours and exhibit diverse bioactive properties, including antimicrobial, anticancer, anti-ageing, antioxidant, and insect-repellent activities [104]. More than 300 types of essential oils have industrial applications. These oils are safe for ingestion, inhalation, and topical use at common doses, provided they are in solution, which explains their widespread incorporation into cosmetic formulations. As previously mentioned, essential oils are poorly soluble in aqueous solutions, unstable, and readily degraded by temperature, light, oxidation, or volatilisation, which greatly limits their use [103]. To overcome these limitations, numerous studies have employed CDs to encapsulate and protect essential oils and their aromas [60,88]. Furthermore, encapsulation in CDs increases the energy required for volatilisation, resulting in slower and longer-lasting fragrance release [10].
Some volatile aroma encapsulated in β-CD include vanillin [74], geraniol [75], jasmone [76], rose oil [79], apple fragrance [78], rosemary oil [77], and linalool (also encapsulated in HP-β-CD) [105], among others. Encapsulation in CDs enables controlled odour release, facilitates conversion of liquid to solid forms, and improves both the stability and solubility of these compounds [106].
4.2. Complexation of Other Cosmetic Ingredients to Mask Their Odour
Dihydroxyacetone (DHA) is used in cosmetics as a sunless tanning agent. DHA is a ketotriose monosaccharide, commercially obtained through the microbial fermentation of glycerol by Gluconobacter oxydans. When applied topically, DHA penetrates and accumulates in the outermost layer of the skin, where it reacts to form melanoidins, brown pigments [107]. This compound is unstable in aqueous solutions and possesses a strong yeast odour difficult to mask with conventional fragrances. Complexation of DHA with CDs can effectively mask this odour. In addition, the slow, controlled release of DHA from CDs results in a more uniform tan. Such formulations have already been commercialised [5].
Glutathione is widely used in cosmetics owing to its potent antioxidant activity, playing a role in the defence against oxidative stress and in redox signalling. It is a tripeptide composed of cysteine, glutamic acid, and glycine, and therefore contains a thiol group. Glutathione is utilised in antioxidant creams, as an anti-aging agent, and to inhibit melanin formation, thereby reducing skin blemishes [108]. Despite its applications, glutathione has an unpleasant odour due to the sulphur in its structure. The glutathione-CD complex is odourless and retains the same biological effects as free glutathione [5,109].
Finally, tea tree oil, extracted from Melaleuca alternifolia, exhibits antimicrobial activity against bacteria, viruses, moulds, and yeasts, and is therefore used in cosmetics for the treatment of acne, athlete’s foot, nail fungus, and mosquito bites [110]. However, its high volatility, low thermostability, and susceptibility to oxidation limit its practical use. In addition, its odour is pungent, strong, harsh and camphoraceous. Encapsulation of tea tree oil with β-CD enhances its stability, masks its odour, and preserves its bioactive properties [111].
4.3. Free Cyclodextrins Used in Cosmetics for Masking Body Odours
Some cosmetic and personal-care products may also contain free CDs to mask body odours, for example, deodorants, shampoos, gels, and diapers.
Deodorants and antiperspirants are employed to neutralise unpleasant odours produced by microbial degradation of sweat. CDs, or mixtures thereof, can be incorporated into deodorants to trap perspiration-related odours. Currently, deodorants with β-CD or HP-β-CD are commercially available [8]. Somes commercial examples of deodorants containing CDs is Procter & Gamble’s Old Spice Clinical Sweat Defence Anti-Perspirant Deodorant [112], Secret’s Aluminium-free Deodorant [113] or Native Deodorant cucumber & mint [114]. CD powders can also be used in diapers, menstrual products, or paper towels to control odour [115].
5. Stabilization of Formulations
The incorporation of CDs into various cosmetic formulations may alter their physical properties, including changes in solubility, texture, viscosity, etc.
In semi-solid formulations, such as creams or gels, the addition of CDs (such as β-CD or M-β-CD) generally leads to a decrease in viscosity. Some authors have suggested that this phenomenon may result from interactions between hydrophobic and CDs, forming ternary complexes. Conversely, the addition of γ-CD can induce a slight increase in viscosity [116]. In addition, emulsions can be stabilised with CDs without using any surface-active agent [117].
CDs can also be employed to convert liquid substances into solid forms. Regardless of the natural state of the guest molecule (gas, liquid, or solid), the resulting inclusion complexes are invariably solid. Stable, water-soluble powders are easier to handle than highly volatile molecules or oils that are insoluble in aqueous solutions [117]. This property underpins the use of CDs in dry shampoos, where they trap sebum and other residues in the hair, forming solid particles that can be removed by brushing. HP-β-CD, for instance, is capable of absorbing grease and dirt, thereby extending the interval between hair washes [54]. A commercial example is FoxyBae®’s Dirty Gal dry shampoo [118].
In addition, cyclodextrins can be added to some cosmetics to act as pearlescent agents. They can be included in shampoos, conditioners, or creams to add shine to hair or skin. Generally, crystals of pearlescent materials or long-chain ester derivatives capable of reflecting light are used for this purpose. However, these products may vary in viscosity, affecting the final appearance of the product by separating into two layers or leaving the hair feeling greasy. The use of cyclodextrins or their derivatives can stabilise the pearling effect of the mixture, as well as stabilising the viscosity of the composition or homogenising it [119,120].
6. Cyclodextrins in the Development of Dermatocosmetic Formulations
Currently, the line that separates the cosmetics industry from the dermopharmaceutical industry is becoming increasingly blurred. Many newly launched formulations contain bioactive compounds intended to treat specific skin conditions, such as psoriasis, acne, or rosacea. However, these formulations are freely available on the market and are not classified as drugs despite their uses. Therefore, these compounds are regulated by cosmetics standards and not by the stricter standards for drugs. As a result, it is increasingly common to find facial routines that include retinoic acid, azelaic acid or vitamin D among their ingredients. In addition, these bioactive compounds are often poorly soluble in water and prone to degradation, making their incorporation into cyclodextrins (CDs) beneficial for protection. An illustrative example is tretinoin, used for the treatment of psoriasis and acne. This compound is photolabile, undergoing approximately 40% degradation within 4 h of topical application. Encapsulation within the β-CD cavity can effectively mitigate this degradation [54].
Similarly, the encapsulation of vitamin D analogues in SBE-β-CD for psoriasis treatment has been shown to increase the apparent solubility of the active compound while reducing skin irritation [54]. Eugenol or quercetin encapsulated in HP-β-CD have also been employed for this purpose, providing sustained release and prolonged activity compared with the free compound [121,122].
Retinoic acid and azelaic acid, both used for the treatment of acne vulgaris, have likewise been encapsulated (retinoic acid in β-CD, azelaic acid in HP-β-CD and SBE-β-CD), thereby enhancing their stability, solubility, controlled release, and skin tolerance, while simultaneously reducing erythema and irritation [123,124].
7. Skin Irritability and Toxicity
In vitro and in vivo studies have demonstrated that CDs authorised for use in cosmetics are generally non-irritating to the skin, eyes, and mucous membranes. However, CDs can interact with certain cell membrane components, such as phospholipids, cholesterol, and some proteins. While this interaction may transiently weaken the skin barrier, it can also enhance the permeability of co-applied bioactive compounds [116,125,126].
Some bioactive compounds used in cosmetics can induce irritation at the application site, causing burning, erythema, or desquamation. Encapsulation of such compounds in CDs offers a solution. The formation of cyclodextrin–guest inclusion complexes provides a protective environment for the bioactive compound, resulting in reduced irritation and increased solubility [2]. For instance, terpenes are susceptible to light- and oxidation-induced degradation, producing p-cymene, which can cause skin irritation. Encapsulation in β-CD mitigates the formation of these undesirable derivatives, thereby reducing irritation while maintaining the bioactive properties of the terpenes [54]. The use of CDs not only decreases irritancy but also increases the concentration and efficacy of bioactive compounds at the site of application [64]. Another example is retinoic acid, whose complexation with β-CD significantly reduces the incidence of topical side effects [54,116,127].
Additionally, essential oils and many bioactive compounds are of a hydrophobic nature and require solubilisation in organic solvents, such as ethanol, for incorporation into cosmetic formulations. These solvents can be irritant and are prone to rapid evaporation. The use of CDs can reduce or eliminate the need for organic solvents, as the resulting inclusion complexes are water-soluble. For example, tretinoin, used in acne treatment, requires 60% ethanol for solubilisation, whereas the tretinoin-β-CD complex is soluble in water [116].
8. Conclusions and Future Perspectives
As demonstrated throughout this review, the use of CDs to encapsulate a wide range of bioactive molecules makes a significant contribution to the cosmetics industry, in some cases becoming essential for the successful formulation of new products. CDs are highly valuable not only for protecting bioactive ingredients from degradation by external factors but also for improving their physicochemical properties, including water solubility, volatility, and permeability, thereby enhancing their delivery. CDs can facilitate the passage of bioactive compounds through the stratum corneum while acting as a reservoir, prolonging the release profile of the encapsulated molecules. They can also be used to preserve fragrances and mask undesirable odours, enhancing their performance in lotions, creams, and perfumes. Being biocompatible and safe, CDs can modify and stabilise formulations, transforming liquid or gaseous molecules into solid inclusion complexes. Moreover, CDs can be employed alone to retain body odours or sebum, demonstrating their versatility as nanomaterials in dermofacial and cosmetic applications.
Although some formulations currently incorporate CDs among their ingredients, the prevalence of such products is expected to increase as consumer awareness grows and further research is conducted. This trend may also facilitate the development of novel cosmetic products with pharmacological activities, offering improved delivery, protection, and preservation of bioactive properties.
The use of cyclodextrins or their polymeric derivatives could result in new cosmetic formulations with novel properties, allowing for even more specific applications, such as the treatment of skin conditions like psoriasis or acne, bringing cosmetics closer to the therapeutic field. However, further studies are needed to develop new formulations that include CDs or their new emerging derivatives among their ingredients. Each CD possesses distinct physicochemical properties, making it suitable for encapsulating specific molecules. Collaboration among research centres, regulatory agencies, and industry is essential to advance preclinical research and develop optimised topical formulations that are both effective and safe for consumers. Overall, the applications of CDs in cosmetics represent a significant advancement, driving the development of innovative and improved products.
Author Contributions
Conceptualization, I.C. and F.J.V.-S.; writing—original draft preparation, I.C., F.J.V.-S., S.N.-O., C.A.-S. and A.M.; writing—review and editing, I.C. and F.J.V.-S.; supervision, I.C. and F.J.V.-S.; project administration, J.M.L.-N.; funding acquisition, J.M.L.-N. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Spanish Ministry of Science and Innovation, project PID2021-122896NB-I00 (MCI/AEI/10.13039/501100011033/FEDER, UE). This work is the result of a predoctoral contract for the training of research staff (for Silvia Navarro-Orcajada, number 21269/FPI/19) financed by the Fundación Séneca (Región de Murcia, Spain), a predoctoral contract (for Irene Conesa) financed by the University of Murcia (Región de Murcia, Spain), a predoctoral contract (for Francisco José Vidal-Sánchez, number FPU21/03503) financed by the Ministry of Universities (Spain) and a Ramon y Cajal Contract funded by MICIU/AEI/10.13039/501100011033 and by the FSE+ (for Adrián Matencio, Grant RYC2023-043196-I).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Crini, G.; Fourmentin, S.; Fenyvesi, É.; Torri, G.; Fourmentin, M.; Morin-Crini, N. Fundamentals and applications of cyclodextrins. In Cyclodextrin Fundamentals, Reactivity and Analysis; Springer Nature: Berlin, Germany, 2018; pp. 1–55. [Google Scholar]
- Dhiman, P.; Bhatia, M. Pharmaceutical applications of cyclodextrins and their derivatives. J. Incl. Phenom. Macrocycl. Chem. 2020, 98, 171–186. [Google Scholar] [CrossRef]
- Bezerra, F.M.; Lis, M.J.; Firmino, H.B.; da Silva, J.G.D.; Valle, R.d.C.S.C.; Valle, J.A.B.; Scacchetti, F.A.P.; Tessaro, A.L. The Role of β-Cyclodextrin in the Textile Industry—Review. Molecules 2020, 25, 3624. [Google Scholar] [CrossRef]
- Matencio, A.; Navarro-Orcajada, S.; Garcia-Carmona, F.; López-Nicolás, J.M. Applications of cyclodextrins in food science. A review. Trends Food Sci. Technol. 2020, 104, 132–143. [Google Scholar] [CrossRef]
- Buschmann, H.-J.; Schollmeyer, E. Applications of cyclodextrins in cosmetic products: A review. J. Cosmet. Sci. 2002, 53, 185–192. [Google Scholar] [PubMed]
- Poulson, B.G.; Alsulami, Q.A.; Sharfalddin, A.; El Agammy, E.F.; Mouffouk, F.; Emwas, A.-H.; Jaremko, L.; Jaremko, M. Cyclodextrins: Structural, Chemical, and Physical Properties, and Applications. Polysaccharides 2022, 3, 1–31. [Google Scholar] [CrossRef]
- Upadhyay, D.; Sharma, S.; Shrivastava, D.; Kulshreshtha, N.M. Production and characterization of β-cyclodextrin glucanotransferase from Bacillus sp. ND1. J. Basic Microbiol. 2019, 59, 192–205. [Google Scholar] [CrossRef] [PubMed]
- Braga, S.S.; Pais, J. Chapter 10—Getting under the skin: Cyclodextrin inclusion for the controlled delivery of active substances to the dermis. In Design of Nanostructures for Versatile Therapeutic Applications; William Andrew: Norwich, NY, USA, 2018; pp. 407–449. [Google Scholar]
- Matencio, A.; Pedrazzo, A.R.; Difalco, A.; Navarro-Orcajada, S.; Monfared, Y.K.; Conesa, I.; Rezayat, A.; López-Nicolás, J.M.; Trotta, F. Advances and Classification of Cyclodextrin-Based Polymers for Food-Related Issues. Polymers 2021, 13, 4226. [Google Scholar] [CrossRef]
- Del Valle, E.M.M. Cyclodextrins and their uses: A review. Process Biochem. 2004, 39, 1033–1046. [Google Scholar] [CrossRef]
- Gonzalez Pereira, A.; Carpena, M.; García Oliveira, P.; Mejuto, J.C.; Prieto, M.A.; Simal Gandara, J. Main Applications of Cyclodextrins in the Food Industry as the Compounds of Choice to Form Host–Guest Complexes. Int. J. Mol. Sci. 2021, 22, 1339. [Google Scholar] [CrossRef]
- FDA. Federal Food, Drug, and Cosmetic Act (FD&C Act). 2018. Available online: https://www.fda.gov/regulatory-information/laws-enforced-fda/federal-food-drug-and-cosmetic-act-fdc-act (accessed on 17 September 2023).
- FDA. Is It a Cosmetic, a Drug, or Both? (Or Is It Soap?). 2022. Available online: https://www.fda.gov/cosmetics/cosmetics-laws-regulations/it-cosmetic-drug-or-both-or-it-soap (accessed on 30 September 2025).
- FDA. Cosmetics & U.S. Law. 2024. Available online: https://www.fda.gov/cosmetics/cosmetics-laws-regulations/cosmetics-us-law (accessed on 29 September 2025).
- EMA/CHMP. Questions and Answers on Cyclodextrins Used as Excipients in Medicinal Products for Human Use; European Medicines Agency: London, UK, 2017. [Google Scholar]
- EMA/CHMP. Background Review for Cyclodextrins Used as Excipients; European Medicines Agency: London, UK, 2014. [Google Scholar]
- Braga, S.S. Cyclodextrins: Emerging Medicines of the New Millennium. Biomolecules 2019, 9, 801. [Google Scholar] [CrossRef]
- Matencio, A.; Hoti, G.; Monfared, Y.K.; Rezayat, A.; Pedrazzo, A.R.; Caldera, F.; Trotta, F. Cyclodextrin Monomers and Polymers for Drug Activity Enhancement. Polymers 2021, 13, 1684. [Google Scholar] [CrossRef]
- Fernández-Romero, A.-M.; Maestrelli, F.; García-Gil, S.; Talero, E.; Mura, P.; Rabasco, A.M.; González-Rodríguez, M.L. Preparation, Characterization and Evaluation of the Anti-Inflammatory Activity of Epichlorohydrin-β-Cyclodextrin/Curcumin Binary Systems Embedded in a Pluronic®/Hyaluronate Hydrogel. Int. J. Mol. Sci. 2021, 22, 13566. [Google Scholar] [CrossRef]
- Rao, M.R.P.; Chaudhari, J.; Trotta, F.; Caldera, F. Investigation of Cyclodextrin-Based Nanosponges for Solubility and Bioavailability Enhancement of Rilpivirine. AAPS PharmSciTech. 2018, 19, 2358–2369. [Google Scholar] [CrossRef]
- Nicolazzi, C.; Venard, V.; Le Faou, A.; Finance, C. In vitro antiviral efficacy of the ganciclovir complexed with be-ta-cyclodextrin on human cytomegalovirus clinical strains. Antivir. Res. 2002, 54, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.H.; Park, J. Encapsulation of flavors by molecular inclusion using β-cyclodextrin: Comparison with spray-drying process using carbohydrate-based wall materials. Food Sci. Biotechnol. 2009, 18, 185–189. [Google Scholar]
- Attoui-Yahia, O.; Khatmi, D.; Kraim, K.; Ferkous, F. Hydrogen bonding investigation in Pyridoxine/β-cyclodextrin complex based on QTAIM and NBO approaches. J. Taiwan Inst. Chem. Eng. 2015, 47, 91–98. [Google Scholar] [CrossRef]
- Köhler, J.E.H.; Grczelschak-Mick, N. The β-cyclodextrin/benzene complex and its hydrogen bonds—A theoretical study using molecular dynamics, quantum mechanics and COSMO-RS. Beilstein J. Org. Chem. 2013, 9, 118–134. [Google Scholar] [CrossRef] [PubMed]
- Matencio, A.; García-Carmona, F.; López-Nicolás, J.M. Encapsulation of piceatannol, a naturally occurring hydroxylated analogue of resveratrol, by natural and modified cyclodextrins. Food Funct. 2016, 7, 2367–2373. [Google Scholar] [CrossRef]
- Navarro-Orcajada, S.; Conesa, I.; Matencio, A.; García-Carmona, F.; López-Nicolás, J.M. Molecular encapsulation and bioactivity of gnetol, a resveratrol analogue, for use in foods. J. Sci. Food Agric. 2022, 102, 4296–4303. [Google Scholar] [CrossRef] [PubMed]
- Fourmentin, S.; Ciobanu, A.; Landy, D.; Wenz, G. Space filling of β-cyclodextrin and β-cyclodextrin derivatives by volatile hydrophobic guests. Beilstein J. Org. Chem. 2013, 9, 1185–1191. [Google Scholar] [CrossRef]
- López-Nicolás, J.M.; Rodríguez-Bonilla, P.; García-Carmona, F. Complexation of Pinosylvin, an Analogue of Resveratrol with High Antifungal and Antimicrobial Activity, by Different Types of Cyclodextrins. J. Agric. Food Chem. 2009, 57, 10175–10180. [Google Scholar] [CrossRef]
- He, J.; Zheng, Z.-P.; Zhu, Q.; Guo, F.; Chen, J. Encapsulation Mechanism of Oxyresveratrol by β-Cyclodextrin and Hydroxypropyl-β-Cyclodextrin and Computational Analysis. Molecules 2017, 22, 1801. [Google Scholar] [CrossRef]
- Conesa, I.; Navarro-Orcajada, S.; Vidal-Sánchez, F.J.; Torralba-Antón, E.; Carrión-Espinosa, M.; Matencio, A.; López-Nicolás, J.M. Pinostilbene as a Potential Cytotoxic Agent in Cancer Cell Lines: Improvement of Solubility and Stability by Cyclodextrin Encapsulation. Pharmaceutics 2025, 17, 1219. [Google Scholar] [CrossRef]
- Decock, G.; Fourmentin, S.; Surpateanu, G.G.; Landy, D.; Decock, P.; Surpateanu, G. Experimental and Theoretical Study on the Inclusion Compounds of Aroma Components with β-Cyclodextrins. Supramol. Chem. 2006, 18, 477–482. [Google Scholar] [CrossRef]
- Rodríguez-Bonilla, P.; López-Nicolás, J.M.; García-Carmona, F. Use of reversed phase high pressure liquid cromatography for the physicochemical and thermodynamic characterization of oxyresveratrol/β-cyclodextrin complexes. J. Chromatogr. B 2010, 878, 1569–1575. [Google Scholar] [CrossRef] [PubMed]
- Matencio, A.; García-Carmona, F.; López-Nicolás, J.M. The inclusion complex of oxyresveratrol in modified cyclodextrins: A thermodynamic, structural, physicochemical, fluorescent and computational study. Food Chem. 2017, 232, 177–184. [Google Scholar] [CrossRef]
- De Gaetano, F.; Margani, F.; Barbera, V.; D’Angelo, V.; Germanò, M.P.; Pistarà, V.; Ventura, C.A. Characterization and In Vivo Antiangiogenic Activity Evaluation of Morin-Based Cyclodextrin Inclusion Complexes. Pharmaceutics 2023, 15, 2209. [Google Scholar] [CrossRef] [PubMed]
- Al Omari, M.M.; Zughul, M.B.; Davies, J.E.D.; Badwan, A.A. Sildenafil/cyclodextrin complexation: Stability constants, thermodynamics, and guest–host interactions probed by 1H NMR and molecular modeling studies. J. Pharm. Biomed. Anal. 2006, 41, 857–865. [Google Scholar] [CrossRef]
- Li, T.; Guo, R.; Zong, Q.; Ling, G. Application of molecular docking in elaborating molecular mechanisms and interactions of supramolecular cyclodextrin. Carbohydr. Polym. 2022, 276, 118644. [Google Scholar] [CrossRef] [PubMed]
- (2-Hidroxipropil)-β-Ciclodextrina Powder, BioReagent, Suitable for Cell Culture|Sigma-Aldrich. Available online: https://www.sigmaaldrich.com/ES/es/product/sigma/c0926?srsltid=AfmBOoob1cysKGkB2_O_oKp2X_Y8GRTI8oxj0dzXoKKsI4SIg0m5TqJc (accessed on 29 September 2025).
- Pharma Virtual Lab by Roquette. KLEPTOSE HP Oral grade. Available online: https://www.roquette.com/innovation-hub/pharma/product-profile-pages/kleptose-hp-oral-grade (accessed on 27 October 2025).
- CYCLOLAB. Available online: https://cyclolab.hu/products/pharma_grade_cyclodextrins-c8/2hydroxypropylbetacyclodextrin_ds45-p42/ (accessed on 27 October 2025).
- PubChem. Methyl Beta-Cyclodextrin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/51051622 (accessed on 29 September 2025).
- Metil-β-Ciclodextrina Powder, BioReagent, Suitable for Cell Culture|Sigma-Aldrich. Available online: https://www.sigmaaldrich.com/ES/es/product/sigma/c4555?srsltid=AfmBOoowPnSMuGGvK8GijNWGzEat46qqI6oubW9q1syrfyJ-tfDU_LJL&utm_source=chatgpt.com (accessed on 29 September 2025).
- PubChem. Hydroxypropyl-Gamma-Cyclodextrin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/2733545 (accessed on 30 September 2025).
- (2-Hidroxipropil)-Gamma-Ciclodextrina, HPGCDCiclodextrina-Shop. Available online: https://www.cyclodextrin-shop.com/product/2-hydroxypropyl-gamma-cyclodextrin-hpgcd/?utm_source=chatgpt.com (accessed on 30 September 2025).
- CYCLOLAB. Available online: https://cyclolab.hu/products/research_grade_cyclodextrins-c23/2hydroxypropylgammacyclodextrin_ds45-p51/ (accessed on 27 October 2025).
- PubChem. (2-Hydroxyethyl)-b-Cyclodextrin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/75412567 (accessed on 30 September 2025).
- Stopilha, R.T.; Xavier-Júnior, F.H.; De Vasconcelos, C.L.; Fonseca, J.L.C. Carboxymethylated-β-cyclodextrin/chitosan particles: Bulk solids and aqueous dispersions. J. Dispers. Sci. Technol. 2020, 41, 717–724. [Google Scholar] [CrossRef]
- SBE-β-CD (Sulfobutylether-Beta-Cyclodextrin). Available online: https://www.glpbio.com/sp/sbe-beta-cd-sulfobutylether-beta-cyclodextrin.html (accessed on 27 October 2025).
- Bharadvaja, N.; Gautam, S.; Singh, H. Natural polyphenols: A promising bioactive compounds for skin care and cosmetics. Mol. Biol. Rep. 2023, 50, 1817–1828. [Google Scholar] [CrossRef]
- De Lima Cherubim, D.J.; Buzanello Martins, C.V.; Oliveira Fariña, L.; Da Silva De Lucca, R.A. Polyphenols as natural antioxidants in cosmetics applications. J. Cosmet. Dermatol. 2020, 19, 33–37. [Google Scholar] [CrossRef]
- Navarro-Orcajada, S.; Conesa, I.; Vidal-Sánchez, F.J.; Matencio, A.; Albaladejo-Maricó, L.; García-Carmona, F.; López-Nicolás, J.M. Stilbenes: Characterization, bioactivity, encapsulation and structural modifications. A review of their current limitations and promising approaches. Crit. Rev. Food Sci. Nutr. 2023, 63, 7269–7287. [Google Scholar] [CrossRef]
- Ratz-Lyko, A.; Arct, J.; Pytkowska, K. Methods for evaluation of cosmetic antioxidant capacity. Skin. Res. Technol. 2012, 18, 421–430. [Google Scholar] [CrossRef]
- Neelam; Khatkar, A.; Sharma, K.K. Phenylpropanoids and its derivatives: Biological activities and its role in food, pharmaceutical and cosmetic industries. Crit. Rev. Food Sci. Nutr. 2020, 60, 2655–2675. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Orcajada, S.; Matencio, A.; Vicente-Herrero, C.; Garcia-Carmona, F.; López-Nicolás, J.M. Study of the fluorescence and interaction between cyclodextrins and neochlorogenic acid, in comparison with chlorogenic acid. Sci. Rep. 2021, 11, 3275. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.; Mascarenhas-Melo, F.; Rabaça, S.; Mathur, A.; Sharma, A.; Giram, P.S.; Pawar, K.D.; Rahdar, A.; Raza, F.; Veiga, F.; et al. Cyclodextrin-based dermatological formulations: Dermopharmaceutical and cosmetic applications. Colloids Surf. B Biointerfaces 2023, 221, 113012. [Google Scholar] [CrossRef] [PubMed]
- López-Nicolás, J.M.; Rodríguez-Bonilla, P.; García-Carmona, F. Cyclodextrins and Antioxidants. Crit. Rev. Food Sci. Nutr. 2014, 54, 251–276. Available online: https://www.tandfonline.com/doi/abs/10.1080/10408398.2011.582544 (accessed on 29 September 2025). [CrossRef]
- Tian, B.; Liu, J. Resveratrol: A review of plant sources, synthesis, stability, modification and food application. J. Sci. Food Agric. 2020, 100, 1392–1404. [Google Scholar] [CrossRef]
- Dhakar, N.K.; Matencio, A.; Caldera, F.; Argenziano, M.; Cavalli, R.; Dianzani, C.; Zanetti, M.; López-Nicolás, J.M.; Trotta, F. Comparative evaluation of solubility, cytotoxicity and photostability studies of resveratrol and oxyresveratrol loaded nanosponges. Pharmaceutics 2019, 11, 545. [Google Scholar] [CrossRef]
- Francioso, A.; Mastromarino, P.; Masci, A.; d’Erme, M.; Mosca, L. Chemistry, stability and bioavailability of resveratrol. Med. Chem. 2014, 10, 237–245. [Google Scholar] [CrossRef] [PubMed]
- López-Nicolás, J.M.; Núñez-Delicado, E.; Pérez-López, A.J.; Barrachina, Á.C.; Cuadra-Crespo, P. Determination of stoichiometric coefficients and apparent formation constants for β-cyclodextrin complexes of trans-resveratrol using reversed-phase liquid chromatography. J. Chromatogr. A 2006, 1135, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Popielec, A.; Loftsson, T. Effects of cyclodextrins on the chemical stability of drugs. Int. J. Pharm. 2017, 531, 532–542. [Google Scholar] [CrossRef]
- Centini, M.; Maggiore, M.; Casolaro, M.; Andreassi, M.; Maffei Facino, R.; Anselmi, C. Cyclodextrins as cosmetic delivery systems. J. Incl. Phenom. Macrocycl. Chem. 2007, 57, 109–112. [Google Scholar] [CrossRef]
- Peres, D.D.; Sarruf, F.D.; de Oliveira, C.A.; Velasco, M.V.R.; Baby, A.R. Ferulic acid photoprotective properties in association with UV filters: Multifunctional sunscreen with improved SPF and UVA-PF. J. Photochem. Photobiol. B 2018, 185, 46–49. [Google Scholar] [CrossRef]
- Navarro-Orcajada, S.; Vidal-Sánchez, F.J.; Conesa, I.; Matencio, A.; López-Nicolás, J.M. Improvement of the Physicochemical Limitations of Rhapontigenin, a Cytotoxic Analogue of Resveratrol against Colon Cancer. Biomolecules 2023, 13, 1270. [Google Scholar] [CrossRef]
- Kfoury, M.; Auezova, L.; Greige-Gerges, H.; Fourmentin, S. Encapsulation in cyclodextrins to widen the applications of essential oils. Environ. Chem. Lett. 2019, 17, 129–143. [Google Scholar] [CrossRef]
- Abril-Sánchez, C.; Matencio, A.; Navarro-Orcajada, S.; García-Carmona, F.; López-Nicolás, J.M. Evaluation of the properties of the essential oil citronellal nanoencapsulated by cyclodextrins. Chem. Phys. Lipids 2019, 219, 72–78. [Google Scholar] [CrossRef]
- Costa, P.; Medronho, B.; Gonçalves, S.; Romano, A. Cyclodextrins enhance the antioxidant activity of essential oils from three Lamiaceae species. Ind. Crops Prod. 2015, 70, 341–346. [Google Scholar] [CrossRef]
- Jiang, L.; Xia, N.; Wang, F.; Xie, C.; Ye, R.; Tang, H.; Zhang, H.; Liu, Y. Preparation and characterization of curcumin/β-cyclodextrin nanoparticles by nanoprecipitation to improve the stability and bioavailability of curcumin. LWT 2022, 171, 114149. [Google Scholar] [CrossRef]
- Knott, A.; Achterberg, V.; Smuda, C.; Mielke, H.; Sperling, G.; Dunckelmann, K.; Vogelsang, A.; Krüger, A.; Schwengler, H.; Behtash, M.; et al. Topical treatment with coenzyme Q 10-containing formulas improves skin’s Q 10 level and provides antioxidative effects. BioFactors 2015, 41, 383–390. [Google Scholar] [CrossRef]
- Fir, M.M.; Smidovnik, A.; Milivojevic, L.; Zmitek, J.; Prosek, M. Studies of CoQ10 and cyclodextrin complexes: Solubility, thermo- and photo-stability. J. Incl. Phenom. Macrocycl. Chem. 2009, 64, 225–232. [Google Scholar] [CrossRef]
- Šmidovnik, A.; Stražišar, M.; Jazbec, P.; Fir, M.M.; Prošek, M. Effect of Complexation Cyclodextrins with Phenolic Acids and Coenzyme Q 10 on their Physico-Chemical Properties and Bioavailability. Acta Chim. Slov. 2010, 57, 9–16. [Google Scholar]
- Celebioglu, A.; Uyar, T. Antioxidant Vitamin E/Cyclodextrin Inclusion Complex Electrospun Nanofibers: Enhanced Water Solubility, Prolonged Shelf Life, and Photostability of Vitamin E. J. Agric. Food Chem. 2017, 65, 5404–5412. [Google Scholar] [CrossRef] [PubMed]
- Kerdpol, K.; Nutho, B.; Krusong, K.; Poo-arporn, R.P.; Rungrotmongkol, T.; Hannongbua, S. Encapsulation of α-tocopherol in large-ring cyclodextrin containing 26 α-D-glucopyranose units: A molecular dynamics study. J. Mol. Liq. 2021, 339, 116802. [Google Scholar] [CrossRef]
- Sangkhawasi, M.; Kerdpol, K.; Ismail, A.; Nutho, B.; Hanpiboon, C.; Wolschann, P.; Krusong, K.; Rungrotmongkol, T.; Hannongbua, S. In vitro and in silico study on the molecular encapsulation of α-tocopherol in a large-ring cyclodextrin. Int. J. Mol. Sci. 2023, 24, 4425. [Google Scholar] [CrossRef] [PubMed]
- Hundre, S.Y.; Karthik, P.; Anandharamakrishnan, C. Effect of whey protein isolate and β-cyclodextrin wall systems on stability of microencapsulated vanillin by spray–freeze drying method. Food Chem. 2015, 174, 16–24. [Google Scholar] [CrossRef]
- Menezes, P.P.; Serafini, M.R.; Santana, B.V.; Nunes, R.S.; Quintans, L.J.; Silva, G.F.; Medeiros, I.A.; Marchioro, M.; Fraga, B.P.; Santos, M.R.V.; et al. Solid-state β-cyclodextrin complexes containing geraniol. Thermochim. Acta 2012, 548, 45–50. [Google Scholar] [CrossRef]
- Ai, L.; Hu, J.; Ji, X.; Zhao, H. Structure confirmation and thermal kinetics of the inclusion of cis-jasmone in β-cyclodextrin. RSC Adv. 2019, 9, 26224–26229. [Google Scholar] [CrossRef] [PubMed]
- Halahlah, A.; Kavetsou, E.; Pitterou, I.; Grigorakis, S.; Loupassaki, S.; Tziveleka, L.-A.; Kikionis, S.; Ioannou, E.; Detsi, A. Synthesis and characterization of inclusion complexes of rosemary essential oil with various β-cyclodextrins and evaluation of their antibacterial activity against Staphylococcus aureus. J. Drug Deliv. Sci. Technol. 2021, 65, 102660. [Google Scholar] [CrossRef]
- Niu, Y.; Deng, J.; Xiao, Z.; Kou, X.; Zhu, G.; Liu, M.; Liu, S. Preparation and slow release kinetics of apple fragrance/β-cyclodextrin inclusion complex. J. Therm. Anal. Calorim. 2021, 143, 3775–3781. [Google Scholar] [CrossRef]
- Hadian, Z.; Kamalabadi, M.; Phimolsiripol, Y.; Balasubramanian, B.; Rodriguez, J.M.L.; Khaneghah, A.M. Preparation, characterization, and antioxidant activity of β-cyclodextrin nanoparticles loaded Rosa damascena essential oil for application in beverage. Food Chem. 2023, 403, 134410. [Google Scholar] [CrossRef]
- Dodero, A.; Schlatter, G.; Hébraud, A.; Vicini, S.; Castellano, M. Polymer-free cyclodextrin and natural polymer-cyclodextrin electrospun nanofibers: A comprehensive review on current applications and future perspectives. Carbohydr. Polym. 2021, 264, 118042. [Google Scholar] [CrossRef]
- Yu, Y.-Q.; Yang, X.; Wu, X.-F.; Fan, Y.-B. Enhancing permeation of drug molecules across the skin via delivery in nanocarriers: Novel strategies for effective transdermal applications. Front. Bioeng. Biotechnol. 2021, 9, 646554. [Google Scholar] [CrossRef]
- Suvarna, P.; Chaudhari, P.; Lewis, S.A. Cyclodextrin-based supramolecular ternary complexes: Emerging role of ternary agents on drug solubility, stability, and bioavailability. Crit. Rev. Ther. Drug Carr. Syst. 2022, 39, 1–50. [Google Scholar] [CrossRef]
- Wink, M. Modes of action of herbal medicines and plant secondary metabolites. Medicines 2015, 2, 251–286. [Google Scholar] [CrossRef]
- De Paula, D.; Oliveira, D.C.R.; Tedesco, A.C.; Bentley, M.V.L.B. Enhancing effect of modified beta-cyclodextrins on in vitro skin permeation of estradiol. Rev. Bras. Ciênc. Farm. 2007, 43, 111–120. [Google Scholar] [CrossRef]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of Curcumin: Problems and Promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef]
- Borel, P.; Hammaz, F.; Lecourt, L.; Marconot, G.; Gillet, G.; Rozier, C.; Desmarchelier, C. The Incorporation of Curcuminoids in Gamma-Cyclodextrins Improves Their Poor Bioaccessibility, Which Is due to Both Their Very Low Incorporation into Mixed Micelles and Their Partial Adsorption on Food. Mol. Nutr. Food Res. 2023, 67, 2200798. [Google Scholar] [CrossRef] [PubMed]
- Ghanghoria, R.; Kesharwani, P.; Agashe, H.B.; Jain, N.K. Transdermal delivery of cyclodextrin-solubilized curcumin. Drug Deliv. Transl. Res. 2013, 3, 272–285. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; Tsuchiya, R.; Doi, M.; Nagatani, N.; Tanaka, T. Solubilization of ultraviolet absorbers by cyclodextrin and their potential application in cosmetics. J. Incl. Phenom. Macrocycl. Chem. 2019, 93, 91–96. [Google Scholar] [CrossRef]
- Braga, S.S. Cyclodextrin superstructures for drug delivery. J. Drug Deliv. Sci. Technol. 2022, 75, 103650. [Google Scholar] [CrossRef]
- Roy, I.; Stoddart, J.F. Cyclodextrin Metal–Organic Frameworks and Their Applications. Acc. Chem. Res. 2021, 54, 1440–1453. [Google Scholar] [CrossRef]
- Liu, Z.; Ye, L.; Xi, J.; Wang, J.; Feng, Z. Cyclodextrin polymers: Structure, synthesis, and use as drug carriers. Prog. Polym. Sci. 2021, 118, 101408. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, Z.; Guo, X.; Zhao, Y.; Ren, S.; Zhang, Z.; Lv, H. Hyaluronic acid-cyclodextrin encapsulating paeonol for treatment of atopic dermatitis. Int. J. Pharm. 2022, 623, 121916. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Wu, A.; Zhou, H.; Huang, Z.; Zang, H. Azelaic acid/β-cyclodextrin loaded hyaluronic acid-based dissolving microneedle for anti-acne application. Colloids Surf. Physicochem. Eng. Asp. 2025, 707, 135890. [Google Scholar] [CrossRef]
- Cutrín-Gómez, E.; Anguiano-Igea, S.; Delgado-Charro, M.B.; Gómez-Amoza, J.L.; Otero-Espinar, F.J. Effect on nail structure and transungual permeability of the ethanol and poloxamer ratio from cyclodextrin-soluble polypseudorotaxanes based nail lacquer. Pharmaceutics 2018, 10, 156. [Google Scholar] [CrossRef] [PubMed]
- Taveira, S.F.; Varela-Garcia, A.; dos Santos Souza, B.; Marreto, R.N.; Martin-Pastor, M.; Concheiro, A.; Alvarez-Lorenzo, C. Cyclodextrin-based poly(pseudo)rotaxanes for transdermal delivery of carvedilol. Carbohydr. Polym. 2018, 200, 278–288. [Google Scholar] [CrossRef]
- Argenziano, M.; Haimhoffer, A.; Bastiancich, C.; Jicsinszky, L.; Caldera, F.; Trotta, F.; Scutera, S.; Alotto, D.; Fumagalli, M.; Musso, T. In vitro enhanced skin permeation and retention of imiquimod loaded in β-cyclodextrin nanosponge hydrogel. Pharmaceutics 2019, 11, 138. [Google Scholar] [CrossRef]
- Hoti, G.; Ferrero, R.; Caldera, F.; Trotta, F.; Corno, M.; Pantaleone, S.; Desoky, M.M.; Brunella, V. A comparison between the molecularly imprinted and non-molecularly imprinted cyclodextrin-based nanosponges for the transdermal delivery of melatonin. Polymers 2023, 15, 1543. [Google Scholar] [CrossRef]
- Bao, Y.; Yang, D.; Liu, H.; Li, S.; Meng, H. Electrospun pullulan nanofibers containing pterostilbene-hydroxypropyl-β-cyclodextrin inclusion complex: Preparation and characterization. Int. J. Biol. Macromol. 2025, 309, 142978. [Google Scholar] [CrossRef]
- Choi, D.-I.; Ju, M.-K.; Park, S.-M.; Lee, S.-Y. Development of anti-aging cosmetics using chitosan/γ-cyclodextrin/fucoidan nanoparticles (nanogel) based on the drug delivery system. Asian J. Beauty Cosmetol. 2023, 21, 441–452. [Google Scholar] [CrossRef]
- Chen, Y.; Su, J.; Dong, W.; Xu, D.; Cheng, L.; Mao, L.; Gao, Y.; Yuan, F. Cyclodextrin-based metal–organic framework nanoparticles as superior carriers for curcumin: Study of encapsulation mechanism, solubility, release kinetics, and antioxidative stability. Food Chem. 2022, 383, 132605. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; McClements, D.J.; Jin, Z.; Qin, Y.; Hu, Y.; Xu, X.; Wang, J. Resveratrol-loaded core-shell nanostructured delivery systems: Cyclodextrin-based metal-organic nanocapsules prepared by ionic gelation. Food Chem. 2020, 317, 126328. [Google Scholar] [CrossRef]
- Beldjoudi, Y.; Taha, S. Ensemble de Récipients Supramoléculaires Organiques Pour Libération Commandée de Médicament. WO2022182945A1, 25 February 2022. Available online: https://patents.google.com/patent/WO2022182945A1/fr (accessed on 27 October 2025).
- Perinelli, D.R.; Palmieri, G.F.; Cespi, M.; Bonacucina, G. Encapsulation of Flavours and Fragrances into Polymeric Capsules and Cyclodextrins Inclusion Complexes: An Update. Molecules 2020, 25, 5878. [Google Scholar] [CrossRef] [PubMed]
- Farouk, A.; Sharaf, S.; Refaie, R.; Abd El-Hady, M.M. Highly Durable Antibacterial Properties of Cellulosic Fabric via β-Cyclodextrin/Essential Oils Inclusion Complex. Polymers 2022, 14, 4899. [Google Scholar] [CrossRef]
- Aytac, Z.; Yildiz, Z.I.; Kayaci-Senirmak, F.; Tekinay, T.; Uyar, T. Electrospinning of cyclodextrin/linalool-inclusion complex nanofibers: Fast-dissolving nanofibrous web with prolonged release and antibacterial activity. Food Chem. 2017, 231, 192–201. [Google Scholar] [CrossRef]
- Xiao, Z.; Zhang, Y.; Niu, Y.; Ke, Q.; Kou, X. Cyclodextrins as carriers for volatile aroma compounds: A review. Carbohydr. Polym. 2021, 269, 118292. [Google Scholar] [CrossRef]
- Turner, J.; O’Loughlin, D.A.; Green, P.; McDonald, T.O.; Hamill, K.J. In search of the perfect tan: Chemical activity, biological effects, business considerations, and consumer implications of dihydroxyacetone sunless tanning products. J. Cosmet. Dermatol. 2023, 22, 79–88. [Google Scholar] [CrossRef]
- Trotta, F.; Caldera, F.; Dianzani, C.; Argenziano, M.; Barrera, G.; Cavalli, R. Glutathione Bioresponsive Cyclodextrin Nanosponges. ChemPlusChem 2016, 81, 439–443. [Google Scholar] [CrossRef]
- Webber, V.; de Siqueira Ferreira, D.; Barreto, P.L.M.; Weiss-Angeli, V.; Vanderlinde, R. Preparation and characterization of microparticles of β-cyclodextrin/glutathione and chitosan/glutathione obtained by spray-drying. Food Res. Int. 2018, 105, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Afsana; Popli, H. A review on efficacy and tolerability of tea tree oil for acne. J. Drug Deliv. Ther. 2019, 9, 609–612. [Google Scholar] [CrossRef]
- Kong, P.; Abe, J.P.; Masuo, S.; Enomae, T. Preparation and characterization of tea tree oil-β-cyclodextrin microcapsules with super-high encapsulation efficiency. J. Bioresour. Bioprod. 2023, 8, 224–234. [Google Scholar] [CrossRef]
- Old Spice. Clinical Sweat Defense Antiperspirant Deodorant for Men, Swagger, 1.7 Oz. Available online: https://oldspice.com/shop-at-retailers/clinical-sweat-defense-antiperspirant-deodorant-for-men-swagger-1-7-oz/ (accessed on 27 October 2025).
- Aluminum Free Deodorant Cotton. Available online: https://secret.com/en-us/shop/aluminum-free-deodorant/cotton (accessed on 27 October 2025).
- Native. Deodorant. Available online: https://www.nativecos.com/products/deodorant (accessed on 27 October 2025).
- Singh, M.; Sharma, R.; Banerjee, U.C. Biotechnological applications of cyclodextrins. Biotechnol. Adv. 2002, 20, 341–359. [Google Scholar] [CrossRef]
- Cal, K.; Centkowska, K. Use of cyclodextrins in topical formulations: Practical aspects. Eur. J. Pharm. Biopharm. 2008, 68, 467–478. [Google Scholar] [CrossRef]
- Bilensoy, E. Cyclodextrins in Pharmaceutics, Cosmetics, and Biomedicine: Current and Future Industrial Applications; John Wiley & Sons: New York, NY, USA, 2011; ISBN 978-0-470-93461-6. [Google Scholar]
- FOXYBAE.COM. Fabulously Fresh Dirty Gal Dry Shampoo + Biotin − FoxyBae. Available online: https://www.foxybae.com/products/dirty-gal-dry-shampoo (accessed on 27 October 2025).
- Muller, R. Use of a Cyclodextrin as Pearlescent Agent and Pearlescent Compositions. US20040033984A1, 19 February 2004. Available online: https://patents.google.com/patent/US20040033984/en (accessed on 27 October 2025).
- Use of Cyclodextrins as Pearlescent Agents and Pearlescent Compositions. JP3986022B2, 3 October 2007. Available online: https://patents.google.com/patent/JP3986022B2/en?oq=JP3986022B2 (accessed on 27 October 2025).
- Guo, Y.; Yue, Y.; Wang, H.; Zhong, Z.; Chen, B.; Shu, L.; Wang, J.; Zhang, Z. Hydroxypropyl-β-cyclodextrin inclusion complex improves the percutaneous therapeutic effect of eugenol on psoriasis mice. Eur. J. Pharmacol. 2025, 1003, 177921. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gong, S.; Liu, L.; Shen, H.; Liu, E.; Pan, L.; Gao, N.; Chen, R.; Huang, Y. Cyclodextrin-Coordinated Liposome-in-Gel for Transcutaneous Quercetin Delivery for Psoriasis Treatment. ACS Appl. Mater. Interfaces 2023, 15, 40228–40240. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xu, Y.; Zhang, J.; Xu, X. Cyclodextrin-based supramolecular dissolving microneedles for enhanced transdermal delivery of azelaic acid in acne vulgaris treatment. J. Drug Deliv. Sci. Technol. 2025, 111, 107108. [Google Scholar] [CrossRef]
- Arpa, M.D.; Biltekin Kaleli, S.N.; Doğan, N. Hydroxypropyl-β-Cyclodextrin-Enhanced Azelaic Acid Hydrogel for Acne Treatment: Evaluation of Antimicrobial, Anti-inflammatory, and Skin Penetration Properties. J. Pharm. Innov. 2025, 20, 106. [Google Scholar] [CrossRef]
- Irie, T.; Uekama, K. Pharmaceutical applications of cyclodextrins. III. Toxicological issues and safety evaluation. J. Pharm. Sci. 1997, 86, 147–162. [Google Scholar] [CrossRef] [PubMed]
- Oktay, A.N.; Celebi, N.; Ilbasmis-Tamer, S.; Kaplanoğlu, G.T. Cyclodextrin-based nanogel of flurbiprofen for dermal application: In vitro studies and in vivo skin irritation evaluation. J. Drug Deliv. Sci. Technol. 2023, 79, 104012. [Google Scholar] [CrossRef]
- Gurita, V.G.; Pavel, I.Z.; Borcan, F.; Moaca, A.; Danciu, C.; Diaconeasa, Z.; Imbrea, I.; Vlad, D.; Dumitrascu, V.; Pop, G. Toxicological evaluation of some essential oils obtained from selected Romania Lamiaceae Species in complex with hydroxypropyl-gamma-cyclodextrin. Rev. Chim. 2019, 70, 3703–3707. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).