Selective Regulatory Effects of Lactobacillus Plantarum Fermented Milk: Enhancing the Growth of Staphylococcus Epidermidis and Inhibiting Staphylococcus aureus and Escherichia coli
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Milk Enzymatic Solution (PM) and Fermentation Solution (FM)
2.3. Effects of PM and FM on the Growth of Bacteria in Monoculture
2.4. Effects of PM and FM on the Growth of Bacteria in Co-Culture
2.5. Metabolomic Analysis
2.5.1. Extraction of Metabolites
2.5.2. Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS) Analysis
2.5.3. Data Preprocessing and Annotation
2.5.4. Metabolite Analysis
2.6. Statistical Analysis
3. Results and Discussion
3.1. Effects of PM and FM on the Growth of Bacteria in Monoculture
3.2. Effects of PM and FM on the Growth of Bacteria in Co-Culture
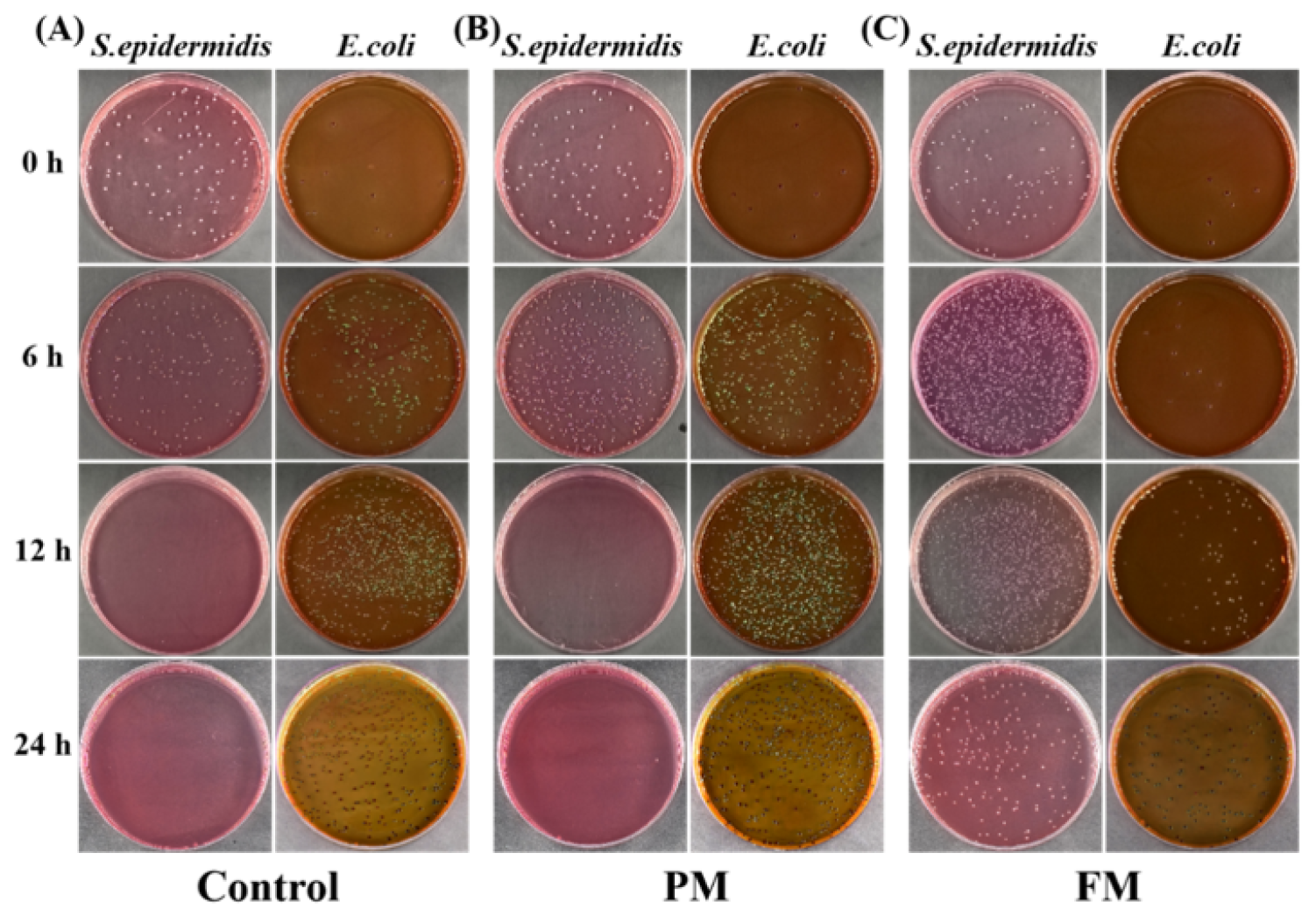
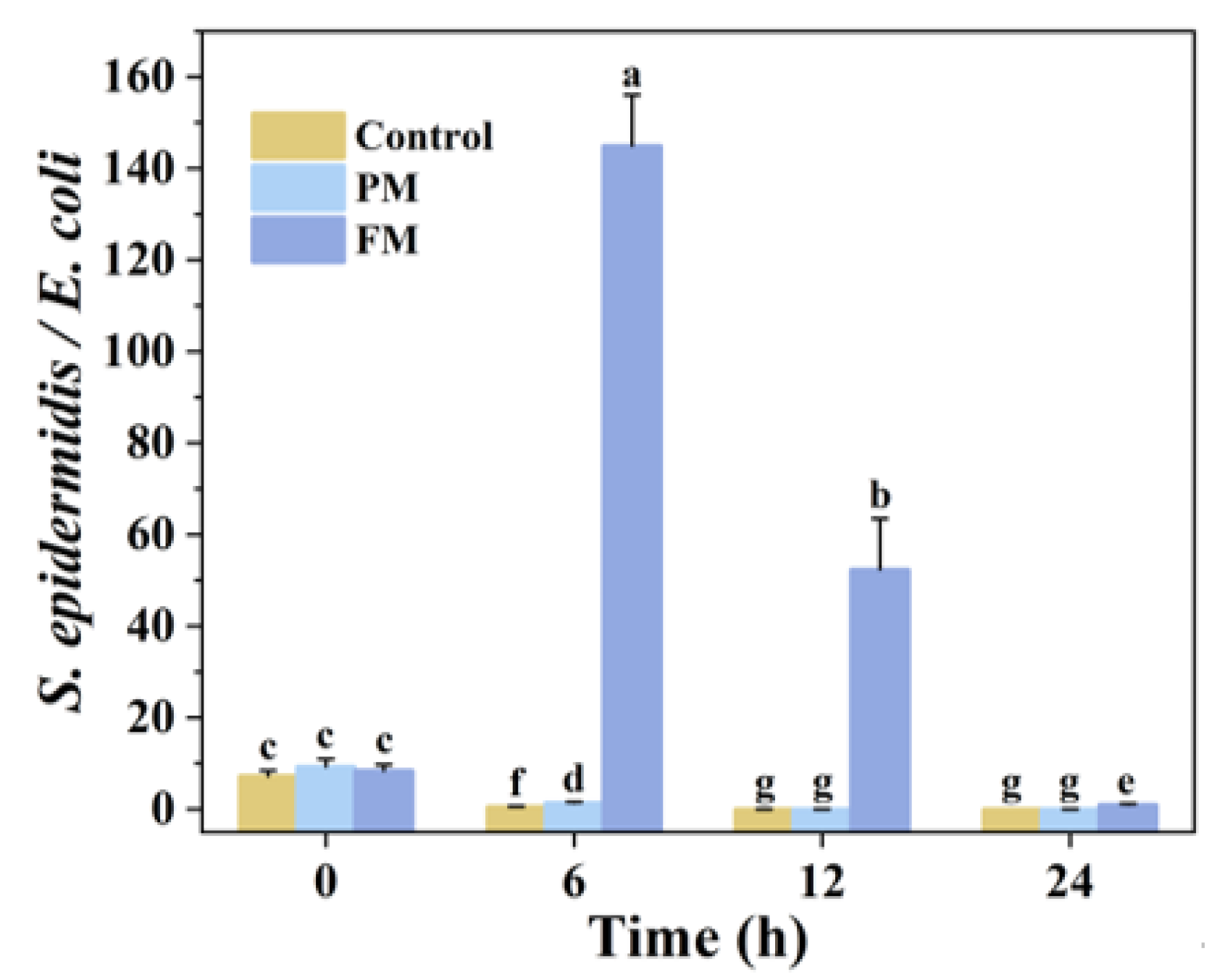
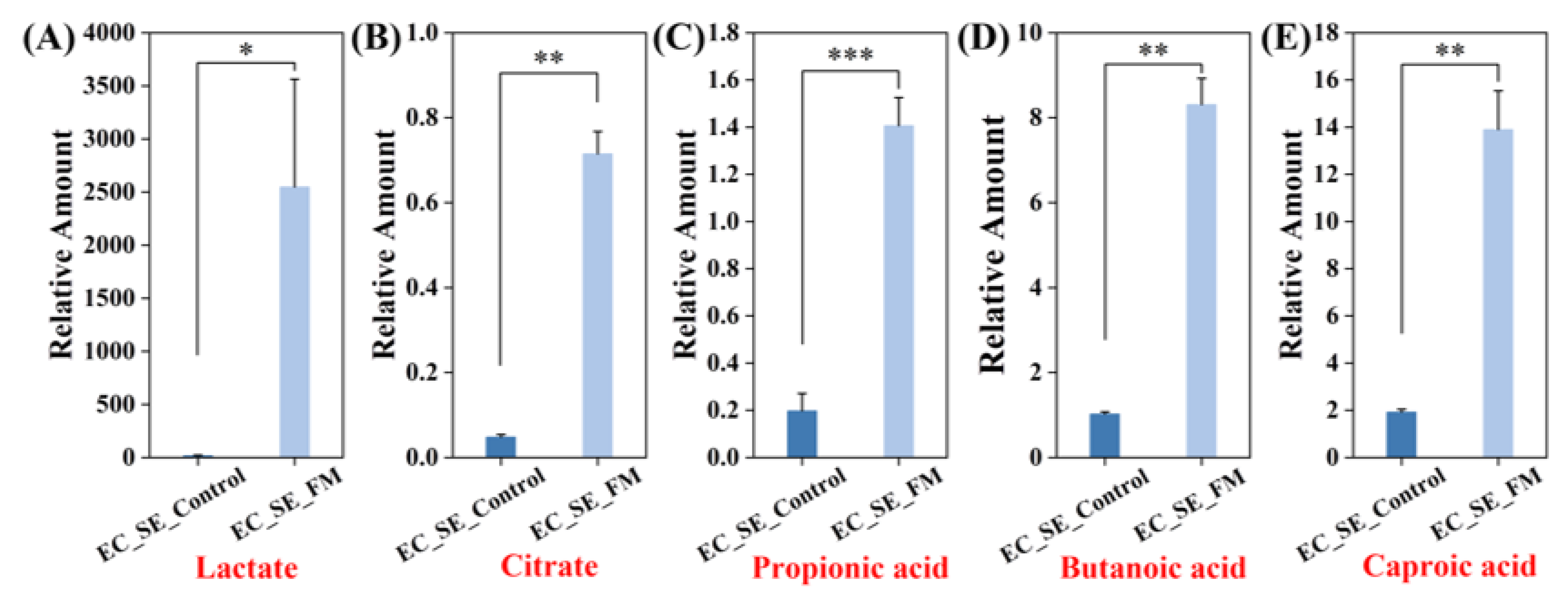
3.2.1. E. coli and S. epidermidis in Co-Culture (EC_SE)
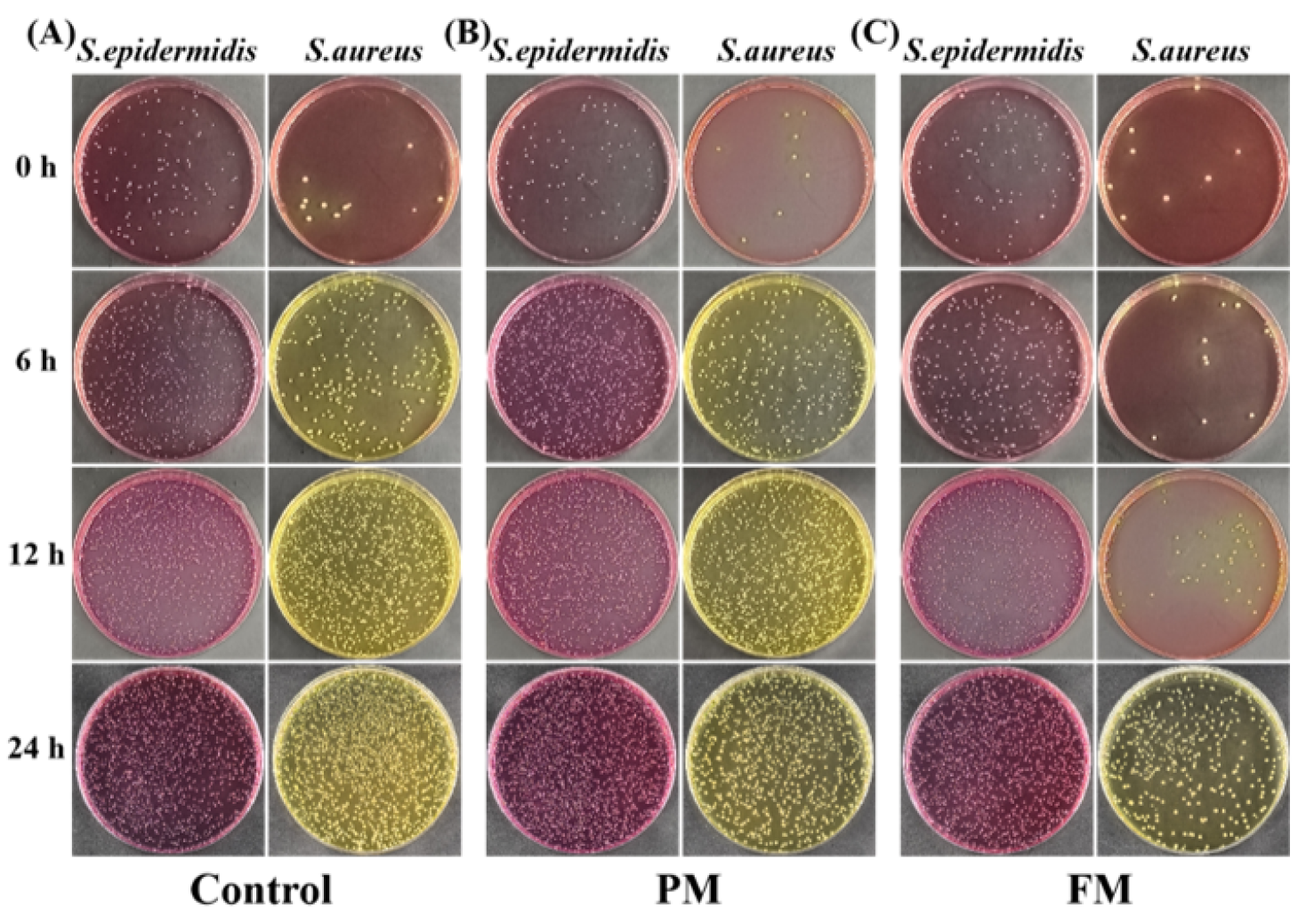
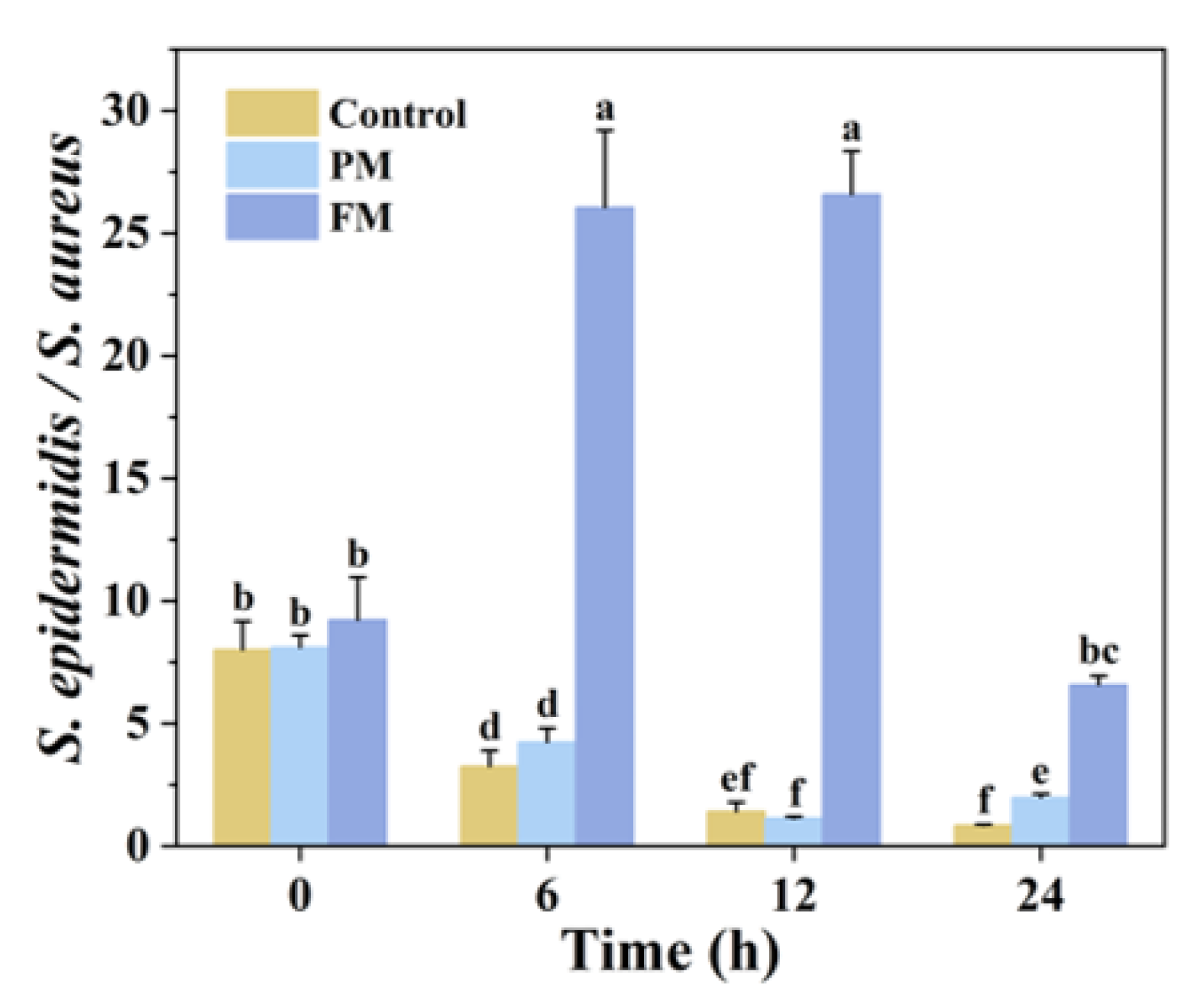
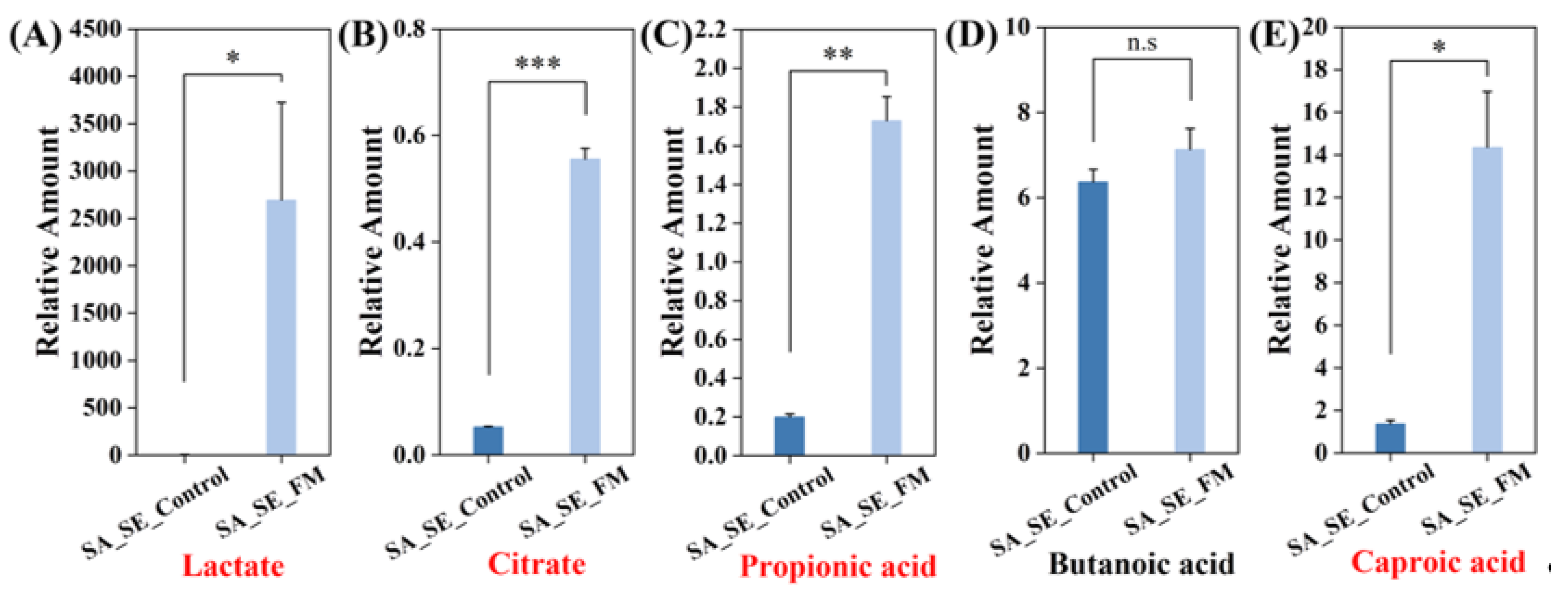
3.2.2. S. aureus and S. epidermidis in Co-Culture (SA_SE)
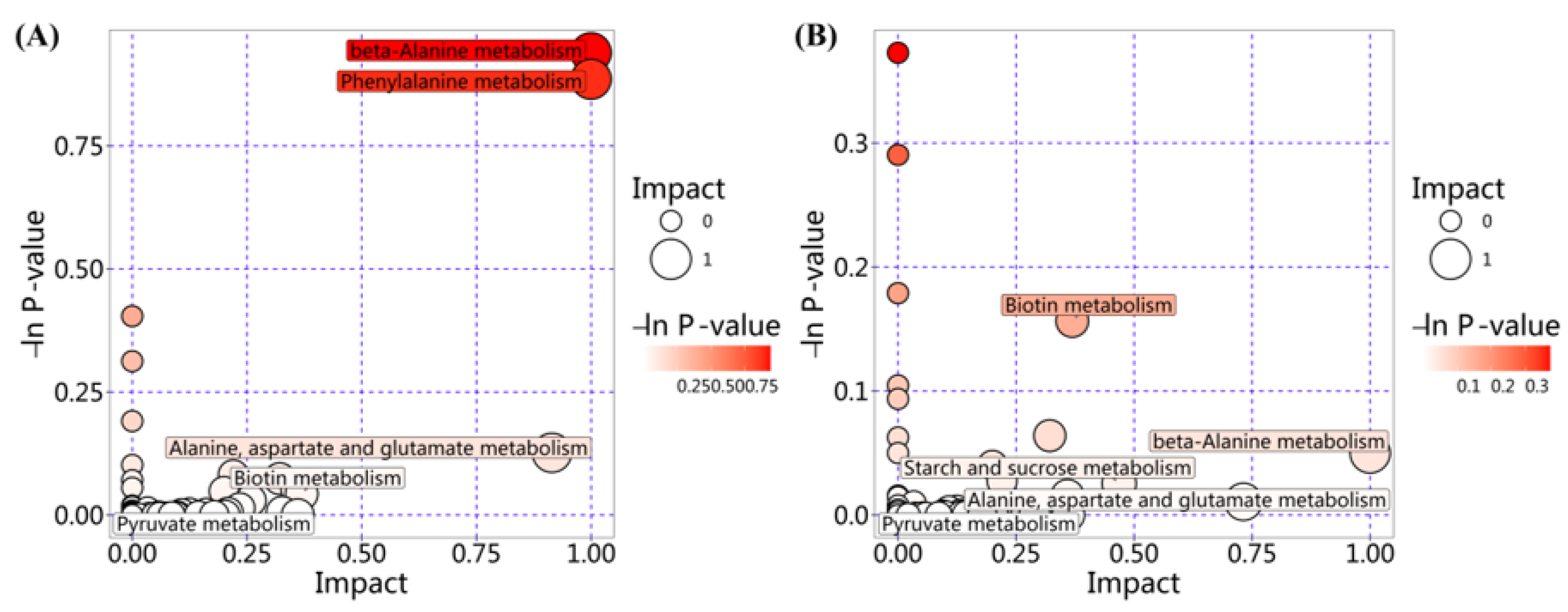
3.3. Identification of Differential Metabolites in the Co-Culture Group
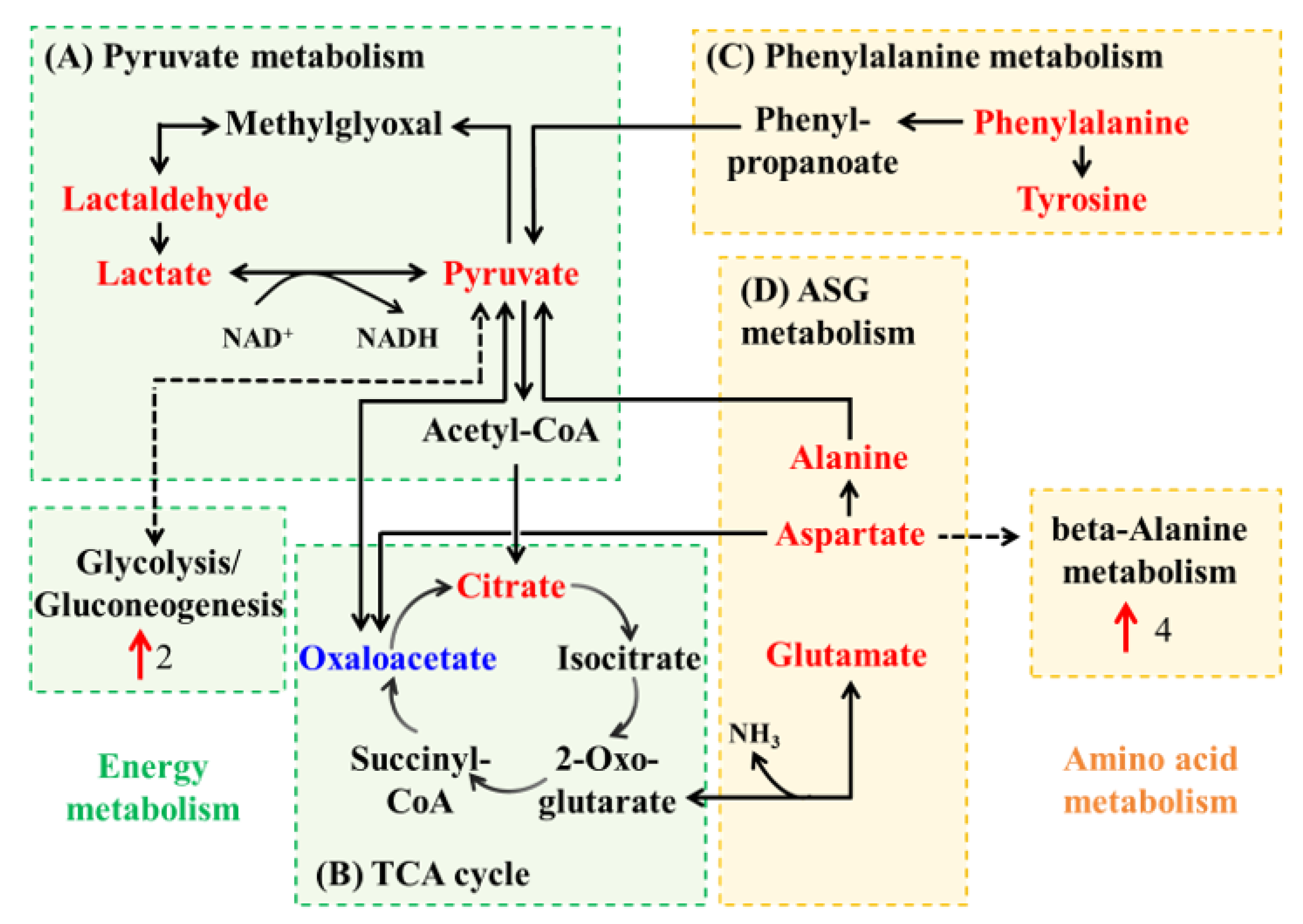
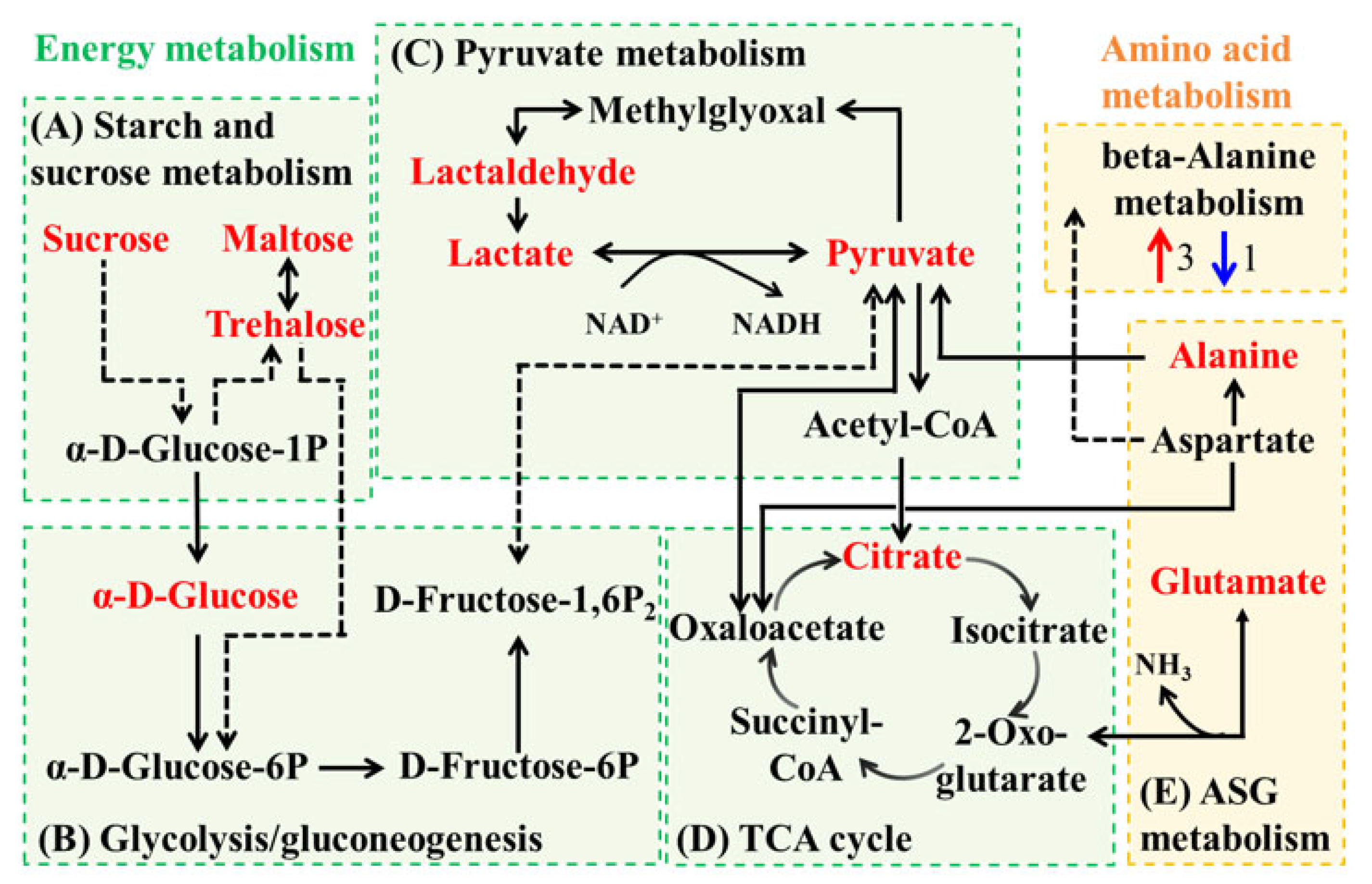
3.4. Metabolic Network Analysis of Co-Culture Groups
3.4.1. Metabolic Network Analysis for Group EC_SE_Control vs. EC_SE_FM
3.4.2. Metabolic Network Analysis for Group SA_SE_Control vs. SA_SE_FM
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Skowron, K.; Bauza-Kaszewska, J.; Kraszewska, Z.; Wiktorczyk-Kapischke, N.; Grudlewska-Buda, K.; Kwiecińska-Piróg, J.; Wałecka-Zacharska, E.; Radtke, L.; Gospodarek-Komkowska, E. Human skin microbiome: Impact of intrinsic and extrinsic factors on skin microbiota. Microorganisms 2021, 9, 543. [Google Scholar] [CrossRef]
- Belkaid, Y.; Tamoutounour, S. The influence of skin microorganisms on cutaneous immunity. Nat. Rev. Immunol. 2016, 16, 353–366. [Google Scholar] [CrossRef]
- Leonel, C.; Sena, I.F.G.; Silva, W.N.; Prazeres, P.H.D.M.; Fernandes, G.R.; Mancha Agresti, P.M.; Drumond, M.M.; Mintz, A.; Azevedo, V.A.C.; Birbrair, A. Staphylococcus epidermidis role in the skin microenvironment. J. Cell Mol. Med. 2019, 23, 5949–5955. [Google Scholar] [CrossRef]
- Williams, M.R.; Gallo, R.L. Evidence that human skin microbiome dysbiosis promotes atopic dermatitis. J. Investig. Dermatol. 2017, 137, 2460–2461. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Wu, Y.; Fischer, J.; Bartels, J.; Schröder, J.M.; Meyer-Hoffert, U. Skin-derived SPINK9 kills Escherichia coli. J. Investig. Dermatol. 2019, 139, 1135–1142. [Google Scholar] [CrossRef]
- Harris-Tryon, T.A.; Grice, E.A. Microbiota and maintenance of skin barrier function. Science 2022, 376, 940–945. [Google Scholar] [CrossRef] [PubMed]
- Byrd, A.L.; Belkaid, Y.; Segre, J.A. The human skin microbiome. Nat. Rev. Microbiol. 2018, 16, 143–155. [Google Scholar] [CrossRef]
- Yang, Y.; Qu, L.; Mijakovic, I.; Wei, Y. Advances in the human skin microbiota and its roles in cutaneous diseases. Microb. Cell Factories 2022, 21, 176–190. [Google Scholar] [CrossRef]
- Boxberger, M.; Cenizo, V.; Cassir, N.; La Scola, B. Challenges in exploring and manipulating the human skin microbiome. Microbiome 2021, 9, 125. [Google Scholar] [CrossRef]
- Ruddell, S.A.; Mostert, D.; Sieber, S.A. Target identification of usnic acid in bacterial and human cells. RSC Chem. Biol. 2024, 5, 617–621. [Google Scholar] [CrossRef] [PubMed]
- Valookolaei, F.S.G.; Sazegar, H.; Rouhi, L. Limonene encapsulated alginate/collagen as antibiofilm drug against Acinetobacter baumannii. BMC Biotechnol. 2024, 24, 86–101. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, X.; Zhao, L.; Gu, Y.; Chen, Y.; Liu, N.; An, L.; Lu, Y.; Cui, S. Effect of leave-on cosmetic antimicrobial preservatives on healthy skin resident Staphylococcus epidermidis. J. Cosmet. Dermatol. 2023, 22, 2115–2121. [Google Scholar]
- De Almeida, C.V.; Antiga, E.; Lulli, M. Oral and topical probiotics and postbiotics in skincare and dermatological therapy: A concise review. Microorganisms 2023, 11, 1420–1437. [Google Scholar] [CrossRef]
- Han, J.H.; Kim, H.S. Skin deep: The potential of microbiome cosmetics. J. Microbiol. 2024, 62, 181–199. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, B.; Ding, Y.; Liu, N.; Yang, C.; Sun, Y. Improved anti-oxidant and anti-bacterial capacities of skim milk fermented by Lactobacillus plantarum. Molecules 2024, 29, 3800–3813. [Google Scholar] [CrossRef] [PubMed]
- Rajapitamahuni, S.; Lyou, E.S.; Kang, B.R.; Lee, T.K. Microbial interaction-induced siderophore dynamics lead to phenotypic differentiation of Staphylococcus aureus. Front. Cell Infect. Microbiol. 2023, 13, 1277176–1277185. [Google Scholar] [CrossRef] [PubMed]
- Pinu, F.; Villas-Boas, S.; Aggio, R. Analysis of intracellular metabolites from microorganisms: Quenching and extraction protocols. Metabolites 2017, 7, 53–72. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Ma, H.; Gan, N.; Wang, H.; Li, Y.; Wang, L.; Song, L. Non-targeted metabolomic profiling of filamentous cyanobacteria aphanizomenon flos-aquae exposed to a concentrated culture filtrate of Microcystis aeruginosa. Harmful Algae 2022, 11, 102170–102178. [Google Scholar] [CrossRef]
- Ohn, H.; Mizuno, T.; Miyoshi, S. Inhibitory effects of Escherichia coli on the formation and development of Staphylococcus epidermidis biofilm. Biocontrol. Sci. 2021, 26, 113–118. [Google Scholar] [CrossRef]
- Kline, K.A.; Costa, F.G.; Horswill, A.R. Overcoming pH defenses on the skin to establish infections. PLoS Pathog. 2022, 18, e1010512. [Google Scholar]
- Wang, Q.Z.; Wu, C.Y.; Chen, T.; Chen, X.; Zhao, X.M. Integrating metabolomics into a systems biology framework to exploit metabolic complexity: Strategies and applications in microorganisms. Appl. Microbiol. Biotechnol. 2006, 70, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Lai, J.L.; Li, J.; Zhang, Y.; Luo, X.G.; Han, M.W.; Zhu, Y.B.; Zhao, S.P. Biodegradation and physiological response mechanism of Bacillus aryabhattai to cyclotetramethylenete-tranitramine (HMX) contamination. J. Environ. Manag. 2021, 288, 112247–112256. [Google Scholar] [CrossRef]
- Wu, R.T.; Chen, J.Y.; Liu, S.; Niu, S.H.; Liao, X.D.; Xing, S.C. Cyclic AMP and biofilms reveal the synergistic proliferation strategy of Pseudomonas aeruginosa and Escherichia coli under the costimulation of high concentrations of microplastics and enrofloxacin. Sci. Total Environ. 2022, 838, 156740–156750. [Google Scholar]
- Hao, H.; Zhang, X.; Chen, S.; Lan, S.; Li, Z.; Liu, S.; Yan, X.; Gao, P.; Chu, Y. Comparative untargeted and targeted metabonomics reveal discriminations in metabolite profiles between Mycoplasma capricolum subsp. capripneumoniae and Mycoplasma capricolum subsp. capricolum. Front. Microbiol. 2023, 14, 1294055–1294067. [Google Scholar] [CrossRef]
- Negari, I.P.; Keshari, S.; Huang, C.M. Probiotic activity of Staphylococcus epidermidis induces collagen Type I production through FFaR2/p-ERK signaling. Int. J. Mol. Sci. 2021, 22, 1414–1427. [Google Scholar] [CrossRef]
- Hwang, J.; Thompson, A.; Jaros, J.; Blackcloud, P.; Hsiao, J.; Shi, V.Y. Updated understanding of Staphylococcus aureus in atopic dermatitis: From virulence factors to commensals and clonal complexes. Exp. Dermatol. 2021, 30, 1532–1545. [Google Scholar] [CrossRef] [PubMed]
- Salgaonkar, N.; Kadamkode, V.; Kumaran, S.; Mallemala, P.; Christy, E.; Appavoo, S.; Majumdar, A.; Mitra, R.; Dasgupta, A. Glycerol fermentation by skin bacteria generates lactic acid and upregulates the expression levels of genes associated with the skin barrier function. Exp. Dermatol. 2022, 31, 1364–1372. [Google Scholar] [CrossRef]
- Krulwich, T.A.; Sachs, G.; Padan, E. Molecular aspects of bacterial pH sensing and homeostasis. Nat. Rev. Microbiol. 2011, 9, 330–343. [Google Scholar] [CrossRef]
- Sfriso, R.; Egert, M.; Gempeler, M.; Voegeli, R.; Campiche, R. Revealing the secret life of skin-with the microbiome you never walk alone. Int. J. Cosmet. Sci. 2019, 42, 116–126. [Google Scholar]
- Su, L.C.; Xie, Z.; Zhang, Y.; Nguyen, K.T.; Yang, J. Study on the antimicrobial properties of citrate-based biodegradable polymers. Front. Bioeng. Biotechnol. 2014, 2, 23–31. [Google Scholar] [CrossRef]
- Loi, V.V.; Busche, T.; Kuropka, B.; Müller, S.; Methling, K.; Lalk, M.; Kalinowski, J.; Antelmann, H. Staphylococcus aureus adapts to the immunometabolite itaconic acid by inducing acid and oxidative stress responses including S-bacillithiolations and S-itaconations. Free Radic. Biol. Med. 2023, 208, 859–876. [Google Scholar] [CrossRef] [PubMed]
- Lund, P.A.; Biase, D.D.; Liran, O.; Scheler, O.; Mira, N.P.; Cetecioglu, Z.; Fernández, E.N.; Bover-Cid, S.; Hall, R.; Sauer, M.; et al. Understanding how microorganisms respond to acid pH is central to their control and successful exploitation. Front. Microbiol. 2020, 11, 556140–556147. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Wang, Y.; Ren, Z.; Wang, S.; Ding, Y.; Liu, N.; Yang, C.; Zhao, B. Selective Regulatory Effects of Lactobacillus Plantarum Fermented Milk: Enhancing the Growth of Staphylococcus Epidermidis and Inhibiting Staphylococcus aureus and Escherichia coli. Cosmetics 2025, 12, 232. https://doi.org/10.3390/cosmetics12050232
Sun Y, Wang Y, Ren Z, Wang S, Ding Y, Liu N, Yang C, Zhao B. Selective Regulatory Effects of Lactobacillus Plantarum Fermented Milk: Enhancing the Growth of Staphylococcus Epidermidis and Inhibiting Staphylococcus aureus and Escherichia coli. Cosmetics. 2025; 12(5):232. https://doi.org/10.3390/cosmetics12050232
Chicago/Turabian StyleSun, Yajuan, Ying Wang, Zixia Ren, Shasha Wang, Yun Ding, Nan Liu, Cheng Yang, and Bingtian Zhao. 2025. "Selective Regulatory Effects of Lactobacillus Plantarum Fermented Milk: Enhancing the Growth of Staphylococcus Epidermidis and Inhibiting Staphylococcus aureus and Escherichia coli" Cosmetics 12, no. 5: 232. https://doi.org/10.3390/cosmetics12050232
APA StyleSun, Y., Wang, Y., Ren, Z., Wang, S., Ding, Y., Liu, N., Yang, C., & Zhao, B. (2025). Selective Regulatory Effects of Lactobacillus Plantarum Fermented Milk: Enhancing the Growth of Staphylococcus Epidermidis and Inhibiting Staphylococcus aureus and Escherichia coli. Cosmetics, 12(5), 232. https://doi.org/10.3390/cosmetics12050232







