Abstract
Polianthes tuberosa L. (PT) flower extracts exhibit considerable bioactivities, yet their application is often constrained by limited bioavailability and efficacy. In this study, fermentation of PT (FPT) using Rhodosporidium toruloides significantly enhanced its phytochemical profile, doubling the total phenol content (697.22 ± 7.51 μg/mL in FPT versus (vs.) 347.61 ± 5.89 μg/mL in non-fermented extract (NF)) and increasing flavonoids by onefold relative to NF (381.44 ± 6.50 μg/mL in FPT vs. 190.25 ± 4.75 μg/mL in NF), resulting in a substantial improvement in radical scavenging capacity (DPPH: 47.59 ± 1.55%; ABTS: 89.87 ± 1.39%). In UVB-irradiated the human keratinocyte cell line, FPT demonstrated superior efficacy over NF by effectively reducing reactive oxygen species and malondialdehyde levels (1.29 ± 0.08 ng/mL at 0.4 mg/mL FPT vs. 1.5 ± 0.1 ng/mL with NF), while concurrently elevating the activity of key antioxidant enzymes. Using human dermal fibroblasts, FPT was further shown to possess notable anti-glycation and anti-carbonylation properties, significantly inhibiting carboxymethyl lysine formation (90.6 ± 3.6% reduction) and protein carbonylation (86.5 ± 2.2% reduction). It also suppressed senescence-associated β-galactosidase activity (67.9 ± 3.0% inhibition), downregulated matrix metalloproteinase-1 expression (62.5 ± 5.1% reduction), and stimulated type I collagen synthesis (166.5 ± 4.2% recovery). Additionally, FPT markedly inhibited UVB-induced melanogenesis in B16F10 melanoma cells by reducing melanin content (36.0 ± 5.3%) and tyrosinase activity (45.7 ± 1.2%), through the downregulation of critical melanogenic genes, including melanocortin 1 receptor, microphthalmia-associated transcription factor, and tyrosinase.
1. Introduction
As the largest organ of the human body, the skin is constantly exposed to environmental stressors such as ultraviolet (UV) radiation and pollution, leading to oxidative stress, which accelerates skin aging and results in various manifestations including wrinkles, loss of elasticity, and hyperpigmentation [1,2]. Oxidative stress, along with glycation, plays a major role in skin aging by promoting the accumulation of reactive oxygen species (ROS), driving the formation of advanced glycation end products (AGEs) and protein carbonylation. These biochemical processes collectively inhibit collagen synthesis, exacerbate inflammatory responses, and disrupt extracellular matrix homeostasis, ultimately compromising skin structural integrity and function [3]. To address these challenges effectively and safely, plant extracts have gained more and more interest owing to their rich natural bioactive compounds that bring multiple functions [4,5,6].
Among various plant extracts, the flower extracts have gained significant attention in cosmetics owing to their rich fragrance as well as various skin care benefits, such as antioxidant, anti-inflammatory, anti-glycation and anti-aging activities [7,8,9,10]. Elfitriani et al. [7] identified that ethanolic extracts from Rosa damascena petals and receptacles had abundant phenolic compounds and flavonoids, exhibiting potent antioxidant capacity and notable collagenase inhibition, suggesting promising anti-aging applications. Research by Song et al. [8] on Rosa gallica petal extracts elucidated their dual functionality; that is, the extracts effectively suppressed melanogenesis through MAPK signaling pathway modulation while concurrently reduced Matrix Metalloproteinase-1 (MMP-1) expression, thereby demonstrating combined skin-lightening and anti-wrinkle effects. Comparative analysis of five chrysanthemum cultivars by Wang et al. [9] revealed that the Boju cultivar extract, characterized by exceptionally high level of total flavonoids, phenolics and chlorogenic acid, exhibited superior antioxidant activity and tyrosinase inhibition. Additionally, Wang et al. [10] found that paeoniflorin monoterpene glycosides in Paeonia lactiflora flower could significantly inhibit melanin production, making it a highly valuable natural whitening agent. Polianthes tuberosa L. (PT), a perennial aromatic plant from the amaryllidaceae family, is renowned for its strong, lasting fragrance. It is widely used in the perfume, essential oil, and traditional spice industries. PT contains a variety of flavonoids and other polyphenolic compounds, which may exhibit potential therapeutic efficacy in the treatment of various diseases associated with oxidative stress, such as diabetes, rheumatoid arthritis, and cardiovascular disorders [11,12]. Extracts from PT flowers have been reported to possess antioxidant [13,14], anti-inflammatory, and antibacterial properties [15]. However, pristine plant extracts often face challenges such as low active ingredient content and limited bioavailability.
Microbial fermentation technology, on one hand, offers an effective strategy to simultaneously enhance the extraction efficiency of target compounds through enzymatic degradation of plant cell walls and augment the bioactivity of plant extracts via generation of low-molecular-weight metabolites. As demonstrated by Matsuzawa et al. [16] solid-state fermentation with Aspergillus oryzae-mediated degradation of insoluble polysaccharides through glycoside hydrolases and lytic polysaccharide monooxygenases, elevating soluble sugars and phenolic yields. Jabłońska-Ryś et al. [17] employed Lactiplantibacillus plantarum EK11 for fermenting edible fungi, resulting in significant pH reduction, elevated lactic acid bacterial counts, and diminished digestible carbohydrate content. On the other hand, microbial fermentation can enhance the profile of functional compounds in fermented products [18,19]. For instance, OuYang et al. [19] developed a synthetic microbial consortium that enriched the vinegar with functional components including acetic acid, chlorogenic acid and hesperidin. Building on these merits, this study focuses on (I) screening optimal microbial strains for PT fermentationan and (II) systematic evaluation of fermented PT extracts for enhanced antioxidant, anti-glycation, anti-carbonylation, anti-photoaging activities, and whitening efficacy.
2. Materials and Methods
2.1. Materials
Fresh PT samples were obtained from the Dounan Flower Market (Kunming, China). Yeast Extract was purchased from OxoidTM (Thermo Fisher Scientific, Waltham, MA, USA). Folin–Ciocalteu reagent was purchased from Meryer Chemical Technology (Shanghai, China). The human keratinocyte cell line (HaCaT) and human dermal fibroblasts (HDF) were obtained from Immocell Biotechnology (Xiamen, China). B16F10 cell was obtained from BeNa Culture Collection (Beijing, China). Cell culture reagents including high-glucose DMEM medium, RPMI-1640 medium, fetal bovine serum (FBS), and 0.25% trypsin were acquired from GibcoTM (Thermo Fisher Scientific, Waltham, MA, USA). Phosphate-buffered saline (PBS) and penicillin-streptomycin solution were obtained from HyClone Laboratories (Logan, UT, USA). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was purchased from Sigma-Aldrich (St. Louis, MO, USA). The RNA extraction kit was procured from Wuhan Servicebio Technology (Wuhan, China). ELISA kits for detecting Interleukin-6 (IL-6), malondialdehyde (MDA), superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), and catalase (CAT) and type I collagen (Col-I) were purchased from Shanghai Universal Biotech (Shanghai, China). Triton X-100, Senescence β-Galactosidase (SA-β-gal) Staining Kit, ROS and MMP-1 Assay Kit, Immunol Staining Fix Solution, Fluorescein-5-thiosemicarbazide (5-FTSC), antifade mounting medium with 4’,6-diamidino-2-phenylindole (DAPI), were purchased from Beyotime Biotechnology (Shanghai, China). Anti-Carboxymethyl Lysine primary antibody, Alexa Fluor® 488-conjugated donkey anti-mouse IgG H&L secondary antibody were purchased from Abcam (Cambridge UK) All other chemicals, unless otherwise specified, were obtained from Sinopharm Chemical Reagent (National Pharmaceutical Group Corporation, Shanghai, China). Bacterial strain (Saccharomycopsis fibuligera CICC 1429, Pichia pastoris CICC 32806, Saccharomyces cerevisiae CICC 32165, Hansenula Anomala CICC 31340, Candida tropicalis CICC 1463, Geotrichum candidum CICC 1315, Rhodosporidium toruloides CICC 31643, Candida utilis CICC 31170, Clavispora lusitaniae CICC 1461, Candida krusei CICC 31748) were obtained from China Center of Industrial Culture Collection (CICC) (Beijing, China).
2.2. Fermentation of PT by Saccharomyces
The activated strains were inoculated into fermentation medium, containing 10 g/L dried PT pollen, 20 g/L peptone, 10 g/L yeast extract, and 20 g/L anhydrous glucose, and cultured at 30 °C for 24 h [20]. After centrifugation at 10,000 rpm for 5 min, the fermented PT (FPT) was subjected to a gradient filtration process through 10, 2, 0.45, and 0.22 μm membrane filters. The final filtrate was collected and stored at −20 °C for future use.
2.3. Determination of Total Phenol Content
The total phenolic content was determined using a modified Folin–Ciocalteu colorimetric method [21]. Initially, 1 mL of the FPT solution was dissolved in 4 mL of deionized water. Then, 100 μL of the test sample was mixed thoroughly with 1.0 mL of Folin–Ciocalteu reagent and 1.0 mL of 10% Na2CO3 solution in a 5 mL centrifuge tube, followed by incubation at room temperature for 90 min. The absorbance was then measured at 760 nm using a UV-Vis spectrophotometer (Hirpyq International Trading Co., Ltd., Shanghai, China). Gallic acid was used as the standard to construct the calibration curve, which was then employed to calculate the total phenolic content in the samples.
2.4. Determination of Total Flavonoid Content
The total flavonoid content was determined using a modified NaNO2-Al(NO3)3 colorimetric method [22]. Initially, 1 mL of FPT was dissolved in 4 mL of deionized water. Then, 1 mL of the sample solution was transferred to a 10 mL centrifuge tube. 0.3 mL of 5% sodium nitrite solution was added in turn, and the reaction was allowed to stand for 6 min after shaking and mixing. Subsequently, 0.3 mL of 10% aluminum nitrate solution was added, shaken and mixed again, and stood for 6 min. Finally, 4 mL 1 mol/L NaOH solution was added, and the solution was diluted to 10 mL scale line with 60% ethanol. The mixture was fully mixed and stood for 15 min. After the completion of the reaction, the absorbance value of the sample solution was determined by an ultraviolet-visible spectrophotometer at 510 nm. The standard curve was prepared with rutin as the standard, and the content of total flavonoids in the sample was calculated by linear regression equation.
2.5. Antioxidant Activities In Vitro
2.5.1. DPPH Radical Scavenging Activity
The DPPH radical scavenging assay was performed with modifications based on previously described methods [23]. Briefly, 5.0 mg of DPPH was accurately weighed and dissolved in anhydrous ethanol with sonication in the dark to ensure complete dissolution. The solution was then brought to a final volume of 100.0 mL with anhydrous ethanol, yielding a working solution at 50.0 μg/mL. The 1 mL FPT sample was dissolved in 4 mL deionized water to prepare the sample solution to be tested.
The experiment was conducted in a 96-well plate, with sample, blank, and control groups. In the sample group, 100 μL of the test sample solution and 100 μL of DPPH ethanol solution were added. In the blank group, 100 μL of anhydrous ethanol replaced DPPH ethanol solution, and in the control group, 100 μL of anhydrous ethanol replaced the sample solution. After thorough mixing, the reactions were incubated in the dark at room temperature for 30 min. The absorbance was then measured at 517 nm using a multifunctional microplate reader (Allsheng Instrument Co., Ltd., Hangzhou, China). The calculation was performed using the following formula:
Scavenging ability (%) = (1 − (Asample − Ablank)/Acontrol) × 100%
2.5.2. ABTS Radical Scavenging Activity
The ABTS radical scavenging assay was adapted from established methods [24] with modifications. First, 76.8 mg of ABTS was dissolved in 20 mL of deionized water to prepare a 7 mmol/L ABTS solution. Then, 13.2 mg of potassium persulfate was dissolved in 20 mL of deionized water to prepare a 2.45 mmol/L potassium persulfate solution. The 7 mmol/L ABTS solution and 2.45 mmol/L potassium persulfate solution were mixed in a 2:1 volume ratio and allowed to react in the dark at room temperature for 16 h to obtain the ABTS stock solution. The ABTS stock solution was then diluted with anhydrous ethanol until the absorbance at 734 nm was approximately 0.7, resulting in the ABTS working solution. For the sample preparation, 1 mL of FPT was dissolved in 9 mL of deionized water to make the test sample solution. The experiment was performed in a 96-well plate with sample, blank, and control groups. In the sample group, 50 μL of the test sample solution and 150 μL of ABTS ethanol solution were added. In the blank group, 150 μL of anhydrous ethanol was used to replace the ABTS solution, and in the control group, 150 μL of anhydrous ethanol replaced the sample solution. After thorough mixing, the reactions were incubated in the dark at room temperature for 15 min. The absorbance was then measured at 734 nm using a multifunctional microplate reader. The calculation was performed using the following formula:
Scavenging ability (%) = (1 − (Asample − Ablank)/Acontrol) × 100%
2.6. Cell Cultures
HaCaT and HDF cells were cultured in DMEM medium, while B16F10 cells were maintained in RPMI-1640 medium. All culture media were supplemented with 10% fetal bovine serum, 1.0 × 105 U/L penicillin, and 100 mg/L streptomycin. Cell cultures were incubated at 37 °C in a humidified atmosphere containing 5% CO2 using an incubator (Bosun Medical, Shanghai, China). Once the cell confluence reached 80–90%, they were treated with a trypsin-EDTA solution containing 0.25% EDTA. After digestion, complete medium was added to stop the digestion process, and the cells were subcultured at a 1:2 to 1:3 ratio.
2.7. Antioxidant Effects of FPT
2.7.1. Cell Viability of Hacat Cells
The cytotoxic effects of FPT on Hacat cells were evaluated using the MTT assay. Prior to the experiment, the FPT solution was filtered through a 0.22 μm filter membrane for sterilization. Hacat cells in logarithmic growth phase were adjusted to a density of 1 × 105 cells/mL and plated in 96-well plates (100 μL per well). Following 24 h incubation at 37 °C to allow cell attachment, the culture medium was replaced with 100 μL of fresh DMEM containing FPT at graded concentrations (0.1–2 mg/mL). Blank wells received medium only. After 24 h treatment, cells were incubated with 100 μL of MTT solution (0.5 mg/mL in DMEM) for 4 h at 37 °C. Subsequently, 100 μL DMSO was added to dissolve the crystal of formazan, and the absorbance value of each hole was measured at 490 nm wavelength using a multifunctional microplate reader.
To assess UVB-induced cytotoxicity, Hacat cells in logarithmic phase (1 × 105 cells/mL) were inoculated in 96-well plates (100 μL/well) and cultured for 24 h. For UVB exposure, experimental groups received graded doses of UVB radiation (10–120 mJ/cm2) through a thin PBS layer after removal of the plate cover. The blank group received DMEM medium treatment without irradiation, and the cells were incubated for another 24 h. Cell viability was then measured using the MTT assay as described previously. Cell viability was calculated by the following Formula.
where ODT and ODB represent the average OD values of the experimental and the blank groups at 490 nm, respectively.
Cell viability (%) = (ODT/ODB) × 100%
2.7.2. Measurement of Intracellular ROS
Cells in the logarithmic growth phase were trypsinized and adjusted to a density of 2 × 105 cells/mL, then seeded in 12-well plates. After 24 h of UVB irradiation and FPT treatment, the DCFH-DA probe was diluted with serum-free medium at a ratio of 1:1000 to a final concentration of 10 μM according to the instructions of the ROS detection ELISA kit. Each well was loaded with 1 mL of the prepared DCFH-DA working solution and incubated at 37 °C for 20 min in the dark. After incubation, cells were gently washed three times with pre-warmed serum-free DMEM medium. Image acquisition was performed using an inverted fluorescence microscope (Motic China Group Co., Ltd., Xiamen, China). Subsequently, cells were trypsinized and resuspended at a density of 5 × 104 cells/mL, then transferred to black 96-well plates. Fluorescence intensity was measured at excitation/emission wavelengths of 488/525 nm using a microplate reader, with triplicate wells for each experimental group [25].
2.7.3. Assessment of Cellular Antioxidant Capacity Against Oxidative Stress
Cells in the logarithmic growth phase were trypsinized and adjusted to a density of 2 × 105 cells/mL, then seeded in 12-well plates. After UVB irradiation and FPT treatment for 24 h, cell suspensions were collected for analysis. The activity of four oxidative stress indicators, namely MDA [26], SOD [27], GSH-Px [28], and CAT [29], was measured strictly according to the instructions of the ELISA kit.
2.8. Anti-Glycation and Anti-Carbonylation Effects of FPT
2.8.1. Cell Viability of HDF Cells
The effects of FPT and UVA on HDF cell viability were assessed using a protocol similar to that described in Section 2.7.1.
2.8.2. Anti-Glycation of FPT
HDF cells at logarithmic growth phase were trypsinized and seeded in 12-well plates at a density of 5 × 104 cells per well. After 48 h of culture, experimental groups were treated with 0.5 mmol/L methylglyoxal (MGO) for 48 h while blank groups remained in standard culture conditions. Following treatment, test compounds were added to experimental groups and fresh medium to control groups for an additional 48 h incubation period. The culture medium was then removed and cells were gently washed twice with PBS.
For immunofluorescence staining, cells were fixed with immunol staining fix solution for 10 min at room temperature, followed by two PBS washes. After blocking with immunostaining buffer for 20 min at room temperature, cells were incubated with anti-Carboxymethyl Lysine primary antibody (1:50 dilution) at 4 °C overnight. The next day, cells were incubated with Alexa Fluor® 488-conjugated donkey anti-mouse IgG H&L secondary antibody (1:1000 dilution) for 2 h at room temperature in the dark, followed by two PBS washes. Finally, cells were counterstained with antifade mounting medium with DAPI for 10 min at room temperature.
Fluorescence images were captured using an inverted fluorescence microscope, with carboxymethyl lysine (CML) staining appearing as green fluorescence and nuclear staining by DAPI as blue fluorescence. Quantitative analysis of CML fluorescence intensity was performed using ImageJ 1.47v software by measuring randomly selected fields from each experimental group [30].
2.8.3. Anti-Carbonylation Effects of FPT
When HDF cells reached the logarithmic growth phase, they were digested and suspended to a density of 2 × 105 cells/well, then seeded into 12-well plates and cultured for 24 h. The culture medium was aspirated, and any residual medium was washed away with PBS. Subsequently, 1 mL of PBS was added, and the cells were irradiated with UVA at an intensity of 12 J/cm2. After irradiation, 1 mL of DMEM culture medium containing prepared samples was added, and the cells were incubated in a CO2 incubator for 24 h. The supernatant was discarded, and the cells were washed three times with PBS. Then, a prepared concentration of 5-FTSC was added for staining, and fluorescence imaging was captured. Fluorescence intensity was detected at 495 nm/517 nm [31].
2.9. Anti-Aging Effects of FPT
2.9.1. Expression of SA-β-Gal in Cells
HDF cells at logarithmic growth phase were detached using 0.25% trypsin and seeded in 12-well plates at a density of 2 × 105 cells/mL. After 24 h of culture to allow cell attachment, cells were exposed to UVA irradiation and treated with FPT for an additional 24 h. The experiment was conducted according to the instructions of ELISA kit. The culture medium was discarded, and the cells were gently washed twice with pre-cooled PBS. Cells were then fixed with 1 mL of fixation buffer at room temperature for 15 min, followed by three PBS washes. Freshly prepared SA-β-gal staining working solution (1 mL/well) was added, and plates were sealed with parafilm before incubation at 37 °C in a CO2-free incubator for 14–16 h in the dark. After staining, the cells were observed under an inverted microscope, and images were captured. The images were processed and analyzed using Image J software to calculate the proportion of the blue-stained area in the images [32].
2.9.2. Determination of MMP-1 Secreted by Cells
HDF cells at logarithmic growth phase were detached using 0.25% trypsin and seeded in 12-well plates at a density of 2 × 105 cells/mL and cultured for 24 h. After the cells adhered, they were subjected to UVA irradiation and FPT treatment. After 24 h of subsequent incubation, culture supernatants were collected and centrifuged at 1000 rpm for 10 min at 4 °C to remove cellular debris, and the supernatant was collected. The procedure was performed strictly according to the instructions of the MMP-1 assay kit to measure the MMP-1 concentration in the supernatant [33].
2.9.3. Determination of Col-I Expression
Cell culture and operation refer to Section 2.9.2. The Col-I expression was quantified using ELISA kits following the manufacturer’s protocols.
2.9.4. Determination of Secretion of Inflammatory Factors IL-6
Cell culture and operation refer to Section 2.9.2. The secretion of inflammatory factors IL-6 was quantified using ELISA kits following the manufacturer’s protocols.
2.10. Anti-Melanogenic Effects of FPT
2.10.1. Cell Viability of B16F10 Cells
The effects of FPT and UVB on B16F10 cell viability were assessed using a protocol similar to that described in Section 2.7.1.
2.10.2. Determination of Melanin Content
The melanin content in B16F10 cells was measured using a modified NaOH lysis method [34]. Cells were seeded in 6-well plates at a density of 2 × 105 cells/mL with 2 mL complete medium per well and cultured for 24 h at 37 °C. After incubation, the control group was treated with 1640 medium alone. The model and experimental groups were exposed to 20 mJ/cm2 UVB irradiation, followed by treatment with 1640 medium or 1640 medium containing 0.2, 0.6, and 1 mg/mL of FPT. After 24 h treatment, cells were collected by trypsinization, followed by centrifugation at 1200 rpm for 5 min. The pellets were washed twice with PBS, and 100 μL of 1 mol/L NaOH solution containing 10% DMSO was added. The samples were then heated at 80 °C for 1 h to lyse the cells. Finally, melanin content was quantified by measuring the absorbance at 405 nm.
2.10.3. Determination of Tyrosinase Activity
The relative activity of intracellular tyrosinase was detected by referring to previous study [35]. Cells were cultured and treated as described in Section 2.10.2. Following treatment, 400 μL of 1% Triton X-100 solution was added to each well and incubated at 4 °C for 30 min to achieve complete cell lysis. The lysates were centrifuged at 12,000 rpm for 5 min to collect the supernatant. For the enzymatic assay, 50 μL of supernatant was transferred to a 96-well plate and mixed with 150 μL of 10 mmol/L L-DOPA solution. After incubation at 37 °C for 30 min, the reaction products were quantified by measuring absorbance at 475 nm.
2.10.4. Reverse Transcription Polymerase Chain Reaction Experiments
Total RNA was extracted from the treated cells using an RNA extraction kit. Nanodrop 2000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA) was used to detect the purity and concentration of isolated RNA samples. The extracted RNA was reverse transcribed into cDNA by PCR instrument (Eastwin Life Sciences Inc., Beijing, China). The reaction system included 5× SweScript All-in-One SuperMix for qPCR (4 μL), gDNA Remover (1 μL), Total RNA (10 μL), RNase free water (Add to 20 μL). The cDNA was used as a template for real-time fluorescence quantitative PCR amplification (Bio-Rad, Hercules, CA, USA). The PCR amplification program was as follows: Pre-denaturation at 95 °C for 30 s; denaturation at 95 °C for 15 s, annealing at 60 °C for 1 min, for 40 cycles; and fluorescence was acquired at 0.5 °C increments, ending at 95 °C for 15 s. The primer sequence and amplified gene information are detailed in Table 1. All experiments were repeated by three techniques, and GAPDH was used as an internal reference gene for normalization analysis. Relative gene expression was calculated using the comparative ΔΔCt method [36]:

Table 1.
Primer design for B16F10 cells.
A = CT (target gene, test sample) − CT (internal standard, test sample)
B = CT (test sample, control sample) − CT (internal standard, control sample)
K = A − B
Fold change = 2−K
2.11. Pre- and Post-Fermentation Metabolomic Profiling of PT Yeast Fermentation Broth
To investigate metabolic compositional changes during fermentation, we employed a non-targeted metabolomics approach using UHPLC-Q-TOF MS. Chromatographic separation was achieved on a Waters ACQUITY UPLC BEH C18 column (2.1 × 100 mm, 1.7 μm) maintained at 40 °C, with a flow rate of 0.4 mL/min. The mobile phase consisted of (A) water with 25 mmol/L ammonium acetate and 0.5% formic acid and (B) methanol. The gradient program was as follows: 5% B (0–0.5 min), increased linearly to 100% B (0.5–10 min), held at 100% B (10–12 min), returned to 5% B (12–12.1 min), and re-equilibrated at 5% B (12.1–16 min). Samples were kept at 4 °C in the autosampler and injected randomly, with quality control (QC) samples interspersed to ensure system stability.
Mass spectrometry was performed on an AB Triple TOF 6600 instrument (SCIEX, Danaher Corporation, Washington, DC, USA) with an ESI source operating in both positive and negative modes. Key parameters were as follows: Gas1 and Gas2: 60 psi; CUR: 30; temperature: 600 °C; ion spray voltage: ±5500 V. Full-scan MS data (60–1000 Da) were acquired in Information Dependent Acquisition mode, with secondary MS/MS fragmentation using a collision energy of 35 ± 15 eV.
Raw data were converted to mzXML format using ProteoWizard (3.0.6428), then processed in MS-DIAL for peak picking, alignment, retention time correction, and peak area extraction. Metabolites were identified by matching accurate mass (mass error < 10 ppm), MS/MS spectra, and retention time against a custom plant metabolome database, with manual validation. Statistical analysis included univariate (fold-change, t-tests) and multivariate (PCA, OPLS-DA) methods. Significantly altered metabolites were selected based on VIP > 1 and p < 0.05, and results were visualized via heatmaps and volcano plots and other forms.
2.12. Statistical Analysis
The data were analyzed by Origin software 2025b, and the data were expressed as mean ± standard deviation. Comparison between groups using analysis of variance (ANOVA) after Tukey test, p value is less than 0.05 as statistically significant.
3. Results and Discussion
3.1. Screening of Microbial Strains
From 27 yeast strains (Table S1), ten strains of good reproductive ability (Table 2) were selected for subsequent fermentation experiments. Based on the total phenols, total flavonoids, and DPPH and ABTS radical scavenging capacities, it is concluded that Rhodosporidium toruloides is the optimal strain for PT fermentation. As shown in Figure 1a,b, the total phenols and flavonoids contents in the FPT were higher than those in the non-fermented one (NF). Moreover, most FPT products demonstrated superior antioxidant activity relative to NF. Among the ten FPT products, the FPT by Rhodosporidium toruloides showed the highest total polyphenols (697.22 ± 7.51 μg/mL) and flavonoids (381.44 ± 6.50 μg/mL), along with the highest antioxidant activity, achieving DPPH and ABTS radical scavenging rates of 47.59 ± 1.55% and 89.87 ± 1.39%, respectively. The increased antioxidant activity of the FPT is primarily attributed to the increase in polyphenols and flavonoids. Previous studies have demonstrated that polyphenols can neutralize free radicals by donating protons and electrons [37] and form stable chelates with metal ions, thereby inhibiting free radical generation [38]. Additionally, microbial fermentation is a complex biochemical process that induces profound alterations in the chemical composition of both substrates and residual components, including phenolic compounds, proteins, and lipids, among others [39].

Table 2.
List of different strains with different reproductive ability indicated by viable cell counts.
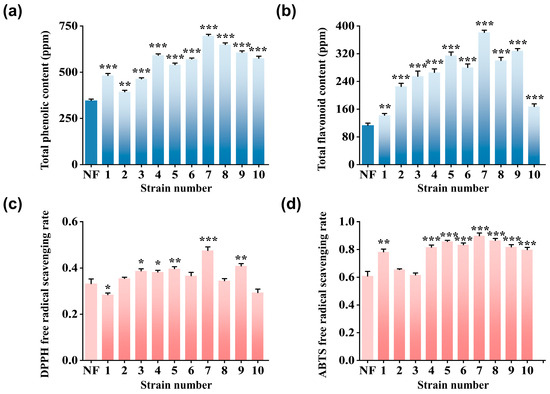
Figure 1.
Screening of microbial strains: (a) Total phenolic content. (b) Total flavonoid content. (c) DPPH radical scavenging activity (%). (d) ABTS radical scavenging activity (%) of NF and FPT fermented with ten representative microbial strains (1 to 10). * p < 0.05, ** p < 0.01, *** p < 0.001 compared with NF.
3.2. Antioxidant Effect of FPT on HaCat Cells in UVB-Induced Oxidative Stress
To establish a UVB-induced oxidative damage model in HaCaT cells, the effect of UVB dosage (0–160 mJ/cm2) on cell viability was initially assessed using the MTT assay. As shown in Figure 2a, UVB irradiation at a dose of 20 mJ/cm2 resulted in a significant reduction in cell viability to approximately 76.4 ± 3.8% compared to the non-irradiated blank (p < 0.01). Consequently, this irradiation dose was selected for all subsequent experiments. Subsequently, the cytotoxicity of FPT on HaCaT cells was evaluated using the MTT assay. As illustrated in Figure 2b, high FPT concentrations of >0.8 mg/mL significantly reduced cell viability to below 80% compared to the blank (p < 0.01). Therefore, a non-cytotoxic concentration range of 0.1–0.8 mg/mL was defined for subsequent assessment of the antioxidant efficacy of FPT. Based on the established UVB-induced oxidative damage model, intracellular ROS levels were quantified using the DCFH-DA fluorescent probe. As depicted in Figure 2c,d, exposure to 20 mJ/cm2 UVB markedly elevated ROS generation compared to the blank group (p < 0.001). Notably, treatment with 0.8 mg/mL NF and FPT significantly attenuated the UVB-induced ROS accumulation (p < 0.001 versus (vs.) model group). Importantly, the ROS suppressive effect observed in the FPT-treated group was significantly more potent than that in the NF-treated group (p < 0.01). These findings indicate that the fermentation process enhances the radical scavenging capacity of PT extract.
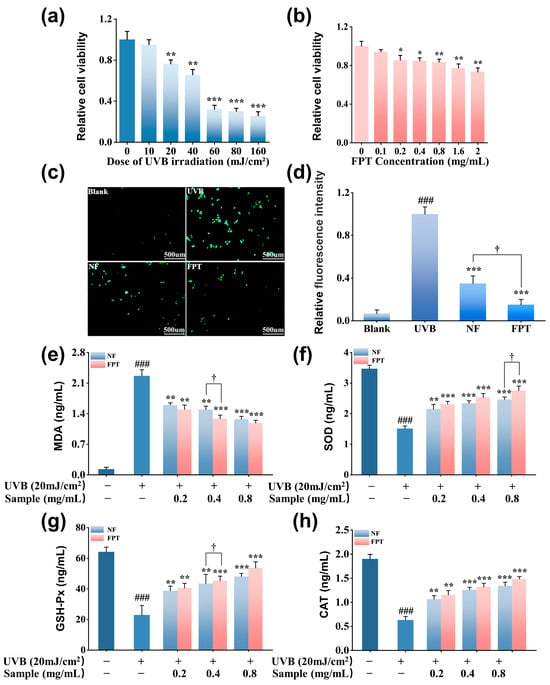
Figure 2.
Evaluation of antioxidant capacity in UVB-irradiated HaCaT cells. (a) Dosage-dependent cytotoxicity of UVB irradiation (0–160 mJ/cm2). (b) Concentration-dependent cytotoxicity of FPT (0.1–2.0 mg/mL) measured by MTT assay. (c) Representative fluorescence micrographs of intracellular ROS levels detected by DCFH-DA probe following 20 mJ/cm2 UVB irradiation (The scale bar is 500 μm). (d) Quantification of DCF fluorescence intensity. Biomarkers of oxidative stress: (e) MDA, (f) SOD, (g) GSH-Px, (h) CAT. * p < 0.05, ** p < 0.01, *** p < 0.001 compared with model group; ### p < 0.001 compared with blank. † p < 0.05 for FPT compared with NF.
Excessive generation of free radicals can overwhelm the cellular antioxidant defense system, leading to the accumulation of MDA, a well-established end-product of lipid peroxidation [40]. Therefore, MDA level serves as a robust biomarker for assessing oxidative damage to cellular membranes. As illustrated in Figure 2e, UVB irradiation significantly elevated intracellular MDA content compared to the non-irradiated blank group (p < 0.01). Both NF and FPT demonstrated a capacity to mitigate this UVB-induced MDA elevation, showing a dose-dependent reduction in MDA levels. Critically, at an equivalent concentration of 0.4 mg/mL, the MDA level in the FPT-treated group (1.29 ± 0.08 ng/mL) was significantly lower than that observed in the NF-treated group (1.5 ± 0.1 ng/mL, p < 0.05). These findings suggest that fermentation likely enhances the ability of PT extract to inhibit UVB-induced lipid peroxidation, potentially through augmenting the bioactivity of its constituent polyphenolic compounds.
To investigate the effect of the fermented extract on the endogenous antioxidant defense system, the content of key antioxidant enzymes—SOD, GSH-Px, and CAT—were assessed (Figure 2f–h). The results revealed that treatment with both NF and FPT extracts significantly restored SOD content (p < 0.01 vs. model group). Moreover, FPT exhibited a significantly greater restorative effect on SOD content than NF (p < 0.05). Similarly, FPT treatment enhanced the contents of GSH-Px and CAT. At a concentration of 0.8 mg/mL, FPT elevated GSH-Px content to 53.9 ± 4.0 ng/mL and CAT content to 1.5 ± 0.1 ng/mL. These levels were significantly higher than those achieved with the same concentration of NF extract (p < 0.05 for both enzymes).
Collectively, this study demonstrates that fermentation significantly enhances the protective effect of PT extract against UVB-induced oxidative damage in HaCaT cells. Compared to the NF, the FPT demonstrated significantly greater capacity for direct ROS scavenging, more potent inhibition of lipid peroxidation, and a stronger ability to restore and activate the endogenous antioxidant defense system, specifically by upregulating the activities of SOD, CAT, and GSH-Px. The enhanced bioactivity of FPT is likely attributable to the microbial biotransformation of polyphenolic compounds during fermentation with Rhodosporidium toruloides, which aligns with the established findings [41] that microbial fermentation can increase the content of free phenolic hydroxyl groups in plant extracts. These findings also reveal that the PT extract exerts its cytoprotective effects through a dual mechanism: not only by directly neutralizing free radicals but also by synergistically bolstering the cellular antioxidant machinery via enhanced enzymatic activity.
3.3. Anti-Glycation and Anti-Carbonylation Activity of FPT
To establish a UVA-induced damage model in HDFs, we first determined the optimal UVA dose by assessing its impact on cell viability via MTT assay (Figure S1a). Exposure to 12 J/cm2 UVA significantly reduced HDF viability to 67.3 ± 4.7% (p < 0.01 vs. Blank), establishing this dose as effective for inducing sub-lethal cellular damage and thus selected for subsequent experiments. Subsequently, the inherent cytotoxicity of FPT on HDFs was assessed using MTT assay (Figure S1b). Results demonstrated that high FPT concentration of >0.8 mg/mL caused a significant decrease in viability to below 80% (p < 0.01). Therefore, a non-cytotoxic concentration range of 0.1–0.8 mg/mL was defined for subsequent assessment of the anti-glycation and anti-carbonylation activity of FPT.
Immunofluorescence staining was employed to evaluate CML (a representative AGE) accumulation (Figure 3a). Compared to the blank group, MGO stimulation significantly enhanced green fluorescence intensity (p < 0.001), confirming successful induction of non-enzymatic glycation. Both NF and FPT extracts at 0.8 mg/mL markedly attenuated CML fluorescence versus the model group (p < 0.001). Crucially, FPT exhibited significantly stronger inhibition than NF, reducing fluorescence by 90.6 ± 3.6% versus 80.3 ± 2.3% (p < 0.05; Figure 3b). This enhanced anti-glycation efficacy of FPT likely stems from the fermentation process through the following mechanism: (1) fermentation-derived enzymes may degrade protein-bound glycation adducts [42]; (2) bioaccessible small-molecule polyphenols generated during fermentation could preferentially neutralize reactive carbonyl species, inhibiting their covalent modification of proteins [43].
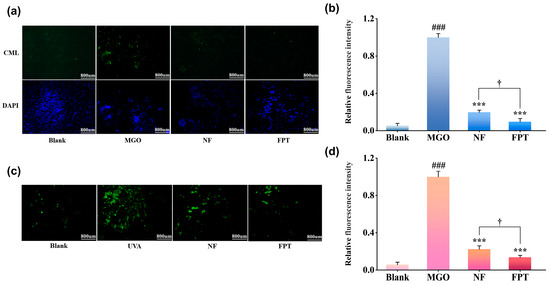
Figure 3.
Superior anti-glycation and anti-carbonylation efficacy of FPT vs. NF. (a) Representative immunofluorescence images of CML (green) and nuclei (DAPI, blue) in human dermal fibroblasts following MGO stimulation with/without 0.8 mg/mL NF or FPT treatment (The scale bar is 800 μm). (b) Quantitative analysis of CML fluorescence intensity. (c) Protein carbonylation detected by 5-FTSC (green) in cells exposed to 12 J/cm2 UVA irradiation with/without 0.8 mg/mL NF or FPT (The scale bar is 800 μm). (d) Quantification of carbonylation fluorescence intensity. *** p < 0.001 compared with model group; ### p < 0.001 compared with blank. † p < 0.05 for FPT compared with NF.
Protein carbonylation was quantified via 5-FTSC staining (Figure 3c). UVA irradiation significantly increased green fluorescence intensity versus the blank control (p < 0.01), confirming substantial protein oxidative damage. Both 0.8 mg/mL NF and FPT treatments significantly attenuated carbonylation levels relative to the model group (p < 0.01). Critically, FPT demonstrated superior efficacy, reducing fluorescence intensity by 86.5 ± 2.2% compared to 77.7 ± 2.3% with NF (p < 0.05; Figure 3d).
This fermentation-enhanced efficacy may be attributed to two key mechanisms: (1) Biotransformation during fermentation generates potent antioxidant metabolites (e.g., phenolic acid derivatives) that preferentially scavenge UVA-induced ROS, limiting protein oxidation [44]; (2) Microbial metabolism produces specific carbonyl quenchers that directly neutralize reactive carbonyl species [45]. To the best of our knowledge, this represents the first systematic demonstration of FPT’s dual inhibitory effects on glycation and protein carbonylation. Crucially, FPT outperformed NF by significantly suppressing AGEs formation (p < 0.05) and providing superior protection against protein carbonylation (86.5 ± 2.2% vs. 77.7 ± 2.3% inhibition, p < 0.05). These findings align with established evidence that microbial fermentation enhances the bioactivity of plant extracts [46,47,48].
3.4. Anti-Aging Activity of FPT
To assess the impact of FPT on UVA-induced senescence, we quantified senescent cells via SA-β-gal staining. UVA irradiation (12 J/cm2) significantly increased SA-β-gal cells versus blank control (p < 0.001; Figure 4a), validating successful senescence induction in our photoaging model. Both FPT and NF at 0.8 mg/mL significantly attenuated SA-β-gal activity (p < 0.01 vs. model). Crucially, FPT demonstrated markedly superior efficacy to NF, achieving 67.9 ± 3.0% versus 46.4 ± 4.9% senescence inhibition (p < 0.05; Figure 4b). These results establish that PT extract effectively counteracts UVA-induced senescence, with microbial fermentation significantly augmenting its anti-senescence capacity.
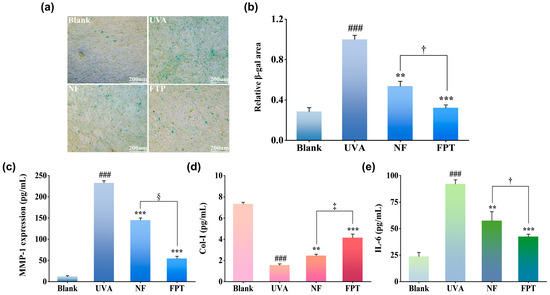
Figure 4.
Fermentation-enhanced protection against UVA-induced photoaging by PT extract. (a) Representative images of SA-β-gal senescent fibroblasts (blue) following 12 J/cm2 UVA irradiation (The scale bar is 200 μm). (b) Quantitative analysis of SA-β-gal area. (c) MMP-1 expression quantified by ELISA. (d) Col-I secretion measured via immunoassay. (e) IL-6 levels determined by multiplex immunoassay. ** p < 0.01, *** p < 0.001 compared with model group; ### p < 0.001 compared with blank. † p < 0.05, ‡ p < 0.01, § p < 0.001 for FPT compared with NF.
Photoaging-induced extracellular matrix (ECM) degradation involves MMP-1-mediated collagen catabolism. Quantitative analysis revealed UVA irradiation significantly upregulated MMP-1 expression versus control (p < 0.001; Figure 4c). Both extracts significantly suppressed MMP-1 hypersecretion (p < 0.01 vs. model), with FPT exhibiting superior inhibition (62.7 ± 5.1% reduction, p < 0.001 vs. NF). Concurrently, Col-I secretion in UVA-exposed cells decreased significantly (p < 0.001; Figure 4d). FPT intervention restored Col-I synthesis more effectively than NF (166.5 ± 4.2% vs. 57.3 ± 4.0% recovery, p < 0.01), demonstrating a 2.3-fold enhancement. These findings indicate FPT mitigates photoaging-associated ECM degradation through dual mechanisms: suppressing collagenolytic MMP-1 activity and promoting Col-I biosynthesis, thereby preserving dermal structural integrity.
Ultraviolet-induced photoaging initiates cutaneous inflammation, characterized by aberrant overexpression of the pro-inflammatory cytokine IL-6. Our data demonstrate UVA irradiation significantly elevated IL-6 secretion in HDFs versus blank (p < 0.001; Figure 4e). Crucially, FPT treatment (0.8 mg/mL) effectively suppressed IL-6 production, achieving 54.0 ± 2.3% inhibition—significantly exceeding the efficacy of non-fermented extract (NF) (p < 0.05). These results indicate FPT mitigates photoaging-associated inflammation through targeted downregulation of key pro-inflammatory mediators, with this anti-inflammatory potency being fermentation-dependent.
This study provides comprehensive evidence for the anti-photoaging efficacy of FPT using a UVA-irradiated HDF cell model. FPT significantly attenuated UVA-induced cellular senescence, as evidenced by reduced SA-β-gal activity (67.9 ± 3.0% vs. NF’s 46.4 ± 4.9% inhibition, p < 0.01). Crucially, FPT rebalanced ECM metabolism by concurrently suppressing collagenolytic MMP-1 expression (62.5 ± 5.1% inhibition) and restoring Col-I synthesis (167.1 ± 4.2% vs. NF’s 57.4 ± 4.0% recovery, p < 0.01). Furthermore, FPT demonstrated potent anti-inflammatory activity, inhibiting IL-6 secretion by 54.0 ± 2.3%—significantly surpassing NF efficacy (p < 0.05). The consistent superiority of FPT over NF across all endpoints highlights fermentation-dependent bioenhancement, likely attributable to biotransformation-mediated liberation of bioactive constituents (e.g., polysaccharides and polyphenols) [49]. These liberated components potentiate multimodal protective effects: enhanced ROS scavenging, carbonyl quenching, anti-inflammatory activity, and collagen stabilization.
3.5. Anti-Melanogenic Activity of FPT
To optimize experimental parameters, B16F10 cell viability was assessed following UVB irradiation and FPT treatment via MTT assay (Figure S2a). UVB doses >20 mJ/cm2 significantly reduced viability in a dose-dependent manner (e.g., 82.3 ± 3.1% at 20 mJ/cm2 vs. blank, p < 0.05; Figure S2b). The 20 mJ/cm2 dose was selected for subsequent melanogenesis study as it maintained >80% viability (non-cytotoxic threshold). Similarly, FPT concentrations >0.8 mg/mL decreased survival to 76.4 ± 2.9% (p < 0.01 vs. blank), establishing a non-cytotoxic window (0.1–0.8 mg/mL) for whitening efficacy assessment.
UVB irradiation significantly stimulated melanogenesis in B16F10 cells, elevating both melanin production and tyrosinase activity (p < 0.001) (Figure 5a). FPT treatment suppressed these effects, reducing melanin content by 36.0 ± 5.3% and tyrosinase activity by 45.7 ± 1.2% at 0.8 mg/mL—significantly exceeding NF’s inhibition (melanin: 24.7 ± 6.1%; tyrosinase: 36.3 ± 4.7%; p < 0.05). These findings indicate FPT inhibits melanogenesis through tyrosinase regulation, with fermentation enhancing its depigmenting efficacy via bioactive component biotransformation.
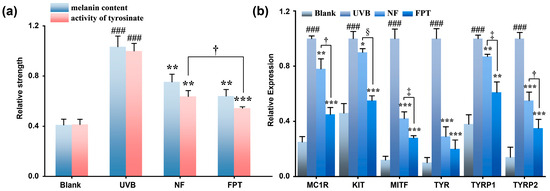
Figure 5.
Antagonism of UVB-stimulated melanogenesis by FPT. (a) Effect of FPT on cellular tyrosinase activity and melanin synthesis in B16F10 cells. (b) Effect of FPT on the expression of melanogenic proteins (MC1R, KIT, MITF, TYR, TYRP1, and TYRP2) in B16F10 cells. * p < 0.05, ** p < 0.01, *** p < 0.001 compared with model group; ### p < 0.001 compared with blank. † p < 0.05, ‡ p < 0.01, § p < 0.001 for FPT compared with NF.
To elucidate the depigmentation mechanism of FPT, q-PCR analysis quantified key melanogenesis-related genes (Melanocortin 1 Receptor (MC1R), Kinase Insert Domain Receptor (KIT), Microphthalmia-Associated Transcription Factor (MITF), Tyrosine (TYR), Tyrosinase Related Protein 1 (TYRP1) and Tyrosinase Related Protein 2 (TYRP2)). UVB irradiation significantly upregulated all target genes, whereas FPT treatment (0.8 mg/mL) effectively reversed these increases, demonstrating significantly greater suppression than NF. Crucially, FPT downregulated MITF, the master transcriptional regulator of melanogenesis, concomitantly reducing expression of its downstream effectors TYR (80 ± 6.5%), TYRP1 (39 ± 7.6%), and TYRP2 (65 ± 6.4%) (p < 0.001 compared with model). These results establish that FPT inhibits melanogenesis primarily through targeted suppression of the MITF signaling axis, with fermentation-driven augmentation of bioactivity.
The results of this study indicate that FPT, within a safe concentration range (0.1–0.8 mg/mL), can effectively inhibit UVB-induced melanin synthesis. The underlying mechanism involves the reduction in tyrosinase activity and downregulation of melanin-related gene expression. Compared to the NF, FPT exhibited a stronger whitening effect, suggesting that the fermentation process may enhance the activity of active ingredients through biotransformation or generate new functional molecules. Furthermore, the inhibition of the MITF-TYR/TYRP signaling pathway may be one of the key mechanisms through which FPT exerts its whitening effect. Future research could further isolate and identify the active components in FPT and explore their molecular targets, providing a theoretical basis for the development of new whitening formulations.
3.6. Changes in Metabolites Before and After PT Fermentation by Rhodosporidium Toruloides
We employed UPLC-Q/TOF-MS characterization to analyze the changes in metabolites before and after PT Fermentation by Rhodosporidium toruloides. Both principal component analysis (PCA) and orthogonal partial least squares discriminant analysis (OPLS-DA) showed that the intragroup data of the fermented and non-fermented groups exhibited strong aggregation, with a clear separation between the groups. These imply significant metabolic changes before and after fermentation (Figure 6a–d).
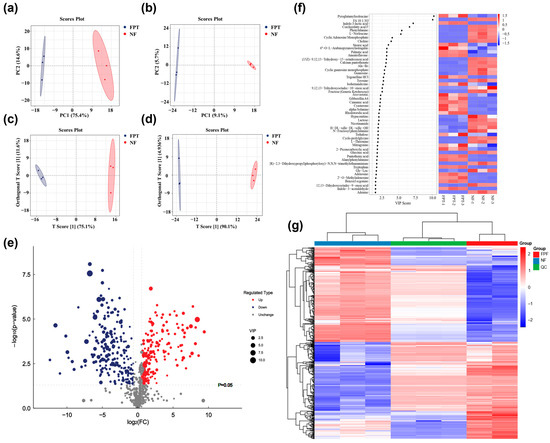
Figure 6.
Untargeted metabolomic analysis in PT fermentation by Rhodosporidium toruloides. PCA score chart of samples in the positive ion mode (a) and negative ion mode (b). OPLS-DA score chart in the positive ion mode (c) and in the negative ion mode (d). (e) Volcano plot of differential metabolites. (f) VIP score calculated by OPLS-DA. (g) Heatmap of differential metabolites.
To further explore the metabolic differences before and after PT fermentation, a total of 432 differential metabolites were identified based on a fold change of >1.5 and p < 0.05. Among them, 205 metabolites were upregulated and 227 were downregulated (Figure 6e). Notably, changes in polyphenolic compounds before and after fermentation were specifically analyzed (Table S2), leading to the identification of 15 upregulated polyphenols. These included xanthohumol, 2,3-dihydroxybenzoic acid, hispidulin and so on, all of which exhibited increased levels post-fermentation. Variable importance in projection (VIP) scores (Figure 6f) and heatmap analysis (Figure 6g) was applied to evaluate these differential metabolites. The top 30 metabolites with the highest VIP values were selected, primarily encompassing nucleotides and their derivatives, alkaloids, fatty acids, amino acids, carbohydrates, phenylpropanoids, and flavonoids.
Among the amino acids and their derivatives, pyroglutamylisoleucine and L-Norleucine showed significant differential expression. Pyroglutamylisoleucine, a cyclic peptide, may exert anti-glycation effects by inhibiting the formation of advanced AGEs [50]. On the other hand, the downregulation of L-Norleucine could reflect a redirection of protein metabolism during fermentation. Additionally, significant changes in phenylpropanoid metabolites, such as the flavonoid glycoside Amentoflavone and Indole-3-lactic acid, further suggest that the FPT may enhance its antioxidant and anti-glycation properties by modulating the levels of phenolic compounds. For example, the upregulation of Amentoflavone, a flavonoid glycoside, may exert a whitening effect by inhibiting tyrosinase activity [51]. Additionally, significant changes in tryptophan and its derivatives could influence the expression of key enzymes in the melanin synthesis pathway [52], further supporting the potential whitening application of the fermented PT product.
4. Conclusions
In conclusion, microbial fermentation profoundly enhances the multifunctional skincare potential of PT flower extracts. Through systematic screening, Rhodosporidium toruloides was identified as the optimal strain for the production of FPT with dramatically increased levels of phenolic and flavonoid compounds. The fermentation process resulted in a superior biological profile across multiple key mechanisms of skin aging and hyperpigmentation. FPT exhibited enhanced radical scavenging capacity and, more importantly, provided comprehensive cellular protection in a UVB-induced keratinocyte model by not only directly neutralizing ROS and inhibiting lipid peroxidation but also potently boosting the endogenous antioxidant defense system. A pivotal finding of this work is the concurrent and potent inhibition of both glycation and protein carbonylation. Furthermore, FPT effectively combated UVA-induced photoaging by attenuating cellular senescence, rebalancing extracellular matrix metabolism through suppressing MMP-1 and promoting Col-I synthesis, and mitigating inflammation. The extract also displayed significant anti-melanogenic effects by inhibiting tyrosinase activity and downregulating the central MITF-mediated signaling pathway. Untargeted metabolomics provided compelling evidence that the significant upregulation of phenylpropanoids and flavonoids, among other bioactive metabolites, offers a chemical basis for the observed superior efficacy.
Although the current in vitro results are promising, this study has several limitations that should be addressed through more systematic and in-depth investigations in the future. Firstly, the reliance solely on cell-based models, while useful for mechanistic insights, falls short of fully replicating the permeation, metabolism, and broader physiological effects of active compounds in human skin. Secondly, although metabolomic analyses indicated a marked upregulation of multiple components following fermentation, the specific compounds responsible for particular bioactivities—such as antioxidant, anti-glycation, and anti-melanogenic effects—remain to be clearly identified. Furthermore, dose–response relationships for certain efficacy endpoints warrant further validation across broader concentration ranges. Future research should focus on comprehensively elucidating the in vivo mechanisms of action of the fermented extract, isolating and identifying key active constituents, and evaluating critical formulation properties including safety, stability, and skin permeation. Additionally, exploring potential synergistic effects among metabolites generated during fermentation could help facilitate the translation of these findings into practical cosmetic and dermatological applications.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cosmetics12060243/s1, Figure S1: (a) Cytotoxic Effects of Different UVA Doses on HDF Cells Measured by MTT Assay. (b) Cytotoxic Effects of Different FPT concentration on HDF Cells Measured by MTT Assay; Figure S2. (a) Cytotoxic Effects of Different FPT concentration on B16F10 Cells Measured by MTT Assay. (b) Cytotoxic Effects of Different UVB Doses on B16F10 Cells Measured by MTT; Table S1: Viable Cell growth status from CP Fermented by Different Strains; Table S2: Differential Metabolites of polyphenols in NF and FPT.
Author Contributions
Writing—original draft, methodology, formal analysis, Q.L. and X.Y.; data curation, investigation, Q.L., H.Z., T.J. and R.H.; resources, software, supervision, T.J. and J.L.; conceptualization, writing—review and editing, supervision, X.Y. and J.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
Hui Zhu, Teng Jiang, and Rubiao Hou, the authors are employees of Brightday and Dream Biotechnology Co., Ltd. The remain authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The funders had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Ansary, T.M.; Hossain, M.R.; Kamiya, K.; Komine, M.; Ohtsuki, M. Inflammatory molecules associated with ultraviolet radiation-mediated skin aging. Int. J. Mol. Sci. 2021, 22, 3974. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Jayawardena, T.U.; Hyun, J.; Wang, K.; Fu, X.; Xu, J.; Gao, X.; Park, Y.; Jeon, Y.J. Antioxidant and anti-photoaging effects of a fucoidan isolated from Turbinaria ornata. Int. J. Biol. Macromol. 2023, 225, 1021–1027. [Google Scholar] [CrossRef]
- Pageon, H.; Zucchi, H.; Dai, Z.; Sell, D.R.; Strauch, C.M.; Monnier, V.M.; Asselineau, D. Biological effects induced by specific advanced glycation end products in the reconstructed skin model of aging. BioRes. Open Access 2015, 4, 54–64. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Z.; Song, X.; Cai, P.; Liu, Q. Ginsenoside CK and retinol on UVA-induced photoaging exert the synergistic effect through antioxidant and antiapoptotic mechanisms. Sci. Rep. 2025, 15, 16664. [Google Scholar] [CrossRef]
- Zeng, Q.; Zhou, F.; Lei, L.; Chen, J.; Lu, J.; Zhou, J.; Cao, K.; Gao, L.; Xia, F.; Ding, S. Ganoderma lucidum polysaccharides protect fibroblasts against UVB-induced photoaging. Mol. Med. Rep. 2017, 15, 111–116. [Google Scholar] [CrossRef]
- Qanash, H.; Alsalamah, S.A.; Bazaid, A.S.; Alghonaim, M.I.; Duhduh, A.; Hudani, I. Therapeutic potential of ozonated Ocimum basilicum L. from Saudi Arabia: Phytochemical characterization and enhanced bioactivities. Pharmaceuticals 2025, 18, 1223. [Google Scholar] [CrossRef]
- Elfitriani, E.; Raif, A.; Ginting, C.N.; Ikhtiari, R. Evaluation of antioxidant and anti-collagenase activity of Rosa damascena L. flower petal and receptacle extract. F1000Research 2020, 9, 716. [Google Scholar] [CrossRef]
- Song, Y.R.; Lim, W.C.; Han, A.; Lee, M.H.; Shin, E.J.; Lee, K.M.; Nam, T.G.; Lim, T.G. Rose petal extract (Rosa gallica) exerts skin whitening and anti-skin wrinkle effects. J. Med. Food 2020, 23, 870–878. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Liu, H.; Huang, Z.; Zhang, Y.; Lu, Y.; Zhou, Y. Evaluation of whitening effects and identification of potentially active compounds based on untargeted metabolomic analysis in different chrysanthemum cultivar extracts. Antioxidants 2024, 13, 1557. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Z.; Chen, S.; Zhang, Q.; Zhu, Y.; Shi, B.; Peng, K.; Pan, Q.; Lu, B. Anti-melanogenesis activity and chemical profiling of herbaceous peony (Paeonia lactiflora pall.) flowers: A natural skin whitening agents containing Paeonia monoterpene glycosides. Ind. Crops Prod. 2025, 225, 120363. [Google Scholar] [CrossRef]
- Maiti, S.; Moon, U.R.; Bera, P.; Samanta, T.; Mitra, A. The in vitro antioxidant capacities of Polianthes tuberosa L. flower extracts. Acta Physiol. Plant. 2014, 36, 2597–2605. [Google Scholar] [CrossRef]
- Rahmatullah, R.N.; Jannat, K.; Islam, M.; Rahman, T.; Jahan, R.; Rahmatullah, M. A short review of Polianthes tuberosa L. considered a medicinal plant in Bangladesh. J. Med. Plants Stud. 2019, 7, 1–3. [Google Scholar]
- Rahul, Y.; Debabandya, M.; Anakkallan, S.; Ahammed, S.T.; Kumar, G.S. Optimization of sequential ultrasound-microwave assisted extraction of polyphenols-rich concrete from tuberose flowers through modelling. Process Biochem. 2023, 134, 175–185. [Google Scholar] [CrossRef]
- Barghout, N.; Chebata, N.; Messgo-Moumene, S.; Khennouf, S.; Yekrelef, A.; El-Hadi, D. Polyphenols from Polianthes tuberosa L. (amaryllidaceae) leaves and their antioxidant properties. Rev. Agrobiol. 2018, 8, 896–901. [Google Scholar]
- Srinivasa Suryakoppa, K.; Appadurai, R.; Byrappa, K.; Khan, M.H.M. Phytochemical analysis of UV active and inactive bioactive compounds present in Polianthes tuberosa (Linn.) flower. J. Sep. Sci. 2021, 44, 3376–3385. [Google Scholar] [CrossRef] [PubMed]
- Matsuzawa, T.; Ishikawa, M.; Fujiwa, S.; Shimada, N.; Kanzaki, H. Solid-state cultivation of Aspergillus oryzae using insoluble plant cell wall polysaccharides and expression analyses of plant polysaccharide degradation-related enzymes. J. Biosci. Bioeng. 2025, 140, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Jabłońska-Ryś, E.; Przygoński, K. Possibilities of using the new Lactiplantibacillus plantarum EK11 Strain as a starter culture for the fermentation of the fruiting bodies of edible mushrooms. Foods 2025, 14, 2833. [Google Scholar] [CrossRef]
- Gong, R.; Zalán, Z.; Song, J.; Suo, H. Microbial fermentation of soybeans: Synergistic enhancement of bioactivity and sensory properties. Food Chem. X 2025, 30, 102924. [Google Scholar] [CrossRef]
- OuYang, Y.; Zou, S.; Liu, P.; Xie, L.; Xiao, Y.; Wang, Y.; Wu, G.; Liu, J.; Liu, B.; Gao, B.; et al. Synthetic microbial consortium enhances acetoin production and functional quality of citrus vinegar via metabolic and process optimization. Front. Microbiol. 2025, 16, 1664794. [Google Scholar] [CrossRef]
- Li, Y.H.; Liu, B.; Zhao, Z.B.; Bai, F.W. Optimized culture medium and fermentation conditions for lipid production by Rhodosporidium toruloides. Chin. J. Biotechnol. 2006, 22, 650–656. [Google Scholar] [CrossRef]
- Moreira, L.; Dias, L.G.; Pereira, J.A.; Estevinho, L. Antioxidant properties, total phenols and pollen analysis of propolis samples from Portugal. Food Chem. Toxicol. 2008, 46, 3482–3485. [Google Scholar] [CrossRef]
- Liu, D.; Han, J.; Lv, Z.; Dai, X. Analysis of flavonoids constituents in herb of air-plant and its antioxidant activity. Med. Plant 2012, 3, 18–22. [Google Scholar] [CrossRef]
- Baliyan, S.; Mukherjee, R.; Priyadarshini, A.; Vibhuti, A.; Gupta, A.; Pandey, R.P.; Chang, C.M. Determination of antioxidants by DPPH radical scavenging activity and quantitative phytochemical analysis of Ficus religiosa. Molecule. 2022, 27, 1326. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, R.P.; Singh, S.P.; Häder, D.P.; Sinha, R.P. Detection of reactive oxygen species (ROS) by the oxidant-sensing probe 2’,7’-dichlorodihydrofluorescein diacetate in the cyanobacterium Anabaena variabilis PCC 7937. Biochem. Biophys. Res. Commun. 2010, 397, 603–607. [Google Scholar] [CrossRef]
- Tsikas, D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal. Biochem. 2017, 524, 13–30. [Google Scholar] [CrossRef]
- Li, J.; Wang, G.; Zhang, Y.; Fan, X.; Yao, M. Protective effects of baicalin against L-glutamate-induced oxidative damage in HT-22 cells by inhibiting NLRP3 inflammasome activation via Nrf2/HO-1 signaling. Iran. J. Basic. Med. Sci. 2023, 26, 351–358. [Google Scholar] [CrossRef]
- Zhang, W.L.; Liu, M.Y.; Zhang, Z.C.; Duan, C.Y. Effect of different anesthesia methods on erythrocyte immune function in mice. Asian Pac. J. Trop. Med. 2013, 6, 995–998. [Google Scholar] [CrossRef]
- Zhao, M.-X.; Wen, J.-L.; Wang, L.; Wang, X.-P.; Chen, T.-S. Intracellular catalase activity instead of glutathione level dominates the resistance of cells to reactive oxygen species. Cell Stress. Chaperones 2019, 24, 609–619. [Google Scholar] [CrossRef]
- Cao, C.; Tan, Q.; Liu, L.; Yang, X.; Chen, H. Cell model research status and application prospects for the evaluation of anti-glycation efficacy. China Surfactant Deterg. Cosmet. 2023, 53, 1451–1458. [Google Scholar] [CrossRef]
- Luo, S.; Wehr, N.B. Protein carbonylation: Avoiding pitfalls in the 2,4-dinitrophenylhydrazine assay. Redox Rep. 2009, 14, 159–166. [Google Scholar] [CrossRef]
- Mohamad Kamal, N.S.; Safuan, S.; Shamsuddin, S.; Foroozandeh, P. Aging of the cells: Insight into cellular senescence and detection methods. Eur. J. Cell Biol. 2020, 99, 151108. [Google Scholar] [CrossRef]
- Beklen, A. Effects of IL-13 on TGF-β and MMP-1 in periodontitis. Biotech. Histochem. 2017, 92, 374–380. [Google Scholar] [CrossRef]
- Petpiroon, N.; Rosena, A.; Pimtong, W.; Charoenlappanit, S.; Koobkokkruad, T.; Roytrakul, S.; Aueviriyavit, S. Protective effects of Thai silk sericins and their related mechanisms on UVA-induced phototoxicity and melanogenesis: Investigation in primary melanocyte cells using a proteomic approach. Int. J. Biol. Macromol. 2022, 201, 75–84. [Google Scholar] [CrossRef]
- Chen, Y.M.; Su, W.C.; Li, C.; Shi, Y.; Chen, Q.X.; Zheng, J.; Tang, D.L.; Chen, S.M.; Wang, Q. Anti-melanogenesis of novel kojic acid derivatives in B16F10 cells and zebrafish. Int. J. Biol. Macromol. 2019, 123, 723–731. [Google Scholar] [CrossRef]
- Guo, X.; Luo, T.; Han, D.; Zhu, D.; Jiang, Z.; Wu, Z. Integrated transcriptomics, proteomics, and metabolomics analysis reveals the mechanism of litchi pulp deterioration during long-term cold storage. Postharvest Biol. Technol. 2023, 195, 112140. [Google Scholar] [CrossRef]
- Cheng, S. Free radical scavenging activities of polyphenols from Prunus humilis bge fruit. Food Sci. 2007, 28, 57–61. [Google Scholar] [CrossRef]
- Ling, Z.; Wan, J.; Hu, M.; Chen, J.; Wei, P. Effects of polyphenol extract from Zanthoxylum bungeanum maxim. on active oxygen species and lipid peroxidation. Lishizhen Med. Mater. Medica Res. 2009, 20, 1941–1943. [Google Scholar] [CrossRef]
- Gülçin, İ.; Huyut, Z.; Elmastaş, M.; Aboul-Enein, H.Y. Radical scavenging and antioxidant activity of tannic acid. Arab. J. Chem. 2010, 3, 43–53. [Google Scholar] [CrossRef]
- Mo, Z.; Dai, Y.; Jiang, N. Mechanism of ultraviolet-induced lipid peroxidation and related skin diseases. Int. J. Dermatol. Venereol. 2017, 43, 231–234. [Google Scholar] [CrossRef]
- Yan, Y.; Su, G.; He, Y. Effects of solid-state fermentation with Aspergillus niger on phenolics release and antioxidant activity of Sugarcane leaves. Food Sci. 2020, 41, 110–116. [Google Scholar] [CrossRef]
- Liu, S.; Li, K.; Wang, S.; Qiao, X.; Ye, M. Glycosylation of natural products catalyzed by microorganisms and their enzymes. Progress. Pharm. Sci. 2018, 42, 14–20. [Google Scholar]
- Lin, D.; Zheng, Z.; Zhou, Y.; Gong, W.; Zhu, Z.; Yan, L.; Hu, Z.; Peng, Y.; Xie, C. Research progress in biotransformation and biological activity of polyphenols in plant-based foods. Food Sci. 2024, 45, 319–327. [Google Scholar] [CrossRef]
- Liu, S.; He, Y.; He, W.; Song, X.; Peng, Y.; Hu, X.; Bian, S.; Li, Y.; Nie, S.; Yin, J.; et al. Exploring the biogenic transformation mechanism of polyphenols by Lactobacillus plantarum NCU137 fermentation and its enhancement of antioxidant properties in wolfberry juice. J. Agric. Food Chem. 2024, 72, 12752–12761. [Google Scholar] [CrossRef]
- Lu, Y.; Lu, M.; Wang, J.; Jiang, X.; Lu, Y.; Qiu, C.; Lv, L.; Dong, W. Inhibitory activity on the formation of reactive carbonyl species in edible oil by synthetic polyphenol antioxidants. J. Agric. Food Chem. 2021, 69, 9025–9033. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Yang, Z.; Wu, Q.; Xia, Y.; Liu, G.; Chu, L.; He, F. Research advance of probiotics in the fermentation of Chinese herbal medicine. J. Univ. Shanghai Sci. Technol. 2024, 46, 357–363. [Google Scholar] [CrossRef]
- Yu, X.; Hu, J.; Huang, X.; Hu, X. Research progress on the applications of active substances from fermented plant materials in cosmetics. Mod. Food Sci. Technol. 2024, 40, 373–378. [Google Scholar] [CrossRef]
- Dong, J.; Cai, L.; Li, X.; Ding, Z. Progress in fermented traditional Chinese medicines with microorganism. J. Yunnan Univ. (Nat. Sci. Ed.) 2018, 40, 1207–1212. [Google Scholar] [CrossRef]
- Blontrock, E.; Lambrechts, E.; Janssen, F.; De Bondt, Y.; Vanhove, S.; Lemoine, J.; Courtin, C.M.; Wouters, A.G.B. Enzyme activity and constituent extractability of kilned and non-kilned oats at pH values relevant for acidic food fermentations. Food Chem. X 2025, 29, 102834. [Google Scholar] [CrossRef]
- Zhu, Y.; Yaylayan, V.A. Interaction of free arginine and guanidine with glucose under thermal processing conditions and formation of Amadori-derived imidazolones. Food Chem. 2017, 220, 87–92. [Google Scholar] [CrossRef]
- Gui, H.; Tang, T.; Yan, H.; Lei, Z. Whitening effect of flavonoid materials in Radix puerariae. China Surfactant Deterg. Cosmet. 2013, 43, 290–293. [Google Scholar] [CrossRef]
- Stephany, F.B.M.; Barros, S.M.B.; Ansanelo, C.G.; Jacqueline, C.; Moraes, B.S.B.d.; Orsati, C.R.; Stuchi, M.S.; Ana, C. Kynurenine inhibits melanogenesis in human melanocyte-keratinocyte co-cultures and in a reconstructed 3D skin model. Exp. Dermatol. 2021, 31, 427–432. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).