Abstract
(1) Background: The pursuit of ingredients that possess both anti-melanogenesis and post-inflammatory hyperpigmentation (PIH) prevention effects has become a new research frontier in cosmetics, though there is little work on plant extract-derived ingredients in this direction. (2) Methods: The study involved evaluating the impact of dark tea extract on melanin content and tyrosinase activity in B16 cells. Meanwhile, Ultraviolet B (UVB)-irradiated assays were conducted on HaCaT cells to assess the secretion of inflammatory factors (IL-1α and IL-1β) and paracrine melanogenic factors (α-MSH, bFGF, and ET-1). Additionally, we performed quantitative real-time polymerase chain reaction (RT-PCR) tests to determine whether the signaling pathways of anti-melanogenesis and PIH punctuation are incorrect. (3) Results: The results showed that dark tea extract significantly inhibited melanin content and tyrosinase activity in B16 cells. In HaCaT cells, the extract reduced the secretion of the aforementioned inflammatory and paracrine melanogenic factors, thereby inhibiting PIH. Moreover, the RT-PCR and the Western Blot results indicated that the dark tea extract could inhibit the melanogenesis signaling pathway of α-MSH/MC1R/MITF and their downstream multiple targets of TYRP-1, TYRP-2, and TYR in B16 cells, while it exerted a PIH inhibition effect by downregulating the p38 MAPK/Nrf2/HO-1 signaling pathway. (4) Conclusions: This study suggests that dark tea extract can not only suppress melanogenesis through multiple targets but also can inhibit UVB-induced PIH, hinting at its skin-brightening efficacy as an agent for the restoration of pigmentation disorders.
1. Introduction
Hyperpigmentation disorders are distressing conditions that are common in all skin types. Among various factors, post-inflammatory hyperpigmentation (PIH) is characterized by an excess secretion of melanin in the epidermis and/or dermis resulting from cutaneous inflammatory insult [1,2], and darkening of the skin is due to the activation of melanogenesis by inflammatory mediators. Therefore, PIH represents a major cosmetic concern associated with conventional skin lightening ingredients, due to both rebound hyperpigmentation and other cutaneous issues—particularly in individuals with darker skin tones—and is often exacerbated by ultraviolet radiation and prolonged sun exposure [3]. Since PIH often develops in sun-exposed areas of the body, especially the face, and can last for several years, it not only compromises skin health/aesthetic appearance but also causes emotional distress, resulting in social and mental health burdens for affected individuals. Common cosmetic agents for post-inflammatory hyperpigmentation (PIH)—including retinoids, topical corticosteroids, arbutin, salicylic acid, mandelic acid, azelaic acid, and vitamin C derivatives—exhibit efficacy but pose challenges. Retinoids and acids may induce irritant dermatitis; topical corticosteroids can cause epidermal atrophy and telangiectasia; some treatments (chemical peels) risk paradoxical hyperpigmentation. Thus, a need persists for novel, well-tolerated ingredients offering enhanced efficacy and stability in skin brightening [4,5,6]. Consequently, the field of cosmetic science continues to actively research and develop novel skin-brightening agents that offer a more favorable profile in terms of efficacy, safety, and stability.
Dark tea (Camellia sinensis (L.) Kuntze, genus Camellia in the family Theaceae) is a traditional fermented tea that has attracted much attention because of its unique processing technology and the richly contained bioactive components. Dark tea extracts (DTE) have been reported to contain a variety of components, including tea polyphenols, theaflavins, thearubigins, etc. [7], with antioxidant, hypolipidemic, hypoglycemic, and other health benefits, thus attracting wide attention in the health field [8,9,10]. Though dark tea has received wide attention in food science [11,12,13], its anti-melanogenesis and PIH amelioration effects for realizing both skin-brightening efficacy and agents for the restoration of pigmentation disorders as a cosmetic ingredient, as well as its underlying mechanism, have not been revealed.
In this study, we investigated the efficacy of extracts in dark tea (from Anhua, Hunan province, a well-known black tea production area in China) for anti-melanogenesis and PIH alleviation effects, as well as their underlying mechanisms, by resorting to in vitro cell experiments. The results indicated that DTE inhibited melanin content and tyrosinase (TYR) activity in B16 cells. Moreover, it reduced the secretion of inflammatory factors (IL-1α and IL-1β) and melanogenic paracrine factors (ET-1, α-MSH, and bFGF) in UVB-irradiated HaCaT keratinocytes, indicating its PIH alleviation effect. The mechanisms by which DTE regulated signaling pathways in B16 cells and HaCaT cells were also investigated by quantitative real-time polymerase chain reaction (qPCR) and Western Blot analysis, validating its anti-oxidative, anti-inflammatory, and anti-melanogenesis pathways.
2. Materials and Methods
2.1. Preparation of DTE
The dark tea (genus Camellia in the family Theaceae, Herbarium of the Institute of Botany, Chinese Academy of Sciences (Beijing, China), PE02222162) (100 g) and deionized water (600 g) were mixed and stirred at 55 °C for 30 min. Then, the extract solution was filtered (by a 600-mesh filter, with 10, 0.8, 0.45, and 0.22 μm filter membranes) for filtrate collection.
2.2. Determination of Total Phenolics in DTE
The polyphenol quantification protocol was modified from Zhang’s method [14]. That is, the gallic acid equivalent (GAE, 10, 20, 30, 40, and 50 μg/mL) was first prepared. Aliquots (1.0 mL) of GAE were mixed with 3.0 mL of 10% Folin–Ciocalteu reagent at room temperature for 5 min. Then, 4.0 mL saturated sodium carbonate was added. After 1 h, the absorbance of the mixed solution was measured at 765 nm using a multimode microplate reader (Aosheng Instrument Co., Hangzhou, China). Thus, the calibration curve was established with results expressed as GAE.
The DTE (0.1 μL) was diluted in deionized water (29.9 mL). Similar detection procedures were conducted as described above by replacing the GAE with a BET solution. The total phenol content in BET was calculated according to the gallic acid standard curve, and the result was expressed as GAE.
2.3. Chemical Composition Determination of DTE
Chromatographic analysis was carried out on a Vanquish UHPLC system (Thermo Fisher Scientific, Erlangen, Germany) equipped with an ACQUITY UPLC HSS T3 column (Waters, Beijing, China) (2.1 mm × 100 mm, 1.8 μm) maintained at 35 °C. The mobile phase consisted of 0.1% formic acid in water (eluent A) and 0.1% formic acid in acetonitrile (eluent B), delivered at a flow rate of 0.3 mL/min.
Mass spectrometric detection was performed using a Q-Exactive HFX mass spectrometer (Thermo Fisher Scientific, Erlangen, Germany) coupled online with the UPLC system. The instrument was operated with electrospray ionization in both positive and negative ion modes, applying spray voltages of 3800 V for ESI+ and 3500 V for ESI−. Other key parameters included the sheath gas flow rate at 45 arbitrary units, auxiliary gas at 20 arbitrary units, capillary temperature set to 320 °C, and vaporizer temperature at 350 °C. Full-scan MS data were acquired at a resolution of 60,000, followed by data-dependent MS2 scans (resolution 15,000) targeting the ten most intense ions.
2.4. Cell Cultures
Human keratinocyte (HaCa, Cell Bank of Type Culture Collection of Chinese Academy of Sciences China (Shanghai, China)) cells were cultured at 37 °C in a humidified CO2 incubator (95% air, 5% CO2) (Bosun Medical Biological Instrument Co., Shanghai, China) in DMEM medium (Gibco, New York, NY, USA) supplemented with 10% fetal bovine serum. Murine B16 melanoma (Cell Bank of Type Culture Collection of Chinese Academy of Sciences China (Shanghai, China)) cells were cultured at 37 °C in a humidified CO2 incubator (95% air, 5% CO2) in 1640 medium (Gibco, New York, NY, USA) supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin.
2.5. Melanin Content and Tyrosinase Activity Assays in B16 Cells
2.5.1. Cell Viability of B16 Cells
The DTE solution was filtered through a 0.22 μm membrane before usage. The B16 cells (2 × 105/mL) were collected and seeded into 96-well plates with 100 µL per well. After being cultured for 24 h, experimental groups were treated with 100 µL of different DTE (0.75, 1, 2.5, 5, 7.5, 10, 25, 50, 75, and 100 μL/mL) concentrations, while control groups were treated with 100 µL of 1640 medium. After incubating them for 24 h and discarding the culture medium, 100 μL of 0.5 mg/mL 3-(4,5-dimethylthiaxolone-2-yl)-2,5-diphenyl tetra-zoliumbromide (MTT, Sigma-Aldrich, St. Louis, MO, USA) solution was added to each well. After 4 h of incubation in the incubator, 100 μL of DMSO (National Pharmaceutical Group Corporation (Sinopharm) Chemical Reagent Co., Beijing, China) solution was added to each well to dissolve the formazan crystals. Finally, the absorbance of each well was measured at an absorbance of 490 nm.
2.5.2. Melanin Content Assay
The detection of melanin content in B16 cells used the reported NaOH lysis method [15]. B16 cells (2 × 105/mL) were seeded in 6-well plates with 2 mL per well. After 24 h of incubation, control groups were treated with the 1640 medium, model groups were treated with 250 nM of α-melanocyte-stimulating hormone (α-MSH), and experimental groups were treated with the mixture of 250 nM α-MSH and DTE (at 2.5, 5.0, or 7.5 μL/mL). After culturing again for 24 h, the cells were collected into a centrifuge tube and centrifuged (Shanghai Luxiangyi Centrifuge Instrument Co, Shanghai, China) twice at 1500 r/min for 10 min. Then, 1.0 mol/L of NaOH solution (10% DMSO) was added, and the tube was water-bathed at 80 °C for 1 h to extract the melanin from cells. Finally, the absorbance of each well was measured at 405 nm.
2.5.3. Tyrosinase Activity Assay
B16 cells (2 × 105/mL) were seeded in 6-well plates with 2 mL per well. After 24 h of incubation, control groups were treated with the 1640 medium, model groups were treated with 250 nM of α-MSH, and experimental groups were treated with the mixture of 250 nM α-MSH and DTE (at 2.5, 5.0, or 7.5 μL/mL). After culturing again for 24 h, B16 cells were solubilized with 400 μL of 0.5% Triton X-100 per well at 4 °C for 1 h. The lysate was centrifuged at 12,000 r/min for 5 min. Then, a 50 μL aliquot of the supernatant was placed into the 96-well plate, followed by the addition of 150 μL of 10 mmol/L L-DOPA solution. After incubation at 37 °C for 30 min, absorbance was measured at 405 nm.
2.6. The PIH Inhibitory Effect of DTE Measured by HaCaT Cells
2.6.1. Cell Viability of HaCaT Cells
The HaCaT cells (2 × 105/mL) were collected and seeded into 96-well plates with 100 μL per well. After being cultured for 24 h, experimental groups were treated with 100 μL of different DTE (0.75, 1, 2.5, 5, 7.5, 10, 25, 50, 75, and 100 μL/mL) concentrations, and control groups were treated with 100 μL of 1640 medium. After being incubated for 24 h, 100 μL of 0.5 mg/mL MTT solution was added to each well. After 4 h of incubation in the incubator, 100 μL of DMSO solution was added to each well, and the absorbance of each well was measured at an absorbance of 490 nm.
Meanwhile, the effect of UVB irradiation on the viability of HaCaT cells was also tested. After being cultured, the control groups were treated with the DMEM culture medium. Experimental groups were irradiated by UVB at different dosages (20, 40, 80, 160, and 320 mJ/cm2) in a thin cover of PBS with the lid open, followed by the treatment with DMEM medium for 24 h. Finally, MTT and DMSO were sequentially added as described above for viability assessment. The experiment was performed in triplicate.
2.6.2. Determination of Secretion of Inflammatory Factors (IL-1α and IL-1β) and Paracrine Melanogenic Factors (ET-1, α-MSH, and bFGF) by ELISA
HaCaT cells (2 × 105/mL) were collected and seeded in 24-well plates with 0.5 mL per well. After the cell culture, the control groups were treated with DMEM medium. Model groups and experimental groups were irradiated with 30 mJ/cm2 UVB with the lid open for irradiation, then treated with the DMEM medium or DTE (at 2.5, 5.0, 7.5 μL/mL) in DMEM. Finally, the inflammatory factors were determined according to the instructions of ELISA kits (Beyotime Biotechnology, Shanghai, China) for IL-1α, IL-1β, ET-1, α-MSH, and bFGF detection.
2.7. RT-PCR Analysis
Total RNA was extracted from the treated cells using an RNA Extraction Kit (Wuhan Servicebio Technology Co., Ltd., Wuhan, China). The purity and concentration of isolated RNA samples were assessed by Nanodrop 2000 (Thermo Fisher Scientific, Erlangen, Germany). Extracted RNA was used to generate cDNA by a PCR machine (Eastwin Life Sciences Inc., Beijing, China). The cDNA was applied as a template for Real-time PCR amplification (Bio-Rad, Hercules, CA, USA), which was carried out in a 40-cycle PCR. The denaturing, annealing, and extension conditions of each PCR cycle were 95 °C for 15 s, 60 °C for 30 s, and 60 °C for 30 s, respectively. The primers were listed in Table 1.

Table 1.
Primers sequence.
2.8. Western Blot Analysis
HaCaT cells incubated under the indicated conditions were lysed, and total protein was extracted by the RIPA buffer, separated by 10% SDS-PAGE, and electro-transferred onto a polyvinylidene difluoride membrane (Wuhan Servicebio Technology Co., Ltd., Wuhan, China). After blocking non-specific antibody binding by incubating the membrane in Tris-buffered saline containing 0.1% Tween-20 and 5% non-fat dried milk for 2 h at room temperature, the membranes were incubated at 4 °C overnight with the following primary antibodies (from Servicebio): Nrf2 (GB115673; 1:1000), HO-1 (GB115713; 1:500), P-P38 (GB153380; 1:400), P38 (GB154685; 1:1000), and β-actin (GB15003; 1:500). Afterward, the membranes were incubated with the appropriate horseradish peroxidase (HRP)-conjugated secondary antibody for 1 h at room temperature. Immunoreactive bands were detected by the Enhanced Chemiluminescence substrate (Wuhan Servicebio Technology Co., Ltd., Wuhan, China) and visualized on the Chemiluminescence Imager (Wuhan Servicebio Technology Co., Ltd., Wuhan, China). Densitometric analysis was performed using the AIWBwellTM software program.
2.9. Statistical Analysis
All results were expressed as mean ± standard deviation (SD). The “Student’s test” referred to in the manuscript is the unpaired t-test. The differences among the three groups were analyzed using one-way analysis of variance (ANOVA) followed by the least square difference (LSD) post-hoc test. The significance levels were indicated as follows: * p < 0.05, considered to be statistically significant.
3. Results
3.1. Determination of Polyphenol Content in DTE
Considering that polyphenol was the most abundant component in tea extracts, we tested the polyphenol content in DTE. The standard curve equation for the determination of polyphenol content from dark tea was y = 0.005x + 0.0518, with a correlation coefficient of R2 = 0.9988, showing a good linear correlation. Compared with the standard curve, the calculation showed that the total phenol content of DTE was 5.46 mg/mL.
3.2. The Determination Results of the Chemical Composition of DTE
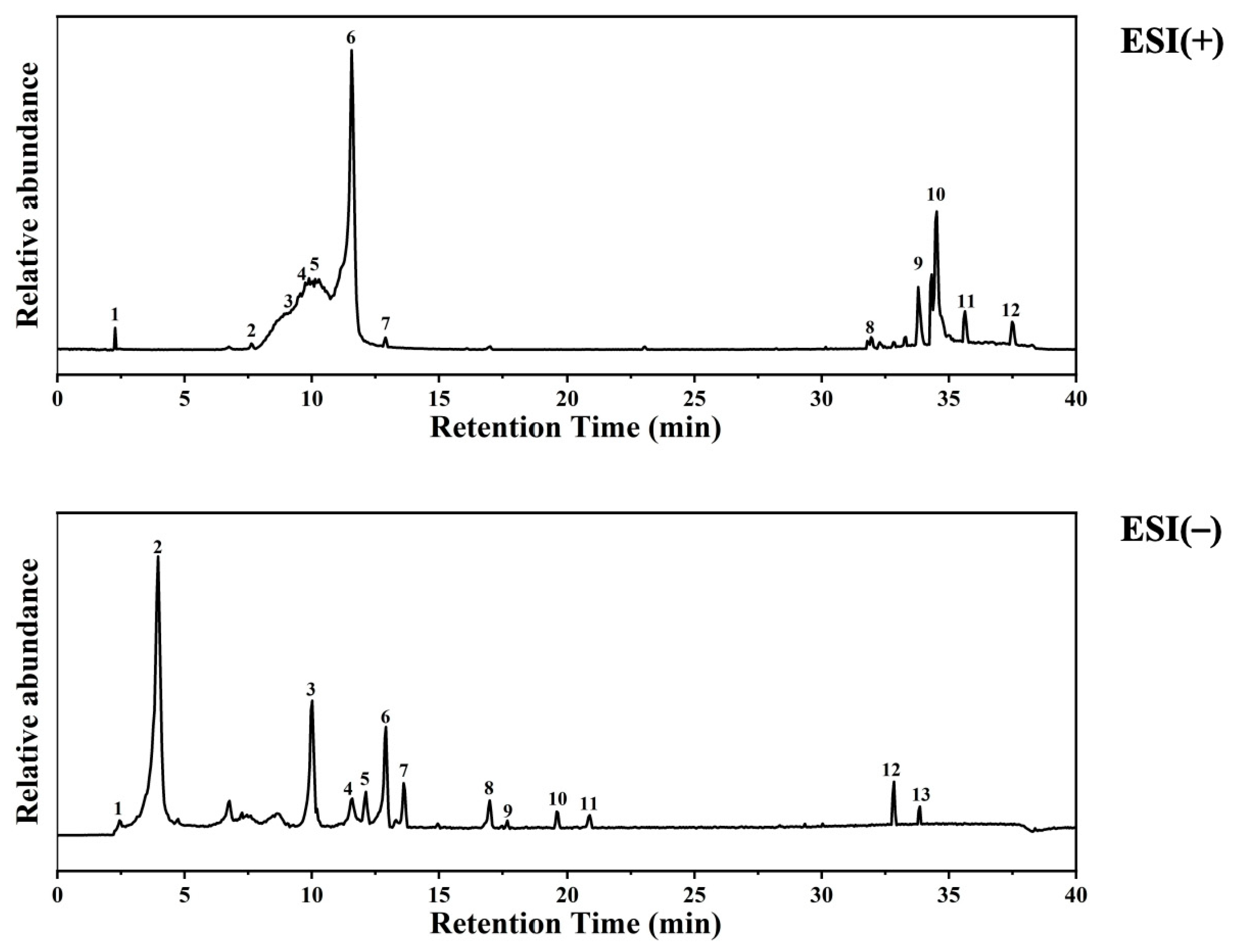
Based on the provided LC-MS dataset, the chemical composition of the DTE was characterized. According to structural classification (Figure 1, Table 2 and Table 3), The extract is rich in purine alkaloids such as caffeine, theobromine, and theophylline; phenolic acids including gallic acid (3.5%) and protocatechuic acid (0.24%); flavan-3-ols like catechin (1.72%) and epigallocatechin gallate (EGCG (0.67%)); other polyphenols such as miquelianin (quercetin glucuronide (0.94%)) and a kaempferol glycoside (0.11%); fatty acid amides (e.g., oleamide, stearamide); organic acids (e.g., azelaic acid); and other miscellaneous compounds like choline and adenosine monophosphate [16,17]. Among these, polyphenolic compounds (various catechins, quercetin, and kaempferol derivatives) and fatty acid amides (oleamide and hexadecanamide) were particularly abundant. The diverse phytochemical profile of DTE, rich in antioxidants, anti-inflammatory agents, and skin-beneficial compounds, provides a substantive material basis for its potential use in cosmetic applications.
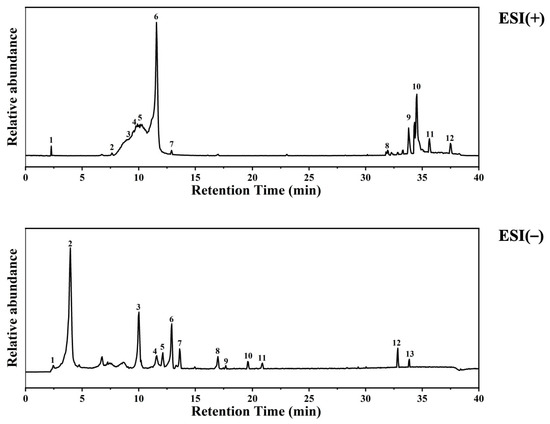
Figure 1.
UPLC-HRMS analysis of ingredients in DTE.

Table 2.
Compounds identified in the positive ion mode by UPLC-HRMS. The table lists the peak number, experimentally observed m/z value, retention time (RT) in minutes, mass error in parts per million (ppm), putative compound identification, and the detected adduct ion form.

Table 3.
Compounds identified in the negative ion mode by UPLC-HRMS. The table lists the peak number, experimentally observed m/z value, retention time (RT) in minutes, mass error in parts per million (ppm), putative compound identification, and the detected adduct ion form.
3.3. Effect of DTE on Melanin Content and Tyrosinase Activity in B16 Cells
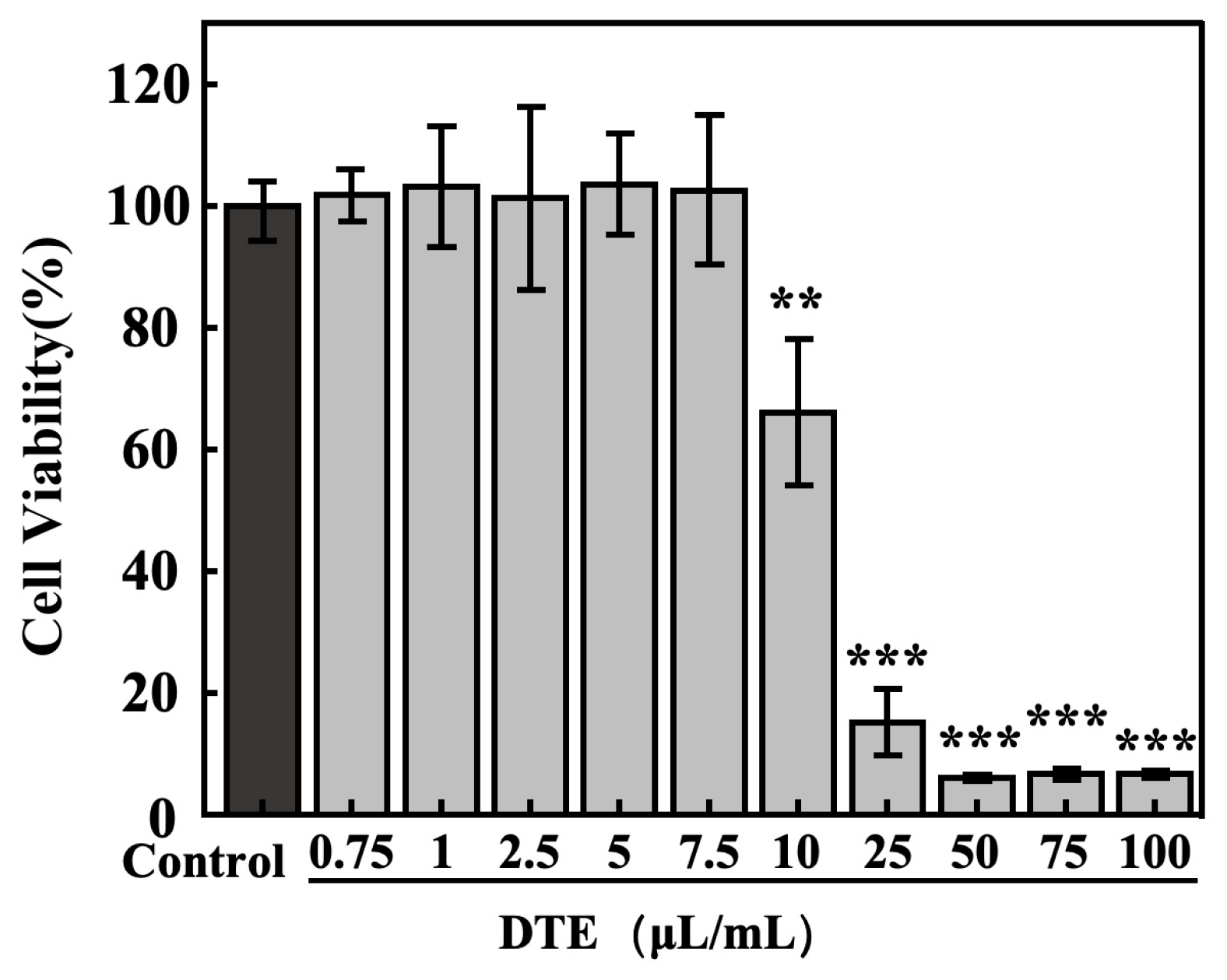
Firstly, to exclude the influence of DTE on cell viability, the concentration–toxicity relationships of DTE on B16 cells were determined using the MTT assay. The cell viabilities of B16 cells (Figure 2) were larger than 95% at lower concentration ranges of DTE from 0.75 to 7.5 μL/mL, and the cell viability ratios were less than 80% at higher concentrations of DTE from 10.0 to 100.0 μL/mL (Figure 1). Therefore, we chose the following concentrations (0.75–7.5 μL/mL) of DTE to investigate their effects on the B16 cells in the subsequent experiments.
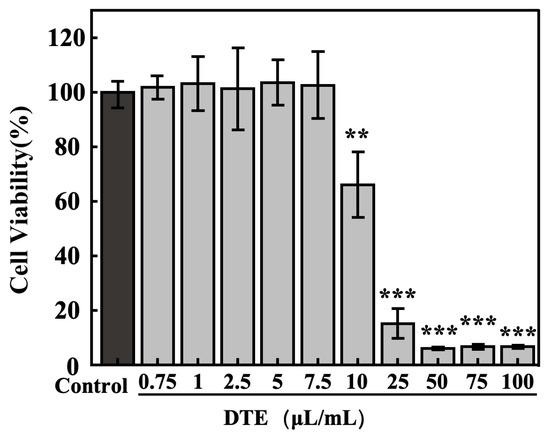
Figure 2.
Effect of DTE on cell viability of B16 cells. All assays were performed in triplicate. Statistical analysis was performed by ANOVA. Error bars indicate the standard deviation of three independent tests in triplicate. (**, *** indicate p < 0.01, 0.001, vs. Control group).
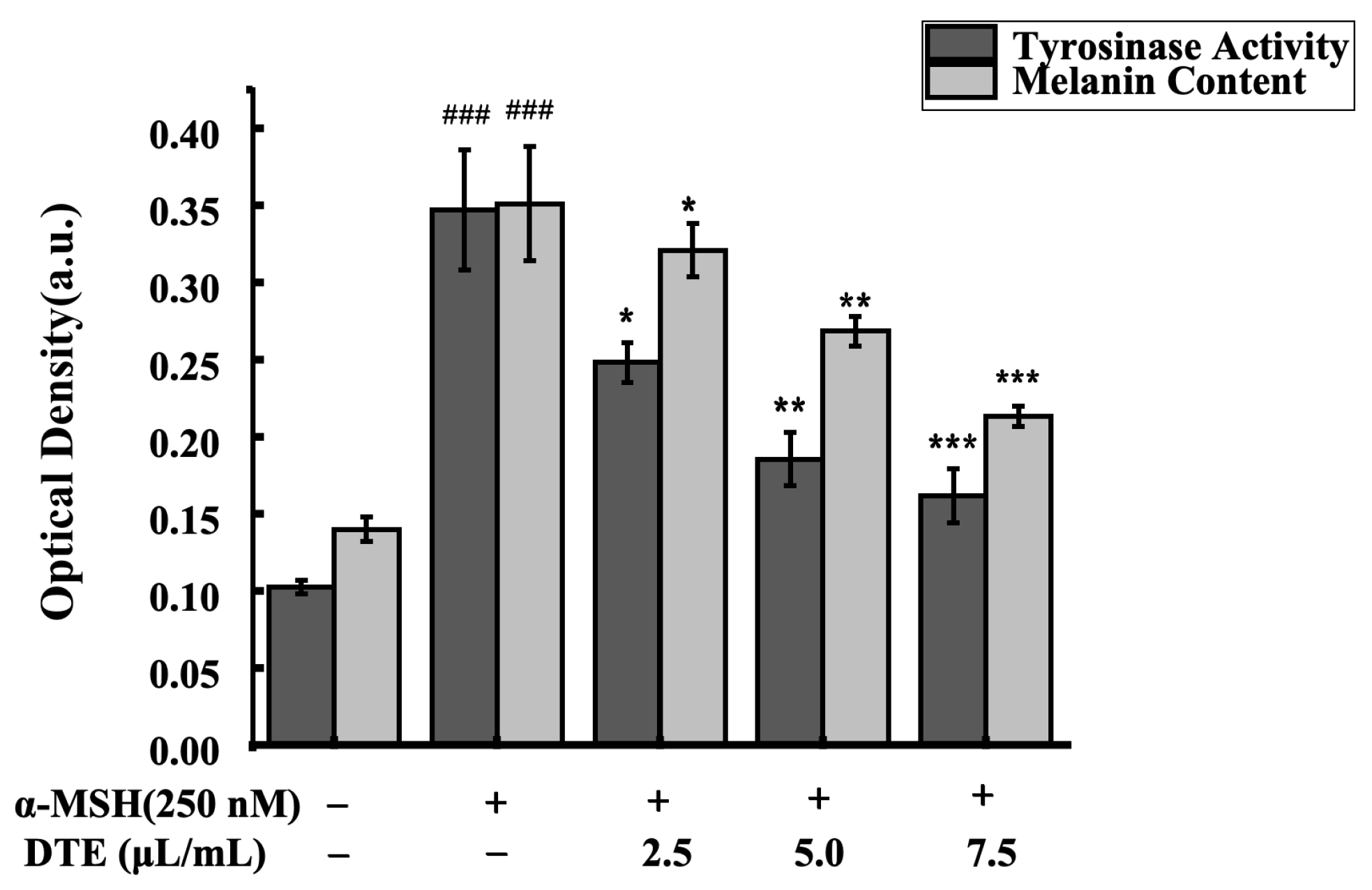
To assess the impacts of DTE on melanin content and tyrosinase activity, the B16 cells were treated with 2.5, 5.0, and 7.5 μL/mL of DTE or stimulated with 250 nM of α-MSH for 24 h. As was observed, the melanin accumulation was greatly reduced and the tyrosinase activity was observably decreased in B16 cells (Figure 3). Specifically, 7.5 μL/mL of DTE significantly decreased the tyrosinase activity by 46%, and 7.5 μL/mL of DTE also significantly decreased the melanin content by 60%. These results showed that DTE can inhibit melanin content and tyrosinase activity, indicating its anti-melanogenesis effect.
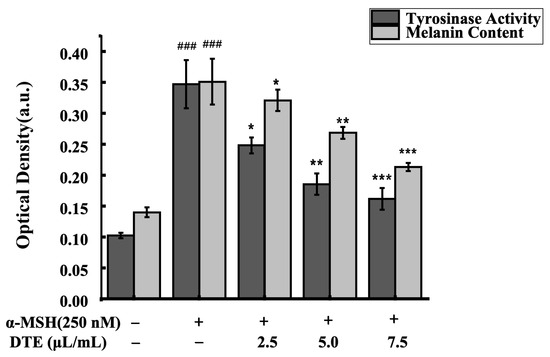
Figure 3.
Effect of DTE on tyrosinase activity and melanin content in B16 cells. All assays were performed in triplicate. Statistical analysis was performed by ANOVA. Error bars indicate the standard deviation of three independent tests in triplicate. (### indicate p < 0.001 vs. Control group; *, ** and *** indicate p < 0.05, 0.01 and 0.001 vs. model group).
3.4. Effects of DTE on Inflammatory and Paracrine Melanogenic Factors in UVB-Irradiated HaCaT Cells
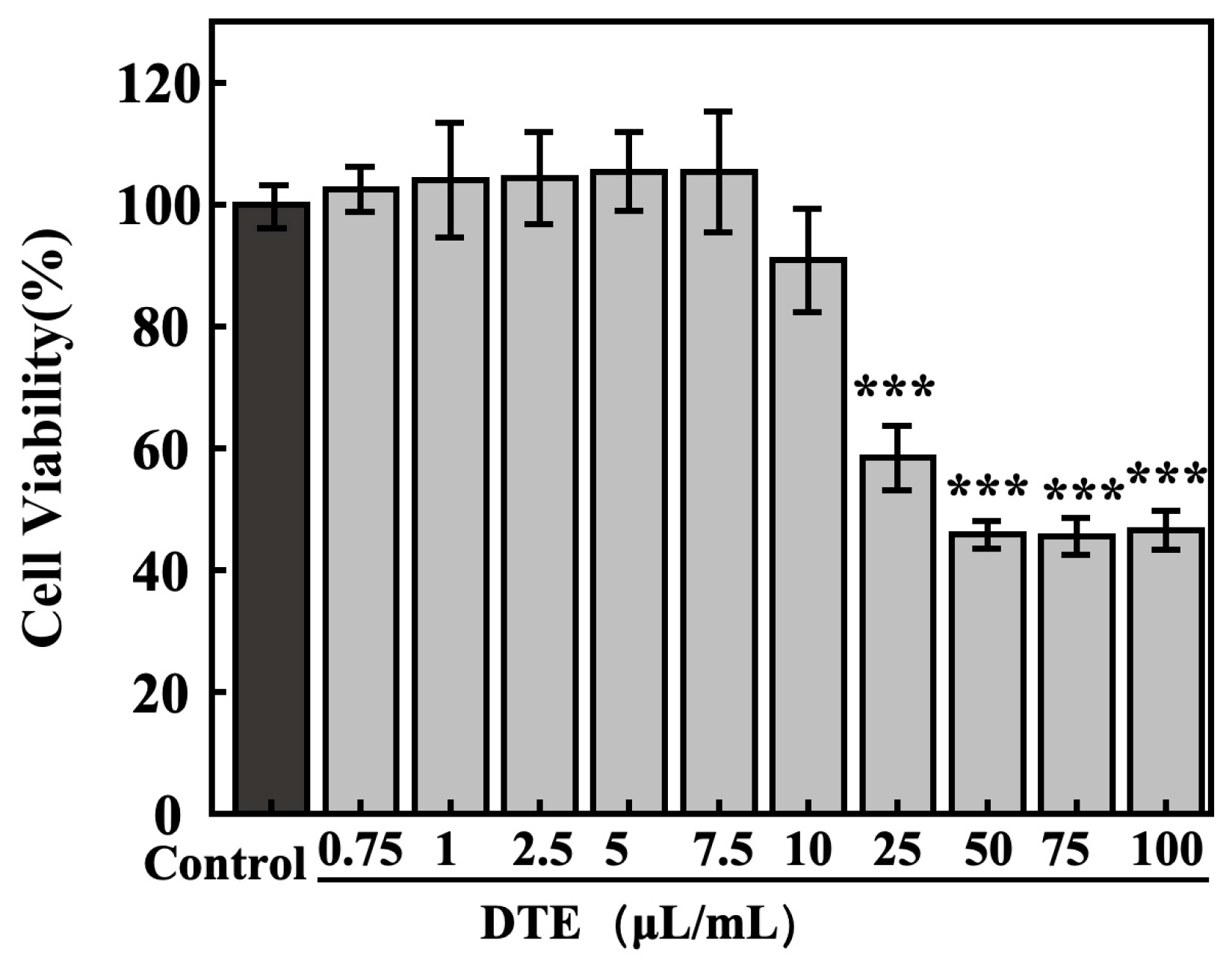
The concentration toxicity relations of DTE on HaCaT cells were determined using MTT assays. As shown in Figure 4, the cell survival ratios of HaCaT cells were greater than 95% at lower concentrations of DTE from 0.75 to 7.5 μL/mL, and the cell viabilities were less than 90% at higher concentrations from 10.0 to 100.0 μL/mL. Therefore, we chose the following concentrations (0.75–7.5 μL/mL of DTE) to investigate the influences of DTE on HaCaT cells in the subsequent experiments.
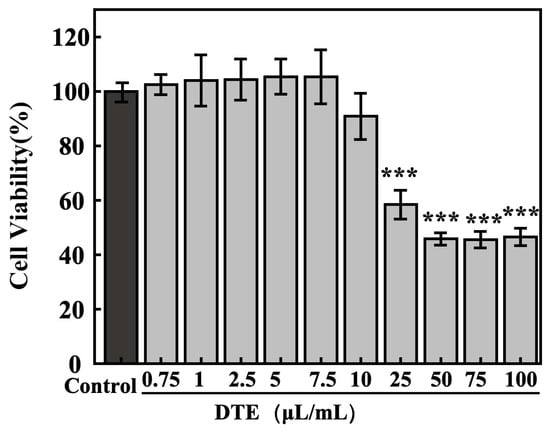
Figure 4.
Effect of DTE on cell viability of HaCaT cells. All assays were performed in triplicate. Statistical analysis was performed by ANOVA. Error bars indicate the standard deviation of three independent tests in triplicate. (*** indicate p < 0.001, vs. Control group).
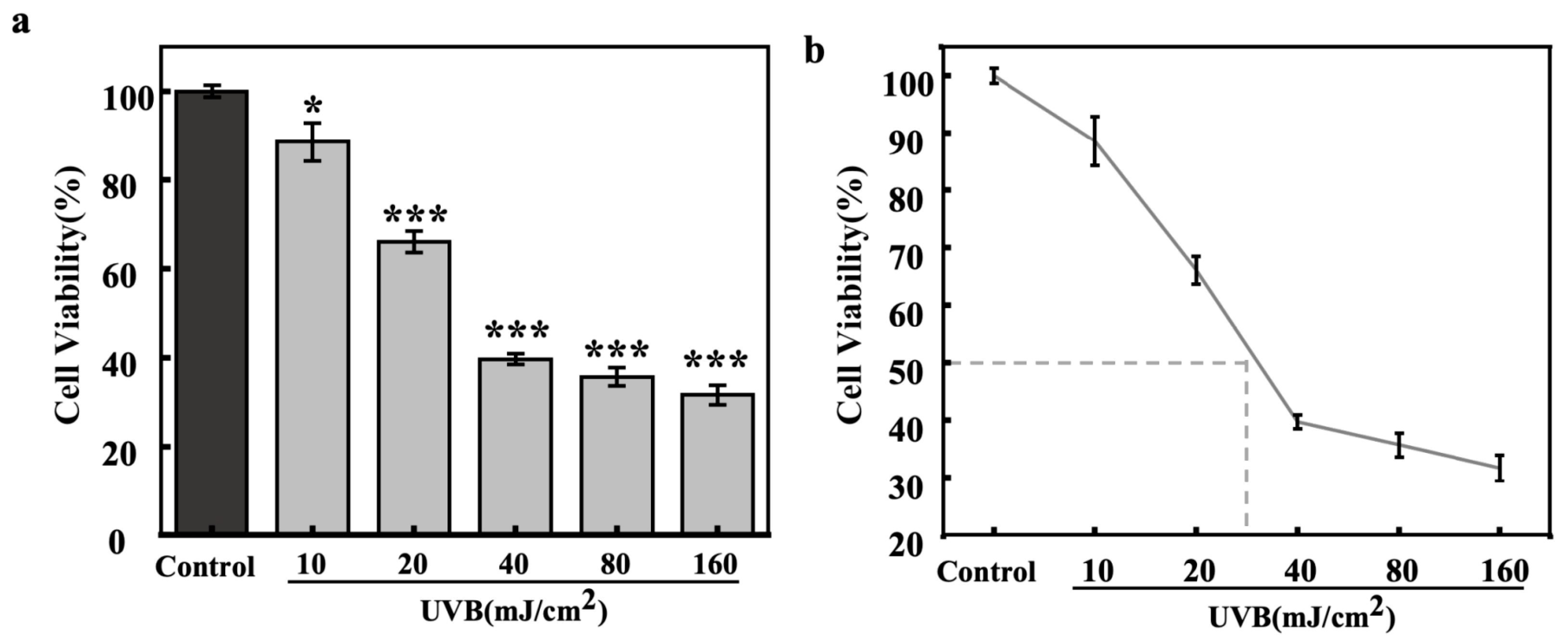
The effects of different UVB irradiation dosages on the viability of HaCaT cells were also evaluated by the MTT method (Figure 5). The experimental data showed that the relative viability of HaCaT cells showed a gradual decrease with increasing UVB irradiation. The IC50 value (Figure 5b) of UVB for HaCaT cells was around 30 mJ/cm2, and this dosage, which showed significant photodamage, was chosen for the investigation of light-induced secretion of inflammatory and paracrine melanogenic factors in HaCaT cells.
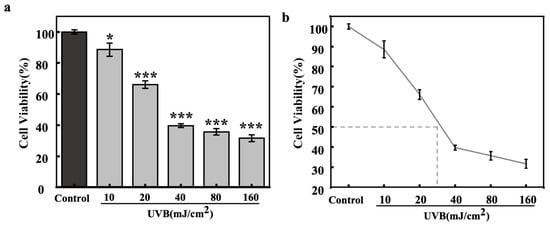
Figure 5.
Effect of UVB dosages on cell viability of HaCaT cells (bar chart (a), dot line chart (b)). All assays were performed in triplicate. Statistical analysis was performed by ANOVA. Error bars indicate the standard deviation of three independent tests in triplicate. (*, and *** indicate p < 0.05, and 0.001, vs. Control group).
To test the anti-inflammatory and anti-melanogenesis agents’ ability of DTE in UVB-irradiated HaCaT cells, we detected the secretion of inflammatory factors (IL-1α and IL-1β) and paracrine melanogenic factors (ET-1, α-MSH, and bFGF).
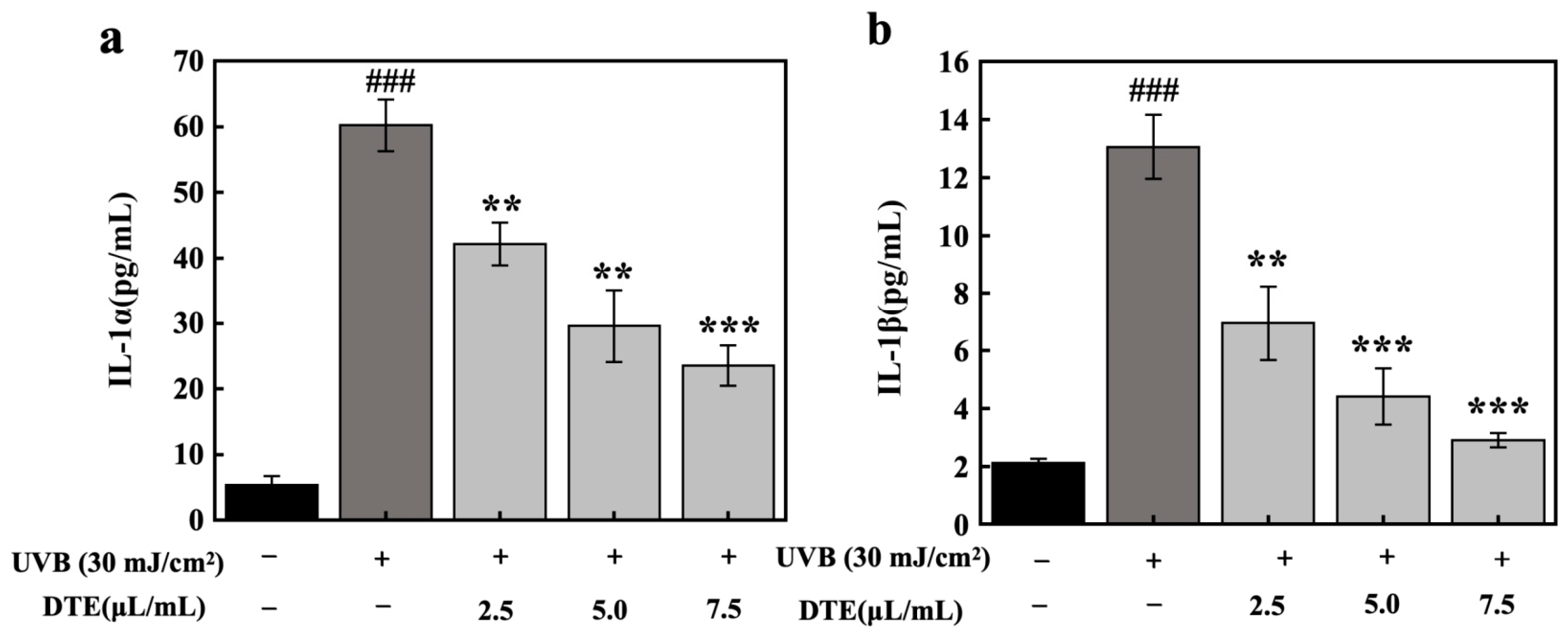
To model PIH, HaCaT cells were exposed to UVB irradiation at a dosage of 30 mJ/cm2, which significantly upregulated secretion levels of IL-1α and IL-1β. Treatment with DTE at increasing concentrations (2.5 μL/mL, 5.0 μL/mL, and 7.5 μL/mL) progressively suppressed the expressions of IL-1α and IL-1β (Figure 6). At the concentration of 7.5 μL/mL, DTE reduced IL-1α and IL-1β generation by 61% and 78%, respectively.
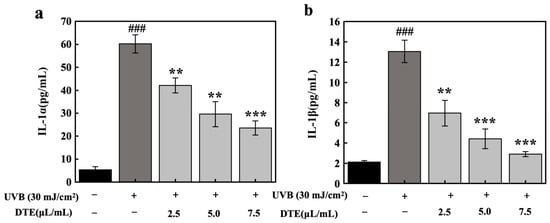
Figure 6.
Effect of DTE on IL-1α (a) and IL-1β (b) content in UVB-irradiated HaCaT cells. All assays were performed in triplicate. Statistical analysis was performed by ANOVA. Error bars indicate the standard deviation of three independent tests in triplicate. (### indicate p < 0.001 vs. Control group, **, *** indicate p < 0.01, 0.001 vs. Model group).
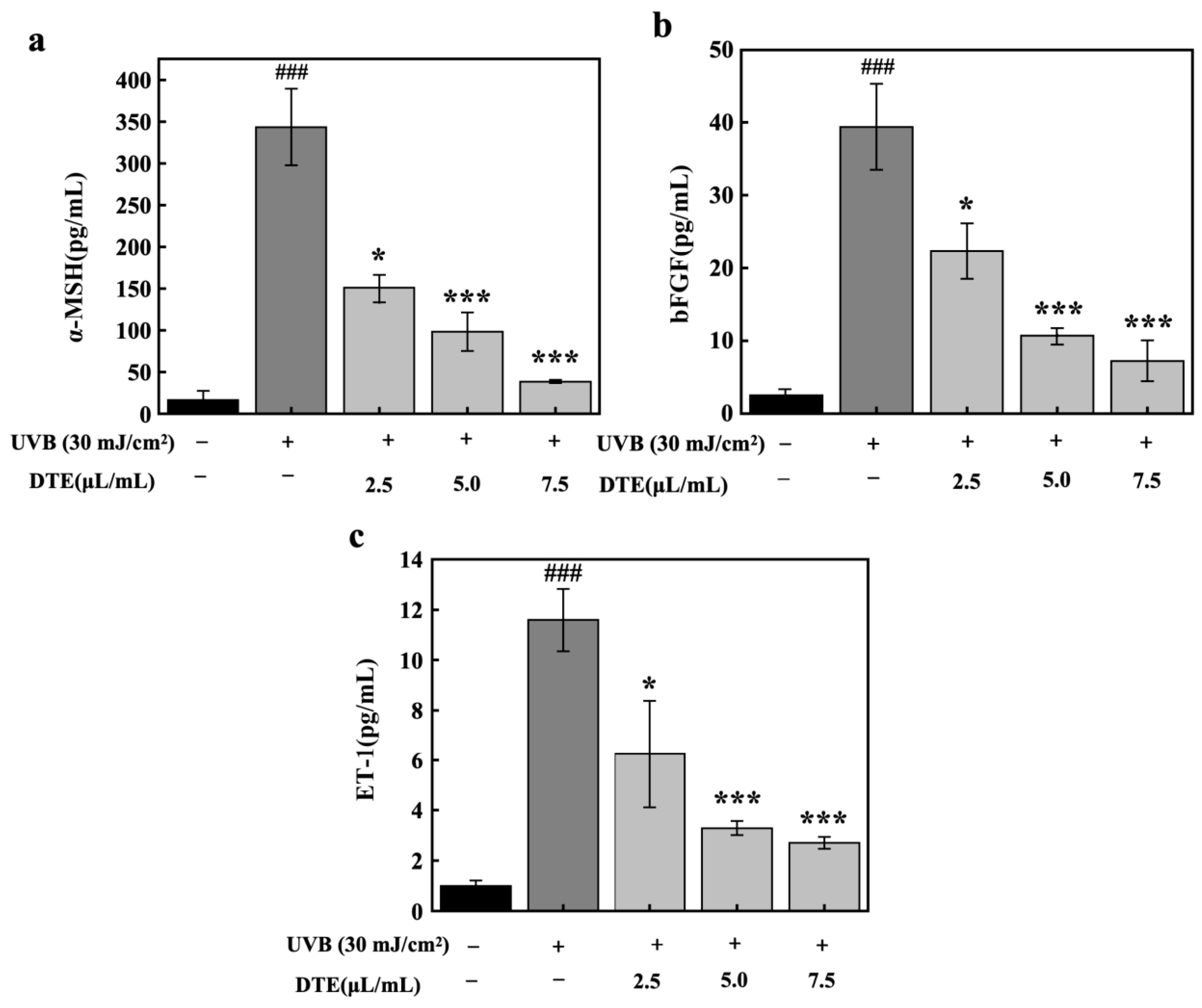
Beyond pro-inflammatory factors, research has indicated that under inflammatory stimuli and UVB irradiation, keratinocytes secrete specific paracrine factors (ET-1, α-MSH, and bFGF). These factors can directly attach to their respective receptors on melanocytes, thereby enhancing melanogenesis [18,19]. HaCaT cells were exposed to UVB irradiation (30 mJ/cm2), which markedly upregulated expression levels of paracrine melanogenic factors of α-MSH, bFGF, and ET-1 (Figure 7). Treatment with DTE at concentrations of 2.5 μL/mL and 7.5 μL/mL progressively suppressed the expressions of α-MSH, bFGF, and ET-1. At a concentration of 7.5 μL/mL, DTE reduced α-MSH, bFGF, and ET-1 levels by 89%, 82%, and 77%, respectively, demonstrating its potent inhibitory effect on UVB-induced secretion of these melanogenic paracrine factors.
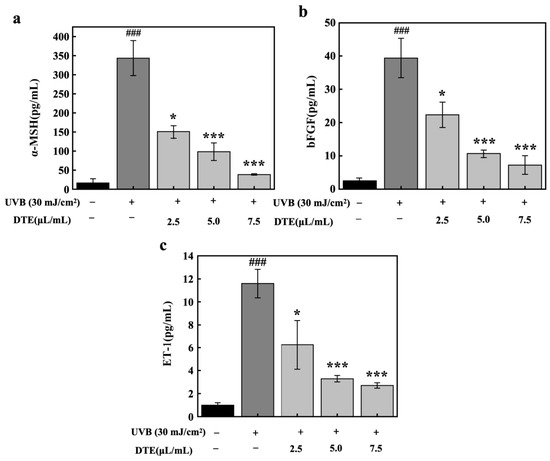
Figure 7.
Effect of DTE on α-MSH (a), bFGF (b), and ET-1 (c) content in UVB-irradiated HaCaT cells. All assays were performed in triplicate. Statistical analysis was performed by ANOVA. Error bars indicate the standard deviation of three independent tests in triplicate (### indicate p < 0.001 vs. Control group, *, and *** indicate p < 0.05, and 0.001 vs. Model group).
3.5. DTE Exerted Anti-Inflammatory Effect Through p38 MAPK/Nrf2/HO-1 Pathway in HaCaT Cells
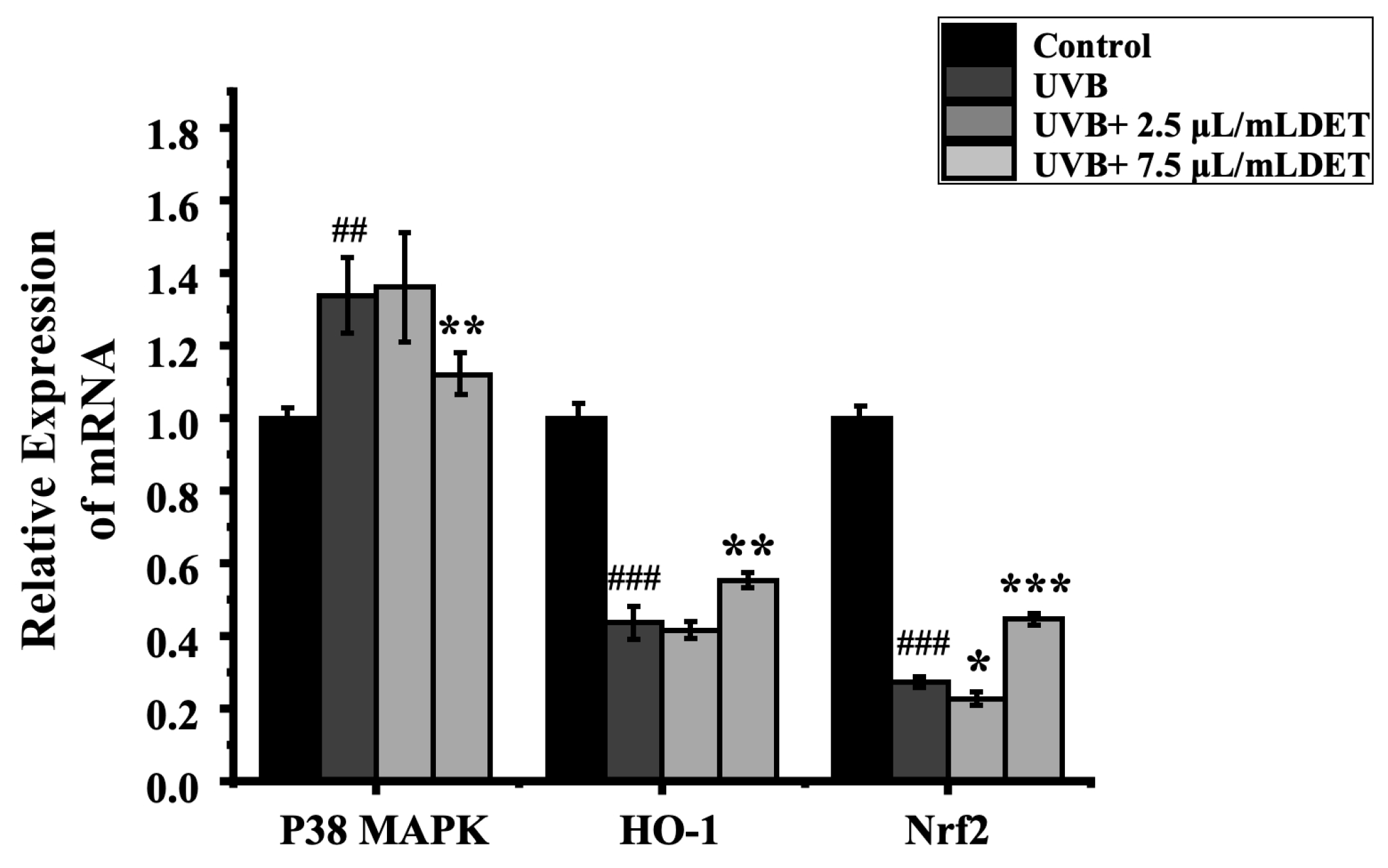
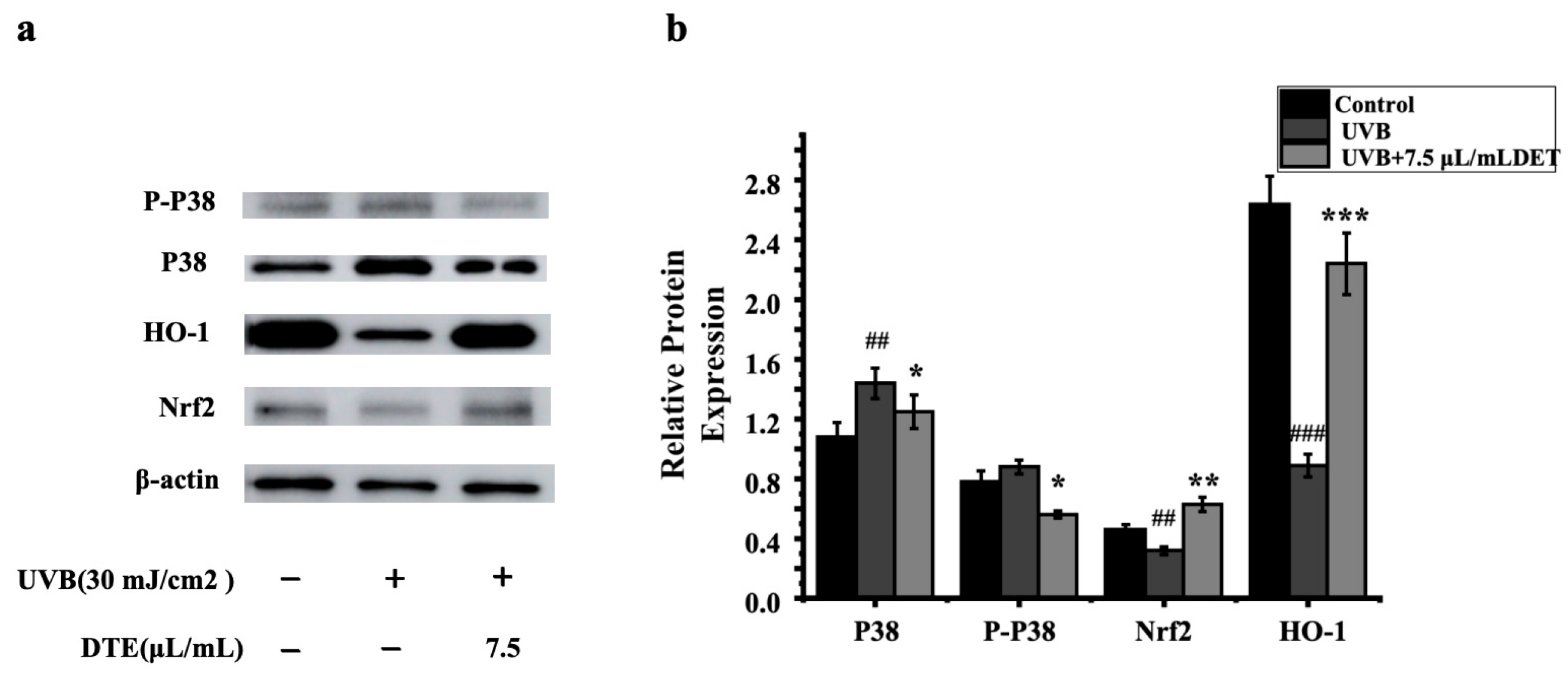
To assess the impacts of DTE on the inflammatory pathway, HaCaT cells were treated with DTE, and the relative expressions of p38 MAPK, Nrf2, and HO-1 were tested. The results of RT-PCR and Western Blot (Figure 8 and Figure 9) showed that UVB irradiation markedly elevated p38 MAPK expression while suppressing the Nrf2/HO-1 signaling pathway when compared to the model group, thus effectively triggering cellular inflammatory responses. Notably, treatment with 7.5 μL/mL of DTE significantly reduced p38 MAPK expression and induced Nrf2/HO-1 pathway activation, indicating its ability to suppress p38 MAPK signaling, activate the Nrf2/HO-1 pathway, and mitigate UVB-induced oxidative stress and inflammatory factor (IL-1α, IL-1β) release.
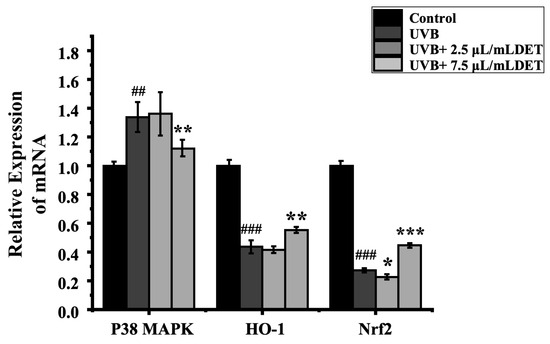
Figure 8.
Effect of DTE (μL/mL) on the mRNA level of p38 MAPK (a), Nrf2 (b), and HO-1 (c) in UVB-irradiated HaCaT cells. All assays were performed in triplicate. Statistical analysis was performed by ANOVA. Error bars indicate the standard deviation of three independent tests in triplicate (##, ### indicate p < 0.01, 0.001 vs. Control group, *, **, *** indicate p < 0.05, 0.01, 0.001 vs. model group).
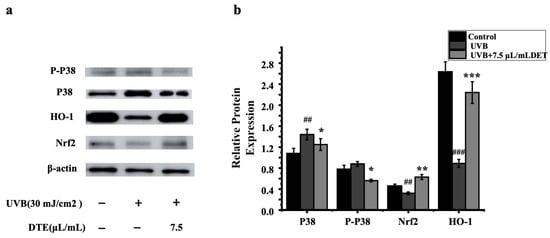
Figure 9.
Western blot assay of P38, P-P38, Nrf2, and HO-1 (a) and effect of DTE (μL/mL) on the protein level of P38, P-P38, Nrf2, and HO-1 (b) in UVB-irradiated HaCaT cells. Statistical analysis was performed by ANOVA. Error bars indicate the standard deviation of three independent tests in triplicate (##, ### indicate p < 0.01, 0.001 vs. Control group, *, **, and *** indicate p < 0.05, 0.01, and 0.001 vs. Model group).
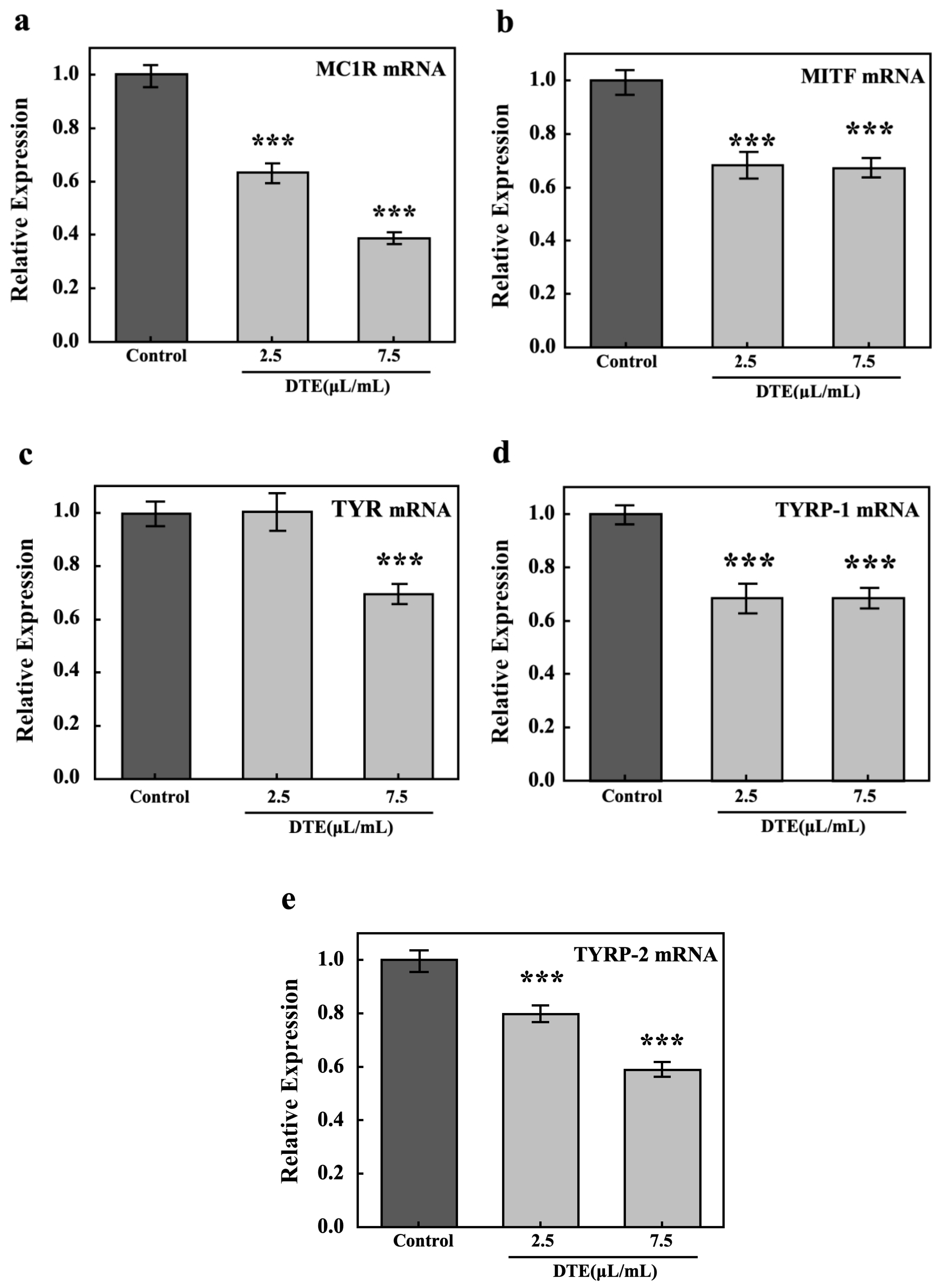
3.6. DTE Exerted Anti-Melanogenesis Effect Through MC1R, MITF, TYR, and TYRP-1/2 Pathway in B16 Cells
To explore DTE’s anti-melanogenesis effect, the key pathways (MC1R, MITF, TYR, and TYRP-1/2) in melanocytes were tested by their relative expressions. The results of RT-PCR showed that the relative expression of melanogenesis-related genes, including MC1R, MITF, TYR, TYRP-1, and TYRP-2, significantly decreased after treatment with DTE, when compared to the model group (Figure 10). It is noteworthy that the levels of MC1R, MITF, TYR, TYRP-1, and TYRP-2 were reduced by 61%, 32%, 30%, 32%, and 33%, respectively, after treatment with 7.5 µL/mL of DTE.
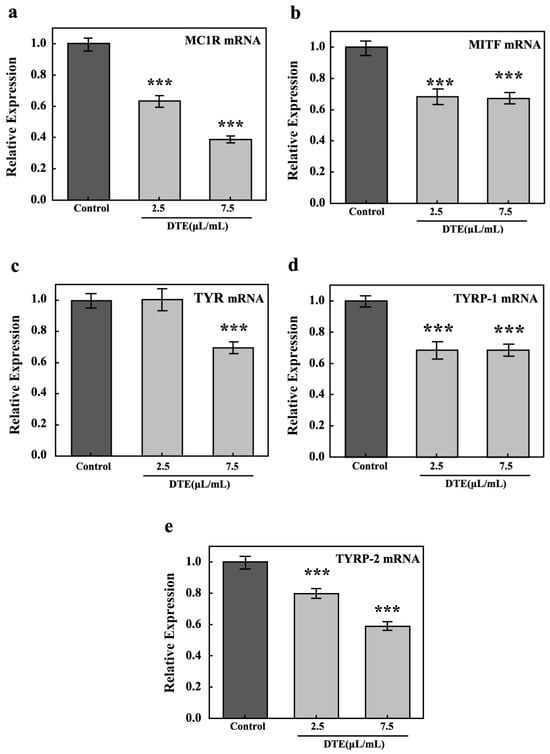
Figure 10.
Effect of DTE on the mRNA level of MC1R (a), MITF (b), TYR (c), TYRP-1 (d), and TYRP-2 (e). Model groups were treated with α-MSH, and the experimental group was treated with the mixture of α-MSH and DTE (2.5 or 7.5 μL/mL). All assays were performed in triplicate. Statistical analysis was performed by ANOVA. Error bars indicate the standard deviation of three independent tests in triplicate (*** indicates p < 0.001 vs. Model group).
4. Discussion
Hyperpigmentation of the skin afflicts many people, which has become one of the most prevalent skin concerns in recent years, which is characterized by excessive melanin secretion in the epidermis and dermis. Common agents for post-inflammatory hyperpigmentation (PIH)—such as retinoids, corticosteroids, arbutin, various acids, and vitamin C derivatives—though effective, are associated with side effects including irritant contact dermatitis, epidermal atrophy, telangiectasia, and paradoxical hyperpigmentation. Consequently, developing novel, well-tolerated brightening ingredients with improved efficacy and stability remains an ongoing research priority. Botanically based raw materials have attracted extensive attention in the cosmetics industry due to their natural, safe, and mild characteristics; however, scarce ingredients have been found to be valid for skin-brightening efficacy and agents for the restoration of pigmentation disorders. Although DTE is rich in various components such as tea polyphenols, theaflavins, and thearubigins, and has been proven to have health benefits such as antioxidation, lipid-lowering, and hypoglycemic effects, the development of its efficacy in cosmetics, especially the effects of anti-melanin production and inhibiting PIH to achieve anti-melanogenesis agent effects, still needs to be clarified.
α-MSH, a known stimulator that can combine with the melanocortin 1 receptor (MC1R) on B16 cells, drives the melanogenic network and promotes tyrosinase activity to induce melanogenesis [20]. Accordingly, to test the skin-brightening effect of DTE, we first applied α-MSH-stimulated B16 cells as a model to evaluate the anti-melanogenesis effect of DTE. From the results, DTE (7.5 μL/mL) significantly decreased the tyrosinase activity by 54% and inhibited the melanin content by 40% in α-MSH-stimulated B16 cells. The effect is comparable to that of 700 μM kojic acid and 150 μM β-arbutin [21]. These results confirmed that DTE can significantly reduce the activity of tyrosinase in B16 cells, which led to a significant reduction in melanin production and demonstrated a skin-brightening effect.
PIH is one of the main causes for rebound pigmentation, during which process darkening of the skin is caused by the activation of melanin production by inflammatory mediators. HaCaT cells are located in the superficial layer of human skin, and when epidermal cells in the skin are stimulated by factors such as UVB (a type of ultraviolet radiation that is particularly harmful to the skin) irradiation, they produce excessive reactive oxygen species (ROS), which overexpress the inflammatory factors of IL-1α and IL-1β and the paracrine melanin factors of ET-1, bFGF, and α-MSH in the cells, thus causing PIH and promoting melanogenesis [22]. α-MSH combines with melanocortin 1 receptor (MC1R) on B16 cells, driving the melanogenic network and enhancing tyrosinase activity to promote melanogenesis. Its regulatory network is a central mechanism in PIH. To establish the PIH model, we irradiated HaCaT cells with 30 mJ/cm2 UVB to elicit inflammation. As the results show, after UVB irradiation, the secretion of inflammatory factors (IL-1α and IL-1β) and paracrine melanin factors (ET-1, bFGF, and α-MSH) increased dramatically in HaCaT cells, but the secretion of inflammatory factors and paracrine melanin factors was greatly reduced in the cell groups treated with DTE. The effect is comparable to that of 20 μM of Curcumin [23]. These results indicated that DTE inhibited inflammatory/paracrine melanin factors’ secretion after UVB irradiation-induced inflammation, which can inhibit melanogenesis from the source to anti-melanogenesis agents, making it a potential candidate for PIH therapy.
Meanwhile, we investigated the mechanism underlying DTE inhibition of the release of inflammatory factors in HaCaT. The p38 MAPK signaling pathway, activated by UVB exposure, plays a pivotal role in melanogenesis by regulating the expression of MITF and its target genes, such as tyrosinase and TRP-2, and is also intricately involved in inflammation through the modulation of inflammatory mediators like cytokines and chemokines [24,25]. The Nrf2/HO-1 axis alleviates inflammaging and oxidative stress by transcriptionally activating antioxidant and anti-inflammatory pathways and enhancing cytoprotection and redox homeostasis [26]. We examined the mRNA and the protein expression levels of p38 MAPK, Nrf2, and the HO-1 pathway after UVB irradiation in HaCaT cells by RT-PCR analysis and Western Blot analysis. Notably, treatment with 7.5 μL/mL of DTE significantly reduced p38 MAPK pathway expression and enhanced Nrf2/HO-1 pathway activation. This indicated that DTE had the ability to alleviate UVB-induced oxidative stress damage and reduce the release of inflammatory cytokines (IL-1α, IL-1β) and paracrine melanin factors (ET-1, bFGF, and α-MSH).
Our previous investigation revealed that DTE effectively attenuated melanogenesis in B16 cells. Further mechanistic studies demonstrated that α-MSH engages with melanocortin 1 receptor (MC1R) on B16 cells, establishing one of the key pathways of melanin synthesis. MC1R activation in B16 cells was found to directly modulate microphthalmia-associated transcription factor (MITF) expression and activity, which governed the transcriptional regulation of tyrosinase (TYR), tyrosinase-related protein 1 (TYRP-1), and tyrosinase-related protein 2 (TYRP-2)—essential components of the melanogenic regulatory network [27]. Analysis via RT-PCR revealed that the mRNA expressions of melanin-producing markers (MC1R, MITF, TYR, TYRP-1, and TYRP-2) were significantly downregulated under 2.5 or 7.5 μL/mL of DTE treatment, indicating that the gene expression of the genuine melanin regulatory network had been inhibited.
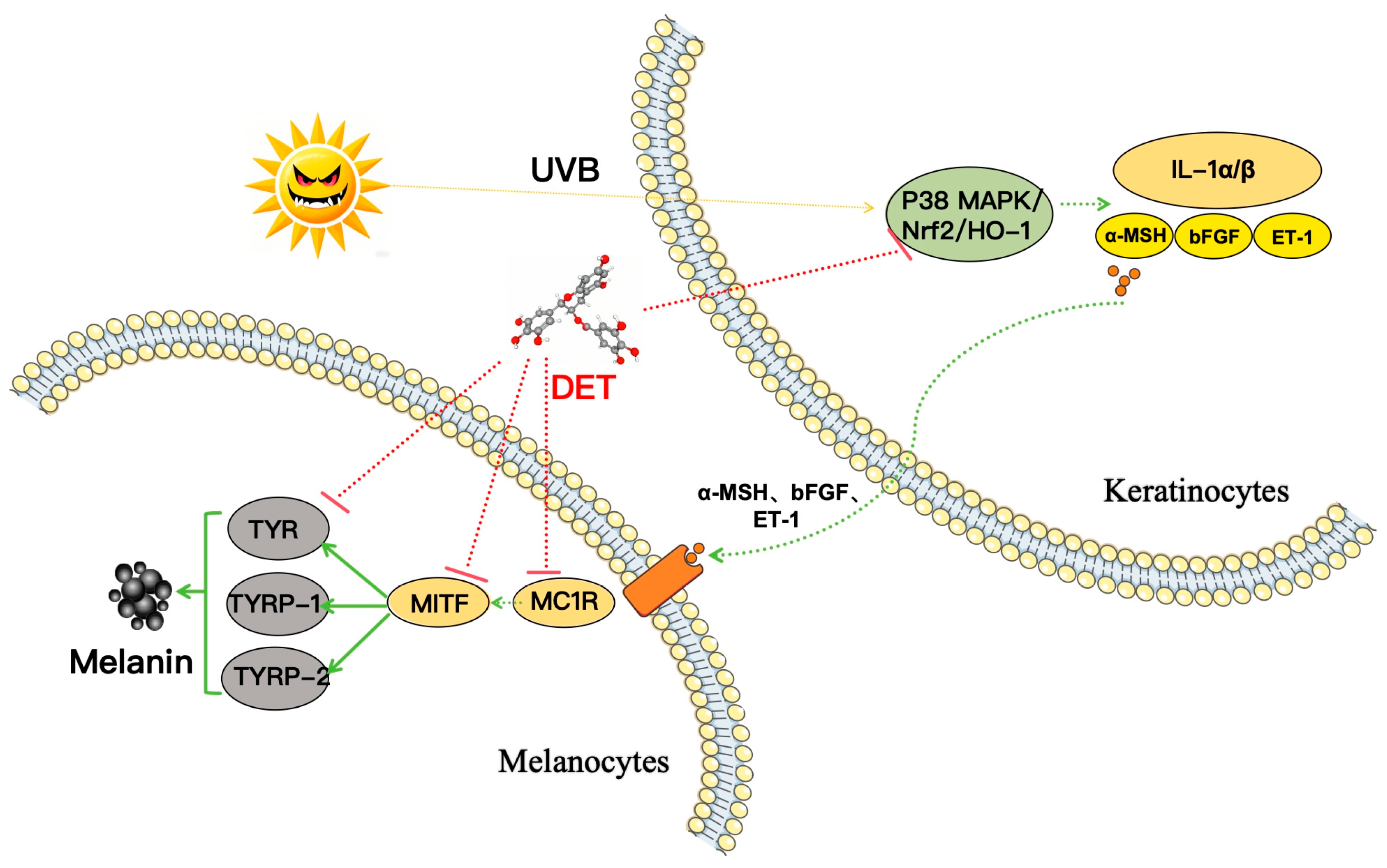
In summary, these RT-PCR findings suggested DTE reduced PIH by inhibiting the expression of the P38 MAPK pathway and upregulating the expression of the Nrf2/HO-1 pathway, thereby decreasing the release of inflammatory factors (IL-1α, IL-1β) and paracrine melanogenic factors (ET-1, bFGF, and α-MSH). Meanwhile, DTE decreased the binding of α-MSH to MC1R receptors, inhibited the activation of MITF, and then reduced TYR, TYRP-1, and TYRP-2 expression, ultimately achieving multi-target action and leading to diminished melanin production (Figure 11).
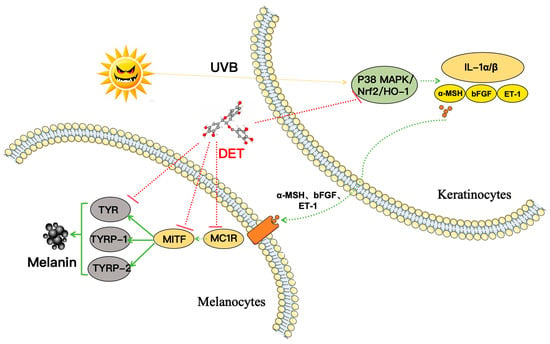
Figure 11.
Schematic presentation of the mechanism underlying the inhibitory actions of DTE on melanogenesis. DTE reduced PIH by inhibiting the P38 MAPK pathway and upregulating the Nrf2/HO-1 pathway, thereby decreasing the release of inflammatory factors and paracrine melanogenic factors. This subsequently decreased the binding of α-MSH to MC1R receptors. Meanwhile, DTE acted on the melanin regulatory network to achieve multi-target (MC1R, MITF, TYR, TYRP-1, and TYRP-2) effects, ultimately leading to diminished melanin production. Generally, DTE can inhibit melanogenesis through multi-target actions; it can also prevent darkening and achieve an anti-melanogenesis effect by inhibiting PIH.
5. Conclusions
This study investigated the anti-melanogenesis and PIH inhibition effects of DTE in α-MSH-stimulated B16 melanoma cells and UVB-exposed HaCaT keratinocytes models, respectively. Experimental data demonstrated that DTE, enriched with polyphenolic constituents, exhibited dual efficacy as skin-brightening efficacy anti-melanogenesis agents. In preventing PIH-induced darkening, DTE suppressed UVB-induced pro-inflammatory cytokine secretion in HaCaT keratinocytes through modulation of the p38 MAPK/Nrf2/HO-1 signaling pathway, concurrently inhibiting paracrine melanogenic mediators (α-MSH, bFGF, and ET-1) associated with the hyperpigmentation cascades. Regarding the anti-melanogenesis effect, the DTE mediated MC1R gene downregulation at the transcriptional level, coupled with multi-target inhibitory mechanisms on melanogenic regulators: MITF expression suppression and subsequent inhibition of tyrosinase family enzymes (TYR, TYRP-1, and TYRP-2), ultimately attenuating melanogenesis in B16 melanocytes. These findings collectively validated DTE’s dual actions in both preventing pigmentation triggers and interrupting melanin biosynthesis pathways, demonstrating its skin-brightening efficacy and agents for the restoration of pigmentation disorder stabilization. Hopefully, DTE with multi-target profiles represents a promising natural active ingredient for developing comprehensive brightening formulations.
Author Contributions
X.D., J.W. (Jingting Wang), C.W., J.D., Y.H., M.Y. and K.R. performed the research. G.W. and J.W. (Jing Wang) designed the research study. X.D. analyzed the data and wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Guangzhou Huashi Cosmetic Technology Co., Ltd.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
Jianming Deng, Yuancheng Huang, and Min Yu were employed by the company Guangzhou Huashi Cosmetic Technology Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The authors declare that this study received funding from Guangzhou Huashi Cosmetic Technology Co., Ltd. The funder had the following involvement with the study: Jianming Deng, Yuancheng Huang, and Min Yu provided the resources, gave useful suggestions, and validated the data. The funder did not affect the experimental process and results in the study. The funding relationship does not affect the scientific quality of the manuscript.
References
- Maghfour, J.; Olayinka, J.; Hamzavi, I.H.; Mohammad, T.F. A focused review on the pathophysiology of post-inflammatory hyperpigmentation. Pigment Cell Melanoma Res. 2022, 35, 320–327. [Google Scholar] [CrossRef]
- Kashetsky, N.; Feschuk, A.; Pratt, M.E. Post-inflammatory hyperpigmentation: A systematic review of treatment outcomes. J. Eur. Acad. Dermatol. Venereol. 2024, 38, 470–479. [Google Scholar] [CrossRef]
- Markiewicz, E.; Karaman-Jurukovska, N.; Mammone, T.; Idowu, O.C. Post-inflammatory hyperpigmentation in dark skin: Molecular mechanism and skincare implications. Clin. Cosmet. Investig. Dermatol. 2022, 15, 2555–2565. [Google Scholar] [CrossRef]
- Alexis, A.F.; Sergay, A.B.; Taylor, S.C. Common dermatologic disorders in skin of color: A comparative practice survey. Cutis 2007, 80, 387–394. [Google Scholar]
- Hu, J.K.; Quinonez, R.L.; Antasiuk, V.; Waibel, J. Treatment of acne vulgaris-associated post-inflammatory dyschromia with combination of non-ablative laser therapy and topical antioxidants. J. Drugs Dermatol. 2024, 23, 769–773. [Google Scholar] [CrossRef]
- Chiang, C.; Ward, M.; Gooderham, M. Dermatology: How to manage acne in skin of colour. Drugs Context 2022, 11, 2021-10-9. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.J.; Wan, X.C.; Bao, G.H. Brick dark tea: A review of the manufacture, chemical constituents and bioconversion of the major chemical components during fermentation. Phytochem. Rev. 2015, 14, 499–523. [Google Scholar] [CrossRef]
- Aloo, O.S.; Kim, D.G.; Vijayalakshmi, S.; Aloo, D.O.; Ochola, C.O.; Oh, D.H. Polyphenol constituents and impacts of fermented teas (Camellia sinensis) in human wellness. Food Biosci. 2024, 60, 104389. [Google Scholar] [CrossRef]
- Albouy, M.; Aubailly, S.; Jeanneton, O.; Marteau, C.; Sobilo, L.; Boulgana, R.; Bru, G. Skin-protective biological activities of bio-fermented Aframomum angustifolium extract by a consortium of microorganisms. Front. Pharmacol. 2023, 14, 1303198. [Google Scholar] [CrossRef]
- Lee, N.Y.; Jo, C.; Sohn, S.H.; Kim, J.K.; Byun, M.W. Effects of gamma irradiation on the biological activity of green tea byproduct extracts and a comparison with green tea leaf extracts. J. Food Sci. 2006, 71, C269–C274. [Google Scholar] [CrossRef]
- Lin, F.J.; Wei, X.L.; Liu, H.Y.; Li, H.; Xia, Y.; Wu, D.T.; Zhang, P.Z.; Gandhi, G.R. State-of-the-art review of dark tea: From chemistry to health benefits. Trends Food Sci. Technol. 2021, 109, 126–138. [Google Scholar] [CrossRef]
- Zhu, M.Z.; Li, N.; Zhou, F.; Ouyang, J.; Lu, D.M.; Xu, W.; Li, J.; Lin, H.Y.; Zhang, Z.; Xiao, J.B.; et al. Microbial bioconversion of the chemical components in dark tea. Food Chem. 2020, 312, 126043. [Google Scholar] [CrossRef]
- Deng, H.; Liu, J.; Xiao, Y.; Wu, J.L.; Jiao, R. Possible mechanisms of dark tea in cancer prevention and management: A comprehensive review. Nutrients 2023, 15, 3903. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, F.J.; Yang, Y.X. Research on the determination of total polyphenol content in grape spirits by Folin-Ciocalteu colorimetric method. China Brew. 2017, 36, 163–166. [Google Scholar]
- Petpiroon, N.; Rosena, A.; Pimtong, W.; Charoenlappanit, S.; Koobkokkruad, T.; Roytrakul, S.; Aueviriyavit, S. Protective effects of Thai silk sericins and their related mechanisms on UVA-induced phototoxicity and melanogenesis: Investigation in primary melanocyte cells using a proteomic approach. Int. J. Biol. Macromol. 2022, 201, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Shi, Y.; Yang, G.; Shi, J.; Ji, J.; Zhang, Y.; Wang, J.; Peng, Q.; Lin, Z.; Lv, H. Hypolipidaemic and antioxidant effects of various Chinese dark tea extracts obtained from the same raw material and their main chemical components. Food Chem. 2022, 375, 131877. [Google Scholar] [CrossRef]
- Peng, S.; Lin, L.; Zhao, M. A comparative study on the bioactivities and chemical compositions of Dancong summer tea and Anhua dark tea: Excavation of glycolipid-lowering functional factors. Food Res. Int. 2025, 204, 115825. [Google Scholar] [CrossRef] [PubMed]
- Serre, C.; Busuttil, V.; Botto, J.M. Intrinsic and extrinsic regulation of human skin melanogenesis and pigmentation. Int. J. Cosmet. Sci. 2018, 40, 328–347. [Google Scholar] [CrossRef]
- Yuan, I.H.; Jin, Z.H. Paracrine regulation of melanogenesis. Br. J. Dermatol. 2018, 178, 632–639. [Google Scholar] [CrossRef]
- Eves, P.C.; MacNeil, S.; Haycock, J.W. α-Melanocyte stimulating hormone, inflammation and human melanoma. Peptides 2006, 27, 444–452. [Google Scholar] [CrossRef]
- Wang, W.; Gao, Y.; Wang, W.W.; Zhang, J.Y.; Yin, J.F.; Le, T.; Xue, J.J.; Engelhardt, U.H.; Jiang, H.Y. Kojic Acid Showed Consistent Inhibitory Activity on Tyrosinase from Mushroom and in Cultured B16F10 Cells Compared with Arbutins. Antioxidants 2022, 11, 502. [Google Scholar] [CrossRef]
- Chen, Y.; Lian, N.; Chen, S.; Xiao, T.; Ke, Y.; Zhang, Y.; Song, C.; Yang, Y.; Xu, S.; Gu, H.; et al. Gsdme deficiency leads to the aggravation of UVB-induced skin inflammation through enhancing recruitment and activation of neutrophils. Cell Death Dis. 2022, 13, 841. [Google Scholar] [CrossRef]
- Kim, L.; Kim, J.Y. Chondroprotective effect of curcumin and lecithin complex in human chondrocytes stimulated by IL-1β via an anti-inflammatory mechanism. Food Sci. Biotechnol. 2019, 28, 547–553. [Google Scholar] [CrossRef]
- Wu, K.C.; Hseu, Y.C.; Shih, Y.C.; Sivakumar, G.; Syu, J.T.; Chen, G.L.; Lu, M.T.; Chu, P.C. Calycosin, a common dietary isoflavonoid, suppresses melanogenesis through the downregulation of PKA/CREB and p38 MAPK signaling pathways. Int. J. Mol. Sci. 2022, 23, 1358. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.C.; Kim, S.; Kim, J.H.; Lee, G.S.; Lee, J.N.; Lee, N.H.; Hyun, C.G. Pratol, an O-methylated flavone, induces melanogenesis in B16F10 melanoma cells via p-p38 and p-JNK upregulation. Molecules 2017, 22, 1704. [Google Scholar] [CrossRef] [PubMed]
- O’Rourke, S.A.; Shanley, L.C.; Dunne, A. The Nrf2-HO-1 system and inflammaging. Front. Immunol. 2024, 15, 1457010. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Ma, W.; Fan, D.; Hu, J.; An, X.; Wang, Z. The biochemistry of melanogenesis: An insight into the function and mechanism of melanogenesis-related proteins. Front. Mol. Biosci. 2024, 11, 1440187. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).